 Open Access
Open Access
ARTICLE
Observation of Flowering Process of Grape (Vitis vinifera L.)—Insight into the Starting of Pollination of Flower Hiding in Calyptras (Cap)
1 College of Horticulture and Landscape Architecture, Yangzhou University, Yangzhou, 225009, China
2
Shandong Academy of Grape, Shandong Academy of Agricultural Sciences, Jinan, 250100, China
* Corresponding Author: Zhaosen Xie. Email:
# These authors contributed equally to this work and should be considered co-first authors
Phyton-International Journal of Experimental Botany 2023, 92(6), 1859-1871. https://doi.org/10.32604/phyton.2023.027067
Received 12 October 2022; Accepted 09 January 2023; Issue published 11 April 2023
Abstract
It is generally agreed that many Vitis vinifera L. cultivars are self-fertile, where self-pollination often occurs before capfall in a process called cleistogamy. Therefore, it is difficult to identify the right time to remove stamens before self-pollination during the cross-breeding of grape. For this paper, we observed the process of grape flowering and measured the pollen viability and stigma receptivity of grape flowers of ‘Shine Muscat’ in order to identify the starting time of self-pollination before capfall and to provide useful information for improving the efficiency of cross-breeding. The results demonstrate that the anther is not cracked during the visible clusters and separated clusters stages. Meanwhile, in the separated floral buds, flowering begins, and full bloom stages, the pollen viability is 60.7%, 73.2% and 80.3%, respectively; however, at the berry set stage, pollen viability drops to zero. The top of the mature stigma is composed of a layer of nearly cylindrical papillary cells, and the stigma receptivity for pollen changes with the development of flowers: in particular, no reaction was observed in the visible clusters stage; weak positive reaction at the separated clusters stage; strong positive reaction at the separated floral buds, flowering begins, and full bloom stages; and no reaction at the berry set stage. In the separated floral buds stage, pollen tubes were seen germinating in the style. In the flowering begins stage, more pollen tubes were observed at the entry of the ovary. During the full bloom stage, most pollen tubes elongated into the ovary base and some entered the pearl hole. At the berry set stage, newborn endosperm nucleus could be seen in the ovule. From the above, we can conclude that the initiation time of closed fertilization for ‘Shine Muscat’ grape can be judged as the separated floral buds stage, and it is best to discard the stamen before the separated floral buds stage when conducting cross-breeding.Keywords
Flowering is an important part of the life cycle of plants. As the reproductive stage of plants, it can achieve the recombination of genetic material and perpetuation of the species. In higher plants, the flowering modes differ with plant species. Grapes are planted all over the world, mostly in the Northern Hemisphere. The flowering type and pollination mode of grapes have been considered as self-pollination by many researchers [1–5]. Many Vitis vinifera L. cultivars are self-fertile, and pollination occurs normally before buds open (Cleistogamy), or later as a tensile cap or calyptra abscises [6]. The shedding of the calyptra is called capfall, but direct pollen release occurs before floral opening for several grape cultivars [7]. For most table grape varieties, stigma receptivity occurs even before capfall, such that wet stigma can be observed before floral opening, and stigma receptivity can be observed in different floral development stages [8–10]. The enzymes in the stigma play an important role in the process of pollen grain germination and penetration of the pollen tube [11]. During flowering, the ability of the stigma to receive pollen can last for a few days, and stigma receptivity is enhanced with the development of stigmatic papillae [12]. Heazlewood et al. [13] have found that pollen was visible on the stigma surface before capfall, indicating that anthesis occurred whilst the calyptra was in place.
Understanding the floral morphology and biology of grapes is fundamental to determine the interactions between pollen grains and the stigma [14]. Although when pollen is transferred from anthers to stigmas has been discussed for Vitis vinifera L. cultivars [13], no study has been carried out on the start time of pollination and changes of flower morphological features in different flowering stages. Study of the flowering process of grapes is helpful not only to improve breeding efficiency, but also to provide useful information for seedless grape berry production. The aims of this study were to characterize the pistil and stamen morphology of ‘Shine Muscat’, as well as to determine the starting time of grape cleistogamy through evaluating differences in floral morphology.
2.1 Plant Materials and Growth Conditions
We used grapevines Vitis vinifera L. cv. ‘Shine Muscat’ (planted in 2018) for sample collection during the flowering season in April of 2021 and 2022. Grapevines were grown in a greenhouse in Jinniu mountain experimental vineyard of Taian City, Shandong Province, China (36.200 N, 117.088 E), with an average elevation of 153 m and an average annual air temperature of 12.9°C.
2.2 Defining of Grape Flowering Stages
According to the morphological characteristics of grape clusters and flowers, the flowering stages were classified into six developmental stages (Fig. 1): Stage 1, visible cluster; stage 2, separated clusters; stage 3, separated floral buds; stage 4, flowering begins; stage 5, full bloom; and stage 6, berry set [15,16].
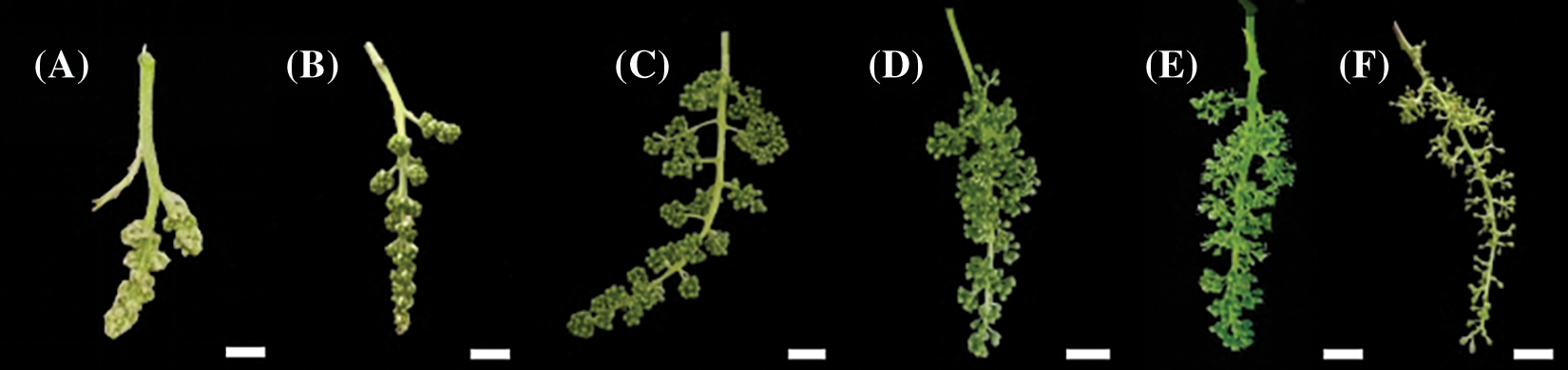
Figure 1: Phenology of the ‘Shine Muscat’ grape flower. (A) Visible clusters: inflorescence clear, five leaves separated; (B) Separated clusters: single flowers in compact groups, eight leaves separated; (C) Separated floral buds: single flowers separated, flower caps still in place, but cap color fading from green, 14 leaves separated; (D) Flowering begins: beginning of flowering, about 16 leaves separated; (E) Full bloom: 50% of caps off, 17–20 leaves separated; (F) Berry set: young berries growing, bunch at right angles to stem. Scale bar = 1 cm
Inflorescences were collected, from the experimental vineyard, from visible cluster (stage 1) to berry set (stage 6). Thirty biological replicates were taken for each sample. We observed the floral organs using an Olympus SZX16 light microscope [17].
2.4 Scanning Electron Microscopy (SEM)
For morphological and anatomical characterization, thirty stigmas were collected from different inflorescences of each stage. The stigmas and anther were fixed in 2.5% glutaraldehyde (buffered with 0.1 M sodium cacodylate). After dehydration in an ethanol series (50%, 75%, 90%, and 100%), the material was submitted to critical point drying. Subsequently, the samples were mounted on metal stubs and sputter-coated with gold, and finally analyzed under a scanning electron microscope (Gemini SEM 300, Carl Zeiss, Germany). Thirty stigmas of each sample were observed and the images of stigmas were analyzed using the ImageJ software [18].
The stigmas were immersed in a solution of reaction mixture (1% benzidine, 3% hydrogen peroxide, and distilled water, 4:11:22 v/v/v) for 3 min. When bubbles appeared around the stigmas, the number of bubbles was recorded, which reflects the strength of stigma receptivity [19,20]. Stigma receptivity was denoted by four grades: (−) no reaction, (+) weak positive reaction, (++) strong positive reaction, and (+++) very strong positive reaction.
Triphenyltetrazolium chloride (TTC) test procedures were used to measure pollen viability. First, the TTC solution was prepared with 0.2 g triphenyltetrazolium chloride, 12 g sucrose, and 20 ml distilled water [21]. Then, 200 μl TTC dye solution was transferred to a PVP tube, into which pollen had been added in advance, and incubated in 37°C water for 20 min. Finally, pollen viability was examined using a light microscope. The viability of pollen was scored according to staining levels: Bold red color, viable; light red color, semi-viable; and yellowish-green color or colorless, non-viable.
In the four development stages of grape flowers, 30 florets were selected for anatomical observation [22]. The samples were fixed in FAA (70% ethanol:acetic acid:formalin, 18:1:1 v/v/v). The samples were dehydrated in increasing concentrations of ethanol (50%, 75%, 90%, and 100%), for 30 min in each ethanol solution. The samples were then embedded in paraffin and 10 μm sections were prepared using a rotary microtome (RM 2125 RTS, Leica, Heidelberg, Germany). The sections were mounted on a glass slide and stained with 1% safranin and 1% fast green. The staining times were 5 min for safranin and 1 min for fast green [23]. Longitudinal section images of grape flowers are presented in Fig. 2.
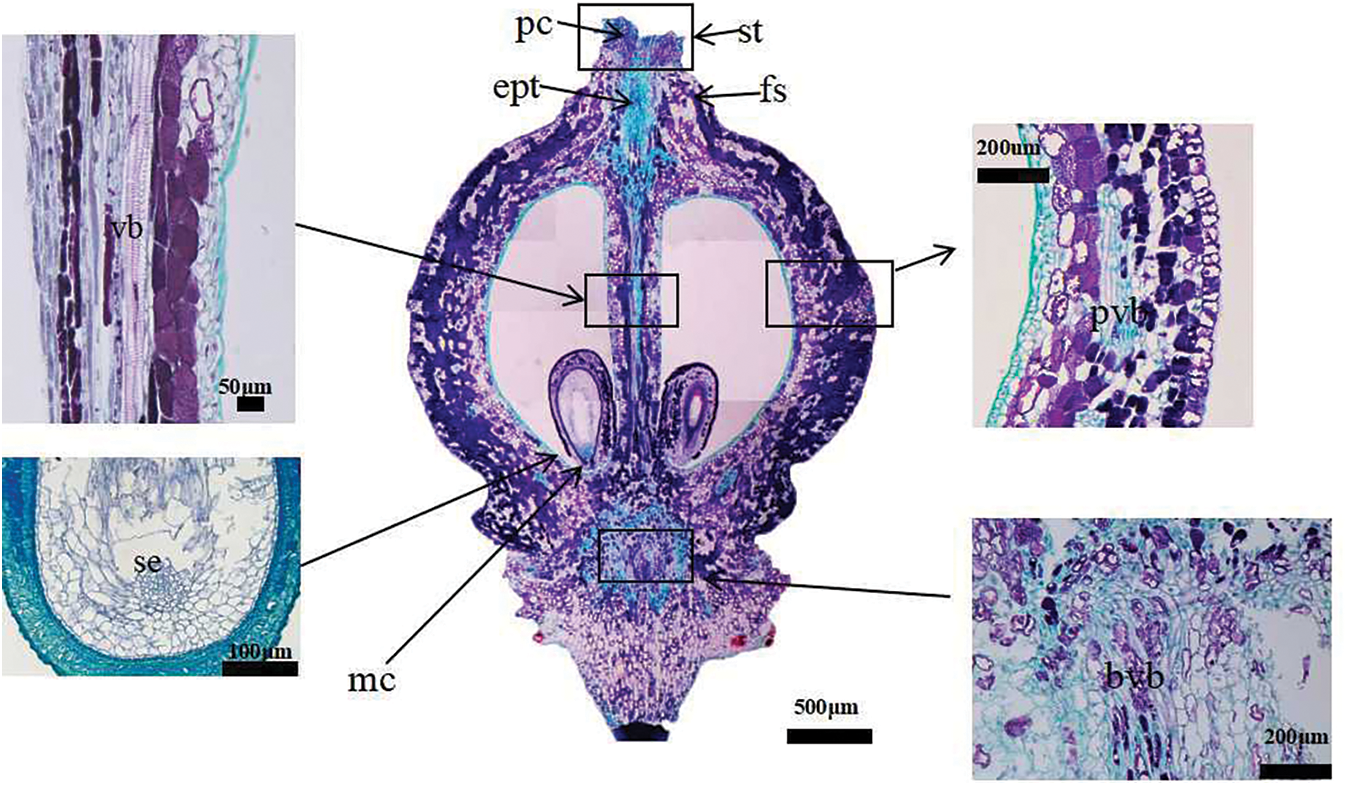
Figure 2: Vertical section diagram of grape single flower structure. At the berry set stage, there is no pollen tube in the style, and the embryo sac and nucellus are well-developed. The inner integument forms a tubular tip near the micropyle, and is twisted. The longitudinal section of ovary shows many vascular bundles. fs: floral style. pc: papillary cell. ept: elongated pollen tube (pollen tube grows in style). st: stigma. vb: central vascular bundle. pvb: peripheral vascular bundles. bvb: brush vascular bundles. mc: micropyle. se: spherical embryonic
2.8 Pollen Tubes in the Ovary and Fertilization
Pollen germination and pollen tube growth were observed by fluorescence microscopy. Samples were prepared for fluorescence microscopy following previously developed methods [24]. A total of 30 pistils were sampled for each stage of flowering. The pistils were put in FAA (70% ethanol:acetic acid:formalin, 18:1:1 v/v/v) for 24 h, then rinsed in distilled water for 5 min, softened for 8–10 h in 5% NaOH, and rinsed again for 4–6 min in distilled water. Finally, the pistils were stained by 0.1% water-soluble aniline blue for 12–24 h. The samples were then covered with cover slips and softly squashed. In each of the five styles, pollen tube growth was followed to the ovules under microscope (Nikon UY200).
3.1 Anatomical Structure of Flower Organs
3.1.1 Dynamic Observation of Stamen Morphological Development
During the visible clusters stage, single flowers can be seen clearly in clusters, but all individual flowers remain close together and, therefore, are difficult to separate out (Fig. 3A). The caps are embedded in the cephalophorum (Fig. 3B), the color of the anther is green, and there is no dehiscence on the surface of anther. Pollen grains are stored in the anther locule. The pollen sac is symmetrical, decoration is clearly visible, and the longitudinal crack is deep in this stage (Fig. 3C). During the separated clusters stage, the single flowers gradually separate (Fig. 3D), and the cap structure is fully developed and becomes longer (Fig. 3E). The color of the anther is yellow–green; the pollen sac is symmetrical, full, and swollen; the sac dent is clearly visible; and the longitudinal crack is deep (Fig. 3F). During the separated floral buds stage, single flowers are completely separated (Fig. 3G), the color of the cap is yellow (Fig. 3H), and some pollen sacs begin to crack longitudinally (Fig. 3I). During the flowering begins stage, the inflorescence continues to separate and grow (Fig. 3J) and the flower caps start to fall off from flowers (Fig. 3K). The color of the anthers is yellow, a large number of pollen sacs are symmetrical and longitudinally split, and yellow pollen grains are found everywhere on the anther locule (Fig. 3L). During the full bloom stage, the inflorescences continue to grow (Fig. 3M) and most of the flower caps fall off (Fig. 3N). Many pollen grains are released from the anthers (Fig. 3O). During the berry set stage, the ovary starts to swell (Fig. 3P) and single flowers change into berries (Fig. 3Q). The anthers start to undergo dehydration, and only a few pollen grains can be seen (Fig. 3R).
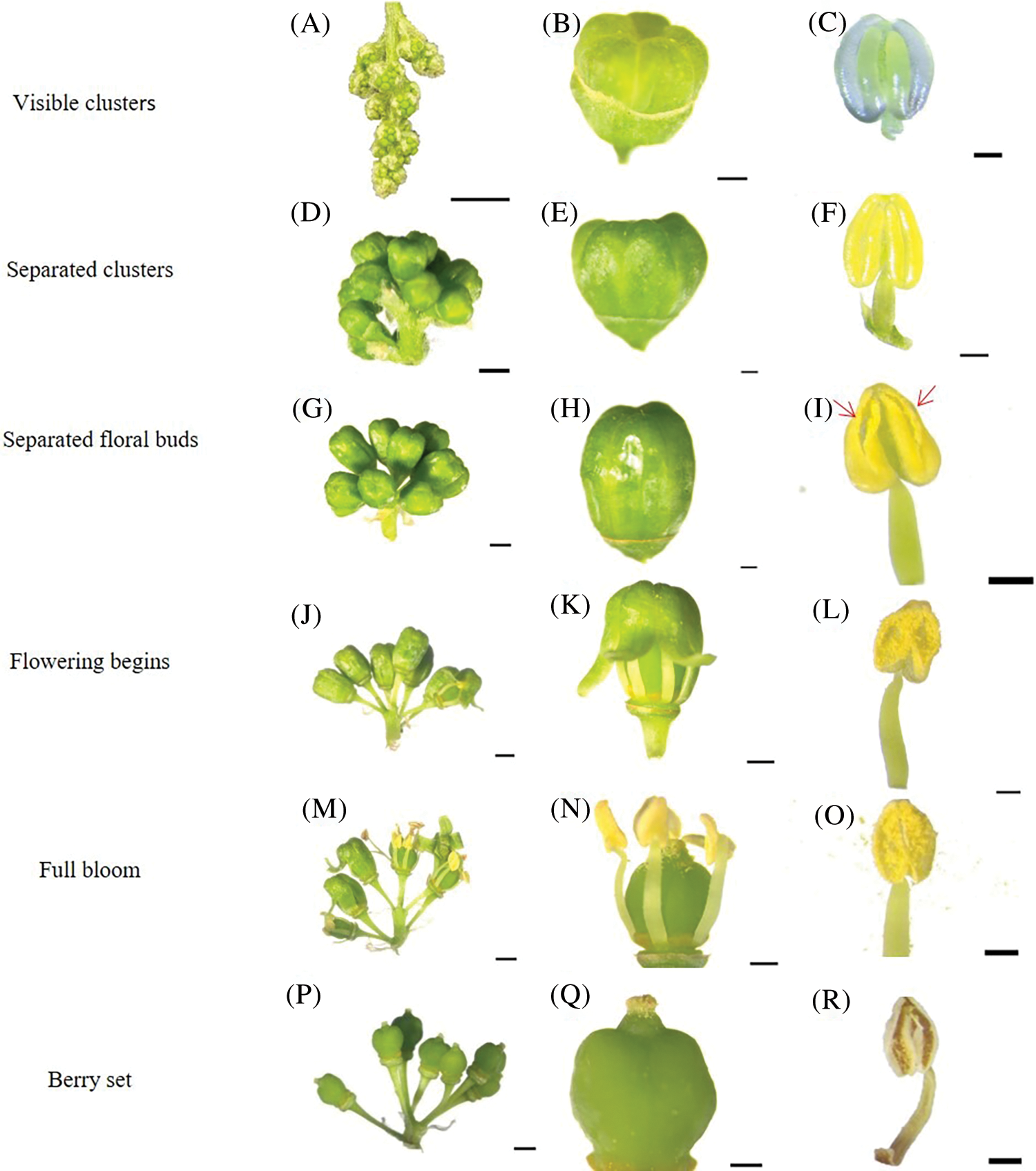
Figure 3: Morphological development of stamens of ‘Shine Muscat’ grape: (A) Inflorescence state during the visible cluster stage; (B) The caps embedded in cephalophorum; (C) The anther is green, pollen sac is symmetrical, decoration is clearly visible, and longitudinal crack is deep; (D) The single flower gradually separates; (E) The cap structure is fully developed and becomes longer; (F) The anther is yellow green, the pollen sac dent is clear, and the longitudinal crack is deep; (G) The single flowers are completely separated; (H) The color of the cap starts changing to yellow; (I) Pollen grains naturally disperse, the flower cap is rolled out; (J) Beginning of flowering; (K) The flower caps start to drop off; (L) Pollen sacs are longitudinally split, and yellow pollen is scattered; (M) Separation of all corolla and receptacle; (N) During the full bloom stage, most of the flower caps fall off; (O) A lot of pollen is scattered around; (P) During the Berry set stage, the single flowers change into berries; (Q) The stigma is dry and brown; (R) Anther senescence
Through observation of anther morphology of the ‘Shine Muscat’ grape by light microscopy, the results indicated that the anthers gradually become mature from stage 3 (Fig. 3); at this stage, the pollen sacs dehisce and the pollen grains are scattered. The mature pollen grains were nearly spherical in polar view, but elliptical in equatorial view. The arrangement of meshes was irregular, with different sizes and uneven distribution. There is a germinating hole in the center of each germinating furrow; the protrusion on the germinating hole is the cytoplasmic sac of the germinating hole (Fig. 4A), the germinating furrow is trifid (Fig. 4B), the groove mark extends to the two poles in a strip shape, the abortive pollen grains present a collapsing structure, and the germinating furrow is seriously depressed (Fig. 4C).

Figure 4: Pollen grain structure of ‘Shine Muscat’ grape: (A) Equatorial view; (B) Polar view; and (C) Abnormal pollen grain morphology. GA: germinal aperture; GF: germinal furrow; EO: exine oration
3.1.2 Dynamic Observation on the Morphological Development of Pistil
Stigma morphology was observed during the six stages. The stigma is covered with papillary cells. With the development of the pistil, the papillae gradually form elongated cells which can accept pollen grains. In addition, the morphological characteristics of the stigma present great changes in color and shape during different flower stages. At the visible clusters stage, the stigma is yellowish-white, no mucus is seen on the stigma, and no pollen grains on it (Fig. 5A). The whole stigma is round, with large intermediate pores in the middle (Fig. 5B), while the papillary cells are small and flat (Fig. 5C). At the separated clusters stage, the stigma is yellowish-green and wet, with a small amount of mucus on it, but still no pollen on it (Fig. 5D). At this stage, the middle hole is still observed, but smaller than in the visible clusters stage. The papillary cells present a smooth and neat structure (Figs. 5E and 5F). At the separated floral buds stage, the stigma is bright white (Fig. 5G). In this stage, the papillary cells on the stigma have fully developed, the surface of the papillary cells being elongated upward (Fig. 5I). With the continuous development of papillary cells, the stigma becomes cylindrical and the middle pore disappears (Fig. 5H). In addition, there is a small amount of pollen grains on the stigma. At the flowering begins stage, the color of the stigma is white, with a large amount of mucus secretion. There are a large number of pollen grains on the ovary wall and stigma (Fig. 5J). The whole stigma is cylindrical, and the cell gaps among papillary cells become larger (Fig. 5K). The size of papillary cells is similar to that in the previous stage, with loose arrangements (Fig. 5L). At the full bloom stage, the color of the stigma is yellow–white, with a large amount of mucus secretion. There are a large number of pollen grains attached to the ovary wall and stigma (Fig. 5M). With the appearance of papillary cells collapsing, the papillary cells start to dry up (Fig. 5N). The surface of papillary cells begins to shrink, and the papillary cells become long and cylindrical (Fig. 5O). These results indicate that some papillary cells have finished water absorption and expansion. In the berry set stage, the color of the stigma is brown, with no mucus secretion, and there are no pollen grains on the ovary wall and stigma (Fig. 5P). The stigma surface is uneven, with no intermediate hole (Fig. 5Q), and the papillary cells collapse and stick together at this stage (Fig. 5R).
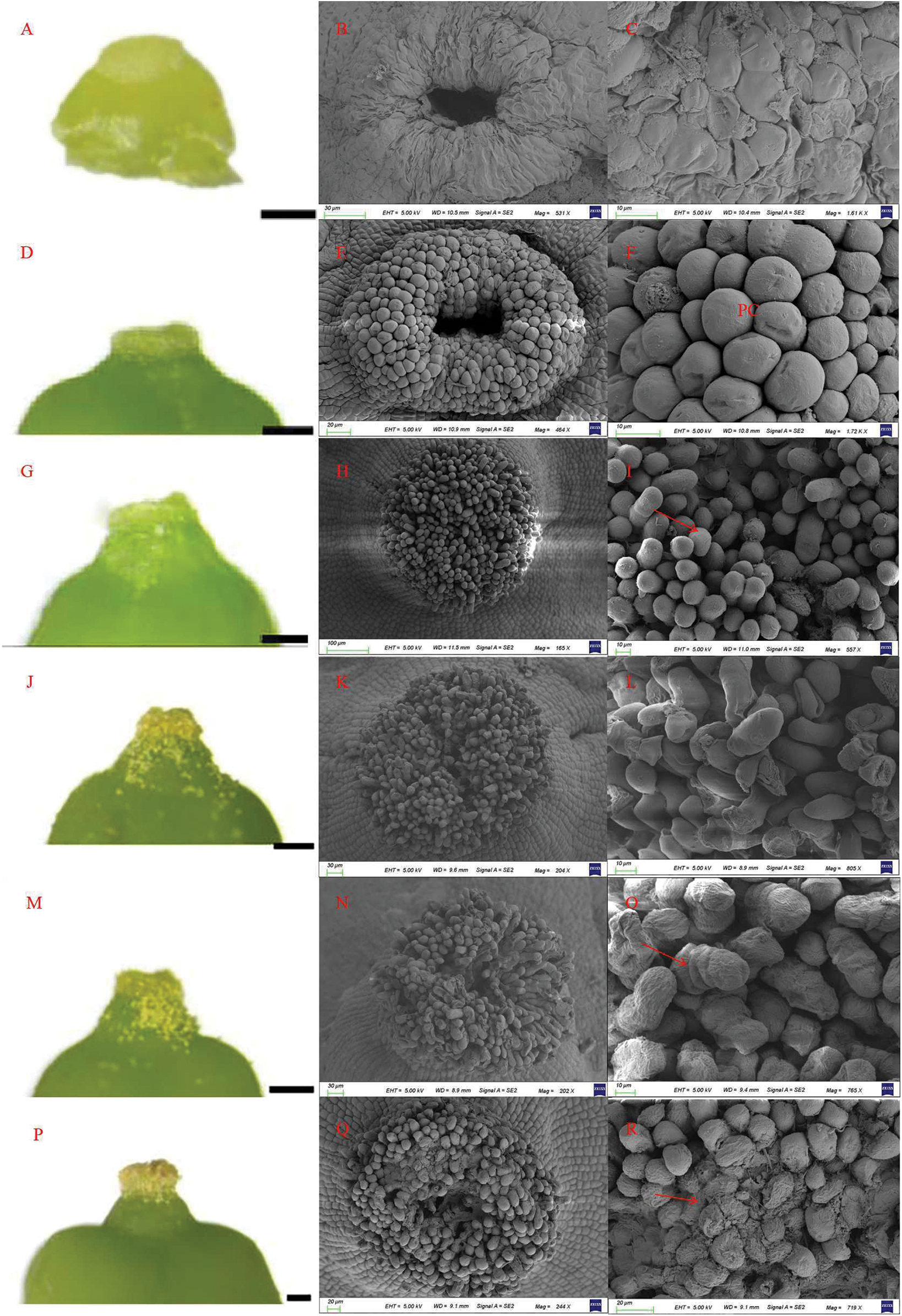
Figure 5: Morpho-anatomy of the stigma and papillary cells in ‘Shine Muscat’ grapes: (A–C) Visible clusters stage, beginning to exhibit cell morphology (A). Observed by light microscope (A, D, G, J, M, P) and papillary cells observed by scanning electron microscope (B, C, E, F, H, I, K, L, N, O, Q, R); (D–F) Separated clusters stage (pc: papillary cell); (G–I) Separated floral buds stage, papillary cells at maturity; (J–L) Bloom beginning; (M–O) Full bloom, papillary cells shrinking; (P–R) Berry set, papillary cells shriveled and disintegrated
With the development of all parts of grape flowers, the length and width of the ovary gradually increase from the visible clusters stage to the berry set stage (Fig. 6). The length and width of the ovary are 478.339 and 579.954 μm, respectively, in the visible clusters stage. In the separated clusters stage, the length and width of ovary increased significantly, with length of 1233.070 μm and width of 1238.930 μm. In the separated floral buds stage, the ovary length and width are 1766.728 and 1424.062 μm, respectively (notably, at this stage, the length of the ovary is larger than the ovary width). From the separated clusters to the full bloom stage, the ovary width increases slowly, but the ovary size reaches a maximum at the berry set stage.
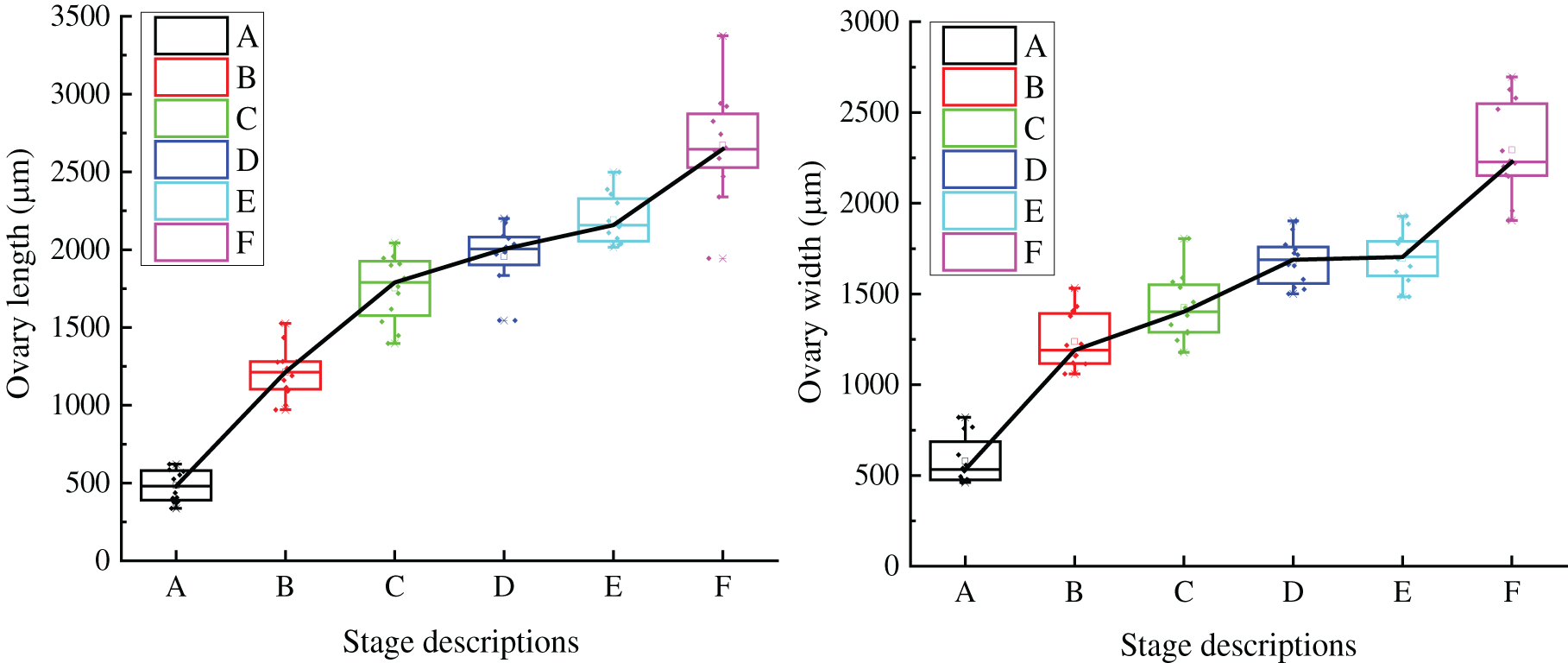
Figure 6: Ovary growth trend of single flower of ‘Shine Muscat’ grape: (A) Visible clusters stage; (B) Separated clusters stage; (C) Separated floral buds stage; (D) Flowering begins stage; (E) Full bloom stage; and (F) Berry set stage
3.2 Quality of Pollen Grains in Flowers
During the flowering process, pollen viability presented great differences among the different stages (Table 1). There was no pollen falling out of anther at the visible clusters and the separated clusters stages. At the separated floral buds stage, pollen grains fell out from the anther locule, and the pollen viability was 60.7%. With advancing flowering stage, a large number of anthers cracked, pollen grains were released from the anther, and the viability of pollen increased gradually. At the flowering begins and full bloom stages, the pollen viability was 73.2% and 80.3%, respectively; however, at the berry set stage, the pollen viability decreased sharply and, at the end of the stage, the pollen viability was zero.
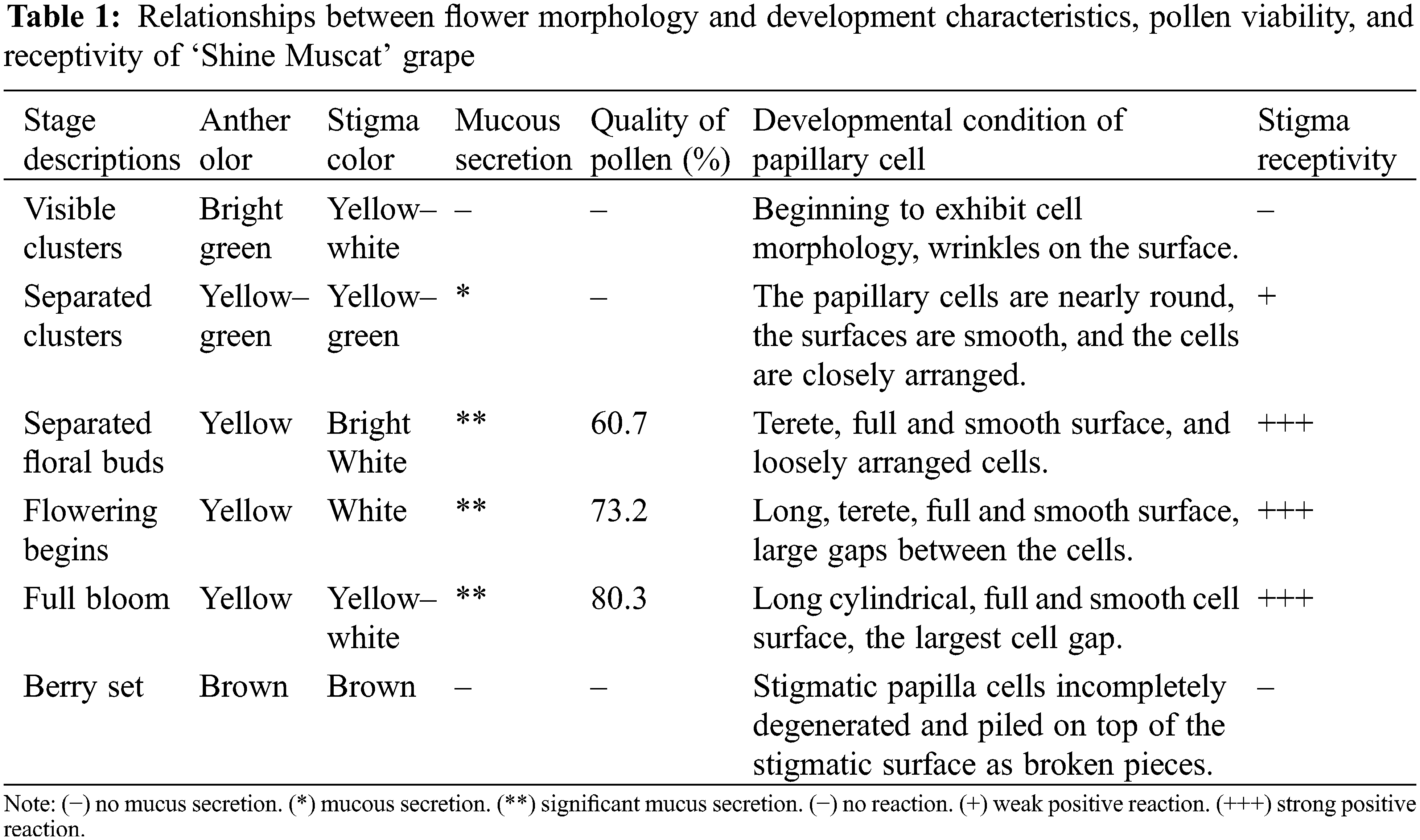
During the visible clusters stage, no bubbles were seen on the stigma, suggesting that the stigma is not receptive to pollen at this stage (Fig. 7A). At the separated clusters stage, there was mucus secretion on the stigma, and there were about 5 bubbles on a single stigma, indicating weak positive reaction to pollen (+) (Fig. 7B). From the separated floral buds stage to the full bloom stage (Figs. 7C–7E), a large amount of mucus secretion could be seen on the stigma, and the number of bubbles on the stigma also increased gradually, suggesting that the stigma has strong receptivity to pollen at these stages. At the berry set stage (Fig. 7F), the stigma is dry and without mucus secretion, and no bubbles were observed on the stigma, indicating that the stigma loses receptivity to pollen at this stage. According to the above results, the stigma of grape flowers is receptive to pollen before capfall.
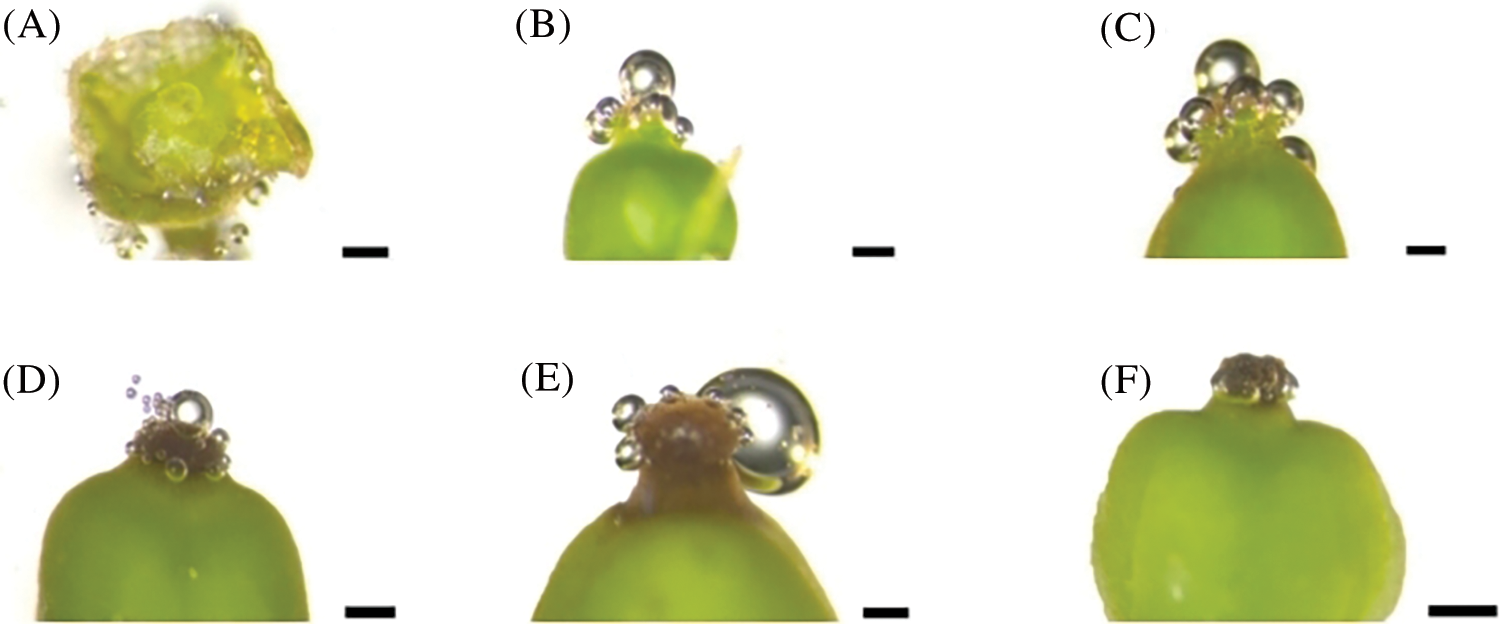
Figure 7: Evaluation of stigma receptivity of ‘Shine Muscat’ grape with hydrogen peroxide: (A) Visible clusters stage, no reaction (−); (B) Separated clusters stage, weak positive reaction (+); (C, D, E) Separated floral buds stage, flowering begins stage, and full bloom stage, Strong positive reaction (+++); and (F) Berry set stage, no reaction (−). Scale bar = 500 μm
3.4 In Vivo Pollen Tube Growth and Ovule Fertilization Process
We observed the development of ovules, embryo sacs, and primary embryo mammary nucleus in four flowering stages. At each stage, there was one inverted ovule in each chamber, located on both sides of the ovary base (Fig. 2). In the visible clusters and separated clusters stages, there was no pollen tube in the style. At the separated floral buds stage, there was obvious pollen tube guide tissue in the center of the stigma (Fig. 8A), the structure of ovule and embryo sac was intact, indicating that they are active to enter next development stage (Fig. 8E). At the same time, pollen tubes begin to germinate (Figs. 8I and 8K). At the flowering begins stage, the arrangement of papilla cells on the stigma appeared relatively loose. Many pollen tubes guide tissues were observed in the center of stigma (Fig. 8B), the size of embryo sac gradually increased at this stage (Fig. 8F), and a large number of pollen tubes elongated into the ovary, most reaching to one-third of the length of the style (Fig. 8J). With the number of pollen tubes increasing in the style, some of them reached the base of the ovary (Fig. 8C). In Fig. 8L, the pollen tubes can be seen to enter the ovule at last (Fig. 8L). At this time, the ovule started to grow, but no split zygote embryo is seen (Fig. 8G). In the berry set stage, the papillary cells shrivel and disintegrate, and the central guide tissue of the stigma was severely depressed (Fig. 8D). The primary endosperm nucleus and cytoplasm gradually form in this stage (Fig. 8H).
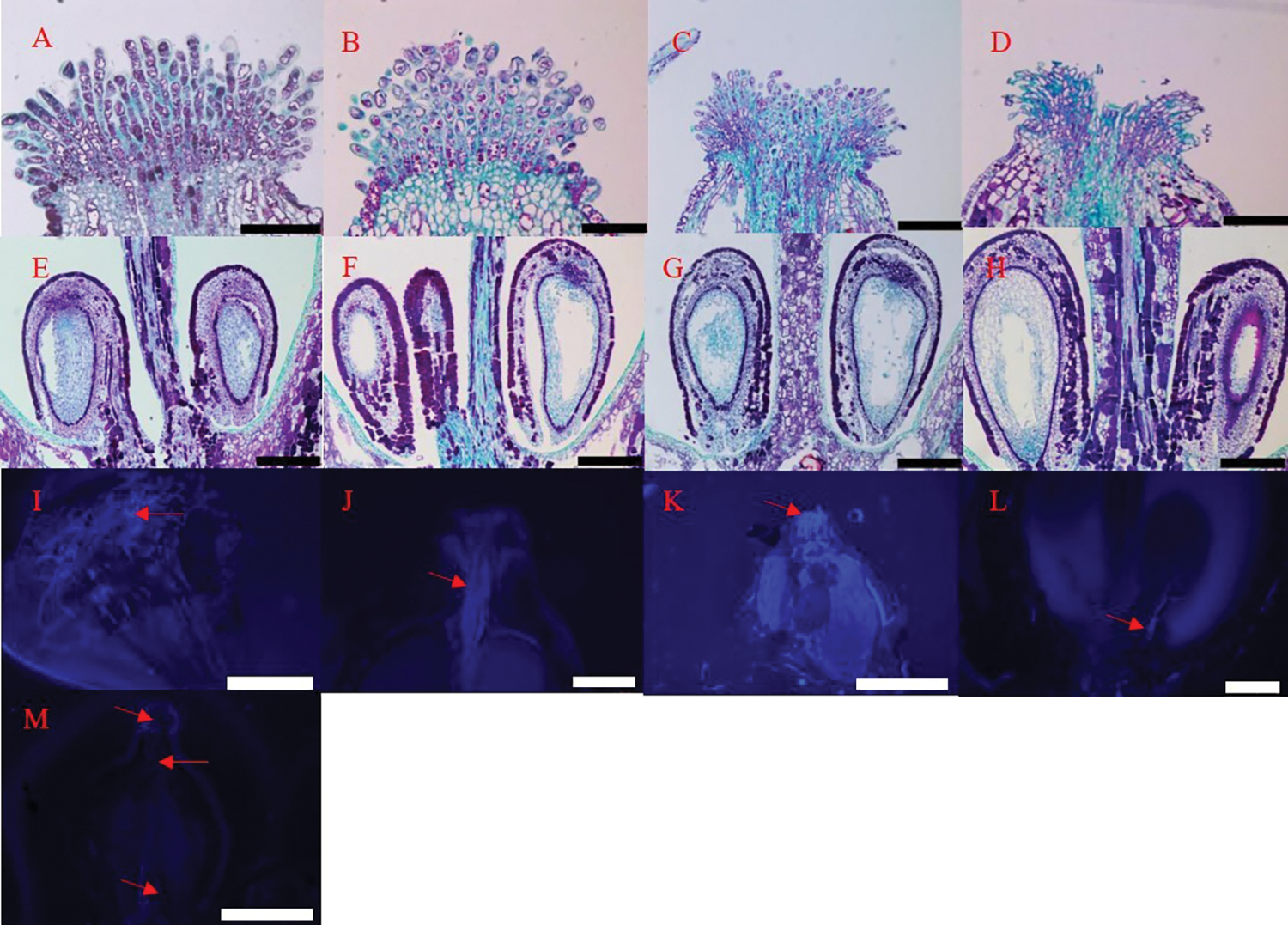
Figure 8: Fertilization and ovule development of ‘Shine Muscat’ grape flower: (A, E) Separated floral buds stage; (B, F) Flowering begins stage; (C, G) Full bloom stage; (D, H) Berry set stage; (I, K) Pollen sprouted pollen tubes on the stigma, which elongated toward the style; (J) Pollen tube germination to one-third the length of the style; (L) Pollen tube into ovule; (M) No pollen tube germinated in stigma and style. Scale bars: (A)–(J), L = 100 μm; K = 1 mm
Cleistogamy in grape is a common biological phenomenon, caused by the early maturation of sexual organs. Many studies have suggested that the male and female gametophytes of grape mature before anthesis, providing the conditions for cleistogamy [8,25]. The morphological characteristics of grape anthers differ between varieties [26]. The pollen grains of Vitis are oblong and spherical [13], and the pollen grains of some seedless grape varieties are also oblong and nearly circular in polar view [27]. He and Zhang have found that the pollen and the female gametophyte of grape matured 2–3 and 1–2 days before blooming, respectively, and that pollen was released to stigma in the flower bud at 1–2 days before blooming [28]. In this study, we found that, during the visible clusters and separated clusters stages, the cap had not fallen off and the anther was not dehiscent, such that the pollen grains were still in the anther locule; however, after the flowering begins stage, the caps fell off from the flower and a large number of pollen grains were seen on the stigma. Therefore, cleistogamy does occur in ‘Shine Muscat’.
The stigma morphology and stigma receptivity of grape flowers change regularly during different developmental stages of flower. The closer to flowering, the stronger the stigma receptivity becomes, while the receptivity of the stigma first enhances, then weakens and, finally, disappears [29,30]. It has been found that the development of stigma in Bromeliaceae and Almond during pollination affects fertilization [30,31]. Assessment of stigma morphology can allow for determination of the best pollination stage of stigma [32]. The cessation of stigma receptivity and rupture of the papillary cell occurs simultaneously [33]. The top surface of the stigma is composed of many papillary cells, which have strong receptivity during flowering. It is generally believed that the stigma surface is uneven, and that growth of the papillary cells is conducive to receive more active pollen grains. The pollen grains enter the style along the thin-walled cell space of the stigma [34–36]. The papillary cells of ‘Shine Muscat’ grape were long and cylindrical while, at the berry set stage, they shriveled and disintegrated. With the development and maturation of papillary cells on the stigma surface, stigma receptivity was also active during the separated clusters stage. The strongest level of stigma receptivity was observed in the separated floral buds, flowering begins, and full bloom stages. These results indicate that stigma receptivity is closely related to the development of papillary cells on the stigma surface. Therefore, the start time of grape cleistogamy can be judged according to the development stage and receptivity of papillary cells.
In the flowering season of grapes, there exists a certain relationship between grape fertilization and embryo development. The style is the main channel for pollen tubes to enter the ovary. The pollen germinates on the stigma, and the pollen tube expands into the style through the stigma guiding tissue, finally expanding into the ovule, as observed through microscopy. In this study, we found that no pollen tube germinated on the stigma during the visible clusters stage and the separated floral buds stage; meanwhile, in the separated floral buds stage, pollen grains began to fall on the stigma, absorb water, and expand, and a few pollen tubes sprouted and fertilized. This result corroborates that the separated floral buds stage is the starting time of the fertilization process. These results confirm the fact that self-pollination of grape flowers usually occurs before flower capfall [13].
The results of this study provide useful information for potential agronomic research on the starting time of pollination of grape flowers hiding in calyptras, which is helpful for grapevine cross-breeding. The flowering process of the cultivar ‘Shine Muscat’ was documented before the opening of the flower, and it was speculated that the separated floral buds stage is the initiation of cleistogamy, which provides a theoretical basis for future research to maximize the quality of cross-breeding while reducing the labor and time requirements in the process.
Acknowledgement: The authors fully appreciate the editors and all anonymous reviewers for their constructive suggestions and comments to improve this manuscript.
Funding Statement: This work was supported by the Grant 31872050 from the National Natural Science Foundation of China, the Agricultural Seed Engineering of Shandong Province (Grant No. 2020LZGC008), the Agricultural Science and Technology Innovation Project for Shandong Academy of Agricultural Sciences (Grant No. CXGC2022A13), and the Key R&D Program of Linyi City (Grant No. 2020ZX010).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Zhaosen Xie, Yue Wang, Xiujie Li; data collection: Zhonghui Cai; analysis and interpretation of results: Bo Li, Yusen Wu; draft manuscript preparation: Yue Wang. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Longbottom, M. L., Dry, P. R., Sedgley, M. (2008). Observations on the morphology and development of star flowers of Vitis vinifera L. cvs Chardonnay and Shiraz. Australian Journal of Grape and Wine Research, 14(3), 203–210. [Google Scholar]
2. Vasconcelos, M. C., Greven, M., Winefield, C. S., Trought, M. C. T., Raw, V. (2009). The flowering process of Vitis vinifera: A review. American Journal of Enology and Viticulture, 60(4), 411–434. https://doi.org/10.5344/ajev.2009.60.4.411 [Google Scholar] [CrossRef]
3. Coito, J. L., Silva, H. G., Ramos, M. J. N., Cunha, J., Eiras-Dias, J. et al. (2019). Vitis flower types: From the wild to crop plants. PeerJ, 7(1), e7879. https://doi.org/10.7717/peerj.7879 [Google Scholar] [PubMed] [CrossRef]
4. García-Breijo, F., Armiñana, J. R., Garmendia, A., Cebrián, N., Beltrán, R. et al. (2020). In vivo pollen tube growth and evidence of self-pollination and prefloral anthesis in cv. Macabeo (Vitis vinifera L.). Agriculture, 10(12), 647. [Google Scholar]
5. Boss, P. K., Buckeridge, E. J., Poole, A., Thomas, M. R. (2003). New insights into grapevine flowering. Functional Plant Biology, 30(6), 593–606. https://doi.org/10.1071/FP02112 [Google Scholar] [PubMed] [CrossRef]
6. Mullins, M. G., Bouquet, A., Williams, L. E. (1992). Biology of the grapevine. Cambridge: Cambridge University Press. [Google Scholar]
7. Sampson, B., Noffsinger, S., Gupton, C., Magee, J. (2001). Pollination biology of the muscadine grape. HortScience, 36(1), 120–124. https://doi.org/10.21273/HORTSCI.36.1.120 [Google Scholar] [CrossRef]
8. Kowalczyk, B. A., Bieniasz, M., Kostecka-Gugała, A. (2022). Flowering biology of selected hybrid grape cultivars under temperate climate conditions. Agriculture, 12(5), 655. https://doi.org/10.3390/agriculture12050655 [Google Scholar] [CrossRef]
9. Heslop-Harrison, Y., Shivanna, K. R. (1977). The receptive surface of the angiosperm stigma. Annals of Botany, 41(6), 1233–1258. https://doi.org/10.1093/oxfordjournals.aob.a085414 [Google Scholar] [CrossRef]
10. Knox, R. B. (1984). Pollen-pistil interactions. In: Cellular interactions, pp. 508–608. Berlin, Heidelberg: Springer. [Google Scholar]
11. Kulloli, S. K., Ramasubbu, R., Sreekala, A. K., Pandurangan, A. G. (2010). Cytochemical localization of stigma-surface esterase in three species of Impatiens (Balsaminaceae) of Western Ghats. Asian Journal of Experimental Sciences, 1, 106–111. [Google Scholar]
12. Yi, W., Law, S. E., McCoy, D., Wetzstein, H. Y. (2006). Stigma development and receptivity in almond (Prunus dulcis). Annals of Botany, 97(1), 57–63. https://doi.org/10.1093/aob/mcj013 [Google Scholar] [PubMed] [CrossRef]
13. Heazlewood, J. E., Wilson, S. (2004). Anthesis, pollination and fruit set in Pinot Noir. Vitis, 43(2), 65–68. [Google Scholar]
14. Lenzi, M., Orth, A. I. (2004). Floral biology of Schinus terebinthifolius raddi (Anacardiaceae) in sandbank areas of santa catarina island, Brazil. Biotemas, 17, 67–89. [Google Scholar]
15. Lebon, G., Duchene, E., Brun, O., Clément, C. (2005). Phenology of flowering and starch accumulation in grape (Vitis vinifera L.) cuttings and vines. Annals of Botany, 95(6), 943–948. https://doi.org/10.1093/aob/mci108 [Google Scholar] [PubMed] [CrossRef]
16. Coombe, B. G. (1995). Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Australian Journal of Grape and Wine Research, 1(2), 104–110. https://doi.org/10.1111/j.1755-0238.1995.tb00086.x [Google Scholar] [CrossRef]
17. Rasband, W. S. (1997–2012). ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA. http://imagej.nih.gov/ij/ [Google Scholar]
18. van der Pluym, A., Hideux, M. J. (1977). Application d’une méthodologie quantitative à la palynologie d’Eryngium maritimum (Umbelliferae). Plant Systematics and Evolution, 127(2), 55–85. https://doi.org/10.1007/BF00984142 [Google Scholar] [CrossRef]
19. Dafni, A. (1992). Pollination ecology: A practical approach. New York, USA: Oxford University Press. [Google Scholar]
20. Dafni, A., Maués, M. M. (1998). A rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction, 11(3), 177–180. https://doi.org/10.1007/s004970050138 [Google Scholar] [CrossRef]
21. Nortin, J. D. (1966). Testing of plum pollen viability with tetrazolium salts. Journal of the American Society for Horticultural Science, 89, 132–134. [Google Scholar]
22. Xie, Z. S., Forney, C. F., Cao, H. M., Li, B. (2014). Changes in water translocation in the vascular tissue of grape during fruit development. Pakistan Journal of Botany, 46, 483–488. [Google Scholar]
23. Heslop-Harrison, J. (1987). Pollen germination and pollen-tube growth. In: International review of cytology, vol. 107, pp. 1–78. USA: Academic Press. [Google Scholar]
24. Abdelgadir, H. A., Johnson, S. D., van Staden, J. (2012). Pollen viability, pollen germination and pollen tube growth in the biofuel seed crop Jatropha curcas (Euphorbiaceae). South African Journal of Botany, 79, 132–139. https://doi.org/10.1016/j.sajb.2011.10.005 [Google Scholar] [CrossRef]
25. Meneghetti, S., Calò, A., Gardiman, M. (2006). Flower biology of grapevine. A review. Advances in Horticultural Science, 20, 317–325. [Google Scholar]
26. Cabello Saénz Santa María, F., de Luis Villota, P., Tortosa Tortola, M. E. (1994). Palynological study of the pollen grain of Vitis vinifera L. cultivars. Some aspects of sculpturing and pollination. Vitis, 33(2), 57–61. [Google Scholar]
27. Burçak, İ. Ş. Ç. İ. (2021). Pollen characteristics of some grape cultivars (Vitis vinifera L.). International Journal of Agriculture Environment and Food Sciences, 5(3), 279–286. [Google Scholar]
28. He, P. C., Zhang, Y. L. (1994). Studies on the gametophytic development and cleistogamy of grapes. Acta Horticulturae Sinia, 21(3), 227–230. [Google Scholar]
29. Wang, L., Liu, L., Zhang, L., Wang, Y., Lian, W. et al. (2011). Stigma receptivity, stigma morphology and fruit set of yantai sweet cherry (Cerasus avium). Chinese Bulletin of Botany, 46(1), 44–49. https://doi.org/10.3724/SP.J.1259.2011.00044 [Google Scholar] [CrossRef]
30. Yi, W., Law, S. E., McCoy, D., Wetzstein, H. Y. (2006). Stigma development and receptivity in almond (Prunus dulcis). Annals of Botany, 97(1), 57–63. https://doi.org/10.1093/aob/mcj013 [Google Scholar] [PubMed] [CrossRef]
31. Brown, G. K., Gilmartin, A. J. (1984). Stigma structure and variation in Bromeliaceae-neglected taxonomic characters. Brittonia, 36(4), 364–374. [Google Scholar]
32. Uwate, W. J., Lin, J. (1981). Development of the stigmatic surface of Prunus avium L., sweet cherry. American Journal of Botany, 68(9), 1165–1176. https://doi.org/10.1002/j.1537-2197.1981.tb07822.x [Google Scholar] [CrossRef]
33. Herrero, M., Arbeloa, A. (1989). Influence of the pistil on pollen tube kinetics in peach (Prunus persica). American Journal of Botany, 76(10), 1441–1447. https://doi.org/10.1002/j.1537-2197.1989.tb15124.x [Google Scholar] [CrossRef]
34. González, M. V., Coque, M., Herrero, M. (1995). Stigmatic receptivity limits the effective pollination stage in kiwifruit. Journal of the American Society for Horticultural Science, 120(2), 199–202. https://doi.org/10.21273/JASHS.120.2.199 [Google Scholar] [CrossRef]
35. Edlund, A. F., Swanson, R., Preuss, D. (2004). Pollen and stigma structure and function: The role of diversity in pollination. The Plant Cell, 16(suppl_1), S84–S97. https://doi.org/10.1105/tpc.015800 [Google Scholar] [PubMed] [CrossRef]
36. Heslop-Harrison, J., Heslop-Harrison, Y., Barber, J. (1975). The stigma surface in incompatibility responses. Proceedings of the Royal Society of London. Series B. Biological Sciences, 188(1092), 287–297. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools