 Open Access
Open Access
ARTICLE
Evaluation of Beta-Lactam Antibiotics on the Regeneration of Peanut Plants and Their Inhibitory Effect on Agrobacterium Growth
1
College of Agronomy, Jilin Agricultural University, Changchun, 130118, China
2
College of Life Science, Jilin Agricultural University, Changchun, 130118, China
3
Department of Biology, College of Natural and Computational Science, Wachemo University, Hossana, 667, Ethiopia
* Corresponding Authors: Abraham Lamboro. Email: ; Jun Zhang. Email:
Phyton-International Journal of Experimental Botany 2023, 92(9), 2489-2501. https://doi.org/10.32604/phyton.2023.029492
Received 22 February 2023; Accepted 26 May 2023; Issue published 28 July 2023
Abstract
The effect of beta-lactam antibiotics on shoot induction and plantlet regeneration from cotyledonary nodes was tested using two peanut cultivars. The culture media contained 4 mg/L 6-benzylaminopurine (BAP) as the main growth regulator. Various concentrations (100–600 mg/L) of cefotaxime, carbenicillin, and timentin were applied in the culture media. In all the tested media, there were no significant differences in the shoot induction as compared to the control. However, little phytotoxic effect was observed at higher concentrations of these antibiotics in the shoot elongation media. Under shoot elongation medium, shoots turned brownish and partly died at higher concentrations where shooting rates were not affected by the treatments. In cefotaxime, timentin, and carbenicillin-containing media, levels of antibiotics greater than 400, 300, and 200 mg/L, respectively resulted in the brown coloration of plantlets. Moreover, the mean shoot number and shoot weight significantly decreased as their dosage increased. The results indicate that maximum levels of antibiotics have an adverse effect on the growth and development of peanuts. Also, cefotaxime (100–300 mg/L) and timentin (100–300 mg/L) will be sufficient in controlling Agrobacterium growth in the culture media with the least phytotoxic effect on the peanut plants.Keywords
Cultivated peanut (Arachis hypogaea L.) is an allotetraploid (2n = 4x = 40) derived from the hybridization of two wild peanut species Arachis duranensis (A-genome) and Arachis ipaensis (B-genome) followed by chromosome duplication [1,2]. It is the second grain legume next to soybean grown in Africa, Asia, and the US [3]. China is the world’s greatest peanut-producing country, accounting for approximately 36% of the total production [4]. Peanuts largely consist of protein, sucrose, and amino acids [5,6]. In the in vitro regeneration of peanuts, cotyledonary nodes have been reported as the most successful explant for the induction of multiple shoots through organogenesis in peanuts [7–10] and grain legumes [11]. In the present study, we used a cotyledonary node for peanut regeneration in the tissue culture system to produce multiple shoots. The cultured media were supplemented with different beta-lactam antibiotics (carbenicillin, cefotaxime, and timentin) to test the shoot regeneration and the phytotoxic effect on the peanuts and their suppression capacity of Agrobacterium overgrowth.
These beta-lactam antibiotics are widely used in plant tissue culture that eliminates Agrobacterium due to their ability to specifically inhibit prokaryotic cell wall synthesis and prevent bacterial growth, with little or no definite effect upon eukaryotic plant cells [12–14]. Nevertheless, some inhibitory effects of beta-lactam antibiotics have been reported for various plants [15–18]. In contrast, significant stimulative effects of timentin [15,19] have been evaluated on callus induction, growth, and organogenesis for plant species. Also, the least phytotoxic effect of timentin has been reported [14]. These reports indicated their effect is controversial [20] and beta-lactam antibiotics may be genotype-specific. Thus, it is important to identify the appropriate concentration with the least phytotoxic effect on peanut growth and toxicity to Agrobacterium.
There have been reports on the effects of antibiotics on in vitro regeneration of plants including Citrus sinensis [21], Chinese cabbage [22], carrot [23], soybean [24], rice [25], Populus euphratica [26], banana [27], sugarcane [28], melon [29], foxtail millet [30], Anthurium andraeanum Linden ex Andre [14], maize [31], walnut [17]. Another report showed the elimination potential of antibiotics on the growth of bacteria and or/Agrobacterium tumefaciens in the culture medium [14,24,32]. Plant sensitivity to antibiotics typically is species-dependent [33]. However, the possible effects of beta-lactam antibiotics on the shoot induction, regeneration, and development via organogenesis of peanuts have not been reported so far, even though such studies are very important for the effective recovery of transgenic peanuts through agrobacterium infection. Thus, the present study aims to evaluate the effect of beta-lactam antibiotics on the regeneration of peanut plants and the inhibition of Agrobacterium growth in the culture medium.
Two peanut cultivars (N3 and JNH8) were used as the donors for the cotyledonary node (CN) explant source. The cultivars were provided by the College of Agronomy, Jilin Agricultural University. Cotyledons were excised from the dry seeds of peanuts and embryo axes were placed in sterilized cold water for about 12 h. Briefly, the cotyledonary surface was sterilized with 5% (w/v) sodium hypochlorite and 75% (w/v) ethanol for 15 and 5 min, respectively followed by three rinses with sterile double distilled water. Then air-dried on the filter paper in an aseptic condition. Finally, cultured in a jar containing Murashige and Skoog media.
The embryonic axes were placed on a glass jar containing solid MS (Murashige and Skoog) media with MS salts and vitamin, sucrose three percent (w/v), pH 5.8 ± 0.3, and 4 mg/L BAP, with agar 0.8% (w/v) and with various levels of antibiotics in a culture vessel for about 3 weeks and incubated under 25/25°C day/night, 16 h photoperiod and 130–150 µmol m−2 s−1 florescent light conditions. The effects of antibiotics on the induction of shoots were evaluated. After approximately 3 weeks of culture, excised cotyledonary nodes were transferred to a glass jar supplemented with a regeneration media and different concentrations of beta-lactam antibiotics. Shoots from the elongation medium were cut out and transferred to a rooting media containing α-naphthaleneacetic acid (1 mg/L NAA) for about one month. Well-rooted plantlets were moved to plastic pots comprising the soil mix, sand: soil: and vermiculite (1:1:1) [10]. The plantlets were covered under transparent plastic bags for retaining humidity and hardened under 25/25°C day/night, 16 h photoperiod, and 130–150 µmol m−2 s−1 florescent light conditions for 15 days. The plastic bags were withdrawn for 2 h for 4 days. The acclimatized plantlets were moved into pots containing a mixture of sand and soil and moved to the greenhouse [10].
Three different kinds of beta-lactam antibiotics namely cefotaxime (cefo), carbenicillin (carb), and timentin (Tim) were used in the experiment. Prior to being introduced to the culture medium, they were dissolved in double distilled sterilized water and filter-sterilized. Working solutions of them were used separately to the cotyledonary nodes culture media at six various levels (100, 200, 300, 400, 500, 600 mg/L).
2.4 Agrobacterium Transformation
Agrobacterium strains harboring pCAMBIA3301 were maintained on a yeast extract peptone (YEP) agar medium (1 L) with 50 mg/L kanamycin. A single colony of EHA105 harboring pCAMBIA3301 was grown in a YEP liquid medium supplemented with 50 mg/L kanamycin on a 200 rpm shaker at 28°C for about 16hr. Bacterial cells at OD600 values of 0.7 were collected and resuspended in the same volume of transformation solution (MS with vitamin, 3% sucrose, pH 5.2). 1/2 ml transformation solution was prepared. The freshly excised CNs from three weeks-old plantlets were added into a transformation solution with Agrobacterium cells for 60 min with moderate shaking, then were dried with sterilized filter paper and moved to shoot elongation media (SEM). After three days of co-cultivation at 25°C in the dark was simultaneously transferred to the fresh medium containing different concentrations of antibiotics in the light condition for about three weeks [8]. After three weeks of shoot elongation shooting percentage, average shoot number, shoot color, and the effect of antibiotics on the elimination of bacteria were observed, recorded, compared, and analyzed.
The responses of peanuts at various concentrations of beta-lactam antibiotics were tested. A completely randomized design was applied with three replications. The experiment was repeated twice. Two way analysis of variance (ANOVA) was performed to compare variances across the mean. Tukey’s Multiple Range Test was used to calculate mean separations and Minitab 17 software was used for statistical analysis.
3.1 Effect of Cefotaxime on Shoot Induction and Elongation Medium
The effects of various levels of Cef on the shoot induction and elongation medium were evaluated on two peanut cultivars (Table 1). For shoot induction, no difference was observed between Cef-supplemented and non-supplemented cultures (Fig. 1). Also, this antibiotic at all evaluated levels did not delay the onset of shoot induction. The shooting rate was best and ranged between 87.5% to 89.3% for JNH8 and 90.0% to 90.8% for N3. There were no significant differences found in shooting rate for both cultivars at all the media treated with Cef antibiotic and non-treated cultures However, shoot numbers (SN) and shoot weight (SW) varied between cultivars. The result showed the frequency of shoot formation in peanuts may be cultivar dependent. Moreover, Cef has a non-detrimental effect on shoot regeneration in both shoot induction and elongation medium tested. On the other hand, a highly significant difference was observed in the SW and SN. Shoot number and shoot weight decreased significantly as the concentration of Cef increased. SN ranged between 1.83 to 3.66 for N3 and 1.83 to 3.2 for JNH8. Besides, SW ranged between 0.18 to 0.53 for N3 and 0.2 to 0.64 for JNH8. As the concentration of Cef exceed 400 mg/L, the SN and SW decreased. There were no significant differences in average SW between non-treated and Cef 100, 200, and 300 mg/L for the cultivar N3 (Table 1). The result indicates higher dosage of this antibiotic has a negative or phytotoxic effect on peanut plants. The preferable shoots and SW were found on a media containing 100 to 300 mg/L Cef (Table 1). The result showed 100 to 300 mg/L Cef has the least phytotoxic effect on the in vitro regeneration of peanuts. The peanut survived and developed into a whole plant at 300 mg/L Cef (Fig. 4).
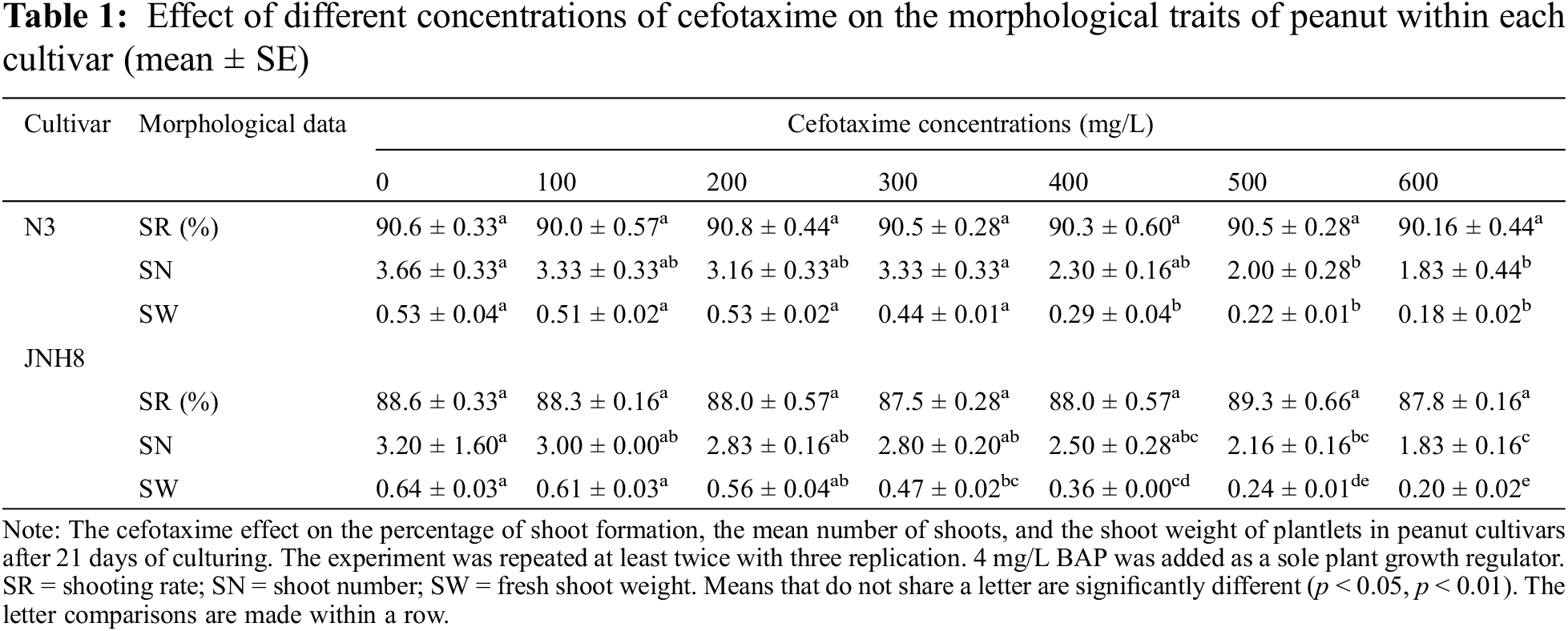
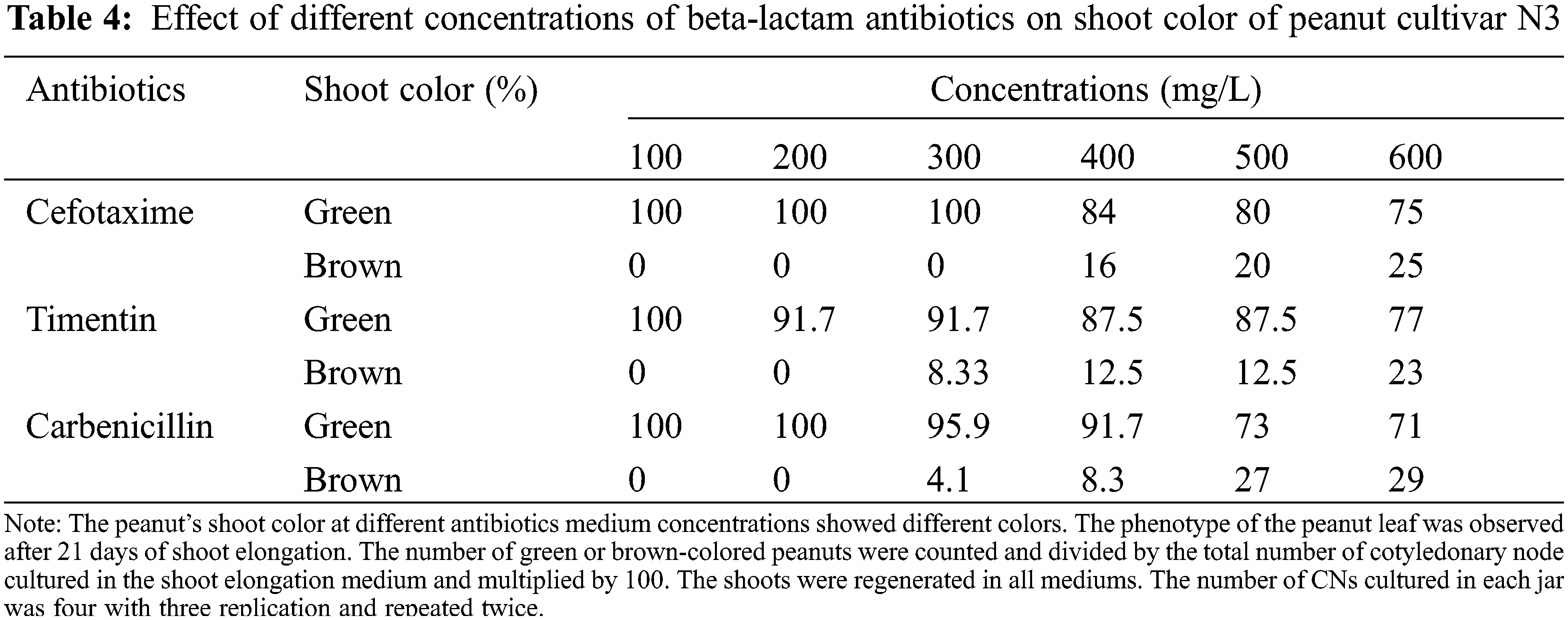
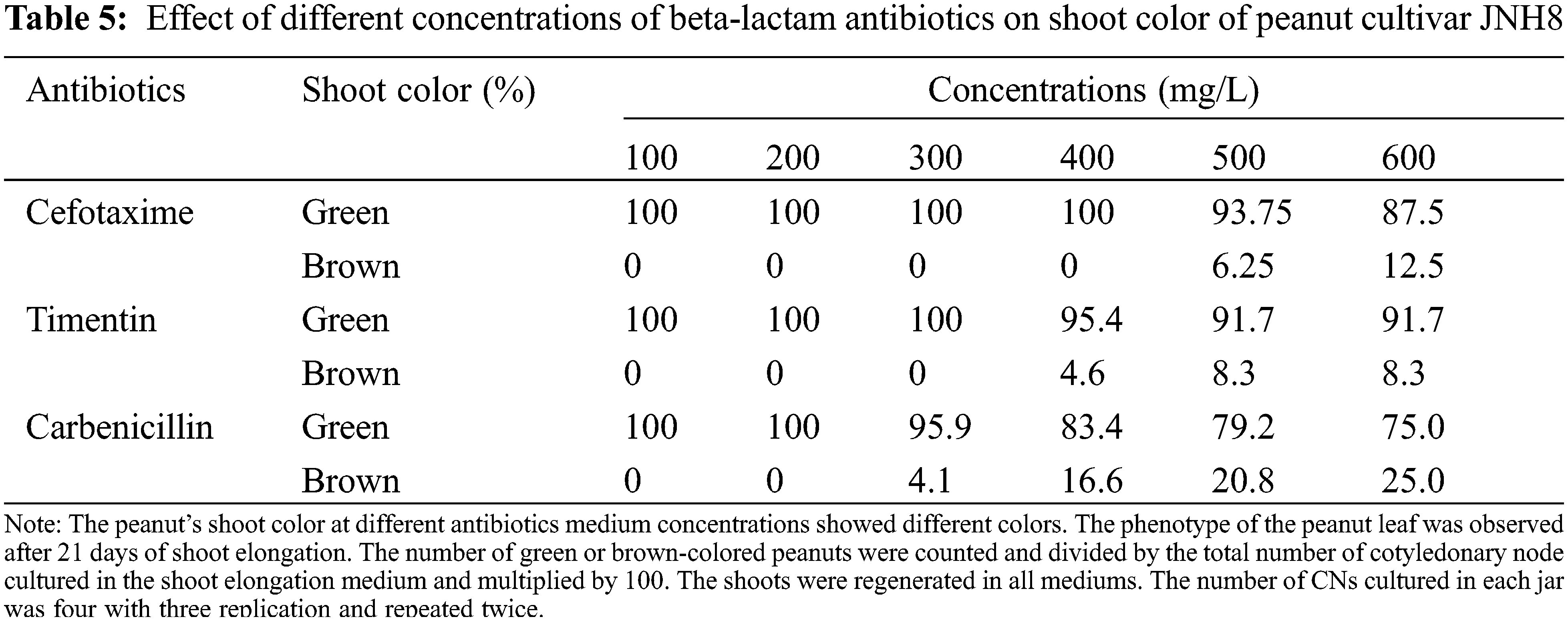
The color of the shoot was found green and normal at dosages of 100 to 300 mg/L. However, the dosage exceeds 300 mg/L some shoots turned brownish. At the concentration of 400, 500, and 600 mg/L Cef, the percentage of brownish color for N3 was 16%, 20%, and 25%, respectively (Tables 4 and 5). A higher percentage of green color was found for the cultivar JNH8 with low brownish color at 500 and 600 mg/L Cef. The brownish-colored shoots partly died.
3.2 Effect of Carbenicillin on Shoot Induction and Elongation Medium
The inclusion of Carb in the culture medium has a non-detrimental effect in the induction of the shoot (Fig. 1). The shoots regenerated at all the concentrations added for both cultivars. However, its influence is clearly observed at the later stage of peanut growth. The induced shoots were excised and transferred to the shoot elongation medium for three weeks. The SR, SN, and SW in control and Carb-treated media after 21 days of culture are shown in Table 2.

Figure 1: The effect of various levels of beta-lactam antibiotics on the regeneration of peanuts in shoot induction media. 10 days old regenerated shoots. The antibiotics have a negligible effect on the regeneration of peanuts on the induction media. C = control; Tim = timentin; Cef = cefotaxime; Carb = carbenicillin. Scale bars = 1.06 cm
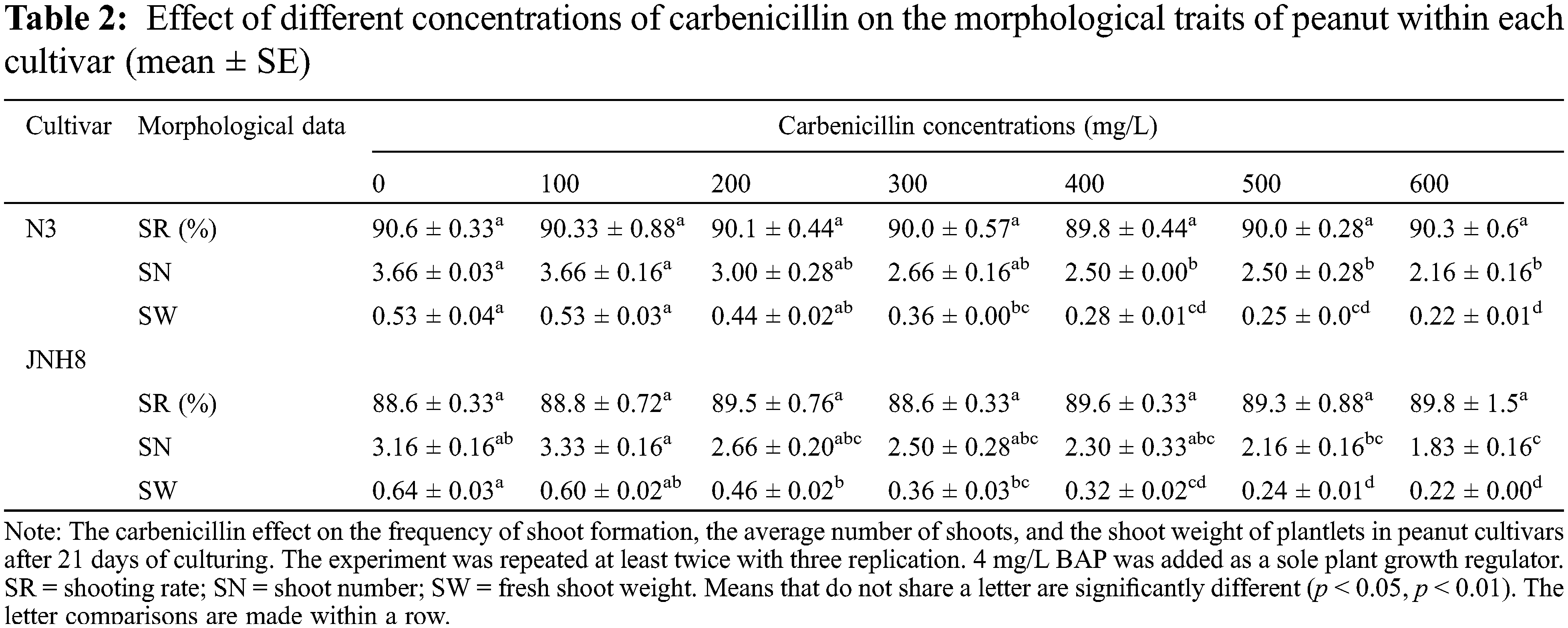
As is presented in Table 2, there were no significant differences in SR. But the SR varied between cultivars. Indicating the shoot formation may be genotype or cultivar-dependent. Besides, multiple shoots formed in most of the cultured media. The SN varied from 2.16 to 3.66 for N3 and 1.83 to 3.33 for JNH8. The maximum SNs were obtained in a media containing 100 mg/L Carb and Carb-free medium. Analysis of the effects of Carb on the regeneration of peanut CNs indicated that there was a negative influence on the number of shoots in the cultured media evaluated, especially when it exceeds 300 mg/L.
The average SW ranged from 0.22 to 0.53 for the cultivar N3 while it ranged from 0.22 to 0.64 for JNH8. The SW highly decreased as the levels of this antibiotic increased, particularly it was reduced 2 to 3 fold at 600 mg/L compared with the control. The result showed that a higher concentration of antibiotic has a phytotoxic effect on the peanut shoot which leads to a reduction in fresh SW. Relative to other concentrations preferable SNs and shoot SW was obtained at 100 to 200 mg/L Carb (Table 2). Also, the least phytotoxic effect was observed at these dosages.
There was a significant difference in the cultivars with respect to the mean number of shoots and fresh SW of the plantlets using various concentrations of Carb in the media. Comparing the effectiveness of the various levels of the antibiotic, 100 mg/L Carb was the best in forming multiple shoots with greater shoot weight. Moreover, better green shoot color was obtained at concentrations of 100 and 200 mg/L. As the concentration of this antibiotic exceeds 200 mg/L, the percentage of brown coloration on the plantlets gradually increased (Tables 4 and 5) and the plantlets with brownish color partly died.
3.3 Effect of Timentin on Shoot Regeneration of Peanuts
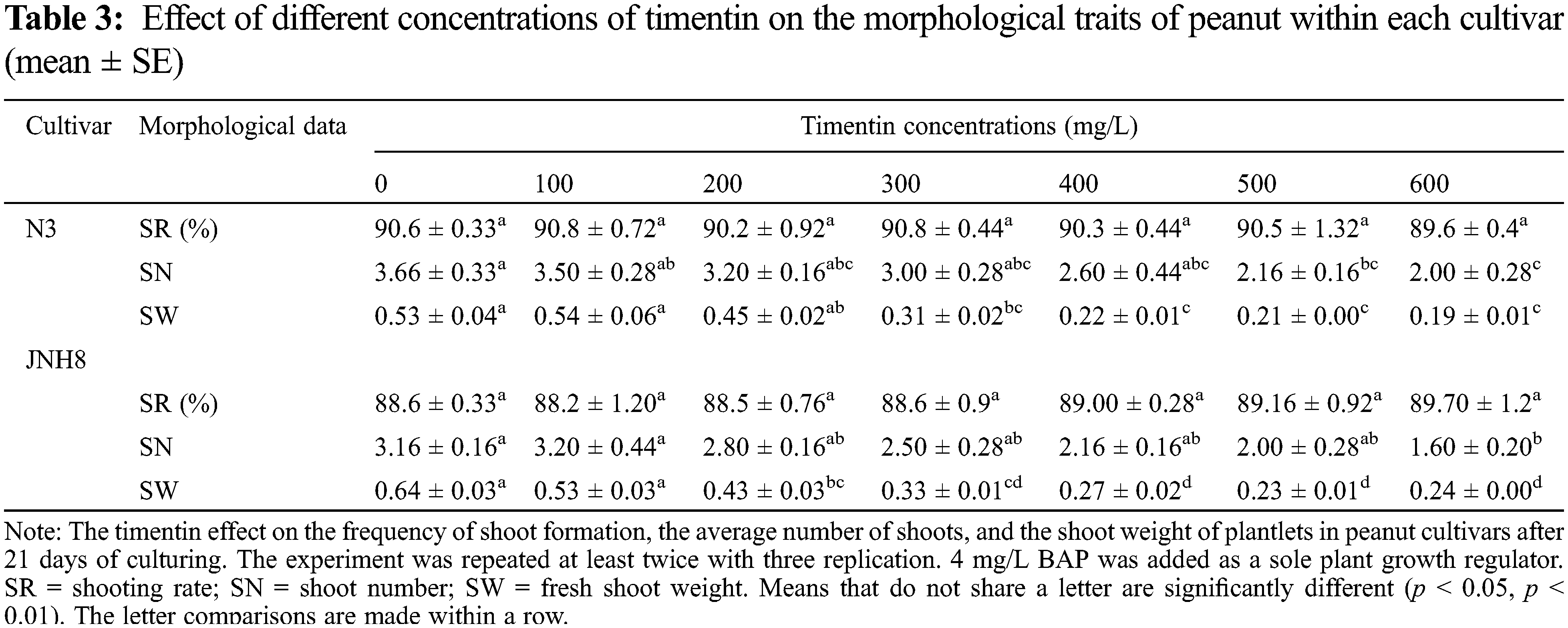
The effect of different concentrations of timentin (Tim) on the regeneration of peanuts shoots are shown in Table 3. The response of two peanut cultivars was tested for this antibiotic. Tim has little effect on the SN and SW of peanuts. It has no negative effect on the induction of shoots evaluated in the shoot induction media. Also, it has no significant effect on the SR of peanuts. The SR varied between 89.6 to 90.8 for N3 and 88.2 to 89.7 for JNH8 cultivars. There were variations in the frequency of shoot formation between cultivars. On the shoot elongation medium shoots regenerated normally similar to antibiotic-free medium. But the number of shoots and SW decreased as the concentrations increased in the cultured medium. The color of the shoots turned brownish in some culture media tested. The result showed that a maximum concentration of Tim negatively affects peanut growth and development.
The average SN and SW varied between 2 to 3.5 and 0.19 to 0.54 g for N3 and 1.6 to 3.2 and 0.23 to 0.53 g for JNH8, respectively (Table 3). The maximum SN (3.5) and SW (0.54 g) were obtained in a culture medium containing 100 mg/L Tim. Both morpholog ical traits decreased at higher concentrations. The shoot color difference was observed at higher concentrations. However, at concentrations of 100 to 300 mg/L Tim better shoots and SW were found. Besides, the least phytotoxic effect was obtained at 100 and 200 mg/L Tim dosages (Table 3 and Fig. 2). The percentage of brown coloration was low at a higher concentration for the cultivar JNH8 whereas the frequency of brown color increased at a higher concentration for N3. The result showed that peanut response to Tim may be genotype-dependent (Tables 4 and 5).

Figure 2: The effect of various levels of beta-lactam antibiotics on in vitro regeneration, shoot phenotype, and their phytotoxic effect on the N3 peanuts. The color of the shoots varied among the antibiotics used. The data and photographs were taken from three weeks old plantlets that were grown in a shoot elongation medium with different antibiotics treatments. The sensitivity of peanuts to the antibiotics was clearly observed. The medium was supplemented with 4 mg/L BAP. Average shoot weight, shoot number, and shooting rate were measured and recorded. Cef = cefotaxime; Tim = Timentin; Carb = carbenicillin. Scale bars = 1.06 cm
3.4 Inhibitory Effect of Antibiotics on the Growth of Agrobacterium
The beta-lactam antibiotics used in the current study substantially eliminated bacterial overgrowth in the culture media (Fig. 3). The plantlets were cultured for about three weeks each in both shoot induction and elongation media containing various concentrations of antibiotics. In all the media tested, no bacterial growth was found. The result showed Cef, Tim and Carb can reduce and/or eliminate bacterial growth. However, their adverse effect on plantlets was found at maximum concentrations (Fig. 3).

Figure 3: Three weeks old elongated shoots cultured in a medium containing 500 mg/L timentin , 500 mg/L carbenicillin and 300 mg/L cefotaxime for the cultivar N3. The plantlets were co-cultivated for three days with Agrobacterium and transferred to antibiotic containing media. There was no bacterial growth found in all the media tested. Scale bars = 1.06 cm

Figure 4: In vitro regeneration of peanut plants via organogenesis with 300 mg/L cefotaxime. (a) Shoot initiation from 10 days old CN. Shoot induction from three weeks old (b--c). Shoot elongation from three weeks old (d). Rooted plantlets survive and developed green multiple shoots (e). Scale bar = 1.06 cm
The impact of beta-lactam antibiotics on the in vitro regeneration of peanuts and their inhibitory action on the Agrobacterium were evaluated. Different concentrations of Carb, Cef, and Tim were applied in the shoot induction and elongation medium for two peanut cultivars. Although beta-lactam antibiotics in various plants have been investigated from the perspective of stimulative [15,19] and non-detrimental effects on plant regeneration [12–14,23,24], their effects on legume crops, specifically on peanut crop varieties have got much less attention.
In the present study, no significant differences were observed between the control and antibiotic-containing medium on the shoot induction medium. Indicating antibiotics have no detrimental effect on the induction of shoots in peanuts. In all the media tested, green and normal shoots were regenerated. Besides, there was no stimulative as well as no phytotoxic effect on the regenerated plantlets in the induction medium. On the contrary, adverse effects of the antibiotics were observed on the shoot elongation media. Under shoot elongation medium, shoots turned brownish and partly died at higher concentrations where shooting rates were not affected by the treatments. Also, the number of shoots and average SW decreased particularly at higher concentrations. In line with the current result, the inhibition of antibiotics on plant growth [21,33,34] was reported. Further studies indicated that antibiotics negatively affect the growth and development of plants [35,36].
In Cef, Tim, and Carb-supplemented media, levels of antibiotics greater than 400, 300, and 200 mg/L, respectively resulted in the brown coloration of plantlets. Which contributed to the reduction of weight in both cultivars. As the level of Carb exceeds 400 mg/L the percentage of brown coloration increased significantly. Among the antibiotics used, the highest phytotoxic effect was recorded for Carb at maximum concentration. The result showed the highest concentration of this antibiotic affects the growth and development of peanut plants. Although little brown coloration was recorded at lower concentrations of Tim, the least phytotoxic effect was found on both peanut cultivars. However, the effects highly varied between cultivars as well as levels of antibiotics. The antibiotics and their dose indicate that the variations in peanut plants showed their response to antibiotics is cultivar-dependent. Moreover, the shoot induction time for shoot formation and elongation from cotyledonary node explant cultured on the media with 100 to 600 mg/L Cef, Carb, and Tim was the same as that of control. These beta-lactam antibiotics reduced SN and SW 2 to 3 fold at maximum concentrations as compared to the control. And also, a very high linear reduction in this trait with rising antibiotic levels was found for both cultivars in all the media tested except for the control. The previous studies indicated that antibiotics can change biomass production, branching patterns, length of shoot, internode length, root-to-shoot ratio, and fresh or dry weight [37–41]. Moreover, the comparatively low cytotoxic effect of direct exposure to antibiotic Tim on in vitro plant tissues has been investigated [15,32,42].
Cephalosporin and penicillin-type antibiotics such as Cef, Carb, or Tim, which are active against gram-negative Agrobacterium, are widely used to eliminate bacterial overgrowth after bactofection [15,43]. Although some effects on plant tissue have been reported, they control bacterial growth [12–14]. Further studies showed antibiotics inhibit the growth of bacteria and/or Agrobacterium tumefaciens in the culture medium [14,24,32]. Beta-lactams prevent peptidoglycan cross-linking cell wall synthesis in bacteria [44]. Besides contamination losses at plantlet regeneration stages may be reduced significantly through the use of antibiotics [45]. Moreover, different authors used various concentrations and types of beta-lactam antibiotics to eliminate the growth of Agrobacterium tumefaciens in Agrobacterium-mediated genetic transformation in peanuts and other plants and used 250 mg/L Cef [8,25], 300 to 500 mg/L Tim [14]. In the present study, various levels of Cef, Tim, and Carb were applied in the culture medium to control the growth of Agrobacterium. The bacterial growth was not observed in the cultured media. Indicating beta-lactam antibiotics are toxic to bacteria and control their growth. However, they inhibit the growth and development of peanuts at maximum dosages.
This study tested the effect of beta-lactam antibiotics such as Cef, Carb and Tim on peanut regeneration using a cotyledonary node as an explant source. The inclusion of 100–600 mg/L of these antibiotics on the culture media have no adverse effect on the shoot induction medium. However, their phytotoxic effect were observed at higher concentrations on the shoot elongation media. Besides, the average shoot number and shoot weight significantly reduced as the concentration of the antibiotics increased. Cef (100–400 mg/L), Carb (100–200 mg/L), and Tim (100–300 mg/L) have the least phytotoxic effect on peanut growth and development. Also, Cef and Tim can be preferable beta-lactam antibiotics in controlling Agrobacterium growth in the culture media with the least phytotoxic to plants. It is concluded that lower doses of antibiotics have little inhibitory effect on the morphology of peanuts. This study will help to develop healthy peanut plants in the future breeding program using Agrobacterium-mediated transformation.
Acknowledgement: The authors are grateful to Dr. Professor Piwu Wang and his research team for kindly allowing the plant biotechnology center to use the materials required during this experimental work.
Funding Statement: This research was financially supported by grants from the Science and Technology Planning Project of Jilin Province (20230202008NC).
Author Contributions: All authors contributed to the study’s conception and design. Conducting the experiment, and writing the original draft preparation were performed by AL. The investigation, resource, data curation, methodology, review, and supervision were done by JZ and SY. Writing, review and editing, software, and formal analysis were performed by XL, DY, and AL. All authors read and approved the final manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Leal-Bertioli, S., José A. C. V., Alves-Freitas, D. M., Moretzsohn, M. C., Guimarães, P. M. et al. (2009). Identification of candidate genome regions controlling disease resistance in Arachis. BMC Plant Biology, 9(1), 1–12. https://doi.org/10.1186/1471-2229-9-112 [Google Scholar] [PubMed] [CrossRef]
2. Seijo, J. G., Lavia, G. I., Fernández, A., Krapovickas, A., Ducasse, D. et al. (2004). Physical mapping of the 5S and 18S-25S rRNA genes by FISH as evidence that Arachis duranensis and A. ipaensis are the wild diploid progenitors of A. hypogaea (Leguminosae). American Journal of Botany, 91(9), 1294–1303. https://doi.org/10.3732/ajb.91.9.1294 [Google Scholar] [PubMed] [CrossRef]
3. King, J. C., Blumberg, J., Ingwersen, L., Jenab, M., Tucker, K. L. (2008). Tree nuts and peanuts as components of a healthy diet. The Journal of Nutrition, 138(9), 1736S–1740S. https://doi.org/10.1093/jn/138.9.1736S [Google Scholar] [PubMed] [CrossRef]
4. FAOSTAT (2019). Food and agricultural organization of the United State. http://www.fao.org/faostat/en/#data [Google Scholar]
5. Liu, H., Shi, A., Liu, L., Wu, H., Ma, T. et al. (2016). Peanut protein processing technology. In: Peanuts: Processing technology and product development, pp. 83–209. Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-12-809595-9.00004-1 [Google Scholar] [CrossRef]
6. Wang, Q., Wang, H. (2018). Peanut processing characteristics and quality evaluation, pp. 97–98. Berlin: Springer. [Google Scholar]
7. Banerjee, P., Maity, S., Maiti, S. S., Banerjee, N. (2007). Influence of genotype on in vitro multiplication potential of Arachis hypogaeaL. Acta Botanica Croatica, 66(1), 15–23. [Google Scholar]
8. Hsieh, Y. F., Jain, M., Wang, J., Gallo, M. (2017). Direct organogenesis from cotyledonary node explants suitable for Agrobacterium-mediated transformation in peanut (Arachis hypogaeaL.). Plant Cell, Tissue and Organ Culture, 128(1), 161–175. https://doi.org/10.1007/s11240-016-1095-1 [Google Scholar] [CrossRef]
9. Lamboro, A., Han, X., Yang, S., Li, X., Yao, D. et al. (2022). High-frequency direct organogenesis from cotyledonary node explants and plantlet regeneration of peanut (Arachis hypogaea) cultivars. International Journal of Agriculture and Biology, 27, 105–114. https://doi.org/10.17957/ijab/15.1906 [Google Scholar] [CrossRef]
10. Lamboro, A., Han, X., Yang, S., Li, X., Yao, D. et al. (2022). Combination of 6-Benzylaminopurine and thidiazuron promotes highly efficient shoot regeneration from cotyledonary node of mature peanut (Arachis hypogaea L.) cultivars. Phyton-International Journal of Experimental Botany, 91(12), 2619–2631. https://doi.org/10.32604/phyton.2022.021404 [Google Scholar] [CrossRef]
11. Chandra, A., Pental, D. (2003). Regeneration and genetic transformation of grain legumes: An overview. Current Science, 84(3), 381–387. [Google Scholar]
12. Asbel, L. E., Levison, M. E. (2000). Cephalosporins, carbapenems, and monobactams. Infectious Disease Clinics of North America, 14(2), 435–447. https://doi.org/10.1016/S0891-5520(05)70256-7 [Google Scholar] [PubMed] [CrossRef]
13. Pollock, K., Barfield, D., Shields, R. (1983). The toxicity of antibiotics to plant cell cultures. Plant Cell Reports, 2(1), 36–39. https://doi.org/10.1007/BF00269232 [Google Scholar] [PubMed] [CrossRef]
14. Zhang, H., Wang, G., Qiao, Y., Chen, C. (2017). Effects of timentin and other β-lactam antibiotics on callus induction, shoot regeneration, and rooting in Anthurium andraeanum Linden ex Andre. In Vitro Cellular and Developmental Biology-Plant, 53(3), 219–225. https://doi.org/10.1007/s11627-017-9823-8 [Google Scholar] [CrossRef]
15. Nauerby, B., Billing, K., Wyndaele, R. (1997). Influence of the antibiotic timentin on plant regeneration compared to carbenicillin and cefotaxime in concentrations suitable for elimination of Agrobacterium tumefaciens. Plant Science, 123(1–2), 169–177. https://doi.org/10.1016/S0168-9452(96)04569-4 [Google Scholar] [CrossRef]
16. Ogawa, Y., Mii, M. (2005). Evaluation of 12 β-lactam antibiotics for Agrobacterium-mediated transformation through in planta antibacterial activities and phytotoxicities. Plant Cell Reports, 23(10), 736–743. https://doi.org/10.1007/s00299-004-0885-9 [Google Scholar] [PubMed] [CrossRef]
17. Tang, H., Ren, Z., Krczal, G. (2000). An evaluation of antibiotics for the elimination of Agrobacterium tumefaciens from walnut somatic embryos and for the effects on the proliferation of somatic embryos and regeneration of transgenic plants. Plant Cell Reports, 19(9), 881–887. https://doi.org/10.1007/s002990000201 [Google Scholar] [PubMed] [CrossRef]
18. Wiebke, B., Ferreira, F., Pasquali, G., Bodanese-Zanettini, M. H., Droste, A. (2006). Influence of antibiotics on embryogenic tissue and Agrobacterium tumefaciens suppression in soybean genetic transformation. Bragantia, 65(4), 543–551. https://doi.org/10.1590/S0006-87052006000400002 [Google Scholar] [CrossRef]
19. Frary, A., Earle, E. D. (1996). An examination of factors affecting the efficiency of Agrobacterium-mediated transformation of tomato. Plant Cell Reports, 16(3), 235–240. https://doi.org/10.1007/BF01890875 [Google Scholar] [PubMed] [CrossRef]
20. Tereso, S., Miguel, C., Maroco, J., Oliveira, M. M. (2006). Susceptibility of embryogenic and organogenic tissues of maritime pine (Pinus pinaster) to antibiotics used in Agrobacterium-mediated genetic transformation. Plant Cell Tissue and Organ Culture, 87(1), 33–40. https://doi.org/10.1007/s11240-006-9130-2 [Google Scholar] [CrossRef]
21. da Silva Mendes, A. F., Cidade, L. C., de Oliveira, M. L. P., Otoni, W. C., Soares-Filho, W. D. S. et al. (2009). Evaluation of novel beta-lactam antibiotics in comparison to cefotaxime on plant regeneration of Citrus sinensisL. Osb. Plant Cell, Tissue and Organ Culture, 97(3), 331–336. https://doi.org/10.1007/s11240-009-9518-x [Google Scholar] [CrossRef]
22. Meng, Q., Liu, Z., Zhang, Y., Liu, C., Ren, F. et al. (2013). Effects of antibiotics on in vitro-cultured cotyledons. Plant Physiology and Biochemistry, 50(4), 436–441. https://doi.org/10.1016/j.plaphy.2013.02.027 [Google Scholar] [CrossRef]
23. Grzebelus, E., Skop, L. (2014). Effect of β-lactam antibiotics on plant regeneration in carrot protoplast cultures. In Vitro Cellular Developmental Biology-Plant, 50(5), 568–575. https://doi.org/10.1007/s11627-014-9626-0 [Google Scholar] [PubMed] [CrossRef]
24. Kim, K. H., Lee, J. E., Kwon, Y. U., Lee, BM. (2009). Influence of antibiotics on shoot regeneration and agrobacteium suppression using cotyledonary node in Korean Soybean cultivars. Korean Journal of Crop Science, 54(3), 307–313. [Google Scholar]
25. Tran, T. N., Sanan-Mishra, N. (2015). Effect of antibiotics on callus regeneration during transformation of IR 64 rice. Biotechnology Reports, 7, 143–149. https://doi.org/10.1016/j.btre.2015.06.004 [Google Scholar] [PubMed] [CrossRef]
26. Ding, X., Chen, X. Y., Li, W., Du, Z. Y. (2006). Effects of antibiotics on plantlet regeneration via organogenesis in Populus euphratica. Forestry Studies in China, 8(1), 27–31. https://doi.org/10.1007/s11632-006-0005-8 [Google Scholar] [CrossRef]
27. Manchanda, P., Kaur, A., Gosal, S. S. (2011). Impact of cefotaxime on in vitro shoot elongation and regeneration in banana (Musa acuminata). Journal of Applied Horticulture, 13(1), 52–56. https://doi.org/10.37855/jah.2011.v13i01.12 [Google Scholar] [CrossRef]
28. Kaur, A., Gill, M., Ruma, D., Gosal, S. S. (2008). Enhanced in vitro shoot multiplication and elongation in sugarcane using cefotaxime. Sugar Tech, 10(1), 60–64. https://doi.org/10.1007/s12355-008-0010-4 [Google Scholar] [CrossRef]
29. Naderi, D., Askari-Khorasgani, O., Mahmoudi, E. (2016). Cefotaxime and benzyladenine improve melon regeneration. Iranian Journal of Biotechnology, 14(1), 56. https://doi.org/10.15171/ijb.1077 [Google Scholar] [PubMed] [CrossRef]
30. Satish, L., Rathinapriya, P., Ceasar, S. A., Rency, A. S., Pandian, S. et al. (2016). Effects of cefotaxime, amino acids and carbon source on somatic embryogenesis and plant regeneration in four Indian genotypes of foxtail millet (Setaria italica L.). In Vitro Cellular and Developmental Biology-Plant, 52(2), 140–153. https://doi.org/10.1007/s11627-015-9724-7 [Google Scholar] [CrossRef]
31. Danilova, S., Dolgikh, Y. I. (2004). The stimulatory effect of the antibiotic cefotaxime on plant regeneration in maize tissue culture. Russian Journal of Plant Physiology, 51(4), 559–562. https://doi.org/10.1023/B:RUPP.0000035752.09295.55 [Google Scholar] [CrossRef]
32. Cheng, Z. M., Schnurr, J., Kapaun, J. (1998). Timentin as an alternative antibiotic for suppression of Agrobacterium tumefaciens in genetic transformation. Plant Cell Reports, 17(8), 646–649. https://doi.org/10.1007/s002990050458 [Google Scholar] [PubMed] [CrossRef]
33. Qin, Y., Teixeira da Silva, J. A., Bi, J., Zhang, S., Hu, G. B. (2011). Response of in vitro strawberry to antibiotics. Plant Growth Regulation, 65(1), 183–193. https://doi.org/10.1007/s10725-011-9587-9 [Google Scholar] [CrossRef]
34. da Silva, J. A. T., Nhut, D. T., Tanaka, M., Fukai, S. (2003). The effect of antibiotics on the in vitro growth response of chrysanthemum and tobacco stem transverse thin cell layers (tTCLs). Scientia Horticulturae, 97(3–4), 397–410. https://doi.org/10.1016/S0304-4238(02)00219-4 [Google Scholar] [CrossRef]
35. Liu, L., Liu, Y. H., Liu, C. X., Wang, Z., Dong, J. et al. (2013). Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecological Engineering, 53, 138–143. https://doi.org/10.1016/j.ecoleng.2012.12.033 [Google Scholar] [CrossRef]
36. Migliore, L., Rotini, A., Cerioli, N. L., Cozzolino, S., Fiori, M. (2010). Phytotoxic antibiotic sulfadimethoxine elicits a complex hormetic response in the weed Lythrum salicaria L. Dose-Response, 8(4), 414–427. https://doi.org/10.2203/dose-response.09-033.Migliore [Google Scholar] [PubMed] [CrossRef]
37. Bradel, B., Preil, W., Jeske, H. (2000). Remission of the free-branching pattern of Euphorbia pulcherrima by tetracycline treatment. Journal of Phytopathology, 148(11–12), 587–590. https://doi.org/10.1046/j.1439-0434.2000.00562.x [Google Scholar] [CrossRef]
38. Liu, F., Ying, G. G., Tao, R., Zhao, J. L., Yang, J. F. et al. (2009). Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environmental Pollution, 157(5), 1636–1642. https://doi.org/10.1016/j.envpol.2008.12.021 [Google Scholar] [PubMed] [CrossRef]
39. Michelini, L., La Rocca, N., Rascio, N., Ghisi, R. (2013). Structural and functional alterations induced by two sulfonamide antibiotics on barley plants. Plant Physiology and Biochemistry, 67, 55–62. https://doi.org/10.1016/j.plaphy.2013.02.027 [Google Scholar] [PubMed] [CrossRef]
40. Yang, Q., Zhang, J., Zhang, W., Wang, Z., Xie, Y. et al. (2010). Influence of tetracycline exposure on the growth of wheat seedlings and the rhizosphere microbial community structure in hydroponic culture. Journal of Environmental Science and Health Part B, 45(3), 190–197. https://doi.org/10.1080/03601231003613492 [Google Scholar] [PubMed] [CrossRef]
41. Li, Z. J., Xie, X. Y., Zhang, S. Q., Liang, Y. C. (2011). Wheat growth and photosynthesis as affected by oxytetracycline as a soil contaminant. Pedosphere, 21(2), 244–250. https://doi.org/10.1016/S1002-0160(11)60124-0 [Google Scholar] [CrossRef]
42. Leone, G. F., Andrade, P. A. M., de Almeida, C. V., de Almeida, C. V., Dini Andreote, F. et al. (2019). Use of antibiotics to control endophytic bacterial growth migration onto culture medium in Eucalyptus cloeziana F. Muell. A micropropagation approach. In Vitro Cellular and Developmental Biology-Plant, 55(4), 421–432. https://doi.org/10.1007/s11627-019-09986-2 [Google Scholar] [CrossRef]
43. Ogawa, Y., Mii, M. (2004). Screening for highly active β-lactam antibiotics against Agrobacterium tumefaciens. Archives of Microbiology, 181(4), 331–336. https://doi.org/10.1007/s00203-004-0650-z [Google Scholar] [PubMed] [CrossRef]
44. Petri, W. A. (2011). Penicillins, cephalosporins, and other β-lactam antibiotics. In: Goodman and Gilman’s the pharmacological basis of therapeutics, 12th edition, pp. 1477–1504. New York: McGraw-Hill. [Google Scholar]
45. Abdi, G., Salehi, H., Khosh-Khui, M. (2008). Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiologiae Plantarum, 30(5), 709–714. https://doi.org/10.1007/s11738-008-0169-z [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools