 Open Access
Open Access
ARTICLE
Analyzing the Cytotoxic and Genetic Impact of Datura stramonium Extract on MCF7 and HT29 Cancer Cells: A Metabolite and Gene Expression Study
1 Department of Biotechnology, College of Science, Taif University, Taif, 21944, Saudi Arabia
2 Department of Biology, College of Science, Taif University, Taif, 21944, Saudi Arabia
3 Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, 21423, Saudi Arabia
4 Department of Biomedical Research, King Abdullah International Medical Research Center, Jeddah, 21423, Saudi Arabia
5 Biology Department, Faculty of Science and Arts, Al-Baha University, Al-Mikhwah, 65731, Saudi Arebia
6 Faculty of Medicine, Mansoura University, Mansoura, 35516, Egypt
7 Department of Chemistry, Collage of Science, Taif University, Taif, 21944, Saudi Arabia
* Corresponding Author: Hadeer Darwish. Email:
Phyton-International Journal of Experimental Botany 2025, 94(1), 181-198. https://doi.org/10.32604/phyton.2025.059387
Received 06 October 2024; Accepted 11 December 2024; Issue published 24 January 2025
Abstract
The interest in using the Datura stramonium plant is due to its natural products, which are used in many pharmaceutical industries. The objective of the current study was to assess the therapeutic and cytotoxic effects of the D. stramonium plant on two types of human cancer cell models (MCF7 and HT29) in vitro. A soxhlet apparatus was used to obtain methanolic extract from dried plant leaves. The recovered crude, after the solvent had evaporated, was then dispersed at varied concentrations of extract 100, 50, 20, and 0.0 µg/mL and tested to see how the cells responded. Also, the cancer-testis antigen (CTA) gene transcription in the two cell types exposed to the plant extract was examined using a semi-quantitative real-time polymerase chain reaction. Gas chromatography–mass spectrometry (GC-MS) results produced the significant main metabolites Nonanoic acid, Tropine N-Oxide, 3,6-Ditigloyloxy-7-hydroxytropane, Hexadecanoic acid, 2-Pentadecanone,6,10,14-trimethyl-, Carvenone, methyl ester, Phytol, Aposcopolamine, Hyoscyamine, 4,8,12,16-Tetramethylheptadecan-4-olide, Scopolamine, Alpha.-Tocospiro A, 1,2-Cycloheptanedione, 3,3,7,7-tetramethyl-, dihydrazone, Campesterol, Stigmasterol, Gamma -Sitosterol and dl-.alpha.-Tocopherol. The results showed that the two types of cell lines impacted by D. stramonium extract, through untreated type 1 cells (MCF7) gave a highly significant transcription according to all applicable genes. All implemented analyses cleared the strong genetic impacts of Datura extract on cancer cells’ genomes. TGIF2LY and C2orf63 transcript accumulation were also significantly elevated when exposed to plant extract at a level of 50 µg/mL in cell line type 2 (HT29), but TGIF2LY and P53 had the lowest relative expression at a level of 100 µg/mL when treated the same cell line type.Keywords
All living species in our world depend heavily on plants to survive. Plants play a significant role in several pharmaceutical businesses, as they produce at least 25% of the medicine in pharmacies, highlighting the immense diversity of plants’ importance in all aspects of life [1]. Promising possibilities for cancer and other disease therapies, plant-derived natural compounds are important resources for drug research and development, with notable potential in avoiding and regulating oxidative stress. The antioxidant properties of phytochemicals like phenolic compounds have been connected to their effects. Given the complexity and abundance of these bio-constituents, plant extracts and preparations must undergo precise in vitro characterization and quantification to potentially translate into in vivo effects and clinical usage [2]. The chemopreventive and anticancer properties of three main classes of secondary metabolites were studied by [3]. These classes included nitrogen-containing secondary metabolites (alkaloids and glucosinolates), polyphenols (curcumin, quercetin, resveratrol, and flavonoids), and terpenoids (monoterpenoids, diterpenoids, sesquiterpenoids, and tetraterpenoids).
The Solanaceae family of plants includes the weed known as Datura. It is commonly used because of its therapeutic and poisonous characteristics [4]. D. stramonium, D. ferox, D. innoxia, and D. metel are among the species in the genus Datura that are valuable for medicine [5]. Since the beginning of time, people have utilized these species for both medical and recreational purposes. Traditional and ethnopharmacological uses of Datura species include anticancer, antiasthmatic, anesthetic, sedative, antihemorrhoidal, expectorant, and demulcent properties [5]. Inflammation, rheumatoid arthritis, wounds, ulcers, gout, asthma and bronchitis, fever, and toothache are just a few of the various illnesses for which it is widely acknowledged as an effective treatment [6].
D. stramonium, which is the most prevalent species in this family and is endemic to Asia, is more prevalent in temperate, tropical, and subtropical temperatures [7]. Atropine, scopolamine, and hyoscyamine are just some of the dangerous tropane alkaloids found in D. stramonium [6]. A GC-MS test of the Datura plant extract was conducted in the current investigation. Liquid, gaseous, or solid materials can all be analyzed using GC-MS. Beginning with the GC, the analysis can be performed [8,9]. Ulcers, wounds, sciatica, rheumatism, inflammation, gout, swellings, bruising, asthma, fever, bronchitis, tumors, toothaches, and cancer disorders have all been treated using D. stramonium [5]. D. stramonium is regarded as poisonous everywhere. Some of the first indications and symptoms to manifest are rapid heartbeat, high heart rate, pupil dilatation, dry mouth, and vision impairment. Decreased body temperature, nausea, severe tremors, aggressive conduct, lack of control over one’s muscles, slow breathing, and a rapid and weak pulse will follow [10]. The hazardous dose for it is four mg for children and ten mg for adults [6]. D. stramonium is involved in criminal behavior in ways beyond the individual use of anesthetic for illicit pleasure [11] when it is blown into the faces of victims as an anesthetic by Datura powder to prevent their free will in crimes such as sexual assaults, robberies, and other crimes [12]. Plants have long been used to cure cancer, as they are still a vital component of modern therapy [13]. Plant extracts can kill a range of cancer cells produced by humans depending on the dosage and the passage of time. To prevent the extracts from harming healthy cells [14]. The effects and eradication of cancer are aided by the immunological, anti-inflammatory, and antioxidant properties of Datura extract [15]. As one of the most significant antioxidants, flavonoids, which influence cancer cells, are phenols. D. stramonium plays a part in the effect and eradication of cancer cells [16]. Alkaloids are crucial secondary metabolites that are necessary to humanity and are known to have therapeutic properties. These therapeutic compounds are significant because they may effectively scavenge free radicals or bind with oxidative reaction catalysts, including certain metal ions, preventing the onset of many degenerative diseases. These compounds are highly valued for usage in pharmaceutical formulations because of their broad spectrum of properties, and they may prove to be useful metabolites that can be utilized to treat deadly illnesses like cancer [17].
The most frequent condition that poses a threat to life is cancer [18]. Cancer can essentially be defined as the uncontrollable expansion of unusual cells in any direct organ [19]. When it deviates from the conventions of cell division, cancer is defined as a condition marked by modifications or mutations in the cell genome. The proteins that are produced by these changes (DNA mutations) upset the delicate biological balance between cell division, leading to malignant cells that continue to divide [20]. Advances in cancer pathobiology research have been made possible by the availability of a wide variety of experimental model systems that examine the various manifestations of this illness, provide an understanding of genetic and epigenetic modifications, and allow the testing of anticancer drugs [21]. Maheshwari and Sharma [22] investigated that most of the phenolic compounds, i.e., quercetin, carvacrol, curcumin, hydroxychavicol, crocin, palmatine, aloin, gingerol, allicin, epigallocatehn, thymol, pyrogallol, and carvone were found to modulate one or more signaling pathways linked with cell survival, cell propagation/differentiation, cell migration, angiogenesis, cell cycle, apoptosis, detoxification enzymes, hormone activities, reactive oxygen species up-and-down regulation, altered gene expression, and immune responses. According to research by Lesetja et al. [23], Tulbaghia violacea extracts show anticancer activities with morphological alterations that facilitate the induction of apoptosis. In contrast to methanolic extract, T. violacea increases apoptosis-related gene expression in all tested cancer cell lines, particularly cervical cancers, while butanolic extract has little to no effect on the selected cancers. Hexane extracts of T. violacea have been demonstrated to be actively involved in the induction of apoptosis.
For studying cancer biology and evaluating the therapeutic impact of anticancer medications in laboratories, human cancer cell lines are useful models [24]. The isolation of each cell line from the illness that the patient has allows for the detection of pathological traits that would otherwise go unnoticed in conventional clinical diagnostic settings as well as the performance of tests that would be hard to carry out in vivo [24]. Artificial reproduction of the therapeutic and cytotoxic effects of wild plants on human cancer cells has been studied using two different types of cancer cells. Human colorectal cancer cell line HT-29 has an epithelial appearance. In vitro studies on intestinal cell uptake, secretion, and translocation [25]. Having estrogen, progesterone, and glucocorticoid receptors, a female patient with metastatic breast cancer’s pleural effusion was used to create the stable epithelioid cell line MCF-7 [26]. A heterogeneous collection of proteins termed the Cancer Testis Antigen (CTA) was created exclusively by the testis tissues, including the germ cells, of a healthy adult male. Leukemia, renal cancer, and colorectal cancer all produce modest quantities of cancer testis antigen, while lung cancer, melanomas, bladder cancer, and ovarian cancer produce significant levels [27]. CTAs play a unique and crucial function in cancer diagnostics and therapeutic targeting since they are made by cancer cells rather than by somatic cells [28]. CTAs’ importance in cancer immunology has increased as a result of their high specificity for malignancies and testis tissues. The modulation of CT genes’ expression and their biological roles in tumor cells are still poorly understood, despite accumulating evidence of their contribution to carcinogenesis. Some CT genes’ roles in cell division and other functions can be identified in normal tissues [29]. There are numerous CTAs, some of which are recognized to have a specific purpose while others are yet unknown in terms of their intended use as cancer marker indications [30]. When cytotoxic T cells detect the MHC peptide complex using seven primers that contain the most tumor-specific antigens, as opposed to the housekeeping Actin gene and the control therapy, tumors are eradicated [31]. This work aims to separate the chemicals from the D. stramonium medicinal plant and test their effects on two cancer cell lines (MCF7 and HT29).
The current investigation was carried out in the Molecular Biology Laboratory and High-Altitude Research Center (HARC) at Taif University in Saudi Arabia.
2.1 Collection of Plant Materials
The region of El Haweia provided the D. stramonium leaves for collection.
2.2 D. stramonium Crude Isolation
The samples of D. stramonium leaves were air-dried before being put through a Soxhlet system to extract secondary compounds while using methanol as a solvent for 6 h. The crude form of D. stramonium was gathered, filtered, and held until analysis at −4°C [32].
2.3 GC-MS Examination of D. stramonium Leaves
2.3.1 Gas Chromatography and Mass Spectrometry Analysis
For the GC-MS analysis of plant crude, an Agilent Technologies (Little Falls, CA, USA) 6890N Network gas chromatographic (GC) system equipped with an Agilent Technologies 5975 inert XL Mass selective detection system and an Agilent Devices 7683B series auto-injector was employed. The HP-5 MS capillary column in Little Falls, California, USA (30 m × 0.25 mm film with a thickness of 0.25 µm) was utilized to separate the compounds. With a 100:1 split ratio, a sample volume of one milliliter was injected in the split mode. An electron ionization system with a 70-eV energy of ionization was implemented for GC/MS monitoring. The GC analysis program that was chosen for the column oven temperature program was. A 1.5 mL min-1 flow rate of helium was utilized as a gas transporter. The mass scanning range was set at 50 to 550 m/z, and the temperatures of the injector and MS transfer line were each maintained at 300°C for 10 min.
2.3.2 Compound Characterization
Considering their retention values in comparison to (C9-C24) n-alkanes or to genuine chemicals, the components of the oil were identified. Moreover, compounds were recognized with their MS data in comparison to published mass spectra and samples from the NIST mass spectral library [33].
2.4 Cultivation of Human Cancer Cell Lines
2.4.1 Developing the Cell Lines
Two of the cell lines MCF7 (Human Caucasian Breast Adenocarcinoma) and HT29 (Human Colon Colorectal Adenocarcinoma) utilized in this experiment were cultivated in Dubeco’s modified Eagle’s medium (DMEM) + GLATMAXMT + 10% FBS at 37°C with 5% CO2. The cell lines passage was based on the dilutions and confluences advised by the American Type Culture Collection (ATCC), who sold them. 10% foetal bovine serum (FBS) was added to the medium in which the cell lines were grown (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA; Lot 41Q6208K). Using the Mycoplasma PCR Detection kit, cultures were routinely tested for mycoplasma contamination (Sigma Aldrich, Saint Louis, MO, USA;MP0035).
2.4.2 Frozen Cancer Cell Lines Regeneration
A container of cells was removed from the liquid nitrogen cylinder and warmed in a bath of water set to 37°C for just over two min. A conical 15 mL tube containing the cells was used that was sterile and filled with 5 mL of full, warmed growth media. After that, to pelletize the cells, they were centrifuged for 5 min at 1000× g. Aspiration was done of the supernatant, next, 10 mL of fresh growth fluid was added to the pellet for re-suspension, and the appropriate flask was then filled with the mixture. The cells were cultured at 37°C for 24 h with the necessary CO2 content in a humidified incubator.
2.4.3 Isolation of Adherent Cells from Culture
The adhering cells were subjected to one wash in Dulbecco’s phosphate saline solution (DPBS; Gibco®, Sigma Aldrich, Saint Louis, MO, USA; 14190-250) when the plates’ medium was aspirated. The cell layer surface of the plate was next covered with trypsin (Gibco®; 25300-054) to allow the cells to disintegrate into suspension. After that, plates were returned to the incubator for 2–5 min based on the cell type. The cells could then be utilized whenever required after warm fresh media containing serum was incorporated to stop the trypsin action.
2.4.4 Generating Stocks of Cancer Cell Lines
1×trypsin-EDTA was used to trypsinize cells after being twice rinsed with 1× DPBS when they reached 80%–90% confluence (GIBCO 1370163). Having gathered the cells in fresh medium (10 mL), they underwent a 5-min centrifugation at 1000 × g. Before being put into a cryotube that had been labeled, in 1 mL of 10% dimethyl sulfoxide (DMSO) and 90% FBS solution, pellets of cells were gently re-dissolved. To achieve successful cell recovery, these vials should then be kept at −80°C for at least 24 h in an isopropanol-filled Nalgene “Mr. Frosty” thawing vial. It strongly suggested maintaining a steady cooling pace of room temperature to 1°C per minute. For temporary storage, the cells could be kept at –80°C for one day, or they could be kept in a liquid nitrogen tank for a longer period.
The slide of a hemocytometer (Z169021-1EA; Sigma-Aldrich) and its cover were thoroughly cleaned via 70% ethanol and then cleaned with deionized water. To provide a good estimate of the actual cell content, stirring the cell suspension helped break up any cell clumps. 10 µL of cell culture were mixed in a clean Eppendorf tube with Trypan blue at 0.4% (Invitrogen; 15250-061). The coverslip was placed over the hemocytometer’s grid and by using a 20P micropipette through V-like wells, 10 µL of the combination was poured into the chamber. Live cells were unstained when a Carl Zeiss Axiostar microscope with 10× objectives was used to read the hemocytometer, whereas blue cells indicated dead cells (with Trypan Blue stains). For both girds, five squares were used to count the average number of cells. Next, the total amount of active cells was calculated following the formula:
Cells/mL–standard number of living cells times 2 (Trypan Blue-based dilution) × dilution factor × 104. Second, the number of viable cells was determined by Biorad’s TC10 cell counter using the trypan blue dye exclusion method.
Depending on the cell line, 6-well plates were used for cell plating at the proper concentration; for example, 103 MCF7 cells per milliliter were adopted for plating. Before the treatment, the cells were incubated for 24 h following treatment with plant extract. The treatment was done three times every 24 h using three different concentrations 100, 50, and 20 µg/mL, after those photos were taken using an inverted microscope for both untreated and treated cells.
The MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) can be used to measure cell viability as described by Darwish et al. [34]. To perform this test in 96-well plates, cells were developed, and three different doses of plant extract were applied. Then each well received 10 µL of MTT ingredient, which was wrapped with aluminum foil and maintained in CO2 for 4 h. After that 100 µL of DMSO was utilized, and 15 min of CO2 incubation followed. The absorbance was read via a microplate reader at 570 nm [35]. The viability of cells was recorded according to the following equation: % viability = Absorbance of sample at 570 nm/Absorbance of untreated at 570 nm × 100, where untreated cells were treated with culture media only. The effect of different concentrations on cell viability was expressed as % inhibition against concentration and used to calculate IC50 (concentration required to kill 50% of the cells). Results were expressed as mean ± SD (n = 5).
2.6 Isolation Procedure for Trizol RNA
Tissues
Tissue samples were homogenized in 1 mL of Trizol reagent per 50 to 100 mg of tissue using a glass-Teflon or power homogenizer (such as the Polytron or Tekmar’s TIS-SUEMIZER). The sample amount shouldn’t be more than 10% of the volume of Trizol reagent employed for homogenization.
Monolayer Grown Cells
Ice-cold PBS was used to rinse the monolayer cell once. Cells in a 3.5 cm culture dish were immediately lysed by a cell scraper and 1 mL of Trizol. The cell lysate was pipetted repeatedly. The area of the culture dish determines how much Trizol reagent was added (10 cm2 of 1 mL), not the entire number of cells. There was a chance that the isolated RNA would pick up DNA if there was not enough Trizol reagent.
Suspension Grown Cells
300× g spined cells for 5 min. The media were removed, and then the cells in icy PBS. Cells were spun to form pellets at 300× g for 5 min. A syringe was used and a needle to repeatedly pipette the Trizol reagent into the cells to lyse them. 5–10 × 106 animal cells should be treated with 1 mL of the reagent.
Nucleoprotein
Nucleoprotein complexes completely dissociated; the homogenized material was kept at room temperature for 5 min to get rid of cell debris, centrifuge, and then the supernatant was placed in a fresh tube.
For every 1 mL of the Trizol reagent, chloroform at 0.2 mL was added. The caps were secured on the sample tubes. Strong vortex sampling for 15 s is followed by an incubation period of 2 to 3 min at room temperature. Between 2°C and 8°C and a maximum of 12,000× g, the samples were centrifuged for 15 min. Centrifugation led to the formation of three separate phases in the mixture: a lower, red, phenol-chloroform phase; interphase; and an upper, white, aqueous phase. Only in the aqueous phase does RNA naturally occur. A new tube was carefully used to transfer the top aqueous phase, paying careful not to disturb the inner phase. During homogenization, the aqueous phase’s volume, which makes up around 60% of the Trizol reagent, was measured.
Isopropyl alcohol was added to the aqueous phase for RNA precipitating. For the beginning of homogenization, 1 mL of Trizol reagent was diluted with 0.5 mL of isopropanol. Samples must be maintained for 10 min at 15°C–30°C before being centrifuged for a maximum of 12,000× g at 2°C–4°C for 10 min. Before centrifugation, the RNA precipitate forms the gel-like pellet on the bottom and side of the tube, which is typically undetectable. The supernatant was eliminated before RNA washing. To wash the RNA pellet once, the Trizol reagent used before was substituted with at least 1 mL of 75% ethanol. The samples were mixed and then centrifuged for 5 min at 2°C to 8°C at no more than 7500× g. The washing process described above was performed once, and then any remaining ethanol was eliminated. To re-dissolve RNA, the RNA pellet was dried for five to ten minutes using air or a vacuum. The solubility of the RNA pellet was significantly reduced if the pellet was allowed to completely dry. The solution was repeatedly pipetted through a pipette tip to dissolve the RNA in DEPC-treated water, resulting in samples with an A260/A280 ratio of less than 1.6.
2.6.4 Spectrophotometric Analysis
39 µL of water treated with DEPC was added to 1 µL of RNA to dilute it (1:40 dilution). OD measurements were determined at 260 and 280 nm in a 10 µL microcuvette to ascertain the material’s purity and concentration. The rule of thumb that 40 µg/mL of RNA = 1 OD at 260 was used.
2.6.5 Primary Strand cDNA Synthesis Reaction
Following the guidelines provided by the manufacturer, cDNAs were created utilizing (First Strand cDNA Synthesis Kit with ThermoScientific Recertification, Lithuania) from 2 µL of total RNA to the 20 µL reaction mixture’s final volume. The cDNA method includes the following components: (dT) 18 Primer, about 1 µL, 200 u/µL of Revert Aid RT, 10 mM dNTP mix (2 u/µL), RiboLock RNase inhibitor (20 u/µL), 5X Reaction Buffer (4 µL), and 9 µL of nuclease-free water were incorporated. Ultimately, adding and combining diethylpyrocarbonate (DEPC) treated water increased the capacity to 20 µL. At 42°C, incubate for 60 min. To stop the process, the mixture was heated at 70°C for five min. Therefore, once obtaining the cDNAs, we chilled them for at least 3–5 min.
Total RNA was extracted using the Promega High-Capacity Access RT-PCR System according to the manufacturer’s instructions, and aliquots of the RNA were reverse transcribed. Sequences of the selected primers for the genes expressing cancer cells (C2orf63, TEX19, GAINTL5, TGIF2LY, RNF17-003, RNF133, P53, and β ACT Housekeeping) were considered from previous studies. This is how the master PCR reaction mix was made: 0.6 µL primer, 2 µL cDNA, and 4 µL PCR volumes by including 12.8 µL ddH2 and employing a negative control, so, the master mix total volume was 20 µL. Thermocycler PXE 0.5 was utilized to conduct semi-quantitative RT-PCR tests. (Thermo Scientific), the cycling program as following: Stage 1: 94°C, 2–4 min; Stage 2 (40 Cycle): 94°C, 30 s, 61.1°C, 1 min; Stage 3: 68°C, 7 min; Stage 4: 4°C. Standard agarose gel electrophoresis was used to evaluate the SqRT-PCR findings. The bands were measured using GelPro32 (Version 4.03).
2.6.7 Agarose Gel Electrophoresis
As previously mentioned, electrophoresis of PCR products was performed by 1.5% Gel that was generated for 90 min at 100 V. UV transelements were used to photograph and show the samples. The reaction products’ sizes were determined with DNA molecular size ranges of 100–1500 bp.
GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA) was utilized in all of the study statistical analyses. The data was analyzed using one-way ANOVA. When the p-values were less than 0.05, significant differences were found. The mean less the standard deviation (SD) represents the results.
3.1 GC-MS Constituents in D. stramonium
GC-MS analysis of D. stramonium methanolic crude extract resulted in eight major secondary products classified as antioxidants that have inhibition properties against tumors and cancer diseases. These products were Nonanoic acid, Tropine N-Oxide, 3,6-Ditigloyloxy-7-hydroxytropane, 2-Pentadecanone,6,10,14-trimethyl-, Carvenone, Hexadecanoic acid, methyl ester, Phytol, Aposcopolamine, Hyoscyamine, 4,8,12,16-Tetramethylheptadecan-4-olide, Scopolamine, Alpha-Tocospiro A, 1,2-Cycloheptanedione, 3,3,7,7-tetramethyl-, dihydrazone, Campesterol, Stigmasterol, Gamma-Sitosterol and dl-alpha-Tocopherol as shown in Table 1.
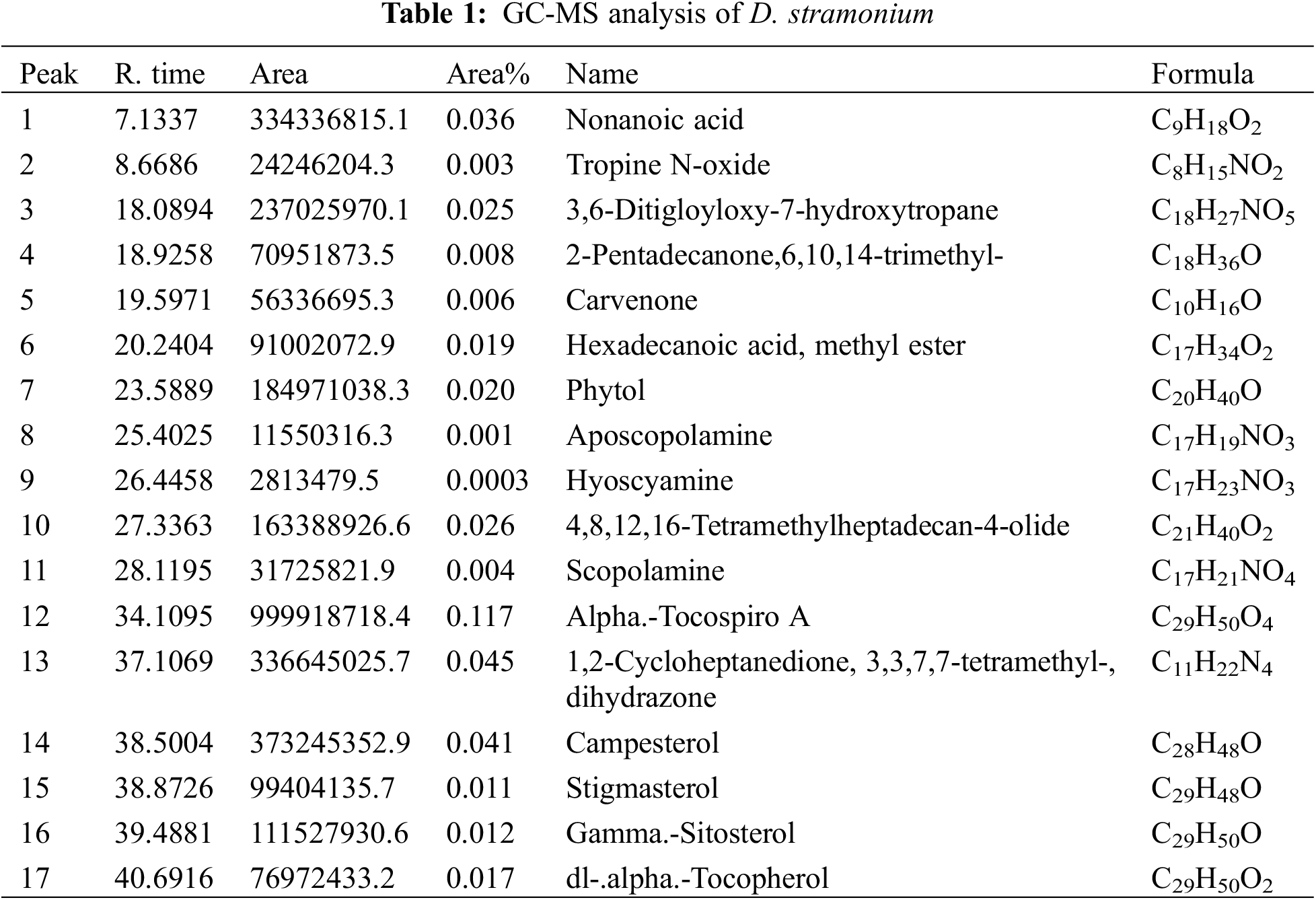
3.2 Impact of D. stramonium Extract on Cancer Cell Lines Morphology
After 24 h of incubation from the last treatment (20 µg/mL) with plant extract, how D. stramonium leaf extract affects cancer cell lines MCF7 and HT29 was evident (Fig. 1a,b). When compared to the samples that were not treated, the treatment had an impact on the density and shape of cancer cells. It is clear that when compared to other concentrations, the effect of concentration at 100 µg/mL is the biggest, followed by concentration at 50 µg/mL. However, compared to other therapies, 20 µg/mL concentration is less efficient. There is no need to perform a negative control sample because the extract has already been dissolved in standard media.
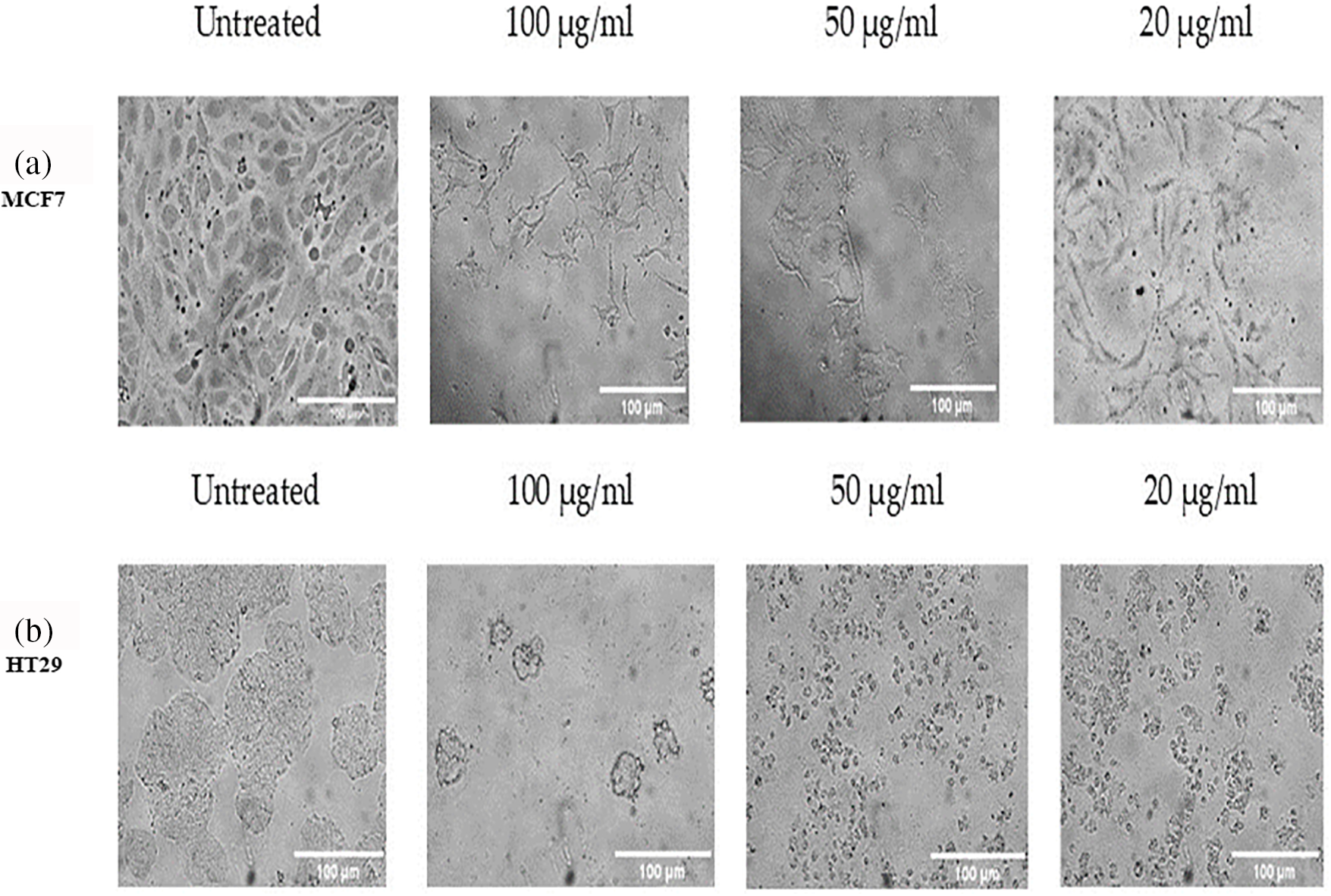
Figure 1: The effect of D. stramonium extract at three concentrations 100, 50 and 20 µg/mL on MCF7 (a), and HT29 (b) cells after 24 h of incubation
3.3 Effects of D. stramonium Extract on the Persistence of Cancer Cell Lines
In two different lines of cancer cells, the potential impact of plant extracts from D. stramonium at various concentrations on cell viability was noted by the MTT Cell Proliferation Assay test (Figs. 2 and 3). When compared to the untreated cells as a positive control, it can be noted that the high concentration (100 µg/mL) significantly recorded 0.134 and 0.259 for MCF7 treated and untreated cells, respectively (Fig. 2), whereas it is more effective than lower concentrations on the HT29 cell line with values of 0.06675 for the extract-treated cells and 0.349 for the untreated ones (Fig. 3). A concentration that kills 50% of the cells (IC50) was determined and used to compare the activity of different preparations. The IC50 values were 63.2 µg/mL for the MCF7 and 47.4 µg/mL for the HT29 groups, respectively. The results prove that the HT29 cell line is the most cytotoxic sensitive one (Figs. 2 and 3).
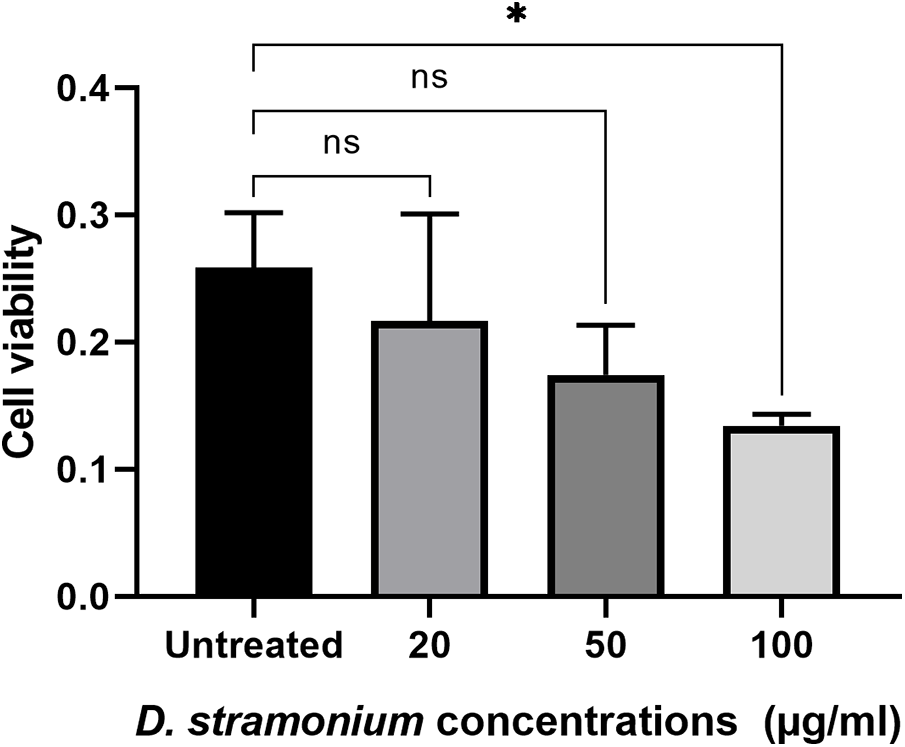
Figure 2: The effect of D. stramonium extract at concentrations (100, 50 and 20 µg/mL) on cell viability of MCF7 cancer cell line using MTT assay. The results are expressed as the mean ± SD (ns, not significant, *p < 0.05)
Figure 3: The effect of D. stramonium extract at concentrations (100, 50 and 20 µg/mL) on cell viability of HT29 cancer cell line using MTT assay. The results are expressed as the mean ± SD (***p < 0.001, ****p < 0.0001)
Expression of the genes C2orf63, TEX19, GAINTL5, TGIF2LY, RNF17-003, RNF133, and P53 was influenced by the impact of different D. stramonium methanolic crude extract dosages on the proliferative activity of MCF7 and HT29 cell lines, demonstrating the relationship between changes in transcription of the cancer antigen (CTA) biosynthesis and the response of two types of the cell line to the addition of D. stramonium crude. So, it was wise to keep an eye on how much cancer would be given priority (Figs. 4 and 5). It has been determined that when compared with the same genes’ expression in cells exposed to the three concentrations of D. stramonium methanolic crude extract, both gene transcript accumulation under untreated cell line type (MCF7) and expression were present. Data in (Fig. 4) demonstrating how the preselected genes affected transcription in cells exposed to various doses of Datura extract revealed that the C2orf63 level of transcripts in cells exposed to 50 µg/mL was 416.97 in comparison to the untreated cells (65.40), whereas cells cultured on medium equipped with 20 µg/mL gave the fold of the GAINTL5 transcript content 175.11 in the cells that received 20 g/mL. Under various rates of exposure to plant extract conditions, the level of RNF17-003 transcript that was gradually increased reached 346.66 with 20 µg/mL. According to the expression patterns of TGIF2LY and P53, the alternative transcript accumulation brought on by exposure to D. stramonium extract was greatly reduced in cell line type 2 HT29 in the cells treated with 100 µg/mL plant extract as shown in (Fig. 5). The C2orf63 transcript level 448.34 increased sharply to 50 µg/mL in the exposed cells, as seen in the expression patterns. Compared to control plants’ transcript levels of 215.25, cells cultivated on medium supplemented with 50 µg/mL of extract produced a P53 transcript value (249.12). Cells treated with D. stramonium methanolic crude extract at 50 and 20 µg/mL concentrations produced greater levels of TEX19 expression, 346.83 and 240.96, respectively. The RNF17-003 gene’s transcript accumulation was observed at various plant extract concentrations, and the control-cultured cells produced a significantly higher level of transcript (251.28) than the cells that were exposed to 100, 50, and 20 µg/mL of plant extract, which managed to score levels of nearly 243.26, 95.01, and 97.28, respectively. It is essential to remember that the level of RNF133 expression (137.64) found in cultured cells grown in media with 50 µg/mL extract was higher than the 110.25 found in control cells. Following recovery, P53 and TGIF2LY transcripts associated with cells maintained on media containing 100 µg/mL data did not show any discernible trend and a non-significant difference when compared to control.
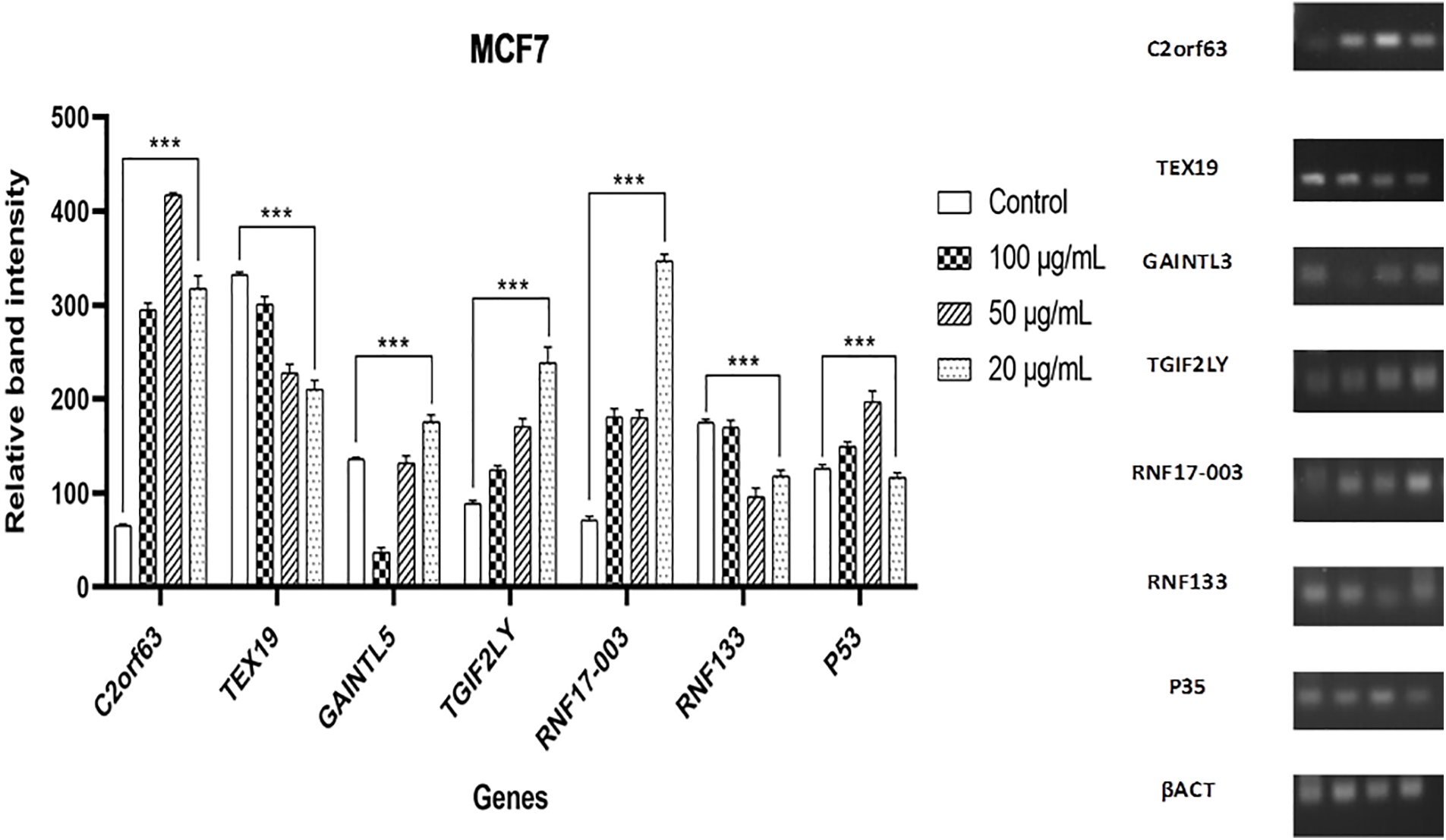
Figure 4: Gene expression of C2orf63, TEX19, GAINTL5, TGIF2LY, RNF17-003, RNF133, and P53 genes in cell line type 1 (MCF7) as determined by RT-PCR. The findings are shown as mean ± SD (***p < 0.001) vs. standard anticancer D. stramonium extract. An internal standard for normalization was actin
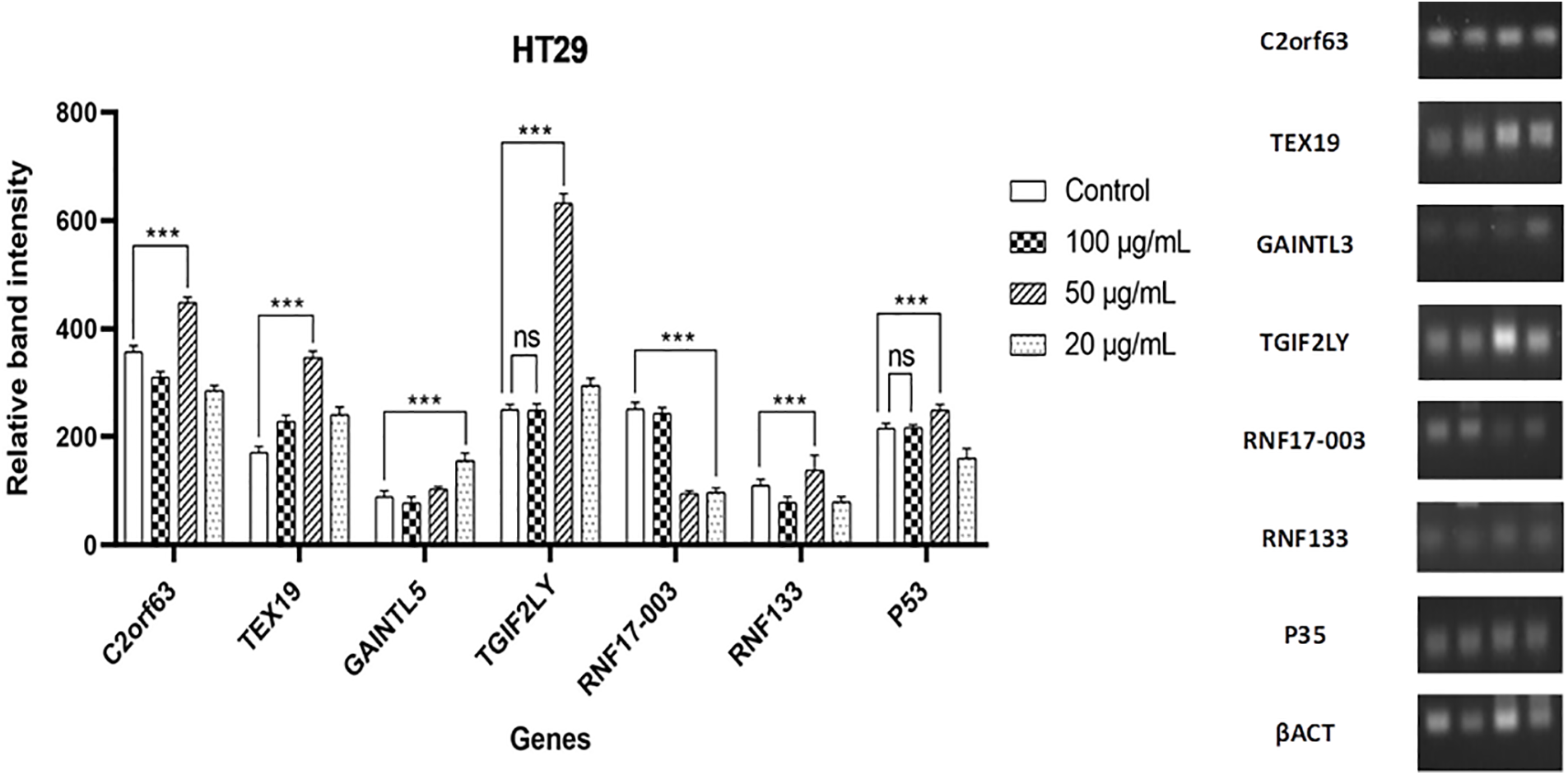
Figure 5: Gene expression of C2orf63, TEX19, GAINTL5, TGIF2LY, RNF17-003, RNF133, and P53 genes in cell line type 2 (HT29) as determined by RT-PCR. The data are presented as the mean ± SD (ns, not significant; ***p < 0.001) vs. standard anticancer D. stramonium extract. Actin was an internal standard for normalization
There were just two tropane alkaloids found in D. stramonium, hyoscyamine, and scopolamine, according to published studies on the substance [36]. A receptor on the plasma membrane or endomembrane surface detects an extracellular or intracellular signal, which triggers a signal transduction network that stimulates transcription factor biosynthesis and regulates the expression of genes involved in plant secondary metabolite biosynthesis. This is the general biological function and regulatory principle for the activation of secondary metabolite biosynthesis in plants [37]. In our knowledge, the three compounds as 2-Pentadecanone, 1,2-Cycloheptanedione, 3,3,7,7-tetramethyl-, dihydrazone, and 6,10,14-trimethyl-, 4,8,12,16-Tetramethylheptadecan-4-olide, have not been reported previously. This result explains the environmental effect of the high altitudes in Taif governorate, which affected the secondary metabolite content in medicinal plants. The capacity of a species to adapt to its environment is essential to its survival in the ecosystem, and most types of plants have evolved a wide variety of metabolic processes to address different environmental challenges [38]. In reaction to environmental changes, plants either modify their physiological and morphological features or adjust their capacity to photosynthesize to preserve a homeostatic rate of photosynthesis [39]. Environmental elements such as soil bacteria, high altitude, high or low temperatures, nutrients, light conditions, drought, soil pH, and water availability are also important, having a significant effect on the quality and quantity of SMs produced by the plants [40]. According to data from the Food and Drug Administration, 74% of the approved molecules are employed in anticancer therapy, and 40% of them are natural substances or compounds inspired by them. The most recent and effective examples of secondary metabolites with strong anticancer potential are described, including compounds of the alkaloid, diterpene, triterpene, and polyphenolic types [41]. Investigating the potential effect of the crude D. stramonium methanolic extract as an anti-cancerous adjuvant, human breast and colon carcinoma cell lines MCF7 and HT29 were performed in this study. The D. stramonium at all concentrations was the most potent on the HT29 cell line regarding cytotoxicity, where the IC50 was 47.4 µg/mL compared to 63.2 µg/mL in the MCF7 group, respectively. Natural substances and some medications can cause proliferation in cancer cells by interfering with actively dividing cells, but not with G0 resting cells [42]. Because of changes in medical thinking, substances derived from plants can now be developed as medicines. Successful cases including paclitaxel and homoharringtonine, which are in clinical usage, and curcumin, and ingenol mebutate which are in clinical trials, were discussed because the focus was on those that are either in clinical trial development or already utilized in anticancer therapy [41]. Datura metel, another species of the genus Datura, has been found to contain several significant phytochemicals, such as withanolides, daturaolone, datumetine, daturglycosides, ophiobolin A, baimantuoluoline A, and many more. These phytochemicals may be responsible for plenty of biological activities, such as wound healing, neurological, anti-inflammatory, anti-microbial, insecticidal, anti-cancer, anti-diabetic, analgesic, antipyretic, anti-inflammatory, anti-inflammatory, anti-microbial, neurological, and anti-diabetic [43]. According to research by [44], breast cancer MCF7 cell lines were utilized to determine the anticancer impact of the plant species D. metel, and the impacts on control Vero cell lines were contrasted, whereas D. stramonium was utilized to treat cancer at a therapeutic dose of 0.05–0.10 mg.
Antioxidants prevent oxidative damage to cells by cooperating with and combating free radicals. The term “free radical scavengers” is another term for antioxidants, which the body produces in some amounts to combat free radicals. Endogenous antioxidants are these compounds. The common pathway for cancer, aging, and many other diseases is cellular damage, which antioxidants play a crucial role in preventing. Because of phytochemical components including beta-damascenone, betaeudesmol, and phytol acetate, which scavenge free radicals and lower the generation of nitric oxide, the Datura plant has antioxidant qualities. By increasing the release of pro-inflammatory cytokines and boosting lymphocyte cytotoxic activity against cancer cells, it also has anti-inflammatory properties. Furthermore, Datura’s anticancer potential relies on its capacity to block cellular signaling pathways that contribute to the formation of cancer, namely in lung and breast tumors, through the action of substances such as cardiac glycoside, flavonoids, alkaloids, and tannins [45]. Besides, several phenolics studied to date possess different pharmacology and pathways to treat different cancers; they are also found to effectively scavenge the multitude of non-biological superoxide radicals, free radicals, nitrogen and chlorine species, hydrogen peroxide, and various reactive oxygen species [22]. These highly reactive ions and molecules are produced during the normal metabolism of cells but are present in higher levels in cancer cells due to increased metabolic activity, mitochondrial dysfunction, peroxisome activity, increased cellular receptor signaling, oncogene activity, increased activity of oxidases, cyclooxygenases, lipoxygenases, and thymidine phosphorylase, or through crosstalk with infiltrating immune cells [2]. Reactive oxygen species (ROS) are managed under normal physiological conditions through detoxification by non-enzymatic molecules such as glutathione or through antioxidant enzymes, which specifically scavenge different kinds of ROS [2].
The maximum superoxide radical inhibition of the D. stramonium seed methanol extract was observed to be 53.17% at a concentration of 60 µg/mL. It was discovered that the methanol extract works well to scavenge superoxide radicals produced by riboflavin’s photoreduction [46]. Aerial sections extracted with methanol of D. metel L. yielded three withanolide glycosides known as daturataturin, daturametelin, and 7,27-dihydroxy-1-oxo with a 2,5,24-trienolide. The antiproliferative effects of each substance were evaluated on the human colorectal cancer cell line (HCT-116). Of the withanolides examined, the highest activity was obtained from the nonglycosidic substance, having 3.2 ± 0.2 µM IC50 [47]. For both Vero and MCF7 cell lines, compared to the stem extract, data revealed that the ethanol extract from the leaves had a higher level of anticancer properties because they had lower IC50 values than the latter. According to reports, the plant’s withanilides, which are steroidal lactones, have a strong anticancer effect on the colorectal cancer (HCT-116) cell line [48]. Calystegines, which have glycosidase inhibitory actions against cancer, are abundant in several Solanaceae species [49]. The findings support the plant’s significant anticancer potential, which has been mentioned in prior papers concerning the species [47]. Our results were by those recorded by [50], who mentioned that by neutralizing free radicals present in different oral mucosal diseases, antioxidants contained in medicinal plants, such as vitamins E, C, and A, lessen mucosal damage. Additionally, the phytochemicals present in medicinal plants can change the cellular defense mechanisms to protect normal cells from ROS and cause oral cancer cells to undergo apoptosis via modifying cell signaling pathways.
Little information is available in the literature on gene expression profile investigations, even though extracts from medicinal plants have been shown to preserve intriguing biological activity, such as anti-inflammatory, anticancer, and antibacterial properties [51–54]. The mRNA was evaluated in the current study using RT-PCR to assess the expression of genes from the cancer-testis (CT) in cancerous cells. Depending on where on the chromosomes they are located, CT genes can be separated into two groups. Genes found on the X chromosome’s X-CT group are normally expressed in both healthy testicles and the placenta during the spermatogonial stage of spermatogenesis. A few X-CT genes that belong to big paralogue families are C2orf63, TEX19, GAINTL5, TGIF2LY, RNF17-003, RNF133, and P53. Of the known CT genes, 52% are X-CT genes [55]. When meiosis occurs, spermatocytes normally express the non-X-CT group that is encoded on the autosomes [56]. SPO11, SCP-1, CT9/3BRDT, BORIS, and BAGE are examples of non-X-CT genes, which are typically discovered as single-copy genes [27]. The results of our investigation were in harmony with the findings of [57], who investigated that the MDA-MB-231 and MCF-7 cell lines were significantly inhibited in their growth by the Kigelia africana ethanol extract, with IC50 values of 20 and 32 μg/mL, respectively, and the mRNA expression levels of apoptotic genes were shown to be influenced by the K. africana ethanolic extract’s IC50 concentration as well, according to quantitative RT-PCR gene expression analysis [57]. Although the majority of spermatogenic germ cells in the testis express CT genes, during several phases of sperm formation, certain CT genes are expressed. Several CT genes have been linked to specific functions in normal tissues, such as cell division [29], and the role of germ cells [58]. For instance, SPO11 is essential for creating DNA double-strand breaks that trigger meiotic homologous recombination [32]. During meiosis 1, synaptonemal complex protein 1 (SYCP1) starts chromosome synapsis [59]. Male germline cells go through meiosis II, during which spermatogenesis regulates the promoter methylation process [60]. One significant cohort of this germ line group is thought to be the CT genes. Although the roles of CT proteins in the testis are not fully understood, several have been implicated in neoplastic processes [61]. Despite having reported that CT genes may be involved in cell development, transcriptional control, potential proto-oncogenes, and genetic instability, there is still a paucity of information about the significance of CT genes in cancer cells [62]. During the development of cancer, CTAs may be essential for controlling complex genes and activating errant genes [63].
According to MTT data recorded by [64], the MCF-7 cancer cells died most when treated with Dioscorea extract at an IC50 concentration (438.35 µg/mL) for 72 h, whereas the control cell line’s cells stayed healthy. The investigation of changes in gene expression also revealed that the rise in Bax gene expression and the reduction in Bcl-2 gene expression after 24 h of treatment with the Dioscorea plant extract expression (2.1 times) at 48 h of therapy, the decrease in Bcl-2 expression was not statistically significant. In contrast to the statistically significant rise in Bax after 72 h of treatment with the plant extract, Bcl-2 gene expression dropped by 0.67 times, and Bax expression rose 2.72 times [64]. Similarly, further evidence that the P53 gene is primarily elevated in cells treated with hexane and methanol Tulbaghia violacea extract raises the possibility that cell death is caused by the P53-dependent pathway [23]. Interestingly, microarrays and macroarrays can also be utilized in toxicogenomic and pharmacogenomic studies, which are designed to analyze in detail how medicinal plant medicines affect target cells’ total gene expression. Thus, Carlo et al. [65] compared the gene expression profile of the K562 cell line with the data from microarrays and discovered that the effects of Emblica officinalis and Moringa oleifera extracts were contrasted.
D. stramonium extract resulted in eight major anticancer inhibition compounds. Nonanoic acid, tropine N-oxide, 3,6-Ditigloyloxy-7-hydroxytropane, 1,2-Cycloheptanedione, 3,3,7,7-tetramethyl-, dihydrazone, hexadecanoic acid, carvenone, 2-pentadecanone,6,10,14-trimethyl-, methyl ester, phytol, aposcopolamine, hyoscyamine, 4,8,12,16-Tetramethylheptadecan-4-olide, Scopolamine, Alpha.-Tocospiro A, Campesterol, Stigmasterol, Gamma. -Sitosterol and dl-.alpha.-Tocopherol. D. stramonium extract confirmed its success in inhibiting cancer cell line development in different stages. Cancer-testis (CT) gene expression was increased in cancer cells (MCF7 and HT29) after exposure to D. stramonium extract, according to our findings. Although D. stramonium extract shows promise in reducing the proliferation of breast and colon cancer cells, additional research is needed to identify the active components of the whole crude extract that target certain cancer cell types. More studies are required to fully understand the various cellular and molecular pathways of cancer cell lines as affected by medicinal plants.
Acknowledgement: The authors extend their appreciations to Taif University, Saudi Arabia, for supporting this work through Project No. (TU-DSPP-2024-74).
Funding Statement: This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2024-74).
Author Contributions: Conceptualization: Fayez Althobaiti, Hadeer Darwish, Saqer S. Alotaibi and Raghad Alruqayb; data curation: Hadeer Darwish, Fahad E. Alharthi, Ibrahim Jafri, Ghadi Alsharif, Jawaher Albaqami and Ahmed Noureldeen, Sarah Awwadh Altalhi; formal analysis: Saqer S. Alotaibi and Ahmed Noureldeen; investigation: Fayez Althobaiti, Hadeer Darwish and Raghad Alruqayb; methodology: Fayez Althobaiti, Hadeer Darwish, Raghad Alruqayb and Ahmed Noureldeen; project administration: Fayez Althobaiti and Hadeer Darwish; resources: Fayez Althobaiti, Hadeer Darwish, Raghad Alruqayb, Sarah Alharthi and Najla Amin T. Al Kashgry; validation: Fayez Althobaiti, Fahad E. Alharthi, Ghadi Alsharif, Hussam Awwadh E Althagafi, Sarah Altalhi and Sarah Alharthi; visualization: Hadeer Darwish, Ibrahim Jafri, Sarah Altalhi, Hesham Noureldeen, Najla Amin T. Al Kashgry and Ahmed Noureldeen; writing—original draft: Fayez Althobaiti, Hadeer Darwish, Raghad Alruqayb and Jawaher Albaqami; writing—review and editing: Hadeer Darwish, Fahad E. Alharthi, Sarah Altalhi, Hussam Awwadh E Althagafi, Hesham Noureldeen and Ahmed Noureldeen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Raven PH. Plants make our existence possible. Plants People Planet. 2021;3(1):2–6. doi:10.1002/ppp3.v3.1. [Google Scholar] [CrossRef]
2. Asuzu PC, Trompeter NS, Cooper CR, Besong SA, Aryee ANA. Cell culture-based assessment of toxicity and therapeutics of phytochemical antioxidants. Molecules. 2022;27(3):1087. doi:10.3390/molecules27031087. [Google Scholar] [PubMed] [CrossRef]
3. Kaur J, Mahey S, Ahluwalia P, Joshi R, Kumar R. Role of plant secondary metabolites as anticancer and chemopreventive agents. In: Sharma AK, Sharma A, editor. Plant secondary metabolites. Singapore: Springer; 2022. p. 97–119. [Google Scholar]
4. Maheshwari NO, Khan A, Chopade BA. Rediscovering the medicinal properties of Datura sp.: a review. J Med Plants Res. 2013;7(39):2885–97. [Google Scholar]
5. Nasir B, Baig MW, Majid M, Ali SM, Khan MZI, Kazmi STB, et al. Preclinical anticancer studies on the ethyl acetate leaf extracts of Datura stramonium and Datura inoxia. BMC Complementary Med Therap. 2020;20:188. doi:10.1186/s12906-020-02975-8. [Google Scholar] [PubMed] [CrossRef]
6. Gaire BP, Subedi L. A review on the pharmacological and toxicological aspects of Datura stramonium L. J Int Med. 2013;11(2):73–9. [Google Scholar]
7. Mukhtar Y, Tukur S, Bashir RA. An overview on Datura stramonium L (jimson weeda notable psychoactive drug plant. Am J Nat Sci. 2019;2(1):1–9. [Google Scholar]
8. Berkov S, Zayed R, Doncheva T. Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia. 2006;77(3):179–82. doi:10.1016/j.fitote.2006.01.002. [Google Scholar] [PubMed] [CrossRef]
9. Hübschmann H. Handbook of GC/MS: Fundamentals and Applications. 3rd ed. Wiley-VCH: Wiley Online Library; 2015. [Google Scholar]
10. Khan S, Haritha CV, Manjusha KM, Banu SA. Toxicological aspects of common plant poisoning in ruminants. Indian Farmer. 2019;6(11):812–22. [Google Scholar]
11. Benítez G, March-Salas M, Villa-Kamel A, Cháves-Jiménez U, Hernández J, Montes-Osuna N, et al. The genus Datura L. (Solanaceae) in mexico and spain-ethnobotanical perspective at the interface of medical and illicit uses. J Ethnopharmacol. 2018;219:133–51. doi:10.1016/j.jep.2018.03.007. [Google Scholar] [PubMed] [CrossRef]
12. Steenkamp PA, Harding NM, van Heerden FR, van Wyk B. Fatal Datura poisoning: identification of atropine and scopolamine by high performance liquid chromatography/photodiode array/mass spectrometry. Forensic Sci Inter. 2004;145(1):31–9. doi:10.1016/j.forsciint.2004.03.011. [Google Scholar] [PubMed] [CrossRef]
13. Monteiro LS, Bastos KX, Barbosa-Filho J, De Athayde-Filho PF, Diniz MM, Sobral MV. Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid Based Complement Altern Med. 2014;604152. doi:10.1155/2014/604152. [Google Scholar] [PubMed] [CrossRef]
14. Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anticancer activity. Sci World J. 2014;721402. doi:10.1155/2014/721402. [Google Scholar] [PubMed] [CrossRef]
15. Chandan G, Kumar C, Verma MK, Satti NK, Saini AK, Saini RV. Datura stramonium essential oil composition and it’s immunostimulatory potential against colon cancer cells. 3Biotech. 2020;10(10):451. [Google Scholar]
16. Al-Snafi A. Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium—a review. IOSR J Pharm. 2017;7(2):43–58. [Google Scholar]
17. Kaur R, Arora S. Alkaliids important therapeutic secondary metabolites of plant origin. J Crit Rev. 2015;3:1–8. [Google Scholar]
18. Mathur G. Cancer: an overview. Acad J Cancer Res. 2015;8:1. [Google Scholar]
19. Kumari M. Cancer notes. In: Unit 1. Nutrition in cancer. India: Amity Institute of Food Technology, AMITY University; 2020. p. 1–15. [Google Scholar]
20. Hejmadi M. Introduction to cancer biology. Denmark: Bookboon, Ventus Publishing; 2014. [Google Scholar]
21. Ferreira D, Adega F, Chaves R. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In: López-Camarillo C, Aréchaga-Ocampo E, editors. Oncogenomics and cancer proteomics-novel approaches in biomarkers discovery and therapeutic targets in cancer. Mexico: IntechOpen; 2013. p. 139–66. [Google Scholar]
22. Maheshwari N, Sharma MC. Anticancer properties of some selected plant phenolic compounds: future leads for therapeutic development. J Herb Med. 2023;42:100801. doi:10.1016/j.hermed.2023.100801. [Google Scholar] [CrossRef]
23. Lesetja RM, Mpho SC, Nonkululeko NM. Anticancer properties of Tulbaghia violacea regulate the expression of p53-dependent mechanisms in cancer cell lines. Sci Rep. 2020;10(1):12924. doi:10.1038/s41598-020-69722-4. [Google Scholar] [PubMed] [CrossRef]
24. Mirabelli P, Coppola L, Salvatore M. Cancer cell lines are useful model systems for medical research. Cancers. 2019;11(8):1098. doi:10.3390/cancers11081098. [Google Scholar] [PubMed] [CrossRef]
25. Chen Z, Hsieh Y, Huang C, Tsai C. Inhibitory effects of probiotic lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules. 2017;22(1):107. doi:10.3390/molecules22010107. [Google Scholar] [PubMed] [CrossRef]
26. Camarillo IG, Xiao F, Madhivanan S, Salameh T, Nichols M, Reece LM, et al. 4-low and high voltage electrochemotherapy for breast cancer: an in vitro model study. In: Sundararajan R, editor. Electroporation-based therapies for cancer. UK: Woodhead Publishing; 2014. p. 55–102. [Google Scholar]
27. Whitehurst AW. Cause and consequence of cancer/testis antigen activation in cancer. Ann Rev Pharmacol Toxicol. 2014;54(1):251–72. doi:10.1146/annurev-pharmtox-011112-140326. [Google Scholar] [PubMed] [CrossRef]
28. McFarlane RJ, Feichtinger J, Larcombe L. Germline/meiotic genes in cancer: new dimensions. Cell Cycle. 2015;14(6):791. doi:10.1080/15384101.2015.1010965. [Google Scholar] [PubMed] [CrossRef]
29. Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 is commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106(1):167–74. doi:10.1182/blood-2004-12-4931. [Google Scholar] [PubMed] [CrossRef]
30. Grizzi F, Franceschini B, Hamrick C, Frezza EE, Cobos E, Chiriva-Internati M. Usefulness of cancer-testis antigens as biomarkers for the diagnosis and treatment of hepatocellular carcinoma. J Trans Med. 2007;5(1):1–11. doi:10.1186/1479-5876-5-3. [Google Scholar] [PubMed] [CrossRef]
31. Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother. 2009;58(4):589–1. doi:10.1007/s00262-008-0582-6. [Google Scholar] [PubMed] [CrossRef]
32. Hussain AI, Anwar F, Sherazi ST, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–95. doi:10.1016/j.foodchem.2007.12.010. [Google Scholar] [PubMed] [CrossRef]
33. Adam GC, Cravatt BF, Sorensen EJ. Profiling the specific reactivity of the proteome with non-directed activity-based probes. Chem Biol. 2001;8:81–95. doi:10.1016/S1074-5521(00)90060-7. [Google Scholar] [PubMed] [CrossRef]
34. Darwish H, Alharthi S, Mehanna RA, Ibrahim SS, Fawzy MA, Alotaibi SS, et al. Evaluation of the anti-cancer potential of Rosa damascena Mill. Callus extracts against the human colorectal adenocarcinoma cell line. Molecules. 2022;27(19):6241. doi:10.3390/molecules27196241. [Google Scholar] [PubMed] [CrossRef]
35. Althobaiti FA. Functional characterisation of SPO11 in human cancer cells (Ph.D. Thesis). University of Bangor: UK; 2017. [Google Scholar]
36. Yamada T, Ohta K. Initiation of meiotic recombination in chromatin structure. J Biochem. 2013;154(2):107–14. doi:10.1093/jb/mvt054. [Google Scholar] [PubMed] [CrossRef]
37. Zhao J, Davis LC, Verporte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–33. doi:10.1016/j.biotechadv.2005.01.003. [Google Scholar] [PubMed] [CrossRef]
38. Jakada BH, Aslam M, Fakher B, Greaves JG, Li Z, Li W, et al. Identification of SWI2/SNF2-related 1 chromatin remodeling complex (SWR1-C) subunits in pineapple and the role of pineapple SWR1 COMPLEX 6 (AcSWC6) in biotic and abiotic stress response. Biomolecules. 2019;9:364. doi:10.3390/biom9080364. [Google Scholar] [PubMed] [CrossRef]
39. Najafabadi M, Ehsanzadeh P. Photosynthetic and antioxidative upregulation in drought-stressed sesame (Sesamum indicum L.) subjected to foliar-applied salicylic acid. Photosynthetica. 2017;55:611–22. doi:10.1007/s11099-017-0673-8. [Google Scholar] [CrossRef]
40. Gong J, Zhang Z, Zhang C, Zhang J, Ran A. Ecophysiological responses of three tree species to a high-altitude environment in the southeastern Tibetan plateau. Forests. 2018;9:48. doi:10.3390/f9020048. [Google Scholar] [CrossRef]
41. Seca AML, Pinto DCGA. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci. 2018;19(1):263. doi:10.3390/ijms19010263. [Google Scholar] [PubMed] [CrossRef]
42. Boik J. Results of therapy at the cellular level. In: Scarpino S, Morena AR, editors. Natural compound cancer therapy. 1st ed. USA: Organ Medical Press; 2001. p. 29–31. [Google Scholar]
43. Islam T, Ara I, Islam T, Sah PK, de Almeida RS, Matias EFF, et al. Ethnobotanical uses and phytochemical, biological, and toxicological profiles of Datura metel L.: a review. Curr Res Toxicol. 2023;4:100106. doi:10.1016/j.crtox.2023.100106. [Google Scholar] [PubMed] [CrossRef]
44. Nazeema B, Julie J, Abirami J, Kumareasan R, Muthukumaran T, Rajasree S, et al. Anti-cancer activity of Datura metel on MCF-7 cell line. Asian J Pharm Clin Res. 2014;7:181–3. [Google Scholar]
45. Chouhan AS, Bharadwaj R, Baxi M, Zhylkybekova A. Evaluating the therapeutic potential of Datura stramonium and Datura inoxia: a mini review. West Kazakhstan Med J. 2024;66(2):119–25. doi:10.18502/wkmj.v66i2.16454. [Google Scholar] [CrossRef]
46. Saleem I, Chandrasekaran S, Krishnasamy G. Antioxidant and anticancer activities of methanol extract of seeds of Datura stramonium. Free Radic Antioxid. 2017;7(2):184–9. doi:10.5530/fra. [Google Scholar] [CrossRef]
47. Ma L, Xie CM, Li J, Lou FC, Hu LH, Daturametelins HI. Three new withanolide glycosides from Datura metel L. Chem Biodiv. 2006;3:180–6. doi:10.1002/cbdv.v3:2. [Google Scholar] [CrossRef]
48. Nash RJ, Rothschild M, Porter EA, Watson A, Waigh RD, Waterman PG. Calystegines in Solanum and Datura species and the death’s-head hawk-moth (Acherontia atropus). Phytochem. 1993;34:1281–3. doi:10.1016/0031-9422(91)80016-T. [Google Scholar] [CrossRef]
49. Jacob GS. Glycosylation inhibitors in biology and medicine. Cur Biol. 1995;5:605–11. [Google Scholar]
50. Prakash S, Radha, Kumar M, Kumari N, Thakur M, Rathour S, et al. Plant-based antioxidant extracts and compounds in the management of oral cancer. Antioxidants. 2021;10(9):1358. doi:10.3390/antiox10091358. [Google Scholar] [PubMed] [CrossRef]
51. Yang P, Zhu W, Xu J, Liu W, Dong Z, Kikuchi T, et al. Sesquiterpenoids and triterpenoids from Secamone lanceolata Blume with inhibitory effects on nitric oxide production. Fitoterapia. 2019;133:5–11. doi:10.1016/j.fitote.2018.11.016. [Google Scholar] [PubMed] [CrossRef]
52. Hajrah NH, Abdul WM, Al-Garni SM, Sheikh A, Ahmed MMM, Hall N, et al. Gene expression profiling to elucidate the pharmacological and toxicological effects of Ricinus communis L. leaf extract in mammalian cells. Biotechnol Biotechnol Equip. 2019;33(1):397–407. doi:10.1080/13102818.2019.1578691. [Google Scholar] [CrossRef]
53. Gupta V, Guleri R, Gupta M, Kaur N, Kaur K, Kumar P, et al. Anti-neuroinflammatory potential of Tylophora indica (Burm. F) Merrill and development of an efficient in vitro propagation system for its clinical use. PLoS One. 2020;15:1–20. [Google Scholar]
54. Chandraa S, Gahlot M, Choudhary AN, Palai S, de Almeida RS, de Vasconcelos JEL, et al. Scientific evidences of anticancer potential of medicinal plants. Food Chem Adv. 2023;2:100239. doi:10.1016/j.focha.2023.100239. [Google Scholar] [CrossRef]
55. Rajagopalan K, Mooney SM, Parekh N, Getzenberg RH, Kulkarni P. A majority of the cancer/testis antigens are intrinsically disordered proteins. J Cell Biochem. 2011;112(11):3256–67. doi:10.1002/jcb.v112.11. [Google Scholar] [CrossRef]
56. Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100(11):2014–21. doi:10.1111/cas.2009.100.issue-11. [Google Scholar] [CrossRef]
57. Aasia K, Awais A, Huma S, Tahir M, Muhammad S, Muhammad IJ, et al. Gene expression and anticancer evaluation of Kigelia africana (Lam.) extracts using MDA-MB-231 and MCF-7 cell lines. PLoS One. 2024;19(6):e0303134. doi:10.1371/journal.pone.0303134. [Google Scholar] [PubMed] [CrossRef]
58. Gedye C, Quirk J, Browning J, Svobodová S, John T, Sluka P, et al. Cancer/testis antigens can be immunological targets in clonogenic CD133 melanoma cells. Cancer Immunol Immunother. 2009;58(10):1635–46. doi:10.1007/s00262-009-0672-0. [Google Scholar] [PubMed] [CrossRef]
59. Schramm S, Fraune J, Naumann R, Hernandez-Hernandez A, Höög C, Cooke HJ, et al. A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination, and fertility. PLoS Genet. 2011;7(5):e1002088. doi:10.1371/journal.pgen.1002088. [Google Scholar] [PubMed] [CrossRef]
60. Klenova EM, Morse HC, Ohlsson R, Lobanenkov VV. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol. 2002;12(5):399–14. doi:10.1016/S1044-579X(02)00060-3. [Google Scholar] [PubMed] [CrossRef]
61. Cheng Y, Wong EW, Cheng CY. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis. 2011;1(3):209–20. doi:10.4161/spmg.1.3.17990. [Google Scholar] [PubMed] [CrossRef]
62. Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen Y. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunolog Rev. 2002;188(1):22–32. doi:10.1034/j.1600-065X.2002.18803.x. [Google Scholar] [PubMed] [CrossRef]
63. Mirandola LJ, Cannon M, Cobos E, Bernardini G, Jenkins MR, Kast WM, et al. Cancer testis antigens: novel biomarkers and targetable proteins for ovarian cancer. Intern Rev Immun. 2011;30(2–3):127–37. [Google Scholar]
64. Fatemeh BD, Mahsa K, Faranak J. Effect of Dioscorea extract on Bax and Bcl-2 gene expression in MCF-7 and HFF cell lines. Egyptian J Med Hum Genet. 2023;24(70):1–11. [Google Scholar]
65. Carlo M, Alessia S, Mahmud THK, Laria L, Roberto G. Effects of plant extracts on gene expression profiling: from macroarrays to microarray technology. Adv Phytomed. 2006;2:21–33. doi:10.1016/S1572-557X(05)02002-7. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools