 Open Access
Open Access
ARTICLE
One Novel Nortriterpenoid from the Mastic (Pistacia lentiscus) and Its Anti-Inflammatory Activity
1 Key Lab of Natural Product Chemistry and Application at Universities of Education Department of Xinjiang Uygur Autonomous Region, School of Chemistry and Chemical Engineering, Yili Normal University, Yining, 835000, China
2 Xinjiang Key Laboratory of Clean Conversion and High Value Utilization of Biomass Resources, School of Chemistry and Chemical Engineering, Yili Normal University, Yining, 835000, China
3 Jiangxi Key Laboratory for Postharvest Technology and Nondestructive Testing of Fruits and Vegetables, College of Agronomy, Jiangxi Agricultural University, Nanchang, 330045, China
* Corresponding Authors: Wei Liu. Email: ; Chunpeng Wan. Email:
Phyton-International Journal of Experimental Botany 2025, 94(1), 199-207. https://doi.org/10.32604/phyton.2025.059581
Received 11 October 2024; Accepted 16 December 2024; Issue published 24 January 2025
Abstract
A novel pair of oleanane nor-triterpenes, with compound 1 featuring a unique 18α-H structure, was isolated from mastic, and this compound represents a noteworthy new entity not previously reported in the literature. The absolute configurations of their structures were further determined using a combination of different analytical methods such as NMR, high-resolution mass spectrometry (HR-MS), ultraviolet (UV), infrared (IR) and single-crystal X-ray diffraction (SXRD). The compound actively mitigated inflammations by efficiently quenching nitric oxide (NO) synthesis within an ex vivo system using lipopolysaccharide activated murine macrophage RAW264.7 cells. Moreover, compound 1 exhibit a better IC50 concentration of 11.69 ± 1.39 µM, surpassing the efficacy of the positive control dexamethasone, which exhibited an IC50 of 23.21 ± 1.17 μM. While compound 2 also demonstrated inhibitory activity, its potency was comparatively weaker, with an IC50 of 26.18 ± 2.66 μM.Keywords
Supplementary Material
Supplementary Material FilePentacyclic triterpenoids, owing to their remarkable pharmacological activities, have emerged as promising structural types for new drug candidates, capturing the attention of researchers worldwide [1,2]. Pentacyclic triterpenoids are found to exert anti-inflammatory influences in the majority of studies. Martín and colleagues have established that oleanolic acid has significant anti-Th1 cell-mediated inflammatory diseases [3]. The following study also showed that glycyrrhizic acid inhibits CVB-3-induced myocarditis by down-regulating tumor necrosis factors [4]. Saikosaponin exhibits strong anti-inflammatory potential, and it could decrease neuroinflammation and its associated neurodegeneration by inhibiting nuclear factor-κB (NF-κB) mediated downstream signaling cascades in vivo [5]. It is observed that lupeol exhibited anti-neuroinflammatory properties in lipopolysaccharide (LPS)-induced microglial neuroinflammation through mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways and has the potential to treat various neuroinflammatory diseases [6]. To date, four triterpenes have been approved for clinical use in humans [7]. However, further research is limited not only by expensive natural product sources but also by the complex procedures of chemical extraction and synthesis [7].
Mastic is a naturally occurring resin isolated from the stem or twig cuts of Pistacia lentiscus L. (Family Anacardiaceae), abundantly found in Southern regions of Chios, Greece [8,9]. Mastic is reported to have great applications to cure gastrointestinal disorders including indigestion, abdominal pain, and ulcer craters in classical Greek medicine. It was also used as a spice in foods, beverages, and cosmetics preparation [10–12]. Since the 1980s, from the literature on mastic, about 200 chemical compounds have been found including monoterpenes, sesquiterpenes, and triterpenes; and current research on its extracts has revealed that it possesses considerable anti-inflammatory, anticancer, and antioxidant [13,14]. Further chemical study of mastic for the anti-inflammatory activity and bioactive constituents [15,16], this research has led to the identification and characterization of a novel 18α-H nor-triterpenoid compound, designated as (1), its diastereoisomer, the known 18β-H nor-triterpenoid (2). Therefore, the isolation, structural identification, plausible biogenetic pathways, and discussion encompass the inhibitory impacts of the isolated compounds on NO synthesis in LPS-induced RAW 264.7 cells.
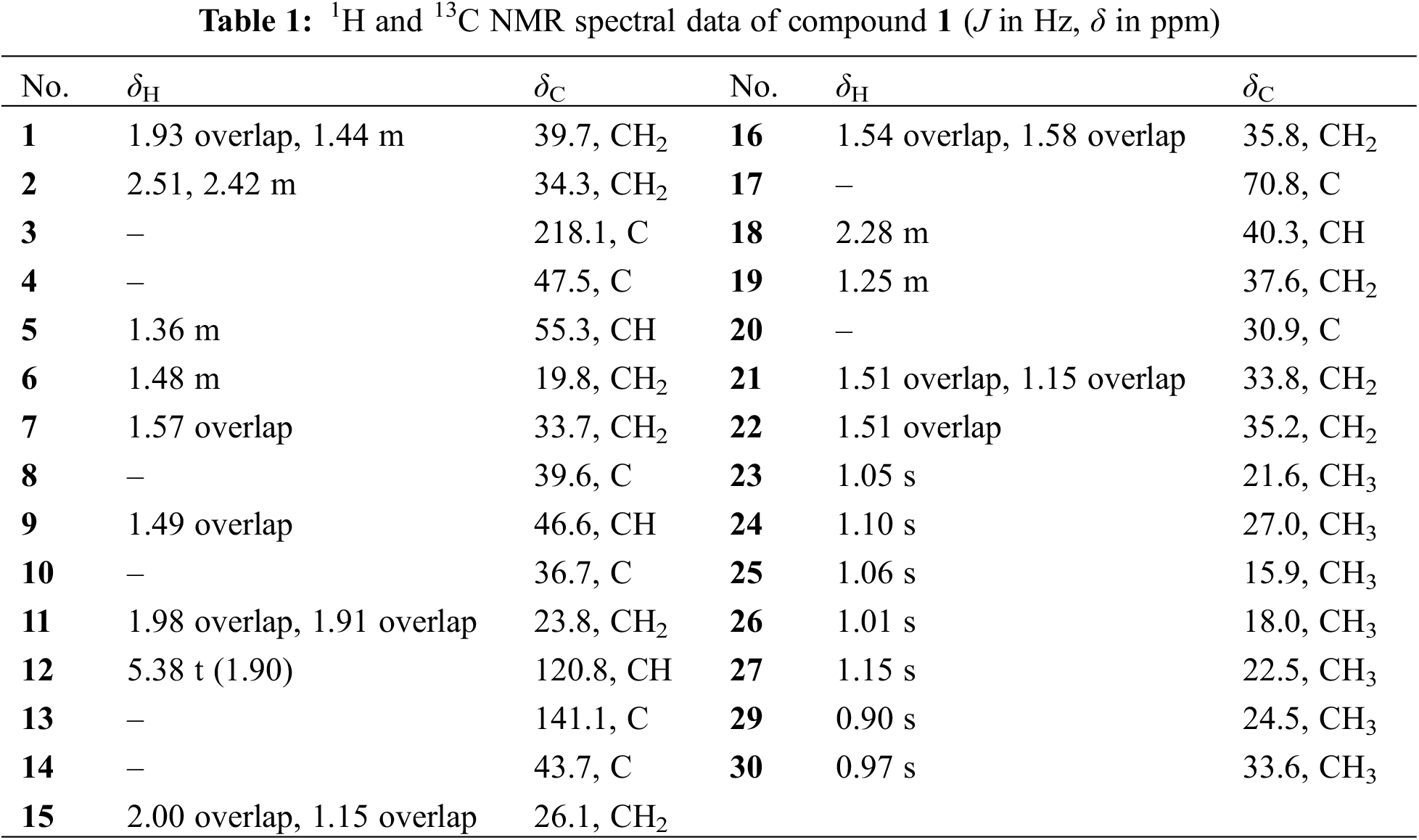
The compound 1 was in the form of colorless needle shaped crystals having the molecular formula of C29H46O2, was confirmed HR-ESI-MS analysis. This exhibited an ion peak at m/z 427.3585 [M + H]+ (calcd for C29H47O2, 427.6576) (Fig. S1), supported by the data obtained from 13C nuclear magnetic resonance (NMR) spectroscopy, with 7 degrees of unsaturation. The diagnostic 1D NMR data (Figs. S2 and S3) implied the presence of an olefin signal (δH5.38, 1H, t, J = 1.9 Hz; δC120.8, 141.1), a carbonyl signal (δC218.1), an oxygenated carbon signal (δC70.8) and 7 methyls (δH 1.15, 1.10, 1.06, 1.05, 1.01, 0.97, 0.90, each 3H, all s). The information presented indicates that compound 1 is an oleanane nor-triterpenoid.
The plane structure of compound 1 was elucidated by interpreting its 2D NMR data, which included HSQC, 1H-1H COSY, and HMBC analyses (Figs. S4–6). 1 was assigned to 7 methyls, 10 methylenes, 4 methines (together with one olefin carbon) and 8 quaternary carbons (including one carbonyl carbon, one oxygen-containing carbon and one olefin carbon) by HSQC spectrum. Fig. 1 illustrates that the 1H-1H COSY spectrum disclosed the existence of six spin-spin coupling segments. The HMBC correlations delineated the connectivity as follows from H3-23/H3-24 to C-3 (δC 218.1), C-4 (δC 47.5), and C-5 (δC 55.3); from H3-25 to C-1 (δC 39.7), C-5, C-9 (δC 46.6) and C-10 (δC 36.7); from H3-26 to C-7 (δC 33.7), C-8 (δC 39.6), C-9 and C-14 (δC 43.7); from H3-27 to C-8, C-13 (δC 141.1), C-14 and C-15 (δC 26.1); from H-12 to C-9, C-14 and C-18 (δC 40.3); from H3-29/H3-30 to C-19 (δC 37.6), C-20 (δC 30.9) and C-21 (δC 33.8); from H2-15, H2-16, H2-19, H2-21 and H2-22 to C-17 (δC 70.8), 1 was established as an oleanane nor-triterpenoid, and its plane configuration was depicted.
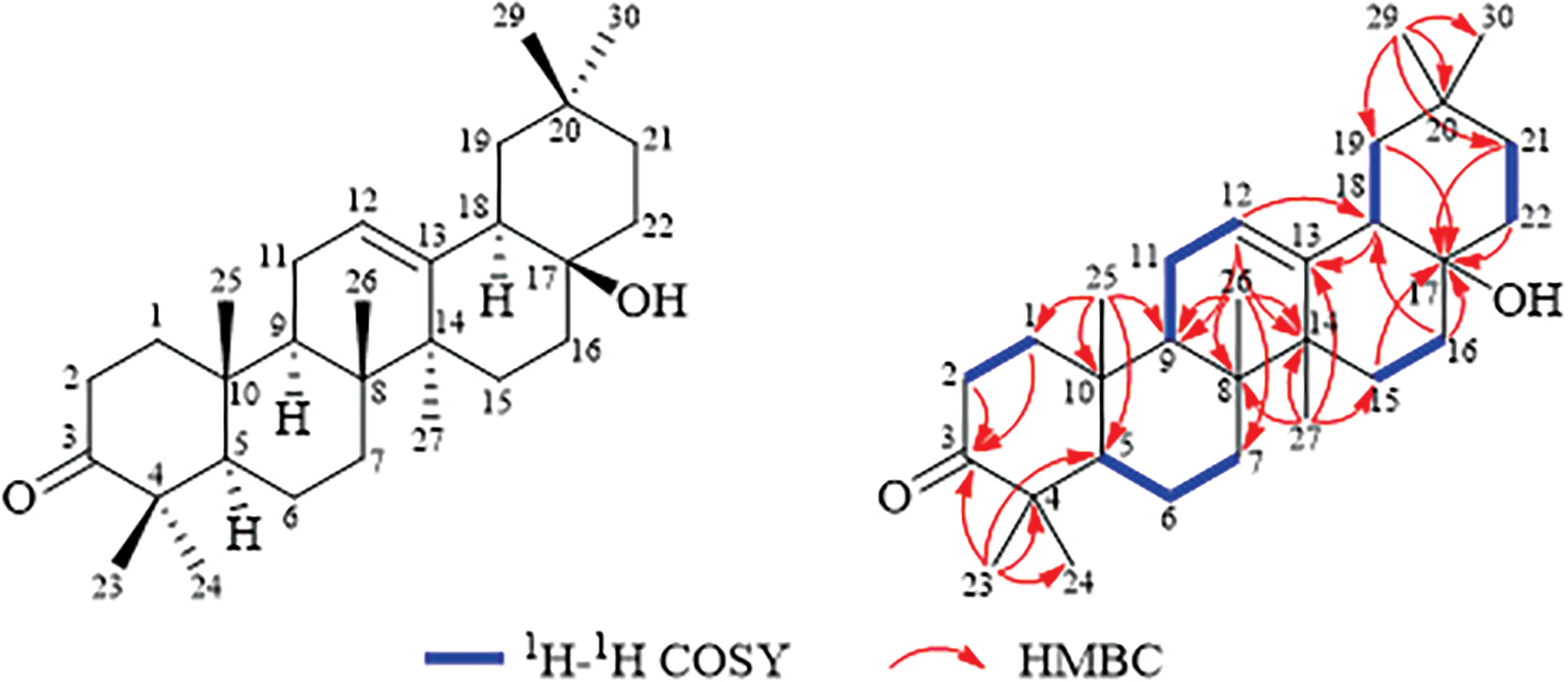
Figure 1: Structure and key COSY, HMBC correlations of compound 1
Compound 1 has the same planar structure as 3-oxoolean-12-en-28-ol [17], known as compound 2 isolated from mastic. The NMR data concerning the region C-16 to C-19 seems contradictory, and it has been identified that additional knowledge regarding the relative configuration of compound 1 is needed. which requires further study to determine the relative configuration of 1. NOESY (Fig. S7) correlations depicted in Fig. 2, which connect H3-27 to H-9 and H-18, H-18 to H3-30, and H-5 to H3-24, suggest that these protons are positioned co-facial to each other and are designated with an α-orientation. Based on these findings, the alignment of H-18 is confirmed to be in the α-configuration for compound 1, contradicting the typical configuration observed in oleanane triterpenes. In addition, the orientation of the 17-OH could not be determined through 2D NMR correlation data.
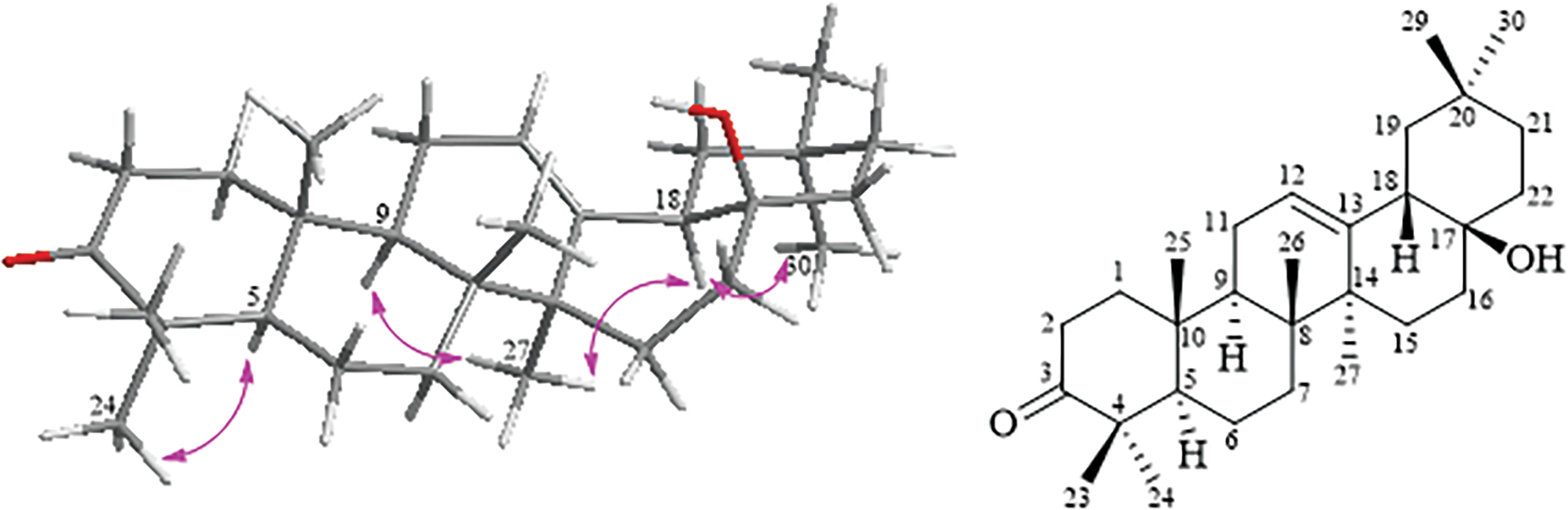
Figure 2: Key NOESY correlations for compound 1 (left) and structural for compound 2 (right)
We were fortunate to acquire single crystals for both compounds 1 and 2 from a methanol solution, which allowed us to definitively determine their absolute configurations via X-ray single-crystal diffraction studies (Fig. 3). Ultimately, the crystallographic analysis via X-ray diffraction of compound 1 disclosed its enantiomeric configuration as 5R, 8R, 9R, 10R, 14S, 17S, 18R, accompanied by a Flack parameter of 0.03(16). Similarly, the β-orientation of both H-18 and OH-17 in 2 was elucidated.
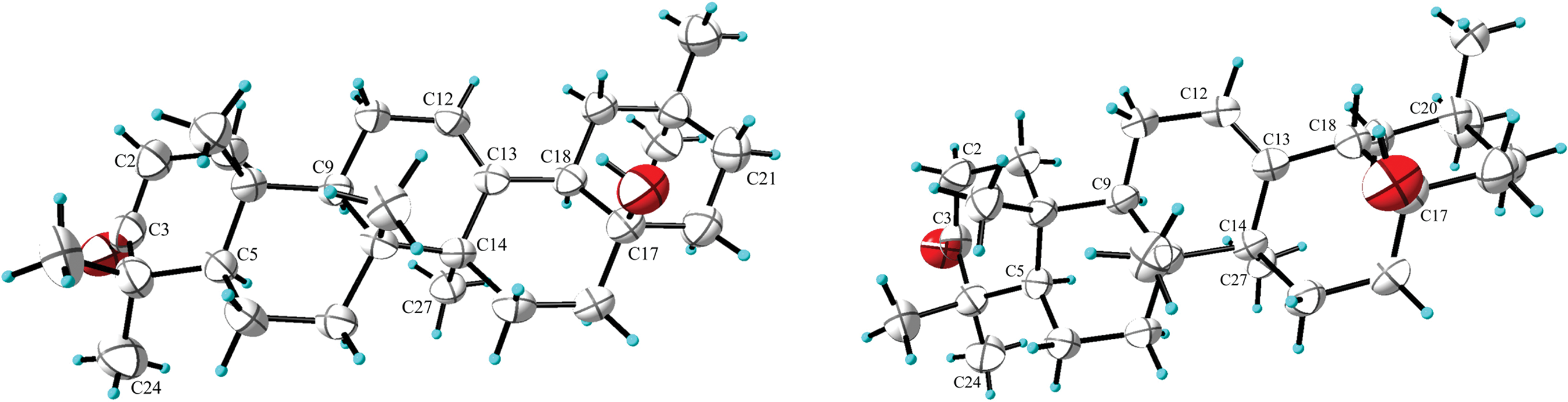
Figure 3: Single crystal diffraction conformation of compounds 1 (left) and 2 (right)
2.2 Elucidation of Potential Biosynthetic Pathways
Within the pentacyclic triterpenes, the α-orientation of H-18 is quite uncommon, with only the 18α and 18β isomers of glycyrrhizinic acid having been identified in Glycyrrhiza glabra [18]. A speculative metabolic pathway for the synthesis of compounds 1 and 2, starting from the assumed squalene precursor, has been delineated in Scheme 1. Initially, squalene synthase catalyzes the dimerization of farnesyl diphosphate (FPP) to yield squalene, which is subsequently converted into 2,3-oxidosqualene through the action of squalene epoxidase (SQE) [19]. In the presence of the NiOSC4 enzyme, 2,3-oxidosqualene undergoes a cyclization reaction to yield the dammarenyl cation [20]. The dammarenyl cation subsequently experiences ring expansion, yielding the 6,6,6,6-baccharccnyl cationin this scenario, π electrons can assail the carbonium ion from either direction [21,22], culminating in the generation of intermediates with distinct H-18 orientations. Subsequently, a set of chiral isomers with distinct 18-H orientations is produced through a sequence of reactions that involve ring expansion, carboxylation, decarboxylation, hydrolysis and oxidation.
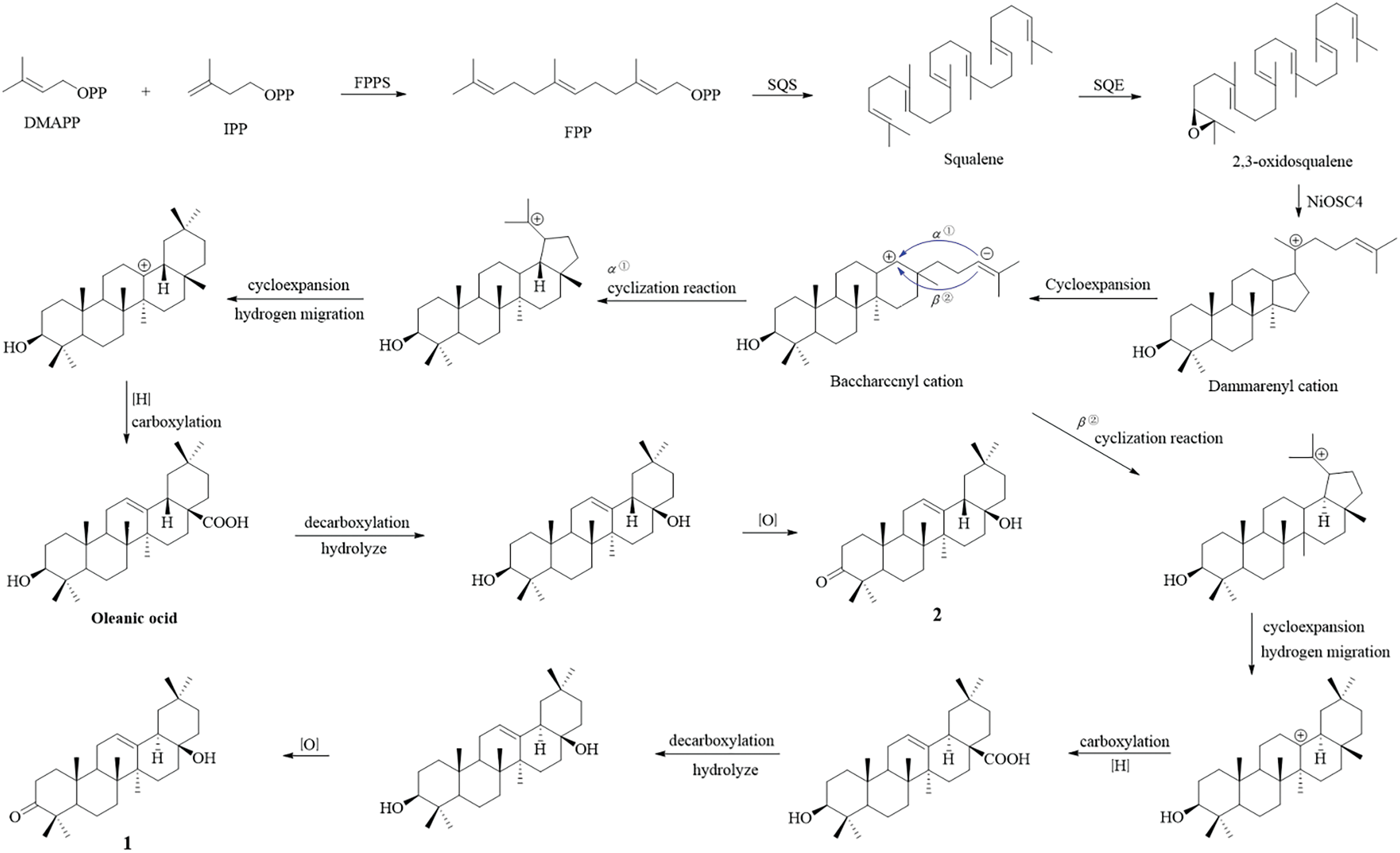
Scheme 1: Plausible biosynthetic pathways for compounds 1 and 2
2.3 Anti-Inflammatory Activity Assay
The subsequent cytotoxicity assay data demonstrated that at a concentration of 80 μM, compounds 1 and 2 yields survival rate of 68.6% and 79.0% in RAW 264.7 cells, respectively, which is similar to the positive control dexamethasone and hence rules out the chance of a false positive results in the inhibition of NO formation. Compound 1 displayed marked anti-inflammatory properties, specifically in the inhibition of NO generation in the RAW264.7 cells, showed an IC50 value of 11.69 ± 1.39 μM, pared with the positive control dexamethasone (IC50 = 23.21 ± 1.17 μM) and compound 2 (IC50 = 26.18 ± 2.66 μM).The obtained results correlate with the literature data that describe compounds possessing the H-18 α-orientation structural characteristic as more active than those with β-H orientation [23,24].
3.1 Routine Experimental Techniques
Optical rotations were obtained through a digital Autopol-IV polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA). Infrared (IR) spectral data was obtained with a Cary 630 spectrometer manufactured (Agilent Technology). The nuclear magnetic resonance (NMR) (Bruker AV-400, Berlin, Germany) spectra were obtained by using TMS standards. X-single-crystal diffraction data were obtained by using XtalABoxford diffraction (Tokyo, Rigaku, Japan). Semi preparative HPLC analyses were done through Hitachi Primaide apparatus equipped with 1110 pump interfaced with a 1430 diode array detector, 1210 autosampler with RP-C18 columns (5 µm, 10 mm × 250 mm dimensions, YMC-pack ODS-A). Q-Exactive tandem mass spectrometer was used to obtain ESI-HR-MS data (Thermo Fisher, Waltham, MA, USA). The optical density (OD) was measured by Multiskan GO full wavelength enzyme labeler (Thermo Fisher). Silica gel (300–400 mesh, Qingdao Marine Chemical Inc., Qingdao, China), MCI gel (GHP20/P120, Mitsubishi Chemicals, Tokyo, Japan), and YMC ODS-A (YMC, Kyoto, Japan) gel was successfully employed during column chromatography. High performance liquid chromatography (HPLC) grade higher purity solvents (Sigma-Aldrich, Saint Louis, MO, USA), and analytical-grade solvents were obtained from the local supplier (Tansoole, Shanghai, China).
Samples of commercial mastic gum used in this study were purchased from Xinjiang Ensar Uyghur Medicine Pieces Co., Ltd. (Hetian, China) on 16 March 2022, and properly authenticated from Xiao-rong Yang, School of Biological Sciences and Technology, Yili Normal University. A specific voucher specimen number (HX-MG-220316) issued and lodged within the Key Lab of Clean Conversion and HighValue Utilization of Biomass Resources, Yili Normal University.
3.3 Extraction and Isolation Procedure
A mass of mastic, precisely 500 g, underwent extraction with a MeOH:EA mixture (3:1 ratio) (total volume 800 mL), employing the maceration method at ambient temperature for a duration of approximately 72 h, yielding 349.6 g of a crude extract. A segment of the initial extract, specifically 337.2 g, was thereafter directed through normal-phase chromatography on a silica gel matrix, utilizing a petroleum ether:ethyl acetate (PE:EA) gradient system established in the ratio from (100:1) to (0:1) was used as the eluent. By employing this procedure, twelve fractions (Fr.1–Fr.12) were obtained from the extract. Fr.2 (13.3 g) was employed to MCI column chromatography (MeOH-H2O, 70:30–100:0) with gradient elution to give five sub-fractions (Fr.2A–Fr.2E). Fr. 2B (449.4 mg) was employed on a silica gel column eluting with a gradient of DCM-MeOH (180:1–0:1) to give seven sub-fractions (Fr. 2B1–Fr. 2B7), Fr. 2B3 (252.0 mg) was run on a silica gel column eluting with a gradient of PE-EA (20:1–0:1) to give four sub-fractions (Fr. 2B3a–Fr. 2B3d), Fr. 2B3d (28.4 mg) was purged by semi preparative HPLC analysis (85% ACN-H2O, 3 mL/min) to give compound 2 (16.7 mg, tR = 37.1 min). Fr. 2C (597.8 mg) was chromatographed on a silica gel column eluting with a gradient of PE-EA (30:1–0:1) to give eight sub-fractions (Fr. 2C1–Fr. 2C8), Fr. 2C6 (262.2 mg) was subjected to ODS column chromatography (MeOH-H2O, 70:30–100:0) with gradient elution to give five subfractions (Fr. 2C6a–Fr. 2C6e). Fr. 2C6e (37.0 mg) was chromatographed on a silica gel column eluting with a gradient of PE-EA (20:1–0:1) to give two sub-fractions (Fr. 2C6e1–Fr. 2C6e2), Fr. 2C6e1 (17.5 mg) was purified by semi preparative HPLC (87% MeOH-H2O, 3 mL/min) to give compound 1 (9.4 mg, tR = 29.3 min).
18α-H-3-oxoolean-12-en-28-ol (1): colorless crystals (methanol),
3.4 X-Ray Crystallographic Analysis
After undergoing recrystallization in methanol at room temperature, compounds 1 and 2 resulted in colorless crystalline forms. X-ray diffraction data were obtained an XtalABoxford diffractometer aligned with Cu Kα radiation (λ = 1.54178 Å). The crystalline structures were determined employing the ShelXT program through intrinsic phasing and refined via the ShelXL package, employing least-squares minimization. These crystalline structures were elucidated by utilizing the ShelXT software for structure solution via intrinsic phasing, followed by refinement with the ShelXL program employing least-squares minimization techniques. The corresponding crystallographic information has been archived at the CCDC under reference numbers 2,386,881 for compound 1 (Fig. S9) and 2,386,883 for compound 2 (Fig. S10).
Crystal data for compound 1. 2(C29H46O2), M = 853.31 Da, orthorhombic, space group P212121, a = 7.43070 (10) Å, b = 19.8885 (4) Å, c = 34.0537 (8) Å, α = 90°, β = 90°, γ = 90°, V = 5032.64 (17)Å, T = 293 (2) K, Z = 4, Cu Kα Radiation (λ = 1.54184), F(000) = 1888, ρcalc = 1.126. A total of 12,856 reflections were noted in the range of 5.07° ≤ 2θ ≤ 133.668°. The final R1 value was 0.0538, and the
Crystal data for compound 2. C29H46O2, M = 426.66 Da, orthorhombic, space group P212121, a = 10.84822 (17) Å, b = 14.5548 (3) Å, c = 15.6281 (3) Å, α = 90°, β = 90°, γ = 90°, V = 2467.58 (8) Å, T = 293 (2) K, Z = 4, Cu Kα Radiation (λ = 1.54184), F(000) = 944, ρcalc = 1.148. A total of 10,273 reflections were measured in the range of 5.658° ≤ 2θ ≤ 136.244°. The final R1 value was 0.0412, and the ωR2 value was 0.1277 (all data). The goodness of fit on F2 was 1.097. Flack parameter = −0.07 (12) (Table S1).
3.5 Cell Culture and Cell Viability Analysis
The Murine RAW 264.7 macrophage cells were cultured and maintained using Dulbico’s Modified Eagle’s Medium (DMEM) contains FBS (10%) and penicillin/streptomycin (1%), in humidified incubator set at 37°C and 5% CO2. Cell stimulation was done using LPS (2.0 µg/mL). Prior to this, dimethylsulfoxide (DMSO) was used for initial solubilization of the compounds, and later further dilutions were prepared in supplemented DMEM.
Following certain modifications in an earlier reported method [25], RAW 264.7 macrophages were seeded (2.0 × 104) in each well using 96-wells plates, and allowed for incubation in fully supplemented DMEM for 24 h. Later on, DMEM was removed, and 100 µL of fresh non-supplemented DMEM mixed with compounds 1 and 2 was poured in each well and allowed for further incubation for 24 h period. Following the guidelines for the CCK-8 assay, the medium was then replaced with 100 µL of CCK-8 reagents + fresh DMEM (1:9 dilution), and covered using aluminum foil. The cells were allowed for 2 h incubation in the dark, the absorbance of the plate was quantified at a wavelength of 450 nm.
3.6 Inhibition of NO Generation
The estimation of the total generation of reactive nitrogen species specifically nitrous oxide (NO) was done uzing Griess reagent following manufacturer’s instructions (Beyotime, Shanghai, China) [26]. Approximately 2.0 × 104 RAW264.7 cells were seeded per well of the 96-well plate, and let them incubate for about 24 h, and then culture medium was replaced with the drug (0, 10, 20, 40, and 80 μM) and positive control media and allowed to incubate for additional 2 h. Next to it, recombinant LPS (2.0 μg/mL) was added into the cultured cells and further incubated for 24 h. For nitrite accumulation, an equal volume of Griess-I and -II reagents was combined with 50 μL aliquots of the supernatant from the media for final reaction. Optical density (OD) of this solution was measured at 540 nm. Dexamethasone served as the benchmark control in the experiment. The IC50 values were subsequently calculated using version 8.0 of the GraphPad Prism software.
In conclusion, a new nor-triterpene with a 18α(H) stereochemistry has been obtained from the extract of mastic for the first time. The pair of oleanane nor-triterpenes from the perspective of biotransformation may be generated through an enzyme-catalyzed series of reactions involving cyclization, oxidation, and decarboxylation starting from a squalene precursor. Anti-inflammatory assays indicated that compound 1 exhibited stronger inhibitory NO production activities (IC50 values 11.69 ± 1.39 μM) than that of 2 (IC50 = 26.18 ± 2.66 μM). Thus, the above data demonstrate the essential contribution of the H-18 orientation to the variability of the structure-activity relationship. The present investigation provides significant information concerning the pharmacophoric features of triterpene derivatives that can be considered as new lead compounds for anti-inflammatory agents.
Acknowledgement: We extend our sincere gratitude to Hengwei Zhou from the College of Physical Science and Technology at Yili Normal University for his invaluable support during the single crystal testing phase of our research.
Funding Statement: This project was funded by National Natural Science Foundation of China (Grant No. 82060774), Key Special Projects for Enhancing Subject Comprehensive Strength at Yili Normal University (Project No. 22XKZZ08), and Open Project of Jiangxi Provinicial Key Laboratory of Natural and Biomimetic Drugs Research (Project No. 2024SSY07051).
Author Contributions: Yan Wu conducted bioactivity testing and authored the initial draft; Xuerui An performed isolations, confirmation and structural explanation of the isolated components; Haofan Lv and Zhiqiang Zhao managed the cultivation and analysis of single crystals; Wei Liu and Chunpeng Wan oversaw the experimental design and manuscript revision. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/phyton.2025.059581.
References
1. Li YL, Wang J, Li LY, Song WH, Li M, Hua X, et al. Natural products of pentacyclic triterpenoids: from discovery to heterologous biosynthesis. Nat Prod Rep. 2023;40(8):1303–53. doi:10.1039/d2np00063f. [Google Scholar] [PubMed] [CrossRef]
2. Yang YH, Dai SY, Deng FH, Peng LH, Li C, Pei YH. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry. 2022;203:113397. doi:10.1016/j.phytochem.2022.113397. [Google Scholar] [PubMed] [CrossRef]
3. Martín R, Cordova C, San Román JA, Gutierrez B, Cachofeiro V, Nieto ML. Oleanolic acid modulates the immune-inflammatory response in mice with experimental autoimmune myocarditis and protects from cardiac injury. Therapeutic implications for the human disease. J Mol Cell Cardiol. 2014;72:250–62. doi:10.1016/j.yjmcc.2014.04.002. [Google Scholar] [PubMed] [CrossRef]
4. Zhang HC, Song YX, Zhang ZC. Glycyrrhizin administration ameliorates coxsackievirus B3-induced myocarditis in mice. Am J Med Sci. 2012;344:206–10. doi:10.1097/MAJ.0b013e31823e2867. [Google Scholar] [PubMed] [CrossRef]
5. Park WH, Kang S, Piao Y, Pak CJ, Oh MS, Kim J, et al. Ethanol extract of Bupleurum falcatum and saikosaponins inhibit neuroinflammation via inhibition of NF-κB. J Ethnopharmacol. 2015;174:37–44. doi:10.1016/j.jep.2015.07.039. [Google Scholar] [PubMed] [CrossRef]
6. Badshah H, Ali T, Rehman S, Amin F, Ullah F, Kim TH, et al. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J Neuroimmune Pharmacol. 2016;11(1):48–60. doi:10.1007/s11481-015-9623-z. [Google Scholar] [PubMed] [CrossRef]
7. Goddard ZR, Searcey M, Osbourn A. Advances in triterpene drug discovery. Trends Pharmacol Sci. 2024;45(11):964–8. doi:10.1016/j.tips.2024.10.003. [Google Scholar] [PubMed] [CrossRef]
8. Soulaidopoulos S, Tsiogka A, Chrysohoou C, Lazarou E, Aznaouridis K, Doundoulakis I, et al. Overview of chios mastic gum (Pistacia lentiscus) effects on human health. Nutrients. 2022;14(3):590. doi:10.3390/nu14030590. [Google Scholar] [PubMed] [CrossRef]
9. Liu W, Liu YS, Chen Y, Qi YR, Yuan T. Research progress of chemical constituents and biological activities ofessential oil of Pistacia lentiscus. Chin J Chin Mater Med. 2019;44(17):3684–94. doi:10.19540/j.cnki.cjcmm.20190529.201. [Google Scholar] [PubMed] [CrossRef]
10. Kishimoto R, Kato N, Koike M, Iwashita N, Takagi Y, Fukuyama T. Topical treatment with mastic (resin from Pistacia lentiscus) elicits anti-inflammatory and anti-pruritic responses by modulating keratinocyte activation in a mouse model of allergic dermatitis. Phytomedicine. 2021;91:153679. doi:10.1016/j.phymed.2021.153679. [Google Scholar] [PubMed] [CrossRef]
11. Papada E, Gioxari A, Amerikanou C, Forbes A, Tzavara C, Smyrnioudis I, et al. Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: a double-blind and placebo-controlled randomised trial. Phytother Res. 2019;33(2):360–9. doi:10.1002/ptr.6229. [Google Scholar] [PubMed] [CrossRef]
12. Papada E, Amerikanou C, Torovic L, Kalogeropoulos N, Tzavara C, Forbes A, et al. Plasma free amino acid profile in quiescent inflammatory bowel disease patients orally administered with mastiha (Pistacia lentiscusa randomised clinical trial. Phytomedicine. 2019;56:40–7. doi:10.1016/j.phymed.2018.08.008. [Google Scholar] [PubMed] [CrossRef]
13. Pachi VK, Mikropoulou EV, Gkiouvetidis P, Siafakas K, Argyropoulou A, Angelis A, et al. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceaea review. J Ethnopharmacol. 2020;254:112485. doi:10.1016/j.jep.2019.112485. [Google Scholar] [PubMed] [CrossRef]
14. Liu W, Gao J, Li MM, Aisa HA, Yuan T. Tirucallane triterpenoids from the mastic (Pistacia lentiscus) and their anti-inflammatory and cytotoxic activities. Phytochemistry. 2021;182:112596. doi:10.1016/j.phytochem.2020.112596. [Google Scholar] [PubMed] [CrossRef]
15. An XR, Wang JL, Sun YW, Yang X, Wu Y, Liu W, et al. Seven new noroleanane triterpenoids from mastic and their no inhibitory and cytotoxic activities. Chem Biodivers. 2024;21(11):e202401585. doi:10.1002/cbdv.202401585. [Google Scholar] [PubMed] [CrossRef]
16. An XR, Feng YP, Sun YW, Yang X, Liu W, Yuan T. A new norolean triterpenoid from mastic. Chin J Chin Mater Med. 2024;49(23):114–23. doi:10.19540/j.cnki.cjcmm.20240910.202. [Google Scholar] [CrossRef]
17. Mehmet D. Phytochemical studies on the mastic gum of Pistacia lentiscus var. chia collected from karaburun peninsula and bioactivities of isolates (M.D. Thesis). Izmir Institute of Technology: Istanbul; Turkey; 2021. [Google Scholar]
18. Deng TM, Peng C, Peng DY, Yu NJ, Chen WD, Wang L. Research progress on chemical constituents and pharmacological effects of Glycyrrhizae Radix et Rhizoma and discussion of Q-markers. Chin J Chin Mater Med. 2021;46(11):2660–76. doi:10.19540/j.cnki.Cjcmm.20210304.201. [Google Scholar] [PubMed] [CrossRef]
19. Tao H, Lauterbach L, Bian GK, Chen R, Hou AW, Mori T, et al. Discovery of non-squalene triterpenes. Nature. 2022;606(7913):414–9. doi:10.1038/s41586-022-04773-3. [Google Scholar] [PubMed] [CrossRef]
20. Li XB, Huang CL, Zhang Y, Ding JY, Xiang GS, Zhang GH, et al. Promiscuous oxidosqualenecyclases from Neoalsomitraintegrifoliola catalyzing the formation of tetracyclic, pentacyclic,and heterocyclic triterpenes. Org Lett. 2024;26(15):3119–23. doi:10.1021/acs.orglett.4c00730. [Google Scholar] [PubMed] [CrossRef]
21. Zhang L, Xiao Y, Liu MZ, Wang W. Progress in the biosynthesis of ursane-type pentacyclic triterpenes. Chin Med Biotechnol. 2024;19(3):223–30. doi:10.3969/j.issn.1673-713X.2024.03.005. [Google Scholar] [CrossRef]
22. Song ZJ, Chen DW, Sui SY, Wang YJ, Cen S, Dai JG. Characterization of a malabaricane-type triterpene synthase from Astragalus membranaceus and enzymatic synthesis of astramalabaricosides. J Nat Prod. 2023;86(7):1815–23. doi:10.1021/acs.jnatprod.3c00331. [Google Scholar] [PubMed] [CrossRef]
23. He JB, Chen K, Hu ZM, Li K, Song W, Yu LY, et al. UGT73F17, a new glycosyltransferase from Glycyrrhiza uralensis, catalyzes the regiospecific glycosylation of pentacyclic triterpenoids. Chem Commun. 2018;54:8594. doi:10.1039/c8cc04215b. [Google Scholar] [PubMed] [CrossRef]
24. Li B, Yang YG, Chen LZ, Chen SC, Zhang J, Tang WJ. 18α-glycyrrhetinic acid monoglucuronide as an anti-inflammatory agent through suppression of the NF-κB and MAPK signaling pathway. MedChemComm. 2017;8(7):1498–504. doi:10.1039/c7md00210f. [Google Scholar] [PubMed] [CrossRef]
25. Yu YH, Feng YP, Liu W, Yuan T. Diverse triterpenoids from mastic produced by Pistacia lentiscus andtheir anti-inflammatory activities. Chem Biodivers. 2022;19(3):e202101012. doi:10.1002/cbdv.202101012. [Google Scholar] [PubMed] [CrossRef]
26. Zeng HT, Yu YH, Zeng X, Li MM, Li X, Xu SS, et al. Anti-inflammatory dimeric benzophenones from an endophyt-icpleosporales species. J Nat Prod. 2022;85(1):162–8. doi:10.1021/acs.jnatprod.1c00900. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools