 Open Access
Open Access
ARTICLE
Assessment of Salinity Tolerance and Ecotypic Variability in Vicia narbonensis L.: Morphological, Physiological, and Biochemical Responses
1 Centre de Recherche en Aménagement du Territoire (CRAT), Campus Zouaghi Slimane, Constantine, 25000, Algérie
2 Laboratoire d’Amélioration et de Développement de la Production Végétale et Animale (LADPVA), Université de Ferhat Abbas, Sétif, 19000, Algérie
3 Department of Geographical Sciences, University of Maryland, College Park, MD 20742, USA
4 Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
5 Centre de Recherche en Agropastoralisme (CRAPast), Djelfa, 17000, Algérie
* Corresponding Author: Salah Hadjout. Email:
(This article belongs to the Special Issue: Abiotic Stress Tolerance in Crop Plants: Physio-biochemical and Molecular Mechanisms)
Phyton-International Journal of Experimental Botany 2025, 94(1), 251-267. https://doi.org/10.32604/phyton.2025.060096
Received 23 October 2024; Accepted 26 December 2024; Issue published 24 January 2025
Abstract
Salinity stress is a major challenge for global agriculture, particularly in arid and semi-arid regions, limiting plant productivity due to water and soil salinity. These conditions particularly affect countries along the southern Mediterranean rim, including Algeria, which primarily focuses on pastoral and forage practices. This study investigates salinity tolerance and ecotypic variability in Vicia narbonensis L., a fodder legume species recognized for its potential to reclaim marginal soils. Morphological, physiological, and biochemical responses were assessed in three ecotypes (eco2, eco9, and eco10) exposed to different salinity levels (low, moderate, and severe). The study was conducted using a completely randomized block design with three blocks per ecotype per dose. The results from the two-way analysis of variance demonstrate significant effects across nearly all attributes studied, revealing distinct ecotypic responses. These findings underscore variations in growth parameters, osmotic regulation mechanisms, and biochemical adjustments. The substantial diversity observed among these ecotypes in their response to salinity provides valuable insights for breeders addressing both agronomic and ecological challenges. Multivariate analyses, including Principal Component Analysis (PCA), revealed key variables distinguishing between ecotypes under salinity stress. Moreover, Classification based on Salinity Tolerance Indices (STI) further differentiated ecotypic performance with more precision, and this is because of the combination of the different parameters studied. These results open up new prospects for the development of strategies to improve the salinity tolerance of forage legumes.Keywords
Ongoing global climate change is exacerbating extreme temperatures, drought, and salinity, which pose significant stressors in agriculture [1]. The increasing global salinity levels are anticipated to escalate into a more severe issue for agricultural production in the forthcoming decades, especially in arid and semi-arid regions [2,3]. Drought, heat, and poor land-water management contribute to salinity problems and challenge agricultural and forestry production in these regions [4–6].
Worldwide, 800 million hectares of land and 32 million hectares of agricultural land are salt-affected [7]. Additionally, Tayyab et al. [8] reported that approximately one-third of cultivated land globally is impacted by salt, highlighting salinity as a significant constraint in food and feed production. Excess salts adversely affect soil structure and fertility, plant growth, crop yield, and microorganisms. This phenomenon is caused by natural processes, such as dry climates and low precipitations, high evaporation rate, poor waterlogging, and human factors, such as inappropriate irrigation practices, poor drainage systems, and excessive use of fertilizers [9,10]. The consequences of salinity are severe, as it can reduce agricultural production by up to 35%, leading to significant food shortages for both humans and animals [11,12]. Agriculture plays a pivotal role in the economic development of many countries. However, salinity represents a significant obstacle that hinders the expansion of agricultural areas and the increased production of many crops [13].
Salinity stress affects many plant species by leading to growth inhibition primarily due to osmotic effects causing water deficits and inducing toxicity and mineral deficiencies [14]. Most plant species, which are glycophytes, cannot survive in high-salinity soils. High salinity causes both osmotic stress and ion toxicity, which in turn induces oxidative stress [15,16]. Osmotic stress depends on the fact that the osmotic potential is more negative outside the roots than inside under a high salt concentration, which makes water uptake more difficult [17].
Among fodder legumes, the annual species of the genus Vicia have been used in agriculture for centuries. These species have been cultivated since Roman times, primarily for use as green manure and as fodder to feed livestock, with Vicia narbonensis being one of the notable species [18]. Given their widespread cultivation and significant production figures, vetches are considered one of the major agricultural crops worldwide, playing a crucial role in sustainable farming systems. In 2022, vetch cultivation covered 322,715 hectares worldwide, with an average yield of 2120.3 kg/ha, resulting in a total production of 684,263.78 tonnes [19]. However, reports on the salinity tolerance of Vicia L. species are limited, leaving a gap in understanding their full potential in saline environments.
Breeding salinity-tolerant genotypes is one of the best strategies to mitigate the adverse effects of high salinity and increase productivity in saline soils [20,21]. Salinity tolerance in plants is not merely a straightforward trait; rather, it emerges from a complex interplay of various physiological characteristics that are intricately intertwined and challenging to pinpoint. Therefore, relying solely on the morphological changes exhibited by a plant in response to salinity may not provide adequate insight into its overall resilience. It is crucial, therefore, to take into account additional physiological and biochemical factors that contribute to the plant’s ability to tolerate salinity [22].
Given the significance of this issue, this study aimed to accomplish several objectives: (1) assess the impact of various salinity levels on the morphological, physiological, and biochemical responses of three ecotypes of Vicia narbonensis L.; (2) identify the key variables that differentiate the Narbonne ecotypes, and (3) classify the ecotypes based on Salinity Tolerance Indices.
This structured approach will provide comprehensive insights into how different ecotypes of Vicia narbonensis L. respond to salinity stress, elucidating crucial factors that influence their adaptability and resilience under adverse conditions.
The study concerns the morphological, physiological, and biochemical response of three ecotypes belonging to the species Vicia narbonensis L. (eco2, eco9, and eco10) subjected to different doses of salt stress (Sodium chloride/Sigma Aldrich). The ecotypes were obtained from ICARDA (International Center for Agricultural Research in the Dry Areas).
The experiment was conducted following a completely randomized block experimental design in a greenhouse at the experimental farm of Ferhat Abbas University 1 in Setif, Algeria (coordinates: 36°12′05.0″ N 5°21′58.5″ E). The study was conducted over a period of 42 days, from January 10 to February 21, 2021, with a design that included three blocks. Each experimental unit consisted of a plastic pot measuring 18 cm in height and 11 cm in diameter, filled with 1 kg of soil that had been sieved through a 3 mm mesh and sterilized by dry heat at 180°C for 1 h (using a Memmert oven). The seeds of Vicia narbonensis L. were germinated in vitro on Petri dishes before being transplanted into pots, with one seed per pot. Twelve seeds per ecotype were replanted. These plants were then subjected to a salinity stress experiment using four NaCl concentrations (0, 4, 8, and 12 g/L). Since there were three ecotypes, this resulted in a total of 36 experimental combinations, calculated as follows: three ecotypes × four salinity levels × three blocks (Fig. 1). A complete nutrient solution of a mixture of macro elements (Hoagland formula) and microelements (Arnon formula) was applied twice a week. The stress was induced after 26 days of growth when the plants reached their branching stage.
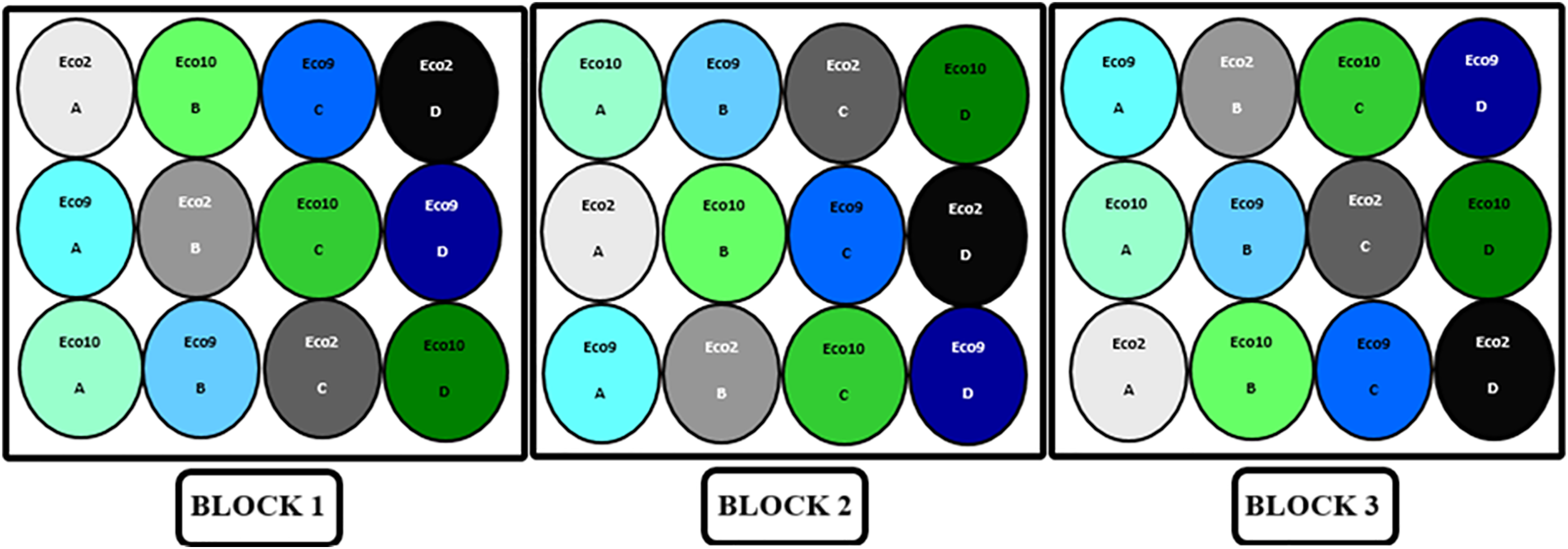
Figure 1: Diagram of the experimental plan of the three ecotypes of Vicia narbonensis L. exposed to different levels of salinity (A: control, B: 4, C: 8, D: 12 g/L)
After 16 days of treatment, several measurements were taken from the three blocks at each stress level, including aerial height (AH) (in centimeters) and growth speed (GS), expressed in milligrams per day, and calculated by dividing the fresh mown weight in milligrams (BIO YIELD) by the vegetative growth phase from sowing to mowing expressed in days (VGP). Leaf area was assessed using ImageJ software, with the average of six leaflets from the top of each plant recorded in square centimeters. The aerial dry weight was also measured in grams.
2.3.2 Physiological Parameters
Leaf relative water content (LRWC) was measured by submerging 100 mg of fresh leaves in 20 mL test tubes of distilled water for 16–18 h in the dark at room temperature. After removing excess water, turgid weight was recorded, and dry weight was determined after steaming the leaves at 70°C for 72 h. LRWC was calculated using the following formula: LRWC = [(fresh weight–dry weight)/(turgid weight–dry weight)] × 100, according to Schonfeld et al. [23]. Meanwhile, electrolyte leakage (EL) was determined by immersing 15 mg of fresh plant material in 15 mL of demineralized water, incubating the samples at room temperature for 2 h, then measuring the initial conductivity (L1). The samples were autoclaved at 100°C for 30 min (Using a BIOBASE vertical autoclave), and the final conductivity (L2) was measured after cooling. EL was calculated as EL (%) = (L1 / L2) × 100, as described by Lutts et al. [24]. Chlorophyll A and B were extracted from 0.3 g of fresh leaves using 7 mL of 80% acetone (Merck), followed by centrifugation at 5000 rpm for 5 min. Absorbance was measured at 663 nm for Chlorophyll A and 645 nm for Chlorophyll B (using a Jenway/6405 UV/Vis spectrophotometer), and chlorophyll content was calculated using the formulas provided by Arnon [25]: Chlorophyll A (mg/g fresh weight) = 12.7 * (A663)−2.69 * (A645) * V/(1000 * W) and Chlorophyll B (mg/g fresh weight) = 22.9 * (A645) − 4.69 * (A663) × V/(1000 * W), where A663 and A645 are absorbance values, V is the final volume of the supernatant (mL), and W is the fresh weight of the sample (g).
To determine proline content, 50 mg of plant material was heated in 2 mL of 40% methanol (Honeywell) at 85°C for 30 min. Proline was quantified using a method involving Solution A (300 mL acetic acid (Honeywell), 80 mL orthophosphoric acid (CLCMlabs), and 120 mL distilled water) and Solution B (3 g ninhydrin dissolved in 100 mL ethanol), as described by Bates et al. [26]. For soluble sugar content, 100 mg of plant material was incubated with 3 mL of 80% ethanol (Honeywell) at room temperature for 48 h, and the total soluble sugars were quantified following the method described by Dubois et al. [27].
The raw data were subjected to a two-way ANOVA with a significance level set at 5%. Subsequently, a post hoc Least Significant Difference (LSD) test was applied to perform pairwise comparisons of the means.
All raw data were transformed into salinity tolerance indices according to the method of Zeng et al. [28] and Rajhi et al. [29]. The tolerance indices were calculated according to the following formula:
STI = Value of the variable under saline conditions/the control average.
Principal Component Analysis (PCA) was conducted on these transformed data to identify key axes and variables contributing significantly to segregation and to assess dissimilarities among individuals exposed to varying salinity levels. Subsequently, the average tolerance indices of the variables contributing most to segregation were subjected to a comparison of means test (LSD) to distinguish homogeneous groups. XLSTAT version 03.5.2014 software was utilized for these analyses.
The analysis of variance at the 5% threshold, displayed a significant effect of the factors “ecotype”, and “dose” and their combination for almost all of the variables studied (Table 1). Leaflet relative water content (LRWC) exhibited a non-significant effect of the “dose” factor. Similarly, aerial dry weight (ADW) displayed a non-significant effect of both “ecotype” and “ecotype*dose” factors. In addition, these variables presented a very high coefficient of determination, which explained that the majority of variability (65%–98%) of the variables to be modeled was explained by the explanatory variables, which were in this case “ecotype”, “dose” and their interaction. These results reflected the magnitude of the diversity of these ecotypes, which constitute a breeding ground for breeders to face both agronomic and ecological challenges. These genetically diverse ecotypes could be used as promising parents for hybridization [30] to develop superior hybrids with desirable combinations of traits [31].

The morphological parameters studied included aerial height (AH), aerial dry weight (ADW), growth speed (GS), and leaf area (LA). The general average taken by the aerial height (AH) of the plants was equal to 13.63 cm. The tallest stem was present in the ecotype “eco2” with 15.81 cm. On the other hand, the dwarfest stem was noted in the ecotype “eco 10” with 11.19 cm (Table 1).
The average dose effect affected enormously the height of almost all of the Vetch plants studied with a reduction that reached almost 50% for the high dose (12 g/L). These results could be explained by previous studies [32,33] indicating that high salinity first reduces elongation and cell division due to the osmotic effect created around the root subsequently causing Na+ toxicity and ionic imbalance, which damages the cells in the transpiring leaves. Therefore, seedling growth decreases in high-salt environments. Bilgili et al. [34] found that the greatest heights of Common Vetch seedlings were obtained in unsalted pots, and the lowest heights were obtained in pots treated with the highest concentration of salt. Additionally, Osuagwu et al. [35] noted significant reductions in the plant height of three varieties of African yambean (Sphenostylis stenocarpa) when exposed to high salinity doses. This is in line with the results obtained during our study concerning eco2 and eco9.
An average value of 0.20 g/plant was recorded for aerial dry weight (ADW). While the growth speed (GS) variable had an average equal to 26.17 mg/day (Table 1). The dose-effect negatively and significantly affected both the aerial biomass and growth speed variable at high doses of salinity, with reductions of 31.33% and 60%, respectively. Reduction in plant growth and dry-matter accumulation under saline conditions has been reported in several grain legumes [36]. Several authors have reported reduced growth and biomass yield in legumes owing to NaCl [37,38]. The reduction in growth and biomass yield due to salinity was attributed to the inhibition of cell division and elongation [39,40]. Simultaneously, a considerable increase (between 30% and 33%) in aerial biomass (dry weight) was displayed under low and moderate salinity conditions. The same trend was observed for eco 10 with respect to the speed of growth. Castroluna et al. [41] explained that this response is a stress avoidance strategy. It involves elongating the aerial parts of the plant while maintaining or altering root production to decrease surface area and reduce permeability.
The general average taken by the leaf area (LA) measured on the ecotypes studied was equal to 5.15 cm2 (Table 1). Overall, our study revealed an exceptional model in which the average dose effect displayed increases in the leaf surface area of most ecotypes subjected to NaCl concentrations (4 and 8 g/L) of NaCl. This contradicts the majority of salt stress results found in the literature where several authors have mentioned that the negative impact of NaCl on leaf surface development was evident at the lowest salt concentration and increased linearly with increasing salt concentration [42,43]. The increase in leaf surface area during our study could only be explained by the avoidance model adopted by certain species which consists of increasing the surface area of the aerial parts after exposure to low and moderate concentrations of salinity, as cited by Castroluna et al. [41]. In the present study, the highest concentration of NaCl caused the maximum inhibitory effect on the leaf areas of eco9 and eco2. The reduction in total leaf area caused by salinity may be linked to the decline in leaf turgor and suppression of cell division [44]. Alzahrani et al. [5] during their studies on two genotypes of Vicia faba L. found that salt stress reduced the leaf area of the two cultivars, and a maximum reduction of 62.39% and 51.63% was reported under the concentration of 150 mM NaCl compared to the control. Our results showed maximum reductions of 12.55% and 25.33% for the two ecotypes eco9 and eco2, respectively, exposed to a salinity concentration of 200 mM NaCl. In light of these results, we can say that our ecotypes (eco2 and eco9) exhibited a much higher tolerance (three times more) than that revealed by Alzahrani et al. [5] regarding leaf area. In addition, the ecotype (eco10) showed to be the most tolerant to salinity and went from 3.84 cm2 in the absence of stress to 4.16 cm2 for the low dose (4 g/L), 4.40 cm2 for the moderate dose (8 g/L) and 4.97 cm2 for the high dose (12 g/L) (Table 1). This ecotype requires more attention and should be exposed to further salt stress tests to confirm its behavior. However, it is important to note that the main difference between our study and that of Alzahrani et al. [5] lies in the duration of stress exposure before measurements were taken. Specifically, we exposed the subjects to stress for 16 days, whereas they did so for 35 days.
The physiological parameters investigated included leaflet relative water content (LRWC), electrolyte leakage (EL), and chlorophyll A and B content (CHL A and B).
Analysis of variance for leaflet relative water content (LRWC) showed that the effect of the “dose” factor was not significant (p value = 0.75, F = 0.4). However, the interaction between ecotype and dose was significant (p value = 0.0039, F = 4.40), suggesting that ecotype behavior was influenced by how each ecotype responds to different doses. Salt stress significantly suppressed the relative water content of leaflets [45]. Additionally, He et al. [46] noted that in their study on Beta vulgaris var. cicla under 0.3% salt stress, LRWC decreased by 2.64% compared to that of the control. However, at 0.5% and 0.7% salt stress levels, LRWC decreased significantly by 13.70% and 18.10%, respectively, compared with the control. Our results diverged from those of these studies, where no trend was observed regarding this variable for the three ecotypes exposed to different salinity levels. Decreased plant growth in saline soils is caused by soil osmotic and water potential, specific toxicity, and nutritional deficits [41]. Often, plant cell membranes are subject to modifications related to increased permeability and loss of integrity under environmental stress [47].
Our results showed that salinity significantly affected membrane permeability (EL) (p value =< 0.0001, F = 109,71), with considerable increases, especially for moderate and high doses. Abdel Motaleb et al. [48] mentioned that salinity deleteriously affected the membrane permeability of beans with significant differences between levels. Cell membrane damage is considered to be among the main causes of decreased yield and its components. Generally, salinity can lead to hyper-ionic and hyper-osmotic stresses and cellular and metabolic dysfunction, including cell membrane damage [49]. This was noticed during our study, particularly for eco2, which displayed a very high value (90.09%) under the high-dose saline treatment, reflecting its high sensitivity to high salinity levels. Similarly, it has been reported that cell membranes are highly sensitive to stress [50]. Therefore, the accumulation of high Na+ and Cl concentrations in chloroplasts has been shown to damage thylakoid membranes [51].
The analysis of variance for chlorophyll A and B content showed a significant effect for all the factors studied (ecotype, dose, and their interaction). Salinity significantly affected chlorophyll A and B content (p value < 0.0001, F = 12.78 for chlorophyll A and p value < 0.0001, F = 17.92 for chlorophyll B) (Table 1). It is formed into two homogeneous treatment groups for the eco2. The first group comprised control, low dose (4 g/L), and moderate dose (8 g/L) treatments, where no significant differences were observed. On the other hand, the second group included the high dose (12 g/L). The chlorophyll A and B content in eco2 exhibited a consistently positive response to the severe salinity dose, reaching approximately 2.32 and 3.18 mg/g, respectively. Chlorophyll is the main pigment found in most oxygenated photosynthetic organisms. Some authors have suggested that variations in pigment content could provide valuable information on the physiological performance of leaves and indicate their photosynthetic capacity as well as the presence of stresses or diseases [51].
While eco10 and eco9 were identified as the most sensitive, showing a generally decreasing response as the salinity concentration increased. This could be explained by the fact that Aydi et al. [52] found that the leaves of the sensitive line were more charged with sodium ion. The excessive accumulation of Na+ in the leaf tissues results in an alteration of the chlorophyll pigments, and/or a restriction of its biosynthesis, which translates into leaf chlorosis. This has been observed in several Fabaceae such as Medicago ciliaris [53], Medicago truncatula [54], and Hedysarum carnosum [55].
Insufficient chlorophyll content can lead to photosystem dysfunction, causing a drop in total CO2 fixation [56]. This has a direct and negative effect on plant growth and productivity [57].
The biochemical parameters measured in our study concerned the content of the two Osmo protectants, proline and soluble sugars. The analysis of variance revealed significant effects of all factors for the two variables, with mean values around 1.72 and 0.3 mg/g, and coefficients of determination of 0.98 and 0.96, respectively.
Salinity induced a significant and consistent synthesis of proline (p value < 0.0001, F = 144.93) under high NaCl doses (12 g/L), especially notable for eco2 (8.05 mg/g), which was multiplied by 4 compared to the control (1.83 mg/g) (Table 1). Proline is the most common endogenous osmolyte that accumulates under various abiotic stresses, including salinity [58]. It is a major source of energy and nitrogen for plant growth under stressful conditions. Not only that, but proline acts as a plant growth regulator by participating in the metabolism and activation of various signaling processes [59]. Proline is a compatible solute compound and its accumulation is an indicator of excessive stress conditions. The accumulation of proline helps plants improve their stress tolerance capabilities and strengthens their antioxidant activity to detoxify elements harmful to the cell [60]. Moreover, Ahmad et al. [61] and Gharsallah et al. [62] reported that salt-tolerant genotypes accumulated more proline than salt-sensitive genotypes. This is what was observed in our case for ecotype “eco2”. Furthermore, it was observed in a previous study by Nasrallah et al. [63] that Vicia faba L. treated with different salinity concentrations accumulated the osmoprotectant proline in large quantities as a protective mechanism to acclimatize to abiotic stress. Moreover, it was documented that proline accumulation protected plants against osmotic stressors by stabilizing several functional units, such as membranes, complex II electron transport, and proteins including enzymes [64]. In harmony with these results, an increase in proline accumulation was documented under salt stress in faba bean [65], wheat [66], Pisum sativum [67], and pearl millet [68].
Our results demonstrate that salinity significantly affected soluble sugar content (p value < 0.0001, F = 120.34). We observed a significant downward trend for both low and moderate salt concentrations, whereas a high concentration produced a substantial upward trend. This aligns with the findings of Sharma et al. [69], indicating an initial decrease followed by an overall increase in the soluble sugar content.
An essential adaptive strategy for salinity tolerance involves the accumulation of osmolytes or Osmo protectants, primarily sugars [70]. These compounds play a critical role in maintaining the intracellular osmotic balance and preserving leaf moisture under environmental stress conditions [71,72]. Rymbai et al. [73] during their studies on mango hybrids mentioned that sugars had high direct and positive effects on yield. This finding aligns with our results, as eco9 displayed the highest yield in dry matter, corresponding to the highest accumulation of proline. According to Ashraf et al. [74], sugars could contribute more than 50% of the osmotic adjustment required by glycophytes exposed to salinity. This highlights the significant role of sugars in enhancing plant resilience to saline environments by regulating the osmotic potential and ensuring optimal cellular hydration levels, as mentioned by several authors [75–78].
3.4 Morphological, Physiological, and Biochemical Response of Ecotypes under Different NaCl Treatments
Salinity tolerance indices were used to perform principal component analysis (PCA) of ecotypes exposed to a low salinity dose (4 g/L), with the first two PCA factors explaining 100% of the variability (Factor 1:64.31%, Factor 2:35.69%). The leaf area (LA) was the most influential variable on the first axis, contributing 15.5% (Table 2). Boussora et al. [79] found significant differences in leaf area under saline conditions, with plants typically showing reduced leaf area, but our results differed, with ecotypes exhibiting opposite trends. Additionally, the leaflet relative water content (LWRC) was strongly correlated with the second factor, contributing to 24%. This was more closely related to genetic behavior than salinity, which showed a non-significant effect, and our findings were consistent with the report by Boussora et al. [79].

The average of the tolerance indices of the most discriminating variables was subjected to a mean comparison test (LSD) to classify the ecotypes according to their tolerance. Individuals with higher values were the most tolerant, while those with lower values were the most sensitive to stress. The dispersion of ecotypes among individual circles and the comparison of average tolerance indices allowed for the differentiation of three groups based on the two most discriminating variables, LA and LWRC (Fig. 2). The first group comprised ecotype 10, positioned at the end of the first factor and simultaneously in the negative range of the second factor, exhibiting the highest tolerance index value (1.4065) (Fig. 3). This ecotype appeared to be the most tolerant to the low NaCl treatment (4 g/L). Additionally, ecotype 2 exhibited a tolerance index of 0.9235, indicating moderate tolerance. Finally, ecotype 9 constituted the last group, with the lowest value (0.7592), which reflected its high sensitivity.
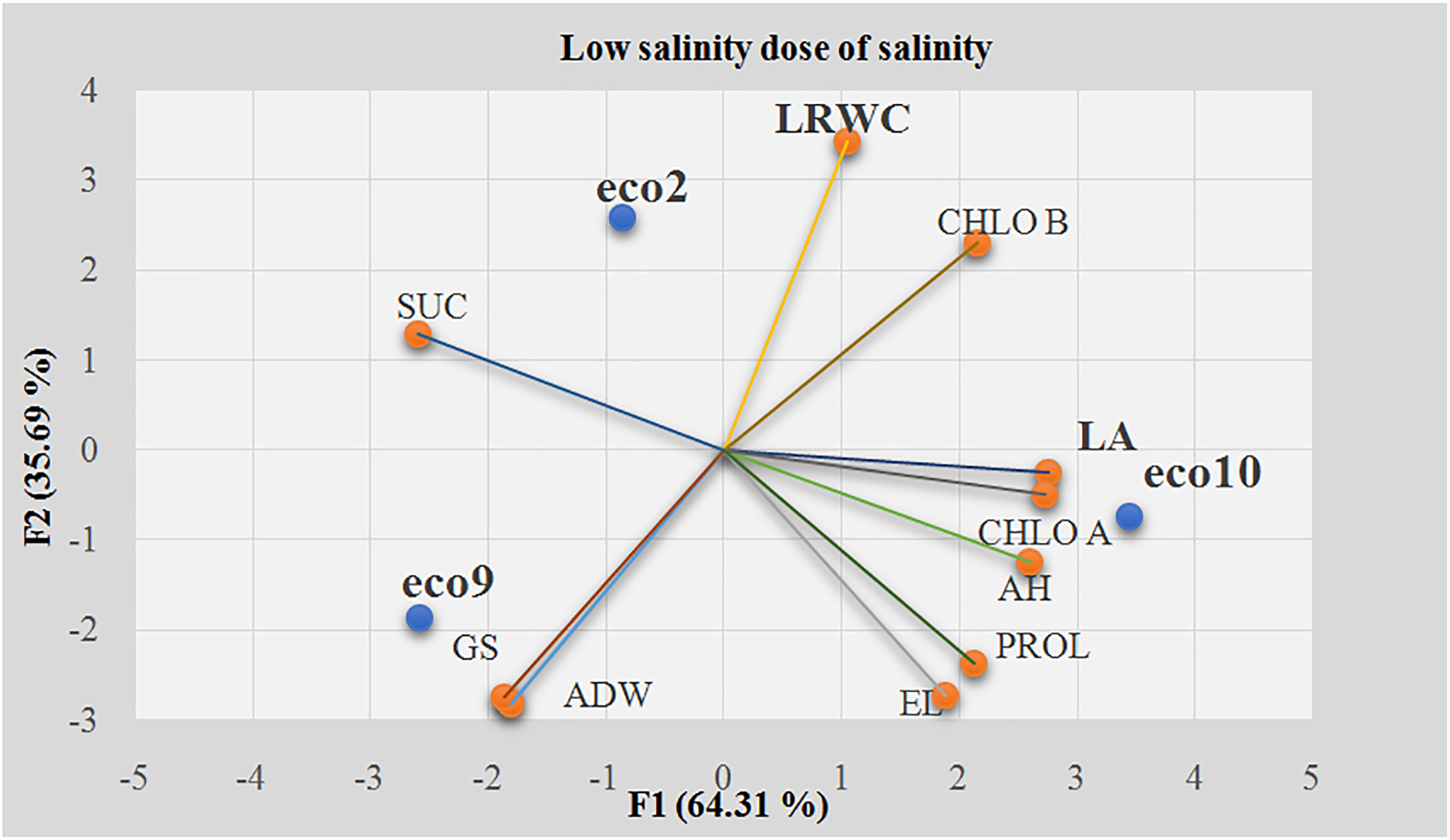
Figure 2: Principal component analysis of all studied variables at the low salinity dose (4 g/L). EL: Electrolyte Leakage; LRWC: Leaf Relative Water Content; AH: Aerial Height; ADW: Aerial Dry Weight; LA: Leaf Area; GS: Growth Speed; CHL A: Chlorophyll A; CHL B: Chlorophyll B; SUC: Soluble Sugar Content; PROL: Proline content; F1: the first axis of PCA; F2: the second axis of PCA
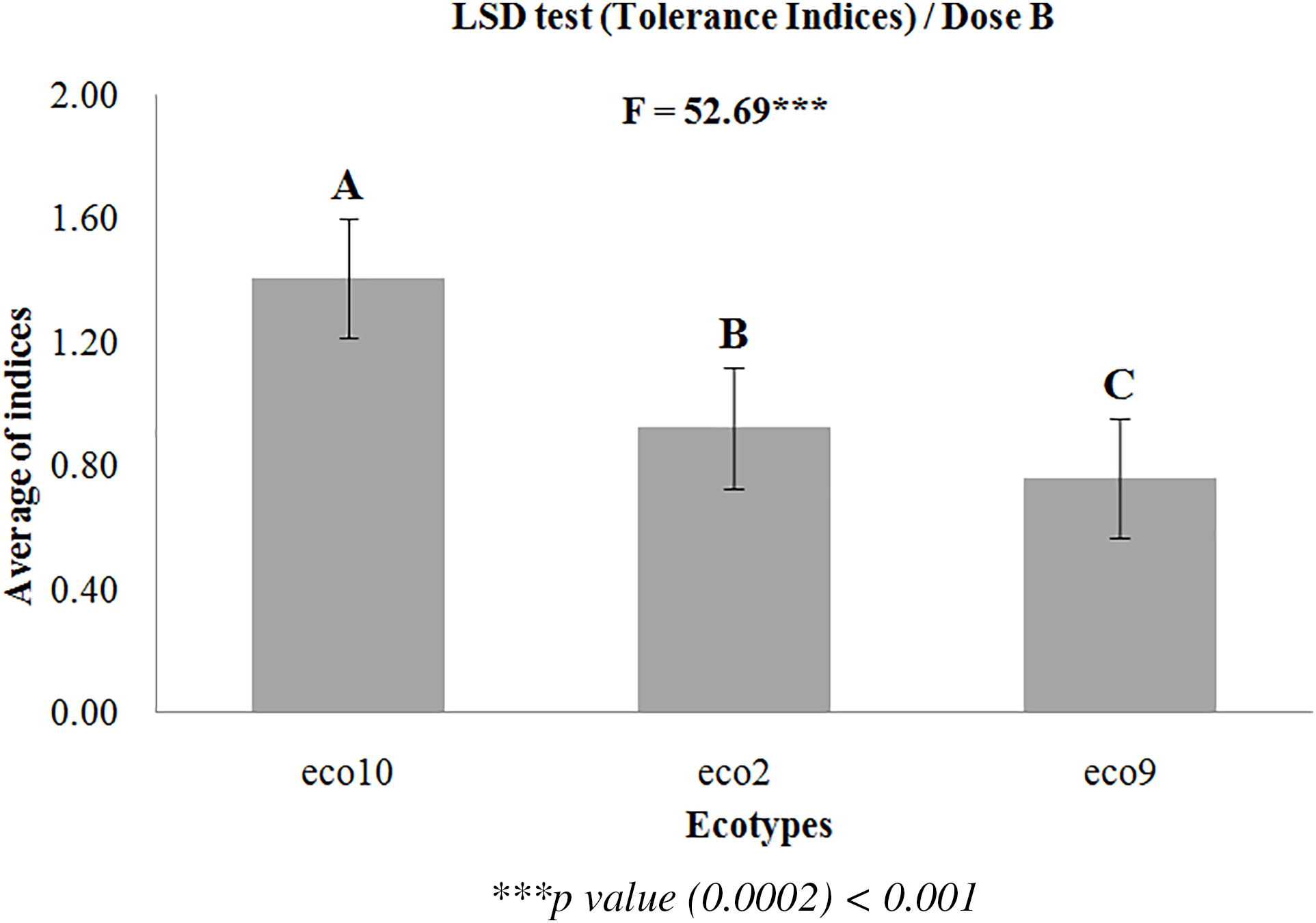
Figure 3: A mean comparison test (LSD) performed on the average tolerance indices of the most discriminating variables at the low salinity dose
At a moderate salinity level (8 g/L), the first two PCA factors (F1 and F2) accounted for 60.39% and 39.61% of the total variability, respectively (Fig. 4). The soluble sugar content (SUC) was a key variable that strongly correlated with the first axis, contributing 16.54% (Table 2). Under high salt stress, soluble sugar levels significantly increased compared to the control, aiding osmotic adjustment [80]. This finding aligns with our results, particularly for eco2 and eco9.
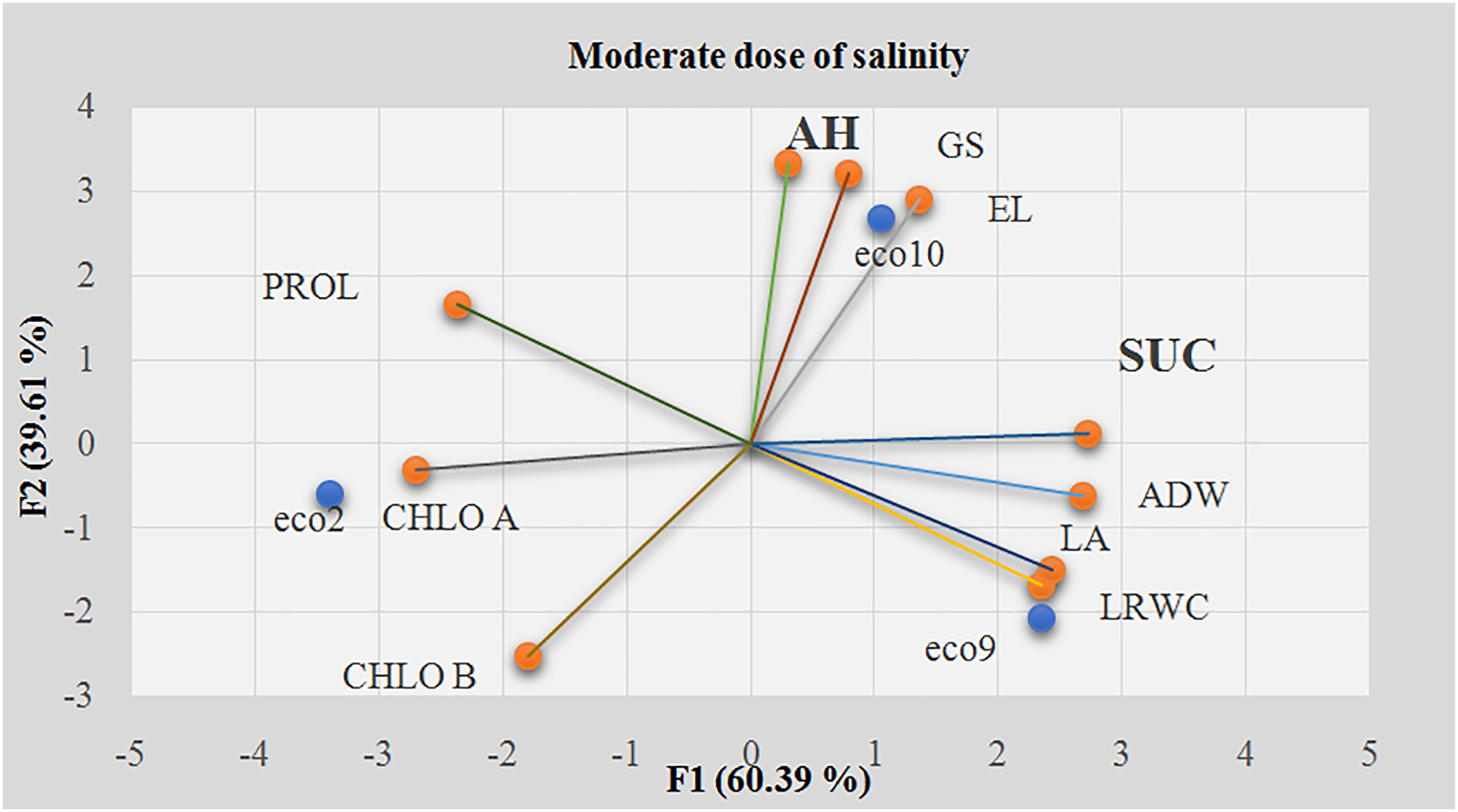
Figure 4: Principal component analysis of all studied variables at the moderate dose of salinity (8 g/L). EL: Electrolyte Leakage; LRWC: Leaf Relative Water Content; AH: Aerial Height; ADW: Aerial Dry Weight; LA: Leaf Area; GS: Growth Speed; CHL A: Chlorophyll A; CHL B: Chlorophyll B; SUC: Soluble Sugar Content; PROL: Proline content; F1: the first axis of PCA; F2: the second axis of PCA
The second PCA factor explained 24.94% of the variability, with plant height (AH) being a major contributing variable. Bhattarai et al. [81] found that salt stress significantly reduced plant height in alfalfa, highlighting the importance of identifying key genes for this trait to develop salt-tolerant cultivars. In our study, the “eco10” ecotype displayed atypical behavior, with the smallest height in the control and the tallest height at a moderate salt concentration of 8 g/L.
A comparison of tolerance indices revealed two homogeneous groups (Fig. 5). The first group, ecotype 10, had the highest tolerance index score of 1.3024, and its position in the PCA circle indicated high efficiency. The second group, ecotypes 9 and 2, had the lowest tolerance indices of 0.6181 and 0.5672, respectively, and was the most sensitive to moderate salinity.

Figure 5: Mean comparison test (LSD) performed on the average tolerance indices of the most discriminating variables at the moderate salinity dose
At a high salinity dose (12 g/L), the main factors in the PCA were related to aerial biomass, especially leaf area (LA) and growth speed (SG) (Fig. 6), contributing 12.75% and 18.48%, respectively (Table 2). The LSD test revealed two homogeneous groups based on tolerance indices. Ecotype 10 showed the highest tolerance to high salinity with a tolerance index of 1.2289, excelling in leaf area and growth rate, whereas ecotypes 2 and 9 were more susceptible, with lower tolerance indices of 0.6058 and 0.5112, respectively (Fig. 7).
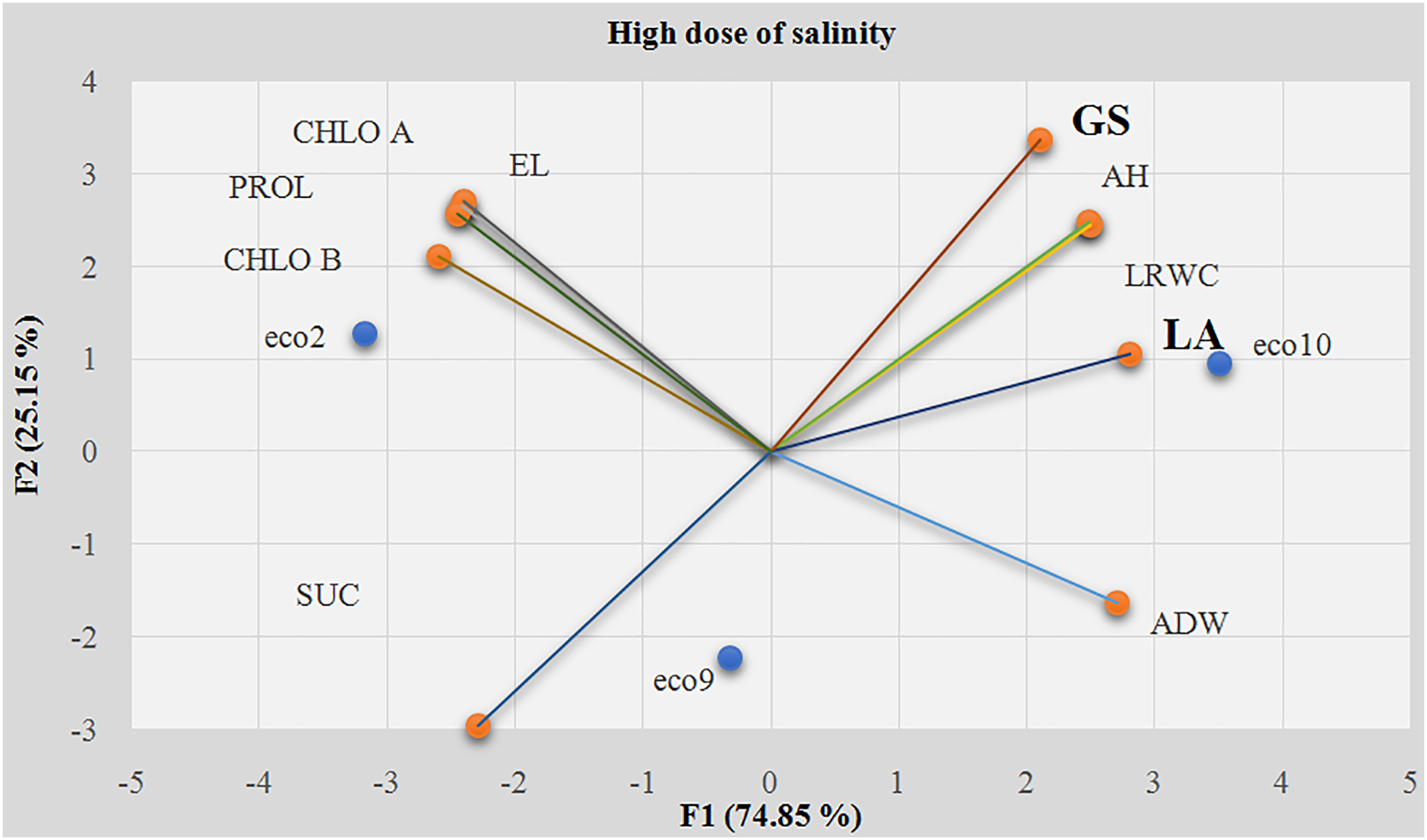
Figure 6: Principal component analysis of all studied variables at the high dose of salinity (8 g/L). EL: Electrolyte Leakage; LRWC: Leaf Relative Water Content; AH: Aerial Height; ADW: Aerial Dry Weight; LA: Leaf Area; GS: Growth Speed; CHL A: Chlorophyll A; CHL B: Chlorophyll B; SUC: Soluble Sugar Content; PROL: Proline content; F1: the first axis of PCA; F2: the second axis of PCA

Figure 7: A mean comparison test (LSD) performed on the average tolerance indices of the most discriminating variables at the high salinity dose
This study found that parameters such as leaf area, aerial height, and growth speed were effective indicators of salt tolerance in vetches, supporting the findings of Ben Chikha et al. [82] for barley and Roshdy et al. [83] for strawberries. Additionally, soluble sugars have been identified as key factors in distinguishing ecotypes, and their accumulation under environmental stress serves as an adaptive trait linked to stress tolerance.
The classification of ecotypes through various statistical analyses (PCA, tolerance indices, and LSD mean comparison test) reveals that ecotype 10 exhibits the highest stress tolerance across all three levels of NaCl application: low, moderate, and severe. This ecotype consistently demonstrated significant tolerance index values across all selected variables in the multivariate analysis (PCA), encompassing morphological, physiological, and biochemical traits. The decision to incorporate it into salt tolerance breeding programs depends on the combination of salt tolerance at different growth stages [30].
Our results showed that traits such as leaf area, growth rate, plant height, and soluble sugar content exhibited significant variation, suggesting their potential as key indicators for identifying salt-tolerant genotypes. Notably, Ecotype 10 demonstrated considerable promise for adaptation to salt stress, maintaining growth under high NaCl concentrations. However, its integration into breeding programs should only be pursued after further validation of its adaptation model through additional experimental trials and more refined approaches. These findings provide valuable insights into the advancement of salt-tolerance breeding in vetches.
Acknowledgment: The authors extend their appreciation to la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT), Algeria, and to Researchers Supporting Project No. (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Funding Statement: This research was funded by la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT), Algeria, and the Researchers Supporting Project No. (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Hocine Bougrine, Mohamed Zouidi, Salah Hadjout, Abdeldjalil Belkendil, Chaouki Boulekdam; data collection: Walid Ouaret, Amar Mebarkia; analysis and interpretation of results: Salah Hadjout, Hocine Bougrine, Amer Zeghmar; draft manuscript preparation: Hocine Bougrine, Salah Hadjout, Fathi Abdellatif Belhouadjeb, Walid Soufan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Aydinoğlu B, Shabani A, Safavi SM. Impact of priming on seed germination, seedling growth and gene expression in common vetch under salinity stress. Cel Mol Biol. 2019;65(3):18–24. doi:10.14715/cmb/2019.65.3.3. [Google Scholar] [CrossRef]
2. Ghobadi M, Khosravi S, Kahrizi D, Shirvani F. Study of water relations, chlorophyll and their correlations with grain yield in wheat (Triticum aestivum L.) genotypes. Inter J Agri Bios Eng. 2011;5(6):353–6. [Google Scholar]
3. De Azevedo Neto AD, da Silva EC. Physiology and biochemistry of salt stress tolerance in plants. In: Abiotic stresses in crop plants. Wallingford, UK: CABI; 2015. p. 81–101. doi:10.1079/9781780643731.0081 [Google Scholar] [CrossRef]
4. De Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Abreu CE, Gomes-Filho E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Envir Exper Bot. 2006;56(1):87–94. doi:10.1016/j.envexpbot.2005.01.008. [Google Scholar] [CrossRef]
5. Alzahrani SM, Alaraidh IA, Migdadi H, Alghamdi S, Khan MA, Ahmad P. Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak J Bot. 2019;51(3):786–98. doi:10.30848/pjb2019-3(3). [Google Scholar] [CrossRef]
6. Zouidi M, Borsali AH, Hachem K, Allam A, Naimi A, Hakmi I. Assessing of the tolerance of Pinus halepensis Mill. seeds to water and saline stress at the germination stage. Forest Ideas. 2019;25:160–70. [Google Scholar]
7. Shahid SA, Zaman M, Heng L. Soil salinity: historical perspectives and a world overview of the problem. In: Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Cham: Springer; 2018. p. 43–53. 10.1007/978-3-319-96190-3_2. [Google Scholar] [CrossRef]
8. Tayyab M, Azeem M, Qasim M, Ahmad N, Ahmad R. Salt stress responses of pigeon pea (Cajanus cajan) on growth, yield and some biochemical attributes. Pak J Bot. 2016;48(4):1353–60. [Google Scholar]
9. Zouidi M, Borsali AH, Allam A, Hadjout S, Hadjadji I, Chikhi D, et al. Effects of crop types on the physicochemical and biological properties of agricultural soils in semi-arid regions (Western Algeria). Malay J Soil Sci. 2023;27:80–96. [Google Scholar]
10. Tarolli P, Luo J, Park E, Barcaccia G, Masin R. Soil salinization in agriculture: mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience. 2024;27(2):1–9. doi:10.1016/j.isci.2024.108830. [Google Scholar] [PubMed] [CrossRef]
11. Koyro HW, Hussain T, Huchzermeyer B, Khan MA. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Env Exp Bot. 2013;91:22–9. doi:10.1016/j.envexpbot.2013.02.007. [Google Scholar] [CrossRef]
12. Türkan I, Demiral T. Recent developments in understanding salinity tolerance. Env Exp Bot. 2009;67(1):2–9. doi:10.1016/j.envexpbot.2009.05.008. [Google Scholar] [CrossRef]
13. Qados AMA. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J Saudi Soc Agri Sci. 2011;10(1):7–15. doi:10.1016/j.jssas.2010.06.002. [Google Scholar] [CrossRef]
14. Praxedes SC, De Lacerda CF, DaMatta FM, Prisco JT, Gomes-Filho E. Salt tolerance is associated with differences in ion accumulation, biomass allocation and photosynthesis in cowpea cultivars. J Agr Crop Sci. 2010;196(3):193–204. doi:10.1111/j.1439-037X.2009.00412.x. [Google Scholar] [CrossRef]
15. Yang Y, Guo Y. Unraveling salt stress signaling in plants. J Integ Plant Bio. 2018;60(9):796–804. doi:10.1111/jipb.12689. [Google Scholar] [PubMed] [CrossRef]
16. Djebbouri M, Terras M, Zouidi M. An initial investigation of Otocarpus virgatus seeds germination an endemic, rare and threatened plant of Algeria. J Stress Physio Biochem. 2022;18(1):10–6. [Google Scholar]
17. Lindberg S, Premkumar A. Ion changes and signaling under salt stress in wheat and other important crops. Plants. 2023;13(1):46. doi:10.3390/plants13010046. [Google Scholar] [PubMed] [CrossRef]
18. Mebarkia A, Bougrine H, Badache F, Mahmah S. Yield and phenology of 10 vetch varieties (Vicia spp.the results of a study carried out on the high plains of Sétif (Algeria) by a North African agricultural network. Fourrage. 2020;241:57–64. [Google Scholar]
19. FAOSTAT. Food and agriculture organization of united nations; 2024 [cited 2024 Mar 05]. Available from: https://www.fao.org/faostat/fr/#data/QCL. [Google Scholar]
20. Kahrizi D, Arminian A, Masumi A. In vitro plant breeding. Iran: Razi University Press Kermanshah; 2011. 205p. [Google Scholar]
21. Campanelli A, Ruta C, Morone-Fortunato I, De Mastro G. Alfalfa (Medicago sativa L.) clones tolerant to salt stress: in vitro selection. Cent Eur J Bio. 2013;8:765–76. doi:10.2478/s11535-013-0194-1. [Google Scholar] [CrossRef]
22. Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Ann Rev Plant Bio. 2000;51(1):463–99. doi:10.1146/annurev.arplant.51.1.463. [Google Scholar] [CrossRef]
23. Schonfeld MA, Johnson RC, Carver BF, Mornhinweg DW. Water relations in winter wheat as drought resistance indicators. Crop Sci. 1988;28(3):526–31. doi:10.2135/cropsci1988.0011183X002800030021x. [Google Scholar] [CrossRef]
24. Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot. 1996;78(3):389–98. doi:10.1006/anbo.1996.0134. [Google Scholar] [CrossRef]
25. Arnon AN. Method of extraction of chlorophyll in the plants. Agro J. 1967;23(1):112–21. [Google Scholar]
26. Bates LS, Waldren RPA, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7. doi:10.1007/BF00018060. [Google Scholar] [CrossRef]
27. Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–6. [Google Scholar]
28. Zeng L, Shannon MC, Grieve CM. Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica. 2002;127(2):235–45. doi:10.1023/A:1020262932277. [Google Scholar] [CrossRef]
29. Rajhi I, Ben Moussa S, Neji I, Baccouri B, Ben Chikha M, Chammakhi, et al. Photosynthetic and physiological responses of small seeded faba bean genotypes (Vicia faba L.) to salinity stress: identification of a contrasting pair towards salinity. Photosynthetica. 2020;58(1):174–85. doi:10.32615/ps.2019.152. [Google Scholar] [CrossRef]
30. Singh S, Sharma VR, Nannuru VKR, Singh B, Kumar M. Phenotypic diversity of pea genotypes (Pisum sativum L.) based on multivariate analysis. Leg Res Inter J. 2021;44(8):875–81. [Google Scholar]
31. Janghel DK, Kumar K, Kumar M, Chhabra AK. Genetic diversity assessment in chickpea (Cicer arietinum L.) through agro-morphological and ISSR molecular markers. Leg Res Inter J. 2021;44(7):751–8. [Google Scholar]
32. Shah SH, Houborg R, McCabe MF. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy. 2017;7(3):61. doi:10.3390/agronomy7030061. [Google Scholar] [CrossRef]
33. Caruso C, Maucieri C, Berruti A, Borin M, Barbera AC. Responses of different Panicum miliaceum L. genotypes to saline and water stress in a marginal Mediterranean environment. Agronomy. 2018;8(1):8. doi:10.3390/agronomy8010008. [Google Scholar] [CrossRef]
34. Bilgili U, Çarpıcı EB, Aşık BB, Celik N. Root and shoot response of common vetch (Vicia sativa L.forage pea (Pisum sativum L.) and canola (Brassica napus L.) to salt stress during early se. Turk J Field Cro. 2011;16(1):33–8. [Google Scholar]
35. Osuagwu AN, Edem UL. Relationship between salt (NaCl) stress and yield components in three varieties of African yam bean (Sphenostylis stenocarpa). Asian J Bio. 2021;13(1):14–21. doi:10.9734/ajob/2021/v13i130176. [Google Scholar] [CrossRef]
36. Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South Afr J Bot. 2016;105:306–12. doi:10.1016/j.sajb.2016.03.011. [Google Scholar] [CrossRef]
37. Dawood MG, El-Awadi ME. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Bio Colom. 2015;20(2):223–35. [Google Scholar]
38. Ahmad P, Alyemeni MN, Ahanger MA, Egamberdieva D, Wijaya L, Alam P. Salicylic acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Rus J Plant Phys. 2018;65(1):104–14. doi:10.1134/S1021443718010132. [Google Scholar] [CrossRef]
39. Ahmad P, Hashem A, Abd-Allah EF, Alqarawi AA, John R, Egamberdieva D, et al. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front Plant Sci. 2015;6:868. doi:10.3389/fpls.2015.00868. [Google Scholar] [PubMed] [CrossRef]
40. Xu Q, Burgess P, Xu J, Meyer W, Huang B. Osmotic stress-and salt stress-inhibition and gibberellin-mitigation of leaf elongation associated with up-regulation of genes controlling cell expansion. Envir Exp Bot. 2016;131:101–9. doi:10.1016/j.envexpbot.2016.06.001. [Google Scholar] [CrossRef]
41. Castroluna A, Ruiz OM, Quiroga AM, Pedranzani HE. Effects of salinity and drought stress on germination, biomass and growth in three varieties of Medicago sativa L. Ava Invest Agro. 2014;18(1):39–50. [Google Scholar]
42. Mezni M, Ghnaya-Chakroun AB, Haffani S. Growth and water status in narbonne vetch (Vicia narbonensis L.) under salt stres. J Agri Veter Sci. 2013;3(3):2319–72. [Google Scholar]
43. Abdelraouf AE, Adss AA, Dakroury ZM. Effect of salinity on growth and genetic diversity of broad bean (Vicia faba L.) cultivars. Alex Sci Exch J. 2016;37:467–79. doi:10.21608/asejaiqjsae.2016.2514. [Google Scholar] [CrossRef]
44. Ali Y, Aslam Z, Ashraf MY, Tahir GR. Effect of salinity on chlorophyll concentration, leaf area, yield and yield components of rice genotypes grown under saline environment. Int J Envir Sci Tech. 2004;1(3):221–5. doi:10.1007/BF03325836. [Google Scholar] [CrossRef]
45. Dadasoglu E, Turan M, Ekinci M, Argin S, Yildirim E. Alleviation mechanism of melatonin in chickpea (Cicer arietinum L.) under the salt stress conditions. Horticulturae. 2022;8(11):1066. doi:10.3390/horticulturae8111066. [Google Scholar] [CrossRef]
46. He H, Zhou W, Lü H, Liang B. Growth, leaf morphological and physiological adaptability of leaf beet (Beta vulgaris var. cicla) to salt stress: a soil culture experiment. Agronomy. 2022;12(6):1393. doi:10.3390/agronomy12061393. [Google Scholar] [CrossRef]
47. Abbasi AR, Sarvestani R, Mohammadi B, Baghery A. Drought stress-induced changes at physiological and biochemical levels in some common vetch (Vicia sativa L.) genotypes. J Agri Sci Tech. 2014;16:505–16. [Google Scholar]
48. Abdel Motaleb N, Elhady SA, Ghoname AA. AMF and Bacillus megaterium neutralize the harmful effects of salt stress on bean plants. Gesunde Pflanz. 2020;72(1):29–39. [Google Scholar]
49. Tlahig S, Bellani L, Karmous I, Barbieri F, Loumerem M, Muccifora S. Response to salinity in legume species: an insight on the effects of salt stress during seed germination and seedling growth. Chem Biodiv. 2021;18(4):e2000917. doi:10.1002/cbdv.202000917. [Google Scholar] [PubMed] [CrossRef]
50. Tayefi-Nasrabadi H, Dehghan G, Daeihassani B, Movafegi A, Samadi A. Some biochemical properties of guaiacol peroxidases as modified by salt stress in leaves of salt-tolerant and salt-sensitive safflower (Carthamus tinctorius L. cv.) cultivars. Afri J Biot. 2011;10(5):751–63. [Google Scholar]
51. Bacha H, Mansour E, Guasmi F, Triki T, Ferchichi A. Proline, glycine bétaïne et composition minérale des plantes de Solanum lycopersicum L. (var Microtom) sous stress salin. J New Sci. 2015;22:1007–13. [Google Scholar]
52. Aydi S, Sassi S, Debouba M, Hessini K, Larrainzar E, Gouia H, et al. Resistance of Medicago truncatula to salt stress is related to glutamine synthetase activity and sodium sequestration. J Plant Nutri Soil Sci. 2010;173(6):892–9. doi:10.1002/jpln.200900235. [Google Scholar] [CrossRef]
53. Ben Salah I, Slatni T, Albacete A, Gandour M, Martínez AC, Houmani H, et al. Salt tolerance of nitrogen fixation in Medicago ciliaris is related to nodule sucrose metabolism performance rather than antioxidant system. Symbiosis. 2010;51(2):187–95. doi:10.1007/s13199-010-0073-3. [Google Scholar] [CrossRef]
54. Najar R, Aydi S, Sassi-Aydi S, Zarai A, Abdelly C. Effect of salt stress on photosynthesis and chlorophyll fluorescence in Medicago truncatula. Plant Biosys Inter J. 2019;153(1):88–97. doi:10.1080/11263504.2018.1461701. [Google Scholar] [CrossRef]
55. Najar R. Physiological aspects of the salt response of a fabaceae with fodder potentiality: Hedysarum carnosum. Tunis: Faculty of Sciences; 2011. 108 p. [Google Scholar]
56. Bashir H, Qureshi MI, Ibrahim MM, Iqbal M. Chloroplast and photosystems: impact of cadmium and iron deficiency. Photosynthetica. 2015;53(3):321–35. doi:10.1007/s11099-015-0152-z. [Google Scholar] [CrossRef]
57. Hussein M, Embiale A, Husen A, Aref IM, Iqbal M. Salinity-induced modulation of plant growth and photosynthetic parameters in faba bean (Vicia faba) cultivars. Pak J Bot. 2017;49(3):867–77. [Google Scholar]
58. Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot. 2015;115(3):433–47. doi:10.1093/aob/mcu239. [Google Scholar] [PubMed] [CrossRef]
59. Sharma S, Villamor JG, Verslue PE. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Phys. 2011;157(1):292–304. doi:10.1104/pp.111.183210. [Google Scholar] [PubMed] [CrossRef]
60. Li J, Essemine J, Shang C, Zhang H, Zhu X, Yu J, et al. Combined proteomics and metabolism analysis unravels prominent roles of antioxidant system in the prevention of alfalfa (Medicago sativa L.) against salt stress. Inter J Mol Sci. 2020;21(3):909. doi:10.3390/ijms21030909. [Google Scholar] [PubMed] [CrossRef]
61. Ahmad P, Kumar A, Ashraf M, Akram NA. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afri J Biot. 2012;11(11):2694. [Google Scholar]
62. Gharsallah C, Fakhfakh H, Grubb D, Gorsane F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants. 2016;8:plw055. doi:10.1093/aobpla/plw055. [Google Scholar] [PubMed] [CrossRef]
63. Nasrallah AK, Atia MA, Abd El-Maksoud RM, Kord MA, Fouad AS. Salt priming as a smart approach to mitigate salt stress in Faba bean (Vicia faba L.). Plants. 2022;11(12):1610. doi:10.3390/plants11121610. [Google Scholar] [PubMed] [CrossRef]
64. Xu D, Wang W, Gao T, Fang X, Gao X, Li J, et al. Calcium alleviates decreases in photosynthesis under salt stress by enhancing antioxidant metabolism and adjusting solute accumulation in Calligonum mongolicum. Conser Phys. 2017;5(1):cox060. doi:10.1093/conphys/cox060. [Google Scholar] [CrossRef]
65. Nasrallah AK, Kheder AA, Kord MA, Fouad AS, El-Mogy MM, Atia MA. Mitigation of salinity stress effects on broad bean productivity using calcium phosphate nanoparticles application. Horticulturae. 2022;8(1):75. [Google Scholar]
66. Alzahrani O, Abouseadaa H, Abdelmoneim TK, Alshehri MA, Mohamed EM, El-Beltagi HS, et al. Agronomical, physiological and molecular evaluation reveals superior salt-tolerance in bread wheat through salt-induced priming approach. Not Bot Hort Agrobot Cluj-Napoca. 2021;49(2):12310. doi:10.15835/nbha49212310. [Google Scholar] [CrossRef]
67. Ahmad F, Kamal A, Singh A, Ashfaque F, Alamri S, Siddiqui MH. Salicylic acid modulates antioxidant system, defense metabolites, and expression of salt transporter genes in Pisum sativum under salinity stress. J Plant Growth Reg. 2022;41(5):1905–18. doi:10.1007/s00344-020-10271-5. [Google Scholar] [CrossRef]
68. Khan I, Raza MA, Awan SA, Shah GA, Rizwan M, Ali B, et al. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPsthe oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Phys Bioch. 2020;156(1):221–32. doi:10.1016/j.plaphy.2020.09.018. [Google Scholar] [PubMed] [CrossRef]
69. Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi:10.3390/biom9070285. [Google Scholar] [PubMed] [CrossRef]
70. Farissi M, Bouizgaren A, Faghire M, Bargaz A, Ghoulam C. Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci Tech. 2011;39(2):389–401. doi:10.15258/sst.2011.39.2.11. [Google Scholar] [CrossRef]
71. Attarzadeh M, Balouchi H, Rajaie M, Dehnavi MM, Salehi A. Improvement of Echinacea purpurea performance by integration of phosphorus with soil microorganisms under different irrigation regimes. Agri Water Manag. 2019;221:238–47. doi:10.1016/j.agwat.2019.04.022. [Google Scholar] [CrossRef]
72. Mehta D, Vyas S. Comparative bio-accumulation of osmoprotectants in saline stress tolerating plants: a review. Plant Stress. 2023;9(5):100177. doi:10.1016/j.stress.2023.100177. [Google Scholar] [CrossRef]
73. Rymbai H, Srivastav M, Singh SK, Singh AK. Growth, flowering and yield attributes of full-sib (Amrapali × Sensation) hybrids of mango. Indian J Horti. 2016;73(2):157–64. [Google Scholar]
74. Ashraf MP, Harris PJ. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166(1):3–16. doi:10.1016/j.plantsci.2003.10.024. [Google Scholar] [CrossRef]
75. Theerawitaya C, Tisarum R, Samphumphuang T, Singh HP, Cha-Um S, Kirdmanee C, et al. Physio-biochemical and morphological characters of halophyte legume shrub, Acacia ampliceps seedlings in response to salt stress under greenhouse. Front Plant Sci. 2015;6:630. doi:10.3389/fpls.2015.00630. [Google Scholar] [PubMed] [CrossRef]
76. El Moukhtari A, Cabassa-Hourton C, Farissi M.Savouré A. How does proline treatment promote salt stress tolerance during crop plant development? Front Plant Sci. 2020;11:553924. doi:10.3389/fpls.2020.01127. [Google Scholar] [PubMed] [CrossRef]
77. Alzahrani F. Metabolic engineering of osmoprotectants to elucidate the mechanism(s) of salt stress tolerance in crop plants. Planta. 2021;253(1):24. doi:10.1007/s00425-020-03550-8. [Google Scholar] [PubMed] [CrossRef]
78. Zulfiqar F, Ashraf M. Proline alleviates abiotic stress induced oxidative stress in plants. J Plant Growth Regul. 2023;42(8):4629–51. doi:10.1007/s00344-022-10839-3. [Google Scholar] [CrossRef]
79. Boussora F, Triki T, Bennani L, Bagues M, Ben Ali S, Ferchichi A, et al. Mineral accumulation, relative water content and gas exchange are the main physiological regulating mechanisms to cope with salt stress in barley. Sci Rep. 2024;14(1):14931. doi:10.1038/s41598-024-65967-5. [Google Scholar] [PubMed] [CrossRef]
80. Beinsan C, Camen D, Sumalan R, Babau M. Study concerning salt stress effect on leaf area dynamics and chlorophyll content in four bean local landraces from Banat area. In: 44th Croatian and 4th International Symposium on Agriculture; 2009. p. 416–9. [Google Scholar]
81. Bhattarai S, Biswas D, Fu YB, Biligetu B. Morphological, physiological, and genetic responses to salt stress in alfalfa: a review. Agronomy. 2020;10(4):577. doi:10.3390/agronomy10040577. [Google Scholar] [CrossRef]
82. Ben Chikha M, Hessini K, Ourteni RN, Ghorbel A, Zoghlami N. Identification of barley landrace genotypes with contrasting salinity tolerance at vegetative growth stage. Plant Biotech. 2016;33(4):287–95. doi:10.5511/plantbiotechnology.16.0515b. [Google Scholar] [PubMed] [CrossRef]
83. Roshdy AED, Alebidi A, Almutairi K, Al-Obeed R, Elsabagh A. The effect of salicylic acid on the performances of salt stressed strawberry plants, enzymes activity, and salt tolerance index. Agronomy. 2021;11(4):775. doi:10.3390/agronomy11040775. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools