 Open Access
Open Access
REVIEW
Nanoparticle Innovations for Mitigating Metal Toxicity in Plants
1 Department of Botany, The Government Sadiq College Women University, Bahawalpur, 63100, Pakistan
2 Department of Arid Land Agriculture, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, 31982, Saudi Arabia
3 College of Agriculture, Northeast Agricultural University, Harbin, 150030, China
4 Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, 63100, Pakistan
5 Department of Horticulture, The University of Agriculture, Dera Ismail Khan, 29220, Pakistan
6 Department of Field Crops, Faculty of Agriculture, Recep Tayyip Erdoğan University, Rize/Pazar, 53300, Türkiye
* Corresponding Author: Ishtiaq Ahmad. Email:
(This article belongs to the Special Issue: Plant Responses to Abiotic Stress Mechanisms)
Phyton-International Journal of Experimental Botany 2025, 94(3), 623-640. https://doi.org/10.32604/phyton.2025.063763
Received 23 January 2025; Accepted 27 February 2025; Issue published 31 March 2025
Abstract
Various environmental stressors, such as salinity, heat, drought, and metals, present significant obstacles to crop productivity. This study delves into the adverse effects of metals, specifically focusing on cadmium (Cd), nickel (Ni), mercury (Hg), chromium (Cr), arsenic (As), lead (Pb), and copper (Cu) on plants. It explores the sources of these metals, examining both natural occurrences and human-induced activities, and investigates the mechanisms through which plants absorb them. Metal pollution, in particular, negatively affects plant and microbiome well-being, producing reactive oxygen species (ROS) that harm essential macromolecules. Traditional stress-resistant plant varieties necessitate substantial development, leading to the exploration of innovative approaches like nanotechnology. This examination underscores the diverse applications of nanoparticles (NPs), such as titanium oxide, copper oxide, zinc oxide, etc., in alleviating metal stress and improving crop resilience. Nanoparticles possess advantageous characteristics, including increased reactivity, small size, and efficient transport within plants. The earlier information underscores the influence of nanoparticles on morpho-physiological and biochemical traits of plants, addressing the limited information in this field, especially under metal toxicity. Mechanisms of NP action encompass chelation, antioxidant enzymatic activity, and the formation of complexes, presenting promising avenues for sustainable agriculture and enhanced food productivity. Future perspectives in nanoparticle strategies for metal toxicity emphasize tailored formulations and long-term ecological studies. Integration with precision agriculture and genetic engineering offers synergies, highlighting collaborative efforts and global cooperation for practical adoption.Keywords
Agriculture plays a crucial role in ensuring food security and providing essential nutrients for the global population. The shift towards monoculture and reliance on a few staple crops has raised concerns about sustainability and food security. This overview highlights the multifaceted contributions of agriculture to food systems, emphasizing the need for sustainable practices to meet future demands. The food industry processes 60%–80% of primary agricultural production, linking agriculture with economic development. Addressing undernourishment and malnutrition requires a commitment to sustainable agriculture that meets current and future food needs [1]. Maintaining crop yield amidst environmental challenges stands as a significant undertaking for scientists. This is due to the substantial impact of diverse abiotic stressors on the environment and climatic conditions, leading to a reduction in crop productivity [2]. Salinity, heat stress, drought, and heavy metals are among the various stresses that pose significant constraints, resulting in yield losses and economic downturns [3]. The presence of heavy metals in the soil hurts both the plant and its associated microbiome. The stress induced by them leads to the generation of (ROS), which, in turn, harms the essential macromolecules inside the organism, impacting the nutritional quality and ecology of the vegetation as well as crops. Cu-contamination in soil can interfere with normal growth processes in plants by detrimentally affecting physiological and biochemical processes in plants [4]. Plants exhibit various physiological and biochemical responses to mitigate the adverse effects of HM stress, including the generation of ROS and the activation of antioxidant defense systems. The following sections detail the mechanisms through which plants cope with heavy metal stress. Plants utilize both enzymatic (e.g., superoxide dismutase, catalase) and non-enzymatic (e.g., ascorbate, glutathione) antioxidants to combat oxidative stress induced by HMs [5]. Catalase (CAT), superoxide dismutase (SOD), peroxidase (POX), glutathione peroxidase (GPXs), malonaldehyde (MDA), and other enzymes encounter a notable hindrance in their antioxidant enzymatic activities due to oxidative stress. Despite this challenge, these enzymes play a crucial role in mitigating stressful conditions while maintaining hydrogen peroxidase (H2O2) homeostasis [6]. Nevertheless, the creation of stress-resistant varieties is a time-intensive endeavor that demands numerous trials in both scientific laboratories and natural settings. Various approaches are employed to enhance resistance to abiotic stresses, and this includes the development of genetically modified varieties specifically designed to withstand such stresses [7].
Nanotechnology has played a pivotal role in overcoming constraints to advance civilization by enhancing oversight and streamlining agricultural contributions. This, in turn, will contribute to increasing crop consumption through the modification of formulations and the reduction of yield losses. Modern generations have witnessed the introduction of pathogen diagnosis, agricultural mechanisms, and advanced cropping systems employing integrated nanomaterials [8]. Nanotechnology has diverse applications in agriculture, materials science, and engineering. The utilization of NPs to enhance resilience against drought and heavy metals (HMs) stress represents an innovative approach to sustainable agriculture and increased food productivity. Nanoparticles effectively counteract the toxicity induced by drought and HMs, thereby restoring crop production in challenging environments [9]. The expansive surface area, diminutive size, capacity to enhance solutes, heightened reactivity, pore size, weight ratio, and transportation within plants constitute advantageous properties of nanoparticles [10]. In molecular biology, nanoparticles are employed to enhance enzymatic activity, increase radical detoxification capacity, and achieve sustainability [11]. Nanoparticles, including titanium oxide (TiO2), molybdenum trioxide (MoO3), copper oxide (CuO), silver (Ag), zinc oxide (ZnO), iron oxide (Fe3O4), silicon (Si) and chromium dioxide (CrO2) are increasingly utilized in the agricultural industry. Nanoparticles are unstable, however, during their synthesis, stabilizing agents including Polyvinyl chloride (PVC), polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP) and cetyltrimethylammonium bromide (CTAB) are being used to increase their efficacy. Nanoparticles fulfill vital roles in the control of biochemical pathways, physiological functioning, and the handling of stress [12]. The application of nanoparticles decreased the uptake of Cadmium (Cd), Manganese (Mn), and Lead (Pb) in Oryza sativa, Triticum aestivum, and Helianthus annuus, as NPs might act as a barrier in heavy metal uptake due uptake competition [13]. Nanoparticles (NPs) play a critical role in plant systems, particularly their mobility and accumulation. These particles can enter plants through both leaves and roots [14]. In the foliage, NPs access the mesophyll cells mainly through stomata and cuticles. Their mobility through cell walls is largely determined by their size and charge. Additionally, plastids and plasmodesmata play key roles in regulating intracellular transport and their distribution within plant cells [15]. By utilizing nanomaterials, researchers have developed innovative strategies to reduce the toxicity of heavy metals in plants, thereby improving agricultural sustainability. The integration of nanotechnology in agrochemicals has shown significant potential in improving nutrient efficiency and reducing heavy metal toxicity [16].
In the empire of plant stress responses, the mitigation of heavy metal stress in plants through nanotechnology has garnered significant attention. However, the literature remains notably deficient in addressing the impact of nanoparticles on morpho-physiological and biochemical attributes of cereals under salinity stress at distinct growth stages. This review aims to fill this knowledge gap by exploring the potential of nanoparticles to ameliorate the dire consequences of heavy metal stress on plants, shed shedding light on an innovative approach to enhance crop resilience and productivity.
2 Heavy Metal Stress Poses Significant Challenges to Crops
Heavy metal stress poses significant encounters to living organisms, primarily due to increased anthropogenic activities leading to environmental contamination. This stress results in various physiological and biochemical alterations, including oxidative stress, which can severely impact growth and productivity. Understanding the mechanisms of heavy metal stress and potential mitigation strategies is crucial for enhancing resilience in affected organisms. Exposure to heavy metals generates (ROS), leading to oxidative damage in plant cells. This could hinder plant development, diminish crop yields, and present a potential hazard by infiltrating the food chain, jeopardizing human health. Heavy metal toxicity in plants poses significant negative impacts on growth, metabolism, and overall health, primarily due to the accumulation of ROS and subsequent oxidative stress (Fig. 1). This toxicity leads to various physiological and biochemical disruptions, ultimately affecting crop yield and food safety [17,18]. Heavy metals disrupt cellular processes, leading to stunted growth and reduced biomass in plants. The accumulation of ROS results in lipid peroxidation and damage to cellular membranes, proteins, and nucleic acids. Heavy metals can inhibit enzyme activity, disrupting metabolic pathways essential for plant health [19].
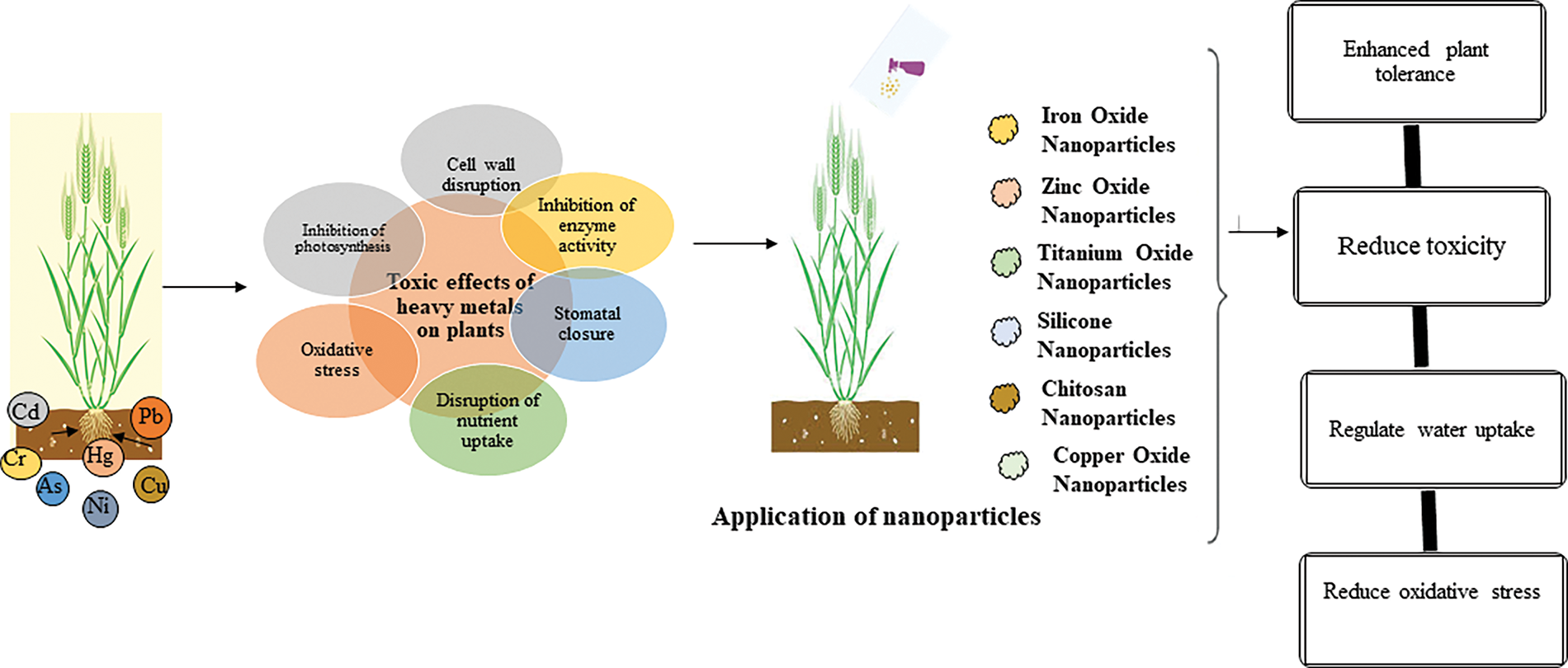
Figure 1: Adverse effects of metal toxicity and its mitigation with nanoparticles
3 Adverse Impacts of Heavy Metals on Plants
3.1 Toxicity of Cadmium (Cd) in Plants
Cadmium (Cd) is one of the most infamous environmental pollutants that showed adverse effects on plants and humans health. In arable terrains and freshwater basins, Cadmium (Cd) is mainly furnished by innate procedures like geogenic influences (resulting from the breakdown of source rocks and volcanic actions) and human-related actions (such as watering with wastewater, application of phosphate fertilizers and pesticides utilization of organic fertilizers, burning of fossil fuels, extraction, refining, and atmospheric precipitation [20]. Cadmium (Cd) toxicity in plants is a significant environmental concern, primarily due to its non-essential nature and harmful effects on plant physiology. Cd enters plants through root uptake and can lead to various detrimental symptoms, including stunted growth, chlorosis, and necrosis. Understanding the mechanisms of Cd toxicity and the strategies for mitigation is crucial for enhancing plant health and productivity. Cd disrupts essential metabolic functions, leading to oxidative stress, DNA damage, and impaired photosynthesis. Cd is absorbed by roots and translocated to aerial parts, affecting nutrient uptake and overall plant health [21]. Cadmium is a prevalent heavy metal contaminant that plant roots absorb, subsequently entering the food chain and posing a threat to human and animal health [22,23].
3.2 Toxicity of Nickel (Ni) in Plants
The advancement of industrial progress has given rise to an increased release of contaminants, affecting ecosystems in the process. One of these pollutants is Nickel (Ni), classified as a transitional element. Nickel and its compounds have various applications in both industrial and business settings. Nickel actively engages in the formation of enzymes, methyl-coenzyme reductase including glyoxalases, superoxide dismutase, peptide ureolysis, ureases, peptide deformylases, and some hydrogenases methyl-coenzyme reductase. Moreover, it plays a crucial role in processes such as acidogenesis, hydrogen metabolism, ureolysis, and methane genesis. Additionally, Nickel contributes significantly to maintaining cellular redox balance, stress resilience/defense, and optimizing nitrogen use efficiency [24]. Nickel (Ni) toxicity poses significant challenges to plant growth and development, primarily due to its accumulation in soils from industrial activities and agricultural practices. While Ni is an essential micronutrient at low concentrations, excessive levels lead to detrimental physiological and biochemical effects on various plant species. The following sections outline the key impacts of Ni toxicity on plants. Nickel (Ni) toxicity poses significant challenges to plant growth and development, primarily due to its accumulation in soils from industrial activities and agricultural practices. While Ni is an essential micronutrient at low concentrations, excessive levels lead to detrimental physiological and biochemical effects on various plant species. The following sections outline the key impacts of Ni toxicity on plants [25]. Excess Ni interferes with the absorption of essential nutrients and phytohormones, further retarding growth and development [26].
3.3 Toxicity of Mercury (Hg) in Plants
Mercury functions as a harmful element for plants and has no positive effects whatsoever. The toxicity of mercury (Hg) in plants significantly impairs their growth, physiological functions, and biochemical processes. Research indicates that even low concentrations of mercury can lead to adverse effects, while higher concentrations result in severe toxicity, affecting plant morphology and reproductive capabilities. The following sections detail the key impacts of mercury on plant health. Mercury exposure leads to reduced plant height and leaf diameter, with severe effects observed at concentrations above 0.10 ppm. Symptoms of toxicity, such as wilting and chlorosis, become evident at higher concentrations, indicating significant stress on the plants [27]. Increased levels of proline and malondialdehyde (MDA) indicate oxidative stress and lipid peroxidation due to mercury accumulation (Table 1). The expression of stress-responsive genes is altered, affecting the plant’s ability to cope with abiotic stress. The accumulation of mercury in plants poses risks to food safety and security, as it can enter the food web and affect human health. Hyperaccumulating plants can exacerbate mercury contamination in agricultural systems, leading to broader ecological consequences [28].
3.4 Toxicity of Chromium (Cr) in Plants
The toxicity of chromium in plants is a significant environmental concern, primarily due to its prevalence in agricultural soils from anthropogenic activities. Chromium exhibits high toxicity towards plants, adversely impacting their growth and development. Toxicity from chromium induces numerous negative impacts on the morpho-physiological characteristics and antioxidant defense system of plants. This results in harm to root tip cells and, in severe cases, can lead to the death of the plant [55]. Chromium exposure leads to reduced growth rates, wilting, and necrosis, particularly in older leaves. Studies indicate that Cr accumulation primarily occurs in roots, hindering the translocation of essential nutrients to aerial parts. Increased levels of ROS due to Cr stress result in oxidative damage, affecting physiological processes like photosynthesis. Cr toxicity disrupts chlorophyll synthesis, leading to diminished photosynthetic efficiency. Enhanced activity of antioxidant enzymes, such as catalase and peroxidase, is observed as a plant response to mitigate oxidative stress. The balance of mineral nutrients is also affected, as Cr competes with essential elements for uptake [56].
3.5 Toxicity of Arsenic (As) in Plants
Arsenic (As) toxicity in plants poses significant challenges to agriculture and food safety due to its widespread environmental presence. The detrimental effects of arsenic on plant health are multifaceted, impacting growth, development, and overall productivity. Understanding these effects is crucial for developing mitigation strategies. Arsenic enters plants primarily through roots, where it can inhibit root growth and nutrient uptake, leading to reduced biomass and chlorophyll degradation [57]. The soil’s As content fluctuates based on its sources, with an average global value of 5 mg kg−1 in soil [58]. The buildup of arsenic in plants has detrimental effects on plant development, such as initiating the generation of ROS, which plays a substantial role in regulating plant physiology and morphology. Furthermore, arsenic disrupts ionic homeostasis and cellular integrity in plants, leading to a reduction in nutrient uptake and consequently hindering plant growth [59]. As in irrigation water and soil components permeate the food chain as the plant absorbs them. Elevated concentrations of As in both soil and irrigation water not only constrain the development and yield of crops but also pose a perilous hazard to human health as they infiltrate the food chain growth [60]. Similar to other metals/metalloids, stress due to toxicity in plants leads to various morpho-physiological disturbances. This includes phenomena such as chlorosis, hindered root proliferation and extension, diminished growth, hindered water absorption, disrupted photosynthesis, respiration, and transpiration. There is also interference with other metabolic processes, ultimately resulting in reduced development and yield through a decrease in biomass accumulation [61].
3.6 Toxicity of Lead (Pb) in Plants
Lead (Pb) toxicity in plants is a significant environmental concern due to its detrimental effects on plant growth and physiological processes. Pb enters plants primarily through root uptake, leading to various toxic responses that hinder growth and yield. The following sections outline the key aspects of Pb toxicity in plants. Pb is absorbed non-selectively by plant roots, facilitated by H+/ATPases, and translocated through apoplastic and symplastic pathways. Factors influencing Pb uptake include soil pH, cation exchange capacity, and root morphology [62]. Pb exposure disrupts seed germination, root and shoot development, and overall biomass yield. It induces oxidative stress by generating ROS, leading to cellular damage and impaired photosynthesis. Pb alters nutrient uptake, water balance, and hormonal regulation, affecting plant health and productivity. Plants employ various strategies to mitigate Pb toxicity, including compartmentalization in vacuoles, chelation with phytochelatins, and enhanced anti-oxidative responses. While the mechanisms of Pb toxicity are well-documented, ongoing research is essential to fully understand the molecular pathways involved and to develop transgenic plants for improved tolerance and remediation strategies [63].
3.7 Toxicity of Copper (Cu) in Plants
Cu toxicity in plants is a significant concern due to its essential role as a micronutrient and the detrimental effects of excessive concentrations. While copper is vital for various physiological processes, including photosynthesis and respiration, elevated levels can lead to physiological and biochemical disorders, ultimately reducing plant growth and health. Cu is a vital micronutrient for the regular growth and maturation of plants [64]. Nevertheless, an excess of copper manifests as elevated toxicity, inducing oxidative stress, augmenting the ROS content within plant cells, and compromising the integrity and functionality of cell membranes [22,23,65,66]. In agriculture, an excess of Cu and Cd results in various adverse consequences for crops. This involves a decrease in seed germination rates, modifications in the development and morphology of crops, and obstacles to the uptake of essential minerals [67]. These unfavorable consequences result in diminished crop yields and inferior quality. The uptake of metal elements by plants depends on the plant type and the specific heavy metal involved. Copper contamination in soil arises from mining activities, excessive use of copper-based fungicides, pesticides, and fertilizers, as well as industrial discharges. Additional sources include sewage sludge, wastewater, and natural processes such as weathering of copper-rich rocks and volcanic activity [68].
4 Nanotechnology Significance in Stress Management
Nanotechnology plays a transformative role in stress management within agriculture, particularly in addressing abiotic stresses such as drought, salinity, and temperature extremes. By leveraging the unique properties of nanoparticles, researchers are developing innovative solutions that enhance plant resilience and productivity. This overview will explore the applications, mechanisms, and potential challenges associated with nanotechnology in stress management. Numerous investigations have been conducted to comprehend the fundamental operational mechanism of nanoparticles for mitigating stress caused by heavy metals. The impacts of heavy metal stress have been observed to affect the physiological, morphological, and biochemical aspects of plant life. Some findings indicate that nanoparticles governing plant growth might pose harm to them when present in elevated [69]. To combat stress induced by heavy metals in plants, diverse strategies have been employed, including the reduction of the bioavailable concentration of heavy metal in the soil [70], enhancing the plant defense system, optimizing physiological functions, formulating protective agents, and regulating the expression of genes associated with the transport of heavy metals are all strategies employed to ameliorate the impact of heavy metal stress [71]. Moreover, the harm induced by heavy metal stress in plants can be partially alleviated by chelating organic acids that accumulate in their cell walls [72]. Nanoparticles have demonstrated the ability to enhance the synthesis of defense compounds Antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), catalase, ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR) and glutathione peroxidase (GPX), play a crucial role in regulating ROS levels within cells. They function to eliminate these reactive species, particularly when present at elevated concentrations. Additionally, the application of nanoparticles (NPs) interacts with plant cell walls and facilitates their breakdown by activating endogenous polyphenols and antioxidant vitamins including vitamin C and vitamin E. Moreover, these molecules enhance the enzyme activities and loosen the cell wall structure. They scavenge ROS generated during nanoparticle penetration, reducing oxidative stress. This dual action allows NPs to penetrate deeper into plant tissues while maintaining cellular homeostasis [73].
4.1 Mechanism of Nanoparticles to Alleviate Heavy Metal Stress
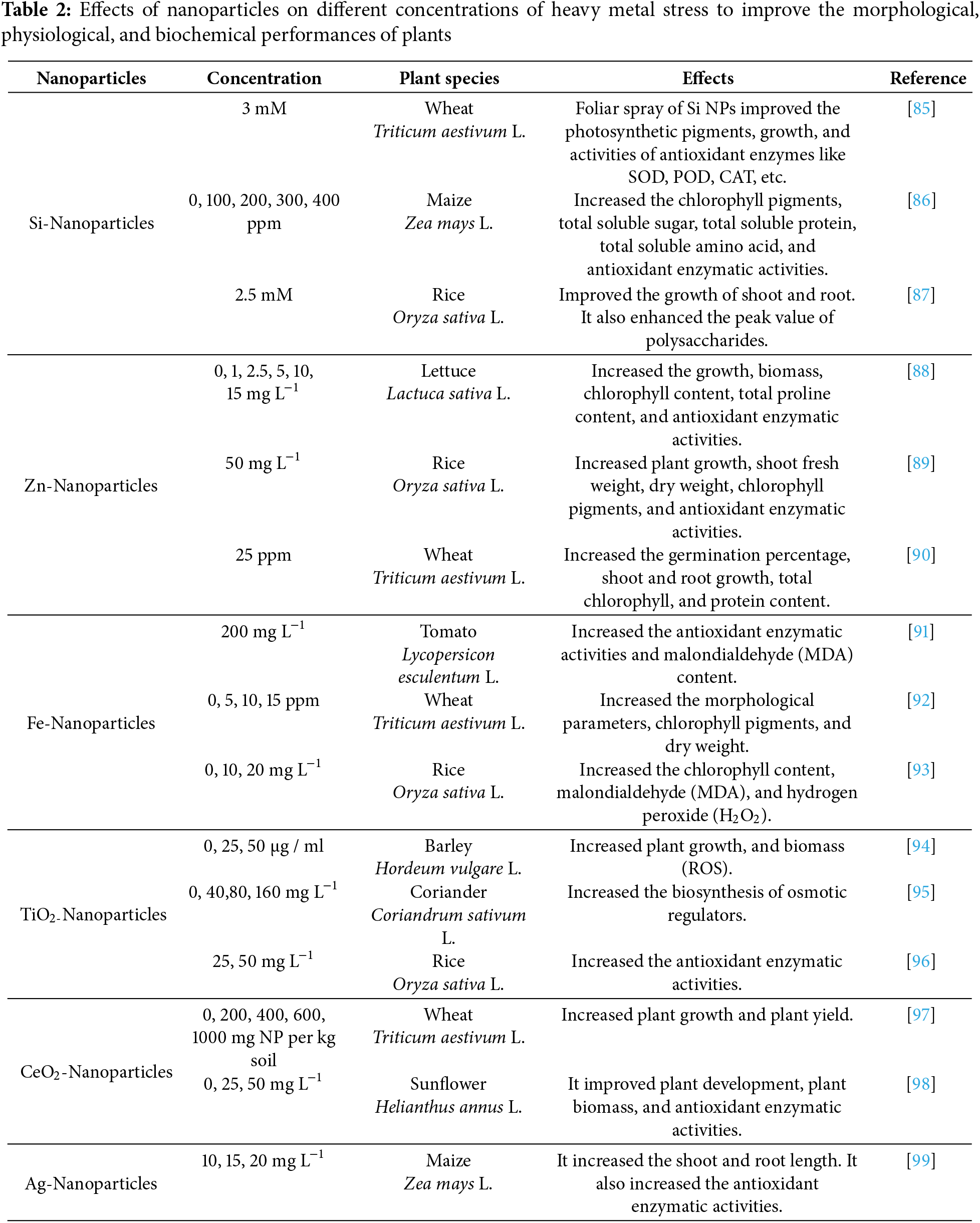
The mechanism of nanoparticles in alleviating heavy metal stress in plants involves several biochemical and physiological pathways that enhance plant resilience. Nanoparticles, particularly silicon nanoparticles, have shown significant potential in mitigating the adverse effects of heavy metals by improving plant growth and antioxidant responses. Nanoparticles can form complexes with heavy metals, reducing their bioavailability and uptake by plants, thereby minimizing toxicity. Enhancement of Antioxidant Activity (Fig. 2): Nanoparticles boost the activity of both enzymatic and non-enzymatic antioxidants, which helps in scavenging reactive oxygen species generated under heavy metal stress [74]. Silicon nanoparticles enhance the uptake of essential nutrients like calcium and potassium, which are crucial for maintaining cellular integrity and function under stress conditions. The application of nanoparticles has been linked to improved growth metrics, such as increased chlorophyll content and biomass, which are vital for plant health [75]. To mitigate the adverse effects of heavy metal stress, plants have developed diverse homeostatic mechanisms to control the accumulation and uptake of metalloids. Nevertheless, the effectiveness of heavy metal detoxification varies and is subject to improvement. Key strategies aimed at enhancing heavy metal resistance in plants encompass reducing the number of bioavailable metal contaminants, regulating the expression of genes involved in metal/metalloid transport, restoring the capability of the apoplastic barrier to intercept metal contaminants, providing additional nutrients to stressed plants, reinforcing both enzymatic and non-enzymatic antioxidant systems and increasing the biosynthesis of protective agents such as organic acids, compatible solutes, phytochelatins, and root secretions [76].
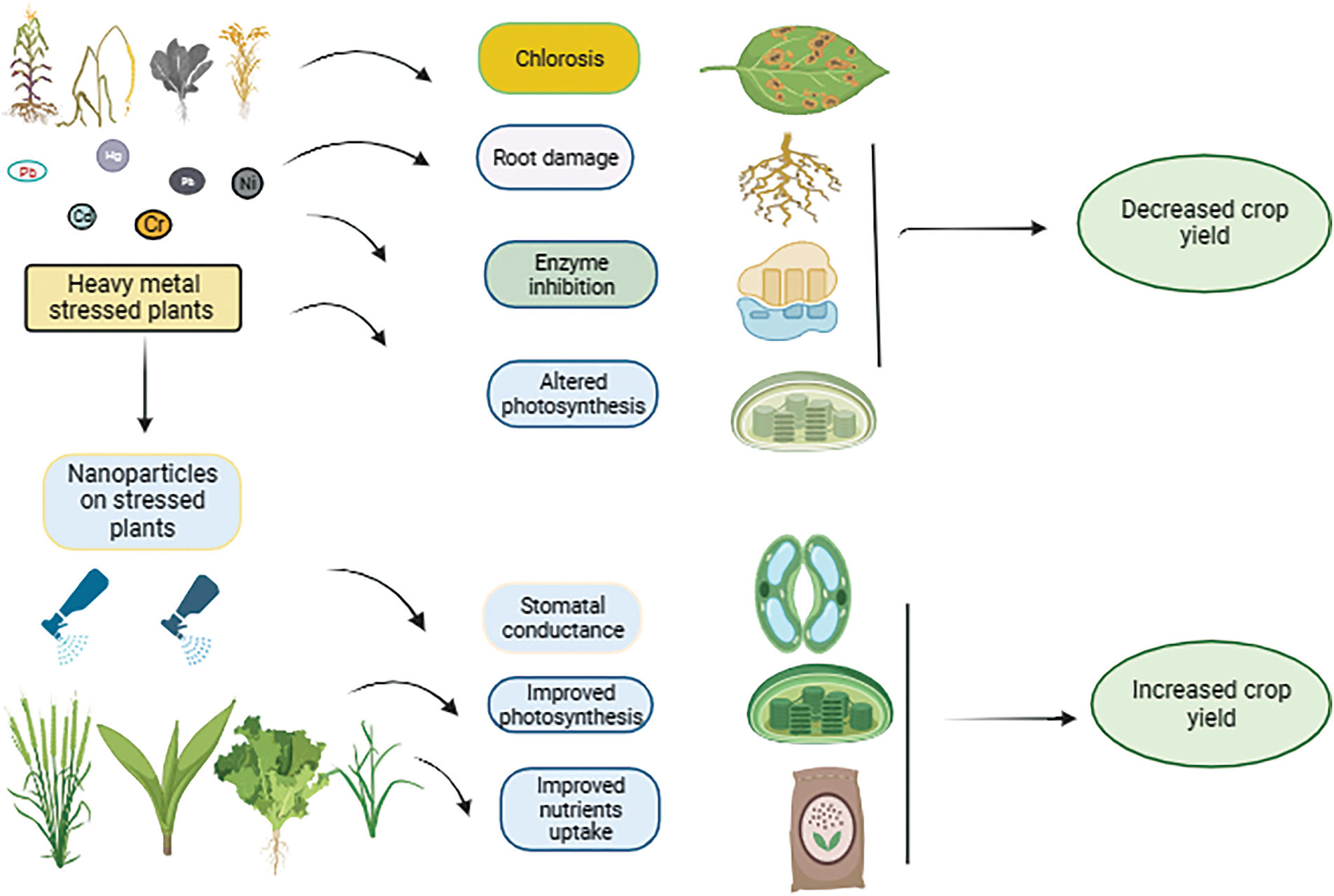
Figure 2: Schematic illustration of the mode of action of nanoparticles to mitigate the effect of heavy metal stress
Nanoparticles have emerged as a promising solution to alleviate heavy metal stress in plants, addressing a significant environmental challenge. Their unique properties enable them to interact with heavy metals in various ways, enhancing plant resilience and promoting growth even in contaminated soils. This response synthesizes findings from recent studies, highlighting the mechanisms through which nanoparticles mitigate heavy metal toxicity and improve plant health. Nanoparticles, particularly silicon nanoparticles, can form complexes with heavy metals, reducing their bioavailability and uptake by plants. This interaction helps in detoxifying heavy metals in the soil, thereby minimizing their harmful effects on plant metabolism [76]. Nanoparticles enhance the activity of both enzymatic and non-enzymatic antioxidants in plants. This boost in antioxidant capacity helps mitigate oxidative stress caused by heavy metals, leading to improved growth and yield [77]. Nanoparticles can alter the physico-chemical properties of the soil, such as pH and microbial activity, which can further reduce heavy metal mobility. This modification enhances the apoplastic barrier in plants, restricting heavy metal movement into plant tissues. The use of nanoparticles in conjunction with plant growth-promoting microorganisms has shown synergistic effects in enhancing phytoremediation. For instance, biogenic iron oxide nanoparticles combined with specific bacterial strains have been found to improve chlorophyll content and growth in plants under heavy metal stress [78]. Silicon nanoparticles are particularly effective in alleviating heavy metal stress due to their natural occurrence and ability to enhance plant resilience. They play a crucial role in detoxifying heavy metals and improving physiological functions in plants. Biogenic iron oxide nanoparticles synthesized from plants like Eichhornia crassipes have demonstrated significant potential in enhancing plant growth and reducing heavy metal toxicity. Their application has been linked to improved antioxidant enzyme activity and reduced electrolyte leakage in stressed plants [79].
The use of environmentally friendly methods to synthesize nanoparticles, such as using plant extracts, has gained attention. These nanoparticles not only mitigate heavy metal stress but also promote sustainable agricultural practices. The use of nanoparticles in agriculture expands, and establishing regulatory frameworks to ensure their safe application is essential. This will help mitigate potential environmental risks associated with nanoparticle use [80].
4.2 Nanoparticles as a Nanophytoremidation
Nano-phytoremediation is an innovative approach that combines the principles of phytoremediation with nanotechnology to address the pressing issue of heavy metal contamination in the environment. This method leverages the unique properties of nanoparticles, which enhance the ability of plants to absorb and detoxify heavy metals from contaminated soils. By utilizing engineered nanomaterials, nano-phytoremediation not only improves the efficiency of pollutant removal but also offers a sustainable and eco-friendly solution to a significant environmental challenge [21,81,82]. Nanophytoremediation operates through various mechanisms that facilitate the removal of heavy metals from the soil. Plants absorb nanoparticles that bind to heavy metals, allowing for their concentration within plant tissues (Table 2). Nanoparticles can catalyze the breakdown of complex organic pollutants into simpler, less harmful compounds. This mechanism involves immobilizing heavy metals in the soil, preventing their leaching into groundwater, and reducing bioavailability to plants and microorganisms [83]. Nanoparticles possess a high surface area and active sites, which increase their reactivity and ability to bind heavy metals effectively. Nanomaterials can penetrate contaminated zones that are otherwise inaccessible, allowing for more thorough remediation efforts [84].
Future viewpoints in the field of nanoparticle-based strategies for alleviating heavy metal toxicity in agriculture involve several key areas of focus. Firstly, further research is needed to optimize nanoparticle formulations, tailoring them to specific soil types, plant species, and heavy metal contaminants. This will enhance the efficacy of these strategies in diverse agricultural settings. Additionally, long-term studies are essential to understand the ecological impacts of nanoparticle applications on soil microbiota, plant-microbe interactions, and overall ecosystem health. Assessing the persistence and bioaccumulation of nanoparticles in the environment is crucial for ensuring sustainable and safe agricultural practices. Future research should also explore the integration of nanotechnology with other innovative approaches, such as precision agriculture and genetic engineering, to create synergistic solutions for heavy metal remediation. Collaborative efforts between multidisciplinary teams, including scientists, engineers, agronomists, and environmentalists, will be essential for addressing the complexity of agricultural systems and developing holistic strategies. Moreover, cost-effective production methods for nanoparticle synthesis need attention to facilitate widespread adoption by farmers. Finally, considering the global nature of heavy metal pollution, international cooperation and knowledge-sharing platforms should be established to accelerate the translation of research findings into practical and accessible solutions for farmers worldwide.
Optimization of nanoparticle formulations for specific environmental contexts and the adapting of applications to diverse soil types and plant species are pivotal for maximizing their efficacy. Long-term ecological studies are imperative to assess the sustained impact of nanoparticle interventions on soil microbiota, plant-microbe interactions, and overall ecosystem health, ensuring the development of sustainable agricultural practices. The integration of nanotechnology with precision agriculture and genetic engineering presents a forward-looking approach, offering synergistic solutions for efficient heavy metal remediation. Collaborative efforts among multidisciplinary teams are essential to navigate the complexity of agricultural systems and devise holistic strategies. Attention to cost-effective nanoparticle synthesis methods is critical for practical adoption by farmers, facilitating the widespread implementation of these strategies. Hence, nanaoparticles are effective for the tolerance against metal toxicity in plants focusing on higher yield.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Maryam: supervision, Mohamed M. El-Mogy: conceptualization, Muhammad Faheem Jan: editing, Iram Naz: writing, Ishtiaq Ahmad: manuscript drafting, Riaz Ahmad: review and Muhammad Tanveer Altaf: formatting and review. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Ahmad R, Muhammad HMD, Naz S, Tiwari RK, Lal MK, Ahmad P, et al. From stress to success: silicon and nano-silicon strategies for enhancing fruit yield and quality in cadmium-exposed trees. S Afr J Bot. 2024;167(2):441–7. doi:10.1016/j.sajb.2024.02.042. [Google Scholar] [CrossRef]
2. Awwad EA, Mohamed IR, El-Hameedb A, Adel M, Zaghloul EA. The co-addition of soil organic amendments and natural bio-stimulants improves the production and defenses of the wheat plant grown under the dual stress of salinity and alkalinity. Egypt J Soil Sci. 2022;62(2):137–53. doi:10.21608/ejss.2022.148406.1513. [Google Scholar] [CrossRef]
3. ElGhamry A, Ghazi DA, Helmy A. Titanium: an element of non-biological atmospheric nitrogen fixation and a regulator of sugar beet plant tolerance to salinity. Egypt J Soil Sci. 2022;62:373–81. doi:10.21608/ejss.2022.165553.1543. [Google Scholar] [CrossRef]
4. Saleem MH, Fahad S, Adnan M, Ali M, Rana MS, Kamran M, et al. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ Sci Pollut Res. 2020;27(29):37121–33. doi:10.1007/s11356-020-09764-3. [Google Scholar] [PubMed] [CrossRef]
5. Ningombam L, Hazarika BN, Yumkhaibam T, Heisnam P, Singh YD. Heavy metal priming plant stress tolerance deciphering through physiological, biochemical, molecular and omics mechanism. S Afr J Bot. 2024;168(5):16–25. doi:10.1016/j.sajb.2024.02.032. [Google Scholar] [CrossRef]
6. Ahmed T, Noman M, Manzoor N, Shahid M, Abdullah M, Ali L, et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol Environ Saf. 2021;209(1):111829. doi:10.1016/j.ecoenv.2020.111829. [Google Scholar] [PubMed] [CrossRef]
7. Jeelani PG, Mulay P, Venkat R, Ramalingam C. Multifaceted application of silica nanoparticles. A review. Silicon. 2020;12(6):1337–54. doi:10.1007/s12633-019-00229-y. [Google Scholar] [CrossRef]
8. Pérez-Labrada F, Hernández-Hernández H, López-Pérez MC, González-Morales S, Benavides-Mendoza A, Juárez-Maldonado A. Nanoparticles in plants: morphophysiological, biochemical, and molecular responses. In: Tripathi DK, Singh VP, Chauhan DK, editors. Plant life under changing environment. Cambridge, MA, USA: Academic Press; 2020. p. 289–322. doi:10.1016/B978-0-12-818204-8.00016-3. [Google Scholar] [CrossRef]
9. El-Ramady H, El-Henawy A, Amer M, Omara AED, Elsakhawy T, Elbasiouny H, et al. Agricultural waste and its nano-management: mini review. Egypt J Soil Sci. 2020;60(4):349–64. doi:10.21608/ejss.2020.46807.1397. [Google Scholar] [CrossRef]
10. Salajegheh M, Yavarzadeh M, Payandeh A, Akbarian MM. Effects of titanium and silicon nanoparticles on antioxidant enzymes activity and some biochemical properties of Cuminum cyminum L. under drought stress. Banat J Biotechnol. 2020;11(21):19–25. doi:10.7904/2068-4738-xi(21)-19. [Google Scholar] [CrossRef]
11. Nejatzadeh F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis l. during in vitro and in vivo germination tests. Heliyon. 2021;7(2):e05981. doi:10.1016/j.heliyon.2021.e05981. [Google Scholar] [PubMed] [CrossRef]
12. Kumari A, Chokheli VA, Lysenko VS, Mandzhieva SS, Minkina TM, Mazarji M, et al. Genotoxic and morpho-physiological responses of ZnO macro-and nano-forms in plants. Environ Geochem Health. 2023;45(12):9345–57. doi:10.1007/s10653-022-01428-0. [Google Scholar] [PubMed] [CrossRef]
13. Ragab GA, Saad-Allah KM. Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol Environ Saf. 2020;191:110242. doi:10.1016/j.ecoenv.2020.110242. [Google Scholar] [PubMed] [CrossRef]
14. Faizan M, Bhat JA, El-Serehy HA, Moustakas M, Ahmad P. Magnesium oxide nanoparticles (MgO-NPs) alleviate arsenic toxicity in soybean by modulating photosynthetic function, nutrient uptake and antioxidant potential. Metals. 2022;12(12):2030. doi:10.3390/met12122030. [Google Scholar] [CrossRef]
15. Wang X, Xie H, Wang P, Yin H. Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Materials. 2023;16(8):3097. doi:10.3390/ma16083097. [Google Scholar] [PubMed] [CrossRef]
16. Ghorbani A, Emamverdian A, Pehlivan N, Zargar M, Razavi SM, Chen M. Nano-enabled agrochemicals: mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J Nanobiotechnol. 2024;22(1):91. doi:10.1186/s12951-024-02371-1. [Google Scholar] [PubMed] [CrossRef]
17. Ansari MKA, Iqbal M, Ahmad M, Munir M, Gaffar SA, Chaachouay N. Heavy metal stress and cellular antioxidant systems of plants: a review. Agric Rev. 2024;45(3):400–9. doi:10.18805/ag.RF-321. [Google Scholar] [CrossRef]
18. Hussain B, Lin Q, Hamid Y, Sanaullah M, Di L, Khan MB, et al. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci Total Environ. 2020;712(2):136497. doi:10.1016/j.scitotenv.2020.136497. [Google Scholar] [PubMed] [CrossRef]
19. Sarma HH, Rajkumar A, Baro A, Das BC, Talukdar N. Impact of heavy metal contamination on soil and crop ecosystem with advanced techniques to mitigate them. J Adv Biol Biotechnol. 2024;27(6):53–63. doi:10.9734/jabb/2024/v27i6865. [Google Scholar] [CrossRef]
20. Adil MF, Sehar S, Chen G, Chen ZH, Jilani G, Chaudhry AN, et al. Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological/ultrastructural adjustments. Ecotoxicol Environ Saf. 2020;190(1):110076. doi:10.1016/j.ecoenv.2019.110076. [Google Scholar] [PubMed] [CrossRef]
21. Abou Fayssal S, Kumar P, Popescu SM, Sardar H, Ahmad R, Gupta D, et al. Health risk assessment of heavy metals in saffron (Crocus sativus L.) cultivated in domestic wastewater and lake water irrigated soils. Heliyon. 2024;10(5):e27138. doi:10.1016/j.heliyon.2024.e27138. [Google Scholar] [PubMed] [CrossRef]
22. Chen D, Chen D, Xue R, Long J, Lin X, Lin Y, et al. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J Hazard Mater. 2019;5:447–55. doi:10.1016/j.jhazmat.2018.12.111. [Google Scholar] [PubMed] [CrossRef]
23. Helal NM, Khattab HI, Emam MM, Niedbała G, Wojciechowski T, Hammami I, et al. Improving yield components and desirable eating quality of two wheat genotypes using Si and NanoSi particles under heat stress. Plants. 2022;11(14):1819. doi:10.3390/plants11141819. [Google Scholar] [PubMed] [CrossRef]
24. Vatansever R, Ozyigit II, Filiz E. Essential and beneficial trace elements in plants, and their transport in roots: a review. Appl Biochem Biotechnol. 2017;181(1):464–82. doi:10.1007/s12010-016-2224-3. [Google Scholar] [PubMed] [CrossRef]
25. Saroy K, Bisht A, Garg N. Physiological and biochemical responses of crop plants to nickel toxicity. Lithium Nickel Contam Plants Environ. 2024;63–94. doi:10.1142/9789811283123_0004. [Google Scholar] [CrossRef]
26. Yu H, Li W, Liu X, Song Q, Li J, Xu J. Physiological and molecular bases of the nickel toxicity responses in tomato. Stress Biol. 2024;4(1):25. doi:10.1007/s44154-024-00162-0. [Google Scholar] [PubMed] [CrossRef]
27. Naz S, Anjum MA, Sadiq B, Ahmad R, Altaf MA, El-Sheikh MA, et al. Purification of sewage wastewater through sand column filter for lessening of heavy metals accumulation in lettuce, carrot, and cauliflower. Water. 2022;14(22):3770. doi:10.3390/w14223770. [Google Scholar] [CrossRef]
28. Maqsood M, Zahra N, Kausar A, Shahzad S, Batool A, Naseer R. Mercury contamination and it’s dynamics in soil-plant systems. In: Kumar N, editor. Mercury toxicity mitigation: sustainable nexus approach. Cham, Switzerland: Springer; 2024. p. 45–63. doi:10.1007/978-3-031-48817-7_2. [Google Scholar] [CrossRef]
29. Lin R, Wang X, Luo Y, Du W, Guo H, Yin D. Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere. 2007;69(1):89–98. doi:10.1016/j.chemosphere.2007.04.041. [Google Scholar] [PubMed] [CrossRef]
30. Saleem MH, Parveen A, Khan SU, Hussain I, Wang X, Alshaya H, et al. Silicon fertigation regimes attenuates cadmium toxicity and phytoremediation potential in two maize (Zea mays L.) cultivars by minimizing its uptake and oxidative stress. Sustainability. 2022;14(3):1462. doi:10.3390/su14031462. [Google Scholar] [CrossRef]
31. Riaz M, Kamran M, Fang Y, Yang G, Rizwan M, Ali S, et al. Boron supply alleviates cadmium toxicity in rice (Oryza sativa L.) by enhancing cadmium adsorption on cell wall and triggering antioxidant defense system in roots. Chemosphere. 2021;266(7):128938. doi:10.1016/j.chemosphere.2020.128938. [Google Scholar] [PubMed] [CrossRef]
32. Jawad Hassan M, Ali Raza M, Ur Rehman S, Ansar M, Gitari H, Khan I, et al. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants. 2020;9(11):1575. doi:10.3390/plants9111575. [Google Scholar] [PubMed] [CrossRef]
33. López-Millán AF, Sagardoy R, Solanas M, Abadía A, Abadía J. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot. 2009;65(2–3):376–85. doi:10.1016/j.envexpbot.2008.11.010. [Google Scholar] [CrossRef]
34. Tanveer K, Ilyas N, Akhtar N, Yasmin H, Hefft DI, El-Sheikh MA, et al. Role of biochar and compost in cadmium immobilization and on the growth of Spinacia oleracea. PLoS One. 2022;17(5):e0263289. doi:10.1371/journal.pone.0263289. [Google Scholar] [PubMed] [CrossRef]
35. Waris Z, Noreen Z, Shah AA, Usman S, Shah AN, Rizwan M, et al. Efficacy of γ-aminobutyric acid (GABA) on physio-biochemical attributes of lettuce (Lactuca sativa L.) under cadmium toxicity. J Plant Growth Regul. 2023;42(8):5041–57. doi:10.1007/s00344-023-11007-x. [Google Scholar] [CrossRef]
36. Ziaf K, Talha HM, Ghani MA, Ahmad I, Anwar R, Ali B, et al. Differential accumulation pattern of cadmium in plant parts of pea varieties in response to varying cadmium levels. S Afr J Bot. 2023;161(3):599–606. doi:10.1016/j.sajb.2023.08.044. [Google Scholar] [CrossRef]
37. Abbasi Q, Pourakbar L, Moghaddam SS. Potential role of apple wood biochar in mitigating mercury toxicity in corn (Zea mays L.). Ecotoxicol Environ Saf. 2023;267(1):115619. doi:10.1016/j.ecoenv.2023.115619. [Google Scholar] [PubMed] [CrossRef]
38. Prajapati R, Kataria S, Gadre R, Landi M, Jain M. Unveiling the mechanisms underpinning alleviation of mercury toxicity by static magnetic field treatment in soybean. J Plant Growth Regul. 2024;43(1):135–51. doi:10.1007/s00344-023-11063-3. [Google Scholar] [CrossRef]
39. Khalid MF, Abou Elezz A, Jawaid MZ, Ahmed T. Salicylic acid restricts mercury translocation by activating strong antioxidant defense mechanisms in sweet pepper (Capsicum annum L.). Environ Technol Innov. 2023;32(6):103283. doi:10.1016/j.eti.2023.103283. [Google Scholar] [CrossRef]
40. Saleem MH, Mfarrej MF, Alatawi B, Mumtaz A, Imran S, Ashraf M, et al. Silicon enhances morpho-physio-biochemical responses in arsenic stressed spinach (Spinacia oleracea L.) by minimizing its uptake. J Plant Growth Regul. 2023;42(3):2053–72. doi:10.1007/s00344-022-10681-7. [Google Scholar] [CrossRef]
41. Singh M, Chakraborty D, Mandal J, Chaudhary DK, Jha AK. Inoculation with Glomus mosseae: an efficient biological management strategy for arsenic mitigation in wheat (Triticum aestivum L.) under arsenic-contaminated soil. Commun Soil Sci Plant Anal. 2023;54(19):2645–56. doi:10.1080/00103624.2023.2240367. [Google Scholar] [CrossRef]
42. Bakhat HF, Zia Z, Fahad S, Abbas S, Hammad HM, Shahzad AN, et al. Arsenic uptake, accumulation and toxicity in rice plants: possible remedies for its detoxification: a review. Environ Sci Pollut Res. 2017;24(10):9142–58. doi:10.1007/s11356-017-8462-2. [Google Scholar] [PubMed] [CrossRef]
43. Martinez-Castillo JI, Ozuna C, Arcibar-Orozco JA, Saldana-Robles A. Arsenic uptake in lettuce: its impact on crop quality and metabolic stress. Appl Ecol Environ Res. 2023;21(6):5285–98. doi:10.15666/aeer/2106_52855298. [Google Scholar] [CrossRef]
44. Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F. Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. Comptes Rendus Biol. 2011;334(2):118–26. doi:10.1016/j.crvi.2010.12.006. [Google Scholar] [PubMed] [CrossRef]
45. Nagar MC, Dotaniya ML, Sharma A, Dotaniya CK, Doutaniya RK, Saha JK. Pressmud overcome lead toxicity by improving spinach biomass in lead-contaminated soils. Environ Monit Assess. 2023;195(1):107. doi:10.1007/s10661-022-10718-0. [Google Scholar] [PubMed] [CrossRef]
46. Osman HE, Fadhlallah RS. Impact of lead on seed germination, seedling growth, chemical composition, and forage quality of different varieties of Sorghum. J Umm Al-Qura Univ Appl Sci. 2023;9(1):77–86. doi:10.1007/s43994-022-00022-5. [Google Scholar] [CrossRef]
47. Dotaniya ML, Pipalde JS, Jain RC, Rajendiran S, Coumar MV, Saha JK, et al. Can lead and nickel interaction affect plant nutrient uptake pattern in Spinach (Spinacia oleracea). Agric Res. 2020;9(3):358–64. doi:10.1007/s40003-019-00428-4. [Google Scholar] [CrossRef]
48. Subhani MA, Amjad M, Iqbal MM, Murtaza B, Imran M, Naeem MA, et al. Nickel toxicity pretreatment attenuates salt stress by activating antioxidative system and ion homeostasis in tomato (Solanum lycopersicon L.an interplay from mild to severe stress. Environ Geochem Health. 2023;45(1):227–46. doi:10.1007/s10653-022-01336-3. [Google Scholar] [PubMed] [CrossRef]
49. Ashraf MA, Hafeez A, Rasheed R, Hussain I, Farooq U, Rizwan M, et al. Effect of exogenous taurine on growth, oxidative defense, and nickel (Ni) uptake in canola (Brassica napus L.) under Ni stress. Physiol Mol Biol Plants. 2023;29(8):1135–52. doi:10.1007/s12298-023-01359-9. [Google Scholar] [PubMed] [CrossRef]
50. Alhammad BA, Seleiman MF, Harrison MT. Hydrogen peroxide mitigates Cu stress in wheat. Agriculture. 2023;13(4):862. doi:10.3390/agriculture13040862. [Google Scholar] [CrossRef]
51. Alves A, Ribeiro R, Azenha M. Effects of exogenously applied copper in tomato plants’ oxidative and nitrogen metabolisms under organic farming conditions. Horticulturae. 2023;9(3):323. doi:10.3390/horticulturae9030323. [Google Scholar] [CrossRef]
52. Kim D, An S, Kim L, Byeon YM, Lee J, Choi MJ, et al. Translocation and chronic effects of microplastics on pea plants (Pisum sativum) in copper-contaminated soil. J Hazard Mater. 2022;436(1):129194. doi:10.1016/j.jhazmat.2022.129194. [Google Scholar] [PubMed] [CrossRef]
53. Raptis S, Gasparatos D, Economou-Eliopoulos M, Petridis A. Chromium uptake by lettuce as affected by the application of organic matter and Cr(VI)-irrigation water: implications to the land use and water management. Chemosphere. 2018;210(Pt 1):597–606. doi:10.1016/j.chemosphere.2018.07.046. [Google Scholar] [PubMed] [CrossRef]
54. Adrees M, Ali S, Iqbal M, Bharwana SA, Siddiqi Z, Farid M, et al. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol Environ Saf. 2015;122:1–8. doi:10.1016/j.ecoenv.2015.07.003. [Google Scholar] [PubMed] [CrossRef]
55. Ahmad R, Ali S, Abid M, Rizwan M, Ali B, Tanveer A, et al. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ Sci Pollut Res. 2020;27(1):1101–11. doi:10.1007/s11356-019-06761-z. [Google Scholar] [PubMed] [CrossRef]
56. Srivast S. Phytotoxicity of chromium (III and VI) on tomato (Lycopersicum esculentum L.) grown in sand culture. Preprint. 2023. doi:10.21203/rs.3.rs-2696066/v1. [Google Scholar] [CrossRef]
57. Zaidi S, Hayat S, Pichtel J. Arsenic-induced plant stress: mitigation strategies and omics approaches to alleviate toxicity. Plant Physiol Biochem. 2024;213(19):108811. doi:10.1016/j.plaphy.2024.108811. [Google Scholar] [PubMed] [CrossRef]
58. Gong Y, Qu Y, Yang S, Tao S, Shi T, Liu Q, et al. Status of arsenic accumulation in agricultural soils across China (1985–2016). Environ Res. 2020;186(3):109525. doi:10.1016/j.envres.2020.109525. [Google Scholar] [PubMed] [CrossRef]
59. Ma X, Sharifan H, Dou F, Sun W. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles. Chem Eng J. 2020;384:123802. doi:10.1016/j.cej.2019.123802. [Google Scholar] [CrossRef]
60. Ghorbani A, Pishkar L, Roodbari N, Tavakoli SA, Jahromi EM, Wu C. Nitrate reductase is needed for methyl jasmonate-mediated arsenic toxicity tolerance of rice by modulating the antioxidant defense system, glyoxalase system and arsenic sequestration mechanism. J Plant Growth Regul. 2023;42(2):1107–19. doi:10.1007/s00344-022-10616-2. [Google Scholar] [CrossRef]
61. Kumar V, Vogelsang L, Schmidt RR, Sharma SS, Seidel T, Dietz KJ. Remodeling of root growth under combined arsenic and hypoxia stress is linked to nutrient deprivation. Front Plant Sci. 2020;23(11):569687. doi:10.3389/fpls.2020.569687. [Google Scholar] [PubMed] [CrossRef]
62. Rahman SU, Qin A, Zain M, Mushtaq Z, Mehmood F, Riaz L, et al. Pb uptake, accumulation, and translocation in plants: plant physiological, biochemical, and molecular response: a review. Heliyon. 2023;10:e27724. doi:10.2139/ssrn.4620053. [Google Scholar] [CrossRef]
63. Hassan SH, Chafik Y, Lebrun M, Sferra G, Bourgerie S, Scippa GS, et al. Lead toxicity and tolerance in plants. In: Hossain MA, Hossain AKMZ, Bourgerie S, Dhankher OP, editors. Heavy metal toxicity and tolerance in plants: a biological, omics, and genetic engineering approach. Hoboken, NJ, USA: Wiley; 2023. p. 373–405. doi:10.1002/9781119906506.ch18. [Google Scholar] [CrossRef]
64. Nazir F, Hussain A, Fariduddin Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere. 2019;230:544–58. doi:10.1016/j.chemosphere.2019.05.001. [Google Scholar] [PubMed] [CrossRef]
65. Chrysargyris A, Papakyriakou E, Petropoulos SA, Tzortzakis N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J Hazard Mater. 2019;15(7):584–93. doi:10.1016/j.jhazmat.2019.01.058. [Google Scholar] [PubMed] [CrossRef]
66. Rather BA, Masood A, Sehar Z, Majid A, Anjum NA, Khan NA. Mechanisms and role of nitric oxide in phytotoxicity-mitigation of copper. Front Plant Sci. 2020;29:675. doi:10.3389/fpls.2020.00675. [Google Scholar] [PubMed] [CrossRef]
67. Yue Z, Chen Y, Chen C, Ma K, Tian E, Wang Y, et al. Endophytic Bacillus altitudinis WR10 alleviates Cu toxicity in wheat by augmenting reactive oxygen species scavenging and phenylpropanoid biosynthesis. J Hazard Mater. 2021;5(1–3):124272. doi:10.1016/j.jhazmat.2020.124272. [Google Scholar] [PubMed] [CrossRef]
68. Neaman A, Schoffer J-T, Navarro-Villarroel C, Pelosi C, Peñaloza P, Dovletyarova E, et al. Copper contamination in agricultural soils: a review of the effects of climate, soil properties, and prolonged copper pesticide application in vineyards and orchards. Plant Soil Env. 2024;70(7):407–17. doi:10.17221/501/2023-pse. [Google Scholar] [CrossRef]
69. Vishwakarma K, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S. Silicon and plant growth promoting rhizobacteria differentially regulate AgNP-induced toxicity in Brassica juncea: implication of nitric oxide. J Hazard Mater. 2020;390(36):121806. doi:10.1016/j.jhazmat.2019.121806. [Google Scholar] [PubMed] [CrossRef]
70. Moharem M, Elkhatib E, Mesalem M. Remediation of chromium and mercury polluted calcareous soils using nanoparticles: sorption-desorption kinetics, speciation and fractionation. Environ Res. 2019;170(36):366–73. doi:10.1016/j.jhazmat.2019.121806. [Google Scholar] [CrossRef]
71. Cao F, Dai H, Hao PF, Wu F. Silicon regulates the expression of vacuolar H+-pyrophosphatase 1 and decreases cadmium accumulation in rice (Oryza sativa L.). Chemosphere. 2020;240:124907. doi:10.1016/j.chemosphere.2019.124907. [Google Scholar] [PubMed] [CrossRef]
72. Zhao L, Lu L, Wang A, Zhang H, Huang M, Wu H, et al. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agric Food Chem. 2020;68(7):1935–47. doi:10.1021/acs.jafc.9b06615. [Google Scholar] [PubMed] [CrossRef]
73. Ullah S, Adeel M, Zain M, Rizwan M, Irshad MK, Jilani G, et al. Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: a full life cycle study. J Environ Manag. 2020;263:110365. doi:10.1016/j.jenvman.2020.110365. [Google Scholar] [PubMed] [CrossRef]
74. Muhammad HMD, Anjum MA, Ahmad R, Ercisli S. Foliar application of moringa and mint leaf extracts enhances carnation growth and flower yield under saline conditions by improving plant defense mechanism. J Plant Growth Regul. 2024;43(9):3254–64. doi:10.1007/s00344-023-11129-2. [Google Scholar] [CrossRef]
75. Gorge WA, Abood MF, Ibade KW. Magnesium oxide nanoparticles synthesized from Solanum elaeagnifolium and their effect against mealybugs Phenacoccus solenopsis tinsley. J Glob Innov Agric Sci. 2025;13:391–5. doi:10.22194/JGIAS/25.1306. [Google Scholar] [CrossRef]
76. Li HB, Li J, Zhao D, Li C, Wang XJ, Sun HJ, et al. Arsenic relative bioavailability in rice using a mouse arsenic urinary excretion bioassay and its application to assess human health risk. Environ Sci Technol. 2017;51(8):4689–96. doi:10.1021/acs.est.7b00495. [Google Scholar] [PubMed] [CrossRef]
77. Singh A, Shrivastava R, Verma S, Agnihotri RK. A review on overcoming plant stress by silicon nanoparticles. J Sci Innov Nat Earth. 2024;5:21–5. doi:10.59436/jsiane264. [Google Scholar] [CrossRef]
78. Kumari N, Rani S, Sharma V. Green agriculture: nanoparticles as tools to mitigate heavy metal toxicity. Rev Environ Contam Toxicol. 2024;262(1):1. doi:10.1007/s44169-023-00053-x. [Google Scholar] [CrossRef]
79. Madhogaria B, Banerjee S, Chakraborty S, Dhak P, Kundu A. Synergistic impacts of plant growth promoting Pseudomonas geniculata TIU16A3 and green-synthesized iron-oxide nanoparticles from Eichhornia crassipes for the amelioration of heavy metal stress in Vigna radiata L. Preprint. 2024;419:126493. doi:10.21203/rs.3.rs-4863542/v1. [Google Scholar] [CrossRef]
80. Abdelgawad KF, Fathy MA, Helal MID, Khater HA, El-Mogy MM, Abdelkader NH. Synthesize of bio-based encapsulated nano urea modified hydroxyapatite for controlling release of nitrogen and enhancing green bean yield. Not Bot Horti Agrobot Cluj-Napoca. 2024;52(2):13751. doi:10.15835/nbha52213751. [Google Scholar] [CrossRef]
81. Nwadinigwe AO, Ugwu EC. Overview of nano-phytoremediation applications. In: Ansari AA, Gill SS, Lanza Gill R,editors. Phytoremediation. Cham, Switzerland: Springer; 2018. p. 377–82. doi:10.1007/978-3-319-99651-6_15. [Google Scholar] [CrossRef]
82. Skinder BM, Nabi M. Non-carbon-based nano-materials used for environmental pollution remediation. In: Tonelli FMP, Bhat RA, Dar GH, Hakeem KR, editors. Nano-phytoremediation and environmental pollution. Boca Raton, FL, USA: CRC; 2024. p. 73–83. doi:10.1201/9781003186298-4. [Google Scholar] [CrossRef]
83. Prakash P, Chandran SS. Nano-phytoremediation of heavy metals from soil: a critical review. Pollutants. 2023;3(3):360–80. doi:10.3390/pollutants3030025. [Google Scholar] [CrossRef]
84. Saleh AM, Al-Najjar MAH, Fadel Alpresem WF. Effect of polyamines and zeolites on the protein profile of leaves of the date palm cuttings Phoenix dactylifera L. grown under heavy metal stress conditions. J Glob Innov Agric Sci. 2023;11:391–6. doi:10.22194/jgias/23.1104. [Google Scholar] [CrossRef]
85. Al-Jaberi MM. Effect of bioremediation or organic treatments of soil contaminated with some heavy metals on the biological and L glutaminase activity. J Glob Innov Agric Sci. 2024;12(1):243–50. doi:10.22194/jgias/12.1143. [Google Scholar] [CrossRef]
86. Ishaq H, Waraich EA, Hussain S, Ahmad M, Ahmad Z, Saifullah. Silicon-mediated growth, physiological, biochemical and root alterations to confer drought and nickel stress tolerance in Maize (Zea mays L. ). Silicon. 2023;15(15):6579–89. doi:10.1007/s12633-023-02536-x. [Google Scholar] [CrossRef]
87. Riaz M, Kamran M, Fahad S, Wang X. Nano-silicon mediated alleviation of cd toxicity by cell wall adsorption and antioxidant defense system in rice seedlings. Plant Soil. 2023;486(1):103–17. doi:10.1007/s11104-022-05588-x. [Google Scholar] [CrossRef]
88. Gao F, Zhang X, Zhang J, Li J, Niu T, Tang C, et al. Zinc oxide nanoparticles improve lettuce (Lactuca sativa L.) plant tolerance to cadmium by stimulating antioxidant defense, enhancing lignin content and reducing the metal accumulation and translocation. Front Plant Sci. 2022;13:1015745. doi:10.3389/fpls.2022.1015745. [Google Scholar] [PubMed] [CrossRef]
89. Dogan Y, Alam P, Sultan H, Sharma R, Soysal S, Baran MF, et al. Zinc oxide nanoparticles for sustainable agriculture: a tool to combat salinity stress in rice (Oryza sativa) by modulating the nutritional profile and redox homeostasis mechanisms. J Agric Food Res. 2025;19(6):101598. doi:10.1016/j.jafr.2024.101598. [Google Scholar] [CrossRef]
90. Rehman A, Khan S, Sun F, Peng Z, Feng K, Wang N, et al. Exploring the nano-wonders: unveiling the role of nanoparticles in enhancing salinity and drought tolerance in plants. Front Plant Sci. 2024;14:1324176. doi:10.3389/fpls.2023.1324176. [Google Scholar] [PubMed] [CrossRef]
91. Kioko MT, Ndirangu S, Nyarindo W. Evaluating effect of climate smart agricultural practices adoption on productivity of drought-tolerant pulses: insights from dryland areas of makueni county, Kenya. J Glob Innov Agric Sci. 2024;12(3):803–13. doi:10.22194/jgias/24.1383. [Google Scholar] [CrossRef]
92. Iannone MF, Groppa MD, de Sousa ME, van Raap MBF, Benavides MP. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: evaluation of oxidative damage. Environ Exp Bot. 2016;131(1):77–88. doi:10.1016/j.envexpbot.2016.07.004. [Google Scholar] [CrossRef]
93. Alharby HF, Ali S. Combined role of Fe nanoparticles (Fe NPs) and Staphylococcus aureus L. in the alleviation of chromium stress in rice plants. Life. 2022;12(3):338. doi:10.3390/life12030338. [Google Scholar] [PubMed] [CrossRef]
94. Ma J, Li Y, Chen F, Sun Y, Zhu Y, Wang L. Bacillus mycoides PM35 in combination with titanium dioxide (TiO2)-nanoparticles enhanced morpho-physio-biochemical attributes in Barley (Hordeum vulgare L.) under cadmium stress. Chemosphere. 2023;323(4):138224. doi:10.1016/j.chemosphere.2023.138224. [Google Scholar] [PubMed] [CrossRef]
95. Sardar R, Ahmed S, Yasin NA. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system stress markers and reducing cadmium uptake. Environ Pollut. 2022;292(4):118373. doi:10.1016/j.envpol.2021.118373. [Google Scholar] [PubMed] [CrossRef]
96. Kiany T, Pishkar L, Sartipnia N, Iranbakhsh A, Barzin G. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ Sci Pollut Res. 2022;29(23):34725–37. doi:10.1007/s11356-021-17927-z. [Google Scholar] [PubMed] [CrossRef]
97. Ayub MA, Ahmad HR, Zia ur Rehman M, Waraich EA. Cerium oxide nanoparticles alleviates stress in wheat grown on Cd contaminated alkaline soil. Chemosphere. 2023;338(2):139561. doi:10.1016/j.chemosphere.2023.139561. [Google Scholar] [PubMed] [CrossRef]
98. Ma J, Alshaya H, Okla MK, Alwasel YA, Chen F, Adrees M, et al. Application of cerium dioxide nanoparticles and chromium-resistant bacteria reduced chromium toxicity in sunflower plants. Front Plant Sci. 2022;13:876119. doi:10.3389/fpls.2022.876119. [Google Scholar] [PubMed] [CrossRef]
99. Adejumo AL, Azeez L, Kolawole TO, Aremu HK, Adedotun IS, Oladeji RD, et al. Silver nanoparticles strengthen Zea mays against toxic metal-related phytotoxicity via enhanced metal phytostabilization and improved antioxidant responses. Int J Phytoremediat. 2023;25(12):1676–86. doi:10.1080/15226514.2023.2187224. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF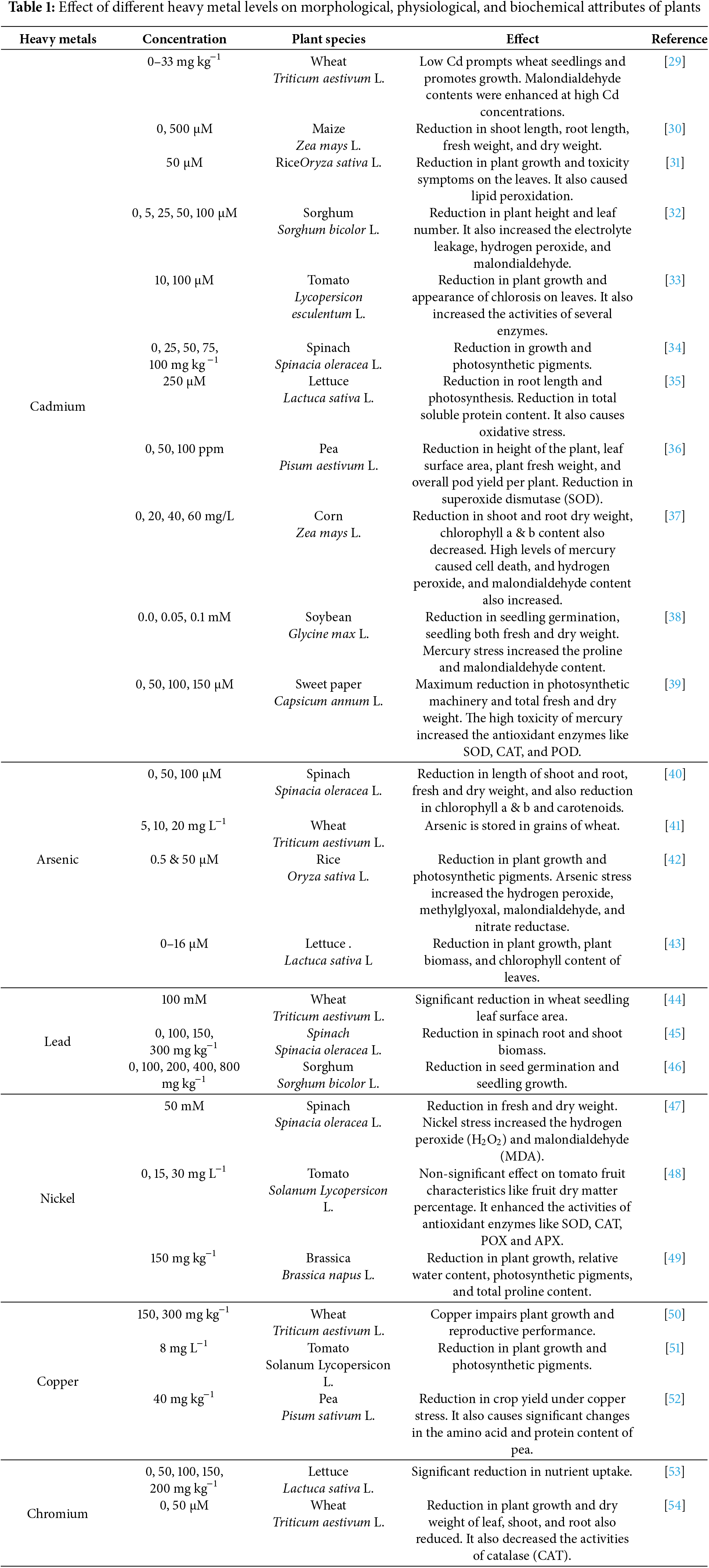
 Downloads
Downloads
 Citation Tools
Citation Tools