 Open Access
Open Access
ARTICLE
Aphicidal and Antimicrobial Activities of Salvia rosmarinus Essential Oil and Its Major Compound, 1,8-Cineole
1 Laboratory of Biology and Health, Faculty of Sciences, University of Ibn Tofail, Kenitra, 14000, Morocco
2 Laboratory of Engineering, Molecular Organometallic Materials and Environment, Faculty of Sciences Dhar El Mehraz, Sidi Mohamed Ben Abdellah University, Fez, 30000, Morocco
3 Higher Institute of Nursing Professions and Health Techniques, Fez, 30000, Morocco
4 UMRT INRAE 1158 BioEcoAgro, Laboratoire BIOPI, University of Picardie Jules Verne, Amiens, 80000, France
5 Biomedical and Translational Research Laboratory, Faculty of Medicine and Pharmacy of Fez, Sidi Mohamed Ben Abdellah University, Fez, 30000, Morocco
6 Laboratory of Epidemiology and Research in Health Sciences, Faculty of Medicine and Pharmacy, Sidi Mohammed Ben Abdellah University, Fez, 30000, Morocco
7 Department of Pharmacognosy, College of Pharmacy, King Saud University (KSU), Riyadh, 11451, Saudi Arabia
8 Laboratory of Microbial Biotechnology and Bioactive Molecules, Sciences and Technologies Faculty, Sidi Mohamed Ben Abdellah University, Fez, 30000, Morocco
9 Laboratoire de Bioressources, Biotechnologie, Ethnopharmacologie et Santé (LBBES), Faculté des Sciences d’Oujda (FSO), Université Mohammed Premier (UMP), Bd Mohamed VI BP 717, Oujda, 60000, Morocco
* Corresponding Authors: Aimad Allali. Email: ; Amine Elbouzidi. Email:
(This article belongs to the Special Issue: Biological Activities of Essential Oils)
Phyton-International Journal of Experimental Botany 2025, 94(4), 1239-1251. https://doi.org/10.32604/phyton.2025.063021
Received 02 January 2025; Accepted 11 March 2025; Issue published 30 April 2025
Abstract
This work uses GC-MS to analyze the bioactive compounds of Salvia rosmarinus essential oils (SREO) and evaluates their antibacterial, antifungal, and insecticidal effects, as well as the major component, 1,8-cineole. Chemical analysis identified 16 compounds accounting for 99.19% of the oil’s total content, with 1,8-cineole (33.17%), camphor (16.53%), α-pinene (14.46%), and camphene (8.14%) as the major constituents. Antimicrobial activities were assessed against pathogenic strains using minimal inhibit concentration (MIC) and minimum bactericidal concentration (MBC) assays. SREO exhibited a minimum MIC of 0.128% against P. aeruginosa, while 1,8-cineole showed a minimum MIC of 2.06% against the same strain, highlighting the higher efficacy of the complete oil compared to the isolated compound. Conversely, for antifungal activity, 1,8-cineole displayed a lower MIC (2.06%) against A. niger and P. digitatum compared to SREO (4.125% against A. niger). Regarding aphicidal activity, results demonstrated the lethal effects of SREO on M. persicae, with an even more pronounced impact observed for 1,8-cineole. At one dose of 40 μL/L air, SREO and 1,8-cineole resulted in 100% insect mortality within 24 h of exposure. After 12 h of exposure to SREO at concentrations of 5, 10, 20, and 40 μL/L air, the mortality rates were 20%, 36.67%, 70%, and 93.33%. 1,8-cineole showed maximum efficacy, achieving complete (100%) mortality within 12 h at 40 μL/L air.Keywords
The rising antibiotic resistance among pathogenic microorganisms and the growing demand for safer, eco-friendly pest control methods have intensified the search for alternative antimicrobial and insecticidal agents [1,2]. Plant-derived essential oils have gotten a lot of interest in this context because of their wide-ranging biological activity and natural origin. Essential oils are complex mixtures of volatile compounds, many of which have antimicrobial, antifungal, and insecticidal properties [3]. These properties are largely attributed to the synergy between their volatile compounds, such as monoterpenes, sesquiterpenes, and phenols. Additionally, essential oils exhibit significant antioxidant activities, enabling them to neutralize free radicals and prevent oxidative damage [4]. They also possess anti-inflammatory [5], antiviral [6], and even anticancer properties [7], making them promising candidates for applications in the pharmaceutical and agricultural industries. Among these, Salvia rosmarinus (Rosmarinus officinalis) essential oil (SREO) has been intensively researched for possible applications in medicine, agriculture, and food preservation [8].
Due to its volatile compounds, the evergreen plant Salvia rosmarinus (S. rosmarinus), cultivated worldwide, is also used in traditional medicine [9]. Its essential oils are rich in terpenes, with camphor and 1,8-cineole being among the most predominant components. These substances are considered characteristic volatile markers of rosemary [9]. Numerous studies on the volatile oils of this plant have reported high concentrations of these compounds, along with others such as α-pinene, camphene, borneol, terpineol, linalool, and caryophyllene.
1,8-cineole, a monoterpene with a distinctive odor, exhibits various biological activities, including antibacterial and insecticidal properties [10], as well as pharmacological effects [11]. Additionally, the food, fragrance, and cosmetics sectors make extensive use of this compound [12]. In order to cure a variety of human ailments, it is also important in traditional medicine in many cultures, including as Chinese, Indian, and Australian Aboriginal practices [13]. Lastly, drugs based on 1,8-cineole have demonstrated significant effectiveness in treating inflammatory respiratory diseases, including chronic obstructive pulmonary disease, sinusitis, bronchitis, colds, and bronchial asthma [14].
Staphylococcus aureus, Listeria innocua, Pseudomonas aeruginosa, Escherichia coli, Aspergillus niger, Candida glabrata, and Penicillium digitatum are among the clinically and agriculturally significant microbial strains against which we examine the antimicrobial efficacy of S. rosmarinus essential oil and its primary constituent, 1,8-cineole. Because of their pathogenicity, resistance to common treatments, and ability to cause spoiling, these microorganisms pose serious problems for the food and healthcare sectors [15–17].
In addition to their antibacterial properties, essential oils and their components have shown promise as natural insecticides. Myzus persicae (M. persicae), commonly referred to as the green peach aphid, is a highly destructive agricultural pest that affects a wide variety of crops. Control of this pest has traditionally relied on chemical insecticides, but increasing insecticide resistance and environmental concerns have driven interest in alternative strategies. SREO and 1,8-cineole have exhibited promising insecticidal activity, making them candidates for integrated pest management approaches.
Although numerous studies have investigated the composition and biological activities of plant extracts, most remain incomplete and do not systematically examine the relationship between the chemical composition of the analyzed extracts and their biological effects. In this context, the aim of this study is to propose an in-depth evaluation of the antibacterial and insecticidal effects of the majority compound of SREO, by comparing it with that of essential oils. We hope to contribute to the development of natural and sustainable solutions for infection management and crop protection by evaluating their efficacy against a diverse group of microbial strains and a key agricultural pest.
This study employed S. rosmarinus leaves gathered in May 2021 from the Boulemane region near Skoura, Morocco. This region is renowned for its botanical diversity and a climate conducive to the growth of various medicinal species. The rosemary specimens were meticulously identified by botanists in the laboratory, using a combination of botanical references and plant catalogues to ensure accurate identification. Following collection, the leaves underwent a rigorous cleaning process to remove impurities such as debris, dust, and insects that might compromise the purity of the extracts. Once cleaned, the leaves were dried under controlled conditions—air-dried in the shade—for a period of 15 days to preserve their chemical integrity.
2.2 Essential Oil Distillation
The hydrodistillation process was used to extract essential oils using a Clevenger-type device, which is well-known for its ability to extract volatile chemicals from plant material. For this extraction, 200 g of pre-dried S. rosmarinus leaves were weighed and placed in a 2-liter flask. To ensure optimal extraction, 1200 mL of distilled water were added to the flask, maintaining an appropriate solid-to-liquid ratio for effective hydro-distillation. The flask containing the plant material and water was then heated, allowing the combination to boil for three hours. After extraction, the essential oil was properly decanted and collected. To preserve its chemical and biological properties, the essential oil was immediately stored at −4°C in airtight vials, protected from light and air. This low-temperature storage is critical to prevent oxidation or degradation of the sensitive components in the essential oil before subsequent analyses and biological testing.
2.3 Chemical Composition Analysis of Essential Oils
The chemical composition of the essential oils was analyzed using the same analytical procedure described by Houzi et al. [18]. Specifically, the analysis was performed with an Agilent 6890 gas chromatograph (GC) coupled to a single quadrupole mass spectrometer. The separation of compounds was carried out using an HP-5MS capillary column. Finally, the identification of the components was based on the comparison of their retention indices (RI) and mass spectra (MS) with reference data from the ADAMS and NIST libraries.
2.4 Microbial Strains and Antimicrobial Testing
In this study, the antimicrobial activity was evaluated using a diverse panel of microorganisms, consisting of four bacterial strains (E. coli, S. aureus, L. innocua, and P. aeruginosa) and three fungal strains (A. niger, C. glabrata, and P. digitatum). The selected bacterial strains include both Gram-positive and Gram-negative species, allowing the assessment of antimicrobial efficacy against different cell wall types. These bacteria represent pathogens of medical and industrial significance. Similarly, the fungal strains comprise molds and yeasts of clinical and environmental relevance, providing valuable insights into antifungal activity. This diverse selection of microorganisms ensures a comprehensive evaluation of the antimicrobial properties of the tested compounds, while highlighting their potential applications in medicine, agriculture, and the food industry. Antimicrobial activity was assessed using a resazurin microtitration assay in 96-well plates. MIC was determined by observing a color change from blue to pink after incubation (37°C for bacteria, 25°C for fungi). MBC and MFC were identified by plating samples from wells without visible growth onto agar and incubating for 24 h at optimal temperatures. The lowest concentrations preventing growth were recorded.
The insecticidal effects of (SREO) and its major compound, 1,8-cineole, were evaluated using different doses: 5, 10, 20, and 40 μL. These doses were applied to Whatman No. 2 filter paper discs (1 × 1 cm), which were then affixed to the inner surface of the lids of 1-liter glass jars. This setup ensured that the concentrations corresponded to 5, 10, 20, and 40 μL/L of air. Twenty adult M. persicae (green peach aphids) were placed on a fresh, healthy citrus leaf inside each jar. The jars were sealed and maintained in an incubator under controlled conditions (27 ± 2°C, photoperiod L:D = 14:10) for 48 h. Adult mortality was assessed at 12-hour intervals following the treatment.
The main chemicals of S. rosmarinus were obtained from the (PubChem database in SDF format). Molecular preparation was carried out using the (LigPrep module in Schrödinger program), which used the OPLS3 force field to assure accurate modeling. Ionization states of the compounds were optimized at a physiological pH of 7.0 ± 2.0 to simulate biological conditions. This process generated up to 32 potential stereoisomers for each compound, accounting for their structural diversity. These prepared molecules were then ready for further computational analysis.
Docking proteins were received from the Protein Data Bank, including Escherichia coli gyrase B (PDB ID: 3G7E), Aspergillus niger β-1,4-endoglucanase (PDB ID: 5I77), and acetylcholinesterase (PDB ID: 6ARY). Protein preparation entailed adding hydrogen atoms, altering bond ordering, eliminating water molecules, defining hydrogen bonds, optimizing receptor atom charges, and minimizing energy using the OPLS3 force field.
Statistical analysis was performed using SPSS for Windows® (version 21.0). A one-way analysis of variance (ANOVA) was conducted to assess differences between the extreme values of the studied groups. The median lethal concentration (LC50) and the maximum lethal concentration (LC95) were determined using the probit method. This approach helps estimate the toxicity of tested substances while considering biological response variability. Confidence intervals were calculated to ensure the reliability of the obtained results. These statistical analyses provide a better interpretation of the effects of the studied compounds.
The essential oil output of SREO from Morocco varies greatly according on the cultivation region, as impacted by climatic circumstances, soil type, harvest season, and extraction methods [19]. Yields vary by geography, but typically range between 1% and 2.5% by weight. Plants growing in mountainous settings, such as the Atlas region, frequently generate higher amounts of essential oils, approaching the upper limit of 2.5%. This is attributed to water stress and altitude, which enhance the concentration of volatile compounds. In this study, the yield was 2.25%. who demonstrated that variations in the structure of secondary metabolism compounds and their natural quantity in medicinal plants are the main causes of variability in their biological activity. These variations are influenced by a variety of external and internal factors, including environmental conditions, the plant’s stage of maturity and the procedures used to prepare and extract the elements from the plant.
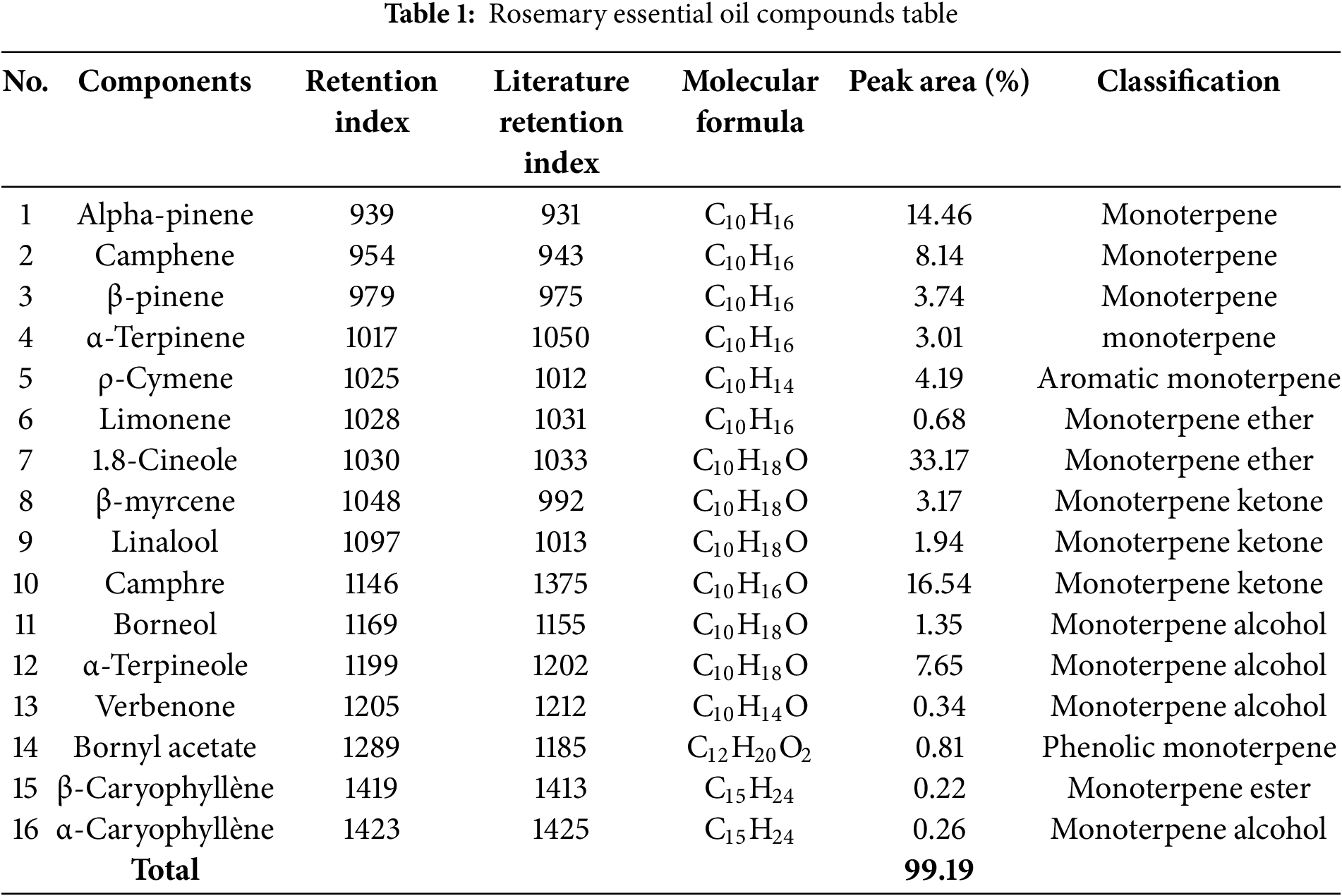
The compounds identified in SREO oils are listed in Table 1. A total of 16 distinct components were detected, representing 99.19% of the total oil content. Of these, the dominant components were 1,8-cineole (33.17%), camphor (16.54%) and α-pinene (14.46%), all classified as monoterpenes. These major compounds contribute significantly to the biological properties of the oil, including its antimicrobial and insecticidal activities. The chemical profile obtained in this study aligns with previous research conducted on S. rosmarinus essential oil in different regions [20,21]. This consistency highlights the reproducibility of our results and reinforces the typical phytochemical composition of Moroccan S. rosmarinus. Such findings are crucial for validating the potential applications of this essential oil in pharmaceutical, agricultural, and industrial fields.
The antibacterial activity tests demonstrated that SREO possesses properties against both Gram+ and Gram– bacteria, with varying minimum inhibitory concentrations (MICs) that reflect the bacteria’s sensitivity to the oil. SREO showed the lowest MIC of 0.128% against P. aeruginosa, indicating stronger antibacterial activity compared to other strains and to results obtained with 1,8-cineole alone, which exhibited a higher MIC of 2.06% against P. aeruginosa (Table 2). In the test (MBC), SREO displayed the highest bactericidal activity at 1.03% against E. coli and P. aeruginosa, whereas the MBCs for S. aureus and L. innocua were 8.75% and 1.125%, respectively. These findings suggest that the antibacterial efficacy of SREO may be due to components other than 1,8-cineole or to the synergistic action of multiple bioactive compounds. The antimicrobial activity of essential oils has long been recognized, with their effectiveness attributed to individual components or their synergistic interactions. For instance, Hendry et al. (2009) [22] reported that crude essential oil exhibited significantly greater efficacy against planktonic microorganisms compared to 1,8-cineole (p < 0.05). The same study found that chlorhexidine digluconate and 1,8-cineole had a synergistic impact against S. aureus, methicillin-resistant S. aureus (MRSA), E. coli, and C. albicans.
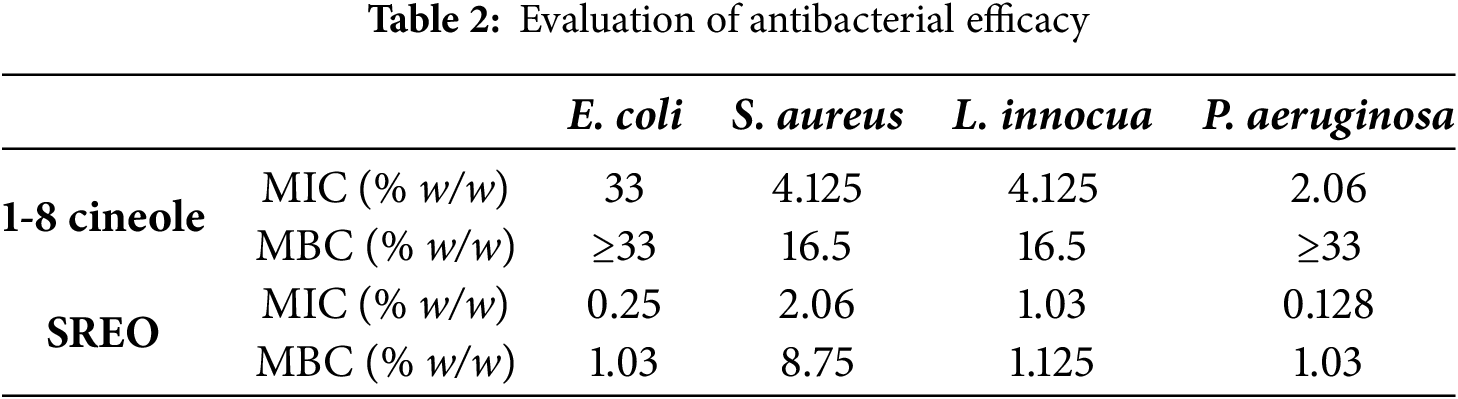
These findings may differ for other bacterial strains, as noted by [23], who found that pure main chemicals were more efficient than essential oils in their investigation. S. mitis was the most susceptible pathogen examined, whereas E. faecalis shown the greatest resistance to the studied substances. Previous study has shown that 1,8-cineole has relatively high antibacterial activity against a variety of pathogens, including S. aureus, P. aeruginosa, E. coli, and B. subtilis [24,25]. However, as demonstrated in our work, the antibacterial activity of 1,8-cineole alone is lower than that of essential oils, most likely due to synergistic interactions between the various bioactive components present [26]. Despite its known antimicrobial properties, the precise mechanisms of 1,8-cineole’s action remain unclear. To explore these mechanisms, an in-silico simulation was conducted to identify potential molecular targets for all essential oil components, including 1,8-cineole.
The results of the antifungal tests differ from those of the antibacterial assays. In particular, 1,8-cineole showed stronger antifungal activity, as shown by its lower (MIC) values for all strains tested (Table 3), indicating high fungal sensitivity to this compound. The MICs for 1,8-cineole were 2.06% for A. niger and P. digitatum, and 4.125% for Candida glabrata. In comparison, the MICs for rosemary essential oils were higher, at 4.125% for A. niger and P. digitatum, and 8.75% for C. glabrata.
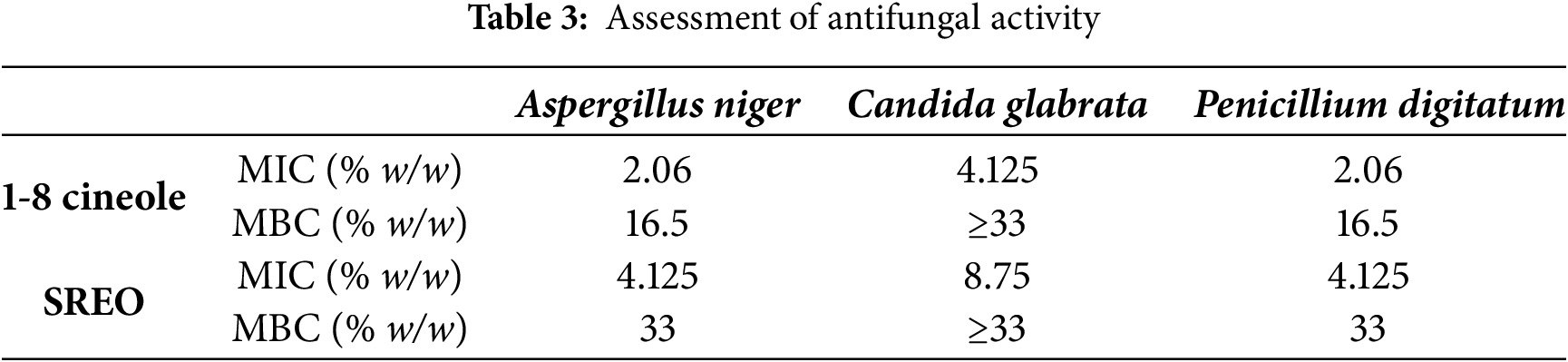
Regarding the minimum fungicidal concentration (MFC), 1,8-cineole exhibited the highest potency, achieving an MFC of 16.5% against A. niger and P. digitatum, outperforming both the other tested strains and rosemary essential oils. These findings highlight the efficacy of 1,8-cineole as a potent antifungal agent.
SREO is well known for its potent natural antifungal effects. For example, reference [18] revealed a strong inhibitory effect on the mycelial growth of several fungi, including full inhibition of B. cinerea. Similarly, reference [27] found that R. officinalis essential oil has substantial inhibitory and fungicidal action against several Candida species, with MIC50 values ranging from 0.5% to 2% and MFC values between 1% and 2%. The study found complete suppression of C. albicans and C. dubliniensis.
The greater antifungal activity of 1,8-cineole compared to rosemary essential oils is consistent with prior findings. For example, reference [28] found that 1,8-cineole shows promising antifungal properties, efficiently suppressing F. oxysporum, F. sporotrichioides, and A. tubingensis. In addition, reference [29] found that a nanoemulsion gel containing 1,8-cineole had substantial inhibitory effects against dermatophyte fungus. Furthermore, reference [30] showed that 1,8-cineole efficiently prevents biofilm formation in the F. solani species complex by inhibiting genes responsible for ergosterol biosynthesis, influencing adhesion, interrupting mitochondrial activity and disrupting extracellular matrix synthesis.
M. persicae mortality rates at concentrations ranging from 5 to 40 μL/L of air were used to assess the aphicidal activity of SREO and its main component, 1,8-cineole. The essential oil had a fatal effect on M. persicae, according to the data, and this effect was exacerbated when 1,8-cineole was used. After 24 h of exposure, both the essential oil and 1,8-cineole killed all of the examined insects at a concentration of 40 μL/L of air. Following 12 h of exposure to the essential oil, the corresponding mortality rates for dosages of 5, 10, 20, and 40 μL/L of air were 20%, 36.67%, 70%, and 93.33%. Notably, 1,8-cineole demonstrated the highest efficacy, achieving 100% mortality at a dose of 40 μL/L of air after just 12 h of exposure (Fig. 1).
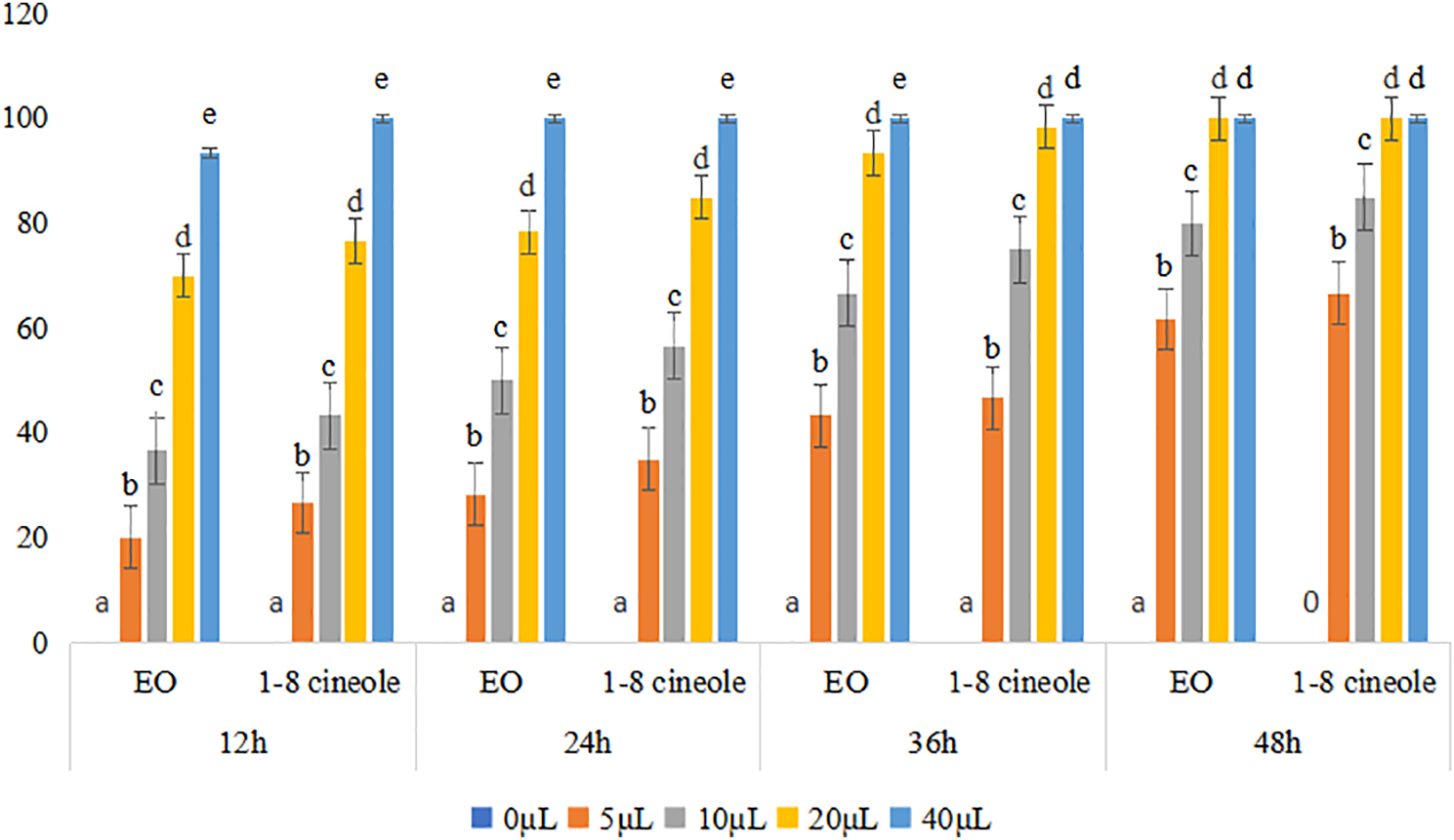
Figure 1: Toxicity of S. rosmarinus essential oil and 1,8-cineole on M. persicae individuals. Histograms show average mortality +/−1 S.D. Values followed by the same letter within each Eo or 1-8 cineole at each sampling time are not significantly different, whereas means followed by different letters are significantly different at p < 0.05
The essential oil (EO) of S. rosmarinus exhibited a lethal concentration (LC50) of 16.3 μL and an LC95 of 36.38 μL after 12 h of exposure (Table 4). In contrast, 1,8-cineole showed an LC50 of 12.52 μL and an LC95 of 26.44 μL, indicating that the effect of 1,8-cineole is stronger than that of the essential oil.
Bioassays of S. rosmarinus essential oil demonstrated significant insecticidal effects against M. persicae, resulting in a marked reduction in adult longevity. To combat the economic losses caused by agricultural pests, several plant essences have been studied for their ability to destroy M. persicae and other pests responsible for significant crop damage. According to [31], the essential oils of L. angustifolia and T. vulgaris, and their principal constituent’s linalool and thymol, are harmful to the green fish lice M. persicae (Hemiptera: Aphididae). The study also revealed that the synergy between imidacloprid and oil compounds was stronger than with linalool or thymol, implying that secondary metabolites may be responsible for the observed synergy. Additionally, Foeniculum vulgare essential oil proved highly effective against M. persicae (LD50 = 0.6 mL/L and LD90 = 2.4 mL/L) without inducing significant mortality in non-target organisms [32]. Similarly, da Silva et al., da Silva et al. (2023) reported that Cymbopogon winterianus essential oil caused high mortality rates in Brevicoryne brassicae (100% at 0.5% in 48 h) and M. persicae (98.99% at 1% in 48 h).
According to these results, reference [33] showed that the most toxic essential oils were those from S. chamaecyparissus and A. millefolium, followed by T. vulgare, T. patula, and A. absinthium. Their LD50 values were 0.34%, 0.34%, 0.47%, 0.61%, and 0.69%, respectively, after a 24-hour treatment period. The bio-insecticidal ability of 1,8-cineole was further confirmed by the same authors, who also noted that the oils of Santolina chamaecyparissus are rich in this chemical.
In antibacterial activity, Camphor, Camphene, and Alpha-Terpineol were the most active molecules against E. coli Gyrase B with a glide score of −5.038, −4.905, and −4.856 kcal/mol, respectively. Whereas in antifungal activity, Camphene, Camphor, and 1,8-Cineole were the most active molecules against the active site of Aspergillus niger with a glide score of −4.75, −4.563, and −4.543 kcal/mol (Table 5). Regarding insecticidal activity, Camphor, Alpha-Terpineol, and Camphene exhibited remarkable inhibitory activity against acetylcholinesterase with a glide gscore of −7.023, −5.926, and −5.923 kcal/mol (Table 5). Camphor established a single hydrogen bond with residue ASN 46 in the active site of E. coli gyrase B (Figs. 2A and 3). This molecule has also established one hydrogen bond with residue CYS 447 in the active site of acetylcholinesterase (Figs. 1c and 2C) Interactions of Camphene the most active molecule in the active sites of Aspergillus niger showed no bond formation (Figs. 1b and 2B).
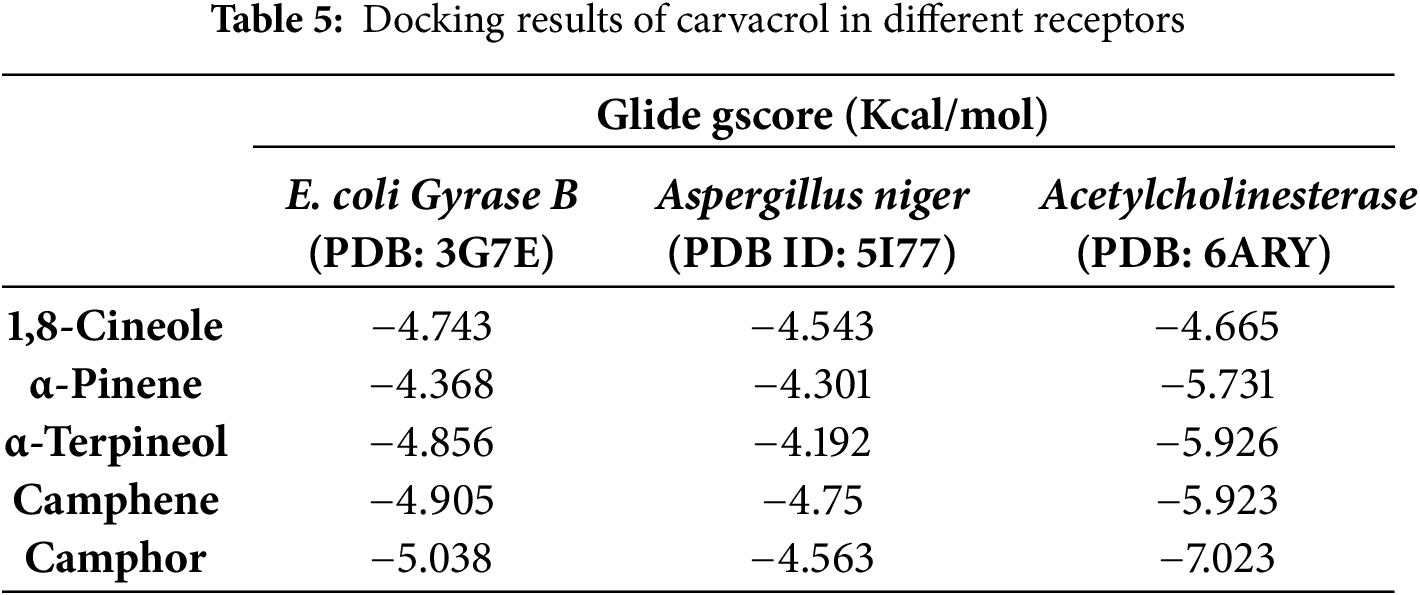
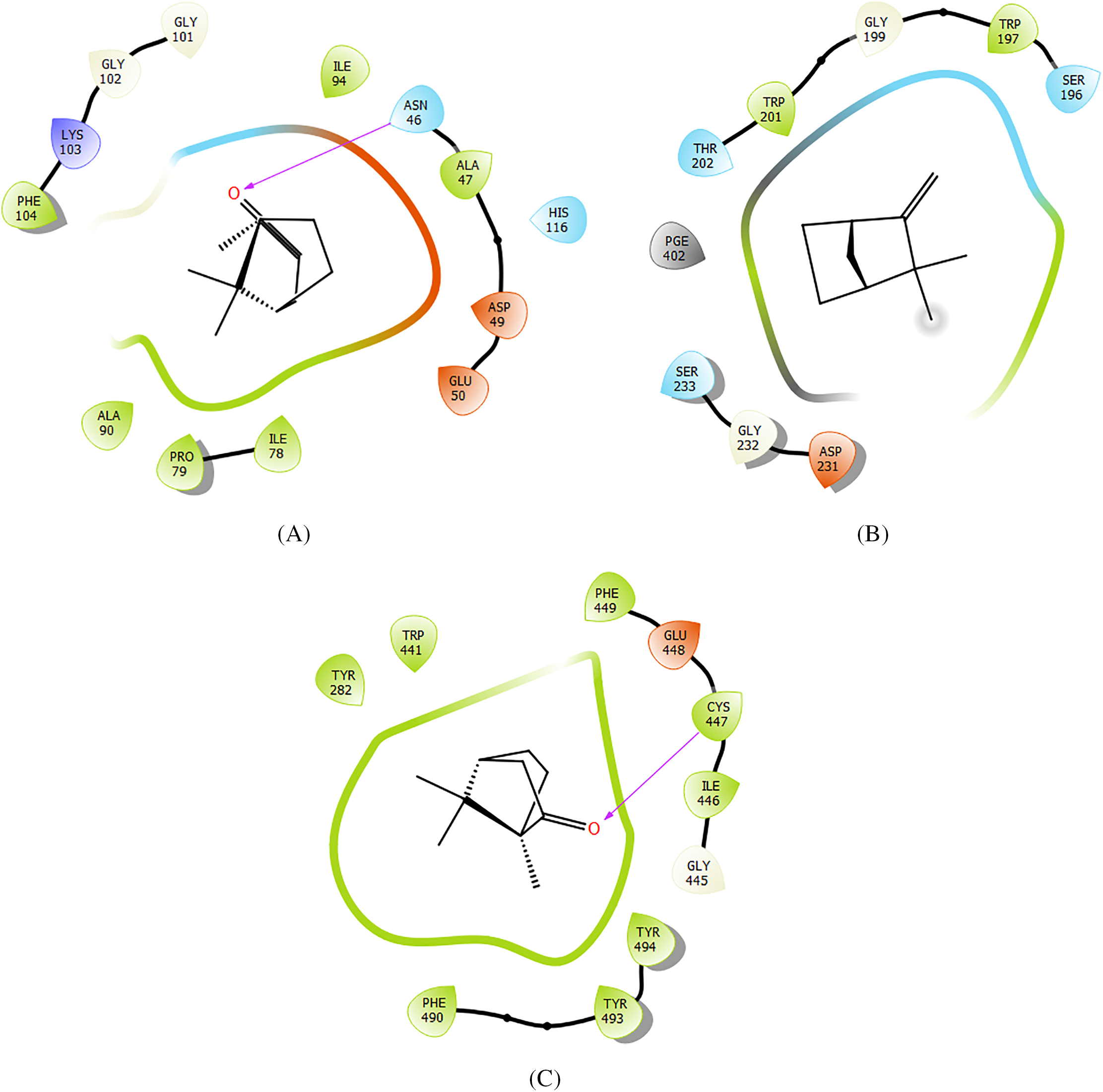
Figure 2: A two-dimensional viewer showing ligand interactions with active sites. (A,C) Camphor interacts with Escherichia coli gyrase B and acetylcholinesterase active sites. (B) Camphene interacts with the active site of beta-1,4-endoglucanase from Aspergillus niger
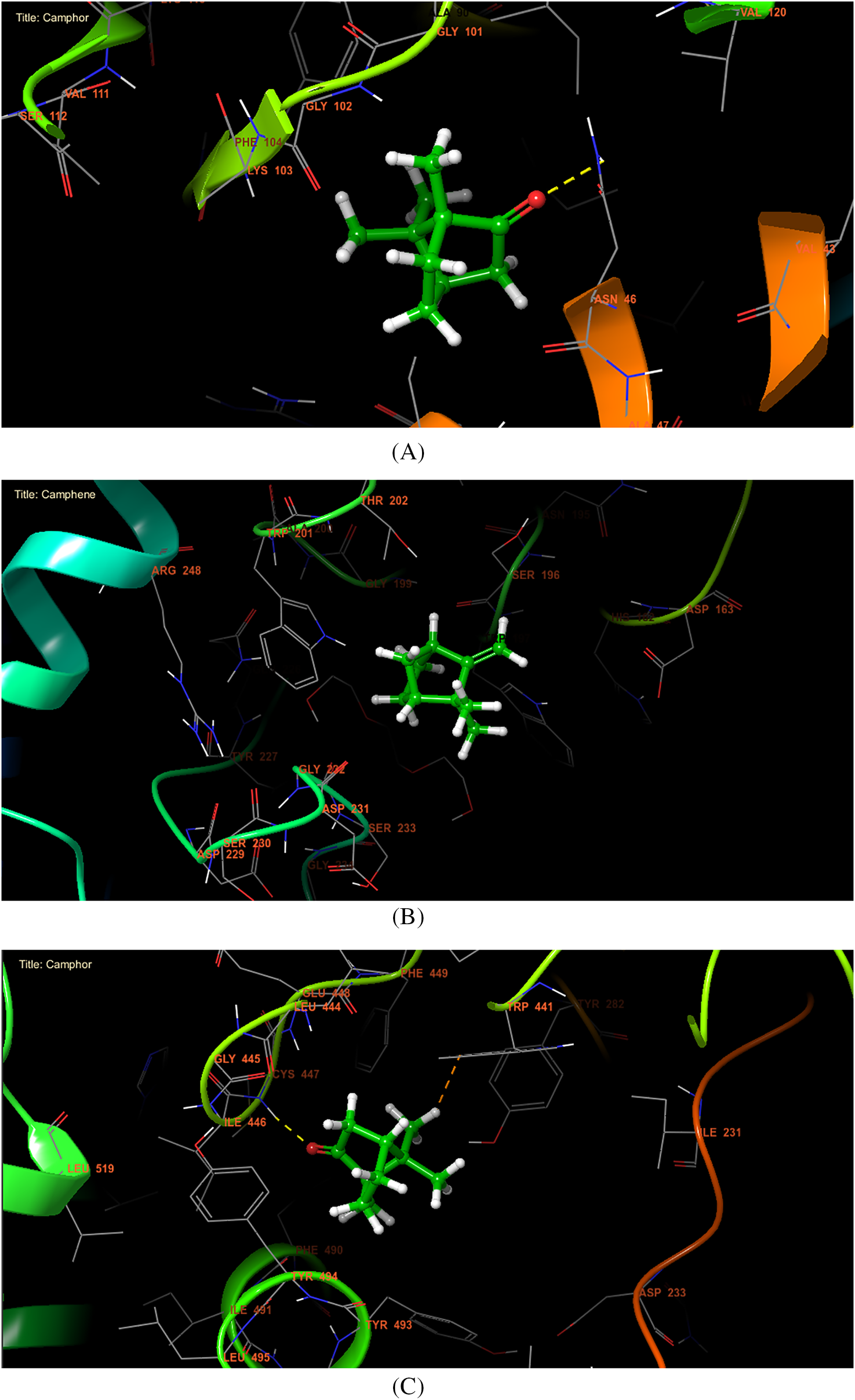
Figure 3: A three-dimensional representation of how ligands interact with active sites. (A and C): Camphor interacts with the active sites of acetylcholinesterase and Escherichia coli gyrase B. (B): Camphene interacts with the As-pergillus niger beta-1,4-endoglucanase active site
This study highlighted the effects of rosemary essential oil and one of its major components, 1,8-cineole, on various bacterial and fungal strains, as well as its aphicidal activity against M. persicae. The findings demonstrate that the efficacy of rosemary oil and 1,8-cineole varies depending on the type of activity tested. Specifically, the essential oils exhibited stronger antibacterial activity compared to 1,8-cineole, while for antifungal activity, 1,8-cineole proved to be more potent. This suggests that the antibacterial properties of the essential oils may be attributed to other secondary compounds, whereas the antifungal effects are primarily linked to 1,8-cineole. In terms of aphicidal activity, 1,8-cineole also showed superior efficacy compared to the whole essential oil, emphasizing the potential of exploring specific molecular components to enhance the applications of aromatic and medicinal plants. Further research is recommended to investigate additional bioactive compounds identified in the chemical composition of rosemary essential oils, with the goal of optimizing and expanding upon the observed results.
Acknowledgement: Not applicable.
Funding Statement: The research was funded by Researchers Supporting Project number (RSP2025R119), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Ghizlane Houzi and Soad Khal-Layoun; methodology, Ghizlane Houzi, Aimad Allali, Amine Elbouzidi, Mohamed Taibi, Mohamed Chebaibi, and Ben Khada Zineb; software, Aimad Allali and Mohamed Chebaibi,; validation, Ramzi A. Mothana, Mohammed F. Hawwal, Rachid Flouchi, Abdeslam Asehraou, Amal Lahkimi, and Soad Khal-Layoun; formal analysis, Aimad Allali, Mohamed Taibi, and Amine Elbouzidi; investigation, Ghizlane Houzi, Aimad Allali, Mohamed Chebaibi, and Ben Khada Zineb; resources, Rachid Flouchi, Abdeslam Asehraou, Amal Lahkimi, and Soad Khal-Layoun; data curation, Aimad Allali; writing—original draft preparation, Ghizlane Houzi, Mohamed Taibi, Aimad Allali, and Mohamed Chebaibi; writing—review and editing, Amine Elbouzidi, Ramzi A. Mothana, Mohammed F. Hawwal, Rachid Flouchi, Abdeslam Asehraou, Amal Lahkimi, and Soad Khal-Layoun; visualization, Ghizlane Houzi; supervision, Soad Khal-Layoun; project administration, Soad Khal-Layoun; funding acquisition, Ramzi A. Mothana and Mohammed F. Hawwal. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the Corresponding Authors, Amine Elbouzidi and Aimad Allali, upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Khursheed A, Rather MA, Jain V, Wani AR, Rasool S, Nazir R, et al. Plant based natural products as potential ecofriendly and safer biopesticides: a comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb Pathog. 2022;173(Pt A):105854. doi:10.1016/j.micpath.2022.105854. [Google Scholar] [PubMed] [CrossRef]
2. Iseppi R, Mariani M, Condò C, Sabia C, Messi P. Essential oils: a natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics. 2021;10(4):417. doi:10.3390/antibiotics10040417. [Google Scholar] [PubMed] [CrossRef]
3. Aimad A, Mssillou I, Siddique F, Mohmmed B, Abdelkrim A, Kara M, et al. Phytochemical analysis and pharmacological activities of essential oils extracted from Zingiber officinale (Roscoe) used in Mediterranean diet: in vitro and in silico studies. Int J Food Prop. 2024;27(1):1180–99. doi:10.1080/10942912.2024.2387937. [Google Scholar] [CrossRef]
4. Abdali El Y, Jalte Meryem M, Agour A, Allali A, Chebaibi M, Bouia A. Chemical composition, free radicals, pathogenic microbes, α-amylase and α-glucosidase suppressant proprieties of essential oil derived from Moroccan Mentha pulegium: in silico and in vitro approaches. J Biol Biomed Res. 2024;1(1):46–61. doi:10.69998/j2br3. [Google Scholar] [CrossRef]
5. Zhu J, Xu Z, Gao P, Liu X. Chemical composition, antioxidant activity, enzyme inhibitory effects, and network pharmacology analysis of essential oil from Bulbophyllum kWangtungense Schltr. S Afr N J Bot. 2024;172:701–9. doi:10.1016/j.sajb.2024.08.005. [Google Scholar] [CrossRef]
6. Ginting B, Chiari W, Duta TF, Hudaa S, Purnama A, Harapan H, et al. COVID-19 pandemic sheds a new research spotlight on antiviral potential of essential oils—a bibliometric study. Heliyon. 2023;9(7):e17703. doi:10.1016/j.heliyon.2023.e17703. [Google Scholar] [PubMed] [CrossRef]
7. Wang Q, Wang XY, Tao J, Nie JT, Zhou YH, Huang J, et al. Exploring the potential anticancer targets and mechanistic pathways of Elsholtzia densa essential oil based on network pharmacology. J Asian Nat Prod Res. 2025;1–20. doi:10.1080/10286020.2024.2446294. [Google Scholar] [PubMed] [CrossRef]
8. Borges RS, Ortiz BLS, Pereira ACM, Keita H, Carvalho JCT. Rosmarinus officinalis essential oil: a review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J Ethnopharmacol. 2019;229:29–45. doi:10.1016/j.jep.2018.09.038. [Google Scholar] [CrossRef]
9. Mohammed HA, Sulaiman GM, Khan RA, Amin MA, Albukhaty S, Elshibani FA, et al. Factors affecting the accumulation and variation of volatile and non-volatile constituents in rosemary, Rosmarinus officinalis L. J Appl Res Med Aromat Plants. 2024;42:100571. doi:10.1016/j.jarmap.2024.100571. [Google Scholar] [CrossRef]
10. Rossi YE, Palacios SM. Insecticidal toxicity of Eucalyptus cinerea essential oil and 1,8-cineole against Musca domestica and possible uses according to the metabolic response of flies. Ind Crops Prod. 2015;63:133–7. doi:10.1016/j.indcrop.2014.10.019. [Google Scholar] [CrossRef]
11. Hoch CC, Petry J, Griesbaum L, Weiser T, Werner K, Ploch M, et al. 1,8-cineole (eucalyptola versatile phytochemical with therapeutic applications across multiple diseases. Biomed Pharmacother. 2023;167:115467. doi:10.1016/j.biopha.2023.115467. [Google Scholar] [PubMed] [CrossRef]
12. Poitou X, Thibon C. Darriet P. 1,8-cineole in French red wines: evidence for a contribution related to its various origins. J Agric Food Chem. 2017;65(2):383–93. doi:10.1021/acs.jafc.6b03042. [Google Scholar] [PubMed] [CrossRef]
13. Chandorkar N, Tambe S, Amin P, Madankar C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phytomed Plus. 2021;1(4):100089. doi:10.1016/j.phyplu.2021.100089. [Google Scholar] [CrossRef]
14. Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1,8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;97(3):250–6. doi:10.1053/rmed.2003.1432. [Google Scholar] [PubMed] [CrossRef]
15. Liu Q, Meng X, Li Y, Zhao CN, Tang GY, Li HB. Antibacterial and antifungal activities of spices. Int J Mol Sci. 2017;18(6):1283. doi:10.3390/ijms18061283. [Google Scholar] [PubMed] [CrossRef]
16. Tran HM, Diep Hong Le, Nguyen VT, Vu TX, Thanh NTK, Giang DH, et al. Penicillium digitatum as a model fungus for detecting antifungal activity of botanicals: an evaluation on Vietnamese medicinal plant extracts. J Fungi. 2022;8(9):956. doi:10.3390/jof8090956. [Google Scholar] [PubMed] [CrossRef]
17. Gervasi T, Ginestra G, Mancuso F, Barreca D, De Luca L, Mandalari G. The in vitro potential of 1-(1 H-indol-3-yl) derivatives against Candida spp. and Aspergillus niger as tyrosinase inhibitors. Microorganisms. 2021;9(10):2070. doi:10.3390/microorganisms9102070. [Google Scholar] [PubMed] [CrossRef]
18. Houzi G, El Abdali Y, Beniaich G, Chebaibi M, Taibi M, Elbouzidi A, et al. Antifungal, insecticidal, and repellent activities of Rosmarinus officinalis essential oil and molecular docking of its constituents against acetylcholinesterase and β-tubulin. Scientifica. 2024;2024:5558041. doi:10.1155/2024/5558041. [Google Scholar] [PubMed] [CrossRef]
19. Llorens L, Llorens-Molina JA, Agnello S, Boira H. Geographical and environment-related variations of essential oils in isolated populations of Thymus richardii Pers. in the Mediterranean basin. Biochem Syst Ecol. 2014;56:246–54. doi:10.1016/j.bse.2014.05.007. [Google Scholar] [CrossRef]
20. da Silva SG, Sant’Ana J, Jahnke SM, dos Santos CDR. Effects of essential oils from the Brazilian pepper tree, Eucalyptus and Citronella on Brassica aphids Brevicoryne brassicae and Myzus persicae (Hemiptera: aphididae) and their parasitoid Diaeretiella rapae (Hymenoptera: braconidae). J Plant Prot Res. 2023;63(3):286–96. doi:10.24425/jppr.2023.146879. [Google Scholar] [CrossRef]
21. Oualdi I, Brahmi F, Mokhtari O, Abdellaoui S, Tahani A, Oussaid A. Rosmarinus officinalis from Morocco, Italy and France: insight into chemical compositions and biological properties. Mater Today Proc. 2021;45:7706–10. doi:10.1016/j.matpr.2021.03.333. [Google Scholar] [CrossRef]
22. Hendry ER, Worthington T, Conway BR, Lambert PA. Antimicrobial efficacy of Eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J Antimicrob Chemother. 2009;64(6):1219–25. doi:10.1093/jac/dkp362. [Google Scholar] [PubMed] [CrossRef]
23. Bernardes WA, Lucarini R, Tozatti MG, Flauzino LG, Souza MGM, Turatti ICC, et al. Antibacterial activity of the essential oil from Rosmarinus officinalis and its major components against oral pathogens. Z Naturforsch C J Biosci. 2010;65(9–10):588–93. doi:10.1515/znc-2010-9-1009. [Google Scholar] [PubMed] [CrossRef]
24. Papadopoulos CJ, Carson CF, Chang BJ, Riley TV. Role of the MexAB-OprM efflux pump of Pseudomonas aeruginosa in tolerance to tea tree (Melaleuca alternifolia) oil and its monoterpene components terpinen-4-ol, 1,8-cineole, and alpha-terpineol. Appl Environ Microbiol. 2008;74(6):1932–5. doi:10.1128/AEM.02334-07. [Google Scholar] [PubMed] [CrossRef]
25. Merghni A, Noumi E, Hadded O, Dridi N, Panwar H, Ceylan O, et al. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb Pathog. 2018;118:74–80. doi:10.1016/j.micpath.2018.03.006. [Google Scholar] [PubMed] [CrossRef]
26. Honório VG, Bezerra J, Souza GT, Carvalho RJ, Gomes-Neto NJ, Figueiredo RCBQ, et al. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Front Microbiol. 2015;6:1223. doi:10.3389/fmicb.2015.01223. [Google Scholar] [PubMed] [CrossRef]
27. Gauch LMR, Pedrosa SS, Esteves RA, Silveira-Gomes F, Gurgel ESC, Arruda AC, et al. Antifungal activity of Rosmarinus officinalis Linn. essential oil against Candida albicans, Candida dubliniensis, Candida parapsilosis and Candida krusei. Rev Pan-Amaz Saude. 2014;5(1):61–6. doi:10.5123/s2176-62232014000100007. [Google Scholar] [CrossRef]
28. Morcia C, Malnati M, Terzi V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(3):415–22. doi:10.1080/19440049.2011.643458. [Google Scholar] [PubMed] [CrossRef]
29. Li L, He M, Fang C, Zhang Y, Wang Y, Song X, et al. Preparation, characterization, ex vivo transdermal properties and skin irritation evaluation of 1,8-cineole nanoemulsion gel. Int J Pharm. 2022;624:121982. doi:10.1016/j.ijpharm.2022.121982. [Google Scholar] [PubMed] [CrossRef]
30. Zhang Y, Wang Y, Zhao X, Liu L, Xing R, Song X, et al. Study on the anti-biofilm mechanism of 1,8-cineole against Fusarium solani species complex. Front Pharmacol. 2022;13:1010593. doi:10.3389/fphar.2022.1010593. [Google Scholar] [PubMed] [CrossRef]
31. Faraone N, Kirk Hillier N, Christopher Cutler G. Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: aphididae). PLoS One. 2015;10(5):e0127774. doi:10.1371/journal.pone.0127774. [Google Scholar] [PubMed] [CrossRef]
32. Pavela R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ Sci Pollut Res Int. 2018;25(11):10904–10. doi:10.1007/s11356-018-1398-3. [Google Scholar] [PubMed] [CrossRef]
33. Czerniewicz P, Chrzanowski G, Sprawka I, Sytykiewicz H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic Biochem Physiol. 2018;145:84–92. doi:10.1016/j.pestbp.2018.01.010. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools