 Open Access
Open Access
REVIEW
Function of Biochar: Alleviation of Heat Stress in Plants and Improvement of Soil Microbial Communities
1 Faculty of Agriculture, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
2 Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
3 Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
4 Department of Textile Engineering, Ahsanullah University of Science and Technology, Dhaka, 1208, Bangladesh
5 Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
* Corresponding Authors: Prodipto Bishnu Angon. Email: ; Md. Arif Sakil. Email:
(This article belongs to the Special Issue: Soil Microbes and Abiotic Stress Factors: Impacts on Root Physiology, Crop Growth, and Hormonal Dynamics)
Phyton-International Journal of Experimental Botany 2025, 94(4), 1177-1210. https://doi.org/10.32604/phyton.2025.063675
Received 21 January 2025; Accepted 01 April 2025; Issue published 30 April 2025
Abstract
Global warming is making plants more susceptible to heat stress. Hence, adjustments to crop production systems are required for global food security. Heat stress (HS) poses a threat to the quality of ecosystems and global food security due to its adverse effects on plant development. The degree to which HS affects physiological disruptions, physical harm, and biochemical changes at various growth stages directly correlates with its effects on physiological functions, plant growth, and crop production. One promising approach is soil modification using biochar, which enhances soil health and promotes the development of microbial communities, ultimately improving plant heat tolerance. Biochar enhances soil structure, improves moisture retention, and increases nutrient availability in hot weather, thereby promoting plant growth and enhancing crop yields. Additionally, biochar, with its porous structure and ability to provide a liming effect, increases the diversity and activity of soil microbes, thereby fostering advantageous symbiotic relationships. These microbial communities support nutrient cycling, root growth, and general soil health, strengthening biochar’s position as a long-term solution for climate-resilient farming. Earlier research concentrated on the connection between biochar and heat stress or microbial populations; however, this review uniquely combines all three elements, providing a fresh viewpoint on their interrelated functions in enhancing plant adaptability. Furthermore, this study demonstrates the potential of biochar as a sustainable component for improving soil and supporting crops that adapt to heat stress. It examines the processes underlying these interactions and provides recommendations for future research strategies.Keywords
The continuous increase in global temperatures brought on by human activity and other means is one of humanity’s main concerns worldwide. When the temperature increases beyond what a plant can withstand, heat stress occurs, which interferes with the plant’s physiological functions (such as photosynthesis rate, transpiration rate, and stomatal conductance) and biochemical functions (phenolic content, carotenoid content, chlorophyll a, and chlorophyll b) [1]. This is triggered by prolonged exposure to high ambient temperatures, particularly during critical growth phases such as fruiting and flowering. Furthermore, in agricultural fields, a lack of shade or protective cover increases direct exposure to sunlight, exacerbating heat damage. The greater severity and frequency of heat waves are predicted as evidence of climate change. Therefore, this decreases the growth, survival, and production of plants [2]. A new analysis by the National Aeronautics and Space Administration (NASA) predicts that climate change will impact wheat and corn output as early as 2030, with a 24% decrease in grain yields [3]. Other research estimated that rice grain output declined by 10% with each 1°C increase, while wheat production is expected to fall by 3%–4% for every 1°C increase [4].
Soil microbiology refers to various microorganisms in soil, including bacteria, fungi, archaea, protozoa, and viruses, which interact with plants and the environment. Although these bacteria, fungi, and archaea are essential for maintaining soil structure, cycling nutrients, and promoting plant development, they can also harm plants and ecosystems in some situations. The majority of the biomass in the soil is composed of bacteria (70%–90%), with fungus coming in second [5]. The third type of life, archaea, lives in extreme conditions essential to maintaining ecological balance and soil health. They release nutrients, establish relationships with plants through plant residues, and directly impact plant development. Additionally, they improve soil fertility, create aggregates, and construct soil structures [6]. Fungi and bacteria are crucial for the breakdown of organic matter, the inhibition of pathogen growth, the enhancement of nutrient cycling, and the texture and structure of soil [7]. However, modifying the soil can help lessen the impacts of heat stress.
Biochar is a porous substance that is made by hydrothermally treating raw biomass or by pyrolyzing it [8]. Pyrolysis is the term for the high-temperature thermal depolymerization of biomass without the involvement of oxygen, where the carbon content of biomass transforms into aromatic carbon compounds as well as amorphous and graphitic-type structures [9]. The biological, chemical, and physical characteristics of soil, like cations, pH, nitrogen (N), phosphorus (P), and calcium (Ca), as well as soil water retention and hydraulic conductivity, are improved by the addition of biochar. Additionally, biochar has outstanding potential for enhancing the soil’s aggregation, porosity, and structure. Additionally, it encourages beneficial bacteria in the soil to proliferate. These qualities make biochar a potentially beneficial solution for reducing both global warming and food insecurity. Biochar can be utilized to enhance nutrient absorption and water-holding capacity. The porous structure of biochar can improve the storage of water as well as the size and distribution of minerals, acting as soil formers [10]. This indirectly supports high microbial activity and survival in general. The liming action of biochar raises the pH and enhances the soil’s capacity to exchange cations. This effect prevents nutrients from leaching by altering their availability. Furthermore, when the pH rises, microbial nitrification increases, resulting in nitrate losses and reduced availability of ammonium, a preferred nitrogen source of plants [11]. This might create adverse circumstances for plants, particularly in calcareous soils, which could reduce output [11]. Biochar is a possible nutritional replenishment agent since its nutrient content changes based on the input material and pyrolysis temperature [12]. The physicochemical characteristics of the soil have changed due to the incorporation of biochar; under heat stress, organic matter has improved, and bulk density has decreased. Furthermore, it was observed that the application of biochar to rice plants increased their surface area, promoted root length, and resulted in a higher dry weight during heat stress due to their lower bulk density of soil compared to the appropriate control [13]. Under stressful circumstances, biochar can help plants thrive by retaining nutrients in contaminated soil, enhancing microbial biomass and soil physicochemical properties, and boosting porosity and surface area, which helps to hold onto soil moisture. These alterations imply that adding biochar improves the uptake of nitrogen and its retention in above-ground tissues while encouraging root architecture, which eventually helps to lessen the detrimental effects of heat stress on plants. It is expected that intense heat waves will occur in the future because of the Earth’s rising temperature. Applying biochar to crops might be one way to increase their resistance to the stress of high temperatures.
Applying biochar to control soil biota is gaining popularity. The microbial population in the soil is impacted by biochar through altering nutrient availability, signaling between plants and microbes, or interactions with different microbial communities [14]. This is because the porous interior of biochar can shield it from physical harm caused by soil compaction or changes in the soil fauna’s diet. In light of their vulnerability to dehydration, the real surface protection of microorganisms within is, therefore, a crucial consideration in handling biochar [10]. Biochar positively impacts the surface binding of gases, such as CO2, generated in the soil. These indirect processes affect the makeup of microbial communities and the microbiological activity of soils [15]. Less competition, a more suitable habitat, higher porosity, an abundance of nutrients and organic materials and nutrients, and enhanced ability of the biochar’s surface to retain water are all potential causes. By its nature, biochar also adjusts the soil’s pH, creating an environment ideal for specific microbial communities. Several studies have been conducted concerning improving agricultural soil microbes and enzymes by optimizing and applying biochar. Thus, knowledge about how biochar mitigates plant heat stress by altering soil biota remains limited and incomplete. Therefore, this review’s goals are to go over (i) heat stress’s adverse impacts on several plant characteristics; (ii) the role of biochar in mitigating these effects; (iii) the way biochar helps soil microorganisms thrive; (iv) how biochar affects soil microbial activity to tolerate heat stress; and (v) how microbial communities react when biochar is added to polluted soils. To close knowledge gaps and create plants that are more resilient to future warming scenarios, we offer recommendations for future research endeavors based on our current understanding.
Biochar production occurs mainly through the pyrolysis process, where biomass breaks down thermally within an atmosphere that limits oxygen availability. Temperature affects biochar’s physicochemical characteristics as the process occurs between 300°C to 700°C [16]. The temperature level during pyrolysis determines char stability through the amount of carbon content [17]. However, substances obtained from low-temperature processes exhibit increased volatility. Different reactions happen during pyrolysis, especially dehydration, depolymerization, and isomerization, which modify the molecular structure of biochar and affect its physical properties [18].
The effective utilization of biochar in soil amendments depends on its physicochemical properties, which include a high surface area, together with cation exchange capacity and stability, because these characteristics are essential for environmental application. The porous nature of biochar enhances its water and nutrient retention capabilities, which makes it an effective solution to enhance soil fertility and plant growth [19]. Biochar possesses stable carbon properties, enabling it to store carbon efficiently and slow the pace of climate change. Research indicates that biochar properties can be optimized by selecting suitable feedstocks and adjusting pyrolysis conditions. This leads to meeting specific environmental requirements such as soil remediation and heavy metal adsorption [20]. Some raw materials show superior abilities for toxin removal, which makes them suitable candidates for pollution control systems [21].
Biochar is derived from various organic feedstocks. Different types of organic waste (Fig. 1) can be used for producing biochar. Generally, waste sources can be classified into forestry, agriculture, and municipal and industrial waste. Forestry wastes comprise leaves of trees, barks, logs, and various types of plants resulting from forestry operations, whereas agricultural wastes include wastes from plant cultivation and animal farming. Plant farming wastes include crop residues, such as stalks, husks, leaves, and straw that remain after harvesting rice, wheat, sugarcane, corn, and other crops. They also include leftover feeds like grains, forages, and other materials, and harvest and processing waste, such as vegetable trimmings, fruit peels, damaged produce, and by-products from food processing [22]. Livestock or animal farming wastes include the manure of different animals and birds, litter, and carcasses. Municipal and industrial waste includes trash from industry, businesses, households, sewage treatment plants, building sites, and hospitals [23].
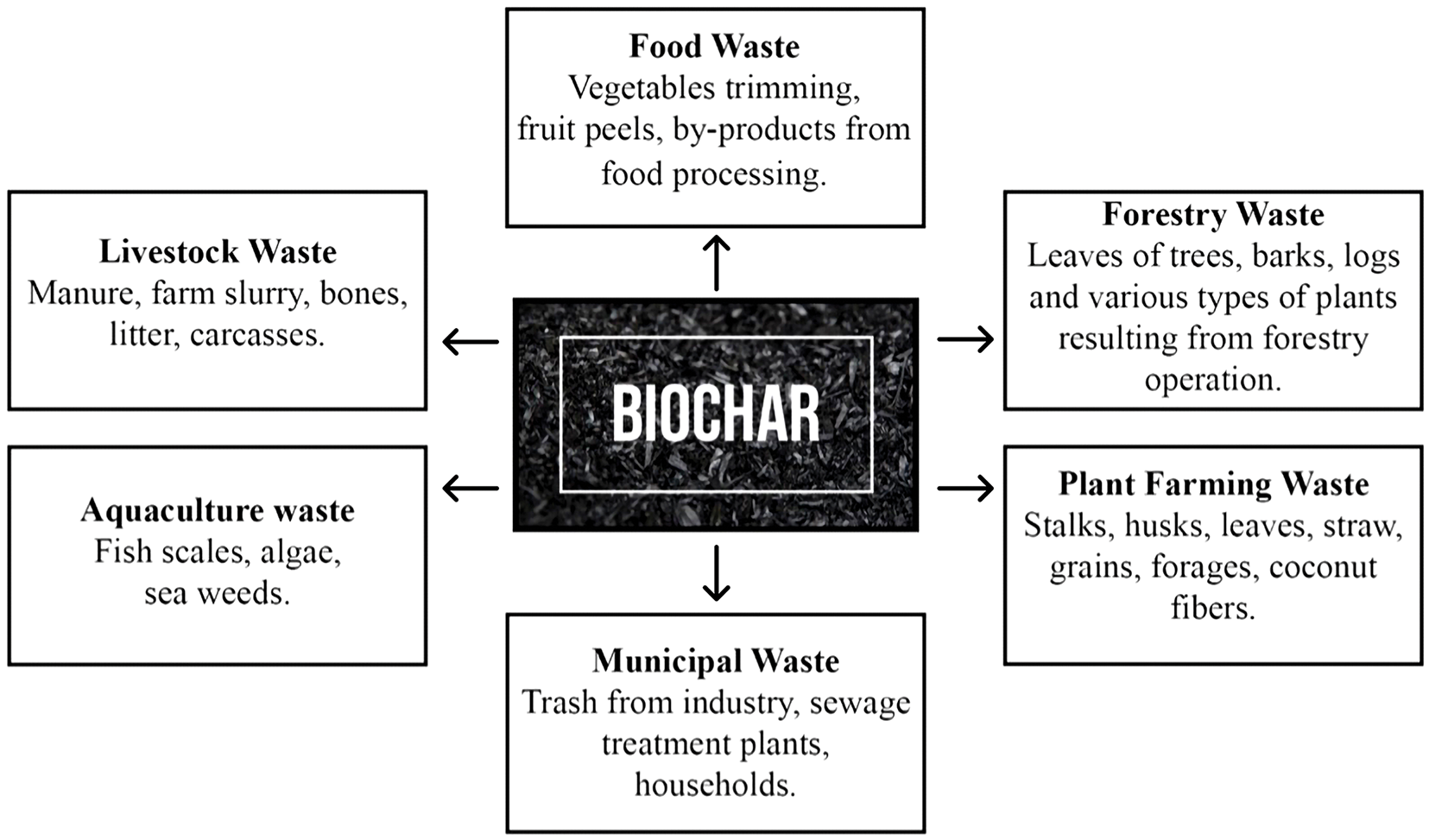
Figure 1: Different waste streams for the production of biochar
3 The Consequences of Heat Stress
Plant growth, development, and reproduction are impacted by the increased frequency, severity, and extended duration of high temperatures brought on by global climate change (Fig. 2) [24]. Heat waves and intermittent spikes in temperature have several detrimental effects on human society as well as the environment, including the potential to affect global agricultural production and future food demand significantly [25]. The rising temperature, when exceeding the threshold border for an extended duration, causes heat stress and impairs the plant’s growth and development [26].
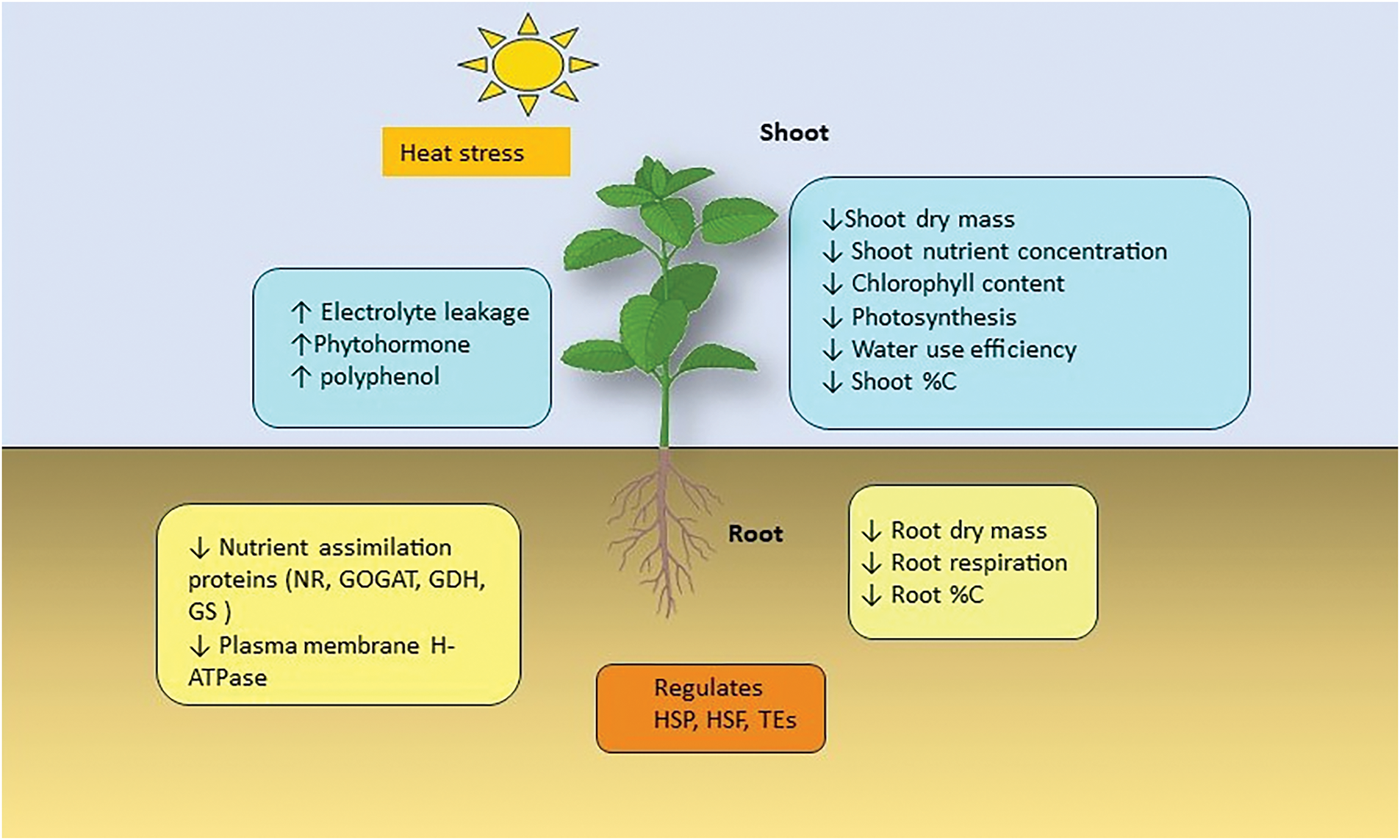
Figure 2: Effects of Heat Stress on Plant Root and Shoot. GDH-glutamate dehydrogenase, GOGAT- glutamine oxoglutarate aminotransferase, GS-glutamine synthetase, HSF-heat shock factors, NR-nitrate reductase, TEs-transposon elements, ↑-increase, ↓-decrease, blue represents an alteration in the shooting process, yellow represents an alteration in the root process, and heat stress-related regulations are represented with orange
3.1 Lowering the Yield of Crops
Although plants can experience heat stress at any stage of development, some growth phases are more susceptible to the effects of heat than others. Particularly in crops, heat stress has significant detrimental effects throughout the thermally sensitive developmental stages of early establishment, blooming, and gametogenesis [27]. Pollen grains are considered the most sensitive plant organ, and anthesis, or the early flowering stage, is deemed the most susceptible developmental stage to heat stress [28]. The final yield of grain is determined by the intricate relationships between several phenophases and their sensitivity to external factors. Reduced fruit set, pollen, seed germination, and yield are some of the negative impacts [29]. Three consecutive days of high temperatures (33°C–40°C) during the anthesis and grain-filling stages of wheat can significantly reduce grain size, weight, and number, resulting in substantial amounts of malformed grains [30]. A single hot day can also severely damage plant organs and reduce grain yield by shortening critical growth stages, such as blooming, anthesis, grain filling, and ripening [31]. Heat stress during reproductive stages leads to significant yield losses. Among the reproductive stages, gametogenesis in common bean [32], and flowering rice [27], sorghum [33], wheat [34], are highly sensitive to heat stress, leading to spikelet sterility and decreased seed and fruit numbers. High temperatures have a direct and detrimental impact on endosperm cellularization during the early stages of seed development, which reduces the sink capacity at the cellular level [29]. Consequently, inadequate seed or fruit filling leads to the sensitivity of storage components’ biosynthesis, transportation, or catabolism to high temperatures. For instance, PGD3, a plastidic 6-phosphogluconate dehydrogenase (6PGDH), is required for normal maize endosperm starch generation, and elevated temperatures suppress PGD3’s activity [35]. Plants undergo physiological changes as a result of heat stress. Plant development is impeded when the photosynthetic machinery is damaged, which lowers transpiration because of stomatal closure and CO2 content, inhibits the rates of enzymes like ATP synthases and photosynthetic enzymes, reduces the enhancement of leaves, and speeds up degression [36]. The metabolism of carbon absorption is changed to counteract the impacts of heat stress, remobilizing the starch stored in the chloroplasts of plants and allowing them to withstand the stress phase and also escape additional injury by producing energy, carbohydrates, as well as their derived metabolites [37,38].
3.2 Decrease in the Rate of Photosynthesis
Photosynthesis is the basis of higher plant growth and production. It is an intricate heat-sensitive physiological process. In addition to serving as a metabolic hub for photosynthesis, chloroplasts play a crucial role in detecting heat stress and utilizing retrograde signaling to trigger the necessary physiological adaptation reactions [39]. Heat stress affects several photosynthesis-related activities, such as electron transport, photophosphorylation, calvin cycle metabolism, photochemical reactions, and thylakoid membrane fluidity. Rubisco activase (RCA) is rendered inactive by HS-induced damage to the chloroplast, and key chloroplast components are downregulated [40]. This leads to a reduction in photosynthetic efficiency [41], a redox imbalance [42], and, in extreme situations, cellular death [43]. Due to the rapid and excessive production of reactive oxygen species (ROS), which damage the photosynthetic apparatus by slowing down electron transport, degrading proteins and pigments, and inactivating photosystem II (PSII) and photosystem I (PSI), HS harms plants and ultimately reduces agricultural production [44]. According to Mustafa et al. [42], wheat treated with HS (37°C ± 2°C) during the heading stage had lower levels of carotenoid (48.27%), chlorophyll a (38.05%), chlorophyll b (56.52%), and total chlorophyll (44.12%). According to Wang et al. [40], HS reduced the amounts of chlorophyll in leaves by raising the activity of chlorophyllase and chlorophyll-degrading peroxidase, resulting in chlorosis or leaf senescence. Heat exposure largely impacts the initial stages of photosynthesis, altering the properties of the chloroplasts’ membranes and blocking the energy transfer process. Heat stress ranging from mild to severe primarily affects carbon metabolism in the stroma of chloroplasts as well as photochemical reactions in the thylakoid lamellae by damaging photosynthetic enzymes and proteins [40].
The disturbance of redox equilibrium caused by high temperature leads to oxidative stress, which produces reactive oxygen species (ROS) as well as disrupts the mechanisms that eliminate these toxic forms of oxygen from different cell compartments [45]. The balance of generation and elimination of ROS within cells is disturbed by elevated temperature, leading to the abundance of oxides such as H2O2 and malondialdehyde (MDA). This, in turn, exposes plants to oxidative damage when they are facing oxidative stress [46]. Apart from non-enzymatic methods such as ascorbate (ASA), glutathione(GSH), α-tocopherol, and flavonoids), the detoxification of excess ROS generated by stressed cells entails the involvement of ROS-scavenging enzymes like superoxide dismutase (SOD), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) [47]. It was widely believed that the build-up of different ROS, like hydroxyl radicals, superoxide (O2-), singlet oxygen (1O2), and hydrogen peroxide (H2O2), beyond the antioxidant capacity of plants, was extremely harmful and might significantly impact plant growth. According to reports, excessive accumulation of ROS causes DNA damage, protein oxidation, lipid peroxidation, as well as cell death [48]. The protein function and activity of fruit cells are altered by oxidation driven by ROS, which also accelerates the ripening process [49]. A viable method for producing heat-tolerant crop plants is to manipulate photosynthesis using enzymes involved in the detoxification of ROS and rubisco activase [50].
3.4 Changes in Hormones and Gene Expression
Thermal stress reduces the synthesis of heat shock proteins (HSPs), their transcription and translation, the generation of phytohormones and antioxidants, and modifications to cell structure that affect hormonal equilibrium [51]. For cells of plants to withstand heat stress, temperature rise promotes the biosynthesis routes of hormones like auxins, ABA, brassinosteroids (BRs), cytokinin (CK), ethylene (ET), jasmonate (JA), and salicylic acid (SA). This leads to a higher accumulation in plant cells [51]. Plant gene products fall into three categories. Heat shock proteins (Hsps), ubiquitin ligases, RNA helicases, ion transporters, and aquaporins (AQPs) are among the proteins in the first group that most likely contribute to tolerance for heat stress [52]. RNA helicase, ubiquitin ligases, and HSPs mostly regulate the metabolism of proteins and RNA during heat stress. Heat stress affects the expression of membrane channel proteins, including AQPs and SUTs, which are involved in transporting water, small solutes, and carbohydrates. The second group comprises transcription factors, mitogen-activated protein kinases, calcium-dependent protein kinases (CDPKs), and other regulatory proteins [46]. They significantly impact the control of the transduction of signals, including the gene expression that responds to heat stress. It also modifies membrane fluidity, activating Ca2+ channels and causing a Ca2+ influx [53]. When plants experience heat stress, signal transduction pathways are activated by Ca2+ signals that are transduced by Ca2+ sensors to various substrates [46]. Low hormone levels can function as a signal molecule under heat stress, regulating various biochemical as well as physiological responses in vegetation. The internal regulatory lncRNA-miRNA-mRNA network is formed by the interaction of miRNAs and lncRNAs with other substances such as DNA, RNA, and proteins, which increases plant heat tolerance [52].
4 Role of Biochar on Plant Physiology and Development under High Temperature
Biochar is a kind of charcoal made by burning biomass or organic material in a low-oxygen atmosphere. Improving soil, carbon sequestration, and reducing the effects of climate change are just a few uses for biochar. In plant physiology, biochar’s enhancement influences root morphology optimization and nitrogen efficiency. Plants can use water and nitrogen (N) more effectively due to this optimized root system, which increases yields [54]. Biochar makes up for yield losses brought on by less N fertilizer application by lowering soil N losses. It supports the partial factor productivity of nitrogen and steady yields of the element [55]. Interestingly, a study team that applied biochar to maize discovered that plant height rose by 4.13%–14.91% across all treatments and that the biomass of the leaves, roots, and steams significantly increased as the amount of biochar applied increased [56]. Researchers discovered that the application of biochar also improved physiological parameters associated with the reduction of drought stress in plants, including electrolyte leakage (–42.5%), peroxidase (–13.14%), water use efficiency (38.41%), transpiration rate (39.17%), stomatal conductance (42.76%), chlorophyll a (19.3%), chlorophyll b (22.24%), photosynthetic rate (24.86%), catalase (24.11%), hydrogen peroxide (–18.03%) and superoxide dismutase (24.66%) [57]. Although not affect the available soil water content, biochar has been demonstrated to enhance the ability of soil to retain water. Applying biochar + P resulted in a 7% increase in the average grain production per plant of rice compared to other temperature treatments and cultivars [58]. Applying biochar and P under extreme temperatures increased antioxidant activity in both Huanghuazhan and IR-64 compared to other treatments. Each antioxidant was tightly controlled in each cultivar leaf and xylem sap. Stress from high temperatures, especially at night, causes plants to grow taller. Putting biochar on the ground, with or without P, improved performance in his experiment [58]. Additionally, rice grain and related traits like the weight of 1000 grains, spike filling rate, rachis dry weight, breadth, and size increased when biochar and P were applied under extreme temperatures [58].
5 Using Biochar to Mitigate Plants’ Heat Stress
To facilitate long-term carbon sequestration, the distinctive characteristics of biochar may impact soil bulk density and porosity [59]. Furthermore, it mitigates the adverse impacts of heat stress on rice, maize, and potato crops [13]. The accumulation patterns of heat-shock proteins in plants, induced by heat stress and crucial for developing heat stress resistance, are strongly linked to nitrogen availability. Applying biochar amendments enhances this availability in soil [13]. Riaz et al. [60] have linked biochar to improvements in soil quality, yields, crop growth and development, and soil fertility, as well as a decrease in the number of abiotic stressors. Its large surface area, reactive functional groups, ability to exchange cations, and susceptible carbon are all thought to contribute to these advantages. Heat stress causes plants to lose more water and release less carbon dioxide, which lowers the photosynthetic rate and biomass output. The soil is supplemented with Biochar to boost fertility and maintain long-term production. Controlling daily and seasonal soil temperatures may also influence the thermal dynamics of the soil environment [59]. Regarding boosting soil fertility and production, biochar is important in mitigating plant heat stress (Table 1).
5.1 Reducing Emissions of Greenhouse Gases
Carbon dioxide, nitrous oxide, and methane are the main gases that contribute to atmospheric radiative forcing. Other sources include wetlands, animals, fertilizers, agricultural practices, and human activities. The amount of greenhouse gases (GHGs) released by biochar-supplemented soil depends on several variables, including biomass types, temperature, pyrolysis conditions, and soil type [70]. The soil’s ability to function as a source of
5.1.1 Nitrous Oxide (N2O) Emission
Through the use of biochar, the emission of nitrogen dioxide during the process of composting is minimized by fixing the NH4+ and NO3– ions and thus diminishing the proportion of the inorganic nitrogen utilized by the nitrifying and denitrifying microorganisms [78]. The outer layer of the biochar can actively accept and lessen N2O by either biotic or abiotic processes [79]. The enzymes involved in N2O formation and reduction during denitrification are the ones encoded by nirK/nirS and nosZ genes [80]. The biochar surface can absorb and reduce N2O through biological or abiotic reactions. The enzymes responsible for N2O production and consumption during denitrification are encoded by the nirK/nirS and nosZ genes [80].
Studies conducted in the field and incubation have shown that soils treated with biochar may reduce
5.1.2 Carbon Dioxide (CO2) Emission
Recently, a study showed that composting biochar derived from pig dung significantly reduces CO2 emissions. Conversely, several studies have shown conflicting findings. For instance, it was discovered a surge of 53.2% was discovered in the trend [83], whereas in another study it was discovered a decline of 26.1% [40]. These differences could be attributed to the fact that biochar enhances external organic matter, accumulates, or provides the microorganisms a better habitat, thus enhancing their activity in capturing carbon [84]. Despite these implications, the reduced availability of O2 sources used by anaerobic bacteria in compost heaps may reduce outputs of CH4 and N2O [78].
According to Luo et al. [85], adding biochar significantly impacts several soil properties, such as pH, porosity, plant productivity, carbon and nitrogen dynamics, water content, and cation exchange capacity. Each of these elements can significantly influence soil
Brassard et al. [89] found that biochar can decrease
5.2 Enhancing the Conditions of the Soil
Biochar improves soil aggregation by adhering to organo-mineral complexes and mycorrhizal fungi and increasing microbial activity [93]. Adding biochar to soil improves its pH, organic matter, porosity, moisture retention, surface area, and electrical conductivity. High temperatures can cause sand soils to fracture [94]. In this case, biochar helps address the microbial aggregation, proliferation, structure, and porosity of wildfire-impacted soils [95]. To prevent wildfires from spreading and to produce biochar that is useful for land reclamation associated with wildfires, dried vegetation like pine needles and leaf litter pine needles can be used to create biomass charcoal [48]. An increase in temperature causes soils to absorb more energy than they expel, which may change the thermal characteristics of the soil, such as reflectance, temperature, diffusivity, and thermal conductivity [96]. The addition of biochar may enhance the thermal characteristics of soils by managing the distribution of heat among soil particles, surface-energy partitioning, and the accumulation or movement of water and heat in the soil; all of this impacts the soil microclimate [97]. Additionally, biochar amendment enhances plant growth by increasing arylsulfatase, acid phosphatase, and urease, which are crucial for the utilization of sulfur phosphates and nitrogen [98]. However, because high-dose amendments contain resistant aromatic compounds, they may inhibit the growth of fungi. Phenolic compounds, metal oxides, and silica promote the formation of exopolysaccharide biofilms by bacteria [99]. Biochar can promote fungal biomass growth by increasing laccase and manganese peroxidase activities (Table 3).
5.3 Strengthening the Soil’s Water Holding Capacity (WHC)
By improving the soil’s ability to store water, biochar additions may increase agricultural productivity in dryland areas without irrigation and lower the need for irrigation water. In sandy loam soils, for example, biochar derived from mango wood enhanced water retention by 11%, but in medium sandy soils, it increased it by 137% [111]. Pine biochar application increases soil water retention and plant available water, improving soil water relations for agricultural land use [112]. In silty loam soils, biochar derived from forest waste increased plant-accessible water by 226%, whereas in other soil types, it dropped by 10% [113]. At other matric potentials, there were no impacts seen, although soil water retention and stress-free available water declined linearly with a biochar application rate. Despite these results, applying biochar to plants exposed to high temperatures improves water retention and water consumption efficiency [114]. Yu et al. [115] reported that the research found that raising the retort temperature from 250°C to 750°C increased water holding capacity from 7% to 16%, with an additional 11% increase. The findings indicate that the potential of biochar to increase the amount of water that can be held may significantly impact areas prone to drying up. These findings also suggest that using biochar benefits increasing crop output and soil health.
5.4 Increasing the Organic Matter (OM) Composition of Soil
Applying biochar to arable soils is an important source of stable organic matter (OM) that might replace conventional organic fertilizers. As biochar is high in OM, it can help increase the amount of OM in soil, improving stability, filtration rate, soil structure, nutrient availability, and water-holding capacity [116]. These enhancements increase the reservoir’s capacity to store nitrogen, phosphate, and sulfur while reducing its susceptibility to erosion [117]. Compared to wood-produced biochar, biochar formed from manure has a lesser surface area [118]. Elevated temperatures result in a decrease in oxygen and hydrogen levels, as well as an increase in carbon content [119]. Numerous biological and chemical processes in soil are impacted by dissolved organic matter (DOM), a highly mobile and active organic matter component. The amount and composition of DOM directly impact microbial activity, pollutant behavior, and the global C-cycle [120].
Furthermore, research has shown that biochar has the potential to both release native DOM into the soil and simultaneously absorb intrinsic DOM [121]. Despite this, research shows that biochar may either have no impact on soil DOM levels at all or significantly decrease DOM leaching by 20% via the adsorption of DOM species in the soil that are similar to humic and have a high molecular weight [122]. The impact of biochar on soil DOM is a complicated matter that depends on many variables, such as soil characteristics, the features of the biochar, as well as the experiment design. Therefore, understanding how biochar releases and adsorbs DOM in soil is crucial to understanding its impact on soil DOM [120].
5.5 Increasing Bulk Density of Soil
The addition of biochar significantly reduced the bulk density of sandy soil after ninety-one (91) days of incubation, likely due to more water in the control columns. As bulk densities drop, plant roots may get denser and longer [123]. This result has to be confirmed in fields with intact soil structure, crops, and residues since several factors interact to alter bulk density, soil water penetration, and drainage [123]. Prober et al. [124] reported that the addition of low bulk density biochar material led to a considerable drop in soil bulk density after two years in mesic forests treated with 20 t h
5.6 Heavy Metal Detoxification
Heavy metals in plants contribute to physiological difficulties that change the metabolism and linkages between plants and nutrients, decreasing production and quality of crops [127]. But researchers have demonstrated that, as a consequence of burning biomass at temperatures higher than 300°C, biochar may retain organic contaminants and heavy metals. Additionally, with co-precipitation, ion exchange, metal ion surface complexation, and physical adsorption, it may be mixed with soil as a remediation and amendment agent to reduce the toxicity and bioavailability of heavy metals in contaminated soils [128]. Silver (Ag), arsenic (As), mercury (Hg), cobalt (Co), nickel (Ni), chromium (Cr), chromium (Cr), lead (Pb), cadmium (Cd) are some of the heavy metals that are considered harmful [129]. Besides, stress conditions due to the presence of Pb, Cd, and As inhibit the formation of secondary metabolites, root elongation, leaf chlorosis, and seed germination [127]. In this case, biochar with higher basal pH (>10), a pyrolysis temperature (between 401°C–600°C), and nano sized particle (<2 mm) appeared to help reduce the absorption of Pb and Cd in plants [130]. When applied to sandy soil, biochar increased soil pH by 4.5 units, reduced bulk density by 3%–31%, and increased soil porosity from 42.5% to 56% when applied to maize straw charcoal at 10–60 t h
6 Biochar as a Microorganism Growth Stimulator of Soil
Changes to the physicochemical conditions of soil can have a beneficial or adverse effect on the microbial population’s activity and structure through habitat modification. Soil microorganisms are directly and primarily promoted to flourish by these alterations in soil characteristics. The content and concentration of accessible nutrients in biochar are determined by the production parameters, including temperature and retention period, as well as the feedstock. Raising the production temperature often results in higher biochar pH, C:N, and C:O ratios, total surface area, while decreasing the concentrations of carbon and organic substances dissolved [136]. By supplying labile C substrates for breakdown, biochar benefits microbial populations. Enhancements to the properties of the soil, like a rise in pH, the availability of nutrients like, C, N, magnesium (Mg), potassium (K), calcium (Ca), phosphorus (P), and, the soil’s water-retention capacity may encourage soil microbiological growth [137].
Biochar provides excellent habitats for soil biota owing to its higher surface area and porosity. Hence, biochar can control the quantity and functions of specific microorganisms that may benefit from the physical characteristics of biochar. Another significant characteristic of biochar that affects soil microbial development is surface area. Thus, higher surface area and porosity provide additional openings for microbial colonization, based on the temperature during pyrolyzing and feedstock type. The specific surface area and enhanced porosity of biochar are linked to a greater capability to hold water in a variety of soil types, giving microorganisms better water retention actions and expansion [138].
The chemical properties of the soil, particularly pH, which is an important component affecting soil microbial population and behavior, may be dramatically changed when an enough quantity of biochar is incorporated into the soil. Adding biochar generated from maize straw reduced N leaching and raised the soil’s electrical conductivity (EC); consequently, alterations were seen in the composition of the bacterial community [139]. A silt loam soil’s total P, total N, and organic C levels were raised by applying 30 t ha−1 of biochar composed of maize straw, increasing the soil’s nutrient availability [140]. Consequently, observations revealed that the AMF/saprotrophic fungus ratio and relative abundances of AMF were greater than those of the control [140]. N-cycling bacterial community structure and abundance in the soil are impacted by soil type and biochar rates [141]. Some effects of applying biochar on the soil’s microbial community are illustrated in Table 4.
7 Impact of Biochar on the Structure and Activity of the Soil Microbial Community
7.1 Architecture of Soil Microbial Populations
The enhancement of both chemical and physical properties (density, macroaggregates strength, pH), as well as the water-air soil regime, are mostly responsible for the beneficial effects of biochar that make it suitable for use as an ameliorant in agricultural practices [125]. The rhizosphere’s microbial community as well as the plant roots are both directly impacted by each of these effects [84,154].
Phospholipid-derived fatty acid (PLFA), was utilized to evaluate the communities of microbes in an acid soil treated with biochar following 431 days of incubation. The outcomes demonstrated that the treated soil’s PLFA levels were greater than those of the control soil [155]. Furthermore, the PLFA showed that biochar Carbon, which is formed at 350°C rather than 700°C, was utilized as a substrate by gram-positive bacteria. Therefore, the research concluded that the biochar’s pyrolyzed temperature has a major influence in managing the composition of the soil microbial community, irrespective of the soil’s pH [155]. However, when the utilization rate of maize biochar increased, there was a corresponding drop in the relative abundances of bacteria and fungus, as well as the PLFA levels [156]. Biochar’s alkalinity, which tends to lower soil microbial community formulation and temporarily suppress activity, is a possible explanation for it.
DGGE (Denaturing Gradient Gel Electrophoresis), qPCR (quantitative Polymerase Chain Reaction) assay, and clone library analysis were used in conjunction with the terminal restriction fragment length polymorphism (T-RFLP) method to identify the 16S and 18S rRNA genes, which were then used to evaluate the structure and abundance of the fungal and bacterial communities in paddy biochar treated soil [157]. The study’s findings demonstrated that while bacterial 16S rRNA gene copy counts increased, fungus 18S rRNA gene copy counts decreased upon the addition of biochar. Applying biochar at greater rates (40 t ha−1) reduced numerous bands associated with the Ascomycota and Glomeromycota, according to the fungal 18S rRNA gene’s DGGE bands. Findings from the research show that although the fungi’s ability to metabolize the very stable organic C in biochar is limited, the bacterial population’s variety may have increased due to the improved soil pH and fertility [157]. PCR amplification, DNA extraction, and DNA sequencing of 16S rRNA gene fragments were used to figure out alterations in the variety of bacteria and community composition in biochar treated soil [139]. The findings of the investigation demonstrated that the variety of bacteria grew along with the utilization rate of biochar. The aforementioned findings from numerous investigations have shown that based on the soil conditions, type of biochar, and time, biochar could change the microbial community’s composition to varying degrees.
7.2 Activities of Soil Enzymes
Soil enzymatic activity is directly affected by a range of biochar impacts on soil microbial population, soil properties, and soil activity. One potential method to watch an enzyme-biochar interaction is to look for the process by which the substrates or enzymes adhere to the biochar [158]. Additions of biochar can repair degraded soil quality by enhancing the biological properties of the soil, like elevating activity of enzyme. Due of its capacity for interacting with the roots of plants and stimulate particular microorganism groups, the enzymatic activity of soil is indirectly impacted by biochar. It has been shown that soils treated with different varieties of biochar and under varied soil conditions have higher dehydrogenase activity [159]. The biochar treatment of a fuvo-aquic soil boosted many extracellular enzymes’ activity linked to the cycling of soil carbon and sulfur (S), like β-D-cellobiosidase, β-glucosidase, α-glucosidase, sulfatase, and β-xylosidase [156]. Nevertheless, this effect appeared erratic and dependent on the pace at which biochar was added. For instance, the enzyme activity in the soil increased when a minimal 0.5% of maize biochar was applied. Still, it dropped as the biochar was added at concentrations higher than 1.0% [156]. It can be tough to prove a clear causal relationship between soil enzyme activity and biochars because of the soil environment’s various kinds of soil, soil enzyme types, and biochar types (Table 5).
7.3 Reaction of Soil Microbes in Polluted Soils
Biochar added to the soil can reduce the levels of various pollutants that are present. Biochar is widely recognized for its capacity to immobilize pollutants, both inorganic and organic in soils through various processes, comprising precipitation, absorption, co-precipitation, ion exchange, and complexation. In soils polluted with heavy metals, the roles and composition of the microbial population of soil may be indirectly impacted by biochar because the constitution of microbial community can be altered, and soil microbial development can be inhibited or prevented by heavy metal toxicity [167].
Field research was conducted in contaminated soil to investigate the impact of biochar on soil microbial activity and heavy metal availability using Cd, Pb, and Cu. Relative to the control, the quantity of soil bacteria was grown 2.8 times by applying 3.0 t ha−1 of biochar produced from bagasse of sugarcane [168]. Microorganisms’ activity, population size, and variety are all impacted by heavy metals (Pb and Cd) in soil. By adding biochar, pH of soil is changed and the bioavailability of heavy metals decreased. Compared to the control, it led to a 39% and 930% rise in the populations of actinomycetes and fungi, respectively [169]. The use of biochar improved the microbial composition of the soil and reduced metal toxicity, as seen by a rising level of phospholipid derived fatty acid (PLFA) and decreased metal toxicity in the soil. Studies on dehydrogenase activity and fatty acid methyl ester (FAME) showed that applying biochar made from vegetable waste at 200°C enhanced the condition of heavy metal contained soils by boosting the soil microbial population and related microbial functions [170].
In Cu-contaminated soil, biochar generated from chicken manure lowered Cu bioavailability and boosted microbial activity because Cu is stored in metal-tolerant soil microbial structures [171]. Soil contaminated with lead and arsenic in low-temperature biochar can elevate the variety of actinobacteria, fungus, and both Gram-positive and Gram-negative bacteria [172]. Therefore, in polluted soils treated with biochar, microbial activity is significantly impacted by biochar’s capacity to change the organic matter concentration, pH, and accessible nutrients [173]. It is essential to determine how soil microorganisms in polluted soils react to biochar amendments; this reaction is summarized in Table 6.
8 Potential Damage to the Soil Ecosystem Due to Biochar Application
Biochar benefits soil ecosystems; however, it also poses risks associated with its use. Biochar is created through the use of totally or partially anoxic biomass pyrolysis. The most common materials used to make biochar are urban sludge, livestock manure, or crop straw. With the application of biochar, pollutants contained in these materials can seep into the soil medium, thereby endangering the soil’s environment [179].
Furthermore, the proportion of heavy metals in the raw material determines the quantity of heavy metals in biochar. It has been discovered that the temperature during pyrolysis affects the amounts of Cd, Zn, and Pb in biochar [180]. Therefore, it is essential to determine the optimal temperature range for pyrolysis before its application. Consequently, biochar’s volatile organic compounds can damage microorganisms when applied to soil. When producing biochar, pyrolysis temperature may impact the presence of the volatile organic component. Typically, the temperature range for pyrolysis is between 300°C to 600°C. These molecules should volatilize as the temperature rises, and certain semi-volatilized organic compounds may accumulate in the biochar [181]. Because the endogenous source of biochar contains volatile organic compounds (VOCs) that hinder the growth of Bacillus mucilaginosus, this bacterium was found in lower quantities in the soil after biochar was added [182].
The incomplete combustion of biomass produces a category of organic pollutants known as polycyclic aromatic hydrocarbons (PAHs), which are poisonous and harmful. However, under the right pyrolysis circumstances, the quantity of PAHs in the biochar formed would be reduced [183]. As biochar may absorb PAHs, the impact of PAHs on soil microorganisms is often minimal. This is because the most quantity of PAHs that might be present in the soil environment is significantly higher than the effective PAHs concentration in biochar. This explains why soil microorganisms are not greatly harmed by endogenous pollutants in biochar [184]. The pyrolysis of biochar produces additional contaminants, such as volatile organic compounds and peroxide-amino hydrocarbons, as well as novel environmental persistent free radical pollutants. Specific oxygen-containing radicals known as Environmentally Persistent Free Radicals (EPFRs) are produced when biochar is pyrolyzed. These radicals include peroxy radicals, semi-quinone, alkoxy, and hydroxyl, and they transfer electrons to the biochar’s transition metals, like Fe, Ni, and Cu. In systems composed of organic matter and particulate matter, EPFRs maintain a more stable state, producing several EPFRs [185]. Plant roots are harmed by EPFRs, which also induce oxidative stress in plants and prevent seed germination. Furthermore, EPFRs trigger oxidative reactions that harm microbial populations and create oxidative stress in soil microorganisms [185]. Soil microorganisms may be harmed by the EPFRs found in biochar, causing changes in their population dynamics and activity levels. This could hinder plant germination in its early stages [186]. A list of heavy metals, organic pollutants, and other harmful substances with their hazardous effects, which may be present in biochar, are mentioned in Table 7.
The application of biochar in farming presents various advantages that strengthen plant resistance to heat stress alongside supporting essential soil microorganisms. This research generates significant value for sustainable agriculture, especially under dual conditions of climate change and soil degradation.
The physical together with chemical properties of biochar enable it to behave as a protective mechanism that allows plants to withstand heat stress. The porous structure of biochar enhances oxygen circulation while boosting the speed of water uptake, resulting in a powerful defense system against heat stress. Scientific studies prove biochar increases soil moisture levels that enable plants to handle temperature variations [189]. Stomatal conductance and photosynthetic efficiency in plants improve during stressful conditions when biochar supplements are used [190]. Additionally, biochar application leads to improved plant development and stress tolerance, as it enhances the availability of water and nutrients for plants [191]. Furthermore, when biochar integrates with soil, it generates dual effects on plant functioning that foster beneficial microbial communities, thereby maintaining soil quality through nutrient processing. Biochar introduction has proven effective in raising microbial diversity while boosting microbial activity, which strengthens both soil fertility and structure [192].
Moreover, biochar enables beneficial microorganisms to flourish while assisting them in metabolically active behavior that improves soil performance as a plant growing environment [193]. Biochar combined with soil microbial communities can enhance plant growth nutrient supplies, especially nitrogen, through this relationship [194]. Soil resilience against abiotic stresses such as heat and salinity receives added strength through this collaborative effect on plant health [195]. Additionally, the implementation of biochar delivers long-term benefits to soil quality, establishing it as a lasting and sustainable approach to enhancing agricultural production. Biochar enhances soil structure by increasing cation exchange capacity and water retention properties, thereby contributing to improved crop yields [196]. Agricultural development depends heavily on these enhancements because climate change produces progressively severe and frequent weather events. Biochar implementation enhances soil health and plant resistance, offering agricultural operators a compact method to reduce climate change effects [197].
10 Future Perspective and Conclusion
To improve the health of the soil, this review gives a general summary of how biochar contributes to soil biological activity and reduces plant heat stress. In light of the increased interest in the benefits of employing biochar as a nutrient and microbial carrier, as well as a C-based soil amendment, we suggest conducting the following study to enhance biochar’s ability to support soil’s biological health and ecological functions: Examine how biochar affects the development of durable microaggregates in different kinds of soil to assess how important these microaggregates are for housing soil microbial development. Investigate how biochar functions in various soil and environmental situations. Assess the real-world effects of biochar on agricultural productivity and its potential future applications. Highlight its shortcomings and offer potential solutions. Examine how carbon (C) and other nutrients are co-located in biochar produced using multiple feedstock types and consider their importance in enhancing microbial functions and activity. Analyse the presence and evolution of contaminants, including PTEs and PAHs, in biochar-derived sources using various feedstock types. These pollutants may impact the soil’s biological health by preventing microbial development and function. Determine the extent to which biochar has immobilized soil pollution to mitigate microbial development and suppress activity.
Biochar soil amendment enhances water retention capacity, nutrient uptake efficiency, and cation exchange capacity, all of which contribute to improved plant health and productivity. The mechanism underlying the aforementioned potential is that it increases the quantity of organic matter and the root-zone surface area while altering the composition of the microbial community. Biochar is essential for mitigating heat stress and other climate change impacts because it reduces greenhouse gas emissions and promotes methanogen activity, thereby enhancing carbon storage [198]. A thorough analysis of the requirements for biochar should be conducted before application, as plant and soil reactions to biochar depend on its dosage, application technique, context (crop, soil chemistry, environment), pyrolysis temperature, and feedstock. A systematic study is necessary to determine how biochar interacts with various plant species and types of soil in diverse environmental settings.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contributions to the paper as follows: Conceptualization, Prodipto Bishnu Angon, Arpita Rani Roy; validation, Md. Arif Sakil, Prodipto Bishnu Angon, Israt Jahan, Arpita Rani Roy; data curation, Arpita Rani Roy, Israt Jahan, Sharah Jabeen Mou, Md. Farhan Hasin, Rebeka Sultana; writing—original draft preparation, Arpita Rani Roy, Israt Jahan, Sharah Jabeen Mou, Md. Farhan Hasin, Rebeka Sultana, Badhon Mazumder; writing—review and editing, Arpita Rani Roy, Israt Jahan, Sharah Jabeen Mou, Prodipto Bishnu Angon, Md. Arif Sakil; visualization, Prodipto Bishnu Angon, Md. Arif Sakil; supervision, Md. Arif Sakil, Prodipto Bishnu Angon. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Dusenge ME, Duarte AG, Way DA. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist. 2019;221(1):32–49. doi:10.1111/nph.15283. [Google Scholar] [PubMed] [CrossRef]
2. Ortiz-Bobea A, Wang H, Carrillo CM, Ault TR. Unpacking the climatic drivers of US agricultural yields. Environ Res Lett. 2019;14(6):064003. doi:10.1088/1748-9326/ab1e75. [Google Scholar] [CrossRef]
3. Jägermeyr J, Müller C, Ruane AC, Elliott J, Balkovic J, Castillo O, et al. Climate impacts on global agriculture emerge earlier in new generation of climate and crop models. Nature Food. 2021;2(11):873–85. doi:10.1038/s43016-021-00400-y. [Google Scholar] [PubMed] [CrossRef]
4. Xu J, Henry A, Sreenivasulu N. Rice yield formation under high day and night temperatures—a prerequisite to ensure future food security. Plant Cell Environ. 2020;43(7):1595–608. doi:10.1111/pce.13748. [Google Scholar] [PubMed] [CrossRef]
5. Bayranvand M, Akbarinia M, Salehi Jouzani G, Gharechahi J, Kooch Y, Baldrian P. Composition of soil bacterial and fungal communities in relation to vegetation composition and soil characteristics along an altitudinal gradient. FEMS Microbiol Ecol. 2021;97(1):fiaa201. doi:10.1093/femsec/fiaa201. [Google Scholar] [PubMed] [CrossRef]
6. Zhang C, Cai Y, Zhang T, He T, Li J, Li X, et al. Litter removal increases the plant carbon input to soil in a Pinus massoniana plantation. Euro J Res. 2022;141(5):833–43. doi:10.1007/s10342-022-01476-2. [Google Scholar] [CrossRef]
7. Zhang Z, Wang Y, Wang S, Zhao L, Zhang B, Jia W, et al. Effects of antibacterial peptide-producing Bacillus subtilis, gallic acid, and cellulase on fermentation quality and bacterial community of whole-plant corn silage. Front Microbiol. 2022;13:311. doi:10.3389/fmicb.2022.1028001. [Google Scholar] [PubMed] [CrossRef]
8. Wang L, O’Connor D, Rinklebe J, Ok YS, Tsang DCW, Shen Z, et al. Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol. 2020;54(23):14797–814. doi:10.1021/acs.est.0c04033. [Google Scholar] [PubMed] [CrossRef]
9. Layek J, Narzari R, Hazarika S, Das A, Rangappa K, Devi S, et al. Prospects of biochar for sustainable agriculture and carbon sequestration: an overview for Eastern Himalayas. Sustainability. 2022;14(11):6684. doi:10.3390/su14116684. [Google Scholar] [CrossRef]
10. Kocsis T, Ringer M, Biró B. Characteristics and applications of biochar in soil-plant systems: a short review of benefits and potential drawbacks. Appl Sci. 2022;12(8):4051. doi:10.3390/app12084051. [Google Scholar] [CrossRef]
11. Xiao L, Meng F. Evaluating the effect of biochar on salt leaching and nutrient retention of Yellow River Delta soil. Soil Use Manag. 2020;36(4):740–50. doi:10.1111/sum.12638. [Google Scholar] [CrossRef]
12. Joseph S, Cowie AL, Van Zwieten L, Bolan N, Budai A, Buss W, et al. How biochar works, and when it doesn’t: a review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy. 2021;13(11):1731–64. doi:10.1111/gcbb.12885. [Google Scholar] [CrossRef]
13. Huang M, Yin X, Chen J, Cao F. Biochar application mitigates the effect of heat stress on rice (Oryza sativa L.) by regulating the root-zone environment. Front Plant Sci. 2021;12:425. doi:10.3389/fpls.2021.711725. [Google Scholar] [PubMed] [CrossRef]
14. Palansooriya KN, Wong JTF, Hashimoto Y, Huang L, Rinklebe J, Chang SX, et al. Response of microbial communities to biochar-amended soils: a critical review. Biochar. 2019;1(1):3–22. doi:10.1007/s42773-019-00009-2. [Google Scholar] [CrossRef]
15. Pariyar P, Kumari K, Jain MK, Jadhao PS. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci Total Environ. 2020;713(11):136433. doi:10.1016/j.scitotenv.2019.136433. [Google Scholar] [PubMed] [CrossRef]
16. Saputra I, Prijono S, Suntari R. Application of oil palm and cacao waste biochar to improve the chemical properties of an Ultisol of Langsa. Aceh J Degrad Min Lands Manag. 2024;12(1):6637–49. doi:10.15243/jdmlm.2024.121.6637. [Google Scholar] [CrossRef]
17. Tomczyk A, Sokołowska Z, Boguta P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Bio/Technol. 2020;19(1):191–215. doi:10.1007/s11157-020-09523-3. [Google Scholar] [CrossRef]
18. Sakhiya AK, Anand A, Kaushal P. Production, activation, and applications of biochar in recent times. Biochar. 2020;2(3):253–85. doi:10.1007/s42773-020-00047-1. [Google Scholar] [CrossRef]
19. Gabhane JW, Bhange VP, Patil PD, Bankar ST, Kumar S. Recent trends in biochar production methods and its application as a soil health conditioner: a review. SN Appl Sci. 2020;2(7):1307. doi:10.1007/s42452-020-3121-5. [Google Scholar] [CrossRef]
20. Su K, Qin Q, Yang J, Li L, Deng S. Recent advance on torrefaction valorization and application of biochar from agricultural waste for soil remediation. J Renew Mater. 2022;10(2):247–61. doi:10.32604/jrm.2022.018146. [Google Scholar] [CrossRef]
21. Mayilswamy N, Nighojkar A, Edirisinghe M, Sundaram S, Kandasubramanian B. Sludge-derived biochar: physicochemical characteristics for environmental remediation. Appl Phys Rev. 2023;10(3):031308. doi:10.1063/5.0137651. [Google Scholar] [CrossRef]
22. Parthasarathy P, Al-Ansari T, Mackey HR, Sheeba Narayanan K, McKay G. A review on prominent animal and municipal wastes as potential feedstocks for solar pyrolysis for biochar production. Fuel. 2022;316:123378. doi:10.1016/j.fuel.2022.123378. [Google Scholar] [CrossRef]
23. Millati R, Cahyono RB, Ariyanto T, Azzahrani IN, Putri RU, Taherzadeh MJ. Chapter 1—agricultural, industrial, municipal, and forest wastes: an overview. In: Taherzadeh MJ, Bolton K, Wong J, Pandey A, editors. Sustainable resource recovery and zero waste. Approaches: Elsevier; 2019. p. 1–22. doi:10.1016/B978-0-444-64200-4.00001-3. [Google Scholar] [CrossRef]
24. Mishra S, Spaccarotella K, Gido J, Samanta I, Chowdhary G. Effects of heat stress on plant-nutrient relations: an update on nutrient uptake, transport, and assimilation. Int J Mol Sci. 2023;24(21):15670. doi:10.3390/ijms242115670. [Google Scholar] [PubMed] [CrossRef]
25. Parker LE, McElrone AJ, Ostoja SM, Forrestel EJ. Extreme heat effects on perennial crops and strategies for sustaining future production. Plant Sci. 2020;295(80):110397. doi:10.1016/j.plantsci.2019.110397. [Google Scholar] [PubMed] [CrossRef]
26. Tiwari M, Kumar R, Min D, Jagadish SVK. Genetic and molecular mechanisms underlying root architecture and function under heat stress—a hidden story. Plant, Cell Environ. 2022;45(3):771–88. doi:10.1111/pce.14266. [Google Scholar] [PubMed] [CrossRef]
27. Jagadish SVK. Heat stress during flowering in cereals-effects and adaptation strategies. New Phytol. 2020;226(6):1567–72. doi:10.1111/nph.16429. [Google Scholar] [PubMed] [CrossRef]
28. Djanaguiraman M, Narayanan S, Erdayani E, Prasad PVV. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020;20(1):268. doi:10.1186/s12870-020-02479-0. [Google Scholar] [PubMed] [CrossRef]
29. Begcy K, Sandhu J, Walia H. Transient heat stress during early seed development primes germination and seedling establishment in rice. Front Plant Sci. 2018;9:1768. doi:10.3389/fpls.2018.01768. [Google Scholar] [PubMed] [CrossRef]
30. Balla K, Karsai I, Bónis P, Kiss T, Berki Z, Horváth Á, et al. Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS One. 2019;14(9):e0222639. doi:10.1371/journal.pone.0222639. [Google Scholar] [PubMed] [CrossRef]
31. Poudel PB, Poudel MR. Heat stress effects and tolerance in wheat: a review. J Biol Today’s World. 2020;9(3):1–6. [Google Scholar]
32. Begcy K, Nosenko T, Zhou L-Z, Fragner L, Weckwerth W, Dresselhaus T. Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiol. 2019;181(2):683–700. doi:10.1104/pp.19.00707. [Google Scholar] [PubMed] [CrossRef]
33. Chiluwal A, Bheemanahalli R, Kanaganahalli V, Boyle D, Perumal R, Pokharel M, et al. Deterioration of ovary plays a key role in heat stress-induced spikelet sterility in sorghum. Plant Cell Environ. 2020;43(2):448–62. doi:10.1111/pce.13673. [Google Scholar] [PubMed] [CrossRef]
34. Bheemanahalli R, Sunoj VSJ, Saripalli G, Prasad PVV, Balyan HS, Gupta PK, et al. Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci. 2019;59(2):684–96. doi:10.2135/cropsci2018.05.0292. [Google Scholar] [CrossRef]
35. Ribeiro C, Hennen-Bierwagen TA, Myers AM, Cline K, Settles AM. Engineering 6-phosphogluconate dehydrogenase improves grain yield in heat-stressed maize. Proc Natl Acad Sci. 2020;117(52):33177–85. doi:10.1073/pnas.2010179117. [Google Scholar] [PubMed] [CrossRef]
36. Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot. 2016;67(18):5381–90. doi:10.1093/jxb/erw299. [Google Scholar] [PubMed] [CrossRef]
37. Wang X, Hou L, Lu Y, Wu B, Gong X, Liu M, et al. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J Exp Bot. 2018;69(22):5531–45. doi:10.1093/jxb/ery303. [Google Scholar] [PubMed] [CrossRef]
38. Raza A. Metabolomics: a systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2022;41(3):741–63. doi:10.1007/s00299-020-02635-8. [Google Scholar] [PubMed] [CrossRef]
39. Hu S, Ding Y, Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci. 2020;11:141. doi:10.3389/fpls.2020.00375. [Google Scholar] [PubMed] [CrossRef]
40. Wang Q, Awasthi MK, Ren X, Zhao J, Li R, Wang Z, et al. Combining biochar, zeolite and wood vinegar for composting of pig manure: the effect on greenhouse gas emission and nitrogen conservation. Waste Manag. 2018;74:221–30. doi:10.1016/j.wasman.2018.01.015. [Google Scholar] [PubMed] [CrossRef]
41. Fatma M, Iqbal N, Sehar Z, Alyemeni MN, Kaushik P, Khan NA, et al. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants. 2021;10(8):1216. doi:10.3390/antiox10081216. [Google Scholar] [PubMed] [CrossRef]
42. Mustafa T, Sattar A, Sher A, Ul-Allah S, Ijaz M, Irfan M, et al. Exogenous application of silicon improves the performance of wheat under terminal heat stress by triggering physio-biochemical mechanisms. Sci Rep. 2021;11(1):23170. doi:10.1038/s41598-021-02594-4. [Google Scholar] [PubMed] [CrossRef]
43. Distéfano AM, Martin MV, Córdoba JP, Bellido AM, D’Ippólito S, Colman SL, et al. Heat stress induces ferroptosis-like cell death in plants. J Cell Biol. 2017;216(2):463–76. doi:10.1083/jcb.201605110. [Google Scholar] [PubMed] [CrossRef]
44. Iqbal N, Umar S, Khan NA, Corpas FJ. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants. 2021;10(1):108. doi:10.3390/antiox10010108. [Google Scholar] [PubMed] [CrossRef]
45. Singh A, Kumar A, Yadav S, Singh IK. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene. 2019;18(14):100173. doi:10.1016/j.plgene.2019.100173. [Google Scholar] [CrossRef]
46. Zhao Y, Du H, Wang Y, Wang H, Yang S, Li C, et al. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J Integr Plant Biol. 2021;63(3):510–27. doi:10.1111/jipb.13056. [Google Scholar] [PubMed] [CrossRef]
47. Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22(1):53–65. doi:10.1016/j.tplants.2016.08.015. [Google Scholar] [PubMed] [CrossRef]
48. Choudhary A, Kumar A, Kaur N. ROS and oxidative burst: roots in plant development. Plant Divers. 2020;42(1):33–43. doi:10.1016/j.pld.2019.10.002. [Google Scholar] [PubMed] [CrossRef]
49. Chen H, Awasthi SK, Liu T, Duan Y, Ren X, Zhang Z, et al. Effects of microbial culture and chicken manure biochar on compost maturity and greenhouse gas emissions during chicken manure composting. J Hazard Mater. 2020;389:121908. doi:10.1016/j.jhazmat.2019.121908. [Google Scholar] [PubMed] [CrossRef]
50. Wang Q, Serban AJ, Wachter RM, Moerner WE. Single-molecule diffusometry reveals the nucleotide-dependent oligomerization pathways of Nicotiana tabacum Rubisco activase. J Chem Phys. 2018;148(12):123319. doi:10.1063/1.5005930. [Google Scholar] [PubMed] [CrossRef]
51. Li N, Euring D, Cha JY, Lin Z, Lu M, Huang L-J, et al. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front Plant Sci. 2021;11:627969. doi:10.3389/fpls.2020.627969. [Google Scholar] [PubMed] [CrossRef]
52. He X, Guo S, Wang Y, Wang L, Shu S, Sun J. Systematic identification and analysis of heat-stress-responsive lncRNAs, circRNAs and miRNAs with associated co-expression and ceRNA networks in cucumber (Cucumis sativus L.). Physiol Plant. 2020;168(3):736–54. doi:10.1111/ppl.12997. [Google Scholar] [PubMed] [CrossRef]
53. Aldon D, Mbengue M, Mazars C, Galaud J-P. Calcium signalling in plant biotic interactions. Int J Mol Sci. 2018;19(3):665. doi:10.3390/ijms19030665. [Google Scholar] [PubMed] [CrossRef]
54. Zhu X, Chen L, Kong X, Bao S, Wu S, Fang L, et al. Biochar alters the morphology of plant roots to enable optimized and reduced nitrogen fertilizer applications. Plant Soil. 2023;492(1):655–73. doi:10.1007/s11104-023-06154-9. [Google Scholar] [CrossRef]
55. Tikoria R, Kumar D, Sharma R, Parkirti P, Jasrotia S, Chowdhary AB, et al. Insights into the role of biochar as potential agent in the management of disease caused by phytopathogens: a review. J Soil Sci Plant Nutr. 2023;23(4):4856–85. doi:10.1007/s42729-023-01489-9. [Google Scholar] [CrossRef]
56. Cong M, Hu Y, Sun X, Yan H, Yu G, Tang G, et al. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front Plant Sci. 2023;14:1172425. doi:10.3389/fpls.2023.1172425. [Google Scholar] [PubMed] [CrossRef]
57. Zulfiqar B, Raza MAS, Saleem MF, Aslam MU, Iqbal R, Muhammad F, et al. Biochar enhances wheat crop productivity by mitigating the effects of drought: insights into physiological and antioxidant defense mechanisms. PLoS One. 2022;17(4):e0267819. doi:10.1371/journal.pone.0267819. [Google Scholar] [PubMed] [CrossRef]
58. Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ, et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem. 2016;103(6):191–8. doi:10.1016/j.plaphy.2016.03.001. [Google Scholar] [PubMed] [CrossRef]
59. Alharbi K, Khan AA, Sakit Alhaithloul HA, Al-Harbi NA, Al-Qahtani SM, Aloufi SS, et al. Synergistic effect of β-sitosterol and biochar application for improving plant growth of Thymus vulgaris under heat stress. Chemosphere. 2023;340:139832. doi:10.1016/j.chemosphere.2023.139832. [Google Scholar] [PubMed] [CrossRef]
60. Riaz M, Arif MS, Hussain Q, Khan SA, Tauqeer HM, Yasmeen T, et al. Application of biochar for the mitigation of abiotic stress-induced damages in plants. In: Plant tolerance to environmental. Stress: CRC Press; 2019. p. 285–304. doi:10.1201/9780203705315-18. [Google Scholar] [CrossRef]
61. Deng B, Bada B, Tammeorg P, Helenius J, Luukkanen O, Starr M. Drought stress and Acacia seyal biochar effects on sorghum gas exchange and yield: a greenhouse experiment. Agric Nat Resour. 2019;53(6):573–80. [Google Scholar]
62. Sousa B, Soares C, Sousa F, Martins M, Mateus P, Rodrigues F, et al. Enhancing tomato plants’ tolerance to combined heat and salt stress—the role of arbuscular mycorrhizae and biochar. Sci Total Environ. 2024;948:174860. doi:10.1016/j.scitotenv.2024.174860. [Google Scholar] [PubMed] [CrossRef]
63. Kumar A, Friedman H, Tsechansky L, Graber ER. Distinctive in-planta acclimation responses to basal growth and acute heat stress were induced in Arabidopsis by cattle manure biochar. Sci Rep. 2021;11(1):9875. doi:10.1038/s41598-021-88856-7. [Google Scholar] [PubMed] [CrossRef]
64. Anwari G, Feng J, Alio Moussa A. Multiple beneficial effects of using biochar (as a Great Organic Material) on tolerance and productivity of rice under abiotic stress. J Mod Mat. 2019;6(1):40–51. doi:10.21467/jmm.6.1.40-51. [Google Scholar] [CrossRef]
65. Murtaza G, Ahmed Z, Eldin SM, Ali B, Bawazeer S, Usman M, et al. Biochar-soil-plant interactions: aa cross talk for sustainable agriculture under changing climate. Front Environ Sci. 2023;11:1059449. doi:10.3389/fenvs.2023.1059449. [Google Scholar] [CrossRef]
66. Zhang Y, Ding J, Wang H, Su L, Zhao C. Biochar addition alleviate the negative effects of drought and salinity stress on soybean productivity and water use efficiency. BMC Plant Biol. 2020;20(1):288. doi:10.1186/s12870-020-02493-2. [Google Scholar] [PubMed] [CrossRef]
67. Liu J, Zhang W, Pang J, Qi J, Lu Y, Yu M, et al. Biochar’s dual impact on soil acidity management and crop yield enhancement: a meta-analysis. 2024;214:25. doi:10.21203/rs.3.rs-4128294/v1. [Google Scholar] [CrossRef]
68. Trupiano D, Cocozza C, Baronti S, Amendola C, Vaccari FP, Lustrato G, et al. The Effects of biochar and its combination with compost on lettuce (Lactuca sativa L.) growth, soil properties, and soil microbial activity and abundance. Int J Agron. 2017;2017(1):3158207. doi:10.1155/2017/3158207. [Google Scholar] [CrossRef]
69. Fidel RB, Laird DA, Parkin TB. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Syst. 2019;3(1):8. doi:10.3390/soilsystems3010008. [Google Scholar] [CrossRef]
70. Mukherjee A, Lal R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy. 2013;3(2):313–39. doi:10.3390/agronomy3020313. [Google Scholar] [CrossRef]
71. Devika OS, Choudhary S, Rani M, Yamini S. Importance of bio-char in climate change mitigation and stress crop production. Magnesium. 2019;4(3):5–8. [Google Scholar]
72. Srinivasarao C, Gopinath K, Venkatesh G, Dubey A, Wakudkar H, Purakayastha T, et al. Use of biochar for soil health management and greenhouse gas mitigation in India: potential and constraints. Hyderabad: The Director, Central Research Institute for Dryland Agriculture; 2013. [Google Scholar]
73. Awasthi MK, Duan Y, Awasthi SK, Liu T, Zhang Z. Influence of bamboo biochar on mitigating greenhouse gas emissions and nitrogen loss during poultry manure composting. Bioresour Technol. 2020;303:122952. doi:10.1016/j.biortech.2020.122952. [Google Scholar] [PubMed] [CrossRef]
74. Chen W, Liao X, Wu Y, Liang JB, Mi J, Huang J, et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017;61:506–15. doi:10.1016/j.wasman.2017.01.014. [Google Scholar] [PubMed] [CrossRef]
75. Agyarko-Mintah E, Cowie A, Singh BP, Joseph S, Van Zwieten L, Cowie A, et al. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017;61(3):138–49. doi:10.1016/j.wasman.2016.11.027. [Google Scholar] [PubMed] [CrossRef]
76. Chowdhury MA, de Neergaard A, Jensen LS. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere. 2014;97:16–25. doi:10.1016/j.chemosphere.2013.10.030. [Google Scholar] [PubMed] [CrossRef]
77. Vandecasteele B, Sinicco T, D’Hose T, Vanden Nest T, Mondini C. Biochar amendment before or after composting affects compost quality and N losses, but not P plant uptake. J Environ Manag. 2016;168:200–9. doi:10.1016/j.jenvman.2015.11.045. [Google Scholar] [PubMed] [CrossRef]
78. He X, Yin H, Han L, Cui R, Fang C, Huang G. Effects of biochar size and type on gaseous emissions during pig manure/wheat straw aerobic composting: insights into multivariate-microscale characterization and microbial mechanism. Bioresour Technol. 2019;271(1):375–82. doi:10.1016/j.biortech.2018.09.104. [Google Scholar] [PubMed] [CrossRef]
79. Harter J, Guzman-Bustamante I, Kuehfuss S, Ruser R, Well R, Spott O, et al. Gas entrapment and microbial N2O reduction reduce N2O emissions from a biochar-amended sandy clay loam soil. Sci Rep. 2016;6(1):39574. doi:10.1038/srep39574. [Google Scholar] [PubMed] [CrossRef]
80. Yin Y, Yang C, Gu J, Wang X, Zheng W, Wang R, et al. Roles of nxrA-like oxidizers and nirS-like reducers in nitrite conversion during swine manure composting. Bioresour Technol. 2020;297:122426. doi:10.1016/j.biortech.2019.122426. [Google Scholar] [PubMed] [CrossRef]
81. Mankasingh U, Choi P-C, Ragnarsdottir V. Biochar application in a tropical, agricultural region: a plot scale study in Tamil Nadu. India Appl Geochem. 2011;26:S218–21. doi:10.1016/j.apgeochem.2011.03.108. [Google Scholar] [CrossRef]
82. Case SDC, McNamara NP, Reay DS, Whitaker J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil—the role of soil aeration. Soil Biol Biochem. 2012;51:125–34. doi:10.1016/j.soilbio.2012.03.017. [Google Scholar] [CrossRef]
83. Mao H, Lv Z, Sun H, Li R, Zhai B, Wang Z, et al. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour Technol. 2018;258:195–202. doi:10.1016/j.biortech.2018.02.082. [Google Scholar] [PubMed] [CrossRef]
84. Liu N, Zhou J, Han L, Ma S, Sun X, Huang G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour Technol. 2017;241(2):190–9. doi:10.1016/j.biortech.2017.03.144. [Google Scholar] [PubMed] [CrossRef]
85. Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, et al. Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem. 2017;106:28–35. doi:10.1016/j.soilbio.2016.12.006. [Google Scholar] [CrossRef]
86. Mitchell PJ, Simpson AJ, Soong R, Simpson MJ. Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol Biochem. 2015;81:244–54. doi:10.1016/j.soilbio.2014.11.017. [Google Scholar] [CrossRef]
87. Hawthorne I, Johnson MS, Jassal RS, Black TA, Grant NJ, Smukler SM. Application of biochar and nitrogen influences fluxes of CO2, CH4 and N2O in a forest soil. J Environ Manag. 2017;192(4):203–14. doi:10.1016/j.jenvman.2016.12.066. [Google Scholar] [PubMed] [CrossRef]
88. Li Y, Hu S, Chen J, Müller K, Li Y, Fu W, et al. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J Soils Sediments. 2018;18(2):546–63. doi:10.1007/s11368-017-1906-y. [Google Scholar] [CrossRef]
89. Brassard P, Godbout S, Raghavan V. Soil biochar amendment as a climate change mitigation tool: key parameters and mechanisms involved. J Environ Manag. 2016;181(4):484–97. doi:10.1016/j.jenvman.2016.06.063. [Google Scholar] [PubMed] [CrossRef]
90. Qi L, Ma Z, Chang SX, Zhou P, Huang R, Wang Y, et al. Biochar decreases methanogenic archaea abundance and methane emissions in a flooded paddy soil. Sci Total Environ. 2021;752:141958. doi:10.1016/j.scitotenv.2020.141958. [Google Scholar] [PubMed] [CrossRef]
91. Sriphirom P, Amnat C, Kazuyuki Y, Sudarut T, Nimaradee B, Towprayoon S. Effects of biochar on methane emission, grain yield, and soil in rice cultivation in Thailand. Carbon Manag. 2021;12(2):109–21. doi:10.1080/17583004.2021.1885257. [Google Scholar] [CrossRef]
92. Jia X, Yan W, Ma H, Shangguan Z. Antagonistic and synergistic interactions dominate GHGs fluxes, soil properties and yield responses to biochar and N addition. Front Environ Sci. 2023;11:1123897. doi:10.3389/fenvs.2023.1123897. [Google Scholar] [CrossRef]
93. Du Z-L, Zhao J-K, Wang Y-D, Zhang Q-Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J Soils Sediments. 2017;17(3):581–9. doi:10.1007/s11368-015-1349-2. [Google Scholar] [CrossRef]
94. Ye-Yang C, Liu Z-H, Zhou D, Wu C, Su J, Luo X-Y. Effect of high temperatures (100°C–600°C) on the soil particle composition and its micro-mechanisms. Eurasian Soil Sci. 2021;54(10):1599–607. doi:10.1134/S1064229321100045. [Google Scholar] [CrossRef]
95. Prats SA, Merino A, Gonzalez-Perez JA, Verheijen FGA, De la Rosa JM. Can straw-biochar mulching mitigate erosion of wildfire-degraded soils under extreme rainfall? Sci Total Environ. 2021;761(6130):143219. doi:10.1016/j.scitotenv.2020.143219. [Google Scholar] [PubMed] [CrossRef]
96. Dong X, Liu S, Yu Y. The variation mechanism of thermal properties of loess with different water contents during freezing. Adv Civil Eng. 2021;2021(1):9990051. doi:10.1155/2021/9990051. [Google Scholar] [CrossRef]
97. Usowicz B, Lipiec J, Łukowski M, Bis Z, Usowicz J, Latawiec AE. Impact of biochar addition on soil thermal properties: modelling approach. Geoderma. 2020;376(8476):114574. doi:10.1016/j.geoderma.2020.114574. [Google Scholar] [CrossRef]
98. Lopes ÉMG, Reis MM, Frazão LA, da Mata Terra LE, Lopes EF, dos Santos MM, et al. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environl Technol Innov. 2021;21:101270. doi:10.1016/j.eti.2020.101270. [Google Scholar] [CrossRef]
99. Taskin E, Branà MT, Altomare C, Loffredo E. Biochar and hydrochar from waste biomass promote the growth and enzyme activity of soil-resident ligninolytic fungi. Heliyon. 2019;5(7):e02051. doi:10.1016/j.heliyon.2019.e02051. [Google Scholar] [PubMed] [CrossRef]
100. Kätterer T, Roobroeck D, Andrén O, Kimutai G, Karltun E, Kirchmann H, et al. Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. Field Crops Res. 2019;235:18–26. doi:10.1016/j.fcr.2019.02.015. [Google Scholar] [CrossRef]
101. Mandal S, Sarkar B, Bolan N, Ok YS, Naidu R. Enhancement of chromate reduction in soils by surface modified biochar. J Environ Manag. 2017;186:277–84. doi:10.1016/j.jenvman.2016.05.034. [Google Scholar] [PubMed] [CrossRef]
102. Yang T, Xu Y, Huang Q, Sun Y, Liang X, Wang L, et al. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ Pollut. 2021;291(1):118129. doi:10.1016/j.envpol.2021.118129. [Google Scholar] [PubMed] [CrossRef]
103. Duan M, Liu G, Zhou B, Chen X, Wang Q, Zhu H, et al. Effects of modified biochar on water and salt distribution and water-stable macro-aggregates in saline-alkaline soil. J Soils Sediments. 2021;21(6):2192–202. doi:10.1007/s11368-021-02913-2. [Google Scholar] [CrossRef]
104. Liu H, Xu F, Xie Y, Wang C, Zhang A, Li L, et al. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci Total Environ. 2018;645:702–9. doi:10.1016/j.scitotenv.2018.07.115. [Google Scholar] [PubMed] [CrossRef]
105. Wen E, Yang X, Chen H, Shaheen SM, Sarkar B, Xu S, et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J Hazard Mater. 2021;407(5):124344. doi:10.1016/j.jhazmat.2020.124344. [Google Scholar] [PubMed] [CrossRef]
106. Wu L, Wei C, Zhang S, Wang Y, Kuzyakov Y, Ding X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J Clean Prod. 2019;235(11):901–9. doi:10.1016/j.jclepro.2019.07.043. [Google Scholar] [CrossRef]
107. Zhen M, Tang J, Li C, Sun H. Rhamnolipid-modified biochar-enhanced bioremediation of crude oil-contaminated soil and mediated regulation of greenhouse gas emission in soil. J Soils Sediments. 2021;21(1):123–33. doi:10.1007/s11368-020-02746-5. [Google Scholar] [CrossRef]
108. Martinsen V, Alling V, Nurida NL, Mulder J, Halse SE, Rita C, et al. pH effects of the addition of three biochars to acidic Indonesian mineral soils. Soil Sci Plant Nutr. 2015;61(5):821–34. doi:10.1080/00380768.2015.1052985. [Google Scholar] [CrossRef]
109. O’Connor D, Peng T, Li G, Wang S, Duan L, Mulder J, et al. Sulfur-modified rice husk biochar: a green method for the remediation of mercury contaminated soil. Sci Total Environ. 2018;621(9):819–26. doi:10.1016/j.scitotenv.2017.11.213. [Google Scholar] [PubMed] [CrossRef]
110. Badr EA, Ibrahim O, Tawfik M, Bahr AA. Management strategy for improving the productivity of wheat in newly reclaimed sandy soil. Management. 2015;8(4):1438–45. [Google Scholar]
111. Villagra-Mendoza K, Horn R. Effect of biochar addition on hydraulic functions of two textural soils. Geoderma. 2018;326:88–95. doi:10.1016/j.geoderma.2018.03.021. [Google Scholar] [CrossRef]
112. Santos RV, Mendes MAA, Alexandre C, Carrott MR, Rodrigues A, Ferreira AF. Assessment of biomass and biochar of maritime pine as a porous medium for water retention in soils. Energies. 2022;15(16):5882. doi:10.3390/en15165882. [Google Scholar] [CrossRef]
113. Baiamonte G, Crescimanno G, Parrino F, De Pasquale C. Effect of biochar on the physical and structural properties of a sandy soil. CATENA. 2019;175(3):294–303. doi:10.1016/j.catena.2018.12.019. [Google Scholar] [CrossRef]
114. Batool A, Taj S, Rashid A, Khalid A, Qadeer S, Saleem AR, et al. Potential of soil amendments (Biochar and Gypsum) in increasing water use efficiency of Abelmoschus esculentus L. Moench. Front Plant Sci. 2015;6:733. doi:10.3389/fpls.2015.00733. [Google Scholar] [PubMed] [CrossRef]
115. Yu O-Y, Raichle B, Sink S. Impact of biochar on the water holding capacity of loamy sand soil. Int J Energy Environ Eng. 2013;4(1):44. doi:10.1186/2251-6832-4-44. [Google Scholar] [CrossRef]
116. Wu Q, Zhang J, Liu X, Chang T, Wang Q, Shaghaleh H, et al. Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front Environ Sci. 2023;10:2399. doi:10.3389/fenvs.2022.1060277. [Google Scholar] [CrossRef]
117. Chang Y, Rossi L, Zotarelli L, Gao B, Shahid MA, Sarkhosh A. Biochar improves soil physical characteristics and strengthens root architecture in Muscadine grape (Vitis rotundifolia L.). Chem Biol Technol Agric. 2021;8(1):7. doi:10.1186/s40538-020-00204-5. [Google Scholar] [CrossRef]
118. Hassan M, Liu Y, Naidu R, Parikh SJ, Du J, Qi F, et al. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: a meta-analysis. Sci Total Environ. 2020;744:140714. doi:10.1016/j.scitotenv.2020.140714. [Google Scholar] [PubMed] [CrossRef]
119. Juriga M, Šimanský V, Horák J, Kondrlová E, Igaz D, Polláková N, et al. The effect of different rates of biochar and biochar in combination with N fertilizer on the parameters of soil organic matter and soil structure. J Ecol Eng. 2018;19(6):153–61. doi:10.12911/22998993/92894. [Google Scholar] [CrossRef]
120. Feng Z, Fan Z, Song H, Li K, Lu H, Liu Y, et al. Biochar induced changes of soil dissolved organic matter: the release and adsorption of dissolved organic matter by biochar and soil. Sci Total Environ. 2021;783(1):147091. doi:10.1016/j.scitotenv.2021.147091. [Google Scholar] [PubMed] [CrossRef]
121. Zhang A, Zhou X, Li M, Wu H. Impacts of biochar addition on soil dissolved organic matter characteristics in a wheat-maize rotation system in Loess Plateau of China. Chemosphere. 2017;186:986–93. doi:10.1016/j.chemosphere.2017.08.074. [Google Scholar] [PubMed] [CrossRef]
122. Dong X, Singh BP, Li G, Lin Q, Zhao X. Biochar has little effect on soil dissolved organic carbon pool 5 years after biochar application under field condition. Soil Use Manag. 2019;35(3):466–77. doi:10.1111/sum.12474. [Google Scholar] [CrossRef]
123. Basso AS, Miguez FE, Laird DA, Horton R, Westgate M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy. 2013;5(2):132–43. doi:10.1111/gcbb.12026. [Google Scholar] [CrossRef]
124. Prober SM, Stol J, Piper M, Gupta VVSR, Cunningham SA. Enhancing soil biophysical condition for climate-resilient restoration in mesic woodlands. Ecol Eng. 2014;71:246–55. doi:10.1016/j.ecoleng.2014.07.019. [Google Scholar] [CrossRef]
125. Blanco-Canqui H. Biochar and soil physical properties. Soil Sci Soc Am J. 2017;81(4):687–711. doi:10.2136/sssaj2017.01.0017. [Google Scholar] [CrossRef]
126. Toková L, Igaz D, Horák J, Aydin E. Effect of biochar application and re-application on soil bulk density, porosity, saturated hydraulic conductivity, water content and soil water availability in a silty loam haplic luvisol. Agronomy. 2020;10(7):1005. doi:10.3390/agronomy10071005. [Google Scholar] [CrossRef]
127. Pandey V, Yadav R, Khare P. Adding mineral-enriched biochar to the rhizosphere reduces heavy metal toxicity on plants and soil microbes. J Environ Chem Eng. 2024;12(5):113972. doi:10.1016/j.jece.2024.113972. [Google Scholar] [CrossRef]
128. Mohamed BA, Ellis N, Kim CS, Bi X. The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ Pollut. 2017;230:329–38. doi:10.1016/j.envpol.2017.06.075. [Google Scholar] [PubMed] [CrossRef]
129. Tang H, Wang S, Liu Y, Umair Hassan M, Song Y, Huang G, et al. Biochar: a promising soil amendment to mitigate heavy metals toxicity in plants. Not Bot Horti Agrobo. 2022;50(3):12778. doi:10.15835/nbha50312778. [Google Scholar] [CrossRef]
130. Albert HA, Li X, Jeyakumar P, Wei L, Huang L, Huang Q, et al. Influence of biochar and soil properties on soil and plant tissue concentrations of Cd and Pb: a meta-analysis. Sci Total Environ. 2021;755:142582. doi:10.1016/j.scitotenv.2020.142582. [Google Scholar] [PubMed] [CrossRef]
131. Wu D, Peng W, La Bao, Yu X, Dong X, Lai M, et al. Biochar alleviating heavy metals phytotoxicity in sludge-amended soil varies with plant adaptability. Environ Res. 2022;215(1–2):114248. doi:10.1016/j.envres.2022.114248. [Google Scholar] [PubMed] [CrossRef]
132. Tan Z, Wang Y, Zhang L, Huang Q. Study of the mechanism of remediation of Cd-contaminated soil by novel biochars. Environ Sci Pollut Res. 2017;24(32):24844–55. doi:10.1007/s11356-017-0109-9. [Google Scholar] [PubMed] [CrossRef]
133. Liang M, Lu L, He H, Li J, Zhu Z, Zhu Y. Applications of biochar and modified biochar in heavy metal contaminated soil: a descriptive review. Sustainability. 2021;13(24):14041. doi:10.3390/su132414041. [Google Scholar] [CrossRef]
134. Mazhar R, Ilyas N, Arshad M, Khalid A. Amelioration potential of biochar for chromium stress in wheat. Pak J Bot. 2020;52(4):1159–68. doi:10.30848/PJB2020-4(19). [Google Scholar] [CrossRef]
135. Naveed M, Tanvir B, Xiukang W, Brtnicky M, Ditta A, Kucerik J, et al. Co-composted biochar enhances growth, physiological, and phytostabilization efficiency of Brassica napus and reduces associated health risks under chromium stress. Front Plant Sci. 2021;12:775785. doi:10.3389/fpls.2021.775785. [Google Scholar] [PubMed] [CrossRef]
136. Huff MD, Kumar S, Lee JW. Comparative analysis of pinewood, peanut shell, and bamboo biomass derived biochars produced via hydrothermal conversion and pyrolysis. J Environ Manag. 2014;146(3):303–8. doi:10.1016/j.jenvman.2014.07.016. [Google Scholar] [PubMed] [CrossRef]
137. Li Y, Zhou C, Qiu Y, Tigabu M, Ma X. Effects of biochar and litter on carbon and nitrogen mineralization and soil microbial community structure in a China fir plantation. J For Res. 2019;30(5):1913–23. doi:10.1007/s11676-018-0731-5. [Google Scholar] [CrossRef]
138. Wong JTF, Chen Z, Chen X, Ng CWW, Wong MH. Soil-water retention behavior of compacted biochar-amended clay: a novel landfill final cover material. J Soils Sediments. 2017;17(3):590–8. doi:10.1007/s11368-016-1401-x. [Google Scholar] [CrossRef]
139. Xu N, Tan G, Wang H, Gai X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol. 2016;74:1–8. doi:10.1016/j.ejsobi.2016.02.004. [Google Scholar] [CrossRef]
140. Luo S, Wang S, Tian L, Li S, Li X, Shen Y, et al. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl Soil Ecol. 2017;117–118(5):10–5. doi:10.1016/j.apsoil.2017.04.024. [Google Scholar] [CrossRef]
141. Abujabhah IS, Doyle RB, Bound SA, Bowman JP. Assessment of bacterial community composition, methanotrophic and nitrogen-cycling bacteria in three soils with different biochar application rates. J Soils Sediments. 2018;18(1):148–58. doi:10.1007/s11368-017-1733-1. [Google Scholar] [CrossRef]
142. Ren H, Guo H, Shafiqul Islam M, Zaki HEM, Wang Z, Wang H, et al. Improvement effect of biochar on soil microbial community structure and metabolites of decline disease bayberry. Front Microbiol. 2023;14:1154886. doi:10.3389/fmicb.2023.1154886. [Google Scholar] [PubMed] [CrossRef]
143. Chen Y, Liu Y, Li Y, Wu Y, Chen Y, Zeng G, et al. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour Technol. 2017;243(3):347–55. doi:10.1016/j.biortech.2017.06.100. [Google Scholar] [PubMed] [CrossRef]
144. Zhang J, Shen J-L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann Microbiol. 2022;72(1):35. doi:10.1186/s13213-022-01689-1. [Google Scholar] [CrossRef]
145. Dominchin MF, Verdenelli RA, Berger MG, Aoki A, Meriles JM. Impact of N-fertilization and peanut shell biochar on soil microbial community structure and enzyme activities in a Typic Haplustoll under different management practices. Eur J Soil Biol. 2021;104:103298. doi:10.1016/j.ejsobi.2021.103298. [Google Scholar] [CrossRef]
146. Yin D, Li H, Wang H, Guo X, Wang Z, Lv Y, et al. Impact of different biochars on microbial community structure in the rhizospheric soil of rice grown in albic soil. Molecules. 2021;26(16):4783. doi:10.3390/molecules26164783. [Google Scholar] [PubMed] [CrossRef]
147. Liu S-J, Liu Y-G, Tan X-F, Zeng G-M, Zhou Y-H, Liu S-B, et al. The effect of several activated biochars on Cd immobilization and microbial community composition during in-situ remediation of heavy metal contaminated sediment. Chemosphere. 2018;208:655–64. doi:10.1016/j.chemosphere.2018.06.023. [Google Scholar] [PubMed] [CrossRef]
148. Zhu X, Mao L, Chen B. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ Int. 2019;133:105211. doi:10.1016/j.envint.2019.105211. [Google Scholar] [PubMed] [CrossRef]
149. Yang C, Liu J, Ying H, Lu S. Soil pore structure changes induced by biochar affect microbial diversity and community structure in an Ultisol. Soil Tillage Res. 2022;224(1):105505. doi:10.1016/j.still.2022.105505. [Google Scholar] [CrossRef]
150. Feng Y, Hu X, Guan Y, Chu Z, Du X, Xie Y, et al. Regulatory effects of different biochar on soil properties and microbial community structure in chrysanthemum continuous cropping soil. Agronomy. 2024;14(9):2034. doi:10.3390/agronomy14092034. [Google Scholar] [CrossRef]
151. Awad YM, Ok YS, Abrigata J, Beiyuan J, Beckers F, Tsang DCW, et al. Pine sawdust biomass and biochars at different pyrolysis temperatures change soil redox processes. Sci Total Environ. 2018;625:147–54. doi:10.1016/j.scitotenv.2017.12.194. [Google Scholar] [PubMed] [CrossRef]
152. Zhou Z, Gao T, Van Zwieten L, Zhu Q, Yan T, Xue J, et al. Soil microbial community structure shifts induced by biochar and biochar-based fertilizer amendment to karst calcareous soil. Soil Sci Soc Am J. 2019;83(2):398–408. doi:10.2136/sssaj2018.08.0297. [Google Scholar] [CrossRef]
153. Sun J, Li H, Wang Y, Du Z, Rengel Z, Zhang A. Biochar and nitrogen fertilizer promote rice yield by altering soil enzyme activity and microbial community structure. GCB Bioenergy. 2022;14(12):1266–80. doi:10.1111/gcbb.12995. [Google Scholar] [CrossRef]
154. Liu Q, Liu B, Zhang Y, Lin Z, Zhu T, Sun R, et al. Can biochar alleviate soil compaction stress on wheat growth and mitigate soil N2O emissions? Soil Biol Biochem. 2017;104(3):8–17. doi:10.1016/j.soilbio.2016.10.006. [Google Scholar] [CrossRef]
155. Luo Y, Dungait JA, Zhao X, Brookes PC, Durenkamp M, Li G, et al. Pyrolysis temperature during biochar production alters its subsequent utilization by microorganisms in an acid arable soil. Land Degrad Dev. 2018;29(7):2183–8. doi:10.1002/ldr.2846. [Google Scholar] [CrossRef]
156. Wang X, Song D, Liang G, Zhang Q, Ai C, Zhou W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl Soil Ecol. 2015;96:265–72. doi:10.1016/j.apsoil.2015.08.018. [Google Scholar] [CrossRef]
157. Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol. 2013;71:33–44. doi:10.1016/j.apsoil.2013.05.003. [Google Scholar] [CrossRef]
158. Bandara T, Herath I, Kumarathilaka P, Seneviratne M, Seneviratne G, Rajakaruna N, et al. Role of woody biochar and fungal-bacterial co-inoculation on enzyme activity and metal immobilization in serpentine soil. J Soils Sediments. 2017;17(3):665–73. doi:10.1007/s11368-015-1243-y. [Google Scholar] [CrossRef]
159. Irfan M, Hussain Q, Khan KS, Akmal M, Ijaz SS, Hayat R, et al. Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: a 2-year field study. Arab J Geosci. 2019;12(3):95. doi:10.1007/s12517-019-4239-x. [Google Scholar] [CrossRef]
160. Yuan F, Li K-Y, Yang H, Deng C-J, Liang H, Song L-H. Effects of biochar application on yellow soil nutrients and enzyme activities. Huan Jing Ke Xue. 2022;43(9):4655–61. [Google Scholar] [PubMed]
161. Foster EJ, Hansen N, Wallenstein M, Cotrufo MF. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric Ecosyst Environ. 2016;233(3):404–14. doi:10.1016/j.agee.2016.09.029. [Google Scholar] [CrossRef]
162. Jiang Y, Wang X, Zhao Y, Zhang C, Jin Z, Shan S, et al. Effects of biochar application on enzyme activities in tea garden soil. Front Bioeng Biotechnol. 2021;9:728530. doi:10.3389/fbioe.2021.728530. [Google Scholar] [PubMed] [CrossRef]
163. Halmi MFA, Simarani K. Responses of soil microbial population and lignocellulolytic enzyme activities to palm kernel shell biochar amendment. Eurasian Soil Sci. 2021;54(12):1903–11. doi:10.1134/S1064229321120073. [Google Scholar] [CrossRef]
164. Pandey B, Suthar S, Chand N. Effect of biochar amendment on metal mobility, phytotoxicity, soil enzymes, and metal-uptakes by wheat (Triticum aestivum) in contaminated soils. Chemosphere. 2022;307(26):135889. doi:10.1016/j.chemosphere.2022.135889. [Google Scholar] [PubMed] [CrossRef]
165. Jin X, Zhang T, Hou Y, Bol R, Zhang X, Zhang M, et al. Review on the effects of biochar amendment on soil microorganisms and enzyme activity. J Soils Sediments. 2024;24(7):2599–612. doi:10.1007/s11368-024-03841-7. [Google Scholar] [CrossRef]
166. Zheng L, Tong C, Gao J, Xiao R. Effects of wetland plant biochars on heavy metal immobilization and enzyme activity in soils from the Yellow River estuary. Environ Sci Pollut Res. 2022;29(27):40796–811. doi:10.1007/s11356-021-18116-8. [Google Scholar] [PubMed] [CrossRef]
167. Xu Y, Seshadri B, Sarkar B, Wang H, Rumpel C, Sparks D, et al. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci Total Environ. 2018;621(2):148–59. doi:10.1016/j.scitotenv.2017.11.214. [Google Scholar] [PubMed] [CrossRef]
168. Nie C, Yang X, Niazi NK, Xu X, Wen Y, Rinklebe J, et al. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: a field study. Chemosphere. 2018;200:274–82. doi:10.1016/j.chemosphere.2018.02.134. [Google Scholar] [PubMed] [CrossRef]
169. Cui L, Yan J, Yang Y, Li L, Quan G, Ding C, et al. Influence of biochar on microbial activities of heavy metals contaminated paddy fields. BioResources. 2013;8(4):5536–48. doi:10.15376/biores.8.4.5536-5548. [Google Scholar] [CrossRef]
170. Meier S, Curaqueo G, Khan N, Bolan N, Rilling J, Vidal C, et al. Effects of biochar on copper immobilization and soil microbial communities in a metal-contaminated soil. J Soils Sediments. 2017;17(5):1237–50. doi:10.1007/s11368-015-1224-1. [Google Scholar] [CrossRef]
171. Meier S, Curaqueo G, Khan N, Bolan N, Cea M, Eugenia GM, et al. Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil. J Soils Sediments. 2017;17(3):741–50. doi:10.1007/s11368-015-1256-6. [Google Scholar] [CrossRef]
172. Ahmad M, Ok YS, Kim B-Y, Ahn J-H, Lee YH, Zhang M, et al. Impact of soybean stover- and pine needle-derived biochars on Pb and As mobility, microbial community, and carbon stability in a contaminated agricultural soil. J Environ Manag. 2016;166:131–9. doi:10.1016/j.jenvman.2015.10.006. [Google Scholar] [PubMed] [CrossRef]
173. Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, et al. The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere. 2017;174:545–53. doi:10.1016/j.chemosphere.2017.01.130. [Google Scholar] [PubMed] [CrossRef]
174. Igalavithana AD, Lee S-E, Lee YH, Tsang DCW, Rinklebe J, Kwon EE, et al. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere. 2017;174:593–603. doi:10.1016/j.chemosphere.2017.01.148. [Google Scholar] [PubMed] [CrossRef]
175. Seneviratne M, Weerasundara L, Ok YS, Rinklebe J, Vithanage M. Phytotoxicity attenuation in Vigna radiata under heavy metal stress at the presence of biochar and N fixing bacteria. J Environ Manag. 2017;186(11):293–300. doi:10.1016/j.jenvman.2016.07.024. [Google Scholar] [PubMed] [CrossRef]
176. Herath I, Iqbal MCM, Al-Wabel MI, Abduljabbar A, Ahmad M, Usman ARA, et al. Bioenergy-derived waste biochar for reducing mobility, bioavailability, and phytotoxicity of chromium in anthropized tannery soil. J Soils Sediments. 2017;17(3):731–40. doi:10.1007/s11368-015-1332-y. [Google Scholar] [CrossRef]
177. Al-Wabel MI, Usman ARA, Al-Farraj AS, Ok YS, Abduljabbar A, Al-Faraj AI, et al. Date palm waste biochars alter a soil respiration, microbial biomass carbon, and heavy metal mobility in contaminated mined soil. Environ Geochem Health. 2019;41(4):1705–22. doi:10.1007/s10653-017-9955-0. [Google Scholar] [PubMed] [CrossRef]
178. Igalavithana AD, Park J, Ryu C, Lee YH, Hashimoto Y, Huang L, et al. Slow pyrolyzed biochars from crop residues for soil metal(loid) immobilization and microbial community abundance in contaminated agricultural soils. Chemosphere. 2017;177:157–66. doi:10.1016/j.chemosphere.2017.02.112. [Google Scholar] [PubMed] [CrossRef]
179. Devi P, Saroha AK. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour Technol. 2014;162:308–15. doi:10.1016/j.biortech.2014.03.093. [Google Scholar] [PubMed] [CrossRef]
180. Zhang Y, Chen Z, Xu W, Liao Q, Zhang H, Hao S, et al. Pyrolysis of various phytoremediation residues for biochars: chemical forms and environmental risk of Cd in biochar. Bioresour Technol. 2020;299(3):122581. doi:10.1016/j.biortech.2019.122581. [Google Scholar] [PubMed] [CrossRef]
181. Lian F, Xing B. Black carbon (Biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk. Environ Sci Technol. 2017;51(23):13517–32. doi:10.1021/acs.est.7b02528. [Google Scholar] [PubMed] [CrossRef]
182. Sun D, Meng J, Liang H, Yang E, Huang Y, Chen W, et al. Effect of volatile organic compounds absorbed to fresh biochar on survival of Bacillus mucilaginosus and structure of soil microbial communities. J Soils Sediments. 2015;15(2):271–81. doi:10.1007/s11368-014-0996-z. [Google Scholar] [CrossRef]
183. Lyu H, He Y, Tang J, Hecker M, Liu Q, Jones PD, et al. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ Pollut. 2016;218:1–7. doi:10.1016/j.envpol.2016.08.014. [Google Scholar] [PubMed] [CrossRef]
184. Xiang L, Liu S, Ye S, Yang H, Song B, Qin F, et al. Potential hazards of biochar: the negative environmental impacts of biochar applications. J Hazard Mater. 2021;420:126611. doi:10.1016/j.jhazmat.2021.126611. [Google Scholar] [PubMed] [CrossRef]
185. Qin Y, Li G, An T, Yang Z. Advances in ecological and health risks of biochar during environmental applications. Chinese Sci Bull. 2021;66(1):5–20. doi:10.1360/TB-2020-0617. [Google Scholar] [CrossRef]
186. Odinga ES, Waigi MG, Gudda FO, Wang J, Yang B, Hu X, et al. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ Int. 2020;134(5):105172. doi:10.1016/j.envint.2019.105172. [Google Scholar] [PubMed] [CrossRef]
187. Anjum R, Krakat N, Toufiq Reza M, Klocke M. Assessment of mutagenic potential of pyrolysis biochars by Ames Salmonella/mammalian-microsomal mutagenicity test. Ecotoxicol Environ Saf. 2014;107:306–12. doi:10.1016/j.ecoenv.2014.06.005. [Google Scholar] [PubMed] [CrossRef]
188. Cai Y, Chen H, Yuan R, Wang F, Chen Z, Zhou B. Metagenomic analysis of soil microbial community under PFOA and PFOS stress. Environ Res. 2020;188:109838. doi:10.1016/j.envres.2020.109838. [Google Scholar] [PubMed] [CrossRef]
189. Das SK, Ghosh GK, Avasthe RK, Sinha K. Compositional heterogeneity of different biochar: effect of pyrolysis temperature and feedstocks. J Environ Manag. 2021;278(7):111501. doi:10.1016/j.jenvman.2020.111501. [Google Scholar] [PubMed] [CrossRef]
190. Murtaza G, Usman M, Iqbal J, Tahir MN, Elshikh MS, Alkahtani J, et al. The impact of biochar addition on morpho-physiological characteristics, yield and water use efficiency of tomato plants under drought and salinity stress. BMC Plant Biol. 2024;24(1):356. doi:10.1186/s12870-024-05058-9. [Google Scholar] [PubMed] [CrossRef]
191. Imran S, Sarker P, Hoque MN, Paul NC, Mahamud MA, Chakrobortty J, et al. Biochar actions for the mitigation of plant abiotic stress. Crop Pasture Sci. 2023;74(2):6–20. doi:10.1071/CP21486. [Google Scholar] [CrossRef]
192. Srivastava PC, Pratap PS, Anand P, Rini L, Kumar SA, Manoj S, et al. Residual effect of micronutrients and sulfur enriched phyto-biochars soil applied to fodder maize on yields, tissue concentration and uptake of nutrients by rice and post-harvest soil properties. J Plant Nutr. 2024;47(16):2753–72. doi:10.1080/01904167.2024.2369064. [Google Scholar] [CrossRef]
193. Alkharabsheh HM, Seleiman MF, Battaglia ML, Shami A, Jalal RS, Alhammad BA, et al. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: a review. Agronomy. 2021;11(5):1–29. doi:10.3390/agronomy11050993. [Google Scholar] [CrossRef]
194. Yang A, Akhtar SS, Li L, Fu Q, Li Q, Naeem MA, et al. Biochar mitigates combined effects of drought and salinity stress in Quinoa. Agronomy. 2020;10(6):993. doi:10.3390/agronomy10060912. [Google Scholar] [CrossRef]
195. Alotaibi KD. Use of biochar for alleviating negative impact of salinity stress in corn grown in arid soil. Can J Soil Sci. 2021;102(1):187–96. doi:10.1139/cjss-2021-0053. [Google Scholar] [CrossRef]
196. Bolan N, Hoang SA, Beiyuan J, Gupta S, Hou D, Karakoti A, et al. Multifunctional applications of biochar beyond carbon storage. Int Mater Rev. 2022;67(2):150–200. doi:10.1080/09506608.2021.1922047. [Google Scholar] [CrossRef]
197. Solaiman Z. Biochar and fertiliser interactions in crop and pasture production. Crop Pasture Sci. 2023;74(2):1–5. doi:10.1071/CP22310. [Google Scholar] [CrossRef]
198. Zeeshan M, Salam A, Afridi MS, Jan M, Ullah A, Hu Y, et al. Biochar for mitigation of heat stress in crop plants. In: Fahad S, Danish S, Datta R, Saud S, Lichtfouse E, editor. Sustainable agriculture reviews 61: biochar to improve crop production and decrease plant stress under a changing climate. Cham: Springer International Publishing; 2023. p. 159–87. doi:10.1007/978-3-031-26983-7_7. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF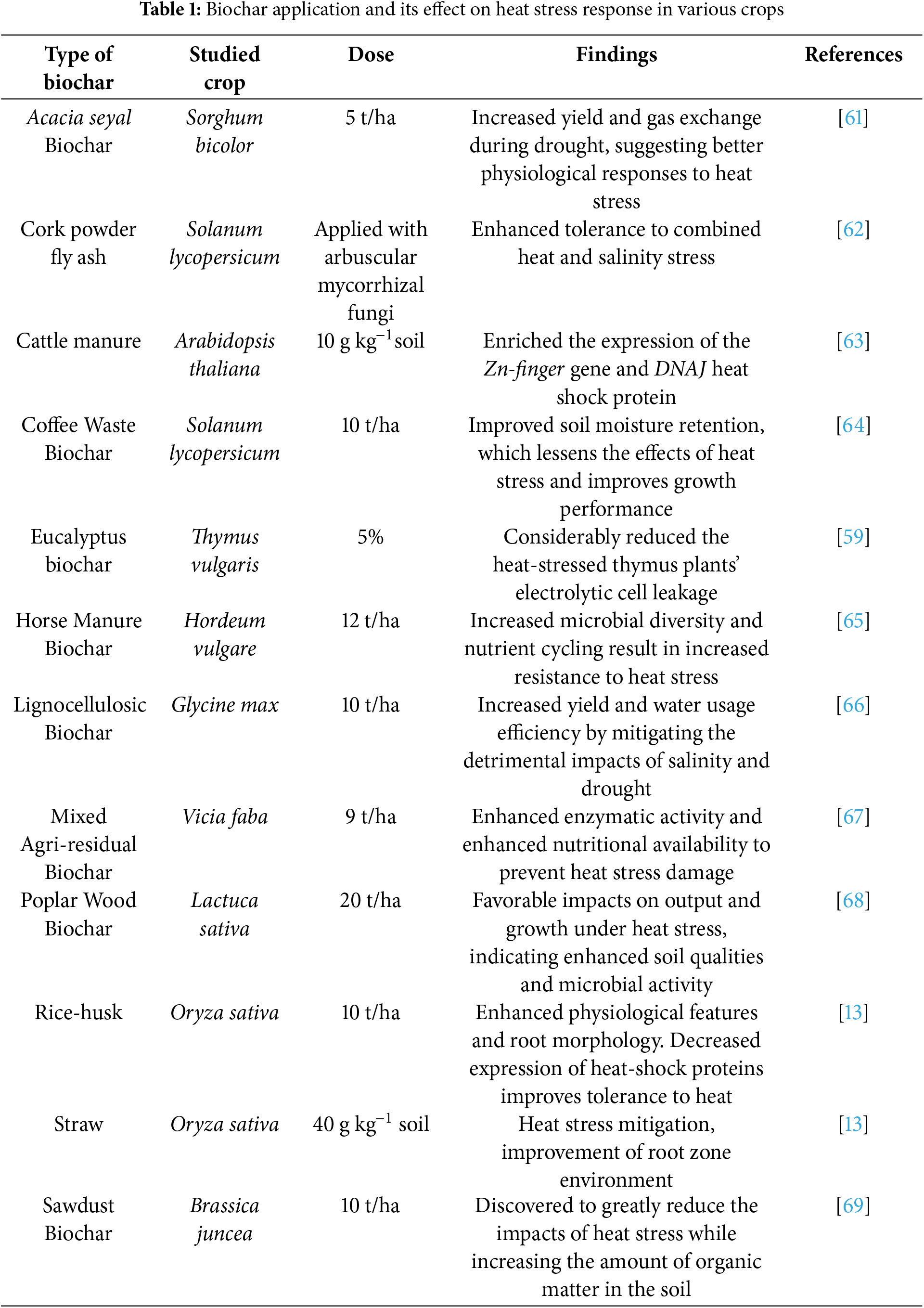
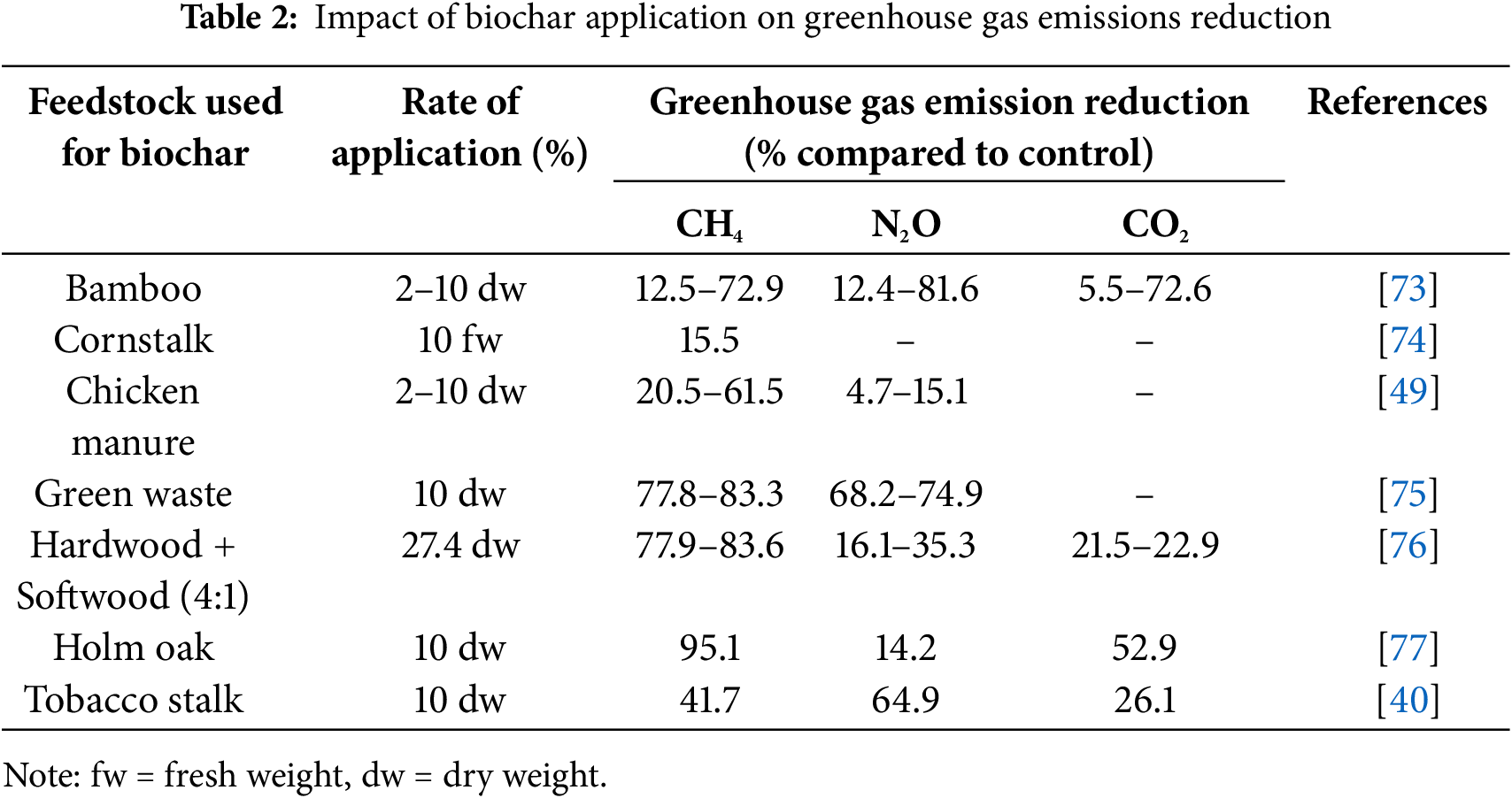
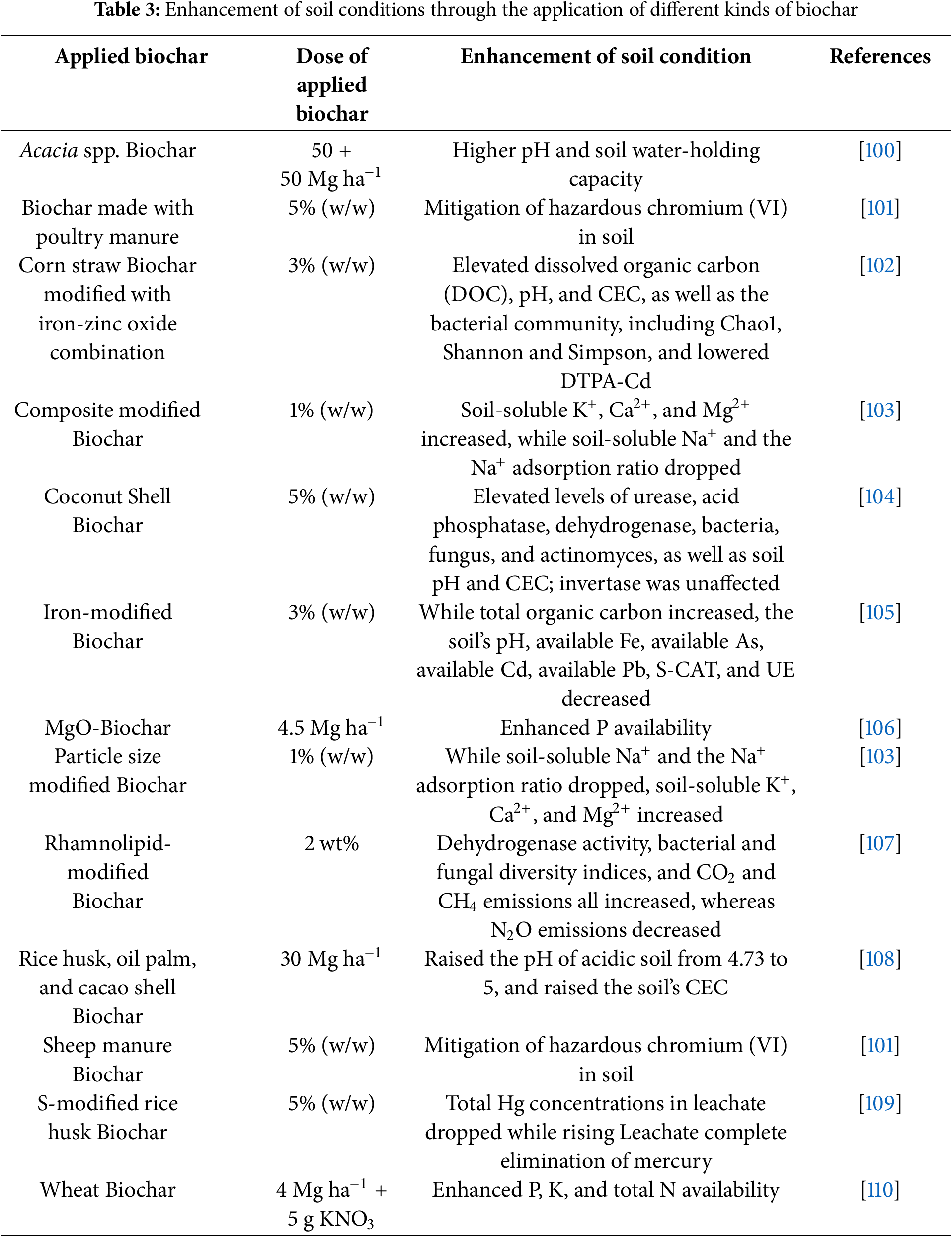

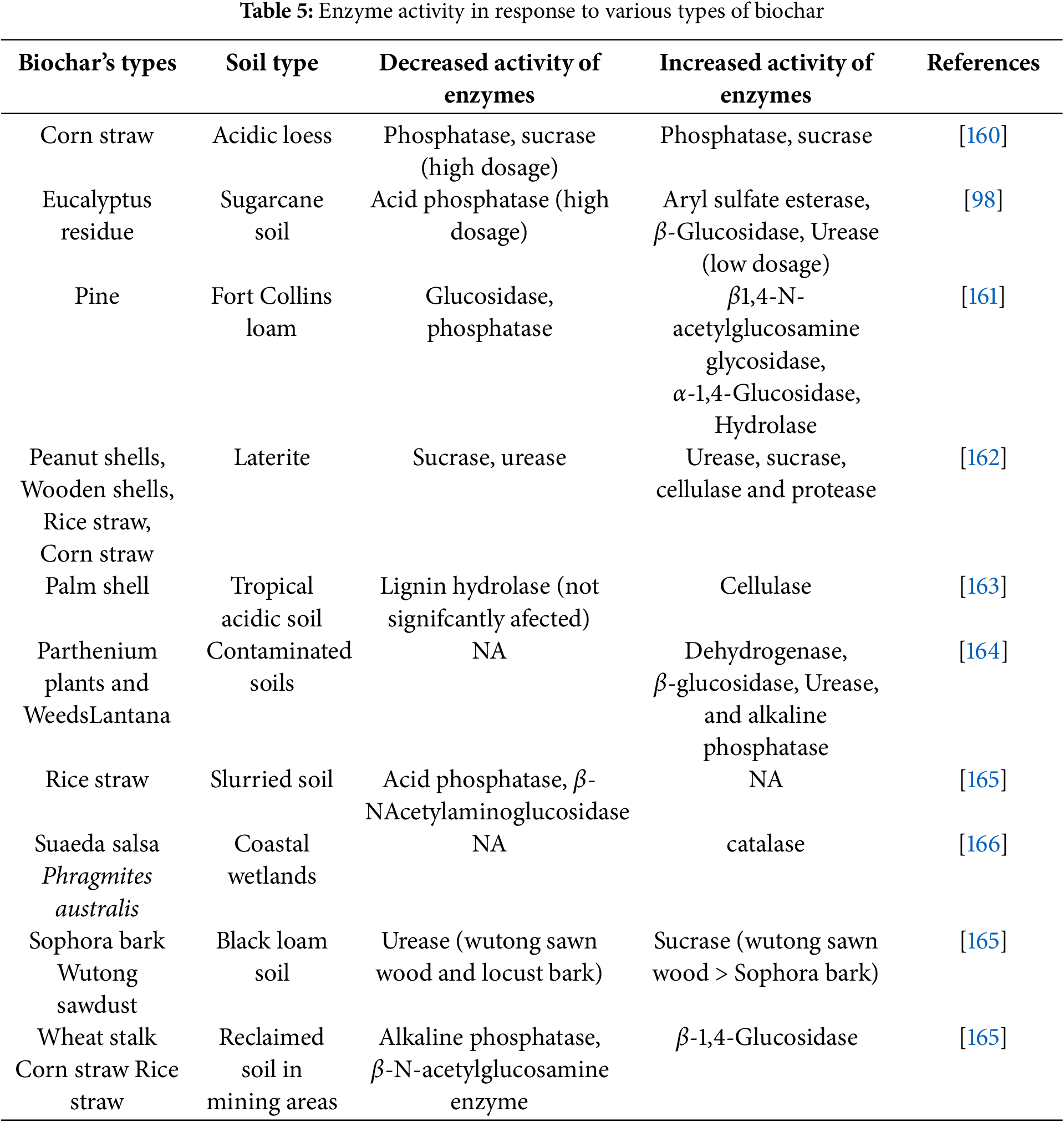
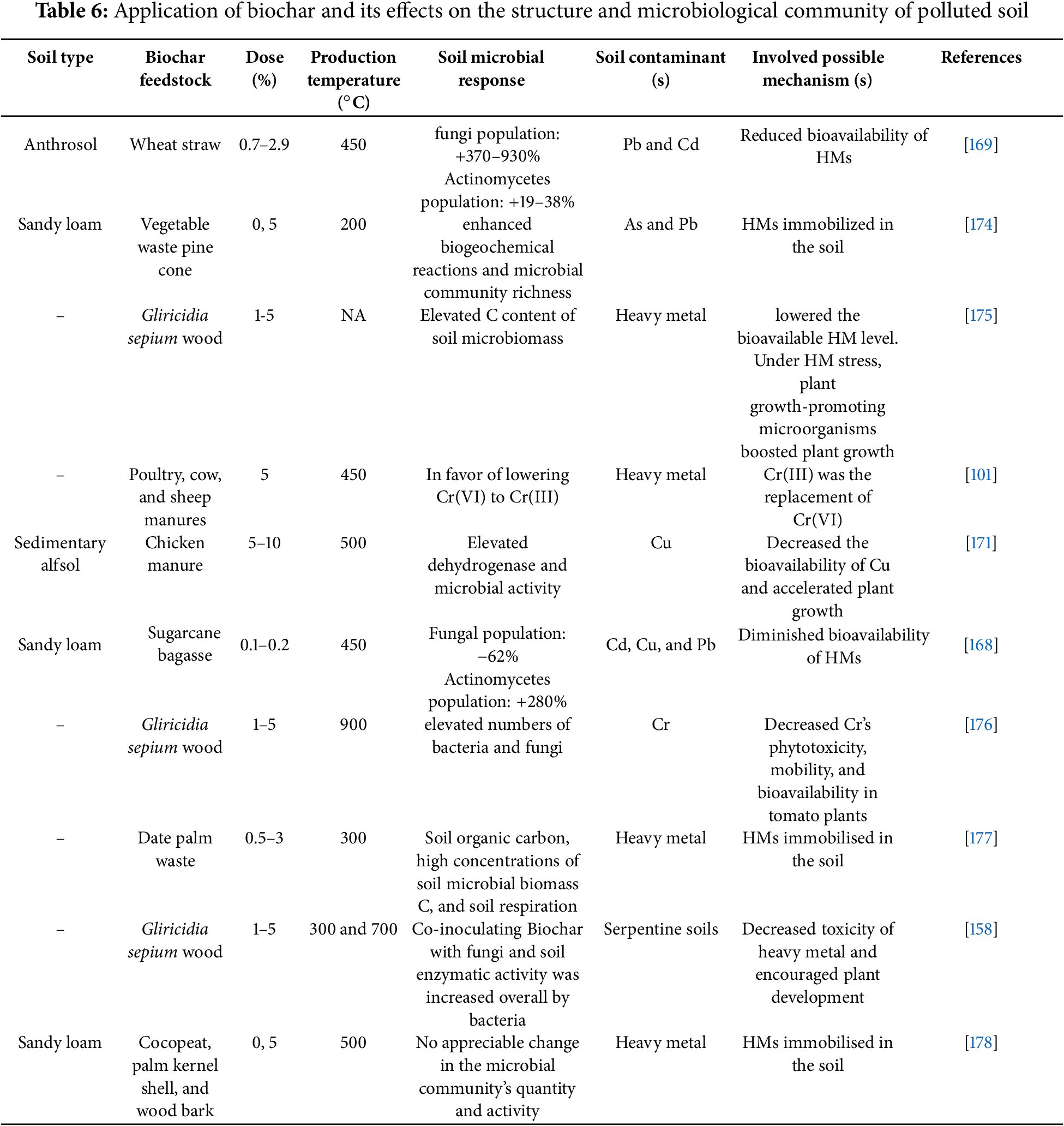
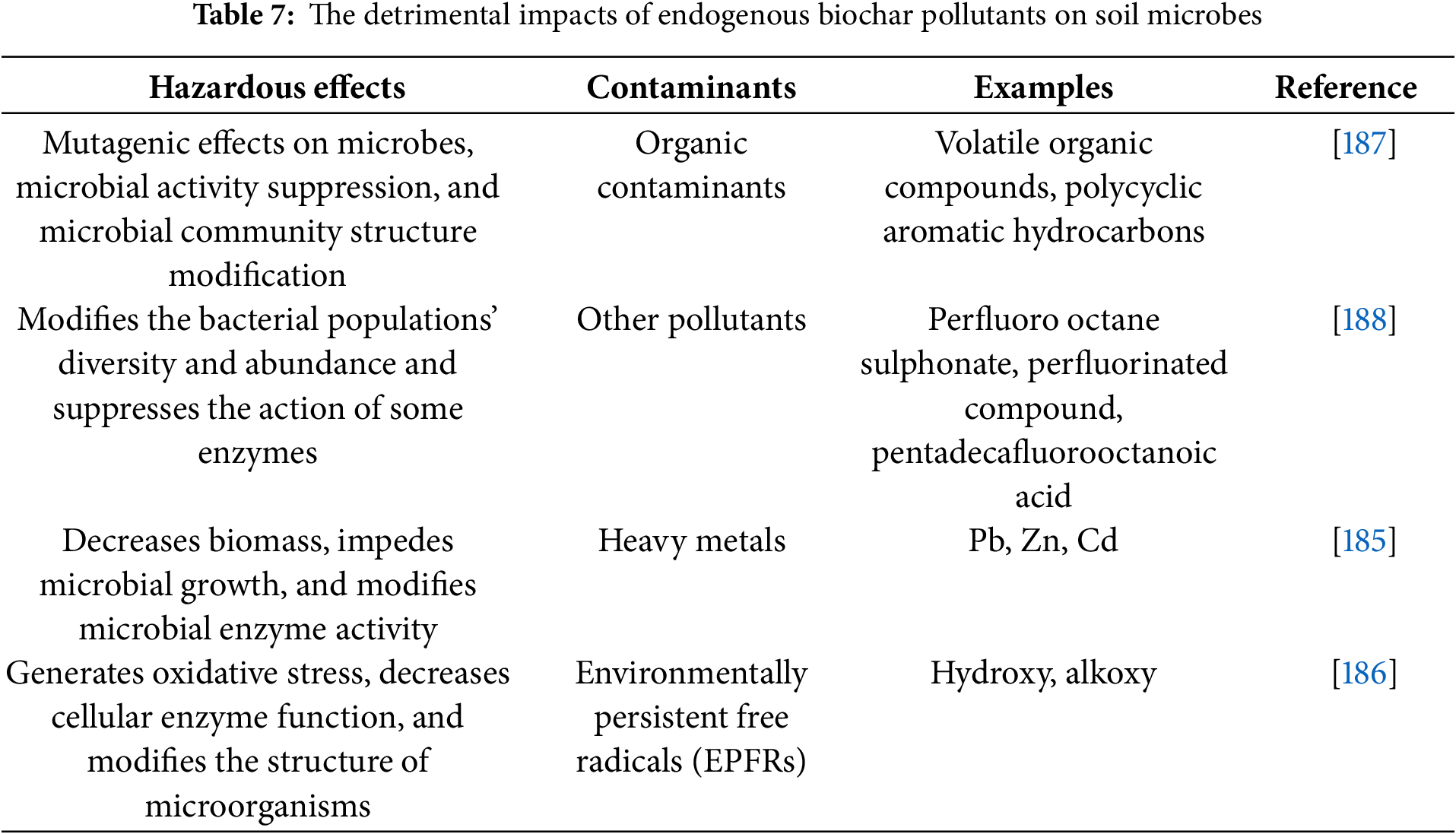
 Downloads
Downloads
 Citation Tools
Citation Tools