 Open Access
Open Access
REVIEW
Biochar Amendments in Soil: A Sustainable Approach for Mitigating Heavy Metal Stress in Plants
1 Department of Agronomy, Khulna Agricultural University, Khulna, 9100, Bangladesh
2 Institute of Plant Science and Resources, Okayama University, Kurashiki, 710-0046, Japan
3 Department of Crop Botany, Khulna Agricultural University, Khulna, 9100, Bangladesh
4 Department of Soil Science, Khulna Agricultural University, Khulna, 9100, Bangladesh
5 Department of Agricultural Chemistry, Khulna Agricultural University, Khulna, 9100, Bangladesh
6 Department of Plant Physiology, Slovak University of Agriculture, Nitra, 94976, Slovakia
7 Department of Seed Science and Technology, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
* Corresponding Authors: Shahin Imran. Email: ; Mohammad Saidur Rhaman. Email:
# Authors contributed equally to this work
(This article belongs to the Special Issue: Plant Responses to Biological and Abiotic Stresses)
Phyton-International Journal of Experimental Botany 2025, 94(4), 1073-1109. https://doi.org/10.32604/phyton.2025.064046
Received 03 February 2025; Accepted 18 March 2025; Issue published 30 April 2025
Abstract
Heavy metal (HM) accumulation in soil poses a major hazard to both ecological health and plant growth progressions. Cadmium (Cd), lead (Pb), copper (Cu), chromium (Cr), arsenic (As), zinc (Zn), and nickel (Ni) are examples of HMs that negatively impact the growth and development of plants, resulting in lower agricultural output and food safety concerns. Biochar (BC), a substance rich in carbon that is formed by pyrolyzing natural biomass, has demonstrated remarkable promise in reducing HM stress in polluted soils. Research has shown that BC effectively lowers plant uptake of metals, and enhances soil qualities, and encourages microbial activity. Besides, BC improves the fertility of soil, retention of water, and nutrient absorption, while it interacts with soil microbes to help mitigate the negative effects. However, a number of variables affect how effective BC is as a soil supplement, including the kind of BC used, the soil’s characteristics, and the metal’s qualities. This review delves into the mechanisms of BC’s interactions with HMs, its potential to mitigate stress caused by different metals, and the factors that influence its efficiency. Furthermore, it draws attention to the drawbacks and difficulties associated with using BC in heavy-metal-contaminated soils, offering suggestions for future studies focused on maximizing its utilization for long-term soil rehabilitation and sustainable agriculture.Keywords
Heavy metal (HM)-contaminated soil is now a major environmental concern that is negatively affecting agricultural systems across the globe. Among the most concerning HMs are cadmium (Cd), Lead (Pb), copper (Cu), chromium (Cr), arsenic (As), zinc (Zn), and nickel (Ni). Of these, trace levels of Cu, Zn, and Ni are thought to be necessary for plant metabolism, as they play a part in growth and enzymatic processes. However, even at low concentrations, Cd, Pb, Cr, and As are toxic and pose serious dangers to species at all levels of the trophic chain. They are also known to have no metabolic function in plants [1]. Numerous human actions, like factory discharges, atmospheric mining, pollutants, production along with processing activities, excessive application of fertilizers and pesticides, and inappropriate trash management, are responsible for the introduction of these metals into the soil [2]. With far-reaching impacts on human health and ecosystems, HMs present serious obstacles to plant growth, food safety, and soil health, after they’re in the ground [3]. Moreover, HMs accumulate in human tissues after passing through the food chain and into the body.
One of the most hazardous HMs is Pb, which accumulates in plants and lingers in the environment, where it caused growth retardation, decreased metabolic processes, and a marked decrease in photosynthetic activity, with a possible 42% drop in root growth [4]. Pb poses serious health concerns to both humans and animals due to its high mobility in the soil-plant system, which raises the possibility that it will enter the food chain. Cd is another very poisonous metal that is readily assimilated by plants, even in minimal concentrations. It is frequently linked to phosphate fertilizers and industrial processes. It inhibits development, oxidative stress, and metabolic processes [5]. Strong carcinogens, such as Cr in its hexavalent form Cr(VI), are present in soils, which has a detrimental effect on the molecular, biochemical, and physiological characteristics of crops [6]. Small amounts of micronutrients are necessary for the development and growth of plants, however, when doses get higher, they can become toxic and interfere with metabolic and physiological functions. Cu, an essential micronutrient, becomes harmful at elevated levels, inhibiting root elongation, reducing nutrient uptake, and causing oxidative damage caused by reactive oxygen species (ROS) production [7]. Another crucial mineral, Zn, can be hazardous in excess and cause stunted root growth, decreased nutrient uptake, and decreased photosynthetic efficiency [8]. Furthermore, tiny levels of Ni, which are necessary, turn poisonous at large doses. This results in oxidative stress, reduced growth, and inhibition of seed germination, all of which lower plant vigor [9]. As, a naturally occurring element that influences the growth of roots, decreases photosynthetic activity and causes health hazards since it accumulates in edible plant portions [10]. Plants exposed to As experience oxidative stress due to the production of ROS from phosphorylation metabolic disruption and redox-driven activities such as methylation and As(V) reduction to As(III) [11]. Humans who consume food containing As on a regular basis run a serious risk of developing cancer, skin sores, and developmental problems [12].
Crop productivity is being impacted by the strain that population increase and global warming are placing on agriculture. Researchers are using methods like applying biochar to improve output and lessen the negative impacts of plant stress [13]. Biochar (BC), a carbon-rich substance, is produced by pyrolyzing organic biomass—such as wood, animal dung, and crop residues—under low oxygen levels [14]. For the creation of biochar, the pyrolysis temperature normally falls between 300°C and 700°C. Depending on the required qualities of the biochar, the heating rate typically ranges from 5°C/min to 30°C/min. Biochar with a higher carbon content and porosity is typically produced at higher temperatures and at quicker heating rates [15]. Recently, soil amendments have gained popularity among researchers and practitioners as a sustainable way to counteract the negative impacts of various HMs [16,17]. The BC offers numerous binding sites due to its porous structure and large surface area, making it particularly promising for various applications [16]. The interactions of BC, for example, hydrogen bonding, electrostatic forces, and hydrophobicity play a complex function in the adsorption of HMs and aromatic substances, particularly influenced by the surface functional groups [18]. Long-term soil remediation is aided by its high cation exchange capacity (CEC), which further stabilizes HMs in the ground by decreasing their leaching and mobility [19]. The HMs can be effectively removed from environments by modifying BC. The removal efficiency can reach up to 99% for Pb and Cr, and >90% for Cd, As, and Hg with BC application [20]. Furthermore, BC enhances the pH, aeration, water retention, and soil structure, which are all more beneficial for the growth of plants. Additionally, it engages in interactions with soil microorganisms, boosting microbial activity and encouraging HMs’ natural abatement [15]. However, the effectiveness of BC can differ based on the variables such as types of BC, HMs characteristics, surrounding factors, and soil qualities. Moreover, the production method significantly influences its adsorption capacity and immobilization potential in plants as well as soil.
This review investigates BC’s effects in alleviating HM stress in plants, emphasizing its potential as an environmentally friendly soil supplement for polluted soils. Although BC has the potential to be used as a soil amendment for contaminated soils, little is known about how it interacts with various HMs. What effects does it have on different metals? What factors influence its effectiveness? Therefore, this review examines the mechanisms by which BC interacts with different HMs and the specific effects on various metals. Additionally, the review addresses the limitations and difficulties in applying BC on soils contaminated with HMs, offering insights into future research directions to enhance its use for soil remediation and sustainable agriculture.
2 Negative Effect of HMs on Plant Development and Growth
HMs such as As, Ni, Zn, Cu, Pb, Cd, and Cr are poisonous to plants and interfere with a number of biochemical and physiological functions, which adversely impact the growth, development, and production of plants (Fig. 1). In this section, we briefly discussed the negative consequences of different HMs in the development and growth of plants.
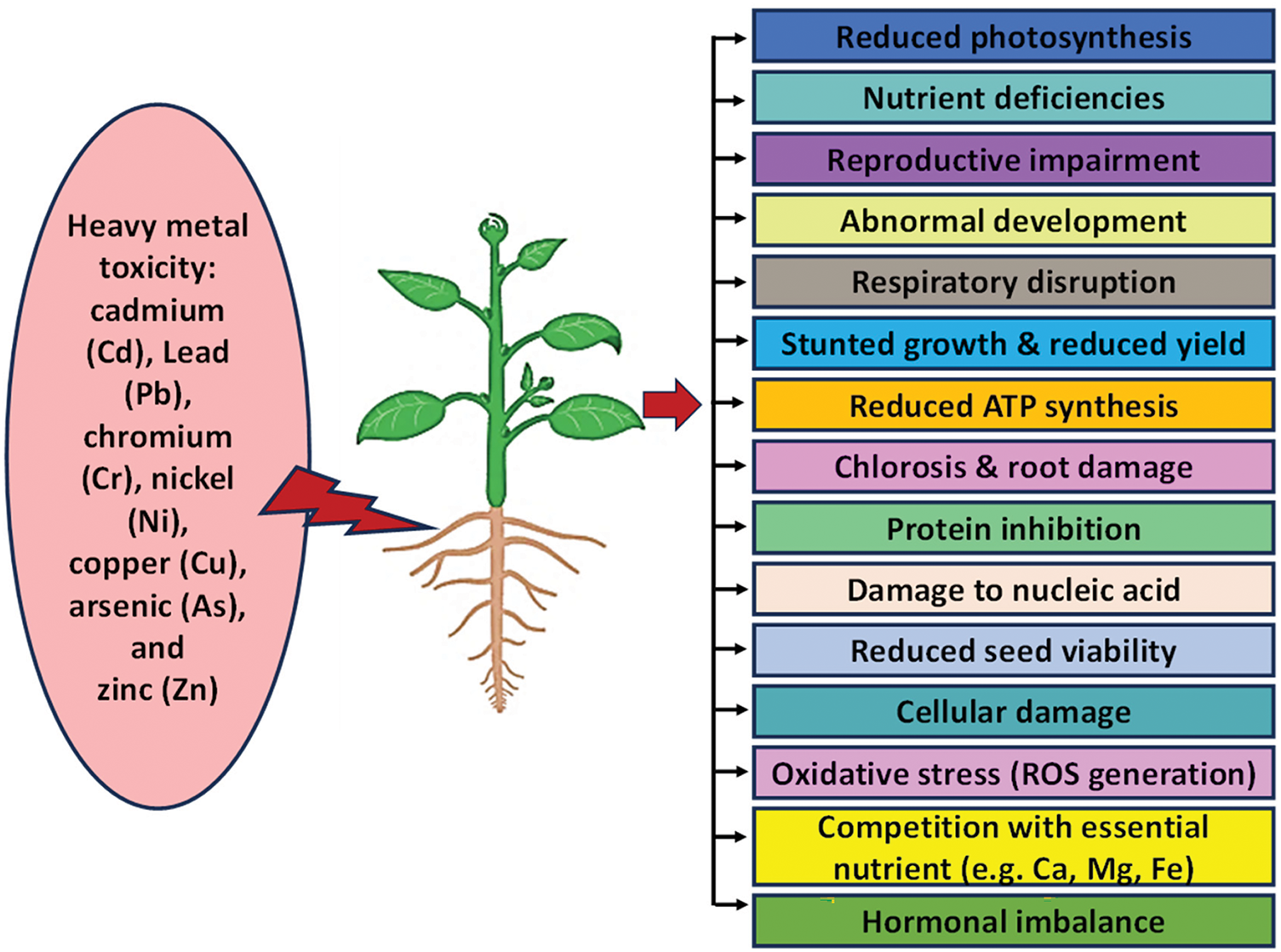
Figure 1: HMs’ detrimental effects on plant development and progress of plant
The Pb is a toxic HM, which hampers nutrient uptake and causes oxidative stress and, consequently, hinders plant growth [4]. The Pb competes with vital elements such as iron and calcium, resulting in deficits that affect several physiological functions, including enzymatic activity, respiration, photosynthesis, and nutritional absorption [4,21]. It has been reported that decreased germination of seeds and unhealthy plants can also result from prolonged Pb pollution [22]. Leaves often display chlorosis due to decreased chlorophyll synthesis, necrosis, and curling, which further diminishes photosynthetic efficiency [23]. Exposure to Pb prevents roots from growing longer, resulting in thicker, shorter roots with darker tips that are less able to absorb nutrients and water [24]. In shoots, Pb toxicity manifests as reduced plant height, smaller and malformed leaves, and overall stunted growth [25]. Visible symptoms such as leaf wilting, darkened root tips, and reduced canopy size are direct morphological indicators of Pb toxicity [26]. Pb exposure frequently causes aberrant development in flowers and fruits, such as decreased size and malformations, which further impairs the plants ability to reproduce [27].
The Pb also inhibits important mitochondrial enzymes, which lowers the synthesis of ATP and impacts cellular respiration in plant cells [28]. By causing oxidative stress and promoting ROS production, Pb damages lipids, proteins, and membranes, disrupting cellular structures and hormonal balance [29].
The Cd, even at extremely minute amounts (0.1–1 µM), can seriously hinder the growth and metabolism of plants [30]. It obstructs the intake and movement of vital nutrients and water, induces ROS production, and disrupts the photosynthetic system, which consequently kills tissues. According to reports, the production of chlorophyll is negatively impacted by Cd poisoning, which also decreases photosynthesis and tampers with water relations, hormonal balance, and nutritional homeostasis [30,31]. In addition, despite defense systems, Cd causes oxidative stress, interferes with nutrient transport, and lowers plant vitality and productivity [32,33].
The Cr is toxic to plants and affects various morpho-physiology in plants. This HM affects plants at different phases of their growth and development, causing serious abiotic stress. It hinders nutrient uptake and translocation, leading to reduced productivity [6]. It affects soil fertility, changes microbial activity, and inhibits plant growth [34]. According to reports, Cr disrupts chloroplast structures, impedes the growth of hypocotyls and epicotyls, and interferes with seed germination [35].
For plants, Cu is a necessary micronutrient. However, the elevated level of Cu may be hazardous and create abnormalities in the plant’s physiology and biochemistry that hinder the growth [36]. It has been reported that Cu reduces the antioxidant enzymes, chlorophyll production, and mineral nutrition, which negatively impacts on photosynthesis, germination, and plant development [37]. According to reports, 25 μM Cu concentrations diminish phosphorus (P) input, hinder P absorption processes, and limit growth in the roots and shoots of plants. Furthermore, Cu poisoning may change the permeability of the root plasma membrane in these plants, further restricting the uptake of P [38].
One hazardous heavy element that seriously threatens plant health is As, adversely affecting physiological, biochemical, and structural processes. Plants exposed to As suffer from a number of negative consequences including disturbance in cell permeability and metabolic pathways, which leads to a reduction in plant biomass [10]. The As induced ROS and disrupted the mitochondria, peroxisomes, cell walls, plasma membranes, and chloroplasts [39].
A high concentration of Zn is considered as HM and its high concentrations in the soil can cause phytotoxicity, which damages plants by stunting growth, hampering photosynthesis and respiration, and lowering yield [40]. Zn is particularly toxic to plants because it is easily absorbed by them and is highly mobile and bioavailable. While less prevalent than Zn shortage, Zn poisoning in plants still carries serious dangers and can negatively impact ecosystem stability and agricultural productivity [8].
Plants suffer severe harm from Ni when it reaches lethal amounts because it disrupts several physiological functions. At poisonous concentrations, Ni damages plants by interfering with physiological processes. Reduced plant growth and production result from its effects on mineral absorption, photosynthesis, root growth, and enzyme activity [9]. The growth, biomass, and root characteristics of sweet potatoes are all severely harmed by high Ni levels. Ni toxicity raises oxidative stress and decreases photosynthetic pigments, gas exchange, and relative water content (RWC) [41].
3 BC Amendments for HM Stress Mitigation
3.1 Lead (Pb) Stress Mitigation
BC amendments have been reported to mitigate Pb stress in different plants via different mechanisms (Table 1). The active functional groups, ion exchange capability, and microporous structure of BCs have all been shown to be significant factors in lowering HMs’ bioavailability and mobility [42,43]. Application of varying BC rates in a Pb-polluted soil considerably decreases the extractability of Pb in ryegrass shoot (Lolium multiflorum Lam.) where the magnitude of the application directly influences the reduction of Pb [44]. According to Almaroai et al. [45], BC lowers maize shoot’s Pb levels by attracting and retaining positively charged ions like Pb on its extremely negatively charged surface. Zheng et al. [46] examined how rice plants’ metal mobility and iron plaque development changed as a result of amending BC in the Pb-contaminated soil and found that BC has the ability to decrease the translocation and accumulation of Pb in rice shoots. In addition, Ahmad et al. [47] found that applying BC was the most effective way to reduce Pb’s phyto-availability and absorption which recommended it as a possible supplement to clean up Pb-contaminated soils [48,49]. Adejumo et al. [50] observed that higher rates of organic amendments, such as BC, and reduced light intensity led to a decrease in both post-cropping soil Pb content and the uptake of Pb by maize. As a result, compost and BC have been widely used to decrease soil metals and agricultural plants’ absorption of Pb. Although BC shows superior efficiency in stabilizing Pb because of its alkaline pH and large surface area, some studies highlight the role of organic amendments like compost and BC in reducing Pb uptake [50,51]. Synergistic effects of BC with chelators like EDTA have also been reported for enhanced phytoextraction of Pb [52]. Hence, applying BC could help create a green layer over the trash to achieve long-term phyto-stabilization by the reduction of the bioavailability of HMs.
3.2 Chromium (Cr) Stress Mitigation
Among the HMs that represent the highest environmental and toxicological hazards, Cr is commonly recognized as one of the most severe and harmful pollutants that result from anthropogenic activity [45]. One kind of Cr that occurs naturally is trivalent chromium (Cr III). It is non-toxic, insoluble, and relatively less mobile [59]. Hexavalent chromium (Cr VI), on the other hand, is extremely soluble, mobile, and poisonous. It is created by human activity rather than occurring naturally. When hexavalent Cr is consumed by contaminated food, the health risks are higher than those of Cr(III). Even at very low concentrations, it is also harmful to animals and plants [60–62]. When oxidizing agents (O2, CO2, NO3, SO4 2−, and/or Mn(VI) are present in the land environment, by removing electrons, Cr(III) is oxidized into its hazardous form, Cr(VI) [63].
BC lessens the harmful effect of Cr by lowering its levels in soil, plants, and water (Table 2). Its large porosity and surface area make it easier for processes including complexation, sorption, precipitation, and immobilization to occur, which lowers the bioavailability of Cr [64,65]. Applying BC to paddy rice reduced soil Cr accumulation and migration coefficients, with residual Cr rising as exchangeable Cr fell [66]. Likewise, BC reduced Cr bioaccumulation in tomato plants and alleviated poisoning symptoms by immobilizing Cr in soils contaminated by tannery waste and serpentine [67,68]. Additionally, research indicates that the combination of elemental sulfur (ES) and BC enhanced the physiological characteristics, stress tolerance, and plant development of maize. This included decreased oxidative stress and improved soil enzymatic activity, as well as increased biomass, chlorophyll content, photosynthesis, and nutrient uptake [63,69].
3.3 Cadmium (Cd) Stress Mitigation
Cd is extremely toxic which is recognized as a carcinogenic element posing a severe health risk to humans even in a minute concentration [81] (Table 3). The Cd contaminations in agricultural lands are mostly caused by human activities like the use of fertilizer, mining, irrigation with wastewater, and sewage sludge application. Given that Cd is comparatively more soluble and mobile in soil-plant systems, it resulted in crop growth retardation, reduced yield, lower grain quality, and increased Cd absorption in the edible parts of the plant [82]. In the human body, Cd mainly enters through the consumption of Cd-accumulated grains and vegetables [83]. There are many proposed methods to mitigate Cd contamination in soil and one of the most effective methods is the addition of organic amendments like BC [84]. The BC additions enhance the physical and biological characteristics of the soil, including its texture, electrical conductivity, structure, pH, bulk density, and cation exchange capacity, in addition to its fertility status [85]. BC has a higher surface area and organic functional groups that sorb HMs from soil that ultimately reducing the phyto-availability of HMs [86]. Once more, various research has shown that applying BC lessens the negative effects of Cd in plants by restricting Cd accumulation via plant roots. Reduced root and shoot Cd content were found in lettuce, barley, wheat, sunflower, etc. through BC application under Cd-contaminated soil [75,87,88]. In order to provide safer food production and environmental sustainability, BC application is a potential method for lowering Cd uptake in crops.
3.4 Copper (Cu) Stress Mitigation
Because of its detrimental effect on ecosystem food and health security, HM contamination in soil has drawn attention from all around the world. In contaminated soils, soil amendments such as BC can lower the bioavailability of HMs and lower the likelihood that they will reach the food chain. The impact of BC use on the buildup of HMs of Cu in several plants showed in Table 4. It has been suggested that incorporating BC into soil could be a significant way to lower emissions of greenhouse gases worldwide [110]. In contaminated soils, BC can reduce the bioavailability of metals, which could result in micronutrient shortages and decreased crop yields in agricultural contexts. The decrease in metal solubility caused by a higher soil pH or the direct binding of metals to BC surfaces could be the cause of this drop in bioavailability [111].
By lowering Cu bioavailability and promoting development, BC efficiently reduces Cu stress in a variety of plants. Citrus wood BC at doses of 1%–4% decreased Cu in sunflower [112], however BC at 1% enhanced enzyme activity and decreased Cu bioavailability and oxidative stress in lettuce [113]. 3% BC improved the development and physiological characteristics of maize cultivated in soils contaminated by tanneries [106,114]. P-loaded BC decreased HM forms in soils and enhanced phosphorus availability [64]. Applications of BC decreased Cu uptake and enhanced biomass and soil conditions in barley, quinoa, black beans, stonecrops, and soybeans [102,115–117]. However, by altering the degree of BC contact with the soil, the BC application techniques—such as integration vs. surface application—had an impact on Cu uptake and plant growth. Because incorporation allowed for better distribution and binding of Cu in the soil, it generally increased Cu immobilization and plant development more efficiently than surface application. Finally, BC provides a sustainable remedy for HM contamination by reducing Cu toxicity, increasing plant growth, and improving soil health.
3.5 Zinc (Zn) Stress Mitigation
In contaminated soils, BC can lower metal bioavailability [126]. This might possibly lock up metals in an agricultural setting, leading to micronutrient shortages and ultimately lowering output. This could be due to the metals adhering directly to the BC surfaces or the higher pH of the soil decreasing metal solubility [127]. Table 5 displays the reaction to organic amendments and how they affect the level of HMs (Zn) in plant tissues. The consequences of lower metal concentrations in plant tissues were noted for treatments using BC and the combination of BC. Several studies demonstrate that applying BC (1%–10% w/w) can immobilize Zn by increasing organic matter, altering the pH of the soil, and changing the form of Zn that is bioavailable into one that is less toxic. For example, BC dramatically boosted plant height, leaf number, water content, and photosynthesis in rubber plants (Ficus elastica Roxb.) under Zn stress, while decreasing lipid peroxidation and Zn bioavailability [128]. Similarly, BC applications in maize (Z. mays) significantly mitigated Zn toxicity, reducing metal concentrations in plant tissues and improving overall plant health [119]. In foxtail millet (Setaria italica L.), BC promoted plant growth and decreased oxidative stress by stabilizing Zn and reducing its bioavailability in the soil [95]. BC’s ability to reduce Zn uptake in crops like tomatoes (S. lycopersicum) led to improved fruit yield and quality, enhancing key parameters like total acidity, vitamin C, and lycopene content [56]. Similarly, in ryegrass (Lolium perenne L.), BC reduced Zn accumulation in shoots, increased soil pH, and bolstered the soil’s acid-neutralizing capacity [44]. In vegetables like radish, lettuce, spinach, and parsley, BC application, either alone or in combination with compost, effectively regulated Zn mobility and decreased HM uptake, promoting healthier crop production [129]. BC from sources like bamboo, rice straw, or sewage sludge shows varying success in stabilizing Zn and improving soil fertility. For example, bamboo and rice straw BC increased plant biomass and reduced Zn solubility in stone crops, while sewage sludge BC decreased Zn availability and promoted healthier soil microbial activity in tomatoes [122,130]. In general, BC consistently improves soil properties, fertility, and mitigates HM toxicity, particularly Zn, making it a valuable tool for rehabilitating contaminated soils and promoting sustainable agriculture.
3.6 Arsenic (As) Stress Mitigation
In addition to having negative effects on plant development, As is a non-essential element that causes cancer [136]. It also has significant deleterious impacts on human health. Arsenite (AsIII) and arsenate (AsV), two forms of As that are commonly found, are generally considered to be quite poisonous to both plants and humans [137]. Both natural and human-caused processes discharge them into the land and water. Furthermore, As pollution causes a variety of physiological, morphological, and biochemical alterations in plants [138]. However, due to these unfavorable effects, numerous scientists have looked at how well BC (BC) works to lower As toxicity in soil and lower its uptake by plants [139] (Table 6).
According to Yu et al. [140] and Irshad et al. [104], applying BC to rice plants improved their biomass production, growth, photosynthetic pigments, and gas exchange properties while lowering the accumulation of As in their edible plant sections [104,140]. In an experiment using mountain brome plants, Strawn et al. [141] discovered decreased root-shoot As concentration, increased plant root biomass, and root growth. According to Beesley et al. [142], tomato plants that had their soil supplemented with BC had increased fruit bulk and decreased fruit As content. Furthermore, when the soil was treated with BC, ryegrass showed considerably higher root-shoot biomass and plant tillering [143]. According to Hakeem et al. [139], BC’s application decreased the amount of As in the leaves and stem as well as the inhibitory effects of As on plant growth. When BC was applied to vegetable plant’s edible portion, the bio-accessibility of As was decreased [144]. When rice plants were grown in soil that had been contaminated with As, it was discovered that certain nutrients, such as P, N, S, and K, and activities of antioxidant enzymes, like as APX and CAT, were significantly elevated [145] (Table 6). However, compared to generic BC, poultry manure-based BC improves nutritional availability (P, S, K, N), increases antioxidant enzyme activities (CAT, APX), and lessens As buildup. Other BC sources, including wood or BC generated from crop residues, may not provide as many nutrient benefits but primarily enhance biomass, root development, and As bioavailability.
3.7 Nickel (Ni) Stress Mitigation
Numerous investigations have shown that BC’s use improves a variety of physio-biochemical and morphological processes in plants, such as germination, enzymatic activity, photosynthesis, and minimizes the detrimental effects of Ni stress on plants (Table 1). Ni is the 24th abundant element in the crust of the planet [151]. For enzymes like glutathione regeneration, urease, SOD, and ROS detoxification in plants, Ni in minute amounts is necessary [152]. However, if the level of Ni exceeds the threshold (20 mmol m−3), it is very phytotoxic [153]. Ni is extremely mobile in soil and water, and excess amounts could quickly be absorbed by edible plant sections, leading to major biochemical and physiological problems in exposed plants [154,155]. Ni is one of the high-risk HM contaminants that poses a harm to the ecosystem, the environment, and food security [156]. Ni toxicity slows plant growth and results in leaf margin black patches, decreased chlorophyll production, Fe mobilization to leaves, decreased rate of transpiration, and decreased water potential of the leaf [157]. Furthermore, Ni buildup in exposed plants impairs the absorption and subsequent essential macro and micronutrients translocation, as well as causing oxidative stress from an excess of ROS [158,159]. Compared to other organic amendments, BC, a cheap porous pyrogenous material made by pyrolyzing organic feedstock under oxygen-deficient conditions, has a higher cation exchange capacity (CEC), a bigger surface area, and a higher porosity [160]. Currently, BC is being used both economically and scientifically for HM cleanup, crop productivity enhancement, and soil amendment in agriculture [158] (Table 7). Application of BC increased stem diameter, plant height, flag LA, number of leaves plant−1, root and shoot dry weight, root and shoot fresh weight, and RWC of maize, rapeseed and summer squash plant under Ni stress condition [53,78,161,162]. In general, BC efficiently reduces Ni stress in plants, fostering development and enhancing physiological processes.
4 Mechanism of BC-Mediated HMs Stress Mitigation
From the above sections, we summarized that BC amendments have many positive effects on HMs stress mitigation. By increasing pH, porosity, CEC, and water retention and decreasing HM stress and availability, BC improves soil health [15,109,169]. BC is rich in nutrients, functional groups, and porosity. It improves the growth and efficacy of microbial inoculants. This promotes development in contaminated soils and increases plant resilience to metal stress [122,170]. Besides, BC is an effective alternative to non-renewable substrates for microbial inoculants. It supports soil biochemical processes, nitrogen cycling, and HM removal [171]. Additionally, BC enhances microbial activity and diversity in the rhizosphere, especially in metal-contaminated soils. Microorganisms help detoxify metals through adsorption, adhesion, electron transport, and ion exchange [109] (Fig. 2).
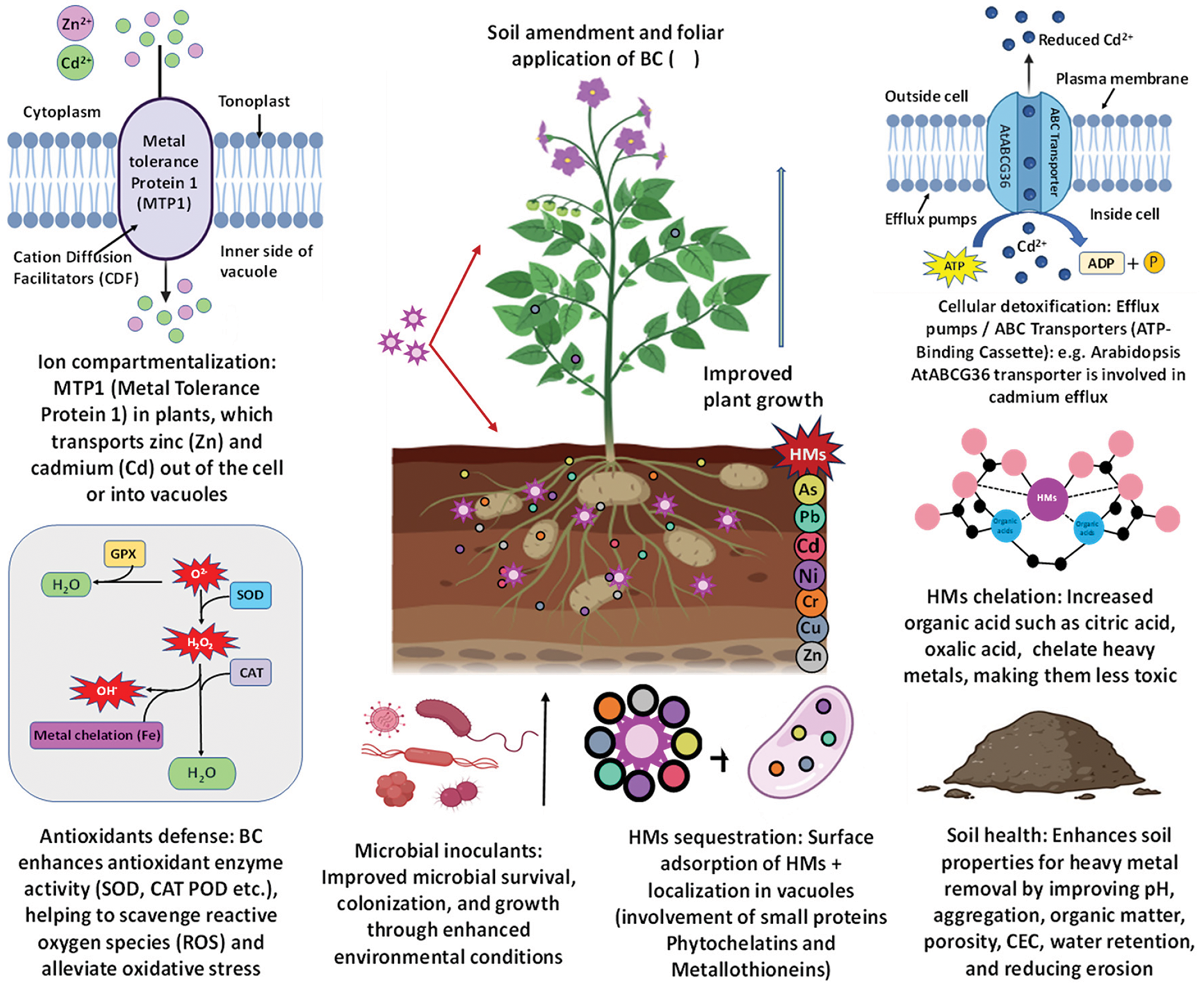
Figure 2: Mechanisms of HM stress mitigation in plants through BC application. BC enhances the detoxification of HMs in plants by influencing efflux pumps and metal transporters. Efflux pumps, such as RND, CDF, and ABC transporters, actively remove metals like Cd from cells. Metal transporters, including NRAMPs and HMAs, move metals across membranes and sequester them in vacuoles. BC reduces metal bioavailability, easing the load on these detoxifying processes and improving plant tolerance to metal toxicity
BC can lessen the solubility of some HMs and decrease their accessibility to plants by controlling the pH of the soil [78]. Again, the effect of BC enhances the number of soil particle’s negatively charged sites, particularly in acidic soils. This leads to the metal’s immobilization and reducing their bioavailability to plants [171]. For instance, Jing et al. [105] found that BC made from wheat straw has been demonstrated to raise soil pH, which lowers rice’s absorption of Cd. Again, BC produced from manure, with its higher Ca content, can attract and immobilize metals like Cd and Cu through ion exchange [172]. BC derived from bamboo residues has been found to effectively adsorb Cr, decreasing its bioavailability to wheat [80]. Advanced BC forms, like nano-engineered versions, are better at trapping metals due to their small pores and surface oxygen groups [173]. The metal-binding effectiveness of BC depends on factors like the material source, surface area, nutrient content, and the presence of functional groups, with advanced BC types offering even greater metal-adsorption capacity. In addition to reducing metal uptake, BC improves root development, nutrient and water availability, general plant vigor, and HM tolerance in plants [174]. Furthermore, BC strengthens plant defense mechanisms against oxidative stress brought on by metals. It has been demonstrated to increase the activity of several antioxidant enzymes, including CAT and SOD, which are essential for reducing oxidative damage in plants subjected to metal stress [175]. Small peptides rich in cysteines, known as phytochelatins (PCs), have the ability to bind metal(loid)s through SH-groups. While a variety of metal(loid)s can trigger PC production in vivo, PCs are primarily implicated in the detoxification of mercury, Zn, Pb, and Cu ions, as well as Cd and As(III), all of which have strong affinity for S-containing ligands [176]. BC stimulates phytochelatins synthesis, metallothionein’s, and other metal-binding peptides that sequester toxic metals, thereby reducing their harmful effects on plant cells. Furthermore, BC modified with transition metals or metal oxides has gained prominence as a catalyst in SR-AOPs due to its large surface area, high stability, and catalytic efficiency [177] (Fig. 2).
BC assists plants in scavenging ROS by releasing free radicals and functional groups involved in ROS neutralization [178]. Furthermore, studies on molecules have demonstrated that BC therapies can regulate the expression of a metal confronting gene (OsFSD1) linked to stress tolerance, including those that code for antioxidant enzymes (OsSOD, OsPOD, OsCAT, and OsAPX) [179]. Bamboo BC, according to Sarraf et al. [15] and Hannan et al. [161], effectively reduced Ni stress by upregulating the levels of CAT, APX, and GR genes and downregulating the expression of SOD genes and Ni transporter genes (BnNi-T, BnNRAMP3, and BnIRT1). It accomplishes this by reducing cellular membrane damage, lowering ROS levels, reducing chlorophyll degradation, and boosting antioxidant activity [180]. Overall, BC is a multipurpose instrument in sustainable agriculture, providing advantages that go beyond improving soil quality, such as lowering the bioavailability of metals and increasing plant resistance to stress caused by metals (Fig. 2).
5 Limitation of BC Application in HMs Contaminated Soil
HM stress in polluted soils can be effectively managed by BC, but there are a number of restrictions and difficulties that need to be taken into account. A number of variables, including the kind of soil, production circumstances, and feedstock, can affect how effective BC is at immobilizing HMs [181]. Different types of BC have varying adsorption capacities, and some may be less effective due to their chemical and physical properties [182]. BC can also interact with soil amendments, which may exceed its metal-binding capacity and release HMs back into the soil. Additionally, BC can alter soil pH, making some HMs more soluble and reducing their effectiveness [183].
Moreover, the heating rate during pyrolysis, and the production conditions are some of the variables that can greatly affect the properties of biochar. The resulting biochar’s chemical makeup, surface area, and nutrient content are significantly influenced by the feedstock material, which might include wood, agricultural waste, or animal dung [184]. The porosity, surface functional groups, and carbon content of the biochar can also be impacted by changes in the heating rates during pyrolysis [185]. Because biochar’s efficacy in soil amendment, heavy metal adsorption, and plant growth promotion might vary based on the source material and manufacturing conditions, it is difficult to estimate how effective it will be in certain applications [110,186].
Excessive application of BC may disrupt nutrient availability in the soil which adversely affects plant growth. Also, uniform distribution of BC can be difficult to achieve and inconsistencies in application may limit its overall effectiveness [187]. The effectiveness of BC in sequestering HMs can also be impacted by variations in production conditions such as the temperature during pyrolysis [188]. Complex interactions exist between BC and the soil matrix, which can modify the pH and other aspects of the soil, thereby influencing the availability of metals and the health of plants. Furthermore, not all HMs can be effectively removed by BC, particularly those that have a poor affinity for its adsorption sites. Other important issues that must be addressed are the long-term stability of BC in the soil, possible nutrient imbalances, and the continuous expenditures associated with manufacturing and application [189]. Furthermore, recent studies indicate that BC’s efficiency in HM remediation may be influenced by its interactions with soil microbial communities; this is an issue that has to be further investigated [190] (Fig. 3). However, the adsorption capacity of BC for HMs can be improved by refining its application techniques, such as controlled incorporation and activation [191]. BC’s effectiveness in reducing soil contamination is increased by using high-carbon feedstocks and adding additives [192,193].
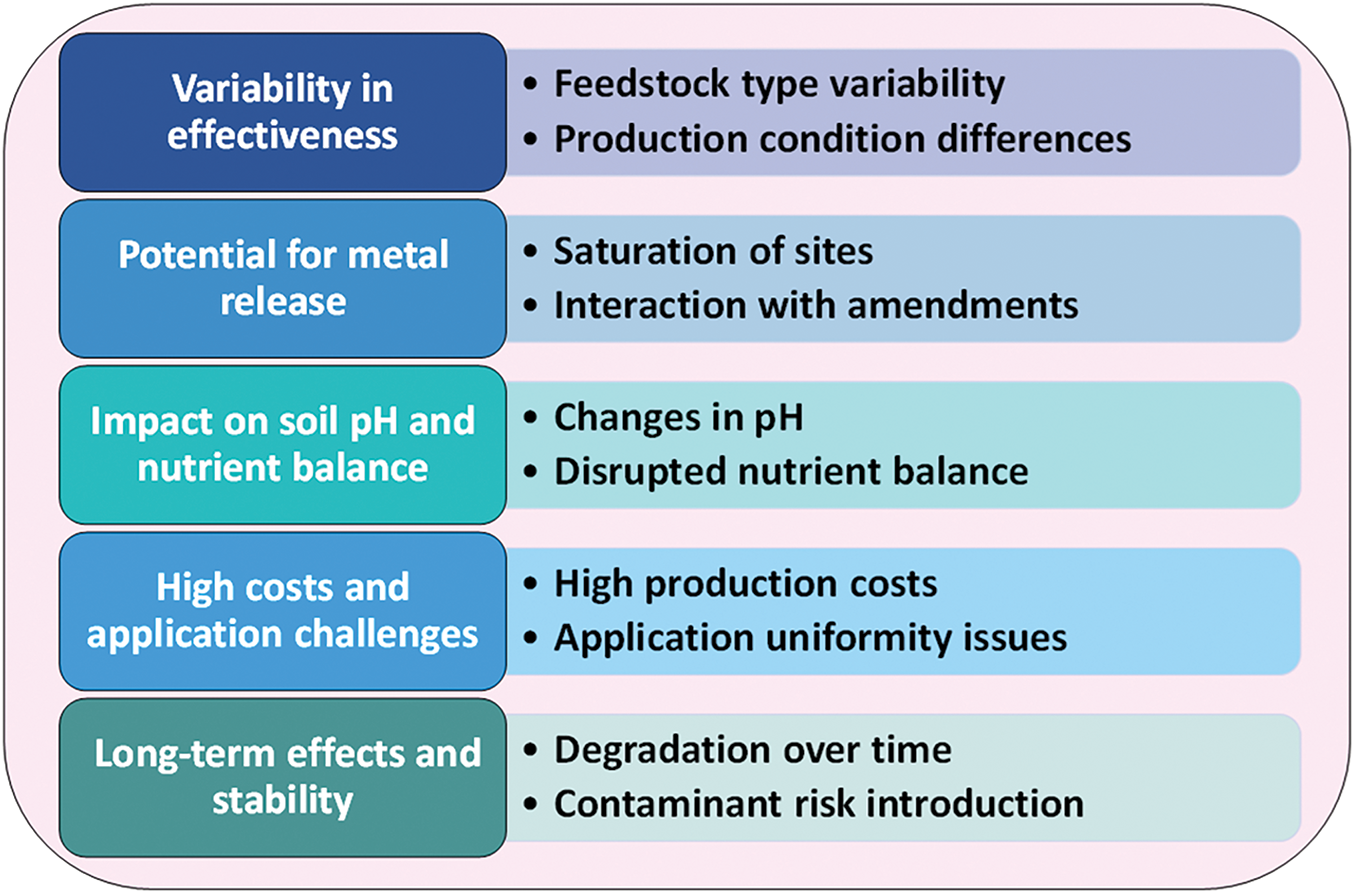
Figure 3: Limitation of BC application in HM contaminated soil
To evaluate the sustainability of this strategy, research on the long-term impacts of applying biochar (BC) on plant productivity and soil health is still crucial. Long-term use of BC may have unforeseen repercussions, including altered microbial populations, changes in soil pH, and possible nutrient imbalances, even while it can improve soil structure, promote nutrient retention, and reduce heavy metal toxicity [194]. Furthermore, BC’s efficacy in soil fertility and carbon sequestration is influenced by its stability and degradation over time [195]. Its long-term effects are further complicated by variations in feedstock type, pyrolysis conditions, and application rates. Therefore, to ascertain if BC treatment continues to be advantageous or presents dangers over time, ongoing monitoring of soil characteristics, plant responses, and ecosystem interactions is crucial.
6 Conclusion and Future Perspectives
BC has emerged as a promising sustainable soil amendment to mitigate HM stress in agricultural systems. Its unique properties, such as high porosity, large surface area, and functional groups, improve soil quality by reducing HM mobility, enhancing pH, and increasing cation exchange capacity. BC effectively immobilizes metals like Pb, As, Cd, and Cr, reducing their bioavailability and uptake by plants, leading to improved growth, enhanced photosynthetic activity, and increased antioxidant defenses. Additionally, BC fosters beneficial microbial activity, aiding in HM detoxification through microbial processes like adsorption and ion exchange. Beyond its chemical stabilization effects, BC enhances nutrient availability, water retention, and soil structure, improving root growth and plant resilience under stress. It also promotes the formation of organo-mineral complexes that reduce HM solubility. These combined effects contribute to sustainable remediation and soil restoration, making BC a vital component of green agricultural practices.
However, several research gaps must be addressed to optimize BC’s effectiveness in HM mitigation. Tailoring BC production for specific metals and soil conditions requires standardizing feedstock types, pyrolysis temperatures, and activation methods. Advanced BC formulations, incorporating metal-oxide nanoparticles or functionalized ligands, could enhance metal immobilization. Molecular studies should explore BC-induced regulation of metal transporter genes, antioxidant enzymes, and peptides like phytochelatins and metallothioneins. While BC impacts microbial communities, long-term studies on its synergistic effects with microbial consortia are needed for sustainable HM detoxification. Field trials should assess BC’s stability, degradation, and secondary pollution risks. Integrating BC with phytoremediation plants offers a hybrid system for better metal uptake and stabilization, while its role in carbon sequestration and HM mitigation under varying climates remains underexplored. Advanced techniques, such as synchrotron-based spectroscopy and nuclear magnetic resonance, could refine our understanding of BC-metal interactions. Future research should explore BC’s impact on soil enzymatic activity and signaling pathways related to HM tolerance. Developing BC composites with biodegradable polymers or bioactive coatings may improve durability and controlled release of beneficial compounds. Integrating BC into circular economy models using agricultural or industrial waste as feedstock promotes sustainability. Additionally, exploring BC’s synergy with other amendments like compost and zeolites could offer broader solutions for soil health. Lastly, establishing global standards for BC quality and safety will accelerate its adoption in environmental cleanup and sustainable farming.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Shahin Imran and Mohammad Saidur Rhaman; writing—original draft preparation, Shahin Imran, Mousumi Jahan Sumi, Israt Jahan Harine, Newton Chandra Paul, Md. Asif Mahamud and Rakibul Hasan Md. Rabbi; writing—review and editing, Shahin Imran, Marian Brestic and Mohammad Saidur Rhaman. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Angon PB, Islam MS, Kc S, Das A, Anjum N, Poudel A, et al. Sources, effects and present perspectives of heavy metals contamination: soil, plants and human food chain. Heliyon. 2024;10(7):e28357. doi:10.1016/j.heliyon.2024.e28357. [Google Scholar] [PubMed] [CrossRef]
2. Haghighizadeh A, Rajabi O, Nezarat A, Hajyani Z, Haghmohammadi M, Hedayatikhah S, et al. Comprehensive analysis of heavy metal soil contamination in mining environments: impacts, monitoring techniques, and remediation strategies. Arab J Chem. 2024;17(6):105777. doi:10.1016/j.arabjc.2024.105777. [Google Scholar] [CrossRef]
3. Omotayo AO, Omotayo OP. Potentials of microbe-plant assisted bioremediation in reclaiming heavy metal polluted soil environments for sustainable agriculture. Environ Sustain Indic. 2024;22:100396. doi:10.1016/j.indic.2024.100396. [Google Scholar] [CrossRef]
4. Collin S, Baskar A, Geevarghese DM, Ali MNVS, Bahubali P, Choudhary R, et al. Bioaccumulation of lead (Pb) and its effects in plants: a review. J Hazard Mater Lett. 2022;3:100064. doi:10.1016/j.hazl.2022.100064. [Google Scholar] [CrossRef]
5. Soni S, Jha AB, Dubey RS, Sharma P. Mitigating cadmium accumulation and toxicity in plants: the promising role of nanoparticles. Sci Total Environ. 2024;912:168826. doi:10.1016/j.scitotenv.2023.168826. [Google Scholar] [PubMed] [CrossRef]
6. Ali S, Mir RA, Tyagi A, Manzar N, Kashyap AS, Mushtaq M, et al. Chromium toxicity in plants: signaling, mitigation, and future perspectives. Plants. 2023;12(7):1502. doi:10.3390/plants12071502. [Google Scholar] [PubMed] [CrossRef]
7. Chen G, Li J, Han H, Du R, Wang X. Physiological and molecular mechanisms of plant responses to copper stress. Int J Mol Sci. 2022;23(21):12950. doi:10.3390/ijms232112950. [Google Scholar] [PubMed] [CrossRef]
8. Kaur H, Srivastava S, Goyal N, Walia S. Behavior of zinc in soils and recent advances on strategies for ameliorating zinc phyto-toxicity. Environ Exp Bot. 2024;220:105676. doi:10.1016/j.envexpbot.2024.105676. [Google Scholar] [CrossRef]
9. Mustafa A, Zulfiqar U, Mumtaz MZ, Radziemska M, Haider FU, Holatko J, et al. Nickel (Ni) phytotoxicity and detoxification mechanisms: a review. Chemosphere. 2023;328:138574. doi:10.1016/j.chemosphere.2023.138574. [Google Scholar] [PubMed] [CrossRef]
10. Sinha D, Datta S, Mishra R, Agarwal P, Kumari T, Adeyemi SB, et al. Negative impacts of arsenic on plants and mitigation strategies. Plants. 2023;12(9):1815. doi:10.3390/plants12091815. [Google Scholar] [PubMed] [CrossRef]
11. Kostecka-Gugała A, Latowski D. Arsenic-induced oxidative stress in plants. In: Hasanuzzaman M, Nahar K, Fujita M, editors. Mechanisms of arsenic toxicity and tolerance in plants. Singapore: Springer; 2018. p. 79–104. doi: 10.1007/978-981-13-1292-2_4. [Google Scholar] [CrossRef]
12. Rahaman MS, Mise N, Ichihara S. Arsenic contamination in food chain in Bangladesh: a review on health hazards, socioeconomic impacts and implications. Hyg Environ Health Adv. 2022;2:100004. doi:10.1016/j.heha.2022.100004. [Google Scholar] [CrossRef]
13. Imran S, Sarker P, Hoque MN, Paul NC, Mahamud MA, Chakrobortty J, et al. Biochar actions for the mitigation of plant abiotic stress. Crop Pasture Sci. 2022;74(2):6–20. doi:10.1071/CP21486. [Google Scholar] [CrossRef]
14. Awasthi G, Nagar V, Mandzhieva S, Minkina T, Sankhla MS, Pandit PP, et al. Sustainable amelioration of heavy metals in soil ecosystem: existing developments to emerging trends. Minerals. 2022;12(1):85. doi:10.3390/min12010085. [Google Scholar] [CrossRef]
15. Sarraf M, Janeeshma E, Arif N, Yadav V, Zahra N, Bouzroud S, et al. Biochar for the mitigation of metal/metalloid stress in plants. J Plant Growth Regul. 2024;43(10):3303–19. doi:10.1007/s00344-024-11335-6. [Google Scholar] [CrossRef]
16. Amalina F, Syukor Abd Razak A, Krishnan S, Sulaiman H, Zularisam AW, Nasrullah M. Advanced techniques in the production of biochar from lignocellulosic biomass and environmental applications. Clean Mater. 2022;6:100137. doi:10.1016/j.clema.2022.100137. [Google Scholar] [CrossRef]
17. Tan XF, Zhu SS, Wang RP, Chen YD, Show PL, Zhang FF, et al. Role of biochar surface characteristics in the adsorption of aromatic compounds: pore structure and functional groups. Chin Chem Lett. 2021;32(10):2939–46. doi:10.1016/j.cclet.2021.04.059. [Google Scholar] [CrossRef]
18. Guo M, Song W, Tian J. Biochar-facilitated soil remediation: mechanisms and efficacy variations. Front Environ Sci. 2020;8:521512. doi:10.3389/fenvs.2020.521512. [Google Scholar] [CrossRef]
19. Tran TK, Huynh L, Nguyen HL, Nguyen MK, Lin C, Hoang TD, et al. Applications of engineered biochar in remediation of heavy metal(loid)s pollution from wastewater: current perspectives toward sustainable development goals. Sci Total Environ. 2024;926:171859. doi:10.1016/j.scitotenv.2024.171859. [Google Scholar] [PubMed] [CrossRef]
20. Khan S, Irshad S, Mehmood K, Hasnain Z, Nawaz M, Rais A, et al. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: a review. Plants. 2024;13(2):166. doi:10.3390/plants13020166. [Google Scholar] [PubMed] [CrossRef]
21. Kabata-Pendias A, Mukherjee AB. Trace elements from soil to human. Berlin/Heidelberg: Springer; 2007. doi:10.1007/978-3-540-32714-1. [Google Scholar] [CrossRef]
22. Rahman SU, Qin A, Zain M, Mushtaq Z, Mehmood F, Riaz L, et al. Pb uptake, accumulation, and translocation in plants: plant physiological, biochemical, and molecular response: a review. Heliyon. 2024;10(6):e27724. doi:10.1016/j.heliyon.2024.e27724. [Google Scholar] [PubMed] [CrossRef]
23. Ali M, Nas FS. The effect of lead on plants in terms of growing and biochemical parameters: a review. MOJ Ecol Environ Sci. 2018;3(4):265–8. doi:10.15406/mojes.2018.03.00098. [Google Scholar] [CrossRef]
24. Zheng S, Ren P, Zhai M, Li C, Chen Q. Identification of genes involved in root growth inhibition under lead stress by transcriptome profiling in Arabidopsis. Plant Mol Biol Report. 2021;39(1):50–9. doi:10.1007/s11105-020-01233-y. [Google Scholar] [CrossRef]
25. Vasilachi IC, Stoleru V, Gavrilescu M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture. 2023;13(10):1983. doi:10.3390/agriculture13101983. [Google Scholar] [CrossRef]
26. Hadi F, Aziz T. A mini review on lead (Pb) toxicity in plants. J Biol Life Sci. 2015;6(2):91. doi:10.5296/jbls.v6i2.7152. [Google Scholar] [CrossRef]
27. Rani M, Vikas, Kumar R, Lathwal M, Kamboj A. Effect and responses of lead toxicity in plants. In: Kumar N, Jha AK, editors. Lead toxicity mitigation: sustainable nexus approaches. Environmental contamination remediation and management. Cham: Springer Nature Switzerland; 2024. p. 211–41. doi: 10.1007/978-3-031-46146-0_10. [Google Scholar] [CrossRef]
28. Chlubek M, Baranowska-Bosiacka I. Selected functions and disorders of mitochondrial metabolism under lead exposure. Cells. 2024;13(14):1182. doi:10.3390/cells13141182. [Google Scholar] [PubMed] [CrossRef]
29. Navabpour S, Yamchi A, Bagherikia S, Kafi H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol Mol Biol Plants. 2020;26(4):793–802. doi:10.1007/s12298-020-00777-3. [Google Scholar] [PubMed] [CrossRef]
30. Qadir S, Jamshieed S, Rasool S, Ashraf M, Akram NA, Ahmad P. Modulation of plant growth and metabolism in cadmium-enriched environments. Rev Environ Contam Toxicol. 2014;229:51–88. doi:10.1007/978-3-319-03777-6_4. [Google Scholar] [PubMed] [CrossRef]
31. Haider FU, Cai L, Coulter JA, Cheema SA, Jun W, Zhang R, et al. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. doi:10.1016/j.ecoenv.2020.111887. [Google Scholar] [PubMed] [CrossRef]
32. Khan Z, Elahi A, Bukhari DA, Rehman A. Cadmium sources, toxicity, resistance and removal by microorganisms-a potential strategy for cadmium eradication. J Saudi Chem Soc. 2022;26(6):101569. doi:10.1016/j.jscs.2022.101569. [Google Scholar] [CrossRef]
33. Moravčíková D, Žiarovská J. The effect of cadmium on plants in terms of the response of gene expression level and activity. Plants. 2023;12(9):1848. doi:10.3390/plants12091848. [Google Scholar] [PubMed] [CrossRef]
34. Ullah S, Liu Q, Wang S, Jan AU, Sharif HMA, Ditta A, et al. Sources, impacts, factors affecting Cr uptake in plants, and mechanisms behind phytoremediation of Cr-contaminated soils. Sci Total Environ. 2023;899:165726. doi:10.1016/j.scitotenv.2023.165726. [Google Scholar] [PubMed] [CrossRef]
35. Saud S, Wang D, Fahad S, Javed T, Jaremko M, Abdelsalam NR, et al. The impact of chromium ion stress on plant growth, developmental physiology, and molecular regulation. Front Plant Sci. 2022;13:994785. doi:10.3389/fpls.2022.994785. [Google Scholar] [PubMed] [CrossRef]
36. Cruz FJR, da Cruz Ferreira RL, Conceição SS, Lima EU, de Oliveira Neto CF, Galvão JR, et al. Copper toxicity in plants: nutritional, physiological, and biochemical aspects. Adv Plant Defense Mech. 2022. doi: 10.5772/intechopen.105212. [Google Scholar] [CrossRef]
37. Mir AR, Pichtel J, Hayat S. Copper: uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals. 2021;34(4):737–59. doi:10.1007/s10534-021-00306-z. [Google Scholar] [PubMed] [CrossRef]
38. Feil SB, Pii Y, Valentinuzzi F, Tiziani R, Mimmo T, Cesco S. Copper toxicity affects phosphorus uptake mechanisms at molecular and physiological levels in Cucumis sativus plants. Plant Physiol Biochem. 2020;157:138–47. doi:10.1016/j.plaphy.2020.10.023. [Google Scholar] [PubMed] [CrossRef]
39. Nahar K, Rhaman MS, Parvin K, Bardhan K, Marques DN, García-Caparrós P, et al. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses. 2022;2(2):179–209. doi:10.3390/stresses2020013. [Google Scholar] [CrossRef]
40. Kaur H, Garg N. Zinc toxicity in plants: a review. Planta. 2021;253(6):129. doi:10.1007/s00425-021-03642-z. [Google Scholar] [PubMed] [CrossRef]
41. Kumar S, Wang M, Liu Y, Fahad S, Qayyum A, Jadoon SA, et al. Nickel toxicity alters growth patterns and induces oxidative stress response in sweetpotato. Front Plant Sci. 2022;13:1054924. doi:10.3389/fpls.2022.1054924. [Google Scholar] [PubMed] [CrossRef]
42. Ahmad M, Ok YS, Rajapaksha AU, Lim JE, Kim BY, Ahn JH, et al. Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: chemical, microbial and spectroscopic assessments. J Hazard Mater. 2016;301:179–86. doi:10.1016/j.jhazmat.2015.08.029. [Google Scholar] [PubMed] [CrossRef]
43. Rahi AA, Younis U, Ahmed N, Ali MA, Fahad S, Sultan H, et al. Toxicity of Cadmium and nickel in the context of applied activated carbon biochar for improvement in soil fertility. Saudi J Biol Sci. 2022;29(2):743–50. doi:10.1016/j.sjbs.2021.09.035. [Google Scholar] [PubMed] [CrossRef]
44. Houben D, Evrard L, Sonnet P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere. 2013;92(11):1450–7. doi:10.1016/j.chemosphere.2013.03.055. [Google Scholar] [PubMed] [CrossRef]
45. Almaroai YA, Usman ARA, Ahmad M, Moon DH, Cho JS, Joo YK, et al. Effects of biochar, cow bone, and eggshell on Pb availability to maize in contaminated soil irrigated with saline water. Environ Earth Sci. 2014;71(3):1289–96. doi:10.1007/s12665-013-2533-6. [Google Scholar] [CrossRef]
46. Zheng RL, Cai C, Liang JH, Huang Q, Chen Z, Huang YZ, et al. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, as in rice (Oryza sativa L.) seedlings. Chemosphere. 2012;89(7):856–62. doi:10.1016/j.chemosphere.2012.05.008. [Google Scholar] [PubMed] [CrossRef]
47. Ahmad M, Lee SS, Lim JE, Lee SE, Cho JS, Moon DH, et al. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere. 2014;95:433–41. doi:10.1016/j.chemosphere.2013.09.077. [Google Scholar] [PubMed] [CrossRef]
48. Ghosh D, Maiti SK. Biochar assisted phytoremediation and biomass disposal in heavy metal contaminated mine soils: a review. Int J Phytoremediation. 2021;23(6):559–76. doi:10.1080/15226514.2020.1840510. [Google Scholar] [PubMed] [CrossRef]
49. Puga AP, Abreu CA, Melo LCA, Beesley L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J Environ Manage. 2015;159:86–93. doi:10.1016/j.jenvman.2015.05.036. [Google Scholar] [PubMed] [CrossRef]
50. Adejumo SA, Owoseni O, Mur LAJ. Low light intensity and compost modified biochar enhanced maize growth on contaminated soil and minimized Pb induced oxidative stress. J Environ Chem Eng. 2021;9(2):104764. doi:10.1016/j.jece.2020.104764. [Google Scholar] [CrossRef]
51. Irfan M, Mudassir M, Khan MJ, Dawar KM, Muhammad D, Ahmad Mian I, et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci Rep. 2021;11:18416. doi:10.1038/s41598-021-97525-8. [Google Scholar] [PubMed] [CrossRef]
52. Rathika R, Srinivasan P, Alkahtani J, Al-Humaid LA, Alwahibi MS, Mythili R, et al. Influence of biochar and EDTA on enhanced phytoremediation of lead contaminated soil by Brassica juncea. Chemosphere. 2021;271:129513. doi:10.1016/j.chemosphere.2020.129513. [Google Scholar] [PubMed] [CrossRef]
53. Abd El-Mageed TA, Abdurrahman HA, Abd El-Mageed SA. Residual acidified biochar modulates growth, physiological responses, and water relations of maize (Zea mays) under heavy metal-contaminated irrigation water. Environ Sci Pollut Res Int. 2020;27(18):22956–66. doi:10.1007/s11356-020-08847-5. [Google Scholar] [PubMed] [CrossRef]
54. Shen X, Huang DY, Ren XF, Zhu HH, Wang S, Xu C, et al. Phytoavailability of Cd and Pb in crop straw biochar-amended soil is related to the heavy metal content of both biochar and soil. J Environ Manage. 2016;168:245–51. doi:10.1016/j.jenvman.2015.12.019. [Google Scholar] [PubMed] [CrossRef]
55. Wagner A, Kaupenjohann M. Biochar addition enhanced growth of Dactylis glomerata L. and immobilized Zn and Cd but mobilized Cu and Pb on a former sewage field soil. Eur J Soil Sci. 2015;66(3):505–15. doi:10.1111/ejss.12246. [Google Scholar] [CrossRef]
56. Almaroai YA, Eissa MA. Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci Hortic. 2020;265:109210. doi:10.1016/j.scienta.2020.109210. [Google Scholar] [CrossRef]
57. Zulqurnain Haider M, Hussain S, Muhammad Adnan Ramzani P, Iqbal M, Iqbal M, Shahzad T, et al. Bentonite and biochar mitigate Pb toxicity in Pisum sativum by reducing plant oxidative stress and Pb translocation. Plants. 2019;8(12):571. doi:10.3390/plants8120571. [Google Scholar] [PubMed] [CrossRef]
58. Puga AP, Abreu CA, Melo LA, Paz-Ferreiro J, Beesley L. Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw biochar. Environ Sci Pollut Res Int. 2015;22(22):17606–14. doi:10.1007/s11356-015-4977-6. [Google Scholar] [PubMed] [CrossRef]
59. Askeland M, Clarke B, Paz-Ferreiro J. Comparative characterization of biochars produced at three selected pyrolysis temperatures from common woody and herbaceous waste streams. PeerJ. 2019;7:e6784. doi:10.7717/peerj.6784. [Google Scholar] [PubMed] [CrossRef]
60. Liu P, Ptacek CJ, Blowes DW, Zou Finfrock Y, Liu Y. Characterization of chromium species and distribution during Cr(VI) removal by biochar using confocal micro-X-ray fluorescence redox mapping and X-ray absorption spectroscopy. Environ Int. 2020;134:105216. doi:10.1016/j.envint.2019.105216. [Google Scholar] [PubMed] [CrossRef]
61. Singh SK, Reddy VR, Fleisher DH, Timlin DJ. Relationship between photosynthetic pigments and chlorophyll fluorescence in soybean under varying phosphorus nutrition at ambient and elevated CO2. Photosynthetica. 2017;55(3):421–33. doi:10.1007/s11099-016-0657-0. [Google Scholar] [CrossRef]
62. Zewdu F, Amare M, Wong BM. Determination of the level of hexavalent, trivalent, and total chromium in the discharged effluent of Bahir Dar tannery using ICP-OES and UV–Visible spectrometry. Cogent Chem. 2018;4(1):1534566. doi:10.1080/23312009.2018.1534566. [Google Scholar] [CrossRef]
63. Bashir MA, Naveed M, Ahmad Z, Gao B, Mustafa A, Núñez-Delgado A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J Environ Manage. 2020;259:110051. doi:10.1016/j.jenvman.2019.110051. [Google Scholar] [PubMed] [CrossRef]
64. Ahmad Z, Gao B, Mosa A, Yu H, Yin X, Bashir A, et al. Removal of Cu(IICd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J Cleaner Prod. 2018;180:437–49. doi:10.1016/j.jclepro.2018.01.133. [Google Scholar] [CrossRef]
65. Igalavithana AD, Mandal S, Niazi NK, Vithanage M, Parikh SJ, Mukome FND, et al. Advances and future directions of biochar characterization methods and applications. Crit Rev Environ Sci Technol. 2017;47(23):2275–330. doi:10.1080/10643389.2017.1421844. [Google Scholar] [CrossRef]
66. Zhu Q, Wu J, Wang L, Yang G, Zhang X. Effect of biochar on heavy metal speciation of paddy soil. Water, Air, Soil Poll. 2015;226(12):429. doi:10.1007/s11270-015-2680-3. [Google Scholar] [CrossRef]
67. Herath I, Kumarathilaka P, Navaratne A, Rajakaruna N, Vithanage M. Immobilization and phytotoxicity reduction of heavy metals in serpentine soil using biochar. J Soils Sediments. 2015;15(1):126–38. doi:10.1007/s11368-014-0967-4. [Google Scholar] [CrossRef]
68. Herath I, Iqbal MCM, Al-Wabel MI, Abduljabbar A, Ahmad M, Usman ARA, et al. Bioenergy-derived waste biochar for reducing mobility, bioavailability, and phytotoxicity of chromium in anthropized tannery soil. J Soils Sediments. 2017;17(3):731–40. doi:10.1007/s11368-015-1332-y. [Google Scholar] [CrossRef]
69. Guo X, Ji Q, Rizwan M, Li H, Li D, Chen G. Effects of biochar and foliar application of selenium on the uptake and subcellular distribution of chromium in Ipomoea aquatica in chromium-polluted soils. Ecotoxicol Environ Saf. 2020;206:111184. doi:10.1016/j.ecoenv.2020.111184. [Google Scholar] [PubMed] [CrossRef]
70. Sarvajeet, Sarvjeet PK, Kumar V, Devanand. Improvement in growth parameters of rice (Oryza sativa L.) in chromium contaminated soil due to BC application. Int J Curr Microbiol Appl Sci. 2020;11:3105–16. [Google Scholar]
71. Xu M, Barbosa da Silva E, Gao P, Liao R, Wu J, Ma J, et al. Biochar impact on chromium accumulation by rice through Fe microbial-induced redox transformation. J Hazard Mater. 2020;388:121807. doi:10.1016/j.jhazmat.2019.121807. [Google Scholar] [PubMed] [CrossRef]
72. Sun P, Chen Y, Li X, Liu L, Guo J, Zheng X, et al. Detoxification mechanisms of biochar on plants in chromium contaminated soil: chromium chemical forms and subcellular distribution. Chemosphere. 2023;327:138505. doi:10.1016/j.chemosphere.2023.138505. [Google Scholar] [PubMed] [CrossRef]
73. Sami H, Ashraf K, Sultan K, Alamri S, Abbas M, Javied S, et al. Remediation potential of biochar and selenium for mitigating chromium-induced stress in spinach to minimize human health risk. S Afr J Bot. 2023;163:237–49. doi:10.1016/j.sajb.2023.10.049. [Google Scholar] [CrossRef]
74. Velli P, Manolikaki I, Diamadopoulos E. Effect of biochar produced from sewage sludge on tomato (Solanum lycopersicum L.) growth, soil chemical properties and heavy metal concentrations. J Environ Manage. 2021;297:113325. doi:10.1016/j.jenvman.2021.113325. [Google Scholar] [PubMed] [CrossRef]
75. Bashir MA, Naveed M, Ashraf S, Mustafa A, Ali Q, Rafique M, et al. Performance of Zea mays L. cultivars in tannery polluted soils: management of chromium phytotoxicity through the application of biochar and compost. Physiol Plantarum. 2021;173(1):129–47. [Google Scholar]
76. Bashir MA, Wang X, Naveed M, Mustafa A, Ashraf S, Samreen T, et al. Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. Int J Environ Res Public Health. 2021;18(9):4461. doi:10.3390/ijerph18094461. [Google Scholar] [PubMed] [CrossRef]
77. Taoze L, Bangyu L, Wei Z. Nutrients and HMs in BC produced by sewage sludge pyrolysis: its application in soil amendment. Pol J Environ Stud. 2014;23(1):271–5. [Google Scholar]
78. Ibrahim EA, El-Sherbini MAA, Selim EM. Effects of biochar on soil properties, heavy metal availability and uptake, and growth of summer squash grown in metal-contaminated soil. Sci Hortic. 2022;301:111097. doi:10.1016/j.scienta.2022.111097. [Google Scholar] [CrossRef]
79. Munir MAM, Liu G, Yousaf B, Mian MM, Ali MU, Ahmed R, et al. Contrasting effects of biochar and hydrothermally treated coal gangue on leachability, bioavailability, speciation and accumulation of heavy metals by rapeseed in copper mine tailings. Ecotoxicol Environ Saf. 2020;191:110244. doi:10.1016/j.ecoenv.2020.110244. [Google Scholar] [PubMed] [CrossRef]
80. Deng P, Wan W, Azeem M, Riaz L, Zhang W, Yang Y, et al. Characterization of biochar derived from bamboo and its application to modulate the toxic effects of chromium on wheat plant. Biomass Conv Bioref. 2024;14(6):7643–58. doi:10.1007/s13399-022-02879-2. [Google Scholar] [CrossRef]
81. Dutta A, Patra A, Jatav HS, Jatav SS, Singh SK, Sathyanarayana E, et al. Toxicity of cadmium in soil-plant-human continuum and its bioremediation techniques. In: Marcelo LL, Sonia S, editors. Soil contamination–threats and sustainable solutions. 3rd ed. London, England; IntechOpen; 2020. doi:10.5772/intechopen.94307. [Google Scholar] [CrossRef]
82. Haider FU, Wang X, Farooq M, Hussain S, Cheema SA, Ain NU, et al. Biochar application for the remediation of trace metals in contaminated soils: implications for stress tolerance and crop production. Ecotoxicol Environ Saf. 2022;230:113165. doi:10.1016/j.ecoenv.2022.113165. [Google Scholar] [PubMed] [CrossRef]
83. Zhuang Z, Niño-Savala AG, Mi ZD, Wan YN, Su DC, Li HF, et al. Cadmium accumulation in wheat and maize grains from China: interaction of soil properties, novel enrichment models and soil thresholds. Environ Pollut. 2021;275:116623. doi:10.1016/j.envpol.2021.116623. [Google Scholar] [PubMed] [CrossRef]
84. Wang F, Zhang S, Cheng P, Zhang S, Sun Y. Effects of soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics. 2020;8(4):102. doi:10.3390/toxics8040102. [Google Scholar] [PubMed] [CrossRef]
85. Rizwan MS, Imtiaz M, Zhu J, Yousaf B, Hussain M, Ali L, et al. Immobilization of Pb and Cu by organic and inorganic amendments in contaminated soil. Geoderma. 2021;385:114803. doi:10.1016/j.geoderma.2020.114803. [Google Scholar] [CrossRef]
86. Hamid Y, Tang L, Sohail MI, Cao X, Hussain B, Aziz MZ, et al. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci Total Environ. 2019;660:80–96. doi:10.1016/j.scitotenv.2018.12.419. [Google Scholar] [PubMed] [CrossRef]
87. Abbas T, Rizwan M, Ali S, Zia-ur-Rehman M, Farooq Qayyum M, Abbas F, et al. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Saf. 2017;140:37–47. doi:10.1016/j.ecoenv.2017.02.028. [Google Scholar] [PubMed] [CrossRef]
88. Elbagory M, Farrag DK, Hashim AM, Omara AE. The combined effect of Pseudomonas stutzeri and biochar on the growth dynamics and tolerance of lettuce plants (Lactuca sativa) to cadmium stress. Horticulturae. 2021;7(11):430. doi:10.3390/horticulturae7110430. [Google Scholar] [CrossRef]
89. Younis U, Qayyum MF, Shah MHR, Danish S, Shahzad AN, Ahmad Malik S, et al. Growth, survival, and heavy metal (Cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J Plant Nutr Soil Sci. 2015;178(2):209–17. doi:10.1002/jpln.201400325. [Google Scholar] [CrossRef]
90. Moreno-Jiménez E, Fernández JM, Puschenreiter M, Williams PN, Plaza C. Availability and transfer to grain of As, Cd, Cu, Ni, Pb and Zn in a barley agri-system: impact of biochar, organic and mineral fertilizers. Agric Ecosyst Environ. 2016;219:171–8. doi:10.1016/j.agee.2015.12.001. [Google Scholar] [CrossRef]
91. Lei M, Li Z, Zhang B, Wang X, Tie B, Ayaz T, et al. Mechanisms of stress alleviation after lime and biochar applications for Brassica napus L. in cadmium-contaminated soil. Adsorption Sci Technol. 2022;2022:4195119. doi:10.1155/2022/4195119. [Google Scholar] [CrossRef]
92. Bashir S, Hussain Q, Shaaban M, Hu H. Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere. 2018;211:632–9. doi:10.1016/j.chemosphere.2018.07.168. [Google Scholar] [PubMed] [CrossRef]
93. Mohamed I, Zhang GS, Li ZG, Liu Y, Chen F, Dai K. Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol Eng. 2015;84:67–76. doi:10.1016/j.ecoleng.2015.07.009. [Google Scholar] [CrossRef]
94. Abedinzadeh M, Etesami H, Alikhani HA, Shafiei S. Combined use of municipal solid waste biochar and bacterial biosorbent synergistically decreases Cd(II) and Pb(II) concentration in edible tissue of forage maize irrigated with heavy metal-spiked water. Heliyon. 2020;6(8):e04688. doi:10.1016/j.heliyon.2020.e04688. [Google Scholar] [PubMed] [CrossRef]
95. Kang X, Geng N, Li X, Yu J, Wang H, Pan H, et al. Biochar alleviates phytotoxicity by minimizing bioavailability and oxidative stress in foxtail millet (Setaria italica L.) cultivated in Cd- and Zn-contaminated soil. Front Plant Sci. 2022;13:782963. doi:10.3389/fpls.2022.782963. [Google Scholar] [PubMed] [CrossRef]
96. Sun J, Fan Q, Ma J, Cui L, Quan G, Yan J, et al. Effects of biochar on cadmium (Cd) uptake in vegetables and its natural downward movement in saline-alkali soil. Environ Pollut Bioavailability. 2020;32(1):36–46. doi:10.1080/26395940.2020.1714487. [Google Scholar] [CrossRef]
97. Hu J, Wu F, Wu S, Lam CL, Lin X, Wong MH. Biochar and Glomus caledonium influence Cd accumulation of upland Kangkong (Ipomoea aquatica Forsk.) intercropped with Alfred stonecrop (Sedum alfredii Hance). Sci Rep. 2014;4:4671. doi:10.1038/srep04671. [Google Scholar] [PubMed] [CrossRef]
98. Kim HS, Kim KR, Kim HJ, Yoon JH, Yang JE, Ok YS, et al. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ Earth Sci. 2015;74(2):1249–59. doi:10.1007/s12665-015-4116-1. [Google Scholar] [CrossRef]
99. Azeem M, Ali A, Arockiam Jeyasundar PGS, Bashir S, Hussain Q, Wahid F, et al. Effects of sheep bone biochar on soil quality, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil. Chemosphere. 2021;282:131016. doi:10.1016/j.chemosphere.2021.131016. [Google Scholar] [PubMed] [CrossRef]
100. Zhang W, Abdelrahman M, Jiu S, Guan L, Han J, Zheng T, et al. VvmiR160s/VvARFs interaction and their spatio-temporal expression/cleavage products during GA-induced grape parthenocarpy. BMC Plant Biol. 2019;19(1):111. doi:10.1186/s12870-019-1719-9. [Google Scholar] [PubMed] [CrossRef]
101. Chen H, Yang R, Zhang X, Chen Y, Xia Y, Xu X. Foliar application of gibberellin inhibits the cadmium uptake and xylem transport in lettuce (Lactuca sativa L.). Sci Hortic. 2021;288:110410. doi:10.1016/j.scienta.2021.110410. [Google Scholar] [CrossRef]
102. Lu H, Li Z, Fu S, Méndez A, Gascó G, Paz-Ferreiro J. Can biochar and phytoextractors be jointly used for cadmium remediation? PLoS One. 2014;9(4):e95218. doi:10.1371/journal.pone.0095218. [Google Scholar] [PubMed] [CrossRef]
103. Li H, Ye X, Geng Z, Zhou H, Guo X, Zhang Y, et al. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J Hazard Mater. 2016;304:40–8. doi:10.1016/j.jhazmat.2015.10.048. [Google Scholar] [PubMed] [CrossRef]
104. Irshad MK, Noman A, Alhaithloul HAS, Adeel M, Rui Y, Shah T, et al. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and as co-contaminated paddy soil. Sci Total Environ. 2020;717:137086. doi:10.1016/j.scitotenv.2020.137086. [Google Scholar] [PubMed] [CrossRef]
105. Jing F, Chen C, Chen X, Liu W, Wen X, Hu S, et al. Effects of wheat straw derived biochar on cadmium availability in a paddy soil and its accumulation in rice. Environ Pollut. 2020;257:113592. doi:10.1016/j.envpol.2019.113592. [Google Scholar] [PubMed] [CrossRef]
106. Bashir S, Qayyum MA, Husain A, Bakhsh A, Ahmed N, Hussain MB, et al. Efficiency of different types of biochars to mitigate Cd stress and growth of sunflower (Helianthus annuus L.) in wastewater irrigated agricultural soil. Saudi J Biol Sci. 2021;28(4):2453–9. doi:10.1016/j.sjbs.2021.01.045. [Google Scholar] [PubMed] [CrossRef]
107. Abid M, Danish S, Zafar-Ul-Hye M, Shaaban M, Iqbal MM, Rehim A, et al. Biochar increased photosynthetic and accessory pigments in tomato (Solanum lycopersicum L.) plants by reducing cadmium concentration under various irrigation waters. Environ Sci Pollut Res Int. 2017;24(27):22111–8. doi:10.1007/s11356-017-9866-8. [Google Scholar] [PubMed] [CrossRef]
108. Hussain S, Irfan M, Sattar A, Hussain S, Ullah S, Abbas T, et al. Alleviation of cadmium stress in wheat through the combined application of boron and biochar via regulating morpho-physiological and antioxidant defense mechanisms. Agronomy. 2022;12(2):434. doi:10.3390/agronomy12020434. [Google Scholar] [CrossRef]
109. Ghassemi-Golezani K, Farhangi-Abriz S. Biochar related treatments improved physiological performance, growth and productivity of Mentha crispa L. plants under fluoride and cadmium toxicities. Ind Crops Prod. 2023;194:116287. doi:10.1016/j.indcrop.2023.116287. [Google Scholar] [CrossRef]
110. Yadav R, Ramakrishna W. Biochar as an environment-friendly alternative for multiple applications. Sustainability. 2023;15(18):13421. doi:10.3390/su151813421. [Google Scholar] [CrossRef]
111. Rehman A, Arif MS, Tufail MA, Shahzad SM, Farooq TH, Ahmed W, et al. Biochar potential to relegate metal toxicity effects is more soil driven than plant system: a global meta-analysis. J Clean Prod. 2021;316:128276. doi:10.1016/j.jclepro.2021.128276. [Google Scholar] [CrossRef]
112. Salmani M, Khorsandi F, Yasrebi J, Karimian N. Biochar effects on copper availability and uptake by sunflower in a copper contaminated calcareous soil. Int J Plant Anim Environ Sci. 2014;4(3):389–94. [Google Scholar]
113. Quartacci MF, Sgherri C, Cardelli R. Biochar amendment reduces oxidative stress in lettuce grown under copper excess. Agrochimica. 2015;2015(2):188–202. doi:10.12871/0021857201527. [Google Scholar] [CrossRef]
114. Abideen Z, Koyro HW, Zulfiqar F, Moosa A, Rasool SG, Ahmad MZ, et al. Impact of biochar amendments on copper mobility, phytotoxicity, photosynthesis and mineral fluxes on (Zea mays L.) in contaminated soils. S Afr J Bot. 2023;158:469–78. doi:10.1016/j.sajb.2023.05.036. [Google Scholar] [CrossRef]
115. Buss W, Kammann C, Koyro HW. Biochar reduces copper toxicity in Chenopodium quinoa Willd. in a sandy soil. J Environ Qual. 2012;41(4):1157–65. doi:10.2134/jeq2011.0022. [Google Scholar] [PubMed] [CrossRef]
116. Fontanive DE, Rafaele DM, Andreola DS, de Oliveira Stumm J, Serafini RF, da Silva DM, et al. Biochar in copper reduction in black beans and soil decontamination. Rev Bras Ciênc Ambient. 2023;58(3):386–94. doi:10.5327/z2176-94781595. [Google Scholar] [CrossRef]
117. Yang W, Pan Y, Yu X, Xiao S, Wang W, Lu M. Biochar and cropping systems changed soil copper speciation and accumulation in sweet corn and soybean. Plants. 2022;11(18):2375. doi:10.3390/plants11182375. [Google Scholar] [PubMed] [CrossRef]
118. Ahmad M, Usman ARA, Al-Faraj AS, Ahmad M, Sallam A, Al-Wabel MI. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere. 2018;194:327–39. doi:10.1016/j.chemosphere.2017.11.156. [Google Scholar] [PubMed] [CrossRef]
119. Al-Wabel MI, Usman ARA, El-Naggar AH, Aly AA, Ibrahim HM, Elmaghraby S, et al. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J Biol Sci. 2015;22(4):503–11. doi:10.1016/j.sjbs.2014.12.003. [Google Scholar] [PubMed] [CrossRef]
120. Gonzaga MIS, Matias MIAS, Andrade KR, Jesus AN, Cunha GDC, Andrade RS, et al. Aged biochar changed copper availability and distribution among soil fractions and influenced corn seed germination in a copper-contaminated soil. Chemosphere. 2020;240:124828. doi:10.1016/j.chemosphere.2019.124828. [Google Scholar] [PubMed] [CrossRef]
121. Medyńska-Juraszek A, Rivier PA, Rasse D, Joner EJ. Biochar affects heavy metal uptake in plants through interactions in the rhizosphere. Appl Sci. 2020;10(15):5105. doi:10.3390/app10155105. [Google Scholar] [CrossRef]
122. Lu K, Yang X, Shen J, Robinson B, Huang H, Liu D, et al. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric Ecosyst Environ. 2014;191:124–32. doi:10.1016/j.agee.2014.04.010. [Google Scholar] [CrossRef]
123. Pir Dad F, Khan WUD, Ijaz U, Sun H, Rafi MN, Alamri S, et al. Potential of amino acids-modified biochar in mitigating the soil Cu and Ni stresses-Targeting the tomato growth, physiology and fruit quality. Plant Physiol Biochem. 2024;211:108711. doi:10.1016/j.plaphy.2024.108711. [Google Scholar] [PubMed] [CrossRef]
124. Rehman M, Liu L, Bashir S, Saleem MH, Chen C, Peng D, et al. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol Biochem. 2019;138:121–9. doi:10.1016/j.plaphy.2019.02.021. [Google Scholar] [PubMed] [CrossRef]
125. Ali A, Guo D, Zhang Y, Sun X, Jiang S, Guo Z, et al. Using bamboo biochar with compost for the stabilization and phytotoxicity reduction of heavy metals in mine-contaminated soils of China. Sci Rep. 2017;7(1):2690. doi:10.1038/s41598-017-03045-9. [Google Scholar] [PubMed] [CrossRef]
126. Ghandali MV, Safarzadeh S, Ghasemi-Fasaei R, Zeinali S. Heavy metals immobilization and bioavailability in multi-metal contaminated soil under ryegrass cultivation as affected by ZnO and MnO2 nanoparticle-modified biochar. Sci Rep. 2024;14(1):10684. doi:10.1038/s41598-024-61270-5. [Google Scholar] [PubMed] [CrossRef]
127. Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int. 2020;137:105576. doi:10.1016/j.envint.2020.105576. [Google Scholar] [PubMed] [CrossRef]
128. Kumar A, Tsechansky L, Lew B, Raveh E, Frenkel O, Graber ER. Biochar alleviates phytotoxicity in Ficus elastica grown in Zn-contaminated soil. Sci Total Environ. 2018;618:188–98. doi:10.1016/j.scitotenv.2017.11.013. [Google Scholar] [PubMed] [CrossRef]
129. Medyńska-Juraszek A, Bednik M, Chohura P. Assessing the influence of compost and biochar amendments on the mobility and uptake of heavy metals by green leafy vegetables. Int J Environ Res Public Health. 2020;17(21):7861. doi:10.3390/ijerph17217861. [Google Scholar] [PubMed] [CrossRef]
130. Waqas M, Li G, Khan S, Shamshad I, Reid BJ, Qamar Z, et al. Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in tomato. Environ Sci Pollut Res. 2015;22(16):12114–23. doi:10.1007/s11356-015-4432-8. [Google Scholar] [PubMed] [CrossRef]
131. Houben D, Sonnet P. Impact of biochar and root-induced changes on metal dynamics in the rhizosphere of Agrostis capillaris and Lupinus albus. Chemosphere. 2015;139:644–51. doi:10.1016/j.chemosphere.2014.12.036. [Google Scholar] [PubMed] [CrossRef]
132. Burachevskaya M, Mandzhieva S, Bauer T, Minkina T, Rajput V, Chaplygin V, et al. The effect of granular activated carbon and biochar on the availability of Cu and Zn to Hordeum sativum distichum in contaminated soil. Plants. 2021;10(5):841. doi:10.3390/plants10050841. [Google Scholar] [PubMed] [CrossRef]
133. Awad M, Moustafa-Farag M, Wei L, Huang Q, Liu Z. Effect of garden waste biochar on the bioavailability of heavy metals and growth of Brassica juncea (L.) in a multi-contaminated soil. Arab J Geosci. 2020;13(12):439. doi:10.1007/s12517-020-05376-w. [Google Scholar] [CrossRef]
134. Wagner A, Kaupenjohann M. Suitability of biochars (pyro- and hydrochars) for metal immobilization on former sewage-field soils. Eur J Soil Sci. 2014;65(1):139–48. doi:10.1111/ejss.12090. [Google Scholar] [CrossRef]
135. Kim HS, Kim KR, Ok YS, Lee YK, Kluge B, Wessolek G, et al. Examination of three different organic waste biochars as soil amendment for metal-contaminated agricultural soils. Water Air Soil Poll. 2015;226(9):282. doi:10.1007/s11270-015-2556-6. [Google Scholar] [CrossRef]
136. Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int. 2019;125:365–85. doi:10.1016/j.envint.2019.01.067. [Google Scholar] [PubMed] [CrossRef]
137. Sarwar T, Khan S, Muhammad S, Amin S. Arsenic speciation, mechanisms, and factors affecting rice uptake and potential human health risk: a systematic review. Environ Technol Innov. 2021;22:101392. doi:10.1016/j.eti.2021.101392. [Google Scholar] [CrossRef]
138. Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, et al. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health. 2018;15(1):59. doi:10.3390/ijerph15010059. [Google Scholar] [PubMed] [CrossRef]
139. Hakeem KR, Alharby HF, Bamagoos AAM, Pirzadah TB. Biochar promotes arsenic (As) immobilization in contaminated soils and alleviates the As-toxicity in soybean (Glycine max (L.) Merr.). Chemosphere. 2022;292:133407. doi:10.1016/j.chemosphere.2021.133407. [Google Scholar] [PubMed] [CrossRef]
140. Yu Z, Qiu W, Wang F, Lei M, Wang D, Song Z. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere. 2017;168:341–9. doi:10.1016/j.chemosphere.2016.10.069. [Google Scholar] [PubMed] [CrossRef]
141. Strawn DG, Rigby AC, Baker LL, Coleman MD, Koch I. Biochar soil amendment effects on arsenic availability to mountain brome (Bromus marginatus). J Environ Qual. 2015;44(4):1315–20. doi:10.2134/jeq2014.11.0477. [Google Scholar] [PubMed] [CrossRef]
142. Beesley L, Marmiroli M, Pagano L, Pigoni V, Fellet G, Fresno T, et al. Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci Total Environ. 2013;454-455:598–603. doi:10.1016/j.scitotenv.2013.02.047. [Google Scholar] [PubMed] [CrossRef]
143. Gregory SJ, Anderson CWN, Camps Arbestain M, McManus MT. Response of plant and soil microbes to biochar amendment of an arsenic-contaminated soil. Agric Ecosyst Environ. 2014;191:133–41. doi:10.1016/j.agee.2014.03.035. [Google Scholar] [CrossRef]
144. Qin J, Niu A, Liu Y, Lin C. Arsenic in leafy vegetable plants grown on mine water-contaminated soils: uptake, human health risk and remedial effects of biochar. J Hazard Mater. 2021;402:123488. doi:10.1016/j.jhazmat.2020.123488. [Google Scholar] [PubMed] [CrossRef]
145. Sahin O, Taskın MB, Kaya E, Taskin H. Poultry manure biochar reduces arsenic induced oxidative stress and arsenic levels in rice plants. J Agric Fac Uludag Univ. 2017;31(1):103–13. [Google Scholar]
146. Namgay T, Singh B, Singh BP. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Soil Res. 2010;48(7):638. doi:10.1071/sr10049. [Google Scholar] [CrossRef]
147. Rahman MM, Das AK, Sultana S, Ghosh PK, Islam MR, Keya SS, et al. Biochar potentially enhances maize tolerance to arsenic toxicity by improving physiological and biochemical responses to excessive arsenate. Biochar. 2023;5(1):71. doi:10.1007/s42773-023-00270-6. [Google Scholar] [CrossRef]
148. Sattar A, Sher A, Abourehab MAS, Ijaz M, Nawaz M, Ul-Allah S, et al. Application of silicon and biochar alleviates the adversities of arsenic stress in maize by triggering the morpho-physiological and antioxidant defense mechanisms. Front Environ Sci. 2022;10:979049. doi:10.3389/fenvs.2022.979049. [Google Scholar] [CrossRef]
149. Liao Y, Ashraf H, Huang S, Ramzan M, Saba R, Baqir M, et al. Unveiling the efficacy of Bacillus faecalis and composted biochar in alleviating arsenic toxicity in maize. BMC Plant Biol. 2024;24(1):660. doi:10.1186/s12870-024-05372-2. [Google Scholar] [PubMed] [CrossRef]
150. Alsamadany H, Alharby HF, Al-Zahrani HS, Alzahrani YM, Almaghamsi AA, Abbas G, et al. Silicon-nanoparticles doped biochar is more effective than biochar for mitigation of arsenic and salinity stress in Quinoa: insight to human health risk assessment. Front Plant Sci. 2022;13:989504. doi:10.3389/fpls.2022.989504. [Google Scholar] [PubMed] [CrossRef]
151. Ghazanfar S, Komal A, Waseem A, Hassan W, Iqbal RJ, Toor S, et al. Physiological effects of nickel contamination on plant growth. Nat Volatiles Essent Oils. 2021;8(5):13457–69. [Google Scholar]
152. Alfano M, Cavazza C. Structure, function, and biosynthesis of nickel-dependent enzymes. Protein Sci. 2020;29(5):1071–89. doi:10.1002/pro.3836. [Google Scholar] [PubMed] [CrossRef]
153. Rehman S, Mansoora N, Al-Dhumri SA, Amjad SF, Al-Shammari WB, Almutari MM, et al. Associative effects of activated carbon biochar and arbuscular mycorrhizal fungi on wheat for reducing nickel food chain bioavailability. Environ Technol Innov. 2022;26:102539. doi:10.1016/j.eti.2022.102539. [Google Scholar] [CrossRef]
154. Hassan MU, Chattha MU, Khan I, Chattha MB, Aamer M, Nawaz M, et al. Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities—a review. Environ Sci Pollut Res Int. 2019;26(13):12673–88. doi:10.1007/s11356-019-04892-x. [Google Scholar] [PubMed] [CrossRef]
155. Shahzad B, Tanveer M, Rehman A, Cheema SA, Fahad S, Rehman S, et al. Nickel; whether toxic or essential for plants and environment—a review. Plant Physiol Biochem. 2018;132:641–51. doi:10.1016/j.plaphy.2018.10.014. [Google Scholar] [PubMed] [CrossRef]
156. Ullah S, Ali I, Liang H, Zhao Q, Wei S, Muhammad I, et al. An approach to sustainable agriculture by untangling the fate of contrasting nitrogen sources in double-season rice grown with and without biochar. GCB Bioenergy. 2021;13(3):382–92. doi:10.1111/gcbb.12789. [Google Scholar] [CrossRef]
157. Ameen N, Amjad M, Murtaza B, Abbas G, Shahid M, Imran M, et al. Biogeochemical behavior of nickel under different abiotic stresses: toxicity and detoxification mechanisms in plants. Environ Sci Pollut Res Int. 2019;26(11):10496–514. doi:10.1007/s11356-019-04540-4. [Google Scholar] [PubMed] [CrossRef]
158. Hasanuzzaman M, Alam MM, Nahar K, Mohsin SM, Bhuyan MHMB, Parvin K, et al. Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology. 2019;28(3):261–76. doi:10.1007/s10646-019-02019-z. [Google Scholar] [PubMed] [CrossRef]
159. Rizwan M, Mostofa MG, Ahmad MZ, Imtiaz M, Mehmood S, Adeel M, et al. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere. 2018;191:23–35. doi:10.1016/j.chemosphere.2017.09.068. [Google Scholar] [PubMed] [CrossRef]
160. Cheng H, Jones DL, Hill P, Bastami MS, Tu CL. Influence of biochar produced from different pyrolysis temperature on nutrient retention and leaching. Arch Agron Soil Sci. 2018;64(6):850–9. doi:10.1080/03650340.2017.1384545. [Google Scholar] [CrossRef]
161. Hannan F, Islam F, Huang Q, Farooq MA, Ayyaz A, Fang R, et al. Interactive effects of biochar and mussel shell activated concoctions on immobilization of nickel and their amelioration on the growth of rapeseed in contaminated aged soil. Chemosphere. 2021;282:130897. doi:10.1016/j.chemosphere.2021.130897. [Google Scholar] [PubMed] [CrossRef]
162. Kamran M, Malik Z, Parveen A, Huang L, Riaz M, Bashir S, et al. Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J Plant Growth Regul. 2020;39(1):266–81. doi:10.1007/s00344-019-09980-3. [Google Scholar] [CrossRef]
163. Qiao Y, Crowley D, Wang K, Zhang H, Li H. Effects of biochar and Arbuscular mycorrhizae on bioavailability of potentially toxic elements in an aged contaminated soil. Environ Pollut. 2015;206:636–43. doi:10.1016/j.envpol.2015.08.029. [Google Scholar] [PubMed] [CrossRef]
164. Younis U, Malik SA, Qayyum MF, Shah MHR, Shahzad AN, Mahmood S. BC affects growth and biochemical activities of fenugreek (Trigonella Corniculata) in cadmium polluted soil. J Appl Bot Food Qual. 2015;18(9):4461. doi:10.3390/ijerph18094461. [Google Scholar] [PubMed] [CrossRef]
165. Younis U, Athar M, Malik SA, Raza Shah MH, Mahmood S. Biochar impact on physiological and biochemical attributes of spinach Spinacia oleracea (L.) in nickel contaminated soil. Global J Environ Sci Manage. 2015;1(3):245–54. [Google Scholar]
166. Younis U, Danish S, Malik SA, Ahmed N, Munir TM, Rasheed MK. Role of cotton sticks biochar in immobilization of nickel under induced toxicity condition and growth indices of Trigonella corniculata L. Environ Sci Pollut Res Int. 2020;27(2):1752–61. doi:10.1007/s11356-019-06466-3. [Google Scholar] [PubMed] [CrossRef]
167. Turan V, Ramzani PMA, Ali Q, Abbas F, Iqbal M, Irum A, et al. Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Arch Agron Soil Sci. 2018;64(8):1053–67. doi:10.1080/03650340.2017.1410542. [Google Scholar] [CrossRef]
168. Shahbaz AK, Lewińska K, Iqbal J, Ali Q, Mahmood-Ur-Rahman, Iqbal M, et al. Improvement in productivity, nutritional quality, and antioxidative defense mechanisms of sunflower (Helianthus annuus L.) and maize (Zea mays L.) in nickel contaminated soil amended with different biochar and zeolite ratios. J Environ Manage. 2018;218:256–70. doi:10.1016/j.jenvman.2018.04.046. [Google Scholar] [PubMed] [CrossRef]
169. Kamali M, Sweygers N, Al-Salem S, Appels L, Aminabhavi TM, Dewil R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem Eng J. 2022;428:131189. doi:10.1016/j.cej.2021.131189. [Google Scholar] [CrossRef]
170. Ahmad M, Ok YS, Kim BY, Ahn JH, Lee YH, Zhang M, et al. Impact of soybean stover- and pine needle-derived biochars on Pb and as mobility, microbial community, and carbon stability in a contaminated agricultural soil. J Environ Manage. 2016;166:131–9. doi:10.1016/j.jenvman.2015.10.006. [Google Scholar] [PubMed] [CrossRef]
171. Bolan S, Hou D, Wang L, Hale L, Egamberdieva D, Tammeorg P, et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci Total Environ. 2023;886:163968. doi:10.1016/j.scitotenv.2023.163968. [Google Scholar] [PubMed] [CrossRef]
172. Wang Z, Shen F, Shen D, Jiang Y, Xiao R. Immobilization of Cu2+ and Cd2+ by earthworm manure derived biochar in acidic circumstance. J Environ Sci. 2017;53:293–300. doi:10.1016/j.jes.2016.05.017. [Google Scholar] [PubMed] [CrossRef]
173. Shahcheraghi N, Golchin H, Sadri Z, Tabari Y, Borhanifar F, Makani S. Nano-biotechnology, an applicable approach for sustainable future. 3 Biotech. 2022;12(3):65. doi:10.1007/s13205-021-03108-9. [Google Scholar] [PubMed] [CrossRef]
174. Sifton MA, Smith SM, Thomas SC. Biochar-biofertilizer combinations enhance growth and nutrient uptake in silver maple grown in an urban soil. PLoS One. 2023;18(7):e0288291. doi:10.1371/journal.pone.0288291. [Google Scholar] [PubMed] [CrossRef]
175. Wang F, Wang X, Song N. Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric, Ecosyst Environ. 2021;315:107425. doi:10.1016/j.agee.2021.107425. [Google Scholar] [CrossRef]
176. Seregin IV, Kozhevnikova AD. Phytochelatins: sulfur-containing metal(loid)-chelating ligands in plants. Int J Mol Sci. 2023;24(3):2430. doi:10.3390/ijms24032430. [Google Scholar] [PubMed] [CrossRef]
177. Shi Q, Deng S, Zheng Y, Du Y, Li L, Yang S, et al. The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ Res. 2022;212:113340. doi:10.1016/j.envres.2022.113340. [Google Scholar] [PubMed] [CrossRef]
178. Rashid MS, Liu G, Yousaf B, Hamid Y, Rehman A, Arif M, et al. Role of biochar-based free radicals in immobilization and speciation of metals in the contaminated soil-plant environment. J Environ Manage. 2023;325(Pt B):116620. doi:10.1016/j.jenvman.2022.116620. [Google Scholar] [PubMed] [CrossRef]
179. Mehmood S, Ahmed W, Ikram M, Imtiaz M, Mahmood S, Tu S, et al. Chitosan modified biochar increases soybean (Glycine max L.) resistance to salt-stress by augmenting root morphology, antioxidant defense mechanisms and the expression of stress-responsive genes. Plants. 2020;9(9):1173. doi:10.3390/plants9091173. [Google Scholar] [PubMed] [CrossRef]
180. Khan Z, Fan X, Khan MN, Khan MA, Zhang K, Fu Y, et al. The toxicity of heavy metals and plant signaling facilitated by biochar application: implications for stress mitigation and crop production. Chemosphere. 2022;308(Pt 3):136466. doi:10.1016/j.chemosphere.2022.136466. [Google Scholar] [PubMed] [CrossRef]
181. Amin MA, Haider G, Rizwan M, Schofield HK, Qayyum MF, Zia-Ur-Rehman M, et al. Different feedstocks of biochar affected the bioavailability and uptake of heavy metals by wheat (Triticum aestivum L.) plants grown in metal contaminated soil. Environ Res. 2023;217:114845. doi:10.1016/j.envres.2022.114845. [Google Scholar] [PubMed] [CrossRef]
182. Zhao M, Dai Y, Zhang M, Feng C, Qin B, Zhang W, et al. Mechanisms of Pb and/or Zn adsorption by different biochars: biochar characteristics, stability, and binding energies. Sci Total Environ. 2020;717:136894. doi:10.1016/j.scitotenv.2020.136894. [Google Scholar] [PubMed] [CrossRef]
183. Bakshe P, Jugade R. Phytostabilization and rhizofiltration of toxic heavy metals by heavy metal accumulator plants for sustainable management of contaminated industrial sites: a comprehensive review. J Hazard Mater Adv. 2023;10:100293. doi:10.1016/j.hazadv.2023.100293. [Google Scholar] [CrossRef]
184. Rizwan M, Murtaza G, Zulfiqar F, Moosa A, Iqbal R, Ahmed Z, et al. Tuning active sites on biochars for remediation of mercury-contaminated soil: a comprehensive review. Ecotoxicol Environ Saf. 2024;270:115916. doi:10.1016/j.ecoenv.2023.115916. [Google Scholar] [PubMed] [CrossRef]
185. Murtaza G, Ahmed Z, Usman M, Iqbal R, Zulfiqar F, Tariq A, et al. Physicochemical properties and performance of non-woody derived biochars for the sustainable removal of aquatic pollutants: a systematic review. Chemosphere. 2024;359:142368. doi:10.1016/j.chemosphere.2024.142368. [Google Scholar] [PubMed] [CrossRef]
186. Rizwan M, Murtaza G, Zulfiqar F, Moosa A, Iqbal R, Ahmed Z, et al. Sustainable manufacture and application of biochar to improve soil properties and remediate soil contaminated with organic impurities: a systematic review. Front Environ Sci. 2023;11:1277240. doi:10.3389/fenvs.2023.1277240. [Google Scholar] [CrossRef]
187. Yang Y, Ahmed W, Ye C, Yang L, Wu L, Dai Z, et al. Exploring the effect of different application rates of biochar on the accumulation of nutrients and growth of flue-cured tobacco (Nicotiana tabacum). Front Plant Sci. 2024;15:1225031. doi:10.3389/fpls.2024.1225031. [Google Scholar] [PubMed] [CrossRef]
188. Campion L, Bekchanova M, Malina R, Kuppens T. The costs and benefits of biochar production and use: a systematic review. J Clean Prod. 2023;408:137138. doi:10.1016/j.jclepro.2023.137138. [Google Scholar] [CrossRef]
189. Godlewska P, Ok YS, Oleszczuk P. The dark side of black gold: ecotoxicological aspects of biochar and biochar-amended soils. J Hazard Mater. 2021;403:123833. doi:10.1016/j.jhazmat.2020.123833. [Google Scholar] [PubMed] [CrossRef]
190. Batool M, Khan WUD, Hamid Y, Farooq MA, Naeem MA, Nadeem F. Interaction of pristine and mineral engineered biochar with microbial community in attenuating the heavy metals toxicity: a review. Appl Soil Ecol. 2022;175:104444. doi:10.1016/j.apsoil.2022.104444. [Google Scholar] [CrossRef]
191. Pathy A, Pokharel P, Chen X, Balasubramanian P, Chang SX. Activation methods increase biochar’s potential for heavy-metal adsorption and environmental remediation: a global meta-analysis. Sci Total Environ. 2023;865:161252. doi:10.1016/j.scitotenv.2022.161252. [Google Scholar] [PubMed] [CrossRef]
192. Rombel A, Różyło K, Ok YS, Oleszczuk P. Influence of biochar characteristics on polycyclic aromatic hydrocarbons content during co-composting of sewage sludge. Bioresour Technol. 2025;423:132220. doi:10.1016/j.biortech.2025.132220. [Google Scholar] [PubMed] [CrossRef]
193. Zulfiqar F, Moosa A, Nazir MM, Ferrante A, Ashraf M, Nafees M, et al. Biochar: an emerging recipe for designing sustainable horticulture under climate change scenarios. Front Plant Sci. 2022;13:1018646. doi:10.3389/fpls.2022.1018646. [Google Scholar] [PubMed] [CrossRef]
194. Hasnain M, Munir N, Abideen Z, Zulfiqar F, Koyro HW, El-Naggar A, et al. Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: a critical review. Ecotoxicol Environ Saf. 2023;249:114408. doi:10.1016/j.ecoenv.2022.114408. [Google Scholar] [PubMed] [CrossRef]
195. Bi Y, Cai S, Wang Y, Zhao X, Wang S, Xing G, et al. Structural and microbial evidence for different soil carbon sequestration after four-year successive biochar application in two different paddy soils. Chemosphere. 2020;254:126881. doi:10.1016/j.chemosphere.2020.126881. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
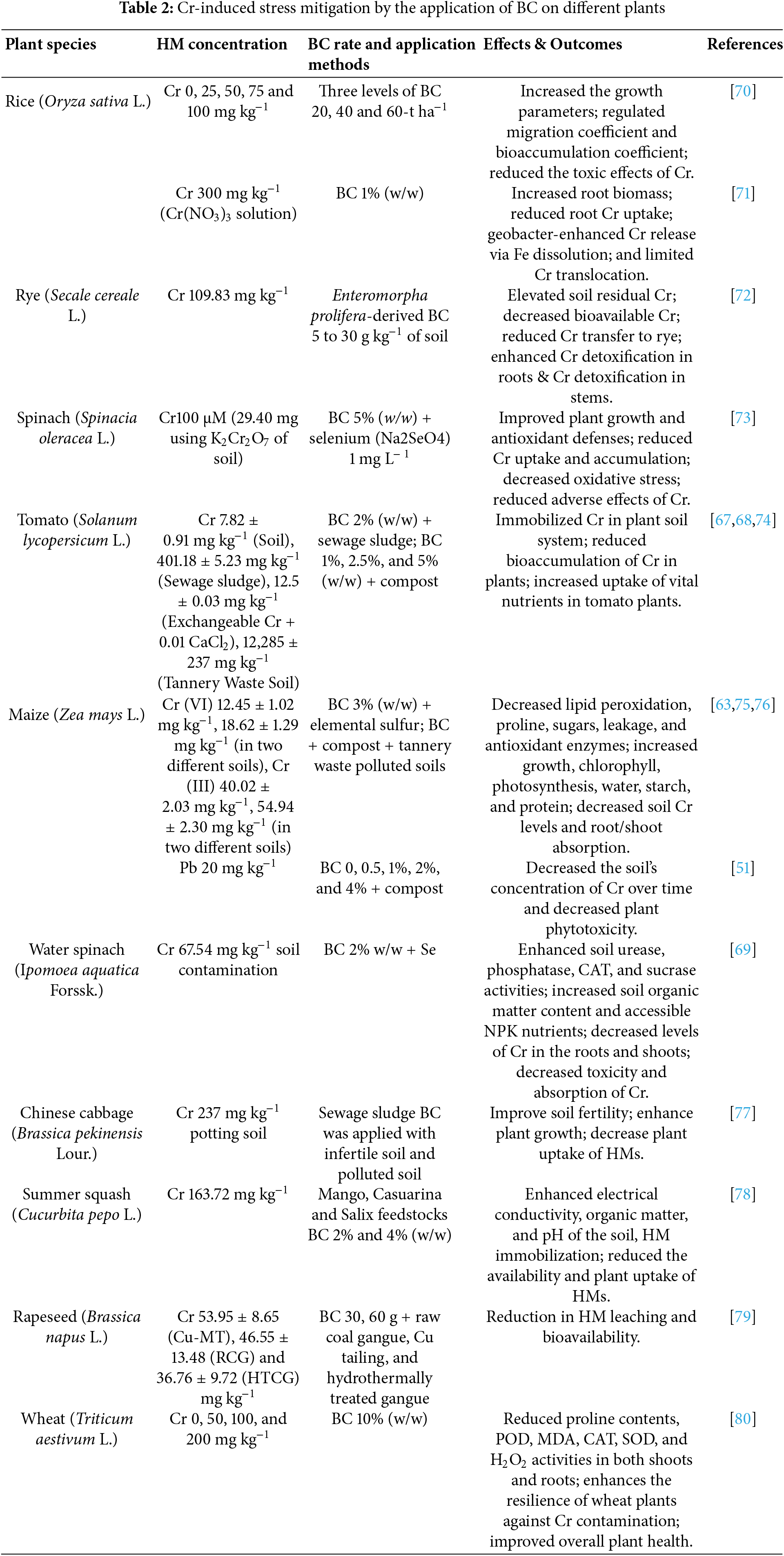




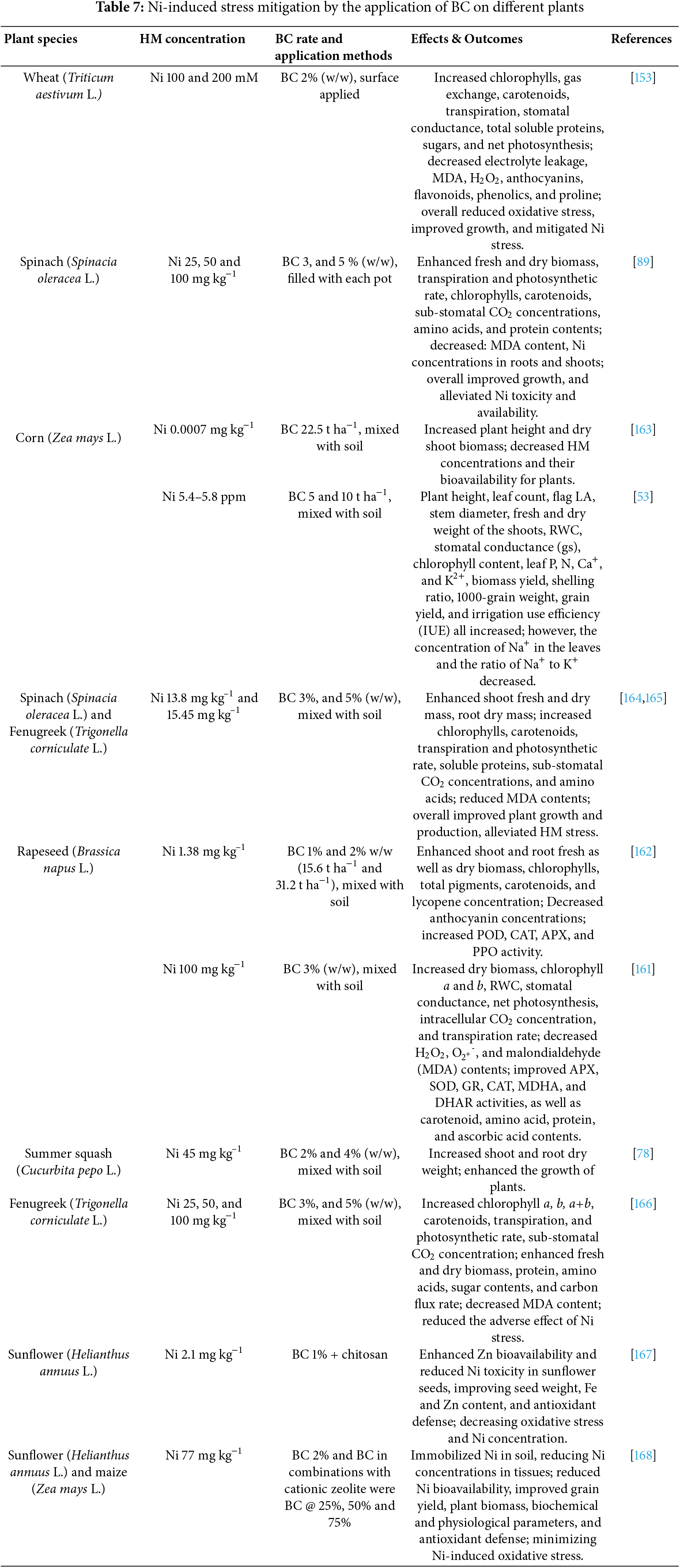
 Downloads
Downloads
 Citation Tools
Citation Tools