 Open Access
Open Access
REVIEW
Zinc Oxide Nanoparticles: Abiotic Stress Tolerance in Fruit Crops Focusing on Sustainable Production
1 Department of Horticulture, Bahauddin Zakariya University, Multan, 60800, Pakistan
2 Department of Botany, Biotechnology and Landscape Architecture, School of Natural Sciences, Tyumen State University, Tyumen, 625003, Russia
3 Department of Botany, The Government Sadiq College Women University, Bahawalpur, 63100, Pakistan
4 Department of Horticulture, The University of Agriculture, Dera Ismail Khan, 29220, Pakistan
* Corresponding Author: Riaz Ahmad. Email:
(This article belongs to the Special Issue: Advances in Fruit Resistance Research and Applications)
Phyton-International Journal of Experimental Botany 2025, 94(5), 1401-1418. https://doi.org/10.32604/phyton.2025.063930
Received 29 January 2025; Accepted 21 April 2025; Issue published 29 May 2025
Abstract
The productivity of fruit crops is badly affected by abrupt changes in climatic conditions. It is a matter of concern for fruit tree researchers to feed the huge population within the available resources. The adverse effects of abiotic stresses are increasing due to fluctuations in climate change. Several abiotic stresses (salinity, drought, water logging, minerals deficiency, temperature extremities and heavy metals) are reducing the overall productivity of crops. Therefore, the application of different management approaches, i.e., phytohormones, nanoparticles, organic amendments, microbes and molecular aspects are effective for the mitigation of abiotic stresses in fruit crops. The aim of the present review was to explore the potential of zinc oxide nanoparticles (ZnO-NPs) to lessen the adverse effects of abiotic stresses in fruit crops. Fruit crops are important sources of minerals and vitamins. ZnO-NPs could improve the tolerance mechanism of fruit crops by reducing oxidative harm. Moreover, these are involved in boosting the antioxidant properties of fruit trees. Regular formation of photosynthetic pigments involved in the regulation of the photosynthesis process through ZnO-NPs applications under adverse conditions. Their use can contribute to the regulation of several metabolic processes that occur in plants subjected to abiotic stresses. The disturbances in photosynthetic pigments, irregular metabolic processes and generation of toxic substances are causing stunted growth, low yield and poor fruit quality. Hence, the application of ZnO-NPs is important for the sustainable production of fruit crops by improving seedlings’ growth and fruit quality via activation of the plant defense system. However, higher concentration of nanoparticles results in growth inhibition and poor yield due to cytotoxicity, oxidative stress, and genotoxicity. Therefore, nanoparticle interaction with fruit crops needs more consideration at the epigenetic level for the mitigation of multiple stresses.Keywords
Fruits are famous valuable crops because they are rich in minerals and vitamin levels necessary for a healthy life [1]. There is a serious global threat to fruit crop productivity [2]. A drastic decline was observed in crop productivity due to abiotic stresses. Abiotic stresses enhance the generation of free radicals and toxic substances, resulting in hampered photosynthesis, impaired metabolic processes, disturbed carbon assimilation and enhanced membrane permeability in plants [3]. Moreover, several developmental processes necessary for sufficient growth are disturbed due to adversities of abiotic stresses [4]. Mitigation of abiotic stresses is necessary for the sustainable productivity of fruit trees.
Different management strategies, i.e., supplementation of phytohormones, microbes, organic amendments and nanotechnology, are effective for the alleviation of abiotic stress tolerance in fruit crops globally [5,6]. All these management strategies have the potential to improve abiotic stress tolerance in fruit crops. The cultivation of tolerant germplasm can enhance the productivity of crops [7]. Therefore, selection, evaluation and identification of tolerant genetic resources is one of the imperative approaches for the cultivation of tolerant germplasm focusing on higher yield and superior quality.
Innovations in nanotechnology enhanced its utilization in the sustainable farming of fruit trees [8]. Nanoparticles had excellent contributions to orchard management due to their smaller size, greater porosity, better mobility and higher surface area [9–11]. These nanoparticles had the potential to boost plant growth and yield due to their small sizes [12]. Several plant researchers are paying greater consideration to metal oxide nanoparticles (NPs) due to their prodigious performance, less expensive, and climate-friendly nature [13]. The optimum use of metal nanoparticles is effective for sufficient growth and yield, while higher levels than the threshold showed adverse effects on growth and yield due to toxicity [14]. However, the selection of nanoparticles depends on several factors, such as type of crop, stress level, cultural practices, and climatic conditions within the growing regions [15].
Zinc (Zn) is a beneficial micronutrient with a significant contribution to plant homeostasis and regulatory mechanisms that occur in numerous horticulture crops [16]. Zn had an excellent contribution to cell membrane rigidity, chloroplast development, photosynthetic pigment synthesis, and improved endogenous hormones [17]. Zn acts as a cofactor for several enzymes, such as ligases, hydrolases, isomerases, and transferases to regulate cellular metabolism [18,19]. Zinc has a significant involvement in the regulatory functioning of minerals absorption and plant-water relations, which further contributes to alleviating abiotic stress tolerance in plants [20,21]. Hence, from previous literature, it has been explored that Zn is significantly involved in the enhancement of abiotic stress tolerance in crops. However, its application on fruit crops is still limited and needs more investigations to explore the beneficial role of Zn on fruit trees growing under adverse climatic conditions.
Severity in temperatures, heavy rainfalls, speedy winds, degraded soils, floods, and rising CO2 levels are majorly due to climate change. However, the fluctuations in climate change are causing the abiotic stresses in plants [22]. Therefore, fluctuations in climate change are becoming much more challenging for plant researchers and farmers to get higher yields from their fruit crops. The vegetative growth of fruit crops is badly affected by adverse climatic conditions. Fruit crops are perennial and mostly propagated from vegetative parts (asexually) to maintain the productivity and quality of fruit crops [23]. Drought conditions disturbed the emergence of vegetative flushes, reduced water level in leaves, stinted flushes length, and poor number of leaves in mango trees [24]. Flower induction was poor due to water deficit conditions in mango trees. Female flowering was disturbed due to adverse climatic conditions [25]. Earlier research suggested that higher temperature and water stress improve the floral stimulation in trees growing under tropical conditions. However, temperature extremities and water stress for longer periods reduced the productivity of crops [26,27]. Climate change drastically reduced the vegetative and reproductive processes due to the occurrence of abiotic stresses (Table 1). It is imperative to explore the vegetative and reproductive processes that occur in fruit trees subjected to abiotic stress conditions. Moreover, the management of abiotic stresses is necessary for sustainable fruit tree production and superior quality for consumption.
Orchard management is necessary to improve the productivity of fruits globally. It is important to enhance the tolerance level of fruit trees against abiotic stress conditions for suitable productivity and quality. The impairing of physiological and biochemical processes is disturbing fruit tree productivity due to adverse climatic conditions. Hence, the regulation of physiological and biochemical processes is imperative for the sustainable farming of fruit crops. The current study aimed to explore the impact of ZnO-NPs on fruit trees subjected to abiotic stresses by the regulation of physiological and biochemical activities.
2 Influence of ZnO-NPs on Vegetative Growth of Fruit Crops
ZnO-NPs had the potential to mitigate adversities that occur from abiotic stress conditions in fruit crops. These had a better contribution in the enhancement of seed germination, seedling growth, roots emergence, leaf anatomy and vegetative expansion. Its beneficial contribution is based on application level, stress conditions, type of crop, and cultivation practices, which may vary. Amirjani et al. [37] revealed the synergistic interactions between Zn, K, and Mn contributed to the beneficial role of Zn in enhancing the K as well as Mn levels in leaf petioles. However, it has also been indicated that rising Zn levels decreased P and Fe levels, which may have been caused by Zn, Fe, and P differing responses [37]. The supplementation of ZnO-NPs as tiny carriers for the plant auxins indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) improved the rooting capability of Pyrus species. Karakeçili et al. [38] indicated that the rooting percentage was found to be greater at 400 mg L−1 of IBA-nZnO (50.0%) as well as IAA-nZnO (41.7%). Gupta et al. [39] stated that strawberry growth was improved by application of different nano-fertilizers. In another study by Bayat et al. [40], it has been studied that strawberry farming is more sustainable via the use of nano-fertilizers. However, ZnO-NPs fertilization of 20 ppm enhanced the tree growth-related traits of the Picual cultivar of olive, as reported by Genaidy et al. [41]. Similarly, Carlesso et al. [42] revealed that ZnO-NPs enhanced the physical attributes of strawberry crops growing within the region. Different concentrationss of ZnO-NPs (0 and 2 g L−1) were applied to the pear seedlings. It has been evaluated that vegetative growth comprising seedlings height, diameter, fresh and dry weights, leaf area, and number of leaves was improved by fertilization of ZnO-NPs [43]. Application of ZnO-NPs (180 mg L−1) enhanced the vegetative growth of grape vines cv. Flame Seedless. Another study by Mahdavi et al. [44] indicated that vegetative growth of grape berries was boosted with ZnO-NPs than control growing under calcareous land.
3 Power of ZnO-NPs on Reproductive Growth of Fruit Crops
Fruits are a vital source of nutrients for humans. Moreover, fruit crops are an important source of earnings for farming communities with higher incomes and a source of employment [45]. Abiotic stresses showed adverse effects on the growth phases of fruit crops subjected to adverse climatic conditions. Fruit crops majorly face salinity, drought, temperature, and heavy metal toxicity. Numerous morphological, anatomical, physiological, and biochemical alterations may occur in fruit crops, which further disturb the crop yield and fruit quality [45]. It is imperative to regulate reproductive growth for fruit crops under adverse climatic conditions (Fig. 1).
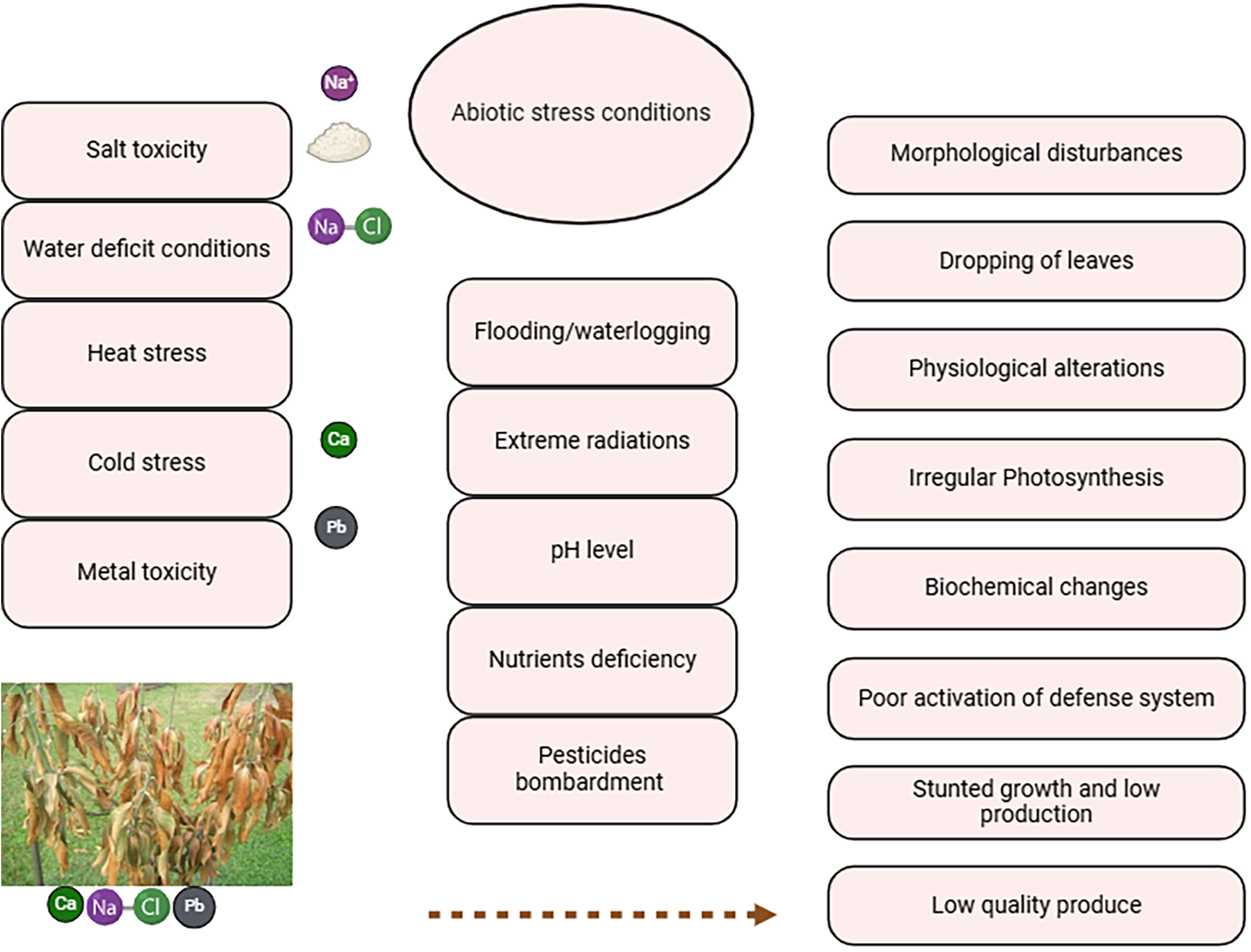
Figure 1: Abiotic stress conditions reduced the growth, yield and fruit quality of fruit crops by alteration in physiological and biochemical changes
Plant reproductive growth can be improved by the application of ZnO-NPs. The innovations in various management practices can be beneficial for sustainable yields of fruit trees. The bud initiation and formation were delayed due to drought conditions. Moreover, floral bud breakage occurred due to water deficit conditions [27]. The emergence of vegetative flushes was poor in mango plants subjected to drought stress. The decreased number of leaves in a flush, flush duration, and leaf water contents were recorded due to water stress conditions. Drought stress poses a significant threat to the floral induction of mango plants because floral induction is stimulated and produced from mature leaves. Flowering initiation can be enhanced on fruit trees by restricted water applications for a short period. However, prolonged water stress conditions may disturb the tree’s developmental processes [26]. The significant delay in flushing was recorded due to water stress, as depicted by Schaffer et al. [27]. Grapevines have excellent xylem arteries; therefore, these are considered more tolerant to drought stress as compared to some other fruit crops [46].
Banana crop production was affected by the low level of available moisture content in the soil. The carbon absorption was disrupted in plants due to restricted leaf water levels [47]. Moreover, the banana was also found to be sensitive to water deficiency at the flowering stage. Banana cultivar ‘Elakki’ productivity was lower due to water stress at the time of floral differentiation [48]. The maximum yield reduction was recorded in Banana cv. ‘Robusta’ when subjected to drought conditions for five weeks at the time of flowering [49].
Three cultivars of banana (Karpuravalli, Robusta, and Rasthali) were subjected to drought stress at the time of flowering. Moreover, the maximum yield reduction was recorded in cvs. Karpuravalli (42.07%), Robusta (25.0%), and Rasthali (18.83%) as illustrated by Ravi et al. [50]. Drought stress in papaya plants resulted in a 50% decrease in leaves, 86% flowering decline, and 58% yield reduction. Moreover, papaya fruit size and yield were also reduced due to drought stress conditions as reported by Masri et al. [51]. Different management practices i.e., growth promoters, use of different mulching materials, modifications managing nutrients, micro-watering, and timely irrigation practices, are important to mitigate adverse conditions that occur from water stress. Selection, characterization, and cultivation of tolerant cultivars need time to mitigate the adverse effects of abiotic stresses in fruit crops. Moreover, rootstock also had a significant contribution to the tolerance mechanism of abiotic stresses. Hence, appropriate management practices can enhance the abiotic stress tolerance mechanism in fruit crops [52].
ZnO-NPs had a positive impact on the improvements of the reproductive phase of fruits subjected to adverse climatic conditions. Different reproductive processes such as floral initiation, pollination and fruit formation of trees were significantly influenced by ZnO-NPs [53]. Similarly, a plant researcher indicated that ZnO-NPs (150 ppm) improved fruit yield and quality of strawberry cv. “Chandler” [54]. Mango yield was enhanced through the supplementation of 100 mg L−1 nZnO by Elsheery et al. [55]. Fruit crops such as pomegranate and mango trees that were sprayed with nano-Zn before flowering showed greater plant resilience to floral malformation disease and improved fruit quality [56,57]. The supplementation of zinc increases fruit retention percentage, fruit set per panicle, and flower biology in mango crops as revealed by the findings of Ahmad et al. [58]. The functionality of many enzymes comprising transphosphorylases, dehydrators, isomers, and aldolases also improved with the supplementation of ZnO-NPs. Furthermore, it has a regulatory contribution in the generation of tryptophan and protein and is occupied in cell division, photosynthetic processes, stomatal regulations, and prevention of membrane integrity [59]. ZnO-NPs boost the endogenous production of auxins. Auxins had a significant contribution in the improvement of reproductive phases of fruit growth and overall tree yield [60,61].
Floral initiation in mango can be influenced by the C/N ratio. Moreover, the accumulation and supply of carbohydrates also depend on the C/N ratio. Several metabolic processes and climatic factors disturbed the C/N ratio in trees, which resulted in the disturbance in the reproductive growth of trees, especially delay and irregular flower initiation observed in mango plants [62]. Modifications in phytohormones, particularly the decrease in gibberellin phases, coincide with a rise in sugars [63]. Hormonal balance is significantly maintained in plants by zinc (Zn) supplementation [64]. In fruit trees, the maximum fruit load on trees in a single year has been observed, while the minimum fruit load in the next year is mostly due to hormonal imbalances within the plants. Therefore, Zn was found to be effective for hormonal regulations in plants. Moreover, management practices can also regulate the fruit load on trees. The thinning of flowering may result in a low number of flowers on a single tree. The lower number of flowers on a tree results in a healthy fruit set and larger fruit size with superior fruit quality. The asymmetrical bearing habit of fruit load in mangoes can be regulated through various adaptive methods that successfully regulate endogenous phytohormones [65]. ZnO-NPs supplementation has improved fruit quality and yield with promising outcomes. Moreover, ZnO-NPs have been shown to improve the reproductive growth of fruit trees. However, its efficacy depends on application level, kind of crop, stress type and numerous other factors.
4 ZnO-NPs Regulate Morphological and Physiological Changes
ZnO-NPs had the potential to regulate morpho-physiological changes in fruit trees subjected to abiotic stresses. The over-generation of ROS is due to adverse climatic conditions in plants. The excess of ROS increased the oxidative harm in plants. The increased level of lipid peroxidation was found to be effective biomarkers for the determination of stress levels in plants. Different antioxidants (enzymatic and non-enzymatic) were regulated by the generation of various osmolytes and metabolites in plants. The regulation of ROS generation is necessary to protect plants from damaging effects that occur from oxidative harm due to adverse climatic conditions. Abd-Allatif et al. [66] determined the adverse effects of salinity stress in plants by disruption of several enzymes, disruption of cell membranes, protein impairment, and death of certain cells, tissues, and impaired functions. ROS overproduction was regulated by the activation of the plant defense system by modulation of several antioxidants and the formation of various osmolytes. These processes are required to maintain a balance in the formation of ROS, and the plants’ potential to scavenge toxic substances and free radicals depends on the robust immune response. Hence, optimum generation of ROS is important for sustainable fruit cultivation because membrane rupturing may reduce with balanced ROS production within the organelles [67,68]. Transgenic plants are tolerant to adverse conditions that occur from abiotic stresses. Moreover, transgenic plants exhibited higher levels of antioxidants and osmolytes which improved stress tolerance in plants against oxidative harm [69]. Fruit plants can effectively mitigate the negative effects of salinity through suitable fertilization. Fruit trees need to produce several bioactive substances which improve plants tolerance against various environmental challenges. Moreover, bioactive substances were boosted with exogenous supplementation of melatonin as exhibited by Chen et al. [70].
Fruit trees with suitable bioactive compound levels boost defense system, photosynthetic apparatus, stomatal regulation, and respiration rates which further contributes to the improvements of tolerance in plants [71]. Drought is the most critical environmental constraint that disturbs tree productivity [72]. Poor hydraulic conductivity was recorded in the xylem vessels of plants due to water deficit conditions. Moreover, stunted growth and low utilization of carbon levels were observed in plants due to drought conditions. However, poor utilization of carbon can also reduce the tree yield [73]. Root architecture is disturbed due to drought stress conditions in mango trees [74]. Moreover, salinity stress also alter the morpho-physiological activities of mango trees [75]. Drought stress enhanced the salt accumulation in the root biosphere of plants. Therefore, long-term exposure to salts is also toxic for higher plants. However, plants faced different salts such as calcium, magnesium, sodium, and chlorides. However, sodium salt is more dangerous for plants because its concentration may be higher in the root zone [76].
Mango is a valuable fruit crop. Excess of salts is toxic to mango trees because mango are sensitive to salt stress [77]. Salinity stress damages plant growth through osmotic imbalances and ionic distress [78,79]. The maximum leaf droppage was recorded in plants due to salinity stress by inhibition in nutrient absorption, carbon assimilation, and chlorophyll contents [80]. The suitable number of leaves contributes to better productivity of crops. The metabolic disturbances in plants were due to the degradation of proteins, lipids, and nucleic acids. Hence, metabolic disturbances allow the overgeneration of ROS in plants. The irregulates in ROS generation disturbed the morpho-physiological activities in plants [81]. The disturbances in morpho-physiological activities result in stunted tree growth, low yield, and inferior fruit quality, as explored by Ansari et al. [82].
The excessive ROS production is due to free radicals and toxic ions which disrupt homeostasis. The disturbances in osmotic pressure were due to excessive ROS generation. The rupturing of membranes, sugar oxidation, photosynthetic pigments degradation, denatured protein, disruption of antioxidants capability and reduced osmolyte formation are due to due to excessive ROS by altering the morpho-physiological activities. Soni et al. [83] revealed the excessive ROS production, and the oxidation of lipids as biomarkers to determine the severity of stress in plants, which disturbed the plant developmental processes. Plant cells and divisions that are subjected to sodium toxicity can reduce the tree yield. Lipid peroxidation assessment indicates that the damage to the membrane was caused by plants being exposed to more saline than normal conditions. The higher degree of damage to the plant cell membranes was indicated by the elevated presence of lipid peroxidation [83].
5 ZnO-NPs Boost Biochemical Activities in Fruit Crops
ZnO-NPs are involved in boosting biochemical activities in fruit trees subjected to abiotic stresses. The antioxidant enzymes and osmoprotectants could regulate and eliminate the excessive ROS within the cell organelles [84,85]. The activation of enzymatic, non-enzymatic, and metabolites contributes to reduce oxidative harm by ROS elimination [86]. Superoxide dismutase (SOD) is one of the most potent cytoplasmic metalloenzymes that contribute to the antioxidant defense network [43]. Moreover, it is comprised of metabolic factors that catalyze the conversion of superoxide anions into dioxygen (O2) and hydrogen peroxide (H2O2). Garg and Manchanda [87] revealed that SOD activity was found to be lower in normal-treated plants as compared to stress-level plants. Therefore, SOD had a significant contribution to the defense system of plants. Catalase (CAT) had the potential to scavenge and eliminate the toxic level of ROS which further reduced the oxidative harm [88]. Moreover, activation of the plant defense system was observed in response to oxidative damage. Hence, elevated SOD, CAT, and POX activities were measured in salt-stressed mango seedlings as compared to non-stressed mango seedlings. Several management approaches have been adopted to enhance the activity of these molecules and purposely over-expressed to control the permeability and ion buildup in mango plants under salt toxicity [89]. Applying NP materials to increase the resilience of plants to abiotic stresses is one of these approaches [90]. Under abiotic stresses, fruit cultivars with a tolerant nature showed greater improvements in antioxidant profiling, whereas sensitive germplasm showed a poor increase in antioxidant profiling [91].
Plants activate their defense system by stimulation of antioxidants within the plant cells and organelles subjected to adverse climatic conditions. Plant metabolism is disturbed due to irregularities in photosynthesis and enzymatic functionality. The regulation of plant metabolic processes is necessary for the activation of antioxidants to scavenge toxic ROS [92]. SOD might eliminate superoxide anions in excess from cells, as proved by the progress of plant defense mechanisms [93]. SOD had a strong chance of balancing O2 from the generation of H2O2. Hence, H2O2 is toxic to plants, as exerted by Dvořák et al. [92]. APX could eliminate the generation of H2O2 [94]. Moreover, APX activation had greater potential to scavenge toxic ROS in plants. Hence, it has been revealed that APX could improve tree tolerance in plants [93]. Fruit trees’ glyoxylic acid-circulating structures and peroxisomes, which effectively convert H2O2 into H2O, are the main locations of CAT. The plants are considered to be more sensitive to H2O2 because CAT significantly lowers H2O2 levels.
The fruit plants had the potential to improve abiotic stresses by the activation of CAT responses across cell divisions. H2O2 generation can be regulated with CAT activity. However, H2O2 is the most reliable indication of abiotic stresses [95]. Moreover, several non-enzymatic activities such as soluble sugars, ascorbic acid, phenols, and tocopherols were reduced under abiotic stresses. ROS regulation is important to reduce oxidative harm (Fig. 2). Fruit tree tolerance is important to improve for higher yield. Ascorbic acid synthesis is effective for improved tolerance levels in fruit crops. The rootstock had a greater contribution to the salinity tolerance of fruit crops by restricting the translocation of excess sodium in plants through roots. Jujube cv. “Gola” was found to be more tolerant due to higher levels of ascorbic acid. Hence, ascorbic acid had a greater contribution to the improvement of salinity tolerance in fruit crops [96].
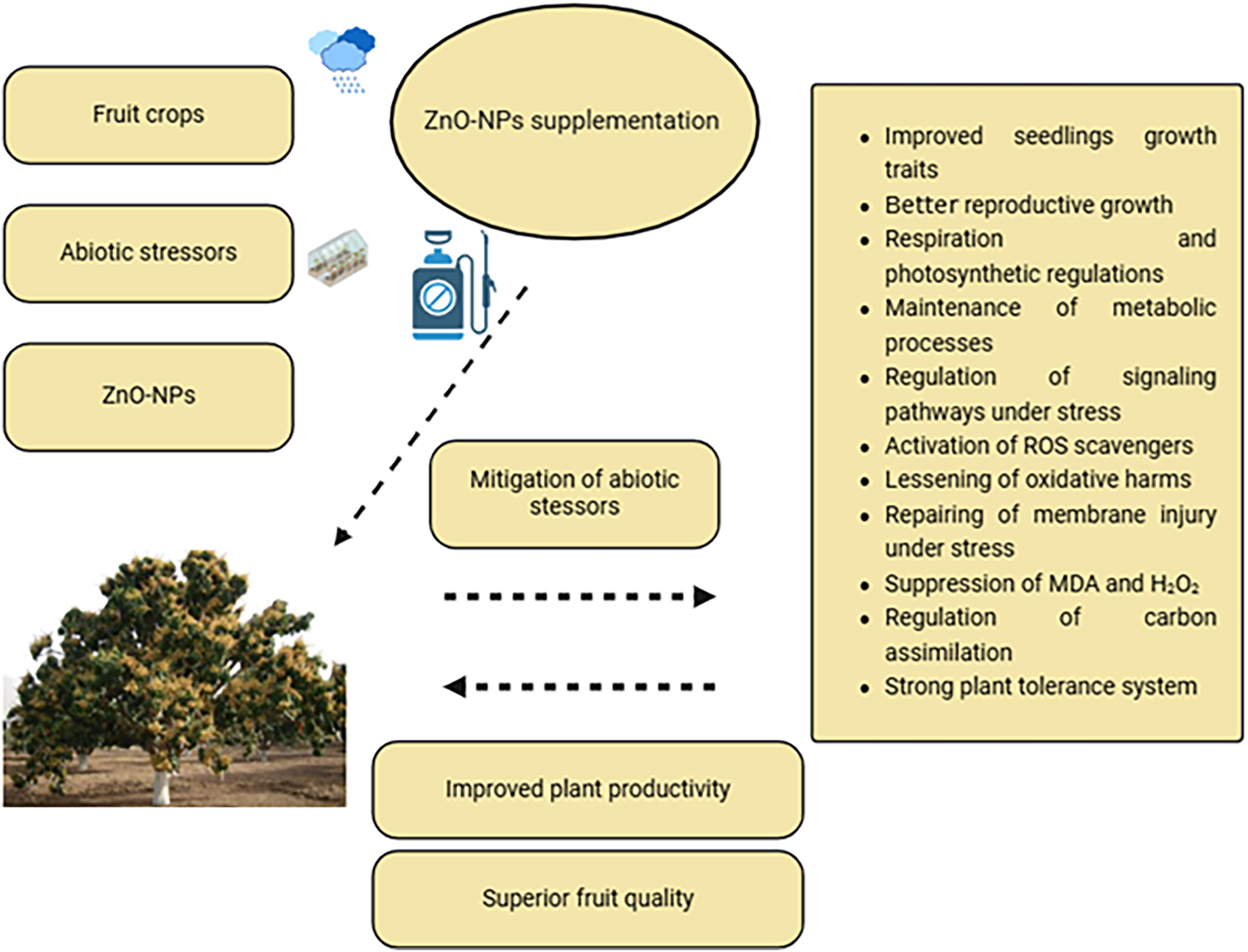
Figure 2: ZnO-NPs mitigate the adverse effects of abiotic stress on fruit crops focusing on the quality production of fruits
Tocopherol is an integral part of the plant because it can limit lipid radical generation and scavenge harmful ROS. The functioning of antioxidants and non-antioxidants is improved by tocopherol generation. Higher plants are comprised of various tocopherol isomers [97]. However, α-tocopherol had stronger antioxidant properties. Moreover, fruit trees have significant levels of α-tocopherol in their chloroplast envelopes [98]. The oxidation of lipids, carotenoids, and tocopherols had the capability to restore oxidized radicals and limit the extent of the signaling channel [99]. These also contribute to strengthening the defense system of plants subjected to abiotic stresses. Moreover, strong thylakoid membranes had the potential to mitigate the oxidative harms that occur in plants due to adverse climatic conditions.
The aerial plant components, i.e., leaf, pollen, flower, and fruit portions are high in flavonoids. Fruit plants commonly store them as glycosides in their vacuoles. In higher-level plants, they are also effective ROS explorers as revealed by Gadi [100]. Moreover, it has been linked to decreased lipid peroxidation and lipoxygenases. A plant researcher explored that flavonoid (1 mM) was very effective at limiting lipoxygenase in saline circumstances as explored by Potapovich and Kostyuk [101].
6 ZnO-NPs Enhance Shelf Life and Fruit Quality
Fruit quality can be enhanced with supplementation of ZnO-NPs. The practical implications are necessary to explore the fruit productivity to feed a huge population in the future. The customers are interested in fresh-cut fruits because of their novel and natural substances. Fruits’ deceased and living tissues are vulnerable to bacterial and fungal invasions, which results in spontaneous decomposition when the fruit is handled and stored. Fruit browning is attributed to the destruction of phenolic molecules that are o-quinone counterparts, which subsequently form polymers into intricate dark-colored pigments [102]. Several kinds of chemical and physiological remedies are used to retard deterioration and extend the freshness of fruit products. Immobilization of the enzyme is a crucial technique to increase their function and longevity. There are two techniques used to immobilize enzymes: covalent adhesion and physical adsorption. There are disadvantages to physical adsorption, including diminished enzyme activity, encapsulation of the active areas, and unwanted polarity of the molecules. However, the enzyme’s covalent immobilization with the particle surface releases the bound active compounds and also leads to an increase in performance [103].
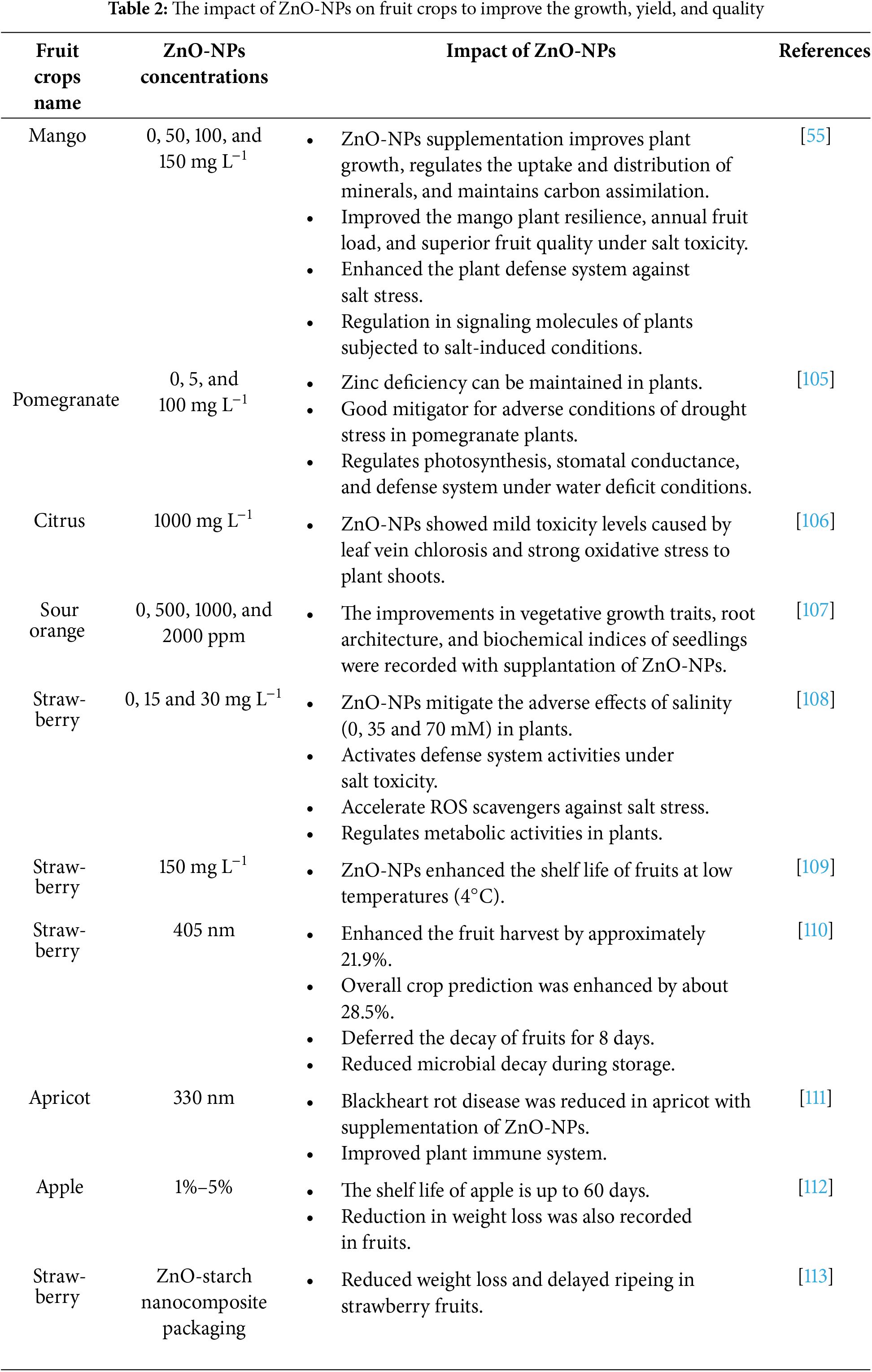
Its thermal resilience was raised by the covalent adherence of GOx on altered iron oxide nanoparticles. The results of the investigation revealed that the greatest activity of both free and immobilized enzymes was observed at 30°C. However, compared to free enzymes, immobilized enzymes remained more resilient when the temperature was enhanced [104]. Because of their fixed authorization, the enzymes affixed to the surface of the nanoparticle demonstrated greater resilience and durability than their free equivalents (Table 2).
The application of Zn-ONPs for the immobilization of enzymes is an intriguing strategy because it offers several advantages. Akbar et al. [114] revealed ZnO-NPs are used in food processing. Their opinion is that ZnO-NPs have a significant antibacterial impact and are a valuable source of Zn for food supplements, making their usage in the food business both acceptable and desired [114]. In light of the commercial significance of GOx hybrids, researchers conceptualized a novel antibacterial strategy to extend fruit shelf life. The GOx/ZnO-NPs bioconjugate was dissolved in a buffer to develop a spraying solution that was used on peach fruit [115]. The results of the treatment were tracked for 15 days at a temperature of 25°C ± 1. Fruit quality after storage was assessed using common quality indicators such as weight loss, fruit hardness, total soluble solids (TSS), and antioxidant activity in removing free radicals [115].
ZnO-NPs management of abiotic stresses in fruit crops seems to be a practical and long-term strategy that could improve production and quality. However, to fully realize this technology’s promise in real-world farming environments, more study and a thorough grasp of the agronomic as well as environmental variables are required.
ZnO-NPs had excellent potential to improve tree yield and fruit quality to combat abiotic stresses by improving the defense system. ZnO-NPs have high reactivity and small size; therefore, fruit crops must strengthen the membranes of plants under adverse climatic conditions. Fruit tree tolerance can be enhanced by supplementation of ZnO-NPs. Moreover, ZnO-NPs supplementation is effective in improving growth, yield, fruit quality, defense system, activation of antioxidants, osmolytes generation, and production of bioactive compounds to improve tree resilient mechanism. However, optimal concentrations of ZnO-NPs can be addressed in the future depending upon the severity of stress, the kind of crops, and their cultivars.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Meryam Manzoor and Konstantin Korolev designed and wrote the paper, Maryam and Riaz Ahmad reviewed and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The availability of data is not applicable and all the required data is included within the manuscript.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Bhardwaj RL, Parashar A, Parewa HP, Vyas L. An alarming decline in the nutritional quality of foods: the biggest challenge for future generations’ health. Foods. 2024;13(6):877. doi:10.3390/foods13060877. [Google Scholar] [PubMed] [CrossRef]
2. Zahid G, Iftikhar S, Shimira F, Ahmad HM, Aka Kaçar Y. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: a review. Sci Hortic. 2023;309(3):111621. doi:10.1016/j.scienta.2022.111621. [Google Scholar] [CrossRef]
3. Rajput VD, Minkina T, Kumari A, Harish, Singh VK, Verma KK, et al. Coping with the challenges of abiotic stress in plants: new dimensions in the field application of nanoparticles. Plants. 2021;10(6):1221. doi:10.3390/plants10061221. [Google Scholar] [PubMed] [CrossRef]
4. Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–31. doi:10.4161/psb.6.11.17613. [Google Scholar] [PubMed] [CrossRef]
5. Collins D, Luxton T, Kumar N, Shah S, Walker VK, Shah V. Assessing the impact of copper and zinc oxide nanoparticles on soil: a field study. PLoS One. 2012;7(8):e42663. doi:10.1371/journal.pone.0042663. [Google Scholar] [PubMed] [CrossRef]
6. Bera K, Dutta P, Sadhukhan S. Plant responses under abiotic stress and mitigation options towards agricultural sustainability. In: Plant stress: challenges and management in the new decade. Cham, Switzerland: Springer International Publishing; 2022. p. 3–28. doi:10.1007/978-3-030-95365-2_1. [Google Scholar] [CrossRef]
7. Fideghelli C, Vitellozzi F, Grassi F, Sartori A. Characterization and evaluation of fruit germplasm for a sustainable use. Acta Hortic. 2003;2003(598):153–60. doi:10.17660/actahortic.2003.598.22. [Google Scholar] [CrossRef]
8. Haq IU. Harnessing nanotechnology for superior fruit crop quality and yield enhancement. Trends Anim Plant Sci. 2024;4:74–81. doi:10.62324/taps/2024.049. [Google Scholar] [CrossRef]
9. Su Y, Ashworth VETM, Geitner NK, Wiesner MR, Ginnan N, Rolshausen P, et al. Delivery, fate, and mobility of silver nanoparticles in Citrus trees. ACS Nano. 2020;14(3):2966–81. doi:10.1021/acsnano.9b07733. [Google Scholar] [PubMed] [CrossRef]
10. Singh A, Tiwari S, Pandey J, Lata C, Singh IK. Role of nanoparticles in crop improvement and abiotic stress management. J Biotechnol. 2021;337(11):57–70. doi:10.1016/j.jbiotec.2021.06.022. [Google Scholar] [PubMed] [CrossRef]
11. Hayat F, Khanum F, Li J, Iqbal S, Khan U, Javed HU, et al. Nanoparticles and their potential role in plant adaptation to abiotic stress in horticultural crops: a review. Sci Hortic. 2023;321:112285. doi:10.1016/j.scienta.2023.112285. [Google Scholar] [CrossRef]
12. Sharma S, Singh SS, Bahuguna A, Yadav B, Barthwal A, Singh Khatana RN, et al. Nanotechnology: an efficient tool in plant nutrition management. In: Ecosystem services: types, management and benefits. Hauppauge, NY, USA: Nova Science Publishers, Inc.; 2022. p. 165–88. [Google Scholar]
13. Shahid M, Ullah UN, Khan WS, Saeed S, Razzaq K. Application of nanotechnology for insect pests management: a review. J Innov Sci. 2021;7(1):28–39. doi:10.17582/journal.jis/2021/7.1.28.39. [Google Scholar] [CrossRef]
14. El-Saadony MT, Saad AM, Soliman SM, Salem HM, Desoky EM, Babalghith AO, et al. Role of nanoparticles in enhancing crop tolerance to abiotic stress: a comprehensive review. Front Plant Sci. 2022;13:946717. doi:10.3389/fpls.2022.946717. [Google Scholar] [PubMed] [CrossRef]
15. Soni S, Jha AB, Dubey RS, Sharma P. Nanowonders in agriculture: unveiling the potential of nanoparticles to boost crop resilience to salinity stress. Sci Total Environ. 2024;925(1):171433. doi:10.1016/j.scitotenv.2024.171433. [Google Scholar] [PubMed] [CrossRef]
16. Song U, Kim J. Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. Hortic Environ Biotechnol. 2020;61(3):625–31. doi:10.1007/s13580-020-00244-8. [Google Scholar] [CrossRef]
17. Vadlamudi K, Upadhyay H, Singh A, Reddy M. Influence of zinc application in plant growth: an overview. Eur J Mol Clinic Medic. 2020;7(7):2321–7. [Google Scholar]
18. Cakmak I. Tansley Review No. 111: possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146(2):185–205. doi:10.1046/j.1469-8137.2000.00630.x. [Google Scholar] [PubMed] [CrossRef]
19. García-López JI, Niño-Medina G, Olivares-Sáenz E, Lira-Saldivar RH, Barriga-Castro ED, Vázquez-Alvarado R, et al. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in Habanero peppers. Plants. 2019;8(8):254. doi:10.3390/plants8080254. [Google Scholar] [PubMed] [CrossRef]
20. Elamawi R, Bassiouni S, Elkhoby W, Zayed B. Effect of zinc oxide nanoparticles on brown spot disease and rice productivity under saline soil. J Plant Prot Pathol. 2016;7(3):171–81. doi:10.21608/jppp.2016.50137. [Google Scholar] [CrossRef]
21. Sturikova H, Krystofova O, Huska D, Adam V. Zinc, zinc nanoparticles and plants. J Hazard Mater. 2018;349:101–10. doi:10.1016/j.jhazmat.2018.01.040. [Google Scholar] [PubMed] [CrossRef]
22. Smith P. Soils and climate change. Curr Opin Environ Sustain. 2012;4(5):539–44. doi:10.1016/j.cosust.2012.06.005. [Google Scholar] [CrossRef]
23. Petri JL, Hawerroth FJ, Fazio G, Francescatto P, Leite GB. Advances in fruit crop propagation in Brazil and worldwide-apple trees. Rev Bras Frutic. 2019;41(3):e4. doi:10.1590/0100-29452019004. [Google Scholar] [CrossRef]
24. Bybordi A. Study effect of salinity on some physiologic and morphologic properties of two grape cultivars. Life Sci J. 2012;9(4):1092–101. [Google Scholar]
25. Singh RN, Majumder PK, Sharma DK, Sinha GC, Bose PC. Effect of de-blossoming on the productivity of mango. Sci Hortic. 1974;2(4):399–403. doi:10.1016/0304-4238(74)90046-6. [Google Scholar] [CrossRef]
26. Scholefield PB, Oag DR, Sedgley M. The relationship between vegetative and reproductive development in the mango in northern Australia. Aust J Agric Res. 1986;37(4):425. doi:10.1071/ar9860425. [Google Scholar] [CrossRef]
27. Schaffer B, Andersen PC. Handbook of environmental physiology of fruit crops. Boca Raton, FL, USA: CRC Press; 2018. doi:10.1201/9780203719299. [Google Scholar] [CrossRef]
28. Syvertsen JP, Garcia-Sanchez F. Multiple abiotic stresses occurring with salinity stress in Citrus. Environ Exp Bot. 2014;103:128–37. doi:10.1016/j.envexpbot.2013.09.015. [Google Scholar] [CrossRef]
29. Nenko NI, Kisileva GK, Ulianovskaya EV, Yablonskaya EK, Karavaeva AV. Physiological-biochemical criteria of the apple-tree resistance to the summer period abiotic stresses. Eurasian J Biosci. 2018;12(1):55–63. [Google Scholar]
30. Gill MS, Jawandha SK. Response of mango (Mangifera indica L.) to abiotic stresses: an overview. Int J Agric Environ Biotechnol. 2012;5(4):459–62. [Google Scholar]
31. Jiang W, Li N, Zhang D, Meinhardt L, Cao B, Li Y, et al. Elevated temperature and drought stress significantly affect fruit quality and activity of anthocyanin-related enzymes in jujube (Ziziphus jujuba Mill. cv ‘Lingwuchangzao’). PLoS One. 2020;15(11):e0241491. doi:10.1371/journal.pone.0241491. [Google Scholar] [PubMed] [CrossRef]
32. Maraghni M, Gorai M, Neffati M, Van Labeke MC. Differential responses to drought stress in leaves and roots of wild jujube, Ziziphus Lotus. Acta Physiol Plant. 2014;36(4):945–53. doi:10.1007/s11738-013-1473-9. [Google Scholar] [CrossRef]
33. Gulen H, Eris A. Some physiological changes in strawberry (Fragaria × ananassa ‘Camarosa’) plants under heat stress. J Hortic Sci Biotechnol. 2003;78(6):894–8. doi:10.1080/14620316.2003.11511715. [Google Scholar] [CrossRef]
34. Elshibli S, Elshibli E, Korpelainen H. Growth and photosynthetic CO2 responses of date palm plants to water availability. Emir J Food Agric. 2016;28(1):58. doi:10.9755/ejfa.2015.05.189. [Google Scholar] [CrossRef]
35. Anjum MA, Hussain S, Arshad P, Hassan A. Irrigation water of different sources affects fruit quality attributes and heavy metals contents of un-grafted and commercial mango cultivars. J Environ Manage. 2021;281:111895. doi:10.1016/j.jenvman.2020.111895. [Google Scholar] [PubMed] [CrossRef]
36. Ang LH, Ng LT. Trace element concentration in mango (Mangifera indica L.seedless guava (Psidium guajava L.) and papaya (Carica papaya L.) grown on agricultural and ex-mining lands of Bidor. Perak Pertanika J Trop Agric Sci. 2000;23(1):15–22. [Google Scholar]
37. Amirjani M, Askary M, Askari F. Investigation of change pigment level, metal uptake and growth characteristics of Madagascar periwinkle (Catharanthus roseus) by nano zinc oxide. J Plant Process Funct. 2016;4(14):17–30. [Google Scholar]
38. Karakeçili A, Korpayev S, Dumanoğlu H, Alizadeh S. Synthesis of indole-3-acetic acid and indole-3-butyric acid loaded zinc oxide nanoparticles: effects on rhizogenesis. J Biotechnol. 2019;303(3):8–15. doi:10.1016/j.jbiotec.2019.07.004. [Google Scholar] [PubMed] [CrossRef]
39. Gupta S, Anusree T, Harini K, Kumar D, Kumar V, Kulshreshtha SK, et al. Effect of nitrogen, calcium and nano fertilizers on growth yield and quality of strawberry (Fragaria × ananassa duch.a review. Int J Environ Clim Change. 2023;13(10):2299–307. doi:10.9734/ijecc/2023/v13i102894. [Google Scholar] [CrossRef]
40. Bayat M, Pakina E, Astarkhanova T, Sediqi AN, Zargar M, Vvedenskiy V. Review on agro-nanotechnology for ameliorating strawberry cultivation. Res Crops. 2019;20(4):731–6. doi:10.31830/2348-7542.2019.108. [Google Scholar] [CrossRef]
41. Genaidy EAE, Abd-Alhamid N, Hassan HSA, Hassan AM, Hagagg LF. Effect of foliar application of boron trioxide and zinc oxide nanoparticles on leaves chemical composition, yield and fruit quality of Olea europaea L. cv. Picual Bull Natl Res Cent. 2020;44(1):106. doi:10.1186/s42269-020-00335-7. [Google Scholar] [CrossRef]
42. Carlesso LC, Luz GLD, Lajus CR, Silva LL, Fiori M, Rossoni C, et al. Physical-chemical properties of strawberry pseudofruits submitted to applications of zinc oxide nanoparticles. Int J Adv Eng Res Sci. 2018;5(7):262–72. doi:10.22161/ijaers.5.7.34. [Google Scholar] [CrossRef]
43. Tarkhan Abo Almeekh M, Lateef Assi S, Kadhum Abdul Ameer H. Effect of spraying with zinc nanoparticles, Humic acid, and adding the mineral fertilizer on the growth of pear seedlings (Hollywood cultivar). IOP Conf Ser: Earth Environ Sci. 2020;553(1):012025. doi:10.1088/1755-1315/553/1/012025. [Google Scholar] [CrossRef]
44. Mahdavi S, Karimi R, Valipouri Goudarzi A. Effect of nano zinc oxide, nano zinc chelate and zinc sulfate on vineyard soil Zn- availability and grapevines (Vitis vinifera L.) yield and quality. J Plant Nutr. 2022;45(13):1961–76. doi:10.1080/01904167.2022.2046081. [Google Scholar] [CrossRef]
45. Hamzah LM, Ibrahim M. Optimizing sour orange growth and chemical properties through foliar application of nano and organic fertilizers. J Glob Innov Agric Sci. 2024;12(3):701–7. doi:10.22194/jgias/24.1303. [Google Scholar] [CrossRef]
46. Serra I, Strever A, Myburgh PA, Deloire A. Review: the interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust J Grape Wine Res. 2014;20(1):1–14. doi:10.1111/ajgw.12054. [Google Scholar] [CrossRef]
47. Thomas DS. The influence of the atmospheric environment and soil drought on the leaf gas exchange of banana (Musa spp) [Ph.D. thesis]. Perth, WA, Australia: The University of Western Australia; 1995. [Google Scholar]
48. Murali K, Srinivas K, Shivakumar HR, Kalyanamurthy KN. Effect of soil moisture stress at different stages on yield and yield parameters of Elakki banana. Adv Plant Sci. 2005;18:817–22. [Google Scholar]
49. Hegde DM, Srinivas K. Yield and quality of banana in relation to post-flowering moisture stress. South Indian Hort. 1989;37(1–2):131–4. doi:10.1016/0378-3774(89)90045-0. [Google Scholar] [CrossRef]
50. Ravi I, Uma S, Vaganan MM, Mustaffa MM. Phenotyping bananas for drought resistance. Front Physiol. 2013;4:9. doi:10.3389/fphys.2013.00009. [Google Scholar] [PubMed] [CrossRef]
51. Masri M, Razak AS, Ghazalli MZ. Response of papaya (Carica papaya L.) to limited soil moisture at reproductive stage. Mardi Res J. 1990;18:191–6. [Google Scholar]
52. Laxman RH, Bhatt RM. Abiotic stress management in fruit crops. In: Abiotic stress management for resilient agriculture. Singapore: Springer Singapore; 2017. p. 399–412. doi:10.1007/978-981-10-5744-1_18. [Google Scholar] [CrossRef]
53. Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr. 2012;35(6):905–27. doi:10.1080/01904167.2012.663443. [Google Scholar] [CrossRef]
54. Kumar UJ, Bahadur V, Prasad VM, Mishra S, Shukla PK. Effect of different concentrations of iron oxide and zinc oxide nanoparticles on growth and yield of strawberry (Fragaria x Ananassa duch) cv. chandler. Int J Curr Microbiol App Sci. 2017;6(8):2440–5. doi:10.20546/ijcmas.2017.608.288. [Google Scholar] [CrossRef]
55. Elsheery NI, Helaly MN, El-Hoseiny HM, Alam-Eldein SM. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy. 2020;10(4):558. doi:10.3390/agronomy10040558. [Google Scholar] [CrossRef]
56. Davarpanah S, Tehranifar A, Davarynejad G, Abadía J, Khorasani R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci Hortic. 2016;210:57–64. doi:10.1016/j.scienta.2016.07.003. [Google Scholar] [CrossRef]
57. Zagzog OA, Gad MM. Improving growth, flowering, fruiting and resistance of malformation of mango trees using nano-zinc. Middle East J Agric. 2017;6(3):673–81. [Google Scholar]
58. Ahmad A, Xie X, Wang L, Muhammad FS, Chen M, Wang L. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011;6(9):2026–32. doi:10.5897/AJAR10.027. [Google Scholar] [CrossRef]
59. Helaly MN, El-Hoseiny H, El-Sheery NI, Rastogi A, Kalaji HM. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol Biochem. 2017;118(6):31–44. doi:10.1016/j.plaphy.2017.05.021. [Google Scholar] [PubMed] [CrossRef]
60. Agusti M, Almela V, Andreu I, Juan M, Zacarias L. Synthetic auxin 3, 5, 6-TPA promotes fruit development and climacteric in Prunus persica L. Batsch. J Hortic Sci Biotechnol. 1999;74(5):556–60. doi:10.1080/14620316.1999.11511152. [Google Scholar] [CrossRef]
61. Brennan RF. Zinc Application and its availability to plants [dissertation]. Perth, WA, Australia: School of Environmental Science, Division of Science and Engineering, Murdoch University; 2005. [Google Scholar]
62. Upreti KK, Reddy YTN, Shivu Prasad SR, Bindu GV, Jayaram HL, Rajan S. Hormonal changes in response to paclobutrazol induced early flowering in mango cv. Totapuri Sci Hortic. 2013;150:414–8. doi:10.1016/j.scienta.2012.11.030. [Google Scholar] [CrossRef]
63. Sandip M, Makwana AN, Barad AV, Nawade BD. Physiology of flowering—the case of mango. Int J Appl Res. 2015;1(11):1008–12. [Google Scholar]
64. Swietlik D. Zinc nutrition in horticultural crops. In: Horticultural reviews. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 1998. p. 109–78. doi:10.1002/9780470650752.ch3. [Google Scholar] [CrossRef]
65. Singh RN. Biennial bearing in fruit trees—accent on mango and apples. Technic Bullet Jodian Council Agric Res. 1971;30:47. [Google Scholar]
66. Abd-Allatif AM, El Kheshin MA, Rashedy AA. Antioxidant Potential of some Mango (Mangifera indica L.) cultivars growing under salinity stress. Egypt J Hort. 2015;42(2):654–65. doi:10.21608/ejoh.2015.1330. [Google Scholar] [CrossRef]
67. Laxmi, Kamal A, Kumar V, Muthukumar M, Bajpai A. Morphological indicators of salinity stress and their relation with osmolyte associated redox regulation in mango cultivars. J Plant Biochem Biotechnol. 2021;30(4):918–29. doi:10.1007/s13562-021-00735-4. [Google Scholar] [CrossRef]
68. Muhammed MAA, Mohamed AKSH, Qayyum MF, Haider G, Ali HAM. Physiological response of mango transplants to phytohormones under salinity stress. Sci Hortic. 2022;296:110918. doi:10.1016/j.scienta.2022.110918. [Google Scholar] [CrossRef]
69. Moazzzam Jazi M, Seyedi SM, Ebrahimie E, Ebrahimi M, De Moro G, Botanga C. A genome-wide transcriptome map of pistachio (Pistacia vera L.) provides novel insights into salinity-related genes and marker discovery. BMC Genomics. 2017;18(1):627. doi:10.1186/s12864-017-3989-7. [Google Scholar] [PubMed] [CrossRef]
70. Chen Z, Gu Q, Yu X, Huang L, Xu S, Wang R, et al. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann Bot. 2018;121(6):1127–36. doi:10.1093/aob/mcx207. [Google Scholar] [PubMed] [CrossRef]
71. Pratama Hamzah AH, Heryadi DY, Pramono SA. Production-optimization of biosurfactant from mangrove sediment bacteria using media salinity, differences in carbon source concentration and pH levels. J Glob Innov Agric Sci. 2024;12(2):391–8. doi:10.22194/jgias/24.1280. [Google Scholar] [CrossRef]
72. Bolat I, Dikilitas M, Ikinci A, Ercisli S, Tonkaz T. Morphological, physiological, biochemical characteristics and bud success responses of myrobolan 29 c plum rootstock subjected to water stress. Can J Plant Sci. 2016;96(3):485–93. doi:10.1139/cjps-2015-0260. [Google Scholar] [CrossRef]
73. Saiki ST, Ishida A, Yoshimura K, Yazaki K. Physiological mechanisms of drought-induced tree die-off in relation to carbon, hydraulic and respiratory stress in a drought-tolerant woody plant. Sci Rep. 2017;7(1):2995. doi:10.1038/s41598-017-03162-5. [Google Scholar] [PubMed] [CrossRef]
74. Tahir FM, Ibrahim M, Hamid K. Effect of drought stress on vegetative and reproductive growth behaviour of mango (Mangifera indica L.). Asian J Plant Sci. 2002;2(1):116–8. doi:10.3923/ajps.2003.116.118. [Google Scholar] [CrossRef]
75. Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 2005;10(12):615–20. doi:10.1016/j.tplants.2005.10.002. [Google Scholar] [PubMed] [CrossRef]
76. Dubey AK, Singh AK, Srivastav M. Salt stress studies in mango—a review. Agric Rev. 2007;28(1):75–8. [Google Scholar]
77. Grieve CM, Grattan SR, Maas EV. Plant salt tolerance. In: Agricultural salinity assessment and management. Reston, VA, USA: American Society of Civil Engineers; 2011. p. 405–59. doi:10.1061/9780784411698.ch13. [Google Scholar] [CrossRef]
78. Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;179(4):945–63. doi:10.1111/j.1469-8137.2008.02531.x. [Google Scholar] [PubMed] [CrossRef]
79. Zhu JK. Plant salt stress. In: Encyclopedia of life science. 2nd edition, Chichester, UK: J. Wiley & Sons, Ltd.; 2007. p. 1–3. [Google Scholar]
80. Jindal PC, Singh JP, Gupta OP. Salt tolerance of mango. a note on phosphorus and magnesium deficiency caused by sodium sulphate. Haryana J Hort Sci. 1976;5:13–4. [Google Scholar]
81. Dayal V, Dubey AK, Awasthi OP, Pandey R, Dahuja A. Growth, lipid peroxidation, antioxidant enzymes and nutrient accumulation in Amrapali mango (Mangifera indica L) grafted on different rootstocks under NaCl stress. Plant Knowledge J. 2014;3(1):15–22. [Google Scholar]
82. Ansari MW, Rani V, Shukla A, Bains G, Pant RC, Tuteja N. Mango (Mangifera indica L.) malformation: a malady of stress ethylene origin. Physiol Mol Biol Plants. 2015;21(1):1–8. doi:10.1007/s12298-014-0258-y. [Google Scholar] [PubMed] [CrossRef]
83. Soni A, Dhakar S, Kumar N. Mechanisms and strategies for improving salinity tolerance in fruit crops. Int J Curr Microbiol App Sci. 2017;6(8):1917–24. doi:10.20546/ijcmas.2017.608.226. [Google Scholar] [CrossRef]
84. Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14(3):89–97. doi:10.1016/0167-7799(96)80929-2. [Google Scholar] [CrossRef]
85. Apel K, Hirt H. REACTIVE OXYGEN SPECIES: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55(1):373–99. doi:10.1146/annurev.arplant.55.031903.141701. [Google Scholar] [PubMed] [CrossRef]
86. Sen A. Oxidative stress studies in plant tissue culture. In: Antioxidant enzyme. Houston, TX, USA: Intech; 2012. p. 59–88. [Google Scholar]
87. Garg N, Manchanda G. ROS generation in plants: boon or bane? Plant Biosyst Int J Deal Aspects Plant Biol. 2009;143(1):81–96. doi:10.1080/11263500802633626. [Google Scholar] [CrossRef]
88. Varjovi MB, Valizadeh M, Bandehagh A. Primary antioxidant enzymes and their important role in oxidative stress in plants and mammalian. Biol Forum Int J. 2015;7:148–54. [Google Scholar]
89. Ahmad R, Sabir MU. Vanadium stress mitigants in plants: from laboratory to field implications. J Hortic Sci Technol. 2023;6(2):16–24. doi:10.46653/jhst23062016. [Google Scholar] [CrossRef]
90. Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–77. doi:10.1016/j.chemosphere.2018.09.120. [Google Scholar] [PubMed] [CrossRef]
91. Wang L, Luo Z, Ban Z, Jiang N, Yang M, Li L. Role of exogenous melatonin involved in phenolic metabolism of Zizyphus jujuba fruit. Food Chem. 2021;341(Pt 2):128268. doi:10.1016/j.foodchem.2020.128268. [Google Scholar] [PubMed] [CrossRef]
92. Dvořák P, Krasylenko Y, Zeiner A, Šamaj J, Takáč T. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front Plant Sci. 2021;11:618835. doi:10.3389/fpls.2020.618835. [Google Scholar] [PubMed] [CrossRef]
93. Alam H, Khattak JZK, Ksiksi TS, Saleem MH, Fahad S, Sohail H, et al. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol Plant. 2021;172(2):1336–51. doi:10.1111/ppl.13273. [Google Scholar] [PubMed] [CrossRef]
94. Sofo A, Scopa A, Nuzzaci M, Vitti A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci. 2015;16(6):13561–78. doi:10.3390/ijms160613561. [Google Scholar] [PubMed] [CrossRef]
95. Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv. 2009;27(1):84–93. doi:10.1016/j.biotechadv.2008.09.003. [Google Scholar] [PubMed] [CrossRef]
96. Bai T, Ma P, Li C, Yin R, Ma F. Role of ascorbic acid in enhancing hypoxia tolerance in roots of sensitive and tolerant apple rootstocks. Sci Hortic. 2013;164:372–9. doi:10.1016/j.scienta.2013.10.003. [Google Scholar] [CrossRef]
97. Ma J, Qiu D, Pang Y, Gao H, Wang X, Qin Y. Diverse roles of tocopherols in response to abiotic and biotic stresses and strategies for genetic biofortification in plants. Mol Breed. 2020;40(2):18. doi:10.1007/s11032-019-1097-x. [Google Scholar] [CrossRef]
98. Shabala S, Munns R. Salinity stress: physiological constraints and adaptive mechanisms. In: Plant stress physiology. Egham, UK: cABI; 2017. p. 24–63. doi:10.1079/9781780647296.0024. [Google Scholar] [CrossRef]
99. Sairam RK. Tyagi A Physiological and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–20. doi:10.1007/1-4020-4225-6. [Google Scholar] [CrossRef]
100. Gadi BR. Effect of fluoride on metabolic patterns and nitrate reductase activity in Ziziphus seedlings. J Global Biosci. 2016;5:3694–8. [Google Scholar]
101. Potapovich AI, Kostyuk VA. Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochem. 2003;68(5):514–9. doi:10.1023/a:1023947424341. [Google Scholar] [PubMed] [CrossRef]
102. Kumar S, Mishra BB, Saxena S, Bandyopadhyay N, More V, Wadhawan S, et al. Inhibition of pericarp browning and shelf life extension of Litchi by combination dip treatment and radiation processing. Food Chem. 2012;131(4):1223–32. doi:10.1016/j.foodchem.2011.09.108. [Google Scholar] [CrossRef]
103. Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal. 2011;353(16):2885–904. doi:10.1002/adsc.201100534. [Google Scholar] [CrossRef]
104. Amzeri A, Adiputra F, Khoiri S. Selection of maize hybrids resulting from line × tester crossing tolerant to drought stress. J Glob Innov Agric Sci. 2024;12(3):575–84. doi:10.22194/jgias/24.1326. [Google Scholar] [CrossRef]
105. Zahedi SM, Harfi T, Marjani M, Vaculík M, Amini M, Sarikhani S. Various forms of foliar applied zinc improve drought acclimation in pomegranate: response of photosynthesis, osmoregulation and antioxidant defense. J Soil Sci Plant Nutr. 2024;24(2):2694–705. doi:10.1007/s42729-024-01694-0. [Google Scholar] [CrossRef]
106. Xiao L, Wang S, Yang D, Zou Z, Li J. Physiological effects of MgO and ZnO nanoparticles on the Citrus maxima. J Wuhan Univ Technol Mater Sci Ed. 2019;34(1):243–53. doi:10.1007/s11595-019-2042-x. [Google Scholar] [CrossRef]
107. Hamed NA, Salah M, Ahmed MF, Shoala T. Physiological assessment of radiation and PVP/Zn-nanoparticles on sour orange seedling. Asian J Agric Hortic Res. 2019;4(4):1–18. doi:10.9734/ajahr/2019/v4i430033. [Google Scholar] [CrossRef]
108. Abu Zeid IM, Mohamed FH, Metwali EMR. Responses of two strawberry cultivars to NaCl-induced salt stress under the influence of ZnO nanoparticles. Saudi J Biol Sci. 2023;30(4):103623. doi:10.1016/j.sjbs.2023.103623. [Google Scholar] [PubMed] [CrossRef]
109. Singh L, Sadawarti RK, Singh SK, Rajput VD, Minkina T, Sushkova S. Efficacy of nano-zinc oxide and iron oxide formulations on shelf life of strawberry. Eurasian J Soil Sci Ejss. 2024;13(3):254–62. doi:10.18393/ejss.1484756. [Google Scholar] [CrossRef]
110. Luksiene Z, Rasiukeviciute N, Zudyte B, Uselis N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: zno nanoparticles. J Photochem Photobiol B. 2020;203(3):111656. doi:10.1016/j.jphotobiol.2019.111656. [Google Scholar] [PubMed] [CrossRef]
111. Farhana, Farooq ABU, Haroon U, Saleem H, Akbar M, Anar M, et al. Bacillus safensis filtrate-based ZnO nanoparticles control black heart rot disease of apricot fruits by maintaining its soluble sugars and carotenoids. World J Microbiol Biotechnol. 2024;40(4):125. doi:10.1007/s11274-024-03944-w. [Google Scholar] [PubMed] [CrossRef]
112. Iqbal T, Afzal M, Ali Al-Asbahi B, Afsheen S, Maryam I, Mushtaq A, et al. Enhancing apple shelf life: a comparative analysis of photocatalytic activity in pure and manganese-doped ZnO nanoparticles. Mater Sci Semicond Process. 2024;173(3):108152. doi:10.1016/j.mssp.2024.108152. [Google Scholar] [CrossRef]
113. Chitena L, Muiva C, Kebaabetswe LP. Application of in situ casted ZnO-starch nanocomposite for packaging of strawberries (Fragaria x Ananassa). Heliyon. 2023;9(11):e22556. doi:10.1016/j.heliyon.2023.e22556. [Google Scholar] [PubMed] [CrossRef]
114. Akbar A, Anal AK. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control. 2014;38(1):88–95. doi:10.1016/j.foodcont.2013.09.065. [Google Scholar] [CrossRef]
115. Batool R, Kazmi SAR, Khurshid S, Saeed M, Ali S, Adnan A, et al. Postharvest shelf life enhancement of peach fruit treated with glucose oxidase immobilized on ZnO nanoparticles. Food Chem. 2022;366:130591. doi:10.1016/j.foodchem.2021.130591. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF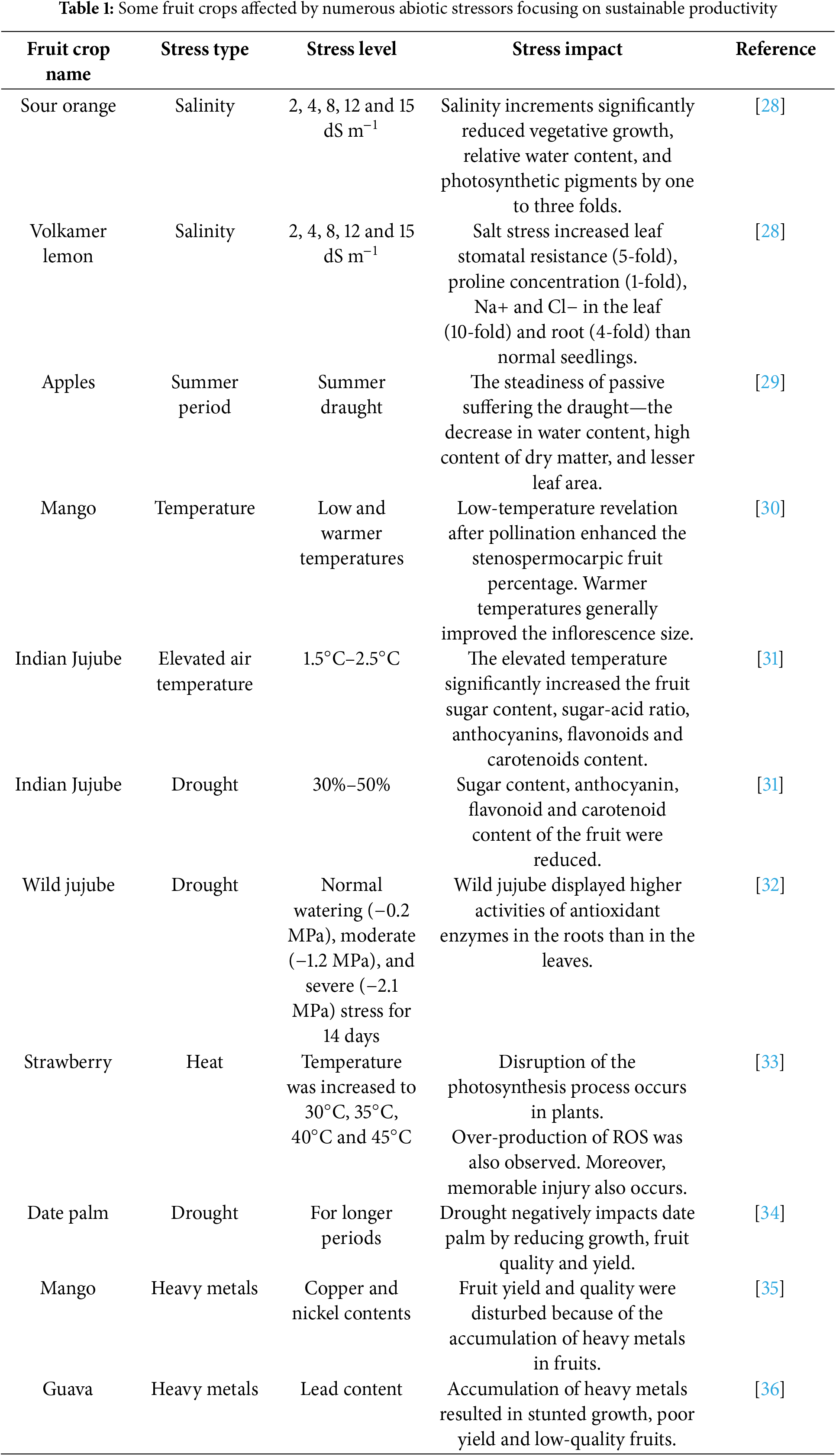
 Downloads
Downloads
 Citation Tools
Citation Tools