 Open Access
Open Access
REVIEW
Innovative Approaches in the Extraction, Identification, and Application of Secondary Metabolites from Plants
1 Ethnopharmacology and Pharmacognosy Team, Department of Biology, Faculty of Sciences and Technology, Errachidia, Moulay Ismaïl University, Meknes, 50000, Morocco
2 Laboratory of Applied Organic Chemistry, Department of Chemistry, Faculty of Sciences and Technologies, Sidi Mohamed Ben Abdellah University, Route d’Imozzer, Fez, 30000, Morocco
3 Biotechnology, Environmental Technology and Valorization of Bio-Resources Team, Department of Biology, Laboratory of Research and Development in Engineering Sciences, Faculty of Sciences and Techniques Al-Hoceima, Abdelmalek Essaadi University, Tétouan, 93000, Morocco
4 Laboratory of Biotechnology, Conservation and Valorization of Natural Resources (LBCVNR), Faculty of Sciences Dhar EI Mehraz, Sidi Mohamed Ben Abdellah University, B. P. 1796, Fez, 30000, Morocco
5 Laboratory of Functional Ecology and Environment, Faculty of Sciences and Technology, Sidi Mohamed Ben Abdellah University, Imouzzer Street, P.O. Box 2202, Fez, 30000, Morocco
* Corresponding Author: Amine Assouguem. Email:
(This article belongs to the Special Issue: Vegetable Resources, Sustainable Plant Protection and Adaptation Strategies to Climate Change)
Phyton-International Journal of Experimental Botany 2025, 94(6), 1631-1668. https://doi.org/10.32604/phyton.2025.065750
Received 21 March 2025; Accepted 16 May 2025; Issue published 27 June 2025
Abstract
Unlike primary metabolites, secondary metabolites serve critical ecological functions, including plant protection, stress tolerance, and symbiosis. This review focuses on extracting, separating, and identifying the major classes of secondary metabolites, including alkaloids, terpenoids, phenolics, glycosides, saponins, and coumarins. It describes optimized methods regarding plant selection, extraction by solvents, and purification of the metabolites, highlighting the latest advancements in chromatographic and spectroscopic techniques. The review also describes some of the most important problems, such as the instability of the compounds or diversity of the structures, and discusses emerging technologies that solve these issues. Moreover, it examines the secondary roles of these metabolites in medicine, such as anticancer and antimicrobial drugs, sustainable agriculture biopesticides, and environmental ecology-also known as allelopathy and bioindicators. It combines traditional ethnobotanical approaches with contemporary science, demonstrating the vital need to protect biodiversity in key ecosystems such as tropical rainforests, mountain regions, coral reefs, and arid zones as a foundation for anticipatory bio-discoveries. It organizes the methodological frameworks and outlines the steps needed to enhance the extraction of bioactive compounds from natural sources.Keywords
Plants are key primary producers of ecosystems throughout the globe and serve as the bedrock of life on Earth. They can synthesize many biological and non-biological compounds representing raw metabolic processes [1,2]. These chemicals are often not regarded as mere metabolites; they are tailored to fulfill distinct functions, promoting survival. Among other things that plants synthesize, secondary metabolites stand out. While primary metabolites, which include carbohydrates, proteins, and nucleic acids, are vital to growth and reproduction, secondary metabolites have a much broader spectrum of specialized functions [3–5]. Alkaloids, terpenoids, flavonoids, tannins, and saponins are only a few examples of the thousands of secondary metabolites of structurally diverse nature. These compounds serve as a chemical barrier to herbivores and pathogens, and as attractants to pollinators or symbiotic organisms. They have additional functions in chemical warfare against neighboring plants. Furthermore, these compounds aid in coping with drought, salinity, and temperature extremes and enhance resilience against abiotic stresses [6,7]. The importance of secondary metabolites is not just limited to plant biology since their diverse biological activities have implications for humanity, agriculture, and industry [8–10]. Since ancient civilizations, plants have been used to treat several ailments and improve well-being. Unbeknownst to humans, they were using the plants’ secondary metabolites. Modern science has revealed the molecular structure of these compounds, resulting in the pharmacological inventions of many life-saving medications, notable examples include taxol (anticancer), vincristine (chemotherapy agent), quinine (antimalarial), galantamine (used in Alzheimer’s treatment), artemisinin (an antimalarial compound from Artemisia annua), morphine (a potent analgesic), and resveratrol (an antioxidant with cardioprotective properties). In agriculture, secondary metabolites are utilized to formulate biopesticides and growth-promoting substances to decrease the use of synthetic chemicals [11–13].
Exploring secondary metabolites may seem fruitful, but it has its challenges. The intricacy of plant matrices, the requirement of highly competent analytical tools, and the low concentrations of target compounds are some challenges one faces [14]. However, technological advances with interdisciplinary research have improved the way these molecules are isolated and characterized [15]. Thus, in this article, we shall attempt to rigorously investigate and analyze the various systems and techniques needed to isolate and characterize plant secondary metabolites to aid scientific discovery and innovation in multiple industries.
2 Importance of Secondary Metabolites
Secondary metabolites are organic molecules that, even though they are not directly engaged in the core processes supporting growth and reproduction, are important for a plant’s prosperity, ecological relationships, and utilization by humans [16]. These are produced through specialized pathways and are classified into three main classes based on their metabolic origins: alkaloids, terpenoids, and phenolics [17]. Their structures, biological activities, and uses vary significantly from each other. These metabolites are critical for the plants that produce them and the economies, ecosystems, and human societies that depend on them [18].
These secondary metabolites of amino acids are synthesized in various organisms, forming a large group of chemical compounds known for their wide-ranging ecological and medicinal purposes. Alkaloids also serve as a potent chemical defense mechanism because they are often toxic to microbes and herbivores. Some of the more popular examples include the following compounds:
Morphine is one of the most popular medicinal analgesics, which derives from the opium poppy (Papaver somniferum) plant as well. For many centuries now, morphine has been prescribed to dull pain, especially for injury or post-surgery recovery [19]. Modern medicine depicts morphine as a centerpiece of any pain management plans especially for terminal diseases like cancer. These unique plants are capable of providing unparalleled pain relief because of their ability to bind to opioid receptors within the human body. The isolation and discovery of morphine is not only a milestone in pharmacology, but also an important step in the isolation of other plant-derived compounds with high efficacy [20].
Quinine is one of the best antimalarials that have been used for centuries and is extracted from the bark of the Cinchona tree. Quinine kills malaria parasites through various mechanisms that interfere with their life cycle [21]. After establishing the infection, this involves inhibiting their growth and juga secara tidak langsung alright. This emphasizes its critical function in malaria management, especially before affordable synthetics emerged. The extraction of quinine led to the creation of synthetic antimalarial medicines and remains an important example of drug development motivated by natural sources. Furthermore, tonic water’s popularity has diverted its use from medicine to more quenching purposes [21].
Atropine is a chemical abundant in Atropa belladonna or deadly nightshade and has many uses in today’s medicine [22]. It is most commonly used for management of slow heartbeat (bradycardia) and as an antidote for organophosphate poisoning as well as mushroom poisoning [23]. Moreover, since atropine blocks the action of acetylcholine at muscarinic parasympathetic sites, it is extremely useful in general medicine. Additionally, atropine is an essential eye medicine that dilates pupils for an eye checkup. This application demonstrates the broad scope of alkaloids in the treatment of many different conditions [24]. The following illustrations show how alkaloids have found use in medicines and other industries due to their potent bioactivities. Their roles in plants are mainly used for deterring herbivores and providing protection from microbial infections. The ecological and economic importance of alkaloids is extremely high since they are some of the world’s most used plant-based compounds [25].
Terpenoids or isoprenoids are the largest and most structurally diverse class of natural products. They are made from isoprene units, and terpenoids perform a wide variety of functions and applications. They act as messengers, pigments, and building blocks for plants and help them survive [26]. Among them, sesquiterpene lactones comprising three isoprene units and a characteristic lactone ring are primarily found in the Asteraceae family. These compounds function in plant defense and display significant pharmacological activities, including anti-inflammatory, anticancer, and antimicrobial effects. Notably, artemisinin, a sesquiterpene lactone from Artemisia annua, has been pivotal in antimalarial treatment, underscoring the therapeutic potential of this compound class.
Essential oils are aromatic compounds made from limonene and menthol, which have a number of applications, from perfumes to food additives and even traditional medicine. Their aroma makes them very desirable in fragrance and flavor-focused industries. They can provide a benefit for plants by acting as a natural pest deterrent, which helps protect the plants from herbivores and pathogens [27,28]. For instance, citronella oil is known for its ability to repel insects and is used as a natural insect repellent. Inhalation of essential oils stimulates the brain and makes the body feel more relaxed by reducing stress while improving mood. These compounds are used in aromatherapy. The psychological and physiological workings of these compounds are still being studied in wellness sciences [29].
Carotenoids are responsible for giving fruits and vegetables such as carrots, tomatoes, and peppers their vibrant colors. They include pigments such as beta-carotene and lycopene. These pigments help attract pollinators and seed dispersers and also protect the plants from damage caused by photooxidation [30]. Carotenoids have many benefits for human nutrition as they serve as precursors to vitamin A and antioxidants. Lack of carotenoids, and specifically vitamin A, can have dire health consequences, which illustrates its necessity in the diet. Carotenoids have also been associated with a decreased occurrence of chronic diseases, such as some cancers and cardiovascular diseases, which further demonstrates their importance for human health [31].
Plant and animal sources contribute to the formation of steroids, which are a class of intricate terpenoids. For instance, in plants, developmental and growth processes are modulated by steroids like brassinosteroids [32]. Various physiological activities, like cell membrane formation and hormone production, require cholesterol, which is a crucial component of steroid hormones. Their derivatives are foundational in human endocrinology. Among the plants, steroids obtained from some Dioscorea species have been employed for the development of drugs such as contraceptive pills and corticosteroids. This achievement emphasizes the potential of terpenoids in pharmacology [33]. The vast diversity of terpenoids underscores their significance in both natural ecosystems and human industries. Their roles in signaling, pigmentation, and defense highlight their ecological importance, while their utility in medicine and cosmetics showcases their economic value. From essential oils to life-saving drugs, terpenoids exemplify the multifunctional nature of secondary metabolites [34].
One or more hydroxyl functional groups attached to an aromatic ring is a defining characteristic of phenolic compounds [35]. Generally, phenolics are available in virtually all plants and have useful biological properties. Phenolics assist in maintaining plant structure and pigmentation as well as help in protecting plants from living and non-living threats [36].
Coumarins are widely distributed plant metabolites known for their defense allelochemical markers in plants. They are indisputably recognized for their biological importance as anticoagulants, antimicrobials, anti-inflammatories, and antioxidant agents [37,38]. A well-known example of a synthetic coumarin is warfarin, a frequently used anticoagulant. Natural coumarins such as umbelliferone and scopoletin exhibit marked biological activities and notable biomedicinal potentials [39]. In addition to pharmacology, coumarins are essential within the cosmetic and perfumer industries as fragrance compounds and filters for ultraviolet radiation. The ecological, medicinal, and industrial significance of these substances demands that adequate attention be devoted to research and conservation of biological diversity [40,41].
Flavonoids are one of the most investigated groups of phenolics due to their antioxidant, anti-inflammatory, and cardiovascular advantages [38]. Various colorful fruits, vegetables, and even drinks such as tea and wine are rich sources of these compounds [42]. These compounds serve significant advantages in conjunction with a plant-based diet and aid in reducing oxidative stress within the body by neutralizing free radicals. Moreover, they enhance ecological activities by attracting pollinators and shielding plants from ultraviolet rays. This reveals how useful these compounds are in applied science and nature on a broader scale [43].
Tannins are classed as astringent compounds that can cover bark, leaves, and unripe fruits. Tannins bind proteins and other organic compounds, thus affecting their antimicrobial and astringent properties [44]. Tannins have wide applications in traditional medicine, most notably in wound and infection treatment. Such a wide application highlights their immense therapeutic potential. Tannins also find application in the leather industry through the tanning of animal hides to enhance the durability of leather. This application speaks to the economic value of phenolic compounds [45].
Lignans are regarded as phytoestrogens present in seeds and grains that carry out basic functions similar to those of estrogen, thereby aiding the human body in more ways than one. Most of their tasks are centered around hormone regulation and cancer prevention, especially breast and prostate cancers, due to their prevalence in all age groups. New research claims that cardiovascular health and metabolic function can also be improved using Lignans [46]. Plants utilize these compounds for structural support by strengthening the plant cell walls and emphasizing their ecological functions. Phenolics may check these and other compounds and other metabolites of the plant are important for plant defense and human health, which makes them important for both natural and applied perspectives. Their multifarious functions and versatility in structure continue to drive creativity in medicine, agriculture, and industry [36].
3 Applications and Implications
The analysis concerned with secondary metabolites has had an undeniable influence on pharmacology, agriculture, and even ecology. These substances have not only propelled technological advancements in medicine and farming, but they have also deepened our grasp of ecological systems and their complex interrelations.
The pharmacological exploration has undergone unprecedented changes, particularly within the field of botanicals and bioactive plant compounds. These compounds are the axis around which a range of therapies, from palliative care to oncology, are based.
Artemisinin, a sesquiterpene lactone compound extracted from the famous herb Artemisia annua L., 1753, belonging to the Asteraceae family, has been a well-known cornerstone of malaria treatment, especially in the case of drug-resistant malaria. The discovery of Artemisinin has saved millions of lives across the globe, bringing forth inspiration for synthetic analogs to be devised [47]. Taxol paclitaxel, extracted from the Pacific yew tree Taxus brevifolia Nutt., 1849, belonging to the Taxaceae family, has been a significant breakthrough in oncology. The mechanism of action of this compound, disrupting the microtubule functions of cancer cells, has transformed the treatment for a myriad of cancers, such as breast and ovarian [48]. Key chemotherapeutic alkaloids vincristine and vinblastine, derived from the Montana periwinkle, have been critical in cancer treatment, particularly in showcasing the importance of plant products in oncology [49]. Different secondary metabolites are being explored with the hopes of obtaining new components that can be used as medicine. New compounds are expected to be discovered as long as people progress in understanding the biological diversity of plants, fungi, and marine creatures, which serve as a rich source of potential chemicals but underline the need for conservation as well [50].
Metabolites such as phenolic compounds and terpenoids are essential in the agricultural industry because they can provide nonchemical methods to tackle issues such as pest control and weed invasion or even improve crop growth [18]. Chrysanthemum cinerariifolium Trevir., 1844, a member of the Asteraceae family, produces a class of compounds known as pyrethrins, which are one of the most potent organic pesticides as well as safe for vertebrates [51]. Their use allows for minimal impact on the environment while practicing agriculture [52]. Furthermore, metabolites such as juglone produced by the black walnut plant are naturally produced chemicals that aid in the suppression of weeds by stunting the growth of neighboring plants and reducing the need for synthetic pesticides that have been proven to be harmful [53]. In addition, terpenoid and phenolic-derived plant growth regulators aid in increasing stress tolerance, flowering, and germination in crops, along with changing climatic conditions, aid in improving the yield of crops. There are also positive impacts on soil due to secondary metabolites, such as increased soil health; for example, plant roots produce flavonoids that enhance nitrogen fixation in rhizobia, benefitting leguminous crops [54].
Secondary metabolites enable multi-dimensional interactions, impacting the dynamics among plants, microorganisms, and insects [55]. These compounds facilitate communication, defense, and adaptation. As with most other volatile organic compounds, some terpenoids immensely affect plant-insect relationships. During herbivory, it is common for plants to emit certain VOCs, which assist in attracting predatory insects or parasitoids that suppress the herbivores, thus minimizing damage [56]. The selective visual and odor stimuli provided by flowers’ phenolic compounds, especially flavonoids, help to attract particular pollinators. This selective attraction increases the overall effectiveness of pollination and assures human reproductive success. Toxic metabolites like alkaloids are harmful to herbivores and pathogens [57]. A classic example is tobacco, where nicotine acts as an insect repellent as well as an antimicrobial. Secondary metabolites also exhibit support for symbiotic relationships. Secondary metabolite signaling between mycorrhizal fungi and plant roots facilitates the transfer of nutrients, which promotes plant growth and nutrient absorption [58].
Secondary metabolites have industrial applications ranging from cosmetics to foods and beverages. Oils are common in perfumes, flavoring, and aromatherapy, and menthol is used extensively for both its flavoring and pharmaceutical properties [59]. Peppermint is a key source of menthol, and is essential for oral hygiene products and cooling agents. Resveratrol, polyphenols, and catechins derived from grapes and green teas, respectively, are used in nutraceuticals to provide anti-inflammatory and antioxidant effects [60]. These compounds support cardiovascular health and may reduce the risk of chronic diseases. In secondary metabolite biotechnology can be an art of bioengineering. With the development of synthetic biology, complex metabolites like artemisinin are produced in microbial systems, which is a great scalable solution to global demand. With climate change, understanding secondary metabolites could help make crops that can withstand for climate (Fig. 1) [61]. These compounds are produced from metabolic pathways, and enhancing them can improve the plant’s stress tolerance, thus making agriculture more sustainable [12].
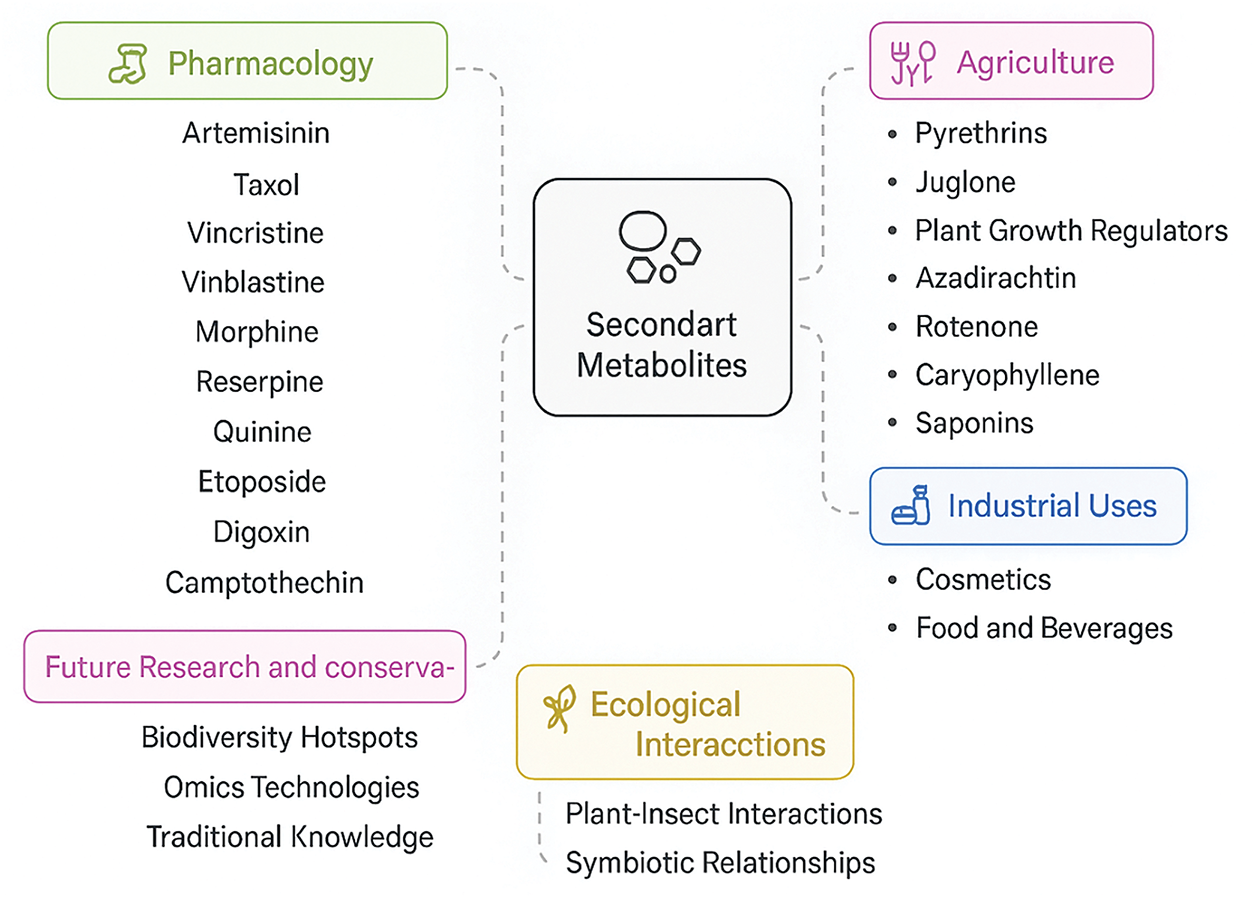
Figure 1: Applications and implications of secondary metabolites: pharmacology, agriculture, ecology, and beyond
3.5 Future Research and Conservation
A massive collection of new secondary metabolites awaits discovery, so biodiversity should be maintained. Many secondary compounds have been found in diverse species of regions like coral reefs and tropical rainforests, which are biodiversity hot spots. These ecosystems, along with their chemical diversity, face the danger of being destroyed due to habitat destruction [62]. The discovery of novel and detailed characterization of secondary metabolites is seeing rapid growth with the emergence of omics technologies such as genomics, metabolomics, and proteomics [63]. Using these technologies, scientists can discover bioactive compounds and the associated biosynthetic pathways with much more efficacy. There is a possibility for much insight to be gained through the fusion of traditional knowledge and modern science to study the use of secondary metabolites. The indigenous knowledge of medicinal plants serves as a strong starting point for new drug discovery. The significance of secondary metabolites and their wide-ranging effects makes them extremely useful in overcoming the world’s entire gamut of challenges. Such technologies are helpful in medical science, agriculture, and several other fields [8].
The extraction of secondary metabolites from plants involves different steps, each critical and needing precision while considering the chemicals’ properties. Below is a detailed breakdown of the workflow, expanded to incorporate more advanced insights.
4.1 Selection of Plant Material
Even within the same geographical area and climate, selecting plant species is of utmost importance regarding metabolite isolation. This choice is always guided by several aspects, which may include:
Ethnobotanical Knowledge: The cultural, medicinal, and nutritional practices that utilize specific plants suggest the possible existence of active compounds and, as such, do contain some form of bioactivity. For example, it is possible for plants used for treating inflammation to possess anti-inflammatory alkaloids or even flavonoids. Ethnobotany provides information on the cultures, foods, and medicinal practices inherited in the community. Modern scientific research greatly benefits from these traditional practices, as they frequently guide the discovery of important pharmacological agents. A classic example is the use of willow bark (Salix spp.) in traditional medicine to reduce pain and fever, eventually leading to the isolation of salicin and the development of aspirin [8–10]. Another important ethnobotanical breakthrough is quinine, derived from the bark of the Cinchona tree. Indigenous communities in South America used Cinchona bark for centuries to treat fever and malaria-like symptoms. This traditional knowledge caught the attention of European colonists and physicians, leading to the isolation of quinine in the 19th century. It became the first effective antimalarial treatment and paved the way for the development of synthetic derivatives and modern antimalarial drugs [8,20].
Ecological Observations: Secondary metabolites having antinutritional properties present in plants are particularly developed in those that survive in arid zones (deserts), extreme altitudes (Alpine), or rainforests as a means of adaptation [64]. For example, Cacti in arid zones produce alkaloids and spines to deter herbivores and reduce water loss. Alpine plants synthesize high levels of flavonoids, which act as UV protectants. In tropical rainforests, many trees produce tannins and saponins that inhibit fungal growth and microbial invasion in the humid environment. The resins of coniferous trees (e.g., pines and firs) are rich in terpenoids, acting as antimicrobial barriers and sealing wounds to prevent decay. Mustard plants produce glucosinolates, which serve both as herbivore deterrents and antimicrobial agents in the rhizosphere. These adaptations ensure plant survival in extreme ecosystems and highlight the ecological importance of secondary metabolites. Understanding these interactions requires detailed ecological studies that examine the relationships between plants, herbivores, pathogens, and neighboring flora [65].
Phytochemical Screening: Depending on the level of secondary metabolites in the species of plants in a collection, primary screening may include colorimetric assays such as the Folin-Ciocalteu method for total phenolics and thin-layer chromatography (TLC) to separate and visualize compound classes like alkaloids, flavonoids, or saponins [66]. Nevertheless, plant collections may also employ spectrophotometer bioinformatics for high-throughput screening as well as for further HPLC and other spectrometric plant assessment; for example, HPLC can separate and quantify curcumin in Curcuma longa or quercetin in onion extracts. Readying resources for the ethnobotanist ensures confidence for the plants deemed as the most useful. Advances in molecular biology, such as metabolomics and transcriptomics, have made phenolic compound screening easy, as results can be anticipated based on the expected biosynthesizing pathways [63].
Extraction is crucial for separating secondary metabolites from highly organized plant structures. Various factors influence the extraction selectivity and efficiency, including solvent choice, extraction method, and, most importantly, the temperature and time parameters.
The choice of solvent plays a crucial role in phytochemical extraction, as it determines the range and efficiency of metabolite recovery. Solvents can be broadly categorized into organic and inorganic types, each selected based on the polarity of the target compounds.
Methanol and Ethanol: These polar solvents are adequate for retrieving water-soluble compounds such as flavonoids, glycosides, and saponins. Ethanol is, along with methanol, widely used in the food and pharmaceutical industries because it is less harmful. Research suggests that ethanol-water mixtures are best for extracting various compounds because of their balance of polarity and solubility [67].
Acetone is useful for extracting a wide range of polar and semi-polar compounds, including phenolic acids and some alkaloids. It evaporates quickly, which helps in drying the extract faster. Acetonitrile: Often used in HPLC-grade extractions due to its excellent solubilizing capacity for polar compounds and compatibility with analytical instruments [67,68].
Chloroform and Hexane: Non-polar solvents such as chloroform and hexane are efficient for extracting lipophilic compounds, which include terms such as terpenoids, sterols, and some essential oils. When used with other solvents in sequential extraction, these non-polar solvents can yield a wider range of metabolites. Diethyl Ether: Suitable for extracting volatile oils and lipophilic flavonoids and often used with other solvents. Petroleum Ether: Commonly used to extract fats, oils, and waxes, particularly during the defatting stage before further phytochemical screening [68].
The water is frequently used for extracting hydrophilic compounds such as tannins, some alkaloids, and polysaccharides. In other cases, water extraction may be for co-extract unwanted substances such as starch or proteines. More advanced filtration systems can solve these problems and enhance the quality of the extract [69].
4.2.4 Modern Extraction Methods
Soxhlet Extraction: Builds on the traditional extraction principles but sets itself apart thanks to its exhaustive nature. This method does exhaust a lot of time. However, implementing automated systems has been shown to speed everything up and bring down the environmental impact. The traditional way of performing Soxhlet removably circulates the solvent effectively [67].
Ultrasonic-Assisted Extraction (UAE): Using ultrasound to extract is much more advanced than using traditional techniques and allows for amplifying metabolite release by destroying cell walls. UAE’s methods are also less prone to degradation which makes it a faster option with less solvent use than the traditional techniques [70].
Supercritical Fluid Extraction (SFE): Besides being one of the most effective methods for extracting nonpolar compounds, supercritical fluid extraction is also environmentally friendly. This is due to the method utilizing supercritical CO2 as a solvent, which leaves minimal residues on the extracted material. Changing the properties of CO2 by adjusting the pressure and temperature makes selective extraction possible [71].
Microwave-Assisted Extraction (MAE): Much like SFE, this method is also energy-saving due to using microwaves to evenly heat plant extracts. The combination of microwaves with ionic liquids is a significant advancement in the field of MAE and widens its range of use [72].
After extraction has been performed in any of these methods, the resulting crude extract typically contains a complex mixture of bioactive and non-bioactive constituents. The purpose of fractionation is to simplify these mixtures by separating compounds based on their distinct physical and chemical properties, such as polarity, molecular weight, and affinity for solvents. This step is essential for the efficient purification and identification of individual secondary metabolites [81].
4.3.1 Liquid-Liquid Partitioning (LLP)
Liquid-Liquid Partitioning: this approach takes advantage of the differing solubility of immiscible solvents, e.g., water and hexane. Separating polar and nonpolar fractions is possible, consequently facilitating efficient purification at later stages. Using several solvent pair combinations in Sequential partitioning improves fractionation efficacy and minimizes contamination [73].
4.3.2 Solid-Phase Extraction (SPE)
SPE involves passing the crude extract through a solid adsorbent material (usually a silica or polymer-based column), which retains specific compound classes based on adsorption and polarity. Solvents of increasing polarity are then used to elute fractions. SPE is widely used for pre-concentration, desalting, and clean-up before HPLC or LC-MS analysis. For example, it can separate flavonoid glycosides from phenolic acids [73].
Thin-Layer Chromatography (TLC): An easy and fast way to evaluate metabolites. Used as a first check to search for bioactive ingredients. Recent TLC devices with automated spotting and densitometry systems can simultaneously offer qualitative and quantitative data [74].
Column Chromatography: Separates mixtures based on the different affinity compounds have for the stationary and mobile phases. The stationary phase is often silica gel or alumina. The resolution of complicated mixtures has been enhanced by new methods, such as gradient elution [75].
High-Performance Liquid Chromatography (HPLC): Highly effective method for separation as well as qualitative determination of metabolites. Combining HPLC with UV, fluorescence, or mass spectrometry analysis enhances resolution. The introduction of ultra-high performance liquid chromatography (UHPLC) has decreased analysis time while increasing resolution [76].
High-Performance Thin-Layer Chromatography (HPTLC)
HPTLC is an advanced form of TLC that offers improved resolution, reproducibility, and sensitivity. It incorporates automated sample application, controlled development chambers, and multi-wavelength scanning, allowing both qualitative and quantitative analysis [82,83]. HPTLC is particularly valuable for fingerprinting plant extracts, detecting adulterants, and standardizing herbal formulations. It serves as a complementary tool to HPLC in quality control and phytochemical profiling [84,85].
Flash Chromatography: one such is a preparative method that utilizes pressurized gas to hasten the separation process for the recovery of large amounts of extract. Systems with integrated detection and fraction collection increase the productivity of the process [77] (Table 1).
Isolation of the secondary metabolite is the goal for these individual compounds and, therefore, must be purified to remove any incidental agents.
Methods to Use
Preparative HPLC: It can isolate minute amounts of the highest purity compounds by offering great resolution separation. More advanced techniques, such as multidimensional HPLC, can isolate closely related isomers [78].
Crystallization: This method takes advantage of differences in solubility. Various compounds, such as certain alkaloids, are able to crystallize easily when subjected to certain solvents. Yield and purity are improved with controlled techniques such as temperature cycling and addition of anti-solvent [79].
Precipitation is a relatively simple and economical method. It occurs when, under certain conditions, a compound becomes insolubly and visible for separation through filtration or centrifugation. This method best serves proteins, polysaccharides, and, in some cases, even alkaloids. Selective precipitation can be triggered by acid-base adjustment, the addition of salt, or the exchange of solvents, such as putting Ethanol into an aqueous extract [86].
Membrane Filtration/ Ultrafiltration: These methods have a semi-permeable filter that can separate a mixture’s components. Ultrafiltration is geared more towards parent molecules like polysaccharides and getting rid of big unwanted materials sitting around in plant extracts. The method is mainly applied with enzymatic hydrolysis or dialysis [86]. Solid-Phase Extraction (SPE): This method employs selective adsorption and elution, which makes it convenient and straightforward. SPE makes it possible to pull metabolites from weak solutions easily. Using molecularly imprinted polymers in SPE enhances the selectivity, making it more useful [80].
Whether a compound is suitable for application in research, medicine, or industry depends greatly on the purity and identity of the isolated compounds. Considerable effort is implemented at a higher level of detail to check and ascertain the quality of the metabolites.
5.1 Methods of Quality Assessment
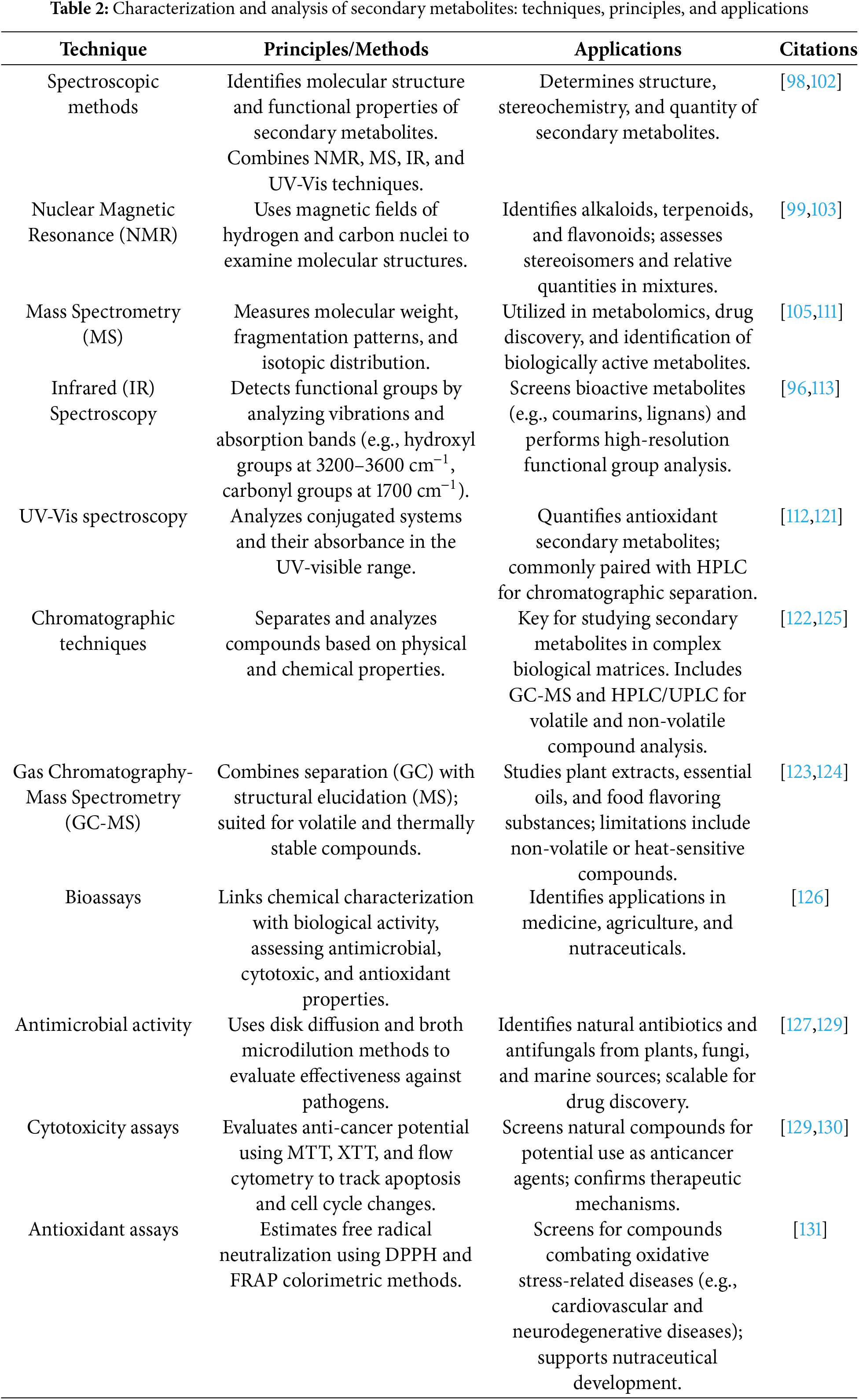
Liquid Chromatography–Mass Spectrometry LC-MS is a powerful technique widely used to analyze complex mixtures and confirm target compounds [77]. While the LC section separates the components in the mix, the MS section provides each component’s molecular weight and structure fragmentation [77]. This method is very valuable in confirming the compound’s identity and purity by providing both qualitative and quantitative analysis. It is also effective in detecting trace compounds and metabolite profiling applications. More detailed structure solutions can be made with tandem MS/MS techniques [79].
Spectral Analysis: Determining the structure and purity of a compound can be performed with characteristics of NMR, MS, and IR spectroscopy. For example, NMR gives detailed information about the molecular structure and the functional groups within a sample. More sophisticated NMR techniques, particularly sensitive low-concentration cryo-probes, are available [81]. The MS gives the molecular weight and fragmentation patterns of the sample. The precision measurement of mass distinguishes a high-resolution type of mass spectrometer commonly referred to as high-resolution MS (HR-MS), making it possible to determine the molecular formula with high accuracy [87]. Infrared spectroscopy is used for looking at a sample with a specific functional group by emphasizing the characteristic absorption bands of the group. Fourier-transform IR (FT-IR) is an infrared spectrometer that enhances the resolution and the speed of data collection [88].
Bioassays: Activity against a biological target fraction or oil extracts of an isolated compound is essential to bioassay test for its bioactivity. In drug discovery, agriculture, and cosmetics, these assays are important. Also, today cell-based assays are available with a possibility for a high-throughput screening platform, so evaluation of bioactivity is much easier [89].
5.2 Establishing a Link between Classical and Contemporary Styles
This link between classical and contemporary styles is critical in understanding the concept of secondary metabolite isolation as it is a dynamic and interdisciplinary process. With the blend of ethnobotanical knowledge and cultural practices, it becomes easier to choose which plants should be selected and studied further based on their therapeutic functions [90,91]. Within the context of scientific endeavors, these traditional methods of investigation follow the basics of efficient ethnobiology, where the best results are guaranteed. In conjunction with these theories, traditional methods have also changed, giving birth to modern methods such as extraction, purification, and analysis of secondary metabolites [92]. There are numerous extraction methods, but with supercritical fluid extraction, ultrasonic-assisted extraction, and microwave-assisted extraction, the guarantee of obtaining the best, efficient, and sustainable result with minimal solvent consumption and time is heightened [93,94]. Along with these are advanced methods of purification that were devised in the form of high-performance liquid chromatography (HPLC), counter-current chromatography (CCC), and preparative chromatography, where the desired compounds are achieved in their purest forms [95]. These techniques of analysis are combined with mass spectrometry (MS), nuclear magnetic resonance (NMR), and Fourier Transform Infrared (FTIR) to check the integrity and quality of the isolated compounds, making these multidisciplinary techniques advanced [96].
Secondary metabolites are important in diverse sectors, including pharmaceuticals and agriculture. These compounds are isolated and introduced into the method, where their structures, properties, and biological activity are determined. These involve different bioassays, chromatographic and spectroscopic techniques, which each provide differing information about the compounds and their possible uses [97]. This part analyzes the techniques, principles, and measures and explains why they are necessary for the study of secondary metabolites.
Spectroscopic techniques are central in identifying secondary metabolites’ molecular structure and functional properties. These methods provide comprehensive details about molecules in terms of the type of elements they include and the arrangement of the atoms. Each method has its advantages and disadvantages. Hence, it is necessary to use them in conjunction [98]. One of the most basic spectroscopic techniques is Nuclear Magnetic Resonance (NMR) spectroscopy [99]. NMR chemistry is primarily used in biochemistry to determine the structure of compounds. It provides multi-dimensional NMR techniques such as two-dimensional doses to realize the blood composition structure. Regular steam proton NMR suggests basic biochemistry molecules contained within blood [100].
Generally speaking, the NMR examines the magnetic fields provided by hydrogen and carbon nuclei and the relative positions of these tissues in their environment, which results in very advanced methods for detecting [101].
Compounds such as alkaloids, terpenoids, flavonoids, and other biologically active compounds use Crescent NMR to determine dissolution’s stereoisomers in situations where they play a significant predominant role in the metabolism. Molecules often utilize submits as the Microwave through Buried NMR [102,103]. Combining areas of the peaks makes it possible to ascertain the relative quantities of various secondary metabolites present in a mixture [102].
Mass spectrometry is one of the important techniques in finding out:
Due to its sensitivity, accuracy, and versatility, mass spectrometry (MS) is an essential analytical technique in natural products and secondary metabolite research. It allows scientists to carefully untangle the chemical intricacy of plant extracts and other biological samples [104].
Determining Molecular Weight
MS is mainly known for its ability to determine molecular weight accurately. This technique is exceptionally beneficial at the preliminary steps of the metabolite detection process. High-resolution MS (HRMS) like Orbitrap or time-of-flight (TOF) systems enable exact mass determination, which greatly assists in calculating molecular formulas with high precision. As an example, the molecular ion peak [M+H]+ in the mass spectrum highlights the value of initiating structural elucidation where the protonated molecular weight of the compound is verified [105,106].
Fragmentation Patterns and Structural Elucidation
With the invention of more advanced MS techniques like tandem mass spectrometry (MS/MS), determining the substructure of a compound through its fragmentation pattern is much easier. Interpreting fragmentation—structural features of a molecule comprising its functional groups and their connections—through CID (collision-induced dissociation) provides numerous details. In the case of flavonoids, characteristic fragments like retro Diels-Alder cleavage products help in subclass differentiation [107]. Mass Frontier and MS-DIAL are examples of software that automate fragmentation interpretation [108].
Isotopic Pattern Analysis and Metabolic Tracing
Patterns of isotopes, which are helpful for isotope labeling studies, can now also be resolved by using high resolution MS. Through the use of isotopic labels (for example 13C, 15N), secondary metabolites can be tracked and their biosynthetic pathways elucidated in real time. This is very important for metabolic flux analysis and in studying how biosynthesis of metabolites changes in response to environmental or genetic perturbations [109,110].
Uses in Drug Development and Metabolomics
Mass spectrometry is integral in metabolomics, which studies minor molecule constituents termed metabolites. In botany, MS metabolomics is often performed to identify bioactive secondary metabolites like alkaloids, phenolics, terpenoids, and saponins. These compounds possess numerous therapeutic activities, such as anticancer, antimicrobial, and anti-inflammatory. For example, LC-MS was used to determine the presence of vincristine and vinblastine, two alkaloid-based anticancer drugs, in Catharanthus roseus [111]. Moreover, targeted MS techniques, such as multiple reaction monitoring (MRM), offer quantitative measurement of metabolites, which is essential in the QC of herbal medicines and phytopharmaceuticals [112].
6.1.2 Infrared (IR) Spectroscopy
Infrared (IR) spectroscopy is an essential analytical technique for functional group detection within organic compounds. IR Absorption Spectroscopy can interrogate molecular bonds and identify the constituent parts of a compound by measuring the absorption of infrared radiation, which causes vibrational transitions in molecular bonds. The method is most suitable for the initial characterization of secondary metabolites owing to its rapidity, low sample requirement, and non-destructive nature [112].
Principle and Functional Group Detection
The functioning of IR spectroscopy is based on detecting vibrational movements of the covalent bonds of the molecules. Every bond has its unique vibrational frequency, depending on the types of atoms and the strength of the bond. If the infrared radiation corresponds to the natural vibrational energy of a bond, the bond would be broken, and an absorption band is formed in the IR spectrum. These absorptions are indicative of particular functional groups and enable the determination of the chemical identity of secondary metabolites.
Hydroxyl (-OH) Groups: Hydroxyl groups are frequent in phenolic compounds and flavonoids. Also known as the O-H stretching vibration, they tend to absorb energy in the 3200–3600 cm−1 range. The IR spectrum of quercetin, a well-known flavonoid, confirms the presence of phenolic hydroxyls as it shows a dominant broadband within this region [96].
Carbonyl (C=O) Groups: Aldehydes, ketones, esters, and carboxylic acids contain carbonyl groups with sharp absorption bands near 1700 cm−1 and C=O vibrations. The position may vary slightly due to hydrogen bonding. For instance, coumarin, an aromatic lactone derivative, possesses a strong carbonyl band at roughly 1715 cm−1, a hallmark of its lactone ring [88].
Uses for Analyzing Secondary Metabolites
IR spectroscopy is the most common method used for screening and confirming the structure of secondary metabolites from plants. IR is especially beneficial for the presence of major functional groups of compounds during secondary metabolite extraction since no extensive sample manipulation is needed.
Fourier Transform Infrared (FTIR) Spectroscopy: FTIR is another IR spectroscope with greater sensitivity and resolution. Despite their complexity, plant extracts can be tested using FTIR, and active constituents like lignans, tannins, and terpenoids can be targeted. FTIR also allows for multivariate chemometric models to be used for quantitative analysis.
Example: Lignans and Coumarins. FTIR spectra of lignans exhibit characteristic absorptions of methoxy groups (C-O stretching near 1100 cm−1), aromatic C=C stretching (around 1600 cm−1), and carbonyl positions (roughly 1700 cm−1). Coumarins, known for their bioactive anticoagulant and anti-inflammatory properties, exhibit combinations of carbonyl and aromatic ring vibrations, which aid in distinguishing simple coumarins from furanocoumarins [100].
6.1.3 Ultrviolet-Visible (UV-Vis) Spectroscopy
Ultraviolet-visible (UV-Vis) spectroscopy has become a standard approach for analyzing secondary metabolites of organisms, especially those possessing conjugated π-electron systems, such as flavonoids and phenolic and polyphenolic compounds. The technique uses UV and visible light from the electromagnetic spectrum, which ranges from 200–700 nm, to measure the amount of light absorbed by a sample, and this corresponds to certain electronic transitions, primarily π → π* and n → π* as well as others [113].
Principle and Analytical Relevance
Secondary metabolites with chromophores (the parts of molecules that contain an atom that can emit or absorb light) display characteristic absorbance spectra, exhibiting significance for both qualitative and quantitative assessments [41].
Conjugated Systems and Aromaticity: In compounds consisting of an extended conjugation, like resonance structures with several alternating double bonds or aromatic rings, they are most prominently visible for absorption in longer wavelengths. Take, for instance, flavonoids, such as quercetin, which have two leading absorption bands [114].
Band I (300–380 nm): Associated with the B-ring cinnamoyl system.
Band II (240–280 nm): Linked to the A-ring benzoyl system.
Other substituents, such as hydroxylation or glycosylation, help differentiate flavonoid subclasses by shifting these bands [115,116].
Quantitative Analysis
Secondary metabolites, and especially antioxidants, are quantitatively measured using UV-Vis spectroscopy. According to the Beer-Lambert Law, concentration can be calculated from absorbance (A) as shown in the relation A = εcl, where: A = absorbance; ε = molar absorptive; c = concentration, l = path length.
This method is appropriately used to determine TPC or TFC (phenolic and flavonoids content) with the reagents like samples: Reagents: Folin–Ciocalteu TPC (measured at about 760 nm) and Aluminum chloride (AlCl3) colorimetric assay for TFC (measured at about 415 nm) [102]. For phenolic content, gallic acid and catechin are standard references often employed [116,117].
Coupling with HPLC (HPLC-DAD/UV)
A Diode Array Detector (DAD) is used on the UV-Vis spectrophotometer, which is key in monitoring the performance of the HPLC system. Thus, real-time compound detection is possible as they elute from the chromatographic column accompanied by spectral collection [118]
For instance, during the polyphenol profiling of green tea (Camellia sinensis), catechins, too, were quantifiably separated using HPLC-UV. Intensely aromatic polyphenols, EGCG, and ECG were detected as expected at 280 nm. For instance, curcuminoids from Curcuma longa were quantifiably measured using UV detection at approximately 420 nm [119,120].
Uses in Antioxidant Studies and Natural Product Investigations
The evaluation of antioxidant activity is particularly facilitated by the use of UV-Vis spectrometry in conjunction with the following assays:
DPPH Radical Scavenging Assay (Absorbance at 517 nm),
ABTS Assay (Absorbance at 734 nm),
Ferric Reducing Antioxidant Power (FRAP) (Absorbance at 593 nm).
These assays serve as rapid approaches in determining the antioxidant activity of secondary metabolites and plant extracts, which is essential in functional food and phytopharmaceutical research [118].
6.2 Chromatographic Techniques
There are different chromatographic methods for different physical and chemical properties of the target compounds, but chromatography is key for analyzing and separating secondary metabolites. The biological matrices are complex, but the versatility of the technique allows it to handle those with ease [121].
6.2.1 Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS combines the powerful separation capacity of gas chromatography with the molecular identification ability of mass spectrometry, enabling both qualitative and quantitative analysis of volatile and thermally stable compounds. In GC, compounds are separated based on volatility and interaction with the column’s stationary phase; in MS, compounds are ionized and fragmented to generate characteristic mass spectra for identification [106,122].
Applications: Commonly used for analyzing essential oils, terpenoids, small organic acids, and volatile alkaloids. Widely applied in the food, fragrance, and pharmaceutical industries to profile aromatic compounds and plant volatiles. First example: GC-MS analysis of Lavandula angustifolia (lavender) oil reveals characteristic peaks for linalool and linalyl acetate, two bioactive volatile terpenes with calming effects. Second example: Screening of volatile constituents in Thymus vulgaris essential oil for antimicrobial studies [28,122].
Advantages: Merges separation power from gas chromatography and structural elucidation from mass spectrometry. Newer GC-MS systems also have automatic sample preparation and spectral compound libraries for quick identification [123].
Limitations: Not particularly useful for secondary metabolites that are non-volatile or heat sensitive, such as saponins.
6.2.2 High-Performance Liquid Chromatography (HPLC) and Ultra-Performance Liquid Chromatography (UPLC)
HPLC and its advanced form, UPLC are liquid-phase techniques suited for non-volatile, thermally sensitive, or polar secondary metabolites. Compounds are separated based on polarity, hydrophobicity, or ionic interactions using different stationary phases (e.g., reverse-phase C18 columns). Detection can be achieved using UV-Vis, DAD, fluorescence, or MS detectors [115,121].
Applications: Ideal for profiling alkaloids, phenolic acids, flavonoids, coumarins, and glycosides. First example: HPLC-DAD used in quantifying curcuminoids (e.g., curcumin, demethoxycurcumin) in Curcuma longa extracts at 420 nm. The second example is UPLC-MS/MS for high-speed profiling of ginsenosides in Panax ginseng.
Advantages: Provide high resolution, sensitivity, and reproducibility. UPLC significantly reduces analysis time (minutes instead of hours) with better peak capacity. It’s compatible with bioassay-guided fractionation of plant extracts [124,125].
Detectors & Enhancements: Diode Array Detectors (DAD) allow multi-wavelength detection, ideal for differentiating overlapping phenolic peaks. Fluorescence Detectors provide high sensitivity for compounds like alkaloids and polyphenols. HPLC-MS/MS is used in pharmacokinetics and metabolite identification studies.
Limitations: More expensive equipment and solvents, and requires careful optimization of mobile phase gradients and column types [41,72,112,121,126].
The importance of assessing the biological activity of secondary metabolites cannot be overemphasized, as it is critical in determining their chemical structure. Such compounds can indeed be useful in medicine, agriculture, and industry. These essays are meant to connect chemical characterization with application [127]. Secondary metabolites possess diverse biological activities with applications in medicine, agriculture, cosmetics, and biotechnology. Medicinally, they exhibit antimicrobial, anticancer, antioxidant, and neuroprotective properties, as seen with paclitaxel and artemisinin. In agriculture, they act as biopesticides and growth promoters, reducing reliance on synthetic chemicals. In cosmetics, flavonoids and phenolics offer anti-aging and UV-protective benefits. Additional effects include antidiabetic, hepatoprotective, and immunomodulatory actions. Their multifunctionality makes them valuable for sustainable solutions. Assessing biological activity alongside chemical structure is vital for discovering new therapeutic and industrial applications [81].
Purpose: Evaluate the effectiveness that varied secondary metabolites have against selected bacterial and fungal pathogens. Some certain naturally occurring compounds, such as some alkaloids and terpenoids, have antimicrobial activity and are of great importance in the fight against antibiotic resistance [128].
Methods: Each screening was performed using disk diffusion assays, which involved inoculating a microorganism on an agar plate and placing the compound in question on dialing wafers that had been cut into discs and placed on the agar plate. The size of the inhibition zone measured the power of the antimicrobial agent. The second method was broth microdilution techniques, which determine the MIC by evaluating the growth of microorganisms in liquid culture media [129].
Applications: The purpose of this assay may involve identifying sources of antibiotics, antifungal, and antibiofilm agents from other sources such as plants, fungi, and even marine organisms. The ability of compounds to inhibit or disrupt biofilm formation, which is a critical factor in microbial resistance and chronic infections, has become a key focus in recent screenings. Typically, these methods are scaled up for high-throughput screening in pharmaceutical pipelines during drug discovery and development [130].
Purpose: Use these assays to evaluate the anticancer potential of secondary metabolites. Because of the ability to alter the middle body parts of living organisms, some secondary metabolites like alkaloids and terpenoids demonstrate cytotoxic activity against cancer cells [130].
Methods: MTT and XTT assays for evaluating cell viability through estimating the metabolic activity of certain cells. Tracking of apoptosis and cell cycle alterations through flow cytometry yields an understanding of how cytotoxic secondary metabolites work within the cell. Uses: For screening natural resources for products with possible utility as anticancer agents with a focus on particular types and stages of cancer. In addition to cytotoxicity assays, the use of secondary metabolites is followed by confirming the therapeutic mechanisms through molecular approaches (Table 2) [131].
Goals: Estimate the capacity of secondary metabolites to neutralize free radicals that are associated with oxidative stress and the development of chronic ailments.
Protocol: Colorimetric methods that depend on DPPH (2,2-diphenyl-1-picrylhydrazyl) to estimate the radical scavenging ability of the tested sample.
The FRAP (Ferric reducing antioxidant power) assay quantifies the use of free electrons from the antioxidants to reduce ferric ions to ferrous ions.
ABTS assay (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)): Generates the ABTS+ radical cation, which is neutralized by antioxidants; suitable for both hydrophilic and lipophilic compounds.
CUPRAC assay (Cupric Ion Reducing Antioxidant Capacity): Involves the reduction of Cu2+ to Cu+, forming a colored complex measured spectrophotometrically.
ORAC assay (Oxygen Radical Absorbance Capacity): Measures the inhibition of peroxyl-radical-induced oxidative degradation of a fluorescent molecule.
Uses: Screening for secondary metabolites that combat diseases associated with oxidative stress such as cardiovascular diseases and neurodegenerative diseases. Antioxidant assays lead nutrition scientists in formulating novel nutraceuticals and functional foods enriched with secondary metabolites [132].
7 Issues in the Isolation and Characterization of Secondary Metabolites
7.1 Plant Extracts Mixes Are Sophisticated
Many plants yield such extracts that are not pure and contain mixtures consisting of thousands of compounds, most of which are similar in their physical chemical attributes. This makes the extraction of individual secondary metabolites particularly challenging. Beyond the elementary obstacles, dealing with these problems sometimes involves tackling a series of such technical and methodological problems [133].
7.2 Comparable Physical Characteristics
Many secondary metabolites in an extract share the same solubility parameters and polarities or the same molecular weights. This poses a challenge for separation techniques like chromatography, which rely on a clear distinction between compounds, as it is hard to obtain. For example, high-performance liquid chromatography (HPLC), ultra-performance liquid chromatography (UPLC), and preparative scale separations usually require several steps to achieve purity. Moreover, the combination of chromatographic techniques and molecular tagging often leads to better separation of closely related compounds [124,125].
Another challenge is the choice of solvent, which can greatly affect the efficiency of the separation. A great amount of work is needed to find a suitable solvent or solvent mix to use; an ideal solvent is highly soluble in the compound but not retained well. In addition, new methods such as supercritical fluid chromatography (SFC) and the use of green solvents have become popular and could be potential solutions to the traditional methods [134].
The co-elution of compounds that have similar retention times is one of the major challenges that are faced during isolation in chromatographic techniques. It is sometimes the case that a compound has similar behavior under different solvent conditions, which makes it essential to apply orthogonal separation techniques {LC-GC} rather than relying on gradient elution strategies. Detectors such as the multi-dimensional chromatography systems LC–MS/MS, or GC–GC–MS are some of the more advanced ones that are proving to be more effective in dealing with these types of co-elution problems as they enable the user to make finer distinctions based on chemical differences rather than physical ones [119].
Capillary electrophoresis and ion mobility spectrometry are the emerging technologies that are being explored to deal with co-elution problems. These methods provide the researchers with another tool as they allow differentiation based on charge and mobility [135]. Moreover, closely eluting compounds can further be investigated with the combination of high resolution mass spectrometry (HRMS) and isotope labeling techniques [136].
Macromolecules, including polysaccharides, lipids, and extracts from plants, tend to interfere with isolation as these extracts often contain pigments [137]. These components make it difficult to identify and purify the compounds of interest as the target metabolites tend to mask of co-elute [138]. To reduce matrix effects, sample pre-treatment, selective precipitation, and membrane filtration techniques are often used. However, in some cases, these techniques tend to lead to loss of targeted compounds, which is unintentional [138].
In an effort to reduce matrix interferences, new techniques and novel adsorbent materials like molecular imprints have been invented [138]. For example, Specific metabolites may be captured by selective ligands coated upon magnetic nanoparticles, which reduces the background signal due to other interfering substances. In the same manner, solid-phase microextraction (SPME) methods are also being modified because of their characteristic of extracting metabolites out of complex matrices without much sample preparation [77].
Matrix modeling is another technique that shows strong potential. With the help of computer techniques, researchers may simulate the target compounds in complex mixtures of a sample. This simulation allows researchers to anticipate the interferences and enhance the separation techniques that can be used. When combined with sophisticated experimental techniques, this technique allows for a more focused and faster method of extraction of secondary metabolites [86].
7.5 Low Abundance of Target Metabolites
The production of secondary metabolites is often significantly low, especially in wild plants or in microorganisms cultivated in a lab environment. Such low yields makes it difficult to acquire enough substrates to analyze deeply into the material. This calls for the necessity of new and advanced methods and technology to overcome the challenges that the low yield poses [139].
7.5.1 Harvesting on a Large Scale
It is common for scientists to request vast amounts of plant tissue to balance low metabolite levels. This not only requires significant amounts of work, but it may also cause ecological damage through excessive collection of wild specimens [139]. Resourceful approaches like the cultivation of the source plant do take time and effort, but they are absolutely necessary. For instance, these plants can be grown using controlled techniques like hydroponic or aeroponic systems, which enhance the conditions for plant growth and consequently improve metabolite production [140]. One alternative is using endophytic microorganisms that can either produce or enhance the specific secondary metabolites needed. If these microorganisms can be isolated and cultured, then large-scale collection of plant tissues may be avoided. In addition, working with local communities for the collection of plant materials can promote ecologically safe practices [141].
The selection of certain methods for their achievement includes bioassay–guided fractionation, solid phase extraction, and solvent partitioning [142]. These methods have trade-offs because they may also result in the precipitation of compounds or contamination with other constituents. However, more recent developments, such as the automated liquid–liquid extraction with solvent extraction systems and new adsorbents like molecularly imprinted polymers (MIPs) have much better recovery rates for compounds [143]. Some advancements in ultrafiltration and nanofiltration enable researchers to selectively concentrate metabolites and remove unwanted matrix components. Integrating these techniques with online monitoring tools such as infrared spectroscopy improves the efficiency of enrichment processes and reduce material loss during alterations [144,145].
7.5.3 Sensitivity of Analytical Instruments
To detect those metabolites, majorly sensitive instruments such as mass spectrometers (MS) or nuclear magnetic resonance (NMR) spectrometers are. Even though some of these sophisticated devices SWIFT the highest level of development, they are not able to detect and quantify some compounds at picomolar or nanomolar concentration. Some Innovations The developments cryogenic NMR and Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) have enhance the sensitivity of analytical techniques significantly [146]. Also, the implementation of microfluidic technologies into sample preparation procedures is servicing detection potential. These systems allow volumetric manipulation at the microliter scale, which lessens dilution and increases signal strength for minuscule components [14]. Certain methods like droplet-based microfluidics have been efficient in obtaining scarce metabolites from limited samples.
7.5.4 Progress in Syntehtic Biology and Metabolic Engineering
Similarly, there is active work in gene editing, and these technologies are considered powerful tools against the scarcity of most secondary metabolites. Constructing and manipulating biosynthetic pathways in microorganisms is a proven way to increase the yield of target compounds [147]. For example, gC9 mAb is designed to insert or delete specific genes from the microbial system to induce metabolic pathways of interest [148]. Besides, the growth of two or more microorganisms together in co-culture systems has demonstrated that interspecies relationships can promote metabolism and metabolite production. This improves productivity and increases the likelihood of obtaining compounds that would not be formed when grown alone [149].
Using a bioreactor has been crucial in increasing the scale of secondary metabolites. The invention of the fed-batch and continuous culture bioreactors allows for increased control of environmental factors like pH, temperature, and nutrient concentration, which may result in significant increases in the yield of metabolites. Moreover, encouraging results have been seen while using elicitors, which are defined as substances that result in signaling or stress response in the biological system to derive secondary metabolites within the bioreactors. Some of these examples can include the addition of jasmonic acid in plant cell cultures or antibiotics in microbial systems to induce biosynthetic processes [150].
7.5.6 High-Throughput Screening Methods
High-throughput screening (HTS) techniques help mitigate the free radical problem by counterfeiting the lack of metabolite abundance. These techniques utilize automated platforms to quickly screen thousands of samples to select the few that contain the targeted compounds in the highest concentration. In addition to advanced imaging technology, HTS enables much more detailed spatial and temporal examination of metabolite distribution [151].
7.5.7 Approaches Based on Computations and Machine Learning
An important contribution can be that machine learning approaches assist in the problem of sparse metabolites. These algorithms are capable of forecasting the most favorable conditions for metabolite biosynthesis, or genetic markers for elite phenotypes, through the analysis of big data obtained from omics studies. Metabolic network modeling similarly predicts bottlenecks in some biosynthetic pathways and these predictions can be used for strategic engineering [152]. Some of these methods are useful for the identification of cryptic gene clusters, which are genomic regions for secondary metabolites that are typically silent during laboratory cultivation. Using these algorithms, it is possible to predict the conditions or changes in the genome that would lead to the expression of otherwise silent clusters, thereby providing access to novel metabolites [153].
7.6 Characterization of the Structural Diversity of Secondary Metabolites
Diverse structural features of secondary metabolites pose a major challenge in their characterization. However, these features would need to be restructured to fully understand their activity in biology and their potential uses. This class of compounds is structurally complex, and a number of techniques and multi-disciplinary approaches will be needed to elucidate the structures and functions of these compounds [154].
7.6.1 Advanced Methods for Spectral Analysis
Several secondary metabolites are characterized by unique stereoisomeric structures with several chiral centers or functional groups, rendering structural elucidation quite difficult. Techniques such as NMR spectroscopy, X-ray crystallography, and advanced forms of mass spectrometry, including tandem MS and high resolution MS, are crucial, and indeed, techniques of these sorts require high-level computational power and special training owing to the complexities in data interpretation [155]. As an illustration, NMR spectroscopy yields high-quality data on a molecular level, but often requires the subject compounds to be isotopically labeled to increase sensitivity [154]. In spite of the above, X-ray crystallography is regarded as supremely accurate as long as the subject compounds can be crystallized, a process that is especially challenging for secondary metabolites that possess non-rigid multi-substituted frameworks [156]. New developments in electron diffraction techniques are emerging as suitable replacements for classic X-ray methods because they enable structure determination of microcrystals that are otherwise too small and unsuitable for traditional methods [157]. The adoption of mass spectrometry, especially in combination with ion mobility separation, tends to be essential when discussing secondary metabolites. This technique not only allows one to analyze mass-to-charge ratios with high resolution but also determines their fragmentation patterns, helping researchers determine the presence of different functional groups and their connectivity within the molecules. The application of these methods in combination with computational spectral deconvolution is proving very beneficial [158].
7.6.2 Stereoisomer Identification
If there are other stereoisomers like structural isomers, characterization may become further complex. For example, chiral enantiomers may differ in their biological activities and seem at times to be indistinguishable without the use of chiral separation procedures or highly sophisticated computational simulations [156]. One of the most convenient methods for resolving enantiomers chiral chromatography remains, but it is rather laborious and frequently requires significant method development [159]. New approaches such as vibrational circular dichroism (VCD) and electronic circular dichroism (ECD) are becoming more common as powerfulestablished techniques for stereochemstry [160]. They offer absolute configuration of the chiral compounds, and provide precise insight to the molecular chiral structures without any method of derivitation or separation. Also the application of more advanced quantum mechanial modeling increased the predict accuracy of determined stereochemical configuration, therefore decrease the experimental burden [156].
Stereoisomer identification is also getting easier with recent updates related to cryogenic electron microscopy (Cryo-EM). By keeping the molecules at cryogenic temperature, simliar to how atoms are frozen at mechanical rooms, the structure of the molecules does not suffer [161]. This method produces stunning visuals for the arrangement of stereoisomers that are globally complex and elaborate [156].
7.6.3 Degradation During Analysis
Some metabolites can undergo analysis-induced degradation, for instance, via light, heat, or solvent exposure. Degradation can cause inaccurate results, so strict control of experimental conditions is necessary [157]. Some of the most effective methods employed today for sample preparation include, temperature-controlled storage and transport, inert gas atmospheres, and stabilizers in the sample [162]. Using in situ flow-through NMR or microfluidic-based MS can examine the sample without removing it from controlled conditions, so the sample is not subjected to any environmental factors that could lead to degradation. These techniques have a lower sample preparation burden, significantly lessening the risk of sample degradation. Chemical modification of labile compounds that render them more stable is also becoming more common to increase the time these compounds can be preserved during testing. UV-active or sensitive metabolites present a different challenge wherein photodegradation can occur. Protective sample containers and UV-filtering solvents can be beneficial techniques that mitigate the issue [163]. Furthermore, techniques without a fixed frequency such as variable fluorescence have been used for studying kinetics of photodegradation [164].
7.6.4 Computational Tools and Structural Databases
The adoption of scientific methods and the development of comprehensive structural databases are increasingly evolving as the most effective ways to solve problems that stem from structural complexity [15]. Metabolite structures and their interactions with biological targets are better understood through molecular docking, quantum chemistry simulations, and machine learning applications [152]. These methods also make it possible to perform structure elucidation in a more efficient manner, as well as to develop hypotheses for compounds that have not yet been characterized [165].
For comparative analysis and spectral matching, freely available databases such as the Human Metabolome Database (HMDB) and METLIN are proving indispensable. Data of this nature enables the researcher to detect metabolites by experimentally acquired data and a library of known compounds. The steady growth and maintenance of sorts of databases is propelling the field single kidney forward significantly [166].
7.7 Environmental and Seasonal Variability
There are different factors ranging from climatic conditions, soil type, and exposure to sunlight that tend to affect secondary metabolite production [153,167]. There are also seasonal and geographical differences that further modify the quantity and quality of the metabolites produced. Accurately identifying and dealing with these variables is significant for reproducibility and consistency in research and industry [4].
Certain regions may have plants that possess distinct secondary metabolite profiles. This type of phenomenon is known as chemotypic variation, and it could make research and industrial applications much harder to replicate [168,169]. Soil characteristics like pH and mineral content, altitude, and climate should be considered in all aspects of metabolite profiling of plants. For instance, high altitudes plants may contain more alkaloIDs because of the increased exposure to UV rays [170]. To tackle these difficulties, researchers are combining geospatial mapping and metabolomics. Combining geographic information with metabolite profiling can allow for predicting and optimizing metabolite yields in a particular region [153]. Also, controlled transplanting experiments where plants are grown in several regions with some controls in place may reveal certain variables affecting metabolite profiling [171].
7.7.2 Seasonal Fluctuations Issues
Many other metabolic products are synthesized by certain species in response to stress or pathogen attack. Due to this, there may be an accumulation in certain months making it difficult to plan for the collection of raw materials [172]. The concentration of some phenolic compounds in some plant species can be high when water is scarce. In contrast, other compounds are produced when the plant is fed on or when fungal pathogens attack [65].
Through field work and seasonal monitoring of the changes coupled with metabolite profiling, researchers are beginning to understand these processes [172]. Moreover, phenological models that correlate changes in season with metabolite production are being designed. These models are particularly useful in understanding when certain compounds can be harvested for the maximum yield [173].
7.7.3 Controlled Cultivation Issues
There is always some degree of variability when using controlled cultivation approaches in the laboratory or greenhouse [174]. This, however, can be reduced by creating the environment needed for metabolite extraction [170]. In nature, certain conditions must be met, for instance light intensity, length of light exposure, nutrition, and the presence of other microbes within the environment have to be controlled. For instance, the generation of some flavonoids needs specific light wavelengths, while others require symbiosis with certain fungi or bacteria [140].
New technologies within precision agriculture and controlled-environment agriculture (CEA) allow these problems to be addressed [175]. Vertical farming, hydroponics, and automated climate control systems allow us to manipulate these parameters with great precision [176]. Furthermore, the application of elicitors, which are agents that induce stress responses in plants, is also being optimized for increased secondary metabolite production. The application of jasmonic acid and salicylic acid, to name a couple, is known to enhance the production of economically important alkaloids and phenolics in a variety of plant species [172].
7.7.4 Microbial and Symbiotic Influences
Increasing attention is given to the role that microbial communities play in the development of secondary metabolites. Various plants rely on their associated microbiota for regulating the synthesis of various metabolites through either direct biosynthetic processes or modulation of plant stress responses. For example, antimicrobial compounds within some plants can be produced naturally through the stimulation of endophytic fungi and bacteria [177].
Research in the field of plant-microbe interactions brings new hope in optimizing metabolite production. In particular, the inoculation of plants with specific microbial strains aimed at enhancing the synthesis of target metabolites is one way of achieving this goal. Also, new advances in microbiome engineering allow for the development of tailored consortia of microbes with the aim of maximizing metabolite production [178].
7.7.5 Innovations in Predictive Modeling
The combination of prediction modeling and environment-related data is a newer technique that can help to solve issues surrounding variability in metabolite production [63]. Models incorporating climate change, soil structures, and plant genetics can help predict metabolite yields, enabling better site selection. This approach helps decide where to cultivate plants and sheds light on how climate changes might affect metabolite availability in the future [179].
Machine learning and artificial intelligence (AI) have been extremely helpful in refining these models. Identifying patterns within complex datasets is a difficult endeavor that AI algorithms are able to perform due to their advanced processing software [180]. For example, certain AI algorithms can calculate the best environmental parameters for producing specific metabolites, thus aiding experimental design and cultivation [181].
To adequately address the environmental and seasonal variability of metabolite production, there needs to be an implementable, efficient plan that adequately conserves the surrounding ecology [182]. Practices such as crop rotation, intercropping, the use of organic fertilizers, and sustainability can increase soil health as well as decrease the variability in metabolite production [183]. Furthermore, integrating TK with modern scientific approaches gives a framework that is more sustainable when it comes to cultivation [182]. It is essential to note the attempts to use selective breeding and genetic engineering to produce climate-resilient plant varieties. Scientists are developing novel strains of plants that can reap high yields in less-than-ideal conditions by identifying and amplifying genes related to metabolite synthesis under stressful conditions [184].
8 Emerging Solutions and Future Directions
The technological and methodological progress being made currently serves as a remedial metric toward emerging issues.
The use of integrated genomics, transcriptomic, and metabolomics approaches renders the biosynthesis of the secondary metabolites much more comprehensible. These methods help in the identification of critical biosynthetic pathways and their regulatory genes, which can be helpful in metabolic engineering or synthetic biology [135]. Moreover, these technologies enable scientists to discover new metabolites that were not previously identified by utilizing the unused biosynthetic capabilities of plant and microbial genomes. For example, new polyketides and nonribosomal peptides have been discovered through genome mining [135].
The emerging issues relative to the composition and structure of various natural products are undergoing adjustments with the introduction of novel systems such as cryo-electron microscopies, high field NMR, and imaging mass spectrometers [90]. Using the above tools, the distribution of metabolites within tissues can be seen, helping scientists in understanding the process of biosynthesis and collateralization [158]. Combined with automation and miniaturization, these tools are proving to make the analysis process more efficient, both in terms of time and samples used [90].
AI and ML finds application in predicting the structure of compounds, optimizing separation methods, and even analyzing more complex datasets [154]. For example, algorithms can predict isolation time for chromatographic processes or retention times and even seem to get the chromatographic procedure straightened out [152]. It can also be used in analyzing the biosynthetic pathways so that such experiments can be done or designed which can help one estimate the outcome of a genetic alteration (Fig. 2) [165].
Figure 2: Emerging technologies and methodologies for addressing challenges in natural product biosynthesis and utilization
8.4 Biotechnological Interventions
The techniques of tissue culture, microbial fermentation, and even genetic changes are making it possible to produce secondary metabolites sustainably [185]. For instance, such engineered yeast or even bacteria can be used as product biofactories. The same goal is aided by advances in synthetic biology that empower scientists to change the configuration of metabolic pathways so that high yield can increase, and applicants of metabolite not produced otherwise can be created. Moreover, co-culture systems and bioreactor optimizations possess scalable solutions for industrial applications [186].
8.5 Sustainable Harvesting Practices
The same concern on balancing biodiversity and human needs for natural products is the primary driving force behind the formulation of sustainable harvesting strategies and the need for conservation efforts. Also, using cylindrical high-yield plant varieties, some agroforestry systems, or even using traditional ecological knowledge can reduce the environmental cost of such changes. In addition, there is a need to establish policies that promote fair trade and equitable benefit sharing to safeguard ethics in the sourced and utilized the natural resources [187].
8.6 Integration of Interdisciplinary Approaches
Blending bioinformatics and environmental science alongside chemical ecology provides novel answers to age-old problems [63]. Working together makes it possible to synthesize information from various omics and field survey sources, leading to designs for studying the dynamics of metabolites. These developments are critical for the forward movement of research and the application of derived results in real-life situations [188].
The study of the secondary metabolites from plants is a lively and appealing cross-disciplinary area of focus of vast importance to science and industry. Such bioactive compounds are made through the complexities of the plant’s metabolic pathways. Engaged plants help us understand the paradigm in which humans seek to improve well as derm advances sustainable agriculture. The application of secondary metabolites to the world goes beyond healing wounds to transforming the planet. The need for medicine and the invention of life-saving drugs such as artemisinin and paclitaxel speak for their importance. These compounds literally changed the game for treating diseases like malaria and cancers, hawking an ever-powerful ml cure. The same goes for the agriculture space, where these compounds enable the making of biopesticides and even allelochemicals, which harm the growth of unwanted plants. These anti-herbicides are powerful tools in the fight against conventional farming. The very same plants used for such things have even greater promise when consumed. Used as antioxidants and flavoring, their uses are as versatile as the term itself. Although they promise a lot of value, transforming plant extracts into useful outputs involves several difficulties. Plant matrices are intricate, and many of the metabolites are present in low concentrations, requiring more advanced extraction and analytical methods. Also, environmental conditions along with seasonal changes can impact metabolite yield, further complicating research design. Technological advancements, particularly in high-performance chromatography and advanced spectroscopic techniques, have significantly enhanced our ability to isolate and characterize these compounds, allowing for a deeper understanding of their structures and functions. The chemical diversity of secondary metabolites, like bioactive compounds, is dependent on the preservation of biodiversity.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design, data collection, and analysis and interpretation of results: Amine Assouguem, draft manuscript preparation: Amine Assouguem, Saoussan Annemer, Mohammed Kara and Abderrahim Lazraq. Writing—review and editing: Amine Assouguem, Saoussan Annemer, Mohammed Kara and Abderrahim Lazraq. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Dickey RM, Forti AM, Kunjapur AM. Advances in engineering microbial biosynthesis of aromatic compounds and related compounds. Bioresour Bioprocess. 2021;8(1):91. doi:10.1186/s40643-021-00434-x. [Google Scholar] [PubMed] [CrossRef]
2. Hou Q, Wang Y, Qu D, Zhao H, Tian L, Zhou J, et al. Microbial communities, functional, and flavor differences among three different-colored high-temperature Daqu: a comprehensive metagenomic, physicochemical, and electronic sensory analysis. Food Res Int. 2024;184(5):114257. doi:10.1016/j.foodres.2024.114257. [Google Scholar] [PubMed] [CrossRef]
3. Adetunji CO, Palai S, Ekwuabu CP, Egbuna C, Adetunji JB, Ehis-Eriakha CB, et al. General principle of primary and secondary plant metabolites: biogenesis, metabolism, and extraction. In: Egbuna C, Mishra AP, Goyal MR, editors. Preparation of phytopharmaceuticals for the management of disorders: the development of nutraceuticals and traditional medicine. Amsterdam, The Netherlands: Elsevier; 2021. p. 3–23. doi:10.1016/B978-0-12-820284-5.00018-6. [Google Scholar] [CrossRef]
4. Salam U, Ullah S, Tang ZH, Elateeq AA, Khan Y, Khan J, et al. Plant metabolomics: an overview of the role of primary and secondary metabolites against different environmental stress factors. Life. 2023;13(3):706. doi:10.3390/life13030706. [Google Scholar] [PubMed] [CrossRef]
5. Ahmed S, Jamil S, Siddiqui MUA. Secondary metabolites-God gifted arsenal for plants. J Pharmacogn Phytochem. 2024;13(1):38–43. doi:10.22271/phyto.2024.v13.i1a.14811. [Google Scholar] [CrossRef]
6. Pandita D, Pandita A. Secondary metabolites in medicinal and aromatic plants (MAPspotent molecules in nature’s arsenal to fight human diseases. In: Aftab T, Hakeem KR, editors. Medicinal and aromatic plants: healthcare and industrial applications. Berlin/Heidelberg, Germany: Springer; 2021. p. 41–84. doi:10.1007/978-3-030-58975-2_2. [Google Scholar] [CrossRef]
7. Setyorini D, Antarlina SS. Secondary metabolites in sorghum and its characteristics. Food Sci Technol. 2022;42(1):e49822. doi:10.1590/fst.49822. [Google Scholar] [CrossRef]
8. Elshafie HS, Camele I, Mohamed AA. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int J Mol Sci. 2023;24(4):3266. doi:10.3390/ijms24043266. [Google Scholar] [PubMed] [CrossRef]
9. Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules. 2022;27(1):313. doi:10.3390/molecules27010313. [Google Scholar] [PubMed] [CrossRef]
10. Chiocchio I, Mandrone M, Tomasi P, Marincich L, Poli F. Plant secondary metabolites: an opportunity for circular economy. Molecules. 2021;26(2):495. doi:10.3390/molecules26020495. [Google Scholar] [PubMed] [CrossRef]
11. Kumar S, Saini R, Suthar P, Kumar V, Sharma R. Plant secondary metabolites: their food and therapeutic importance. In: Sharma AK, Sharma A, editors. Plant secondary metabolites: physico-chemical properties and therapeutic applications. Berlin/Heidelberg, Germany: Springer; 2022. p. 371–413. doi:10.1007/978-981-16-4779-6_12. [Google Scholar] [CrossRef]
12. Koza NA, Adedayo AA, Babalola OO, Kappo AP. Microorganisms in plant growth and development: roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms. 2022;10(8):1528. doi:10.3390/microorganisms10081528. [Google Scholar] [PubMed] [CrossRef]
13. Elhamouly NA, Hewedy OA, Zaitoon A, Miraples A, Elshorbagy OT, Hussien S, et al. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front Plant Sci. 2022;13:1044896. doi:10.3389/fpls.2022.1044896. [Google Scholar] [PubMed] [CrossRef]
14. Caesar LK, Montaser R, Keller NP, Kelleher NL. Metabolomics and genomics in natural products research: complementary tools for targeting new chemical entities. Nat Prod Rep. 2021;38(11):2041–65. doi:10.1039/d1np00036e. [Google Scholar] [PubMed] [CrossRef]
15. Gaudêncio SP, Bayram E, Lukić Bilela L, Cueto M, Díaz-Marrero AR, Haznedaroglu BZ, et al. Advanced methods for natural products discovery: bioactivity screening, dereplication, metabolomics profiling, genomic sequencing, databases and informatic tools, and structure elucidation. Mar Drugs. 2023;21(5):308. doi:10.3390/md21050308. [Google Scholar] [PubMed] [CrossRef]
16. Kussmann M, Abe Cunha DH, Berciano S. Bioactive compounds for human and planetary health. Front Nutr. 2023;10:1193848. doi:10.3389/fnut.2023.1193848. [Google Scholar] [PubMed] [CrossRef]
17. Hilal B, Khan MM, Fariduddin Q. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: phenolics, terpenes, and alkaloids. Plant Physiol Biochem. 2024;211(8):108674. doi:10.1016/j.plaphy.2024.108674. [Google Scholar] [PubMed] [CrossRef]
18. Bano A, Qadri TA, Mahnoor, Khan N. Bioactive metabolites of plants and microbes and their role in agricultural sustainability and mitigation of plant stress. S Afr J Bot. 2023;159(1):98–109. doi:10.1016/j.sajb.2023.05.049. [Google Scholar] [CrossRef]
19. Kaboudin B, Sohrabi M. Chemistry and synthesis of major opium alkaloids: a comprehensive review. J Iran Chem Soc. 2021;18(12):3177–218. doi:10.1007/s13738-021-02268-y. [Google Scholar] [CrossRef]
20. Chaachouay N, Zidane L. Plant-derived natural products: a source for drug discovery and development. Drugs Drug Candidates. 2024;3(1):184–207. doi:10.3390/ddc3010011. [Google Scholar] [CrossRef]
21. Kingston DGI, Cassera MB. Antimalarial natural products. In: Kinghorn AD, Falk H, Gibbons S, Asakawa Y, Liu JK, Dirsch VM, editors. Antimalarial natural products. Berlin/Heidelberg, Germany: Springer; 2022. p. 1–106. doi:10.1007/978-3-030-89873-1_1. [Google Scholar] [CrossRef]
22. Malik M, Hussain S, Sajjad N, Nazir S, Gondal MU, Arshad J. Physicochemical analysis, qualitative and quantitative investigation of atropine in medicinal plant Atropa belladonna. Eur J Pharm Med Res. 2021;8:149–57. [Google Scholar]
23. Karunarathna I, Bandara S, Karunarathna A. Comprehensive management of organophosphate poisoning: pathophysiology, diagnosis, and treatment. 2024. doi:10.13140/RG.2.2.27398.59201. [Google Scholar] [CrossRef]
24. García Del Valle I, Alvarez-Lorenzo C. Atropine in topical formulations for the management of anterior and posterior segment ocular diseases. Expert Opin Drug Deliv. 2021;18(9):1245–60. doi:10.1080/17425247.2021.1909568. [Google Scholar] [PubMed] [CrossRef]
25. Heinrich M, Mah J, Amirkia V. Alkaloids used as medicines: structural phytochemistry meets biodiversity—an update and forward look. Molecules. 2021;26(7):1836. doi:10.3390/molecules26071836. [Google Scholar] [PubMed] [CrossRef]
26. Maswal M, Pandita M, Bashir S. Terpenes, terpenoids and steroids: properties, biosynthesis and functions. In: Banday AH, editor. Steroids and their medicinal potential. Sharjah, United Arab Emirates: Bentham Science Publishers; 2023. p. 1–38. doi:10.2174/789815049336123010003. [Google Scholar] [CrossRef]
27. Sharmeen JB, Mahomoodally FM, Zengin G, Maggi F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26(3):666. doi:10.3390/molecules26030666. [Google Scholar] [PubMed] [CrossRef]
28. Bolouri P, Salami R, Kouhi S, Kordi M, Asgari Lajayer B, Hadian J, et al. Applications of essential oils and plant extracts in different industries. Molecules. 2022;27(24):8999. doi:10.3390/molecules27248999. [Google Scholar] [PubMed] [CrossRef]
29. Cui J, Li M, Wei Y, Li H, He X, Yang Q, et al. Inhalation aromatherapy via brain-targeted nasal delivery: natural volatiles or essential oils on mood disorders. Front Pharmacol. 2022;13:860043. doi:10.3389/fphar.2022.860043. [Google Scholar] [PubMed] [CrossRef]
30. Liang MH, He YJ, Liu DM, Jiang JG. Regulation of carotenoid degradation and production of apocarotenoids in natural and engineered organisms. Crit Rev Biotechnol. 2021;41(4):513–34. doi:10.1080/07388551.2021.1873242. [Google Scholar] [PubMed] [CrossRef]
31. Bas TG. Bioactivity and bioavailability of carotenoids applied in human health: technological advances and innovation. Int J Mol Sci. 2024;25(14):7603. doi:10.3390/ijms25147603. [Google Scholar] [PubMed] [CrossRef]
32. Arif Y, Singh P, Bajguz A, Hayat S. Phytoecdysteroids: distribution, structural diversity, biosynthesis, activity, and crosstalk with phytohormones. Int J Mol Sci. 2022;23(15):8664. doi:10.3390/ijms23158664. [Google Scholar] [PubMed] [CrossRef]
33. Naseem N, Khaliq T, Jan S, Nabi S, Sultan P, Hassan QP, et al. An overview on pharmacological significance, phytochemical potential, traditional importance and conservation strategies of Dioscorea deltoidea: a high valued endangered medicinal plant. Heliyon. 2024;10(10):e31245. doi:10.1016/j.heliyon.2024.e31245. [Google Scholar] [PubMed] [CrossRef]
34. Viskelis J, Surguchov A. Essential oils: recent advances, new perspectives and applications. Cambridge, UK: IntechOpen; 2024. 186 p. [Google Scholar]
35. Chen Z, Świsłocka R, Choińska R, Marszałek K, Dąbrowska A, Lewandowski W, et al. Exploring the correlation between the molecular structure and biological activities of metal-phenolic compound complexes: research and description of the role of metal ions in improving the antioxidant activities of phenolic compounds. Int J Mol Sci. 2024;25(21):11775. doi:10.3390/ijms252111775. [Google Scholar] [PubMed] [CrossRef]
36. Sun W, Shahrajabian MH. Therapeutic potential of phenolic compounds in medicinal plants-natural health products for human health. Molecules. 2023;28(4):1845. doi:10.3390/molecules28041845. [Google Scholar] [PubMed] [CrossRef]
37. Kumar A, Kumar P, Shravya H, Pai A. Coumarins as potential anticoagulant agents. Res J Pharm Technol. 2022;15:1659–63. doi:10.52711/0974-360X.2022.00277. [Google Scholar] [CrossRef]
38. Afnan, Saleem A, Akhtar MF, Sharif A, Akhtar B, Siddique R, et al. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules. 2022;27(21):7286. doi:10.3390/molecules27217286. [Google Scholar] [PubMed] [CrossRef]
39. Elmusa F, Elmusa M. Mini-review on coumarins: sources, biosynthesis, bioactivity, extraction and toxicology. J Turk Chem Soc Sect A Chem. 2024;11(3):933–44. doi:10.18596/jotcsa.1419322. [Google Scholar] [CrossRef]
40. Sharifi-Rad J, Cruz-Martins N, López-Jornet P, Lopez EP, Harun N, Yeskaliyeva B, et al. Natural coumarins: exploring the pharmacological complexity and underlying molecular mechanisms. Oxid Med Cell Longev. 2021;2021(1):6492346. doi:10.1155/2021/6492346. [Google Scholar] [PubMed] [CrossRef]
41. Cafeo G, Irrera E, Russo M, Dugo P. Extraction and chromatographic approaches for coumarin, furocoumarin, and polymethoxyflavone characterization in foods. Foods. 2024;13(16):2517. doi:10.3390/foods13162517. [Google Scholar] [PubMed] [CrossRef]
42. Ruiz Rodríguez LG, Zamora Gasga VM, Pescuma M, Van Nieuwenhove C, Mozzi F, Sánchez Burgos JA. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res Int. 2021;140(9):109854. doi:10.1016/j.foodres.2020.109854. [Google Scholar] [PubMed] [CrossRef]
43. Giurranna E, Nencini F, Bettiol A, Borghi S, Argento FR, Emmi G, et al. Dietary antioxidants and natural compounds in preventing thrombosis and cardiovascular disease. Int J Mol Sci. 2024;25(21):11457. doi:10.3390/ijms252111457. [Google Scholar] [PubMed] [CrossRef]
44. Ucella-Filho JGM, Freire ASM, Carréra JC, Lucas FMF, Zucolotto SM, Júnior AFD, et al. Tannin-rich bark extract of plants as a source of antimicrobial bioactive compounds: a bibliometric analysis. S Afr J Bot. 2022;150:1038–50. doi:10.1016/j.sajb.2022.09.018. [Google Scholar] [CrossRef]
45. Das RK, Mizan A, Zohra FT, Ahmed S, Ahmed KS, Hossain H. Extraction of a novel tanning agent from indigenous plant bark and its application in leather processing. J Leather Sci Eng. 2022;4(1):18. doi:10.1186/s42825-022-00092-5. [Google Scholar] [CrossRef]
46. Jang WY, Kim MY, Cho JY. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int J Mol Sci. 2022;23(24):15482. doi:10.3390/ijms232415482. [Google Scholar] [PubMed] [CrossRef]
47. Khursheed A, Rather MA, Jain V, Wani AR, Rasool S, Nazir R, et al. Plant based natural products as potential ecofriendly and safer biopesticides: a comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb Pathog. 2022;173(2):105854. doi:10.1016/j.micpath.2022.105854. [Google Scholar] [PubMed] [CrossRef]
48. Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, et al. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. 2021;2021(1):3687700. doi:10.1155/2021/3687700. [Google Scholar] [PubMed] [CrossRef]
49. Bajalia EM, Azzouz FB, Chism DA, Giansiracusa DM, Wong CG, Plaskett KN, et al. Phytochemicals for the prevention and treatment of renal cell carcinoma: preclinical and clinical evidence and molecular mechanisms. Cancers. 2022;14(13):3278. doi:10.3390/cancers14133278. [Google Scholar] [PubMed] [CrossRef]
50. Balkrishna A, Yadav P, Yadav P, Saini A, Kumar B, Singh N, et al. Factors affecting biodiversity and conservation of medicinal plants. In: Nandave M, Joshi R, Upadhyay J, editors. Ethnopharmacology and OMICS advances in medicinal plants volume 1: uncovering diversity and ethnopharmacological aspects. Berlin/Heidelberg, Germany: Springer; 2024. p. 1–16. doi:10.1007/978-981-97-2367-6_1. [Google Scholar] [CrossRef]
51. Dabiri M, Majdi M, Bahramnejad B. Spatial and developmental regulation of putative genes associated with the biosynthesis of sesquiterpenes and pyrethrin I in Chrysanthemum cinerariaefolium. Biologia. 2021;76(5):1603–16. doi:10.1007/s11756-021-00710-3. [Google Scholar] [CrossRef]
52. Kamaruzaman NH, Mohd Noor NN, Radin Mohamed RMS, Al-Gheethi A, Ponnusamy SK, Sharma A, et al. Applicability of bio-synthesized nanoparticles in fungal secondary metabolites products and plant extracts for eliminating antibiotic-resistant bacteria risks in non-clinical environments. Environ Res. 2022;209(6):112831. doi:10.1016/j.envres.2022.112831. [Google Scholar] [PubMed] [CrossRef]
53. Souto AL, Sylvestre M, Tölke ED, Tavares JF, Barbosa-Filho JM, Cebrián-Torrejón G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: prospects, applications and challenges. Molecules. 2021;26(16):4835. doi:10.3390/molecules26164835. [Google Scholar] [PubMed] [CrossRef]
54. Assaf M, Korkmaz A, Karaman Ş, Kulak M. Effect of plant growth regulators and salt stress on secondary metabolite composition in Lamiaceae species. S Afr J Bot. 2022;144(4):480–93. doi:10.1016/j.sajb.2021.10.030. [Google Scholar] [CrossRef]
55. Liu R, Bao ZX, Zhao PJ, Li GH. Advances in the study of metabolomics and metabolites in some species interactions. Molecules. 2021;26(11):3311. doi:10.3390/molecules26113311. [Google Scholar] [PubMed] [CrossRef]
56. Makhlouf L, El Fakhouri K, Kemal SA, Maafa I, Meftah Kadmiri I, El Bouhssini M. Potential of volatile organic compounds in the management of insect pests and diseases of food legumes: a comprehensive review. Front Plant Sci. 2024;15:1430863. doi:10.3389/fpls.2024.1430863. [Google Scholar] [PubMed] [CrossRef]
57. Divekar PA, Narayana S, Divekar BA, Kumar R, Gadratagi BG, Ray A, et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int J Mol Sci. 2022;23(5):2690. doi:10.3390/ijms23052690. [Google Scholar] [PubMed] [CrossRef]
58. Dang H, Zhang T, Wang Z, Li G, Zhao W, Lv X, et al. Succession of endophytic fungi and arbuscular mycorrhizal fungi associated with the growth of plant and their correlation with secondary metabolites in the roots of plants. BMC Plant Biol. 2021;21(1):165. doi:10.1186/s12870-021-02942-6. [Google Scholar] [PubMed] [CrossRef]
59. Sarathambal C, Srinivasan V, Jeevalatha A, Sivaranjani R, Alagupalamuthirsolai M, Peeran MF, et al. Unravelling the synergistic effects of arbuscular mycorrhizal fungi and vermicompost on improving plant growth, nutrient absorption, and secondary metabolite production in ginger (Zingiber officinale Rosc.). Front Sustain Food Syst. 2024;8:1412610. doi:10.3389/fsufs.2024.1412610. [Google Scholar] [CrossRef]
60. Hudz N, Kobylinska L, Pokajewicz K, Horčinová Sedláčková V, Fedin R, Voloshyn M, et al. Mentha piperita: essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules. 2023;28(21):7444. doi:10.3390/molecules28217444. [Google Scholar] [CrossRef]
61. Alami MM, Guo S, Mei Z, Yang G, Wang X. Environmental factors on secondary metabolism in medicinal plants: exploring accelerating factors. Med Plant Biol. 2024;3(1):e016. doi:10.48130/mpb-0024-0016. [Google Scholar] [CrossRef]
62. Barthwal R, Mahar R. Exploring the significance, extraction, and characterization of plant-derived secondary metabolites in complex mixtures. Metabolites. 2024;14(2):119. doi:10.3390/metabo14020119. [Google Scholar] [PubMed] [CrossRef]
63. Mashabela MD, Masamba P, Kappo AP. Metabolomics and chemoinformatics in agricultural biotechnology research: complementary probes in unravelling new metabolites for crop improvement. Biology. 2022;11(8):1156. doi:10.3390/biology11081156. [Google Scholar] [PubMed] [CrossRef]
64. Samal I, Bhoi TK, Raj MN, Majhi PK, Murmu S, Pradhan AK, et al. Underutilized legumes: nutrient status and advanced breeding approaches for qualitative and quantitative enhancement. Front Nutr. 2023;10:1110750. doi:10.3389/fnut.2023.1110750. [Google Scholar] [PubMed] [CrossRef]
65. Al-Khayri JM, Rashmi R, Toppo V, Chole PB, Banadka A, Sudheer WN, et al. Plant secondary metabolites: the weapons for biotic stress management. Metabolites. 2023;13(6):716. doi:10.3390/metabo13060716. [Google Scholar] [PubMed] [CrossRef]
66. Jović MD, Agatonovic-Kustrin S, Ristivojević PM, Trifković JĐ, Morton DW. Bioassay-guided assessment of antioxidative, anti-inflammatory and antimicrobial activities of extracts from medicinal plants via high-performance thin-layer chromatography. Molecules. 2023;28(21):7346. doi:10.3390/molecules28217346. [Google Scholar] [PubMed] [CrossRef]
67. Kumari K, Adhikari P, Pandey A, Samant SS, Lal M, Pande V. Influence of solvent polarity on phytochemicals, antioxidants, and antimicrobial properties of Delphinium denudatum: a medicinal herb from sainj valley, Himachal pradesh. India Bioactivities. 2024;2(1):30–40. doi:10.47352/bioactivities.2963-654x.214. [Google Scholar] [CrossRef]
68. Siddiqui T, Khan MU, Sharma V, Gupta K. Terpenoids in essential oils: chemistry, classification, and potential impact on human health and industry. Phytomed Plus. 2024;4(2):100549. doi:10.1016/j.phyplu.2024.100549. [Google Scholar] [CrossRef]
69. Berlinck RGS, Crnkovic CM, Gubiani JR, Bernardi DI, Ióca LP, Quintana-Bulla JI. The isolation of water-soluble natural products—challenges, strategies and perspectives. Nat Prod Rep. 2022;39(3):596–669. doi:10.1039/d1np00037c. [Google Scholar] [PubMed] [CrossRef]
70. More PR, Jambrak AR, Arya SS. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci Technol. 2022;128:296–315. doi:10.1016/j.tifs.2022.08.016. [Google Scholar] [CrossRef]
71. Singh S, Verma DK, Thakur M, Tripathy S, Patel AR, Shah N, et al. Supercritical fluid extraction (SCFE) as green extraction technology for high-value metabolites of algae, its potential trends in food and human health. Food Res Int. 2021;150(19):110746. doi:10.1016/j.foodres.2021.110746. [Google Scholar] [PubMed] [CrossRef]
72. Moussa H, Dahmoune F, Mróz M, Remini H, Kadri N, Hamid S, et al. Efficient optimization approaches for microwave assisted extraction of high-quality antioxidant compounds from Salvia officinalis L. uhplc-HRMS differential analysis of phenolic profiles obtained by ultrasound and microwave extraction. Sustain Chem Pharm. 2023;35(9):101194. doi:10.1016/j.scp.2023.101194. [Google Scholar] [CrossRef]
73. Zhang A, Yan H, Yin Q, Cao J, Wang Y, Li Q. Liquid-liquid phase equilibrium measurement and thermodynamic modeling for the separation of n-hexane and methanol with four solvents. J Mol Liq. 2023;372:121169. doi:10.1016/j.molliq.2022.121169. [Google Scholar] [CrossRef]
74. Kowalska T, Sajewicz M. Thin-layer chromatography (TLC) in the screening of botanicals-its versatile potential and selected applications. Molecules. 2022;27(19):6607. doi:10.3390/molecules27196607. [Google Scholar] [PubMed] [CrossRef]
75. Waksmundzka-Hajnos M, Hawrył M, Hawrył A, Jóżwiak G. Thin layer chromatography in phytochemical analysis. In: Buszewski B, Baranowska I, editors. Handbook of bioanalytics. Berlin/Heidelberg, Germany: Springer; 2022. p. 1–31. doi:10.1007/978-3-030-63957-0_24-1. [Google Scholar] [CrossRef]
76. Hameedat F, Hawamdeh S, Alnabulsi S, Zayed A. High performance liquid chromatography (HPLC) with fluorescence detection for quantification of steroids in clinical, pharmaceutical, and environmental samples: a review. Molecules. 2022;27(6):1807. doi:10.3390/molecules27061807. [Google Scholar] [PubMed] [CrossRef]
77. Badawy MEI, El-Nouby MAM, Kimani PK, Lim LW, Rabea EI. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal Sci. 2022;38(12):1457–87. doi:10.1007/s44211-022-00190-8. [Google Scholar] [PubMed] [CrossRef]
78. Queiroz EF, Guillarme D, Wolfender JL. Advanced high-resolution chromatographic strategies for efficient isolation of natural products from complex biological matrices: from metabolite profiling to pure chemical entities. Phytochem Rev. 2024;23(5):1415–42. doi:10.1007/s11101-024-09928-w. [Google Scholar] [PubMed] [CrossRef]
79. Sruthi D, Dhanalakshmi M, Yashavantha Rao HC, Parthasarathy R, Jayabaskaran C. Extraction, isolation, and characterization of phytochemicals, the bioactive compounds of plants. In: Pati S, Sarkar T, Lahiri D, editors. Recent frontiers of phytochemicals. Amsterdam, The Netherlands: Elsevier; 2023. p. 1–8. doi:10.1016/b978-0-443-19143-5.00033-5. [Google Scholar] [CrossRef]
80. Wan Q, Liu H, Deng Z, Bu J, Li T, Yang Y, et al. A critical review of molecularly imprinted solid phase extraction technology. J Polym Res. 2021;28(10):401. doi:10.1007/s10965-021-02744-2. [Google Scholar] [CrossRef]
81. Alqahtani SS, Moni SS, Sultan MH, Ali Bakkari M, Madkhali OA, Alshahrani S, et al. Potential bioactive secondary metabolites of Actinomycetes sp. isolated from rocky soils of the heritage village Rijal Alma, Saudi Arabia. Arab J Chem. 2022;15(5):103793. doi:10.1016/j.arabjc.2022.103793. [Google Scholar] [CrossRef]
82. Khorshed AA, Abdelnaeem FM, Oraby M, Nagy DM, Derayea SM. Dual pH-optimized HPTLC method for simultaneous quantification and enhanced fluorescence detection of triamterene and losartan in human plasma: toward achieving maximum separate fluorescence intensity. Microchem J. 2025;212(3):113407. doi:10.1016/j.microc.2025.113407. [Google Scholar] [CrossRef]
83. Balekundri A, Ahire ED, Shelke RU, Rishipathak DD, Kshirsagar SJ. Eco-friendly HPTLC method for trifluridine and tipiracil determination: quality-by-design meets green analytical chemistry. Green Anal Chem. 2025;13(2):100234. doi:10.1016/j.greeac.2025.100234. [Google Scholar] [CrossRef]
84. Vázquez A, Tabanca N. Development of a combined 2D-MGD TLC/HPTLC method for the separation of terpinen-4-ol and α-terpineol from tea tree, Melaleuca alternifolia, essential oil. Biomolecules. 2025;15(1):147. doi:10.3390/biom15010147. [Google Scholar] [PubMed] [CrossRef]
85. Rabadiya VA, Shah N, Akabari AH. Eco-friendly and stability-indicating HPTLC method for the estimation of Carvedilol in pharmaceutical dosage forms: a greenness assessment using NEMI scale, AGREE, and white analytical chemistry. Green Anal Chem. 2025;13:100237. doi:10.1016/j.greeac.2025.100237. [Google Scholar] [CrossRef]
86. Jha AK, Sit N. Extraction of bioactive compounds from plant materials using combination of various novel methods: a review. Trends Food Sci Technol. 2022;119(8):579–91. doi:10.1016/j.tifs.2021.11.019. [Google Scholar] [CrossRef]
87. Dary Guerra-Fajardo L, Pavón-Pérez J, Vallejos-Almirall A, Jorquera-Pereira D. Advances in analytical techniques coupled to in vitro bioassays in the search for new peptides with functional activity in effect-directed analysis. Food Chem. 2022;397(19):133784. doi:10.1016/j.foodchem.2022.133784. [Google Scholar] [PubMed] [CrossRef]
88. Yadav MK, Song JH, Vasquez R, Lee JS, Kim IH, Kang DK. Methods for detection, extraction, purification, and characterization of exopolysaccharides of lactic acid bacteria—a systematic review. Foods. 2024;13(22):3687. doi:10.3390/foods13223687. [Google Scholar] [PubMed] [CrossRef]
89. Dar AN, Shahzad J, Ali JS, Sarwar U, Sajjad A, Zia M. Bioassay guided triterpene isolation and its biological evaluation using branches extract of a significant medicinal plant; Monotheca buxifolia. Pharmacol Res Nat Prod. 2024;3(1):100026. doi:10.1016/j.prenap.2024.100026. [Google Scholar] [CrossRef]
90. Dong SH, Duan ZK, Bai M, Huang XX, Song SJ. Advanced technologies targeting isolation and characterization of natural products. Trac Trends Anal Chem. 2024;175:117711. doi:10.1016/j.trac.2024.117711. [Google Scholar] [CrossRef]
91. Alemu M, Asfaw Z, Lulekal E, Warkineh B, Debella A, Sisay B, et al. Ethnobotanical study of traditional medicinal plants used by the local people in Habru District, North Wollo Zone, Ethiopia. J Ethnobiol Ethnomed. 2024;20(1):4. doi:10.1186/s13002-023-00644-x. [Google Scholar] [PubMed] [CrossRef]
92. Krakowska-Sieprawska A, Kiełbasa A, Rafińska K, Ligor M, Buszewski B. Modern methods of pre-treatment of plant material for the extraction of bioactive compounds. Molecules. 2022;27(3):730. doi:10.3390/molecules27030730. [Google Scholar] [PubMed] [CrossRef]
93. Shrivastav G, Jyoti TP, Chandel S, Singh R. Eco-friendly extraction: innovations, principles, and comparison with traditional methods. Sep Purif Rev. 2024;1–17. doi:10.1080/15422119.2024.2381605. [Google Scholar] [CrossRef]
94. Buvaneshwaran M, Radhakrishnan M, Natarajan V. Influence of ultrasound-assisted extraction techniques on the valorization of agro-based industrial organic waste—a review. J Food Process Eng. 2023;46(6):e14012. doi:10.1111/jfpe.14012. [Google Scholar] [CrossRef]
95. Han T, Cao X, Pei H, Fan C. Overview of solvent system selection strategies for countercurrent chromatography. Sep Purif Rev. 2023;52(4):305–25. doi:10.1080/15422119.2022.2096472. [Google Scholar] [CrossRef]
96. Cherniienko A, Lesyk R, Zaprutko L, Pawełczyk A. IR-EcoSpectra: exploring sustainable ex situ and in situ FTIR applications for green chemical and pharmaceutical analysis. J Pharm Anal. 2024;14(9):100951. doi:10.1016/j.jpha.2024.02.005. [Google Scholar] [PubMed] [CrossRef]
97. Phetkul U, Vongkul A, Chaichan K, Paosen S, Voravuthikunchai SP, Daus M, et al. Isolation and structural elucidation of coumarin and flavonoids from Citrus grandis Linn. (Tubtim Siam Pomelo) and their biological activities. Trends Sci. 2024;21(2):7129. doi:10.48048/tis.2024.7129. [Google Scholar] [CrossRef]
98. Krysa M, Szymańska-Chargot M, Zdunek A. FT-IR and FT-Raman fingerprints of flavonoids—a review. Food Chem. 2022;393(2):133430. doi:10.1016/j.foodchem.2022.133430. [Google Scholar] [PubMed] [CrossRef]
99. Khalil A, Kashif M. Nuclear magnetic resonance spectroscopy for quantitative analysis: a review for its application in the chemical, pharmaceutical and medicinal domains. Crit Rev Anal Chem. 2023;53(5):997–1011. doi:10.1080/10408347.2021.2000359. [Google Scholar] [PubMed] [CrossRef]
100. Zhao J, Wang M, Saroja SG, Khan IA. NMR technique and methodology in botanical health product analysis and quality control. J Pharm Biomed Anal. 2022;207(18):114376. doi:10.1016/j.jpba.2021.114376. [Google Scholar] [PubMed] [CrossRef]
101. Letertre MPM, Giraudeau P, de Tullio P. Nuclear magnetic resonance spectroscopy in clinical metabolomics and personalized medicine: current challenges and perspectives. Front Mol Biosci. 2021;8:698337. doi:10.3389/fmolb.2021.698337. [Google Scholar] [PubMed] [CrossRef]
102. Chen W, Yu HQ. Advances in the characterization and monitoring of natural organic matter using spectroscopic approaches. Water Res. 2021;190(21):116759. doi:10.1016/j.watres.2020.116759. [Google Scholar] [PubMed] [CrossRef]
103. Blanck JJ, Huebner TM, Rolls AM, Cornell JS, Hwang CS. Comprehensive review of the components in cat’s claw (Uncaria tomentosa) and their antibacterial activity. AppliedChem. 2022;2(1):1–29. doi:10.3390/appliedchem2010001. [Google Scholar] [CrossRef]
104. Zalidis AP, Kalogiouri NP, Mourtzinos I, Sarris D, Gkatzionis K. A novel liquid chromatographic time-of-flight tandem mass spectrometric method for the determination of secondary metabolites in functional flours produced from grape seed and olive stone waste. Molecules. 2025;30(7):1527. doi:10.3390/molecules30071527. [Google Scholar] [PubMed] [CrossRef]
105. Mattoli L, Gianni M, Burico M. Mass spectrometry-based metabolomic analysis as a tool for quality control of natural complex products. Mass Spectrom Rev. 2023;42(4):1358–96. doi:10.1002/mas.21773. [Google Scholar] [PubMed] [CrossRef]
106. Yergey AL, Edmonds CG, Lewis IAS, Vestal ML. Liquid chromatography/mass spectrometry: techniques and applications. Berlin/Heidelberg, Germany: Springer; 2013. 306 p. [Google Scholar]
107. Liu W, Zhou L, Hu J. Developing a new method for analyzing carbonate-associated phosphate through inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS). J Anal Atom Spectrom. 2025;40(4):967–74. doi:10.1039/d4ja00429a. [Google Scholar] [CrossRef]
108. Alseekh S, Aharoni A, Brotman Y, Contrepois K, D’Auria J, Ewald J, et al. Mass spectrometry-based metabolomics: a guide for annotation, quantification and best reporting practices. Nat Methods. 2021;18(7):747–56. doi:10.1038/s41592-021-01197-1. [Google Scholar] [PubMed] [CrossRef]
109. Li D, Gaquerel E. Next-generation mass spectrometry metabolomics revives the functional analysis of plant metabolic diversity. Annu Rev Plant Biol. 2021;72(1):867–91. doi:10.1146/annurev-arplant-071720-114836. [Google Scholar] [PubMed] [CrossRef]
110. Secilmis D, Begzati A, Grankvist N, Roci I, Watrous J, Majithia AR, et al. Isotope tracing-based metabolite identification for mass spectrometry metabolomics. bioRxiv. doi:10.1101/2025.04.07.647691. [Google Scholar] [PubMed] [CrossRef]
111. Ahmad F, Nadeem H. Mass spectroscopy as an analytical tool to harness the production of secondary plant metabolites: the way forward for drug discovery. In: Gene, drug, and tissue engineering. Berlin/Heidelberg, Germany: Springer; 2022. p. 77–103. doi:10.1007/978-1-0716-2716-7_5. [Google Scholar] [CrossRef]
112. Ahmed R. Harnessing tandem mass spectrometry for rational medication use in pharmaceutical sciences. Radinka J Health Sci. 2025;2(3):356–65. doi:10.56778/rjhs.v2i3.419. [Google Scholar] [CrossRef]
113. Goupy J, Creighton L. Mixture designs. In: Goupy J, Creighton L, editors. Introduction to design of experiments. Cary, NC, USA: Sas Institute Inc.; 2007. p. 287–306. [Google Scholar]
114. Li J, Ding Y, Lu Y, Liu J, Zhou C, Shao Z. The influence and compensation of environmental factors (pH, temperature, and conductivity) on the detection of chemical oxygen demand in water by UV-vis spectroscopy. Appl Sci. 2025;15(4):1694. doi:10.3390/app15041694. [Google Scholar] [CrossRef]
115. Mabasa XE, Mathomu LM, Madala NE, Musie EM, Sigidi MT. Molecular spectroscopic (FTIR and UV-vis) and hyphenated chromatographic (UHPLC-qTOF-MS) analysis and in vitro bioactivities of the Momordica balsamina leaf extract. Biochem Res Int. 2021;2021(1):2854217. doi:10.1155/2021/2854217. [Google Scholar] [PubMed] [CrossRef]
116. Li J, Lu Y, Ding Y, Zhou C, Liu J, Shao Z, et al. Prediction of water chemical oxygen demand with multi-scale one-dimensional convolutional neural network fusion and ultraviolet-visible spectroscopy. Biomimetics. 2025;10(3):191. doi:10.3390/biomimetics10030191. [Google Scholar] [PubMed] [CrossRef]
117. Cavalcante Minho LA, Lopes dos Santo WN. Heterogeneous ensemble learning applied to UV-VIS identification of multi-class pesticides by high-performance liquid chromatography with diode array detector (HPLC/DAD). Chemom Intell Lab Syst. 2025;261(2):105385. doi:10.1016/j.chemolab.2025.105385. [Google Scholar] [CrossRef]
118. Sarker SD, Nahar L. Computational phytochemistry. Amsterdam, The Netherlands: Elsevier; 2024. 450 p. [Google Scholar]
119. Rahman M. Application of computational methods for the isolation of plant secondary metabolites. In: Sarker SD, Nahar L, editors. Computational phytochemistry. Amsterdam, The Netherlands: Elsevier; 2024. p. 147–86. doi:10.1016/b978-0-443-16102-5.00001-8. [Google Scholar] [CrossRef]
120. Botto JM, Loffredo L, Menon GK, Champy P, Hadji-Minaglou F. Exhaustive analytical profiling of phytocompounds in botanical active ingredients: fighting the global prevalence of adulterated botanical ingredients for cosmetics. Cosmetics. 2025;12(2):63. doi:10.3390/cosmetics12020063. [Google Scholar] [CrossRef]
121. de Souza ALC, do Rego Pires A, Moraes CAF, de Matos CHC, dos Santos KIP, Campos e Silva R, et al. Chromatographic methods for separation and identification of bioactive compounds. In: Cruz JN, editor. Drug discovery and design using natural products. Berlin/Heidelberg, Germany: Springer; 2023. p. 153–76. doi:10.1007/978-3-031-35205-8_6. [Google Scholar] [CrossRef]
122. Ranjan S, Chaitali ROY, Sinha SK. Gas chromatography-mass spectrometry (GC-MSa comprehensive review of synergistic combinations and their applications in the past two decades. J Anal Sci Appl Biotechnol. 2023;5:2–5. [Google Scholar]
123. Sadgrove NJ, Padilla-González GF, Phumthum M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants. 2022;11(6):789. doi:10.3390/plants11060789. [Google Scholar] [PubMed] [CrossRef]
124. Lefebvre T, Destandau E, Lesellier E. Selective extraction of bioactive compounds from plants using recent extraction techniques: a review. J Chromatogr A. 2021;1635:461770. doi:10.1016/j.chroma.2020.461770. [Google Scholar] [PubMed] [CrossRef]
125. Acquavia M, Pascale R, Foti L, Carlucci G, Scrano L, Martelli G, et al. Analytical methods for extraction and identification of primary and secondary metabolites of apple (Malus domestica) fruits: a review. Separations. 2021;8(7):91. doi:10.3390/separations8070091. [Google Scholar] [CrossRef]
126. Li W, Zhang X, Wang S, Gao X, Zhang X. Research progress on extraction and detection technologies of flavonoid compounds in foods. Foods. 2024;13(4):628. doi:10.3390/foods13040628. [Google Scholar] [PubMed] [CrossRef]
127. Razzaq MA, Imran M, Khan MK, Azeem A, Raza MH, Afzal MA, et al. Essential oils as alternative treatments for common parasitic infections in animals. In: Zafar MA, Abbas RZ, Imran M, Tahir S, Qamar W, editors. Complementary and alternative medicine: essential oils. Faisalabad, Pakistan: Unique Scientific Publishers; 2024. p. 276–82. doi:10.47278/book.CAM/2024.475. [Google Scholar] [CrossRef]
128. Zheng R, Li S, Zhang X, Zhao C. Biological activities of some new secondary metabolites isolated from endophytic fungi: a review study. Int J Mol Sci. 2021;22(2):959. doi:10.3390/ijms22020959. [Google Scholar] [PubMed] [CrossRef]
129. Hulankova R. Methods for determination of antimicrobial activity of essential oils in vitro—a review. Plants. 2024;13(19):2784. doi:10.3390/plants13192784. [Google Scholar] [PubMed] [CrossRef]
130. Thawabteh AM, Swaileh Z, Ammar M, Jaghama W, Yousef M, Karaman R, et al. Antifungal and antibacterial activities of isolated marine compounds. Toxins. 2023;15(2):93. doi:10.3390/toxins15020093. [Google Scholar] [PubMed] [CrossRef]
131. Ye G, Sun X, Li J, Mai Y, Gao R, Zhang J. Secondary metabolites of mulberry leaves exert anti-lung cancer activity through regulating the PD-L1/PD-1 signaling pathway. J Pharm Anal. 2024;14(6):100926. doi:10.1016/j.jpha.2023.12.016. [Google Scholar] [PubMed] [CrossRef]
132. Kabir MT, Rahman MH, Shah M, Jamiruddin MR, Basak D, Al-Harrasi A, et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed Pharmacother. 2022;146(2021):112610. doi:10.1016/j.biopha.2021.112610. [Google Scholar] [PubMed] [CrossRef]
133. de Oliveira I, Albuquerque B, Ueda JM, Alves MJ, Ferreira ICFR, Barros L, et al. New challenges and opportunities from secondary metabolites. In: Carocho M, Heleno SA, Barros L, editors. Natural secondary metabolites. Berlin/Heidelberg, Germany: Springer; 2023. p. 925–65. doi:10.1007/978-3-031-18587-8_29. [Google Scholar] [CrossRef]
134. Ganzera M, Zwerger M. Analysis of natural products by SFC-applications from 2015 to 2021. Trac Trends Anal Chem. 2021;145:116463. doi:10.1016/j.trac.2021.116463. [Google Scholar] [CrossRef]
135. Paglia G, Smith AJ, Astarita G. Ion mobility mass spectrometry in the omics era: challenges and opportunities for metabolomics and lipidomics. Mass Spectrom Rev. 2022;41(5):722–65. doi:10.1002/mas.21686. [Google Scholar] [PubMed] [CrossRef]
136. Gao S, Zhou X, Yue M, Zhu S, Liu Q, Zhao XE. Advances and perspectives in chemical isotope labeling-based mass spectrometry methods for metabolome and exposome analysis. Trac Trends Anal Chem. 2023;162(6476):117022. doi:10.1016/j.trac.2023.117022. [Google Scholar] [CrossRef]
137. Ahmad MM, Ali Shahid Chatha S, Iqbal Y, Hussain AI, Khan I, Xie F. Recent trends in extraction, purification, and antioxidant activity evaluation of plant leaf-extract polysaccharides. Biofuels Bioprod Biorefin. 2022;16(6):1820–48. doi:10.1002/bbb.2405. [Google Scholar] [CrossRef]
138. Xin A, Fry SC. Cutin: xyloglucan transacylase (CXT) activity covalently links cutin to a plant cell-wall polysaccharide. J Plant Physiol. 2021;262:153446. doi:10.1016/j.jplph.2021.153446. [Google Scholar] [PubMed] [CrossRef]
139. López-Yerena A, Domínguez-López I, Vallverdú-Queralt A, Pérez M, Jáuregui O, Escribano-Ferrer E, et al. Metabolomics technologies for the identification and quantification of dietary phenolic compound metabolites: an overview. Antioxidants. 2021;10(6):846. doi:10.3390/antiox10060846. [Google Scholar] [PubMed] [CrossRef]
140. Selwal N, Rahayu F, Herwati A, Latifah E, Suhara C, Suastika IBK, et al. Enhancing secondary metabolite production in plants: exploring traditional and modern strategies. J Agric Food Res. 2023;14:100702. doi:10.1016/j.jafr.2023.100702. [Google Scholar] [CrossRef]
141. Atherton HR, Li P. Hydroponic cultivation of medicinal plants—plant organs and hydroponic systems: techniques and trends. Horticulturae. 2023;9(3):349. doi:10.3390/horticulturae9030349. [Google Scholar] [CrossRef]
142. Montaño-Pérez CA, Yáñez-Ibarra G. Phytochemical compounds of plants: extraction, purification, and identification methods. In: Improving health and nutrition through bioactive compounds. Amsterdam, The Netherlands: Elsevier; 2025. p. 323–35. doi:10.1016/b978-0-443-21873-6.00027-0. [Google Scholar] [CrossRef]
143. Sanyal D, Mathur P. Advanced adsorbent mediated extraction techniques for the separation of antibiotics from food, biological, and environmental matrices. Sep Purif Rev. 2022;51(3):373–407. doi:10.1080/15422119.2021.1954950. [Google Scholar] [CrossRef]
144. Park M, Somborn A, Schlehuber D, Keuter V, Deerberg G. Raman spectroscopy in crop quality assessment: focusing on sensing secondary metabolites: a review. Hortic Res. 2023;10(5):uhad074. doi:10.1093/hr/uhad074. [Google Scholar] [PubMed] [CrossRef]
145. Agarwal A, Jaiswal N, Tripathi AD, Paul V. Downstream processing; applications and recent updates. In: Bioprocessing for biofuel production. Berlin/Heidelberg, Germany: Springer; 2020. p. 29–55. doi:10.1007/978-981-15-7070-4_2. [Google Scholar] [CrossRef]
146. Ye D, Li X, Shen J, Xia X. Microbial metabolomics: from novel technologies to diversified applications. Trac Trends Anal Chem. 2022;148:116540. doi:10.1016/j.trac.2022.116540. [Google Scholar] [CrossRef]
147. Ning Y, Xu Y, Jiao B, Lu X. Application of gene knockout and heterologous expression strategy in fungal secondary metabolites biosynthesis. Mar Drugs. 2022;20(11):705. doi:10.3390/md20110705. [Google Scholar] [PubMed] [CrossRef]
148. Wang J, Wang K, Deng Z, Zhong Z, Sun G, Mei Q, et al. Engineered cytosine base editor enabling broad-scope and high-fidelity gene editing in Streptomyces. Nat Commun. 2024;15(1):5687. doi:10.1038/s41467-024-49987-3. [Google Scholar] [PubMed] [CrossRef]
149. Guo L, Xi B, Lu L. Strategies to enhance production of metabolites in microbial co-culture systems. Bioresour Technol. 2024;406(1):131049. doi:10.1016/j.biortech.2024.131049. [Google Scholar] [PubMed] [CrossRef]
150. Mohaddab M, El Goumi Y, Gallo M, Montesano D, Zengin G, Bouyahya A, et al. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules. 2022;27(22):8093. doi:10.3390/molecules27228093. [Google Scholar] [PubMed] [CrossRef]
151. Liu Y, Yuan H, Ding D, Dong H, Wang Q, Zhang D. Establishment of a biosensor-based high-throughput screening platform for tryptophan overproduction. ACS Synth Biol. 2021;10(6):1373–83. doi:10.1021/acssynbio.0c00647. [Google Scholar] [PubMed] [CrossRef]
152. Bao H, Zhao J, Zhao X, Zhao C, Lu X, Xu G. Prediction of plant secondary metabolic pathways using deep transfer learning. BMC Bioinformatics. 2023;24(1):348. doi:10.1186/s12859-023-05485-9. [Google Scholar] [PubMed] [CrossRef]
153. Annemer S, Ez zoubi Y, Ramzi A, El Hadrami EM, El Ouali Lalami A, Satrani B, et al. Variations in saffron quality in Morocco (Taliouine and Taznakht) according to altitude and provenance: chemometric investigation. J Food Process Preserv. 2022;46(2):e16292. doi:10.1111/jfpp.16292. [Google Scholar] [CrossRef]
154. Singh VK, Kumar A. Secondary metabolites from endophytic fungi: production, methods of analysis, and diverse pharmaceutical potential. Symbiosis. 2023;90(2):111–25. doi:10.1007/s13199-023-00925-9. [Google Scholar] [PubMed] [CrossRef]
155. Rawat P, Singh Y, Bisht M, Pal M. Modern analytical techniques for extraction, purification, and structural characterization of microbial bioactive compounds. In: Microbial bioactive compounds. Berlin/Heidelberg, Germany: Springer; 2023. p. 85–102. doi:10.1007/978-3-031-40082-7_5. [Google Scholar] [CrossRef]
156. Pereda-Miranda R, Bautista E, Martínez-Fructuoso L, Fragoso-Serrano M. From relative to absolute stereochemistry of secondary metabolites: applications in plant chemistry. Rev Bras De Farmacogn. 2023;33(1):1–48. doi:10.1007/s43450-022-00333-y. [Google Scholar] [CrossRef]
157. Ivănescu B, Burlec AF, Crivoi F, Roşu C, Corciovă A. Secondary metabolites from Artemisia genus as biopesticides and innovative nano-based application strategies. Molecules. 2021;26(10):3061. doi:10.3390/molecules26103061. [Google Scholar] [PubMed] [CrossRef]
158. Cai Y, Zhou Z, Zhu ZJ. Advanced analytical and informatic strategies for metabolite annotation in untargeted metabolomics. Trac Trends Anal Chem. 2023;158:116903. doi:10.1016/j.trac.2022.116903. [Google Scholar] [CrossRef]
159. Ibrahim AE, El Gohary NA, Aboushady D, Samir L, Karim SEA, Herz M, et al. Recent advances in chiral selectors immobilization and chiral mobile phase additives in liquid chromatographic enantio-separations: a review. J Chromatogr A. 2023;1706(2):464214. doi:10.1016/j.chroma.2023.464214. [Google Scholar] [PubMed] [CrossRef]
160. Batista ANL, Valverde AL, Nafie LA, Batista JMJr. Stereochemistry of natural products from vibrational circular dichroism. Chem Commun. 2024;60(76):10439–50. doi:10.1039/d4cc02481h. [Google Scholar] [PubMed] [CrossRef]
161. Shanbhag AP. Stairway to stereoisomers: engineering short- and medium-chain ketoreductases to produce chiral alcohols. ChemBioChem. 2023;24(6):e202200687. doi:10.1002/cbic.202200687. [Google Scholar] [PubMed] [CrossRef]
162. Aswathi VP, Meera S, Ann Maria CG, Nidhin M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: a critical review. Nanotechnol Environ Eng. 2023;8(2):377–97. doi:10.1007/s41204-022-00276-8. [Google Scholar] [CrossRef]
163. Kang MJ, Suh JH. Metabolomics as a tool to evaluate nut quality and safety. Trends Food Sci Technol. 2022;129:528–43. doi:10.1016/j.tifs.2022.11.002. [Google Scholar] [CrossRef]
164. Kiki C, Yan X, Elimian EA, Jiang B, Sun Q. Deciphering the role of microbial extracellular and intracellular organic matter in antibiotic photodissipation: molecular and fluorescent profiling under natural radiation. Environ Sci Technol. 2024;58(26):11661–74. doi:10.1021/acs.est.4c01141. [Google Scholar] [PubMed] [CrossRef]
165. Najmi A, Javed SA, Al Bratty M, Alhazmi HA. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules. 2022;27(2):349. doi:10.3390/molecules27020349. [Google Scholar] [PubMed] [CrossRef]
166. Chen L, Lu W, Wang L, Xing X, Chen Z, Teng X, et al. Metabolite discovery through global annotation of untargeted metabolomics data. Nat Methods. 2021;18(11):1377–85. doi:10.1038/s41592-021-01303-3. [Google Scholar] [PubMed] [CrossRef]
167. Annemer S, Farah A, Stambouli H, Assouguem A, Almutairi MH, Sayed AA, et al. Chemometric investigation and antimicrobial activity of Salvia rosmarinus spenn essential oils. Molecules. 2022;27(9):2914. doi:10.3390/molecules27092914. [Google Scholar] [PubMed] [CrossRef]
168. Laftouhi A, Eloutassi N, Ech-Chihbi E, Kara M, Assouguem A, Ali EA, et al. Impact of climatic disturbances on the chemical compositions and metabolites of Salvia officinalis. Open Chem. 2023;21(1):20230115. doi:10.1515/chem-2023-0115. [Google Scholar] [CrossRef]
169. Laftouhi A, Eloutassi N, Ech-Chihbi E, Rais Z, Taleb A, Assouguem A, et al. Impact of climate change on the chemical compositions and antioxidant activity of Mentha pulegium L. ACS Omega. 2023;8(49):46598–607. doi:10.1021/acsomega.3c05564. [Google Scholar] [PubMed] [CrossRef]
170. Pant P, Pandey S, Dall’Acqua S. The influence of environmental conditions on secondary metabolites in medicinal plants: a literature review. Chem Biodivers. 2021;18(11):e2100345. doi:10.1002/cbdv.202100345. [Google Scholar] [PubMed] [CrossRef]
171. Xu W, Cheng Y, Guo Y, Yao W, Qian H. Effects of geographical location and environmental factors on metabolite content and immune activity of Echinacea purpurea in China based on metabolomics analysis. Ind Crops Prod. 2022;189:115782. doi:10.1016/j.indcrop.2022.115782. [Google Scholar] [CrossRef]
172. Jan R, Asaf S, Numan M, Lubna, Kim K-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11(5):968. doi:10.3390/agronomy11050968. [Google Scholar] [CrossRef]
173. Ciocan AG, Tecuceanu V, Enache-Preoteasa C, Mitoi EM, Helepciuc FE, Dimov TV, et al. Phenological and environmental factors' impact on secondary metabolites in medicinal plant Cotinus coggygria scop. Plants. 2023;12(9):1762. doi:10.3390/plants12091762. [Google Scholar] [PubMed] [CrossRef]
174. Kumar V, Ahluwalia V, Saran S, Kumar J, Patel AK, Singhania RR. Recent developments on solid-state fermentation for production of microbial secondary metabolites: challenges and solutions. Bioresour Technol. 2021;323(4):124566. doi:10.1016/j.biortech.2020.124566. [Google Scholar] [PubMed] [CrossRef]
175. Hamilton AN, Gibson KE, Amalaradjou MA, Callahan CW, Millner PD, Ilic S, et al. Cultivating food safety together: insights about the future of produce safety in the U.S. controlled environment agriculture sector. J Food Prot. 2023;86(12):100190. doi:10.1016/j.jfp.2023.100190. [Google Scholar] [PubMed] [CrossRef]
176. Ahmed N, Zhang B, Deng L, Bozdar B, Li J, Chachar S, et al. Advancing horizons in vegetable cultivation: a journey from ageold practices to high-tech greenhouse cultivation—a review. Front Plant Sci. 2024;15:1357153. doi:10.3389/fpls.2024.1357153. [Google Scholar] [PubMed] [CrossRef]
177. Lv J, Yang S, Zhou W, Liu Z, Tan J, Wei M. Microbial regulation of plant secondary metabolites: impact, mechanisms and prospects. Microbiol Res. 2024;283:127688. doi:10.1016/j.micres.2024.127688. [Google Scholar] [PubMed] [CrossRef]
178. Aggarwal N, Pham HL, Ranjan B, Saini M, Liang Y, Hossain GS, et al. Microbial engineering strategies to utilize waste feedstock for sustainable bioproduction. Nat Rev Bioeng. 2024;2(2):155–74. doi:10.1038/s44222-023-00129-2. [Google Scholar] [CrossRef]
179. Fadil M, Farah A, Ihssane B, Haloui T, Lebrazi S, Zghari B, et al. Chemometric investigation of light-shade effects on essential oil yield and morphology of Moroccan Myrtus communis L. SpringerPlus. 2016;5(1):1062. doi:10.1186/s40064-016-2749-5. [Google Scholar] [PubMed] [CrossRef]
180. Zameer R, Tariq S, Noreen S, Sadaqat M, Azeem F. Role of transcriptomics and artificial intelligence approaches for the selection of bioactive compounds. Drug Des Using Mach Learn. 2022;63:283–317. doi:10.1002/9781394167258.ch10. [Google Scholar] [CrossRef]
181. Sriprateep K, Sala-Ngam S, Srithep Y, Khonjun S, Golinska-Dawson P, Srichok T, et al. Plant production yield optimization and cost-effectiveness using an innovative artificial multiple intelligence system. Ann Oper Res. 2024;1–36. doi:10.1007/s10479-024-05835-7. [Google Scholar] [CrossRef]
182. Edo GI, Samuel PO, Jikah AN, Yousif E, Onyibe PN, Opiti AR, et al. Sensitivity ecology and evolution, toxicology organism assessment model in the use of chemical applications for the management of toxic substances. Ecol Front. 2024;44(5):890–908. doi:10.1016/j.ecofro.2024.06.001. [Google Scholar] [CrossRef]
183. Alaoui I, Zahri A, El Kamari F, El Ghadraoui O, Nekhla H, Goubi A, et al. Olive mill pomace impact on the phytochemical content and antioxidant activity of Rosmarinus officinalis L. Trop J Nat Prod Res. 2023;7:4186–92. [Google Scholar]
184. Yadav B, Jogawat A, Rahman MS, Narayan OP. Secondary metabolites in the drought stress tolerance of crop plants: a review. Gene Rep. 2021;23(3):101040. doi:10.1016/j.genrep.2021.101040. [Google Scholar] [CrossRef]
185. Rusu AV, Trif M, Rocha JM. Microbial secondary metabolites via fermentation approaches for dietary supplementation formulations. Molecules. 2023;28(16):6020. doi:10.3390/molecules28166020. [Google Scholar] [PubMed] [CrossRef]
186. Ozyigit II, Dogan I, Hocaoglu-Ozyigit A, Yalcin B, Erdogan A, Yalcin IE, et al. Production of secondary metabolites using tissue culture-based biotechnological applications. Front Plant Sci. 2023;14:1132555. doi:10.3389/fpls.2023.1132555. [Google Scholar] [PubMed] [CrossRef]
187. Hu B, Peng N, Zhu G. Sustainable finance in natural resource extraction: navigating green recovery pathways. Resour Policy. 2024;96(1):105197. doi:10.1016/j.resourpol.2024.105197. [Google Scholar] [CrossRef]
188. Singh KS, van der Hooft JJJ, van Wees SCM, Medema MH. Integrative omics approaches for biosynthetic pathway discovery in plants. Nat Prod Rep. 2022;39(9):1876–96. doi:10.1039/d2np00032f. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF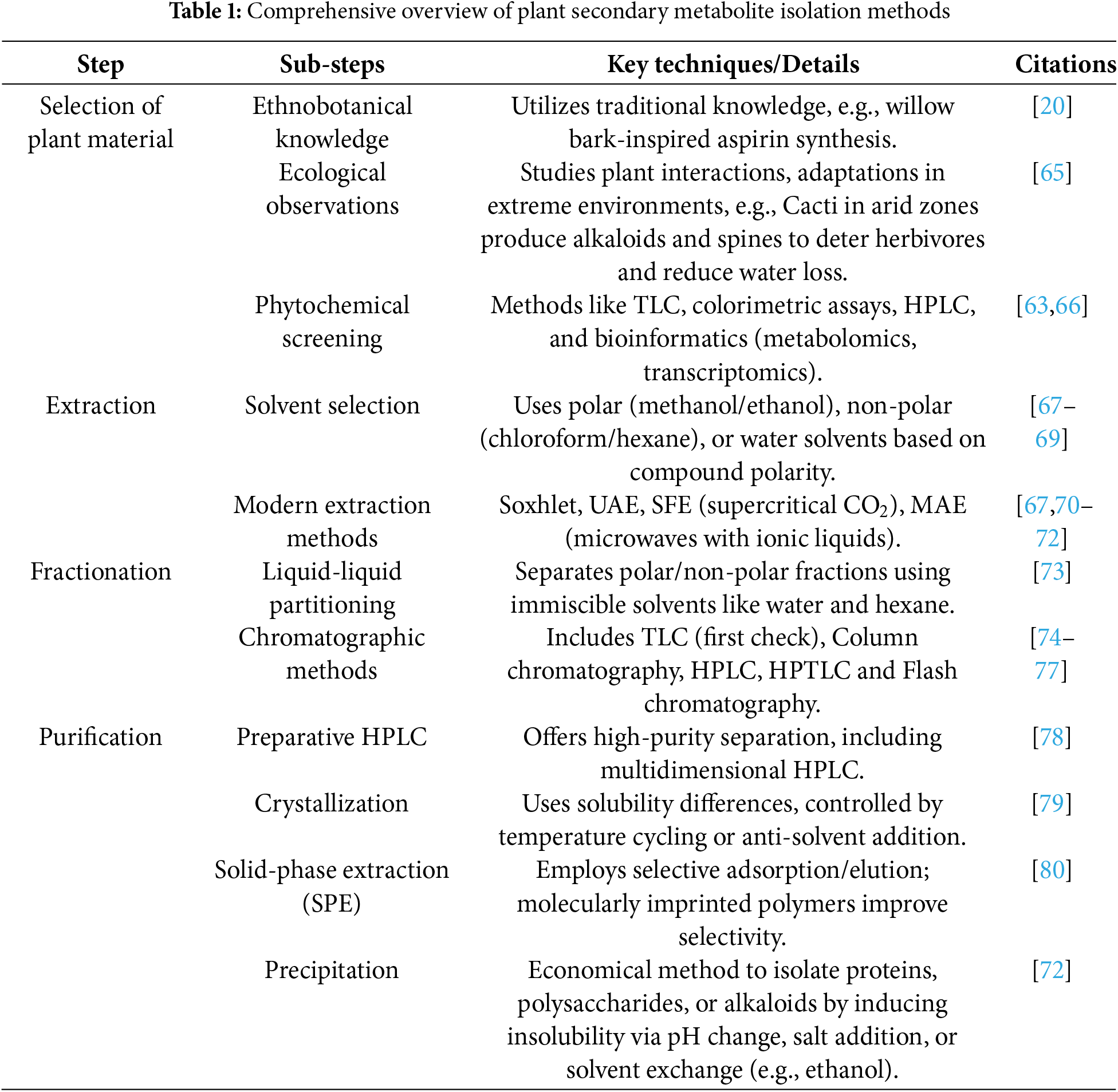
 Downloads
Downloads
 Citation Tools
Citation Tools