 Open Access
Open Access
ARTICLE
The Addition of Calcium and Strontium Improves Salt Tolerance of Chinese Cabbage at the Germination Stage
1 College of Life Sciences, Qufu Normal University, Qufu, 273165, China
2 Integrated Department, Jining Confucius High School, Jining, 272106, China
3 College of Agriculture and Forestry Science, Linyi University, Linyi, 276000, China
* Corresponding Authors: Nianwei Qiu. Email: ; Hongxia Zhang. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Plant Responses to Stress Factors)
Phyton-International Journal of Experimental Botany 2025, 94(6), 1811-1826. https://doi.org/10.32604/phyton.2025.065751
Received 21 March 2025; Accepted 15 May 2025; Issue published 27 June 2025
Abstract
Strontium has similar chemical properties to calcium and has recently been recognized as a non-essential beneficial element for plants. In order to compare the effects of strontium and calcium on improving salt tolerance of Chinese cabbage during the germination stage, 2, 4, and 8 mmol/L of SrCl2, CaCl2 or an equimolar mixture of both were added separately to a 150 mmol/L NaCl solution. The results showed that Ca-Sr addition significantly increased seed viability, seed vigor, seed germination rate and seed germination uniformity of Chinese cabbage compared with the salt-control group. The differences in germination percentage (GP) and germination energy (GE) among the Ca-addition and Sr-addition groups were not significant, and the differences in coefficient of rate of germination (CRG), index of rate of germination (IRG) and coefficient of variation of the germination time (CVT) were relatively small, but clear differences were observed in germination index (GI), vigor index (VI) and coefficient of uniformity of germination (CUG). The results of GI and VI indicated that the higher the concentration of Ca-addition or Sr-addition, the more significant the enhancement of seed vigor. Under saline stress (150 mmol/L NaCl), the Ca-Sr co-addition outperformed Sr-treatment alone, and Ca-addition achieved the highest seed vigor at equivalent concentrations. Furthermore, all Ca-Sr treatments significantly enhanced the uniformity of Chinese cabbage sprouts exposed to 150 mM NaCl, with the best uniformity improved by the addition of 2 and 4 mmol/L SrCl2. Ca-Sr treatments increased the salt tolerance of Chinese cabbage sprouts during the germination stage mainly because the Ca2+ and Sr2+ significantly enhanced plasma membrane stability and reduced oxidative stress (as indicated by decreased contents of malondialdehyde and O2·− contents) in sprouts. The decrease of soluble sugar and proline content caused by Ca-Sr addition implies that elevated levels of these osmolytes were not the primary contributors to improved seed germinability in Chinese cabbage. These findings demonstrate that Sr is a beneficial element for enhancing salt tolerance in plants, laying a theoretical foundation for the development and application of strontium in agriculture.Keywords
More than 800 million hectares of land are affected by salinity and alkalinity worldwide [1]. Cultivating salt-tolerant crops is an important strategy for the utilization of saline-alkali wasteland. Although substantial progress has been made in breeding salt-tolerant grain crops, their economic returns remain low in high salinity soils. This is primarily due to the high demand for freshwater and input costs [2,3]. Salt-tolerant vegetables with high economic yield and profitability offer a more viable approach to developing saline-alkali land. Many salt-tolerant vegetables have been successfully cultivated and adopted. For example, tomatoes not only grow well under saline conditions, but may also exhibit enhanced quality attributes [4]. Seed germination is the initial stage of agricultural production, yet it is also the most salt-sensitive phase in the crop life cycle [5]. Enhancing salt tolerance during seed germination is therefore critical for successful vegetable cultivation in saline-alkali soils.
Our previous results showed that the seeds of the salt-tolerant Chinese cabbage variety, Juhong 65, had a low germination percentage and could not survive under 300–400 mmol/L NaCl, whereas its seedlings could survive for up to two months under the same salinity level [6]. Halophyte seeds are also sensitive to salt stress during germination and typically initiate germination only after soil salinity is reduced by rainfall [7]. Therefore, vegetable cultivation in saline-alkali soil commonly employs seedling transplantation or direct sowing following freshwater irrigation. To further improve seed germination and survival rate under saline conditions, various physiological interventions have been explored. Soil inoculation with Streptomyces avidinii, Bacillus proteolyticus and Bacillus safensis has been shown to significantly improve the germination rate of mung beans, peppers, cucumbers and common bean under saline conditions [8,9]. Microbial seed coatings using beneficial bacteria also exhibit a comparable promoting effect [10]. The application of exogenous silicon has also been found to effectively improve the germination ability of seeds under saline conditions [11]. A growing number of studies have focused on the use of plant growth regulators, such as gibberellin, brassinolide, ethylene, kinetin, melatonin, and salicylic acid to improve seed germinability [12–16]. In recent years, modern nanomaterials (such as carbon nanoparticles and nano-selenium) and physical methods (such as constant-frequency and variable-frequency ultrasonic treatments) have also been employed to improve seed germinability under saline conditions [17–19]. Since high salinity disrupts ionic balance and impairs nutrient uptake, supplementation with organic or mineral nutrients in the saline medium can also enhance seed germinability [20,21]. For example, the exogenous application of amino acids, including Gly, Cys, Ser and Met, can significantly alleviate the inhibition of salt stress on seed germination of Arabidopsis thaliana [22,23]. Soaking seeds with glucose and sucrose under salt stress significantly enhances antioxidant capacity, thereby improving seed germination and early seedling growth [23]. The exogenous addition of phosphorus, potassium, calcium, magnesium and other mineral nutrients is particularly practical in saline-alkali agriculture, and demonstrates a pronounced effect on promoting seed germination and seedling growth [24–27]. Among mineral nutrients, calcium is most commonly reported to enhance seed germinability under salt stress [27–35]. Ca-addition can also improve plant salt tolerance throughout all developmental stages [36,37]. Strontium and calcium belong to the same group of elements in the periodic table and exhibit similar chemical properties. Both strontium and calcium are essential nutrients for humans and animals. Recently, strontium has been identified as a new non-essential beneficial element for plants [38,39].
To explore the beneficial role of strontium in plant salt tolerance, this study compared the effects of Ca and Sr supplementation on seed germinability of Chinese cabbage (a widely cultivated vegetable in East Asia) under saline conditions, which would lay a solid foundation for further agricultural applications of strontium.
2.1 Plant Material and Experimental Design
The seeds of Chinese cabbage (Brassica rapa L. ssp. Pekinensis. Cultivar name: Qingmaye) were purchased from Tianjin Kerun Seed Co., Ltd. (Tianjin, China). The seed germination experiment included a blank-control group (B-CK), a salt-control group (S-CK) and a calcium-strontium addition group (Ca-Sr addition). The B-CK group received half-strength Hoagland nutrient solution without Ca(NO3)2 (hereinafter referred to as “nutrient solution”). The S-CK group was treated with 150 mmol/L NaCl dissolved in the nutrient solution (hereinafter referred to as “salt solution”). In the Ca-Sr addition groups, CaCl2 or SrCl2 was added to the salt solution to the final concentration of 2, 4 or 8 mmol/L (labeled as “2Ca”, “4Ca”, “8Ca” and “2Sr”, “4Sr”, “8Sr”, respectively). In Ca-Sr mixed addition group, equimolar concentrations of CaCl2 and SrCl2 were added to the salt solution to the total concentration of 2, 4 or 8 mmol/L (labeled as “1Ca + 1Sr”, “2Ca + 2Sr”, “4Ca + 4Sr”, respectively). Five glass Petri dishes (15 cm in diameter, 2 cm in height) were prepared for each treatment group with 2 layers of filter paper on the bottom. The corresponding treatment solution (30 mL) was poured into each Petri dish to moisten the filter paper, and the solution depth was about 1 mm. One hundred uniform, viable seeds were evenly distributed in each Petri dish, which was then covered to minimize evaporation. The Petri dishes were incubated in an artificial climate chamber at 22°C with a light intensity of 50 μmol/m2/s and a 16 h/8 h light/dark photoperiod. The solution in Petri dishes was renewed daily to maintain constant solute concentrations. The number of newly germinated seeds in each Petri dish was recorded every 24 h. Germination was defined as radicle protrusion of at least 2 mm through the seed coat. The germination experiment was ended on the 6th day when no additional germination was observed. Germination indices of Chinese cabbage seeds were calculated based on the recorded data. After the germination experiment, all sprouts from each Petri dish were collected to measure total fresh weight and calculate the average fresh weight of each sprout (g FW/plant). The sprouts were washed twice with distilled water and blotted dry with absorbent paper. The prepared sprouts were used directly or frozen with liquid nitrogen for subsequent physiological analyses.
2.2 Calculation of Seed Germination Indices
Germination indices were calculated using the following formulas [40].
Average germination days (
The symbols in the formulas are described as follows: N2: Total number of germinated seeds on the 2nd day; N6: Total number of germinated seeds on the 6th day; NT: Total number of seeds in each Petri dish (100 seeds); ni: The number of newly germinated seeds on the ith day; di: Corresponding germination days (ith day); k: Total germination days (6 days); FW: The average fresh weight per sprout (g); Ni: Total number of germinated seeds on the ith day.
2.3 Determination of Plasma Membrane Permeability
A total of 0.2 g fresh sprouts, washed with distilled water, were placed in a test tube (containing 20 mL distilled water). The tubes were vacuum-infiltrated for 15 min and subsequently kept at room temperature (25°C) for 30 min. The original conductivity (OC) of the supernatant was measured with a conductivity meter. The test tube was subsequently immersed in boiling water for 15 min. The total conductivity (TC) was measured after the tube was cooled to 25°C. Plasma membrane permeability was calculated as OC/TC × 100%.
2.4 Determination of Malondialdehyde and Superoxide Anion (O2·−)
Lipid peroxidation was quantified by measuring the malondialdehyde (MDA) content. MDA was extracted from 1 g frozen sprouts of Chinese cabbage by grinding with 10 mL 10% trichloroacetic acid. The MDA content was determined following the method of Hodges et al. [41]. Superoxide anion radical (O2·−) was extracted from 0.5 g fresh sprouts by grinding with 5 mL 50 mM phosphate buffer (K2HPO4-KH2PO4, pH7.8). The O2·− concentration was determined by monitoring the formation of nitrite, the product of hydroxylamine reaction with O2·− [42].
2.5 Determination of Soluble Sugar and Proline
Anthrone reagent was added to the MDA extract to develop color, and the absorbance was determined at 620 nm. The soluble sugar concentration was calculated using a standard curve of glucose.
Proline was extracted from 0.5 g frozen sprouts by grinding with 5 mL 3% sulfosalicylic acid. Proline concentration was determined using acid-ninhydrin binding methods [43].
A randomized block design was used in this research. All values were expressed as mean ± standard deviation (SD) based on five replicates. One-way ANOVA and Duncan’s LSR test were used to assess the differences among treatments. Means were considered significantly different at p < 0.05, as indicated by different lowercase letters.
3.1 Ca-Sr Addition Improves the Seed Viability of Chinese Cabbage under Saline Conditions
Seed viability refers to the potential of seeds to germinate, or the vitality of embryos, which is commonly expressed as germination percentage (GP). In agriculture, a GP value above 85% is typically required for qualified vegetable seeds. Under non-saline condition (B-CK), the GP of Chinese cabbage seeds reached 98.2%, indicating high-quality seed vigor (Fig. 1). The GP values of Chinese cabbage seeds under 50 and 100 mmol/L NaCl treatments showed no significant difference compared with that of the B-CK group. Under the condition of 150 mmol/L NaCl, the germination rate decreased to 56.8%, which falls below agricultural production standards. Under the condition of 200 mmol/L NaCl, the GP value further dropped to 18.6%, rendering the seeds agriculturally unviable. Therefore, 150 mmol/L NaCl was selected as the salt stress level for the salt-control group (S-CK) and subsequent treatments of Ca-Sr addition.
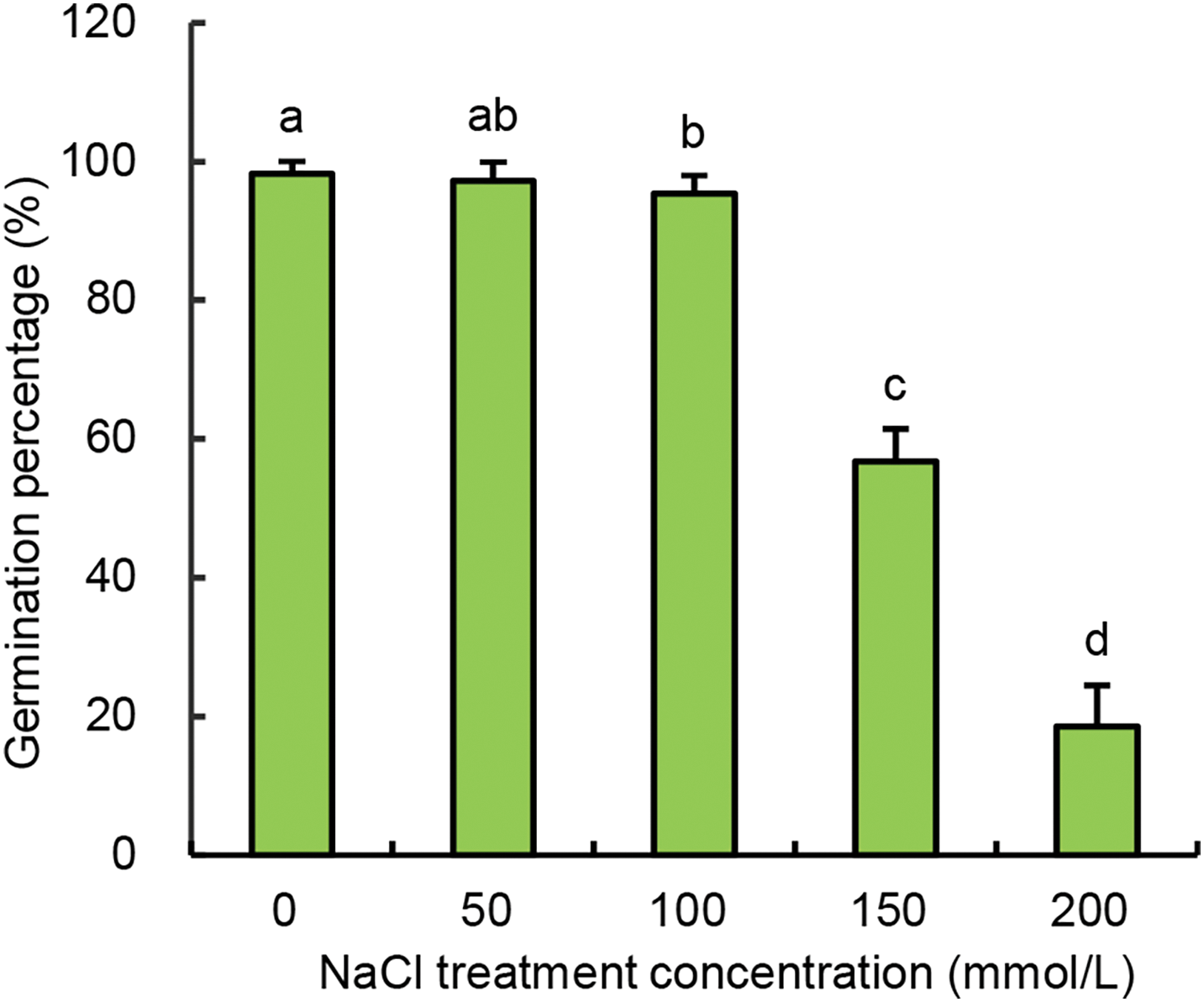
Figure 1: Effects of different NaCl concentrations on the germination percentage of Chinese cabbage seeds. Means were separated at a significant level (p < 0.05) by different lowercase letters
Under the condition of 150 mmol/L NaCl, the GP values of Chinese cabbage seeds increased to over 90% following Ca-addition, Sr-addition or the Ca-Sr mixed addition (Fig. 2A). Therefore, Sr-addition exhibited a comparable promoting effect on seed germination to Ca-addition under saline conditions. The germination energy (GE) exhibited a similar trend (Fig. 2B). The difference between GP and GE in the Ca-Sr addition groups was only 1.6%–3.4%, indicating rapid and synchronized germination, with most seeds germinating within 2 days. In contrast, the difference between GP and GE of the S-CK group was 9.8%, indicating slower and more dispersed germination. However, GP and GE were not sensitive enough to distinguish the differences among different Ca-Sr treatments in their promotive effects on germination.
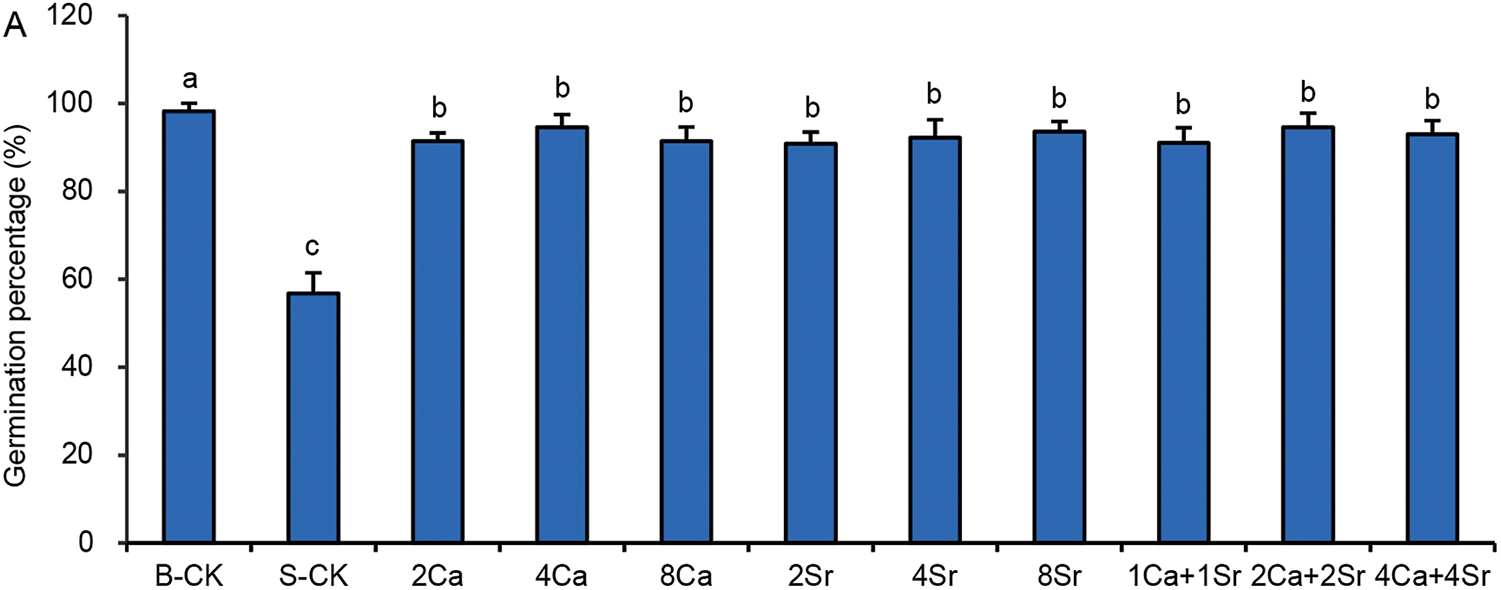
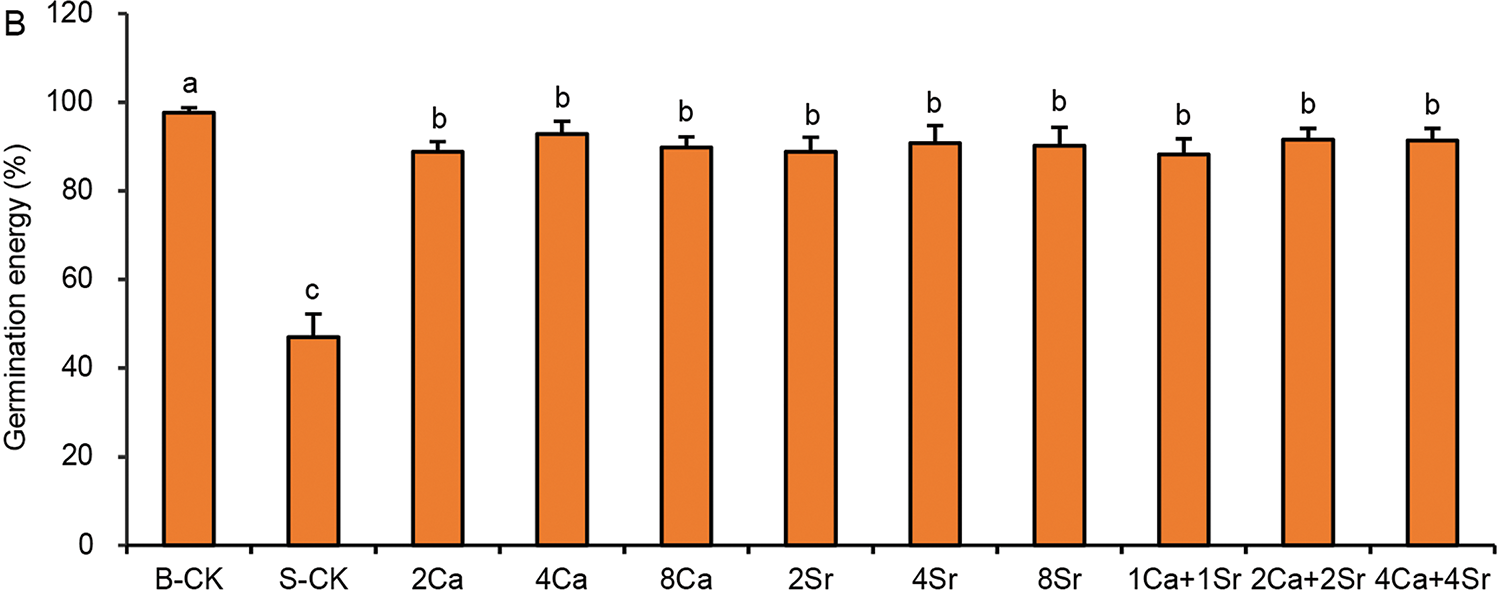
Figure 2: Effects of calcium-strontium addition on germination percentage (A) and germination energy (B) of Chinese cabbage seeds under 150 mmol/L NaCl. The B-CK group received half-strength Hoagland nutrient solution without Ca(NO3)2. The S-CK group was treated with 150 mmol/L NaCl dissolved in the nutrient solution. In the Ca-Sr addition groups, CaCl2 or SrCl2 was added to the salt solution to the final concentration of 2, 4 or 8 mmol/L, labeled as “2Ca”, “4Ca”, “8Ca” and “2Sr”, “4Sr”, “8Sr”, respectively. In Ca-Sr mixed addition group, equimolar concentrations of CaCl2 and SrCl2 were added to the salt solution to the total concentration of 2, 4 or 8 mmol/L, labeled as “1Ca + 1Sr”, “2Ca + 2Sr”, “4Ca + 4Sr”, respectively. Means were separated at a significant level (p < 0.05) by different lowercase letters
3.2 Ca-Sr Addition Enhances Seed Vigor of Chinese Cabbage under Saline Condition
Seed vigor is defined as the capacity of seeds to germinate rapidly and uniformly, and to produce robust seedlings, reflecting their growth potential and adaptability under various environmental conditions. Germination index (GI) is one of the most commonly used indicators of seed vigor. As shown in Fig. 3A, the GI of Chinese cabbage seeds was 74.5 seeds/d under the B-CK condition, indicating strong seed vigor. Under the 150 mmol/L NaCl condition, the GI rapidly decreased to 24.6 seeds/d, approximately one-third of that of the B-CK group. All Ca-Sr treatments resulted in a GI more than doubled that of the S-CK group. The promoting effects of 2Sr and 4Sr addition on GI were slightly lower than those of the other Ca-Sr addition treatments, yielding GI values of 50.1 and 51.33 seeds/d, respectively.
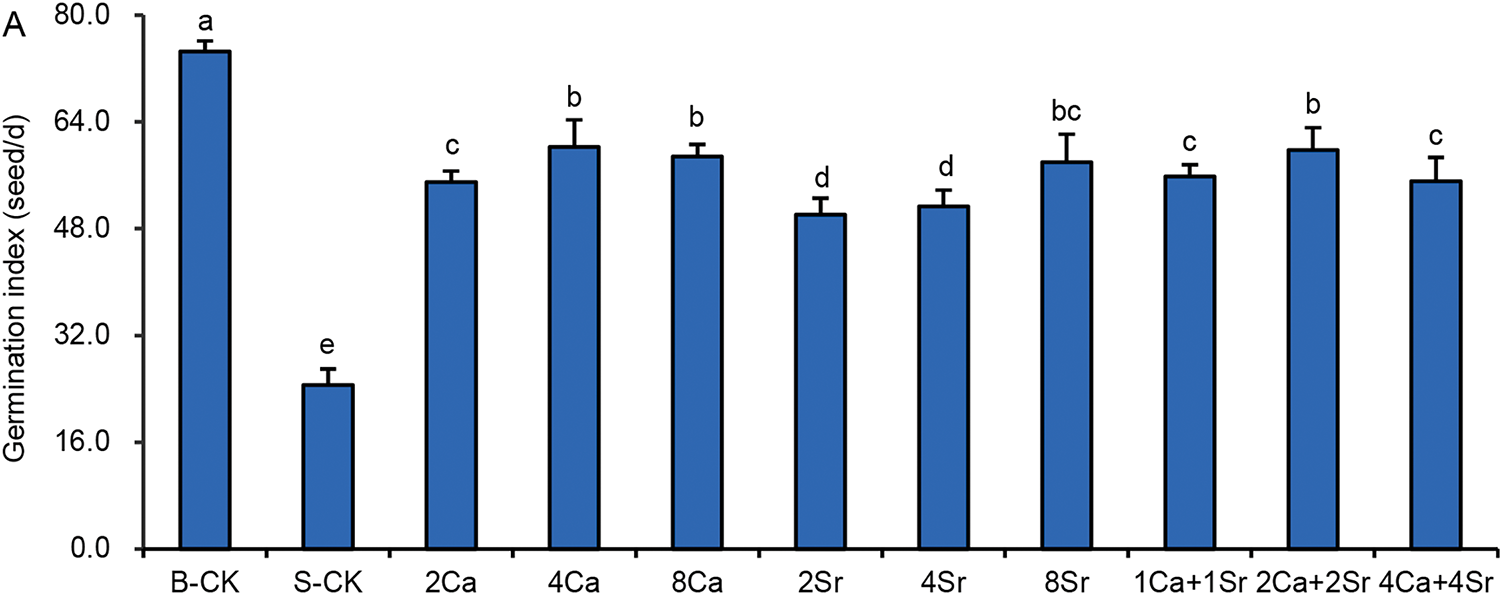
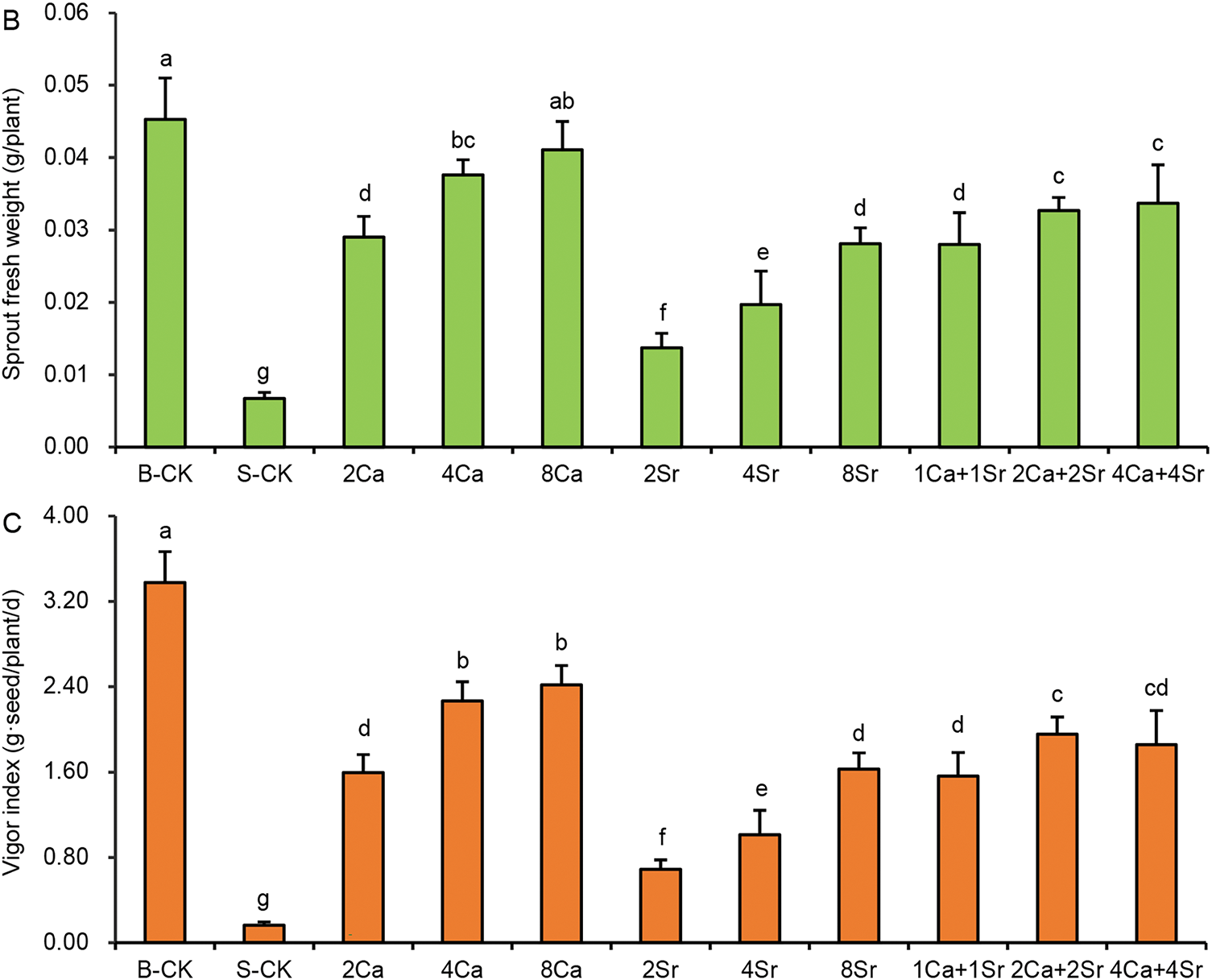
Figure 3: Effects of calcium-strontium addition on germination index (A), sprout fresh weight (B) and vigor index (C) of Chinese cabbage seeds under 150 mmol/L NaCl. The B-CK group received half-strength Hoagland nutrient solution without Ca(NO3)2. The S-CK group was treated with 150 mmol/L NaCl dissolved in the nutrient solution. In the Ca-Sr addition groups, CaCl2 or SrCl2 was added to the salt solution to the final concentration of 2, 4 or 8 mmol/L, labeled as “2Ca”, “4Ca”, “8Ca” and “2Sr”, “4Sr”, “8Sr”, respectively. In Ca-Sr mixed addition group, equimolar concentrations of CaCl2 and SrCl2 were added to the salt solution to the total concentration of 2, 4 or 8 mmol/L, labeled as “1Ca + 1Sr”, “2Ca + 2Sr”, “4Ca + 4Sr”, respectively. Means were separated at a significant level (p < 0.05) by different lowercase letters
Another key index of seed vigor is the vigor index (VI), calculated by multiplying GI by the fresh weight per sprout. Fresh weight measurement on the 6th day of germination showed increasing Ca concentration resulted in larger Chinese cabbage sprouts (Fig. 3B). The fresh weight of individual sprout treated with 2, 4 and 8 mmol/L CaCl2 was 4.33, 5.61 and 6.13 times as much as that of the S-CK group, respectively. Ca-addition significantly improved the growth of Chinese cabbage sprouts under saline condition. Sr-addition exhibited a similar effect on the sprouts, although its growth promoting effects were consistently lower than those of CaCl2 at equivalent concentrations. The fresh weight of sprouts treated with 2, 4 and 8 mmol/L SrCl2 was 2.04, 2.94 and 4.19 times as much as that of the S-CK group, respectively. The growth promoting effect of Ca-Sr mixed addition fell in between the effects of Ca and Sr treatment alone at the same concentrations. Among all Ca-Sr treatments, 4–8 mmol/L CaCl2 had the best growth promoting effect under 150 mmol/L NaCl. Consistent with GI trends, VI is more clearly differentiated among Ca-Sr treatments (Fig. 3C), primarily due to the increase in sprout fresh weight. GI and VI were more sensitive indicators for evaluating seed germinability of Chinese cabbage than GP and GE under saline conditions.
3.3 Ca-Sr Addition Increases Seed Germination Rate of Chinese Cabbage under Saline Condition
Seed germinability can also be assessed by the germination rate. The coefficient of rate of germination (CRG), the reciprocal of the average germination time (
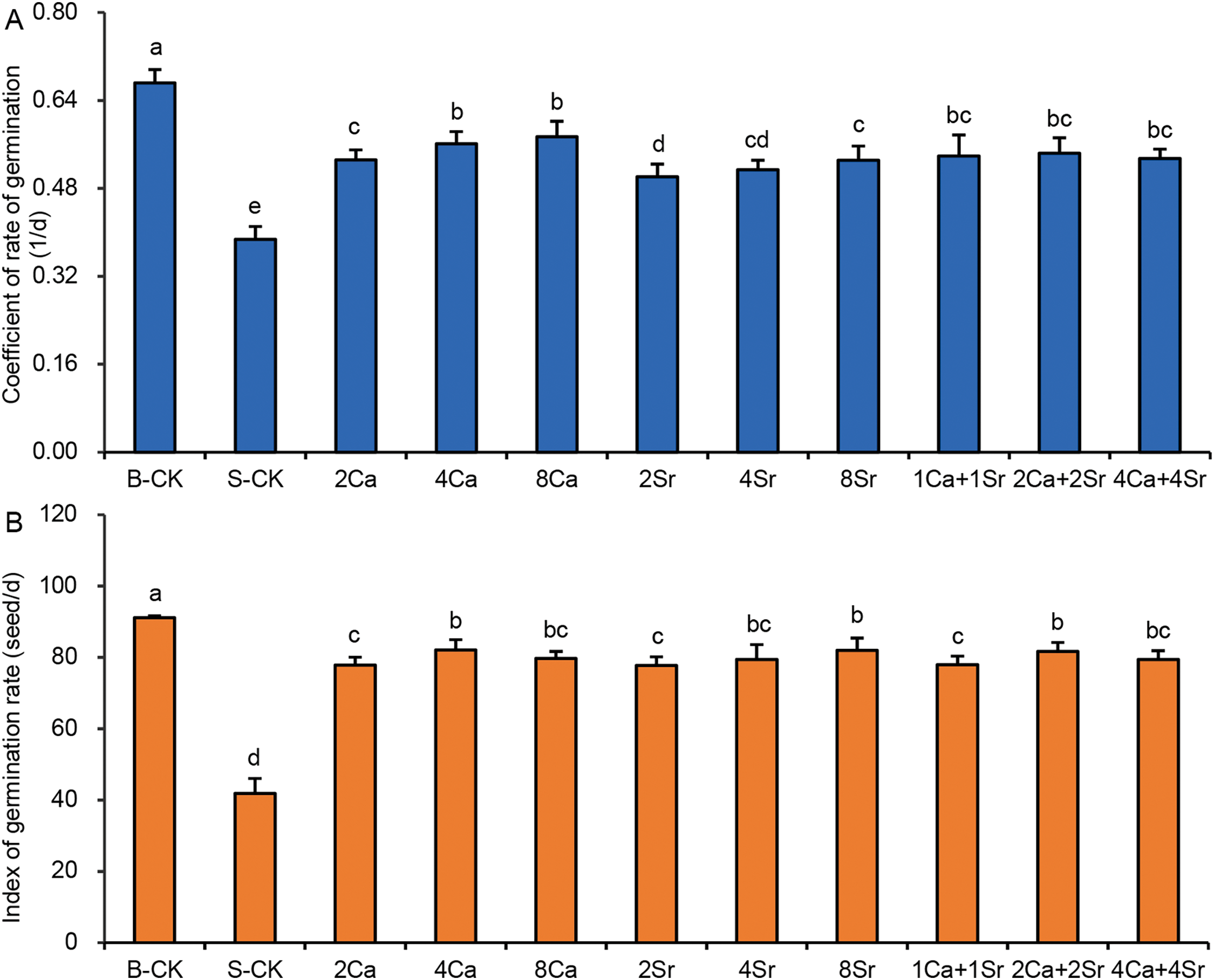
Figure 4: Effects of calcium-strontium addition on coefficient of rate of germination (A) and index of rate of germination (B) of Chinese cabbage seeds under 150 mmol/L NaCl. The B-CK group received half-strength Hoagland nutrient solution without Ca(NO3)2. The S-CK group was treated with 150 mmol/L NaCl dissolved in the nutrient solution. In the Ca-Sr addition groups, CaCl2 or SrCl2 was added to the salt solution to the final concentration of 2, 4 or 8 mmol/L, labeled as “2Ca”, “4Ca”, “8Ca” and “2Sr”, “4Sr”, “8Sr”, respectively. In Ca-Sr mixed addition group, equimolar concentrations of CaCl2 and SrCl2 were added to the salt solution to the total concentration of 2, 4 or 8 mmol/L, labeled as “1Ca + 1Sr”, “2Ca + 2Sr”, “4Ca + 4Sr”, respectively. Means were separated at a significant level (p < 0.05) by different lowercase letters
3.4 Ca-Sr Addition Improves Seed Germination Uniformity of Chinese Cabbage under Saline Condition
The uniformity of germination is an important indicator in vegetable cultivation, which can be quantified by the coefficient of uniformity of germination (CUG) and the coefficient of variation of germination time (di) (CVT). A higher CUG indicates greater germination uniformity, whereas a lower CVT value reflects more synchronized germination. The CUG results showed that all Ca-Sr treatments enhanced the germination uniformity of Chinese cabbage seeds, among which, 2Sr, 4Sr and 8Ca addition groups exhibited the same germination uniformity as the B-CK group (Fig. 5A). CUG was more sensitive than CVT in reflecting treatment differences. The CUG values in all Ca-Sr treatments were more than double that of the S-CK group, with clear differences observed under different treatment concentrations. Although the CVT value of all Ca-Sr addition groups was significantly lower than that of the S-CK group, CVT did not show a significant difference between the S-CK group and the B-CK group (Fig. 5B). Therefore, CUG is recommended as a reliable indicator for evaluating seed germination uniformity under saline conditions.
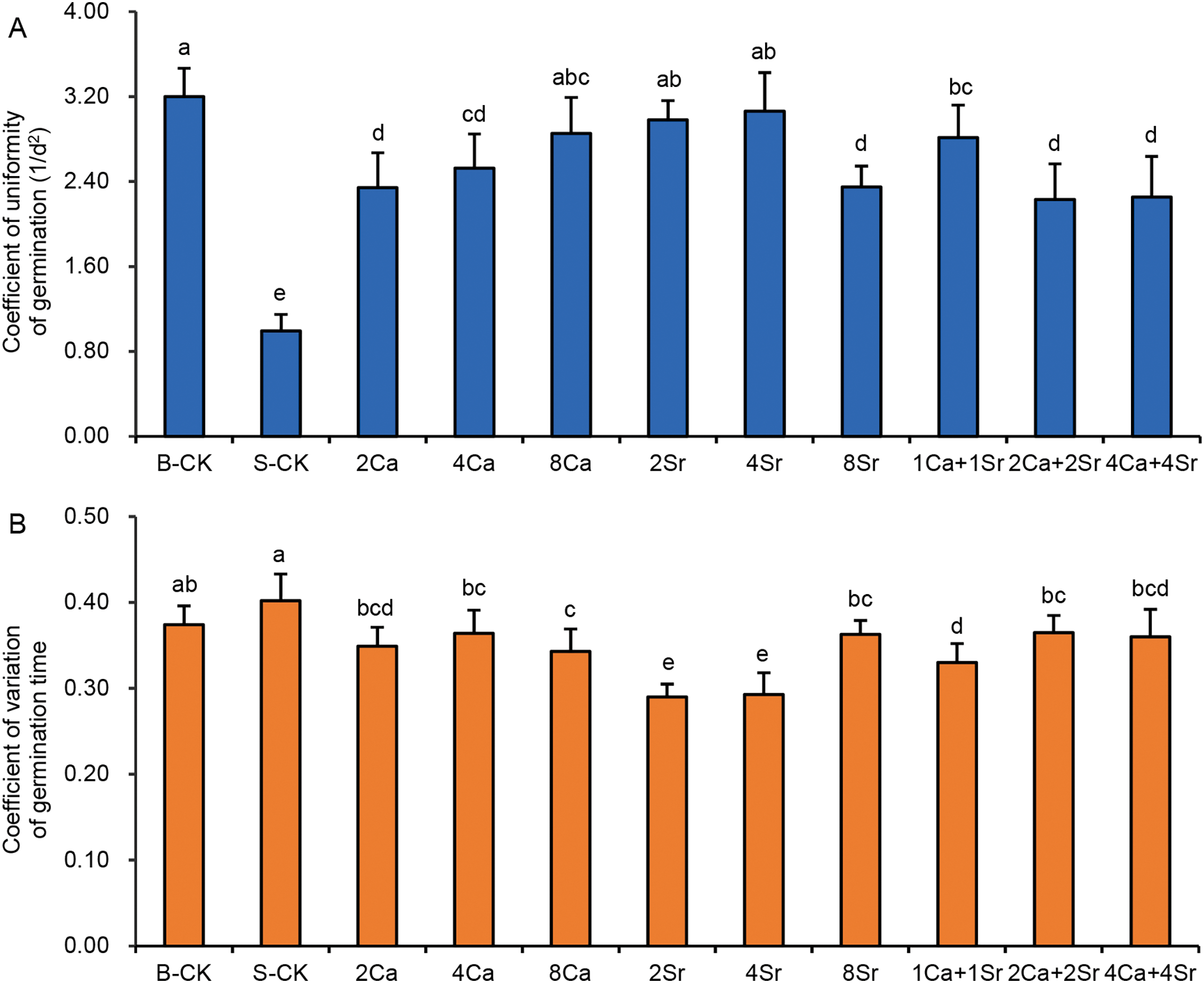
Figure 5: Effects of calcium-strontium addition on coefficient of uniformity of germination (A) and coefficient of variation of the germination time (B) of Chinese cabbage seeds under 150 mmol/L NaCl. The B-CK group received half-strength Hoagland nutrient solution without Ca(NO3)2. The S-CK group was treated with 150 mmol/L NaCl dissolved in the nutrient solution. In the Ca-Sr addition groups, CaCl2 or SrCl2 was added to the salt solution to the final concentration of 2, 4 or 8 mmol/L, labeled as “2Ca”, “4Ca”, “8Ca” and “2Sr”, “4Sr”, “8Sr”, respectively. In Ca-Sr mixed addition group, equimolar concentrations of CaCl2 and SrCl2 were added to the salt solution to the total concentration of 2, 4 or 8 mmol/L, labeled as “1Ca + 1Sr”, “2Ca + 2Sr”, “4Ca + 4Sr”, respectively. Means were separated at a significant level (p < 0.05) by different lowercase letters
3.5 Ca-Sr Addition Enhances Plasma Membrane Stability of Chinese Cabbage Sprouts under Saline Condition
Among all treatments, 8Ca-addition treatment showed the greatest effect in promoting seed vigor under saline conditions. Therefore, the addition treatments of 8Ca, 8Sr, and “4Ca + 4Sr” were selected to investigate the physiological mechanisms by which Ca-Sr addition enhanced salt tolerance of Chinese cabbage sprouts during the germination stage. As shown in Fig. 6A, plasma membrane permeability of Chinese cabbage sprouts increased significantly under 150 mmol/L NaCl, reaching 3.51 times the level of the B-CK group. Following treatments with 8Ca, 8Sr, and 4Ca + 4Sr, the plasma membrane permeability of Chinese cabbage sprouts significantly decreased to 1.15, 1.89, and 1.25 times that of the B-CK group, respectively. At equivalent concentrations, Sr-addition was less effective than Ca-addition or the Ca-Sr combination in stabilizing membrane integrity.
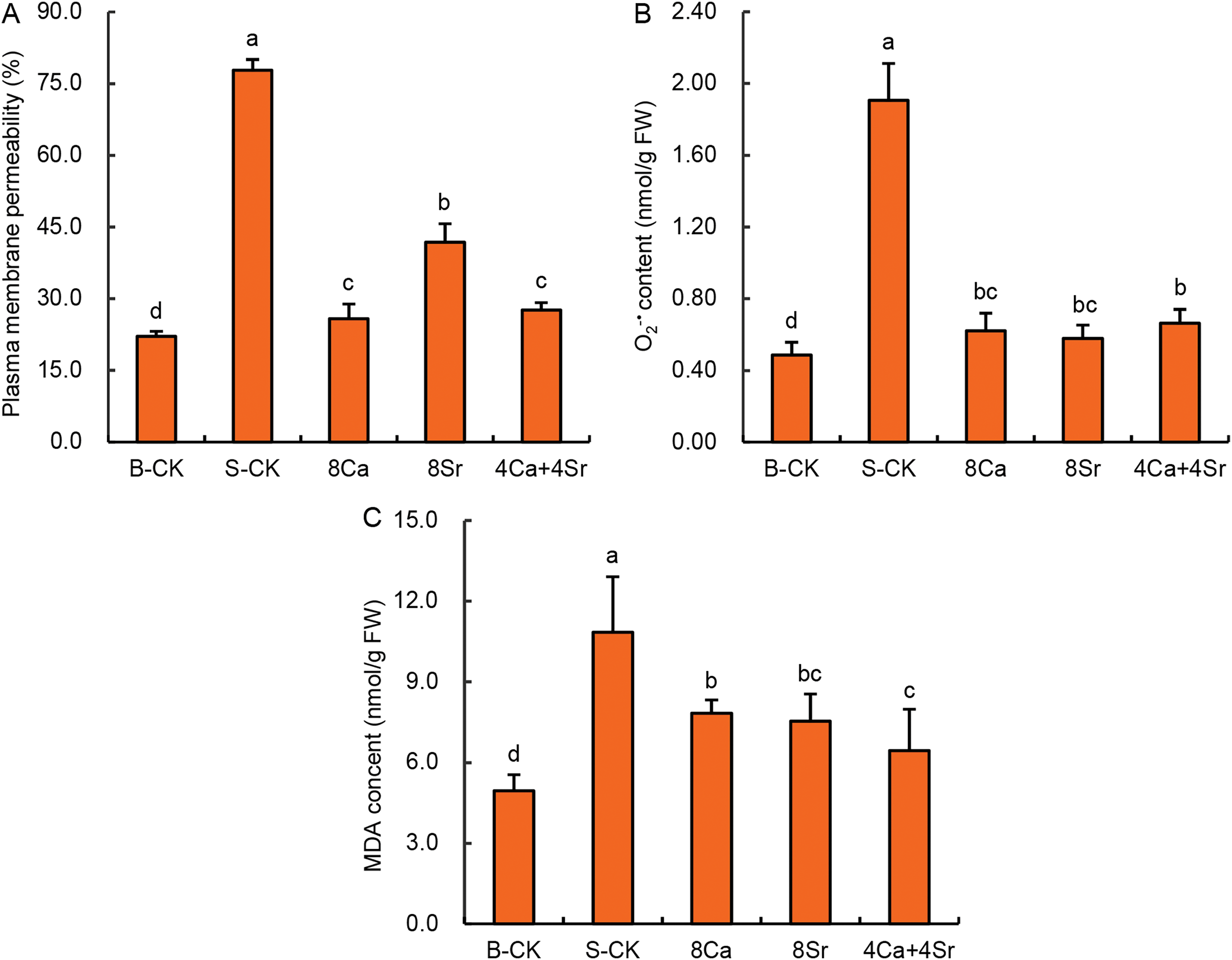
Figure 6: Effects of calcium-strontium addition on plasma membrane permeability (A), O2·− content (B) and malondialdehyde (MDA) content (C) of Chinese cabbage sprouts under 150 mmol/L NaCl. Means were separated at a significant level (p < 0.05) by different lowercase letters
3.6 Ca-Sr Addition Alleviates Oxidative Stress in Chinese Cabbage Sprouts under Saline Condition
Salt stress often induces secondary oxidative stress, leading to elevated production of reactive oxygen species within cells. As shown in Fig. 6B, the content of O2·− in Chinese cabbage sprouts treated with 150 mmol/L NaCl was 3.92 times that of the B-CK group. The addition of 8Ca, 8Sr, and 4Ca + 4Sr in 150 mmol/L NaCl significantly reduced the accumulation of O2·− in the sprouts, which were 1.28, 1.15, and 1.37 times that of the B-CK group, respectively (Fig. 6B). Malondialdehyde (MDA), a product of membrane lipid peroxidation, also confirmed that Ca-Sr addition significantly reduced oxidative damage in Chinese cabbage sprouts treated with 150 mmol/L NaCl (Fig. 6C). The MDA content in sprouts treated with 150 mmol/L NaCl was 2.19 times that of the B-CK group. Following the addition of 8Ca, 8Sr, and 4Ca + 4Sr in 150 mmol/L NaCl, the MDA contents in the sprout decreased to 1.58, 1.52, and 1.30 times that of the B-CK group, respectively. The above results indicate that Ca-Sr treatments effectively alleviated the oxidative damage of membrane lipids in the sprouts under salt stress.
3.7 Ca-Sr Addition Decreases the Accumulation of Organic Osmotytes Regulation Substances in Chinese Cabbage Sprouts
Under salt stress, plants generally accumulate low-molecular-weight organic osmolytes, such as proline and soluble sugars to maintain cellular osmotic balance. As shown in Fig. 7A, the proline content in Chinese cabbage sprouts treated with 150 mmol/L NaCl increased dramatically, reaching 57.8 times that of the B-CK group. With the addition of 8Ca, 8Sr, and 4Ca + 4Sr in 150 mmol/L NaCl, the proline content in the sprouts decreased to 19.6, 44.1, and 33.3 times that of the B-CK group, respectively. The increase multiple in the content of soluble sugars in the sprouts was relatively small after exposure to 150 mmol/L NaCl, increasing to only 2.33 times that of the B-CK control group. Following the addition of 8Ca, 8Sr, and 4Ca + 4Sr in 150 mmol/L NaCl, the soluble sugar contents in the sprouts decreased to 1.40, 1.60, and 1.19 times that of B-CK group, respectively (Fig. 7B). These results indicate that Ca-Sr-enhanced salt tolerance in sprouts was not associated with elevated proline or soluble sugar accumulation.
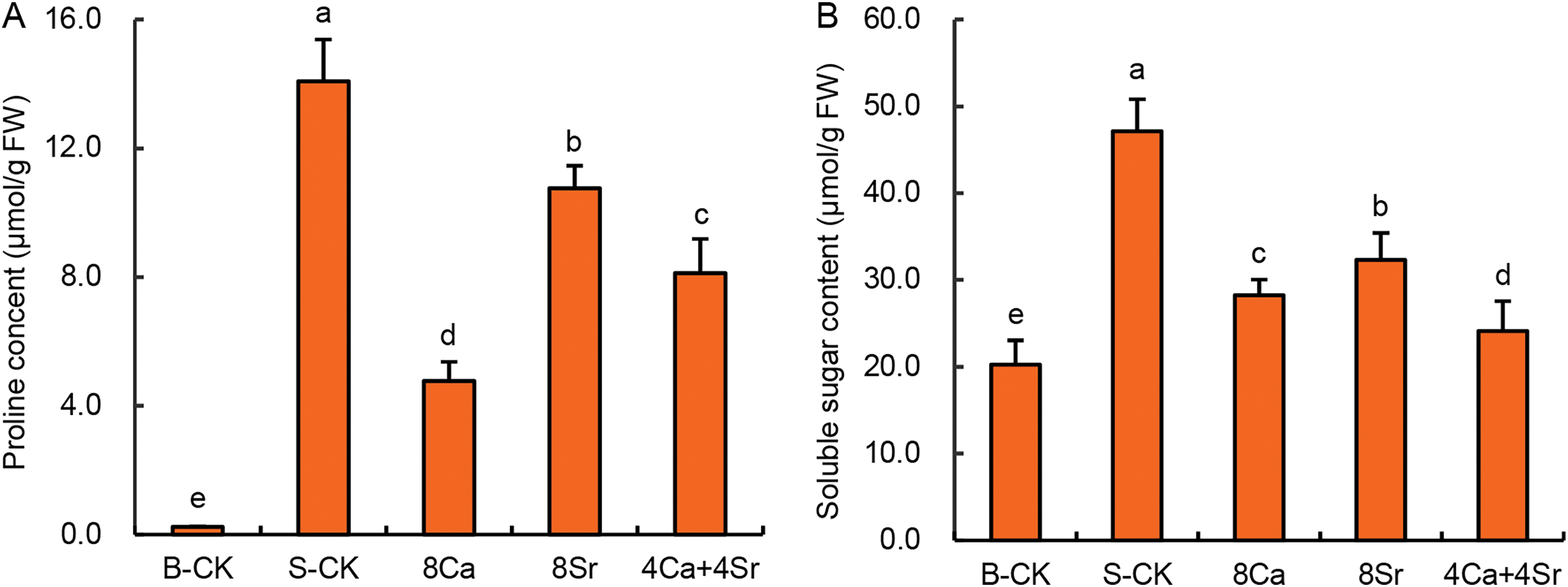
Figure 7: Effects of calcium-strontium addition on soluble sugar content (A) and proline content (B) of Chinese cabbage sprouts under 150 mmol/L NaCl. Means were separated at a significant level (p < 0.05) by different lowercase letters
The germination stage is the most critical and sensitive period in the life cycle of crops under environmental stress conditions [44]. For example, although cv. Qingmaye is a relatively salt-tolerant Chinese cabbage cultivar, its seed germinability was significantly inhibited under 150 mmol/L NaCl (Fig. 1). There are several key reasons why the germination period is particularly sensitive to salt stress. Firstly, salt-resistant genes are not fully expressed during the germination stage [45], or salinity may suppress the expression of both germination and salt-tolerant genes [32,46]. Secondly, salt-resistant anatomical structures are not yet developed, leaving cells vulnerable to salt-induced damage [47]. Thirdly, sprouts have limited osmotic regulation and water absorption capacity [24]. Fourth, salt stress disrupts nutrient uptake and leads to ionic imbalances in sprouting tissues [20,48]. Fifth, photosynthesis of the sprouts is at a low level. So sprouts mainly rely on stored reserves, which are limited and rapidly depleted under stress. Therefore, main physiological measures to improve salt tolerance during the germination stage include the exogenous application of organic osmotic regulatory substances, plant hormones, mineral nutrients or biostimulants [49]. Among these measures, the addition of exogenous Ca is the most widely used. Sr and Ca are both group IIA elements, and Sr exhibits chemical properties very similar to Ca. It is hypothesized that the addition of Sr may also enhance seed germinability under salt stress, similar to calcium.
This study demonstrated that Ca-addition, Sr-addition, and Ca-Sr mixed addition all significantly improved seed germinability of Chinese cabbage under saline condition (Fig. 2). According to the VI value, seed vigor increased with rising concentrations of Ca and Sr under salt stress. Since the difference between the 4Ca group and the 8Ca group was not statistically significant, 4–8 mmol/L CaCl2 was identified as the optimal concentration in this study. In previous studies involving other plants species, the optimal calcium addition concentration ranges from 5–10 mmol/L CaCl2, which aligns well with the findings of our study [28,29,34,35,50]. In some studies, CaCl2 was applied as a seed priming agent to improve seed germinability prior to salt exposure. In these cases, the seeds were soaked in CaCl2 solution for 12 h [30,32,46]. This priming method requires a higher concentration of CaCl2 (6–15 g/L, or 54–135 mmol/L). The promoting effect of Ca2+ on seed germination under saline conditions is universal for plants. However, most studies have focused on the effects of calcium on GP, GE, GI and VI, while relatively few studies have examined its effects on germination rate and germination uniformity [32]. In this study, seed viability, seed vigor, seed germination rate and seed germination uniformity of salt-treated Chinese cabbage seeds were systematically compared among Ca-addition and Sr-addition treatments. VI was the most sensitive index among these germination indices. The VI results showed that calcium was more effective than strontium at equivalent concentrations (Fig. 3). From the CRG results, the germination rates of Ca-addition groups were also slightly higher than those of Sr-addition groups at the same concentration, while IRG did not differ significantly (Fig. 4). Based on CUG and CVT, the 2 and 4 mmol/L SrCl2 treatments showed greater improvement in germination uniformity than the corresponding CaCl2 treatments (Fig. 5). The overall effects of Sr-addition and Ca-addition on salt-tolerance of Chinese cabbage were comparable during the germination stage, indicating that strontium is beneficial to seed germination under salt stress. These findings provide new evidence supporting strontium as a beneficial element for plants.
Several mechanisms explain how calcium enhances seed germinability of Chinese cabbage under saline conditions. Calcium integrates the cell wall in the form of calcium pectin, which can slow salt absorption and stabilize cellular membranes [51]. Strontium had a similar effect to calcium in enhancing the stability of plasma membrane of Chinese cabbage sprout under saline conditions (Fig. 6A). As a signal substance, calcium can directly activate the metabolic activity of seeds (such as increasing the activity of amylase and upregulating the expression of glucose metabolism genes), thereby improving seed vigor and germination rate [32]. Calcium signal is also involved in regulating the expression of salt-resistant genes. CaCl2-addition has been shown to upregulate the expression of NHX2, NHX4, SOS1, AKT1, AKT2, HKT1, HAK1 and KUP genes [34,46], enhancing K+ uptake and decreasing the Na+/K+ ratio to maintain ion homeostasis under salt stress. Our results showed that Ca-Sr addition significantly reduced the content of O2·− and the level of membrane lipid peroxidation (MDA content) under 150 mmol/L NaCl (Fig. 6B,C). The effect of calcium addition on alleviating the oxidative damage caused by salt stress is well documented [30,46]. Nevertheless, we propose that the alleviation of oxidative damage by Ca-addition is mainly due to the decreased production of reactive oxygen species. Kamran et al. found that Ca-addition increased the contents of soluble sugar and proline in the sprouts of Chinese cabbage, thereby enhancing osmotic protection ability of cells [31]. Similarly, nitric oxide (NO) improved the germination of wheat seeds exposed to salinity via modulating sugar and proline metabolism [52]. In contrast, we found that Ca-Sr addition significantly reduced soluble sugar and proline contents in Chinese cabbage sprouts under 150 mmol/L NaCl (Fig. 7). A marked increase in proline contents may be a consequence of salt stress rather than a cause [53], because exogenous application of proline did not improve salt tolerance of Kochia Scoparia during the germination stage [54]. The mechanism by which strontium enhances salt tolerance during germination may resemble that of calcium, but further investigation is needed to confirm this hypothesis.
Under 150 mmol/L NaCl, the addition of 2–8 mmol/L CaCl2 and SrCl2 significantly enhanced seed viability, seed vigor, seed germination rate and seed germination uniformity of Chinese cabbage, mainly because calcium and strontium could significantly enhance the stability of biomembrane under salt stress. When comparing the effects of Ca-addition and Sr-addition, GI, VI and CUG were the more sensitive indices, followed by CRG, IRG, and CVT. GP and GE did not significantly differ among the various Ca-Sr addition treatments. Therefore, GI, VI and CUG are more informative for evaluating seed germinability under salt stress. At equal concentrations, calcium was more effective than strontium in enhancing seed vigor, while strontium outperformed calcium in improving germination uniformity. These results provide strong evidence that strontium is a beneficial element for enhancing salt tolerance. The application of calcium and strontium represents a feasible strategy to promote seed germination and sprout growth in saline–alkaline soils.
Acknowledgement: We are grateful for the support provided by the Department of Plant Physiology, Qufu Normal University.
Funding Statement: This research was funded by the Natural Science Foundation of Shandong Province (ZR2020MC144) and the Scientific Research Training Program for Undergraduates of Qufu Normal University (XJ2024016).
Author Contributions: Experimental design, conceptualization and methodology: Nianwei Qiu and Hongxia Zhang; Determination of germination indices: Bingxuan Fan, Yuqi Gao, Yunshu Tang and Yamin Xing; Determination of salt-resistance indices: Shiyang Li and Jia Song; Data analysis and figures: Shiyang Li and Jia Song; Review and editing the manuscript: Nianwei Qiu and Hongxia Zhang; Project administration: Nianwei Qiu. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Bijalwan P, Jeddi K, Saini I, Sharma M, Kaushik P, Hessini K. Mitigation of saline conditions in watermelon with mycorrhiza and silicon application. Saudi J Biol Sci. 2021;28(7):3678–84. doi:10.1016/j.sjbs.2021.05.019. [Google Scholar] [PubMed] [CrossRef]
2. Munns R, Gilliham M. Salinity tolerance of crops—what is the cost? New Phytol. 2015;208(3):668–73. doi:10.1111/nph.13519. [Google Scholar] [PubMed] [CrossRef]
3. Liu XW, Feike T, Chen SY, Shao LW, Sun HY, Zhang XY. Effects of saline irrigation on soil salt accumulation and grain yield in the winter wheat-summer maize double cropping system in the low plain of North China. J Integr Agric. 2016;15(12):2886–98. doi:10.1016/S2095-3119(15)61328-4. [Google Scholar] [CrossRef]
4. Oliveira CED, Zoz T, Seron CD, Boleta EHM, de Lima BH, Souza LRR, et al. Can saline irrigation improve the quality of tomato fruits? Agron J. 2022;114(2):900–14. doi:10.1002/agj2.21003. [Google Scholar] [CrossRef]
5. Moles TM, Guglielminetti L, Reyes TH. Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Sci Hortic. 2019;257(9):108730. doi:10.1016/j.scienta.2019.108730. [Google Scholar] [CrossRef]
6. Qiu NW, Liu Q, Wang FD, Zhao N, Sun KY, Miao XM, et al. Long-term observation on salt tolerance of Chinese cabbage at different development stages. Plant Physiol J. 2015;51:1597–603. (In Chinese). doi:10.13592/j.cnki.ppj.2015.1007. [Google Scholar] [CrossRef]
7. Gul B, Ansari R, Flowers TJ, Khan MA. Germination strategies of halophyte seeds under salinity. Envrion Exp Bot. 2013;92:4–18. doi:10.1016/j.envexpbot.2012.11.006. [Google Scholar] [CrossRef]
8. Meza C, Valenzuela F, Echeverría-Vega A, Gomez A, Sarkar S, Cabeza RA, et al. Plant-growth-promoting bacteria from rhizosphere of Chilean common bean ecotype (Phaseolus vulgaris L.) supporting seed germination and growth against salinity stress. Front Plant Sci. 2022;13:1052263. doi:10.3389/fpls.2022.1052263. [Google Scholar] [PubMed] [CrossRef]
9. Zhang H, Bai X, Han YJ, Han LZ. Stress-resistance and growth-promoting characteristics and effects on vegetable seed germination of Streptomyces sp. strains isolated from wetland plant rhizospheres. Curr Microbiol. 2023;80(5):190. doi:10.1007/s00284-023-03297-x. [Google Scholar] [PubMed] [CrossRef]
10. Gong M, He JX, Kong M, Huo QY, Jiang YW, Song JQ, et al. A microencapsulation approach to design microbial seed coatings to boost wheat seed germination and seedling growth under salt stress. Front Plant Sci. 2023;14:1283590. doi:10.3389/fpls.2023.1283590. [Google Scholar] [PubMed] [CrossRef]
11. El-Moukhtari A, Ksiaa M, Zorrig W, Cabassa C, Abdelly C, Farissi M, et al. How silicon alleviates the effect of abiotic stresses during seed germination: a review. J Plant Growth Regul. 2023;42(6):3323–41. doi:10.1007/s00344-022-10794-z. [Google Scholar] [CrossRef]
12. Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust J Agr Res. 2007;58(8):811–5. doi:10.1071/AR06253. [Google Scholar] [CrossRef]
13. Shahba MA, Qlan YL, Lair KD. Improving seed germination of saltgrass under saline conditions. Crop Sci. 2008;48(2):756–62. doi:10.2135/cropsci2007.07.0382. [Google Scholar] [CrossRef]
14. Li HP, Sun HC, Ping WC, Liu LT, Zhang YJ, Zhang K, et al. Exogenous ethylene promotes the germination of cotton seeds under salt stress. J Plant Growth Regul. 2023;42(6):3923–33. doi:10.1007/s00344-022-10859-z. [Google Scholar] [CrossRef]
15. Ben Youssef R, Jelali N, Motos JRA, Abdelly C, Albacete A. Salicylic acid seed priming: a key frontier in conferring salt stress tolerance in barley seed germination and seedling growth. Agronomy. 2025;15(1):154. doi:10.3390/agronomy15010154. [Google Scholar] [CrossRef]
16. Wang L, Tanveer M, Wang HL, Arnao MB. Melatonin as a key regulator in seed germination under abiotic stress. J Pineal Res. 2024;76(1):e12937. doi:10.1111/jpi.12937. [Google Scholar] [PubMed] [CrossRef]
17. Baz H, Creech M, Chen JJ, Gong HJ, Bradford K, Huo HQ. Water-soluble carbon nanoparticles improve seed germination and post-germination growth of lettuce under salinity stress. Agronomy. 2020;10(8):1192. doi:10.3390/agronomy10081192. [Google Scholar] [CrossRef]
18. Ghazi AA, El-Nahrawy S, El-Ramady H, Ling WT. Biosynthesis of nano-selenium and its impact on germination of wheat under salt stress for sustainable production. Sustainability. 2022;14(3):1784. doi:10.3390/su14031784. [Google Scholar] [CrossRef]
19. Gong M, Kong M, Huo QY, He JX, He J, Yan ZS, et al. Ultrasonic treatment can improve maize seed germination and abiotic stress resistance. BMC Plant Bio. 2024;24(1):758. doi:10.1186/s12870-024-05474-x. [Google Scholar] [PubMed] [CrossRef]
20. Guo R, Shi LX, Ding XM, Hu YJ, Tian SY, Yan DF, et al. Effects of saline and alkaline stress on germination, seedling growth, and ion balance in wheat. Agron J. 2010;102(4):1252–60. doi:10.2134/agronj2010.0022. [Google Scholar] [CrossRef]
21. Akgün I, Kara B, Atlindal D. Effect of salinity (NaCl) on germination, seedling growth and nutrient uptake of different triticale genotypes. Turk J Field Crops. 2011;16(2):225–32. [Google Scholar]
22. Cheng Y, Tian QY, Zhang WH. Glutamate receptors are involved in mitigating effects of amino acids on seed germination of Arabidopsis thaliana under salt stress. Environ Exp Bot. 2016;130:68–78. doi:10.1016/j.envexpbot.2016.05.004. [Google Scholar] [CrossRef]
23. Zhao Y, Yang KJ, Li ZT, Zhao CJ, Xu JY, Hu XW, et al. Alleviation of salt stress during maize seed germination by presoaking with exogenous sugar. Chin J Appl Ecol. 2015;26(9):2735–42. (In Chinese). doi:10.13287/j.1001-9332.20150630.016. [Google Scholar] [CrossRef]
24. Yagmur M, Kaydan D. Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. Afr J Biotechnol. 2008;7(3):2156–62. doi:10.5897/AJB08.170. [Google Scholar] [CrossRef]
25. Uçgun K, Ferreira JFS, Liu X, da Silva JB, Suarez DL, de Lacerda CF, et al. Germination and growth of spinach under potassium deficiency and irrigation with high-salinity water. Plants. 2021;9(12):1739. doi:10.3390/plants9121739. [Google Scholar] [PubMed] [CrossRef]
26. Chen XF, Zhang RD, Xing YF, Jiang B, Li B, Xu XX, et al. The efficacy of different seed priming agents for promoting sorghum germination under salt stress. PLoS One. 2021;16(1):e0245505. doi:10.1371/journal.pone.0245505. [Google Scholar] [PubMed] [CrossRef]
27. Gao RR, Wei XY, He Z, Zhao RH, Wang K, Yang XJ, et al. Soil salt and NaCl have different effects on seed germination of the halophyte Suaeda salsa. J Plant Nutr Soil Sc. 2018;181(4):488–97. doi:10.1002/jpln.201700544. [Google Scholar] [CrossRef]
28. Salahshoor F, Kazemi F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016;62(10):460–6. doi:10.17221/319/2016-PSE. [Google Scholar] [CrossRef]
29. Mulaudzi T, Hendricks K, Mabiya T, Muthevhuli M, Ajayi RF, Mayedwa N, et al. Calcium improves germination and growth of Sorghum bicolor seedlings under salt stress. Plants. 2020;91(6):730. doi:10.3390/plants9060730. [Google Scholar] [PubMed] [CrossRef]
30. Jiang XW, Zhang CR, Wang WH, Xu GH, Zhang HY. Seed priming improves seed germination and seedling growth of Isatis indigotica Fort. under salt stress. Hortscience. 2020;55(5):647–50. doi:10.21273/HORTSCI14854-20. [Google Scholar] [PubMed] [CrossRef]
31. Kamran M, Wang D, Xie KZ, Lu YS, Shi CH, EL-Sabagh A, et al. Pre-sowing seed treatment with kinetin and calcium mitigates salt induced inhibition of seed germination and seedling growth of choysum (Brassica rapa var. parachinensis). Ecotox Environ Safe. 2021;227(1):112921. doi:10.1016/j.ecoenv.2021.112921. [Google Scholar] [PubMed] [CrossRef]
32. Xing YF, Chen XF, Zhang M, Li B, Cui T, Liu C, et al. CaCl2 priming promotes sorghum seed germination under salt stress by activating sugar metabolism. Plant Growth Regul. 2023;101(2):385–97. doi:10.1007/s10725-023-01025-w. [Google Scholar] [CrossRef]
33. Danial AW, Basset RA. Amelioration of NaCl stress on germination, growth, and nitrogen fixation of Viciafaba at isosmotic Na-Ca combinations and Rhizobium. Planta. 2024;259(3):69. doi:10.1007/s00425-024-04343-z. [Google Scholar] [PubMed] [CrossRef]
34. Chandran AEJ, Finkler A, Hait TA, Kiere Y, David S, Pasmanik-Chor M, et al. Calcium regulation of the Arabidopsis Na+/K+ transporter HKT1;1 improves seed germination under salt stress. Plant Physiol. 2024;194(3):1834–52. doi:10.1093/plphys/kiad651. [Google Scholar] [PubMed] [CrossRef]
35. Zehra A, Gul B, Ansari R, Khan MA. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S Afr J Bot. 2012;78:122–28. doi:10.1016/j.sajb.2011.05.016. [Google Scholar] [CrossRef]
36. Hadi MR, Karimi N. The role of calcium in plants’ salt tolerance. J Plant Nutr. 2012;35(13):2037–54. doi:10.1080/01904167.2012.717158. [Google Scholar] [CrossRef]
37. Strzyz P. Decoding the Ca2+ signal for salt tolerance. Nat Rev Mol Cell Bio. 2022;23(10):643. doi:10.1038/s41580-022-00539-x. [Google Scholar] [PubMed] [CrossRef]
38. Zhang WR, Kang Z, Wang Q, Qiu NW, Chen M, Zhou F. The biological effects of strontium (88Sr) on Chinese cabbage. Plant Soil Environ. 2020;66(4):149–54. doi:10.17221/108/2020-PSE. [Google Scholar] [CrossRef]
39. Chao EK, Wu MM, Yue DX, Yuan YX, Qiu NW, Zhou F. Promoting effect of low concentration strontium on photosynthetic performance of Chinese cabbage seedlings: combined leaf characteristics, photosynthetic carbon assimilation and chlorophyll fluorescence. Ecotox Environ Safe. 2024;274(5):116200. doi:10.1016/j.ecoenv.2024.116200. [Google Scholar] [PubMed] [CrossRef]
40. Wang SN, Sun J, Guo JY, Han LY, Qiu NW, Zhou F. Overview of seed germination indices and their determination methods. J Triticeae Crops. 2023;43(2):190–96. (In Chinese). doi:10.7606/j.issn.1009-1041.2023.02.08. [Google Scholar] [CrossRef]
41. Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–11. doi:10.1007/s004250050524. [Google Scholar] [CrossRef]
42. Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammonium-chloride: a simple assay for superoxide dismutase. Anal Biochem. 1976;70(2):616–20. doi:10.1016/0003-2697(76)90488-7. [Google Scholar] [PubMed] [CrossRef]
43. Shabnam N, Tripathi I, Sharmila P, Pardha-Saradhi P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma. 2016;253(6):1577–82. doi:10.1007/s00709-015-0910-6. [Google Scholar] [PubMed] [CrossRef]
44. Weitbrecht K, Mueller K, Leubner-Metzger G. First off the mark: early seed germination. J Exp Bot. 2011;62(10):3289–309. doi:10.1093/jxb/err030. [Google Scholar] [PubMed] [CrossRef]
45. Tan M, Liao F, Hou LT, Wang J, Wei LJ, Jian HJ, et al. Genome-wide association analysis of seed germination percentage and germination index in Brassica napus L. under salt and drought stresses. Euphytica. 2017;213(2):40. doi:10.1007/s10681-016-1832-x. [Google Scholar] [CrossRef]
46. Chen XF, Zhang RD, Li B, Cui T, Liu C, Liu CJ, et al. Alleviation of oxidative damage induced by CaCl2 priming is related to osmotic and ion stress reduction rather than enhanced antioxidant capacity during germination under salt stress in sorghum. Front Plant Sci. 2022;13:881039. doi:10.3389/fpls.2022.881039. [Google Scholar] [PubMed] [CrossRef]
47. Liu CY, Jiang XB, Yuan ZH. Plant responses and adaptations to salt stress: a review. Horticulturae. 2024;10(11):1221. doi:10.3390/horticulturae10111221. [Google Scholar] [CrossRef]
48. Guo JR, Liu LL, Du M, Tian HY, Wang BS. Cation and Zn accumulation in brown seeds of the euhalophyte Suaeda salsa improves germination under saline conditions. Front Plant Sci. 2020;11:602427. doi:10.3389/fpls.2020.602427. [Google Scholar] [PubMed] [CrossRef]
49. Sun CW, Chen J, Wang LL, Li JJ, Shi ZQ, Yang LF, et al. Thymol deploys multiple antioxidative systems to suppress ROS accumulation in Chinese cabbage seedlings under saline stress. Agronomy. 2024;14(5):1059. doi:10.3390/agronomy14051059. [Google Scholar] [CrossRef]
50. Zhang DW, Vu TS, Huang J, Chi CY, Xing Y, Fu DD, et al. Effects of calcium on germination and seedling growth in Melilotus officinalis L. (Fabaceae) under salt stress. Pak J Bot. 2019;51(1):1–9. doi:10.30848/PJB2019-1(44). [Google Scholar] [CrossRef]
51. An P, Li XJ, Zheng YR, Eneji AE, Inanaga S. Calcium effects on root cell wall composition and ion contents in two soybean cultivars under salinity stress. Can J Plant Sci. 2014;94(4):733–40. doi:10.4141/CJPS2013-291. [Google Scholar] [CrossRef]
52. Çatav SS. Nitric oxide improves the germination of wheat seeds exposed to salinity via modulating sugar and proline metabolism. Biologia. 2023;78(11):3073–83. doi:10.1007/s11756-023-01507-2. [Google Scholar] [CrossRef]
53. Çelik Ö, Atak C. The effect of salt stress on antioxidative enzymes and proline content of two Turkish tobacco varieties. Turk J Biol. 2012;36:339–56. doi:10.3906/biy-1108-11. [Google Scholar] [CrossRef]
54. Khan MA, Gul B, Weber DJ. Seed germination of Kochia Scoparia under saline conditions: responses with germination regulating chemicals. Pak J Bot. 2009;41(6):2933–41. doi:10.3417/2007161. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools