 Open Access
Open Access
ARTICLE
Compound Fertilizer Application Improves the Growth of Herbaceous Peony (Paeonia lactiflora Pall.) under High Temperature in Summer
College of Horticulture and Landscape Architecture, Yangzhou University, Yangzhou, 225009, China
* Corresponding Author: Daqiu Zhao. Email:
(This article belongs to the Special Issue: Plant Responses to Abiotic Stress Mechanisms)
Phyton-International Journal of Experimental Botany 2025, 94(6), 1751-1767. https://doi.org/10.32604/phyton.2025.065874
Received 24 March 2025; Accepted 12 May 2025; Issue published 27 June 2025
Abstract
Summer high temperatures have severely impaired the growth of herbaceous peony (Paeonia lactiflora Pall.) in East China. While compound fertilizer application enhances soil fertility and promotes plant growth, its efficacy in maintaining optimal plant performance under summer heat stress remains poorly understood. This study investigated the effects of compound fertilizer application on herbaceous peony growth during summer thermal stress. Results demonstrated that compound fertilizer supplementation significantly improved plant growth under elevated temperatures, manifesting enhanced phenotypic characteristics, elevated antioxidant enzyme activities, and increased nutrient accumulation. Compared to untreated controls, fertilized plants exhibited three key responses: (1) increased chlorophyll content coupled with reduced relative conductivity, malondialdehyde levels, and reactive oxygen species (ROS) accumulation; (2) upregulated activities of four critical antioxidant enzymes and augmented nitrogen, phosphorus, and potassium assimilation, collectively enhancing photosynthetic efficiency; and (3) stimulated expression of chlorophyll biosynthesis-related genes alongside suppressed transcription of chlorophyll degradation-associated genes. These findings establish a theoretical framework for optimizing compound fertilizer strategies to mitigate summer heat stress in herbaceous peony cultivation across East China.Keywords
Herbaceous peony (Paeonia lactiflora Pall.) is a perennial herbaceous species within the genus Paeonia of the family Paeoniaceae. Previously classified under Ranunculaceae, modern taxonomic revisions have established its placement in the Ranunculaceae. The plant exhibits an erect stem, with fleshy tuberous roots subterraneously developed. The terminal leaves are characteristically bipinnately compound (biternate), comprising ovate leaflets with entire margins and a dark green adaxial surface. This morphological configuration aligns with its adaptation to temperate climates and seasonal growth patterns. It’s a perennial herbaceous plant native to temperate East Asia including China, Mongolia, and Siberia, thrives in well-drained fertile soils with neutral to slightly alkaline pH (6.5–7.5). This heliophilous species exhibits high cold tolerance and is widely cultivated in temperate gardens worldwide for its diverse colors and flower forms. In Traditional Chinese Medicine, its root is highly valued for its anti-inflammatory, antispasmodic, and analgesic properties, with clinical applications including menstrual regulation, pain relief, and treatment of arthritis and hepatic disorders. The Herbaceous peony is a culturally significant ornamental plant in China, recently achieving unprecedented commercial recognition by ranking among the top three in China’s 2024 Plant Variety Rights list released by the National Forestry and Grassland Administration. It’s well-known that Beijing, Luoyang (Henan Province), and Yangzhou (Jiangsu Province)—where it serves as the city flower—had the most developed herbaceous peony industry. Due to the intensification of the global greenhouse effect, summer temperatures in the middle and lower reaches of the Yangtze River have been rising annually. Specifically, summer heatwaves in the Yangtze River Basin have become increasingly severe, with temperatures exceeding 40°C for prolonged periods in 2024, far exceeding its optimal growth range of 18–22°C [1]. These extreme conditions disrupt photosynthesis, nutrient allocation, and flower bud differentiation, compromising current-year growth and next-season ornamental quality.
There are generally three strategies to solve this phenomenon. First, genetic engineering techniques can be used for gene editing to enhance plants’ high-temperature tolerance to survive high temperatures in summer. Liao et al. [2] discovered a new strategy for responding to high temperatures, wherein the immune activator (Enhanced Disease Susceptibility 1) OsEDS1 regulates ROS homeostasis, thus improving the resistance of rice to high temperature in summer. However, genetic engineering approaches to enhance thermotolerance in herbaceous peony remain limited. Zhang et al. [3] found that PlTOE3 could bind to the promoter of tryptophan decarboxylase (PlTDC) to promote PlTDC transcription and increase melatonin content in herbaceous peony, which is more beneficial to its growth and development in summer. However, inefficient transformation systems and technical barriers hinder the practical implementation of transgenic/CRISPR approaches in herbaceous peony. While hybridization offers potential for thermotolerant cultivar development, herbaceous peony’s 4–5-year generation time, strict vernalization requirements, and seasonal germination dependency [1] severely limit progress. Lastly, exogenous treatments like plant growth regulators are widely used. Wen et al. [4] found that exogenous brassinosteroids (BR) could improve the activity of key enzymes in wheat involved in starch synthesis under high temperatures in summer, increasing amylose and amylopectin content by 25.34% and 11.57% over two years, respectively, thereby increasing yield. Sheng et al. [5] showed that application of biochar to cultivation environment could improve the physicochemical properties of the medium, soil enzyme activity, and nitrogen, phosphorus, and potassium content, this also increased rhizosphere microbial abundance, promoting the growth of roots and enhancing its tolerance to face high temperature in summer. Also, exogenous salicylic acid improves the crop’s photosynthetic properties and antioxidant capacity during the grain filling stage to reduce the damage caused by high summer temperatures [6].
However, studies on exogenous treatments or improved cultivation substrates for better growth of herbaceous peony in summer are scarce. Only a few methods have been effectively applied, such as exogenous paclobutrazol treatment [7], exogenous trehalose treatment [8], and shading during summer [9]. It is worth noting that, these methods are mostly suited for potted herbaceous peony, and considering the economic and labor costs, the above techniques seem unsuitable for field-grown herbaceous peony. Therefore, it is crucial to identify suitable strategies to improve the growth of field-grown herbaceous peony under high temperature in summer.
Fertilization is a key factor that influences plant growth. Appropriate fertilization can improve both yield and quality. According to the type of nutrients, fertilizers can be divided into three types: Single-nutrient fertilizers, compound (mixed) fertilizer, and complete fertilizer. Single-nutrient fertilizers typically contain only one nutrient in each particle, for example, urea particles only contain nitrogen, and potassium chloride particles only contain potassium [10]. Compound fertilizer includes various nutrients (e.g., nitrogen, phosphorus, and potassium) in each granule and has the advantages of high nutrient content, fewer components, and good physical properties. On the one hand, it plays a vital role in balanced fertilization, improving fertilizer utilization rate, and promoting a high and stable crop yield. Proper use of fertilizers has been shown to improve the yield and quality of crops such as sugar beet [11] and Platostoma palustre [12]. Compound fertilizer is also widely used to alleviate plant damage caused by high temperature in summer. Fahad et al. [13] found that appropriate concentrations of compound fertilizers significantly reduced the adverse effects of high temperature on pollen and maintained pollen viability. However, few studies have been conducted on applying compound fertilizers in the flower industry, especially in ornamental flowers. Regmi et al. [14] found that fertilizer application could counteract some of the adverse effects of biochar on Viola cornuta, thereby improving the nutritional quality and increasing the production of bioactive compounds, improving ornamental quality. In addition, applying compound fertilizers and slow-release fertilizers can effectively enhance the number of blooms and the quality of finished freesia flowers [15]. Rational fertilisation is an effective strategy for remediation of Cd and Pb contamination in chrysanthemums, and the amended soil pH, organic matter, cation exchange capacity and nutrient effectiveness were increased, reducing the damage caused by Cd and Pb to chrysanthemums [16].
Although studies on improving plant quality and abiotic stress resistance through applying complex fertilizers have been widely reported, few studies have been reported using ornamental plants as the subject. As a perennial herb, high temperature in summer seriously affects photosynthesis and nutrient accumulation after anthesis, which will directly affect the ornamental value of herbaceous peony in the following year. This study aims to investigate whether compound fertilizer treatments can mitigate the effects of high summer temperatures on field-grown herbaceous peony and improve the ornamental quality of Paeonia lactiflora in the following year.
2.1 Plant Materials and Treatments
Herbaceous peony ‘Hongyan Zhenghui’ were introduced in 2009 and ramet in 2017, planted at a row spacing of 1 × 1 m2 in the National Herbaceous Peony Germplasm Resources base of Yangzhou University (32°30′ N, 119°25′ E). The experimental site is characterized by a subtropical monsoon climate, with an average annual precipitation of 991 mm and a mean annual temperature of 15.2°C. The physicochemical properties of cultivated soils have been reported in previous study [17]. Plants were divided into Control, Group T1, and Group T2. Control was planted in a normal environment, Group T1 was treated with compound fertilizer at 0.2 kg/m2, and Group T2 was treated with compound fertilizer at 0.4 kg/m2. The compound fertilizer in this study is a commercial product for sale; its primary nutrients are nitrogen, phosphorus, and potassium. Treatments were applied once during the plant dormancy period (November 2023), before flowering (March 2024), and after flowering (May 2024); all treatments were using hole fertilization. We also monitored the precipitation throughout the fertiliser application cycle, which was 38.3, 26.6, 67.8, 22.5, 19.3, 83.8 and 28 mm from November 2023 to May 2024, respectively. From 5 June to 5 August 2024, physiological indicators such as plant phenotype, photosynthetic characteristics, and ROS accumulation were measured every 20 days. The remaining leaf samples were stored in a −80°C refrigerator after being frozen with liquid nitrogen for the detection of antioxidant enzyme activities, the relevant expression level of genes related to chlorophyll biosynthesis and degradation, etc.
2.2 Determination of Color Indices and Chlorophyll Content
Based on the leaf distribution characteristics of paeoniae, the leaves are concentrated at the top of the plant, with fewer leaves growing in the middle and at the base, so the top leaves were used for sampling and index determination. The top leaves were used to detect SPAD values SPAD-502 (Koinca Minolia Sensing, Japan). Three biological replicates were tested for each treatment.
Chlorophyll content was detected using a kit (Suzhou Corning Biotechnology Co., Ltd., Suzhou, China). A tissue sample of 0.1 g out of the leaf veins was taken, distilled water and acetone were added and ground thoroughly. The milled sample was fixed with the extract and left to stand for 3 h away from light, and the absorbance at 663 and 645 nm was measured after the sample was whitened entirely, and the chlorophyll a, chlorophyll b, and total chlorophyll contents were calculated.
2.3 Determination of REC and MDA Content
REC (relative electrical conductivity) was determined according to the method reported by Sheng et al. [5]. Take 0.1 g of fresh sample and add 20 mL of distilled water, after standing at room temperature for about 3 h, test the REC of the solution C1, after boiling water bath for 30 min, test the REC of the solution C2 again, than calculate the final REC of the sample.
Malondialdehyde (MDA) content was then measured by the kit (Beijing Solarbio Biotechnology Co., Ltd., Beijing, China). The leaf tissue was submerged in the extract, homogenised and processed, centrifuged at high speed and the supernatant was taken, added to the corresponding solution in the kit respectively, boiled water bath for 1 h, centrifuged at high speed and the supernatant was taken in the cuvette, the absorbance of the samples was detected at 532 and 600 nm and the MDA content was calculated.
2.4 Determination of ROS Measurements
The accumulation of H2O2 was observed by diaminoaniline (DAB) staining. The leaves were immersed in DAB solution and stained at room temperature in the dark for 2–6 h, then 95% ethanol was added, and the samples were immersed. Samples were decololated in a water bath at 80°C, and 95% ethanol was changed every 10 min. The H2O2 content was measured according to a kit (Beijing Solarbio Biotechnology Co., Ltd., Beijing, China).
The accumulation of
2.5 Determination of Antioxidant Enzyme Activities
The main antioxidant enzymes used in plants to measure abiotic stress include Superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), and contents of those were assayed by the kit (Suzhou Corning Biotechnology Co., Ltd., Suzhou, China). Homogenize plant tissues and centrifuge to collect the supernatant as the test sample. Mix the sample with specific substrates (e.g., nitroblue tetrazolium for SOD, guaiacol for POD) at the ratio specified in the kit, followed by incubation under dark conditions or at designated temperatures for a defined duration. Add termination reagents (e.g., sulfuric acid for CAT, ascorbic acid for APX) to halt the reaction. Measure absorbance at characteristic wavelengths (e.g., 560 nm for SOD, 470 nm for POD, 240 nm for CAT, 290 nm for APX) using a spectrophotometer. Calculate enzyme activity (expressed as U/g fresh weight or per protein content) based on standard curves or formulas, quantifying substrate conversion rates per unit time.
2.6 Determination of Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters
The portable photosynthesizer (LI-6800, Li-Cor, Lincoln, NE, USA) was used to detect plant photosynthesis parameters between 8:30 and 9:00 a.m., mainly including the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr).
The plants to be tested were protected from light for at least 4 h, and then a chlorophyll fluorescence spectrometer (Heinz Walz GmbH, Effeltrich, Germany) was used to detect the chlorophyll fluorescence parameters of the plants, mainly including Fv/Fm, qN, Y(NO), and Y(II), and the above data were processed by PAM WIN software.
2.7 Determination of Nutrient Accumulation
Plant tissues were digested according to a method proposed by Guo et al. [18]. 0.05 g of each sample was digested with HNO3/H2O in a microwave digester (MARS5, CEM, Matthews, NC, USA). The concentrations of nitrogen (N), phosphorus (P), potassium (K) was determined by inductively coupled plasma optical emission spectrometry (ICP OES, iCAP6300, Thermo Scientific, Waltham, MA, USA). Standard N solution (53,638, MerControl KGaA, Darmstadt, Germany), standard P solution (1.25046, MerControl KGaA, Darmstadt, Germany), standard K solution (53,337, MerControl KGaA, Darmstadt, Germany) were used for standards.
2.8 RNA Extraction and Gene Expression Analysis
MiniBEST Plant RNA Extraction Kit (TaKaRa, Kusatsu, Shiga, Japan) was used to RNA extraction. First-strand cDNA was synthesized using the HiScript II qRT SuperMix with a gDNA wiper (Vazyme, Nanjing, China). RT-qPCR was performed with ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) on the BioRad CFX Connect Real-Time PCR Detection System (Bio Rad, Hercules, CA, USA). Herbaceous peony Actin (PlActin, JN105299) with stable expression levels was used as an internal control. The specific primers utilized are listed in Table 1.
Data are presented as mean ± standard deviation (SD), except they are presented as mean ± standard error (SE) in the gene expression analysis, shown by error bars. Statistical analyses were performed using GraphPad Prism 9.0 by Student’s t-test or one-way ANOVA followed by Dunn’s test at p < 0.05.
3.1 Effects of Compound Fertilizer Treatment on Leaf Color under High Temperature in Summer
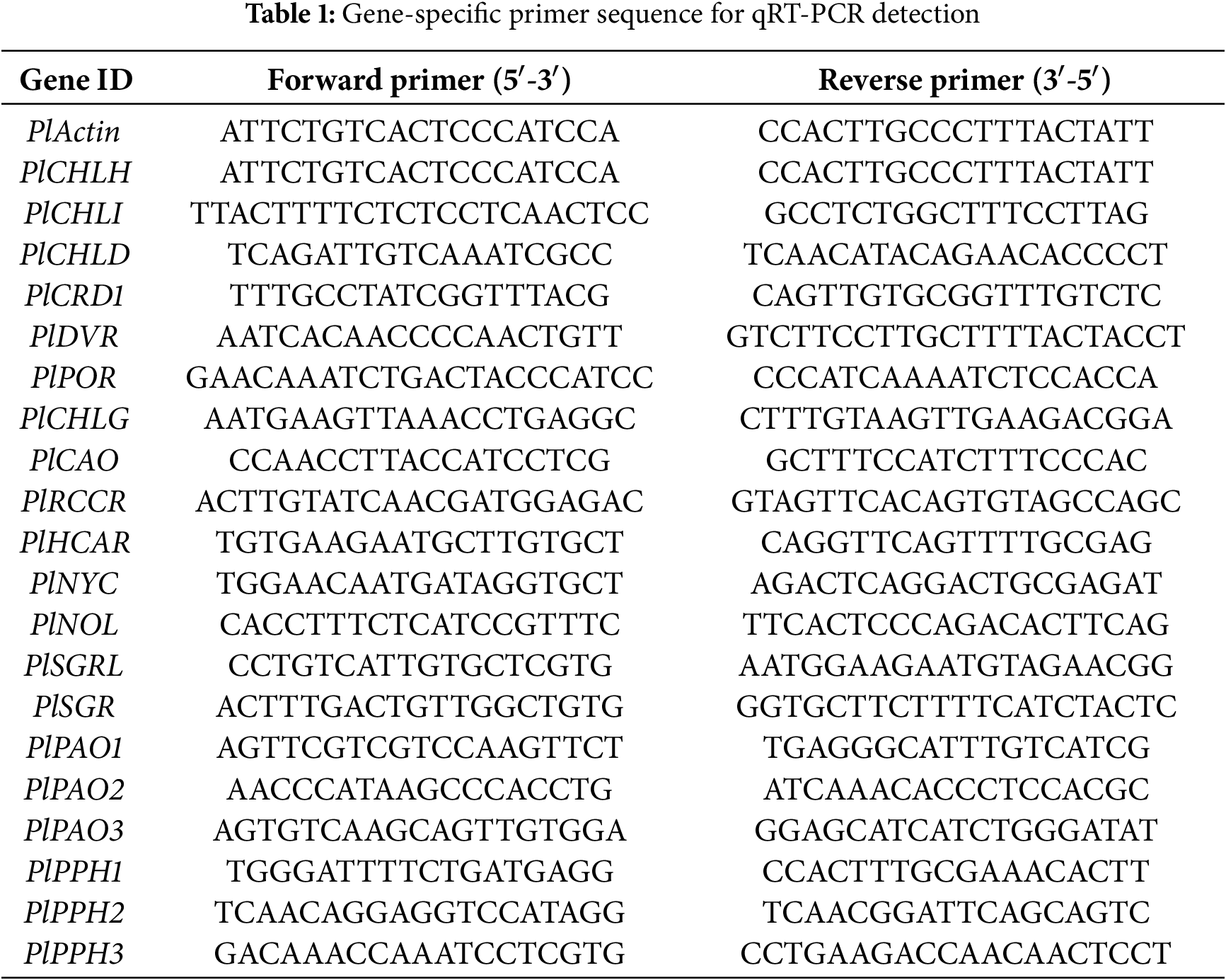
The temperature conditions from 5 June to 5 August 2024 were analyzed and found an overall increasing trend (Fig. 1A). No significant phenotypic differences were observed between 5 June and 25 June among Control, Groups T1, and T2 (Fig. 1B). There was no significant difference between b*, h°, and L* values. By 15 July, there were no significant differences between Control and Group T1, but Group T2 seemed greener. The b* values of Group T2 were significantly lower than those of Control and Group T1, while the h° and L* values of Control were lower than those of T1 and T2. However, with the increase in temperature, the leaf color of Groups T1 and T2 was significantly greener than that of the Control, while the Control gradually turned yellow and grew worse (Fig. 1C).
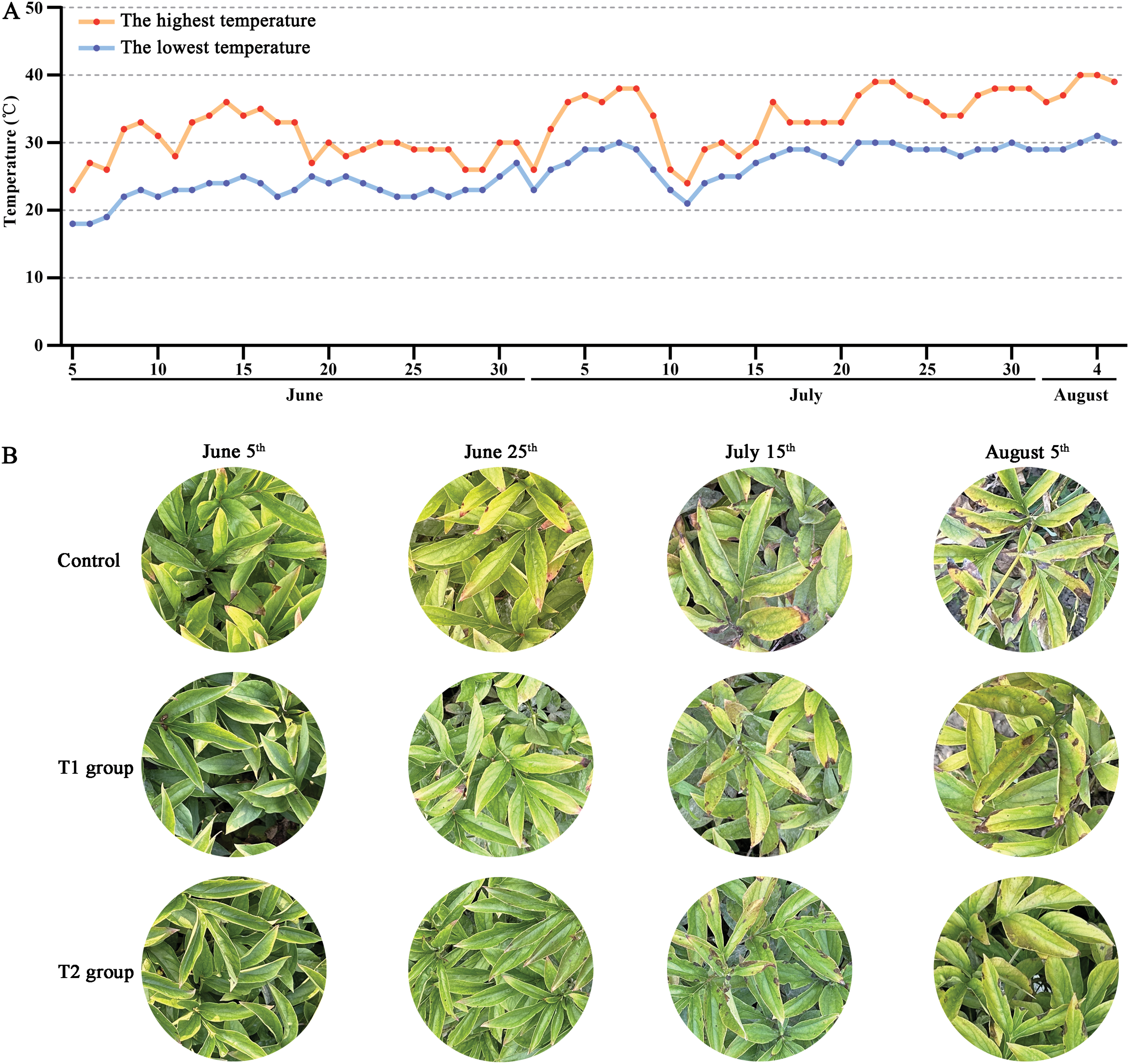
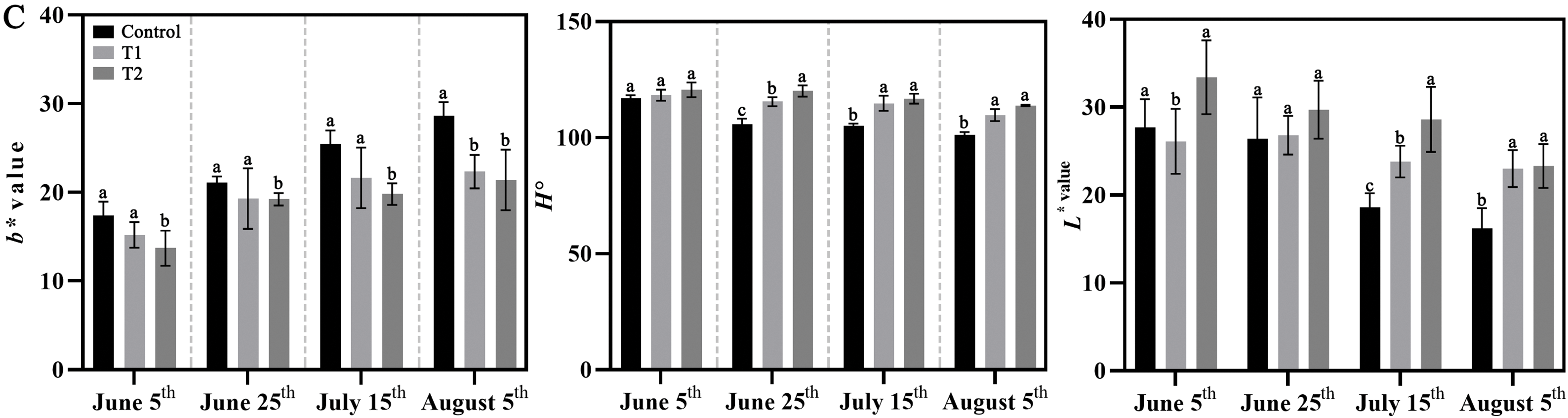
Figure 1: Effects of compound fertilizer on leaf color of herbaceous peony under high temperature in summer. (A) Daily temperature difference between day and night from 5 June to 5 August. (B) The phenotype of herbaceous peony treated with compound fertilizer. (C) The b*, h° and L* values of herbaceous peony treated with compound fertilizer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
SPAD value and chlorophyll contents also characterized the changes in leaf color of herbaceous peony caused by compound fertilizer treatment under high temperature in summer (Fig. 2). With the summer temperature increase, the leaves of three groups gradually lost green, but Groups T1 and T2 generally presented a better state with greener leaves. The results showed that the contents of chlorophyll a, chlorophyll b, and chlorophyll (a + b) in Groups T1 and T2 were higher than those in the Control at different stages, and the content of chlorophyll b in Group T2 was more sensitive to high temperature. By 5 August, the content of chlorophyll b in Group T2 was about 30% higher than that in Group and about 3 times the Control.
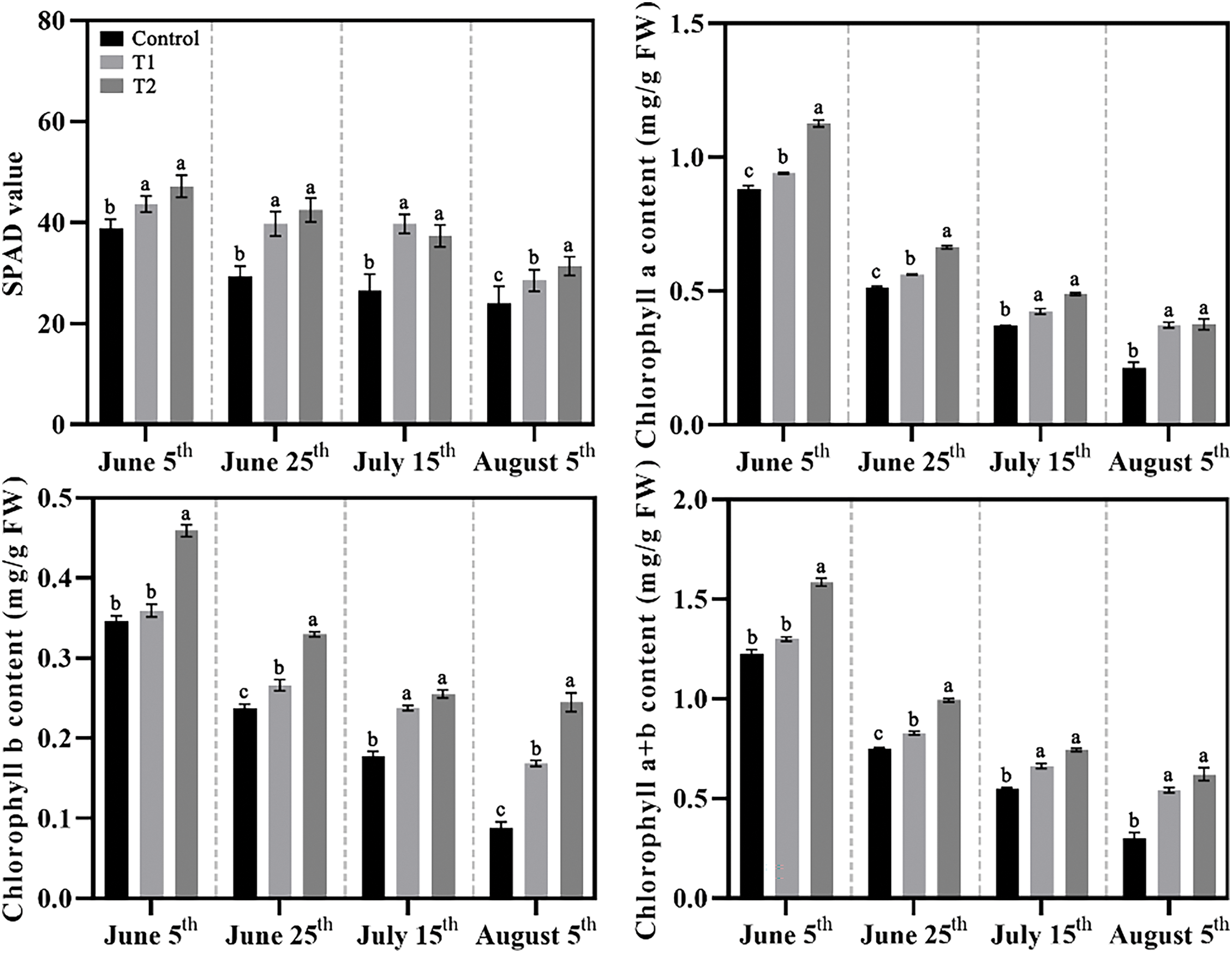
Figure 2: Effects of compound fertilizer on the chlorophyll of herbaceous peony under high temperature in summer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
3.2 Effects of Compound Fertilizer on Membrane Lipid Peroxidation under High Temperature in Summer
Overall, the REC and MDA content in the Control, Groups T1, and T2 increased with rising temperatures, with the Control showing tremendous variation. By 25 June, no significant changes in REC were observed across the three groups. However, by 15 July, the REC in Control was approximately 10% higher than that of Groups T1 and T2; by 5 August, it was approximately 20% higher. Meanwhile, REC changes in T2 remained insignificant (Fig. 3A). The MDA content showed a more sensitive response to high temperature in summer, with significant increases by 25 June in Control, which remained significantly higher than the treated groups. By 5 August, the MDA content in Groups T1 and T2 was about 60% and 50%, respectively, of that in Control (Fig. 3B).
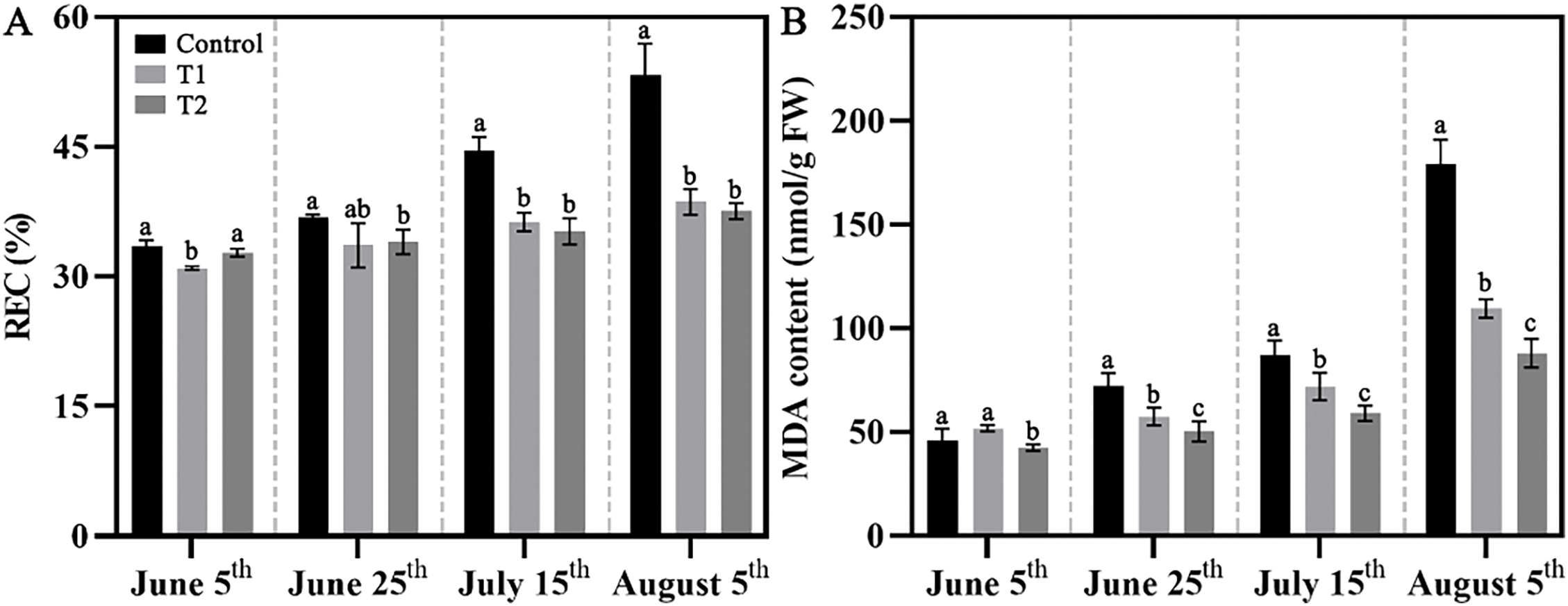
Figure 3: Effects of compound fertilizer treatment on the membrane lipid peroxidation level of herbaceous peony under high temperature in summer. (A) The REC of herbaceous peony treated with compound fertilizer. (B) The MDA content of herbaceous peony treated with compound fertilizer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
3.3 Effects of Compound Fertilizer Treatment on ROS Accumulation under High Temperature in Summer
Firstly, the accumulation of H2O2 in herbaceous peony was observed by DAB staining (Fig. 4A). Before 15 June, there was no significant difference in leaf staining results among the three groups, but on 5 August, it was obvious that the staining of the Control was more obvious, the leaves were darker, and the accumulation of H2O2 was significantly more. At the same time, we also quantitatively measured the H2O2 content (Fig. 4B), and the results were consistent with the visual observation; the H2O2 content of Control was higher, about twice that of Groups T1 and T2.
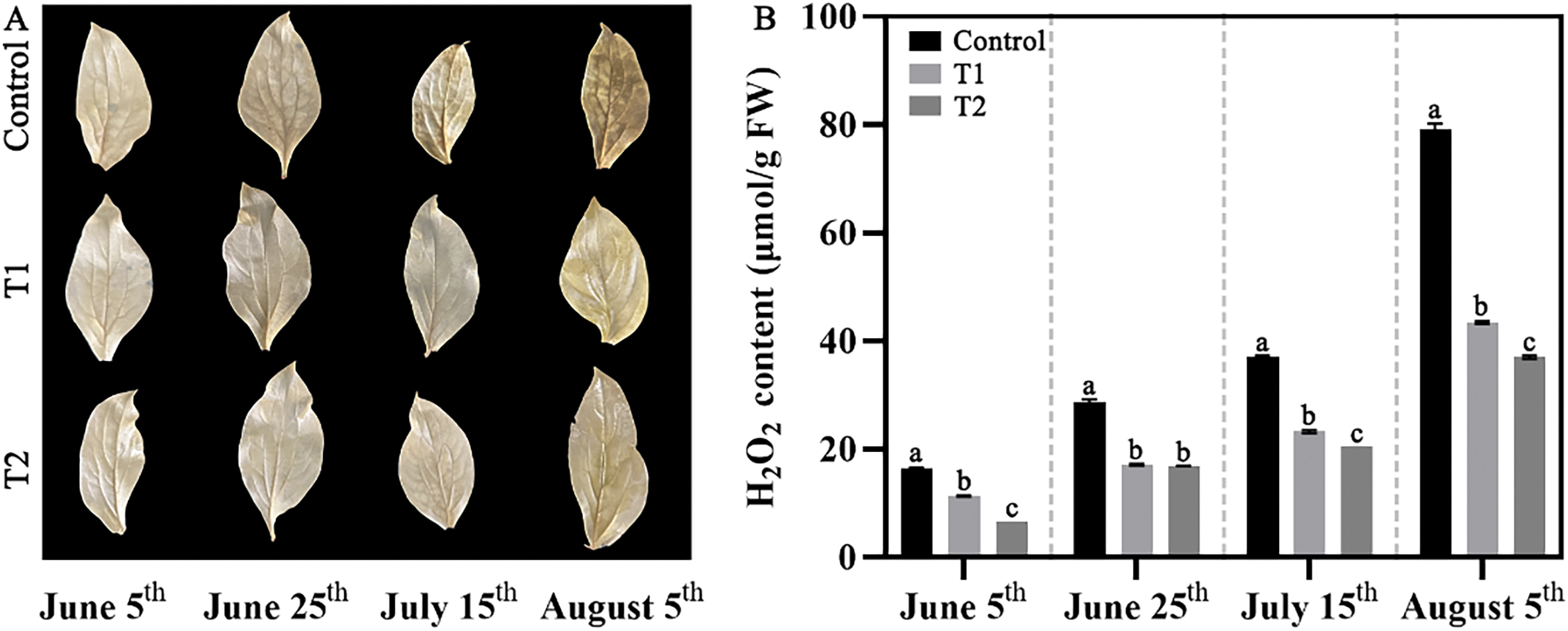
Figure 4: Effects of compound fertilizer treatment on H2O2 accumulation of herbaceous peony under high temperature in summer. (A) The DAB staining of herbaceous peony treated with compound fertilizer. (B) The H2O2 content of herbaceous peony treated with compound fertilizer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
After that, NBT staining was performed to observe the accumulation of O2.− (Fig. 5A). The blue dots represent the accumulation of O2.−. With the increase of summer temperature, the accumulation of O2.− in the three groups gradually increased, and the accumulation of O2.− in Groups T1 and T2 were lower than that of Control at all stages. The quantitative analysis of O2.− accumulation level was also conducted (Fig. 5B), the results showed that compound fertilizer treatment could significantly inhibit the accumulation of O2.− in herbaceous peony, and the O2.− content of Groups T1 and T2 was significantly lower than that of the Control, only about half of that of Control.
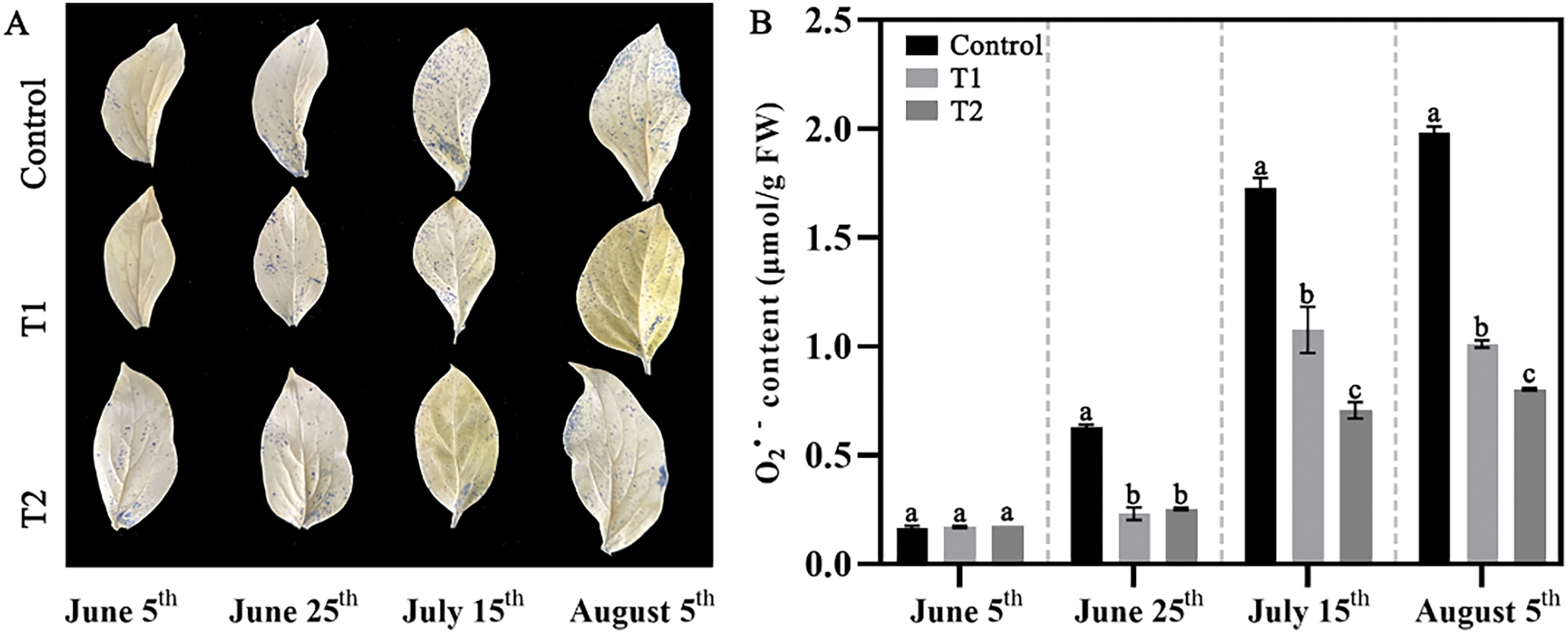
Figure 5: Effects of compound fertilizer treatment on O2.− accumulation of herbaceous peony under high temperature in summer. (A) The NBT staining of herbaceous peony treated with compound fertilizer. (B) The O2.− content of herbaceous peony treated with compound fertilizer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
3.4 Effects of Compound Fertilizer Treatment on Antioxidant Enzyme Activities under High Temperature in Summer
The content of antioxidant enzymes reflects the plant’s ability to resist high temperature in summer (Fig. 6). The SOD content in the three groups of herbaceous peonies exhibited a trend of first increasing and then decreasing, with the SOD content in Control being consistently lower than in Groups T1 and T2 at all stages. POD content did not show a significant response to high temperature, with the Control showing no changes before 15 July, followed by a substantial decrease. By 5 August, it was reduced to about half of the previous period. Group T1 showed a relatively high accumulation of POD until 5 June, after which it decreased by about one-third but remained nearly twice as high as Control. Group T2 showed a slow increase in POD accumulation before 25 June, followed by a significant decrease in the subsequent 20 days, and then increased again by 5 August, reaching about 140% of the Control level and 30% higher than Group T1. The CAT content in all groups increased first and decreased in the final stage, with the treatment groups maintaining higher levels than the Control. APX appeared to be the most sensitive antioxidant enzyme under high temperature in summer, with the APX content in all three groups increasing steadily, and the treated groups showing levels approximately twice that of Control by the final stage.
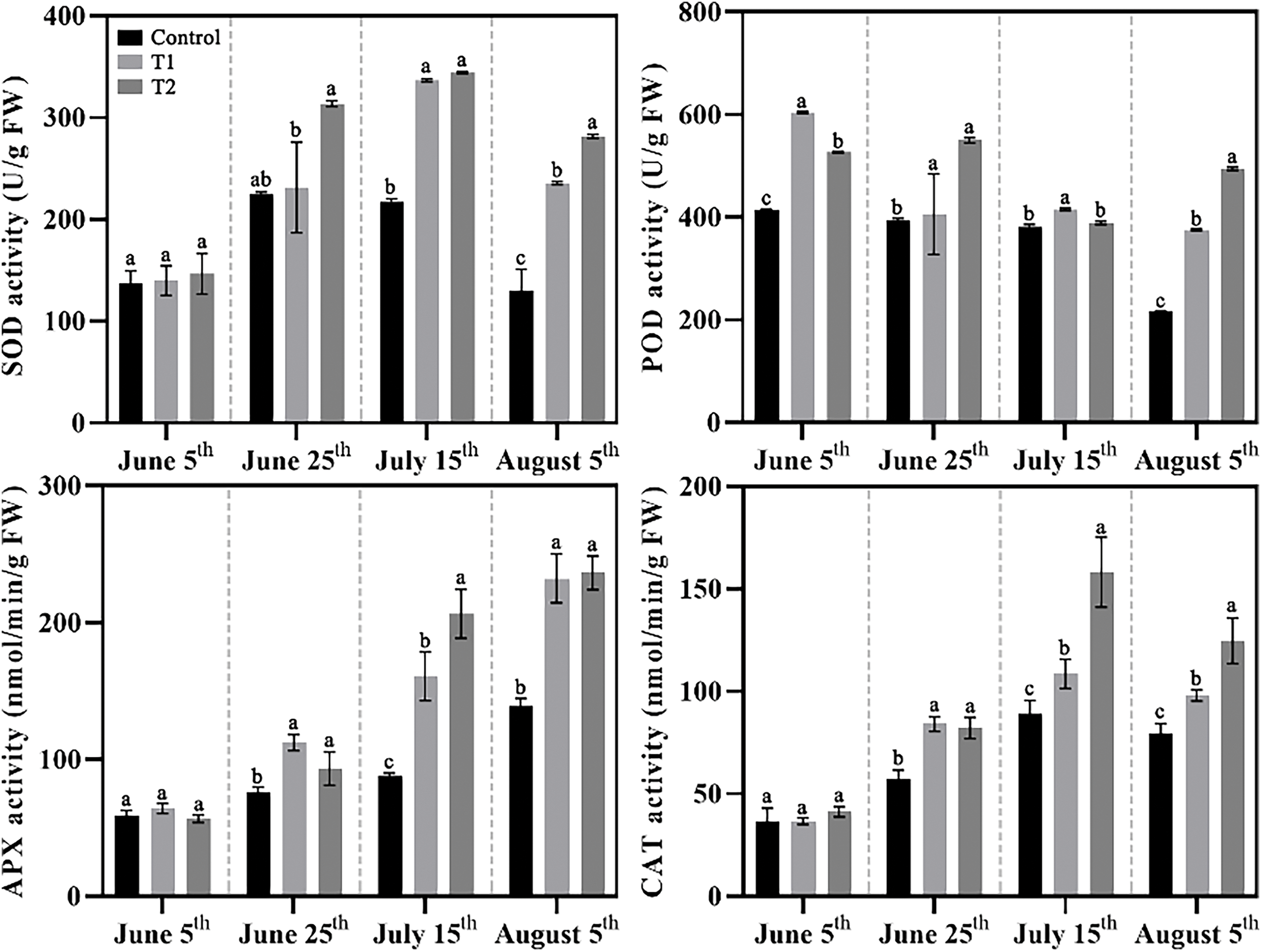
Figure 6: Effects of compound fertilizer treatment on the antioxidant enzyme activity of field-grown herbaceous peony under high temperature in summer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
3.5 Effects of Compound Fertilizer Treatment on Photosynthetic Characteristics under High Temperature in Summer
The Ci, Pn, and Gs in all three groups significantly decreased under high temperature in summer, but both Ci and Pn were still higher in Groups T1 and T2 compared to Control. The Tr in Control and T1 decreased with increasing temperature duration, while Tr reached a stable level after 40 days of high-temperature exposure in Group T2 (Fig. 7).
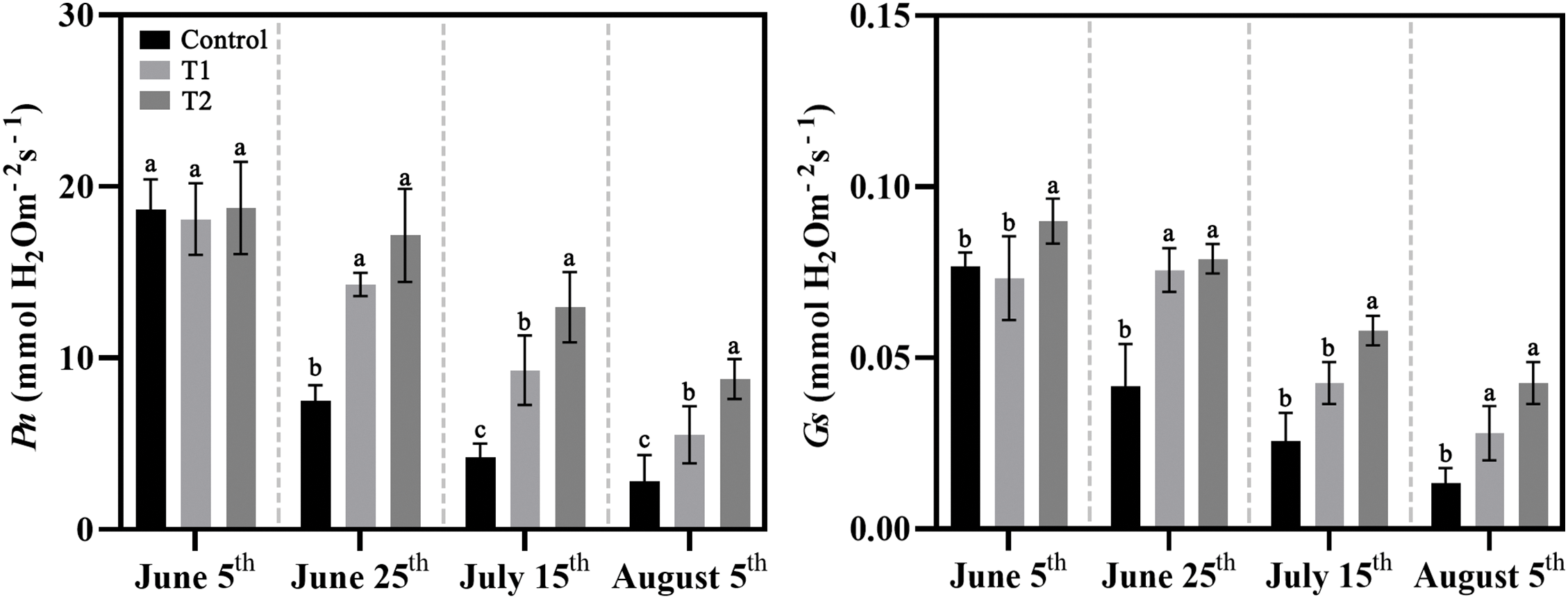
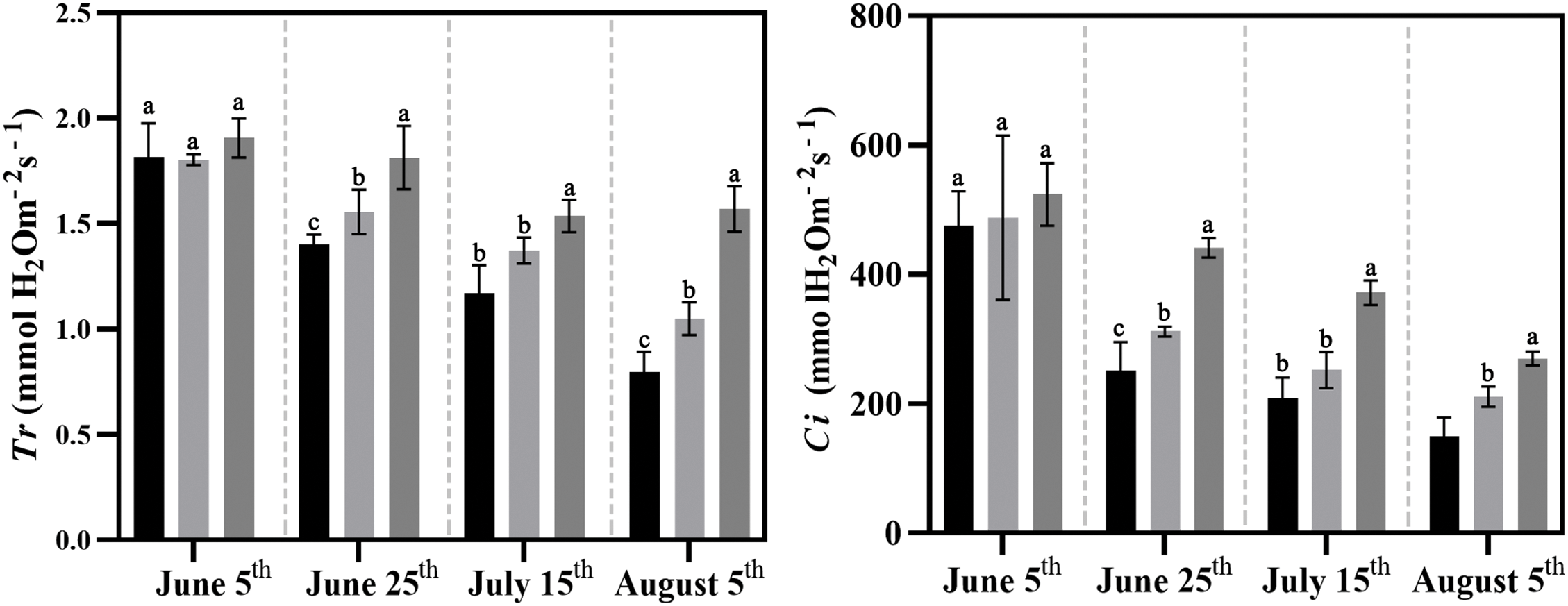
Figure 7: Effects of compound fertilizer treatment on photosynthetic characteristics of herbaceous peony under high temperature in summer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
3.6 Effects of Compound Fertilizer Treatment on Chlorophyll Fluorescence Parameters under High Temperature in Summer
Chlorophyll fluorescence parameters, which can reflect photosynthetic capacity. The result showed significant changes in Fv/Fm and Y(II) in Groups T1 and T2, which remained stable even under high temperature in summer, while Control showed significant reductions in contrast. Y(NO) and qN increased gradually in Groups T1 and T2, while Control significantly rose (Fig. 8). Overall, the photosynthetic and chlorophyll fluorescence parameters indicate that Groups T1 and T2 significantly improved the photosynthesis of herbaceous peony under high temperature in summer.
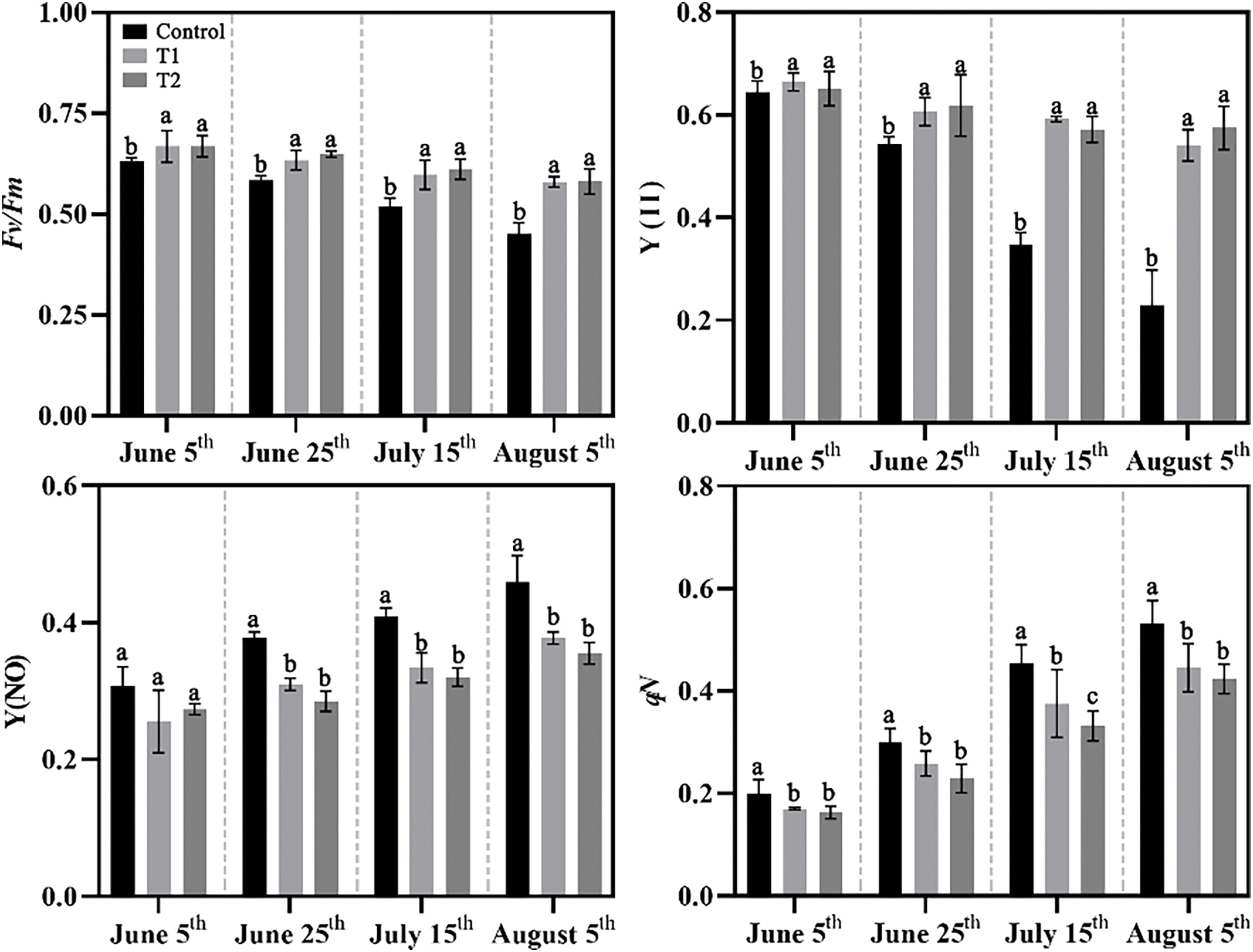
Figure 8: Effects of compound fertilizer treatment on chlorophyll fluorescence parameters of herbaceous peony under high temperature in summer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test
3.7 Effects of Compound Fertilizer Treatment on Nutrient Accumulation under High Temperature in Summer
The N, P, and K contents in the leaves of the three groups were quantitatively analyzed. The results showed that N content in Control declined, remaining nearly unchanged after 25 June. In T1, nitrogen content decreased initially and then increased, while T2 showed almost no change in N content throughout the measurement stage (Fig. 9A). P content in Control remained stable during the first three stages, but significantly increased in the final stage, with the phosphorus content on 5 August being about twice that of 15 July. In contrast, Groups T1 and T2 showed a more significant response to P content, with a trend of decrease followed by an increase during the growth period, recovering to the level of 5 June by 5 August (Fig. 9B). As the phenological stages progressed, the K content in Control gradually decreased, while it steadily increased in Group T1, reaching about twice that of Control by 5 August. The K content in Group T2 fluctuated and remained generally higher throughout (Fig. 9C).
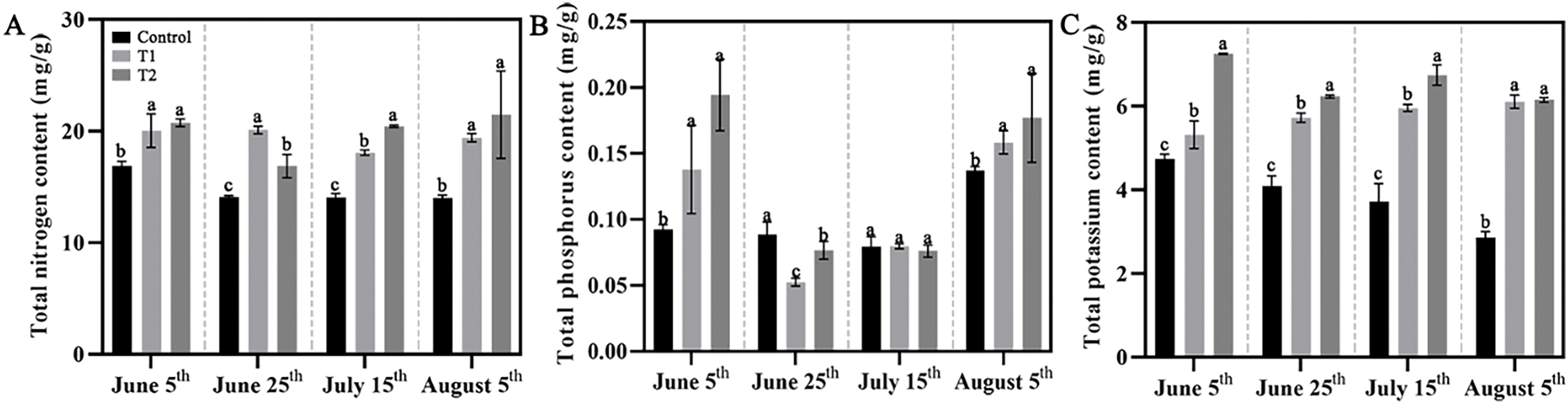
Figure 9: Effects of compound fertilizer treatment on nutrient accumulation of herbaceous peony under high temperature in summer. (A). The N content of herbaceous peony treated with compound fertilizer. (B). The P content of herbaceous peony treated with compound fertilizer. (C). The K content of herbaceous peony treated with compound fertilizer. Values represent mean ± standard deviation (SD), and different letters indicate significant differences according to Duncan’s multiple range test (p < 0.05)
3.8 Effects of Compound Fertilizer Treatment on the Expression of Genes Related to Chlorophyll Biosynthesis under High Temperature in Summer
The expression levels of eight chlorophyll biosynthesis-related genes were analyzed by using qRT-PCR, namely PlCAO, PlPOR, PlDVR, PlDRI1, PlCHLD, PlCHLI, PlCHLH, and PlCHLG (Fig. 10). The results showed that the expression levels of PlCAO, PlPOR, PlCHLD, and PlCHLG in all groups first increased and then decreased, with Groups T1 and T2 showing higher expression levels of these genes than Control. The expression of PlDVR, PlCHLH, and PlCRD1 also exhibited an increase followed by a decrease in Groups T1 and T2, whereas the expression of PlDVR and PlCHLH in Control increased again in the final phase. The expression of PlCRD1 remained stable after 20 days of high-temperature exposure in Control.
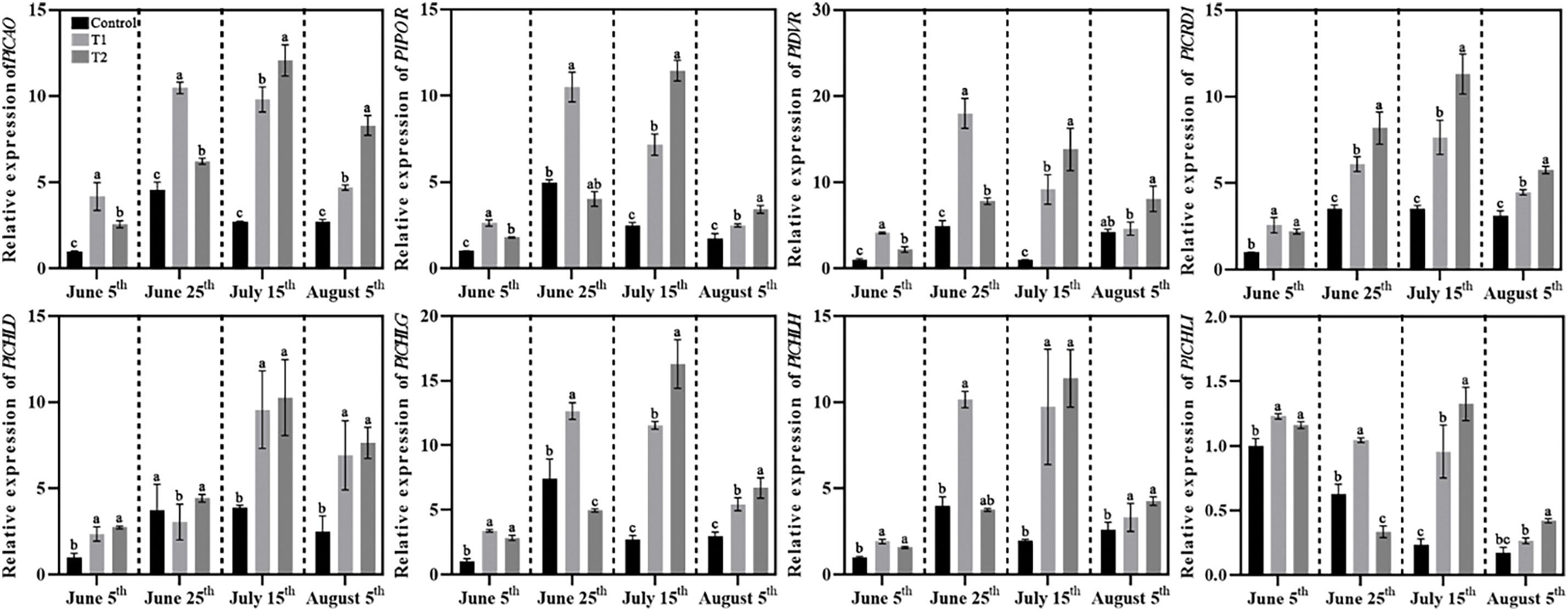
Figure 10: Effects of compound fertilizer treatment on expression of genes related to chlorophyll biosynthesis of herbaceous peony under high temperature in summer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test.
3.9 Effects of Compound Fertilizer Treatment on the Expression of Genes Related to Chlorophyll Degradation under High Temperature in Summer
Additionally, the expression of twelve genes involved in chlorophyll degradation was also analyzed (Fig. 11). The results indicated two main trends. The first group included the PlPAOs (PlPAO1, PlPAO2, PlPAO3) and PlPPHs (PlPPH1, PlPPH2, PlPPH3), where the expression of these genes in all groups followed a pattern of first increasing and then decreasing. The second group, which consisted of six other genes (PlSGR, PlSGRL, PlHCAR, PlNOL, PlNYC), showed significant increases in Control as high-temperature exposure continued, leading to leaf chlorosis, while in Groups T1 and T2, the expression levels of these genes increased and then decreased, suggesting that the compound fertilizer treatments helped suppress chlorophyll degradation even under high temperature in summer.
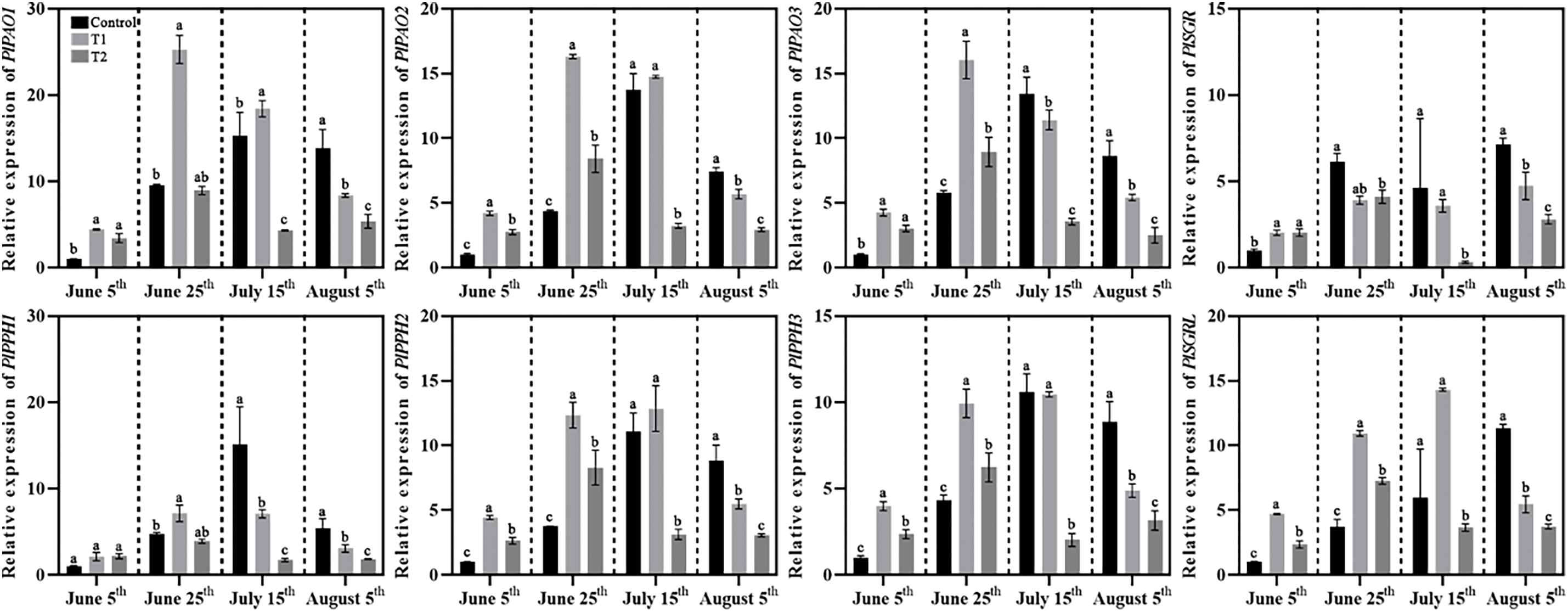
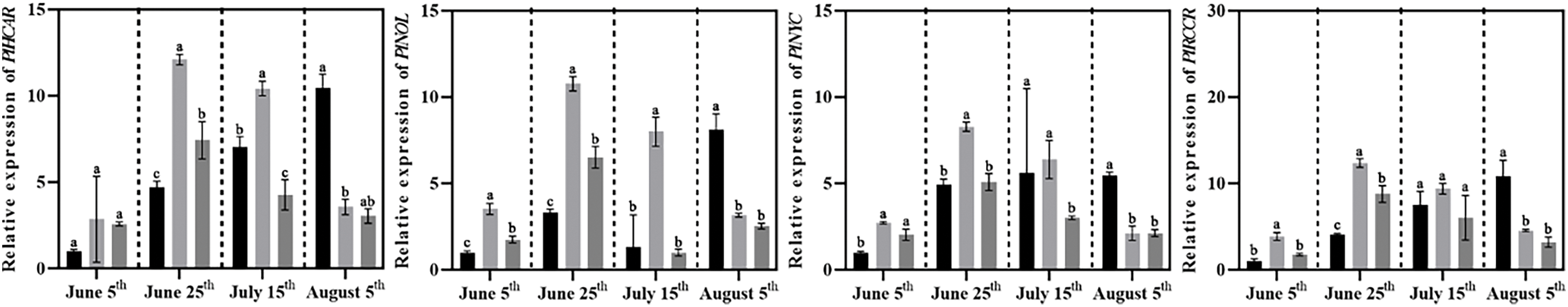
Figure 11: Effects of compound fertilizer treatment on expression of genes related to chlorophyll degradation of herbaceous peony under high temperature in summer. Different letters indicate a significant difference at p < 0.05 according to Duncan’s multiple range test.
Fertilization strategies are critical for optimizing plant nutrient status and stress resilience in large-scale cultivation systems. Soil microorganisms and enzymes facilitate nutrient transformation into plant-available forms [19]. Our previous studies demonstrated that adding biochar to the soil can promote nutrient accumulation in plants, effectively increasing the nitrogen, phosphorus, and potassium contents [5]. Similarly, Du et al. [20] confirmed that the exogenous application of nitrogen fertilizer promotes the accumulation of endogenous nitrogen in plants. In this study, we applied compound fertilizer with three primary elements—nitrogen, phosphorus, and potassium on the field-grown herbaceous peony. We found that compound fertilizer treatment enhanced nutrient accumulation in herbaceous peony plants by measuring the nutrient content in T1 and T2. It is well established that N, P, and K are crucial elements during plant growth. When nitrogen is sufficient, plants can synthesize more proteins, promoting cell division and growth, thereby increasing leaf area, which enhances photosynthesis [21]. Phosphorus is involved in photosynthesis, respiration, and other metabolic processes [22], while potassium is distributed in metabolically active organs and tissues, ensuring the smooth operation of various metabolic processes [23].
The synergistic roles of these macronutrients in abiotic stress tolerance have been widely documented. Guo et al. [24] studied the sorghum yield response and nutrient use efficiency to nitrogen and phosphorus in saline soils, finding that applying nitrogen and phosphorus in saline-alkali soils improved sorghum’s SPAD values and nutrient accumulation during maturation. Similarly, humic acid can be used as a fertilizer, stimulating rice root morphology, upregulated phosphorus transporter genes, and promoting phosphorus absorption and accumulation [25]. Zhang et al. [26] found that under salt stress, the dry weight, stomatal conductance, transpiration rate, photosynthetic rate, and K content in zoysiagrass were significantly lower than those without salt stress. However, adding potassium increased dry weight and significantly enhanced stomatal conductance, transpiration rate, and photosynthetic rate. Our results align with previous studies, showing that Groups T1 and T2 can reduce the physiological response of plants to high temperatures in summer, as evidenced by decreased REC and MDA levels and reduced accumulation of H2O2 and O2.−, and increased antioxidant enzyme activity. Notably, POD showed a relatively modest response, suggesting differential enzyme regulation under combined nutrient and heat stress.
Photosynthesis allows chlorophyll-containing plants to convert light energy into chemical energy, synthesizing organic compounds from carbon dioxide and water while releasing O2 as a byproduct [27]. In this study, compound fertilizer application significantly improved photosynthetic performance in heat-stressed herbaceous peony, with Pn in T1/T2 treatments 5–10 times higher than controls. Besides, chlorophyll content is often used to measure the efficiency of photosynthesis, and the control plants exposed to prolonged heat stress exhibited a significant decline in chlorophyll content, whereas T1 and T2 treatments maintained higher chlorophyll levels with attenuated degradation rates. This result is consistent with our previous research [28].
Abiotic stress significantly modulates the expression of genes and enzymes involved in chlorophyll biosynthesis, directly influencing plant growth, development, and crop productivity [27]. Under summer heat stress, chlorophyll biosynthesis-related genes (PlCAO, PlDVR, PlPOR, PlCRD1) showed a marked downregulation in control plants. In contrast, treatment groups exhibited an attenuated decline in expression levels. This observation aligns with findings in Sichuan pepper, where drought stress upregulated PlCRD1 [29], and in salt-stressed rice, where OsCAO played a critical role in maintaining chlorophyll content [30].
Chlorophyll degradation is equally essential to chlorophyll biosynthesis. Initially, chlorophyll degradation was considered the final response to senescence. However, chlorophyll degradation is crucial in promoting senescence, breaking down chlorophyll-protein complexes, forming photosystem II, and maintaining seed quality. Controlling chlorophyll degradation also holds significant agricultural value [31]. Sakuraba et al. [32] found that SGRL could form complexes with three CCEs on LHCII, accelerating the metabolic pathways of chlorophyll degradation intermediates to prevent accelerated leaf yellowing and senescence after chlorophyll degradation. In our study, we conducted qRT-PCR analysis of twelve genes involved in the chlorophyll degradation pathway. The expression levels of PlSGR, PlSGRL, PlNYC, PlNOL, and PlHCAR genes in Control increased as the high temperature continued in summer. In contrast, the expression levels of these genes in the T1 and T2 significantly decreased between 15 July and 5 August, indicating that chlorophyll degradation was suppressed. Notably, the expression levels of PlPAOs and PlPPHs, exhibited an initial increase followed by a decrease across all treatments, suggesting potential involvement in alternative chlorophyll catabolic pathways.
This study investigated the efficacy of two compound fertilizer concentrations in mitigating summer thermal stress in field-grown herbaceous peony (Paeonia lactiflora Pall.). Results revealed that elevated temperatures disrupted multiple physiological processes, including osmotic regulation, ROS homeostasis, antioxidant enzyme activities, photosynthetic efficiency, and nutrient assimilation, while altering chlorophyll metabolism-related genes’ expression patterns Notably, compound fertilizer application counteracted heat-induced damage through three interconnected mechanisms: (1) preservation of chlorophyll content via transcriptional reprogramming—upregulating chlorophyll biosynthesis genes (e.g., PlPOR, PlCHLH) while suppressing degradation-associated regulators (e.g., PlPAO, PlSGR); (2) enhancement of ROS scavenging capacity through coordinated activation of SOD, POD, CAT and APX, accompanied by reduced malondialdehyde accumulation and membrane permeability; (3) improved photosynthetic performance and nutrient acquisition, particularly nitrogen, phosphorus, and potassium compared to non-fertilized controls. Compound fertiliser treatments positively affect flower quality, number of adult flowers, and flowering time during the following year’s paeoniae blooming period. These findings establish an integrated fertilizer-mediated thermotolerance framework combining physiological adaptation and molecular regulation, providing actionable strategies for the sustainable cultivation of herbaceous peony in East China.
Acknowledgement: Not applicable.
Funding Statement: This work was supported by the Jiangsu Province Seed Industry Revitalization Unveiled Project (JBGS(2021)020), Forestry Science, Technology Innovation and Promotion Project of Jiangsu Province (LYKJ [2021]01), National Forest and Grass Science and Technology Innovation and Development Research Project (2023132012).
Author Contributions: Daqiu Zhao conceived the experiments; Zhaoyu Hou and Zhipeng Sheng conducted the experiments; Zhipeng Sheng and Zhaoyu Hou collected and analyzed the results; Zhipeng Sheng and Zhaoyu Hou wrote the original draft; Jun Tao and Daqiu Zhao reviewed and revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Shen M, Wang Q, Yu X, Teixeira Da Silva JA. Micropropagation of herbaceous peony (Paeonia lactiflora Pall.). Sci Hortic. 2012;148:30–8. doi:10.1016/j.scienta.2012.09.017. [Google Scholar] [CrossRef]
2. Liao M, Ma Z, Kang Y, Zhang B, Gao X, Yu F, et al. Enhanced disease susceptibility 1 promotes hydrogen peroxide scavenging to enhance rice thermotolerance. Plant Physiol. 2023;192(4):3106–19. doi:10.1093/plphys/kiad257. [Google Scholar] [PubMed] [CrossRef]
3. Zhang TT, Tang YH, Luan YT, Cheng ZY, Wang XX, Tao J, et al. Herbaceous peony AP2/ERF transcription factor binds the promoter of the tryptophan decarboxylase gene to enhance high-temperature stress tolerance. Plant Cell Environ. 2022;45(9):2729–43. doi:10.1111/pce.14357. [Google Scholar] [PubMed] [CrossRef]
4. Wen J, Tang Y, Li J, He T, Xiao J, Nangia V, et al. Effects of exogenous brassinosteroids on the starch structure, physicochemical properties, and digestibility of wheat under high-temperature stress at the rarly grain-filling stage. Int J Biol Macromol. 2024;283(3):137690. doi:10.1016/j.ijbiomac.2024.137690. [Google Scholar] [PubMed] [CrossRef]
5. Sheng ZP, Qian Y, Meng JS, Tao J, Zhao DQ. Rice hull biochar improved the growth of tree peony (Paeonia suffruticosa Andr.) by altering plant physiology and rhizosphere microbial communities. Sci Hortic. 2023;322:112204. doi:10.1016/j.scienta.2023.112204. [Google Scholar] [CrossRef]
6. Wang ZT, Guo J, Luo WX, Niu SD, Qu LL, Li J, et al. Salicylic acid cooperates with lignin and sucrose signals to alleviate waxy maize leaf senescence under heat stress. Plant Cell Environ. 2025;48(6):4341–55. doi:10.1111/pce.15437. [Google Scholar] [PubMed] [CrossRef]
7. Meng JS, Li M, Hao ZJ, Zhao DQ, Tao J. Paclobutrazol can enhance the thermal-tolerance on herbaceous peony (Paeonia lactiflora). Russ J Plant Physiol. 2022;69(3):57. doi:10.1134/S1021443722030104. [Google Scholar] [CrossRef]
8. Zhao DQ, Li TT, Hao ZJ, Cheng ML, Tao J. Exogenous trehalose confers high-temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperones. 2019;24(1):247–57. doi:10.1007/s12192-018-00961-1. [Google Scholar] [PubMed] [CrossRef]
9. Xie A, Lv M, Zhang D, Shi Y, Yang L, Yang X, et al. Effects of slight shading in summer on the leaf senescence and endogenous hormone and polyamine contents in herbaceous peony. Sci Rep. 2023;13(1):18714. doi:10.1038/s41598-023-46192-y. [Google Scholar] [PubMed] [CrossRef]
10. Li N, Yang Y, Wu Y, Liu B, Tao L, Zhan Y, et al. Better performance of compound fertilizers than bulk-blend fertilizers on reducing ammonia emission and improving wheat productivity. Agric Ecosyst Environ. 2022;335:108018. doi:10.1016/j.agee.2022.108018. [Google Scholar] [CrossRef]
11. Piskin A. Effect of zinc applied together with compound fertilizer on yield and quality of sugar beet (Beta vulgaris L.). J Plant Nutr. 2017;40(18):2521–31. doi:10.1080/01904167.2017.1380815. [Google Scholar] [CrossRef]
12. Huang S, Chen H, Wei F, Quan C, Xu M, Chen Z, et al. Comparative analysis of organic and compound fertilizers on the yield and metabolites of Platostoma palustre. Phyton-Int J Exp Bot. 2024;93:2645–62. doi:10.32604/phyton.2024.053492. [Google Scholar] [CrossRef]
13. Fahad S, Hussain S, Saud S, Tanveer M, Bajwa AA, Hassan S, et al. A biochar application protects rice pollen from high-temperature stress. Plant Physiol Biochem. 2015;96:281–7. doi:10.1016/j.plaphy.2015.08.009. [Google Scholar] [PubMed] [CrossRef]
14. Regmi A, Poudyal S, Singh S, Coldren C, Moustaid-Moussa N, Simpson C. Biochar influences phytochemical concentrations of Viola cornuta flowers. Sustainability. 2023;15(5):3882. doi:10.3390/su15053882. [Google Scholar] [CrossRef]
15. AL-Zurfi MTH, Mashkoor SA, AL-Soltani SAT, Al-Janabi ASA. Response of Freesia hubrida plant to spraying with organic fertilizer extract and vitamin E in grotw and floweringtraits. Int J Agric Stat Sci. 2023;19(1):167–72. doi:10.59467/IJASS.2023.19.167. [Google Scholar] [CrossRef]
16. Zhou BC, Liao YQ, Zheng XJ, Wang ZL, Li Q, Chen M. The effects of amendments on Cd and Pb under different fertilizer application conditions. Sci Rep. 2025;15(1):5385. doi:10.1038/s41598-025-90063-7. [Google Scholar] [PubMed] [CrossRef]
17. Zu MT, Yuan YD, Zuo JJ, Sun LP, Tao J. Microbiota associated with the rhizosphere of Paeonia lactiflora Pall. (ornamental cultivar). Apply Soil Ecol. 2022;169:104214. doi:10.1016/j.apsoil.2021.104214. [Google Scholar] [CrossRef]
18. Guo N, Fan LY, Cao Y, Ling H, Xu GH, Zhou J, et al. Comparison of two willow genotypes reveals potential roles of iron-regulated transporter 9 and heavy-metal ATPase 1 in cadmium accumulation and resistance in Salix suchowensis. Ecotoxicol Environ Saf. 2022;244:114065. doi:10.1016/j.ecoenv.2022.114065. [Google Scholar] [PubMed] [CrossRef]
19. Xia L, Lam SK, Chen D, Wang J, Tang Q, Yan X. Can Knowledge-based nitrogen management produce more staple grain with lower greenhouse gas emission and reactive nitrogen pollution? A meta-analysis. Glob Change Biol. 2017;23(5):1917–25. doi:10.1111/gcb.13455. [Google Scholar] [PubMed] [CrossRef]
20. Du L, Zhong H, Guo X, Li H, Xia J, Chen Q. Nitrogen fertilization and soil nitrogen cycling: unraveling the links among multiple environmental factors, functional genes, and transformation rates. Sci Total Environ. 2024;951:175561. doi:10.1016/j.scitotenv.2024.175561. [Google Scholar] [PubMed] [CrossRef]
21. Mu X, Chen Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol Biochem. 2021;158:76–82. doi:10.1016/j.plaphy.2020.11.019. [Google Scholar] [PubMed] [CrossRef]
22. Liu D. Root developmental responses to phosphorus nutrition. J Integr Plant Biol. 2021;63(6):1065–90. doi:10.1111/jipb.13090. [Google Scholar] [PubMed] [CrossRef]
23. Ullah A, Ali M, Shahzad K, Ahmad F, Iqbal S, Rahman MHU, et al. Impact of seed dressing and soil application of potassium humate on cotton plants productivity and fiber quality. Plants. 2020;9:1444. doi:10.3390/plants9111444. [Google Scholar] [PubMed] [CrossRef]
24. Guo X, Wu Q, Wang L, Zhou G, Zhu G, Suliman MSE, et al. Optimum nitrogen and phosphorus combination improved yield and nutrient use efficiency of sorghum in saline soil. Plants. 2025;14:102. doi:10.3390/plants14010102. [Google Scholar] [PubMed] [CrossRef]
25. Lv X, Li Q, Deng X, Ding S, Sun R, Chen S, et al. Fulvic acid application increases rice seedlings performance under low phosphorus stress. BMC Plant Biol. 2024;24:703. doi:10.1186/s12870-024-05435-4. [Google Scholar] [PubMed] [CrossRef]
26. Zhang L, Jiang Q, Zong J, Guo H, Liu J, Chen J. Effects of supplemental potassium on the growth, photosynthetic characteristics, and ion content of Zoysia matrella under salt stress. Horticulturae. 2023;10:31. doi:10.3390/horticulturae10010031. [Google Scholar] [CrossRef]
27. Morales F, Ancín M, Fakhet D, González-Torralba J, Gámez AL, Seminario A, et al. Photosynthetic metabolism under stressful growth conditions as a basis for crop breeding and yield improvement. Plants. 2020;9:88. doi:10.3390/plants9010088. [Google Scholar] [PubMed] [CrossRef]
28. Sheng ZP, Meng JS, Tao J, Zhao DQ. Osmotic regulation, antioxidant enzyme activities, and photosynthetic characteristics of tree peony (Paeonia suffruticosa Andr.) in response to high-temperature stress. Phyton-Int J Exp Bot. 2023;92:3133–47. doi:10.32604/phyton.2023.028818. [Google Scholar] [CrossRef]
29. Hu H, He B, Ma L, Chen X, Han P, Luo Y, et al. Physiological and transcriptome analyses reveal the photosynthetic response to drought stress in drought-sensitive (Fengjiao) and drought-tolerant (Hanjiao) Zanthoxylum bungeanum cultivars. Front Plant Sci. 2022;13:968714. doi:10.3389/fpls.2022.968714. [Google Scholar] [PubMed] [CrossRef]
30. Chen WM, Jin N, Shi Y, Su YQ, Fei BJ, Li W, et al. Coordinate expression of light-harvesting chlorophyll a/b gene family of photosystem II and chlorophyll a oxygenase gene regulated by salt-induced phosphorylation in Dunaliella salina. Photosynthetica. 2010;48:355–60. doi:10.1007/s11099-010-0046-z. [Google Scholar] [CrossRef]
31. Tanaka A, Ito H. Chlorophyll degradation and its physiological function. Plant Cell Physiol. 2025;66:139–52. doi:10.1093/pcp/pcae093. [Google Scholar] [PubMed] [CrossRef]
32. Sakuraba Y, Kim D, Kim YS, Hörtensteiner S, Paek NC. Arabidopsis staygreen-like (SGRL) promotes abiotic stress-induced leaf yellowing during vegetative growth. FEBS Lett. 2014;588:3830–7. doi:10.1016/j.febslet.2014.09.018. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools