 Open Access
Open Access
VIEWPOINT
Future of the current anticoronaviral agents: A viewpoint on the validation for the next COVIDs and pandemics
Drug Discovery & Clinical Research Department, Dikernis General Hospital (DGH), Dikernis, 35744, Egypt
* Corresponding Author: AMGAD M. RABIE. Email: Array
BIOCELL 2023, 47(10), 2133-2139. https://doi.org/10.32604/biocell.2023.030057
Received 20 March 2023; Accepted 27 May 2023; Issue published 08 November 2023
Abstract
Despite the global decline in the severity of the coronavirus disease 2019 (COVID-19) cases, the disease still represents a major concern to the relevant scientific and medical communities. The primary concern of drug scientists, virologists, and other concerned specialists in this respect is to find ready-to-use suitable and potent anticoronaviral therapies that are broadly effective against the different species/strains of the coronaviruses in general, not only against the current and previous coronaviruses (e.g., the recently-appeared severe acute respiratory syndrome coronavirus 2 “SARS-CoV-2”), i.e., effective antiviral agents for treatment and/or prophylaxis of any coronaviral infections, including those of the coming ones from the next species and strains (if any). As an expert in this field, I tried, in this up-to-date perspective “viewpoint” article, to evaluate the suitability and applicability of using the currently-available anticoronaviral agents for the next coronavirus diseases (COVIDs) and coronaviral pandemics, highlighting the most important general guidelines that should be considered in the next pandemics from the therapeutic points of view.Graphic Abstract
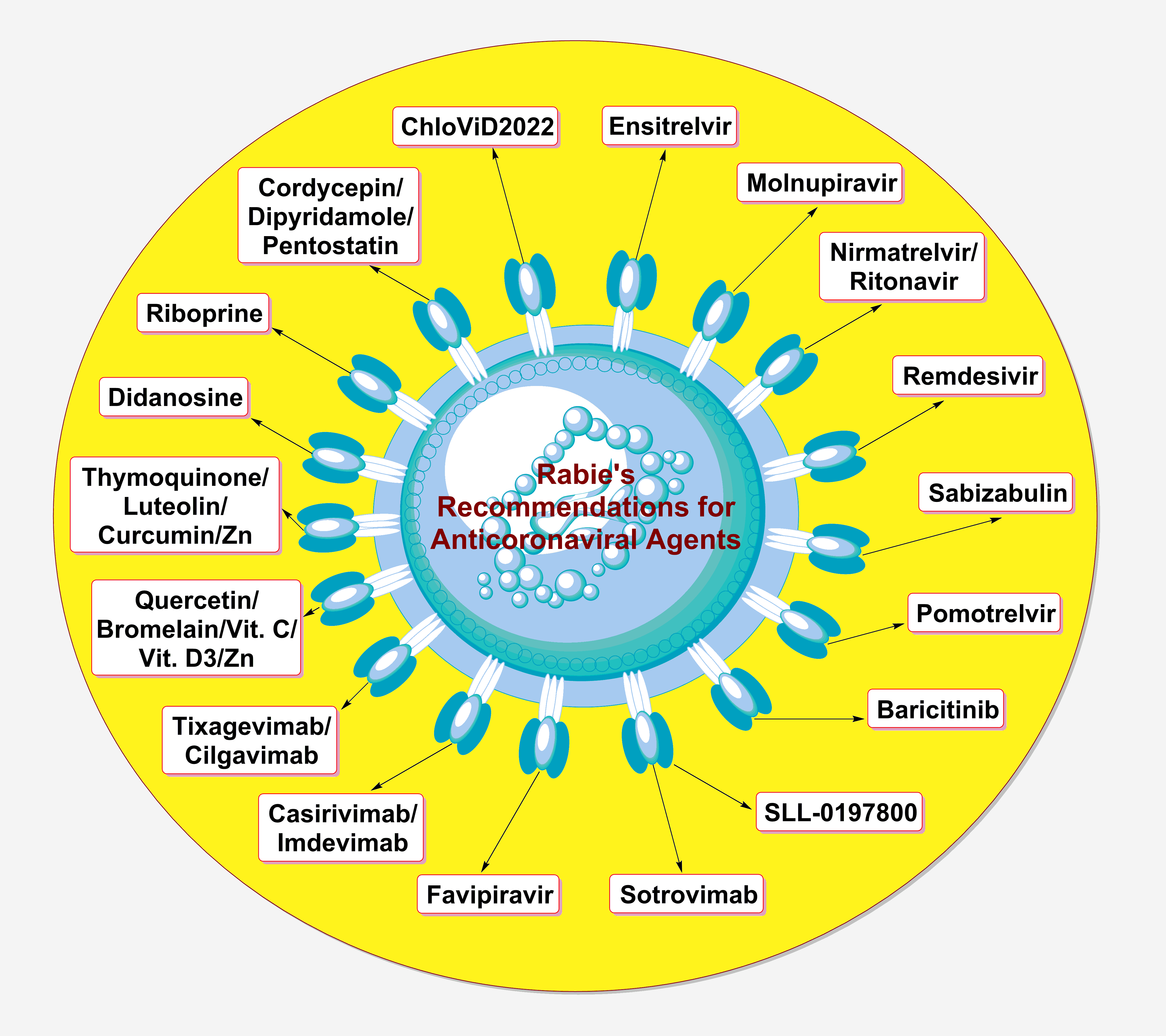
Keywords
It has been more than three years since the emergence of the fatal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and the coronavirus disease 2019 (COVID-19) outbreak. Despite the relative success of safe and effective vaccines for COVID-19, people still suffer severely from illness due to this viral infection and its ensuing unlimited complications. With the continual evolution of more resistant pathogenic SARS-CoV-2 variants of interest (VOI), the threat is far from over. Mystery still clouds the functional roles of a few protein components of the virus; hence, no definitive, clear, and 100%-effective therapy against all the strains of the virus. That is why scientists are still discussing the most effective pharmacological and clinical strategies that can be employed to develop new efficient potent therapies against COVID-19, along with discussing the latest updates about the known anti-COVID-19 agents from the clinical and toxicological points of view. Bringing a new life to shelved drugs (i.e., evaluation of the repurposing potentials and capacities of old drugs) against the new infectious disease “COVID-19” is considered one of the most important strategies tried for COVID-19 therapy since 2020. However, the traditional novel-drug design/discovery strategy still remains a prominent strategy for having therapies specifically targeting the virus, not the COVID-19 and its associated complications as a whole.
In general, there is a tetragonal therapeutic approach that must be efficiently applied and achieved for successful and comprehensive management and treatment of the COVID-19 disease (Rabie and Abdalla, 2022a): (I) potent antiviral/virucidal medications that significantly limit SARS-CoV-2 transmission, cell entry, replication, intercellular spread, and pathogenicity (this is the focal point of COVID-19 treatment); (II) effective immunoregulatory medications that immediately mitigate the acute nonproductive immune responses and thence considerably diminish end-organ damage; (III) direct-action antifibrotic medications that possess strong effects in COVID-19 patients having acute respiratory distress syndrome (ARDS) and accordingly withstand the long-term sequelae of this irritating disease; and (IV) rapid-action thrombolytic medications that effectively resist the induced blood clotting throughout the entire cardiovascular system. There is a clear shortage of medicines that act to meet principally the first requirement among the four requirements; thus, the current expert-opinion editorial article specifically covers this pivotal key point.
The scarcity of therapies targeting the previously-mentioned first requirement may be significantly attributed to the unsuccessful outcomes of many inappropriate clinical trials of COVID-19 (Rabie and Abdalla, 2022b; Battaglini et al., 2022; Demasi, 2022). Some of these trials, e.g., the DisCoVeRy clinical trial (Vanden Eynde, 2021), faced complete failures, while others, e.g., the PINETREE clinical trial (Gottlieb et al., 2022), faced partial failures. In my personal standpoint, the significant fails (key pitfalls) of many COVID-19 clinical trials could be principally imputed to (in a decreasing order of incidence frequency) (Rabie and Abdalla, 2022b; Battaglini et al., 2022; Demasi, 2022): (I) actual inefficacies of the investigated target compounds, especially those repurposed/repositioned ones, (II) inappropriate and inconvenient trial designs, (III) apparent time picking biases, considering various time trend scenarios, different COVID-19 stages/severities, as well as chronological bias in the treatment effect estimate, (IV) considerable population selection favoritisms induced by the picking of individuals/groups/data for analysis with improper selection randomization and unclear or ineffective exclusion criteria, (V) dose biases due to imprecise/unclear study design characteristics, unplanned random dosage changes during the course of the trial, and/or ineffective equivalence dosages, (VI) variable “inconstant” treatment durations, (VII) short follow-up periods (in most cases), (VIII) very low fragility indices, rendering the study outcomes less robust in terms of final statistical significance, (IX) absence of standard proven effective treatment, i.e., lack of a very potent/efficacious positive-control reference anti-COVID-19 drug, (X) insufficient personnel and low morale (specifically during the pandemics), (XI) increased rate of unethical acts within COVID-19 clinical trials, such as falsifying data, failing to gain informed consents, and/or failing to disclose adverse effects in a proper and timely manner, (XII) challenge and difficulty in unifying the immunological status and previous immunization status among all participants in most trials, (XIII) apparent various effects of geographical and environmental differences on the SARS-CoV-2 virus and COVID-19 patient status, and (XIV) rapid evolutionary nature of the SARS-CoV-2 virus (i.e., continuous rapid emergence of new SARS-CoV-2 variants/lineages), which might expose the previous, recent, and ongoing trials results to relative future invalidity since the new SARS-CoV-2 strains were not examined in these trials.
Major Targeted SARS-CoV-2 Proteins
The drug targets for SARS-CoV-2 were verified and confirmed employing a number of logic approaches based on the previous relevant species/strains of the virus (but with some modifications related mainly to the new genome of the current coronavirus, SARS-CoV-2). These approaches include, for instance, evaluating the highly potential chemical agents against the virus’s structural/binding proteins, nonstructural/functional proteins (almost all are enzymes), and/or virulence factors/pathogenic enzymes (Rabie and Abdalla, 2022a, 2022b). Although a considerable number of structural and nonstructural proteins form the entire SARS-CoV-2 particle, mainly three crucial coronaviral proteins among these that have been specifically targetable and druggable at high rates until now. The first is the spike (S) protein, which is the coronaviral-2 key required for binding to and opening any accessible host gate (cell) (Huang et al., 2020). The intracellular entry could be considered the first step in the identified life cycle of the SARS-CoV-2 virus. The S protein is a clove-shaped trimeric glycoprotein having two functionally distinct subunits symbolized as S1 (the receptor binding domain) and S2 (the membrane fusion domain) (Huang et al., 2020). It is worth mentioning that the S1 domain is substantially involved in binding with the host cells by effectively interacting with the host angiotensin-converting enzyme 2 (ACE2) (Huang et al., 2020). On the other hand, the S2 domain (which is anchored mainly via the S1 domain) significantly aids in the fusion of the viral contents, e.g., RNA genomes, in the host cells (Huang et al., 2020). Due to the crucial role of the S1 domain, it could be considered a priceless nonenzymatic therapeutic target in anticoronaviral drug design strategies for hindering virus entry into human cells.
The second protein is the main protease (Mpro) enzyme, the vital enzyme that SARS-CoV-2 relies on to induce the replication and, accordingly, pathogenesis of the coronaviral particles (Hu et al., 2022). It is the nonstructural protein 5 (nsp5; also called 3-chymotrypsin-like protease “3CLpro”), encoded by SARS-CoV-2 to cleave the entire coronaviral polyprotein at more than 11 fixed locations to release most of the functional proteins of the virus (mainly the nonstructural proteins from 4 to 16 “nsp4 to nsp16”) (Hu et al., 2022). It is important to know that this cysteine protease, Mpro, comprises three successive domains (subunits), domains I, II, and III (Hu et al., 2022). In addition, this enzyme has an active cysteine-histidine catalytic dyad at its known binding site, which is mainly responsible for cleaving certain peptide linkages (Hu et al., 2022). Blocking this active site could lead to the disruption of all biological processes responsible for the multiplication and pathogenesis of the virus. The active catalytic binding site of Mpro is specifically located in a particular cleft between domain I and domain II (Hu et al., 2022). The last protein is the RNA-dependent/directed RNA polymerase (RdRp). This coronaviral-2 RNA replicase is the final active or functional form of the nonstructural protein 12 (nsp12); it stimulates and activates the synthesis of a new RNA strand complementary to a certain coronaviral-2 genomic RNA template in any SARS-CoV-2 particle to initiate the replication process (Rabie, 2022a). This synthetic procedure requires some cofactors like the two nonstructural proteins 7 and 8 (nsp7 and nsp8) (Rabie and Abdalla, 2022a, 2022b; Rabie, 2022a). This highly essential enzyme “RdRp” could be considered one of the most druggable proteinous targets in SARS-CoV-2 particles (that could be used in nonspecific anticoronaviral drug design strategies to efficiently stop mass virus invasion and propagation) due to its high conservation nature, since it is nearly the most structurally-fixed protein in all coronaviruses and has an insignificant mutation tendency (Rabie and Abdalla, 2022a, 2022b). Therapeutic targeting plans for any of the three principal druggable proteins of SARS-CoV-2 usually begin with computational assessments (Rabie, 2021). Natural products/ingredients occupy a large part of these speculative preparatory studies (Rabie, 2022b; Chandra et al., 2023; Zrieq et al., 2021); however, not all of them reached the final pharmacological and clinical confirmations.
Available Approved and Investigational Anti-SARS-CoV-2/Anti-COVID-19 Therapies/Agents
Until now, only a few chemical agents have been confirmed to have certain acceptable or good degrees of effectiveness against the SARS-CoV-2 particles (belonging to different strains). Almost all of these agents act mainly on one of the aforementioned three major coronaviral-2 proteins. Very few of them have already been approved, few are on their way for official approval, and some are still under comprehensive investigation. They are either synthetic or natural, and also either new or repurposed. The most important compounds (including engineered antibodies) expected to act nonspecifically against all the newer SARS-CoV-2 strains and potential newer coronavirus species (in general) in the current and next pandemics, from my own viewpoint and actual experience as an expert in therapeutic coronavirology (an expert opinion), are summarized and presented in Table 1 (these expert recommendations include anticoronaviral drugs from all discovered generations; also, most of the suggested therapies are available orally).
Key Therapeutic Points (10 Important General Guidelines) to Be Considered in the Expected Next Coronavirus Diseases (COVIDs) and Coronaviral Pandemics
a) Any emerging coronavirus should be treated through the relevant healthcare management regimens and protocols as an emergent chemicobiological weapon, not a simple bacterium-like particle or microorganism.
b) Detecting, recognizing, and tracking the RNA genomic sequences and their modifications (mutations) should be among the first critical steps in facing any newly-emerged coronavirus species.
c) In the next potential COVIDs and coronaviral pandemics, people should be prepared for the coronaviral infection earlier, not later, by taking prophylactic agents (not only vaccines) that act mainly against the S proteins (or even against all the three major targets, the S/Mpro/RdRp proteins) in the form of easy-to-use aerosols, drops, gels, and creams.
d) After the occurrence of any confirmed coronaviral infections, the focus must primarily be on disrupting the master step in the virus life cycle, the replication phase, by slowing its rate to the maximum via using effective anti-RdRp and/or anti-Mpro agents.
e) For rapid and successful treatment of the infection, using combination therapy that acts on the three principal phases (or at least two of them) of the virus life cycle (including the damaging pathogenic phase) is much more recommended than using monotherapy (that acts only on just one phase).
f) All pathogenic sequelae of the coronaviral infection in the diverse tissues of the human body should be carefully tracked and accordingly managed and cured with specific relevant remedies, side by side with the primary anticoronaviral therapy administration in the first days/weeks of infection.
g) After the viral titer declines to approximately zero values, anticoronaviral therapy (especially drugs acting on Mpro/RdRp) should not be discontinued for at least one week to ten days to prevent relapse.
h) When establishing a fixed anticoronaviral treatment pro-tocol at any certain hospital, city, or location, the endemic and epidemic situations of the newly-emerged coronaviral strain, as well as the sensitivity and resistance degrees of this strain to the different available anticoronaviral drugs, should be accurately considered.
i) Expected adverse off-target effects of anticoronaviral drugs (if any) should be continuously monitored, managed, and also updated in the relevant literature.
j) Overall, therapeutic plans for the diverse coronaviral infections cannot work in isolation from other constituents of healthcare systems, since managing viral pandemics requires a comprehensive and coordinated approach, including early detection and diagnosis, available vaccination, effective broad-spectrum antiviral therapy, supportive care, health system strengthening, continuous research and development, and good preparedness planning. Accurate and early detection and diagnosis are among the critical factors in managing any outbreak. Rapid diagnostic tests and increased surveillance systems which can help to detect and isolate cases promptly, preventing further transmission, are very important in this context. Vaccination is one of the most effective ways to prevent infections and reduce severe disease and death. The development of safe and effective vaccines remains the initial tool to control viral pandemics. During such fatal pandemics, the efforts of governments, health organizations, and individuals towards increasing vaccine accessibility and coverage worldwide should be very proactive and consistent. Several antiviral therapies have been developed and authorized for the treatment of COVID-19. Providing detailed information about these anticoronaviral drugs and discussing those research efforts must continue to identify and develop new highly effective antivirals. Patients with severe or very severe COVID-19 require intensive care and support to manage complications such as respiratory failure, sepsis, and shock; therefore, relevant hospitals must be prepared with respect to several related parameters such as the requirement of adequate oxygen supply, mechanical ventilation centers, and other supportive measures like fluid management, nutrition, and pain management. All these can improve outcomes and reduce mortality. Most importantly, the COVID-19 pandemic has highlighted the importance of strengthening therapeutic and healthcare systems, particularly in resource-limited settings. Concerned officials and scientists should discuss this issue regularly and highlight the need for increasing the capacity of healthcare workers, enhancing laboratory diagnostic capabilities, improving infection control measures, and ensuring a robust supply chain of essential medical commodities. We also know that continued research and development are essential to developing effective therapeutics, vaccines, and diagnostics. As a priority, the policies of governments, academic institutions, and the private sector regarding continuing research, development, and innovation to develop effective therapeutics, vaccines, and diagnostics to combat future coronaviral pandemics should be discussed and updated on a regular basis. Last but not least, the preparedness planning and the plans at the national and international levels for similar future pandemics (such as with regard to providing essential medical supplies and personal protective equipment, establishing surge capacity in healthcare facilities, and developing communication strategies to inform the public and healthcare providers about the outbreak) should be discussed.
In general, for almost 100% effective management and treatment of any coronaviral infection, the three principal phases of infection (the entry, multiplication, and pathogenic phases) should be successfully targeted by a combination therapy or, at least, a very potent multitarget monotherapy. Targeting only any one or two phases of the three, though effective, could not provide more than 85% successful control of the infection at most, along with the possibility of prolonging the primary treatment interval from very few days (in the case of the triple-target therapy) to some weeks. Many natural compounds have shown very promising anticoronaviral activities, rendering them ideal drug candidates to be included in the therapeutic management protocols in the next similar coronaviral pandemics. Examples of these drugs are riboprine, thymoquinone, cordycepin, quercetin, luteolin, curcumin, bromelain, and vitamin D.
Ensitrelvir fumarate, on the other hand, could be considered the most promising clinically-effective synthetic anticoronaviral agent (up to date) that may be highly efficient against the imminent invasions of the newer coronavirus species (if any). The available anticoronaviral remedies are provided in different types of formulations, e.g., oral, intravenous, subcutaneous, and nasal-spray, allowing for broad and unrestricted use of these therapies in all ages/conditions and at all levels. Continuing to track the coronaviral particles deeply in the diverse human tissues and organs, along with monitoring all scientifically-unexplained effects of the virus at the molecular, cellular, and extracellular levels, is very important to reveal the remaining mysterious secrets of the coronavirus species (including the next species) and also to potentially provide novel therapeutic targets against the virus.
Acknowledgement: The author appreciates and is very thankful for the kind and sincere efforts and advice of the relevant respectable Editors and Reviewers of the BIOCELL journal for helping in reviewing and presenting this paper in the most accurate, descriptive, and informative form.
Funding Statement: The author received no specific funding for this study.
Author Contributions: The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of literature results, and manuscript preparation.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The author declares that he has no conflicts of interest to report regarding the present study.
References
Barnette KG, Gordon MS, Rodriguez D, Bird TG, Skolnick A et al. (2022). Oral sabizabulin for high-risk, hospitalized adults with COVID-19: Interim analysis. New England Journal of Medicine Evidence 1: 1–11. https://doi.org/10.1056/EVIDoa2200145 [Google Scholar] [CrossRef]
Battaglini D, Cruz F, Robba C, Pelosi P, Rocco PRM (2022). Failed clinical trials on COVID-19 acute respiratory distress syndrome in hospitalized patients: Common oversights and streamlining the development of clinically effective therapeutics. Expert Opinion on Investigational Drugs 31: 995–1015. https://doi.org/10.1080/13543784.2022.2120801 [Google Scholar] [PubMed] [CrossRef]
Chandra S, Palai S, Ferreira-Matias EF, Pita-Neto IC, Gomes-Ramalho CL, de Andrade EM, de Almeida RS, Iriti M, Melo-Coutinho HD (2023). Indian medicinal plants are effective in the treatment and management of COVID-19. BIOCELL 47: 677–695. https://doi.org/10.32604/biocell.2023.026081 [Google Scholar] [CrossRef]
Cheng MM, Reyes C, Satram S, Birch H, Gibbons DC et al. (2023). Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA. Infectious Diseases and Therapy 12: 607–621. https://doi.org/10.1007/s40121-022-00755-0 [Google Scholar] [PubMed] [CrossRef]
ClinicalTrials.gov (2023). The study of quadruple therapy zinc, quercetin, bromelain and vitamin C on the clinical outcomes of patients infected with COVID-19. ClinicalTrials.gov Identifier: NCT04468139. https://clinicaltrials.gov/ct2/show/NCT04468139 [Google Scholar]
Demasi M (2022). FDA oversight of clinical trials is “grossly inadequate,” say experts. BMJ 379: o2628. https://doi.org/10.1136/bmj.o2628 [Google Scholar] [PubMed] [CrossRef]
Eltayb WA, Abdalla M, Rabie AM (2023). Novel investigational anti-SARS-CoV-2 agent ensitrelvir “S-217622”: A very promising potential universal broad-spectrum antiviral at the therapeutic frontline of coronavirus species. ACS Omega 8: 5234–5246. https://doi.org/10.1021/acsomega.2c03881 [Google Scholar] [PubMed] [CrossRef]
Gonzales TL, Skarda P, Bird TG, Schnaus M, Steiner M et al. (2022). LB1530. Clinical benefit of oral sabizabulin for hospitalized adults with COVID-19 on supplemental oxygen. Open Forum Infectious Diseases 9: ofac492.1876. https://doi.org/10.1093/ofid/ofac492.1876 [Google Scholar] [CrossRef]
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ et al. (2022). Early remdesivir to prevent progression to severe COVID-19 in outpatients. New England Journal of Medicine 386: 305–315. https://doi.org/10.1056/NEJMoa2116846 [Google Scholar] [PubMed] [CrossRef]
Gupta A, Gonzalez-Rojas Y, Juarez E, Casal MC, Moya J et al. (2022). Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 327: 1236–1246. https://doi.org/10.1001/jama.2022.2832 [Google Scholar] [PubMed] [CrossRef]
Hu Q, Xiong Y, Zhu GH, Zhang YN, Zhang YW, Huang P, Ge GB (2022). The SARS-CoV-2 main protease (MproStructure, function, and emerging therapies for COVID-19. MedComm 3: e151. https://doi.org/10.1002/mco2.151 [Google Scholar] [PubMed] [CrossRef]
Huang Y, Yang C, Xu XF, Xu W, Liu SW (2020). Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica 41: 1141–1149. https://doi.org/10.1038/s41401-020-0485-4 [Google Scholar] [PubMed] [CrossRef]
Jalbert JJ, Hussein M, Mastey V, Sanchez RJ, Wang D et al. (2022). Effectiveness of subcutaneous casirivimab and imdevimab in ambulatory patients with COVID-19. Infectious Diseases and Therapy 11: 2125–2139. https://doi.org/10.1007/s40121-022-00691-z [Google Scholar] [PubMed] [CrossRef]
Kawashima S, Matsui Y, Adachi T, Morikawa Y, Inoue K, Takebayashi S, Nobori H, Rokushima M, Tachibana Y, Kato T (2023). Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally. Biochemical and Biophysical Research Communications 645: 132–136. https://doi.org/10.1016/j.bbrc.2023.01.040 [Google Scholar] [PubMed] [CrossRef]
Kearney BP, Plummer A, Wolfgang G, Turnquist D, Marshall MR, Denison M, Schwabe C, Lopatin U, Arnold L (2022). PBI-0451 an orally administered 3CL protease inhibitor of SARS-CoV-2 for COVID-19. Topics in Antiviral Medicine 30: 179–180. https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1880428 [Google Scholar]
Khan A, Iqtadar S, Mumtaz SU, Heinrich M, Pascual-Figal DA, Livingstone S, Abaidullah S (2022). Oral co-supplementation of curcumin, quercetin, and vitamin D3 as an adjuvant therapy for mild to moderate symptoms of COVID-19—Results from a pilot open-label, randomized controlled trial. Frontiers in Pharmacology 13: 898062. https://doi.org/10.3389/fphar.2022.898062 [Google Scholar] [PubMed] [CrossRef]
Lee CC, Hsieh CC, Ko WC (2021). Molnupiravir—A novel oral anti-SARS-CoV-2 agent. Antibiotics 10: 1294. https://doi.org/10.3390/antibiotics10111294 [Google Scholar] [PubMed] [CrossRef]
Luttens A, Gullberg H, Abdurakhmanov E, Vo DD, Akaberi D et al. (2022). Ultralarge virtual screening identifies SARS-CoV-2 main protease inhibitors with broad-spectrum activity against coronaviruses. Journal of the American Chemical Society 144: 2905–2920. https://doi.org/10.1021/jacs.1c08402 [Google Scholar] [PubMed] [CrossRef]
Mahase E (2021). COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 375: n2713. https://doi.org/10.1136/bmj.n2713 [Google Scholar] [PubMed] [CrossRef]
Munafò F, Donati E, Brindani N, Ottonello G, Armirotti A, De Vivo M (2022). Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Scientific Reports 12: 10571. https://doi.org/10.1038/s41598-022-14664-2 [Google Scholar] [PubMed] [CrossRef]
Rabie AM (2021). Teriflunomide: A possible effective drug for the comprehensive treatment of COVID-19. Current Research in Pharmacology and Drug Discovery 2: 100055. https://doi.org/10.1016/j.crphar.2021.100055 [Google Scholar] [PubMed] [CrossRef]
Rabie AM (2022a). Potent inhibitory activities of the adenosine analogue cordycepin on SARS-CoV-2 replication. ACS Omega 7: 2960–2969. https://doi.org/10.1021/acsomega.1c05998 [Google Scholar] [PubMed] [CrossRef]
Rabie AM (2022b). New potential inhibitors of coronaviral main protease (CoV-MproStrychnine bush, pineapple, and ginger could be natural enemies of COVID-19. International Journal of New Chemistry 9: 225–237. https://doi.org/10.22034/ijnc.2022.3.10 [Google Scholar] [CrossRef]
Rabie AM (2022c). Efficacious preclinical repurposing of the nucleoside analogue didanosine against COVID-19 polymerase and exonuclease. ACS Omega 7: 21385–21396. https://doi.org/10.1021/acsomega.1c07095 [Google Scholar] [PubMed] [CrossRef]
Rabie AM, Abdalla M (2022a). Forodesine and riboprine exhibit strong anti-SARS-CoV-2 repurposing potential: In silico and in vitro studies. ACS Bio & Med Chem Au 2: 565–585. https://doi.org/10.1021/acsbiomedchemau.2c00039 [Google Scholar] [PubMed] [CrossRef]
Rabie AM, Abdalla M (2022b). A series of adenosine analogs as the first efficacious anti-SARS-CoV-2 drugs against the B.1.1.529.4 lineage: A preclinical repurposing research study. ChemistrySelect 7: e202201912. https://doi.org/10.1002/slct.202201912 [Google Scholar] [PubMed] [CrossRef]
Rabie AM, Abdel-Dayem MA, Abdalla M (2023). Promising experimental anti-SARS-CoV-2 agent “SLL-0197800”: The prospective universal inhibitory properties against the coming versions of the coronavirus. ACS Omega 8: 35538–35554. https://doi.org/10.1021/acsomega.2c08073 [Google Scholar] [PubMed] [CrossRef]
Rabie AM, Eltayb WA (2023). Potent dual polymerase/exonuclease inhibitory activities of antioxidant aminothiadiazoles against the COVID-19 Omicron virus: A promising in silico/in vitro repositioning research study. Molecular Biotechnology. https://doi.org/10.1007/s12033-022-00551-8 [Google Scholar] [PubMed] [CrossRef]
Selvaraj V, Finn A, Lal A, Khan MS, Dapaah-Afriyie K, Carino GP (2022). Baricitinib in hospitalised patients with COVID-19: A meta-analysis of randomised controlled trials. eClinicalMedicine 49: 101489. https://doi.org/10.1016/j.eclinm.2022.101489 [Google Scholar] [PubMed] [CrossRef]
Shinkai M, Tsushima K, Tanaka S, Hagiwara E, Tarumoto N et al. (2021). Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: A randomized, phase III clinical trial. Infectious Diseases and Therapy 10: 2489–2509. https://doi.org/10.1007/s40121-021-00517-4 [Google Scholar] [PubMed] [CrossRef]
Soeroto AY, Yanto TA, Kurniawan A, Hariyanto TI (2023). Efficacy and safety of tixagevimab-cilgavimab as pre-exposure prophylaxis for COVID-19: A systematic review and meta-analysis. Reviews in Medical Virology 33: e2420. https://doi.org/10.1002/rmv.2420 [Google Scholar] [PubMed] [CrossRef]
Vanden Eynde JJ (2021). COVID-19: Failure of the DisCoVeRy clinical trial, and now-new hopes? Pharmaceuticals 14: 664. https://doi.org/10.3390/ph14070664 [Google Scholar] [PubMed] [CrossRef]
Xu H, Liu B, Xiao Z, Zhou M, Ge L et al. (2021). Computational and experimental studies reveal that thymoquinone blocks the entry of coronaviruses into in vitro cells. Infectious Diseases and Therapy 10: 483–494. https://doi.org/10.1007/s40121-021-00400-2 [Google Scholar] [PubMed] [CrossRef]
Zrieq R, Ahmad I, Snoussi M, Noumi E, Iriti M et al. (2021). Tomatidine and patchouli alcohol as inhibitors of SARS-CoV-2 enzymes (3CLpro, PLpro and NSP15) by molecular docking and molecular dynamics simulations. International Journal of Molecular Sciences 22: 10693. https://doi.org/10.3390/ijms221910693 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools