 Open Access
Open Access
REVIEW
Therapeutic application of mesenchymal stem cells-derived extracellular vesicles in colorectal cancer
1 Department of Biochemistry, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
2 Solid Tumor Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia, Iran
* Corresponding Author: JAFAR REZAIE. Email:
(This article belongs to the Special Issue: Extracellular Vesicles and Cancer)
BIOCELL 2023, 47(3), 455-464. https://doi.org/10.32604/biocell.2023.025603
Received 21 July 2022; Accepted 13 September 2022; Issue published 03 January 2023
Abstract
Colorectal cancer (CRC) is the third most common cancer and the leading cause of cancer death globally. Resistance to therapy is a challenge for CRC treatment. Mesenchymal stem cells (MSCs) have become one of the furthermost effective approaches for tumor treatment due to their specific feature; however, their therapeutic function is controversial. Recently, extracellular vesicles (EVs) derived from MSCs (MSCs-EVs) have attracted extensive research attention due to their promising role in CRC treatment. EVs are cell-derived vesicles that transfer different biomolecules between cells, contributing to intracellular communication. MSCs-EVs can suppress CRC by delivering therapeutic agents to tumor cells. Several studies indicate that MSCs-EVs can serve as a drug delivery system for the treatment of different cancers. Various methods are used to modify (engineer) MSCs-EVs for loading therapeutic agents. Modified MSCs-EVs have improved specificity, targeting ability, and immunogenicity compared to synthetic carriers. Furthermore, CRC-EVs participate in regulating different cells, such as immune cells, fibroblasts, and endothelial cells, promoting tumorigenesis. MSCs-EVs-based therapy indicates a high potential in the treatment of cancer; however, the majority of studies have been conducted in the pre-clinical, and their clinical applications need further scrutiny. In this review, we describe the biogenesis of EVs, focusing on the effect of MSCs-EVs on CRC cells and CRC-derived EVs on other cells. Furthermore, MSCs-EVs as a drug delivery system for cancers is also reviewed, and perspectives regarding the therapeutic application of MSCs-EVs are discussed.Keywords
Colorectal cancer (CRC), a heterogeneous disease, develops malignant tumors in the inner walls of the colon and rectum in the form of polyps (Fanelli et al., 2020). It is the third most prevalent malignant tumor and the third most lethal cancer worldwide. In 2018, 1.8 million new cases of CRC and 881,000 deaths were reported, accounting for approximately 10% of new cancer cases and deaths worldwide (Bray et al., 2018). The number of new cases is estimated to increase to 2.5 million in 2035 (Xie et al., 2020). For benign stage CRC, no treatment is needed, and in case of metastatic invasion, surgery can be used to eliminate lymph nodes and malignant tumors (Hashiguchi et al., 2020). Chemotherapy is another treatment for CRC as a targeted therapy through the use of ramucirumab and bevacizumab to prevent some specific protein functions involved in CRC development (Bennouna et al., 2019; Kanat and Ertas, 2019; Modest et al., 2019). In addition, radiation therapy is another common treatment method for the treatment of CRC by applying high-energy radiation beams (Klement et al., 2019). However, efficient therapy remains a challenge for clinicians. The evidence indicates that MSCs have a wide range of applications in the treatment of many diseases, including cancer (Fayazi et al., 2021; You et al., 2022). They are present within bone marrows and other tissues like dental pulp, umbilical cord blood, and adipose and help in homeostasis in healthy tissues in the regeneration and wound healing (Bernardo and Fibbe, 2013; Abdyazdani et al., 2017; Mirershadi et al., 2020). MSCs, as non-hematopoietic precursor cells, have several characteristics, such as their capability to differentiate to produce cells like osteocytes, adipocytes, neurocytes, and fibroblasts (Abdyazdani et al., 2017; Wang et al., 2018). Evidence indicates that MSCs can inhibit tumor cells through both direct contact and paracrine (François et al., 2019; Li et al., 2021). In recent years, nanomedicine has developed to improve the pharmacokinetics and pharmacological patterns of unstable anti-cancer drugs (Patra et al., 2018). The nanocarrier-based approach has various properties, including enhancement of drug delivery efficacy to cancer cells and reduction of the side-effects and non-targeting effects (Hou et al., 2022). In this regard, various studies indicate that extracellular vesicles (EVs) have numerous advantages over conventional synthetic carriers, making them suitable for drug delivery systems (Herrmann et al., 2021). EVs are heterogeneous vesicles released from cells; they contain various biomolecules such as DNAs, proteins, and RNAs that contribute to intracellular communication. They are classified depending on the mechanism of generation and their size, into apoptotic bodies (2–5 μm), exosomes (30–150 nm), and microvesicles (100–1000 nm) (Basso and Bonetto, 2016; Gurung et al., 2021). Among them, microvesicles and exosomes are released from living cells and are involved in many processes, including angiogenesis, proliferation, differentiation, and intercellular communication (Burrello et al., 2016; Todorova et al., 2017; Phan et al., 2018). The therapeutic effects of EVs have increasingly been indicated in various diseases (Ahmadi et al., 2018; Akbari et al., 2020, Hassanpour et al. 2020b). MSCs-derived EVs (MSCs-EVs) have unique advantages as carriers for anti-cancer therapy (Weng et al., 2021). Naseri et al. (2018) reported that MSCs-EVs could migrate to the tumor sites, just like the MSCs. MSCs-EVs are therapeutic tools in regenerative medicine (van Niel et al., 2018) and advance an emerging strategy for CRC therapy due to their roles in metastasis and growth of cancer cells (Xing et al., 2020). For example, in one study, these vesicles could be loaded with doxorubicin, where they could target CRC cells (Bagheri et al., 2020). Therefore, MSCs-EVs can transfer therapeutic agents to tumor cells. The present study discussed EVs biogenesis, the effect of MSCs-EVs on CRC, and the effect of CRC-EVs on other cells, and reviewed targeted cancer therapy by modified MSCs-EVs, and perspectives regarding the application of MSCs-EVs in cancer therapy.
Many types of cells produce EVs to transfer biomolecules, communicating with other cells (Hessvik and Llorente, 2018). EVs are phospholipid membrane vesicles that contain bioactive molecules such as RNAs, DNA strands, proteins, signaling molecules, and lipids, therefore, can regulate the fate and behavior of target cells located near or away (Abels and Breakefield, 2016; Latifkar et al., 2019). A growing body of evidence indicates that EVs can be found in various body fluids, including blood, milk, bile, saliva, cerebrospinal fluid (CSF), bronchoalveolar lavage fluid (BALF), and urine (Abels and Breakefield, 2016; Latifkar et al., 2019). EVs are classically divided into three types such as exosomes, microvesicles, and apoptotic bodies, based on their size and origin (Raposo and Stahl, 2019) (Table 1, Fig. 1). Exosomes refer to 30–150 nm vesicles that are originated from endosomal compartments namely multivesicular bodies (MVBs) located in the cell cytoplasm, while microvesicles are 100–1000 nm vesicles originating from the plasma membrane a process resembling virus outward from infected cells (Abels and Breakefield, 2016; Latifkar et al., 2019). Finally, apoptotic bodies are the largest EVs (200–5000 nm) generated when a cell goes through apoptosis (Abels and Breakefield, 2016; Latifkar et al., 2019) (Fig. 1). Exosomes are initially generated from the inward budding of MVBs membrane where different molecules contribute to the sorting, loading, abscission, and formation of exosomes (Zhang et al., 2019; Jafari et al., 2020). If MVBs fuse with the plasma membrane, exosomes are released into the extracellular space; alternatively, MVBs may fuse with the lysosomes, and exosomes are degraded (Zhang et al., 2019; Babaie, 2020) (Fig. 1). Some common molecules such as CD63, CD81, CD82, and Alix are present on the exosomal membrane and are known as exosomal markers (Kowal et al., 2014; Zhang et al., 2019; Feghhi et al., 2021). Once released into the extracellular matrix, EVs can interact with target cells and participate in different cellular events. According to previous studies, three proposed mechanisms through which EVs reach target cells include receptor-ligand, endocytosis, and direct fusion with the cellular membrane (Mulcahy et al., 2014; Gurung et al., 2021). Thus, they are involved in several physiological and pathological processes (Hassanpour et al. 2020a, Soraya et al., 2021). Regardless of the development in the field of EVs, additional research, considering the International Society for Extracellular Vesicles (ISEV) guidelines, is a necessary requirement for the progress of EVs terms, methodology, and study and also proposes more appropriate determination of the cargo and role of EVs. Different cell types release EVs containing numerous biomolecules with varying functions. In recent years, some databases were established to organize and present components of EVs from various sources such as Vesiclepedia (http://www.microvesicles.org), Exocarta (http://www.exocarta.org), and a Bioinformatics lab from China (http://bioinfo.life.hust.edu.cn).

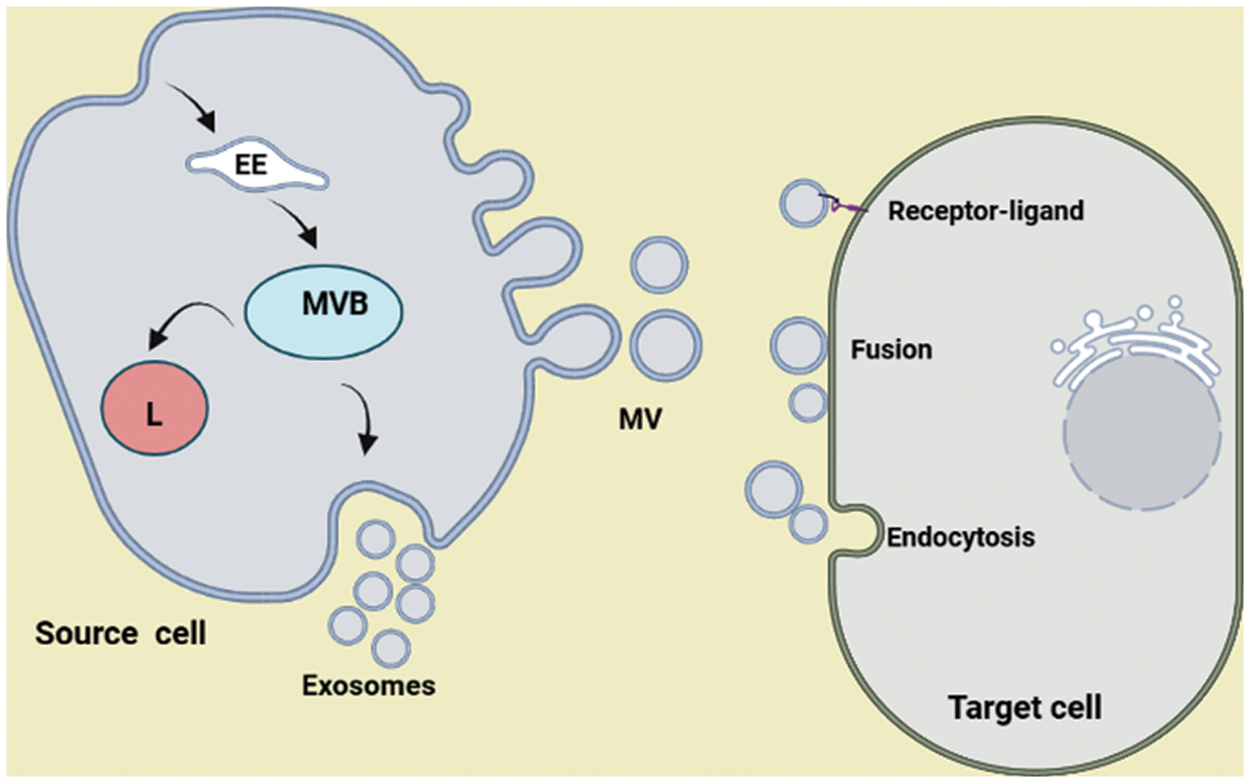
Figure 1: Biogenesis of extracellular vesicles (EVs). The release of EVs from cells occurs either through the inward budding of the membrane of multivesicular bodies (exosomes) or through the outward budding of the plasma membrane (microvesicles, MVs). When MVBs fuse with the plasma membrane, exosomes are released into the extracellular matrix. Once EVs are released, they can affect/reach target cells in three possible ways, including endocytosis, direct fusion, and receptor-ligand interaction. MVB: multivesicular body; L: lysosome; EE: early endosome.
Effect of mesenchymal stem cells derived extracellular vesicles on colorectal cancer
In the case of cancer, MSCs have been shown to act as a double-edged sword where they may promote and/or suppress cancer progress (Tian et al., 2020; Zhang et al., 2022). EVs released by MSCs play a significant role in tumor development, proliferation, invasion, angiogenesis, and drug resistance. Nevertheless, contradictory findings have shown that MSCs-EVs can also inhibit tumors through different mechanisms, including intercellular signaling, and immune responses. Therefore, the association between MSCs-EVs and tumors is controversial. In this section, we discuss the distinct role of MSCs-EVs in CRC (Fig. 2). Luetzkendorf et al. (2010) produced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL-MSCs) by third-generation lentiviral vector system and then co-cultured them with TRAIL-sensitive CRC-cell lines (HCT-15 and DLD-1) and resistant CRC-cell lines (SW480 and HCT-8). They found that these cells promoted apoptosis in CRC cells in vitro. In xenograft models, TRAIL-MSCs suppressed CRC-tumor growth by increasing apoptosis. When TRAIL-MSCs were administrated systemically, the growth of CRC was not affected, which may be due to pulmonary entrapment and a low rate of tumor absorption (Luetzkendorf et al., 2010). Another study demonstrated that C-X-C motif chemokine receptor 4 overexpression-MSCs improved the homing ability of cells in the intestine. These cells recovered colitis-related tumors in the mice model through decreasing tumor load, pro-inflammatory cytokines, and signal transducer and activator of transcription 3 (STAT3) phosphorylation level (Zheng et al., 2018). Conversely, Nishikawa et al. (2019) reported that MSCs can communicate with CRC cells through chemokine (C-C motif) ligand 3/4/5-CC chemokine receptor 5 signaling and increase the growth of CRC tumors in vivo (Nishikawa et al., 2019). Similarly, de Boeck et al. (2013) showed that MSCs from bone marrow induced the survival, invasion, and growth of CRC cells by producing soluble NRG1 and activating human epidermal growth factor receptor 2 (HER2)/HER3-dependent phosphatidylinositol-3-kinase/protein kinase B (Akt) signaling pathway in CRC cells. Besides MSCs, EVs from MSCs have been shown to transfer miRNAs to CRC cells, inhibiting tumor growth. For instance, EVs derived from miR-16-5p-overexpression MSCs could deliver miR-16-5p to CRC cells and inhibit proliferation, migration, and invasion of cells and induce apoptosis through downregulating integrin subunit alpha 2 (ITGA2) expression (Xu et al., 2019) (Fig. 2). Moreover, in vivo experiments showed that the MSCs-overexpressing miR-16-5p suppresses CRC growth. MSCs-EVs containing miR-4461 could decrease proliferation, migration, and invasion of CRC cells in vitro by inhibiting coat complex subunit beta 2 (COPB2) expression, proposing that miR-4461 may be a possible target for the diagnosis and treatment of CRC (Chen et al., 2020). Li et al. (2021) showed that miR-3940-5p cargo of MSCs-EVs inhibited the invasion of cells and repressed the tumor growth and metastasis in vivo. MiR-3940-5p can directly target ITGA6 and suppress tumor cells. Recently, in an in vivo tumorigenesis experiment, researchers showed that MSCs-EVs overexpressing miR-15a could suppress the proliferation, migration, and invasion of cells. Further scrutiny indicated that these EVs increased the apoptosis of CRC cells through the down-regulation of lysine demethylase 4B (KDM4B) (Liu et al., 2021). On the contrary, Li et al. (2021) found that EVs from MSCs contain miR-222 that could reach CRC cells and target activating transcription factor 3 (ATF3) binding and inhibits the activity of AKT1, increasing tumor invasion and immunosuppression of CRC cells. These results indicate that MSCs-EVs can inhibit tumorigenesis; however, some findings show that MSCs and their EVs may promote tumorigenesis. Therefore, the clinical application of MSCs-EVs in CRC treatment remains controversial. Despite the controversial results of MSCs-EVs therapy, MSCs-EVs can serve as a drug delivery system by delivering therapeutic agents to CRC cells.

Figure 2: Therapeutic effect of mesenchymal stem cells derived extracellular vesicles (MSCs-EVs) on colorectal cancer (CRC) cells. Micro RNAs transferred by MSCs-EVs can target genes in CRC cells, inducing apoptosis and arrest in CRC cells, and suppressing tumorigenesis. (ITGA2: Integrin alpha 2; COPB2: COPI coat complex subunit beta 2; ITGA6: integrin alpha 6; ATF3: activating transcription factor 3; KDM4B: lysine demethylase 4B).
Effect of CRC-EVs on other cells
Popēna et al. (2018) reported that CRC cell line-derived EVs regulate the immunophenotype and secretory profile in monocytes and inactive macrophages, inducing mixed M1 and M2 cytokine responses. THP-1 monocytes and M0 macrophages efficiently take up SW480 and SW620-derived EVs, and dynamin-dependent endocytic pathways may be involved. Interestingly, SW480 and SW620-derived EVs enhanced CD14 expression in M0 macrophages, while SW480-derived EVs reduced HLA-DR expression in M1 and M2 polarized macrophages. Furthermore, SW480-derived EVs significantly enhanced C-X-C motif chemokine ligand 10 (CXCL10) expression in M0 macrophages and monocytes. In contrast, SW620-derived EVs result in the secretion of CXCL10, interleukin (IL)-6, IL-10, and IL-23 in M0 macrophages. However, the addition of CRC cell line-derived EVs together with IFN-γ, LPS (M1), and IL-13, IL-4 (M2) stimuli during macrophage polarization had no additional influence on cytokine expression in M1 and M2 macrophages (Popēna et al., 2018). Profilin-1 (PFN1) is a direct target of miR-375 and is positively regulated by HLA-F antisense RNA 1 (HLA-F-AS1) by binding to miR-375. Overexpression of HLA-F-AS1 suppressed miR-375 and enhanced the PFN1 expression pattern in CRC cells and CRC EVs, further increasing the M2 polarization of macrophages. In addition, macrophages treated with PFN1 in CRC EVs induced CRC in vivo and in vitro cell migration and proliferation. Therefore, application of HLA-F-AS1 in EVs may serve as a promising therapeutic strategy for CRC (Zhang et al., 2021). Additionally, CRC small EVs can be specifically targeted to liver tissue and result in liver macrophage polarization toward an IL-6-secreting pro-inflammatory phenotype (Shao et al., 2018). Also, EVs from CRC taken up by macrophages result in M2-like polarization and programmed death-ligand 1 (PD-L1) expression, then enhance PD-L1+CD206+ macrophage abundance and reduce T cell activity in the CRC tumor microenvironment. EVs-derived miR-21-5p and miR-200a are key signaling molecules that mediate the regulatory function of CRC on macrophages. CRC-derived miR-21-5p and miR-200a synergistically make macrophage M2-like polarization and PD-L1 expression by regulating the phosphatase and TENsin homolog deleted on chromosome 10/AKT and the suppressor of cytokine signaling 1/STAT1 pathways, resulting in reduced CD8+ T cell activity and enhanced tumor growth. So, inhibiting the secretion of specific sEV-miRNAs from CRC and targeting PD-L1 in tumor-associated macrophages may serve as new means for CRC treatment as well as a sensitization method for anti-PD-L1 therapy in CRC (Yin et al., 2022). CRC-EVs can regulate the CD8 T cells; in individuals with low body mass index (BMI)-CRC EVs, the rate of apoptosis in CD8 T cells was higher than in those with high BMI-CRC EVs. IL-10, IL-17A, granulysin, granzyme A, and perforin, for instance, were increased in the non-CRC EVs-treated CD8T cells (Abu et al., 2020). On the other hand, Yamada et al. (2016) reported that EVs can impair T cell function. The CRC EVs alter the phenotype of the T cells to Treg-like cells by inactivating the stress-activated protein kinases signaling and stimulating the transforming growth factor-β/ suppressor of mothers against decapentaplegic (Smad) signaling. In addition, the CRC EVs-induced-Treg-like cells had a remarkable tumor-growth-enhancing function in vivo and in vitro (Yamada et al., 2016). Fibroblast is one of the cells that influence by CRC EVs. CRC EVs are uptake by human fibroblasts that stimulate migration through the Rho-focal adhesion kinase signaling in co-incubated human fibroblasts. In addition, HT29 cell-derived EVs are more effective in activating human fibroblasts than cancer-associated fibroblasts (Clerici et al., 2021). Suppressor of cytokine signaling 3 (SOCS3) is a direct target of miR-221-3p and the secreted miR-221-3p shuttled by CRC EVs has regulatory function on the STAT3/ vascular endothelial growth factor receptor-2 signaling axis by targeting SOCS3 in endothelial cells. CRC EVs increased endothelial cell migration, proliferation, and the formation of vessel-like structures. The proangiogenic effect of CRC EVs on the cells was recapitulated by miR-221-3p overexpression, indicating the importance of EVs-derived miR-221-3p in enhancing endothelial cell angiogenesis (Dokhanchi et al., 2021).
Mesenchymal stem cells derived extracellular vesicles as a drug delivery system for cancer
MSCs-EVs can deliver therapeutic agents to tumor cells like pancreatic ductal adenocarcinoma (PDAC), CRC, hepatocellular carcinoma (HCC), breast cancer, and glioma. Generally, two methods are used to load therapeutic agents into MSCs-EVs (i) direct method, in which therapeutic agents are directly sorted into isolated EVs by different loading methods and (ii) the indirect method, in which EVs-producing cells (e.g., MSCs) are genetically manipulated to express distinct biomolecules (miRs, proteins) or co-cultured with therapeutic agents in which EVs derived from them would be contained with therapeutic agents (Tukmechi et al., 2014; Patil et al., 2020; Vahabi et al., 2022) (Fig. 3). For example, Lou (2015) transfected MSCs with a miR-122 expression plasmid and then isolated EVs. After co-culturing with HCC cells in vitro, MSCs-EVs delivered miR-122 to the HCC cancer cells and augmented the sensitivity of HCC to sorafenib as the chemotherapy drug. Moreover, intra-tumor administration of these EVs considerably stimulated the antitumor efficiency of sorafenib on HCC in animal models. They proposed that miR-122 could target genes coding for insulin-like growth factor1 receptor, A disintegrin and metalloproteinase 10, and cyclin G1, and therefore, induce cell cycle arrest and cell death, improving the sensitivity of tumor cells to chemotherapy (Lou, 2015). In another study, Lou et al. (2020) sorted miR-199a into EVs using miR-199a lentivirus infection to inhibit mTOR signaling in HCC cells and found that the sensitivity of HCC cells was augmented after cultivating with MSCs-EVs containing miR-199a. The administration of these EVs significantly increased the effect of doxorubicin on HCC in vivo. In one study, (Kanat and Ertas, 2019), anti-miR-142-3p oligonucleotides were incorporated into MSCs-EVs to be delivered to breast cancer cells to increase the expression of miR-142-3p and miR-150 in these cells (Naseri, 2018; Naseri, 2020). Results showed successful delivery of MSCs-EVs to cancer cells both in vitro and in vivo and up-regulated target RNA expression, improving the expression of APC and P2X7R genes. Furthermore, these EVs inhibited tumor growth and clone-formation abilities of the MCF7 cells. The MSCs-EVs exhibited a great bio-distribution capacity and successfully repressed tumor mass growth (Naseri, 2018; Naseri, 2020). Ding (2019) used MSCs-EVs to deliver miR-145-5p to PDAC cells. They observed that these EVs were efficiently distributed miR-145-5p to PDAC cells, suppressed growth and invasion, and increased apoptosis and cell cycle arrest associated with a low level of Smad3 mRNAs in vitro. Moreover, MSCs-EVs reduced the growth and invasion of cancer cells in the xerograph model. Seemingly, miR-145-5p inhibited Smad3 expression levels. MSCs-EVs can deliver various drugs, such as doxorubicin, paclitaxel, and magnolol, selectively to tumor cells. For instance, Gomari et al. (2018) demonstrated that doxorubicin carried by MSCs-EVs can significantly impede the growth of tumor cells in an animal breast cancer model. The surface of MSCs-EVs was modified to increase the targeting ability of EVs in glioma. Jia and co-workers linked neuropilin-1-targeted peptide to MSCs-EVs by click chemistry and then incorporated superparamagnetic iron oxide nanoparticles and curcumin into them. These MSCs-EVs successfully delivered the therapeutic agents to the targeting area and induced anti-cancer effects (Jia, 2018). Similarly, Zhaung et al. (2020) produced MSCs-EVs with modified surfaces and loaded them with superparamagnetic iron oxide nanoparticles. Proteins of cell-penetrating peptides (CPP) and TNF-α (CTNF-α)-anchored were linked to EVs containing superparamagnetic iron oxide nanoparticles. These EVs showed a targeting antitumor role and considerably suppressed tumor cell growth by inducing apoptosis by the TNFR I pathway, in both in vitro and in vivo mic melanoma subcutaneous cancer models. In clinical trials, exosomes derived from MSCs have been registered to load and deliver the KrasG12D siRNA to pancreatic adenocarcinoma cancer cells (gov Identifier: NCT03608631). The findings show that EVs can deliver therapeutic agents to cancer cells, inhibit tumor cell proliferation, and sensitize the tumor cells to chemotherapy. MSC-EV could induce various effects on cancer cells and tumor stromal cells; thus, it is necessary to limit the endogenous impact when used for drug delivery. In this context, one approach seems to inhibit/decrease the factor/s that support tumor cells. Another approach is to load EVs with relative inhibitors. For example, MSCs-EVs contain proangiogenic factors that may induce angiogenesis in tumor cells (Zhang et al., 2022). So, by using a relative siRNA in EVs or down-regulating targeted gene/s in EVs-producing MSCs, it is possible to inhibit/decrease the supportive impact of MSCs-EVs in cancer.

Figure 3: Application of mesenchymal stem cells derived extracellular vesicles (MSCs-EVs) for drug delivery system. In general, modified EVs can be produced through two approaches, including a direct method where EVs derived from MSCs are directly modified to load therapeutic agents and the indirect method in which MSCs are genetically or exogenously modified to load/express therapeutic agents. Therefore, EVs from these cells contain therapeutic agents.
Platelets-derived extracellular vesicles (p-EVs) for drug delivery
The number of platelets-derived EVs (platelet microparticles) in the blood rises on activation, shear stress, inflammation, and during apoptosis (Burnouf et al., 2014; Melki et al., 2017). These vesicles receive features from their parental cells: the expression of CD41, CD31, CD42, CD63 CD62, and CD61 platelet membrane surface antigens, which activate inherent interactions with the surrounding environment (Franco et al., 2015; Chimen et al., 2020), and physiological loading with complex functional components. These p-EVs bear cytokines, growth factors, chemokines, anticoagulant, pro-coagulant, anti-inflammatory, pro-inflammatory, and proangiogenic, antiangiogenic factors, lipids, and nucleic acids (mRNA and miRNA) (Melki et al., 2017; Boilard, 2018). The structure and composition of p-EVs, as well as their implications in some pathologies, support their prospective therapeutic application in hemostasis, tissue regeneration, and immunomodulation, and as drug-delivery vehicles (Burnouf et al., 2018; Kerris et al., 2020; Wu and Zhou, 2020). These features resemble those of MSCs-EVs. Platelets have numerous advantages as an EV source. They are anucleated (opposite to MSCs), thus alleviating safety concerns related to possible teratogenic risks. Membranes with p-EVs express integrins that can be used to target recipient cells and tissues and may simplify the crossing of biological barriers (Burnouf et al., 2014). Compared to MSCs-EVs, the production of clinical-grade allogenic platelets is already in place in many countries, including as a source of human platelet lysates (Burnouf et al., 2016), therefore providing a readily accessible national resource. The collection of platelet concentrates, and thus the production of EVs, can be done from autologous or allogeneic sources as reasoned appropriate, thereby creating greater opportunities for clinical applications in a given regulatory and clinical trials. Similar to MSCs, platelets have a high ability to produce EVs through several physiological and biophysical mechanisms in vivo and in vitro (Sung et al., 2019; Wu and Zhou, 2020). EVs of platelets can be abundantly isolated from blood because it has long been thought to contribute to the majority (up to 70%–90%) of the pool of EVs (Berckmans et al., 2019). Importantly, these EVs can be directly produced from collected platelet concentrates, in contrast to MSCs that require a phase of isolation and ex vivo incubation and expansion to prepare clinically relevant EVs doses. Therefore, in comparison to MSCs-EVs, bypassing the necessity for a GMP cell culture facility saves time required for facility design, qualification, validation, and operator training and decreases the capital and operational costs required to reach clinical phases and the market (Agrahari et al., 2019; Burnouf et al., 2019). The avoidance of such ex vivo processing also circumvents the preparation and regulatory issues in the quality control of growth medium supplements, including potential ‘contamination’ by the EVs present in fetal bovine serum or human platelet lysates (Agrahari et al., 2019; Barro et al., 2020). Major possible limiting issues in the use of platelets as a source of EVs include the dependence on blood donors or blood collection organizations for a robust source of the starting material and risks of pathogen contamination. Therefore, compared to producing and using MSCs-EVs, platelet concentrates are an established, licensed medicine in most countries and are listed as essential medicines by the World Health Organization (Johnson et al., 2021). Platelet collection is under the supervision of national regulatory consultants. Medical devices for platelet collection are licensed by national regulatory authorities and can be used to prepare allogeneic or autologous platelet concentrates (Johnson et al., 2021). However, there is a risk of contamination by blood-borne infectious agents resistant to existing pathogen-reduction processes. Most importantly, possible variability among platelet donors may affect the features and function of EVs (Johnson et al., 2021). Also, isolation, purification, and characterization methods still lack standardization, and no guidelines for the application of platelets-derived-EVs based therapeutic exist, as is also the case for MSC-EVs (Lener et al., 2015).
Altogether, regardless of the discrepancies in the function of natural MSCs-EVs in CRC studies, their modification or loading and application as carriers for the delivery of therapeutic agents are promising in cancer therapy. MSCs are harmless and advantageous source cells for the production of EVs, and modification of their content may be a promising tool for cancer treatment (Rezaie et al., 2022) (Fig. 3). Modified MSCs-EVs can deliver therapeutic agents to cancer cells effectively. MSCs-EVs represent very low immunogenicity with high biocompatibility, making them ideal for therapeutic goals. Finally, the content and surface of MSCs-EVs can be covalently or genetically modified (Rezaie et al., 2022; Yang and Zhang, 2022). However, this field faces challenges, such as selecting an assured and suitable source of MSCs for delivering therapeutic agents is a serious step; consequently, various MSCs may yield different EVs with variations in size, cargo, and roles (Zhang et al., 2022). EVs are heterogeneous regarding sizes or contents; therefore, the modifying process must not create more heterogeneity and membrane modification, which may negatively impact EVs loading and targeting potential. The side and unwanted effects of modifying EVs remain to be revealed in further studies (Théry et al., 2018; Rezaie et al., 2021). This field is proceeding and requests a deep understanding of the EVs kinetics and developments about modifying and loading methods of EBs to acquire better cancer treatment. The majority of studies were performed in a pre-clinical setting, and the results of clinical application of modified EVs remain a problem, as this field faces some challenges essential to be considered in clinical translation studies. The biology and role of EVs are not yet fully discovered. Several questions are associated with the biogenesis pathway and uptake, nomenclature, characterizations, and purification of EVs, which affect methods and programs that deal with their modifications and loading methods (Théry et al., 2018). Large-scale production of EVs is another challenge and needs standardization for their isolation, purification, loading, and modification. Large-scale production of EVs, especially from MSCs, is very problematic because purification and incubation of human autologous MSCs are laborious and challenging in vitro in a short time. Similar to other EVs, MSCs-EVs may be cleaned by the liver, spleen, and lungs when intravenously injected; consequently, these are not effectively concentrated in the target tissue (Rani et al., 2015).
CRC is the third most common cancer and the leading cause of death due to cancer worldwide. MSCs have become one of the furthermost effective tools for tumor treatment own to their unique properties; however, their therapeutic effects are controversial. MSCs-EVs are a promising tool for the treatment of CRC and other cancers. These vesicles also transfer certain RNAs and biomolecules that contribute to the inhibition of growth and development of CRC through different signaling pathways. On the other hand, CRC-EVs can target other cells and induce tumorigenesis. Modified MSCS-EVs offer a novel therapeutic avenue for the delivery of numerous synthetic and biological molecules to cancer cells. These vesicles represent very low immunogenicity with high biocompatibility, which makes them ideal for therapeutic objectives. However, this field of study is novel and has not yet reached adequate maturity to translate into clinical application, and more studies are desirable to recognize all therapeutic features of MSCs-EVs in CRC.
Acknowledgement: All figures were created by BioRender.com.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contribution: Study conception and design: R. Jafar; data collection: N. Mohedeseh, R. Yousef; draft manuscript preparation: R. Jafar, N. Mohedeseh. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Abdyazdani N, Nourazarian A, Charoudeh HN, Kazemi M, Feizy N, Akbarzade M, Mehdizadeh A, Rezaie J, Rahbarghazi R (2017). The role of morphine on rat neural stem cells viability, neuro-angiogenesis and neuro-steroidgenesis properties. Neuroscience Letters 636: 205–212. DOI 10.1016/j.neulet.2016.11.025. [Google Scholar] [CrossRef]
Abels ER, Breakefield XO (2016). Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cellular and Molecular Neurobiology 36: 301–312. DOI 10.1007/s10571-016-0366-z. [Google Scholar] [CrossRef]
Abu N, Othman N, Ab Razak NS, Bakarurraini NAAR, Nasir SN, Soh JEC, Mazlan L, Azman ZAM, Jamal R (2020). Extracellular vesicles derived from colorectal cancer affects CD8 T cells: An analysis based on body Mass index. Frontiers in Cell and Developmental Biology 8: 564648. DOI 10.3389/fcell.2020.564648. [Google Scholar] [CrossRef]
Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T (2019). Extracellular microvesicles as new industrial therapeutic frontiers. Trends in Biotechnology 37: 707–729. DOI 10.1016/j.tibtech.2018.11.012. [Google Scholar] [CrossRef]
Ahmadi M, Rahbarghazi R, Shahbazfar AA, Keyhanmanesh R (2018). Monitoring IL-13 expression in relation to miRNA-155 and miRNA-133 changes following intra-tracheal administration of mesenchymal stem cells and conditioned media in ovalbumin-sensitized rats. The Thai Journal of Veterinary Medicine 48: 347–355. [Google Scholar]
Akbari A, Jabbari N, Sharifi R, Ahmadi M, Vahhabi A, Seyedzadeh SJ, Nawaz M, Szafert S, Mahmoodi M, Jabbari E (2020). Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sciences 249: 117447. DOI 10.1016/j.lfs.2020.117447. [Google Scholar] [CrossRef]
Babaie M (2020). The effect of health indicators and macroeconomic variables on the level of life expectancy: Comparison of developing and developed countries with the panel data approach. Studies in Medical Sciences 31: 568–575. [Google Scholar]
Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M (2020). Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sciences 261: 118369. DOI 10.1016/j.lfs.2020.118369. [Google Scholar] [CrossRef]
Barro L, Nebie O, Chen MS, Wu YW, Koh MBC, Knutson F, Watanabe N, Takahara M, Burnouf T (2020). Nanofiltration of growth media supplemented with human platelet lysates for pathogen-safe xeno-free expansion of mesenchymal stromal cells. Cytotherapy 22: 458–472. DOI 10.1016/j.jcyt.2020.04.099. [Google Scholar] [CrossRef]
Basso M, Bonetto V (2016). Extracellular vesicles and a novel form of communication in the brain. Frontiers in Neuroscience 10: 127. DOI 10.3389/fnins.2016.00127. [Google Scholar] [CrossRef]
Bennouna J, Hiret S, Bertaut A, Bouché O, Deplanque G, Borel C, François E, Conroy T, Ghiringhelli F, des Guetz G (2019). Continuation of bevacizumab vs cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal cancer: The UNICANCER PRODIGE18 randomized clinical trial. JAMA Oncology 5: 83–90. DOI 10.1001/jamaoncol.2018.4465. [Google Scholar] [CrossRef]
Berckmans RJ, Lacroix R, Hau CM, Sturk A, Nieuwland R (2019). Extracellular vesicles and coagulation in blood from healthy humans revisited. Journal of Extracellular Vesicles 8: 1688936. DOI 10.1080/20013078.2019.1688936. [Google Scholar] [CrossRef]
Bernardo ME, Fibbe WE (2013). Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 13: 392–402. DOI 10.1016/j.stem.2013.09.006. [Google Scholar] [CrossRef]
Boilard E (2018). Thematic review series: Exosomes and microvesicles: Lipids as key components of their biogenesis and functions extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. Journal of Lipid Research 59: 2037–2046. DOI 10.1194/jlr.R084640. [Google Scholar] [CrossRef]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
Burnouf T, Agrahari V, Agrahari V (2019). Extracellular vesicles as nanomedicine: Hopes and hurdles in clinical translation. International Journal of Nanomedicine 14: 8847–8859. DOI 10.2147/IJN.S225453. [Google Scholar] [CrossRef]
Burnouf T, Burnouf PA, Wu YW, Chuang EY, Lu LS, Goubran H (2018). Circulatory-cell-mediated nanotherapeutic approaches in disease targeting. Drug Discovery Today 23: 934–943. DOI 10.1016/j.drudis.2017.08.012. [Google Scholar] [CrossRef]
Burnouf T, Goubran HA, Chou ML, Devos D, Radosevic M (2014). Platelet microparticles: Detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Reviews 28: 155–166. DOI 10.1016/j.blre.2014.04.002. [Google Scholar] [CrossRef]
Burnouf T, Strunk D, Koh MB, Schallmoser K. (2016). Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 76: 371–387. [Google Scholar]
Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G (2016). Stem cell-derived extracellular vesicles and immune-modulation. Frontiers in Cell and Developmental Biology 4: 83. DOI 10.3389/fcell.2016.00083. [Google Scholar] [CrossRef]
Chen HL, Li JJ, Jiang F, Shi WJ, Chang GY (2020). MicroRNA-4461 derived from bone marrow mesenchymal stem cell exosomes inhibits tumorigenesis by downregulating COPB2 expression in colorectal cancer. Bioscience, Biotechnology, and Biochemistry 84: 338–346. DOI 10.1080/09168451.2019.1677452. [Google Scholar] [CrossRef]
Chimen M, Evryviadou A, Box CL, Harrison MJ, Hazeldine J, Dib LH, Kuravi SJ, Payne H, Price JM, Kavanagh D (2020). Appropriation of GPIbα from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica 105: 1248–1261. DOI 10.3324/haematol.2018.215145. [Google Scholar] [CrossRef]
Clerici SP, Peppelenbosch M, Fuhler G, Consonni SR, Ferreira-Halder CV (2021). Colorectal cancer cell-derived small extracellular vesicles educate human fibroblasts to stimulate migratory capacity. Frontiers in Cell and Developmental Biology 9: 696373. DOI 10.3389/fcell.2021.696373. [Google Scholar] [CrossRef]
de Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems G (2013). Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62: 550–560. DOI 10.1136/gutjnl-2011-301393. [Google Scholar] [CrossRef]
Ding Y (2019). Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Letters 442: 351–361. DOI 10.1016/j.canlet.2018.10.039. [Google Scholar] [CrossRef]
Dokhanchi M, Pakravan K, Zareian S, Hussen BM, Farid M, Razmara E, Mossahebi-Mohammadi M, Cho WC, Babashah S (2021). Colorectal cancer cell-derived extracellular vesicles transfer miR-221-3p to promote endothelial cell angiogenesis via targeting suppressor of cytokine signaling 3. Life Sciences 285: 119937. DOI 10.1016/j.lfs.2021.119937. [Google Scholar] [CrossRef]
Fanelli GN, Dal Pozzo CA, Depetris I, Schirripa M, Brignola S, Biason P, Balistreri M, Dal Santo L, Lonardi S, Munari G (2020). The heterogeneous clinical and pathological landscapes of metastatic Braf-mutated colorectal cancer. Cancer Cell International 20: 1–12. DOI 10.1186/s12935-020-1117-2. [Google Scholar] [CrossRef]
Fayazi N, Sheykhhasan M, Soleimani Asl S, Najafi R (2021). Stem cell-derived exosomes: A new strategy of neurodegenerative disease treatment. Molecular Neurobiology 58: 3494–3514. DOI 10.1007/s12035-021-02324-x. [Google Scholar] [CrossRef]
Feghhi M, Rezaie J, Akbari A, Jabbari N, Jafari H, Seidi F, Szafert S (2021). Effect of multi-functional polyhydroxylated polyhedral oligomeric silsesquioxane (POSS) nanoparticles on the angiogenesis and exosome biogenesis in human umbilical vein endothelial cells (HUVECs). Materials & Design 197: 109227. DOI 10.1016/j.matdes.2020.109227. [Google Scholar] [CrossRef]
Franco AT, Corken A, Ware J (2015). Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126: 582–588. DOI 10.1182/blood-2014-08-531582. [Google Scholar] [CrossRef]
François S, Usunier B, Forgue-Lafitte ME, L’Homme B, Benderitter M, Douay L, Gorin NC, Larsen AK, Chapel A (2019). Mesenchymal stem cell administration attenuates colon cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem Cells Translational Medicine 8: 285–300. DOI 10.1002/sctm.18-0117. [Google Scholar] [CrossRef]
Gomari H, Moghadam MF, Soleimani M (2018). Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. OncoTargets and therapy 11: 5753–5762. [Google Scholar]
Gurung S, Perocheau D, Touramanidou L, Baruteau J (2021). The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Communication and Signaling 19: 1–19. DOI 10.1186/s12964-021-00730-1. [Google Scholar] [CrossRef]
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M (2020). Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. International Journal of Clinical Oncology 25: 1–42. DOI 10.1007/s10147-019-01485-z. [Google Scholar] [CrossRef]
Hassanpour M, Rezaie J, Darabi M, Hiradfar A, Rahbarghazi R, Nouri M (2020a). Autophagy modulation altered differentiation capacity of CD146+ cells toward endothelial cells, pericytes, and cardiomyocytes. Stem Cell Research & Therapy 11: 1–14. DOI 10.1186/s13287-020-01656-0. [Google Scholar] [CrossRef]
Hassanpour M, Rezaie J, Nouri M, Panahi Y (2020b). The role of extracellular vesicles in COVID-19 virus infection. Infection, Genetics and Evolution 85: 104422. DOI 10.1016/j.meegid.2020.104422. [Google Scholar] [CrossRef]
Herrmann IK, Wood MJA, Fuhrmann G (2021). Extracellular vesicles as a next-generation drug delivery platform. Nature Nanotechnology 16: 748–759. DOI 10.1038/s41565-021-00931-2. [Google Scholar] [CrossRef]
Hessvik NP, Llorente A (2018). Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Sciences 75: 193–208. DOI 10.1007/s00018-017-2595-9. [Google Scholar] [CrossRef]
Hou S, Hasnat M, Chen Z, Liu Y, Faran Ashraf Baig MM, Liu F, Chen Z (2022). Application perspectives of nanomedicine in cancer treatment. Frontiers in Pharmacology 13: 909526. DOI 10.3389/fphar.2022.909526. [Google Scholar] [CrossRef]
Jafari R, Rahbarghazi R, Ahmadi M, Hassanpour M, Rezaie J (2020). Hypoxic exosomes orchestrate tumorigenesis: Molecular mechanisms and therapeutic implications. Journal of Translational Medicine 18: 1–14. DOI 10.1186/s12967-020-02662-9. [Google Scholar] [CrossRef]
Jia G (2018). NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 178: 302–316. DOI 10.1016/j.biomaterials.2018.06.029. [Google Scholar] [CrossRef]
Johnson J, Wu YW, Blyth C, Lichtfuss G, Goubran H, Burnouf T (2021). Prospective therapeutic applications of platelet extracellular vesicles. Trends in Biotechnology 39: 598–612. DOI 10.1016/j.tibtech.2020.10.004. [Google Scholar] [CrossRef]
Kanat O, Ertas H (2019). Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy. World Journal of Clinical Oncology 10: 52–61. DOI 10.5306/wjco.v10.i2.52. [Google Scholar] [CrossRef]
Kerris EWJ, Hoptay C, Calderon T, Freishtat RJ (2020). Platelets and platelet extracellular vesicles in hemostasis and sepsis. Journal of Investigative Medicine 68: 813–820. DOI 10.1136/jim-2019-001195. [Google Scholar] [CrossRef]
Klement RJ, Abbasi-Senger N, Adebahr S, Alheid H, Allgaeuer M, Becker G, Blanck O, Boda-Heggemann J, Brunner T, Duma M (2019). The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: A combined analysis of 388 patients with 500 metastases. BMC Cancer 19: 1–12. DOI 10.1186/s12885-019-5362-5. [Google Scholar] [CrossRef]
Kowal J, Tkach M, Théry C (2014). Biogenesis and secretion of exosomes. Current Opinion in Cell Biology 29: 116–125. DOI 10.1016/j.ceb.2014.05.004. [Google Scholar] [CrossRef]
Latifkar A, Hur YH, Sanchez JC, Cerione RA, Antonyak MA (2019). New insights into extracellular vesicle biogenesis and function. Journal of Cell Science 132: jcs222406. DOI 10.1242/jcs.222406. [Google Scholar] [CrossRef]
Lener T, Gimona M, Aigner L, Börger V, Buzas E et al. (2015). Applying extracellular vesicles based therapeutics in clinical trials–An ISEV position paper. Journal of Extracellular Vesicles 4: 30087. DOI 10.3402/jev.v4.30087. [Google Scholar] [CrossRef]
Li S, Yan G, Yue M, Wang L (2021). Extracellular vesicles-derived microRNA-222 promotes immune escape via interacting with ATF3 to regulate AKT1 transcription in colorectal cancer. BMC Cancer 21: 1–13. DOI 10.1186/s12885-021-08063-5. [Google Scholar] [CrossRef]
Liu L, Yu T, Jin Y, Mai W, Zhou J, Zhao C (2021). MicroRNA-15a carried by mesenchymal stem cell-derived extracellular vesicles inhibits the immune evasion of colorectal cancer cells by regulating the KDM4B/HOXC4/PD-L1 axis. Frontiers in Cell and Developmental Biology 9: 629893. DOI 10.3389/fcell.2021.629893. [Google Scholar] [CrossRef]
Lou G (2015). Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. Journal of Hematology & Oncology 8: 122. DOI 10.1186/s13045-015-0220-7. [Google Scholar] [CrossRef]
Lou G, Chen L, Xia C, Wang W, Qi J, Li A, Liu Y (2020). MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. Journal of Experimental & Clinical Cancer Research 39: 1–9. [Google Scholar]
Luetzkendorf J, Mueller LP, Mueller T, Caysa H, Nerger K, Schmoll HJ (2010). Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. Journal of Cellular and Molecular Medicine 14: 2292–2304. DOI 10.1111/j.1582-4934.2009.00794.x. [Google Scholar] [CrossRef]
Melki I, Tessandier N, Zufferey A, Boilard E (2017). Platelet microvesicles in health and disease. Platelets 28: 214–221. DOI 10.1080/09537104.2016.1265924. [Google Scholar] [CrossRef]
Mirershadi F, Ahmadi M, Rezabakhsh A, Rajabi H, Rahbarghazi R, Keyhanmanesh R (2020). Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Research & Therapy 11: 1–12. DOI 10.1186/s13287-020-01921-2. [Google Scholar] [CrossRef]
Modest D, Pant S, Sartore-Bianchi A (2019). Treatment sequencing in metastatic colorectal cancer. European Journal of Cancer 109: 70–83. DOI 10.1016/j.ejca.2018.12.019. [Google Scholar] [CrossRef]
Mulcahy LA, Pink RC, Carter DRF (2014). Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles 3: 24641. DOI 10.3402/jev.v3.24641. [Google Scholar] [CrossRef]
Naseri Z (2018). Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. International Journal of Nanomedicine 13: 7727–7747. DOI 10.2147/IJN. [Google Scholar] [CrossRef]
Naseri Z (2020). Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Reviews and Reports 16: 541–556. DOI 10.1007/s12015-019-09944-w. [Google Scholar] [CrossRef]
Naseri Z, Oskuee RK, Jaafari MR, Moghadam MF (2018). Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. International Journal of Nanomedicine 13: 7727. DOI 10.2147/IJN. [Google Scholar] [CrossRef]
Nishikawa G, Kawada K, Nakagawa J, Toda K, Ogawa R, Inamoto S, Mizuno R, Itatani Y, Sakai Y (2019). Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death & Disease 10: 264. DOI 10.1038/s41419-019-1508-2. [Google Scholar] [CrossRef]
Patil SM, Sawant SS, Kunda NK (2020). Exosomes as drug delivery systems: A brief overview and progress update. European Journal of Pharmaceutics and Biopharmaceutics 154: 259–269. DOI 10.1016/j.ejpb.2020.07.026. [Google Scholar] [CrossRef]
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP et al. (2018). Nano based drug delivery systems: Recent developments and future prospects. Journal of Nanobiotechnology 16: 71. DOI 10.1186/s12951-018-0392-8. [Google Scholar] [CrossRef]
Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A (2018). Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. Journal of Extracellular Vesicles 7: 1522236. DOI 10.1080/20013078.2018.1522236. [Google Scholar] [CrossRef]
Popēna I, Ābols A, Saulīte L, Pleiko K, Zandberga E, Jēkabsons K, Endzeliņš E, Llorente A, Linē A, Riekstiņa U (2018). Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Communication and Signaling 16: 1–12. [Google Scholar]
Rani S, Ryan AE, Griffin MD, Ritter T (2015). Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Molecular Therapy 23: 812–823. DOI 10.1038/mt.2015.44. [Google Scholar] [CrossRef]
Raposo G, Stahl PD (2019). Extracellular vesicles: A new communication paradigm? Nature Reviews Molecular Cell Biology 20: 509–510. DOI 10.1038/s41580-019-0158-7. [Google Scholar] [CrossRef]
Rezaie J, Ahmadi M, Ravanbakhsh R, Mojarad B, Mahbubfam S, Shaban SA, Shadi K, Berenjabad NJ, Etemadi T (2021). Tumor-derived extracellular vesicles: The metastatic organotropism drivers. Life Sciences: 120216. DOI 10.1016/j.lfs.2021.120216. [Google Scholar] [CrossRef]
Rezaie J, Nejati V, Mahmoodi M, Ahmadi M (2022). Mesenchymal stem cells derived extracellular vesicles: A promising nanomedicine for drug delivery system. Biochemical Pharmacology 203: 115167. DOI 10.1016/j.bcp.2022.115167. [Google Scholar] [CrossRef]
Shao Y, Chen T, Zheng X, Yang S, Xu K, Chen X, Xu F, Wang L, Shen Y, Wang T (2018). Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis 39: 1368–1379. DOI 10.1093/carcin/bgy115. [Google Scholar] [CrossRef]
Soraya H, Sani NA, Jabbari N, Rezaie J (2021). Metformin increases exosome biogenesis and secretion in U87 MG human glioblastoma cells: A possible mechanism of therapeutic resistance. Archives of Medical Research 52: 151–162. DOI 10.1016/j.arcmed.2020.10.007. [Google Scholar] [CrossRef]
Sung PS, Huang TF, Hsieh SL (2019). Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nature Communications 10: 1–13. DOI 10.1038/s41467-019-10360-4. [Google Scholar] [CrossRef]
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 7: 1535750. DOI 10.1080/20013078.2018.1535750. [Google Scholar] [CrossRef]
Tian J, Zhu Q, Zhang Y, Bian Q, Hong Y, Shen Z, Xu H, Rui K, Yin K, Wang S (2020). Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and Treg cell responses. Frontiers in Immunology 11: 598322. DOI 10.3389/fimmu.2020.598322. [Google Scholar] [CrossRef]
Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F (2017). Extracellular vesicles in angiogenesis. Circulation Research 120: 1658–1673. DOI 10.1161/CIRCRESAHA.117.309681. [Google Scholar] [CrossRef]
Tukmechi A, Rezaee J, Nejati V, Sheikhzadeh N (2014). Effect of acute and chronic toxicity of paraquat on immune system and growth performance in rainbow trout, Oncorhynchus mykiss. Aquaculture Research 45: 1737–1743. DOI 10.1111/are.12118. [Google Scholar] [CrossRef]
Vahabi A, Rezaie J, Hassanpour M, Panahi Y, Nemati M, Rasmi Y, Nemati M (2022). Tumor cells-derived exosomal CircRNAs: Novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochemical Pharmacology 200: 115038. DOI 10.1016/j.bcp.2022.115038. [Google Scholar] [CrossRef]
van Niel G, D’Angelo G, Raposo G (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology 19: 213–228. DOI 10.1038/nrm.2017.125. [Google Scholar] [CrossRef]
Wang S, Miao Z, Yang Q, Wang Y, Zhang J (2018). The dynamic roles of mesenchymal stem cells in colon cancer. Canadian Journal of Gastroenterology and Hepatology 2018: 1–8. DOI 10.1155/2018/7628763. [Google Scholar] [CrossRef]
Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, Li L (2021). Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. Journal of Hematology & Oncology 14: 1–22. DOI 10.1186/s13045-021-01141-y. [Google Scholar] [CrossRef]
Wu Y, Zhou X (2020). Addressing information discrepancies in conversation: bú shì…ma? Interrogatives as account solicitations in Mandarin Chinese. Journal of Pragmatics 162: 45–61. DOI 10.1016/j.pragma.2020.03.005. [Google Scholar] [CrossRef]
Xie YH, Chen YX, Fang JY (2020). Comprehensive review of targeted therapy for colorectal cancer. Signal transduction and targeted therapy 5: 1–30. [Google Scholar]
Xing H, Liang C, Xu X, Sun H, Ma X, Jiang Z (2020). Mesenchymal stroma/stem-like cells of GARP knockdown inhibits cell proliferation and invasion of mouse colon cancer cells (MC38) through exosomes. Journal of Cellular and Molecular Medicine 24: 13984–13990. DOI 10.1111/jcmm.16008. [Google Scholar] [CrossRef]
Xu Y, Shen L, Li F, Yang J, Wan X, Ouyang M (2019). microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. Journal of Cellular Physiology 234: 21380–21394. DOI 10.1002/jcp.28747. [Google Scholar] [CrossRef]
Yamada N, Kuranaga Y, Kumazaki M, Shinohara H, Taniguchi K, Akao Y (2016). Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget 7: 27033–27043. DOI 10.18632/oncotarget.7041. [Google Scholar] [CrossRef]
Yang J, Zhang L (2022). The roles and therapeutic approaches of MSC-derived exosomes in colorectal cancer. Clinical and Translational Oncology 24: 1–9. DOI 10.1007/s12094-021-02750-2. [Google Scholar] [CrossRef]
Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G, Qin Y, Chen Y, Cui K, Zhou L (2022). Colorectal cancer-derived small extracellular vesicles promote tumor immune evasion by upregulating PD-L1 expression in tumor-associated macrophages. Advanced Science 9: 2102620. DOI 10.1002/advs.202102620. [Google Scholar] [CrossRef]
You B, Jin C, Zhang J, Xu M, Xu W, Sun Z, Qian H (2022). MSC-derived extracellular vesicle-delivered L-PGDS inhibit gastric cancer progression by suppressing cancer cell stemness and STAT3 phosphorylation. Stem Cells International 2022: 9668239. DOI 10.1155/2022/9668239. [Google Scholar] [CrossRef]
Zhang F, Guo J, Zhang Z, Qian Y, Wang G, Duan M, Zhao H, Yang Z, Jiang X (2022). Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Letters 526: 29–40. DOI 10.1016/j.canlet.2021.11.015. [Google Scholar] [CrossRef]
Zhang J, Li S, Zhang X, Li C, Zhang J, Zhou W (2021). LncRNA HLA-F-AS1 promotes colorectal cancer metastasis by inducing PFN1 in colorectal cancer-derived extracellular vesicles and mediating macrophage polarization. Cancer Gene Therapy 28: 1269–1284. DOI 10.1038/s41417-020-00276-3. [Google Scholar] [CrossRef]
Zhang Y, Liu Y, Liu H, Tang WH (2019). Exosomes: Biogenesis, biologic function and clinical potential. Cell & Bioscience 9: 1–18. DOI 10.1186/s13578-019-0282-2. [Google Scholar] [CrossRef]
Zhuang M, Chen X, Du D, Shi J, Deng M, Long Q, Rao L (2020). SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale 12: 173–188. [Google Scholar]
Zheng XB, He XW, Zhang LJ, Qin HB, Lin XT et al. (2018). Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterology Report 7: 127–138. DOI 10.1093/gastro/goy017. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools