 Open Access
Open Access
REVIEW
Development and application prospect of stem cell combined with 3D printing technology for oral disease
1 Center of Infectious Diseases, West China Hospital of Sichuan University, Chengdu, 610041, China
2 West China College of Stomatology, Sichuan University, Chengdu, 610041, China
3 West China School of Medicine, Sichuan University, Chengdu, 610041, China
4 Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, Chengdu, 610041, China
* Corresponding Authors: HUA ZHANG. Email: ; DONGBO WU. Email:
# Yixian You and Yihung Lee contributed equally to this work
BIOCELL 2025, 49(1), 45-59. https://doi.org/10.32604/biocell.2024.057259
Received 13 August 2024; Accepted 11 November 2024; Issue published 24 January 2025
Abstract
With organ transplantation facing many dilemmas, tissue and organ regeneration as an alternative has bright prospects. In regenerative medicine, Three-dimensional (3D) printing technology and stem cells has been widely applied to the treatment of diseases related to tissue or organ replacement in dentistry, respectively. However, there are very few studies on the combination of the two, and even fewer clinical studies have been reported in dentistry. In this review, the current oral tissue engineering in vivo and in vitro based on 3D printing and stem cell technology will be summarized, and the discussion on the development prospects of this research direction will be given. Besides, the working principles and advantages & disadvantages of several types of 3D printers, as well as the mechanism of stem cells in tissue engineering will be elucidated. This review provides clinicians and researchers with the current state of research and trends in the combination of stem cells and 3D printing technology to treat oral-related diseases. In the future, 3D bioprinters are poised for ongoing innovation with the advancement of relevant technologies, catalyzing an increase in clinical studies focused on treating oral diseases using stem cells and 3D scaffolds. Consequently, these developments will further advance the field of oral tissue engineering.Keywords
List of Abbreviations
| 3D | Three-dimensional |
| MSCs | Mesenchymal stem cells |
| CAD-CAM | Computer-aided manufacturing technology |
| NNT | Neural network tissue |
| TrkC | Tropomyosin receptor kinase C |
| NSCs | Neural stem cells |
| ESCs | Embryonic stem cells |
| iPSCs | Induced pluripotent stem cells |
| ALP | Alkaline phosphatase |
| hDPSCs | Human dental pulp stem cells |
| pTDM | Porcine treated dentin matrix |
| xTDM/PCL | Xenogeneic TDM-polycaprolactone |
| CNPs | Ceria nanoparticles |
| ICAM-1 | Intercellular cell adhesion molecule-1 |
| ECM | Extracellular matrix |
| IGF-1 | Insulin-like growth factor 1 |
| TNF-α | Tumor necrosis factor-α |
| SDF-1 | Stromal cell-derived factor |
| SHED | Stem cells from human exfoliated deciduous teeth |
| FBR | Foreign body reaction |
| HA | Hyaluronic acid |
| ITOP | Integrated tissue–organ printer |
| HUVECs | Human umbilical vein endothelial cells |
| KC | Keratinocytes |
| FB | Fibroblasts |
| PCL | Polycaprolactone |
| Cs | Carbon steel |
| CP | Calcium phosphate ceramic |
| IPCABs | Inkjet-printed custom-made artificial bones |
| TCP | Tribasic calcium phosphate |
| BMSCs | Bone marrow mesenchymal stem cells |
| ADMSCs | Adipose-derived mesenchymal stem cells |
| SLS | Selective laser sintering |
| PDLSCs | Periodontal ligament stem/progenitor cells |
| ABSCs | Alveolar bone stem/progenitor cells |
| hAFSCs | Human amniotic fluid–derived stem cells |
| ASCs | Adipose-derived stem cells |
| VML | Volumetric muscle loss |
| GMSCs | Gingiva-derived MSCs |
| BMP | Bone morphogenetic protein |
| PDGF-AB | Platelet-derived growth factor-AB |
| Tregs | Regulatory T cell |
| IL | Interleukin |
| VEGF | Vascular endothelial growth factor |
| ISSCR | International society for stem cell research |
Organ and tissue loss or severe dysfunction caused by disease and(or) injury usually need transplantations or regenerations [1]. While organ transplantation is a well-established treatment for organ and tissue failure, significant challenges such as donor shortages, immune rejection, high costs, and societal issues persist and need to be addressed [2,3]. In response, regenerative medicine has emerged as a promising alternative [4], with tissue engineering pioneering the field since the 1980s [5]. Advances in technologies like stem cells, additive manufacturing, biomaterials, and microfluidics have enabled significant progress in reconstructing damaged tissues and organs such as bone [6], skeletal muscle [7], blood vessels [8], etc. These advancements have laid a solid foundation and made critical strides in regenerating oral tissues [9,10]. However, clear and important challenges remain in the development of reproducible and clinically safe methods for tissue repair and regeneration [11]. In recent years, stem cells have been frequently used in tissue engineering studies. Different stem cells have a variety of differentiation potentials, such as bone tissue and adipose tissue [12]. With biological characteristics, including secreting growth factors and cytokines, immunoregulation, anti-inflammation, anti-apoptosis, promoting angiogenesis, tissue repair, and other regulatory functions. Among the various stem cells, mesenchymal stem cells (MSCs) are among the most frequently used in tissue engineering due to their easy acquisition and the absence of significant ethical concerns. The mechanism of regenerating tissues of MSCs may be related to intercellular interactions and immunoregulation [13,14].
Furthermore, the successive development of biomaterials has also injected new impetus into tissue engineering: newly developed biomaterials not only have good biocompatibility but also can interact with cells. However, traditional methods of manufacturing scaffolds, such as freeze-drying, solvent casting and particle leaching, gas foaming, etc., cannot be customized for complex tissues, which limits the application of biomaterials in tissue engineering [15].
Tissue engineering based on stem cell technology and three-dimensional (3D) printing technology can easily solve that problem, which combines high precision and high-resolution printing equipment with stem cells for refined and customized tissue repair and regeneration [16]. In dentistry, 3D bioprinting offers advantages over conventional techniques by better simulating natural tissue’s 3D structure and cellular interactions [17,18]. Coupled with mathematical modeling and computer-aided technologies (CAD-CAM), this approach enables more accurate tissue repairs [16]. Currently, 3D bioprinting is widely used in other biomedical fields [19]. For a example, Li et al. [20] transplanted neural network tissue (NNT) derived from tropomyosin receptor kinase C (TrkC)-modified neural stem cells (NSCs) into rats after seeding of NSCs on the 3D engineering scaffold, and the results showed the NNT survived for up to 8 weeks and significant recovery of paralyzed limb motor function. In addition, many studies have used stem cells combined with 3D printing technology to produce heart patches, which have been shown to promote cardiomyocyte proliferation, angiogenesis, and improvement of cardiac function in rat models of myocardial infarction [21,22]. Likewise, 3D bioprinting using stem cells has already been used to treat oral diseases, current applications include regeneration of various oral tissues (Fig. 1). However, the technology remains stuck in experimental research, and it is rarely used in clinical trials. This review highlights recent research progress and existing challenges in utilizing 3D printing and stem cell engineering for oral diseases. By summarizing current advancements and obstacles, it aims to provide a theoretical framework for advancing basic research and facilitating future clinical applications in this evolving field.
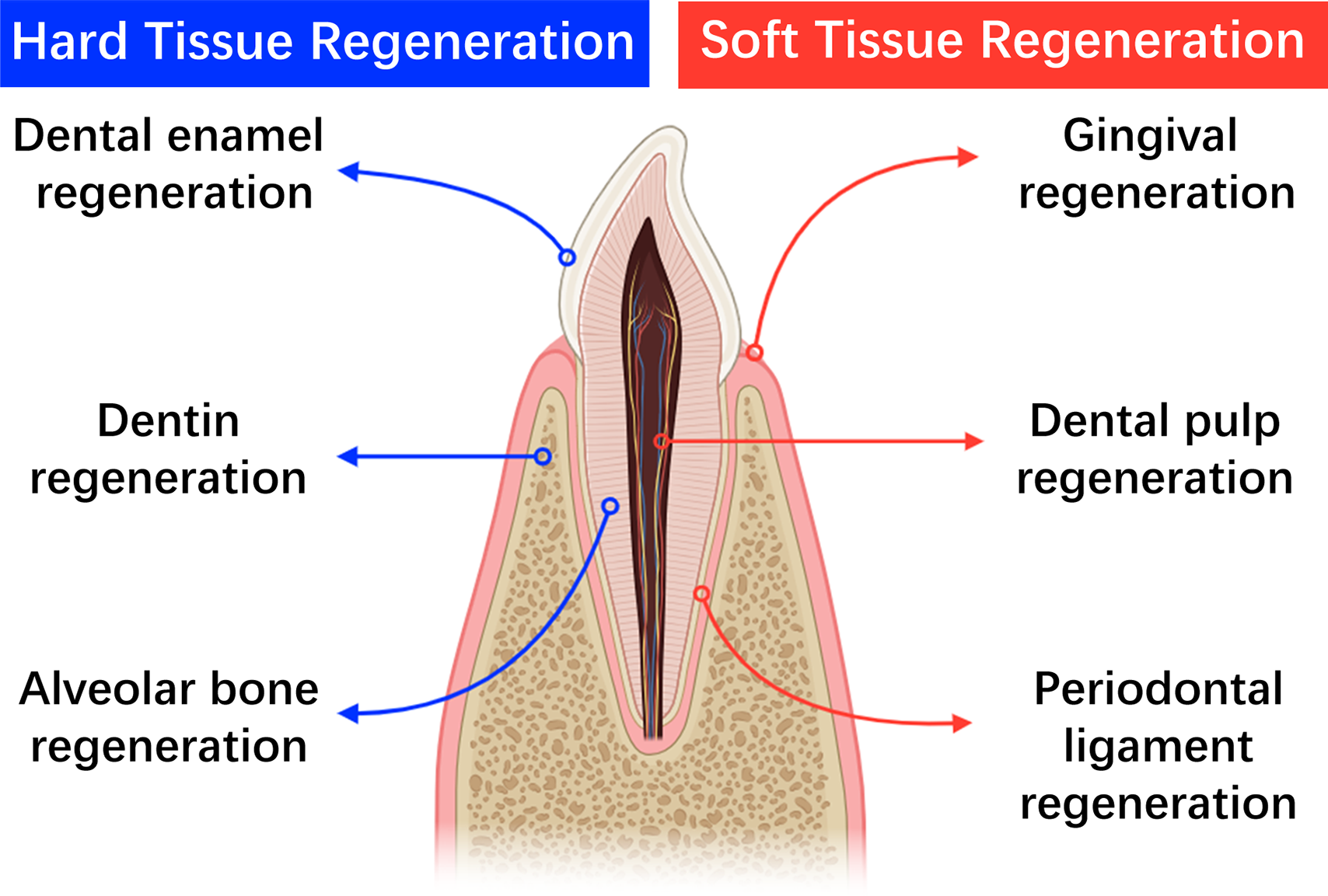
Figure 1: Current applications of 3D-bioprinting in oral diseases (created by adobe photoshop).
3D Bioprinter-Based Tissue Engineering
The advent and dissemination of stereolithography apparatus, commonly referred to as 3D bioprinters, catalyzed a transformative epoch in the domain of tissue engineering during the late 20th century [16]. This technological innovation, grounded in the principles of additive manufacturing, enables the precise deposition and assembly of biomaterials or cellular constructs, guided by a digital 3D blueprint. The resultant constructs span a spectrum from medical devices to tissue-engineered scaffolds and organs, heralding a new era of personalized medicine and regenerative therapy. Typically, a 3D bioprinter comprises a printhead, a construction platform, control systems, material supply systems, cooling systems, a computer and relevant software, working together for fabricating complex biological structures. Bioprinters print tissues or organs with bioink made from biomaterials, cells, growth factors, and other materials. The process hinges on the use of bioinks, a composite of biomaterials, cellular components, growth factors, and ancillary substances. These bioinks are meticulously layered to construct a computer-assisted biological engineering structure, leveraging the additive manufacturing process [23,24]. In the realm of tissue engineering, stem cells form the cornerstone of bioinks. Pluripotent stem cells, notably embryonic stem cells (ESCs), MSCs, dental pulp stem cells (DPSCs), stem cells from apical papilla (SCAPs) [25] and Periodontal ligament stem cells (PDLSCs) [26] are pivotal due to their inherent capacity for differentiation and regenerative potential [27,28]. Stem cells from human exfoliated deciduous teeth (SHEDs), with high proliferation rate and multilineage differentiation potential, the noninvasive harvesting procedure, also have a promising prospect [29]. Bioinks, typically hydrogel-based, encapsulate these cellular elements within a supportive matrix, facilitating their viability and functionality during the printing process [30–32]. Contrary to conventional biomaterials, bioinks are uniquely characterized by their filamentous deposition during additive manufacturing [33]. Yet, this deposition necessitates a delicate balance of conditions, particularly in terms of temperature, to preserve the biological integrity of the cellular components. The bio-3D printed microenvironment is closely related to the processes of cell proliferation, differentiation adhesion and so on. Temperature is one of the factors that affect the printing material. Suitable temperature helps to improve the stability of the printing material for in vitro preforming, and it can also serve as part of energy and plays an essential role in the formation of cell-laden materials. 34°C is similar to the ambient temperature in homeostasis and is favorable in regenerative medicine [34]. High-temperature intolerance of living cells underscores the requirement for mild printing environments, ensuring the bioink maintains its biological attributes throughout the additive manufacturing process [30]. Liquid environment also plays an important role as liquid itself singly stimulates tissues or organs to manufacture or transformation. Lipid-responsive materials transform in spatial and temporal manner thus they probably mimic the normal hormone-like effects [34].
Three principal methodologies underpin the construction of 3D bioprinter constructs capable of emulating the complexity of living tissue or organs, namely, biomimicry, autonomous self-assembly, and mini-tissue building blocks [16]. The application of 3D bioprinting in basic biological research and biomedical science should meet the following requirements: the cells in the printed structure must represent the cell types in the real tissue, and the cell structure of the artificial tissue should best reproduce the physiological tissue [35]. Diverse 3D printing techniques exist, with laser-assisted bioprinting, inkjet printing, and extrusion-based bioprinting standing as the most prevalent applications. Laser-assisted bioprinting harnesses laser energy to precisely target and manipulate a layer of material containing a laser absorption layer. The energy imparted by the laser pulse causes the targeted material, embedded in matrices such as collagen or plasma, to be propelled forward without compromising cellular integrity [27,36]. This technique has been successfully employed in various bioprinting applications, encompassing skin and bone tissue regeneration [36,37]. Inkjet bioprinting operates through dual methodologies: direct printing and powder-based printing. Direct printing involves direct printing, where materials are gathered at the nozzle and the ink or material is maintained in a liquid state and held within the nozzle. When the pressure within the nozzle changes, it triggers the ejection of droplets, which are then deposited onto the surface in a precise and controlled manner. Powder-based printing utilizes a powdered form of the target material, which is positioned under the nozzle. This process relies on an adhesive or binder that is dispensed from the nozzle. Upon mix with the powdered material, the adhesive causes the particles to coalesce and form a solid structure. The polymerization or solidification of the material is achieved through the interaction between the adhesive and the powder, resulting in a strong and cohesive structure. There are two factors that can promote the change in nozzle pressure: piezoelectric induction and thermal induction [38,39]. This technology can be used to customize an artificial bone of a special size and shape to repair bone defects [40]. Extrusion-based bioprinting, distinguished by its efficiency in material delivery and parallel printing capabilities, is particularly adept at rapidly printing large constructs [39]. However, its utility is somewhat constrained by the requirement for thermoplastic materials, which may not be entirely conducive to cellular viability [27]. Notwithstanding, recent advancements have demonstrated the successful survival of human dental pulp stem cells (hDPSCs) in bioinks printed using this technology [41]. A comparative overview of the salient features of these three printing methodologies is delineated in Table 1, elucidating their respective advantages, limitations, and applications in the burgeoning field of 3D bioprinting.
Practical Applications of 3D Bioprinting Using Stem Cells in Oral Diseases
At present, there are two primary approaches to leveraging stem cells within the context of 3D bioprinting for medical applications, distinguished by the inclusion or exclusion of supportive biomaterials that act as scaffolds. Alveolar ridge defects are often caused by bone loss, trauma, infection or severe periodontitis [48], hard tissues such as alveolar bone and teeth usually have complex morphology and structure, which brings great difficulties to autologous bone transplantation, and 3D bioprinting has unique advantages in this respect. These scaffolds are crucial as they offer the necessary physical structure and living conditions for the stem cells. An overview of how 3D printing technology, combined with stem cells, is being applied in the treatment of oral diseases will be provided in Table 2.
Scaffolds based on 3D bioprinting using stem cells in oral diseases
In previous research, a prevalent approach in integrating 3D printing with stem cells involves embedding the stem cells with a 3D-printed scaffold to establish a conducive milieu for cellular proliferation [6,59]. In these studies, researchers are apt to immobilize stem cells on scaffolds using the gel method (stem cells attached to 3D printed scaffolds using gel as a medium) [60–62] rather than incubating them directly [63], as considerations include biocompatibility and precise control of cell distribution. Within stomatology, this methodology parallels scaffold-based tissue regeneration applications for oral diseases. Lee et al. [49] conducted studies cultivating periodontal complexes, encompassing dentin/cementum, periodontal ligament, alveolar bone, and other tissues in vitro using multiphase region-specific microscaffolds. These scaffolds incorporated spatiotemporal delivery of bioactive cues in conjunction with DPSCs, effectively mitigating issues such as delamination commonly associated with traditional methods. In another in vitro study, Han et al. [41] employed hDPSCs, a fibrin-based bioink, and polycaprolactone (PCL) to successfully fabricate a dentin-pulp complex via 3D printing. This approach not only maintained structural integrity over an extended period but also exhibited robust cellular adhesion, optimal oxygenation, and efficient fluid transport capabilities. Furthermore, the versatility of customization according to patient-specific requirements was demonstrated.
Although significant advancements have been achieved in bone regeneration through 3D bioprinting using stem cells, controlling the directional differentiation of these cells remains a formidable challenge, often resulting in suboptimal tissue regeneration outcomes. Consequently, various strategies, such as scaffold modifications and material enhancements, have been devised to address these limitations [51–53]. Likewise in oral diseases, researchers have improved the performance of scaffolds by changing the raw materials and modifying scaffolds, and these studies have shown that the scaffolds made progress in osteogenic differentiation [28,64,65], porosity of bone [66], cell viability [28], angiogenesis [67], cementogenesis [68]. These investigations underscore ongoing advancements in the application of 3D-printed scaffolds and stem cells in stomatology. While numerous in vitro and animal studies have been conducted, clinical trials remain largely unexplored. To date, limited clinical study has been documented involving surgical treatment of alveolar clefts using 3D-printed scaffolds seeded with autologous bone marrow mesenchymal stem cells (BMSCs) [69]. Employing PCL scaffolds fabricated via micro-extrusion 3D printing, previously reported for periodontal repair in 2015 [70], this study [69] capitalized on PCL’s biocompatibility and the participant-derived nature of the stem cells to avoid immune rejection and ensure favorable postoperative healing outcomes. The process of treatment is illustrated by Fig. 2. It is noteworthy, however, that the average bone mineral density of the regenerated teeth did not match that of the subjects’ normal teeth, suggesting room for optimization in bone regeneration protocols.
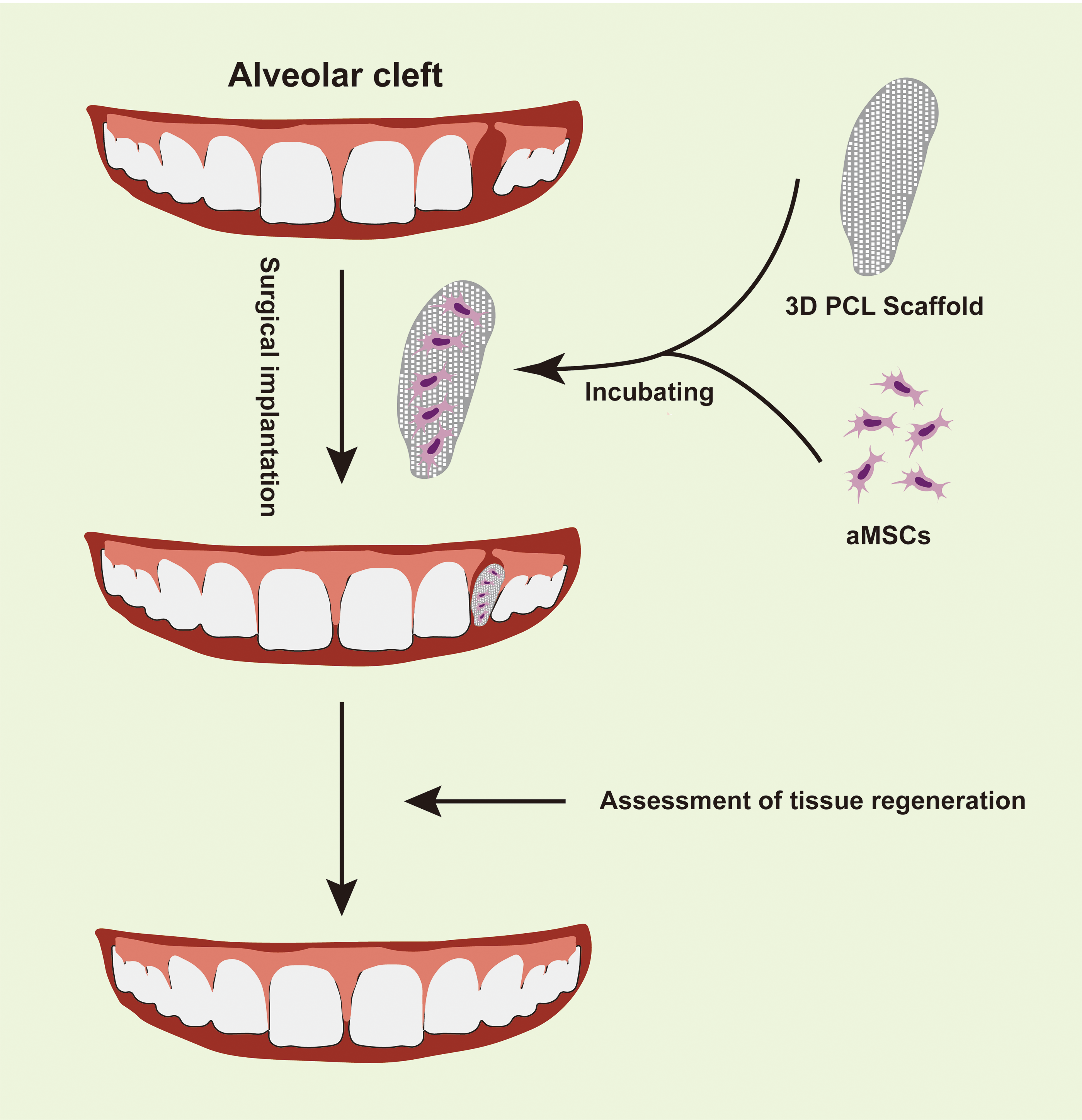
Figure 2: Illustration of surgical treatment of alveolar cleft with the mesenchymal stem cells-seeded 3D printed scaffold [69]. 3D: Three-dimensional; PCL: Polycaprolactone; aMSCs: Autologous mesenchymal stem cells (created by adobe illustrator).
This clinical case underscores the promising future of in situ stem cell engineering utilizing minimally manipulated stem cells (cell populations obtained and used with minimal alteration of their natural state [71,72]. The autologous stem cells not only exhibit no immune rejection but also eliminate the concerns of ethical and moral issues. In addition, the stem cells isolated during the surgical procedure maintain high viability and a stable phenotype because of the minimal intervention [71]. Bajuri, MY and co-workers [73] reported on the application of autologous stem cells combined with 3D bioprinted scaffolds for the treatment of diabetic foot ulcers. Owing to the benefits mentioned above, their novel therapeutics enhanced efficacy in treating diabetic foot ulcers, accelerating wound healing and significantly reducing the size of the ulcers. During a typical treatment plan, stem cells are extracted and subsequently combined with a 3D bioprinted scaffold. The scaffold provides both physical support for the stem cells and a microenvironment conducive to their survival, growth, and differentiation, closely resembling physiological conditions in vivo. This microenvironment simulates the natural niche of stem cells in the body to ensure their survival, proliferation and differentiation [74]. After seeding on the scaffold, the entirety is surgically implanted into the body. Within the host environment, these stem cells engage with other cell types and the extracellular matrix through signaling molecules, facilitating proliferation and differentiation in vivo supported by an adequate blood supply. This interaction activates or represses specific gene expressions responsible for various functions related to directional differentiation [75–77] of stem cells and tissue formation for clinical treatment. While in situ tissue engineering technology offers significant advantages, it is not without its drawbacks. Autologous stem cells are typically obtained in smaller quantities [72], as they undergo minimal (20 min incubation [69]) in vitro manipulation for proliferation. This limited proliferation is due to the inherent risks associated with in vitro expansion, which include unsuitable culture conditions, compromised stem cell proliferation, unpredictable differentiation outcomes, and complications arising from labeling procedures. All these factors significantly contribute to the phenomenon of “senescent drift” in stem cells during in vitro cell culture. In other words, uncontrollable factors and non-standardized in vitro culture of stem cells on scaffolds may lead to genotype-phenotype relationship changes in stem cells cultivation.
In 3D-bioprinted scaffolds for stem cells cultivation, there are two primary modes: synchronized printing of stem cells with scaffolds and sequential printing of scaffolds followed by implantation of stem cells [78]. When stem cells are cultivated on 3D bioprinted scaffolds in vitro over extended periods, they undergo a complex and transformative process marked by a series of intricate phenotypic changes. This metamorphic process encompasses the adaptation of stem cells to their surrounding microenvironment, their subsequent proliferation and differentiation, culminating in the functional maturation that is pivotal for tissue regeneration. Within this microenvironment, 3D scaffolds [79,80], enriched with bioactive media, serve as the physical substratum for stem cell anchorage. These cells secrete adhesion molecules, such as ICAM-1 and integrins, enabling their adhesion to the scaffold’s surface. Simultaneously, they are influenced by a myriad of factors such as growth factors, oxygen tension, intercellular signaling, and the extracellular matrix (ECM) [81], which collectively orchestrate the migration, proliferation, and differentiation of stem cells within the scaffold (details described in the next part). Eventually, the stem cells differentiate into target cells and form tissue, which is able to replace the diseased tissue normally and perform its normal function.
However, the in vitro culture of stem cells has its inherent limitations. For instance, “adherent wall” culture methods can lead to the emergence of heterogeneous stem cell populations [82]. The proliferation rate of stem cells is highly sensitive to changes in environmental oxygen concentration [83]. Inadequately managed freezing and thawing processes may adversely affect the phenotype and functionality of stem cells [84]. Additionally, certain components present in the serum of cultured cells can influence the proliferation of stem cells. Smith et al. reported that certain platelet concentrations can enhance stem cell proliferation and eliminate the xenogeneic serum components [85]. When considering stem cell differentiation, challenges such as uncontrolled differentiation pathways and the complete loss of differentiation potential arise. Besides, there exists a risk of senescence and transformation among stem cells cultured in vitro. Numerous reports highlight that prolonged expansion of MSCs can induce cellular senescence [86]. Finally, it is noteworthy that for stem cells subjected to labeling, the efficiency of proliferation [87] and even cell viability may be compromised [88], as many exogenous tracers used for labeling are not optimal for stem cell health. It is believed that these problems are caused by the absence of standardization. Therefore, it is essential to establish a standardized protocol for culture of stem cells on 3D bioprinted scaffolds designed for culturing in clinical treatments.
Most current studies involving 3D-printed scaffolds and stem cells are primarily conducted in vitro and in animal models, each with inherent limitations. For instance, while successful bone tissue culturing using scaffolds has been achieved in vitro, further investigations are necessary to assess the scaffolds’ capability for craniofacial applications [54]. In addition, Rodriguez et al. [89] utilized tissue engineering techniques, excluding 3D printing, to address craniofacial volumetric muscle loss (VML) in a large animal model (sheep), revealing comparable functional outcomes between treated and untreated groups, unlike previous successes in repairing VML in sheep hindlimbs. This disparity may stem from severe craniofacial denervation and ischemia, intensifying repair challenges. Applying the same experimental methods used for repairing cranial and facial bone defects in rats to larger animals or humans may lead to issues such as inadequate oxygen supply during bone repair [90]. Consequently, the nutritional needs of cells must be considered when regenerating larger tissues or organs, necessitating further investigation into vascular and neural regeneration [91,92]. Induction of osteogenesis, angiogenesis and inhibition of inflammatory response is another difficulty in tissue engineering of oral diseases, as well as a research hotspot. Emerging new materials and sights has brought new hope to overcome these problems. The layered bioceramic scaffold can simulate the structure and biological characteristics of normal bone tissue. Bredigite (BRT, Ca7MgSi4O16) is a ceramic biomaterial that can induce bone and angiogenesis and can be manufactured by 3D printing technology. Its combination with 3D printing technology has great application prospects in onlay graft [93].
Furthermore, the immune response of the implanted scaffold to the participant should also be taken into account. Because implantation of poor biocompatible biomaterials can trigger severe foreign body reaction (FBR) [94] in the body. For 3D printed scaffolds, specifically, different components within 3D printed scaffolds can trigger various immune responses. Studies have shown the surface properties of biomaterials, their physicochemical properties, and their interactions with cells affect the behavior of immune cells. Natural polymers, such as gelatin, collagen [95] and highly purified alginate [96], have the characteristics of low immunogenicity. However, chitosan and hyaluronic acid (HA) are the exceptions. The former leads to activation of macrophages and dendritic cells [97] and aggregation of neutrophils [98], which is related to the physicochemical properties of chitosan, and its immunogenicity can be mitigated by changing the deacetylation degree, molecular weight and positive charge [99]. The latter’s fragment (<200 kDa) can recognize HA-binding receptors TLR-2 and TLR-4 and triggers pro-inflammatory responses, while high molecular (>1000 kDa) weight HA does not cause immune response [100].
Scaffold-free based 3D bioprinting using stem cells in oral diseases
Scaffold-free bioprinting is a technology that circumvents the use of artificial scaffolds to support biological tissue or organ structures during the 3D bioprinting process. This approach has garnered considerable interest in recent years. Scaffold-free bioprinting offers potential advantages over scaffold-based methods, including mitigating issues such as rejection and tissue failure associated with scaffolds. It also promotes enhanced biocompatibility, facilitates intercellular interactions, supports long-term functionality, and improves cell viability [101–103].
For repairing cranial and maxillofacial bones, Kang et al. [55] utilized integrated tissue–organ printer (ITOP) to fabricate the defected mandible using human amniotic fluid-derived stem cells (hAFSCs) as cell sources. Their study demonstrated osteogenic differentiation and stimulated calcium deposition within the printed structure. Moreover, hAFSCs were employed to print a skull structure and implanted into rat cranial defects, resulting in the formation of vascularized bone tissue surpassing scaffold-only treatments. While inkjet, laser, and extrusion-based bioprinters can transport living cells and biological materials to fabricate 3D structures, achieving surgical implants with precise size, shape, and tissue structure remains challenging. ITOP technology offers a promising solution to this issue.
The maxillofacial nerve may be injured due to trauma, surgery, or other reasons. Zhang et al. [104] simulated fundamental development processes via tissue self-assembly. Human gingiva-derived mesenchymal stem cells (GMSCs), a subgroup of MSCs isolated from gingiva, have been employed for 3D printing and culture. Implantation of GMSC spheroids has demonstrated efficacy in promoting facial nerve repair and regeneration.
Tooth loss, stemming from dental caries, periodontitis, or trauma, has spurred interest in whole-tooth regeneration within oral regenerative medicine. Park et al. [56] successfully utilized hDPSCs and a bone morphogenetic protein (BMP) mimetic peptide tethering bioink to bioprint a structurally sound tooth substitute. However, these studies remain predominantly confined to in vitro settings and primarily focus on partial tooth tissue regeneration, highlighting significant avenues for further research in this domain.
Despite the advantages of scaffold-free bioprinting, such as avoiding scaffold-related biocompatibility concerns, it faces inherent limitations. Achieving tissue maturation and the transition from liquid to solid phases rapidly is crucial to maintaining product composition and integrity [105]. Furthermore, scaffold-free bioprinted constructs may exhibit inferior mechanical properties compared to scaffold-based methods [106]. Additionally, the unpredictable behavior of cells and the extended preparation time required for stable assembly pose significant challenges [35]. Recent efforts have explored mathematical modeling to predict and optimize scaffold-free constructs, aiming to mitigate these limitations [101].
The Mechanism of Stem Cells in Tissue Engineering
The application of stem cells in 3D bioprinting for oral diseases shares similarities with their application in other diseases. In stem cell bioengineering, the primary goal is to achieve precise and strategic modulation of stem cell populations, ensuring consistent and predictable outcomes. This objective is heavily influenced by microenvironmental cues perceived by stem cells, which encompass both biochemical and biophysical signals. Biophysical signals include intercellular interactions, as well as the shape, topology, compliance, and interactions of extracellular matrix proteins [107].
Stem cells are known to secrete various cytokines and growth factors [60,108]. Previous research has demonstrated that adult BMSCs can effectively repair many tissues and migrate in response to chemokines and growth factors secreted by cells. Among these factors, platelet-derived growth factor-AB (PDGF-AB) and insulin-like growth factor 1 (IGF-1) are particularly potent. Chemokines have also shown effectiveness on tumor necrosis factor-α (TNF-α)-primed cells, suggesting that MSC mobilization and subsequent homing (incorporation into damaged tissue via the extracellular matrix) are linked to both systemic and local inflammation [108]. Cytokines also play an important role in osteogenic differentiation, Kim et al. constructed a scaffold composed of PCL and HA with a combination of stromal cell-derived factor 1 (SDF-1) and BMP-7. Their results suggest that SDF-1 and BMP-7 are critical in attracting endogenous cells, including MSCs and endothelial cells, to promote vascularization [109].
In addition to cytokines and growth factors, exosomes secreted by stem cells have garnered increasing attention in recent years [110,111]. Numerous studies have indicated that MSC-secreted exosomes possess therapeutic effects akin to those of MSCs themselves. The primary mechanism is thought to involve the horizontal transfer of proteins, mRNAs, and regulatory microRNAs that alter the activity of target cells to exert their effects. MSC-derived exosomes are also believed to modulate the activity of immune cells such as B cells, T cells, natural killer cells, dendritic cells, and macrophages, mimicking the effects of MSCs [112]. In one study involving THP-1 cells treated with MSC exosomes, pro-inflammatory cytokine responses induced by these exosomes were reduced, while anti-inflammatory interleukin (IL)-10 expression was significantly enhanced. Furthermore, treatment with activated CD4+ T cells enabled THP-1 cells to induce regulatory T cell (Treg) polarization, confirming that MSC-derived exosomes can attenuate immune activity in vivo by promoting IL-10 production and Treg differentiation [113].
Angiogenesis is a major topic in tissue engineering, with poor angiogenesis, inadequate oxygen transport will lead to the death of cells in the central area. This is due to the lack of oxygen in the center of the tissue, and if they are far from the blood vessel >200 μm, they will begin to necrosis [114]. In the realm of stem cell therapy, significant attention has been directed towards angiogenesis. For instance, Schlosser et al. [115] conducted studies where BMSCs were transplanted into ischemic mice tails, resulting in notable enhancements in functional capillary density and blood flow at the ischemic loci. This improvement is attributed to the secretion of proangiogenic cytokines by bone marrow-derived circulating MSCs, particularly vascular endothelial growth factor (VEGF), recognized as pivotal in angiogenesis [116]. VEGF also serves as a prominent therapeutic target for anti-angiogenic treatments in various diseases, including tumors, underscoring its crucial role in vascular development.
Among the mechanisms exploited by stem cells in tissue engineering, immunoregulation, anti-inflammation, and anti-apoptosis are paramount (Fig. 3). MSCs exemplify these functions by secreting diverse soluble factors that suppress the activation, proliferation, and differentiation of immune cell subsets such as T cells, B cells, and macrophages [117]. MSCs also interact with regulatory T cells (Tregs) and monocytes, pivotal in their immune-modulatory capacities [118]. Notably, the specific immunoregulatory roles of distinct stem cell types, such as dental pulp stem cells (DPSCs) vs. BMSCs, warrant further investigation [117]. Bone immune regulation and microenvironment are the focus of research. Macrophage polarization can promote bone regeneration [93,119]. The biological scaffold material can influence the microenvironment, thus inducing osteogenesis and soft tissue differentiation. For instance, Chen et al. fabricated a xenogeneic hard tissue scaffold based on Porcine treated dentin matrix (pTDM) xenogeneic TDM-polycaprolactone (xTDM/PCL) and modified the scaffolds by Ceria nanoparticles (CNPs) (xTDM/PCL/CNPs). Regeneration of teeth evaluated on beagles demonstrated that xTDM/PCL/CNPs scaffold expedited the calcification inside the scaffolds and helped form periodontal ligament-like tissues surrounding the scaffolds [119].
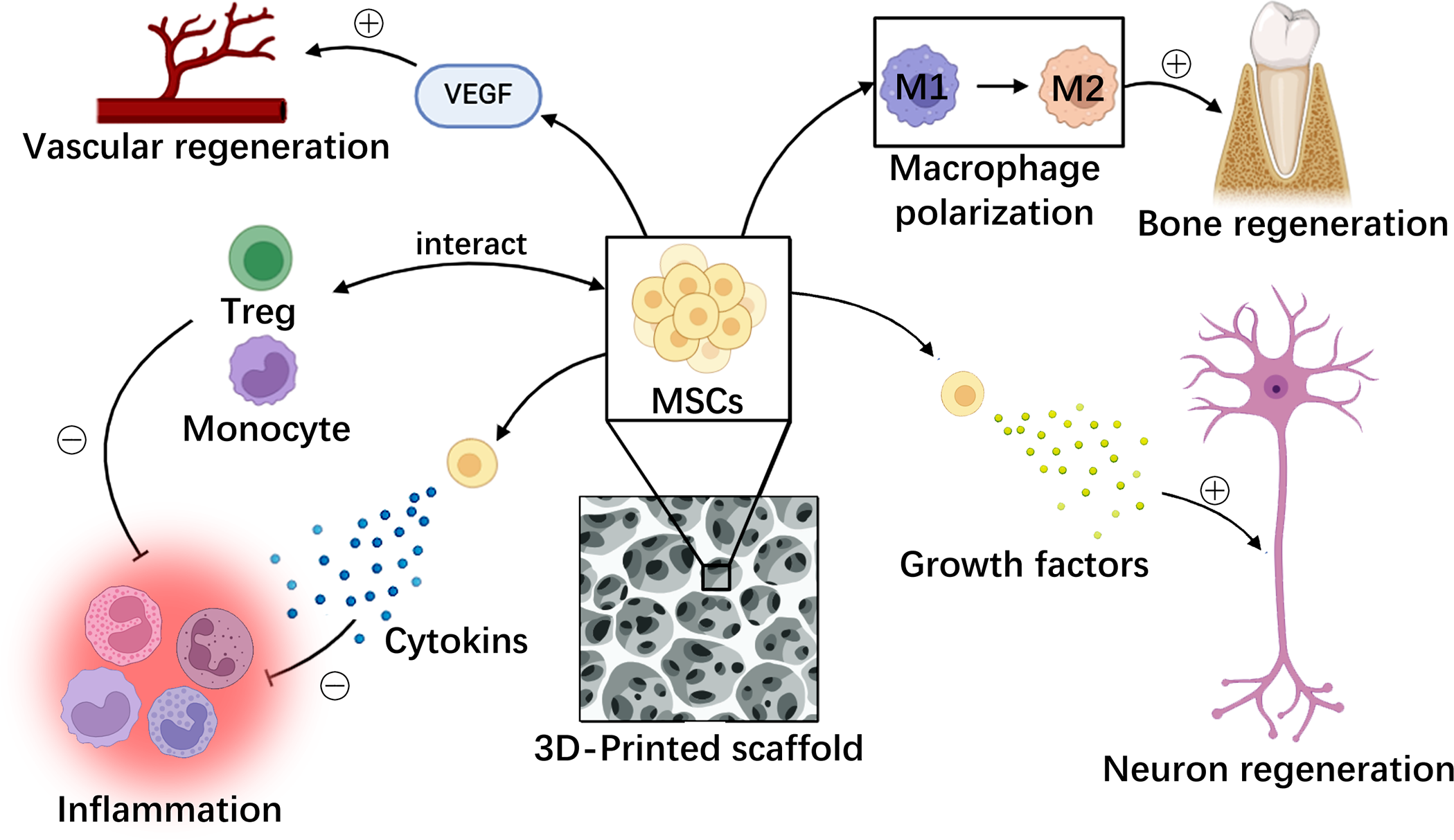
Figure 3: MSCs function in various aspects of tissue regeneration. MSCs promote multiple tissue regeneration and inhibit local inflammatory responses through different mechanisms. MSCs: mesenchymal stem cells, VEGF: vascular endothelial growth factor, Treg: regulatory T cells (created by adobe photoshop).
Dentin is rich in calcium structure, so ossification and stem osteogenic differentiation are important for dentin regeneration. During the process of dentine regeneration, new bone tissue invades the dentine tubules and genes associated with odontogenesis would be upregulated.
In Macalester’s work, a hMSC-laden hydrogel-human dentine interface model is developed using extrusion 3D bioprinting, they found that dentine surface could generate changes in cell morphology and to induce osteogenic differentiation of hMSCs. They also noted that dentine surface stimulates ECM production and tissue maturation, as well as collagenous structures [120].
Levels of components in bio-ink also influence the effect of osteogenesis. Wang et al. [64] used gelatin methacryloyl of different concentrations as the carrier of DPSC and founded that DPSCs in 10% GelMA bioprinted structures showed better osteogenic differentiation potential, which was accompanied by higher ephrinB2/EphB4 signal expression levels. Incorporating bioactive glass microsphere (BGM) into hydrogel could improve its bioactivity, and could promote the formation of mineralized nodules and osteogenic differentiation of mBMSCs. The incorporation of BGM into the scaffold resulted in enhanced cell adhesion, proliferation, alkaline phosphatase (ALP) activity, calcium nodule formation and the promotion of osteogenesis-related gene expression (OPN and Runx2). In vivo periodontal repair experiment revealed that mBMSCs and growth factor-laden 3D-bioprinted scaffolds promoted the reconstruction of periodontal tissue significantly [121].
MSCs additionally exhibit anti-inflammatory effects by releasing IL-1, TNF-α, and interferon-γ, thereby mitigating inflammatory responses in target areas [13,60]. In studies on myocardial infarction, BMSCs have been observed to upregulate the cell survival gene Akt during ischemia, particularly potent following transient hypoxia, and confer protective effects via paracrine mediators, including inhibition of left ventricular remodeling [122].
In addition, stem cells contribute to tissue repair by secreting growth factors like glia-derived neurotrophic factor, brain-derived neurotrophic factor, neurotrophin-3, and nerve growth factor b, promoting neurite outgrowth and neuronal survival in vitro [123]. This capability underscores their potential in facilitating tissue regeneration.
The application of stem cells in tissue engineering may undergo modifications when employed in 3D bioprinting. While the self-assembling process shares foundational developmental pathways akin to natural biological processes [124]. Recent studies have highlighted distinct outcomes. For instance, research has demonstrated that MSCs often aggregate into 3D spheres within bioprinted constructs, thereby exhibiting heightened differentiation capabilities [104]. Meanwhile, the utilization of BMP-mimetic peptide-tethered bioinks has been shown to significantly bolster the viability, proliferation, and odontogenic differentiation of hDPSCs [56]. The above studies show that 3D bioprinting may enhance the occurrence of the above mechanisms in oral diseases.
In the field of stomatology, the regeneration of human teeth and oral tissues poses significant challenges due to their complex organization and specialized environment. The application of 3D bioprinting utilizing stem cells offers promising therapeutic avenues for patient treatment. In this research direction, preliminary successes have been noted in addressing craniofacial bone defects and regenerating periodontal tissues. Regarding craniofacial bone defects, 3D bioprinting leverages materials conducive to cellular growth and tissue formation, thereby promoting effective tissue regeneration while meeting the mechanical demands of hard bone tissues [125]. Various innovative materials have emerged in this context. Furthermore, enhancing angiogenesis is crucial, particularly in treating large bone defects. Consequently, research efforts have focused on technologies to stimulate angiogenesis and vascular anastomosis [52]. In terms of the application of periodontal tissue regeneration, although it is difficult to cultivate a variety of tissues with correct structures at the same time, successful examples exist where scaffolds have facilitated the cultivation of the periodontium complex, encompassing alveolar bone, periodontal membrane, and periodontal ligament [49]. These reports indicate that 3D bioprinting using stem cells also has good application prospects in the field of stomatology. Beyond oral diseases, Liu et al. [126] demonstrated the feasibility of in situ 3D bioprinting using BMSCs and methacrylated hyaluronic acid/polycaprolactone incorporating kartogenin/β-TCP for osteochondral defect repair in rats osteoarthritis.
The utilization of bioink in 3D bioprinting plays a pivotal role in enhancing cell differentiation and tissue formation, thereby significantly augmenting the efficacy of tissue repair. Notably, the hydrogel component within bioink serves dual purposes: it acts as a scaffold matrix for structural support and provides vital protection to living cells throughout the printing process, distinguishing 3D bioprinting technology in its capability. By integrating stem cells and biomaterials, such as hydrogels, with precision, this technology can more accurately replicate designed tissues and organs, thereby enhancing the functionality of stem cells and advancing tissue repair outcomes beyond those achievable through traditional bioengineering methods.
Presently, limitations in stem cell application primarily revolve around ethical concerns, cell quality control, specific usage standards, and associated controversies. The International Society for Stem Cell Research (ISSCR) guidelines serve as critical references, offering researchers guidance on ethical considerations and other pertinent issues in stem cell research [127]. Additionally, the adoption of induced pluripotent stem cells (iPSCs) presents a promising avenue. iPSCs, derived without ethical quandaries, possess self-renewal capabilities and the potential to differentiate into all three germ layers (ectoderm, mesoderm, and endoderm) [128]. Efforts towards iPSC banking and rigorous quality control initiatives in many countries ensure a stable and accessible source for research. The emergence of iPSC banks also makes their source more stable and easier to obtain than other stem cells [129]. Nonetheless, due to unique cellular attributes, iPSCs present vast opportunities for advancement in their application within 3D bioprinting compared to other stem cell types [35].
Finally, the current studies on 3D bioprinting using stem cells are almost all in vitro and in animal experiments. Rare studies and clinical studies are needed to confirm the effectiveness of such therapies in the future. However, a large number of studies have confirmed that stem cells have the ability to promote tissue repair and have important application prospects in tissue repair. In addition, the emergence of new materials opens up the prospect of treating more oral diseases, for example, Xin et al. reported a photoactive nanomaterial that can be applied to the surface of tooth enamel and can eliminate bacteria and biofilms, which may play a role in the treatment of dental plaques, enamel demineralization, dental caries, and periodontitis [130]. However, at present, the application of 3D bioprinting technology in nanomaterials is limited, and new breakthroughs are still needed [131]. With the deepening of research, the ethical problems of cells, the quality control of cells, and the continuous improvement of printing technology, 3D bioprinting using stem cells technology will have good application prospects in human oral diseases (Fig. 4).
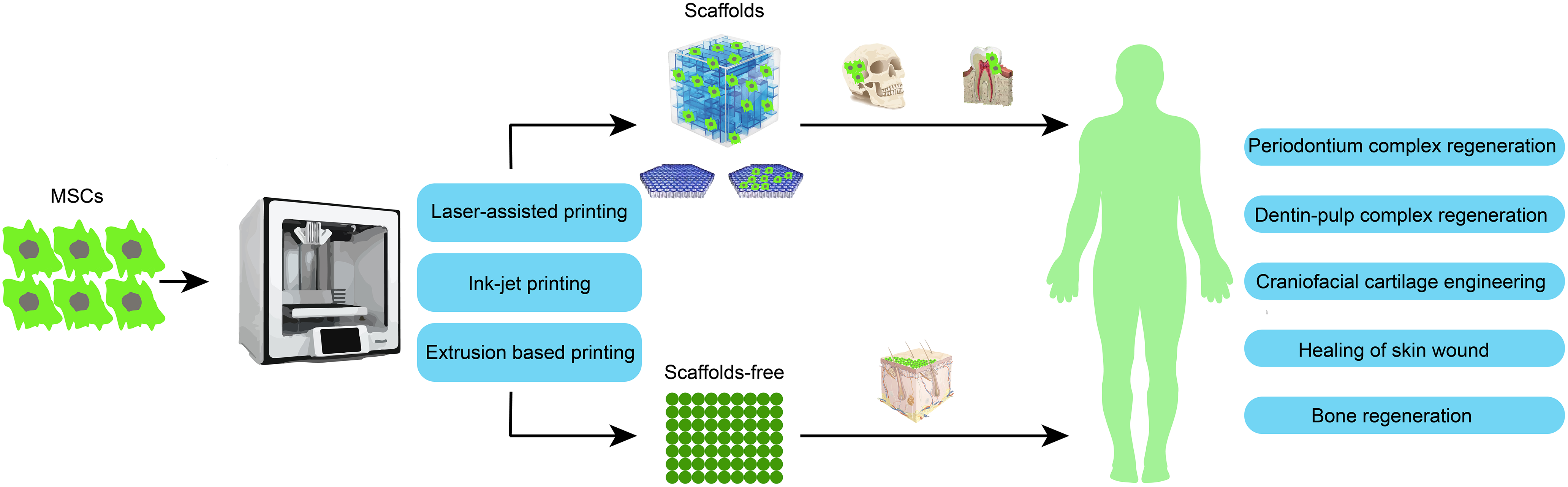
Figure 4: Overview of 3D bioprinting mainstream methods and applications in oral diseases (created by adobe illustrator).
Acknowledgement: We extend our gratitude to Ye Zhou for creating Fig. 4 and kindly providing it to us.
Funding Statement: This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD23030); National Natural Science Foundation of China (No. 82172254); Science and Technological Supports Project of Sichuan Province, China (No. 2024YFFK0214).
Author Contributions: Study conception and design: Dongbo Wu, Hua Zhang; draft manuscript preparation: Yixian You, Yihung Lee, Yushin Hu; review and editing: Wei Jiang, Enqiang Chen, Hong Tang; visualization: Youhui Xu, Jouchen Chen, Changhai Liu, Yixian You, Yihung Lee; supervision: Dongbo Wu, Hua Zhang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112(47):14452–9. doi:10.1073/pnas.1508520112. [Google Scholar] [PubMed] [CrossRef]
2. Pruett TL, Chandraker A. The white house organ summit: what it means for our field. Am J Transplant. 2016;16(8):2245–6. doi:10.1111/ajt.13947. [Google Scholar] [PubMed] [CrossRef]
3. Vanholder R, Dominguez-Gil B, Busic M, Cortez-Pinto H, Craig JC, Jager KJ, et al. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021;17(8):554–68. doi:10.1038/s41581-021-00425-3. [Google Scholar] [PubMed] [CrossRef]
4. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6. doi:10.1126/science.8493529. [Google Scholar] [PubMed] [CrossRef]
5. Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23(1):3–9. doi:10.1016/s0022-3468(88)80529-3. [Google Scholar] [PubMed] [CrossRef]
6. Wang C, Huang W, Zhou Y, He L, He Z, Chen Z, et al. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020;5(1):82–91. doi:10.1016/j.bioactmat.2020.01.004. [Google Scholar] [PubMed] [CrossRef]
7. Fan T, Wang S, Jiang Z, Ji S, Cao W, Liu W, et al. Controllable assembly of skeletal muscle-like bundles through 3D bioprinting. Biofabrication. 2022;14:015009. doi:10.1088/1758-5090/ac3aca. [Google Scholar] [PubMed] [CrossRef]
8. Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22(3):340–54. doi:10.1016/j.stem.2018.02.009. [Google Scholar] [PubMed] [CrossRef]
9. Moharamzadeh K, Colley H, Murdoch C, Hearnden V, Chai WL, Brook IM, et al. Tissue-engineered oral mucosa. J Dent Res. 2012;91(7):642–50. doi:10.1177/0022034511435702. [Google Scholar] [PubMed] [CrossRef]
10. Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009;36(5):368–89. doi:10.1111/j.1365-2842.2009.01939.x. [Google Scholar] [PubMed] [CrossRef]
11. Abou Neel EA, Chrzanowski W, Salih VM, Kim HW, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42(8):915–28. doi:10.1016/j.jdent.2014.05.008. [Google Scholar] [PubMed] [CrossRef]
12. Matichescu A, Ardelean LC, Rusu LC, Craciun D, Bratu EA, Babucea M, et al. Advanced biomaterials and techniques for oral tissue engineering and regeneration–a review. Materials. 2020;13(22):5303. doi:10.3390/ma13225303. [Google Scholar] [PubMed] [CrossRef]
13. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi:10.1038/emm.2013.94. [Google Scholar] [PubMed] [CrossRef]
14. Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int. 2017:5173732. doi:10.1155/2017/5173732. [Google Scholar] [PubMed] [CrossRef]
15. Farag MM. Recent trends on biomaterials for tissue regeneration applications: review. J Mater Sci. 2023;58(2):527–58. doi:10.1007/s10853-022-08102-x. [Google Scholar] [CrossRef]
16. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85. doi:10.1038/nbt.2958. [Google Scholar] [PubMed] [CrossRef]
17. Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res. 2015;94((9 Suppl)):143S–52S. doi:10.1177/0022034515588885. [Google Scholar] [PubMed] [CrossRef]
18. Lee UL, Yun S, Cao HL, Ahn G, Shim JH, Woo SH, et al. Bioprinting on 3D printed titanium scaffolds for periodontal ligament regeneration. Cells. 2021;10(6):1337. doi:10.3390/cells10061337. [Google Scholar] [PubMed] [CrossRef]
19. Kačarević Ž. P, Rider PM. An introduction to 3D Bioprinting: possibilities. Chall Future Asp. 2018;11(11):2199. doi:10.3390/ma11112199. [Google Scholar] [PubMed] [CrossRef]
20. Li G, Zhang B, Sun JH, Shi LY, Huang MY, Huang LJ, et al. An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact Mater. 2021;6(11):3766–81. doi:10.1016/j.bioactmat.2021.03.036. [Google Scholar] [PubMed] [CrossRef]
21. Mirdamadi E, Tashman JW, Shiwarski DJ, Palchesko RN, Feinberg AW. FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater Sci Eng. 2020;6(11):6453–9. doi:10.1021/acsbiomaterials.0c01133. [Google Scholar] [PubMed] [CrossRef]
22. Asulin M, Michael I, Shapira A, Dvir T. One-step 3D printing of heart patches with built-in electronics for performance regulation. Adv Sci. 2021;8(9):2004205. doi:10.1002/advs.202004205. [Google Scholar] [PubMed] [CrossRef]
23. Ozbolat IT. Scaffold-based or scaffold-free bioprinting: competing or complementing approaches? J Nanotechnol Eng Med. 2015;6(2):024701. doi:10.1115/1.4030414. [Google Scholar] [CrossRef]
24. Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3):032002. doi:10.1088/1758-5090/8/3/032002. [Google Scholar] [PubMed] [CrossRef]
25. Ebadi M, Miresmaeili A, Rajabi S, Shojaei S, Farhadi S. Isolation and characterization of apical papilla cells from root end of human third molar and their differentiation into cementoblast cells: an in vitro study. Biol Proced Online. 2023;25(1):2. doi:10.1186/s12575-023-00190-6. [Google Scholar] [PubMed] [CrossRef]
26. Daghrery A, Ferreira JA, Xu JP, Golafshan N, Kaigler D, Bhaduri SB, et al. Tissue-specific melt electrowritten polymeric scaffolds for coordinated regeneration of soft and hard periodontal tissues. Bioact Mater. 2023;19:268–81. doi:10.1016/j.bioactmat.2022.04.013. [Google Scholar] [PubMed] [CrossRef]
27. Dwivedi R, Mehrotra D. 3D bioprinting and craniofacial regeneration. J Oral Biol Craniofac Res. 2020;10(4):650–9. doi:10.1016/j.jobcr.2020.08.011. [Google Scholar] [PubMed] [CrossRef]
28. Buyuksungur S, Hasirci V, Hasirci N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J Biomed Mater Res A. 2021;109(12):2425–37. doi:10.1002/jbm.a.37235. [Google Scholar] [PubMed] [CrossRef]
29. Mahdavi-Jouibari F, Parseh B, Kazeminejad E, Khosravi A. Hopes and opportunities of stem cells from human exfoliated deciduous teeth (SHED) in cartilage tissue regeneration. Front Bioeng Biotech. 2023;11:1021024. doi:10.3389/fbioe.2023.1021024. [Google Scholar] [PubMed] [CrossRef]
30. Puppi D, Chiellini F. Biodegradable polymers for biomedical additive manufacturing. Appl Mater Today. 2020;20:100700. doi:10.1016/j.apmt.2020.100700. [Google Scholar] [CrossRef]
31. Zhang S, Li Q, Liu P, Lin C, Tang Z, Wang HL. Three-dimensional cell printed lock-key structure for oral soft and hard tissue regeneration. Tissue Eng Part A. 2021. doi:10.1089/ten.TEA.2021.0022. [Google Scholar] [PubMed] [CrossRef]
32. Neufurth M, Wang S, Schröder HC, Al-Nawas B, Wang X, Müller WEG. 3D bioprinting of tissue units with mesenchymal stem cells, retaining their proliferative and differentiating potential, in polyphosphate-containing bio-ink. Biofabrication. 2021;14(1):015016. doi:10.1088/1758-5090/ac3f29. [Google Scholar] [PubMed] [CrossRef]
33. Unagolla JM, Jayasuriya AC. Hydrogel-based 3D bioprinting: a comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl Mater Today. 2020;18:100479. doi:10.1016/j.apmt.2019.100479. [Google Scholar] [PubMed] [CrossRef]
34. Wang Z, Xiang L, Lin F, Tang Y, Cui W. 3D bioprinting of emulating homeostasis regulation for regenerative medicine applications. J Control Release. 2023;353:147–65. doi:10.1016/j.jconrel.2022.11.035. [Google Scholar] [PubMed] [CrossRef]
35. Salaris F, Rosa A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: challenges and opportunities. Brain Res. 2019;1723:146393. doi:10.1016/j.brainres.2019.146393. [Google Scholar] [PubMed] [CrossRef]
36. Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, et al. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109(7):1855–63. doi:10.1002/bit.24455. [Google Scholar] [PubMed] [CrossRef]
37. Keriquel V, Oliveira H, Remy M, Ziane S, Delmond S, Rousseau B, et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci Rep. 2017;7(1):1778. doi:10.1038/s41598-017-01914-x. [Google Scholar] [PubMed] [CrossRef]
38. Shirazi SF, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HS, Adib Kadri N, et al. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater. 2015;16(3):033502. doi:10.1088/1468-6996/16/3/033502. [Google Scholar] [PubMed] [CrossRef]
39. Pantermehl S, Emmert S, Foth A, Grabow N, Alkildani S, Bader R, et al. 3D printing for soft tissue regeneration and applications in medicine. Biomedicines. 2021;9(4):336. doi:10.3390/biomedicines9040336. [Google Scholar] [PubMed] [CrossRef]
40. Saijo H, Igawa K, Kanno Y, Mori Y, Kondo K, Shimizu K, et al. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J Artif Organs. 2009;12(3):200–5. doi:10.1007/s10047-009-0462-7. [Google Scholar] [PubMed] [CrossRef]
41. Han J, Kim DS, Jang H, Kim HR, Kang HW. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J Tissue Eng. 2019;10:2041731419845849. doi:10.1177/2041731419845849. [Google Scholar] [PubMed] [CrossRef]
42. Kérourédan O, Hakobyan D, Rémy M, Ziane S, Dusserre N, Fricain JC, et al. In situ prevascularization designed by laser-assisted bioprinting: effect on bone regeneration. Biofabrication. 2019;11(4):045002. doi:10.1088/1758-5090/ab2620. [Google Scholar] [PubMed] [CrossRef]
43. Cooper GM, Miller ED, Decesare GE, Usas A, Lensie EL, Bykowski MR, et al. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Eng Part A. 2010;16(5):1749–59. doi:10.1089/ten.TEA.2009.0650. [Google Scholar] [PubMed] [CrossRef]
44. Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9(10):1304–11. doi:10.1002/biot.201400305. [Google Scholar] [PubMed] [CrossRef]
45. Kim SE, Shim KM, Jang K, Shim JH, Kang SS. Three-dimensional printing-based reconstruction of a maxillary bone defect in a dog following tumor removal. In vivo. 2018;32(1):63–70. doi:10.21873/invivo.11205. [Google Scholar] [PubMed] [CrossRef]
46. Kundu J, Shim JH, Jang J, Kim SW, Cho DW. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9(11):1286–97. doi:10.1002/term.1682. [Google Scholar] [PubMed] [CrossRef]
47. Jensen MB, Slots C, Ditzel N, Kolstrup S, Kassem M, Thygesen T, et al. Treating mouse skull defects with 3D-printed fatty acid and tricalcium phosphate implants. J Tissue Eng Regen Med. 2020;14(12):1858–68. doi:10.1002/term.3146. [Google Scholar] [PubMed] [CrossRef]
48. Dragan E, Nemtoi A. Review of the long-term outcomes of guided bone regeneration and autologous bone block augmentation for vertical dental restoration of dental implants. Med Sci Monit. 2022;28:e937433. doi:10.12659/MSM.937433. [Google Scholar] [PubMed] [CrossRef]
49. Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014;20(7–8):1342–51. doi:10.1089/ten.tea.2013.0386. [Google Scholar] [PubMed] [CrossRef]
50. Morrison RJ, Nasser HB, Kashlan KN, Zopf DA, Milner DJ, Flanangan CL, et al. Co-culture of adipose-derived stem cells and chondrocytes on three-dimensionally printed bioscaffolds for craniofacial cartilage engineering. Laryngoscope. 2018;128(7):E251–7. doi:10.1002/lary.27200. [Google Scholar] [PubMed] [CrossRef]
51. Moncal KK, Aydin RST, Abu-Laban M, Heo DN, Rizk E, Tucker SM, et al. Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater Sci Eng C Mater Biol Appl. 2019;105(11):110128. doi:10.1016/j.msec.2019.110128. [Google Scholar] [PubMed] [CrossRef]
52. Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomed Mater Res B Appl Biomater. 2018;106(5):1788–98. doi:10.1002/jbm.b.33994. [Google Scholar] [PubMed] [CrossRef]
53. Tu C, Chen J, Huang C, Xiao Y, Tang X, Li H, et al. Effects of electromagnetic fields treatment on rat critical-sized calvarial defects with a 3D-printed composite scaffold. Stem Cell Res Ther. 2020;11(1):433. doi:10.1186/s13287-020-01954-7. [Google Scholar] [PubMed] [CrossRef]
54. Roskies M, Jordan JO, Fang D, Abdallah MN, Hier MP, Mlynarek A, et al. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J Biomater Appl. 2016;31(1):132–9. doi:10.1177/0885328216638636. [Google Scholar] [PubMed] [CrossRef]
55. Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–9. doi:10.1038/nbt.3413. [Google Scholar] [PubMed] [CrossRef]
56. Park JH, Gillispie GJ, Copus JS, Zhang W, Atala A, Yoo JJ, et al. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication. 2020;12(3):035029. doi:10.1088/1758-5090/ab9492. [Google Scholar] [PubMed] [CrossRef]
57. Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1(11):792–802. doi:10.5966/sctm.2012-0088. [Google Scholar] [PubMed] [CrossRef]
58. Kim BS, Kwon YW, Kong JS, Park GT, Gao G, Han W, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. doi:10.1016/j.biomaterials.2018.03.040. [Google Scholar] [PubMed] [CrossRef]
59. Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15–31. doi:10.1016/j.bioactmat.2021.09.014. [Google Scholar] [PubMed] [CrossRef]
60. Soriente A, Fasolino I, Gomez-Sánchez A, Prokhorov E, Buonocore GG, Luna-Barcenas G, et al. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J Biomed Mater Res A. 2022;110(2):266–72. doi:10.1002/jbm.a.37283. [Google Scholar] [PubMed] [CrossRef]
61. Hwangbo H, Lee H, Jin EJ, Lee J, Jo Y, Ryu D, et al. Bio-printing of aligned GelMa-based cell-laden structure for muscle tissue regeneration. Bioact Mater. 2022;8:57–70. doi:10.1016/j.bioactmat.2021.06.031. [Google Scholar] [PubMed] [CrossRef]
62. Liu Y, Fan L, Lin X, Zou L, Li Y, Ge X, et al. Functionalized self-assembled peptide RAD/Dentonin hydrogel scaffold promotes dental pulp regeneration. Biomed Mater. 2021;17(1):015009. doi:10.1088/1748-605X/ac3928. [Google Scholar] [PubMed] [CrossRef]
63. Chen CY, Shie MY, Lee AK, Chou YT, Chiang C, Lin CP. 3D-printed ginsenoside Rb1-loaded mesoporous calcium silicate/calcium sulfate scaffolds for inflammation inhibition and bone regeneration. Biomedicines. 2021;9(8):907. doi:10.3390/biomedicines9080907. [Google Scholar] [PubMed] [CrossRef]
64. Wang W, Zhu Y, Liu Y, Chen B, Li M, Yuan C, et al. 3D bioprinting of DPSCs with GelMA hydrogel of various concentrations for bone regeneration. Tissue Cell. 2024;88:102418. doi:10.1016/j.tice.2024.102418. [Google Scholar] [PubMed] [CrossRef]
65. Wang W, Zhu Y, Li J, Geng T, Jia J, Wang X, et al. Bioprinting EphrinB2-modified dental pulp stem cells with enhanced osteogenic capacity for alveolar bone engineering. Tissue Eng Part A. 2023;29(7–8):244–55. doi:10.1089/ten.tea.2022.0180. [Google Scholar] [PubMed] [CrossRef]
66. Tian Y, Liu M, Liu Y, Shi C, Wang Y, Liu T, et al. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J Biomed Mater Res A. 2021;109(7):1209–19. doi:10.1002/jbm.a.37114. [Google Scholar] [PubMed] [CrossRef]
67. Sajad Daneshi S, Tayebi L, Talaei-Khozani T, Tavanafar S, Hadaegh AH, Rasoulianboroujeni M, et al. Reconstructing critical-sized mandibular defects in a rabbit model: enhancing angiogenesis and facilitating bone regeneration via a cell-loaded 3D-printed hydrogel-ceramic scaffold application. ACS Biomater Sci Eng. 2024;10(5):3316–30. doi:10.1021/acsbiomaterials.4c00580. [Google Scholar] [PubMed] [CrossRef]
68. Cho H, Tarafder S, Fogge M, Kao K, Lee CH. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect Tissue Res. 2016;57(6):488–95. doi:10.1080/03008207.2016.1191478. [Google Scholar] [PubMed] [CrossRef]
69. Ahn G, Lee JS, Yun WS, Shim JH, Lee UL. Cleft Alveolus reconstruction using a three-dimensional printed bioresorbable scaffold with human bone marrow cells. J Craniofac Surg. 2018;29(7):1880–3. doi:10.1097/SCS.0000000000004747. [Google Scholar] [PubMed] [CrossRef]
70. Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94(9 Suppl):153S–7S. doi:10.1177/0022034515588303. [Google Scholar] [PubMed] [CrossRef]
71. Klabukov I, Baranovskii D. Stem cells and their derivatives: unlocking the promising potential of minimally manipulated cells for in situ tissue engineering. Cell Transplant. 2024;33:9636897231221846. doi:10.1177/09636897231221846. [Google Scholar] [PubMed] [CrossRef]
72. Krasilnikova OA, Baranovskii DS, Yakimova AO, Arguchinskaya N, Kisel A, Sosin D, et al. Intraoperative creation of tissue-engineered grafts with minimally manipulated cells: new concept of bone tissue engineering in situ. Bioengineering. 2022;9(11):704. doi:10.3390/bioengineering9110704. [Google Scholar] [PubMed] [CrossRef]
73. Bajuri MY, Kim J, Yu Y, Shahul Hameed MS. New paradigm in diabetic foot ulcer grafting techniques using 3D-bioprinted autologous minimally manipulated homologous adipose tissue (3D-amhat) with fibrin gel acting as a biodegradable scaffold. Gels. 2023;9(1):66. doi:10.3390/gels9010066. [Google Scholar] [PubMed] [CrossRef]
74. Li J, Liu Y, Zhang Y, Yao B, Enhejirigala, Li Z, et al. Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Front Cell Dev Biol. 2021;9:640388. doi:10.3389/fcell.2021.640388. [Google Scholar] [PubMed] [CrossRef]
75. Jia X, Song J, Lv W, Hill JP, Nakanishi J, Ariga K. Adaptive liquid interfaces induce neuronal differentiation of mesenchymal stem cells through lipid raft assembly. Nat Commun. 2022;13(1):3110. doi:10.1038/s41467-022-30622-y. [Google Scholar] [PubMed] [CrossRef]
76. Qian J, Wang Y, Li X, Lu J. Hydrogel microenvironment contributes to chemical-induced differentiation of mesenchymal stem cells: single-cell infrared microspectroscopy characterization. Anal Bioanal Chem. 2023;415(17):3305–12. doi:10.1007/s00216-023-04746-z. [Google Scholar] [PubMed] [CrossRef]
77. Xue G, Zhang J, Wu L, Sun S, Wu H, Hou Y, et al. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes with CYP450 metabolic enzyme activity induced by a liver injury microenvironment. Biochemical and BioO, 2023;647:47–54. doi:10.1016/j.bbrc.2023.01.065. [Google Scholar] [PubMed] [CrossRef]
78. Irvine SA, Venkatraman SS. Bioprinting and differentiation of stem cells. Molecules. 2016;21(9):1188. doi:10.3390/molecules21091188. [Google Scholar] [PubMed] [CrossRef]
79. Abuwatfa WH, Pitt WG, Husseini GA. Scaffold-based 3D cell culture models in cancer research. J Biomed Sci. 2024;31(1):7. doi:10.1186/s12929-024-00994-y. [Google Scholar] [PubMed] [CrossRef]
80. Huang X, Huang Z, Gao W, Gao W, He R, Li Y, et al. Current advances in 3D dynamic cell culture systems. Gels. 2022;8(12):829. doi:10.3390/gels8120829. [Google Scholar] [PubMed] [CrossRef]
81. Khlusov IA, Litvinova LS, Yurova KA, Khlusova MY. Precise tissue bioengineering and niches of mesenchymal stem cells: their size and hierarchy matter. Biocell. 2022;46(6):1365–73. doi:10.32604/biocell.2022.018917. [Google Scholar] [CrossRef]
82. Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11(3):176–87. doi:10.1038/nrn2761. [Google Scholar] [PubMed] [CrossRef]
83. Samal JRK, Rangasami VK, Samanta S, Varghese OP, Oommen OP. Discrepancies on the role of oxygen gradient and culture condition on mesenchymal stem cell fate. Adv Healthc Mater. 2021;10(6):e2002058. doi:10.1002/adhm.202002058. [Google Scholar] [PubMed] [CrossRef]
84. Adamzyk C, Emonds T, Falkenstein J, Tolba R, Jahnen-Dechent W, Lethaus B, et al. Different culture media affect proliferation, surface epitope expression, and differentiation of ovine MSC. Stem Cells Int. 2013;2013:387324. doi:10.1155/2013/387324. [Google Scholar] [PubMed] [CrossRef]
85. Smith JR, Pfeifer K, Petry F, Powell N, Delzeit J, Weiss ML. Standardizing umbilical cord mesenchymal stromal cells for translation to clinical use: selection of GMP-compliant medium and a simplified isolation method. Stem Cells Int. 2016;2016:6810980. doi:10.1155/2016/6810980. [Google Scholar] [PubMed] [CrossRef]
86. Legzdina D, Romanauska A, Nikulshin S, Kozlovska T, Berzins U. Characterization of senescence of culture-expanded human adipose-derived mesenchymal stem cells. Int J Stem Cells. 2016;9(1):124–36. doi:10.15283/ijsc.2016.9.1.124. [Google Scholar] [PubMed] [CrossRef]
87. Ji F, Duan HG, Zheng CQ, Li J. Comparison of chloromethyl-dialkylcarbocyanine and green fluorescent protein for labeling human umbilical mesenchymal stem cells. Biotechnol Lett. 2015;37(2):437–47. doi:10.1007/s10529-014-1692-1. [Google Scholar] [PubMed] [CrossRef]
88. Nagyova M, Slovinska L, Blasko J, Grulova I, Kuricova M, Cigankova V, et al. A comparative study of PKH67, DiI, and BrdU labeling techniques for tracing rat mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2014;50(7):656–63. doi:10.1007/s11626-014-9750-5. [Google Scholar] [PubMed] [CrossRef]
89. Rodriguez BL, Vega-Soto EE, Kennedy CS, Nguyen MH, Cederna PS, Larkin LM. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLoS One. 2020;15(9):e0239152. doi:10.1371/journal.pone.0239152. [Google Scholar] [PubMed] [CrossRef]
90. Pare A, Charbonnier B, Tournier P, Vignes C, Veziers J, Lesoeur J, et al. Tailored three-dimensionally printed triply periodic calcium phosphate implants: a preclinical study for craniofacial bone repair. ACS Biomater Sci Eng. 2020;6(1):553–63. doi:10.1021/acsbiomaterials.9b01241. [Google Scholar] [PubMed] [CrossRef]
91. Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, et al. Dental pulp stem cells: their potential in reinnervation and angiogenesis by using scaffolds. J Endod. 2017;43(9s):S12–6. doi:10.1016/j.joen.2017.06.001. [Google Scholar] [PubMed] [CrossRef]
92. Hsiao D, Hsu SH, Chen RS, Chen MH. Characterization of designed directional polylactic acid 3D scaffolds for neural differentiation of human dental pulp stem cells. J Formos Med Assoc. 2020;119:268–75. doi:10.1016/j.jfma.2019.05.011. [Google Scholar] [PubMed] [CrossRef]
93. Xuan YW, Guo YB, Li L, Zhang CP, Yin XL, Zhang Z. 3D-printed bredigite scaffolds with ordered arrangement structures promote bone regeneration by inducing macrophage polarization in onlay grafts. J Nanobiotechnol. 2024;22(1):102. doi:10.1186/s12951-024-02362-2. [Google Scholar] [PubMed] [CrossRef]
94. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi:10.1016/j.smim.2007.11.004. [Google Scholar] [PubMed] [CrossRef]
95. Naomi R, Bahari H, Ridzuan PM, Othman F. Natural-based biomaterial for skin wound healing (Gelatin vs. Collagen). Expert Review. Polymers. 2021;13(14):2319. doi:10.3390/polym13142319. [Google Scholar] [PubMed] [CrossRef]
96. Frent OD, Vicas LG, Duteanu N, Morgovan CM, Jurca T, Pallag A, et al. Sodium alginate-natural microencapsulation material of polymeric microparticles. Int J Mol Sci. 2022;23(20):12108. doi:10.3390/ijms232012108. [Google Scholar] [PubMed] [CrossRef]
97. Oliveira MI, Santos SG, Oliveira MJ, Torres AL, Barbosa MA. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur Cell Mater. 2012;24:136–52. doi:10.22203/ecm.v024a10. [Google Scholar] [PubMed] [CrossRef]
98. VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59(3):585–90. doi:10.1002/jbm.1270. [Google Scholar] [PubMed] [CrossRef]
99. Li X, Xing R, Xu C, Liu S, Qin Y, Li K, et al. Immunostimulatory effect of chitosan and quaternary chitosan: a review of potential vaccine adjuvants. Carbohydr Polym. 2021;264:118050. doi:10.1016/j.carbpol.2021.118050. [Google Scholar] [PubMed] [CrossRef]
100. Fan C, Wang H, editors Connection between hyaluronic acid and toll-like receptor in signal transduction. In: Joint Conferences of International Conference on Computer Science and Engineering Technology (CSET)/International Conference on Medical Science and Biological Engineering (MSBE), 2016; Hong Kong, China. [Google Scholar]
101. Alblawi A, Ranjani AS, Yasmin H, Gupta S, Bit A, Rahimi-Gorji M. Scaffold-free: a developing technique in field of tissue engineering. Comput Meth Prog Bio. 2020;185:105148. doi:10.1016/j.cmpb.2019.105148. [Google Scholar] [PubMed] [CrossRef]
102. De Pieri A, Rochev Y, Zeugolis DI. Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. npj Regen Med. 2021;6(1):18. doi:10.1038/s41536-021-00133-3. [Google Scholar] [PubMed] [CrossRef]
103. Breathwaite EK, Weaver JR, Murchison AC, Treadwell ML, Odanga JJ, Lee JB. Scaffold-free bioprinted osteogenic and chondrogenic systems to model osteochondral physiology. Biomed Mater. 2019;14(6):065010. doi:10.1088/1748-605X/ab4243. [Google Scholar] [PubMed] [CrossRef]
104. Zhang Q, Nguyen PD, Shi S, Burrell JC, Cullen DK, Le AD. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep. 2018;8(1):6634. doi:10.1038/s41598-018-24888-w. [Google Scholar] [PubMed] [CrossRef]
105. Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–74. doi:10.1016/j.biomaterials.2008.12.084. [Google Scholar] [PubMed] [CrossRef]
106. Lee JK, Link JM, Hu JCY, Athanasiou KA. The self-assembling process and applications in tissue engineering. Cold Spring Harb Perspect Med. 2017;7(11). doi:10.1101/cshperspect.a025668. [Google Scholar] [PubMed] [CrossRef]
107. Tewary M, Shakiba N, Zandstra PW. Stem cell bioengineering: building from stem cell biology. Nat Rev Genet. 2018;19(10):595–614. doi:10.1038/s41576-018-0040-z. [Google Scholar] [PubMed] [CrossRef]
108. Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–45. doi:10.1634/stemcells.2007-0054. [Google Scholar] [PubMed] [CrossRef]
109. Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89(8):842–7. doi:10.1177/0022034510370803. [Google Scholar] [PubMed] [CrossRef]
110. Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9(1):17. doi:10.1038/s41392-023-01704-0. [Google Scholar] [PubMed] [CrossRef]
111. Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG, et al. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11:590470. doi:10.3389/fphar.2020.590470. [Google Scholar] [PubMed] [CrossRef]
112. Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol. 2016;4(556):83. doi:10.3389/fcell.2016.00083. [Google Scholar] [PubMed] [CrossRef]
113. Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–44. doi:10.1089/scd.2013.0479. [Google Scholar] [PubMed] [CrossRef]
114. Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357(6353):4266. doi:10.1126/science.aal2379. [Google Scholar] [PubMed] [CrossRef]
115. Schlosser S, Dennler C, Schweizer R, Eberli D, Stein JV, Enzmann V, et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res. 2012;83(3):267–75. doi:10.1016/j.mvr.2012.02.011. [Google Scholar] [PubMed] [CrossRef]
116. Zha Y, Li Y, Lin T, Chen J, Zhang S, Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11(1):397–409. doi:10.7150/thno.50741. [Google Scholar] [PubMed] [CrossRef]
117. Fang J, Feng C, Chen W, Hou P, Liu Z, Zuo M, et al. Redressing the interactions between stem cells and immune system in tissue regeneration. Biol Direct. 2021;16(1). doi:10.1186/s13062-021-00306-6. [Google Scholar] [PubMed] [CrossRef]
118. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41(9):653–64. doi:10.1016/j.tips.2020.06.009. [Google Scholar] [PubMed] [CrossRef]
119. Chen JH, Huang YB, Tang HL, Qiao XC, Sima X, Guo WH. A xenogeneic extracellular matrix-based 3D printing scaffold modified by ceria nanoparticles for craniomaxillofacial hard tissue regeneration via osteo-immunomodulation. Biomed Mater. 2024;19(4). doi:10.1088/1748-605X/ad475c. [Google Scholar] [PubMed] [CrossRef]
120. Macalester W, Boussahel A, Moreno-Tortolero RO, Shannon MR, West N, Hill D, et al. A 3D in-vitro model of the human dentine interface shows long-range osteoinduction from the dentine surface. Int J Oral Sci. 2024;16(1). doi:10.1038/s41368-024-00298-9. [Google Scholar] [PubMed] [CrossRef]
121. Miao G, Liang L, Li W, Ma C, Pan Y, Zhao H, et al. 3D bioprinting of a bioactive composite scaffold for cell delivery in periodontal tissue regeneration. Biomolecules. 2023;13(7). doi:10.3390/biom13071062. [Google Scholar] [PubMed] [CrossRef]
122. Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–21. doi:10.1161/01.res.0000225952.61196.39. [Google Scholar] [PubMed] [CrossRef]
123. Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, et al. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. Faseb j. 2014;28(4):1634–43. doi:10.1096/fj.13-243980. [Google Scholar] [PubMed] [CrossRef]
124. Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC. Self-organization and the self-assembling process in tissue engineering. Annu Rev Biomed Eng. 2013;15(1):115–36. doi:10.1146/annurev-bioeng-071812-152423. [Google Scholar] [PubMed] [CrossRef]
125. Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020;4(4):370–80. doi:10.1038/s41551-019-0471-7. [Google Scholar] [PubMed] [CrossRef]
126. Liu Y, Peng L, Li L, Huang C, Shi K, Meng X, et al. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279. doi:10.1016/j.biomaterials.2021.121216. [Google Scholar] [PubMed] [CrossRef]
127. Lovell-Badge R, Anthony E, Barker RA, Bubela T, Brivanlou AH, Carpenter M, et al. ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Rep. 2021;16(6):1398–408. doi:10.1016/j.stemcr.2021.05.012. [Google Scholar] [PubMed] [CrossRef]
128. Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCsmolecular mechanisms of induction and applications. Signal Transduct Target Ther. 2024;9(1):112. doi:10.1038/s41392-024-01809-0. [Google Scholar] [PubMed] [CrossRef]
129. Huang CY, Liu CL, Ting CY, Chiu YT, Cheng YC, Nicholson MW, et al. Human iPSC banking: barriers and opportunities. J Biomed Sci. 2019;26(1):87. doi:10.1186/s12929-019-0578-x. [Google Scholar] [PubMed] [CrossRef]
130. Xin HL, Liu YY, Xiao YN, Wen M, Sheng LY, Jia ZJ. Design and nanoengineering of photoactive antimicrobials for bioapplications: from fundamentals to advanced strategies. Adv Funct Mater. 2024. doi:10.1002/adfm.202402607. [Google Scholar] [CrossRef]
131. Zhao FX, Zhang ZJ, Guo WH. The 3-dimensional printing for dental tissue regeneration: the state of the art and future challenges. Front Bioeng Biotech. 2024;12. doi:10.3389/fbioe.2024.1356580. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF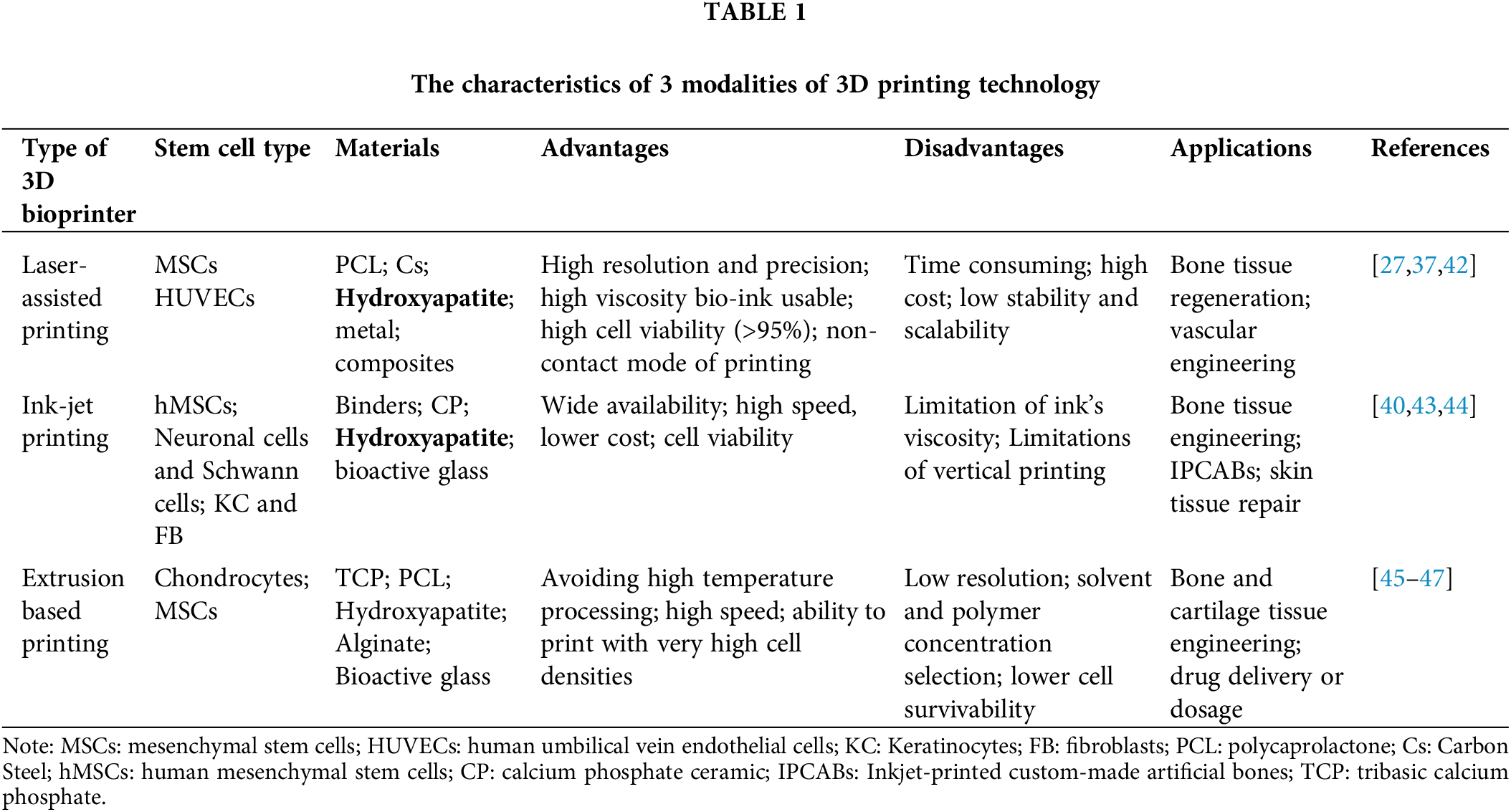
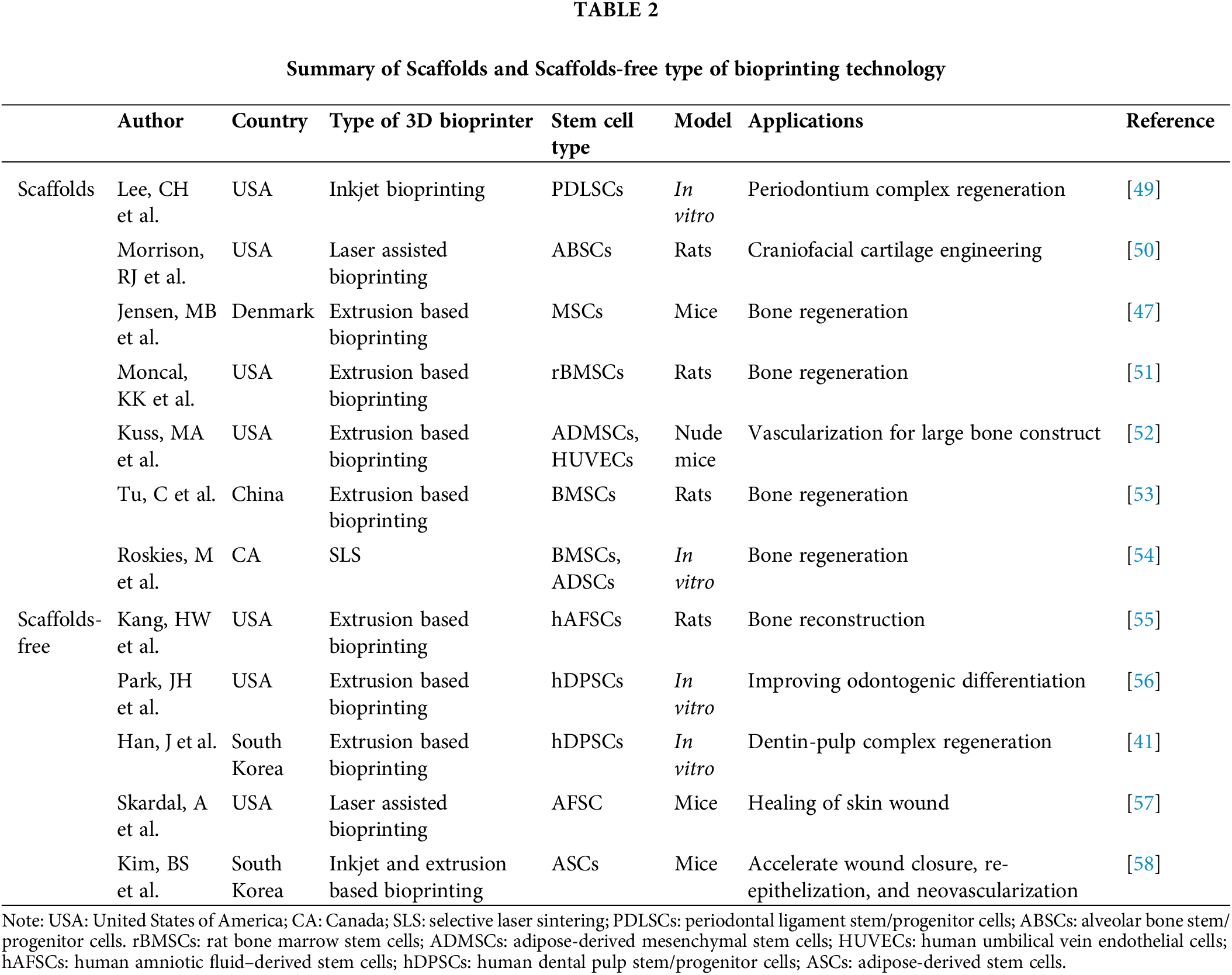
 Downloads
Downloads
 Citation Tools
Citation Tools