 Open Access
Open Access
ARTICLE
Tetramethylpyrazine Alleviates Pancreatitis Progression by Regulating Inflammation and Autophagy through the YAP-RIPK1-NF-κB Axis
General Surgery Department, The First Hospital of Yulin, No. 59, Wenhua Road, Suide County, Yulin, 718000, China
* Corresponding Author: Yang Liu. Email:
BIOCELL 2025, 49(10), 1985-2006. https://doi.org/10.32604/biocell.2025.069527
Received 25 June 2025; Accepted 29 August 2025; Issue published 22 October 2025
Abstract
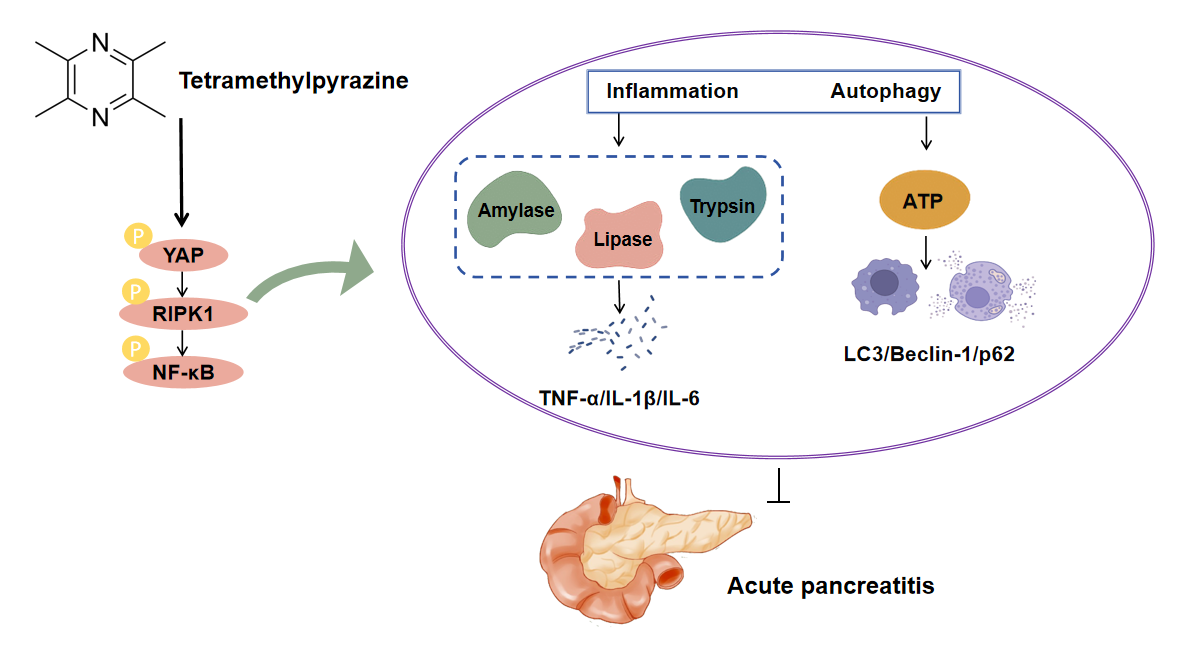
Background: Acute pancreatitis (AP) is a serious gastrointestinal disorder. Tetramethylpyrazine (TMP), a bioactive alkaloid extracted from Ligusticum chuanxiong, exhibits various pharmacological effects, but its protective mechanisms against AP remain unclear. This study aimed to investigate the protective effects and underlying mechanisms of TMP in AP. Methods: The study utilized Cerulein (CER) to model pancreatitis across experimental systems. Cellular responses were characterized through functional assays (CCK-8 viability, EdU proliferation, Transwell migration, flow cytometric apoptosis, Fluo-3/AM calcium imaging) and inflammatory profiling (ELISA for trypsin, CRP, TNF-α, IL-1β, IL-6). Autophagy was monitored via mRFP-GFP-LC3 flux and LysoTracker staining, with parallel LDH measurements. Mechanistic studies examined autophagy-related proteins (LAMP-1, p62, LC3) and YAP-RIPK1-NF-κB signaling intermediates by Western blot, supported by YAP genetic manipulation. In vivo assessments included HE-stained histology, TUNEL apoptosis detection, and MPO-based neutrophil evaluation. Results: TMP dose-dependently improved CER-induced cell viability and ATP levels, inhibited cell apoptosis, reduced Ca2+ concentration, LDH release, and inflammatory responses (trypsin, CRP, TNF-α, IL-1β, IL-6, and NLRP3). TMP also upregulated LAMP-1 protein expression, downregulating p62, Beclin-1, Atg5, LC3 protein levels, promoting autophagosome-lysosome fusion, and enhancing lysosomal activity, thus alleviating pancreatic damage. Additionally, TMP inhibited YAP, RIPK1, and NF-κB p65 phosphorylation, with YAP knockdown enhancing TMP effects while YAP overexpression partially counteracted TMP actions. In the rat AP model, TMP significantly improved tissue pathological changes, reduced inflammatory infiltration and cell apoptosis, decreased serum inflammatory marker levels, and inhibited YAP-RIPK1-NF-κB axis activation. Conclusion: This study reveals that TMP alleviates AP progression by inhibiting YAP-RIPK1-NF-κB axis activation, achieving dual effects of anti-inflammation and promoting autophagy.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools