 Open Access
Open Access
COMMENTARY
Exendin-4 and Wound Healing
Research Unit of Histology and Embryology, Department of Biology, Florence, 50134, Italy
* Corresponding Author: Stefano Bacci. Email:
(This article belongs to the Special Issue: Understanding Cellular Mechanisms in Wound Healing During Therapeutic Interventions)
BIOCELL 2025, 49(11), 2137-2145. https://doi.org/10.32604/biocell.2025.069216
Received 17 June 2025; Accepted 19 September 2025; Issue published 24 November 2025
Abstract
This commentary analyzes the effect of Exendin-4 on wound healing. After introducing information about the drug, considerations are added regarding its impact on this process. To accelerate wound healing, the drug’s combined effects with stem cells and other factors are examined, aiming to enhance and thus accelerate the process. Finally, the clinical potential of this drug’s effect is considered, not only for wound healing but also in other diseases. Therefore, reading this commentary may provide research perspectives that can stimulate the future reader’s thinking.Keywords
Exendin-4, an insulinotropic intestinal peptide, is a bioactive polypeptide peptide of 39 amino acid residues, isolated from the saliva of the Gila monster (Heloderma suspectum) that shares 53% homology with Glucagon-like peptide-1 (GLP-1). It activates the GLP-1 receptor (GLP-1R), emulating the majority of GLP-1’s effects, such as internalization and phosphorylation (Figs. 1 and 2).

Figure 1: Gila Monster [1]
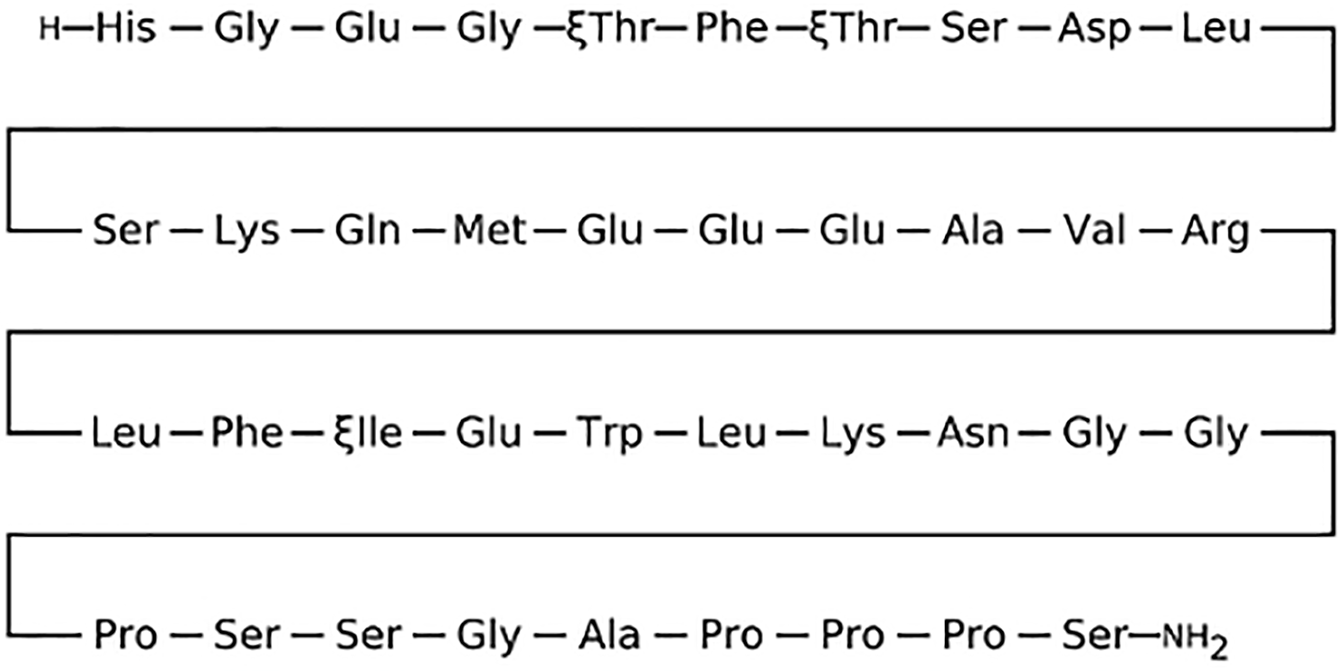
Figure 2: Amino acid structure of Exendin-4 [2]
GLP-1 stimulates insulin release from pancreatic beta cells, secreting approximately 60% of post-prandial insulin. In type 2 diabetes patients, GLP-1’s insulin-secreting efficiency is strongly reduced. Exendin-4, an anti-diabetic compound, elevates cyclic AMP levels in pancreatic acinar cells and functions as a GLP-1 agonist and incretin mimic, promoting insulin production and inhibiting incorrect glucagon release. It further inhibits stomach emptying and emulates the majority of GLP-1 actions [3,4].
Exendin-4 has been employed in anti-diabetic therapy, offering benefits such as reducing the risk of hypoglycemia, preventing cardiovascular and neurological complications, enhancing foot ulcer recovery, and alleviating pain. Nevertheless, it is uncertain whether these effects are secondary to hyperglycemia correction or if they are contingent upon extra-pancreatic GLP-1R activation. Exendin-4 (9-39), a peptide that lacks intrinsic activity at the GLP-1R, is classified as a competitive antagonist. The binding of Exendin-4 and Exendin-4 (9-39) to the GLP-1R would be mutually exclusive. Exendin-4 and Exendin-4 (9-39) are both critical experimental instruments for the study of the pharmacology of GLP-1R [3,4].
Wound healing is a multifaceted process including four primary phases: hemostasis, inflammation, proliferation, and remodeling [5,6].
Hemostasis begins when endothelial cells release von Willebrand factor, facilitating platelet adhesion and mediator release, culminating in a fibrin clot that halts hemorrhage. Rapid arterial constriction occurs via smooth muscle contraction, diminishing flow and generating vasoactive metabolites [5,6].
During the inflammation phase, mast cells (MC) release histamine or serotonin, which facilitates, through diapedesis, the movement of neutrophils and monocytes to the affected area. Within the wound, phagocytosis is boosted to fight off bacteria or damaged cells. Leukocytes start the proliferative process by releasing cytokines and growth factors. Other cell types, like keratinocytes, also help by releasing cytokines that cause inflammation [5,6].
The proliferation phase involves fibroblasts in the development of granulation tissue and the modulation of keratinocyte migration and proliferation. Macrophages and MC perpetually release growth factors implicated in this process [5,6].
The maturation phase differentiates between collagen restoration and wound contraction, with myofibroblasts derived from fibroblasts being pivotal. Growth factors govern transitions between mesenchymal-mesenchymal and endothelial-mesenchymal phenotypes via the Transforming Growth Factor (TGF) beta or Notch signaling pathways. Beta2AR is a pivotal molecule that facilitates the epithelial-mesenchymal transition process [5,6].
The ensuing development of the scar entails the remodeling of granulation tissue, in which matrix metalloproteinases (MMPs) and their inhibitors are pivotal. This leads to diminished extracellular matrix production and alteration of its constituents, including the substitution of type III collagen for type I collagen. The demise of certain cell types within granulation tissue is regarded as a crucial event in wound resolution [5,6].
Exendin-4 enhances wound healing by boosting angiogenesis, collagen production, and cell proliferation (see Table 1 and Fig. 3) [7]. Via GLP-1R signaling, Exendin-4 directly generates a quantitative increase in fibroblasts/myofibroblasts relative to non-treated wounds. These effects are inhibited when this drug is given to wounds in the presence of Exendin-4 (9-39), an antagonist of GLP-1R [8]. In a murine model, after 48 h, MC (expressing GLP-1R) generate Tumour Necrosis Factor (TNF) alpha, HSP47, and raise their degranulation index. These cells move, minimizing distance to dendritic cells (DC) or vasculature and improving intercellular interactions with granulocytes. M2 macrophages grow in quantity in the dermis, whereas M1 macrophages exclusively express Tumour Growth Factor (TGF) beta. Plasmacytoid DC rise and express CD25 or interact with Treg cells. The positive effects of Exendin-4 on wound regeneration may entail MC’s important function in the release of TNFalpha, TGFbeta, and M2 macrophages [9]. However, these studies need further verification before being applied to humans.
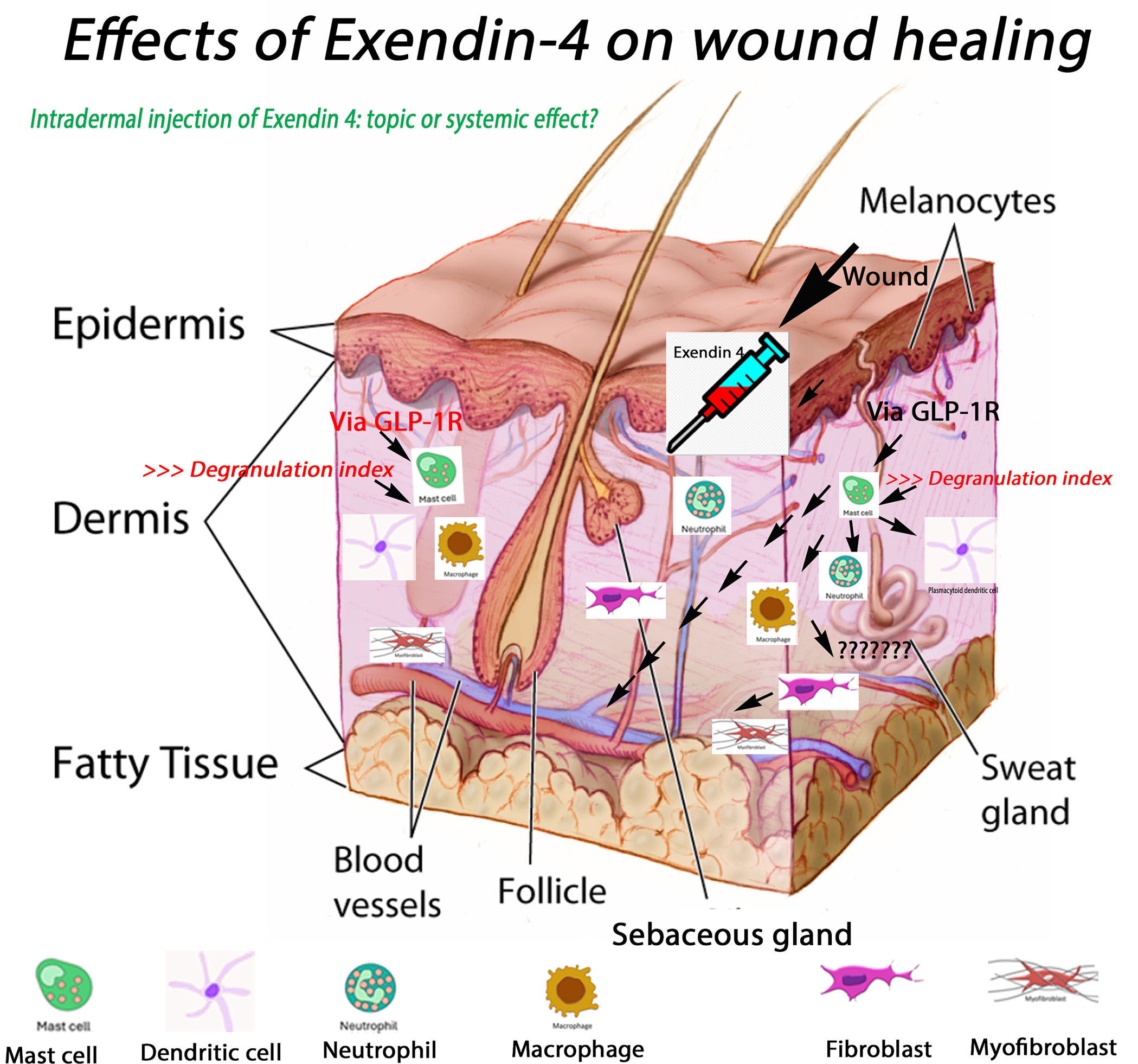
Figure 3: Presumable effects of Exendin-4 on wound healing: central role of mast cells [10–15] (see the commentary for details). Exendin-4 injection activates mast cells, thanks to the presence of GLP-1 receptor, causing an elevated degranulation index and histamine release. This stimulates angiogenesis and changes capillary permeability. Macrophages and neutrophils migrate to the infected area, initiating an inflammatory response. This process leads to early development of granulation tissue and re-epithelialization. Fibroblasts transform into myofibroblasts, facilitating wound closure. Additional cell types, such as dendritic cells and Tregs, are crucial in initiating the inflammatory response
4 Exendin-4 in Diabetic Wounds
Diabetic skin ulcers are characterized by inadequate granulation tissue growth and remodeling, leading to prolonged inflammation during healing. Inadequate angiogenesis in diabetic wounds impedes wound healing due to a lack of critical proangiogenic factors, increased antiangiogenic factors, and decreased capillary maturation factors, miRNAs, and MMPs [16–19].
Exendin-4 has a variable impact on the critical activities of human dermal fibroblasts in both normoglycemic and hyperglycemic states in humans [20]. Exendin-4 has been discovered to accelerate wound healing in diabetic rodents by increasing epidermal and dermal regeneration, cell proliferation, and tissue regeneration indicators [21–23]. In a mouse model, it has been shown that the topical administration of Exendin-4 had favorable effects on the acceleration of the healing process of cutaneous lesions in hyperglycemic rodents. These effects are likely due to the activation of GLP-1R through MC in the proliferative phase [24].
Exendin-4 can be combined with adipose-derived stem cells (ADSCs) to improve wound healing. A combination of Exendin-4 and ADSCs showed significantly better therapeutic effects than either treatment alone. In vitro angiogenesis assays showed that both Exendin-4 and ADSC-conditioned media improved endothelial cell migration, invasion, and proliferation, as well as increased keratinocyte migration and proliferation. Co-culture with ADSCs also increased cell migration and proliferation [25]. Besides, endoplasmic reticulum stress is regulated by Exendin-4, which protects endothelial progenitor cells from the damage caused by high glucose levels [26].
There is a correlation between the interaction of Exendin-4 with growth factors such as Platelet-Derived Growth Factor and the stimulation of cell proliferation, chemotaxis (the attraction of cells to the wound site), and differentiation, which ultimately results in a more rapid healing of the damaged tissue [27]. Additionally, Exendin-4 is able to collaborate with other peptides that play a role in the different phases of wound healing, including those that possess the ability to impact inflammation and the synthesis of collagen [27].
It has been shown that neurogenic stimuli have a substantial impact on the process of wound regeneration that happens after an injury. This is demonstrated by the fact that delayed wound healing occurs in animal models after the surgical ablation of cutaneous nerves [28]. It is therefore appropriate in this context to observe how histamine secreted by MCs that are located near nerves and play a role in wound healing activates acetylcholine and thus elevates these cell types to a coordinating role in neuromodulation [29].
7 Possible Uses in Wound Dressings
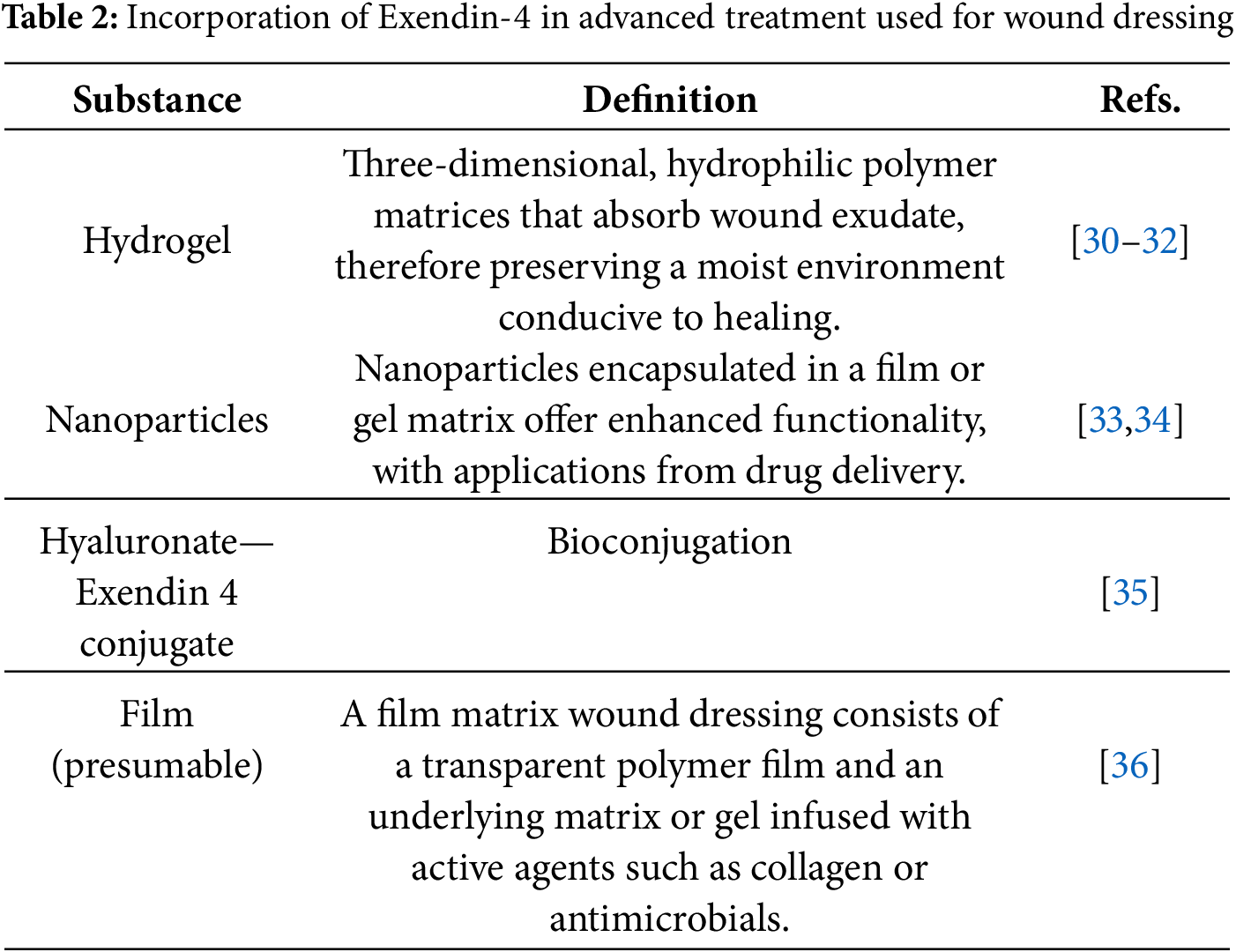
Exendin-4 may be included in wound dressings to enhance healing in diverse wound types, particularly in diabetic individuals. Its capacity to expedite wound healing, promote skin regeneration, and regulate inflammation makes it a compelling choice for incorporation into advanced wound care solutions [30]. Exendin-4 can be used in hydrogels; besides, presumably, the drug can be integrated into a film matrix for targeted, prolonged release, reducing injection frequency and enhancing patient adherence. Other methods for achieving sustained and localized release include nanoparticles encapsulated in films or gels, or chemically linked to polymers like hyaluronic acid (see Table 2) [31–36].
Exending-4 may be used with other treatments to enhance results, as in chronic gastric ulcer [37]. Exendin-4 injection significantly decreased stomach ulcer area without altering blood glucose levels. This drug also reinstated pro-angiogenic factors, mitigated inflammation, and reduced superoxide anions. The enhancement in ulcer healing was associated with elevated MMP-2 expression and granulation tissue development. The research supports the prospective clinical use of GLP-1 analogues as adjunctive hypoglycemic medicines in the treatment of diabetes-related ulcers [37].
Exendin-4 has also shown promise in preclinical studies for neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, by reducing amyloid beta protein levels, improving motor function, promoting neurogenesis, and exhibiting anti-inflammatory properties. Nonetheless, prevalent side effects, particularly with injectable forms, may include nausea and vomiting. Therefore, further clinical trials are necessary to thoroughly evaluate the effectiveness and safety of Exendin-4 as a treatment for neurodegenerative illnesses [27,38].
Exendin-4 is a potential therapeutic agent for wound healing, especially in diabetic patients, since it enhances angiogenesis, collagen production, and cellular proliferation. While Exendin-4 has beneficial effects on wound healing, it may also have cytotoxic effects in some situations.
Research continues to explore the optimal use of Exendin-4 in wound healing, including its potential as a therapeutic agent for diabetic foot ulcers and other chronic wounds. Certainly, critical gaps that have emerged in certain studies regarding both acute wounds and human diabetic ulcers (e.g., pharmacokinetics of topical Exendin-4) need to be overcome. This step is essential for a better understanding of the underlying cellular mechanism. In this direction, the study of neuromodulation undoubtedly takes on promising research perspectives. From a clinical perspective, the therapeutic potential of combining Exendin-4 with ADSCs should be viewed with great interest by the clinical community as a new direction.
Acknowledgement: The author thanks Stefania Alexandra Brandusescu for their skillful assistance and revision of the manuscript. Besides, the author wants to know the professional capacity of Virginia Lotti, Gaetano DeSiena, and all the students that in the past have worked at this project.
Funding Statement: The author received no specific funding for this study.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The author declares no conflicts of interest to report regarding the present study.
References
1. By NSRW gila monster.jpg [Internet]. 1941 [cited 2025 Aug 28]. Available from: https://commons.wikimedia.org/w/index.php?title=File:NSRW_Gila_Monster.jpg&oldid=440106358. [Google Scholar]
2. PubChem compound summary for CID 56927919, Exendin 4.: national center for biotechnology information [Internet]. 2012 [cited 2025 Aug 28]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Exendin-4. [Google Scholar]
3. Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30(Suppl. 2):72–130. doi:10.1016/j.molmet.2019.09.010. [Google Scholar] [PubMed] [CrossRef]
4. Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):234. doi:10.1038/s41392-024-01931-z. [Google Scholar] [PubMed] [CrossRef]
5. Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25(8):599–616. doi:10.1038/s41580-024-00715-1. [Google Scholar] [PubMed] [CrossRef]
6. Fernández-Guarino M, Hernández-Bule ML, Bacci S. Cellular and molecular processes in wound healing. Biomedicines. 2023;11(9):2526. doi:10.3390/biomedicines11092526. [Google Scholar] [PubMed] [CrossRef]
7. Kang HM, Kang Y, Chun HJ, Jeong JW, Park C. Evaluation of the in vitro and in vivo angiogenic effects of Exendin-4. Biochem Biophys Res Commun. 2013;434(1):150–4. doi:10.1016/j.bbrc.2013.03.053. [Google Scholar] [PubMed] [CrossRef]
8. Bacci S, Laurino A, Manni ME, Landucci E, Musilli C, De Siena G, et al. The pro-healing effect of Exendin-4 on wounds produced by abrasion in normoglycemic mice. Eur J Pharmacol. 2015;764:346–52. doi:10.1016/j.ejphar.2015.06.056. [Google Scholar] [PubMed] [CrossRef]
9. Paroli G, Murciano N, Mancini C, Soldaini M, Rijli S, DeSiena G, et al. The role of mast cells in cellular modifications evoked by Exendin-4 in treated wounds: a preclinical study. J Wound Care. 2022;31(8):701–8. doi:10.12968/jowc.2022.31.8.701. [Google Scholar] [PubMed] [CrossRef]
10. By anatomy the skin—NCI visuals online esp.jpg [Internet]. 2019 [cited 2025 Aug 27]. Available from: https://commons.wikimedia.org/w/index.php?title=File:Anatomy_The_Skin_-_NCI_Visuals_Online_esp.jpg&oldid=1053566163. [Google Scholar]
11. By types of granulocytes.png [Internet]. 2025 [cited 2025 Aug 27]. Available from: https://commons.wikimedia.org/w/index.php?title=File:Types_of_granulocytes.png&oldid=998325198. [Google Scholar]
12. By fibroblasts—smart-servier.jpg [Internet]. 2019 [cited 2025 Aug 27]. Available from: https://commons.wikimedia.org/w/index.php?title=File:Fibroblasts_–_Smart-Servier.jpg&oldid=822286179. [Google Scholar]
13. By myofibroblasts multicellular origin.png [Internet]. 2021 [cited 2025 Aug 27]. Available from: https://commons.wikimedia.org/w/index.php?title=File:Myofibroblasts_multicellular_origin.png&oldid=1002229590. [Google Scholar]
14. By dendritic cell.svg [Internet]. 2008 [cited 2025 Aug 27]. Available from: https://commons.wikimedia.org/w/index.php?title=File:Dendritic_cell.svg&oldid=713711577. [Google Scholar]
15. By Filled Syringe icon.svg [Internet]. 2009 [cited 2025 Aug 27]. Available from: https://commons.wikimedia.org/w/index.php?title=File:Filled_Syringe_icon.svg&oldid=897167345. [Google Scholar]
16. Wolf SJ, Melvin WJ, Gallagher K. Macrophage-mediated inflammation in diabetic wound repair. Semin Cell Dev Biol. 2021;119(1):111–8. doi:10.1016/j.semcdb.2021.06.013. [Google Scholar] [PubMed] [CrossRef]
17. Dasari N, Jiang A, Skochdopole A, Chung J, Reece EM, Vorstenbosh J, et al. Updates in diabetic wound healing, inflammation, and scarring. Semin Plast Surg. 2021;35(3):153–8. doi:10.1055/s-0041-1731460. [Google Scholar] [PubMed] [CrossRef]
18. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol. 2017;199(1):17–24. doi:10.4049/jimmunol.1700223. [Google Scholar] [PubMed] [CrossRef]
19. Wan R, Weissman JP, Grundman K, Lang L, Grybowski DJ, Galiano RD. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair Regen. 2021;29(4):573–81. doi:10.1111/wrr.12954. [Google Scholar] [PubMed] [CrossRef]
20. Wolak M, Drobnik J, Bojanowska E. Exendin-4 differentially modulates essential functions of human dermal fibroblasts under normoglycemic and hyperglycemic conditions. J Physiol Pharmacol. 2021;72(3):461–7. doi:10.26402/jpp.2021.3.14. [Google Scholar] [PubMed] [CrossRef]
21. Yang C, Zhu P, Yan L, Chen L, Meng R, Lao G. Dynamic changes in matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 levels during wound healing in diabetic rats. J Am Podiatr Med Assoc. 2009;99(6):489–96. doi:10.7547/0990489. [Google Scholar] [PubMed] [CrossRef]
22. Wolak M, Staszewska T, Juszczak M, Gałdyszyńska M, Bojanowska E. Anti-inflammatory and pro-healing impacts of Exendin-4 treatment in Zucker diabetic rats: effects on skin wound fibroblasts. Eur J Pharmacol. 2019;842:262–9. doi:10.1016/j.ejphar.2018.10.053. [Google Scholar] [PubMed] [CrossRef]
23. Roan JN, Cheng HN, Young CC, Lee CJ, Yeh ML, Luo CY, et al. Exendin-4, a glucagon-like peptide-1 analogue, accelerates diabetic wound healing. J Surg Res. 2017;208:93–103. doi:10.1016/j.jss.2016.09.024. [Google Scholar] [PubMed] [CrossRef]
24. Lotti V, De Siena G, Bacci S. Effects of Exendin-4 on diabetic wounds: direct action on proliferative phase of wound healing. BIOCELL. 2024;48(12):1751–9. doi:10.32604/biocell.2024.057904. [Google Scholar] [CrossRef]
25. Seo E, Lim JS, Jun JB, Choi W, Hong IS, Jun HS. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J Transl Med. 2017;15(1):35. doi:10.1186/s12967-017-1145-4. [Google Scholar] [PubMed] [CrossRef]
26. Yang Y, Zhou Y, Wang Y, Wei X, Wang T, Ma A. Exendin-4 regulates endoplasmic reticulum stress to protect endothelial progenitor cells from high-glucose damage. Mol Cell Probes. 2020;51(4):101527. doi:10.1016/j.mcp.2020.101527. [Google Scholar] [PubMed] [CrossRef]
27. Figat M, Kardas G, Kuna P, Panek MG. Beneficial influence of Exendin-4 on specific organs and mechanisms favourable for the elderly with concomitant obstructive lung diseases. Brain Sci. 2022;12(8):1090. doi:10.3390/brainsci1208109. [Google Scholar] [CrossRef]
28. Nardini P, Bacci S. Neuroimmunomodulation in chronic wounds: an opinion. Front Cell Dev Biol. 2025;13:1562346. doi:10.3389/fcell.2025.1562346. [Google Scholar] [PubMed] [CrossRef]
29. Fantozzi R, Masini E, Blandina P, Mannaioni PF, Bani-Sacchi T. Release of histamine from rat mast cells by acetylcholine. Nature. 1978;273(5662):473–4. doi:10.1038/273473a0. [Google Scholar] [PubMed] [CrossRef]
30. Hu Y, Xiong Y, Tao R, Xue H, Chen L, Lin Z, et al. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing. Biomater Transl. 2022;3(3):188–200. doi:10.12336/biomatertransl.2022.03.003. [Google Scholar] [PubMed] [CrossRef]
31. Nizam AAK, Masri S, Fadilah NIM, Maarof M, Fauzi MB. Current insight of peptide-based hydrogels for chronic wound healing applications: a concise review. Pharmaceuticals. 2025;18(1):58. doi:10.3390/ph18010058. [Google Scholar] [PubMed] [CrossRef]
32. Klapcia A, Domalik-Pyzik P. Hydrogel dressing an insulin delivery systems for diabetic wounds. Front Biosci. 2025;17(1):26446. doi:10.31083/FBE26446. [Google Scholar] [PubMed] [CrossRef]
33. Shi Y, Lu A, Wang X, Belhadj Z, Wang J, Zhang Q. A review of existing strategies for designing long-acting parenteral formulations: focus on underlying mechanisms, and future perspectives. Acta Pharm Sin B. 2021;11(8):2396–415. doi:10.1016/j.apsb.2021.05.002. [Google Scholar] [PubMed] [CrossRef]
34. Amatya R, Joseph A, Roh GS, Benmokadem Y, Min KA, Shin MC. Long-lasting Exendin-4-coated gold nanoparticles: synthesis and in vivo evaluation of hypoglycemic activity. Pharmaceuticals. 2024;17(11):1475. doi:10.3390/ph17111475. [Google Scholar] [PubMed] [CrossRef]
35. Kong JH, Oh EJ, Chae SY, Lee KC, Hahn SK. Long acting hyaluronate—Exendin 4 conjugate for the treatment of type 2 diabetes. Biomaterials. 2010;31(14):4121–8. doi:10.1016/j.biomaterials.2010.01.091. [Google Scholar] [PubMed] [CrossRef]
36. Rani Raju N, Silina E, Stupin V, Manturova N, Chidambaram SB, Achar RR. Multifunctional and smart wound dressings—a review on recent research advancements in skin regenerative medicine. Pharmaceutics. 2022;14(8):1574. doi:10.3390/pharmaceutics14081574. [Google Scholar] [PubMed] [CrossRef]
37. Chen YC, Ho CC, Yi CH, Liu XZ, Cheng TT, Lam CF. Exendin-4, a glucagon-like peptide-1 analogue accelerates healing of chronic gastric ulcer in diabetic rats. PLoS One. 2017;12(11):e0187434. doi:10.1371/journal.pone.0187434. [Google Scholar] [PubMed] [CrossRef]
38. Verma A, Chaudhary S, Solanki K, Goyal A, Yadav HN. Exendin-4: a potential therapeutic strategy for Alzheimer’s disease and Parkinson’s disease. Chem Biol Drug Des. 2024;103(1):e14426. doi:10.1111/cbdd.14426. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF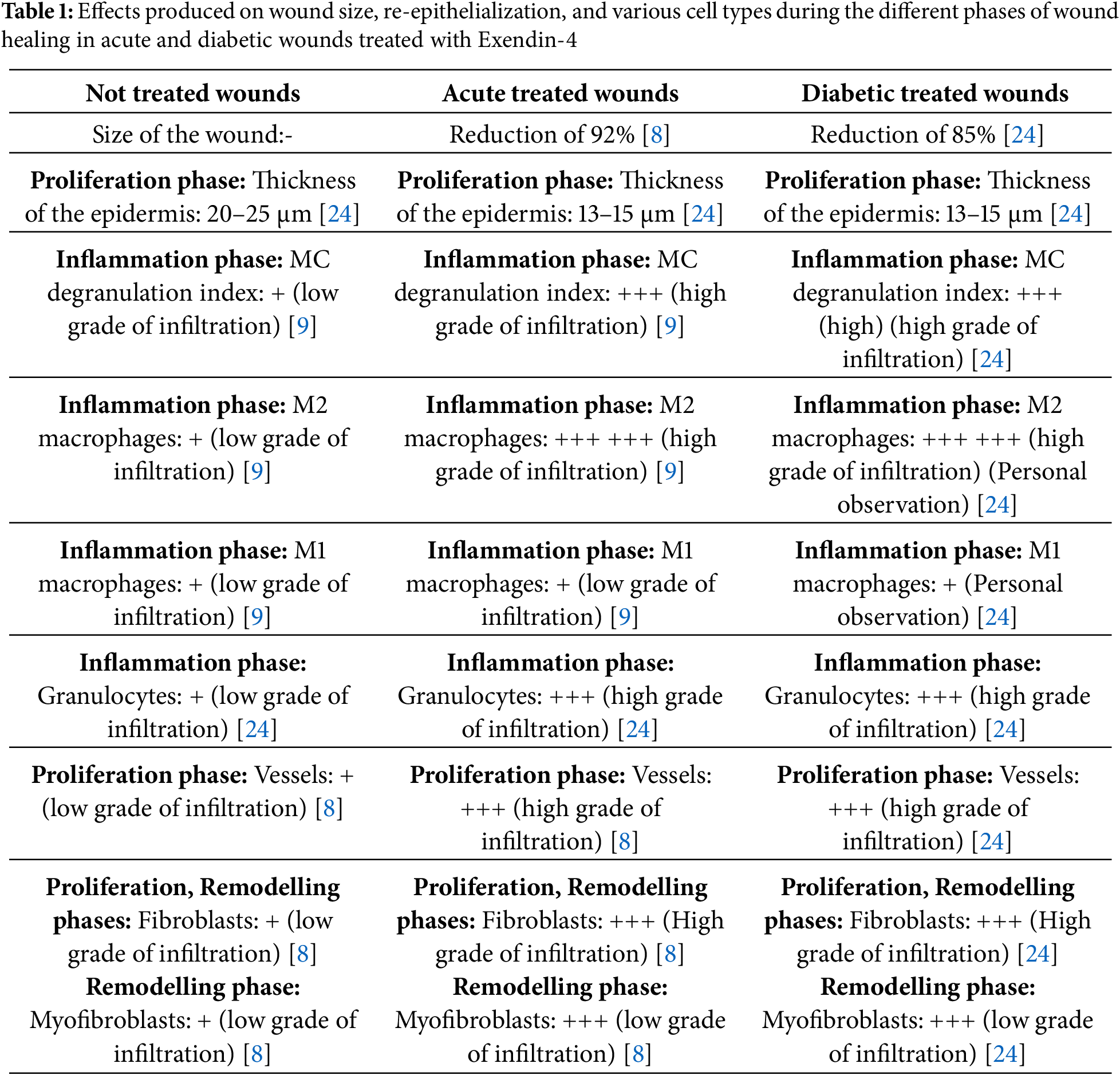
 Downloads
Downloads
 Citation Tools
Citation Tools