 Open Access
Open Access
ARTICLE
N-Acetyl Cysteine Inhibits Weaning Stress-Induced Intestinal Cell Cycle Arrest in Piglets through Decreasing ERK, JNK, and p38 Phosphorylation
1 School of Biology and Pharmaceutical Engineering, Jilin Agricultural Science and Technology University, Jilin, 132101, China
2 School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, 200240, China
* Corresponding Author: Jianxiong Xu. Email:
BIOCELL 2025, 49(5), 907-924. https://doi.org/10.32604/biocell.2025.063568
Received 18 January 2025; Accepted 24 April 2025; Issue published 27 May 2025
Abstract
Objectives: Weaning induces oxidative stress in pigs, increasing the risk of diarrhea and death. Intestinal damage is associated with obstructed intestinal cell cycles. To stop damage caused by reactive oxygen species (ROS), N-acetyl cysteine (NAC) has been widely employed. In this study, we examined changes in the intestinal cyclin of weaning piglets and assessed the impact of NAC on intestinal cell cycle arrest and intracellular signaling pathways. Methods: We conducted two animal experiments. In the first, we divided 12 litters of 120 newborn piglets into two groups: a control group and a weaning group. The control piglets were allowed to suckle normally. The weaning group was weaned after 3 weeks and fed a normal diet for piglets. We slaughtered six piglets from the control group and six from the weaning group. We observed cyclin changes and intestinal development at days 0, 1, 4, and 7 after weaning. In the second experiment, we divided 15 litters of 150 piglets that were 2 weeks old into three groups: the control group, the weaning group, and the NAC group. Control piglets were allowed to suckle normally. Piglets in the weaning and NAC groups were weaned when they were 21 days old. The NAC group was fed a basal diet supplemented with 500 mg/kg NAC, and the weaning group was fed the basal diet alone. The experimental period was 14–25 days of age. Four days after weaning, we slaughtered one piglet from each litter. We then analyzed intestinal cell cycle indexes, intestinal oxidative stress, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 phosphorylation. Results: Weaning decreased the piglets’ feed intake and daily gain, reduced the serum antioxidant capacity, and increased the intestinal ROS level. Furthermore, the jejunum histology and barrier development of the jejunum exhibited damage after weaning, the microvilli displayed hypoplasia, and the p21 and p27 protein expression levels of the jejunum were significantly elevated. We did not observe any significant differences in cyclin D and E after days 1, 4, and 7 post-weaning compared with the control group. We observed, however, significantly increased cyclin D and E expression, lower ERK, JNK, and p38 kinase phosphorylation; villus atrophy alleviation; decreased p21 and p27 expression; and increased average daily intake of feed and weight gain. Conclusion: This research demonstrates that weaning stress inhibits piglet intestinal proliferation by reducing cyclin D and cyclin E expression. NAC downregulates p21 and p27 through modulating mitogen-activated protein kinases (MAPKase) phosphorylation, thereby promoting cell proliferation. The results indicate that NAC promotes intestinal function and the integrity of enterocytes and holds promise as a new feed additive for animal health.Keywords
During the growth and development of mammals, weaning is an essential process. In the traditional pig industry, the weaning age of piglets is usually 50–60 days after birth. Using methods like improved annual productivity and efficiency of feed utilization in sows make disease transmission reduction, growth and development promotion, and utilization rate improvement for penned piglets, it is possible to increase the economic benefits of pig production. Intensive pig farming often weans piglets early to achieve these goals, weaning them when they are only 3–4 weeks old. Because this process is stressful for piglets and causes oxidative stress, it increases oxidative damage to the intestinal mucosa [1,2]. ROS, an excess free radical, can slow cell migration, cause cell cycle arrest and apoptosis, and block the intestinal epithelial barrier development [2]. To alleviate ROS production, inhibit apoptosis, and protect the function and integrity of intestinal cells, the regulation of antioxidants is necessary [3]. The cell cycle is responsible for the fundamental progression of cell life. During cell cycle progression, cells move into physiological states of proliferation, differentiation, senescence, and apoptosis [4]. The redox state is known to regulate cell cycle progression through the cyclin-CDK-CKI network [5,6].
To regulate the cell cycle, cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors (CKIs) work together. Cyclin D and cyclin E act as positive regulators during cell cycle progression, while CKIs such as p21 and p27 can inhibit cyclin-CDK complex activity. Cell proliferation is regulated by mitogen-activated protein kinase (MAPK) signaling pathways, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK), and p38 pathways [7]. The MAPK signaling pathway can be activated by higher ROS levels. These kinases may then translocate to the nucleus, where they are responsible for regulating p21 and p27 transcription [8,9]. The homeostasis of free radical metabolism in the intestine is maintained by antioxidant enzymes and the reduced glutathione/glutathione disulphide (GSH/GSSG) redox system [10]. N-acetylcysteine (NAC), the glutathione precursor and effective antioxidant with sulfhydryl groups, processes antioxidant, anti-inflammatory, and cytoprotective properties that are essential for animal health [11]. NAC can inhibit kinase phosphorylation associated with the activation of cell signaling pathways, thereby modulating downstream gene expression [12]. Its potential effect on animal gastrointestinal health has been recognized [13]. The regulatory impacts of the antioxidant NAC on enterocyte cycle arrest in piglets that are weaned. However, as well as the underlying molecular mechanisms remain unclear.
In weaning piglets, regulatory strategies to alleviate weaning stress have mainly focused on supplementing feed with functional amino acids, plant extracts, and intestinal microbiota [14–16]. Weaning stress is associated with alterations in intestinal morphology and function. Intestinal cell proliferation plays a crucial role in maintaining intestinal morphology and function. To date, few studies have examined the impact of ROS on intestinal cell proliferation in weaned piglets. The present study was conducted to investigate the changes in intestinal cyclin and the effects of NAC on intestinal cell cycle arrest and intracellular signaling pathways in weaning piglets.
2.1 Animals and Experimental Design
Xian County WENZ Husbandry Co., Ltd. (Cangzhou, China) provided 120 3-week-old piglets (Duroc × Landrace × Large white, body weight 6.00 ± 0.05 kg). We randomly divided the 12 litters into two groups of six litters each: a control group and a weaning group. The control pigs remained in the farrowing pens until the end of the experiment. We weaned piglets in the weaning group at which point they were 3 weeks age. We moved them to the nursery pen from the farrowing pen and fed them a basal diet. The farrowing and nursery building was maintained at a temperature of 30°C. The piglets that were weaned had free access to a normal diet for piglets. Using a nipple waterer, all piglets had free access to water. We formulated the piglet diets following the National Research Council’s recommended swine nutrition requirements. Table 1 presents the nutrient contents and dietary composition. The Ethics Committee of Shanghai Jiao Tong University (Approval No. 201801124) approved this study.

We selected and killed one piglet from each replicate following days 0, 1, 4, and 7 after weaning. To kill the piglets, we used an intramuscular injection composed of 4% sodium pentobarbital solution at 40 mg/kg body weight. Before sampling, the piglets were fasted for 12 h. We recorded the body weight of the piglets on the day they were killed. We collected samples of blood from each piglet’s precaval vein. To obtain serum, we centrifuged the blood for 10 min at 4°C (3000× g). To remove blood, we rinsed the jejunum thoroughly using ice-cold phosphate-buffered saline. We immediately froze the serum and jejunum samples in liquid nitrogen, which we stored at −80°C. At the beginning and end of the experiment, we recorded the body weight and feed intake. We calculated the following: average daily feed intake (ADFI), average daily gain (ADG), and feed/gain (F/G).
To elucidate the regulatory effect of NAC on enterocyte proliferation in weaning piglets and the intracellular signaling pathway, a second animal experiment was conducted.
A total of 150 14-day-old piglets (Duroc × Landrace × Large White) from 15 litters were randomly allocated into control, weaning, and NAC groups, with five litters per group. When the piglets were between 14 and 25 days old, the weaning and control piglets had free access to a normal diet for piglets. The NAC group ate the same diet, but 500 mg/kg NAC was added to their diet. According to a study by Zhu et al. [17], we set the dose of NAC. After 3 weeks, we weaned the NAC and the weaning groups. These piglets were moved to the nursery pens, but the control group was allowed to feed and suckle until they were 25 days old. On the fourth day after weaning (25 days of age), we randomly selected and slaughtered one piglet from each of the litters. We collected jejunal tissue and blood. We weighed the piglets when they were 21 days old and when they were 25 days old, and we recorded the amount of feed they ate. The piglets’ diet, the feeding and management methods, and the sampling techniques were the same as those utilized in the first animal experiment.
2.2.1 Antioxidant Capacity Analysis
We used commercially available kits to assay the activities of catalase (CAT) activities (A007-2-1); glutathione peroxidase (GSH-Px) (A005-1-2); hydroxyl radical inhibition capacity (IHR) (A0018-1-1); malondialdehyde (MDA) (A003-1-1); and serum superoxide dismutase (SOD) (A001-3-2). We followed the recommendations of the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). We used a free-radical analyzer (World Precision Instruments, TBR 4100, Sarasota, FL, USA) to assay the content of NO and H2O2.
2.2.2 Measurement of Oxidative Damage and Redox State
The kit has been coated with purified protein carbonylation (PC)(A131-1-1), 8-hydroxydeoxyguanosine (8OH-dG)(A121-1-2), and 8-isoprostaglandin F2α (8-ISO-PGF2α)(A111-2-0) antibodies on microporous plates. We used commercially available kits to assay the indexes. We followed the recommendations of the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). We used a Biotek GK3 microplate reader (Winooski, VT, USA) to measure absorbance at 450 nm. We used a standard curve to assess the samples.
On the basis of the oxidation of 2,7-dichlorofluoresce diacetate (DCFH-DA) (Beyotime, S0035s, Hangzhou, China) to 2,7-dichlorofluorescein (DCFH), we measured ROS. In the presence of ROS, DCFH was able to oxidize quickly and could form 2,7-dichlorofluorescein, which was highly fluorescent. The results were calculated as the fluorescence intensity/mg protein and the fluorescence intensity/L.
Total glutathione (GSH + GSSG) in the samples was determined using a kit (Beyotime, S0053, Hangzhou, China). The samples underwent pre-treatment for GSSG determination as follows: the sample and the GSSG standard were mixed at 37°C for 30 min, and a 50 μL aliquot was collected. We used the GSSG standard to mix the pretreatment aliquot and read the value of absorbance at 405 nm. The GSH content = glutathione (GSH + GSSG) − 2 × GSSG. The GSSG/GSH ratio was further calculated based on the GSH content.
2.2.3 Intestinal Histomorphology
The jejunum samples were fixed in 4% neutral buffered formalin for 48 h and processed for paraffin embedding. The paraffin blocks were sectioned into 5 μm slices, and the slices were stained with hematoxylin and eosin (Beyotime, C0105S, Hangzhou, China) for morphological analysis and the assessment of histological damage following the method put forward by Shu [18]. The scoring standards for histological damage set by Shu were as follows: 0, normal; 1, one-third loss of crypt glands; 2, two-thirds loss of crypt glands; and 3, total loss of crypt glands plus epithelial cells and inflammatory cell infiltration.
2.3 Transmission Electron Microscopy
We used 2.5% glutaraldehyde to fix the mucosal specimens of the jejunum sections for 2 h. We used phosphate buffer (0.1 M) to wash the specimens three times. We used osmic acid to fix the washed samples. We used uranyl acetate to stain the samples, and ethanol for progressive dehydration. Then, we used an embedding solution to soak the samples and used uranium and lead to double-stain the ultrathin sections. To observe the ultrastructure of tight junctions and the microvilli of the jejunum, we used transmission electron microscopy (Hitachi, H-600, Tokyo, Japan).
We homogenized the intestine samples in 500 μL of ice-cold RIPA lysis buffer (Sangon, C500005, Shanghai, China) containing 1 mM PMSF (Sangon, A610425, Shanghai, China) and protease inhibitor (Sangon, C610005, Shanghai, China), and we incubated the suspension on ice for 30 min. Then we centrifuge the suspension for 10 min at 12,000× g and collect supernatant lysates. The bicinchoninic acid (BCA) protein assay kit (Beyotime, S0034, Hangzhou, China) was utilized to measure protein concentration. We used sodium dodecyl sulfate (SDS)-polyacrylamide gels for electrophoresis of 20–40 μg of protein. We used polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA) to transfer the sample.
We used Tris-buffered saline/Tween (TBST) with 5% skimmed milk powder or bovine serum albumin (BSA) for blocking. Then, we incubated the membranes at 4°C overnight using the following primary antibodies: cyclin D (1:200, SC-753), cyclin E (1:200, SC-481), p21 (1:200, SC-528), p-p21 (phosphorylated p21) (1:100, SC-377514), p27 (1:200, SC-397), p-p27 (phosphorylated p27) (1:100, SC-129139), p38 (1:200, SC-535), p-p38 (phosphorylated p38) (1:200, SC-7973), and Smad4 (1:200, SC-7154), all of which were from Santa Cruz Biotechnology (Santa Cruz, Paso Robles, CA, USA). We also used ERK (1:1000, #9102) and p-ERK (phosphorylated ERK) (1:1000, #4370) from Cell Signaling Technology Inc., (Danvers, MA, USA). We used JNK (1:200, SC-571, Santa Cruz Biotechnology, Paso Robles, CA, USA) and p-JNK (phosphorylated JNK) (1:100, #10915) from Biorbyt (Cambridge, UK). To perform secondary antibody binding, we used an anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP) (1:2000, sc-2005) from Santa Cruz Biotechnology for 2 h at 4°C. We used Sangton ECL detection reagents (Shanghai, China) for detection and enhanced image acquisition with a Tanon enhanced chemiluminescence detection system (Shanghai, China). Beta-actin was used as a loading control (42 kDa) (Affinity Biosciences, AF7108, Cincinnati, OH, USA).
To compare the control and weaning groups’ values in the first animal experiment, we followed the independent sample t-test method. To compare all three groups, we used one-way analysis of variance (ANOVA). For the second animal experiment, we applied a least significant difference (LSD) post-hoc test. All of the data are presented as mean ± SEM, and the difference was considered statistically significant when p < 0.05. Analysis was performed with SPSS 20.0 (SPSS Statistics, Armonk, NY, USA).
3.1.1 Average Daily Feed Intake and Average Daily Gain
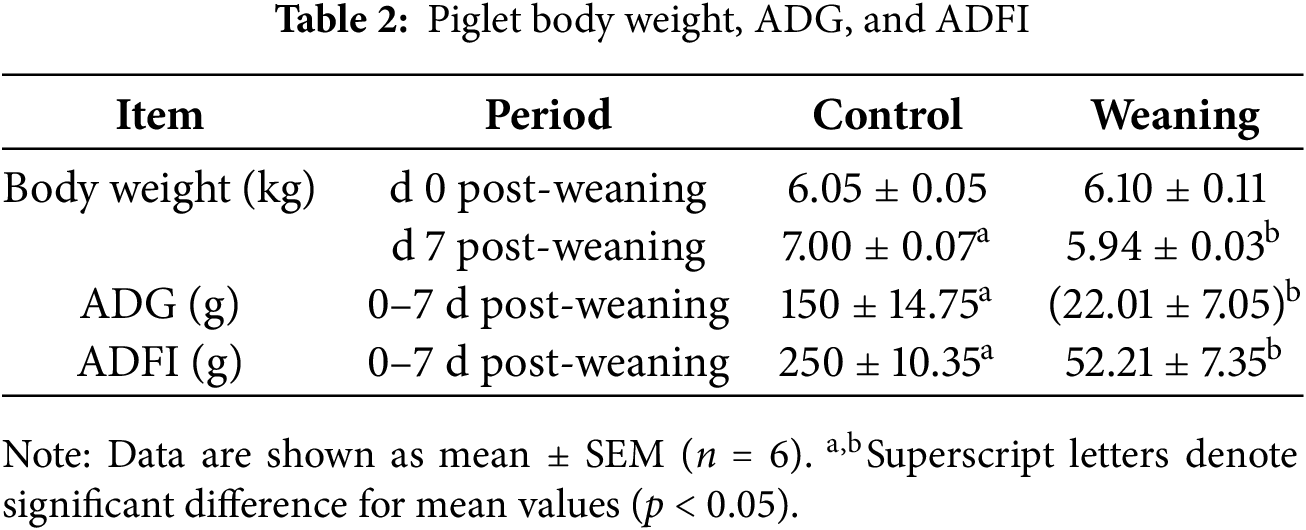
Table 2 presents the results for the piglets’ ADFI and ADG after 0–7 days of weaning. Weaning decreased the ADFI and ADG of the piglets relative to the control group (p < 0.05).
3.1.2 Antioxidant Capacity Analysis
Fig. 1 shows that the antioxidant enzyme activity of piglets that were weaned was lower than that of the control group (p < 0.05) four days after weaning, but it recovered seven days after weaning. The MDA content was significantly increased, and IHR was significantly reduced in weaning piglets on post-weaning day 4 and day 7 (p < 0.05).
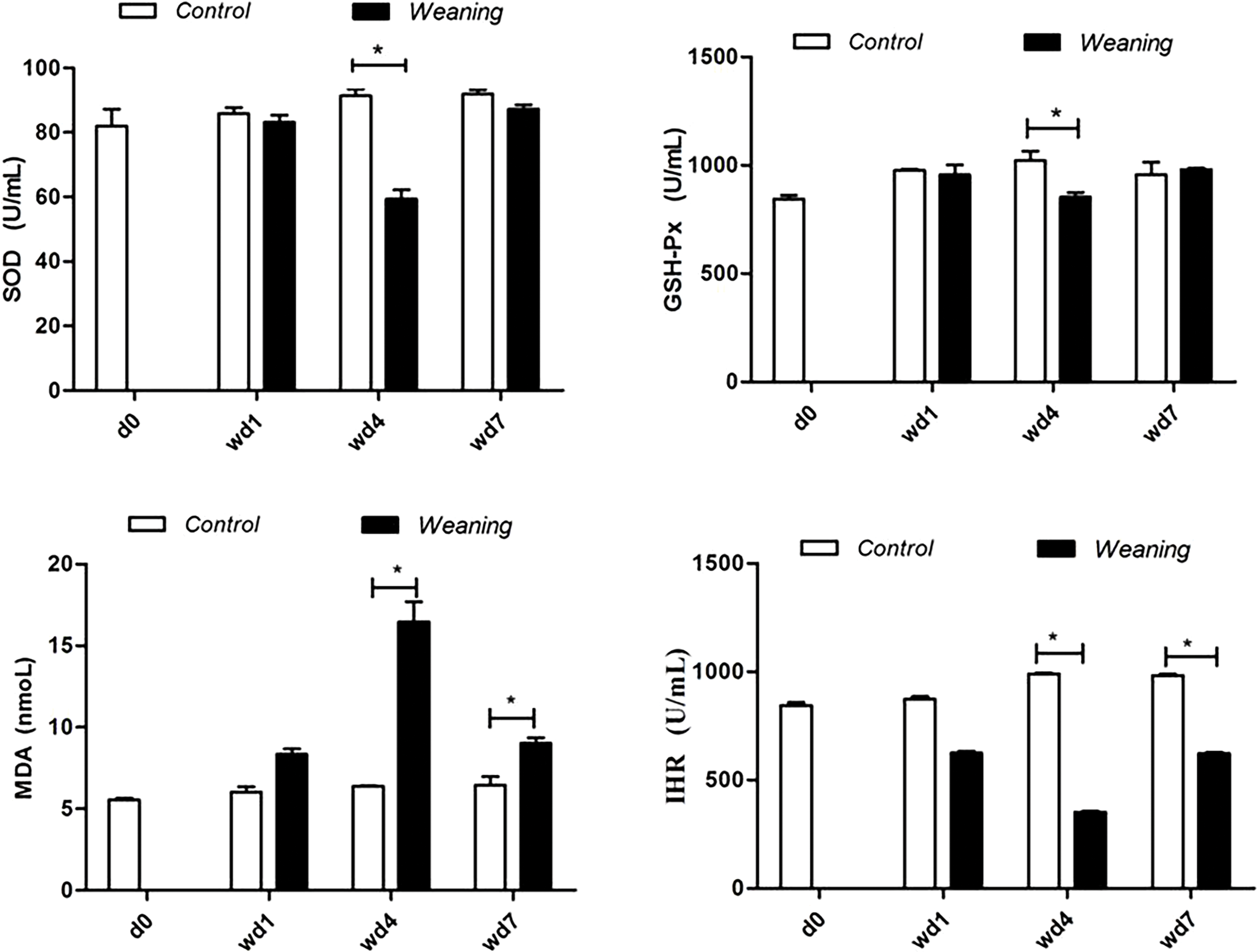
Figure 1: Serum antioxidative parameters of piglets. d0, 0 days post-weaning; wd1, 1 day post-weaning; wd4, 4 days post-weaning; wd7, 7 days post-weaning. CAT, catalase; GSH-Px, glutathione peroxidase; IHR, hydroxyl radical inhibition capacity; MDA, malondialdehyde; SOD, serum superoxide dismutase. *p < 0.05; data are given as mean ± SEM (n = 6)
Fig. 2 shows that serum H2O2 and NO content increased in the weaning group compared with the control group four days after weaning (p < 0.05).
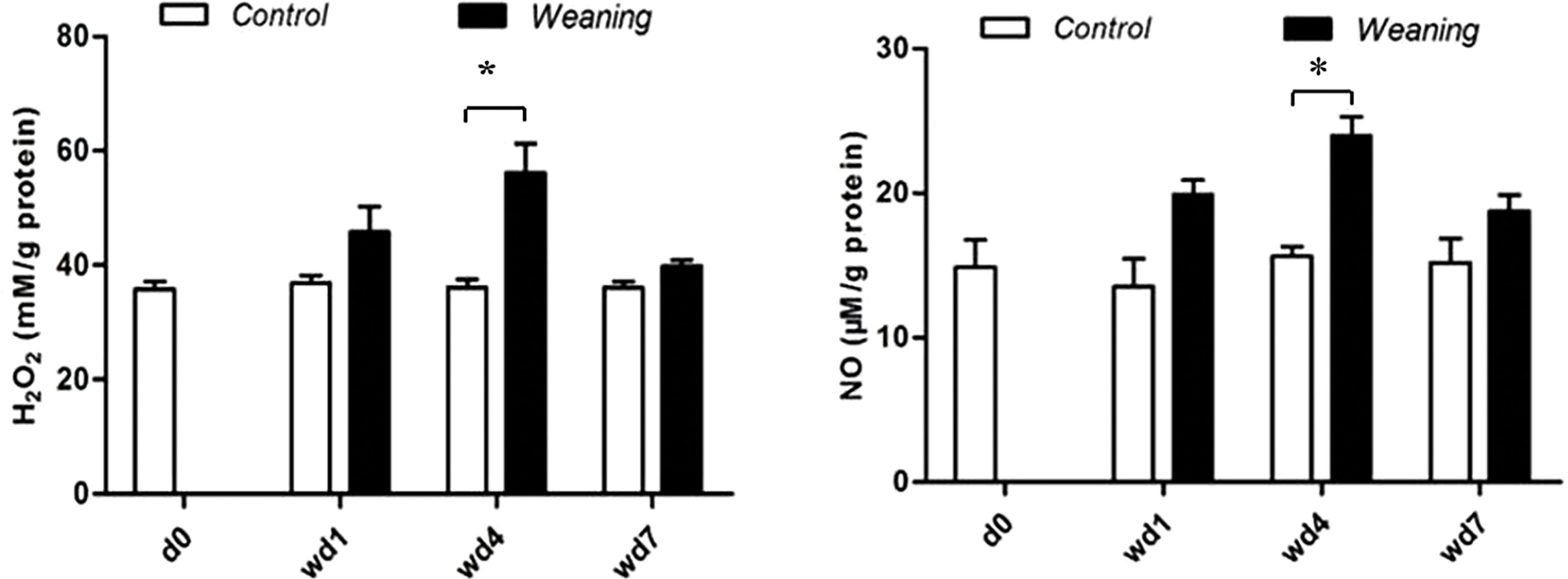
Figure 2: Jejunal concentrations of NO and H2O2 of piglets. d0, 0 days post-weaning; wd1, 1 day post-weaning; wd4, 4 days post-weaning; wd7, 7 days post-weaning. *p < 0.05; data are given as mean ± SEM (n = 6)
3.1.3 Intestinal Histomorphology
We did not observe any inflammatory cell infiltration of the jejunal villi in the control group. We did observe inflammatory cell infiltration in the intestinal villi in the weaning group one day after weaning. At post-weaning day 4, the inflammatory cell infiltration of the villus was increased, concentrated in the lamina propria. At post-weaning day 7, the number of inflammatory cell infiltrations was decreased (Fig. 3A). The jejunal histology damage was severe in weaning piglets at post-weaning day 4 (Fig. 3B).
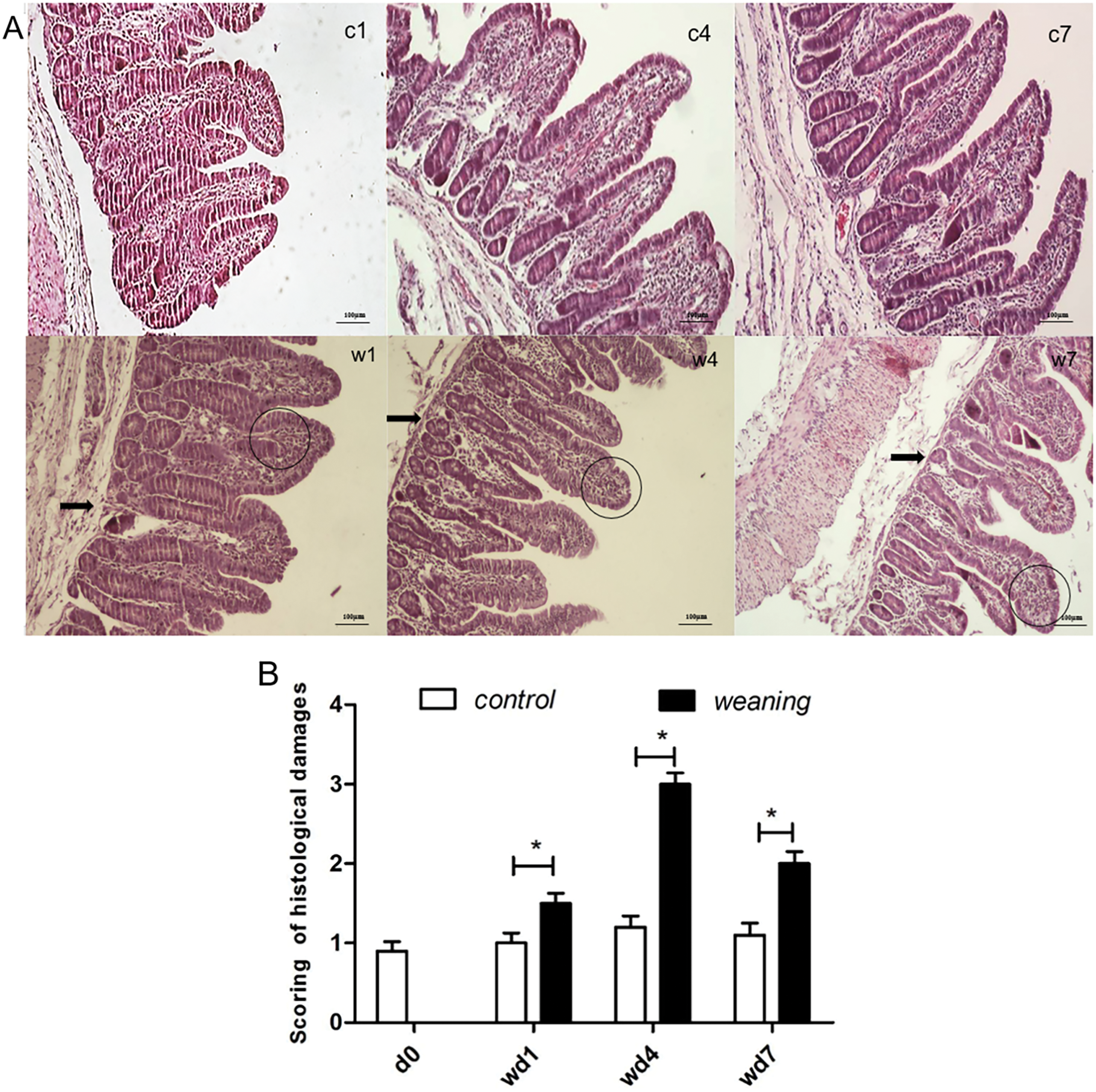
Figure 3: Scored the jejunum histological damage of piglets. In Fig. 3A, (c1,c4,c7) are the control group on 1, 4, and 7 days post weaning, (w1,w4,w7) are the weaning group on 1, 4, and 7 days post-weaning, respectively (n = 6). Thick black arrows indicate gland damage in the form of the loss of villus crypts, while a circle indicates the increased inflammatory cell infiltration in the villous lamina propria. Magnification: 100×. In Fig. 3B, the jejunum histological damage of piglets is scored. Data are presented as mean ± SEM (n = 6). *p < 0.05;. d0, wd1, wd4, and wd7 indicate 0, 1, 4, and 7 days post-weaning, respectively
The tight junctions and microvilli were well-developed in the control group (Fig. 4C1,C4,C7). At post-weaning day 1, the tight junctions and microvilli were comparably well-developed in the weaning piglets (Fig. 4W1). At post-weaning day 4, the cell linkage was less tight, some enterocytes exhibited compaction, and the microvilli were underdeveloped (Fig. 4W4). At postweaning day 7, the development of microvilli was recovered (Fig. 4W7).
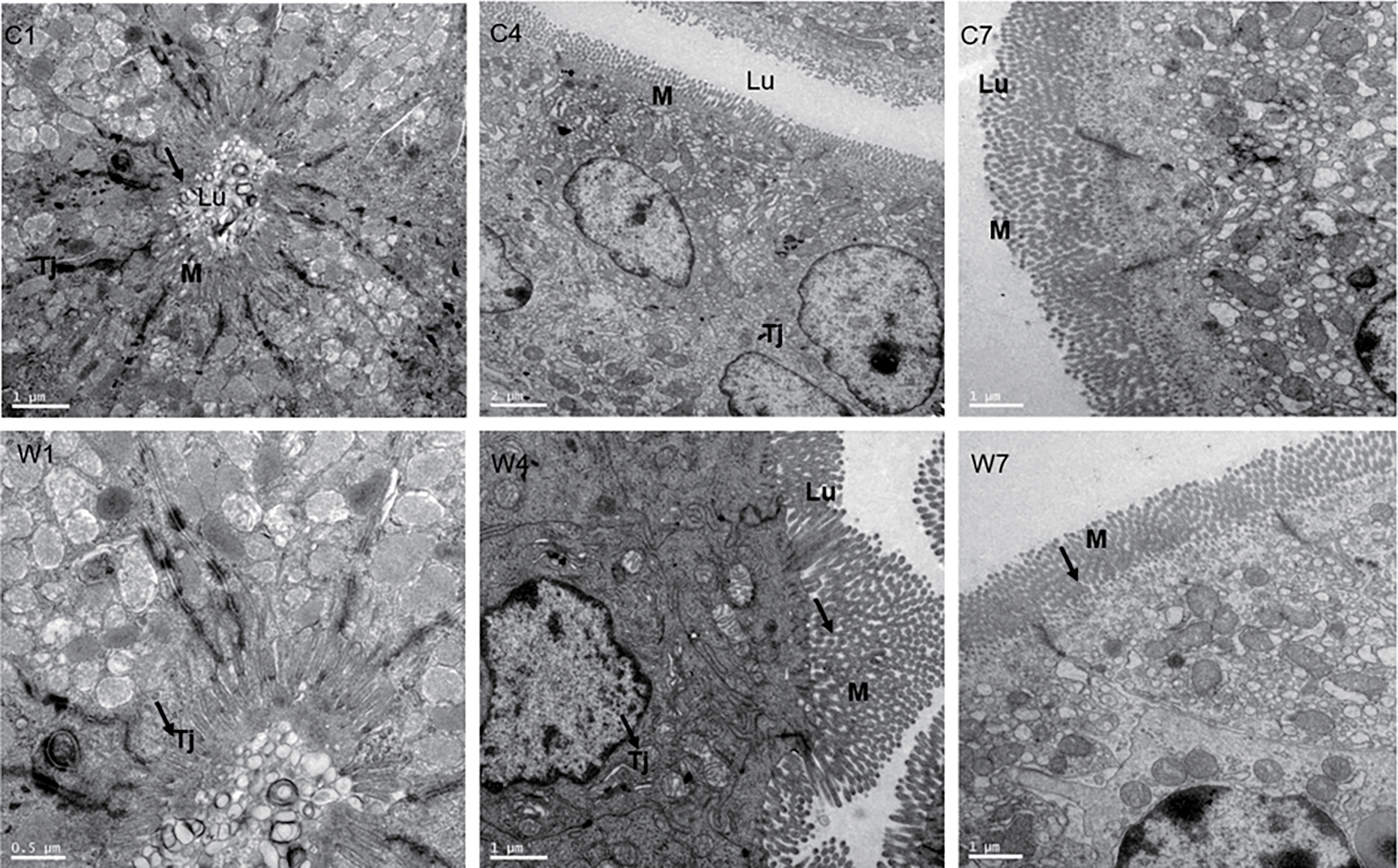
Figure 4: Jejunum microvilli and tight junctions were observed using transmission electron microscopy. (C1,C4,C7) are 1, 4, and 7 days post-weaning in the control group. (W1,W4,W7) are 1, 4, and 7 days post-weaning in the weaning group, respectively. M: microvilli. Tj: tight junction, Lu: lumen. Thick black arrows indicate intestinal microvilli. Magnification: ×5000 (C4), ×10,000 (C1,C7,W4,W7), ×20,000 (W1). (C4,C7,W4,W7) are cross sections, while (C1,W1) are vertical sections
3.1.4 Expression of Intestinal Cell Cycle Regulating Proteins
Compared with the piglets that were weaned, expression of cyclin D and E increased in the control group (p > 0.05). Four and seven days after weaning, levels of p21 and p27 decreased significantly in the control group compared with the piglets that were weaned (p < 0.05) (Fig. 5A,B). In the control group, Smad4 expression increased significantly compared with the piglets that were weaned four days after weaning day (p < 0.05) but decreased significantly seven days after weaning (p < 0.05).
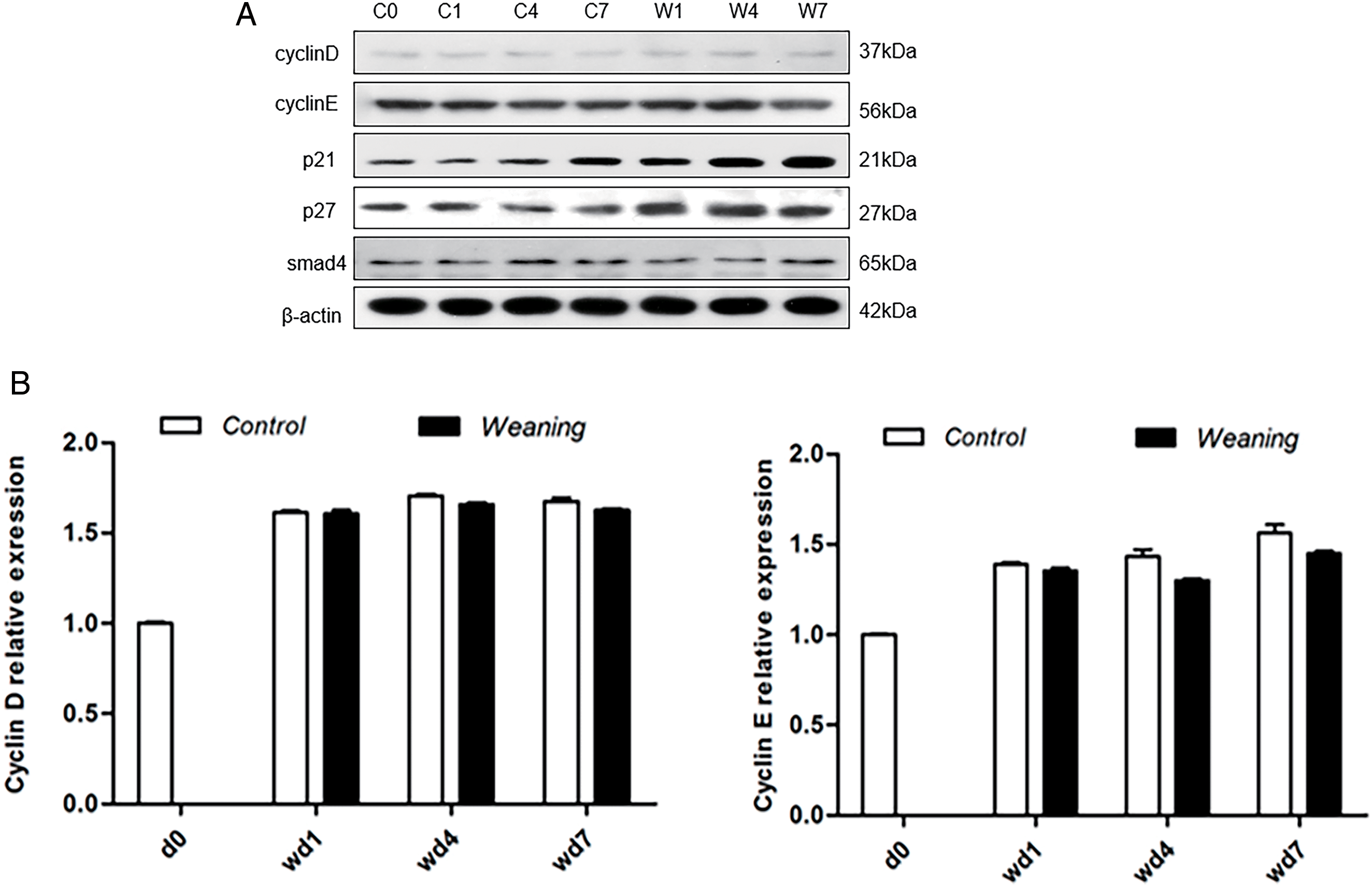
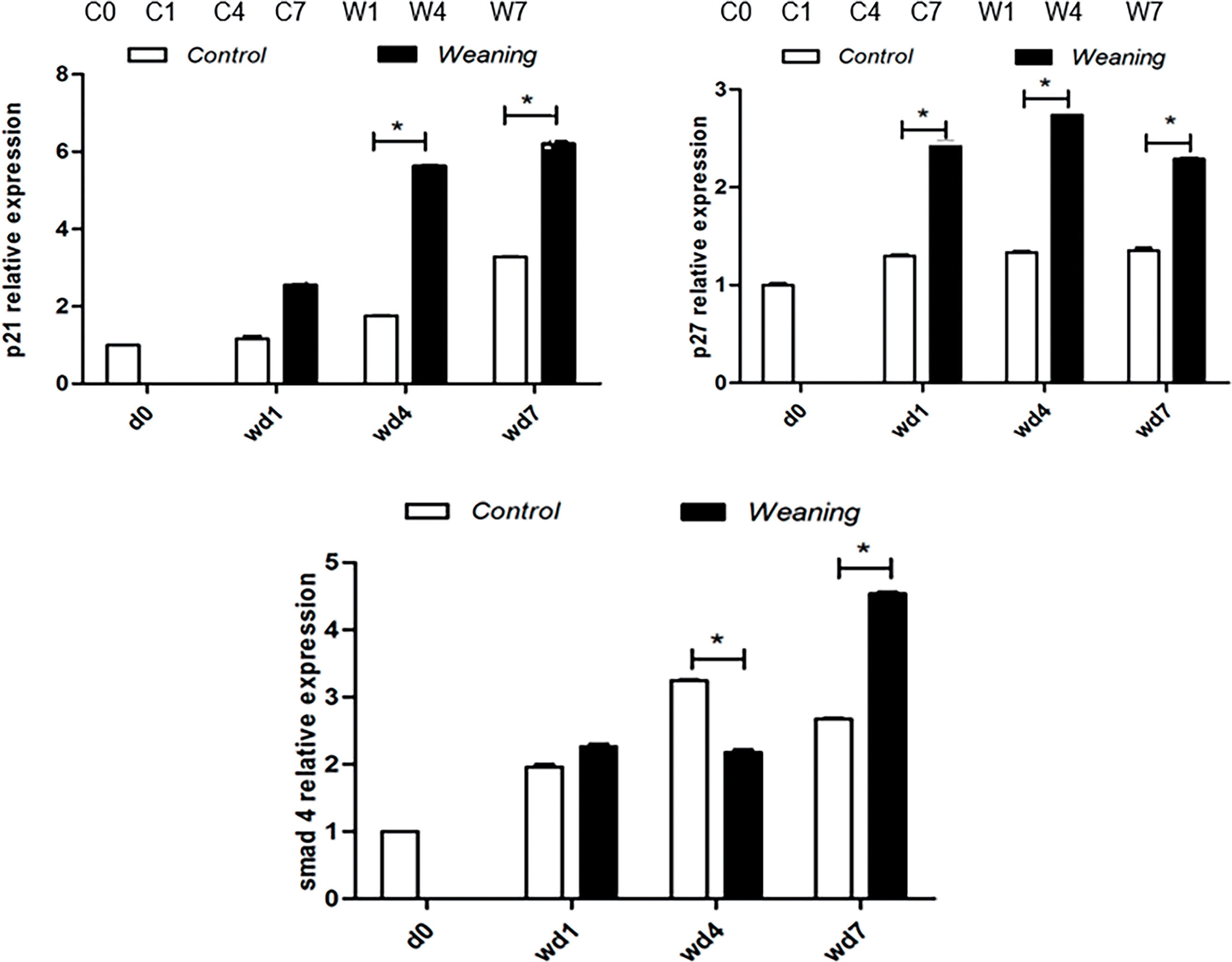
Figure 5: Piglet jejunum showing protein expression of cell cycle regulation. (A): Regulation of the cell cycle by protein expression in the control group and the weaning group at days 0, 1, 4, and 7 after weaning. C0, 1, 4, and 7 represent the control group at 0, 1, 4, and 7 days post-weaning, respectively. W1, W4, and W7 represent the weaning groups at 1, 4, and 7 days post-weaning, respectively. (B): Protein levels of cell cycle regulators of piglets determined by western blotting. The protein expression levels of the (day 0) d0 were set to 1.0. *p < 0.05; data were shown as mean ± SEM (n = 6)
3.2.1 Body Weight, ADFI, and ADG
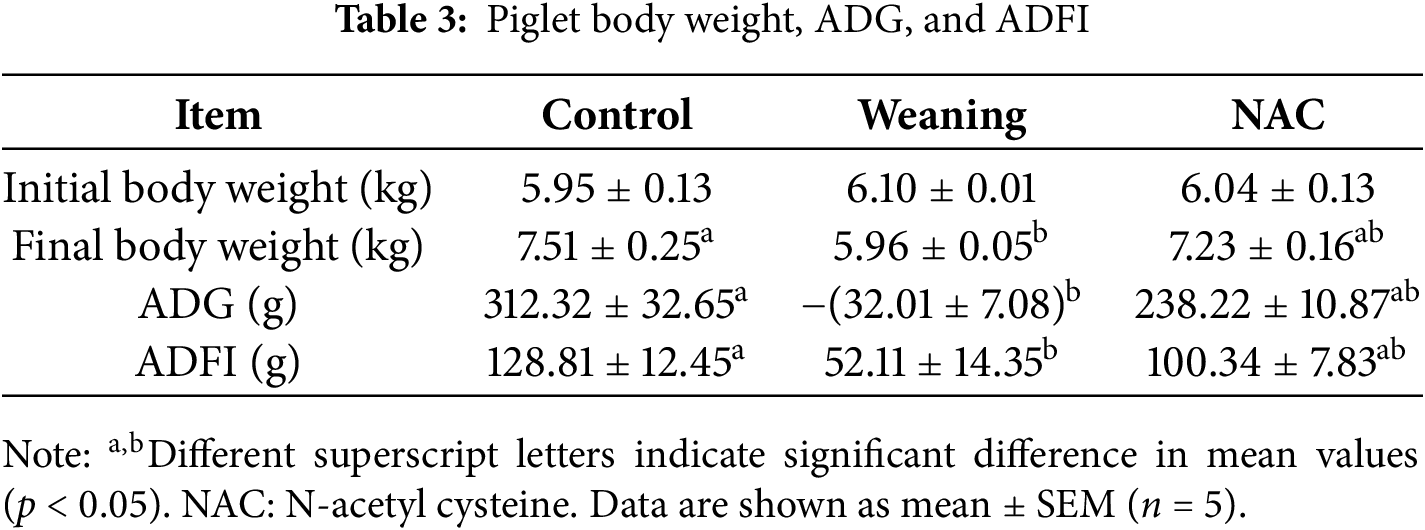
Compared with the control group, body weight, ADFI, and ADG decreased significantly in the piglets that were weaned (p < 0.05). Results are given in Table 3. Compared with the piglets that were weaned, adding NAC to the piglet diet significantly increased the ADFI and ADG of the piglets four days after weaning (p < 0.05).
3.2.2 Oxidative Damage and Redox State Indexes
Fig. 6 shows that compared with the control group, the content of 8OH-dG, 8-ISO-PGF-2α, and PC significantly increased in the piglets that were weaned (p < 0.05). Compared with the weaning group, the 8OH-dG, 8-ISO-PGF2α, and PC contents of piglets supplemented with NAC were significantly decreased (p < 0.05).
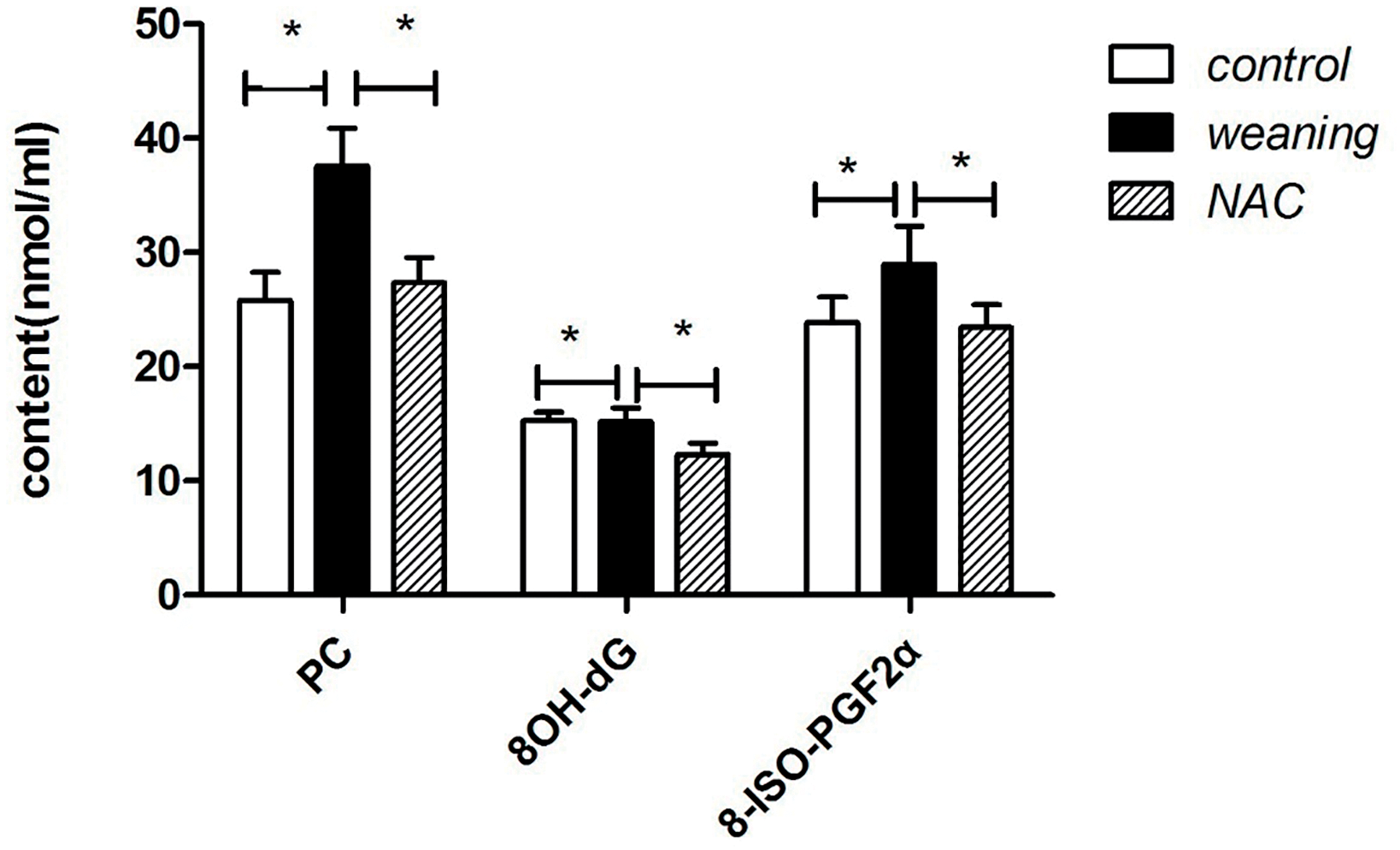
Figure 6: Serum oxidative damage of piglets. PC: protein carbonylation; 8OH-dG: 8-hydroxydeoxyguanosine; 8-ISO-PGF2α: 8-isoprostaglandin F2α; NAC: N-acetyl cysteine. *p < 0.05; data are shown as mean ± SEM (n = 5)
Compared with piglets that were weaned, the serum ROS significantly decreased (p < 0.05) and the GSH content significantly increased (p < 0.05) in the control group. The results are given in Table 4. In the control group, the content of ROS and GSSG in the intestine significantly decreased (p < 0.05), the GSH content significantly increased (p < 0.05), and the GSSG/GSH significantly decreased (p < 0.05).
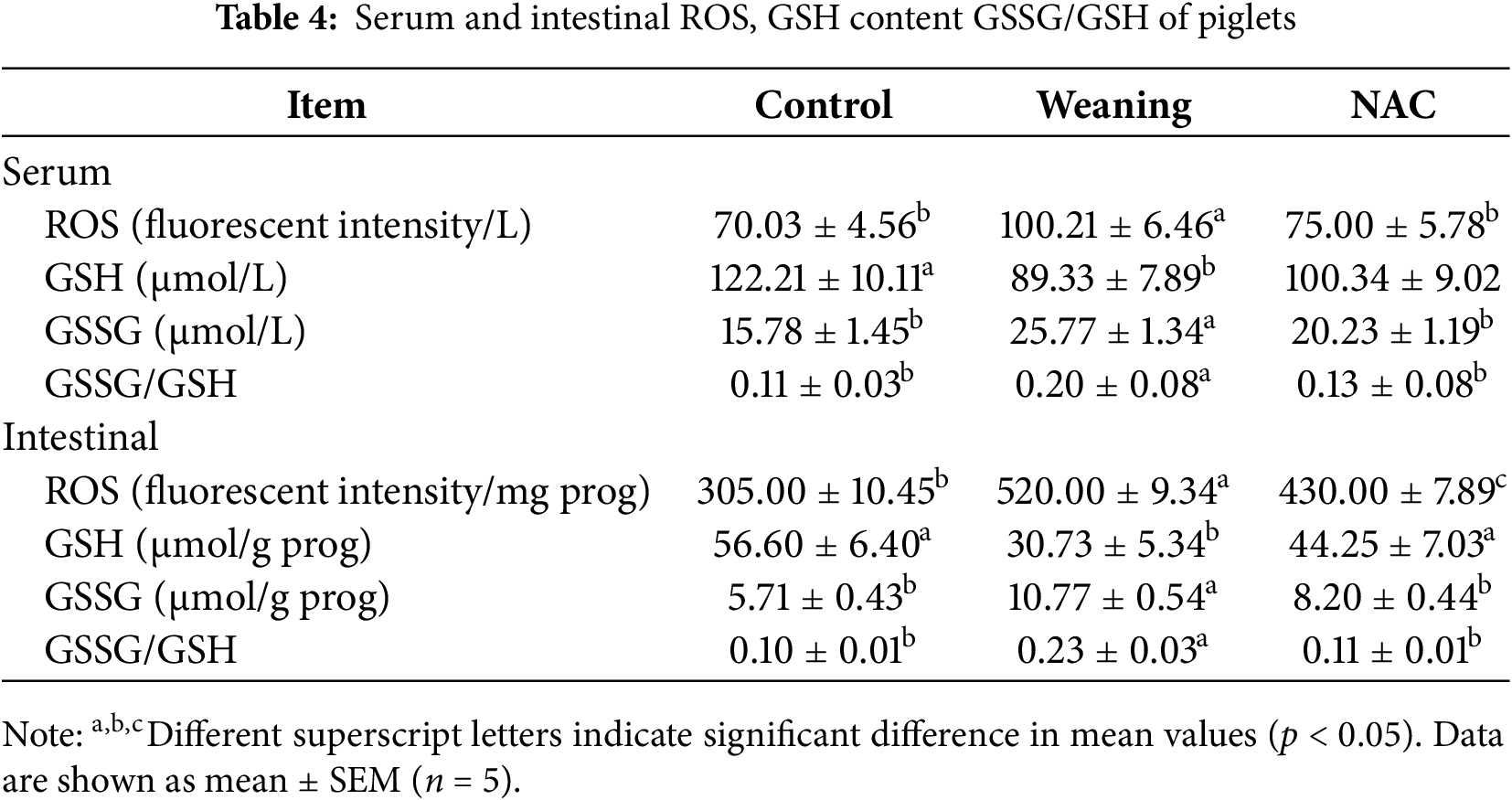
Compared with the piglets that were weaned, the ROS levels decreased significantly (p < 0.05), the GSH content increased (p > 0.05), the content of GSSG/ROS in the intestine decreased (p < 0.05), the content of GSH significantly increased (p < 0.05), and the GSH/GSSG significantly decreased (p < 0.05) in the NAC group.
Compared with the piglets that were weaned, the jejunal villus width and height significantly increased (p < 0.05), and the jejunal crypt depth significantly decreased (p < 0.05) compared with the control group. The results are given in Table 5.
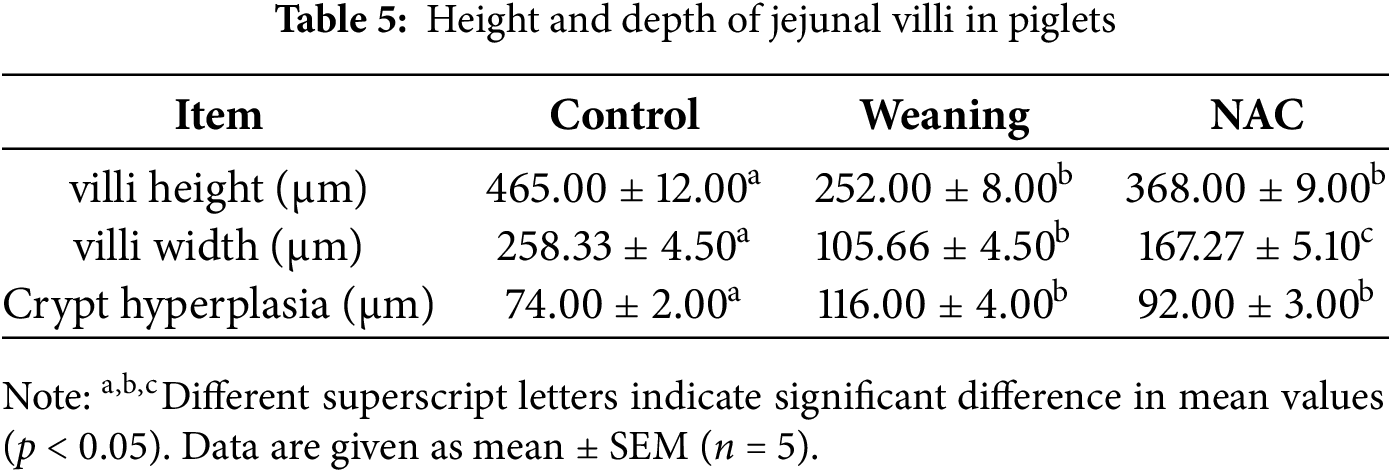
Compared with the control group, the jejunal villus width and height decreased (p < 0.05), and crypt hyperplasia increased (p < 0.05) compared with the NAC group.
3.2.4 Cyclin D, Cyclin E, and Smad4 Protein Expression
Compared with the piglets that were weaned, cyclin D and E expression significantly increased, and Smad4 expression increased in the control group (p < 0.05). Results are shown in Fig. 7A,B. Compared with piglets that were fed NAC, cyclin D and E protein expression significantly decreased (p < 0.05), and Smad4 expression significantly decreased in piglets that were weaned.
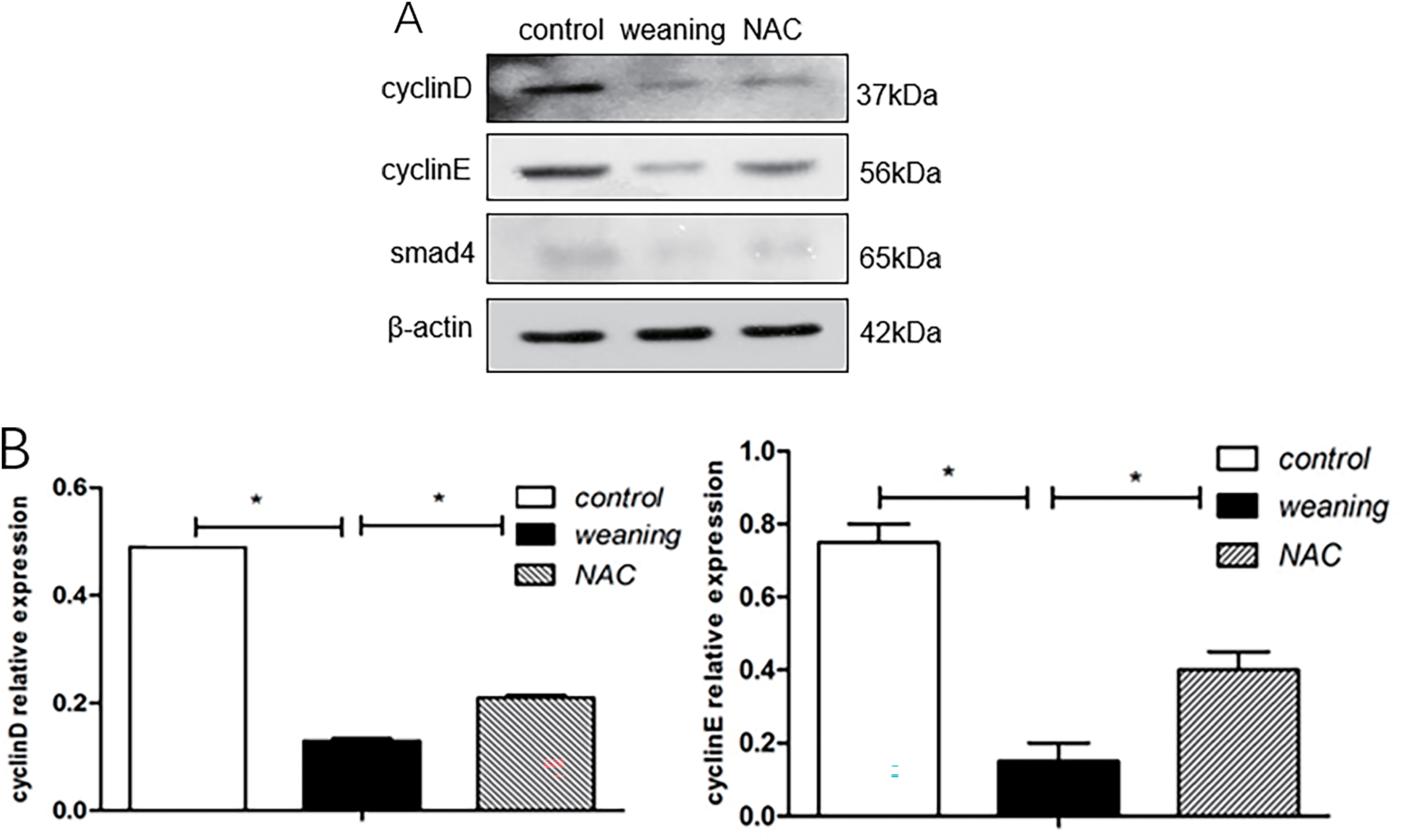
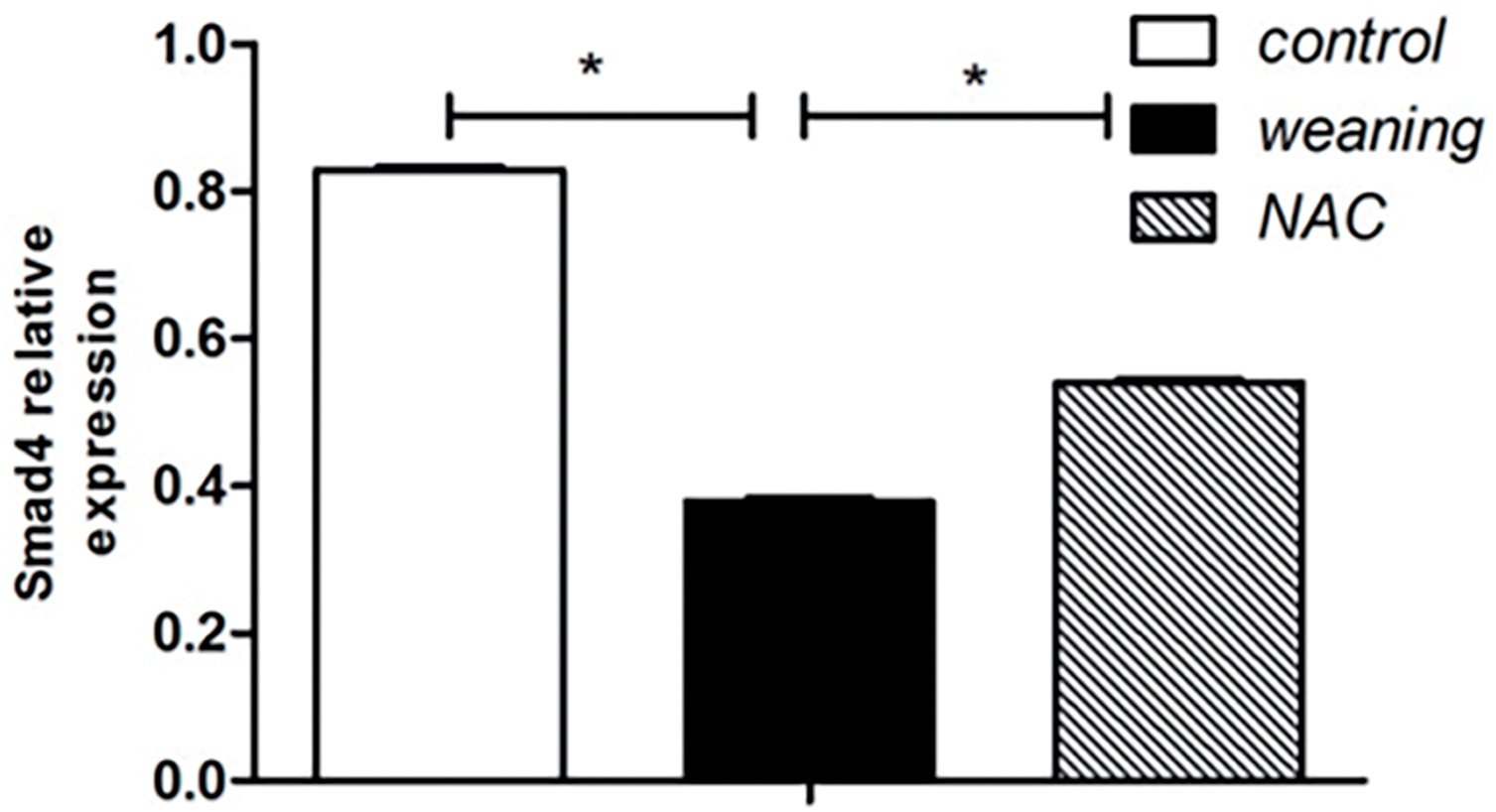
Figure 7: Cyclin D, cyclin E, and smad4 protein expression levels of piglets. (A): Cell cycle regulator protein expression of the dietary supplementation of NAC. (B): Protein levels of cell cycle regulators in piglets were determined using western blotting. Data are presented as mean ± SEM (n = 5). *p < 0.05
3.2.5 Phosphorylation Levels and Total Levels of p21 and p27
The total levels of p21 and p27 in the weaning group and their phosphorylation levels were significantly increased (p < 0.05) compared with the control group (Fig. 8A,B). Compared with the weaning group, NAC supplementation decreased the total levels and phosphorylation levels of p21 and p27.
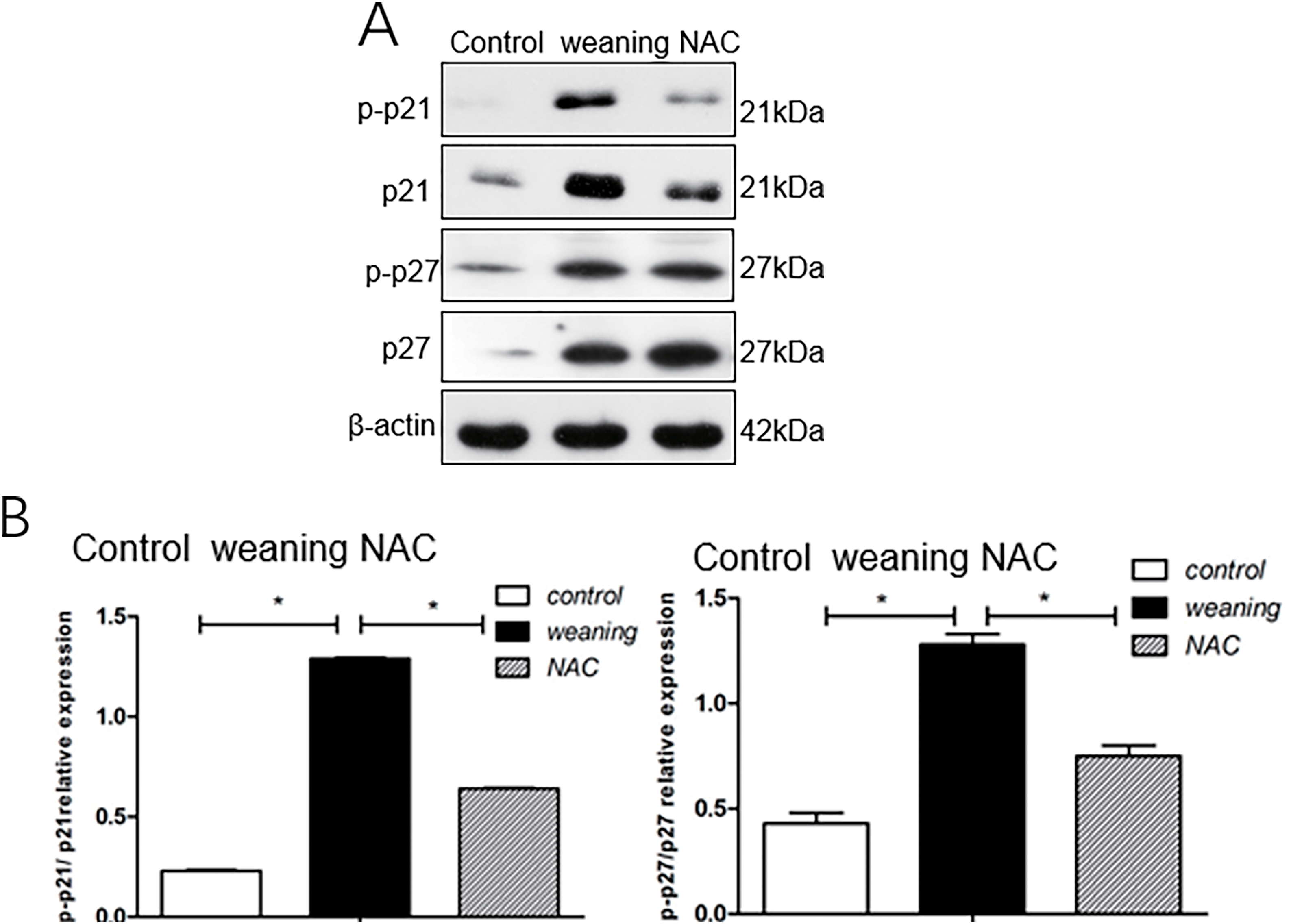
Figure 8: Phosphorylation levels and the total levels of p21 and p27 in the intestine of piglets. (A): Cell cycle inhibitor protein expression in piglets fed diets supplemented with N-acetyl cysteine (NAC). (B): Results from western blotting show levels of cell cycle inhibition. *p < 0.05; data are shown as mean ± SEM (n = 5)
3.2.6 Phosphorylation Levels and Total Levels of p38, JNK, and ERK
Compared with the control group, p-ERK/ERK, p-JNK/JNK, and p-p38/p38 ratios were significantly decreased (p < 0.05) in piglets that were weaned (Fig. 9A,B). Compared with the group fed NAC, p38, JNK, and ERK phosphorylation significantly increased (p < 0.05) in piglets that were weaned.
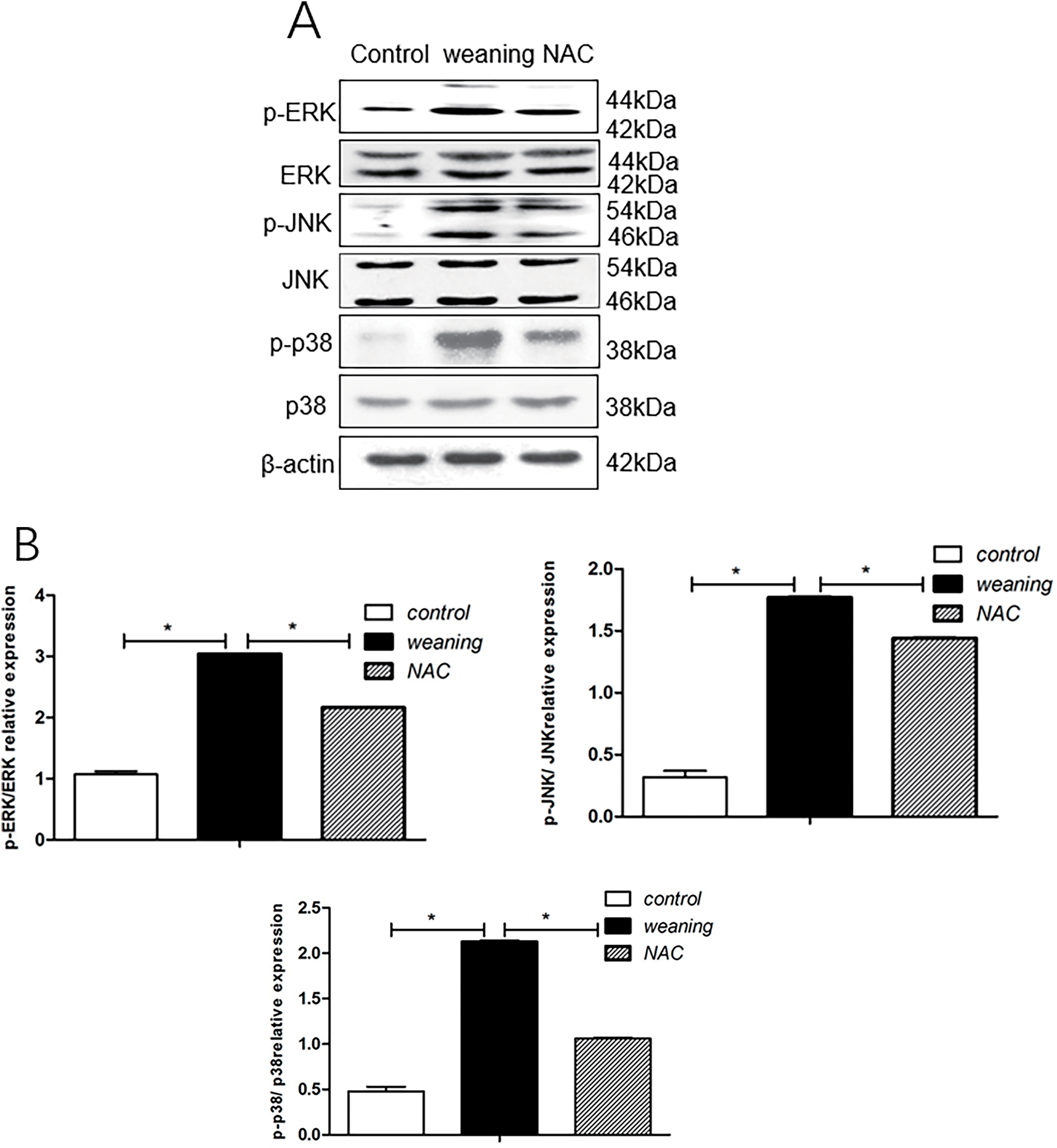
Figure 9: Phosphorylation levels and total levels of p38, JNK, and ERK. (A): p38, JNK, and ERK protein expression of piglets supplemented with N-acetyl cysteine (NAC). (B): Results from western blotting show protein levels of cell cycle inhibition. *p < 0.05; data are shown as mean ± SEM (n = 5)
The most important period in piglet development occurs during early weaning. Oxidative stress caused by early weaning in piglets has an impact on intestinal function development [19,20]. Intestinal cell proliferation is an important foundation for the development of intestinal function [21]. Excessive ROS due to oxidative stress leads to a series of phenomena that are deleterious to cell proliferation and development, such as cell cycle progression arrest, autophagy, and apoptosis [22–26]. Cyclins are a group of proteins that exhibit simultaneous cyclic expression changes during the cell cycle. These proteins work with CDKs to drive and control the progression of the cell cycle. ROS acts as a signal to induce the phosphorylation of kinases involved in MAPK signal transduction events [24]. The MAPK signalling pathway contains a network of protein kinases: MAPKKK, MAPKK, and MAPK. In response to ROS, the serine/threorine terminal of MAPK is activated. MAPK stimulates MAPKK phosphorylation, and the MAPKKK activity of Raf 1 operates downstream of transcription factors and gene expression [27–30].
ERK, JNK, and p38 are initiated by Raf1 phosphorylation. Expression of c-Fos and c-Jun is stimulated at the transcriptional level by ERK, JNK, and p38 phosphorylation.
Expression of the downstream target is induced by c-Fos and c-Jun activation [31]. Thus, inhibition of cyclin D and E complexes is triggered by ROS, which increases p21 and p27 expression [32]. In experiment I, weaning reduced the expression of cyclin E and cyclin D (p > 0.05), while the expression of p21 and p27 was significantly increased from post-weaning days 1 to 7 (p < 0.05). Cyclin D and cyclin E are key regulatory proteins for the G1 phase and the transition from the G1→ S phase in the cell cycle. Their expression levels play a critical role in whether cell cycle progression moves forward. The findings of this study indicate that the cell cycle progression in the intestines of early-weaning piglets may be arrested in the G1 phase. According to the results of the intestinal morphological analysis, piglets that were weaned had increased depth of intestinal crypts and elevated p21 and p27 expression. The potential mechanism may be that the accumulation of intestinal stem cells, which do not divide promptly due to cell cycle arrest, leads to crypt hyperplasia.
The expression of CDK4 has been reported to show no evident changes induced by ROS, indicating a lag in response [4]. Therefore, cyclin D and E expression play a crucial role. In the second animal experiment, we found that weaning significantly decreased cyclin D and E expression (p < 0.05). We attributed the statistical difference in cyclin D and E expression between the first and second animal experiments to the differences in the sample sizes. According to the second animal experiment’s morphological results, the villus in the piglets that were weaned were not as well developed as the villus in the NAC group. This result indicated that NAC regulated p21 and p27 expression by controlling ROS, thus encouraging cell proliferation and blocking cell cycle arrest. Compared with normal suckled piglets, the cytoprotective effect of the NAC diet improved the redox state, relieved the intestinal damage, and maintained the intestinal health of the piglets.
In this study, we found that the expression of p-ERK, p-JNK, and p-p38 in the intestines was significantly upregulated as a result of stress caused by early weaning of piglets. This result aligned with research by Hu et al. [33], which showed increased ratios of ERK, JNK, and p38 phosphorylation in the intestines of piglets that were weaned.
Our results showed that p-ERK, p-JNK, and p-p38 triggered carboxyl-terminal phosphorylation of transcription factor Sp1 and regulated downstream target gene expression (e.g., p21), and ROS activated the MAPK signaling pathways [34]. In the second animal experiment, ERK, JNK, and p38 phosphorylation significantly decreased in the piglets that were weaned, and p21 and p27 expression significantly decreased (p < 0.05) compared with the NAC group.
Stress caused by early weaning triggered the ERK, JNK, and p38 signaling pathways. By decreasing ERK, JNK, and p38 phosphorylation, NAC was able to modulate p21 and p27 expression through ROS regulation. Zhao (2018) reported that the down-regulation of smad4 could directly induce increased expression of p21, which affected cell growth arrest [35]. Cell cycle arrest is a primary cause of reduced intestinal function induced as a result of stress in piglets caused by weaning. We found that NAC promoted cell proliferation and alleviated cell cycle arrest mediated by ROS. Supplementing the piglet diet with NAC also increased ADG and ADFI in piglets, which improved intestinal function after weaning. This study reveals the changes in the intestinal cell cycle during early weaning in piglets, their relationship with the MAPK signaling pathway, and the influence of NAC on this process. These experiments were preliminary, and the sample sizes were small. In future research, further experiments can be conducted to examine the expression of cyclin-cdk complex and p21 and p27 genes and compare them with protein levels to clarify the role of MAPK in intestinal cell cycle changes and the regulation mechanism of NAC during early weaning.
In this study, we verified that stress caused by weaning downregulated the cyclin D and E expression in the intestines, which stagnated cell proliferation and caused intestinal barrier dysfunction in piglets. By supplementing the piglet diet with NAC, excess ROS induced by the stress of weaning could be scavenged, and p21 and p27 expression could be regulated by decreasing ERK, JNK, and p38 phosphorylation. As a result, cell cycle arrest was inhibited in piglets’ intestines. NAC can promote intestinal function and intestinal cell integrity and is expected to represent a promising new type of feed additive.
Acknowledgement: The authors would like to acknowledge Dr. Lihui Zhu, Dr. Wenli Luo providing suggestions for experimental design in Shanghai Jiao Tong University.
Funding Statement: This research was supported by the Jilin Agricultural Science and Technology University under the Scientific Startup Foundation for Doctors ((2022)733) and Shanghai Jiao Tong University under the National Natural Science Foundation of China (30972103).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Jianxiong Xu, Qi Guo; data collection: Xuan Cai; analysis and interpretation of results: Jiaojiao Xie; draft manuscript preparation: Siqi Wu. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: The experimental protocol received approval from the Ethics Committee of Shanghai Jiao Tong University (Approval No. 201801124). All animals were treated humanely.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| ERK | Extracellular signal-regulated kinase |
| p38 | Mitogen-activated protein kinase p38 |
| JNK | c-Jun N-terminal kinases |
| SOD | Superoxide dismutase |
| GSH-px | Glutathione peroxidase |
| ROS | Reactive oxygen species |
| NAC | N-acetylcysteine |
| MAPK | Mitogen-activated protein kinase |
| CDKs | Cyclin-dependent kinases |
| CKIs | CDK inhibitors |
| PC | Protein carbonylation |
| 8OH-dG | 8-hydroxydeoxyguanosine |
| 8-ISO-PGF2α | 8-isoprostaglandin F2α |
| ADG | Average daily gain |
| ADFI | Average daily feed intake |
References
1. Zhu L, Zhao K, Chen X, Xu J. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 2013;9(8):2581–9. doi:10.2527/jas.2012-4444. [Google Scholar] [PubMed] [CrossRef]
2. Xiong X, Yang H, Tan B, Yang C, Wu M, Liu G, et al. Differential expression of proteins involved in energy production along the crypt-villus axis in early-weaning pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2015;309(4):G229–37. doi:10.1152/ajpgi.00095.2015. [Google Scholar] [PubMed] [CrossRef]
3. Ziegler AL, Pridgen TA, Blikslager AT. Environmental stressors affect intestinal permeability and repair responses in a pig intestinal ischemia model. Tisse Barriers. 2020;8(4):e1832421. doi:10.1080/21688370.2020.1832421. [Google Scholar] [PubMed] [CrossRef]
4. Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26(8):1101–9. doi:10.1038/sj.onc.1209895. [Google Scholar] [PubMed] [CrossRef]
5. Menon SG, Goswami P. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007;67(13):6392–9. doi:10.1158/0008-5472.CAN-07-0225. [Google Scholar] [PubMed] [CrossRef]
6. Hodder S, Fox M, Mokhtar AMBA, Mott HR, Owen D. Acknowledging the role of the Activated-Cdc42 associated kinase (ACK) in regulating protein stability in cancer. Small GTPases. 2023;14(1):14–25. doi:10.1080/21541248.2023.2212573. [Google Scholar] [PubMed] [CrossRef]
7. Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAPkinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60(3):261–310. doi:10.1124/pr.107.00106. [Google Scholar] [PubMed] [CrossRef]
8. Chakraborti S, Chakraborti T. Oxidant-mediated activation of mitogen-activated protein kinases and nuclear transcription factors in the cardiovascular system: a brief overview. Cell Signal. 1998;10(10):675–83. doi:10.1016/S0898-6568(98)00014-X. [Google Scholar] [PubMed] [CrossRef]
9. Qi T, Luo Y, Cui W, Zhou Y, Ma X, Wang D, et al. Crosstalk between the CBM complex/NF-κB and MAPK/P27 signaling pathways of regulatory T cells contributes to the tumor microenvironment. Front Cell Dev Biol. 2022;10:e911811. doi:10.3389/fcell.2022.911811. [Google Scholar] [PubMed] [CrossRef]
10. Haddad JJ, Harb HL. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s). Mol Immunol. 2005;42(9):987–1014. doi:10.1016/j.molimm.2004.09.029. [Google Scholar] [PubMed] [CrossRef]
11. Giancarlo A, Alessandra A, Giovanna B, Giulio V, Marina C, Liusa B. N-acetyl cysteine as an antioxidant and disulphide breaking agent: the reason why. Free Radic Res. 2018;52(7):751–62. doi:10.1080/10715762.2018.1468564. [Google Scholar] [PubMed] [CrossRef]
12. Huang F, Liu QY, Xie SJ, Xu J, Huang B, Wu YH, et al. Cypermethrin induces macrophages death through cell cycle arrest and oxidative stress-mediated JNK/ERK signaling regulated apoptosis extracellular MUC3 mucin secretion follows adherence of lactobacillus strains to intestinal epithelial cells in vitro. Int J Mol Sci. 2016;17(6):885. doi:10.3390/ijms17060885. [Google Scholar] [PubMed] [CrossRef]
13. Lee SI, Kang KS. N-acetylcyteine modulates lipopolysaccharide-induced intestinal dysfunction. Sci Rep. 2019;9(1):1004. doi:10.1038/s41598-018-37296-x. [Google Scholar] [PubMed] [CrossRef]
14. Qi M, Jing W, Tan B, Li J, Liao S, Liu Y, et al. Dietary glutamine, glutamate, and aspartate supplementation improves hepatic lipid metabolism in post-weaning piglets. Anim Nutr. 2020;6(2):124–9. doi:10.1016/j.aninu.2019.12.002. [Google Scholar] [PubMed] [CrossRef]
15. Meng Q, Sun S, Luo Z, Shi B, Shan A, Cheng B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019;10(9):5626–43. doi:10.1039/C9FO00637K. [Google Scholar] [PubMed] [CrossRef]
16. Li J. Effect of dietary added Cordyceps militaris on growth performance and intestinal health of weaned piglets [dissertation]. Xianyang, China: Northwest Agricutural and Forestry Technology University; 2023. [Google Scholar]
17. Zhu LH, Cai X, Guo Q, Chen XL, Zhu SW, Xu JX. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Brit J Nutr. 2013;110(11):1938–47. doi:10.1017/S0007114513001608. [Google Scholar] [PubMed] [CrossRef]
18. Shu DZ. Effect of berberine hydrochloride on experimental colitis in mice [dissertation]. Chongqing, China: Chongqing Medical University; 2005. [Google Scholar]
19. Price KL, Lin X, van Heugten E, Odle R, Willis G, Odle J. Diet physical form, fatty acid chain length, and emulsification alter fat utilization and growth of newly weaned pigs. J Anim Sci. 2013;91(2):783–92. doi:10.2527/jas.2012-5307. [Google Scholar] [PubMed] [CrossRef]
20. Zhu LH. Molecular mechanism and nutritional regulation of intestinal cell apoptosis in early weaned piglets [dissertation]. Shanghai, China: Shanghai Jiao Tong University; 2014. [Google Scholar]
21. Chen X, Yang Z, Hu H, Duan W, Wang A, Dong Y, et al. Differentiation and proliferation of intestinal stem cells and its underlying regulated mechanisms during weaning. Curr Protein PepSci. 2019;20(7):690–5. doi:10.2174/1389203720666190125101834. [Google Scholar] [PubMed] [CrossRef]
22. Burhans WC, Heintz NH. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med. 2009;47(9):1282–93. doi:10.1016/j.freeradbiomed.2009.05.026. [Google Scholar] [PubMed] [CrossRef]
23. Sun WY, Liu J, Shi X, Bi YJ, Liu HY, Xu T. Emamectin benzoate and microplastics led to skeletal muscle atrophy in common carp via induced oxidative stress, mitochondrial dysfunction, and protein synthesis and degradation imbalance. J Agric Food Chem. 2025;73(5):3106–16. doi:10.1021/acs.jafc.4c10479. [Google Scholar] [PubMed] [CrossRef]
24. Xue Q, Kang R, Klionsky DJ, Tang DL, Liu JB et al., Copper metabolism in cell death and autophagy. Autophagy. 2023;19(8):2175–95. doi:10.1080/15548627.2023.2200554. [Google Scholar] [PubMed] [CrossRef]
25. Lu HM, Hou LL, Zhang Y, Gao TT, Wang Y, Xing MW. Polystyrene microplastics mediate cell cycle arrest, apoptosis, and autophagy in the G2/M phase through ROS in grass carp kidney cells. Environ Toxicol. 2024;39(4):1923–35. doi:10.1002/tox.24068. [Google Scholar] [PubMed] [CrossRef]
26. Zhao HJ, Wang Y, Liu YC, Yin K, Wang DX, Li BY, et al. ROS-induced hepatotoxicity under cypermethrin: involvement of the crosstalk between Nrf2/Keap1 and NF-κB/iκB-α pathways regulated by proteasome. Env Sci Technol. 2021;55(9):6171–83. doi:10.1021/acs.est.1c00515. [Google Scholar] [PubMed] [CrossRef]
27. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–83. doi:10.1038/s41580-020-0230-3. [Google Scholar] [PubMed] [CrossRef]
28. Winterbourn CC. Hydrogen peroxide reactivity and specificity in thiol-based cell signalling. Biochem Soc Trans. 2020;48(3):745–54. doi:10.1042/BST20190049. [Google Scholar] [PubMed] [CrossRef]
29. Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97(10):2499–574. doi:10.1007/s00204-023-03562-9. [Google Scholar] [PubMed] [CrossRef]
30. Mohammadi Zonouz A, Ghasemzadeh Rahbardar M, Hosseinzadeh H. The molecular mechanisms of ginkgo (Ginkgo biloba) activity in signaling pathways: a comprehensive review. Phytomedicine. 2024;126:155352. doi:10.1016/j.phymed.2024.155352. [Google Scholar] [PubMed] [CrossRef]
31. Averill-Bates D. Reactive oxygen species and cell signalling review. BBA Mol Cell Res. 2024;1871(2):119573. doi:10.1016/j.bbamcr.2023.119573. [Google Scholar] [PubMed] [CrossRef]
32. Ionica M, Samantha C, de Petra JV, Paul B, Aneela M, Wan M, et al. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem. 2012;287(13):9845–54. doi:10.1074/jbc.M111.250357. [Google Scholar] [PubMed] [CrossRef]
33. Hu CH, Xiao K, Luan ZS, Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91(3):1094–101. doi:10.2527/jas.2012-5796. [Google Scholar] [PubMed] [CrossRef]
34. Zhang X. Molecular Mechanism studies of neferine induced G1 cell cycle arrest in human osteosarcoma [dissertation]. Jinan, China: Shandong University; 2012. [Google Scholar]
35. Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 2018;12;14(2):111–23. doi:10.7150/ijbs.23230. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools