 Open Access
Open Access
REVIEW
The Role of Ginsenoside Rg3 in Modulating Oxidative Stress, Apoptosis, and Angiogenesis: Implications for Skincare and Anticancer Therapies
Product Development Team, Agricultural Corporation Korean Master Ginseng Co., Ltd., 30 Digital-ro 32-gil, Guro-gu, Seoul, 08390, Republic of Korea
* Corresponding Author: Tae Hyon Kim. Email:
BIOCELL 2025, 49(7), 1141-1168. https://doi.org/10.32604/biocell.2025.065464
Received 13 March 2025; Accepted 27 May 2025; Issue published 25 July 2025
Abstract
Ginsenosides, the bioactive saponins primary found in Panax ginseng, possess a complex structure that underlies their diverse pharmacological properties. Ginsenoside Rg3 stands out for its broad therapeutic potential, including anticancer, anti-inflammatory, neuroprotective, and cardiovascular effects. This review provides a comprehensive overview of the cellular and molecular mechanisms of Rg3, emphasizing its roles in regulating apoptosis, inflammation, oxidative stress, and metabolic pathways relevant to skincare and anticancer applications. The unique biological activities of its isomeric forms, 20(S)-Rg3 and 20(R)-Rg3, are highlighted, alongside strategies to enhance its bioavailability, such as nanoencapsulation and prodrug design. Additionally, the synergistic effects of Rg3 when combined with other treatments are discussed, underscoring its promise as a bridging agent between conventional and emerging therapies in dermatology and oncology. Finally, the current research gaps are identified, and future directions are proposed to further optimize the clinical application of Rg3.Keywords
Ginsenosides are a class of saponins predominantly found in Panax ginseng and other ginseng species [1]. These compounds possess a complex structure comprising a steroid-like aglycone (the non-sugar portion) linked to one or more sugar moieties (glycosides) [2]. The aglycone component is a triterpenoid, and the sugar molecules are typically glucose, rhamnose, or other sugars with the aglycone backbone connected via a glycosidic bond [3]. Sugars enhance the solubility, bioavailability, and pharmacological activity of ginsenoside [4]. Consequently, the structure of ginsenosides is pivotal for their biological and pharmacological effects. Generally, ginsenosides are categorized into two primary groups based on the position and type of sugar attached to the aglycone: protopanaxadiol-type (PPD) and protopanaxatriol-type (PPT) [5]. PPD-type ginsenosides feature a hydroxyl group at the 6-position of the aglycone, whereas PPT-type ginsenosides have a hydroxyl group at the 20-position [6]. The presence of different sugars and functional groups at specific positions further defines the specific type of ginsenoside (such as Rg1, Rg3, Re, Rh1, etc.) [7].
Among these, ginsenoside Rg3 is a notable active compound found in Panax ginseng and has been recognized for its various therapeutic properties [8]. Ginsenoside Rg3 exists in two isomeric forms, 20(S)-Rg3 and 20(R)-Rg3, which differ in the stereochemistry of their hydroxyl groups at the 20th carbon position (Fig. 1) [9]. These two isomers exhibit distinct biological activities, with 20(S)-Rg3 often demonstrating greater biological activity than 20(R)-Rg3 [9,10]. The fundamental skeleton is typically of the dammarane type, which constitutes the core structure of most ginsenosides, including Rg3 [11]. This backbone comprises carbon atoms arranged in a specific configuration, which can be modified by functional groups such as hydroxyl (-OH), carbonyl, and acetoxy groups [12].
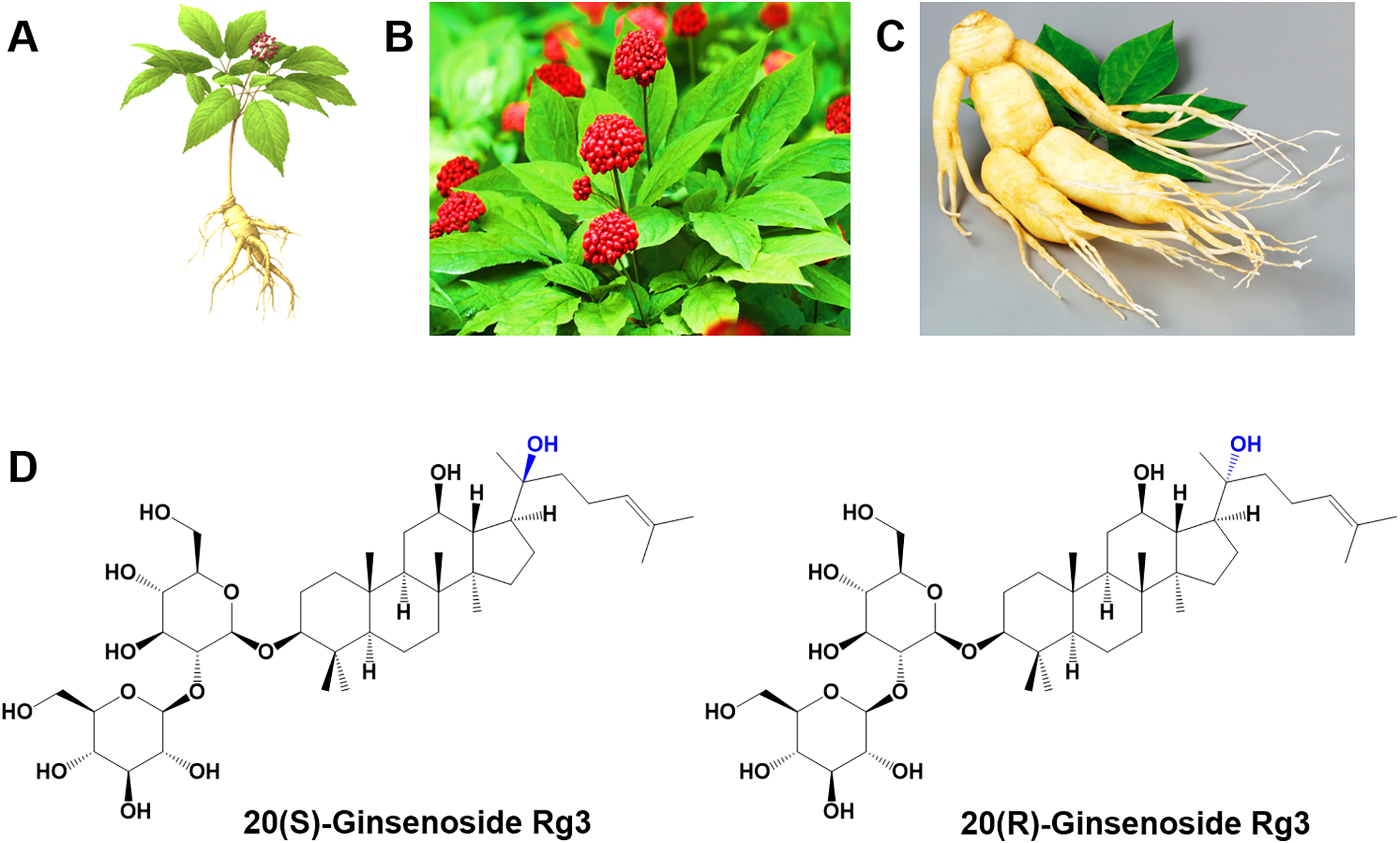
Figure 1: Overview of the morphology and stereochemistry of Panax ginseng and its active components. (A) Micrograph depicting the morphological structure of Panax ginseng. (B) Close-up image of the berry from Panax ginseng. (C) Characteristic anthropomorphic shape of the Panax ginseng root, commonly referred to as the “man-root” due to its resemblance to a human figure. (D) Chemical structures of two stereoisomers, 20(R)-ginsenoside Rg3 and 20(S)-ginsenoside Rg3, illustrating their distinct configurations. Reprinted with permission from Ref. [2]. 2022, Multidisciplinary Digital Publishing Institute
Ginsenoside Rg3 is recognized for its extensive pharmacological activities, including anticancer, anti-inflammatory, neuroprotective, and cardiovascular effects [9]. Nevertheless, its pharmacokinetic properties pose significant challenges, particularly owing to its low bioavailability. Upon oral administration, Rg3 is absorbed in the gastrointestinal tract; however, its large molecular structure and glycosidic linkage (with sugar groups) result in poor absorption [13]. The compound undergoes extensive first-pass metabolism in the liver, where it is transformed into various metabolites, some of which may retain their pharmacological effects [14]. The absorption process is further impeded by the conversion of Rg3 to its aglycone form by intestinal microbes [15]. Although this conversion can enhance the absorption, it occurs relatively slowly. Additionally, Rg3 is rapidly eliminated from the body, primarily through urine, contributing to its short half-life and limiting its therapeutic efficacy [16].
To enhance its bioavailability, researchers are investigating various strategies, such as nanoencapsulation, lipid-based formulations, and prodrug designs [17–21]. These approaches aim to improve the solubility, stability, and controlled release of Rg3 into the body, thereby increasing its therapeutic effectiveness [22]. Biologically, Rg3 demonstrates potent anticancer activity by promoting apoptosis in various cancer cells and inhibiting tumor growth [23]. It can suppress angiogenesis, the process of new blood vessel formation required by tumors for growth, and also reduce cancer cell migration and invasion, thereby preventing metastasis [24]. Rg3 is also known for its anti-inflammatory effects, as it suppresses the production of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and modulates pathways such as nuclear factor kabba B (NF-κB), which play a central role in inflammatory responses [25]. Furthermore, Rg3 scavenges reactive oxygen species (ROS), thereby reducing oxidative stress and protecting cells from damage [26]. This antioxidant activity contributes to its neuroprotective, cardioprotective, and anti-aging effects.
Ginsenoside Rg3 exhibits neuroprotective properties, including enhancement of cognitive function and memory, which may be beneficial in the management of neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases [27,28]. It facilitates neurogenesis and mitigates neuroinflammation, thereby protecting the brain against neurodegenerative damage [29]. In the context of cardiovascular health, Rg3 contributes to the reduction of blood pressure, improvement of blood circulation, and prevention of myocardial injury resulting from ischemia or heart attack [30]. Furthermore, Rg3 has been shown to modulate the immune system by enhancing the activity of immune cells and improving the body’s response to infections or cancer therapies [31]. Some studies indicate that Rg3 may enhance insulin sensitivity and regulate blood glucose levels, which could be advantageous for the management of diabetes [32]. Additionally, Rg3 may assist in hormone regulation, particularly in menopausal women, by alleviating symptoms, such as hot flashes [33]. Despite its significant therapeutic potential, the low bioavailability of Rg3 necessitates careful formulation to augment its clinical efficacy.
This review provides a comprehensive analysis of the anticancer and skincare applications of Ginsenoside Rg3, emphasizing its ability to modulate key cellular and molecular pathways. By focusing on mechanisms such as oxidative stress, apoptosis, inflammation, angiogenesis, and cellular metabolism, we highlight Rg3’s potential as a multifaceted therapeutic agent in both oncology and dermatology (Fig. 2). Moreover, we explore its synergistic effects when combined with conventional treatments, positioning Rg3 as a promising integrative approach that bridges natural compounds and modern therapies. Finally, we identify current research gaps and propose future directions to enhance the clinical translation of Rg3-based interventions.
Figure 2: Various applications of Rg3. Created with BioRender and ChemDraw
2 Skin Care Applications of Ginsenoside Rg3
Ginsenoside Rg3, a potent bioactive compound derived from ginseng, confers numerous skin care benefits owing to its anti-aging, anti-inflammatory, and regenerative properties [34]. It functions as a powerful antioxidant that mitigates oxidative stress and prevents premature signs of aging, such as wrinkles and loss of elasticity [35]. Additionally, Rg3 inhibits inflammation, rendering it effective for treating conditions such as acne and eczema. It also promotes collagen synthesis, thereby enhancing skin firmness and elasticity, while supporting skin regeneration and wound healing [36]. Its photoprotective effects help shield the skin from UV damage and may also regulate pigmentation, contributing to skin brightening and addressing hyperpigmentation.
Ginsenoside Rg3 plays a significant role in mitigating skin aging through its multifaceted effects on collagen metabolism and oxidative stress [37]. It contributes to the maintenance of the skin structure by inhibiting the activity of matrix metalloproteinases (MMPs), enzymes responsible for the degradation of collagen and elastin in the skin [38]. MMPs are often upregulated by UV radiation and environmental stressors, leading to skin aging (Fig. 3). By inhibiting these enzymes, Rg3 helps to preserve the integrity of the extracellular matrix of the skin, thereby preventing the breakdown of essential structural proteins. For instance, when the stereoisomers of both 20(S)-ginsenoside Rg3 and 20(R)-ginsenoside Rg3 were co-cultured with HaCaT cells (human immortalized keratinocytes) and human dermal fibroblast cells prior to UV-B irradiation at 70 mJ/cm2, a greater reduction in UV-B-induced ROS levels was observed for 20(S)-ginsenoside Rg3 [39]. In addition, 20(S)-ginsenoside Rg3 suppressed MMP-2 activity in HaCaT cells. These findings suggest that ginsenoside Rg3 has stereoselective anti-photoaging properties.
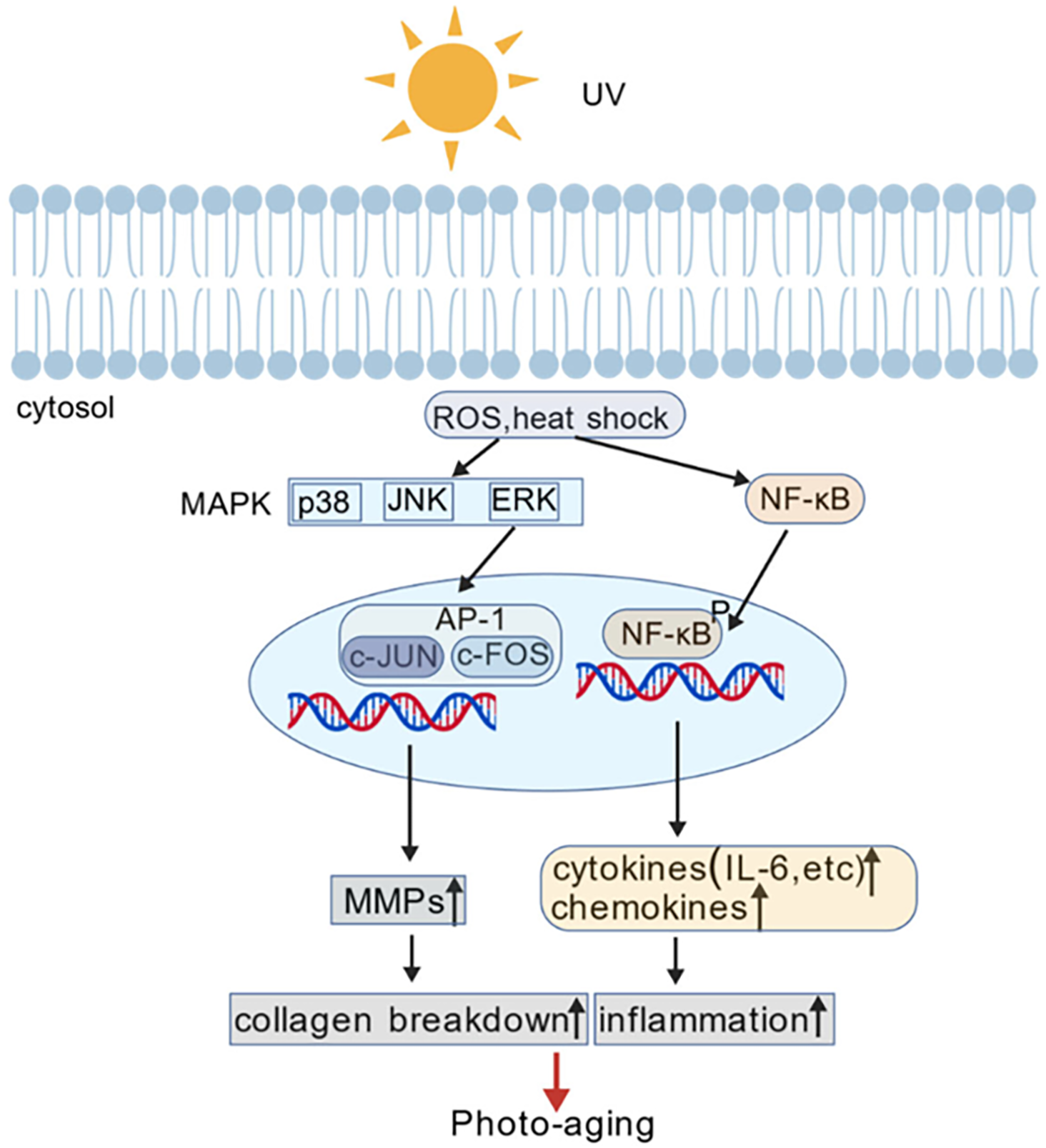
Figure 3: UV-induced MMP activation and its role in skin photoaging. Exposure to UV radiation stimulates ROS production, leading to the activation of MAPK signaling pathways. This, in turn, upregulates MMPs, which degrade collagen and other extracellular matrix components, contributing to skin photoaging characterized by wrinkles, loss of elasticity, and impaired barrier function. Reprinted with permission from Ref. [38]. 2024, Wiley Online Library. Abbreviations: UV, ultraviolet; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; AP-1, activator protein-1; c-Jun and c-Fos, components of AP-1 transcription factor; MMPs, matrix metalloproteinases
Additionally, Rg3 stimulates collagen production, a crucial component for maintaining skin elasticity and firmness [40]. Collagen production declines naturally with age, contributing to the development of wrinkles and loss of skin elasticity [41]. By enhancing collagen synthesis, Rg3 helps counteract these aging signs, promote a youthful appearance, and improve skin texture. Treatment of UV-exposed human dermal fibroblasts with Rg3 results in an increase in extracellular matrix (ECM) proteins, including collagen and elastin, while reducing the activity of inhibitory collagenases such as MMP-2 and MMP-3 [42]. Furthermore, Rg3 enhanced the expression of antioxidant proteins, such as heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) [43].
One of Rg3’s most potent attributes is its antioxidant activity [44]. Rg3 neutralizes free radicals, which are unstable molecules that induce oxidative stress, and accelerates skin aging by damaging cellular components, proteins, and DNA. Ding et al. prepared Rg3-loaded gel and examined the change in oxidative stress of HaCaT cells exposed to UVB irradiation [45]. Rg3 formulation effectively mitigated UVB-induced oxidative stress, pro-inflammatory responses, and apoptotic activity. Moreover, Rg3-Gel reduced the generation of intracellular ROS and malondialdehyde (MDA) while improving the expression of antioxidant enzymes such as glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) [45]. Additionally, elevated levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), TNF-α, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2), caused by UVB irradiation were significantly suppressed by Rg3-Gel treatment.
This antioxidant effect reduces oxidative damage to skin cells, helping to slow down the appearance of fine lines, wrinkles, and age spots. Collectively, these effects render Rg3 a valuable compound in anti-aging skincare, working synergistically to both prevent and repair the visible signs of aging by protecting and enhancing the skin collagen network and reducing oxidative stress.
4 UV Protection and Skin Barrier
Ginsenoside Rg3 has demonstrated significant potential in enhancing skin barrier function and protecting the skin from UV-induced damage and environmental stressors [46]. One of the primary mechanisms by which Rg3 offers protection is the reduction in oxidative damage caused by UV exposure [47]. UV radiation generates free radicals and ROS, which can damage skin cells, accelerate aging, and impair barrier function in the skin [48]. The antioxidant properties of Rg3 neutralize these harmful molecules, thereby reducing oxidative stress and preserving the skin integrity.
Ginsenoside Rg3 has been shown to repair UVB-induced skin barrier damage in a BALB/c hairless mouse model (Fig. 4) [49]. Its protective effects were evident in the mitigation of UVB-induced epidermal barrier dysfunction, characterized by increased epidermal thickness, elevated transepidermal water loss, and diminished water content in the stratum corneum, without causing weight changes. Rg3 also inhibited the phosphorylation of JNK, p38, and ERK in UVB-irradiated HaCaT cells. A mixture of ginsenosides exhibited similar protective effects by inhibiting the MAPK pathway and enhancing the upregulation of involucrin and aquaporin 3 expression, comparable to treatment with intact Rg3.
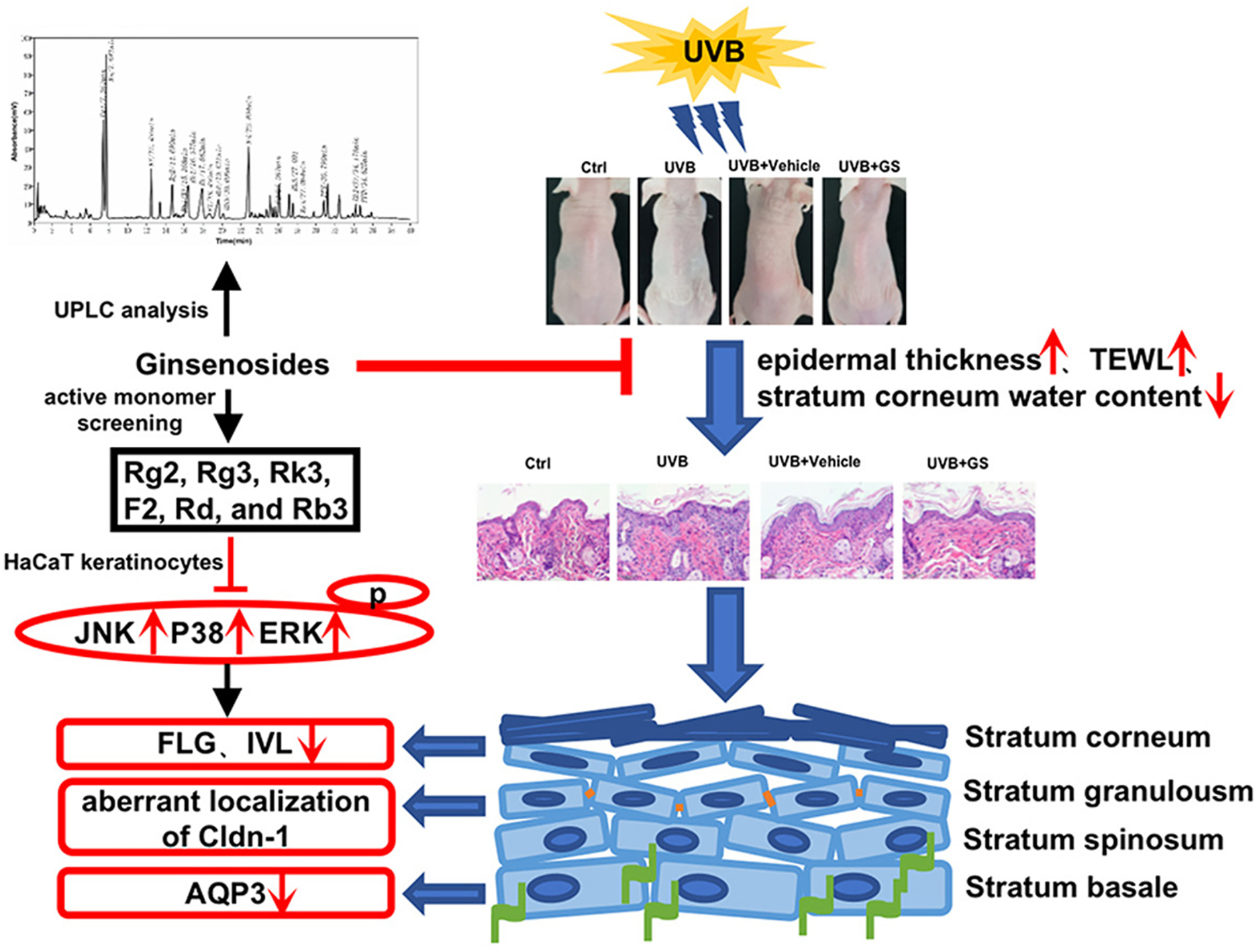
Figure 4: Workflow summarizing the study on the protective effects of ginsenosides against UVB-induced skin barrier damage. This background underscores the potential cosmetic value of ginsenosides in preventing skin photoaging and the unknown protective mechanisms of their monomeric constituents. This methodology involves the identification of ginsenoside monomer types. Reprinted with permission from Ref. [49]. 2022, Elsevier Ltd. Abbreviations: UPLC, ultra-performance liquid chromatography; JNK, c-Jun N-terminal kinase; p38, p38 mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; FLG, filaggrin; IVL, involucrin; Cldn-1, claudin-1; AQP3, aquaporin 3; ctrl, control; UVB, ultraviolet B; GS, ginsenoside; TEWL, trans-epidermal water loss
Furthermore, Rg3 strengthens the skin’s natural defense mechanisms by improving skin barrier function. The skin barrier is crucial for maintaining hydration, preventing the penetration of harmful microorganisms, and protecting the skin against environmental pollutants. Rg3 aids in regulating the expression of proteins involved in maintaining the skin barrier, thereby enhancing its capacity to retain moisture and protect against environmental stressors.
Han et al. demonstrated that Rg3 suppresses thymic stromal lymphopoietin (TSLP) production by downregulating the murine double minute 2/hypoxia-inducible factor 1-α (MDM2/HIF1α) signaling pathway in an in vitro model of phorbol 12-myristate 13-acetate plus calcium ionophore A23187 (PMACI)-stimulated human mast cell line (HMC-1 cell line) and a phorbol 12-myristate 13-acetate (PMA)-induced mouse ear edema model of inflammation [50]. This dual action of protecting against oxidative stress and supporting barrier function renders Rg3 particularly beneficial for shielding the skin from both UV damage and other environmental stressors, such as pollution and harsh weather conditions. By strengthening the resilience of the skin to external damage and promoting a healthier barrier, Rg3 can reduce the risk of inflammation, irritation, and photoaging, making it a valuable ingredient in products designed for skin protection and repair.
Ginsenoside Rg3 demonstrates substantial anti-inflammatory properties, rendering it advantageous for mitigating skin redness, sensitivity, and inflammation associated with dermatological conditions such as acne, eczema, and other related conditions [51]. Rg3 modulates various inflammatory pathways by inhibiting the activation of proinflammatory mediators and cytokines [52]. The principal mechanism of Rg3 is its ability to suppress the production of inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which are typically elevated during inflammatory responses in the skin [53]. These cytokines are responsible for initiating inflammation, redness, and swelling in conditions, such as acne and psoriasis. By downregulating these cytokines, Rg3 contributes to the reduction in the overall inflammatory response in the skin, leading to decreased irritation and sensitivity.
Furthermore, Rg3 inhibits the activation of NF-κB, a critical transcription factor involved in inflammatory process [54]. By suppressing NF-κB, Rg3 diminishes the expression of various proinflammatory enzymes and proteins, thereby effectively reducing the overall inflammatory response. This action aids in calming irritated skin, reducing redness, and preventing flare-ups, which are commonly observed in inflammatory skin conditions. Through these mechanisms, Rg3 can be efficacious in alleviating the symptoms of inflammatory skin disorders, making it a promising ingredient in formulations aimed at soothing sensitive or acne-prone skin. Its ability to modulate inflammatory pathways without causing irritation is an ideal candidate for products targeting both the symptoms and the underlying causes of skin inflammation. The beneficial effects of Ginsenoside Rg3 on various skin-related applications, including anti-aging, wound healing, and UV protection, are summarized in Table 1.
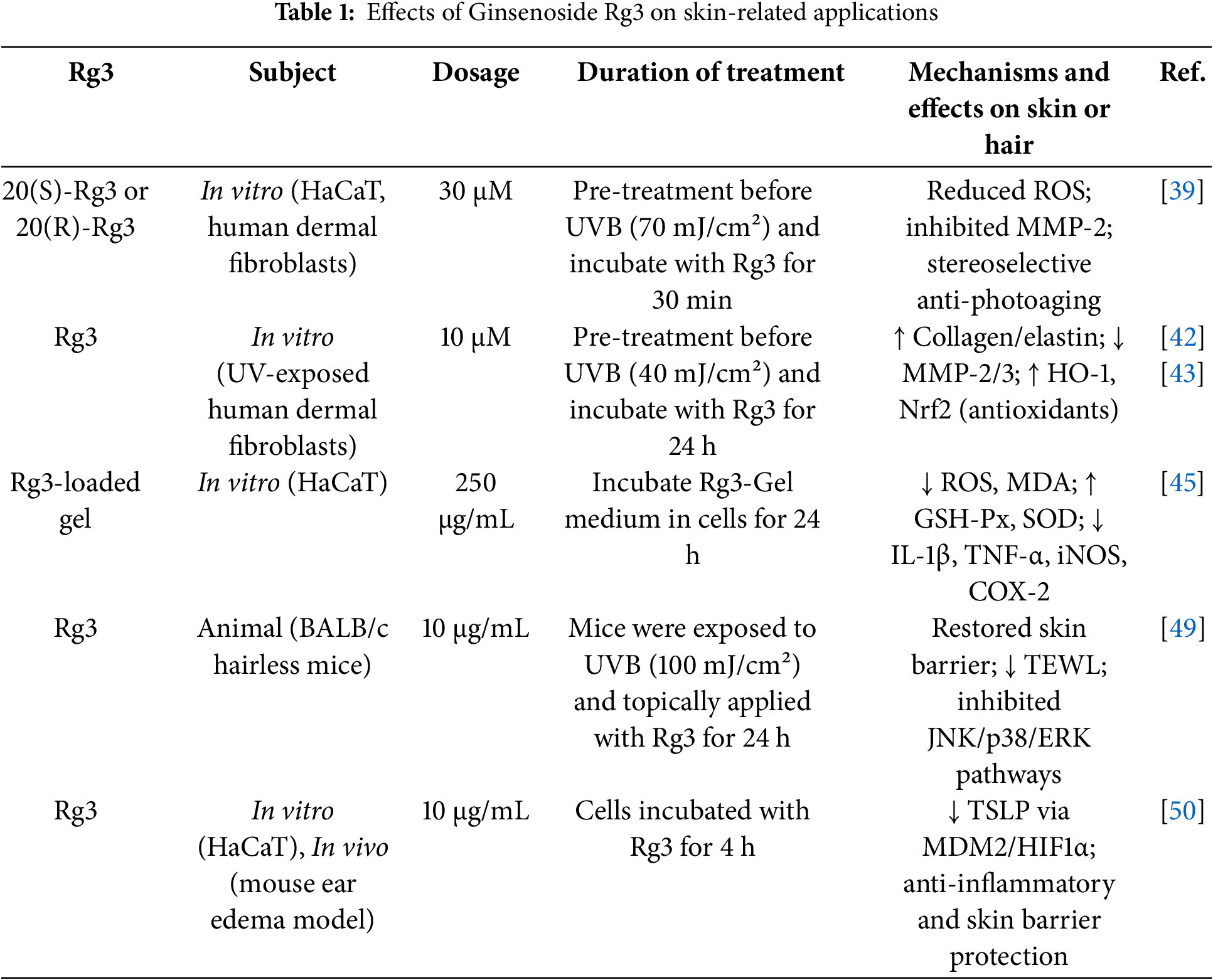
6 Anticancer Applications of Ginsenoside Rg3
Ginsenoside Rg3, a bioactive compound derived from Panax ginseng, exhibits significant anticancer properties via various mechanisms. It induces apoptosis in cancer cells by activating proapoptotic proteins and inhibiting antiapoptotic factors. Additionally, Rg3 suppresses tumor angiogenesis by inhibiting vascular endothelial growth factor (VEGF) and associated signaling pathways, while reducing cancer cell migration and invasion by targeting MMPs and epithelial-to-mesenchymal transition (EMT) [55]. Furthermore, it modulates inflammatory pathways, reduces oxidative stress through antioxidant activity, and arrests the cell cycle at critical checkpoints. Ginsenoside Rg3 also enhances the immune system by activating natural killer (NK) cells, which are essential components of the innate immune system responsible for directly attacking tumor cells. Moreover, Rg3 stimulates other immune cells such as T lymphocytes and macrophages to augment their tumor-targeting capabilities, thereby fostering a more coordinated and effective immune response against cancer [56]. These combined actions indicate that Rg3 is a promising candidate for cancer therapy.
7 Modulation of Apoptosis Pathways by Ginsenoside Rg3
Ginsenoside Rg3 has demonstrated potential as a potent inducer of apoptosis in cancer cells through its regulation of key apoptotic signaling molecules, including B-cell lymphoma 2 (Bcl-2) family proteins, caspases, and other critical components involved in programmed cell death [57]. One of the primary mechanisms by which Rg3 induces apoptosis is by modulating the balance between pro-apoptotic and anti-apoptotic proteins in the Bcl-2 family [58]. The Bcl-2 family comprises pro-survival proteins, such as Bcl-2 and Bcl-xL, as well as pro-apoptotic proteins, such as Bax, Bak, and Bid [59]. In healthy cells, pro-survival Bcl-2 proteins prevent apoptosis by inhibiting the activation of proapoptotic proteins. Rg3 disrupts this balance by downregulating the expression of anti-apoptotic Bcl-2 proteins and upregulating pro-apoptotic proteins such as Bax and Bak.
For instance, Rg3 upregulates the expression of Bax and downregulates Bcl-2 in osteosarcoma cells [57]. Increased Bax expression facilitates the formation of Bax-Bcl-2 heterodimers, which activate caspase-9 and subsequently cleave the downstream factor procaspase-3, ultimately promoting apoptosis. Additionally, the phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) signaling pathway, which plays a critical role in oncogene activation, tumor cell proliferation, and inactivation of osteosarcoma-suppressing mechanisms, is involved in this process. This shift promotes mitochondrial membrane permeabilization, an essential stage in the apoptotic process [60]. Once the mitochondria are compromised, cytochrome c is released into the cytoplasm where it activates caspases, a family of protease enzymes essential for executing apoptosis.
Kim et al. demonstrated that MDA-MB-231 breast cancer cells treated with 30 μM Rg3 had an increased proportion of apoptotic cells [58]. Treatment with Rg3 increased the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2, induced depolarization of mitochondrial membrane potential, and initiated the release of cytochrome c from the mitochondria. Rg3 enhanced the activation of caspase-9, an initiator caspase that initiates the apoptotic cascade, and caspase-3, an effector caspase responsible for the breakdown of cellular components. These caspases cleave various cellular substrates, leading to cell death and the characteristic morphological changes observed in apoptosis, such as cell shrinkage, DNA fragmentation, and membrane blebbing.
In addition to its effects on the Bcl-2 family and caspases, Rg3 may also modulate other apoptotic signaling pathways, including the activation of p53, a tumor suppressor protein that can induce apoptosis in response to DNA damage [61]. By promoting the activation of p53 and other stress-induced signaling molecules, Rg3 further enhances the apoptotic response in tumor cells.
Kim et al. investigated the mechanisms underlying the anti-proliferative and pro-apoptotic effects of Rg3 in MDA-MB-231 breast cancer cells, which exhibit constitutive NF-κB activation and a mutant p53 form (Fig. 5) [62]. Their findings indicated that Rg3 suppressed NF-κB signaling, potentially by inactivating ERK and Akt, and destabilizing mutant p53. These effects likely contributed to the downregulation of Bcl-2 expression, ultimately triggering apoptosis in MDA-MB-231 breast cancer cells. Through these mechanisms, Rg3 induces apoptosis in cancer cells by disrupting the regulatory networks that control cell survival and death, making it a promising candidate for cancer therapy, particularly as an adjuvant treatment to enhance the effects of conventional chemotherapies.
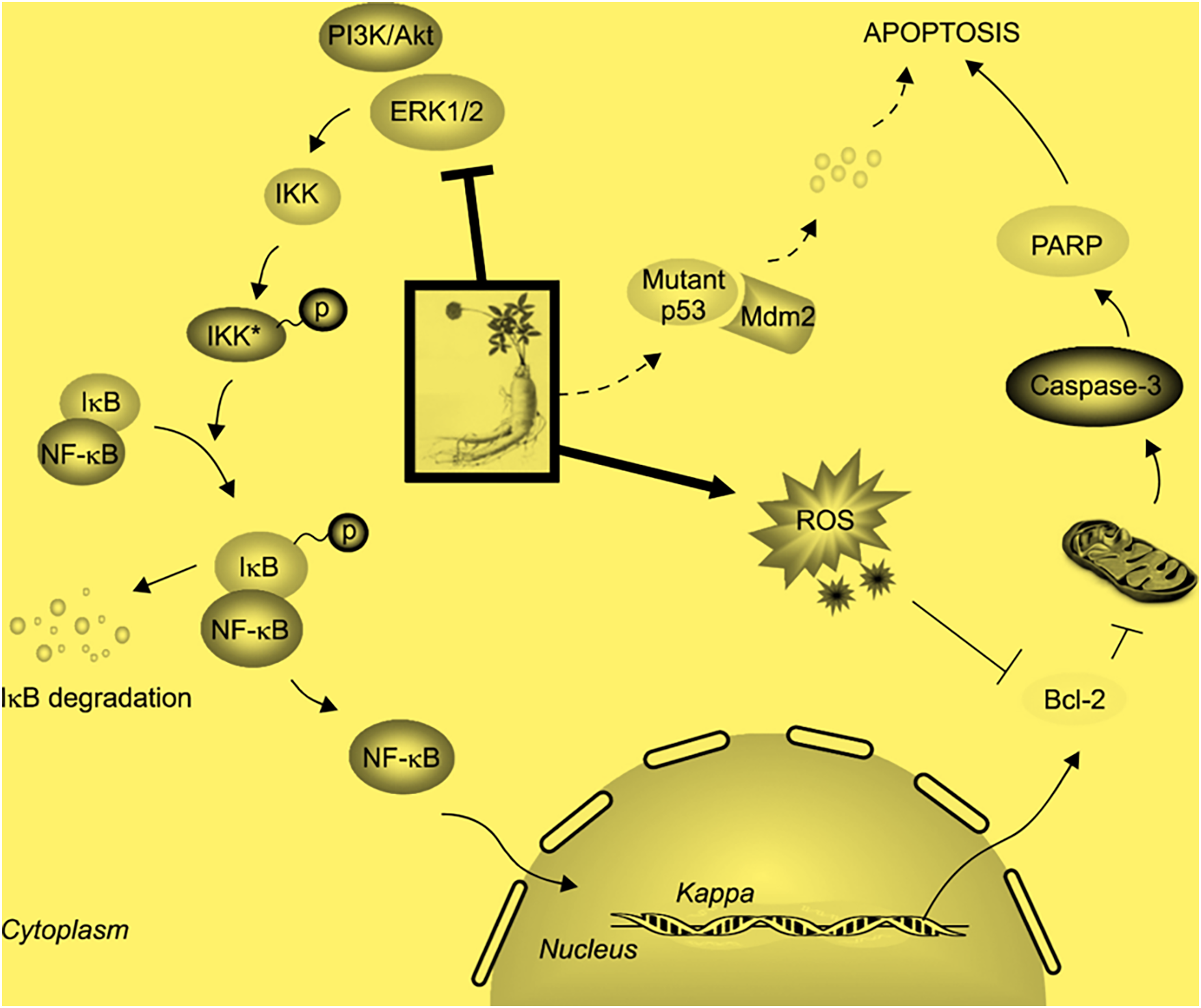
Figure 5: Proposed molecular mechanisms by which Rg3 suppresses NF-κB signaling and induces apoptosis in MDA-MB-231 cells. Rg3 inhibits NF-κB activation and promotes apoptosis by modulating key apoptotic proteins. Reprinted with permission from Ref. [62]. 2014; Korean Society for Cancer Prevention
8 Ginsenoside Rg3-Mediated Inhibition of Metastasis: EMT Reversal and MMP Suppression
Ginsenoside Rg3 has demonstrated significant anti-metastatic properties, particularly in the inhibition of cancer cell migration and invasion, which are critical stages in the progression of metastasis. These effects are primarily mediated by the regulation of EMT and inhibition of MMPs.
Inhibition of EMT: EMT is a crucial process in cancer metastasis, wherein epithelial cells transition by losing their cell-cell adhesion properties and acquiring mesenchymal traits, enabling them to migrate and invade the surrounding tissues [63]. Rg3 modulates EMT by reversing molecular changes that promote this transition. It reduces the expression of mesenchymal markers, such as N-cadherin, vimentin, and fibronectin, while promoting the expression of epithelial markers, such as E-cadherin. By restoring epithelial characteristics, Rg3 helps prevent the detachment of cancer cells from the primary tumor, thereby inhibiting their ability to spread to distant organs.
Tian et al. confirmed that Rg3 inhibits EMT and invasion in lung cancer through a glycobiological mechanism [64]. In their study, researchers observed that treatment with Ginsenoside Rg3 at 25, 50, and 100 μg/mL effectively suppressed cell migration and invasion. These inhibitory effects were confirmed through wound healing assays, which measure the closure of artificial gaps in cell monolayers, and transwell assays, which assess the ability of cells to migrate through a porous membrane. Furthermore, Rg3 significantly influenced the expression of EMT marker proteins, which is a key process in cancer metastasis. Specifically, it upregulated E-cadherin, a marker of the epithelial phenotype associated with reduced metastatic potential, while downregulating Snail, N-cadherin, and vimentin, all markers linked to the mesenchymal phenotype and enhanced cell motility. Consequently, these molecular changes translated to tangible in vivo benefits, as Rg3 treatment led to a notable reduction in tumor volume and weight in a xenograft mouse model. Additionally, when tumor cells were introduced via tail vein injection to mimic metastatic spread, Rg3 significantly reduced the number of metastatic nodules in lung tissues.
Inhibition of MMPs: MMPs are enzymes that degrade the ECM, a crucial step in the invasion of cancer cells through tissue barriers [65]. Rg3 has been shown to suppress the activity of MMPs, particularly MMP-2 and MMP-9, which are critical for degrading type IV collagen and other ECM components [66]. By inhibiting MMP production and activation, Rg3 reduces the capacity of cancer cells to infiltrate the surrounding tissue and migrate to distant sites, which is a fundamental aspect of metastasis. Through these mechanisms, Rg3 exerts a dual effect on metastatic progression by preventing the acquisition of invasive properties through EMT inhibition and by limiting the physical ability of cancer cells to invade the ECM through MMP inhibition. These anti-metastatic effects make Rg3 a promising candidate for cancer therapy, particularly in combination with other treatments aimed at reducing the spread of cancer and improving patient prognosis.
Wang et al. demonstrated that Rg3 effectively inhibits cell migration and invasion in both HNE1 and CNE2 nasopharyngeal carcinoma cell lines, underscoring its potential as an anti-metastatic agent [67]. Rg3 reduced the levels of MMP-2 and MMP-9 in both HNE1 and CNE2 nasopharyngeal carcinoma cell lines, thereby inhibiting extracellular matrix degradation associated with tumor progression. Moreover, Rg3 significantly modulated the expression of EMT markers, increasing E-cadherin and decreasing Vimentin and N-cadherin levels, indicating reversal of the mesenchymal phenotype. Additionally, Rg3 suppressed the expression of key EMT-related transcription factors, particularly zinc finger E-box-binding homeobox 1 (ZEB1), further reinforcing its role in mitigating cancer cell migration and invasion. These findings suggest that Rg3 regulates MMP-2 and MMP-9 expression and inhibits EMT in nasopharyngeal carcinoma.
9 Ginsenoside Rg3 as an Inhibitor of Tumor Angiogenesis: Mechanisms Involving VEGF, MMPs, and Hypoxia
Ginsenoside Rg3 has demonstrated potential in inhibiting tumor angiogenesis, the process by which new blood vessels are formed to supply oxygen and nutrients to growing tumors [68]. Tumor angiogenesis is driven by several pro-angiogenic factors, with VEGF being the most critical. Rg3 interferes with this process by suppressing the expression of VEGF and other key angiogenic factors, thereby limiting the ability of tumors to establish blood supply.
Suppression of VEGF Expression: VEGF is a key regulator of angiogenesis and plays a crucial role in promoting endothelial cell proliferation, migration, and formation of new blood vessels [69–71]. Rg3 inhibits the expression of VEGF at both transcriptional and translational levels, reducing the activation of VEGF receptors on endothelial cells, which in turn diminishes their ability to form new blood vessels. By downregulating VEGF, Rg3 starves the tumor, restricting its growth and ability to spread.
For example, Lv et al. demonstrated that Rg3 at concentrations of 0, 25, 50, and 100 μg/mL significantly inhibited cell viability, migration, invasion, angiogenesis, and the protein expression of N-cadherin, Vimentin, COX2, and VEGF in A549 and H1299 lung cancer cells in a dose-dependent manner [72]. Conversely, Rg3 treatment promoted E-cadherin expression, suggesting its potential to suppress EMT and tumor progression. COX2 overexpression significantly reversed the effects of Rg3 on cell viability, migration, invasion, angiogenesis, and expression of EMT-related proteins in lung cancer cells. However, these reversed effects were counteracted by VEGF knockdown, indicating that VEGF plays an essential role in mediating the effect of COX2 on tumor progression. In conclusion, Rg3 reduced migration, invasion, and angiogenesis in lung cancer cells by inhibiting the expression of COX2 and VEGF, highlighting its potential as a therapeutic agent to target both inflammatory and angiogenic pathways in cancer.
Modulation of Angiogenic Signaling Pathways: In addition to VEGF, other angiogenic signaling molecules such as platelet-derived growth factors (PDGFs), fibroblast growth factors (FGFs), and MMPs play a key role in tumor angiogenesis. Rg3 has been shown to modulates several of these factors. For instance, it may reduce the secretion of MMPs, which are involved in the breakdown of the ECM and creation of pathways for endothelial cell migration. By suppressing these angiogenic mediators, Rg3 further inhibits the formation of new blood vessels within tumors.
Inhibition of Angiogenesis through Hypoxia-Inducible Factors (HIFs): Under low-oxygen conditions (hypoxia), tumors often activate hypoxia-inducible factors (HIFs), which subsequently stimulate the expression of VEGF and other angiogenic factors [73–75]. Rg3 has been shown to reduce the activity of HIFs, thereby preventing the upregulation of VEGF and other proangiogenic molecules under hypoxic conditions [76]. This additional layer of regulation further enhances Rg3’s ability to limit tumor angiogenesis.
By inhibiting VEGF and other key angiogenic factors, Rg3 effectively disrupts the tumor’s ability to establish and maintain blood supply, rendering it a promising candidate for anti-cancer therapies, particularly in limiting tumor growth and metastasis by targeting angiogenesis. For instance, Zeng et al. demonstrated that Rg3 suppresses the synthesis of HIF-1α and VEGF proteins in bone marrow stem cells derived from patients with acute leukemia [77]. Rg3 treatment resulted in a reduction in serum levels of HIF-1α and VEGF in patients, indicating its potential to inhibit key factors involved in tumor progression and angiogenesis. Mechanistically, Rg3 downregulated the phosphorylation of Akt and ERK1/2 in BMSCs from acute leukemia patients. These findings suggest that Rg3 interferes with the activation of Akt and MAPK pathways in BMSCs, thereby regulating HIF-1α and VEGF expression by inhibiting the activity of Akt and ERK kinases. This underscores the role of Rg3 in targeting critical signaling pathways to modulate the tumor microenvironment and inhibit leukemia progression. The effects of Ginsenoside Rg3 on cancer progression, angiogenesis, oxidative stress, and its involvement in key molecular pathways are summarized in Table 2.
10 Role in Cellular Metabolism
Ginsenoside Rg3 plays a pivotal role in the regulation of cellular metabolism by modulating the key pathways involved in energy production and nutrient utilization. It inhibits glycolysis in cancer cells, thereby reducing their energy supply and enhancing mitochondrial function to improve the cellular energy balance [78]. Rg3 activates AMP-activated protein kinase (AMPK), which promotes fatty acid oxidation, glucose uptake, and mitochondrial biogenesis while concurrently inhibiting anabolic processes [79]. In addition, it improves insulin sensitivity, regulates fatty acid metabolism, and modulates protein metabolism, thereby contributing to enhanced metabolic health. These results suggest that Rg3 is a promising therapeutic agent for various diseases, including cancer and metabolic disorders.
11 Glycolytic Pathway and Warburg Effect
Ginsenoside Rg3 has demonstrated potential in modulating cellular energy metabolism, particularly within cancer cells, by influencing key metabolic pathways, such as glycolysis and mitochondrial function. This is particularly pertinent in the context of the Warburg effect, a phenomenon wherein cancer cells predominantly rely on aerobic glycolysis (the conversion of glucose to lactate) rather than oxidative phosphorylation for energy production, even in the presence of oxygen [80,81].
Inhibition of Glycolysis: The Warburg effect is a hallmark metabolic shift observed in numerous cancer cells, characterized by a preference for glycolysis over oxidative phosphorylation, even in the presence of oxygen. This metabolic reprogramming results in the upregulation of key glycolytic enzymes including lactate dehydrogenase A, hexokinase 2 (HK2), and pyruvate kinase M2 (PKM2) [82]. These enzymes facilitate the accelerated conversion of glucose to lactate, thereby providing cancer cells with the requisite energy and biosynthetic intermediates to support rapid growth and proliferation. Rg3 inhibits key glycolytic enzymes, thereby suppressing the glycolytic pathway. This inhibition leads to a reduction in lactate production, depriving cancer cells of a crucial source of energy and metabolic intermediates, rendering them less capable of sustaining rapid proliferation and survival.
Zhou et al. demonstrated the inhibition of glycolysis in SKOV3 ovarian cancer cell lines through the application of 20(S)-Rg3, which downregulated the expression of key glycolytic enzymes such as HK2 and PKM2 [83]. Their study further revealed that 20(S)-Rg3 modulated HK2 expression via the DNMT3A/miR-532-3p signaling axis. This pathway alteration contributed to the suppression of the Warburg effect in ovarian cancer cells. Moreover, in vivo experiments using nude mouse xenograft models confirmed that 20(S)-Rg3 treatment reduced HK2 expression, supporting its potential as a metabolic regulator in ovarian cancer therapy.
Mitochondrial Function and Oxidative Phosphorylation: Rg3 also influences mitochondrial function. Cancer cells frequently exhibit compromised mitochondrial function due to a metabolic shift towards glycolysis. Rg3 has been reported to enhance mitochondrial function by promoting mitochondrial biogenesis, augmenting oxidative phosphorylation, and increasing ATP production via mitochondria [84]. This transition towards oxidative metabolism can help restore the normal balance of cellular energy production, thereby limiting the survival advantage conferred by glycolytic metabolism in cancer cells.
Treatment with Rg3 in mouse-derived myoblast cell line (C2C12) activated key proteins in the insulin signaling pathway, such as insulin receptor substrate-1 (IRS-1) and Akt [85]. Rg3 was found to elevate ATP production and increase the oxygen consumption rate, indicating enhanced mitochondrial function. Additionally, Rg3 upregulated the expression of key transcription factors involved in mitochondrial biogenesis, including peroxisome proliferator-activated receptor γ coactivator 1α, nuclear respiratory factor 1 (NRF-1), and mitochondrial transcription factor A. These findings suggest that Rg3 promotes mitochondrial function and biogenesis, which may contribute to improved cellular energy metabolism and potentially enhance therapeutic response in cancer cells. This led to a subsequent increase in the expression of mitochondrial complex IV and V.
Regulation of Key Metabolic Pathways: Rg3 also influences critical metabolic regulators, such as AMPK and the mTOR pathway, which are essential for cellular energy sensing and metabolic reprogramming in cancer cells [86]. By activating AMPK, Rg3 can inhibit the mTOR signaling pathway, which is frequently overexpressed in cancer and promotes anabolic processes including protein and lipid synthesis [87]. This inhibition reduces cancer cell metabolism and growth, further limiting their ability to thrive under the altered metabolic conditions that are characteristic of the Warburg effect.
Treatment with ginsenoside Rg3 in transverse aortic constriction (TAC)-induced mouse models enhanced heart function while preserving both the mitochondrial structure and function (Fig. 6) [88]. An integrated analysis of metabolomics, proteomics, and targeted metabolomics data revealed that Rg3 regulates glycolysis, influences glucose uptake, and alleviates myocardial insulin resistance. The molecular mechanism underlying Rg3’s regulation of glucose metabolism was further investigated by examining the interaction pathways involving AMPK, insulin resistance, and glucose metabolism. Rg3 promoted glucose absorption in insulin-resistant H9c2 cells, a rat cardiomyoblast cell line, through the stimulation of AMPK, and this effect relied on the insulin signaling pathway, highlighting Rg3’s potential as a therapeutic agent for promoting myocardial glucose metabolism under conditions of insulin resistance.
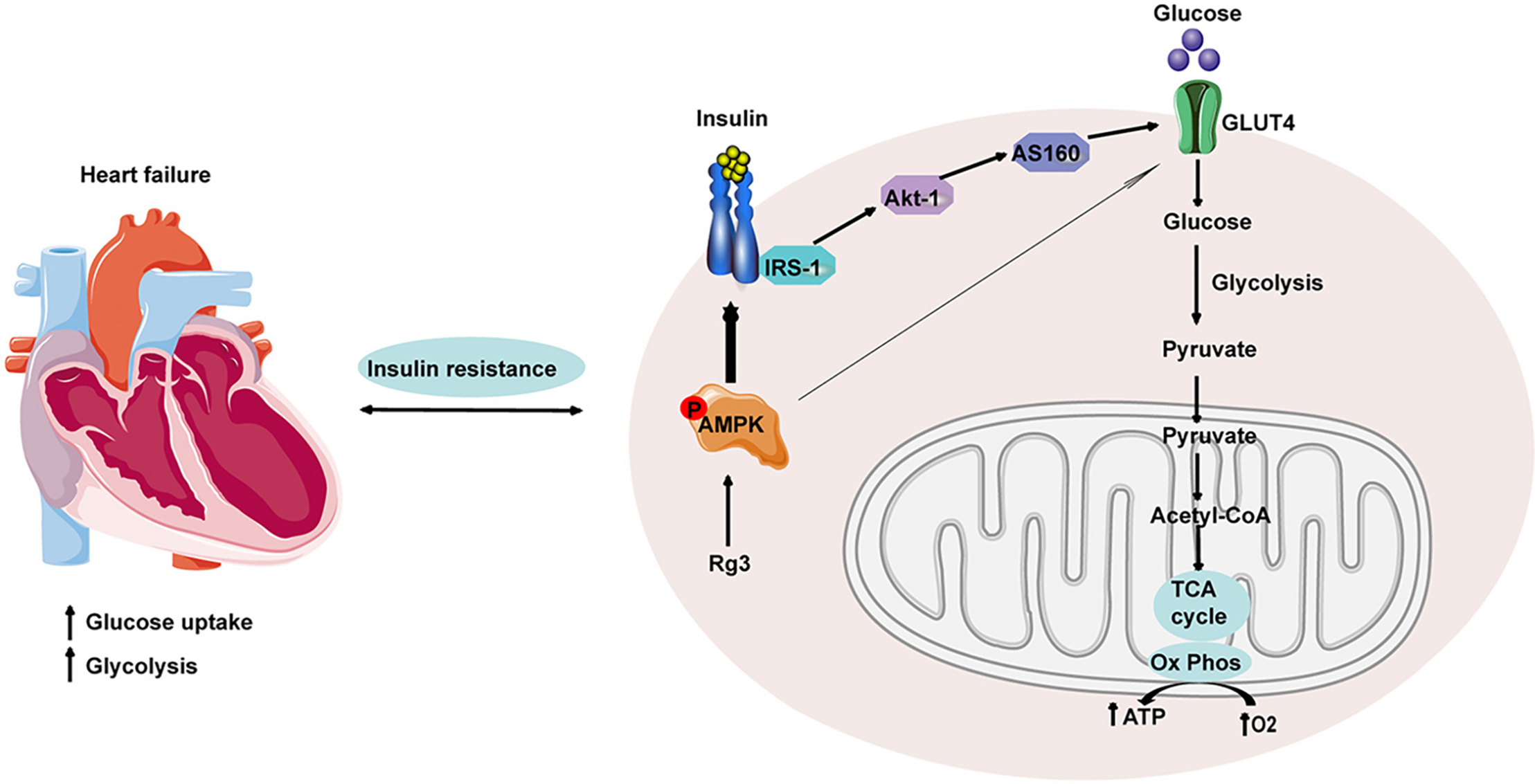
Figure 6: Ginsenoside Rg3 enhanced myocardial glucose metabolism and mitigated insulin resistance by activating the AMPK signaling pathway. AMPK activation promotes glucose uptake and utilization, diminishes insulin resistance, and reestablishes metabolic homeostasis in myocardial tissues, underscoring its therapeutic potential for metabolic disorders. Reprinted with permission from Ref. [88]. 2022, Elsevier Ltd.
Overall Impact on Cancer Metabolism: Rg3 disrupts the metabolic flexibility that cancer cells depend on for rapid growth by simultaneously targeting both the glycolytic pathway and mitochondrial function. This essentially compels cancer cells to revert to more normal metabolic processes, thereby reducing their efficiency in producing the energy required for continued survival and proliferation. This dual action on glycolysis and mitochondrial function represents a promising strategy for targeting cancer metabolism and is increasingly being recognized as an effective therapeutic approach in cancer treatment.
12 Rg3 in Oxidative Stress and Antioxidant Defense
Ginsenoside Rg3 has been extensively reported to mitigate oxidative stress, a central factor in both skin aging and tumor progression, by orchestrating multiple antioxidant mechanisms. Oxidative stress arises from an imbalance between ROS generation and the cellular antioxidant defense system, leading to damage of lipids, proteins, and DNA. Rg3 contributes to redox homeostasis by directly scavenging ROS and downregulating their intracellular accumulation, thus protecting skin cells from photoaging and inflammation while simultaneously inhibiting ROS-driven proliferation in cancer cells. Mechanistically, Rg3 enhances the activity and expression of key antioxidant enzymes, including catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx), which together neutralize harmful oxidants and preserve cellular integrity. For instance, studies have demonstrated that Rg3 treatment significantly elevated these enzyme levels in cyclophosphamide-induced oxidative injury models, while reducing markers of oxidative damage such as malondialdehyde and nitric oxide [89]. In addition to enzyme activation, Rg3 activates the Nrf2/Keap1 signaling pathway, a master regulatory axis of antioxidant response. Upon activation, Nrf2 translocates into the nucleus and upregulates a battery of cytoprotective genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), and glutathione peroxidase 4 (GPX4), reinforcing the cellular defense system. Notably, Rg3-mediated Nrf2 activation has also been linked to the suppression of ferroptosis and ischemia-induced tissue injury, illustrating its potential beyond classical ROS neutralization. Furthermore, the modulation of oxidative stress by Rg3 has downstream effects on inflammatory and apoptotic signaling pathways, such as NF-κB and caspase cascades, thereby integrating redox regulation with immune modulation and cell survival. These antioxidant properties position Rg3 as a promising therapeutic agent that not only preserves skin health but also counteracts the oxidative microenvironment that supports tumor growth and metastasis.
Reduction of ROS: Rg3 aids in reducing oxidative stress by directly scavenging ROS and lowering intracellular levels [90]. ROS are highly reactive molecules that are capable of damaging lipids, proteins, and DNA, leading to cell dysfunction and death. In skin cells, this can result in accelerated aging, inflammation, and pigmentation, whereas in cancer cells, ROS accumulation can promote tumor growth and metastasis. By neutralizing ROS, Rg3 protects the cells from oxidative damage and mitigates its deleterious effects. For instance, Sun et al. reported that ROS production increased in Lewis lung carcinoma (LLC) cells grown in serum-containing media compared with LLC cells cultured in serum-free media [91]. Elevated ROS levels were found to induce the proliferation of LLC cells. However, treatment with Rg3 (200 ng/mL) reduced ROS levels, leading to significant inhibition of cell proliferation. Rg3 treatment also notably decreased the expression of cyclins and cyclin-dependent kinases, which are crucial for cell cycle progression in LLC cells. Additionally, Rg3 suppressed the activation of MAPKs and induced apoptosis in LLC cells by activating pro-apoptotic proteins and suppressing anti-apoptotic proteins. These results suggest that Rg3 inhibits tumor cell proliferation and promotes apoptosis by modulating key molecular pathways.
Upregulation of antioxidant enzymes Rg3 enhances the cellular antioxidant defense system by upregulating the expression and activity of key antioxidant enzymes such as catalase, SOD, and GPx [92]. These enzymes play a pivotal role in neutralizing ROS and maintaining cellular redox balance. SOD converts superoxide radicals into hydrogen peroxide (H2O2), which is subsequently decomposed into water by catalase and GPx, thereby detoxifying harmful ROS and preventing cellular damage. By boosting the activity of these enzymes, Rg3 strengthens the cell’s natural antioxidant defenses and reduces the burden of oxidative stress. Wei et al. demonstrated that Rg3 inhibited cyclophosphamide (Cy)-induced oxidative stress in mice by increasing the indices of the spleen and thymus as well as enhancing the overall antioxidant capacity [89]. Rg3 treatment elevated the activities of key antioxidant enzymes, including catalase, superoxide dismutase, and lysozyme, while simultaneously decreasing xanthine oxidase activity. Additionally, Rg3 reduced the levels of malondialdehyde and nitric oxide, which are markers of oxidative damage. These findings suggest that Rg3 possesses potent antioxidant properties, providing defense against oxidative stress induced by cyclophosphamide.
Activation of the Nrf2 Signaling Pathway: The Nrf2 pathway serves as a pivotal regulator of antioxidant responses [93]. Rg3 activates the Nrf2 pathway, resulting in elevated expression of various antioxidant and cytoprotective genes [94]. The activation of Nrf2 enhances cellular resilience against oxidative stress by upregulating genes involved in detoxification, ROS scavenging, and glutathione synthesis. This extensive antioxidant response provides comprehensive defense against oxidative damage, thereby benefiting both skin health and cancer prevention. Zhong et al. reported that Rg3 upregulated the expression of the ferroptosis-related protein GPX4 and reduced iron accumulation in mice with myocardial ischemia/reperfusion (MI/R) injury (Fig. 7) [95]. Furthermore, Rg3 activates the Nrf2 signaling pathway, which plays a crucial role in mitigating myocardial ischemia-induced ferroptosis. Notably, Rg3 modulated the keap1/Nrf2 signaling pathway to alleviate oxygen-glucose deprivation/reperfusion (OGD/R)-induced ferroptosis in H9C2 cells. These findings suggest that Rg3 mitigates myocardial ischemia-induced ferroptosis through the keap1/Nrf2/GPX4 signaling pathway, underscoring its potential as a protective agent against oxidative and iron-induced cellular damage.
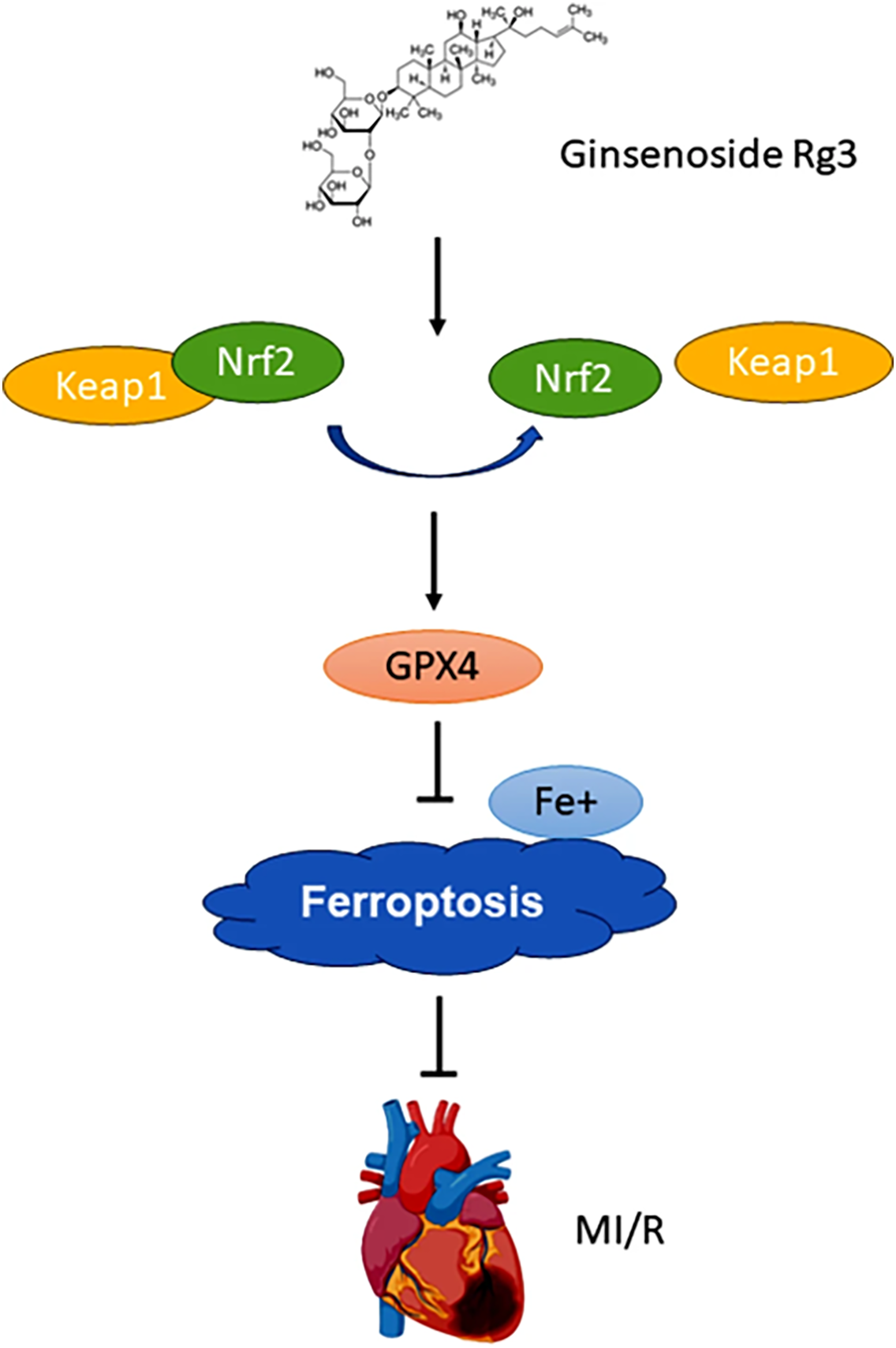
Figure 7: Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion-induced ferroptosis via the Keap1/Nrf2/GPX4 signaling pathway. Rg3 activates Nrf2 by disrupting its interaction with Keap1, leading to the upregulation of GPX4 expression and reduction in lipid peroxidation, thereby safeguarding myocardial tissues from ferroptotic damage. Reprinted with permission from Ref. [95]. 2024, Springer Nature
Protective Effects in Skin Care and Cancer: In the realm of skin care, the antioxidant properties of Rg3 are instrumental in combating photoaging, reducing inflammation, and protecting against UV-induced skin damage. Thus, Rg3 is a valuable ingredient in formulations aimed at mitigating the signs of aging and promoting healthy, resilient skin. In the context of cancer prevention and therapy, Rg3’s ability to reduce oxidative stress can limit damage to normal cells, protect cellular DNA from mutations, and suppress pathways that drive tumorigenesis. Furthermore, in cancer cells that thrive under oxidative conditions, Rg3’s antioxidant activity may diminish their survival advantage.
By decreasing oxidative stress and enhancing antioxidant defenses, Rg3 contributes to both skin protection and cancer suppression, underscoring its potential as a therapeutic agent for preventing oxidative damage across various biological systems.
13 Synergistic Effects of Ginsenoside Rg3 in Combination with Chemotherapy, Radiotherapy, and Natural Compounds
Ginsenoside Rg3 exhibits potential synergistic effects when combined with other therapeutic agents, thereby enhancing its application in both skincare and anticancer treatments. Synergy in this context refers to the phenomenon in which the combined use of Rg3 with other compounds or treatments results in a greater overall effect than when each is used individually [96]. This property renders Rg3 particularly promising for augmenting the efficacy of various treatments, including traditional cancer therapies and advanced skin care formulations.
Enhanced Chemotherapy and Radiotherapy Efficacy: Rg3 has been shown to improve the effectiveness of chemotherapeutic agents such as paclitaxel, cisplatin, and doxorubicin in eradicating cancer cells [97]. This is achieved by sensitizing cancer cells to these drugs, potentially allowing for lower dosages, which can mitigate the side effects associated with high-dose chemotherapy. For instance, Jiang et al. reported that Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer through the downregulation of PD-L1 [98]. Additionally, Rg3 can inhibit multidrug resistance (MDR) proteins, such as P-glycoprotein, which cancer cells often overexpress to expel chemotherapeutic drugs. This inhibition increases drug accumulation within the cells, thereby enhancing their sensitivity to treatment. Furthermore, Rg3 has been found to mitigate oxidative damage in normal cells caused by chemotherapy and radiotherapy, thus protecting healthy tissues while potentiating the anticancer effects of these treatments [99].
Combination with Other Natural Compounds in Skin Care: In skincare, Rg3 can act synergistically with other natural antioxidants, such as vitamin C, vitamin E, and green tea polyphenols, to enhance skin resilience against environmental stressors [100]. These combinations bolster skin defense mechanisms, reducing oxidative damage from UV exposure, pollution, and other environmental stressors. Rg3’s antioxidant effects complement those of other compounds by providing a broader range of ROS scavenging and anti-inflammatory benefits, which are particularly beneficial in formulations designed to protect and rejuvenate aging skin.
Inhibition of Tumor Progression with Immune Checkpoint Inhibitors and Targeted Therapies: Rg3 may synergize with immune checkpoint inhibitors (ICIs) and targeted therapies in cancer treatment [101]. For instance, Ginsenoside Rg3, in conjunction with a stimulator of interferon genes (STING) agonist, inhibited the proliferation of triple-negative breast cancer (TNBC) cells, reduced TNBC cell stemness and the EMT process, and promoted apoptosis in TNBC cells (Fig. 8) [102]. Additionally, the combination of Rg3 and a STING agonist modified the tumor microenvironment by inducing tumor-associated macrophage (TAM) M1 polarization and reversing TAM M2 polarization. Studies have suggested that Rg3 can modulate immune responses, potentially enhancing the antitumor effects of ICIs by reducing inflammation and improving immune surveillance. Similarly, Rg3’s inhibition of angiogenesis and metastasis complements targeted therapies that focus on specific tumor growth pathways, potentially leading to improved outcomes in tumor control and regression.
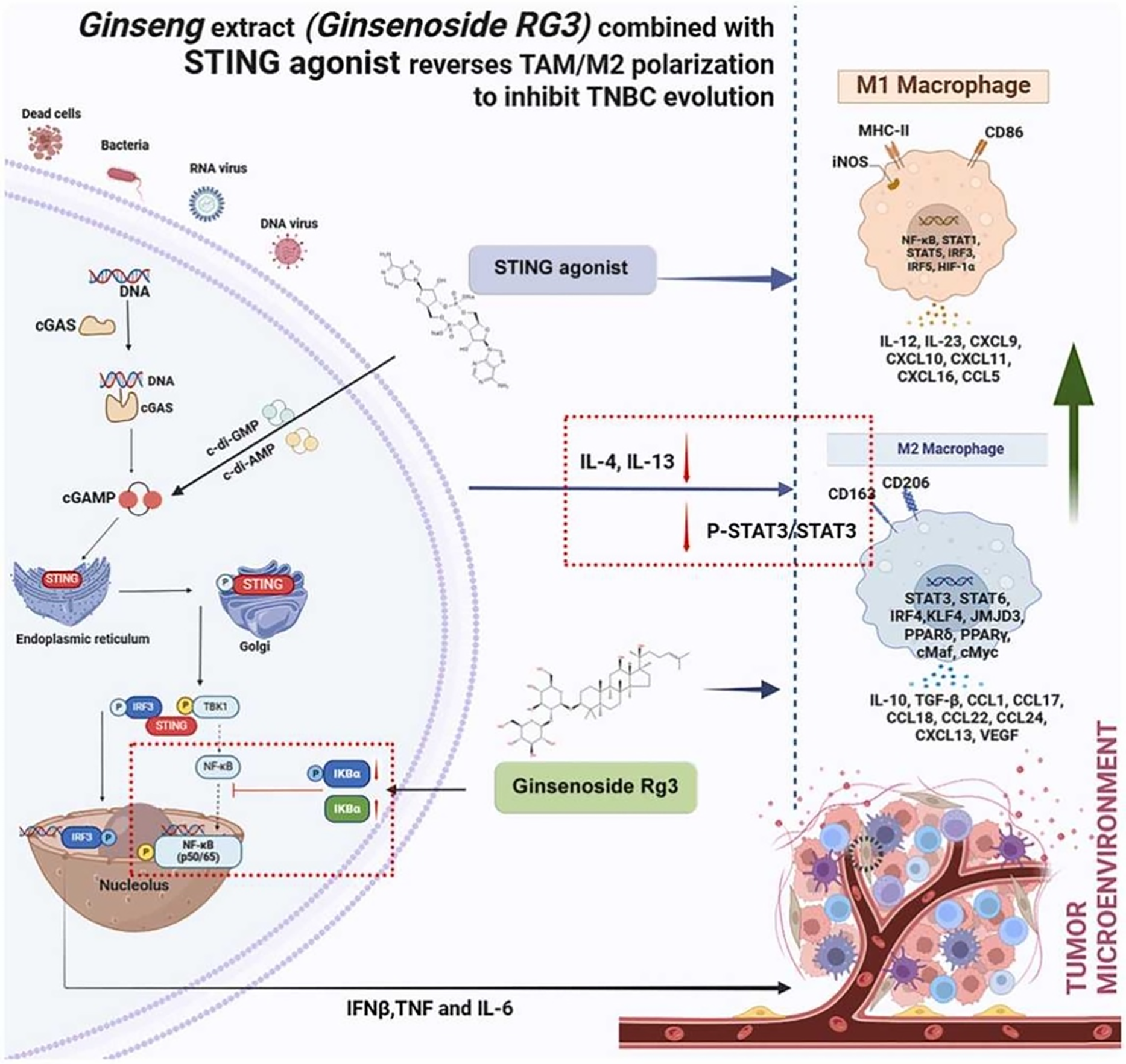
Figure 8: The combination of ginseng extract (Ginsenoside Rg3) and a STING agonist reverses TAM/M2 macrophage polarization, thereby shifting the tumor microenvironment to a pro-inflammatory state and inhibiting the progression of triple-negative breast cancer (TNBC). Reprinted with permission from Ref. [102]. 2024, Elsevier Ltd.
Enhanced Bioavailability and Efficacy of Co-Administered Agents: Rg3 is frequently incorporated into nanoformulations or co-administered with other agents to enhance its bioavailability and stability [103]. When utilized in conjunction with nanoparticle systems or encapsulated alongside other therapeutic agents, Rg3 facilitates improved cellular uptake and more effective delivery to target tissues, particularly tumors. These systems also offer protection against Rg3 degradation, thereby extending its activity and augmenting its therapeutic effects in combination with other compounds. For example, Zhang et al. developed Rg3-PLGA@TMVs by encapsulating ginsenoside Rg3 within poly(lactic-co-glycolic acid) (PLGA) nanoparticles and subsequently coating them with tumor cell-derived microvesicle (TMV) membranes [104]. Rg3-PLGA@TMVs exhibited several key advantages, including controlled drug release, enhanced storage stability, high drug-loading efficiency, and capacity to effectively activate dendritic cells. This activation was characterized by an increase in CD86+CD80+ dendritic cells, a reduction in phagocytic activity, and lower acid phosphatase levels, indicating a shift towards a more mature and activated dendritic cell phenotype. When used in combination with doxorubicin (DOX), Rg3-PLGA@TMVs demonstrated a synergistic effect, significantly inhibiting 4T1 tumor growth, a murine mammary carcinoma cell line model of triple-negative breast cancer, and promoting antitumor immunity in tumor-bearing mice. Notably, Rg3-PLGA@TMVs also mitigated the deleterious effects of DOX on normal cells and organs, particularly cardiotoxicity, thereby providing a safer and more effective therapeutic option. Overall, the synergistic potential of Rg3 across therapeutic domains renders it a versatile candidate for enhancing drug therapies and skincare solutions. Whether combined with pharmaceuticals or natural bioactives, Rg3 can amplify desired outcomes, reduce side effects, and offer a more comprehensive approach to managing skin health and cancer treatment.
14 Molecular Targets and Mechanisms
Ginsenoside Rg3 exerts therapeutic effects by targeting a range of molecular pathways that regulate cellular survival, proliferation, and apoptosis, rendering it effective for both skin care and cancer treatment. In cancer cells, Rg3 modulates critical signaling pathways implicated in cell cycle control, including PI3K/Akt and MAPK, which are frequently overexpressed in tumors and drive cancer cell growth and resistance to apoptosis [105]. By inhibiting these pathways, Rg3 inhibits cancer cell survival and proliferation. It also targets pro-apoptotic and anti-apoptotic proteins within the Bcl-2 family, thereby enhancing apoptosis by promoting caspase activation and mitochondrial dysfunction. The effect of Rg3 on angiogenesis involves the downregulation of VEGF and suppression of MMPs, thereby limiting the tumor’s ability to grow new blood vessels and invade the surrounding tissues. Additionally, in skincare, Rg3 activates antioxidant pathways, particularly the Nrf2 pathway, upregulating the expression of antioxidant enzymes that neutralize ROS and reduce oxidative stress. Collectively, these mechanisms make Rg3 a versatile agent with potential benefits across multiple molecular targets, enabling both anticancer and protective effects.
14.1 Gene Expression and Epigenetic Modulation
Ginsenoside Rg3 exerts its influence on gene expression and signaling pathways through both direct modulation and epigenetic mechanisms, significantly affecting skin and cancer cells. In cancer cells, Rg3 downregulates genes involved in cell proliferation, survival, and invasion by modulating signaling pathways, such as PI3K/Akt, NF-κB, and MAPK. This modulation results in the suppression of oncogenic processes including cell growth, angiogenesis, and metastasis. Furthermore, Rg3 has been demonstrated to upregulates genes associated with apoptosis by increasing the expression of pro-apoptotic proteins, such as Bax, and decreasing anti-apoptotic proteins, such as Bcl-2, thereby enhancing cancer cell death.
Rg3 also affects gene expression through epigenetic modification. It influences DNA methylation and histone acetylation, two key epigenetic mechanisms that regulate gene activity and expression. For instance, Rg3 has been linked to the demethylation of tumor suppressor genes, restoring their expression and thus aiding in the suppression of cancer progression [106]. Similarly, it may influence histone acetylation patterns to increase the availability of chromatin to the transcriptional machinery, thereby enhancing the expression of genes that promote cell cycle arrest and apoptosis.
In skin cells, Rg3 activates the Nrf2 pathway, a key regulator of antioxidant defense, by promoting the expression of genes encoding antioxidant enzymes such as SOD and catalase. This activation helps skin cells counteract oxidative stress, a major factor in skin aging and damage. Through gene expression and epigenetic modifications, Rg3 enhances protective, anti-inflammatory, and anti-cancer responses in both skin and cancer cells, contributing to its therapeutic potential across diverse applications.
14.2 Targeted Signaling Pathways
Ginsenoside Rg3 modulates several key signaling pathways pertinent to its skincare and anticancer activities, including AMPK, NF-κB, and JAK/STAT, which are involved in the regulation of cellular metabolism, inflammation, and proliferation.
AMPK Pathway: The AMPK pathway is a crucial regulator of cellular energy homeostasis. In cancer cells, Rg3 activates AMPK, leading to the inhibition of the mTOR pathway, thereby reducing cell growth and proliferation [86]. This pathway also induces autophagy, which facilitates the clearance of damaged cellular components and contributes to decreased cancer cell survival. In skin cells, AMPK activation by Rg3 promotes lipid metabolism and helps maintain skin barrier function, providing a protective effect beneficial for skin care.
NF-κB Pathway: Rg3 significantly influences the NF-κB pathway, which governs inflammation and immune response [107]. In cancer, Rg3 inhibits NF-κB activation, resulting in the downregulation of pro-inflammatory cytokines (e.g., TNF-α and IL-6) and a reduction in inflammation, which can promote tumor growth and metastasis. Suppression of NF-κB also sensitizes cancer cells to apoptosis. In skin cells, NF-κB inhibition by Rg3 helps mitigate inflammatory responses and protects against redness, sensitivity, and conditions, such as acne, thereby making it advantageous for anti-inflammatory skincare formulations.
JAK/STAT Pathway: The JAK/STAT pathway is essential for cell growth and immune regulation. Rg3 downregulates the JAK/STAT pathway in cancer cells, decreases cancer cell proliferation and resistance to apoptosis, and inhibits immune evasion tactics employed by tumors [108]. Additionally, this pathway modulation plays a role in reducing angiogenesis, thereby limiting the blood supply necessary for tumor growth. In skin care, modulating JAK/STAT can help manage inflammation and skin cell proliferation, potentially aiding in conditions such as psoriasis or skin aging.
By targeting these pathways—AMPK for energy balance, NF-κB for inflammation control, and JAK/STAT for growth regulation—Rg3 exerts both anticancer and protective effects. This multi-pathway modulation allows a comprehensive approach to address oxidative damage, inflammation, and abnormal cell proliferation in various tissue types.
15 Current Research Gaps of Rg3
Despite promising preclinical results, significant gaps persist in research on Rg3, particularly regarding its application in clinical settings. One of the primary limitations is the paucity of large-scale, well-controlled clinical trials that evaluate the efficacy, safety, and optimal dosage of Rg3 in humans, for both skincare and anticancer applications. The pharmacokinetics and metabolism of Rg3 in humans remain poorly understood, particularly in terms of bioavailability, absorption, and elimination. Although Rg3 demonstrates strong bioactivity in vitro and in animal models, its bioavailability in humans is low owing to poor water solubility and rapid metabolism; therefore, innovative delivery systems or formulation improvements are necessary to enhance its bioavailability. Furthermore, the pathways of Rg3 metabolism in humans and their potential interactions with other drugs or bioactive compounds require further investigation to ensure safe and effective therapeutic use. Additionally, research on the long-term effects and potential toxicity of high doses of Rg3 is limited, particularly when used in combination with other therapies.
16 Future Directions for Rg3 Research
Future research on ginsenoside Rg3 should focus on several critical areas to fully realize its therapeutic potential. Clinical trials are essential to confirm Rg3’s efficacy and safety in human subjects, with particular emphasis on optimal dosing, side effects, and drug interactions, especially when combined with other treatments like chemotherapy and radiotherapy. These studies will be pivotal in establishing Rg3 as a safe and effective therapeutic option. Additionally, investigating Rg3’s molecular targets in human cancer and skin cells will provide deeper insights into its mechanistic pathways, refining its therapeutic effects and guiding its application in personalized medicine. To maximize its clinical utility, developing advanced delivery systems such as nanoparticle carriers, liposomes, or other novel formulations is crucial to improve Rg3’s bioavailability and ensure targeted delivery. These systems will enhance its systemic absorption for anticancer therapy and percutaneous absorption for topical applications, making Rg3 more effective in various treatment contexts. Furthermore, exploring the synergy of Rg3 with other therapies—such as chemotherapy, immune checkpoint inhibitors, and targeted therapies—can lead to enhanced therapeutic outcomes, particularly in cancer treatment, by boosting efficacy and reducing side effects. Finally, regulatory and formulation studies are necessary to evaluate Rg3’s pharmacokinetics, pharmacodynamics, and stability across different formulations, ensuring its safe and effective use in clinical practice.
To address the current research gaps, future studies should focus on specific clinical investigations that confirm the therapeutic efficacy and safety of Rg3 in human subjects, with a focus on dose optimization and assessing potential drug-drug interactions and side effects. Research examining the mechanistic pathways of Rg3 in human cancer and skin cells would deepen our understanding of its therapeutic effects and refine its targeted applications. Improving the bioavailability of Rg3 is also a crucial direction, which could be achieved through the development of advanced formulations such as nanoparticle carriers, liposomes, or other novel delivery systems designed to enhance percutaneous absorption for topical applications or systemic delivery for anticancer therapy. Additionally, research could explore the role of Rg3 as a combination agent in both skincare and oncology by examining how it interacts with other active compounds or treatments to maximize therapeutic efficacy while minimizing side effects. These directions would bring Rg3 closer to being a reliable therapeutic option with clinically proven efficacy for human applications.
Furthermore, exploring the synergistic effects of Rg3 with other therapeutic agents could open new avenues for combination treatments, particularly in oncology. Finally, advancements in biotechnology could enable the production of Rg3 in larger quantities, making it more accessible for research and clinical applications. Overall, while significant progress has been made, continued research into ginsenoside Rg3 promises to unlock new therapeutic potentials and improve health outcomes.
In conclusion, ginsenoside Rg3 exhibits substantial therapeutic potential across a broad spectrum of medical applications, particularly in anticancer, anti-inflammatory, neuroprotective, and cardiovascular health domains. The distinct biological activities of its isomeric forms, 20(S)-Rg3 and 20(R)-Rg3, provide valuable insights into their multifaceted mechanisms of action, including the regulation of apoptosis, inflammation, and oxidative stress. Despite its potential, challenges such as the persistence of bioavailability can be addressed through innovative strategies such as nanoencapsulation and prodrug development. Furthermore, Rg3’s ability to synergize with other therapeutic agents suggests its role in bridging conventional and emerging treatment modalities in both oncology and dermatology. To fully realize its clinical potential, future research should prioritize refining delivery methods, understanding its precise molecular targets, and conducting rigorous clinical trials to validate its efficacy in diverse therapeutic settings.
Acknowledgement: Not applicable.
Funding Statement: This research was supported by the Agricultural Corporation Korean Master Ginseng Co., Ltd., Republic of Korea.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Young Mae Ko and Tae Hyon Kim; data collection: Young Mae Ko and Tae Hyon Kim; analysis and interpretation: Young Mae Ko and Tae Hyon Kim; draft manuscript preparation: Young Mae Ko and Tae Hyon Kim. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: Young Mae Ko and Tae Hyon Kim are full-time employees of Agricultural Corporation Korean Master Ginseng Co., Ltd., which also provided funding for this study. The authors affirm that they have no additional financial or personal conflicts of interest that could have influenced the work reported in this manuscript.
References
1. Shin BK, Kwon SW, Park JH. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39(4):287–98. doi:10.1016/j.jgr.2014.12.005. [Google Scholar] [PubMed] [CrossRef]
2. Murugesan M, Mathiyalagan R, Boopathi V, Kong BM, Choi SK, Lee CS, et al. Production of minor ginsenoside CK from major ginsenosides by biotransformation and its advances in targeted delivery to tumor tissues using nanoformulations. Nanomaterials. 2022;12(19):3427. doi:10.3390/nano12193427. [Google Scholar] [PubMed] [CrossRef]
3. Jia L, Zhao Y. Current evaluation of the millennium phytomedicine–ginseng (Ietymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009;16(19):2475–84. doi:10.2174/092986709788682146. [Google Scholar] [PubMed] [CrossRef]
4. Niu T, Smith DL, Yang Z, Gao S, Yin T, Jiang ZH, et al. Bioactivity and bioavailability of ginsenosides are dependent on the glycosidase activities of the A/J mouse intestinal microbiome defined by pyrosequencing. Pharm Res. 2013;30(3):836–46. doi:10.1007/s11095-012-0925-z. [Google Scholar] [PubMed] [CrossRef]
5. Yang EJ, Shin KC, Lee DY, Oh DK. Complete biotransformation of protopanaxatriol-type ginsenosides in Panax ginseng leaf extract to aglycon protopanaxatriol by β-glycosidases from Dictyoglomus turgidum and Pyrococcus furiosus. J Microbiol Biotechnol. 2018;28(2):255–61. doi:10.4014/jmb.1709.09053. [Google Scholar] [PubMed] [CrossRef]
6. Wang HP, Wang ZJ, Du J, Lin ZZ, Zhao C, Zhang R, et al. Comprehensive identification of ginsenosides in the roots and rhizomes of Panax ginseng based on their molecular features-oriented precursor ions selection and targeted MS/MS analysis. Molecules. 2023;28(3):941. doi:10.3390/molecules28030941. [Google Scholar] [PubMed] [CrossRef]
7. Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72(8):689–99. doi:10.1016/j.phytochem.2011.02.012. [Google Scholar] [PubMed] [CrossRef]
8. Wu L, Bai L, Dai W, Wu Y, Xi P, Zhang J, et al. Ginsenoside Rg3: a review of its anticancer mechanisms and potential therapeutic applications. Curr Top Med Chem. 2024;24(10):869–84. doi:10.2174/0115680266283661240226052054. [Google Scholar] [PubMed] [CrossRef]
9. Wang Y, Li G, Chen T, Wu W, Yan Z, Li X. Anticancer effect and molecular mechanism of ginsenoside Rg3 in various cancer types. Intell Pharm. 2023;1(2):52–63. doi:10.1016/j.ipha.2023.04.012. [Google Scholar] [CrossRef]
10. Yang KE, Jang HJ, Hwang IH, Hong EM, Lee MG, Lee S, et al. Stereoisomer-specific ginsenoside 20(S)-Rg3 reverses replicative senescence of human diploid fibroblasts via Akt-mTOR-Sirtuin signaling. J Ginseng Res. 2020;44(2):341–9. doi:10.1016/j.jgr.2019.08.002. [Google Scholar] [PubMed] [CrossRef]
11. Kang DI, Lee JY, Yang J, Jeong SM, Lee JH, Nah SY, et al. Evidence that the tertiary structure of 20(S)-ginsenoside Rg3 with tight hydrophobic packing near the chiral center is important for Na+ channel regulation. Biochem Biophys Res Commun. 2005;333(4):1194–201. doi:10.1016/j.bbrc.2005.06.026. [Google Scholar] [PubMed] [CrossRef]
12. Tong Y, Song X, Zhang Y, Xu Y, Liu Q. Insight on structural modification, biological activity, structure-activity relationship of PPD-type ginsenoside derivatives. Fitoterapia. 2022;158(1):105135. doi:10.1016/j.fitote.2022.105135. [Google Scholar] [PubMed] [CrossRef]
13. Won HJ, Kim HI, Park T, Kim H, Jo K, Jeon H, et al. Non-clinical pharmacokinetic behavior of ginsenosides. J Ginseng Res. 2019;43(3):354–60. doi:10.1016/j.jgr.2018.06.001. [Google Scholar] [PubMed] [CrossRef]
14. Li Z, Li J, Sun M, Men L, Wang E, Zhao Y, et al. Analysis of metabolites and metabolism-mediated biological activity assessment of ginsenosides on microfluidic co-culture system. Front Pharmacol. 2023;14:1046722. doi:10.3389/fphar.2023.1046722. [Google Scholar] [PubMed] [CrossRef]
15. Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42(2):123–32. doi:10.1016/j.jgr.2017.01.008. [Google Scholar] [PubMed] [CrossRef]
16. Qian T, Cai Z, Wong RNS, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B. 2005;816(1-2):223–32. doi:10.1016/j.jchromb.2004.11.036. [Google Scholar] [CrossRef]
17. Hu QR, Hong H, Zhang ZH, Feng H, Luo T, Li J, et al. Methods on improvements of the poor oral bioavailability of ginsenosides: pre-processing, structural modification, drug combination, and micro- or nano- delivery system. J Ginseng Res. 2023;47(6):694–705. doi:10.1016/j.jgr.2023.07.005. [Google Scholar] [PubMed] [CrossRef]
18. Mathiyalagan R, Murugesan M, Ramadhania ZM, Nahar J, Manivasagan P, Boopathi V, et al. Triterpenoid saponin-based supramolecular host-guest injectable hydrogels inhibit the growth of melanoma via ROS-mediated apoptosis. Mat Sci Eng R. 2024;160:100824. doi:10.1016/j.mser.2024.100824. [Google Scholar] [CrossRef]
19. Phan VHG, Murugesan M, Nguyen PPT, Luu CH, Le NHH, Nguyen HT, et al. Biomimetic injectable hydrogel based on silk fibroin/hyaluronic acid embedded with methylprednisolone for cartilage regeneration. Coll Surf B: Biointerf. 2022;219:112859. doi:10.1016/j.colsurfb.2022.112859. [Google Scholar] [PubMed] [CrossRef]
20. Phan VHG, Duong HS, Le QGT, Janarthanan G, Vijayavenkataraman S, Nguyen HNH, et al. Nanoengineered injectable hydrogels derived from layered double hydroxides and alginate for sustained release of protein therapeutics in tissue engineering applications. J Nanobiotechnol. 2023;21(1):405. doi:10.1186/s12951-023-02160-2. [Google Scholar] [PubMed] [CrossRef]
21. Thambi T, You DG, Han HS, Deepagan VG, Jeon SM, Suh YD, et al. Bioreducible carboxymethyl dextran nanoparticles for tumor-targeted drug delivery. Adv Healthc Mater. 2014;3(11):1829–38. doi:10.1002/adhm.201300691. [Google Scholar] [PubMed] [CrossRef]
22. Shen Y, Zhong B, Zheng W, Wang D, Chen L, Song H et al. Rg3-lipo biomimetic delivery of paclitaxel enhances targeting of tumors and myeloid-derived suppressor cells. J Clin Invest. 2024;134(22):e178617. doi:10.1172/jci178617. [Google Scholar] [PubMed] [CrossRef]
23. Chen H, Yang H, Fan D, Deng J. The anticancer activity and mechanisms of ginsenosides: an updated review. eFood. 2020;1(3):226–41. doi:10.2991/efood.k.200512.001. [Google Scholar] [CrossRef]
24. Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY, Gong FR, et al. Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo. Int J Oncol. 2018;52:127–38. doi:10.3892/ijo.2017.4183. [Google Scholar] [CrossRef]
25. Ma CH, Chou WC, Wu CH, Jou IM, Tu YK, Hsieh PL, et al. Ginsenoside Rg3 attenuates TNF-α-induced damage in chondrocytes through regulating SIRT1-mediated anti-apoptotic and anti-inflammatory mechanisms. Antioxidants. 2021;10(12):1972. doi:10.3390/antiox10121972. [Google Scholar] [CrossRef]
26. Park HM, Kim SJ, Kim JS, Kang HS. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem Toxicol. 2012;50(8):2736–41. doi:10.1016/j.fct.2012.05.027. [Google Scholar] [PubMed] [CrossRef]
27. Jakaria M, Haque ME, Kim J, Cho DY, Kim IS, Choi DK. Active ginseng components in cognitive impairment: therapeutic potential and prospects for delivery and clinical study. Oncotarget. 2018;9(71):33601–20. doi:10.18632/oncotarget.26035. [Google Scholar] [PubMed] [CrossRef]
28. Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, et al. Ginsenoside Rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther. 2013;21(5):381–90. doi:10.4062/biomolther.2013.053. [Google Scholar] [PubMed] [CrossRef]
29. Lee MY, Kim M. Effects of Red ginseng on neuroinflammation in neurodegenerative diseases. J Ginseng Res. 2024;48(1):20–30. doi:10.1016/j.jgr.2023.08.003. [Google Scholar] [PubMed] [CrossRef]
30. Shi L, Luo J, Wei X, Xu X, Tu L. The protective role of ginsenoside Rg3 in heart diseases and mental disorders. Front Pharmacol. 2024;15:1327033. doi:10.3389/fphar.2024.1327033. [Google Scholar] [PubMed] [CrossRef]
31. Gao S, Fang C, Wang T, Lu W, Wang N, Sun L, et al. The effect of ginsenoside Rg3 combined with chemotherapy on immune function in non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2023;102(14):e33463. doi:10.1097/md.0000000000033463. [Google Scholar] [PubMed] [CrossRef]
32. Kim TH. Ginsenosides for the treatment of insulin resistance and diabetes: therapeutic perspectives and mechanistic insights. J Ginseng Res. 2024;48(3):276–85. doi:10.1016/j.jgr.2024.03.002. [Google Scholar] [PubMed] [CrossRef]
33. Song J, Lee N, Yang HJ, Lee MS, Kopalli SR, Kim YU, et al. The beneficial potential of ginseng for menopause. J Ginseng Res. 2024;48(5):449–53. doi:10.1016/j.jgr.2024.05.008. [Google Scholar] [PubMed] [CrossRef]
34. Cong L, Ma J, Zhang Y, Zhou Y, Cong X, Hao M. Effect of anti-skin disorders of ginsenosides—a systematic review. J Ginseng Res. 2023;47(5):605–14. doi:10.1016/j.jgr.2023.04.005. [Google Scholar] [PubMed] [CrossRef]
35. Ramadhania ZM, Yang DU, Moektiwardojo M, Han Y, Park JK, Rupa EJ, et al. Enhanced anti-skin aging effects of fermented black ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1. Appl Sci. 2023;13(1):550. doi:10.3390/app13010550. [Google Scholar] [CrossRef]
36. Costa EF, Magalhães WV, Di Stasi LC. Recent advances in herbal-derived products with skin anti-aging properties and cosmetic applications. Molecules. 2022;27(21):7518. doi:10.3390/molecules27217518. [Google Scholar] [PubMed] [CrossRef]
37. Su J, Su Q, Hu S, Ruan X, Ouyang S. Research progress on the anti-aging potential of the active components of ginseng. Nutrients. 2023;15(15):3286. doi:10.3390/nu15153286. [Google Scholar] [PubMed] [CrossRef]
38. Feng C, Chen X, Yin X, Jiang Y, Zhao C. Matrix metalloproteinases on skin photoaging. J Cosmet Dermatol. 2024;23(12):3847–62. doi:10.1111/jocd.16558. [Google Scholar] [PubMed] [CrossRef]
39. Lim CJ, Choi WY, Jung HJ. Stereoselective skin anti-photoaging properties of ginsenoside Rg3 in UV-B-irradiated keratinocytes. Biol Pharm Bull. 2014;37(10):1583–90. doi:10.1248/bpb.b14-00167. [Google Scholar] [PubMed] [CrossRef]
40. Kim MK, Lee SK, Park JH, Lee JH, Yun BH, Park JH, et al. Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies. Sci Rep. 2017;7(1):17670. doi:10.1038/s41598-017-17956-0. [Google Scholar] [PubMed] [CrossRef]
41. Al-Atif H. Collagen supplements for aging and wrinkles: a paradigm shift in the fields of dermatology and cosmetics. Dermatol Pract Concept. 2022;12:e2022018. doi:10.5826/dpc.1201a18. [Google Scholar] [PubMed] [CrossRef]
42. Lee H, Hong Y, Tran Q, Cho H, Kim M, Kim C, et al. A new role for the ginsenoside RG3 in antiaging via mitochondria function in ultraviolet-irradiated human dermal fibroblasts. J Ginseng Res. 2019;43(3):431–41. doi:10.1016/j.jgr.2018.07.003. [Google Scholar] [PubMed] [CrossRef]
43. O’Rourke SA, Shanley LC, Dunne A. The Nrf2-HO-1 system and inflammaging. Front Immunol. 2024;15:1457010. doi:10.3389/fimmu.2024.1457010. [Google Scholar] [PubMed] [CrossRef]
44. Seo BY, Choi MJ, Kim JS, Park E. Comparative analysis of ginsenoside profiles: antioxidant, antiproliferative, and antigenotoxic activities of ginseng extracts of fine and main roots. Prev Nutr Food Sci. 2019;24(2):128–35. doi:10.3746/pnf.2019.24.2.128. [Google Scholar] [PubMed] [CrossRef]
45. Ding C, Peng X, Yang J, Chen K, Liu X, Zhao Y, et al. Rg3-loaded P407/CS/HA hydrogel inhibits UVB-induced oxidative stress, inflammation and apoptosis in HaCaT cells. Biomed Pharmacother. 2023;165:115177. doi:10.1016/j.biopha.2023.115177. [Google Scholar] [PubMed] [CrossRef]
46. You L, Cho JY. The regulatory role of Korean ginseng in skin cells. J Ginseng Res. 2021;45(3):363–70. doi:10.1016/j.jgr.2020.08.004. [Google Scholar] [PubMed] [CrossRef]
47. Hu S, Huo L, He J, Jin Y, Deng Y, Liu D. Ginseng glycoprotein and ginsenoside facilitate anti UV damage effects in diabetic rats. Front Pharmacol. 2022;13:1075594. doi:10.3389/fphar.2022.1075594. [Google Scholar] [PubMed] [CrossRef]
48. Poljšak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206. doi:10.1155/2012/135206. [Google Scholar] [PubMed] [CrossRef]
49. Li Z, Jiang R, Wang M, Zhai L, Liu J, Xu X, et al. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J Ginseng Res. 2022;46(1):115–25. doi:10.1016/j.jgr.2021.05.001. [Google Scholar] [PubMed] [CrossRef]
50. Han NR, Ko SG, Moon PD, Park HJ. Ginsenoside Rg3 attenuates skin disorders via down-regulation of MDM2/HIF1α signaling pathway. J Ginseng Res. 2021;45(5):610–6. doi:10.1016/j.jgr.2021.06.008. [Google Scholar] [PubMed] [CrossRef]
51. Yang J, Guo J, Tang P, Yan S, Wang X, Li H, et al. Insights from traditional chinese medicine for restoring skin barrier functions. Pharmaceuticals. 2024;17(9):1176. doi:10.3390/ph17091176. [Google Scholar] [PubMed] [CrossRef]
52. Kee JY, Hong SH. Ginsenoside Rg3 suppresses mast cell-mediated allergic inflammation via mitogen-activated protein kinase signaling pathway. J Ginseng Res. 2019;43(2):282–90. doi:10.1016/j.jgr.2018.02.008. [Google Scholar] [PubMed] [CrossRef]
53. Lee K, Pan Y, Lee Y, Song W, Park KY. Anti-inflammatory effects and immunomodulatory efficacy of unitein (Fermented Glycine max, Panax ginseng, and Chenpi Mixture) in RAW 264.7 macrophages and mice splenocytes along with component analysis. J Food Biochem. 2024;2024:9998250. doi:10.1155/2024/9998250. [Google Scholar] [CrossRef]
54. Jang WY, Hwang JY, Cho JY. Ginsenosides from Panax ginseng as key modulators of NF-κB signaling are powerful anti-inflammatory and anticancer agents. Int J Mol Sci. 2023;24(7):6119. doi:10.3390/ijms24076119. [Google Scholar] [PubMed] [CrossRef]
55. Chen QJ, Zhang MZ, Wang LX. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell Physiol Biochem. 2011;26(6):849–58. doi:10.1159/000323994. [Google Scholar] [PubMed] [CrossRef]
56. Lee Y, Park A, Park YJ, Jung H, Kim TD, Noh JY, et al. Ginsenoside 20(R)-Rg3 enhances natural killer cell activity by increasing activating receptor expression through the MAPK/ERK signaling pathway. Int Immunopharmacol. 2022;107(5):108618. doi:10.1016/j.intimp.2022.108618. [Google Scholar] [PubMed] [CrossRef]
57. Li Y, Lu J, Bai F, Xiao Y, Guo Y, Dong Z. Ginsenoside Rg3 suppresses proliferation and induces apoptosis in human osteosarcoma. Biomed Res Int. 2018;2018(12):4306579. doi:10.1155/2018/4306579. [Google Scholar] [PubMed] [CrossRef]
58. Kim BM, Kim DH, Park JH, Na HK, Surh YJ. Ginsenoside Rg3 induces apoptosis of human breast cancer (MDA-MB-231) cells. J Cancer Prev. 2013;18(2):177–85. doi:10.15430/jcp.2013.18.2.177. [Google Scholar] [PubMed] [CrossRef]
59. Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25(1):65–80. doi:10.1038/cdd.2017.186. [Google Scholar] [PubMed] [CrossRef]
60. Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13(8):1396–402. doi:10.1038/sj.cdd.4401963. [Google Scholar] [PubMed] [CrossRef]
61. Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X, et al. 20(S)-ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Des Devel Ther. 2015;9:3969–87. doi:10.2147/dddt.S84527. [Google Scholar] [PubMed] [CrossRef]
62. Kim BM, Kim DH, Park JH, Surh YJ, Na HK. Ginsenoside Rg3 inhibits constitutive activation of NF-κB signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as potential upstream targets. J Cancer Prev. 2014;19(1):23–30. doi:10.15430/jcp.2014.19.1.23. [Google Scholar] [PubMed] [CrossRef]
63. Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15(1):129. doi:10.1186/s13045-022-01347-8. [Google Scholar] [PubMed] [CrossRef]
64. Tian L, Shen D, Li X, Shan X, Wang X, Yan Q, et al. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget. 2016;7:1619–32. doi:10.18632/oncotarget.6451. [Google Scholar] [PubMed] [CrossRef]
65. Mustafa S, Koran S, AlOmair L. Insights into the role of matrix metalloproteinases in cancer and its various therapeutic aspects: a review. Front Mol Biosci. 2022;9:896099. doi:10.3389/fmolb.2022.896099. [Google Scholar] [PubMed] [CrossRef]
66. Lee SY. Anti-metastatic and anti-inflammatory effects of matrix metalloproteinase inhibition by ginsenosides. Biomedicines. 2021;9(2):198. doi:10.3390/biomedicines9020198. [Google Scholar] [PubMed] [CrossRef]
67. Wang D, Wu C, Liu D, Zhang L, Long G, Hu G, et al. Ginsenoside Rg3 inhibits migration and invasion of nasopharyngeal carcinoma cells and suppresses epithelial mesenchymal transition. Biomed Res Int. 2019;2019:8407683. doi:10.1155/2019/8407683. [Google Scholar] [PubMed] [CrossRef]
68. Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM, Lee BY, et al. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012;13(7):504–15. doi:10.4161/cbt.19599. [Google Scholar] [PubMed] [CrossRef]
69. Kim SH, Thambi T, Lym JS, Giang Phan VH, Lee DS. Tunable engineering of heparinized injectable hydrogels for affinity-based sustained delivery of bioactive factors. Macromol Mater Eng. 2019;304(9):1900279. doi:10.1002/mame.201900279. [Google Scholar] [CrossRef]
70. Turabee MH, Thambi T, Lee DS. Development of an injectable tissue adhesive hybrid hydrogel for growth factor-free tissue integration in advanced wound regeneration. ACS Appl Bio Mater. 2019;2(6):2500–10. doi:10.1021/acsabm.9b00204. [Google Scholar] [PubMed] [CrossRef]
71. Phan VHG, Le TMD, Janarthanan G, Ngo PKT, Lee DS, Thambi T. Development of bioresorbable smart injectable hydrogels based on thermo-responsive copolymer integrated bovine serum albumin bioconjugates for accelerated healing of excisional wounds. J Ind Eng Chem. 2021;96(19):345–55. doi:10.1016/j.jiec.2021.01.041. [Google Scholar] [CrossRef]
72. Lv Q, Xia Z, Huang Y, Ruan Z, Wang J, Huang Z. Ginsenoside Rg3 alleviates the migration, invasion, and angiogenesis of lung cancer cells by inhibiting the expressions of cyclooxygenase-2 and vascular endothelial growth factor. Chem Biol Drug Des. 2023;101(4):937–51. doi:10.1111/cbdd.14203. [Google Scholar] [PubMed] [CrossRef]
73. Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35(5):1735–43. doi:10.1016/j.biomaterials.2013.11.022. [Google Scholar] [PubMed] [CrossRef]
74. Thambi T, Park JH, Lee DS. Hypoxia-responsive nanocarriers for cancer imaging and therapy: recent approaches and future perspectives. Chem Commun. 2016;52(55):8492–500. doi:10.1039/C6CC02972H. [Google Scholar] [PubMed] [CrossRef]
75. Thambi T, Son S, Lee DS, Park JH. Poly(ethylene glycol)-b-poly(lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomat. 2016;29:261–70. doi:10.1016/j.actbio.2015.10.011. [Google Scholar] [PubMed] [CrossRef]
76. Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou H, et al. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 2014;9:e103887. doi:10.1371/journal.pone.0103887. [Google Scholar] [PubMed] [CrossRef]
77. Zeng D, Wang J, Kong P, Chang C, Li J, Li J. Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 2014;7:2172–8. [Google Scholar] [PubMed]
78. Jiang RY, Fang ZR, Zhang HP, Xu JY, Zhu JY, Chen KY, et al. Ginsenosides: changing the basic hallmarks of cancer cells to achieve the purpose of treating breast cancer. Chin Med. 2023;18(1):125. doi:10.1186/s13020-023-00822-9. [Google Scholar] [PubMed] [CrossRef]
79. Jeong KJ, Kim GW, Chung SH. AMP-activated protein kinase: an emerging target for ginseng. J Ginseng Res. 2014;38(2):83–8. doi:10.1016/j.jgr.2013.11.014. [Google Scholar] [PubMed] [CrossRef]
80. Thambi T, Deepagan VG, Yoo CK, Park JH. Synthesis and physicochemical characterization of amphiphilic block copolymers bearing acid-sensitive orthoester linkage as the drug carrier. Polymer. 2011;52(21):4753–9. doi:10.1016/j.polymer.2011.08.024. [Google Scholar] [CrossRef]
81. Thambi T, Park JH. Recent advances in shell-sheddable nanoparticles for cancer therapy. J Biomed Nanotechnol. 2014;10(9):1841–62. doi:10.1166/jbn.2014.1977. [Google Scholar] [PubMed] [CrossRef]
82. Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang J, et al. Targeting the Warburg effect: a revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm Sin B. 2024;14(3):953–1008. doi:10.1016/j.apsb.2023.12.003. [Google Scholar] [PubMed] [CrossRef]
83. Zhou Y, Zheng X, Lu J, Chen W, Li X, Zhao L. Ginsenoside 20(S)-Rg3 inhibits the warburg effect via modulating DNMT3A/MiR-532-3p/HK2 pathway in ovarian cancer cells. Cell Physiol Biochem. 2018;45(6):2548–59. doi:10.1159/000488273. [Google Scholar] [PubMed] [CrossRef]
84. Huang Q, Gao S, Zhao D, Li X. Review of ginsenosides targeting mitochondrial function to treat multiple disorders: current status and perspectives. J Ginseng Res. 2021;45(3):371–9. doi:10.1016/j.jgr.2020.12.004. [Google Scholar] [PubMed] [CrossRef]
85. Kim MJ, Koo YD, Kim M, Lim S, Park YJ, Chung SS, et al. Rg3 improves mitochondrial function and the expression of key genes involved in mitochondrial biogenesis in C2C12 myotubes. Diabetes Metab J. 2016;40(5):406–13. doi:10.4093/dmj.2016.40.5.406. [Google Scholar] [PubMed] [CrossRef]
86. Zhang C, Shu X, Yin C, Hu S, Liu P. The role of the mTOR pathway in breast cancer stem cells (BCSCsmechanisms and therapeutic potentials. Stem Cell Res Ther. 2025;16(1):156. doi:10.1186/s13287-025-04218-4. [Google Scholar] [PubMed] [CrossRef]
87. Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10(1):31. doi:10.1186/s13578-020-00396-1. [Google Scholar] [PubMed] [CrossRef]
88. Ni J, Liu Z, Jiang M, Li L, Deng J, Wang X, et al. Ginsenoside Rg3 ameliorates myocardial glucose metabolism and insulin resistance via activating the AMPK signaling pathway. J Ginseng Res. 2022;46(2):235–47. doi:10.1016/j.jgr.2021.06.001. [Google Scholar] [PubMed] [CrossRef]
89. Wei X, Su F, Su X, Hu T, Hu S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83(4):636–42. doi:10.1016/j.fitote.2012.01.006. [Google Scholar] [PubMed] [CrossRef]
90. Morshed MN, Ahn JC, Mathiyalagan R, Rupa EJ, Akter R, Karim MR, et al. Antioxidant activity of Panax ginseng to regulate ROS in various chronic diseases. Appl Sci. 2023;13(5):2893. doi:10.3390/app13052893. [Google Scholar] [CrossRef]
91. Sun HY, Lee JH, Han YS, Yoon YM, Yun CW, Kim JH, et al. Pivotal roles of ginsenoside Rg3 in tumor apoptosis through regulation of reactive oxygen species. Anticancer Res. 2016;36:4647–54. doi:10.21873/anticanres.11015. [Google Scholar] [PubMed] [CrossRef]
92. Lee YM, Yoon H, Park HM, Song BC, Yeum KJ. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J Ginseng Res. 2017;41(2):113–9. doi:10.1016/j.jgr.2016.03.003. [Google Scholar] [PubMed] [CrossRef]
93. Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690(1-2):12–23. doi:10.1016/j.mrfmmm.2009.09.007. [Google Scholar] [PubMed] [CrossRef]
94. Li WL, Li K, Chang WG, Shi H, Zhang WX, Wang Z, et al. 20(R)-ginsenoside Rg3 alleviates diabetic retinal injury in T2DM mice by attenuating ROS-mediated ER stress through the activation of the Nrf2/HO-1 axis. Phytomedicine. 2024;135(6):156202. doi:10.1016/j.phymed.2024.156202. [Google Scholar] [PubMed] [CrossRef]
95. Zhong G, Chen J, Li Y, Han Y, Wang M, Nie Q, et al. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway. BMC Complement Med Ther. 2024;24(1):247. doi:10.1186/s12906-024-04492-4. [Google Scholar] [PubMed] [CrossRef]
96. Thambi T, Deepagan VG, Ko H, Lee DS, Park JH. Bioreducible polymersomes for intracellular dual-drug delivery. J Mater Chem. 2012;22(41):22028–36. doi:10.1039/C2JM34546C. [Google Scholar] [CrossRef]
97. Zhu Y, Liang J, Gao C, Wang A, Xia J, Hong C, et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J Control Release. 2021;330(1):641–57. doi:10.1016/j.jconrel.2020.12.036. [Google Scholar] [PubMed] [CrossRef]
98. Jiang Z, Yang Y, Yang Y, Zhang Y, Yue Z, Pan Z, et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed Pharmacother. 2017;96(2):378–83. doi:10.1016/j.biopha.2017.09.129. [Google Scholar] [PubMed] [CrossRef]
99. Li L, Ni J, Li M, Chen J, Han L, Zhu Y, et al. Ginsenoside Rg3 micelles mitigate doxorubicin-induced cardiotoxicity and enhance its anticancer efficacy. Drug Deliv. 2017;24(1):1617–30. doi:10.1080/10717544.2017.1391893. [Google Scholar] [PubMed] [CrossRef]
100. Tomas M, Gunal-Koroglu D, Kamiloglu S, Ozdal T, Capanoglu E. The state of the art in anti-aging: plant-based phytochemicals for skin care. Immunity & Ageing. 2025;22(1):5. doi:10.1186/s12979-025-00498-9. [Google Scholar] [PubMed] [CrossRef]
101. Wang W, Kong M, Shen F, Li P, Chen C, Li Y, et al. Ginsenoside Rg3 targets glycosylation of PD-L1 to enhance anti-tumor immunity in non-small cell lung cancer. Front Immunol. 2024;15:1434078. doi:10.3389/fimmu.2024.1434078. [Google Scholar] [PubMed] [CrossRef]
102. Fu Q, Lu Z, Chang Y, Jin T, Zhang M. Ginseng extract (Ginsenoside RG3) combined with STING agonist reverses TAM/M2 polarization to inhibit TNBC evolution. Ind Crops Prod. 2024;222:119589. doi:10.1016/j.indcrop.2024.119589. [Google Scholar] [CrossRef]
103. Shang Q, Liu W, Leslie F, Yang J, Guo M, Sun M, et al. Nano-formulated delivery of active ingredients from traditional Chinese herbal medicines for cancer immunotherapy. Acta Pharm Sin B. 2024;14(4):1525–41. doi:10.1016/j.apsb.2023.12.008. [Google Scholar] [PubMed] [CrossRef]
104. Zhang S, Zheng B, Wei Y, Liu Y, Yang L, Qiu Y, et al. Bioinspired ginsenoside Rg3 PLGA nanoparticles coated with tumor-derived microvesicles to improve chemotherapy efficacy and alleviate toxicity. Biomater Sci. 2024;12(10):2672–88. doi:10.1039/D4BM00159A. [Google Scholar] [PubMed] [CrossRef]
105. Xie Q, Wen H, Zhang Q, Zhou W, Lin X, Xie D, et al. Inhibiting PI3K-AKt signaling pathway is involved in antitumor effects of ginsenoside Rg3 in lung cancer cell. Biomed Pharmacother. 2017;85:16–21. doi:10.1016/j.biopha.2016.11.096. [Google Scholar] [PubMed] [CrossRef]
106. Yang Y, Nan Y, Du Y, Liu W, Ning N, Chen G, et al. Ginsenosides in cancer: proliferation, metastasis, and drug resistance. Biomed Pharmacother. 2024;177:117049. doi:10.1016/j.biopha.2024.117049. [Google Scholar] [PubMed] [CrossRef]
107. Lee IS, Uh I, Kim KS, Kim KH, Park J, Kim Y, et al. Anti-inflammatory effects of ginsenoside Rg3 via NF-κB pathway in A549 cells and human asthmatic lung tissue. J Immunol Res. 2016;2016(4):7521601. doi:10.1155/2016/7521601. [Google Scholar] [PubMed] [CrossRef]
108. Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharmaceutica Sinica B. 2022;12(3):1163–85. doi:10.1016/j.apsb.2021.08.020. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools