 Open Access
Open Access
ARTICLE
ScRNA-seq and Experimental Analyses Unveil Lrg1 Regulating the Oxidative Phosphorylation Pathway to Affect Neutrophil Accumulation after Cerebral Ischemia-Reperfusion
1 Neurology Department, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, 330006, China
2 Department of Pharmacy, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, 330006, China
3 School of Pharmacy, Jiangxi Medical College, Nanchang University, Nanchang, 330019, China
4 College of Pharmacy, Hubei University of Chinese Medicine, Wuhan, 430065, China
5 Key Laboratory of Rare Neurological Diseases of Jiangxi Provincial Health Commission, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, 330006, China
6 Jiangxi Key Laboratory of Neurological Diseases, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, 330006, China
* Corresponding Authors: Jin Chen. Email: ; Yanni LV. Email:
# These authors contributed equally to this work
BIOCELL 2025, 49(9), 1749-1769. https://doi.org/10.32604/biocell.2025.068507
Received 30 May 2025; Accepted 13 August 2025; Issue published 25 September 2025
Abstract
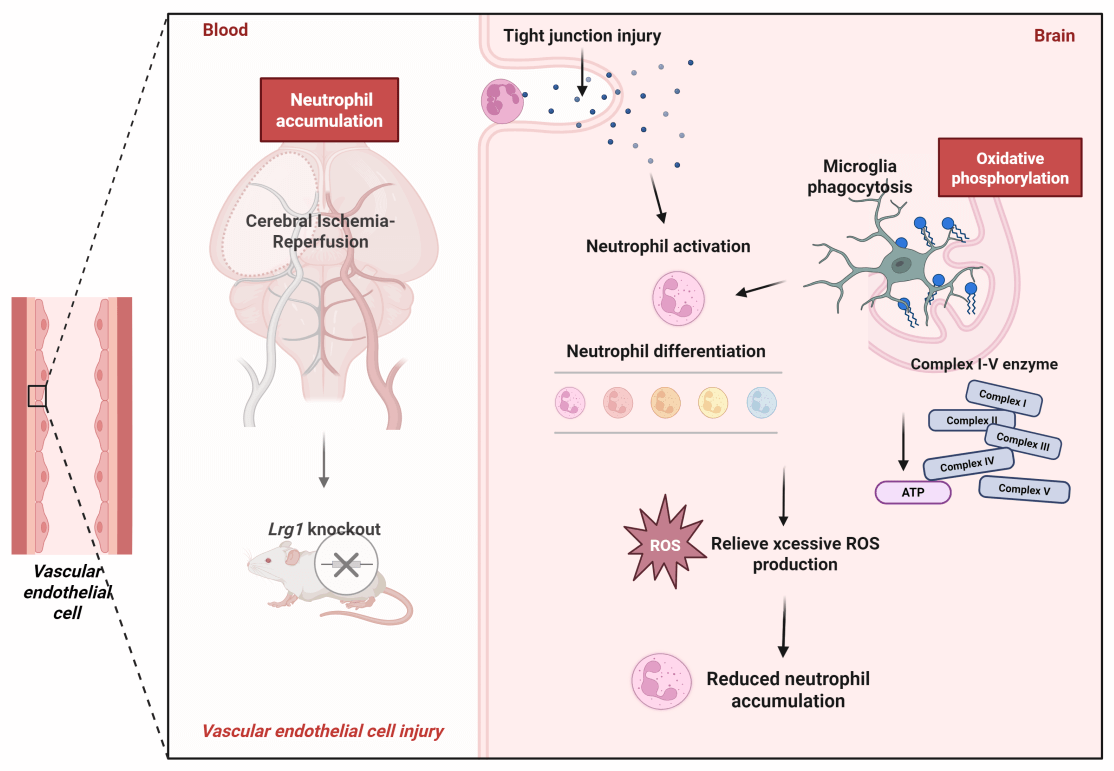
Background: After ischemic stroke, neutrophils hyperactivate, increasing in number and worsening inflammation, causing neural damage. Prior scRNA-seq showed Lrg1 modulates cells subsentence to cerebral ischemia-reperfusion injury, but its mechanism in regulating neutrophil accumulation/differentiation post-injury is unclear. Methods: Lrg1 knockout impact on neutrophil accumulation was assessed via immunofluorescence and western blot. Three-dimensional reconstruction of immunofluorescent staining analyzed cell-cell interactions among neutrophils and microglia. scRNA-seq of WT and Lrg1-/- mice from GSE245386 and GSE279462 was conducted. Each group conducted oxidative phosphorylation scoring via Gene Set Enrichment Analysis (GSEA), while Metascape was employed to perform GO and KEGG enrichment analyses for elucidating functional mechanisms. CellChat exhibited cell-cell communication. Furthermore, alterations in microglial phagocytic activity were evaluated by immunostaining for CD68, a well-established marker of phagolysosomal activity in phagocytic cells. Brain energy metabolism was evaluated via glutamate dehydrogenase activity and ATP levels with ELISA, and enzyme expression was analyzed by immunofluorescence and western blot. Results: Lrg1 knockout decreased neutrophil accumulation and NET formation in mice. 3D immunofluorescence reconstruction confirmed neutrophil co-localization with endothelial cells/microglia. scRNA-seq revealed that the oxidative phosphorylation score was significantly higher in the MCAO/R+WT group compared to both the Sham-operated+WT and Lrg1-/- groups. Notably, the oxidative phosphorylation score was further elevated in the MCAO/R+Lrg1-/- group. Immunostaining showed that Lrg1 knockout elevated CD68+ lysosome expression post-MCAO/R, with TMEM119 colocalizing with these lysosomes. MCAO/R raised CD68 expression in ischemic brains, an effect further intensified by Lrg1 knockout. KEGG analysis linked differential genes to oxidative phosphorylation pathways. Validation in MCAO/R vs. sham groups revealed increased ROS production and reduced expression of complex enzymes I-V (NDUFB8, SDHB, UQCRC1, MTCO2, ATP5A1). Lrg1 intervention increased enzyme expression. Immunofluorescence and western blot in brain tissue showed similar patterns in microglia and enzymes I-V. Conclusions: Lrg1 knockout significantly enhances microglial phagocytic activity towards neutrophils subsequent to cerebral ischemia-reperfusion injury, through its regulatory effect on the oxidative phosphorylation pathway. This finding accentuates Lrg1 as a highly potential therapeutic target for intervening in and modulating post-ischemic inflammatory responses.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools