 Open Access
Open Access
ARTICLE
Analysis of Risk Factors for Early Mortality in Surgical Shunt Palliation: Time for a Change?
1 Department of Cardiovascular and Thoracic Surgery, Université catholique de Louvain (UCLouvain)-Cliniques Universitaires Saint-Luc, Brussels, Belgium
2 Department of Pediatric Cardiology, Université catholique de Louvain (UCLouvain)-Cliniques Universitaires Saint-Luc, Brussels, Belgium
3 Department of Anesthesiology, Université catholique de Louvain (UCLouvain)-Cliniques Universitaires Saint-Luc, Brussels, Belgium
4 Department of Pediatric Intensive Care, Université catholique de Louvain (UCLouvain)-Cliniques Universitaires Saint-Luc, Brussels, Belgium
* Corresponding Author: Alain J. Poncelet. Email:
Congenital Heart Disease 2023, 18(5), 539-550. https://doi.org/10.32604/chd.2023.042344
Received 26 May 2023; Accepted 05 September 2023; Issue published 10 November 2023
Abstract
Objectives: Over the last decade, neonatal repair has been advocated for many congenital heart diseases. However, specific subgroups of complex congenital heart disease still require temporary palliation for which both surgical and endovascular techniques are currently available. We reviewed our institutional experience with shunt palliation with an emphasis on risk factors for early mortality. Methods: This is a single-center retrospective study on 175 patients undergoing surgery for central shunt or modified Blalock-Taussig shunt. All data were extracted from a prospectively collected computerized database. We identified risk factors for early mortality by uni- and multi-variable analysis. All data were censored at the time of death or shunt take-down operation. Results: Mean age and weight at surgery were 24 days (IQR [7–95]) and 3.4 kg (IQR [2.9–4.8]), respectively, with 96 neonates (55%). Most patients had a biventricular heart disease (115 patients, 66%), and 51 patients (29.1%) had univentricular heart disease. Thoracotomy was performed in 129 patients (74%). Cardiopulmonary bypass was used in 23 patients (13%). The median intensive care and overall length of stay were 4 days (IQR [2–9]) and 18 days (IQR [13–29]), respectively. In-hospital mortality was 8.6% (15/175). By multivariable regression analysis, prematurity (HR 5.6 [2.1–14.7]), CPB use (HR 6.7 [2.2–18.6]), unplanned <30-day reoperation (HR 3.5 [1.2–10]) or catheterization (HR 4.5 [1.2–16.9]) were all significant predictors of early mortality. Conclusions: Procedural-related mortality remains high (8.6%) in surgical shunt palliation. For patients with prematurity, low weight at birth, or if the use of cardiopulmonary bypass is contemplated, alternative endovascular techniques of palliation should be considered together with longitudinal follow-up studies.Keywords
Supplementary Material
Supplementary Material FileAbbreviations and Acronyms
| CHD | Congenital Heart Disease |
| CPB | Cardio-Pulmonary Bypass |
| CUSL | Cliniques Universitaires Saint-Luc |
| DORV | Double Outlet Right Ventricle |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| LCOS | Low Cardiac Output State |
| MBTS | Modified Blalock-Taussig shunt |
| MVO2 | Mixed Venous Oxygen Saturation |
| NEC | Necrotizing Enterocolitis |
| PA | Pulmonary Artery |
| PA-VSD | Pulmonary Atresia-Ventricular Septal Defect |
| PDA | Patent Ductus Arteriosus |
| POC | Pulmonary Over Circulation |
| SaO2 | Arterial Oxygen Saturation |
| SWR | Shunt-to-Weight Ratio |
| UVH | Univentricular Heart |
Over the last decade, neonatal repair has been advocated for many congenital heart diseases (CHD). However, specific subgroups of complex CHD still require temporary palliation to provide time for pulmonary resistance to fall and for Pulmonary Artery (PA) branches to grow [1].
Despite the refinements in neonatal care, surgical techniques and Cardio-Pulmonary Bypass (CPB) technology, early mortality in surgical palliation (modified-Blalock-Taussig shunt (MBTS) and central shunts) has not decreased over the years, ranging from 6.5% to 12% in centers of excellence [2–6].
Accordingly, ductal and right ventricular outflow tract stenting has emerged lately as attractive alternative endovascular approaches in high-risk neonates with encouraging results [7,8].
Initially performed in the center of expertise for high-risk patients, patent ductus arteriosus (PDA) stenting has been more liberally attempted recently. Interestingly, Bauser-Heaton et al. reported a retrospective US multicentric study [9] in which risk-adjusted mortality was similar with each approach (14.3% PDA stent vs. 12.8% MBTS), with a higher interstage reintervention rate in the interventional arm (51.3% PDA stent vs. 21.3% MBTS). A recent meta-analysis comparing the two modalities of palliation found no difference in early mortality, while duration of ventilation and hospital length of stay favored the endovascular approach [10].
The aim of this study was to review our 20-year experience of surgical shunt palliation with a specific interest in analyzing patient-related and procedure-related risk factors for in-hospital mortality and to identify specific subgroup(s) of patients to whom we could offer non-surgical alternatives. As for other centers, those results should stand as the benchmark for future comparison between these alternative choices of palliation.
This retrospective analysis was approved by our Institutional Ethical Board (Cliniques Universitaires Saint-Luc (CUSL), Brussels, Belgium, IRB 2015\11-01\ID 327).
2.2 Data Collection and Follow-Up
This study is a single-center retrospective case study series which includes all patients (no exclusion criteria) who underwent palliation with a surgical shunt (MBTS or central shunt) from 1997 until 2019 (n = 175).
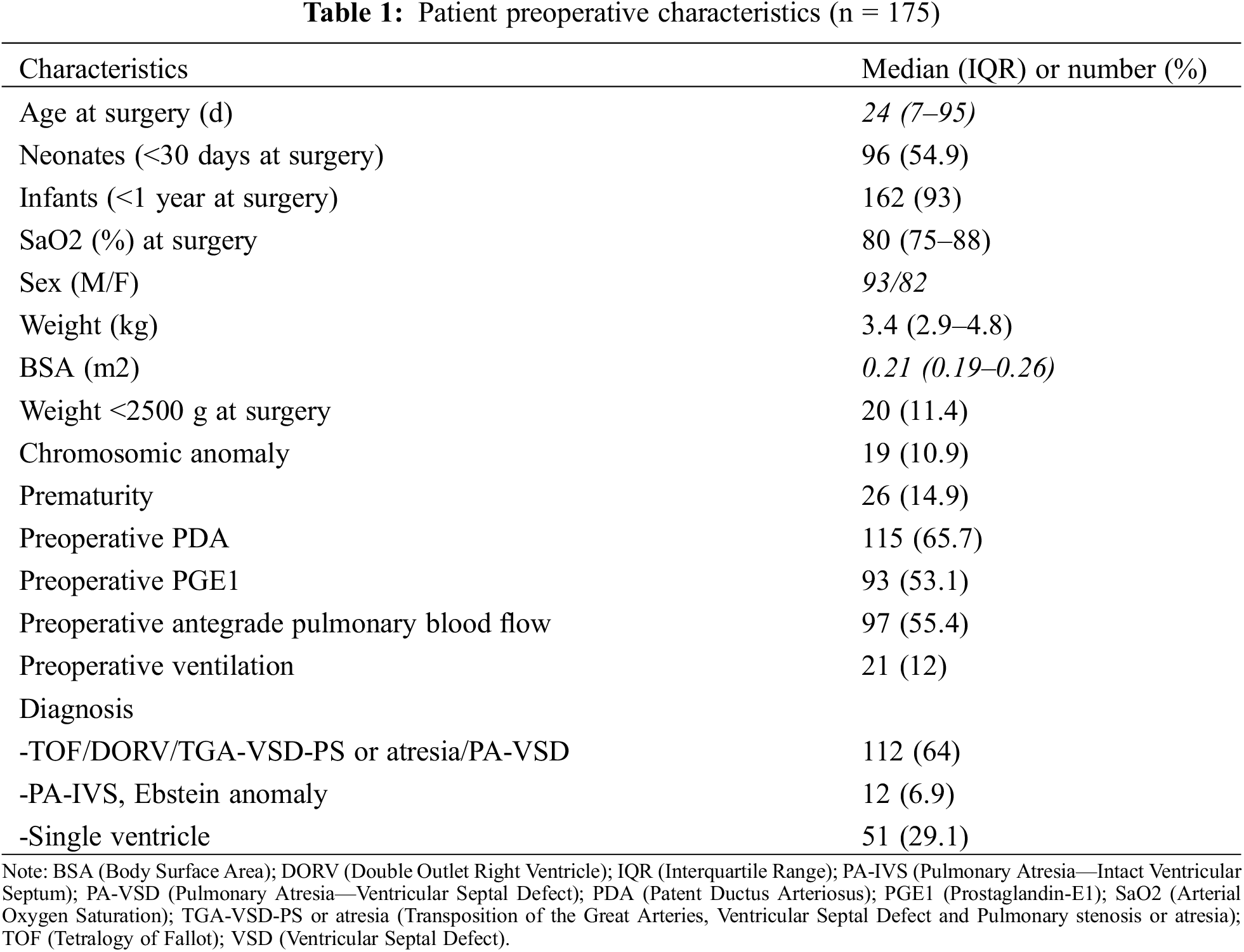
It represents a heterogeneous group of patients: neonates with ductal-dependant pulmonary or systemic circulation, tetralogy of Fallot with restrictive pulmonary blood flow and/or cyanotic spells, complex transposition of the great arteries with LVOTO, Ebstein anomaly and other rare conditions (see Table 1).
Over this 20-year period, our center’s policy was to provide surgical additional pulmonary blood flow in any patient with O2 saturation on room air below 80% in whom a primary curative repair (biventricular) was not deemed feasible or for those entering a classic single-ventricle pathway.
All data were collected from patient’s medical record, operative notes and catheterization studies.
We defined pulmonary over circulation (POC) as an oxygen saturation of >85% in a fraction inhaled oxygen ≤25%, and two or more of the following during the immediate postoperative Intensive Care Unit stay: (a) need to modify systemic and pulmonary vascular resistance (red blood cell transfusion), (b) evidence of decreased cardiac output (decreasing mixed venous oxygen saturation (MVO2), widening gap between arterial oxygen saturation (SaO2) to MVO2, increasing lactate >2 mmol/L), and (c) the need for diuretics.
Postoperative low cardiac output syndrome was defined as a combination of clinical (e.g., decrease urinary output), haemodynamic and biochemical parameters (decreasing MVO2, widening SaO2 to MVO2, and increasing lactate >2 mmol/L).
In addition to the shunt size, two other variables were considered to assess the effect of shunt size on outcomes: the shunt size/weight ratio and the graft index defined as the graft cross sectional area/patient body surface area.
The observation period for shunt thrombosis or stenosis was from insertion to takedown of the shunt at the planned surgery or autopsy. Diagnosis of shunt thrombosis required confirmation angiographically, at surgery or autopsy. All variables of interest selected for this study are listed in Supplementary Table S1.
Continuous data are presented as mean ± standard deviation, or median and IQR (interquartile range) for nonparametric data. Normality of the distribution was assessed with the Shapiro-Wilk test. Categorical data are presented as numbers and proportions and compared with the Chi-square test or the Fisher’s exact test, if appropriate. Differences between means or medians were compared using unpaired Student’s t-test or Mann—Whitney U test, according to the distribution. Survival was calculated from the date of the shunt procedure to the time of death or to the time of the following shunt take-down procedure, which ever came first.
Survival curves were generated with the Kaplan—Meier estimator and were compared using the log-rank test.
To assess the factors associated with survival, a Cox proportional hazards model was generated by including pre-, intra- and post-operative variables. To determine the independent predictors of each outcome of interest, variables with a p-value < 0.20 in the univariate analysis were entered into a multivariate conditional forward stepwise selection procedure. Results are displayed as hazard ratio (HR) or adjusted HR with 95% confidence interval. All tests were two-sided, with significance set at the 0.05 probability level (See Supplementary Table S1 for a complete list of variables assessed in the logistic regression models).
All statistical analyses were performed using the IBM SPSS Statistic version 26 (IBM Corp., Armonk, New York, USA).
3.1 Demographic and Operative Details
The median age and weight at shunt palliation were 24 days (IQR 7–95) and 3.4 kg (IQR 2.9–4.8).
Most of the patients (162/175, 93%) were either neonates or infants. Prior to surgery, median oxygen saturation was 80% (IQR 75%–88%). Antegrade pulmonary blood flow was present in 97 patients (55%). The most common diagnosis were patients with functional uni-ventricular heart (n = 51), followed by pulmonary atresia-ventricular septal defect (PA-VSD) (n = 41), tetralogy of Fallot (n = 30), d-Transposition of the Great Arteries/Ventricular Septal Defect/Pulmonary stenosis or atresia (n = 20), double outlet right ventricle (DORV)—Fallot type (n = 14), and PA-IVS (n = 10). As seen in Table 1, we pooled altogether patients palliated prior to biventricular repair and distinguish them from the univentricular repair patients and the undetermined ones (bi-V or 1 1/2V or single V).
Over the study period, our center’s surgical policy has been to favor the thoracotomy approach (contra-lateral to the aortic arch) and to avoid sternotomy for a surgical shunt whenever the target pulmonary vessel was 3 mm of more in diameter as measured by preoperative transthoracic echocardiography.
In neonates, the ductus, if patent, was left intact while prostaglandin was discontinued at the start of surgery. Prior to proximal target vessel clamping, intravenous unfractionated heparin (100 U/kg) was routinely administered.
When sternotomy was performed, the use of CPB was mostly based on patient’s hemodynamics after test-clamping of both the pulmonary and the systemic vessel.
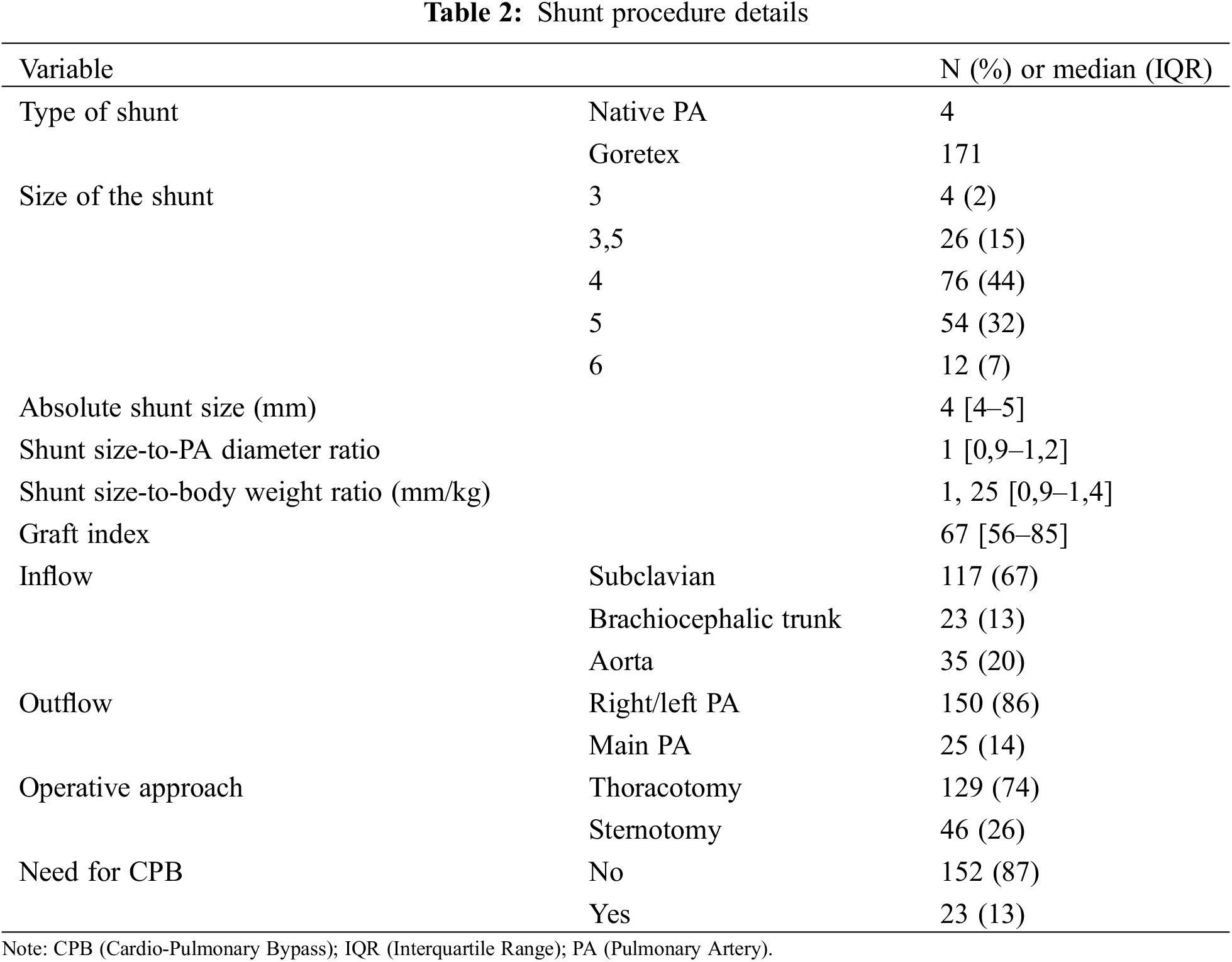
The operation was performed by thoracotomy or sternotomy in 129 (74%) and 46 (26%) patients, respectively. Cardiopulmonary bypass was required in 23 patients (13%).
Proximal anastomosis came from the subclavian artery, the brachiocephalic trunk or the ascending aorta in 117, 23 and 35 patients, respectively. Distal anastomosis was performed on the right PA (n = 134, 77%) or left PA (n = 16, 9%), and on the main PA (n = 25, 14%).
Goretex stretch vascular grafts (W.L.Gore & Associates, Inc., AZ, USA) were used in 171 patients, 4 patients had direct main PA to aorta anastomosis without interposing graft. The most frequent shunt sizes (graft or native PA) were 3.5 mm (n = 26), 4 mm (n = 77) and 5 mm (n = 56). The median shunt-weight ratio was 1.25 (IQR 0.92–1.43), the median graft index was 67 mm2/m2 (IQR 56–85) and the median shunt-to-PA diameter was 1 (IQR 0.9–1.2) (Table 2).
Patients were routinely placed under unfractionated heparin on Pediatric Intensive Care Unit arrival, and started on acetylsalicylic acid during the immediate postoperative period (together with enteral feeding).
The median interval time from initial palliation to the shunt takedown procedure was 345 days (IQR 202–755).
3.2 In-Hospital Morbidity and Mortality
The median time to extubation was 1 day (IQR [0.3–3]). The median intensive care and overall length of stay were 4 (IQR [2–9]) and 18 (IQR [13–29]) days, respectively.
Peri-operative inotropic support was needed to maintain stable hemodynamics in 48 patients (27%). Two patients required extra-corporeal membrane oxygenation support (1.1%). POC and low cardiac output state (LCOS) were observed in 63 and 15 patients (36% and 8.6%, respectively). Early shunt thrombosis was diagnosed in 12 patients (6.9%). Of those, 3 deaths were directly related to shunt thrombosis (early mortality 25%).
Other major morbidities such as necrotizing enterocolitis (NEC), bacteriemia or bleeding are detailed in Table 3.
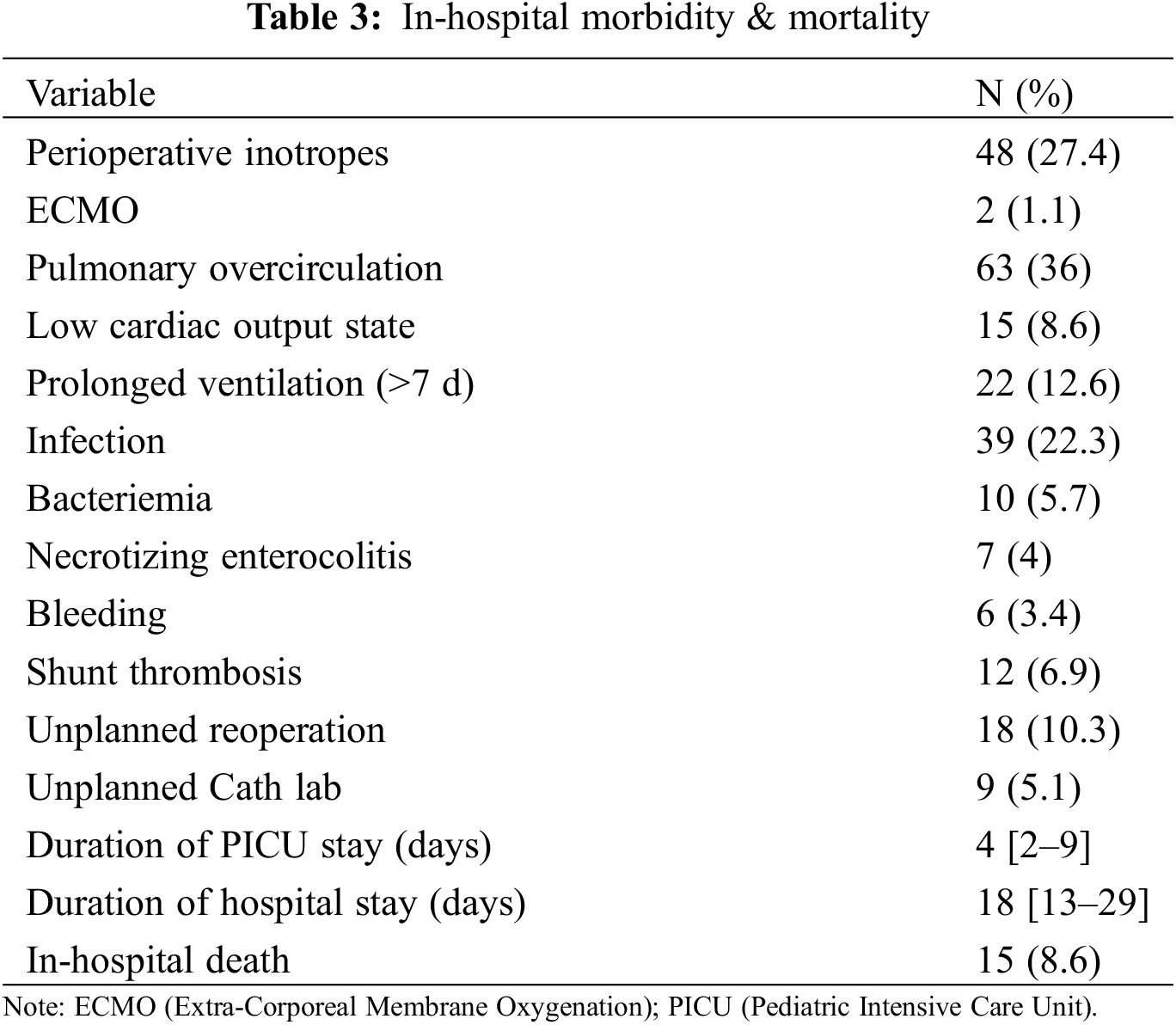
Unplanned early catheterization and unplanned early surgical reintervention were performed in 9 and 18 patients, respectively (5.1% and 10.3%). Twelve reinterventions were shunt-related (5 shunt thrombosis, 2 flow reduction, 5 insufficient shunt) and 6 were shunt-unrelated (surgical wound revision n = 3, patent ductus arteriosus ligation, pulmonary coarctation repair and staged contralateral unifocalisation n = 1 each).
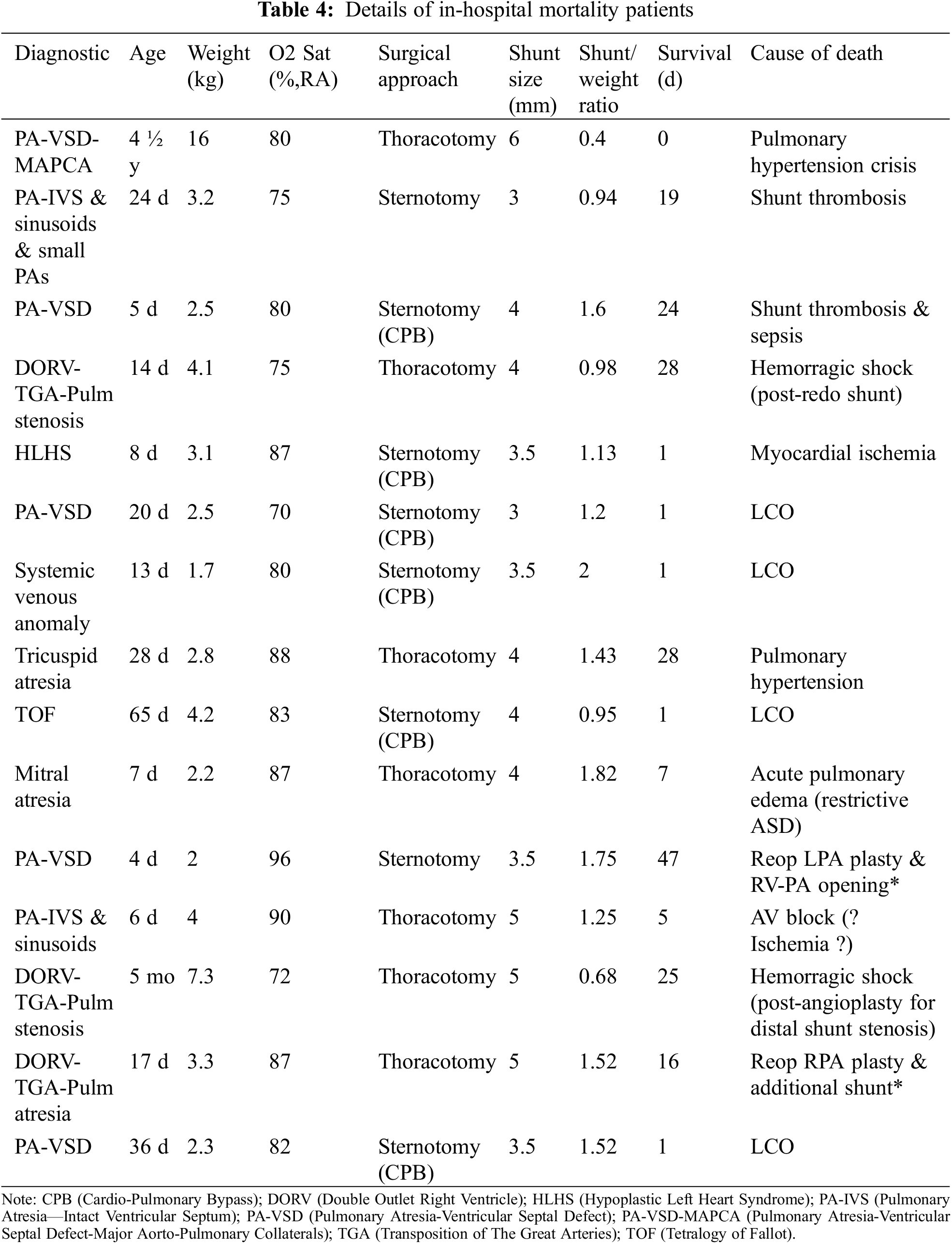
Overall, in-hospital mortality was 8.6% (15/175). A detailed analysis of this subgroup is provided in Table 4. All deaths secondary to LCOS were encountered in the CPB subgroup.
3.3 Predictors of Early Mortality
For the entire cohort, by univariable analysis, preoperative variables such as weight <2500 g (HR 4 95CI [1.5–10.4]) (p = 0.006) and prematurity (<37 w-GA) (HR 4.3 95CI [1.7–10.8]) (p = 0.002) were strong predictors of in-hospital death. However, neither neonatal surgery, univentricular anatomy nor genetic syndrome were related to a poorer outcome (p = 0.58, p = 0.89, and p = 0.18, respectively). Dividing the cohort in terciles, and considering the oldest subgroup (1997–2004) as the reference, the era effect was neutral for 2005–2012 (HR 2.1 95CI [0.7–6.2]) (p = 0.19) and for 2013–2019 (HR 1 95CI [0.3–3.7]) (p = 0.98) (Table 5).
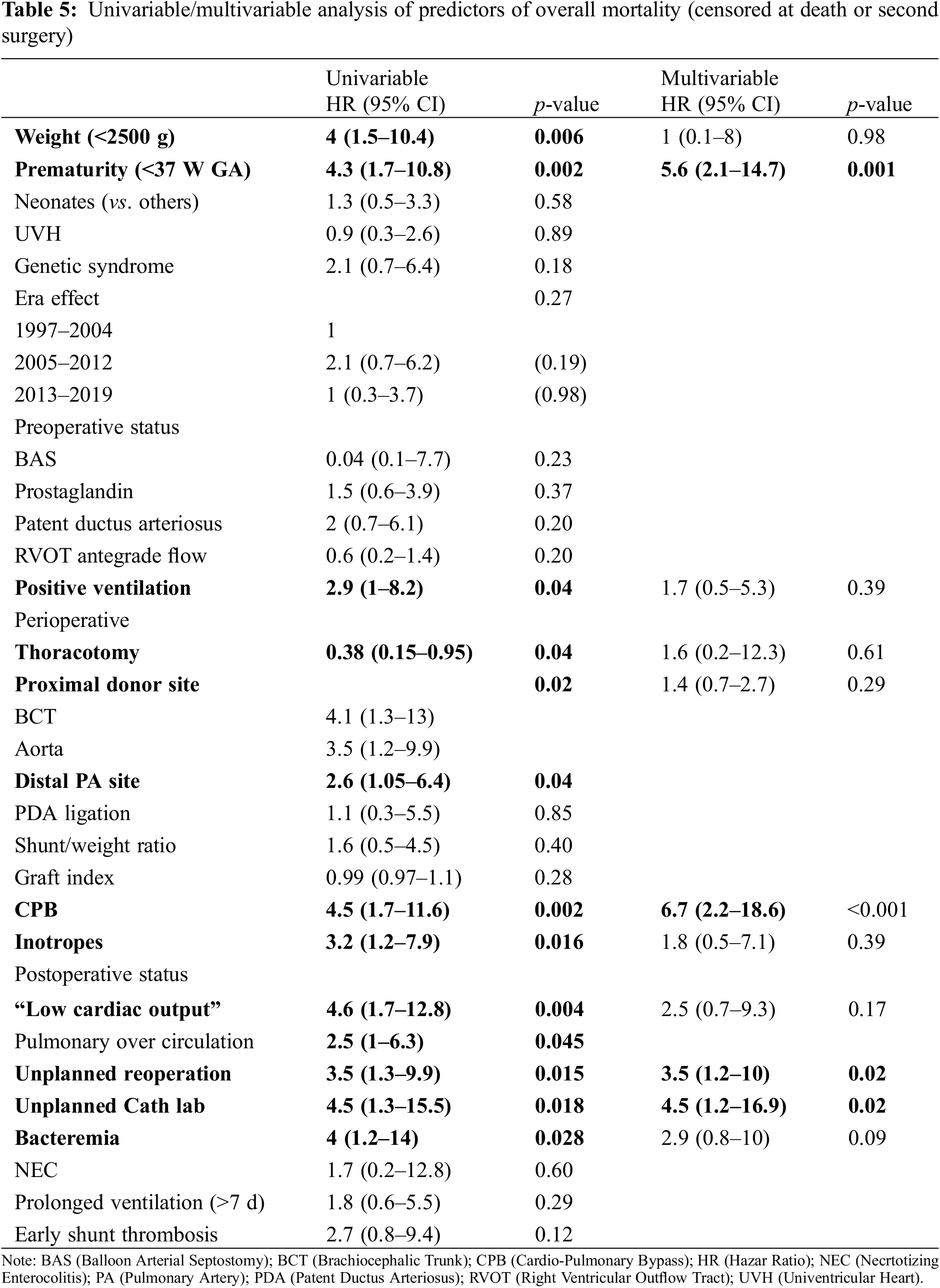
Relative to patient condition prior to surgery, positive ventilation (HR 2.9, 95CI [1–8.2]) (p = 0.04) was the only significant predictor of death.
Among the operative variables, surgical approach (HR 0.4, 95CI [0.15–0.95]) (p = 0.04), proximal site of shunt implantation (p = 0.02), distal site of shunt implantation (HR 2.6, 95CI [1.05–6.4]), the need for CPB (HR 4.5, 95CI [1.7–11.6]) (p = 0.002), the need for vasoactive inotropic support (HR 3.2, 95CI [1.2–7.9]) (p = 0.016), were all strong predictors of death.
Among other variables, intraoperative ductus ligation (p = 0.85), the shunt/weight ratio (p = 0.40) and the graft index (p = 0.28) did not influence early survival. Though not statistically significant, we encountered a two-fold increase in mortality in patients with a shunt-to-weight ratio (SWR) >1.4 (12.5 vs. 6.7%, p = 0.20).
Post-operatively, POC (HR 2.5, 95CI [1–6.3]) (p = 0.045), LCOS (HR 4.6, 95CI [1.7–12.8]) (p = 0.004), bacteremia (HR 4, 95CI [1.2–14]) (p = 0.028), unplanned reoperation (HR 3.5, 95CI [1.3–9.9]) (p = 0.015) or catheterization (HR 4.5, 95CI [1.3–15.5]) (p = 0.018) were all significant predictors of death. Neither prolonged ventilation (HR 1.8, 95CI [0.6–5.5]) (p = 0.29) nor early shunt thrombosis (HR 2.7, 95CI [0.9–10.3]) (p = 0.09) were found to be significant.
In multivariable analysis, prematurity (HR 5.6, 95CI [2.1–14.7], p = 0.001), the need for CPB (HR 6.7, 95CI [2.2–18.6]) (p < 0.001), unplanned reoperation (HR 3.5, 95CI [1.2–10]) (p = 0.02) or catheterization (HR 4.5, 95CI [1.2–17]) (p = 0.02) remained the only predictors of in-hospital death (Table 5).
Following hospital discharge, late shunt thrombosis occurred in 5 patients (2.9%). The rate of inter-stage reoperations and catheterization were 15.4% (27/175) and 13.7% (24/175).
Additional shunt reinterventions were mostly related to the development of pulmonary coarctation (n = 14) or to delay complete repair surgery in complex CHD (n = 10).
As for catheterization, angioplasty or stenting of pulmonary arteries was required in 19 patients (12.3%).
Four patients died during the inter-stage period (2.5%), two were shunt-unrelated (viral pneumonia, n = 1 and recurrence of pulmonary vein stenosis after total abnormal pulmonary venous return repair, n = 1) and two were shunt-related: one sudden death following shunt thrombosis and one death of “unknown cause” (with shunt and pulmonary arteries thrombosis at autopsy) (1.3%).
This study underscores that despite improvements in surgical techniques and neonatal intensive care management, surgical shunt palliation in neonates and infants remains among the most life-threatening surgical cardiac procedures. Our in-hospital mortality of 8.6% is in agreement with recent series reporting from 6.5% to 12% [3,6,11,12].
The large heterogeneity of patient diagnosis across studies as well as the relative proportion of patients with univentricular heart (UVH) disease could explain the observed differences in mortality.
As reported by McKenzie et al. in 2015 [6] and by Petrucci et al. in the STS-CHSD registry [2], our data analysis on preoperative and perioperative variables highlights that the most powerful predictors of early demise were dysmaturity, weight <2.500 g at surgery, ventilator-dependency, need for CPB and bacteremia. All of these are established predictors of mortality in neonatal surgery, regardless of the placement of a systemic-to-pulmonary shunt [13].
More specifically, our study underscores that some surgical technical aspects also influenced the early outcome: sternotomy and central shunt originating from the ascending aorta increased the in-hospital mortality by 2- to 3-fold, independent of the site of distal anastomosis (RPA or MPA). Of note, CPB was used in 37% of patients when the aorta was the inflow site (vs. 13% in the entire cohort). We speculate that it indirectly reflected either the size of the donor site, the size of the branch pulmonary arteries, or the clinical status of the patient at the time of surgery. McKenzie et al. [6] reported similar findings in their sternotomy group (HR of in-H death = 3.2).
In contrast, our study did not support the negative role of a higher graft index reported by Sasikumar et al. [3]. Our cohort had a median graft index of 58 mm2/m2 (only 26 patients receiving a 3.5 mm shunt). In their series, the graft index was 44.4 mm2/m2 and the most frequent graft size was 3.5 mm. It is likely that with our favored surgical approach (74% of thoracotomy), a longer shunt resulted in greater resistance to the blood flow, which could explain these contradictory results.
In earlier reports, a higher SWR was also found to have a negative effect on early survival.
However, larger shunts were implanted in those studies (62% 5-mm shunt or greater in Bove’s series) [12]. Our data did not corroborate their findings, and the few patients with very high SWR in our cohort (17 patients with SWR >1.6) limited the power of our statistical analysis. Indeed, over the years, we opted to move away from oversizing shunts as reflected by a SWR of 1.25 and a shunt-to-PA size of 1 for our entire cohort.
LCOS and POC were both predictors of early death in univariable analysis, but not confirmed in multivariable analysis. It is likely that the statistical weight of CPB use (HR 6.7) overshadowed the former variables as they occurred mostly in neonates operated under CPB.
Consistent with Dorobantu et al. [11], we found that unplanned reoperation and/or catheterization were strong predictors of in-hospital mortality in uni- and multi-variable analysis. They were required in 9.1% (<30-days reoperation) and 5.1% (<30-days catheterization), respectively, which compares favorably to other series [3].
Importantly, early shunt thrombosis was only of borderline significance in univariable analysis. It is likely that the proportion of patients with residual antegrade pulmonary blood flow (55%) reduced the clinical impact of shunt thrombosis in this cohort.
The rates of inter-stage reoperations and catheterization were 15.4% (27/175) and 13.7% (24/175), and are in agreement with the literature [3,11]. Late shunt thrombosis occurred in 5 patients (2.9%).
In this study, inter-stage mortality was 2.3% (4 patients) with two shunt-related deaths (1.1%), a lower rate compared to the 5%–6% recently reported [3,12]. As mentioned earlier, residual antegrade pulmonary blood flow in more than half of our patients, a lower proportion of UVH diagnosis (29%), and a low threshold to stent a previously placed MBTS when desaturation was noticed could all have contributed to those favorable results.
Finally, as neonatal ductal stenting has emerged as an alternative to initial surgical palliation [14], we as surgeons should support this developing field of interventional cardiology for those neonates who pay the highest burden to surgical shunt palliation. Based on our data, most efforts should be directed towards those low-weight neonates (<2500 g) with/without prematurity in whom a surgical shunt interposition could not be contemplated without the use of concomitant CPB.
Finally, at a time when congenital cardiac surgery programs are initiated in lower-income developing countries, our study corroborates the fact that surgical shunt palliation remains a potentially life-threatening procedure. Such mortality figures could be their optimal target when self-reflecting on their own experience [15]. It could help those pioneering centers to find ways to improvement in surgical palliation, or to stimulate them to embrace alternative palliation procedures as currently proposed by many interventional cardiologists [9,10,14].
The small number of patients, the extensive period of recruitment and shunt palliation being performed by only two experienced surgeons may not allow us to extrapolate our results.
As mentioned in the discussion, our series included a larger proportion of patients with bi-ventricular anatomy, as well as numerous patients with some degree of antegrade pulmonary blood flow. Both characteristics could have influenced our results in terms of morbidity and mortality during the inter-stage period.
The current study demonstrates that early mortality remains high (8.6%) in surgical shunt palliation. Multivariable analysis identified prematurity, the need for CPB, unplanned reoperation or catheterization as strong predictors of in-hospital death. Alternative endovascular techniques of palliation should be considered in this specific subset of high-risk patients together with longitudinal follow-up studies to validate their beneficial effect.
Acknowledgement: The authors thank Dr. D. Castanares Zapatero for his help in statistical reviewing and Mrs. P. Segers for her expertise in editorial help.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: FXVV: Conceptualization, data curation, formal analysis, investigation, methodology, writing original draft. KC: Investigation, writing-review & editing. JH: Investigation, writing-review & editing. GDB: Investigation, writing-review & editing. JER: Investigation, writing-review & editing. MM: Investigation, writing-review & editing. TD: Investigation, writing-review & editing. AJP: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, writing original draft, writing-review & editing.
Availability of Data and Materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics Approval: This retrospective analysis was approved by our Institutional Ethical Board (Cliniques Universitaires Saint-Luc (CUSL), Brussels, Belgium, IRB 2015\11-01\ID 327).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/chd.2023.042344.
References
1. Holtby, H. M. (2014). Anesthetic considerations for neonates undergoing modified Blalock-Taussig shunt and variations. Paediatric Anaesthesia, 24(1), 114–119. [Google Scholar] [PubMed]
2. Petrucci, O., O’Brien, S. M., Jacobs, M. L., Jacobs, J. P., Manning, P. B. et al. (2011). Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Annals of Thoracic Surgery, 92(2), 642–651. [Google Scholar] [PubMed]
3. Sasikumar, N., Hermuzi, A., Fan, C. S., Lee, K. J., Chaturvedi, R. et al. (2017). Outcomes of Blalock-Taussig shunts in current era: A single center experience. Congenital Heart Disease, 12(6), 808–814. [Google Scholar] [PubMed]
4. Dirks, V., Pretre, R., Knirsch, W., Valsangiacomo Buechel, E. R., Seifert, B. et al. (2013). Modified Blalock Taussig shunt: A not-so-simple palliative procedure. European Journal of Cardiothoracic Surgery, 44(6), 1096–1102. [Google Scholar] [PubMed]
5. Patregnani, J. T., Sochet, A. A., Zurakowski, D., Klugman, D., Diab, Y. et al. (2018). Cardiopulmonary bypass reduces early thrombosis of systemic-to-pulmonary Artery shunts. World Journal of Pediatric and Congenital Heart Surgery, 9(3), 276–282. [Google Scholar]
6. McKenzie, E. D., Khan, M. S., Samayoa, A. X., Vener, D. S., Ishak, Y. M. et al. (2016). The Blalock-Taussig shunt revisited: A contemporary experience. Journal of American College of Surgeons, 216(4), 699–704. [Google Scholar]
7. Alwi, M., Mood, M. C. (2013). Stenting of Lesions in patent ductus arteriosus with Duct-Dependent pulmonary blood flow: Focus on case selection, techniques and outcome. Interventional Cardiology Clinics, 2(1), 93–113. [Google Scholar] [PubMed]
8. Quandt, D., Ramchandani, B., Stickley, J., Mehta, C., Bhole, V. et al. (2017). Stenting of the right ventricular outflow tract promotes better pulmonary arterial growth compared with modified Blalock-Taussig shunt palliation in tetralogy of Fallot-type Lesions. JACC: Cardiovascular Interventions, 10(17), 1774–1784. [Google Scholar] [PubMed]
9. Bauser-Heaton, H., Qureshi, A. M., Goldstein, B. H., Glatz, A. C., Ligon, R. A. et al. (2022). Comparison of patent ductus arteriosus stent and Blalock-Taussig shunt as palliation for neonates with sole source Ductal-Dependent pulmonary blood flow: Results from the congenital catheterization research collaborative. Pediatric Cardiology, 43(1), 121–131. [Google Scholar] [PubMed]
10. Tseng, S. Y., Truong, V. T., Peck, D., Kandi, S., Brayer, S. et al. (2022). Patent ductus arteriosus stent versus surgical aortopulmonary shunt for initial palliation of cyanotic congenital heart disease with Ductal-Dependent pulmonary blood flow: A systematic review and meta-analysis. Journal of The American Heart Association, 11(13), e024721. [Google Scholar] [PubMed]
11. Dorobantu, D. M., Pandey, R., Sharabiani, M. T., Mahani, A. S., Angelini, G. D. et al. (2016). Indications and results of systemic to pulmonary shunts: Results from a national database. European Journal of Cardiothoracic Surgery, 49(6), 1553–1563. [Google Scholar] [PubMed]
12. Bove, T., Vandekerckhove, K., Panzer, J., De Groote, K., De Wolf, D. et al. (2015). Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World Journal of Pediatric and Congenital Heart Surgery, 6(1), 67–74. [Google Scholar]
13. Curzon, C. L., Milford-Beland, S., Li, J. S., O’Brien, S. M., Jacobs, J. P. et al. (2008). Cardiac surgery in infants with low birth weight is associated with increased mortality: Analysis of the society of thoracic surgeons congenital heart database. Journal of Thoracic and Cardiovascular Surgery, 135(3), 546–551. [Google Scholar] [PubMed]
14. Glatz, A. C., Petit, C. J., Goldstein, B. H., Kelleman, M. S., McCracken, C. E. et al. (2018). Comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with Ductal-Dependent pulmonary blood flow: Insights from the congenital catheterization research collaborative. Circulation, 137(6), 589–601. [Google Scholar] [PubMed]
15. Manuel, V., Morais, H., Turquetto, A. L. R., Miguel, G., Miana, L. A. et al. (2019). Single ventricle palliation in a developing sub-saharan African country: What should be improved? World Journal for Pediatric and Congenital Heart Surgery, 10(2), 164–170. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools