 Open Access
Open Access
ARTICLE
Loss to Specialized Cardiology Follow-Up in Adults Living with Congenital Heart Disease
1 Department of Cardiology, Royal Prince Alfred Hospital, Sydney, Australia
2 Department of Cardiothoracic Surgery, Westmead Hospital, Sydney, Australia
3 Susan Wakil School of Nursing and Midwifery, The University of Sydney, Sydney, Australia
4 Sydney Medical School, The University of Sydney, Sydney, Australia
* Corresponding Author: Rachael Cordina. Email:
Congenital Heart Disease 2024, 19(1), 49-63. https://doi.org/10.32604/chd.2023.044874
Received 10 August 2023; Accepted 27 September 2023; Issue published 20 March 2024
Abstract
Background: Much has been written about the loss to follow-up in the transition between pediatric and adult Congenital Heart Disease (CHD) care centers. Much less is understood about the loss to follow-up (LTF) after a successful transition. This is critical too, as patients lost to specialised care are more likely to experience morbidity and premature mortality. Aims: To understand the prevalence and reasons for loss to follow-up (LTF) at a large Australian Adult Congenital Heart Disease (ACHD) centre. Methods: Patients with moderate or highly complex CHD and gaps in care of >3 years (defined as LTF) were identified from a comprehensive ACHD database. Structured telephone interviews examined current care and barriers to clinic attendance. Results: Overall, 407 (22%) of ACHD patients (n = 1842) were LTF. The mean age at LTF was 31 (SD 11.5) years and 54% were male; 311 (76%) were uncontactable. Compared to adults seen regularly, lost patients were younger, with a greater socio-economic disadvantage, and had less complex CHD (p < 0.05 for all). We interviewed 59 patients (14%). The top 3 responses for care absences were “feeling well” (61%), losing track of time (36%), and not needing follow-up care (25%). Conclusions: A large proportion of the ACHD population becomes lost to specialised cardiac care, even after a successful transition. This Australian study reports younger age, moderate complexity defects, and socio-economic disadvantage as predictive of loss to follow-up. This study highlights the need for novel approaches to patient-centered service delivery even beyond the age of transition and resources to maintain patient engagement within the ACHD service.Keywords
Nomenclature
| ACHD | Adult Congenital Heart Disease |
| ADL | Activities of daily living |
| LTF | Lost to follow-up |
Adults with congenital heart defects experience longer and fuller lives credited to advances in prenatal screening and medical and surgical care. Now more adults live with ACHD than children, and they continue to survive, with complex medical and psychosocial needs [1–4]. An essential part of living longer with ACHD is the requirement for consistent life-long follow-up with specialized clinicians to recognize and address progressive changes associated with cardiac defects [5,6]. The frequency of specialist care generally varies from every one to three years, depending on the ACHD classification.
Loss to follow-up (LTF), also referred to as gaps in care or lapses in specialty care, are absences from a specialized congenital healthcare provider for three or more years [7]. Patients with gaps in their specialist care are more likely to experience higher morbidity and mortality rates than those with regular, guideline-directed care [8,9]. Without continuous specialized care, there is more preventable death, loss of cardiac function, reduced life expectancy, unnecessary intervention, and delays in timely intervention [10–13]. Ultimately these factors lead to unplanned or emergency treatment in hospitals, increased health resource utilization, and poor quality of life [14,15]. Loss to follow-up rates differs between regions ranging from 4% to 63% of people with ACHD internationally, predominantly reported after the transition from paediatric care [16–19]. No studies examine ACHD follow-up experiences post-transition, within a universal health care system, thus highlighting the research gap addressed by this Australian study. In this study, we identify the prevalence, causes, and predictors of gaps in specialist ACHD care in an outpatient cohort. We examine the proportion of patients lost to follow-up, the barriers to care, and the reasons for return to care.
We conducted a single-centre cohort study of the largest Adult Congenital Heart Disease (ACHD) service, embedded in a tertiary referral centre in New South Wales, Australia.
The outpatient clinic receives medical referrals for adults with ACHD, from paediatric and adult generalists and specialists, encompassing a population of 8.16 million people over a geographical area of 801,150 km².
All adults registered to the ACHD clinic database with moderately or severely complex anatomy were included in the study. ACHD anatomical complexities were categorized according to the 2018 AHA Guidelines. Researchers examined database attendance records in May 2019 to identify patients who had not attended follow-up at the clinic for three years or more. Follow-up attendance was determined by the frequency of care plans documented in the medical records. Patients were excluded if they were non-English speaking, aged 16 years or younger, intellectually disabled or had ACHD anatomy classified as simple complexity. We also excluded people over 75 years as that group were confounded by other issues such as nursing home placement and difficulty travelling to appointments. This study was approved by the Human Research and Ethics Committee Sydney Local Health District (Protocol X18-0189 HREC/11/RPAH/625). Additionally, all people surveyed provided informed verbal consent at the time of telephone contact.
Loss to follow-up was defined as absence from any form of ACHD cardiology care for greater than 3 years or being unable to be located. Patients were telephoned at various times of the day and evening, across all weekdays, including weekends. Voicemail or text messages were left on valid numbers to locate and engage missing patients. Further hand searching of electronic and paper medical records for alternate contacts was undertaken for patients with invalid contact details.
Patients who gave verbal consent were invited to participate in the survey which was conducted by an experienced clinician not working at the ACHD clinic to reduce response bias. Telephone questionnaires were administered between May 2019 and May 2020 to understand current care, barriers to clinic attendance and to offer specialty care reconnection if desired. Participants without a current ACHD specialist were invited to return to the adult CHD clinic or another appropriate specialist centre. The inclusion process is shown in Fig. 1.
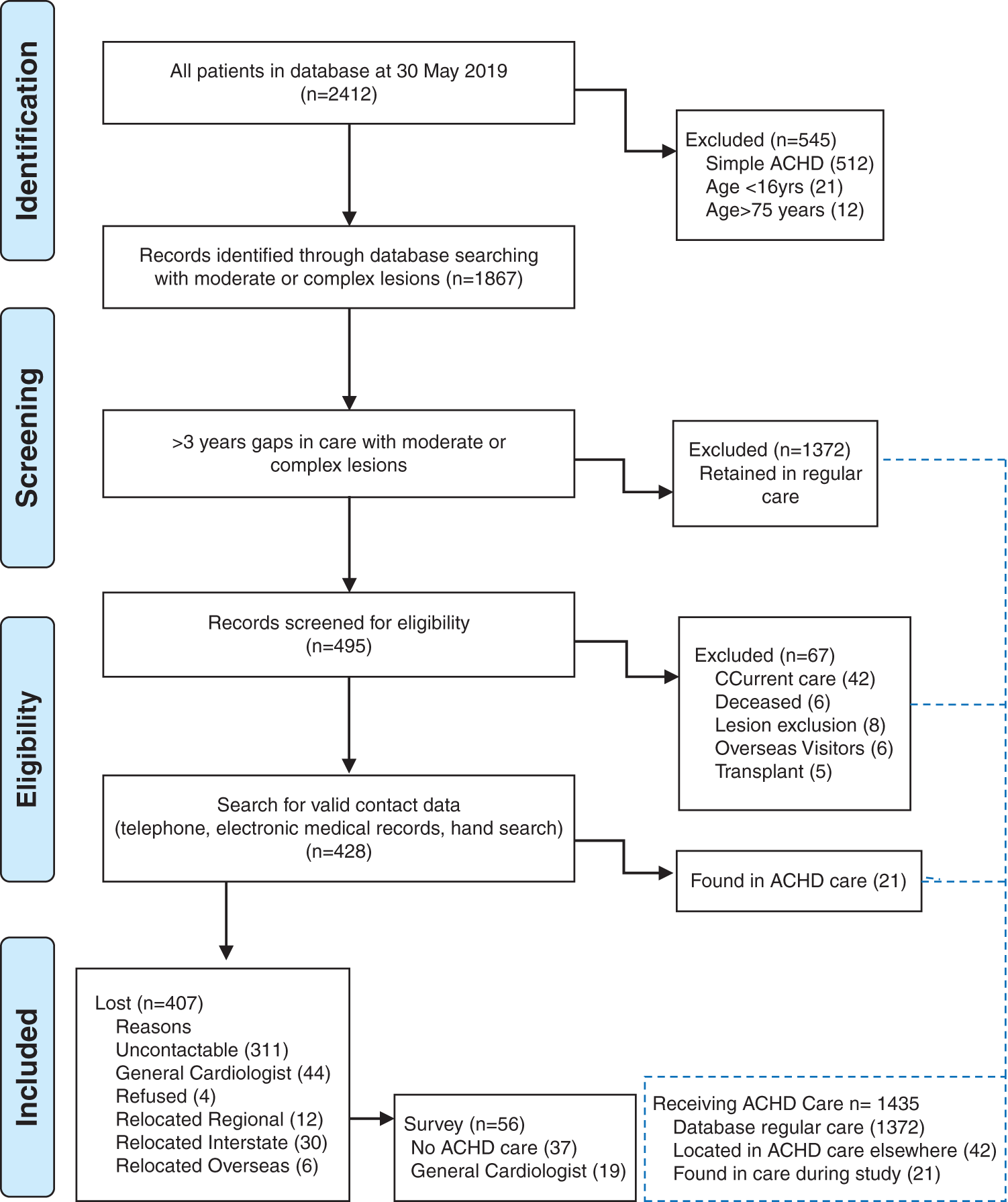
Figure 1: Flowsheet with screening for gaps in care, eligibility and inclusion for survey
Data were collected directly from the database, electronic medical record (eMR) and survey tool. Demographic and clinical characteristics of the patients were collected, including gender, age at last clinic review, socio-economic status, geographical location and ACHD anatomy. Socio-economic status was sourced from the Socio-Economic Indexes for Areas (SEIFA) by the Australian Bureau of Statistics that ranks areas in Australia according to relative socio-economic advantage and disadvantage [20]. An area with a high score on this index has a relatively high incidence of advantage and a relatively low incidence of disadvantage. Geographical remoteness was derived from the Accessibility/Remoteness Index of Australia (ARIA) which categorizes areas according to their distance from “service centers” [21]. ARIA defines five categories of remoteness from very remote, remote, outer regional, inner regional to major city by utilizing postcodes. Remote and very remote categories were condensed.
The survey tool was adapted from the HEART-ACHD trial published by Gurvitz et al. [18], to assess reasons for returning to or abstaining from specialized ACHD care. The self-reported survey of approximately ten (10) minutes duration was administered over the telephone to consenting people. The survey examined demographics (7 fields; gender, nationality, ethnicity, education level, English fluency, language spoken at home, independence in ADL), ACHD care arrangements (5 fields with yes, no or numeric responses); reasons for absence from care (Likert 6 points scale with 19 items). People were asked to rate their reasons for ceasing ACHD care from 5 strongly agree to 1 strongly disagree and 0 as not relevant if no choices were appropriate. Patients who had returned to general cardiology care were asked what prompted this to occur (Likert 6 points scale with 14 items). The results are reported as frequencies.
Data were analyzed using IBM SPSS Statistics version 27. Data are presented as means and standard deviation (SD) or frequency and proportions. Characteristics of the subjects were compared using t-tests for continuous variables if normally distributed, Mann-Whitney if non-normally distributed and categorical data were analyzed using Pearson’s χ2 test and two sample z tests (https://epitools.ausvet.com.au/ztesttwo).
The predictors of loss to follow-up were examined with univariable and multivariable analyses using Cox Hazard regression. The Cox Regression was modelled with the event LTF from the last clinic review date, time as the years in care and covariates included gender, geographical remoteness, socio-economic disadvantage and ACHD lesion. Age was not included as a covariate as it linked to the “event” and forms part of the model and consequently every iteration of analysis shows age as having a very strong relationship. All variables were retained in the model to identify the multivariate predictors.
Qualitative comments from the participant survey were analyzed thematically and patterns reported.
In May 2019, a total of 2412 records were extracted from the database. The records were screened to exclude all patients with simple complexity disease or in current care. Four hundred and ninety-five people were identified as being lost to follow-up and are the subjects of this study, as shown in Fig. 1.
3.1 Prevalence of Loss to Follow-Up
Of people with care gaps in our database, 407 were lost to follow-up. This translates to 22% of our cohort.
3.2 Characteristics of Lost to Follow-Up
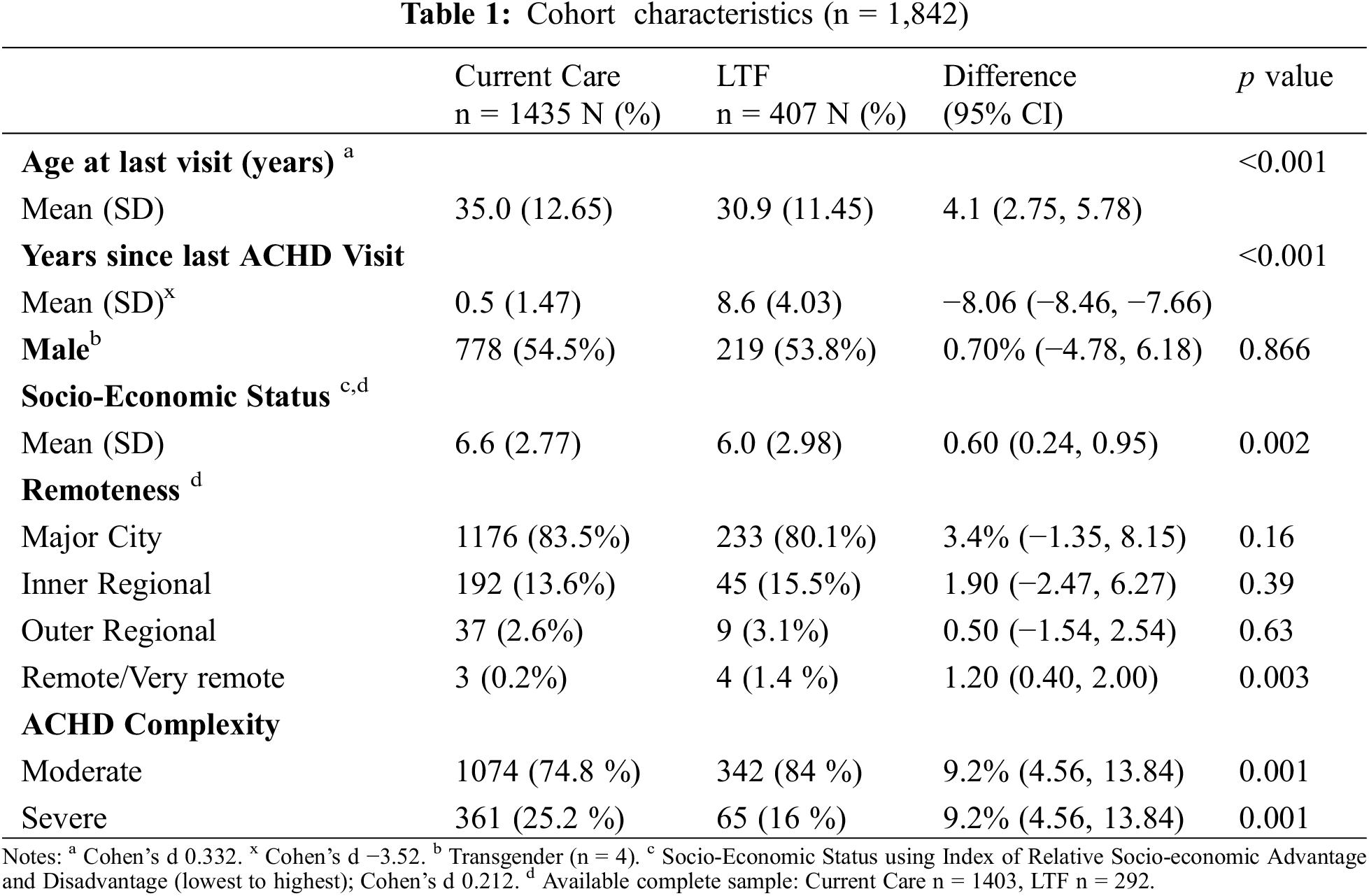
We compared the patients with LTF to those in current care and found differences between the groups in age, socio-economic status, remoteness and disease complexity (Table 1). Patients LTF were younger (30.9, SD 11.4 years) than those in current care (35.0, SD 12.65 years) based on age at their last clinic visit (p < 0.001). There were no differences in gender between the groups; males with LTF and those in current care were 53.8% and 54.5% (p = 0.87), respectively. Geographical remoteness scores showed some differences between the groups, with the majority of people in both groups residing close to a major city (80.1 % vs. 83.5%, p = 0.16). A higher proportion of LTF compared to current care resided in remote/very remote areas (1.4% vs. 0.2%, p = 0.003). We found that patients LTF had lower relative socio-economic advantage and disadvantage indices (6.0 SD 2.98) compared to those in the current care group (6.6 SD 2.77, p = 0.002). In the moderate ACHD category, more people were LTF than in current care (84% vs. 74.8%, p < 0.001), while in the severe category, more were in current care than LTF (25.2% vs. 16%, p < 0.001).
3.3 Predictors of Loss to Follow-Up
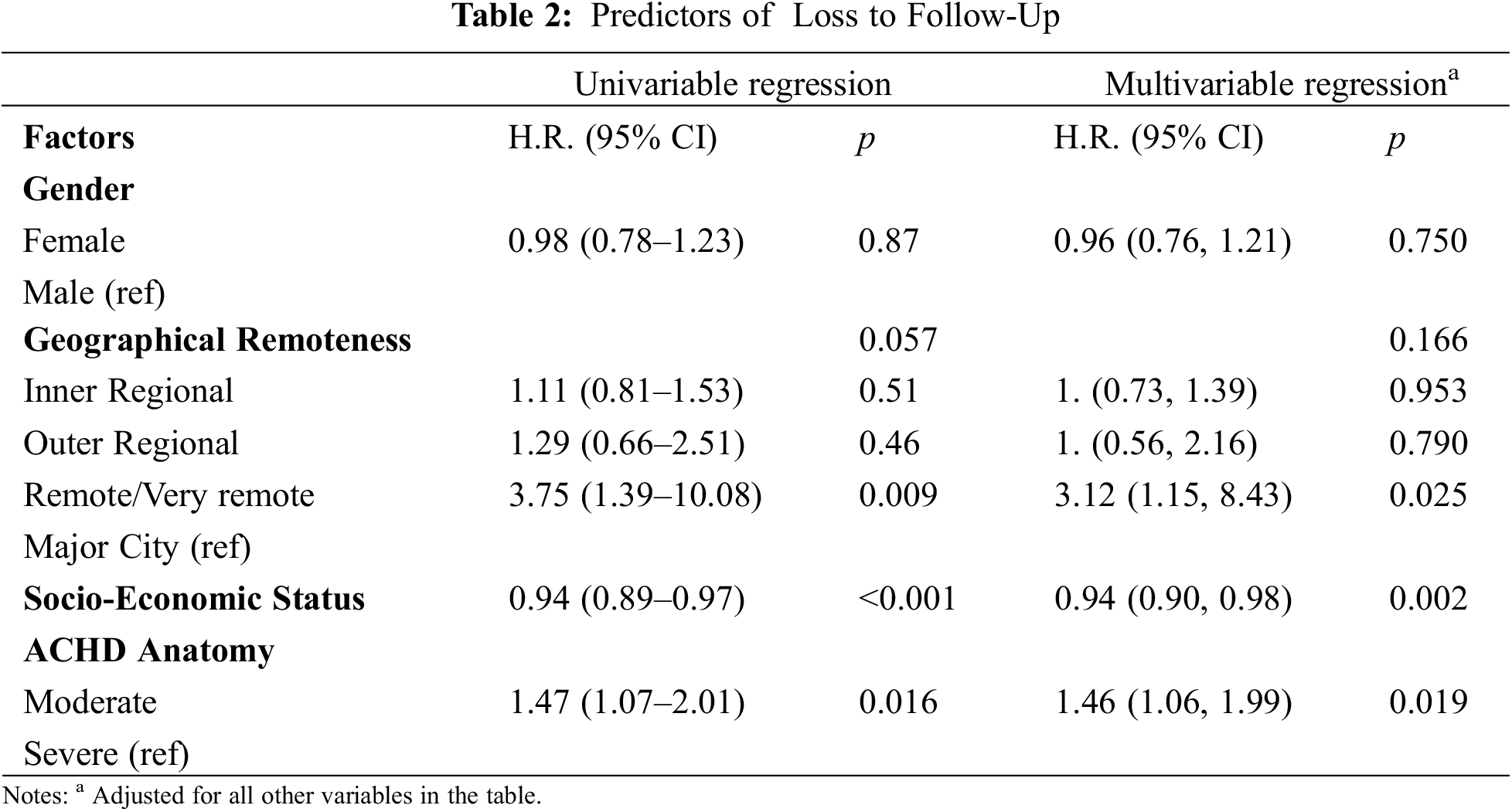
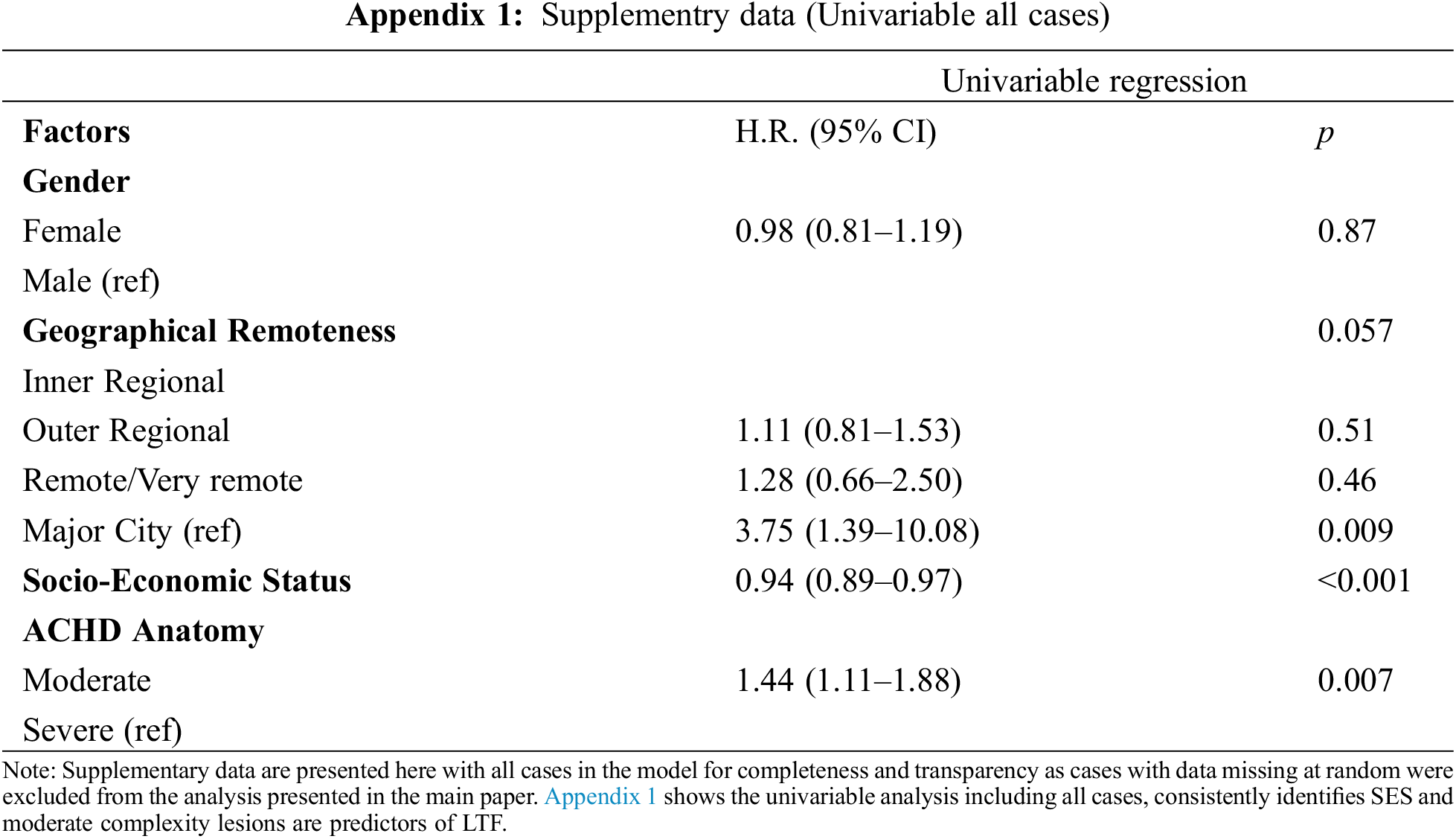
Predictors of loss to follow-up in our cohort are detailed in Table 2. The same variables were predictive in both the univariable and multivariable analyses. People who lived in remote/very remote areas were 3.12 (95% CI: 1.15, 8.43) times more likely to be lost to follow-up than those living in a major city. The risk of becoming lost to follow-up reduced by 6% (95% CI: 0.90, 0.98) for every one-point increase in the level of socio-economic advantage (p = 0.002). People with moderately complex lesions were 1.46 (95% CI: 1.06, 1.99) times more likely to be LTF than those with severely complex lesions. Table 2 reports results calculated from cases with complete data only to enable comparison between univariate and multivariate analyses. Appendix 1 provides supplementary univariate analysis of all cases, demonstrating consistent predictors of LTF, irrespective of missing data for gender, SES and geographical remoteness.
Telephoning, digital medical record and hand searches determined the remaining care status and survey inclusions accordingly as shown in Fig. 1. More than 1500 phone calls were placed in search of lost patients and to engage the survey participants, with 3–5 phone attempts required to connect with 75% of people, 4–9 call attempts to reach 23%, and 10–15 call attempts for the remaining 1%. Fifty-six eligible participants were invited to complete the survey. Further analysis was undertaken in the survey sub-group who responded to our phone calls. Call duration averaged 10 minutes, however 39% of calls were longer due to participant comments (20–30 minutes).
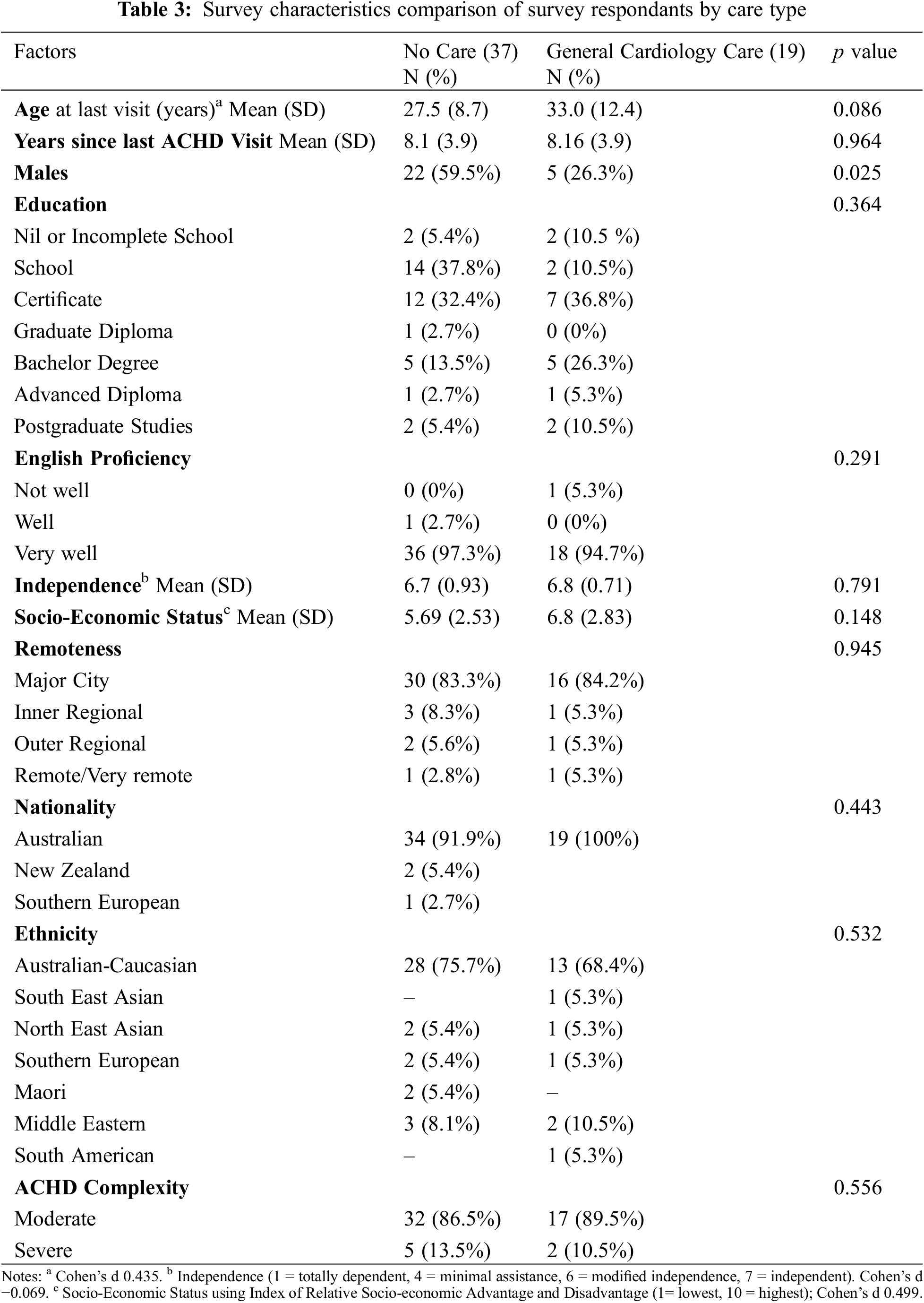
We stratified the survey responses into “no ACHD care” (n = 37) and “general cardiology” (n = 19) summarized in Table 3.
3.6 Self-Reported No ACHD Care
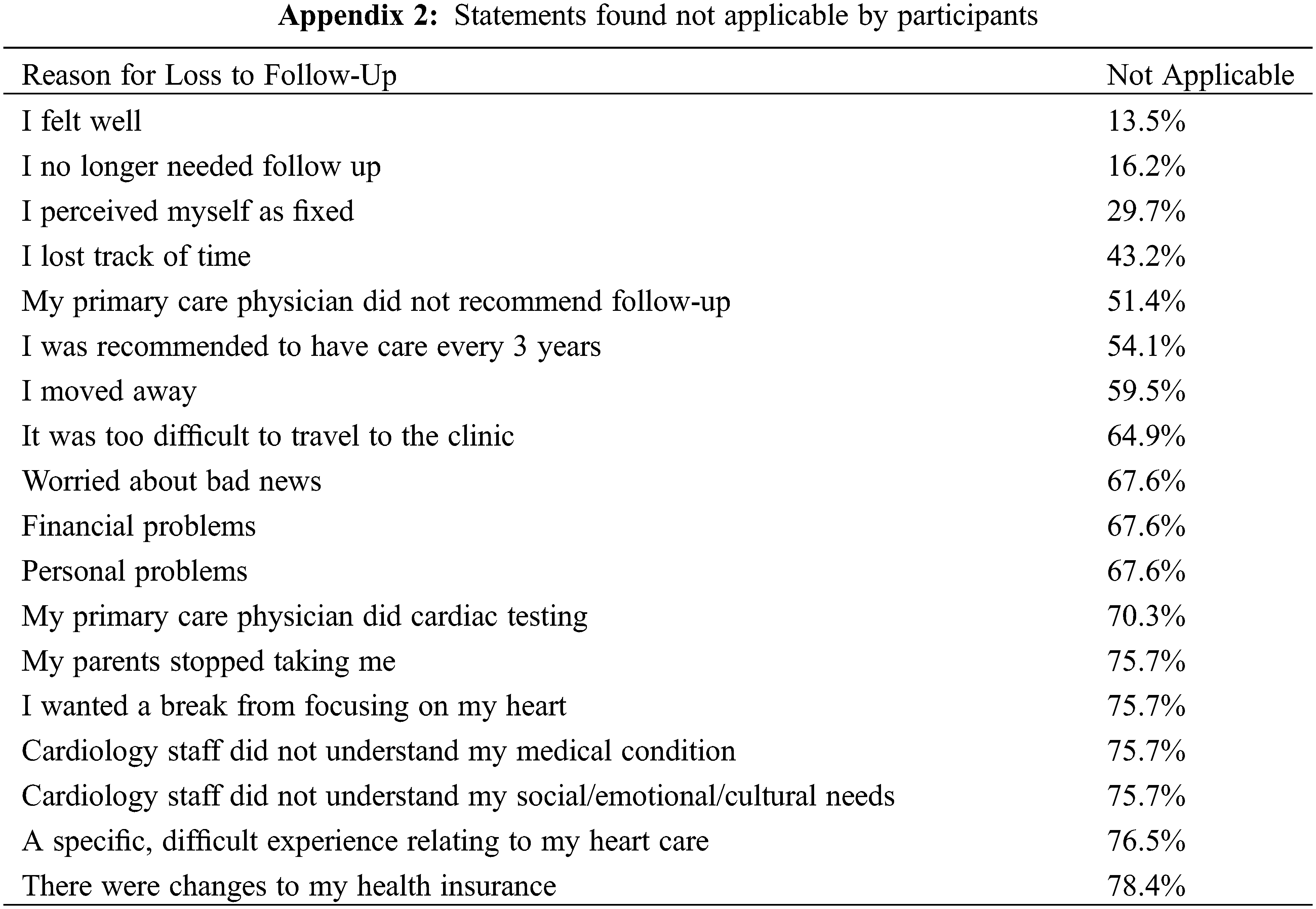
Thirty-seven people were absent from ACHD care citing positive health status as a reason; 62% stated they felt well, 35% had lost track of time and 24% believed they no longer needed follow-up. A further 24% stated their GP did not recommend any ongoing cardiac care and 19% perceived their condition to be fixed, as seen in Fig. 2. Several people were worried about receiving bad news (11%), had moved away from the ACHD centre (11%) and had been recommended to have care every 3 years (19%). There were several statements that did not resonate with the cohort and deemed “not applicable” outlined in Appendix 2.
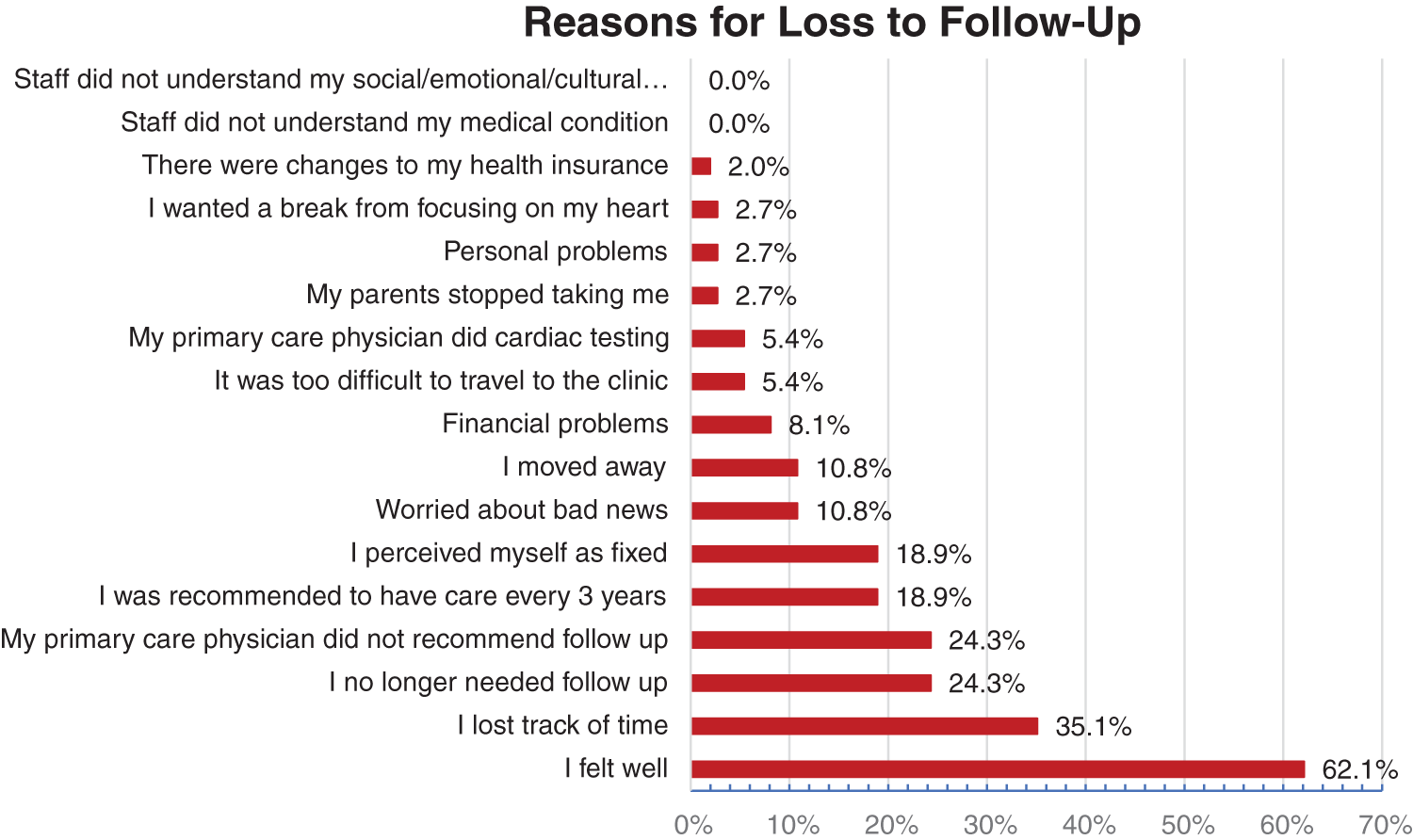
Figure 2: Reasons for loss to follow-up
% Agree and Strongly Agree with reasons for loss to follow-up statements.
3.7 Self-Reported General Cardiology Care
General cardiology care was motivated by several factors shown in Fig. 3 with recommendation from other health care providers (32%) and a desire to prevent potential problems (32%) being the top responses for statement agreement. The onset of new symptoms or health problems (16%), an interest in getting pregnant (11%), concern about deterioration (5%) and an emergency department visit (5%) were the only other affirmative responses.
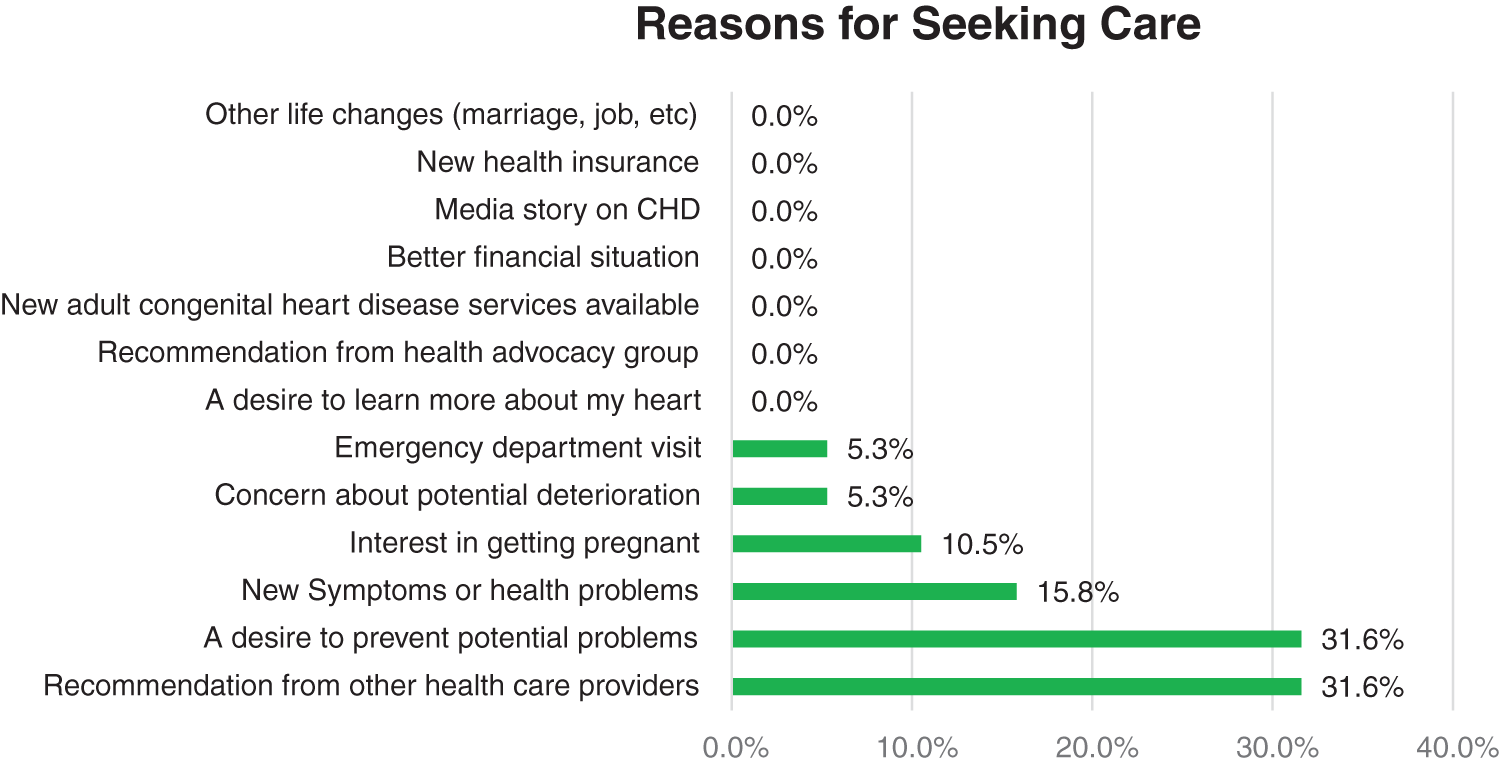
Figure 3: Reasons for returning to general cardiology care
% Agree and Strongly Agree with the return to care statements.
As with the “no care” group, a number of statements did not resonate with the cohort and deemed “not applicable” outlined in Appendix 3.
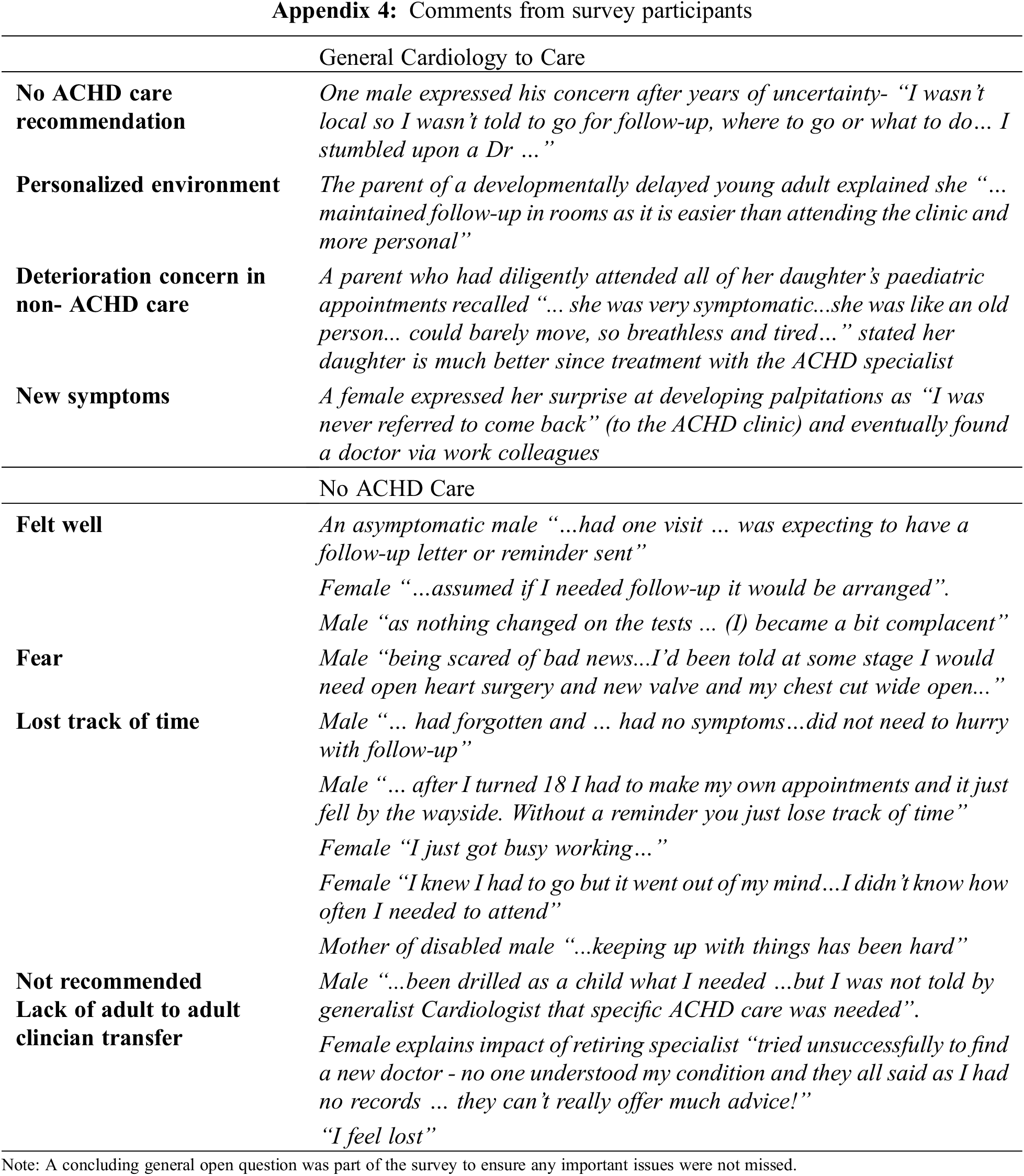
Self-reports showed that 34% were being seen by a general cardiologist, the remaining 66% were not under any care. People reported independently finding a general cardiology specialist, with some selected because they were local or offered private rooms consultations which were preferable for some to a public clinic. Several people shared their fears of surgery, previous bad experiences, lack of understanding, the difficulty of navigating their own way back to care, re-engaging in generalist care rather, as an alternative to no ongoing cardiac care in Appendix 4.
Our study examined the prevalence and predictors of LTF in an Australian outpatient ACHD population, finding that 22% of patients with moderate or severely complex ACHD were lost. Whilst in the lower range when compared to European, Canadian, and American studies reporting LTF at 4–61% [17,18,22–24], it is concerning that one-fifth of people did not access guideline-based care.
Predictors for loss to follow-up included younger age, moderately complex lesions, socio-economic disadvantage, and geographical remoteness which are consistent with other studies [10,18,25–27]. In our ACHD cohort LTF occurred in young people in their early 30s who had already transitioned from pediatric care to our ACHD centre. Although the transition is a well-recognized time of risk for LTF to occur [27–30] our data suggest that measures are important throughout life to maintain engagement in ACHD care.
Our study highlights that even adults with a high school education, living close to a major city, and with English language skills are not being retained in care. People with moderately complex lesions were more likely to experience LTF compared to people who had more severe disease. The reasons for this observation are likely multifactorial, due to periods of “wellness” between interventions, less health anxiety, and non-ACHD clinicians providing care. Gender did not influence loss to follow-up in our study, although previous studies have shown higher care gaps in males [24,29]. Low SES scores predicted LTF, likely reflecting the impact of disadvantage in terms of people’s health literacy and social resources. Geographic remoteness was a predictive factor for people in living remote/very remote locations although the majority resided in major cities. Australia is not densely populated, and patients travel considerable distances that exceed many European countries’ land borders to access specialized services [31,32]. This may be related to the fact that we only considered patients who had been seen at least once at our ACHD service-it is likely that some children had been lost to the system before reaching adult care and thus unrepresentative of the broader ACHD population in Australia, particularly considering the health disparities in rural and remote areas and amongst Aboriginal and multi-cultural people [33].
In our cohort, people did not remain engaged in ACHD care due to feeling well, because of a perception that care was not needed or losing track of time. Worldwide these are recurrent themes that reflect challenges in transition preparation, ACHD knowledge for patients as well as primary health care teams, and the need for lifelong care messaging. Lack of patient transfer by retiring clinicians was an important factor highlighted in survey comments, that led to a perception that no care was needed and subsequent failure to maintain care continuity. There is a strong likelihood that as many as 20% of lost people will eventually present as emergency admissions and be at risk for catastrophic complications due to a lack of close monitoring in specialty care [13]. Missed referral opportunities at both primary and specialist health levels to return to or retain ACHD care were highlighted by over a third of people.
Conversely, we found most people returned to care due to a recommendation from other health care providers. The ACHD health promotion role of community-based providers such as dentists, youth health and community nurses, pharmacists, and primary health may be under-recognized. For example, identification of ACHD during a health encounter could trigger an action to promote re-engagement or a referral to the nearest ACHD centre. Whilst studies have examined the economic and health benefits of shared care relationships with ACHD specialists, these have been at the medical specialist level [34,35]. In Australia, the design and implementation of an ACHD health pathway framework could provide information for general practice teams, together with patients, at the point of care to guide decisions and facilitate local ACHD service referrals. Any CHD history would activate the pathway, then recommend (via electronic record or visual aid) management, referrals, education, and resources for any patient.
The decision to return to ACHD care was not influenced by concerns about health insurance and finances, appointment schedules, or the location of the ACHD centre which differs from the findings in North America [18]. We found that several causes of LTF in USA and Canadian studies were not perceived as relevant by participants. Finances and health insurance were not reasons for an absence from care and this difference may be attributed to the universal health care system present in Australia.
Our sample is drawn from a single-site study albeit from the largest ACHD service in NSW. The LTF proportion in our study may be linked to the high volume of ACHD outpatient centre which have been shown to be predictive of care continuity [36]. It is also possible that the LTF rates are underestimated as the true prevalence of ACHD is imprecise in the absence of a national database [37–39]. Our data describes only people seen in our service and possibly does not reflect those lost from paediatric to adult care.
Retention to consistent, lifelong specialized ACHD care positively influences morbidity and mortality. Loss to follow-up has previously been demonstrated in the teenage and early 20’s population, attributed to the inadequate transition from paediatric to adult services. This study identifies that even after a successful transition; younger age, moderate complexity defects, SES disadvantage, and geographical remoteness are predictors of loss to follow-up. Fear, lack of specific health knowledge, and communication contribute to LTF. Conversely, stronger connections with knowledgeable general practitioners and uptake of consumer-orientated digital applications promoting ACHD health are potentially protective pillars [40]. Opportunities for novel communication and engagement modalities and maintaining connections between patients and providers must be explored to enhance the retention of specialty ACHD care beyond the period of transition.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Cordina and Celermajer; data collection: Dickson; analysis and interpretation of results: Dickson & Fethney; draft manuscript preparation: Dickson. All authors reviewed the results and approved the final draft.
Availability of Data and Materials: The datasets are not publicly available due to ethical considerations, though can be made available upon reasonable request.
Ethics Approval: Human Research and Ethics Committee Sydney Local Health District (Protocol X18-0189 HREC/11/RPAH/625). Additionally, all people surveyed provided informed verbal consent at the time of telephone contact.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circ. 2010;122(22):2264–72. [Google Scholar]
2. Mylotte D, Pilote L, Ionescu-Ittu R, Abrahamowicz M, Khairy P, Therrien J, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circ. 2014;129(18):1804–12. doi:10.1161/CIRCULATIONAHA.113.005817. [Google Scholar] [PubMed] [CrossRef]
3. Khan A, Gurvitz M. Epidemiology of ACHD: what has changed and what is changing? Prog Cardiovasc Dis. 2018;61(3):275–81. doi:10.1016/j.pcad.2018.08.004. [Google Scholar] [PubMed] [CrossRef]
4. Australian Institute of Health and Welfare. Congenital heart disease in Australia. Cat. no. CDK 14. Canberra: AIHW; 2019. [Google Scholar]
5. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. AHA/ACC guideline for the management of adults with congenital heart disease. J Am Coll Cardiol. 2018. doi:10.1016/j.jacc.2018.08.1029. [Google Scholar] [PubMed] [CrossRef]
6. Brida M, Šimkova I, Jovović L, Prokšelj K, Antonová P, Olga Balint H, et al. European society of cardiology working group on adult congenital heart disease and study group for adult congenital heart care in central and south eastern european countries consensus paper: current status, provision gaps and investment required. Eur J Heart Fail. 2021;23(3):445–53. doi:10.1002/ejhf.2040. [Google Scholar] [PubMed] [CrossRef]
7. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease. Syst Rev. 2015;10(5):413–27. doi:10.1111/chd.12251. [Google Scholar] [PubMed] [CrossRef]
8. Cordina R, Nasir Ahmad S, Kotchetkova I, Eveborn G, Pressley L, Ayer J, et al. Management errors in adults with congenital heart disease: prevalence, sources, and consequences. Eur Heart J. 2017;39(12):982–9. doi:10.1093/eurheartj/ehx685. [Google Scholar] [PubMed] [CrossRef]
9. Baumgartner H. Does frequently inadequate adult care threaten the outcome of congenital heart disease after successful paediatric treatment? Eur Heart J. 2018;39(12):990–2. doi:10.1093/eurheartj/ehy035. [Google Scholar] [PubMed] [CrossRef]
10. Gerardin J, Raskind-Hood C, Rodriguez F, Hoffman T, Kalogeropoulos A, Hogue C, et al. Lost in the system? Transfer to adult congenital heart disease care—challenges and solutions. Congenit Heart Dis. 2019;14(4):541–8. doi:10.1111/chd.12780. [Google Scholar] [PubMed] [CrossRef]
11. Fernandes SM, Khairy P, Fishman L, Melvin P, O’Sullivan-Oliveira J, Sawicki GS, et al. Referral patterns and perceived barriers to adult congenital heart disease care: results of a survey of U.S. pediatric cardiologists. J Am Coll Cardiol. 2012;60(23):2411–8. doi:10.1016/j.jacc.2012.09.015. [Google Scholar] [PubMed] [CrossRef]
12. Iversen K, Vejlstrup NG, Sondergaard L, Nielsen OW. Screening of adults with congenital cardiac disease lost for follow-up. Cardiol Young. 2007;17(6):601–8. doi:10.1017/S1047951107001436. [Google Scholar] [PubMed] [CrossRef]
13. Kaemmerer H, Fratz S, Bauer U, Oechslin E, Brodherr-Heberlein S, Zrenner B, et al. Emergency hospital admissions and three-year survival of adults with and without cardiovascular surgery for congenital cardiac disease. J Thorac Cardiovasc Surg. 2003;126(4):1048–52. doi:10.1016/S0022-5223(03)00737-2. [Google Scholar] [PubMed] [CrossRef]
14. Mackie AS, Tran DT, Marelli AJ, Kaul P. Cost of congenital heart disease hospitalizations in Canada: a population-based study. Can J Cardiol. 2017;33(6):792–8. doi:10.1016/j.cjca.2017.01.024. [Google Scholar] [PubMed] [CrossRef]
15. Apers S, Kovacs AH, Luyckx K, Thomet C, Budts W, Enomoto J, et al. Quality of life of adults with congenital heart disease in 15 countries: evaluating country-specific characteristics. J Am Coll Cardiol. 2016;67(19):2237–45. doi:10.1016/j.jacc.2016.03.477. [Google Scholar] [PubMed] [CrossRef]
16. Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ, et al. Children and adults with congenital heart disease lost to follow-up: who and when? Circ. 2009;120(4):302–9. [Google Scholar]
17. Wray J, Frigiola A, Bull C. Network, adult congenital heart disease research network, 2013, Loss to specialist follow-up in congenital heart disease; out of sight, out of mind. Heart. 2013;99(7):485–90. [Google Scholar] [PubMed]
18. Gurvitz M, Valente AM, Borberg C, Cook S, Kay J, et al. Alliance for Adult Research in Congenital Cardiology (AARCC) and Adult Congenital Heart Association. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61(21):2180–4. doi:10.1016/j.jacc.2013.02.048. [Google Scholar] [PubMed] [CrossRef]
19. Moons P, Skogby S, Bratt E, Zühlke L, Marelli A, Goossens E, et al. Discontinuity of cardiac follow-up in young people with congenital heart disease transitioning to adulthood: a systematic review and meta-analysis. J Am Heart Assoc. 2021;10(6):e019552. [Google Scholar] [PubMed]
20. Australian Bureau of Statistics. Socio-economic indices for areas (SEIFA) 2016; 2018. [Google Scholar]
21. Australian Bureau of Statistics. Accessibility/Remoteness Index of Australia (ARIA). Australian Bureau of Statistics. Available from: https://www.abs.gov.au/statistics/standards/australian-statistical-geography-standard-asgsedition-3/jul2021-jun2026/remoteness-structure/remoteness-areas [Accessed 2023]. [Google Scholar]
22. Caruana M, Aquilina O, Grech V. Can the inevitable be prevented?–An analysis of loss to follow-up among grown-ups with congenital heart disease in Malta. Malta Med J. 2018;30(1):13–21. [Google Scholar]
23. Gaydos SS, Chowdhury SM, Judd RN, McHugh KE. A transition clinic intervention to improve follow-up rates in adolescents and young adults with congenital heart disease. Cardiol Young. 2020;30(5):633–40. doi:10.1017/S1047951120000682. [Google Scholar] [PubMed] [CrossRef]
24. Moore JA, Sheth SS, Lam WW, Alexander AJ, Shabosky JC, Espaillat A, et al. Hope is no plan: uncovering actively missing transition-aged youth with congenital heart disease. Pediatr Cardiol. 2022;43(5):1046–53. [Google Scholar] [PubMed]
25. Goossens E, van Deyk K, Budts W, Moons P. Are missed appointments in an outpatient clinic for adults with congenital heart disease the harbinger for care gaps? Eur J Cardiovasc Nur. 2021;21(2):127–34. doi:10.1093/eurjcn/zvab012. [Google Scholar] [PubMed] [CrossRef]
26. Ko JM, Yanek LR, Cedars AM. Factors associated with a lower chance of having gaps in care in adult congenital heart disease. Cardiol Young. 2021;31(10):1576–81. doi:10.1017/S1047951121000524. [Google Scholar] [PubMed] [CrossRef]
27. Kollengode MS, Daniels CJ, Zaidi AN. Loss of follow-up in transition to adult CHD: a single- centre experience. Cardiol Young. 2018;28(8):1001–8. doi:10.1017/S1047951118000690. [Google Scholar] [PubMed] [CrossRef]
28. Kempny A, Diller GP, Dimopoulos K, Alonso-Gonzalez R, Uebing A, Li W, et al. Determinants of outpatient clinic attendance amongst adults with congenital heart disease and outcome. Int J Cardiol. 2016;203:245–50. doi:10.1016/j.ijcard.2015.10.081. [Google Scholar] [PubMed] [CrossRef]
29. Norris MD, Webb G, Drotar D, Lisec A, Pratt J, Akanbi F, et al. Risk factors associated with loss to follow-up in young adults with congeital heart disease. J Am Coll Cardiol. 2012;59(13):E790. doi:10.1016/S0735-1097(12)60791-8. [Google Scholar] [CrossRef]
30. Sonneveld HM, Strating MMH, van Staa AL, Nieboer AP. Gaps in transitional care: what are the perceptions of adolescents, parents and providers? Child: care. Health Dev. 2013;39(1):69–80. doi:10.1111/j.1365-2214.2011.01354.x. [Google Scholar] [PubMed] [CrossRef]
31. McGrath L, Taunton M, Levy S, Kovacs AH, Broberg C, Khan A, et al. Barriers to care in urban and rural dwelling adults with congenital heart disease. Cardiol Young. 2022;32(4):612–7. doi:10.1017/S1047951121002766. [Google Scholar] [PubMed] [CrossRef]
32. Salciccioli KB, Oluyomi A, Lupo PJ, Ermis PR, Lopez KN. A model for geographic and sociodemographic access to care disparities for adults with congenital heart disease. Congenit Heart Dis. 2019;14(5):752–9. doi:10.1111/chd.12819. [Google Scholar] [PubMed] [CrossRef]
33. Jackson JL, Morack J, Harris M, DeSalvo J, Daniels CJ, Chisolm DJ, et al. Racial disparities in clinic follow-up early in life among survivors of congenital heart disease. Congenit Heart Dis. 2019;14(2):305–10. doi:10.1111/chd.12732. [Google Scholar] [PubMed] [CrossRef]
34. Willems R, Ombelet F, Goossens E, de Groote K, Budts W, Moniotte S, et al. Different levels of care for follow- up of adults with congenital heart disease: a cost analysis scrutinizing the impact on medical costs, hospitalizations, and emergency department visits. Eur J Health Econ. 2021;22(6):951–60. doi:10.1007/s10198-021-01300-5. [Google Scholar] [PubMed] [CrossRef]
35. Hardy RY, Keller D, Gurvitz M, McManus B, Varda D, Lindrooth RC, et al. Patient sharing and health care utilization among young adults with congenital heart disease. Med Care Res Rev. 2021;78(5):561–71. doi:10.1177/1077558720945925. [Google Scholar] [PubMed] [CrossRef]
36. Skogby S, Moons P, Johansson B, Sunnegårdh J, Christersson C, Nagy E, et al. Outpatient volumes and medical staffing resources as predictors for continuity of follow-up care during transfer of adolescents with congenital heart disease. Int J Cardiol. 2020;310:51–7. [Google Scholar] [PubMed]
37. Nicolae M, Gentles T, Strange G, Tanous D, Disney P, Bullock A, et al. Adult congenital heart disease in Australia and New Zealand: a call for optimal care. Heart Lung Circ. 2019;28(4):521–9. doi:10.1016/j.hlc.2018.10.015. [Google Scholar] [PubMed] [CrossRef]
38. Celermajer D, Strange G, Cordina R, Selbie L, Sholler G, Winlaw D, et al. Congenital heart disease requires a lifetime continuum of care: a call for a regional registry. Heart Lung Circ. 2016;25(8):750–4. doi:10.1016/j.hlc.2016.03.018. [Google Scholar] [PubMed] [CrossRef]
39. Jenkins K, Botto LD, Correa A, Foster E. Public health approach to improve outcomes for congenital heart disease across the life span. J Am Heart Assoc. 2019;8(8):e009450. doi:10.1161/JAHA.118.009450. [Google Scholar] [PubMed] [CrossRef]
40. Bassareo PP, Chessa M, Di Salvo G, Walsh KP, Mcmahon CJ. Strategies to aid successful transition of adolescents with congenital heart disease: a systematic review. Children. 2023;10(3):423. [Google Scholar] [PubMed]
Appendices

Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools