 Open Access
Open Access
ARTICLE
Construction of Synergistic and Efficient Flame-Retardant Polyamide 6 Composites by Incorporating Aluminum Diethylphosphinate and Fly Ash
Heilongjiang Key Laboratory of Molecular Design and Preparation of Flame Retarded Materials, College of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University, Harbin, 150040, China
* Corresponding Authors: Miaojun Xu. Email: ; Xiaoli Li. Email:
; Bin Li. Email:
(This article belongs to the Special Issue: Polymer-based Functional Composite Materials: Cutting-edge Advances and Innovative Applications)
Journal of Polymer Materials 2025, 42(4), 1035-1049. https://doi.org/10.32604/jpm.2025.073108
Received 10 September 2025; Accepted 11 November 2025; Issue published 26 December 2025
Abstract
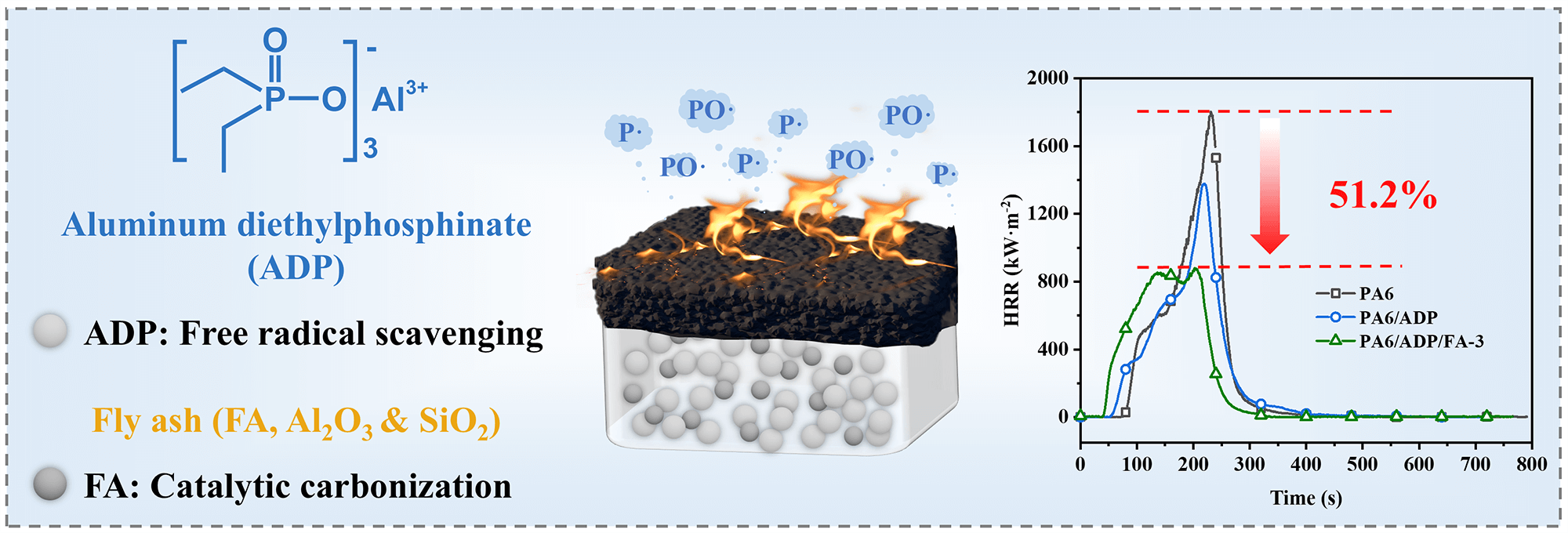
The fabrication of highly flame-retardant polyamide 6 (PA6) composites is of great significance for expanding their practical applications. Herein, a new flame-retardant system (ADP/FA) was developed by combining aluminum diethylphosphinate (ADP) with excellent flame retardancy and fly ash (FA), an economical and environmentally friendly industrial waste. Due to the synergistic flame-retardant effect of ADP/FA in the condensed phase and gas phase, the PA6 composite containing only 11 wt% of ADP/FA (mass ratio 93:7) obtained vertical burning (UL-94) tests V-0 rating with a limiting oxygen index (LOI) of 30.9%. To obtain the same flame-retardant level of PA6/ADP/FA-3, the loading amount of ADP alone was required 14 wt%. Compared with the PA6/ADP, the introduction of FA not only reduced the amount of flame retardant added but also inhibited the formation of molten droplets during combustion, greatly enhancing the fire safety of the PA6 composites. The flame-retardant performance of the ADP/FA system is superior to that of most current ADP-based synergistic strategies. In the meantime, the introduction of FA also significantly reduced the high smoke release caused by ADP flame retardant. The peak smoke production rate (pSPR) of the PA6 composite, from 0.221 m2·s−1 (PA6/ADP) to 0.116 m2·s−1, represents a 47.5% decrease. This work provides a feasible solution for fabricating PA6 composites with excellent flame retardancy.Graphic Abstract
Keywords
Polyamide 6 (PA6), as an engineering plastic, has been broadly applied to automotive industry, electrical and electronic devices, transportation and aerospace fields on account of its excellent processability, electrical insulation, corrosion resistance and superior mechanical property [1–3]. However, due to its inherent structural characteristics, PA6 exhibits high flammability and severe melt dripping accompanied by rapid flame propagation and substantial heat release, which significantly limits its applications in these fields [4–7]. Therefore, the development of PA6 composites with high flame retardancy is of critical significance for expanding their practical applications and ensuring life and property safety.
Currently, there are two primary methods for fabricating flame-retardant PA6: copolymerization and blending modification [8]. Out of consideration for cost-effectiveness and practicability, the incorporation of flame retardants into PA6 has become the dominant approach in industrial production, which has been proven to be an effective and straightforward method for enhancing its flame retardancy [9]. Various flame retardants, including halogenated [10,11], phosphorus-based [12,13], nitrogen-based [14,15], silicon-containing [16,17] and metal oxide [18,19], enhance the fire safety of materials through distinct mechanisms. Halogen-free flame retardants have gradually supplanted halogenated flame retardants due to health and environmental concerns. Owing to the high fire-resistant performance and environmental friendliness, phosphorus-based flame retardants have attracted considerable interest [20]. It generated phosphoric acid/polyphosphoric acid and phosphorus-containing free radicals during combustion, thereby exerting flame-retardant effects in both the condensed phase and gas phase [21,22]. As a phosphorus-based flame retardant with excellent thermal stability and a high phosphorus content, aluminum diethylphosphinate (ADP) has been employed in flame-retardant PA6 systems and demonstrated certain flame retardancy [23]. It primarily functions in the gas phase by decomposing to generate free radicals. Nevertheless, the lack of efficient char formation and insufficient condensed phase flame-retardant effect of ADP lead to substantial smoke generation upon burning [24,25]. Consequently, standalone application of it shows insufficient efficacy to achieve the desired flame-retardant performance.
To overcome the limitations of ADP in practical applications, it is commonly combined with synergistic agents such as melamine cyanurate (MCA) [26], melamine polyphosphate (MPP) [27], montmorillonite (MMT) [28,29], metal oxide [18] and SiO2 [30], which facilitate the development of a more stable and cohesive char layer. This strengthened its condensed-phase flame retardancy and effectively suppressed material combustion. Lu et al. [31] fabricated a flame retardant (MTP) containing phosphorus-nitrogen components through electrostatic interaction between the groups of melamine (MA) and amino trimethylene phosphonic acid (ATMP) in aqueous solution. When the mass ratio of MTP to ADP was 1:3 with a total loading of 20 wt%, the PA6 composite obtained UL-94 V-0 rating in the vertical burning tests and exhibited a limiting oxygen index (LOI) value of 28.6%. Subsequently, Lu et al. [32] further developed an environmentally friendly boron-containing route to synthesize a phosphorus-nitrogen-boron ternary compound (MBA) based on this approach. The glass-forming effect of boric acid during combustion enhanced the compactness of the char layer, enabling the PA6 composite containing 5 wt% MBA and 10 wt% ADP to attain UL-94 V-0 rating with a LOI value of 26.8%. Although the synthesized synergist displays favorable cooperative effects with ADP, its relatively high cost and limited flame-retardant efficiency require further improvement. Hence, developing a low-cost and highly flame-retardant ADP-based system remains an attractive approach to advance its practical applications.
Fly ash (FA), a fine-particulate byproduct resulting from the combustion of coal in power plants, is primarily composed of Al2O3 and SiO2 [33]. Compared with traditional inorganic hydroxides (Al (OH)3, Mg (OH)2) [34], layered double hydroxide (LDH) [35] and nano-clays [36], it has a wide range of sources, low cost, environmental friendliness, and possesses a unique hollow spherical structure. Furthermore, it facilitates the formation of a high-quality char layer during combustion. However, it should be noted that the physicochemical properties of FA such as particle size distribution, surface chemistry, and composition may vary depending on its source and combustion conditions, which could influence its dispersion and interfacial compatibility in polymer matrices. Considering these, this research prepared a new flame-retardant system (ADP/FA) for PA6 through a synergistic strategy combining FA with ADP, achieving high-efficiency flame retardancy. As a result of the gas-phase radical trapping effect of ADP, the ADP/FA system effectively suppressed flame propagation during combustion. Additionally, because of the excellent self-supporting property and catalytic char-forming capability of FA, the ADP/FA system improved the melt viscosity of the material, facilitating the development of compact and thermally stable char layer upon combustion. The collaborative flame-retardant action occurring in both the condensed and gaseous phases enabled the PA6 composite to achieve a LOI of 30.9% with merely 11 wt% ADP/FA (mass ratio 93:7) addition and reduced the peak heat release rate by 51.2%, surpassing some existing ADP-based flame-retardant systems. Besides, it effectively contained the high smoke emission associated with ADP combustion. This work provided a simple yet efficient strategy to enhance the fire safety of PA6, demonstrating significant promise for broad industrial application.
The supplier of aluminum diethylphosphinate (ADP) was Zhejiang Xinhua Chemical Co., Ltd. (Jiande, China). Fly ash (FA) was supplied by Asus Mineral Processing Plant in Lingshou County (Shijiazhuang, China). The particle size of FA is 5–50 μm and the BET value is 374 m2/kg. Polyamide 6 (PA6) was supported by DuPont Co., Ltd (Wilmington, DE, USA).
2.2 Preparation of PA6 Composites
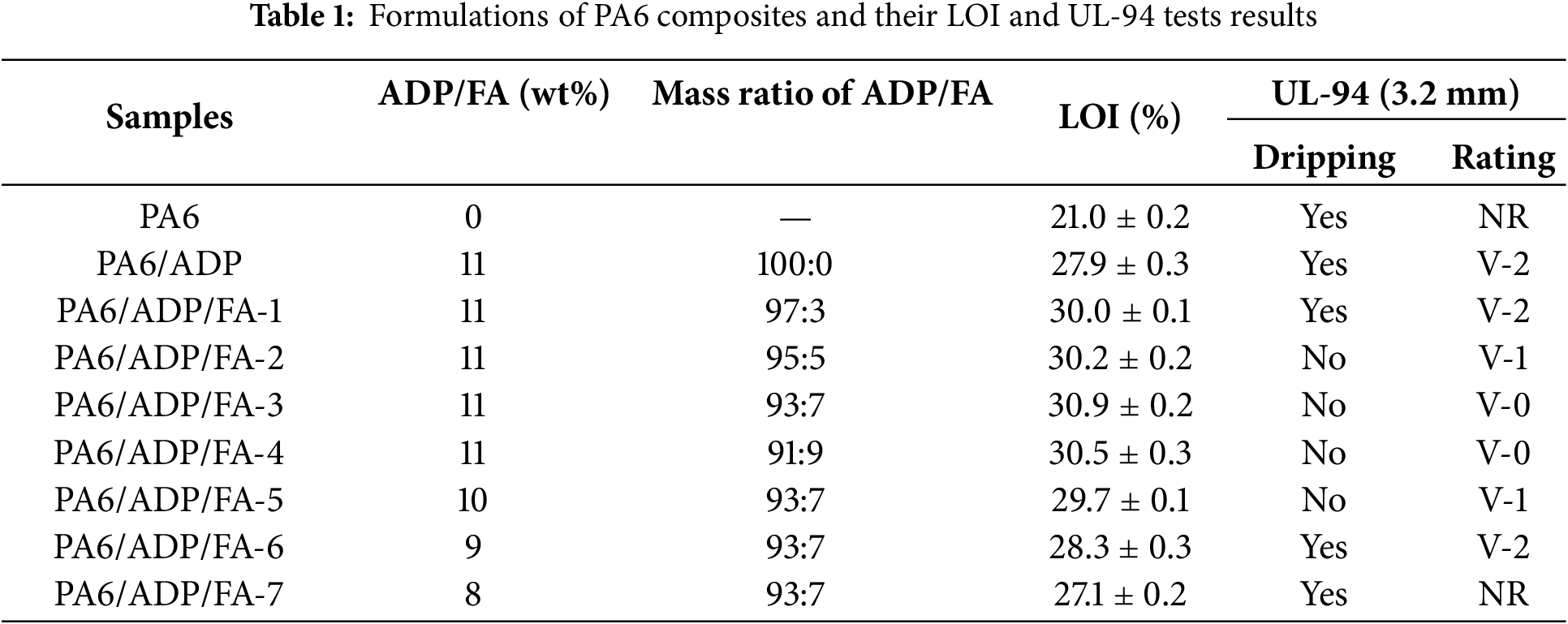
Firstly, PA6, ADP and FA were vacuum-dried at 80°C for 8 h for subsequent use. After that, a high-speed mixing blender was used to evenly combine ADP and FA in a specific ratio. A twin-screw extruder was then applied to combine the dried PA6 with the previously indicated mixture. The temperature zones from the feed zone to the end zone were set at 210°C, 230°C, 230°C, 235°C and 230°C, respectively. The shear rate was 32 s−1, the screw speed was 60 r/min, and the material residence time was 90 s. Whereafter, the extruded samples were pelletized and vacuum-dried for 8 h at 80°C. Lastly, an injection molding machine operating at 230°C was utilized to prepare the test specimens. A summary of the various sample formulations was provided in Table 1.
The thermal stability of the samples was examined using a thermogravimetric analyzer (TGA, PerkinElmer TGA 8000) in a nitrogen (N2) atmosphere at a heating rate of 10°C·min−1. The CZF-2 apparatus (Jiangning, China) was used to perform the vertical burning tests on samples that were 130 mm × 13 mm × 3.2 mm in accordance with ASTM D 3801. The JF-3 oxygen index device (Jiangning, China) was applied to test the limiting oxygen index (LOI) in accordance with ASTM D 2863. The dimension of the samples was 130 mm × 6.5 mm × 3 mm. In accordance with ISO 5660, samples with the dimension of 100 mm × 100 mm × 4 mm were subjected to the cone calorimeter test (CCT) (West Sussex, UK) at the heat flux of 50 kW·m−2 to assess their combustion behavior. Rheological measurements were conducted at 230°C using an AR2000ex rheometer with angular frequency ranging 0.1–100 s−1. Field emission scanning electron microscopy (SEM, JSM-7500F, JEOL) was employed to analyze the morphology. The Lab RAM HR800 laser Raman spectrometer (Horiba Jobin Yvon, France) was utilized to perform the Laser Raman spectra at room temperature using a 532 nm laser. X-ray photoelectron spectroscopy (XPS) was tested by Thermo Scientific K-Alpha system with a monochromated AlKα source. The gas-phase products of the samples were analyzed by using a thermogravimetric analyzer (TG 209 F3, Netzsch, Germany) in combination with an FT-IR spectrometry (TG-IR). The samples were heated from 30°C to 800°C at a rate of 10°C·min−1 under the N2 atmosphere and flow rate of 20 mL·min−1.
The thermal stability and decomposition behavior of PA6 and its composites under N2 atmosphere were studied by TGA tests, and the corresponding results were exhibited in Fig. 1 and Table 2. Pure PA6 presented excellent thermal stability with the temperature at 5 wt% mass loss (T5%) and the temperature at maximum mass loss rate (Tmax) being 397.9°C and 454.9°C, respectively. However, its char-forming ability was limited, yielding a mere 1.3% residual char at 800°C. Upon incorporation of 11 wt% ADP, the T5% and Tmax of PA6/ADP slightly decreased due to the catalytic effect of metal hypophosphite. Despite the reduction in the maximum thermal decomposition rate (Rmax) of PA6/ADP from −2.43%·min−1 of PA6 to −1.07%·min−1, the char yield showed no notable improvement. With the introduction of FA, the thermal decomposition temperature of PA6 composite further decreased, but its char yield increased to 5.0%. This suggests that the incorporation of FA enhanced the carbonization capability of PA6 composite, which is anticipated to improve its flame retardancy. It should be noted that although a kinetic analysis of thermal degradation (e.g., Kissinger, FWO, or KAS methods) could further clarify the catalytic effect of FA on the decomposition process, such modeling requires TGA data at multiple heating rates and extensive parameter fitting. Since the main purpose of this work is to demonstrate the synergistic flame-retardant mechanism of FA, the detailed kinetic modeling will be carried out in our future study for further publication.
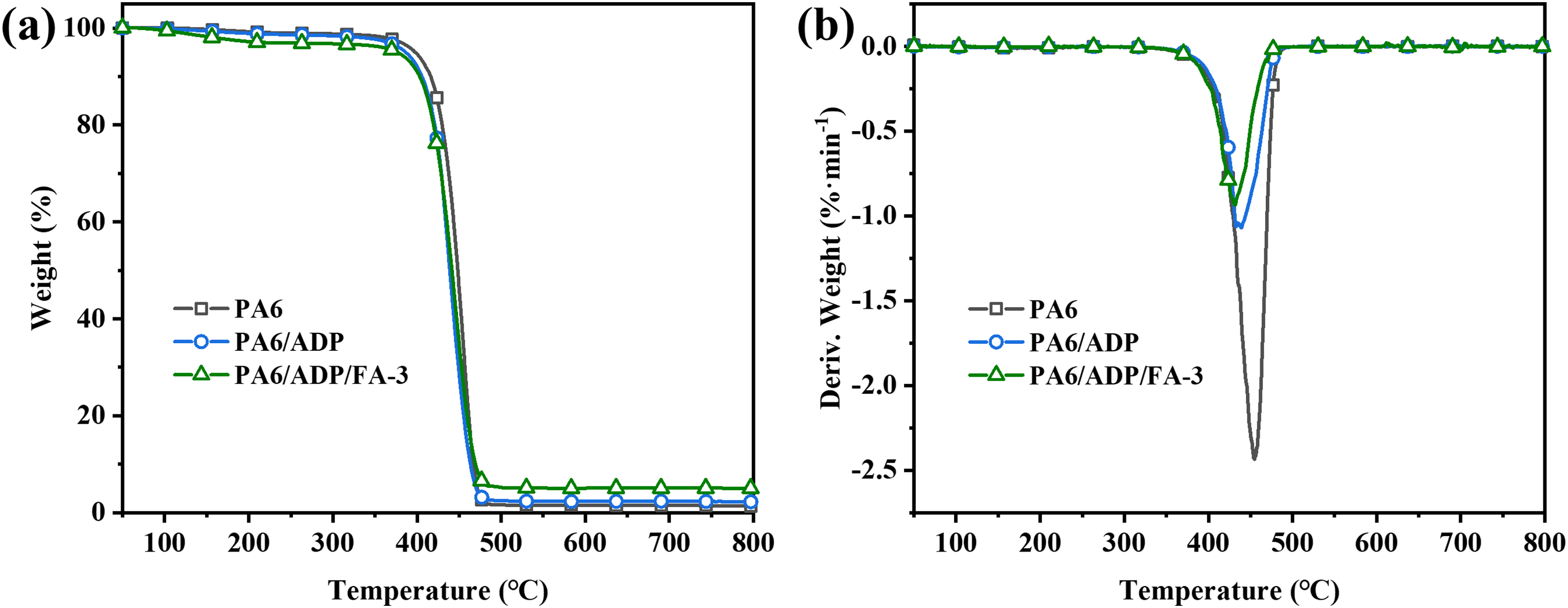
Figure 1: (a) TGA and (b) DTG curves of PA6 and its composites under N2 atmosphere

The limiting oxygen index (LOI) and vertical burning (UL-94) tests were utilized to assess the fire safety of PA6 and its composites. Table 1 illustrated the high flammability of pure PA6, which has an LOI of merely 21.0% and no rating in UL-94 tests. With the incorporation of 11 wt% ADP, the PA6 composite achieved a LOI of 27.9% and obtained a V-2 rating in UL-94 tests, demonstrating the effective flame-retardant enhancement by ADP. It is noteworthy that the introduction of a small amount of FA into the system significantly improved the flame retardancy of PA6 composites. Particularly, the PA6 composite demonstrated optimal flame retardancy at an ADP/FA mass ratio of 93:7. The introduction of a small amount of FA enabled the PA6/ADP/FA-3 to rapidly form a dense char layer on the surface during combustion. This char layer acted as a physical barrier, effectively blocking the transfer of oxygen and heat. Meanwhile, it significantly increases the melt viscosity of the polymer melt, anchoring the polymer melt within the char layer framework, thereby completely preventing the generation of burning droplets (Fig. 2). Therefore, the PA6/ADP/FA-3 composite not only passed V-0 rating in UL-94 tests but also attained a LOI of 30.9%, indicating outstanding synergistic flame-retardant effect between ADP and FA.
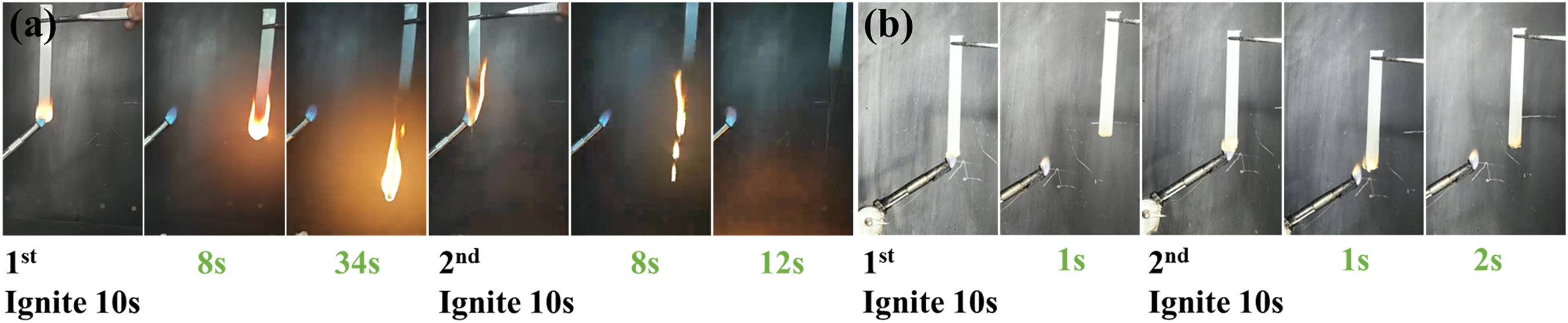
Figure 2: Video snapshots during UL-94 vertical burning tests for (a) PA6/ADP and (b) PA6/ADP/FA-3
The combustion behavior of PA6 and its composites under circumstances of a near-real fire was surveyed by the cone calorimeter test (CCT), and the Table 3 provided the pertinent data. As portrayed in Fig. 3a, pure PA6 burned rapidly and fiercely after being ignited, showing a sharp peak in heat release rate (HRR) at 231 s with a value of 1802.7 kW·m−2. Ultimately, its total heat release (THR) reached as high as 169.0 MJ·m−2 (Fig. 3b). The HRR trend of PA6/ADP was consistent with that of pure PA6, but with a slightly reduced combustion intensity. And a sharp HRR peak of 1377.4 kW·m−2 was observed at 220 s, manifesting that ADP exhibits certain flame retardancy in PA6. In contrast, PA6/ADP/FA-3 displayed a faster HRR during the initial combustion stage, which is likely attributed to the catalytic degradation of the polymer matrix induced by metal oxides in FA. Subsequently, a broad and stable plateau period emerged (Fig. 3a), probably caused by the char layer formed through early-stage catalytic degradation covering the matrix surface. The peak HRR of PA6/ADP/FA-3 was only 879.1 kW·m−2, representing reductions of 51.2% and 36.2% compared to pure PA6 and PA6/ADP, respectively. Additionally, it also had a lower THR than both pure PA6 and PA6/ADP (Fig. 3b). To objectively assess the fire risks of polymers, the flame performance index (FPI) and flame growth index (FGI) were calculated by Eqs. (1) and (2), respectively [37].
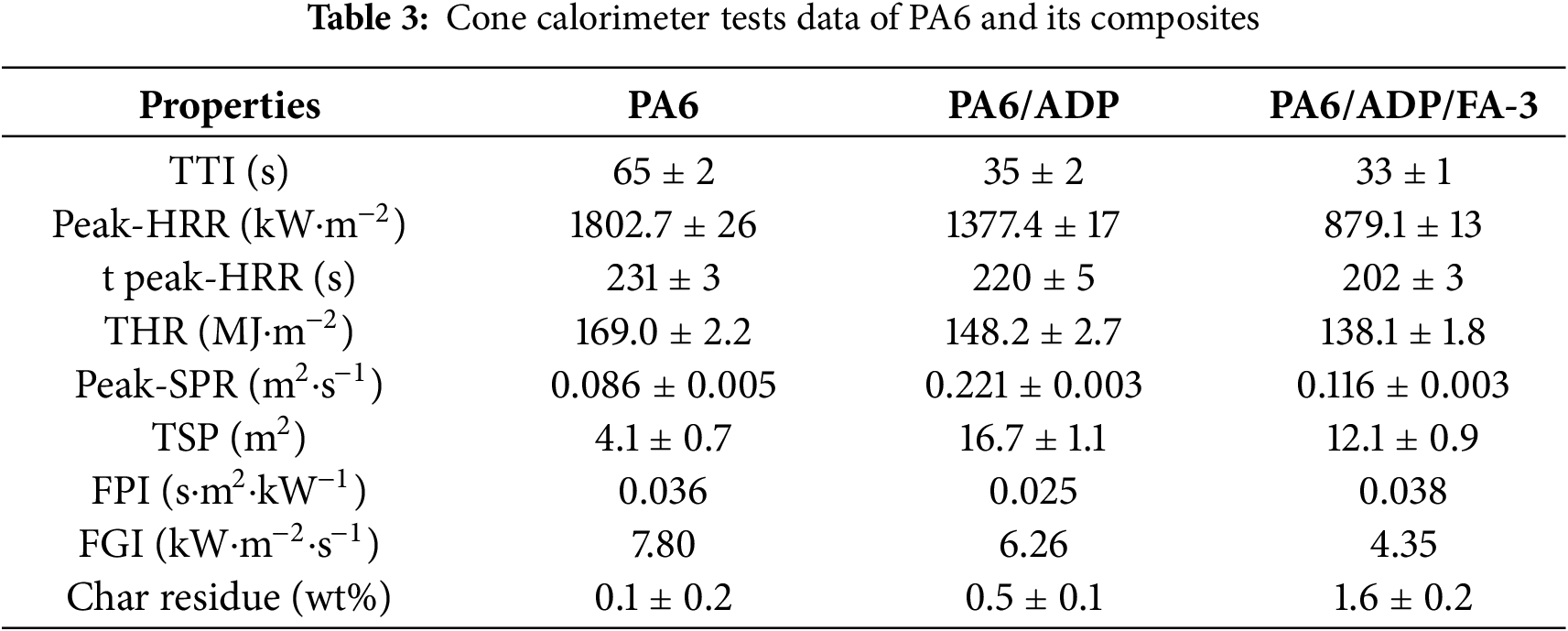
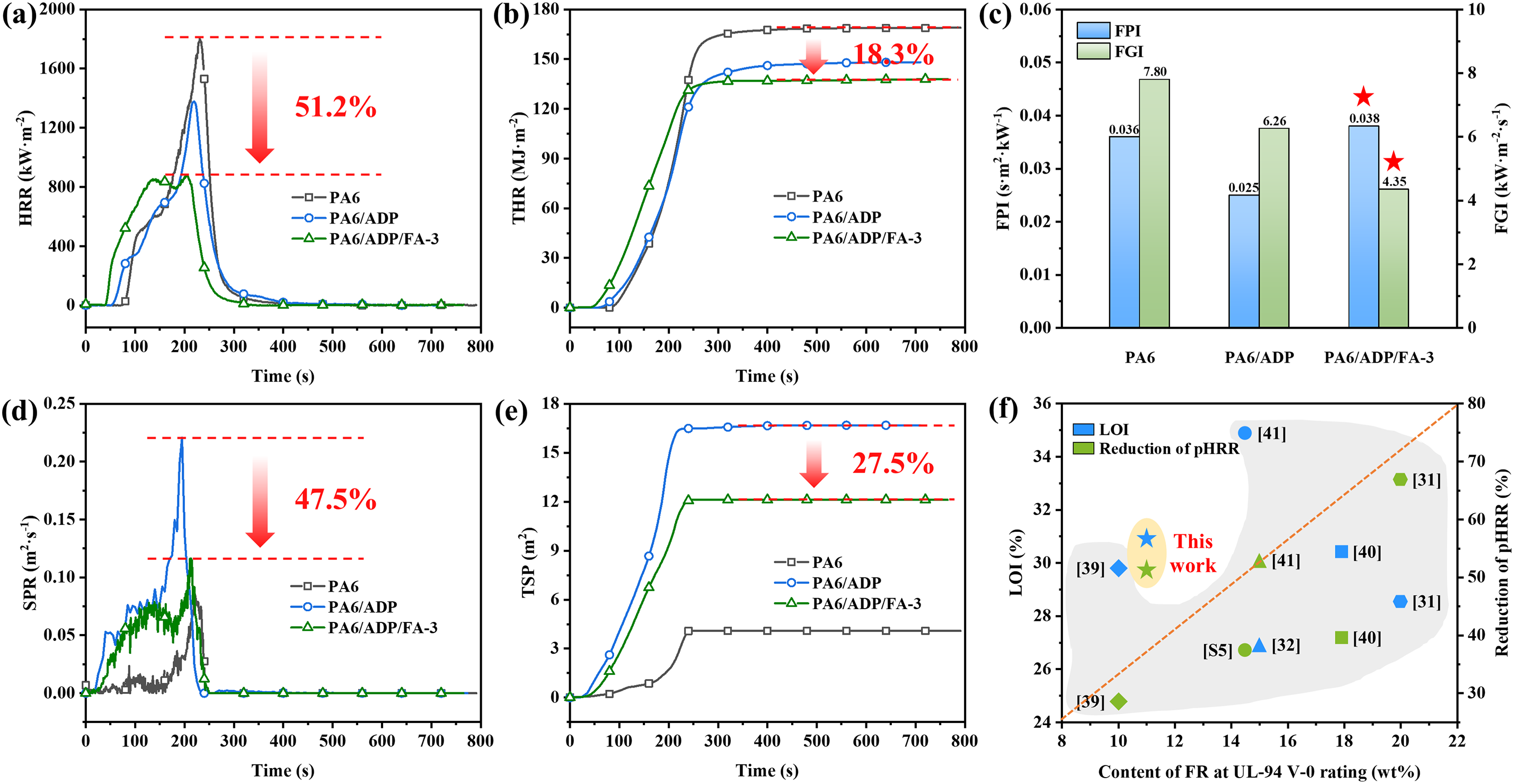
Figure 3: (a) Heat release rate, (b) total heat release, (c) FPI and FGI, (d) smoke production rate and (e) total smoke release of PA6 and its composites. (f) The flame retardancy of PA6/ADP/FA-3 and previously reported ADP-based flame-retardant PA6 composites [31,32,39,40,41]
Typically, the higher FPI and the lower FGI, the better fire safety of the polymers [38]. As depicted in Fig. 3c, the PA6/ADP/FA-3 attained optimal fire safety with the highest FPI (0.038 s·m2·kW−1) and lowest FGI (4.35 kW·m−2·s−1) values. These results demonstrates that ADP and FA present excellent synergistic flame-retardant effect, thereby imparting superior fire resistance to PA6.
Moreover, smoke release production is also a critical factor in measuring fire safety. As revealed in Fig. 3d,e, pure PA6 presented the lowest smoke release production due to relatively complete combustion in the CCT. The introduction of ADP resulted in a dramatic increase in the smoke release production of PA6 composite, which is in agreement with the intrinsic gas-phase flame retardant mechanism of ADP [39]. The phosphorus-containing radicals generated from the thermal decomposition of ADP can scavenge active radicals H and HO in the gas phase, leading to incomplete combustion of the material and substantial smoke production [40]. However, compared to PA6/ADP, the peak smoke production rate (pSPR) and total smoke production (TSP) of PA6/ADP/FA-3 decrease by 47.5% and 27.5%, respectively, when just a minor quantity of ADP was substituted by FA. It indicated that the incorporation of FA strengthens the condensed-phase flame retardant effect of the system. This conclusion is further supported by the residual char content measured via CCT (Table 3). The recent developments in ADP-modified PA6 systems were summarized in Fig. 3f and Table 4. The upper-left region of the dashed line in the figure indicates a superior flame-retardant strategy. Clearly, this work achieved excellent comprehensive performance at a relatively low loading amount. This manifests that the ADP/FA system has significant advantages and promising potential for enhancing the fire safety of PA6.
The complicated viscosity (𝜂*) of PA6 and its composites was first tested over the whole frequency range to investigate the flame-retardant mechanism of the ADP/FA system (Fig. 4). The 𝜂* of PA6/ADP is higher than that of pure PA6 owing to the hydrogen bonding interaction between ADP and the PA6 matrix, which boosts molecular chain friction and restricts their mobility. Apparently, the presence of FA further increased the 𝜂* of the PA6 composite [41]. The higher 𝜂* of PA6/ADP/FA-3 is presumably attributed to the hollow spherical structure of FA, which enabled PA6 molecular chains to penetrate and form an intricate network during the melting process. In general, a higher 𝜂* of the material correlates with reduced volatilization of degradation products during combustion [44], thereby heightening its flame retardancy. To further quantify the shear-thinning behavior and structural evolution of the PA6/ADP/FA composites, the viscosity–frequency data could be fitted using the Carreau–Yasuda model, which is widely applied to describe non-Newtonian polymer melts. This model expresses the viscosity as a function of shear rate and allows the extraction of key parameters such as the zero-shear viscosity (η0), relaxation time (λ), and power-law index (n). These parameters can reflect the degree of molecular chain entanglement and filler–matrix interactions within the system. In future work, Carreau–Yasuda fitting will be carried out to provide a more quantitative understanding of how FA incorporation influences the rheological behavior and flow dynamics of the flame-retardant composites.
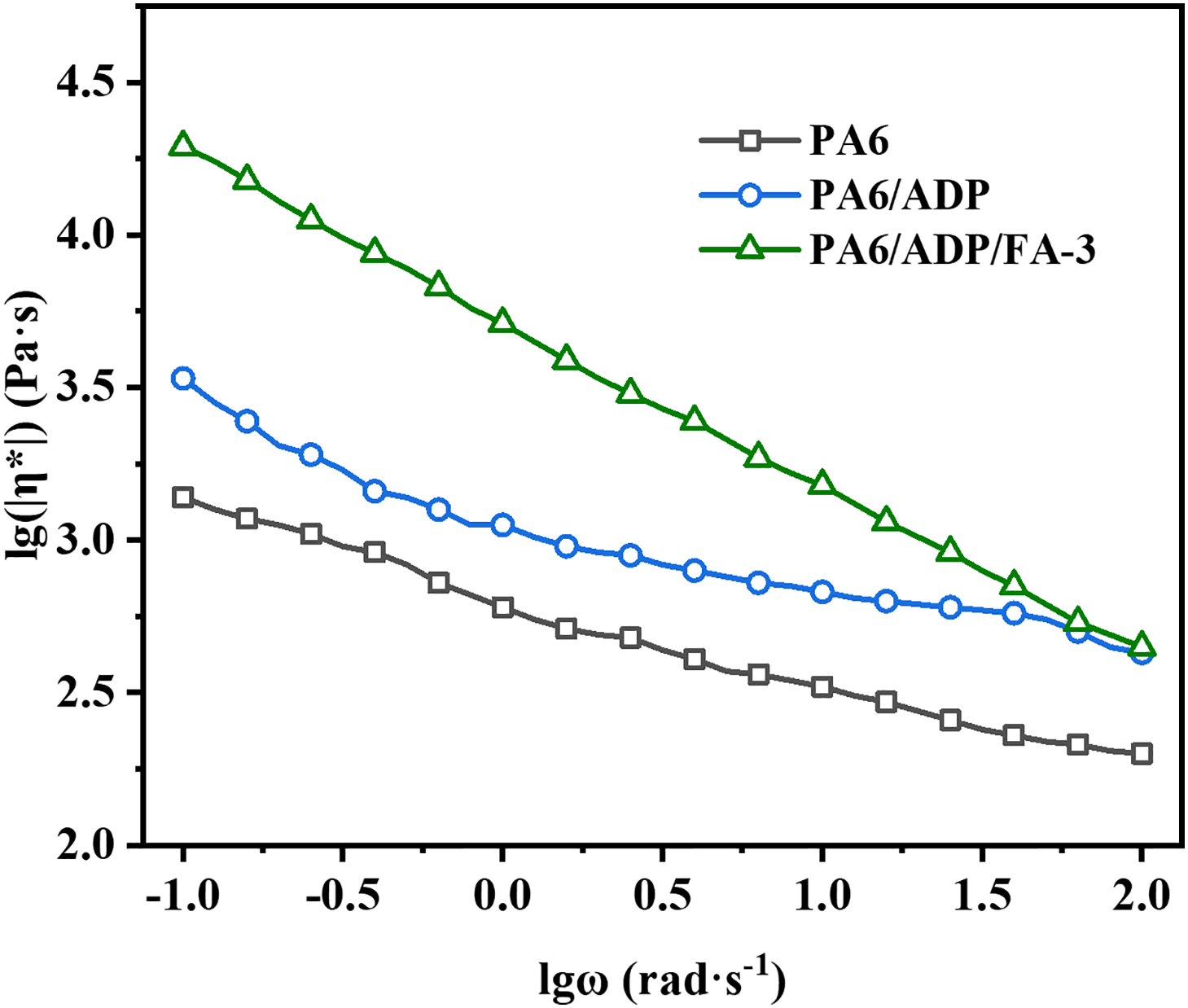
Figure 4: Complex viscosity as a function of angular frequency for PA6 and its composites fluid
To elucidate the mechanism of the ADP/FA system in the condensed phase, the residual char after CCT was analyzed. The residual char morphologies of PA6 and its composites recorded by digital camera and SEM were exhibited in Fig. 5. Compared to the non-charring behavior of pure PA6, PA6/ADP retained a small amount of fragile and loose char after combustion (Fig. 5a). The SEM-obtained microstructural morphology of the residual char revealed numerous distinct pores and tunnel-like structures on its surface (Fig. 5b), which account for its high heat release and smoke production. In stark contrast, PA6/ADP/FA-3 left behind a more intact, rigid, and higher-yield residual char after combustion (Fig. 5c). Besides, its microstructure appeared more denser with no visible pores on the surface, and even revealed raised bubble-like structures (Fig. 5d). This suggests that FA suppresses the transmission of heat, oxygen and flammable volatiles during combustion by promoting the development of a compact char layer and retaining more material in the condensed phase.
Figure 5: The surface morphologies of the residual char for (a,b) PA6/ADP and (c,d) PA6/ADP/FA-3 at the end of CCT
The graphitization degree of the residual char was then quantitatively examined by Raman spectroscopy. The vibrational modes observed at approximately 1370 cm−1 (D band) and 1600 cm−1 (G band) are attributed to disordered sp3 carbon and ordered graphitic sp2 carbon domains, respectively [42]. The area ratio of the D band to G band (ID/IG) reflects the graphitization degree of the residual char. A lower ID/IG ratio indicates a higher graphitization degree of the residual char. Notably, PA6/ADP/FA-3 displayed a lower ID/IG ratio compared to PA6/ADP (Fig. 6a,b), illustrating that its residual char possesses higher thermal stability.
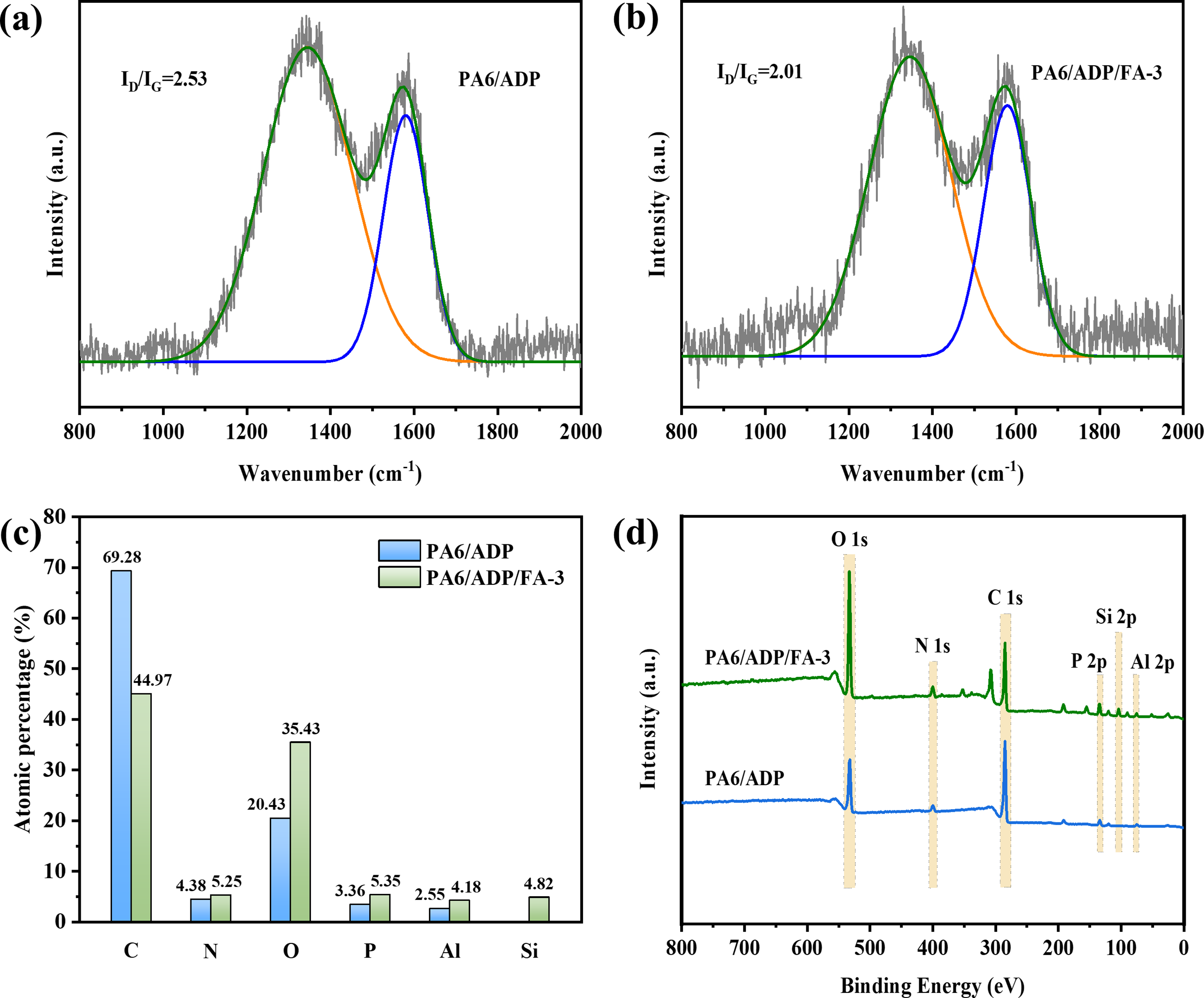
Figure 6: Raman spectra of residual char for (a) PA6/ADP and (b) PA6/ADP/FA-3. (c) Element contents and (d) XPS spectra of PA6 composites
The chemical composition of the residual char was further surveyed by XPS. As shown in Fig. 6c, the residual char of PA6/ADP/FA-3 presented distinctly higher P and Al contents than that of PA6/ADP. Additionally, the presence of Si was detected in the residual char of PA6/ADP/FA-3 (Fig. 6d). This shows that FA can catalyze the char formation of the matrix during combustion, thereby retaining more phosphorus-containing compounds in the condensed phase. The presence of Si and increased Al content in the residual char of PA6/ADP/FA-3 can be put down to the retention of Al2O3 and SiO2 from FA in the condensed phase after combustion.
To clarify the mechanism of the ADP/FA system in the gas phase, the gaseous products generated during the thermal degradation of PA6 and PA6/ADP/FA-3 were investigated by TG-IR. The 3D spectra analysis disclosed a notable reduction in the intensity of pyrolytic volatiles for PA6/ADP/FA-3 compared to pure PA6 and PA6/ADP (Fig. 7a–c), demonstrating the effective suppression of volatile emission. The details at different temperatures were further uncovered by IR spectra. No significant gaseous products were detected from pure PA6 and PA6/ADP below 400°C, while apparent C-H (2940 cm−1), CO2 (2354 cm−1), carbonyl (1710 cm−1) and NH3 (965 cm−1) absorption peaks emerged at 500°C (Fig. 7d,e) [24]. The incorporation of 11 wt% ADP/FA led to a marked decrease in the intensity of these characteristic peaks in the PA6 composite. In addition, the appearance of P=O (1154 and 1084 cm−1) absorption peak in PA6/ADP/FA-3 (Fig. 7f) illustrated the generation of phosphorus-containing radicals with radical quenching capability in the gas phase [25]. As portrayed in Fig. 7g–i, the release of NH3 from PA6/ADP/FA-3 occurred slightly earlier than that from PA6/ADP due to the catalytic carbonization effect of FA, which is consistent with the results of TGA test. Furthermore, the release of hydrocarbons and CO2 has also been reduced to a certain extent, which further indicated the synergistic flame-retardant effect of ADP and FA.
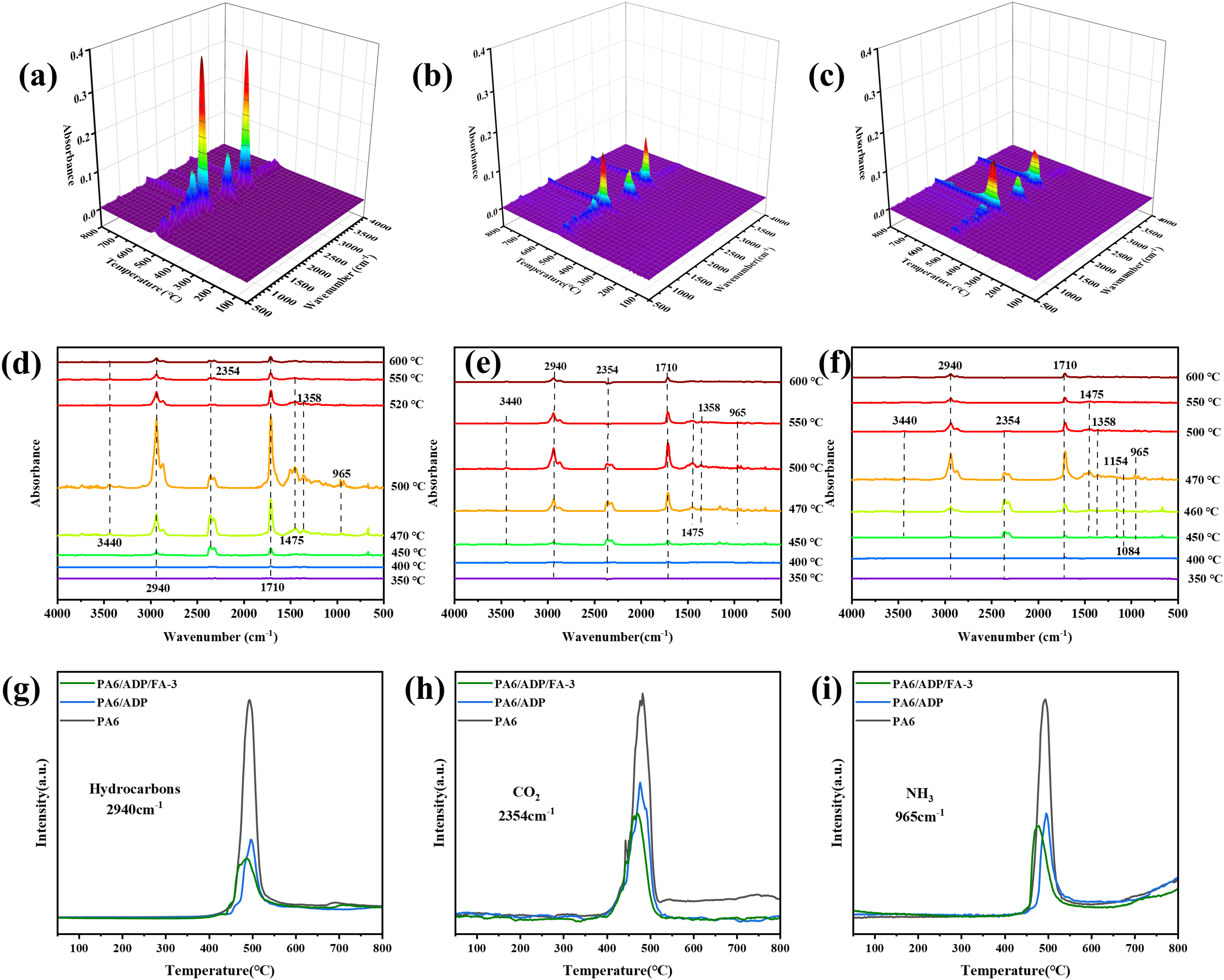
Figure 7: 3D TG-IR and IR spectra of pyrolysis gaseous products for (a,d) PA6, (b,e) PA6/ADP and (c,f) PA6/ADP/FA-3. Real-time absorption peak curves of (g) hydrocarbons, (h) CO2 and (i) NH3
As evidenced by the analytical results, Fig. 8 schematically presented the flame-retardant mechanism of the ADP/FA system. In the gas phase, the decomposition of ADP generated phosphorus radicals, which effectively terminate active radicals H and HO, inhibiting the propagation of combustion chain reactions. In the condensed phase, the hollow spherical structure of FA enhanced the melt viscosity of the composite, which restricted the release of pyrolytic volatiles during combustion. The component such as Al2O3 in FA has certain Lewis acid property, which worked in synergy with the Brønsted acid provided by ADP during the combustion process to catalyze the rapid cross-linking and dehydration of the PA6 matrix, thereby forming a char layer. Furthermore, the particles in the FA serve as a framework to embed within the char layer, strengthening its structure. The strengthened char layer effectively blocked heat and oxygen and captured smoke particles, achieving efficient fire resistance and smoke suppression effects. By simultaneously functioning in the condensed and gas phases, the ADP/FA system achieved a synergistic flame-retardant effect, thereby effectively improving the fire safety of PA6.
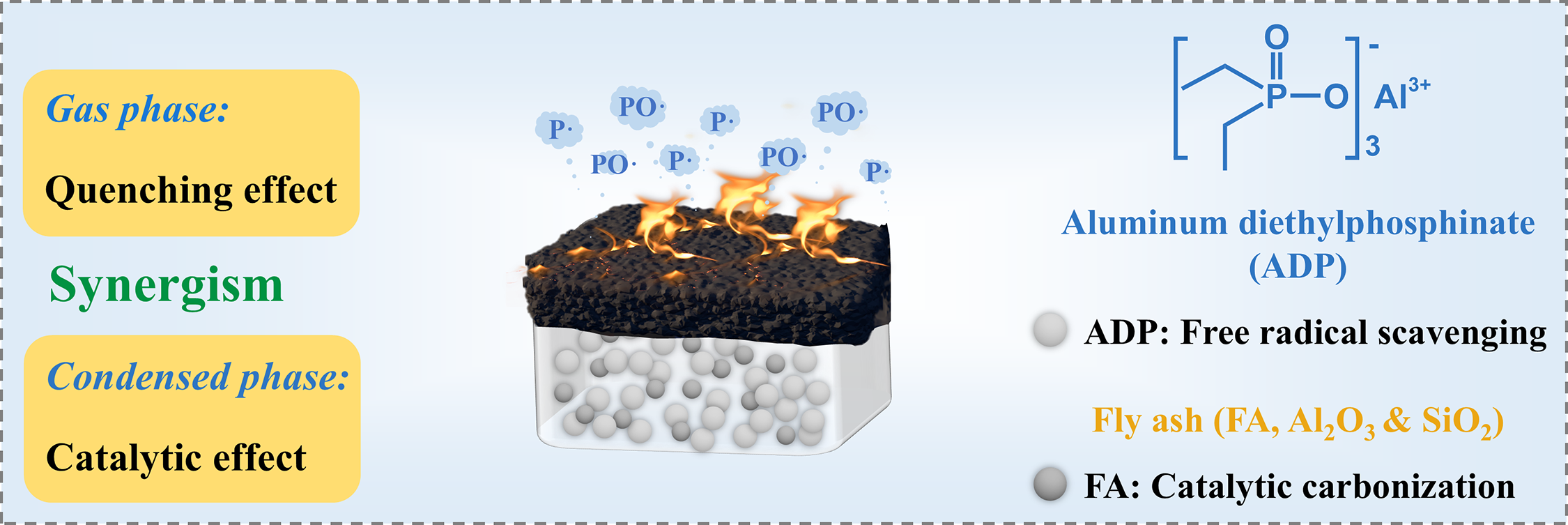
Figure 8: The proposed flame-retardant mechanism of PA6/ADP/FA-3 composite
3.4 Mechanical Properties of PA6 Composites
In addition to the investigation of the flame retardancy of PA6 composites, we also studied the changes in their mechanical properties. As shown in Fig. 9, the weak interfacial bonding caused by the poor compatibility of ADP and FA with the matrix, as well as the early failure triggered by it as a stress concentration point, led to a certain degree of reduction in the tensile strength, flexural strength, and Izod impact strength of the PA6 composites. Therefore, the next research work will focus on improving the limitations of this content.
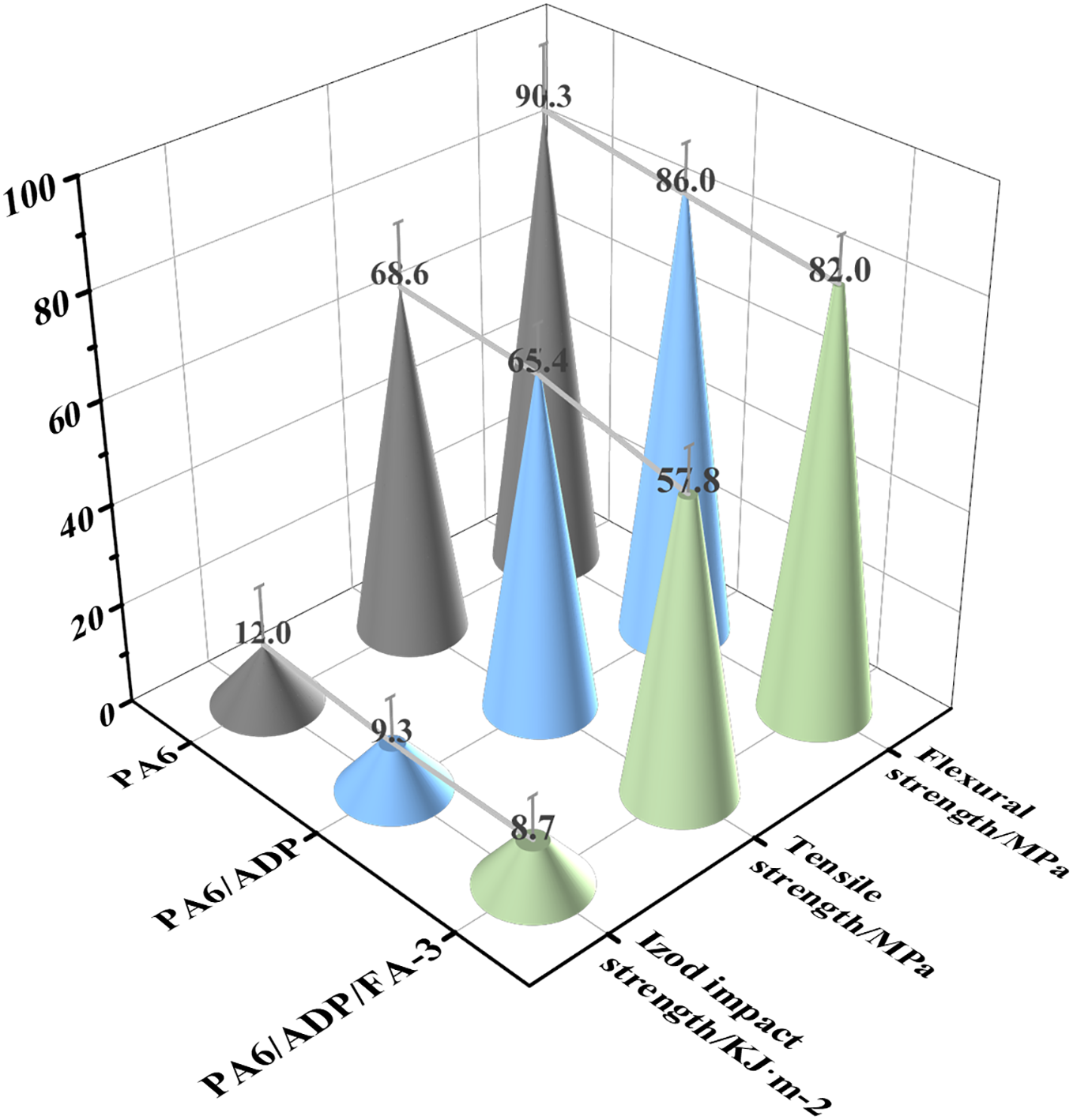
Figure 9: The mechanical properties of PA6, PA6/ADP and PA6/ADP/FA
The presented ADP/FA flame-retardant system exhibited efficient flame retardancy for PA6 composites, and the loading amount of flame-retardant additives was reduced from 14 wt% ADP to 11 wt% ADP/FA with the mass fraction of 97:3 when the PA6 composites achieved UL-94 V-0 rating. The provided ADP/FA system exhibited excellent synergistic flame retarded effect for PA6, and not only interrupted the free radicals chain reaction but also facilitated the formation of high-quality char layer in condensed phase during combustion. Moreover, the incorporation of FA efficiently increased the melt viscosity, and promoted the carbonization of PA6. Consequently, the release of decomposition products was efficiently restrained. As a result, the released of heat and smoke of PA6/ADP/FA was obviously reduced compared with PA6/ADP, especially the peak smoke production rate and total smoke production values were reduced by 47.5% and 27.5%, respectively. As a result, the ADP/FA endowed PA6 composites with more excellent fire safety performance, and it benefited to preparing flame retardant PA6 composites with highly comprehensive properties. The mechanical properties and other aspects of PA6 composites should be further investigated in the future and improved to promote the preparation and development of PA6 composites with excellent comprehensive performance. Meanwhile, the research will also conduct tests on different sources of FA and explore various PA6 blend systems to further expand its application prospects.
Acknowledgement: Not applicable.
Funding Statement: This work was financially supported by the Natural Science Foundation of China (52173069), the Key Research and Development Projects in Heilongjiang Province (2024ZXDXA29), the Natural Science Foundation of Heilongjiang Province (LH2024B004), and the Fundamental Research Funds for the Central Universities (ZHLJZR241700006).
Author Contributions: The authors confirm contribution to the paper as follows: Writing—original draft, Ruiping Wang; investigation, Ruiping Wang and Chuang He; visualization, Shuo Zhang; writing—review & editing, Miaojun Xu; validation, Zhuo Wang, Bin Tao and Suliang Gao; resources, Miaojun Xu and Bin Li; funding acquisition, Miaojun Xu, Xiaoli Li and Bin Li; supervision, Bin Li. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Gao J, Wu Y, Li J, Peng X, Yin D, Jin H, et al. A review of the recent developments in flame-retardant nylon composites. Compos Part C-Open. 2022;9:100297. doi:10.1016/j.jcomc.2022.100297. [Google Scholar] [CrossRef]
2. Li Y, Wang J, Xue B, Wang S, Qi P, Sun J, et al. Enhancing the flame retardancy and UV resistance of polyamide 6 by introducing ternary supramolecular aggregates. Chemosphere. 2022;287:132100. doi:10.1016/j.chemosphere.2021.132100. [Google Scholar] [PubMed] [CrossRef]
3. He W, Xu H, Song P, Xiang Y, Qin SP. N-decorated halloysite nanotubes for flame retardancy enhancement of polyamide 6/aluminum diethylphosphinate. Polym Degrad Stabil. 2022;196:109847. doi:10.1016/j.polymdegradstab.2022.109847. [Google Scholar] [CrossRef]
4. Wang W, Wang F, Li H, Liu Y. Synthesis of phosphorus-nitrogen hybrid flame retardant and investigation of its efficient flame-retardant behavior in PA6/PA66. J Appl Polym Sci. 2022;140(8):e53536. doi:10.1002/app.53536. [Google Scholar] [CrossRef]
5. Zhang Q, Zhu G, Xiao X, Liu Q, Jiang M, Guo D, et al. Controllable micro cross-linking towards multifunctional flame-retardant aliphatic polyamide. Chem Eng J. 2023;472(9):144983. doi:10.1016/j.cej.2023.144983. [Google Scholar] [CrossRef]
6. Zheng X, Li Y, Tang J, Yu G. Structure and properties of PVDF/PA6 blends compatibilized by ionic liquid-grafted PA6. ACS Omega. 2022;7(15):12772–8. doi:10.1021/acsomega.1c07341. [Google Scholar] [PubMed] [CrossRef]
7. Fan S, Peng B, Yuan R, Wu D, Wang X, Yu J, et al. A novel schiff base-containing branched polysiloxane as a self-crosslinking flame retardant for PA6 with low heat release and excellent anti-dripping performance. Compos Pt B-Eng. 2020;183(15):107684. doi:10.1016/j.compositesb.2019.107684. [Google Scholar] [CrossRef]
8. Qiu X, Wu C, Lin J, Wang Y, Ding L, Hu J, et al. Construction of MOFs-based nanocomposites and their application in flame retardant polymers: a review. Polym Degrad Stabil. 2024;229(34):110982. doi:10.1016/j.polymdegradstab.2024.110982. [Google Scholar] [CrossRef]
9. Tawiah B, Ullah S, Cheng Z, Rahman M, Ming Y, Chen D, et al. Microporous transition metal phosphide flame retardant toughened PA6 composites with excellent thermal conductivity and ferroelectric response. Compos Pt B-Eng. 2025;300(3):112502. doi:10.1016/j.compositesb.2025.112502. [Google Scholar] [CrossRef]
10. Mourgas G, Giebel E, Schneck T, Unold J, Buchmeiser MR. Syntheses of intrinsically flame-retardant polyamide 6 fibers and fabrics. J Appl Polym Sci. 2019;136(31):app47829. doi:10.1002/app.47829. [Google Scholar] [CrossRef]
11. Weil E, Levchik S. Current practice and recent commercial developments in flame retardancy of polyamides. J Fire Sci. 2004;22(3):251–64. doi:10.1177/0734904104040546. [Google Scholar] [CrossRef]
12. Zhang A, Zhang J, Liu L, Dai J, Lu X, Huo S, et al. Engineering phosphorus-containing lignin for epoxy biocomposites with enhanced thermal stability, fire retardancy and mechanical properties. J Mater Sci Technol. 2023;167:82–93. doi:10.1016/j.jmst.2023.06.004. [Google Scholar] [CrossRef]
13. Xue Y, Shen M, Zheng Y, Tao W, Han Y, Li W, et al. One-pot scalable fabrication of an oligomeric phosphoramide towards high-performance flame retardant polylactic acid with a submicron-grained structure. Compos Pt B-Eng. 2020;183:107695. doi:10.1016/j.compositesb.2019.107695. [Google Scholar] [CrossRef]
14. Zhu H, Xu S. Preparation of flame-retardant rigid polyurethane foams by combining modified melamine-formaldehyde resin and phosphorus flame retardants. ACS Omega. 2020;5(17):9658–67. doi:10.1021/acsomega.9b03659. [Google Scholar] [PubMed] [CrossRef]
15. Tao W, Li J. Melamine cyanurate tailored by base and its multi effects on flame retardancy of polyamide 6. Appl Surf Sci. 2018;456:751–62. doi:10.1016/j.apsusc.2018.06.215. [Google Scholar] [CrossRef]
16. Wang R, Zhang S, Du S, Xu M, Wang Z, Song P, et al. Glass-blowing-inspired constructing a novel ceramizable intumescent flame retardant for realizing superior flame retardancy, smoke suppression and water resistance of polyethylene composites. Compos Pt A-Appl Sci Manuf. 2025;190(2):108687. doi:10.1016/j.compositesa.2024.108687. [Google Scholar] [CrossRef]
17. Li Y, Xue B, Qi P, Gu X, Sun J, Li H, et al. The synergistic effect between bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate and polysiloxane on the photo-aging resistance and flame retardancy of polypropylene. Compos Pt B-Eng. 2022;234(11):109666. doi:10.1016/j.compositesb.2022.109666. [Google Scholar] [CrossRef]
18. Xu Z, Duan L, Hou Y, Chu F, Jiang S, Hu W, et al. The influence of carbon-encapsulated transition metal oxide microparticles on reducing toxic gases release and smoke suppression of rigid polyurethane foam composites. Compos Pt A-Appl Sci Manuf. 2020;131(5):105815. doi:10.1016/j.compositesa.2020.105815. [Google Scholar] [CrossRef]
19. Liu J, Wang R, Liang D, Zhang S, Feng J, Xu M, et al. Highly efficient polyethylene composites with flame-retardant, smoke and toxicity suppression properties by incorporating urchin-like ZnO@C-N and intumescent flame retardant. Polym Degrad Stabil. 2025;233(6):111190. doi:10.1016/j.polymdegradstab.2025.111190. [Google Scholar] [CrossRef]
20. Wang R, Zhang S, Hu X, Leng Y, Li X, Tao B, et al. Constructing piperazine pyrophosphate@LDH@rGO with hierarchical core-shell structure for improving thermal conductivity, flame retardancy and smoke suppression of epoxy resin thermosets. Compos Pt B-Eng. 2024;287(5):111870. doi:10.1016/j.compositesb.2024.111870. [Google Scholar] [CrossRef]
21. Jiang S, Liu L, Yang X, Li B, Xu M. Nickel-aluminum hydrotalcite for improving flame retardancy and smoke suppression of intumescent flame retardant polypropylene: preparation, synergy and mechanism study. Macromol Mater Eng. 2022;308(4):2200533. doi:10.1002/mame.202200533. [Google Scholar] [CrossRef]
22. Huo S, Song P, Yu B, Ran S, Chevali VS, Liu L, et al. Phosphorus-containing flame retardant epoxy thermosets: recent advances and future perspectives. Prog Polym Sci. 2021;114:101366. doi:10.1016/j.progpolymsci.2021.101366. [Google Scholar] [CrossRef]
23. Li J, Qian L, Wang X, Qiu Y, Tang W, Li S. Alloying synergistic flame retardant effect improving fire resistance and mechanical properties of polyamide 6. J Appl Polym Sci. 2022;139(48):e53226. doi:10.1002/app.53226. [Google Scholar] [CrossRef]
24. Li J, Qian L, Wang X, Wang J, Qiu Y, Chen Y, et al. Alloying synergistic flame retardant effect on PA6 by polyimide containing alkyl hypophosphate structure. Eur Polym J. 2024;211(3):113033. doi:10.1016/j.eurpolymj.2024.113033. [Google Scholar] [CrossRef]
25. Wang Y, Cui L, Zhang J, Shen J, Xu H, Zhou Z, et al. Fire retardant treatments for polyamide 6 and 66: advances and trends over the last five years. ACS Appl Polym Mater. 2025;7(8):4677–93. doi:10.1021/acsapm.5c00291. [Google Scholar] [CrossRef]
26. Shan H, Yan L, Xu B, Wang D, Wu M. Polyphosphamide containing triazine and melamine cyanurate for flame-retardant PA6. ACS Appl Polym Mater. 2023;5(7):5322–33. doi:10.1021/acsapm.3c00732. [Google Scholar] [CrossRef]
27. Wang Y, Qu C, Yu K, Si Z, Zhang J. PTFE-based flame retardant coatings optimized by melamine polyphosphate/aluminum diethyl hypophosphite/anhydrous transparent powder through orthogonal experiment. Prog Org Coat. 2024;191(Part B):108423. doi:10.1016/j.porgcoat.2024.108423. [Google Scholar] [CrossRef]
28. Zhang D, Liu J, Williams B, Hou Z, Bodin J, Lofink B, et al. Enhancing flame retardancy of flexible polyurethane foams through one-step coassembled nanocoatings. Adv Compos Hybrid Mater. 2024;7(6):230. doi:10.1007/s42114-024-01024-z. [Google Scholar] [CrossRef]
29. Zhang D, Williams B, Becher E, Shrestha S, Nasir Z, Lofink B, et al. Flame retardant and hydrophobic cotton fabrics from intumescent coatings. Adv Compos Hybrid Mater. 2018;1(1):177–84. doi:10.1007/s42114-017-0006-1. [Google Scholar] [CrossRef]
30. Zhou R, Sun X, Xie J, Ma G, Li W, Jiang J, et al. A series of novel flame retardants produced with nanosilica, melamine, and aluminum diethylphosphinate to improve the flame retardancy of phenolic resin. ACS Omega. 2022;7(20):16980–9. doi:10.1021/acsomega.1c07246. [Google Scholar] [PubMed] [CrossRef]
31. Lu Y, Feng J, Yi D, Xie H, Xu Z, Cao C, et al. Strong synergistic effects between P/N-containing supramolecular microplates and aluminum diethylphosphinate for fire-retardant PA6. Compos Pt A-Appl Sci Manuf. 2024;176(2017):107834. doi:10.1016/j.compositesa.2023.107834. [Google Scholar] [CrossRef]
32. Lu Y, Chu T, Huo S, Huang G, Xu Z, Feng J, et al. Green synthesis of a P/N/B-containing aggregate for boosting fire-retardancy of PA6/aluminum diethylphosphinate composites. Polym Degrad Stabil. 2024;229(Pt 2):110949. doi:10.1016/j.polymdegradstab.2024.110949. [Google Scholar] [CrossRef]
33. Al-Shmaisani S, Kalina R, Douglas Ferron R, Juenger MCG. Assessment of blended coal source fly ashes and blended fly ashes. Constr Build Mater. 2022;342(1999):127918. doi:10.1016/j.conbuildmat.2022.127918. [Google Scholar] [CrossRef]
34. Zhang Z, Wu W, Zhang M, Qu J, Shi L, Qu H, et al. Hydrothermal synthesis of 4ZnO·B2O3·H2O/RGO hybrid material and its flame retardant behavior in flexible PVC and magnesium hydroxide composites. Appl Surf Sci. 2017;425:896–904. doi:10.1016/j.apsusc.2017.07.101. [Google Scholar] [CrossRef]
35. Priyadharshini A, Xavier J. Recent innovations in graphene-based nanocomposite coatings for enhanced flame retardancy in industrial applications. Polym Degrad Stabil. 2025;240(3):111479. doi:10.1016/j.polymdegradstab.2025.111479. [Google Scholar] [CrossRef]
36. Kaur R, Verma SK, Mehta R. Tailoring the properties of polyurethane composites: a comprehensive review. Polym Plast Technol Mater. 2025;64(13):2004–18. doi:10.1080/25740881.2025.2493869. [Google Scholar] [CrossRef]
37. Liu L, Yao M, Zhang H, Zhang Y, Feng J, Fang Z, et al. Aqueous self-assembly of bio-based flame retardants for fire-retardant, smoke-suppressive, and toughened polylactic acid. ACS Sustain Chem Eng. 2022;10(49):16313–23. doi:10.1021/acssuschemeng.2c05298. [Google Scholar] [CrossRef]
38. Chen C, Su S, Sun M, Wang Z, Zhang X, Tang L. Synergistic flame retardancy of ZnO with piperazine pyrophosphate/melamine polyphosphate in PP. Polym Test. 2023;117(3):107878. doi:10.1016/j.polymertesting.2022.107878. [Google Scholar] [CrossRef]
39. Pan Y, Song L, Wang W, Zhao H. Polydimethylsiloxane wrapped aluminum diethylphosphinate for enhancing the flame retardancy of polyamide 6. J Appl Polym Sci. 2020;137(35):e49027. doi:10.1002/app.49027. [Google Scholar] [CrossRef]
40. Feng H, Li D, Cheng B, Song T, Yang R. A cross-linked charring strategy for mitigating the hazards of smoke and heat of aluminum diethylphosphonate/polyamide 6 by caged octaphenyl polyhedral oligomeric silsesquioxanes. J Hazard Mater. 2022;424(7):127420. doi:10.1016/j.jhazmat.2021.127420. [Google Scholar] [PubMed] [CrossRef]
41. Xu M, Ma K, Jiang D, Zhang J, Zhao M, Guo X, et al. Hexa-[4-(glycidyloxycarbonyl) phenoxy]cyclotriphosphazene chain extender for preparing high-performance flame retardant polyamide 6 composites. Polymer. 2018;146:63–72. doi:10.1016/j.polymer.2018.05.018. [Google Scholar] [CrossRef]
42. Wang C, Huo S, Ye G, Song P, Wang H, Liu Z. A P/Si-containing polyethylenimine curing agent towards transparent, durable fire-safe, mechanically-robust and tough epoxy resins. Chem Eng J. 2023;451(19):138768. doi:10.1016/j.cej.2022.138768. [Google Scholar] [CrossRef]
43. Liang T, Cai J, Liu S, Lai H, Zhao J. Chain extension and synergistic flame-retardant effect of aromatic schiff base diepoxide on polyamide 6/aluminum diethylphosphinate composites. Materials. 2019;12(14):2217. doi:10.3390/ma12142217. [Google Scholar] [PubMed] [CrossRef]
44. Song P, Liu H, Shen Y, Du B, Fang Z, Wu Y. Fabrication of dendrimerlike fullerene (C60)-decorated oligomeric intumescent flame retardant for reducing the thermal oxidation and flammability of polypropylene nanocomposites. J Mater Chem. 2009;19(9):1305–13. doi:10.1039/b815610g. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF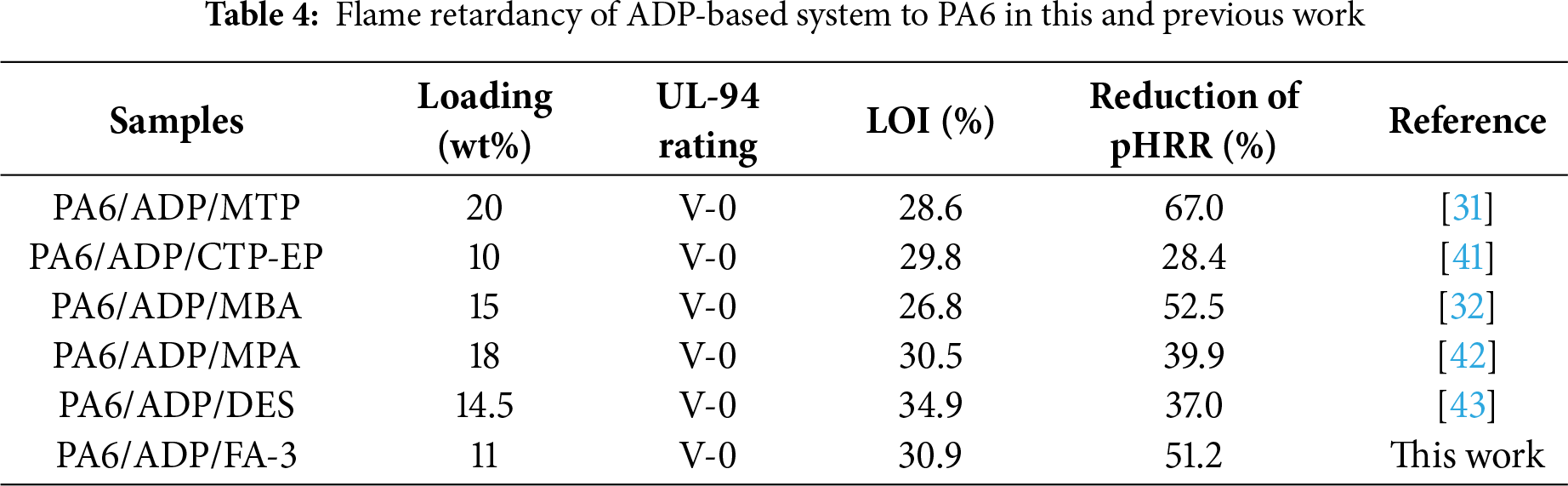
 Downloads
Downloads
 Citation Tools
Citation Tools