 Open Access
Open Access
REVIEW
Recent Developments in Bioadhesives and Binders
1 College of Chemistry and Material Engineering, Zhejiang A&F University, Hangzhou, 311300, China
2 Yunnan Key Laboratory of Wood Adhesives and Glued Products, College of Material Science and Engineering, Southwest Forestry University, Kunming, 650224, China
3 Laboratoire d’Etude et de Recherche sur le MAteriau Bois (LERMAB), University of Lorraine, Blvd. des Aiguillettes, Nancy, 54000, France
* Corresponding Authors: Antonio Pizzi. Email: ; Guanben Du. Email:
(This article belongs to the Special Issue: Renewable and Biosourced Adhesives-2023)
Journal of Renewable Materials 2025, 13(2), 199-249. https://doi.org/10.32604/jrm.2025.02024-0048
Received 27 November 2024; Accepted 03 January 2025; Issue published 20 February 2025
Abstract
This review is composed of three main parts each of which is written by well-known top specialists that have been, in a way or other, also the main participants of the majority of the developments reported. Thus, after a general part covering the grand lines and more in-depth views of more recent tannin, lignin, carbohydrate and soy bioadhesives, some mix of the other bio raw materials with soy protein and soy flour and some other differently sourced bioadhesives for wood, this review presents a more in-depth part on starch-based wood adhesives and a more in-depth part covering plant protein-based adhesives. It must be kept in mind that the review is focused on completely or almost completely biosourced adhesives, the fashionable adhesives derived from mixes of biosourced materials with synthetic resins having been intentionally excluded. This choice was made as the latter constitute only an intermediate interval, possibly temporary if even for a somewhat long times, towards a final full bioeconomy of scale in this field. This review also focuses on more recent results, mainly obtained in the last 10–20 years, thus on adhesive formulations really innovative and sometimes even non-traditional. In all these fields there is still a lot of possibility of innovation for relevant formulation as this field is still in rapid growth.Graphic Abstract
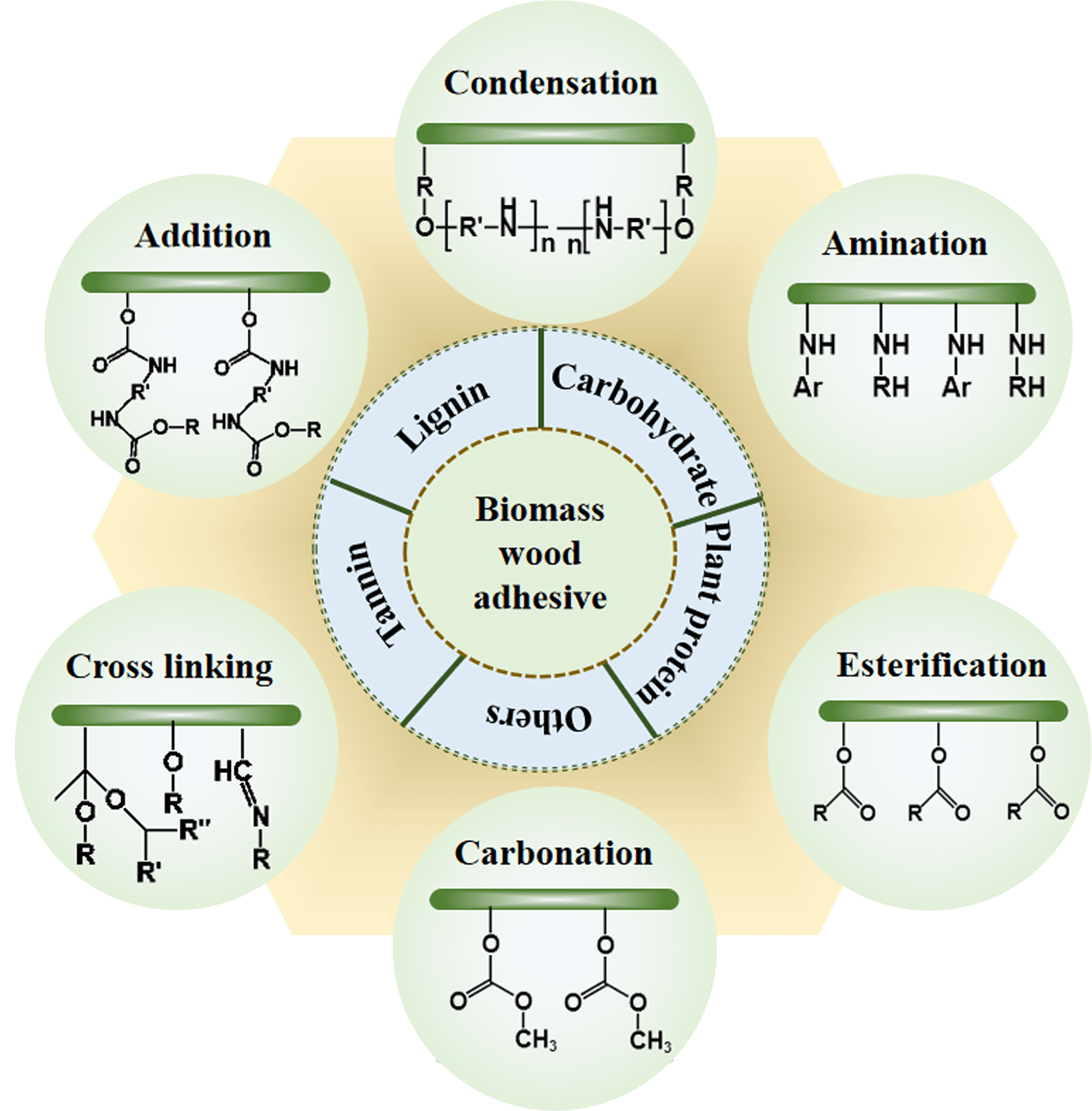
Keywords
It must be clearly pointed out that the formulations presented in this review are at the forefront of the field of bioadhesives and bioresins for wood, thus stressing their novelty and their innovative nature. It must be equally pointed out that the great majority of the formulations presented are tested and obtained in the laboratory, and only few of them have been tried under effective industrial conditions but all show great potential for industrial application. Industrially used bioresins and bioadhesives for wood are at present limited to tannin adhesives using older technologies being hardened with formaldehyde or hexamine, these having been in extensive operation in several countries for many years, and to some soy protein adhesives being used industrially in North America and China for the same purpose. These are not described in this review because extensive literature on their formulation and application exists already. In this review, only novelty bioadhesives and resins are described, thus products that can take the place in the future of the present bioadhesives for wood and that can advance the effectiveness, performance, ecological worth and cost of these resins. Tannin biobinders for metal bonding are also in limited industrial application. This has been clearly indicated in the relevant text in this review.
Quite a few advances have been made in this field quite recently, either by combining carbohydrates with other types of biosourced materials and resin, as for example tannins of different kinds, soy protein isolates and soy flour, and even lignin. Four main approaches can be taken for using carbohydrates for wood panel adhesives: (1) to modify synthetic wood adhesives such as urea-formaldehyde (UF) and phenol-formaldehyde (PF) adhesives. A great amount of relevant exists on such a more traditional approach [1–7]. For this reason it will not be expanded in this review. (2) By degrading and modifying carbohydrates to simpler compounds to be used as adhesives building blocks. (3) By using them unaltered as wood adhesives, and finally (4) to use carbohydrates to react with other renewable biomaterials such as soy, tannins, lignin and soy protein and flour. Approach 2 above leads to furanic resins, the building blocks of which, namely furfuraldehyde (furfural) and furfuryl alcohol, are obtained by treating with acids carbohydrates originating from vegetal waste. Such resins are erroneously thought to be synthetic resins [8]. This is false because they are real bioresins from renewable materials, very much in industrial use for a number of applications different from wood panels adhesives. Relevant reviews on furanic resin binders do exist [8]. Their drawbacks are that both their building blocks give dark-colored, almost black resins and are also relatively expensive. This notwithstanding, furanic resins are extensively used industrially for applications where their higher cost is not a drawback such as binders for foundry sand cores. They are perfectly usable as thermosetting wood adhesives but furfuryl alcohol toxicity before the resin is hardened is a problem that needs to be looked over and solved. While still considering these limitations, wood panel adhesives prepared by reacting furanic materials with glyoxal and glutaraldehyde, aldehydes of much lower toxicity of formaldehyde and moreover, non-volatile, have also been reported [9].
Several research groups [10,11] have reported recently the use of liquefied wood holocellulosic costituents, to obtain good wood adhesives. Liquefaction of holocellulosic materials by sulfuric acid with the aid of phenol or ethylene glycol have also been recently reported, considering however this original approach is much older [12].
The most recently innovative and encouraging approach for using monomeric and oligomeric carbohydrates as wood adhesives is by specific oxidation by sodium periodate, or other more effective specific oxidants such as persulfates [13], or even by progressively less specific oxidants such as potassium permanganate and hydrogen peroxide. Periodate ions cleavage of vicinal 1,2–glycols generate two aldehyde groups from the glycol C-OHs. This is a long time known, and used, carbohydrate chemistry reaction [14]. Such a reaction is relatively mild and the fact of being able to carry it out in water under mild conditions renders it particularly suitable on carbohydrates that are soluble in water. This reaction extensive utilization in carbohydrates chemistry is due to the advantage of its great selectivity [14–19]. The periodate specific oxidation reaction can and has been used successfully on both polymeric carbohydrates such as starch, cellulose and hemicelluloses [14–19] (Fig. 1) and on carbohydrate monomers [14–19] such as glucose, and dimers [14–19] such as sucrose (Fig. 2). For both long oligomers and up to cellulose the reaction leads to the generated aldehydes condensing and cross-linking with other carbohydrate chains, for cellulose even forming solid panels (Fig. 1) [17].

Figure 1: Scheme of sodium periodate carbohydrates oxidation yielding bioaldehydes with no toxicity and of no volatility and to different cross-linked products depending [15,17,18]

Figure 2: An example of different lower molecular weight biosourced aldehydes being obtained through different mechanisms as the consequence of periodate oxidation followed by further rearrangements [15,16,19]
Further addition of periodate increases the degree of oxidation allowing the generated aldehyde groups to react with other biomaterials such as soy or other proteins [15,16], condensed tannins [19] or even lignin [20], leading also to cross-linking and thus to applicable wood adhesives [15–20].
The action of sodium periodate for 1 h at 120°C on sucrose and particularly glucose yielded a variety of bioaldehydes by a number of mechanisms. Such an aldehyde generation behavior is also evident by periodate oxidation of the soy flour insoluble carbohydrates fraction, thus yielding soy flour based wood adhesives of acceptable performance [15,16]. The same takes place with the hemicellulose oligomer fragments in commercial tannin extracts [19]. An example of these aldehydes is shown in Fig. 2.
Much higher molecular weight aldehydes on carbohydrate oligomers of higher molecular weight are also produced, as for example from soy flour [15] which present one or more aldehyde groups (Fig. 3). Such an approach has been successfully taken also for tannin and lignin adhesives [19,20].

Figure 3: One example of one of the number of aldehydes of high molecular weight generated from carbohydrates polymers by sodium periodate specific oxidation [15]
The periodate specific oxidation approach has also been used to prepare chitosan-based adhesives to bond three veneer layer plywood and moreover also by addition of other carbohydrates, such as glucose, sucrose and starch [21] (Fig. 4). The effect on bonding performance of different degrees of oxidation achieved by varying the level of addition of sodium periodate has been determined by also further explaining its mechanism. Chitosan adhesives water resistance and bonding strength was improved by adding oxidized starch performing even better than oxidized sucrose or glucose. Addition at ambient temperature to a chitosan adhesive solution of 8% by weight of starch oxidized by 10% sodium periodate yielded the best performance formulation. TMA showed the chitosan adhesive added of oxidized starch appearing to cure at lower temperatures and with better bonding strength than other adhesives under test. Even glucose as model compound, after oxidation to yield aldehyde groups, led to elimination of water by two aldehyde groups by condensation with the chitosan.

Figure 4: One of the compounds detected by condensation of a chitosan fractions with oxidized glucose [21]. Copyright © 2022, Elsevier
A research work based on the Maillard reaction developed a sucrose adhesive coupled with hyperbranched cross-linked structures, containing no formaldehyde and yielding a good bonding performance [22]. For this oxidized sucrose was reacted with polyamines yielding a hardened network of high cross-linked density. The results were much superior to adhesives prepared by just oxidizing sucrose both for dry and especially wet strength. This was ascribed to the highly branched, hardened cross-linked network obtained by the induced Maillard reaction condensation between oxidized sucrose and the polyamine.
Several articles have appeared using in some way the specific oxidation system on cellulose to prepare adhesives of good performance. One of this discloses a resin prepared by polyethyleneimine (PEI), urea, and dialdehyde cellulose by Schiff base reaction. Chemical analysis determined Schiff base bridging between aldehyde groups in dialdehyde cellulose (DAC) and amino groups in polyurea which built a hardened cross-linked network, with good water resistance but also obtained by a simple and low cost preparation procedure. The improved performance of such an adhesive adhesive has been attributed to the formation of hyperbranched cross-linked structures yielding a higher cross-linking density of the hardened network [23].
A second article presented a synthesis based on the Maillard reaction of dialdehyde cellulose and polyamines yielding a hyperbranched chemically cross-linked cellulose with covalent and hydrogen bonds interactions [24]. The active aldehyde sites of the dialdehyde cellulose skeletal chain anchor the polyamines amino groups to form covalent bonds consuming a significant proportion of hydrophilic groups, the remaining aliphatic segments of polyamines criss-cross to knit a hydrophobic network endowing the adhesive with the ability to resist water erosion. integrant-exposed hydrophilic groups form intermolecular hydrogen bonds preferentially after curing and clustering due to the agglomeration effect of cellulose, which reduces the opportunity of forming hydrogen bonds with water molecules. The adhesive presented an outstanding water resistance [24].
Another oxidized cellulose adhesive based on the coreaction of cellulose-hexamethylene diamine-urea has been disclosed giving good bonding and good water resistance [25]. To increase the cellulose solubility it was at first hydrolyzed enzymatically thus exposing an larger number of hydroxyl groups. The hydrolyzed cellulose then underwent sodium periodate (NaIO4) thus forming a series of oligomer chains richly endowed of aldehyde groups. The adhesive itself was prepared by crosslinking oxidized cellulose with a synthetic reactive polyurea polymer, which obtained by hexamethylenediamine and urea undergoing a deamidation reaction. This adhesive so prepared showed good dry, wet and hot water strength performance. It also showed an increase of 60% of wet bonding strength in relation to a simpler oxidized cellulose-hexamethylenediamine adhesive.
A more unusual but very interesting approach consisted in using sodium periodate to specifically oxidize wood veneers to be used for plywood preparation [26]. These present a heterocyclic structure with a large number of ureido groups. Such an adhesive used as a formaldehyde-free wood binder showed excellent bond strength and thermal resistance after hardening with moreover binding well a variety of materials and tissues. Of particular note by oxidizing polar wood veneer surfaces with sodium periodate generates on their surfaces aldehyde and carboxyl groups which then can covalently cross-link with the ureido polymer adhesives. This resulted in an improved plywood bond strength higher than the China National Standard (GB/T 9846-2015) requirements for Type II plywood. Moreover, of even greater interest was the marked decrease in the needed temperature of hot pressing for curing from 200 down o 120°C.
Sucrose and glucose were recently used to prepare NIPU (non-isocyanate polyurethanes) adhesives and resins by reacting them with dimethyl carbonate followed by reaction with a diamine or polyamine [27,28] (Fig. 5).

Figure 5: Higher molecular weight of linear and branched NIPUs (non-isocyanate polyurethanes) oligomers obtained and detected by reacting dimethyl carbonate and a biosourced diamine [27]
Zhao et al. [29] have presented a particularly interesting approach by preparing a renewable urea-oxidized starch resin, eco-friendly and with no formaldehyde emission by condensing a majority of urea and oxidized starch by adding some nano-titanium dioxyde (nano-TiO2). As replacement of formaldehyde oxidized starch was used thus avoiding formaldehyde vapours emission from the a nano-TiO2-catalyzed oxidized starch-urea resin [29]. The schematic synthesis of this resin is shown in Fig. 6.
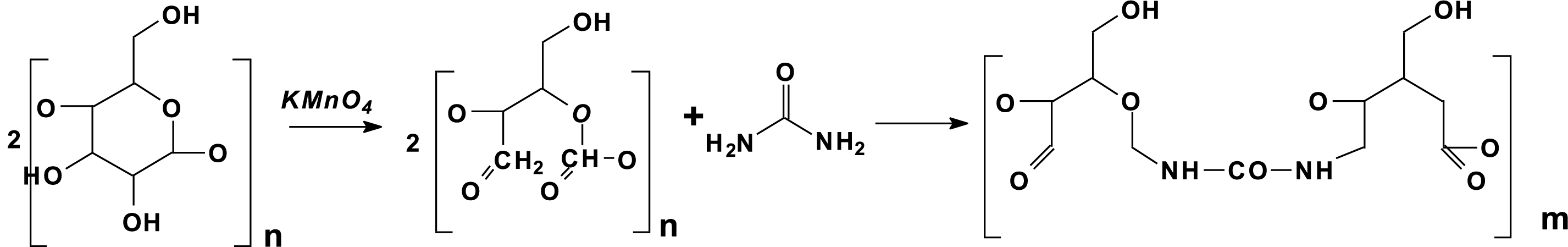
Figure 6: Cross-linking by urea of dialdehyde starch obtained by permanganate oxidation [29]
The utilization of citric acid (CA) for bonding wood panels has been an equally unexpected and revolutionary concept. Several different approaches were eventually explored, based on glucose, sucrose and other carbohydrates. Citric acid thermoset bonding for particleboard is a concept first proposed by Japanese scientists giving good results [30,31]. Such an approach has later been utilized for a number of different application, like improving waterproofing of the weldline of wood joints during wood welding [32] and to bond with citric acid alone flat plywood and LVL (laminated veneer lumber) veneers [33]. It was shown that wood carbohydrates and lignin were bound in situ in the wood by citric acid. Bonding appeared even to occur across the interface between two veneers closely joined [33], indicating the acid to function as available plywood adhesive at the molecular covalent level (Fig. 7). Later, simple sugars such as sucrose + citric acid adhesives were shown to be acceptable wood panel adhesives [34–37]. In these the pH was much lower and thus furanic compounds such as furfural and 5-hydroxymethyl furfural (5-HMF) were generated from the sugars by the residual acid and by their and the polymeric wood constituents cross-linking by the acid (Fig. 8). This constituted further confirmation of the identical cross-linking manner determined for LVL and plywood by Del Menezzi et al. [33].

Figure 7: Examples of citric acid bridging glucose or/and holocellulose chains (left), and lignin (right) among the many compounds identified [33]

Figure 8: Proposed bridging of sucrose by citric acid and linked reactive furanic moieties generated by action of the acid on sucrose [36,37]
Very recently Umemura demonstrated the use of glutaric acid, less acid than citric acid, for esterification of wood constituents, confirming for all carboxylic acids, both glutaric and citric acid were used [38], do esterify wood constituents sites as already demonstrated for citric acid alone [33].
Umemura et al. confirmed the reactions of carbohydrates with citric acid as well as formation of furanic compounds generated by the effect of the acid on heating from the sugar of sugar-citric acid adhesives in particleboard [36,37]. At the very low pH values of operation used, these reactions seemed to be the only reactions which occurred. The literature reports several types of wood composites obtained by bonding with citric acid, like wood molding, particleboard, fibreboard, and veneered wood panels. Of these the most widely investigated is wood particleboard. One grave drawback never considered at the beginning of the use of citric acid as a binder either alone or in combination with glucose or other carbohydrates is the low pH at which this type of bonding does occur.
Later work by Umemura et al. [39] disclosed on using glucose and p-toluen sulfonic acid showed that all these acid +carbohydrare systems, citric acid included, operate and harden at pH much lower than 4, even as low as pHs 2 to 3. Such low pHs unfortunately preclude the use of such systems, at least for wood adhesives, first because pHs lower than 4 on hardening are not admitted by most world wood panels standards, second because this rule is due to the heavy surface hydrolysis and degradation of the polymeric wood constituents, in particular cellulose and hemicelluloses, at the wood-adhesive interface induced by the strong residual acidity of the adhesive. Several research work on this effect on different fields of wood science are known such in CCA treated timber and on the disastrous use of acid phenol-formaldehyde resins. This drawback has been in great part solved by using citric acid systems coupled with self-neutralizing systems added to the adhesive system [40] based on a similar system conceived in the past to self-neutralize acid setting phenol-formaldehyde adhesives for wood bonding [41] and more recently with a second and different buffering system for MUF adhesives reinforced by citric acid [42]. The system used for self-neutralizing the starch-citric acid bioadhesive for example is based on the principle showed in Fig. 9.

Figure 9: The self- neutralizing system used in starch–citric acid wood adhesives [31]
These systems however have not as yet been proven valid for glucose +citric acid. Although such systems are now well known and work well, the fear of the danger induced by the presence of residual acid on the glue line interface still militate against their industrial use.
Citric acid used for binding cotton cellulose was already well known for various applications, well before the work on wood adhesives, for example in the field of textiles for finishing cotton cellulose and textiles [43]. The carbohydrates esterification was shown to occur passing through the stage of an anhydride intermediate [44], although this latter being unstable such a result is even open to discussion as direct esterification without such an intermediate is just as probable. Conversely, lignin in situ esterification in wood has only been detected and demonstrated later [33,38]. Several drawbacks do however haunt citric acid wood bioadhesives, like (i) the acid low viscosity being overcome by using with the acid sugar or other biosourced materials such as starch or tannin; (ii) its high cost and the still to-day relative short supply and consequent high cost of citric acid, which nonetheless seems likely to decrease in future as the number and capacity of production facilities is increased. The reader is advised to also consult a good review on the reactions of citric acid with wood and cellulose which does exist and well worth to read [45].
Other approaches to thermosetting bioadhesives have also been tried and reported. Among the more unusual ones, adhesives based on the reaction of glycerol triacetate and a diamine with either sucrose or glucose yielding oligomer chains of different molecular weights (Fig. 10) and performing well as plywood bioadhesives. Such an adhesive is a clear example of serendipity having been discovered by chance. However, it works, with the chemical species formed being identified and characterized [46].

Figure 10: Cross-linkable oligomer types obtained by reacting glucose and sucrose with hexanediamine and triacetin [39]. Copyright © 2023, Springer
3 Recent Developments on Different Approaches with Predominant Proportions of Lignin
3.1 Lignin Demethylation Coupled with Specific Oxidation
A biobased wood adhesive was developed successfully by modifying lignin first by demetilating it and second oxidizing it by a periodate oxidation.
Specific oxidation treatments already used to generate aldehydes from bio-oligomers with vicinal –C-OH groups by cleaving the C-C bond, as for carbohydrates, tannins, proteins and others, have also been applied to generate resins from lignin (see Carbohydrates) (Fig. 11).

Figure 11: A schematic example of vicinal hydroxyl groups oxidation and C-C cleaving
Carbohydrates treated with specific oxidants undergo rearrangements by a long time well-known reaction [14]. A number of different biomaterials have been recently shown to undergo the same reaction [15–20,47,48] and so do also lignins. This oxidation mechanism in lignin is similar but not exactly comparable to what that occurs for other types of biomaterials (Fig. 12).

Figure 12: Periodate specific oxidation brought to its completeness of a lignin unit
The in-between lignin units cleavage then occurs in two C-C bonds of the Beta-O-4 linkages (Fig. 13).

Figure 13: Sequential localization of β-O-4 cleavages by periodate specific oxidation in lignin
The aldehydes so generated are still linked to lignin oligomers and their residues. A hardened network is then obtained by the aromatic sites of pre-demethylated lignin then reacting with the aldehydes generated by oxidation.
Different instrumental analysis techniques, namely FTIR, MALDI-ToF and TMA, were used in combination for characterizing the lignin bio-adhesives developed. Moreover, the shear strength in tension of the bonded products was used to determine and characterize how the lignin bio-adhesives developed performed. The dry strength obtained using demethylated lignin yielded acceptable shear strength of the bonded wood joints, but their shear strength wet was very poor. The results then showed that sodium peroxide (NaIO4) oxidant does improve wood joints bond strength of bio-adhesives using lignin. Addition of sodium periodate at a level of 20% on demethylated lignin proved to yield optimal results. Thus, this lignin bio-adhesive contained no added aldehydes, is high in biomass level, presents a certain ease of preparation, a low cost and a good performance to bond interior-grade plywood.
The wet shear strength tested wet of plywood bonded with bio-adhesives generated from demethylated oxidized lignin however, was shown to not satisfy the China national standard requirements (GB/T 9846–2015, ≥0.7 MPa). Such a lignin adhesive will thus require additional modifications in future to reach the level of wet strength required by the relevant standards.
By oxidizing starch to dialdehyde starch with hydrogen peroxide a bio-adhesive has been developed based on pre-glyoxalated lignin cross-linking with dialdehyde starch to a hardened state through a urea bridge, with urea being covalently linked to both the aldehyde groups of dialdehyde starch and the lignin though its reactive dihydroxyethyl groups [48]. Fig. 14 shows the scheme of the reactions occurring.

Figure 14: Scheme of urea cross-linking glyoxalated lignin with dialdehyde starch according to Chen et al. [48]
Particleboards were bonded with such an adhesive coupled with tests by DSC (differential scanning calorimetry) and TMA (thermomechanical analysis). This adhesive yielded particleboard presenting internal bond (IB) strengths of the level required by the appropriate European Norms. The addition of just 5% of biosourced glycerol glycidyl ether on resin solids even improved further the adhesive IB strength and its resin density of cross-linking.
3.2 Non-Isocyanate Polyurethane (NIPU) Bioadhesives Based on Lignin
Two consecutive reactions lead to the preparation of lignin NIPU bioadhesives [49]. Carbonating the hydroxyl groups of any biosourced compound comes first. Carbonation can be achieved by a number of different reactions based for example on cyclic carbonates or CO2. Recently an easier synthesis and swifter rate of carbonation of bio-sourced materials presenting –OH groups has been reported by using a simple aliphatic carbonate, namely dimethyl carbonate (DMC). DMC is a good compound for the carbonation first step in the synthesis of NIPU. It is easily available, has no irritating or other toxic effects either on contact or inhalation, of low cost, and presents an agreeable [50].
The conditions of hydroxygroups carbonation using DMC is in general effected at 90°C, thus near to the boiling point of DMC. It is a bimolecular nucleophilic substitution, acyl-cleaving, under basic catalysis [50]. It has been used recently to prepare glucose, sucrose and tannin-based bio-NIPU resins presenting excellent bonding properties [27,28] both for coatings and adhesives for wood [51–53].
The second step of NIPU bio-resins preparation involves reacting the carbonated material with a diamine or multiamine. A scheme describing succinctly such a two steps sequence of the synthesis of a NIPU bio-adhesive is shown in Fig. 15.

Figure 15: Sequence of reactions of carbonation and amination leading to a non-isocyanate urethane bridge
NIPU organosolv lignin bio-adhesives have been used to successfully bond wood particleboard [53] but just with hot presses at 220°C–230°C. Modern particleboard factories use comparable temperatures when continuous presses are used, thus such press temperatures are not unusual, but may be problematic in older plants. Adding 10%–15% of an epoxidized silane (Fig. 16) coupling additive has been reported to partially solve this drawback, being sufficient enough to decrease down to 180°C the press temperature while still obtaining acceptable results.

Figure 16: Epoxidized silane coupling additive [53]
The additive epoxy group increases the cross-linking of the cured adhesive while the silane group renders adhesion to the substrate easier. Lignin NIPU adhesives have shown on analysis to be composed of a variety of oligomer species, with some presenting two urethane bonds within a same unit of lignin and of two lignin units connected with a urethane bridge. A large number of these oligomers are linear ad can be represented by a structure as shown in Fig. 17.
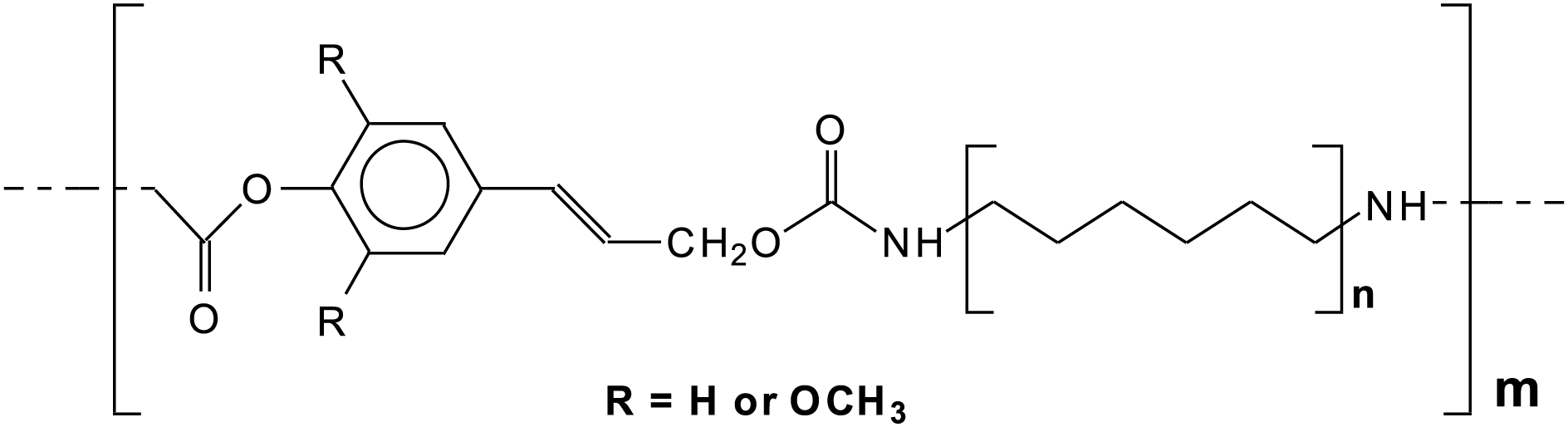
Figure 17: An example of a general linear structure for oligomers obtained by reaction with DMC and diamine of lignin aromatic and aliphatic–hydroxyl groups [53]
The mechanism by which NIPU lignin adhesives harden by cross-linking into a tridimensional cured network involves curing by just heating. A variety of different species tridimensionally branched have been detected by MALDI ToF mass spectrometry indicating the capability of NIPU adhesives to cross-link to hardened networks. Two categories of oligomers appear to be formed: (i) oligomers branched but not cross-linked in which just the reaction of diamine and DMC induces branching without any further reaction with lignin units, and (ii) oligomers which after branching have cross-linked with a number of lignin units forming tridimensional structures confirming how cross-linking of these resins occurs by heating thus leading to hardened networks. Fig. 18 shows an example of one such networked oligomeric NIPU structure detected by MALDI ToF.

Figure 18: An example of a NIPU lignin oligomer structure detected by MALDI ToF mass spectrometry [53]
Consequently, the branched NIPU lignin resins evolving into cross-linked structures can be represented by a general structural formula as shown in Fig. 19.

Figure 19: An example of a urethane cross-linked NIPU tridimensional network structure obtained by reacting with DMC and diamine all types of lignin’s hydroxyl groups [53]
The occurrence of unwanted side reactions is also ascertained. An example is the formation of oligomers produced by DMC reacting with just the diamine such as a DMC linking two diamine molecules, and others [53].
The formation of urethane bridges has also been confirmed by FTIR spectrometry.
The proportion of the “bio” content of these resins can even be considerably increased by a reaction of amination of all or a majority of the hydroxyl groups of demethylated lignin oligomers by reaction with ammonia, thus transforming them in aminogroups, thus transforming the lignin oligomers themselves in diamines or/and multiamines. Other biological polyphenols have already been shown to succesfullyand with ease undergo such a reaction, for example flavonoid tannins [54,55]. Hexamethylene diamine and multiamines have hence been eliminated by this route in the formation of NIPU resins with just the DMC –C=O residue remaining in the urethane bridges in the structures and reaching in this manner a NIPU “bio” content of up to 95% as shown in Fig. 20.

Figure 20: Example of structures observed by reacting aminated lignin with carbonated lignin [55]. Copyright © 2015, Elsevier
3.3 Reactions of Lignin with Amines
The well-known low reactivity with aldehydes of lignin aromatic rings to yield resins has always been a considerable limitation to its utilization as an adhesive. Thus, an alternative reaction had to be used to overcome this drawback. Isocyanates were then used to react with the hydroxymethyl groups in lignin-formaldehyde resins. Although such adhesives are not “green” this led to the realization that the notoriety for low reactivity and poor cross-linking of lignin was unjust as it depended only on the traditional phenol-aldehyde approach, incorrect for lignin, and that its disadvantage to form resins could be easily overcome by using reactions different from the traditional type. The use of isocyanates was then an eye opener to realize that the route to viable lignin resins was through alternative reaction systems, although toxic isocyanates themselves are now an approach to lignin resins that is unacceptable to be considered “green”. This opened the search for alternative routes for reaction and cross-linking of lignin to viable resins.
This background needs to be appreciated to understand some of the more recent developmental approaches in cross-linking other natural polyphenols by alternative polycondensation routes [56–60]. It caused the application of such alternative reaction routes to yield new lignin resins capable of cross-linking and hardening with relatively more ease. One of these routes led to hardened resins by involving the reaction of diamines with a no sulfur kraft lignin [56] and also this latter reaction with polyamines [60]. By this approach (a) reaction of amine groups substitution of the lignin phenolic hydroxyl groups yielded covalent bonds, and (b) substitution reaction by the amine groups of the lignin alcoholic hydroxyl groups of its aliphatic side chain also yielding covalent bonds; (c) some ionic bonds still subsisted nonetheless between the amine groups of aminated lignin with its aliphatic and phenolic hydroxyl groups at both 180°C and 100°C. (d) Hardened lignin resin networks are also obtained at 180°C by its reaction with hexamethylene diamine, but to a much lower level at 100°C.
The amination reaction of polyphenols was already known for ammonia reacting with phenolic hydroxyl groups [54,55].
3.4 Wood Surface Coatings and Metals/Teflon Biobinders by Triethyl Phosphate Cross-Linking of Lignin
Triethyl phosphate (TEP) has been recently shown to react with lignin through a novel reaction yielding a thermosetting binder suitable to be developed for a number of bio-based applications such as heat-resistant varnishes, surface coatings, adhesives and other resins [61]. The reaction between the two materials is a polycondensation occurring by the TEP esyerifying both types of lignin hydroxyl groups. Higher temperatures and ammonia catalysis favors the progress of the reaction. Simple model compounds of lignin hydroxyl groups, such as glycerol and guaiacol, were initially used to characterize the reaction. This was followed by confirming the results by reacting TEP with Biochoice lignin, a no sulfur kraft lignin.
The resins obtained by the reaction of lignin with TEP at 180°C and 220°C yielded hard and rigid black solids that were insoluble in acetone. Conversely, when the mixture was just heated at 90°C it did yield a black flexible semisolid resin, insoluble in water but still soluble in acetone. MALDI ToF, CP-MAS 13C NMR and FTIR spectrometries were used to characterize the resins and determine the structures been formed. The instrumental analysis indicated both the presence of some still unreacted lignin and of TEP reacted with the phenolic moieties of lignin, in structure as shown in Fig. 21.

Figure 21: A schematic example of a bound structure of triethyl phosphate with a phenolic –OH of a lignin unit [61]
TEP did also react with the –CH2OH of lignin units aliphatic side chains.
A really large variety of structures formed in the reaction were identified by combined MALDI ToF and 13C NMR analysis, such as those shown in Fig. 22.

Figure 22: Examples of some of the diverse structures formed by TEP reaction with lignin units [61]
Substitution by ammonia of the lignin phenolic –OHs also sometime does occur even linking in this manner two lignin units. The reaction occurs with relative ease and is known to occur for other natural polyphenols [61–63].
Several structures have been identified to have formed in this manner (Fig. 23).
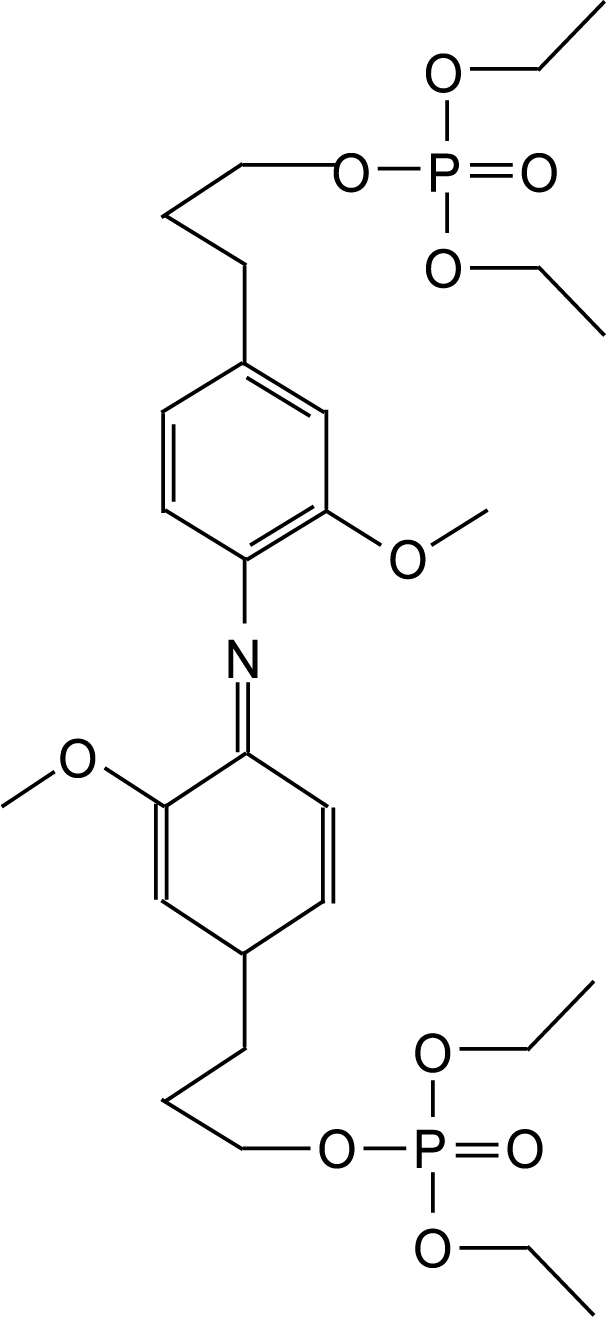
Figure 23: An example of a structure formed during the reaction of lignin with triethyl phosphate where the ammonia used as catalyst has substituted a phenolic –OH [61]
The sessile water drop test was used to ascertain how effective these TEP-lignin wood surface coatings were Fig. 24 shows the effectiveness of such a surface coating with the results attesting their suitability for wood application.

Figure 24: (Right) Variation as a function of time of a water drop contact angle in the water sessile drop test on a untreated beech wood control and on a TEP-Lignin surface coating on beech wood. (left) Aspect in the water sessile drop test at 60 s on: (a) the non treated beech wood control), and (b) on a TEP-Lignin surface coating on beech wood [61]
The initial contact angles of a drop of water in the sessile drop tests in Fig. 24 indicate that a much higher contact angle occurs if the wood surface is coated with a TEP-lignin resin rather than on the uncoated beech wood surface. Furthermore, the value of the contact angle of the water drop is much more stable as function of the time of permanence for the treated wood surface than for the untreated control.
The reaction is temperature-dependent, hence a temperature from 180°C favors it to reach the state of a hardened, not soluble resin. The presence of ammonia also favors the reaction ammonia as it yields a higher density of cross-linking.
The resins based on the reaction of TEP with both lignin and with other renewable polyphenols such as tannins, has been originally developed to prepare a Teflon natural binder on aluminum and steel for non-stick frying pans. Some examples of this are shown in Fig. 25 [62–64].

Figure 25: Non-stick pan metal base showing the applied lignin-TEP coating before Teflon covering (left) and the same non-stick pan metal base with the Teflon coat layer bonded with the underlying lignin-TEP binder by a proprietary process [64]
These bio-sourced binders were developed to eliminate polluting phenol-formaldehyde adhesives. The Resins was tested to be able to adhere well to both the metallic pan base and to the non-stick Teflon overlayer. Fig. 24 shows the case in which the polyphenols-TEP coat was initially adhering to a steel or aluminum pan base before Teflon being applied (left) and after Teflon application (right), according to a proprietary process. The factory requirement test which was passed very satisfactorily was to be capable to withstand repeatedly each time for 11 min to a temperature higher than 400°C. Such an application is of course patented [63] and has been tested industrially.
3.5 Citric Acid and Wood Lignin Bonding
Citric acid alone is capable of directly bonding wood panels [30–33,38]. It can also improve the bonding performance to wood when added to either commercial synthetic adhesives or new bio-based adhesive combinations [33]. Wood panels can be bonded by just citric acid alone by extensive esterification of both wood lignin and thholocellulose constituents forming direct cross-links between substrate surfaces in contact [33]. Model compounds have at first be used to show the sites on lignin and carbohydrate constituents likely of reacting directly with citric acid and leading to identify the esterified sites as shown in Fig. 26 for lignin and for the wood substrate carbohydrates constituents as shown in Fig. 27.

Figure 26: Example of lignin units multi esterified by citric acid by both reacting with phenolic and aliphatic hydroxyl groups [33]

Figure 27: Example of monosaccharides multiesterified by citric acid [33]
Direct cross-linking of polymeric wood carbohydrates and lignin also appear to occur on flat wood veneer substrates increasing cross-linking density on and of the wood. Thus, joint strength of laminated veneer lumber (LVL) and plywood is improved. Some lignin internal rearrangements do also occur simultaneous to its esterification by citric acid. The performance of direct bonding by citric acid alone for LVL and plywood, hence with just the acid applied to flat wood veneers, gave excellent bond strength results. However, higher hot press temperatures than what is usual were needed for this application (180°C for 10 min). X-ray densitometry examination of the interfacial bond line between veneers determined that very high densities of the interfaces resulted with density values as high as 1015 kg/m3, thus 3 times higher than the veneers core density (Fig. 28) which were practically as low than that of the veneers not pressed. Thus, the thickness density profile of plywood and LVL bonded directly by citric acid alone shows an alternation of low and high density zones through the panel thickness [33,65].

Figure 28: X-Ray panel thickness density profile of a 5-layer LVL panel bonded directly by citric acid alone (ρ = 610 kg/m3) [33]
SEM micrographs (80x) observation indicated that even at 3000x magnification it was not possible to identify the bond line. This confirmed that the veneers surfaces themselves were unduly densified at the bond line. The appearance that the veneers bond line surface boundaries did either melt or somewhat flow to densify as such by the action of citric acid under heat. It is even possible that materials from the veneers surfaces became entangled by partially flowing into each other. This appears to infer that the reaction between the untreated veneer surface and the citric acid treated veneer surface caused some partial wood melting and flowing, leading to easier veneers compaction and consequently increasing interfacial density.
Adding a citric acid solution on the wood surfaces in wood friction welding also induced some clear wood surfaces melting and flowing at the interface improving the water resistance of the welding line. As wood welding causes wood intercellular material, hence ignin and hemicelluloses, to partially melt and flow the above my well show the important effect of either citric acid grafted lignin or citric acid grafted hemiceluloses or both to further improve the welded joint water resistance [32].
It is worth noting that in all this flurry of novelties in lignin adhesives the use of lignosulfonates for water proofing other mostly biomass wood adhesives has not been forgotten either. Thus, a recent resin adhesives was prepared by oxidizing sodium lignosulfonate with hydrogen peroxide to obtain oxidized lignin presenting quinone and aldehyde structures. This material was then mixed with soy meal to yield a biomass resin presenting much improved water resistance than soy meal alone for the plywood bonded with it [66]. The condensation reaction was shown to occur between the oxidized lignin-generated quinone and aldehyde groups with the amino groups present in the soy protein side chains yielding a hardened network of markedly improved water resistance of the bonded joint. A similar approach was taken by condensing sodium lignosulfonate with the aldehydes generated by oxidizing sucrose [67]. In this approach the optimal conditions of preparation were of 13% oxidizing compound. Chemical analysis confirmed addition reactions between lignosulfonate and oxidized glucose yielding on curing a density network, thus enhancing the cured adhesive and the bonded joint water resistance.
4 Recent Developments in Tannin Adhesives
Tannins do react and polycondense covalently by applying heat with both soy flour and soy protein hydrolysate even without the need to add a hardener or other cross-linking additive. This without any rearranging treatment to improve the reactivity of the tannin. This approach has led to the formulation of wood panels adhesives with either soy flour or soy protein hydrolysate presenting good bonding performance [68–71]. The indication that this was possible was first obtained by the reaction of a condensed flavonoid tannin extract with collagen [72]. An example of one of the higher molecular weight structures detected is shown in Fig. 29.

Figure 29: Example of a structure detected by direct covalent reaction between flavonoid tannin monomers and oligomers with the side chain groups of a short sequence of linked collagen aminoacids [72,73]. No hardeners added
The structures are obtained by bonds directly formed between the tannin and the soy protein [73], and other proteins such as collagen [72] side chains with the formation of a relatively small proportion of covalent and ionic linkages at ambient temperature but a considerable proportion of covalent linkages tannin-protein amino acids and the disappearance of ionic bonds at higher temperature, namely already at 80°C [72]. No hardeners either synthetic or biosourced are added [72,73]. The linkages between the two materials appeared to occur by amination of the phenolic –OHs of the tannin by the amino groups of the non-skeletal side chains of arginine, and by esterification by the –COOH groups of glutamic and aspartic acid of at least the aliphatic alcohol-OH on the C3 site of the flavonoid units heterocycle of the tannin. The proportion of covalent linkages increases markedly and predominated with increasing temperatures.
The adhesive tested for plywood was composed exclusively of the two vegetable biomaterials, soy protein isolate and a condensed tannin, and thus was totally bio-sourced and non-toxic, soy protein being a food grade material, and tannins having been classified as being not at all toxic by the European Commission REACH program. The pre-reaction between the two yielded the best plywood bonding results when limited to a temperature of 40°C (Fig. 30), final cross-linking being achieved during the plywood higher temperature hot pressing procedure, as for any other thermosetting adhesive. Pre-reaction at higher temperatures, namely 60°C and 80°C, achieved extensive premature cross-linking, pre-curing and hardening of the combination of the two materials as any capability to cross-link further was lost when hot pressed for preparing plywood (Fig. 30). The plywood shear strength tested dry, after 24 h cold water soak and 1 h in boiling water satisfied the requirements of relevant standards.

Figure 30: Effect of pre-heating treatment (40°C, 60°C, and 80°C) on shear strength of plywood specimens in different conditions (dry, cold water soaking, and boiling water soaking). Control: Ambient temperature (20 ± 2). EN 314-2 specifies 1 N/mm2 for dry shear strength of plywood [73]
Previous work on this system had used addition of hardeners such as paraformaldehyde, or better hexamethylenetetramine (hexamine), as at that time the use of a hardener was considered necessary [68–71] as only the low temperature predominant ionic bonds between soy and tannin had been detected by MALDI mass spectrometry [69,70]. The protein-tannin direct covalent bonding reaction [72] at higher temperature had not yet being detected. Even this approach gave good bonding results but the use of formaldehyde and formaldehyde yielding compounds as hardener tended to deny the full biobased nature of such adhesives.
4.1 Development of Tannin-Based Non-Isocyanate Polyurethane (NIPU) Adhesives
Tannin extract-based NIPU resins have been the first fully bio-NIPUs ever prepared for wood surface coatings and foams [74]. Glucose based NIPU adhesives have been produced but they need a curing temperature as high as 200°C to obtain acceptable results for the relevant standards [27,28]. The same temperature is needed for tannin NIPU adhesives for wood panels. In the preparation of Tannin-based NIPU adhesives bio-sourced glycerol diglycidyl ether (GDE) had to be added, on top of the same reaction also used to form the glucose and lignin NIPU resins, to decrease the temperature of hot pressing, upgrade bonding, and decrease the volatile organic compounds emission that might be harmful, such as any residual excess of hexamethylene diamine. The GDE epoxy group is capable of cross-linking with ease with the diamine amino groups or other chemical species also containing amino groups. Thus, the cross-link is so eased that a lower hardening temperature can be afforded to be used. Simultaneously, esterification by the GDE epoxy group of the alcoholic character hydroxyl groups on the flavonoids C3 also occurs. Addition of the GDE helped reduce the temperature of curing down to 180°C from the 220°C of the same NIPU resin without GDE addition. he reduced. Fracture surface morphology resulted to be improved as well as the thermal stability. Addition of the GDE improved considerably the bond strength of the plywood bonded with such a resin at the lower hot press temperature used, the panels strength well exceeding the standard requirements for interior grade plywood (≥0.7 MPa) in relation to the tannin NIPU resin without such an additive. A further refinement of this approach was also tested by substitute to different extents the hexamethylene diamine with polyethylene imine [75] yielding a decrease in the adhesive hexamethylene diamine content from 31% down to 19%. This adhesive too passed the relevant requirements the European Norm EN 312–2010 [76] for 12-mm particleboards of type P2.
4.2 Tannins with Renewable Hardeners and Other Resins
While paraformaldehyde or hexamine have been in the past the original hardeners of choice for tannin adhesives the substitution of these has been a strong incentive to find more environment friendly hardeners. One interesting very recent example is the use of hardeners based on renewable polymeric carbohydrate-based sap bio-exudates obtained from African trees. These present furanic moieties linked to the carbohydrate chain that added to the tannin resin release on hot-pressing in the particleboard 5-Hydroxymethyl furfural (5-HMF) and furan 2,5-dialdehyde hardening the resin [77]. Even the fast reacting procyanidin-type tannins, such as pine bark tannin extract, harden well with the furanic materials both released from, or still linked to, the carbohydrate oligomer chain [77] (Fig. 30), with good wood panels bond performance. The reactions occurring are schematically shown in Fig. 31.

Figure 31: Polymeric carbohydrate-based sap bio-exudates from african tree species proposed as procyanindin-type tannin extracts hardeners by reactive furanics released by neutral pH hydrolysis. Sometime direct reaction of the furanic moieties still linked to the carbohydrate chain of the exudate with the tannin have been shown to occur, without any detachment of any furanic compounds from the carbohydrate chain [77]. Copyright © 2019, Elsevier
The relatively limited world production of tannins to-day, around 220–240 thousand tons yearly, in relation to the much higher proportion of synthetic wood adhesives indicates that alternative solutions to this drawback must be sought. While the potential availability of tannins in the world runs into the millions of tons, more extraction factories would need to be erected to fully exploit this resource. While some new extraction factories have indeed appeared or have been in planning in the last decades these this movement is ongoing but still too slow and the existing facilities are still not enough to satisfy the potential demand for tannin adhesives. This situation has thus dictated the need to extend the tannin resource with other renewable biomaterials. Under this scenario extension of the tannin with other more abundant biomaterials has become an urgent necessity. Such an approach has also been taken for the formulation of mixed biomaterials-based wood adhesives by preparing either tannins-lignin adhesives copolymerized in situ in the wood panel during hot-pressing (Fig. 32) [78] or tannin-soy copolymers [68–71,73,79], and the use of tannin–furfuryl alcohol adhesive formulations, furfuryl alcohol being also a bio-based material [80,81].
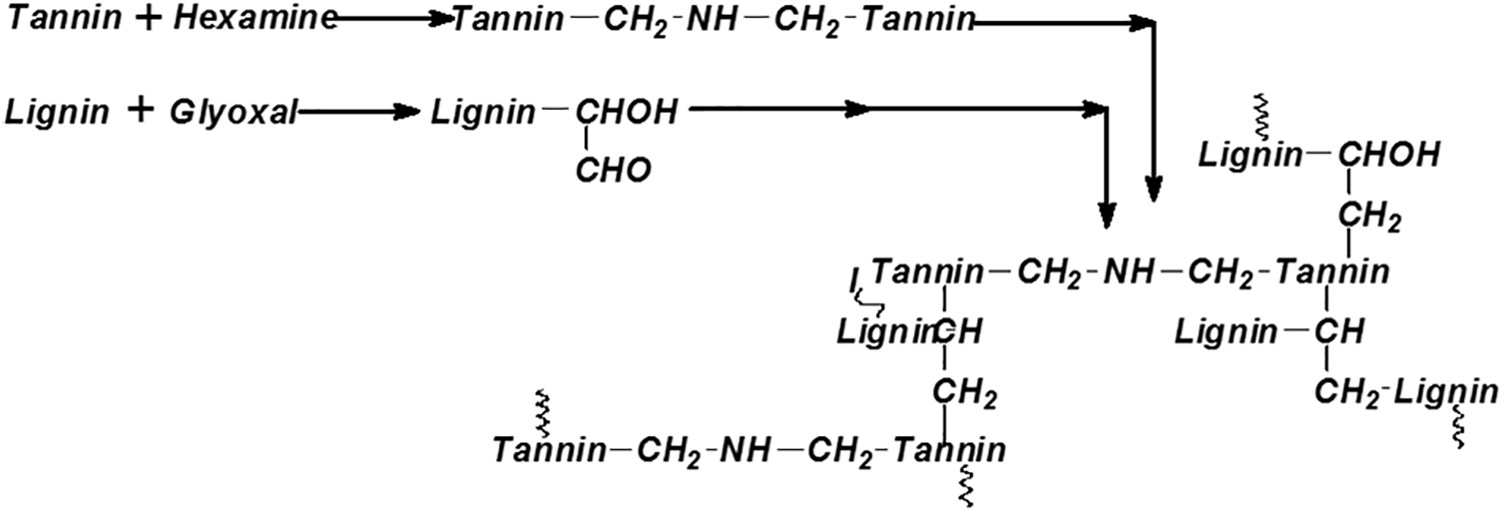
Figure 32: Scheme of the sequence of reactions of a flavonoid tannin with glyoxalated lignin [78]. Copyright © 2010, Taylor and Francis
The second new constraint is the demand of most companies to eliminate formaldehyde emissions from tannin adhesives. Several routes have been used to achieve this: (1) using aldehydes of lower or no toxicity for substitution and complete elimination of formaldehyde [19,82–84]. The aldehydes used for this were glyoxal, glutaraldehyde, and even vanillin which thus yielded a tannin adhesive completely biosourced, Equally, even aldehydes generated by periodate oxidation of monomeric and polymeric carbohydrates, non-volatile, were used in this approach [84]. It must be pointed out that oxidation of the oligomeric hemicelluloses residues always present in tannin extract were also successfully used for this purpose without the need to add any other carbohydrates to the tannin extract. (2) Non-aldehyde hardeners like trishydroxymethylnitromethane [85] and trishydroxymethylaminomethane [86] were also successfully used. (3) Addition to the tannin extract of furfuryl alcohol which functioned as both a hardener and linked comonomer [87]. (4) Forming –CH2–NH–CH2– bridges linking tannin chains by using hexamine, with even the formaldehyde emission generated by just heating the wood or other formaldehyde emission being absorbed by the secondary amine groups of the –CH2–NH–CH2– to yield panels that are really truly at zero-formaldehyde emission [88–91]. (5) Tannins autocondensation curing without hardeners lastly, even promoted by the catalytic action on the adhesive by the lignocellulosis substrate for the procyanidin-types like tannins from pine bark, and catalyzed by a number of different accelerators like silicates and SiO2 smoke for the slower reacting tannin types [92–97] also lead to preparing interior grade particleboard panels.
Coreaction with non-furanic and furanic humins of tannins [98] yield NIPU adhesives too. Both humin types are renewable and bio-sourced. The commercial non-furanic types have yielded NIPU predominantly humin-based wood panels binders comprising both tannin-humins and humins only NIPU adhesives with the former performing better.
Paraformaldehyde, and hexamethylenetetramine (hexamine) are still to-day the hardeners of choice being used industrially for tannin adhesives [99] although tannins being phenolics the proportions of aldehyde used and the formaldehyde emitted are very much lower than the for the corresponding oil-derived synthetic wood adhesives.
Condensed tannin-polyethyleneimine adhesives have also been experimented with yielding good bonding but the polyethyleneimine synthesis being rather complex, the monomers used for it being toxic, and such a product having a high cost alternatives were sought. Thus, melamine-based (melamine being biosourced from trimerization of urea) highly branched polyamine have been synthesized through an easy route and was shown to be an ideal substitute to replace polyethyleneimine in this type of tannin adhesive [100]. The plywood bonding obtained with these adhesives was good, and the water resistance also. The proposed mechanism for polyamine-catechol crosslinking reactions was verified also with for the melamine polyamine, confirming that both Schiff base formation and Michael addition contribute to the formation of crosslinked network.
A biomass adhesive composed of chitosan and tannin has also been developed by a rather easy procedure [101] to bond bamboo, wood and bamboo-wood substrates and has been successfully tested. The hot press temperature used was of 160°C. This adhesive was shown to present good water resistance, high wet shear strength even at the low curing temperature reported, although a higher temperature of curing yielded even better results.
Finally, condensed tannin copolymerized with hyperbranched tris(2-aminoethyl)amine-urea formed by amine-amido deamination were found to yield a thermoset adhesive for particleboard with no aldehydes but satisfying the requirements of relevant standards for particleboard internal bond strength [102]. The tannin-triamine-urea cures well at the 180°C, a relatively low temperature for to-day’s particleboard manufacturing lines. Formaldehyde emission was tested to be zero as no aldehyde was used, not even its traces generated by wood heating. The tannin seems to act as an additional cross-linking agent, almost like a nucleating molecule, for the triamine-urea hyperbranched oligomers. The predominant cross-linking reaction is that of the substitution of the tannin phenolic hydroxyl groups by the amino groups of the triamine appeared to be the predominant reaction (Fig. 33). Reaction of the tannin with the still free urea amide groups seems to be rather rare but it may occur with the rarer tannin flavonoid units with an opened heterocyclic ring.

Figure 33: An example of a predominant initial species starting to form the final hyperbranched networks leading to good strength wood adhesives in the tannin-hyperbranched tris(2-aminoethyl)amine-urea [102]
The combination of the three materials forms hyperbranched networks leading to good strength wood adhesives.
5 Starch and Starch Hydrolysate Adhesives
Starch, as a raw material for biomass adhesives, is a semi-crystalline substance consisting of amylose and amylopectin in specific proportions [103–106]. Being originated from the roots, stems, leaves, and fruits of plants, starch shows some advantages, such as sustainability, large reserves, and easy to isolate and obtain. Its basic structural unit is primarily glucose. Hydrolyzed products of starch like glucose, sucrose, and soluble starch are commonly utilized in biomass adhesive formulations. Starch can serve as an adhesive after gelatinization, with its bonding provided by the formation of hydrogen bonds between the hydroxyl groups of the starch and those on the wood at the interface of starch and wood [107]. However, the breaking of hydrogen bonds to moisture can lead to reduce the water resistance of starch or starch hydrolysate-based adhesives. To enhance the water resistance of starch-based adhesives, some modification methods such as esterification [108–110], oxidation [111–115], grafting [116], copolymerization, and cross-linking [109,117,118] are employed. Esterification and oxidation are particularly effective methods which have attracted more attention during the last five years. Numerous studies have successfully utilized raw materials like glucose, sucrose, and starch to develop wood adhesives with improved water resistance through esterification and oxidation.
Oxidative modification is a method in which the hydroxyl group in glucose or glucose unit has oxidized to form an aldehyde group or carboxyl group under the action of an oxidant. Commonly utilized oxidants for this process include KMnO4 [29], H2O2 [111], NaClO, (NH4)2S2O8, and NaIO4. The oxidized starch can then interact with compounds that contain hydroxyl, phenolic hydroxyl, or amino groups, leading to the formation of a cross-linked structure. The hydrogen bond has been replaced by more stable chemical bonds, thereby enhancing the water resistance of starch adhesives. To gain insights into the reaction mechanism, Song et al. [13] employed sucrose as the raw material, subjecting it to oxidative modification. The resulting product was then combined with hexamethylenediamine in a Schiff base reaction to produce an adhesive with high water resistance. The study further investigated the impact of different oxidants (KMnO4, H2O2, NaIO4, (NH4)2S2O8, and NH4NO3), oxidation duration, and curing temperature on the properties of the oxidized sucrose-based adhesive. The findings revealed that NH4NO3 exhibited the most effective oxidation. Optimal dry and wet adhesive strengths of the oxidized sucrose adhesive were achieved when the oxidation time, oxidant dosage, and curing temperature were set at 45 min, 13 wt.%, and 160°C, respectively, resulting in values of 1.52 and 0.93 MPa. Feng et al. [118] introduced an innovative oxidant-free air oxidation method for producing an oxidized sucrose-based adhesive. The process involves hydrolyzing sucrose in an acidic environment (pH approximately 3), followed by oxidation in the air. Subsequently, polyamine compounds were added for curing and cross-linking to enhance its water resistance. Experimental results indicate that the optimal conditions for achieving the highest bonding strength include a sucrose/polyamine molar ratio of 5:1, an acid hydrolysis time of 2 h, a reaction time of 1 h, and a hot-pressing temperature of 200°C. Under these conditions, the adhesive exhibited dry and wet bonding strengths of 2.4 and 1.6 MPa, respectively. However, it is important to note that the study lacks evidence regarding the conversion of sugars into furans in an acidic environment.
Compared to small molecule sucrose, starch has a more complex chemical structure, presenting new challenges for oxidative modification. Zhao et al. [29] utilized KMnO4 as an oxidant to modify corn starch, followed by the introduction of urea as a cross-linking agent to prepare a urea-oxidized starch adhesive. The addition of nano-titanium dioxide was a novel approach to enhance the bonding strength of the resulting adhesive. In a separate study by Song et al. [119], oxidized starch was prepared using NaIO4 as the oxidant. The oxidized starch was then reacted with hexamethylenediamine through a Schiff base reaction to produce an oxidized starch adhesive with excellent water resistance. Another study by Liu et al. [113] involved the use of Fenton’s reagent to oxidize starch, followed by the addition of stabilizer polyvinyl alcohol (PVA) and cross-linking agent isocyanate (PM-200) to create a starch-based adhesive with a network cross-linked structure (SFA). The formation of this cross-linked structure significantly improved the water resistance of the starch adhesive. Based on these studies, the oxidative modification mechanism of starch adhesive is illustrated in Fig. 34. Starch undergoes oxidation by oxidants to generate aldehyde groups or carboxyl groups. Subsequently, a cross-linking agent capable of reacting with oxidized starch, such as polyamines or isocyanates, is introduced into the system to form a cross-linked network structure through condensation reactions, Schiff base reactions, etc.

Figure 34: The oxidative modification mechanism of starch adhesive [29]. Copyright © 2018, Elsevier
5.2 Esterification Modification
Esterification modification is a viable method for enhancing the performance of starch adhesives. In comparison to oxidative modification, the preparation process for esterification modification is more straightforward. These esterifying agents like polybasic acids/esters [119–123], long-chain fatty acids [124–127], acid anhydrides [128], and phosphates [129,130] react with –OH groups of starch to create ester bonds, introducing new active groups such as saturated long carbon chains, –C=C-, and –COOH into the starch structure. This results in starch acquiring new chemical properties and improving the mechanical properties and water resistance of starch products. Fig. 35 illustrates common esterification modifications for starch.

Figure 35: Esterification modification of starch [131]. Copyright © 2019, Elsevier
Sun et al. [108] utilized dodecyl succinic anhydride to esterify with starch, incorporating long hydrophobic chains into the starch structure and introducing a significant amount of isocyanate during the curing phase to synergistically enhance the hydrophobicity of the starch adhesive. Zia-ud-Din et al. [132] employed vinyl acetate to modify hydrolyzed corn starch by esterification reaction under nitrogen protection, introducing –C=C- into the starch structure. During the curing process the initiator, ammonium persulfate, was added and reacted with oxidized starch to form a cross-linked network via free radical polymerization, enhancing the wet bonding strength of the starch adhesive. Building upon this, sucrose fatty acid was grafted onto the starch structure through esterification. The sucrose fatty acid acts as a side chain to prevent starch adhesive aggregation, which is conducive to the occurrence of esterification reactions between vinyl acetate and starch, enhancing the wet bonding strength of the obtained starch adhesive. Jin et al. [127] grafted corn starch with itaconic acid (IA) and N-hydroxyethylacrylamide (HEAA) to develop a renewable, biodegradable, formaldehyde-free starch-based adhesive for particleboard production. The study revealed that IA enveloped and coated the surface of starch granules, undergoing an esterification reaction with -OH on the starch surface, introducing active –C=C- into the starch structure. HEAA was unevenly distributed on the starch surface as cross-linked main chains. These two components formed a complex three-dimensional cross-linked structure through self- and mutual cross-linking during the hot-pressing process, strengthening the water resistance of the starch adhesive and effectively preventing board disintegration due to excessive expansion during soaking. In these works, esterification reaction was employed to incorporate –C=C- groups into the starch structure, followed by cross-linking via free radical initiation to create a network structure that enhances the water resistance and bonding strength of starch adhesives. Nevertheless, the establishment of the cross-linked network is mainly dependent on the free radical polymerization involving –C=C- in the modified structure, leading to challenges in the formation of network. Additionally, the abundant active –OH groups in the starch structure remain underutilized.
Inspired by esterification modification, polybasic acid/ester can serve as an esterification modifier for starch. This allows for the esterification and cross-linking with –OH groups in starch or starch hydrolyzate, resulting in a stable cross-linked structure to enhance water resistance in starch adhesives. Common polybasic acids/esters used for this purpose include citric acid, maleic acid, phosphoric acid, maleic anhydride, polylactic acid [125], etc.
In previous research, sucrose and citric acid were dissolved at room temperature to create an adhesive for the production of particleboards [34–36,133,134]. These studies have shown that during hot pressing, sucrose was converted into 5-hydroxymethylfurfural (5-HMF) with the participation of citric acid. The 5-HMF and remaining sucrose can both react with citric acid to form a cross-linked structure, enhancing the water resistance of the obtained adhesives [36]. Additionally, citric acid acts as a cross-linking agent, and can also react with hydroxyl groups from wood surfaces to boost mechanical strength. The transformation reaction of 5-HMF and the esterification reaction of the sucrose-citric acid solution can only be completed during hot pressing [37]. This means that the adhesive requires relatively harsh curing conditions (200°C, 10 min). Furthermore, the rapid penetration of the solvent water was not conducive to the esterification reaction between sucrose and citric acid. Wibowo et al. [135] introduced ZnCl2 as a catalyst to accelerate sucrose hydrolysis into glucose and fructose, and then convert into 5-HMF. 5-HMF polymerized into humin polymers under ZnCl2 catalysis. Citric acid was introduced and esterified with humin polymers to form a cross-linked structure. In this study, the self-polymerization of 5-HMF and the esterification reaction between humin polymers and citric acid were found to jointly contribute to the formation of the cross-linked network. As a result, the introduction of ZnCl2 was observed to have a positive impact on reducing the curing conditions of sucrose-citric acid adhesive compared to previous research. This cure time by 40% at 200°C. However, the high reactivity of 5-HMF limited the adhesive’s storage period.
Being different from previous studies, Li et al. [136] employed glucose and citric acid as raw materials and established suitable reaction conditions to create a more stable citric acid-glucose-based (CAG) adhesive through an esterification reaction in an aqueous phase. The esterified glucose molecules with different molecular weights in the adhesive contain numerous active groups, which can further esterify during the hot-pressing stage to form a stable cross-linked structure. The esterification reaction between citric acid and glucose can take place during both the preparation of the adhesive and the hot-pressing process, rather than just during the hot-pressing process. It is anticipated that the CAG adhesive, compared to citric acid-sucrose adhesive simply mixed at room temperature, can develop a cross-linked structure with a greater degree of esterification after curing, which exhibits high water resistance and binding strength. The results indicated that a molar ratio of citric acid to glucose above 0.6 and a reaction time of 1 h resulted in a CAG adhesive possessing a wet adhesive strength that met the requirement of China National Standard of GB/T 9846-2015 [137]. Li et al. [138] continued this research to explore the preparation of various polyester glucose-based adhesives using different polybasic acids (phosphoric acid, maleic acid, and citric acid) and glucose, analyzing the reactions between these acids and glucose in depth. By examining the similarities and differences in mechanisms, they were able to evaluate the performance variations among the polyester glucose-based adhesives obtained. This study offers insights to develop water-resistant polyester adhesives using polysaccharides like starch as raw materials.
The molecular weight of starch is higher than that of glucose, suggesting potentially better bonding performance. However, the semi-crystalline structure of starch presents challenges for esterification modification. In a study by Widyorini et al. [139], citric acid was initially used in the preparation of particleboards. Due to the small molecular size of citric acid, effective cross-linking between citric acid and -OH groups on the particle surface was limited. To address this, starch was introduced into citric acid to leverage its macromolecular structure and adhesion properties, compensating for the molecular size limitation of citric acid. Through an esterification reaction, a network structure was formed between starch and citric acid, enhancing the mechanical properties of the resulting particleboard. Results indicated that the optimal dry strength was achieved at a citric acid/starch mass ratio of 87.5:12.5, yielding approximately 1.21 MPa. However, its wet bonding strength was not investigated. In another study by Kusumah et al. [140], a citric acid-modified starch adhesive was prepared by directly mixing citric acid and starch at room temperature for particleboard production. While the resulting starch adhesive exhibited good dry strength, the wet strength was not reported. Amini et al. [141] utilized citric acid and corn starch, along with sodium hypophosphite as a catalyst, to create a citric acid-esterified starch adhesive at low temperature (50°C). However, the addition of phenolic resin was necessary to achieve bonding strength meeting usage standards.
The water resistance and bonding strength of the polyester starch-based adhesive prepared in these studies showed significant improvements compared to unmodified starch adhesive. However, to achieve the wet bonding strength of GB/T 9846-2015 [137], it was found necessary to incorporate auxiliary reinforcing substances such as phenolic resin or isocyanate. This is because the esterification modification of starch was hindered by its semi-crystalline structure of starch. The limited penetration of the modifier into the dense crystalline regions results in the esterification reaction primarily occurring on the surface of the amorphous and crystallized regions. These crystallized regions are susceptible to damage from water penetration in humid conditions, causing the cross-linked structure to deteriorate and reducing the water resistance of the adhesive. Therefore, activating starch during the esterification modification process is crucial for enhancing the overall performance of the starch adhesive.
Li et al. [142] observed the acidolysis and esterification reactions between citric acid and starch. They utilized citric acid as a modifier to react with starch to create a citric acid-starch (CASt) adhesive. This modification method is not limited by the kind of starch. The prepared polyester starch adhesive has excellent water resistance and bonding strength, without the need for introducing reinforcing substances. Its reaction mechanism is shown in Fig. 36. The acidolysis reaction breaks down the crystal structure within the starch, revealing numerous active groups that facilitate the esterification reaction. The structure of the CASt adhesive, containing active groups, allows for continuous esterification during the hot-pressing process. Citric acid was grafted into the starch structure via an esterification reaction to act as an active side chain, thereby preventing the starch adhesive from gelling as a result of hydrogen bond reorganization. Additionally, the inherent antibacterial properties of citric acid enhance the mildew resistance of starch adhesives, extending their service life. Even after 5 months of storage at room temperature, the resulting CASt adhesive maintains good fluidity and does not exhibit mold growth. Its wet bonding strength of 1.05 ± 0.02 MPa was only 20% lower than that of freshly prepared adhesive.
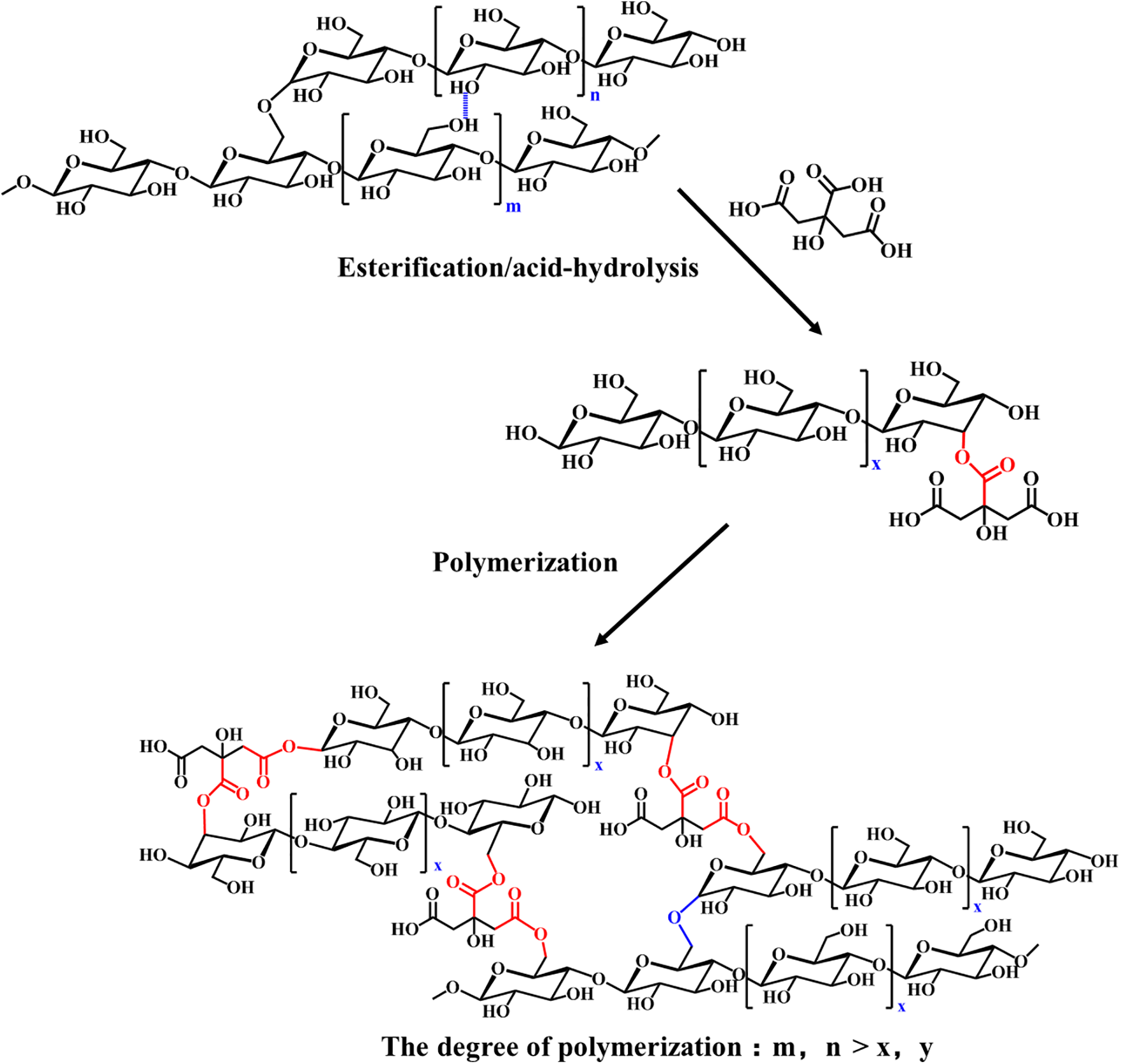
Figure 36: The esterification modification mechanism of starch adhesive [142]. Copyright © 2023, Elsevier
6 Modification and Research Progress of Plant Protein Adhesives
Traditional formaldehyde-based adhesives used in the production of wood-based panels pose potential formaldehyde pollution risks to the living environment. Plant protein adhesives, with their abundant sources, environmental friendliness, and renewability, have shown significant application potential and have become a research hotspot. Recent studies have addressed the issue of poor water resistance in plant protein adhesives. However, challenges such as high viscosity, poor coating performance, low pre-pressing strength of the panel, susceptibility to mildew, high cost, poor functionality, poor water retention, and brittleness remain key obstacles to their widespread application. Therefore, this review deals the innovative research on the modification and industrial application of plant protein adhesives in recent years, focusing on the causes of these issues, modification strategies, innovative methods, and their implementation effects. Additionally, it explores the future directions for the development of plant protein adhesive modification and application.
In recent years, due to the advantages of low cost and high strength, formaldehyde-based adhesives have dominated the wood processing industry. However, the raw materials for formaldehyde-based adhesives are mostly derived from non-renewable resources such as petroleum, coal, and natural gas. During production, transportation, and use, it is difficult to avoid the release of formaldehyde and phenol, which can cause potential pollution to the living environment and harm human health. As consumers’ concerns about indoor air pollution continue to rise, the development and application of formaldehyde-free wood adhesives have become a research hotspot in the wood-based panel industry.
Biomass adhesives, due to their non-toxic and renewable characteristics, have attracted significant attention. These include adhesives based on starch, plant proteins, lignin, vegetable oils, and tannins. Among them, plant protein-based adhesives have become a research focus in the field of biomass adhesives in recent years due to their abundant sources and ease of modification. However, plant protein adhesives have limitations such as low bonding strength and poor water resistance due to strong intermolecular forces and the abundance of hydrophilic groups in their molecular chains, which restrict their practical application. To address these issues, researchers have explored various modification techniques, including protein molecular design, biomimetic structure construction, branched structure construction, multi-network structure design, and chemical crosslinking. Among these techniques, chemical crosslinking modification, especially epoxy modification, is considered one of the most effective and practical methods to improve the water resistance and bonding strength of plant protein adhesives. Adhesives prepared using these methods have been successfully applied in the production of plywood and blockboard.
However, in actual industrial production applications, plant protein adhesives still face many challenges. For instance, the relatively large molecular weight of plant proteins leads to low solid content and high viscosity of the adhesives, causing difficulties in veneer coating. During industrial production, after the adhesive is applied and the veneer is assembled, cold pressing is required for shaping. The low pre-pressing bonding performance results in difficulty in forming the panel, especially for veneers with high moisture content, where pre-pressing performance decreases sharply. Additionally, wood-based panels are flammable, and their use in interior decoration may pose fire hazards, leading to significant property loss and threats to human life. This paper mainly reviews and discusses the recent progress in the modification research of plant protein adhesives to address issues such as high viscosity, poor coating performance, low pre-pressing strength of the panel, brittleness, susceptibility to mildew, poor water retention, and poor functionality in practical applications. Furthermore, it explores the future development prospects of plant protein adhesives.
6.2 Low Viscosity Modification
Viscosity determines the flowing ability of adhesives. If the viscosity is too low, plant protein adhesives may excessively penetrate into the veneer, hindering the formation of an effective adhesive layer. Conversely, if the viscosity is too high, the adhesive cannot be uniformly applied to the veneer surface, resulting in poor bonding uniformity and stability. Plant protein molecules have a higher relative molecular weight compared to formaldehyde-based adhesives, leading to molecular chain entanglement and strong intermolecular forces. When mixed with water, the resulting adhesive exhibits excessively high viscosity, causing difficulties in application, increased coating amounts, and unstable mechanical properties of the final product.
Adding small oily molecules and metal ions can effectively reduce intermolecular forces, thereby lowering the viscosity of plant protein adhesives. Li et al. [143] prepared a plant protein adhesive by blending peanut meal and soybean meal, and added low molecular weight oily triglycidylamine (TGA) to the system. TGA effectively reduced the intermolecular or inter-aggregate forces of protein molecules, lowering the initial viscosity of the adhesive through molecular lubrication. Inspired by the strong adhesion behavior of gecko toes and mussels, Zhang et al. [144] synthesized a brush polymer with a catechol structure, mixed it with calcium phosphate-deposited soybean meal powder, and added TGA to prepare an adhesive for wood bonding. They found that the molecular lubrication effect of TGA significantly reduced the adhesive viscosity by 27.60%, enhancing the adhesive’s flowing ability and significantly improving its coating performance. Ionic strength also greatly affects the water absorption, swelling, and solubility of proteins, with multivalent ions being superior to monovalent ions. Metal ions can form multiple ionic bond networks, acting as sacrificial bonds to enhance adhesive bonding strength while reducing viscosity. Li et al. [145] added oxidized sodium alginate and calcium chloride to a plant protein system and adjusted the crosslinking structure of the adhesive by varying the Ca2+ concentration. When an appropriate amount of Ca2+ was added, the adhesive viscosity decreased by 50.85%, as high concentrations of Ca2+ complexed with oxidized sodium alginate and plant proteins, reducing intermolecular forces and solubility, thereby lowering viscosity. Additionally, the bonding strength of the cured adhesive increased by 65.3%.
Biological modification can disrupt the hierarchical structure of plant proteins, thereby reducing the viscosity of plant protein adhesives. Biological modification utilizes proteases to catalyze protein hydrolysis under mild conditions, reducing protein molecular weight and thus decreasing adhesive viscosity. Xu et al. [146] used the protease bromelain to disrupt the molecular structure and intermolecular forces of plant proteins, and then used TGA to recombine the degraded peptide chains, successfully reducing the adhesive viscosity. When 0.1% bromelain and 3% TGA were added, the viscosity of the resulting adhesive was reduced by 95% compared to the unmodified adhesive, while its water-resistant bonding strength reached 1.1 MPa, a 76.2% improvement over the unmodified adhesive.
6.3 Toughening and Strengthening Modifications
The inherent brittleness of plant protein adhesives, especially after crosslinking modification, results in higher brittleness, making them prone to brittle fracture and poor impact resistance. This leads to issues such as local cracking during post-processing of wood-based products, like grooving, which hinders their industrial application. Additionally, their brittleness causes loss of adhesive strength when bonding harder wood materials, leading to delamination [147]. Therefore, mimicking the tough structures found in nature and constructing toughened and strengthened structural systems is an important approach to enhance the toughness and post-processing performance of plant protein adhesives.
There are many tough structures in nature [148], such as insect wings, nacre, arthropod exoskeletons, spider silk, and gecko feet. These structures provide significant insights, particularly in constructing adhesive systems with different soft and hard phases to improve toughness through stress transfer and dispersion. Constructing similar structures in plant protein adhesive systems can effectively enhance their toughness and bonding performance, with the key being to improve the interfacial compatibility of different phases. Inspired by the tough microstructures of dragonfly wings and the irregular polygonal skeleton of plant cell walls, Zeng et al. [149] used tannic acid-modified molybdenum disulfide hybrids tightly bound to the SM framework through Schiff base and strong hydrogen bonds. This setup dissipated stress energy through crack deflection. Additionally, incorporating a small amount of phenylboronic acid/quaternary ammonium bifunctional chitosan optimized interfacial interactions within the adhesive, promoting B-O bonding, B-N coordination bonds, and electrostatic interactions, all of which facilitated energy dissipation. The adhesive’s shear strength and fracture toughness reached 1.58 MPa and 0.87 J, respectively, which were 409.7% and 866.7% higher than those of the unmodified adhesive. Inspired by the highly organized layered structure and unique fracture mechanism of dragonfly wings, Zhou et al. [150] created a dynamic network composed of a lignin-polyurea (LPU) framework and plant proteins, forming a dual crosslinked network of dynamic imine and hydrogen bonds to maximize the strong interactions between LPU and proteins. The LPU framework acted as a rigid nerve to slow crack propagation and transfer stress, while the interactions between hydrogen bonds and imine bonds with protein molecules dissipated strain energy. This bio-adhesive’s fracture toughness improved by approximately seven times compared to the unmodified version. Inspired by the brick-and-mortar hierarchical tough structure of nacre, Jiang et al. [151] used hydroxyl-functionalized boron nitride (BN-OH) nanosheets as the “brick” part and protein chains as the “mortar” part. BN-OH not only enhanced toughness through interlayer interface sliding but also achieved synergistic toughening with the nano effect. Additionally, glutaraldehyde was added as a “bridge” between protein chains and BN-OH, reacting with hydroxyl groups in BN-OH or active amino groups in protein chains to form dynamic covalent bonds for energy dissipation. The toughness of the adhesive increased by 1577% compared to the unmodified adhesive. Inspired by the microphase separation structure of spider silk, Zhang et al. [152] used 2,2,6,6-tetramethylpiperidine-1-oxyl radicals (TOCNFs) as the hard phase, uniformly distributed in the continuous protein (soft) phase, and grafted self-synthesized hydroxymethyl melamine prepolymer onto TOCNFs as a bond connecting the two phases, forming a microphase separation structure. This microphase structure effectively improved the toughness of the soy-based adhesive. Inspired by the brush-like structure of gecko toes and the strong adhesive properties of mussel proteins, Zhang et al. [153] synthesized a brush polymer with a catechol structure and combined it with Ca3(PO4)2 deposited on soybean powder to construct a biomineralized structure. The cured adhesive layer interface was tightly continuous, and the internal toughening of the brush polymer and energy dissipation from Ca3(PO4)2 deposition improved bonding toughness. Compared to the unmodified adhesive, the dry and wet shear strengths of the resulting plywood increased by 118.8% and 750%, respectively, and the bonding work increased from 0.31 to 4.05 J, an improvement of 1206.5%.
Organic-inorganic hybridization [154] is also an important method to improve the performance of plant protein adhesives. Adding inorganic materials to the plant protein adhesive system and enhancing the degree of organic-inorganic hybrid interface fusion through surface modification can construct stress transfer and dispersion systems, significantly improving adhesive toughness. Inspired by the reinforcement mechanism of arthropod cuticle phenolamine crosslinking and mineral enhancement, Zhang et al. [154] synthesized a polymer by reacting 3,4-dihydroxybenzaldehyde with long-chain polyamines and grafted it onto acid-stabilized montmorillonite, then introduced it into an amino-rich soy protein isolate matrix. Using Fe3+ as an oxidant induced phenolamine crosslinking. This phenolamine crosslinking and montmorillonite enhancement created dynamic sacrificial bonds and microphase separation structures in the adhesive system, with the adhesive work reaching 2.13 J, an increase of 192%.
Designing and regulating crosslinked structures to form covalent-subvalent multiple bonding systems and constructing subvalent bond networks as sacrificial bonds is also an important approach to improve the toughness of plant protein adhesives [155]. Researchers have introduced hydrogen bonds, ionic bonds, or constructed dual cross-linked network structures with dynamic covalent bonds and coordination bonds into the adhesive covalent bond network. These can rearrange or reorganize under stress, providing excellent toughness to the adhesive. Xu et al. [156] introduced 1,2,3-propanetriol diglycidyl ether, catechol-based tannic acid, and metal ion Zn2+ into soy protein adhesives to construct a triple network structure composed of covalent bonds, hydrogen bonds, and ionic bonds. Covalent bonds and hydrogen bonds dispersed internal stress, while hydrogen bonds and ionic bonds formed dynamic networks, creating dynamic bonds during adhesive fracture to effectively dissipate energy. The adhesive’s fracture strain reached 30%, a 36.4% improvement over the unmodified adhesive. Xu et al. [157] used epoxy cross-linked soy protein isolate as a covalent bond network, synthesized dopamine-functionalized waste paper fibers as catechol group donors to form a hydrogen bond network, and physically enhanced the system with copper hydroxide as an inorganic material to form an ionic bond network with catechol groups. This created an organic-inorganic hybrid multiple bonding structure in the soy protein adhesive. The results showed that compared to the unmodified adhesive, the dry/wet shear strengths of the plywood prepared with the modified adhesive increased by 46% and 144%, reaching 2.0 and 1.22 MPa, respectively, and the fracture toughness improved by 145%.
6.4 Enhancing Coating Performance Modifications
The coating performance of adhesives determines their practical application in the industrial production of wood-based panels. Industrial plant protein adhesives use soybean meal as a raw material, which has poor solubility and high molecular weight, leading to poor adhesion to veneers. This results in issues such as coating peeling, inconsistent coating thickness, and surface peeling. Poor coating performance of plant protein adhesives directly affects the bonding strength and stability of wood-based panels, as well as their durability, making them susceptible to environmental factors (e.g., humidity, temperature, and chemicals), thereby reducing the lifespan of the panels. Improving the initial tackiness of the adhesive is a primary method for enhancing the coating performance of plant protein adhesives.
The catechol structure in mussel adhesive proteins in nature provides strong initial tackiness, allowing adhesion to any surface in wet environments. Therefore, introducing catechol structures into adhesive systems can improve the coating performance of plant protein adhesives. Xu et al. [158] proposed preparing a nano-hybrid by grafting 3,4-dihydroxybenzaldehyde onto polyethyleneimine and nano-silica through electrostatic interactions and Schiff base reactions. This hybrid was then mixed with multifunctional crosslinker hexachlorocyclotriphosphazene and soy protein isolate to prepare a plant protein adhesive. The nano-hybrid with catechol structures formed strong hydrogen bonds with the wood surface, significantly enhancing the initial tackiness of the adhesive. Compared to the unmodified adhesive, the modified adhesive showed a significant improvement in coating uniformity, with a 90% reduction in roller residue. Liu et al. [159] pretreated lignin using ultrasound and other methods to demethoxylate the guaiacyl units on lignin, preparing lignin containing catechol adhesive groups (MAL). By mixing MAL with plant proteins to prepare adhesives, the introduction of catechol structures into the adhesive system resulted in coating performance comparable to that of urea-formaldehyde resin. Additionally, the catechol structures could oxidize to quinones and react with amino groups in the protein, improving the water-resistant bonding performance of the adhesive. Liu et al. [160] also grafted 3,4-dihydroxybenzaldehyde onto hyperbranched polyamide, preparing a hyperbranched catechol polymer. Additionally, a core-shell structured polymer with hyperbranched polyamide as the core and tannin as the shell was prepared using tannin and hyperbranched polyamide. Introducing these into the adhesive system improved both the coating performance and water-resistant bonding performance of the adhesive.
Constructing molecular topology structures can effectively improve the coating performance of adhesives. In 2018, Professor Zhigang Suo’s team at Harvard University innovatively proposed a new approach and method, constructing “bond-stitch” molecular topology structures to achieve ultra-strong adhesion between hydrogels and various materials [161]. The basic principle includes two aspects: on one hand, the polymer chains used as “stitching” materials have specific functional groups that match the functional groups on the wood surface to form chemical bonds. On the other hand, these polymer chains can diffuse into the adhesive materials (such as hydrogels, biological tissues, pre-gels, etc.) and form topological entanglements with the polymer network of the adhesive material, creating a “bond-stitch” molecular topology structure. Under this topological adhesion, at least one polymer network or interfacial chemical bond must be broken to achieve interface debonding. As long as the interfacial chemical bonds and the chemical bonds forming the polymer network are strong enough, strong and tough adhesion between the two materials can be achieved. Plant protein adhesives, similar to hydrogel systems before curing, provide a good basis for constructing “bond-stitch” molecular topology structures within their system, thereby improving adhesive performance. Huang et al. designed a “bond-stitch” topology structure with urushiol catechol groups as the bonding functional groups and poly(urushiol-acrylamide) (PUAM) as the stitching material. PUAM stitched into the soy protein network formed topological entanglements, enhancing the cohesive strength of the adhesive. The abundant catechol groups on PUAM improved the initial tackiness of the adhesive, significantly enhancing its coating uniformity on poplar veneer. The pre-pressing strength of the modified adhesive with the veneer increased by 40% to 0.42 MPa, and the wet bonding strength of the plywood increased by 230% to 1.17 MPa. Huang et al. [162] also constructed a “bond-stitch” topology structure with dopamine catechol groups as the bonding functional groups and dopamine-grafted sodium alginate (SD) as the stitching material. SD stitched into the soy protein network formed strong hydrogen bonds with the wood and protein, improving the interfacial adhesion and cohesion of the adhesive. The wet bonding strength of the prepared plywood increased from 0.11 to 1.09 MPa.
6.5 Modifications to Improve Pre-Pressing Strength of Veneer Panels
After the veneer is coated with adhesive, the panel needs to be placed in a cold press before entering the hot press. This step uses the adhesive’s tackiness to bond the veneers together, achieving preliminary shaping in a short time and laying the foundation for subsequent surface repairs and hot pressing without a cushion. The pre-pressing process is crucial for the widely adopted cushion-free hot pressing technique, as it determines whether the adhesive can be used on the production line. The duration of pre-pressing also affects the production efficiency of plywood. Ideally, the pre-pressing time should be between 1–3 h. A shorter pre-pressing time can effectively improve product quality, reduce auxiliary time during the hot pressing process, shorten the hot pressing cycle, and increase the productivity of wood-based panels. To achieve good pre-pressing performance, the adhesive needs to have excellent tackiness or the ability to react at low temperatures. However, plant protein adhesives inherently have poor initial tackiness due to the poor solubility and high molecular weight of proteins. Additionally, their reaction activity with epoxy cross-linkers at room temperature is low, resulting in no pre-curing performance. Consequently, plant protein adhesives exhibit poor pre-pressing performance. This issue is exacerbated when dealing with high moisture content veneers, as plant proteins cannot remove the hydration layer on the veneer surface, leading to a significant decline in pre-pressing performance, making it difficult to achieve shaping even after 12 h.
Introducing catechol structures is a promising approach to address the poor pre-pressing performance of adhesives. Liu et al. [163] used radical grafting to combine tannic acid (TA) with chitosan (CS) molecular chains, inducing TA to self-assemble into chitosan-based nanospheres (CS@TA). By chelating Fe(III) ions with the catechol groups of CS@TA, the cohesive strength of the adhesive was further enhanced, increasing the pre-pressing strength to 0.71 MPa. Inspired by the wet adhesion of mussels and the organic-inorganic mineralization enhancement of oysters, Zeng et al. [164] constructed a radical polymerization system. They grafted allyl glycidyl ether (A) onto plant protein (SP) to form double-bond SP molecules (SPA). Then, through radical polymerization, they grafted urushiol (U) onto SPA, obtaining plant protein molecules with catechol structures (SPU). By adding modified magnesium hydroxide (MH@A172) to SPU, the cohesive strength and pre-pressing strength of the adhesive were improved. At a pre-pressing temperature of 20°C and a wood moisture content of 100%, the pre-pressing strength reached 0.53 MPa, an enhancement of 231.25% compared to the unmodified adhesive.
Constructing a radical polymerization system to improve the pre-curing performance of adhesives can effectively enhance the pre-pressing performance of veneer panels. Molecular topology structures not only increase the adhesive strength between the adhesive and wood but also form strength comparable to cross-linked networks through topological entanglement. Huang et al. [165] designed and constructed a “bond-stitch” topology structure by radical copolymerizing acrylic acid and methacrylic acid zwitterionic sulfobetaine into a polymer and stitching it into plant protein molecules. This structure increased the cohesive strength of the adhesive and promoted the formation of chain entanglements through non-covalent interactions, achieving a pre-pressing strength of 0.42 MPa.
Plant protein adhesives contain abundant nutrients and polysaccharides, making them susceptible to fungal and bacterial attacks in humid environments. This results in a short shelf life for the adhesive [164], decreased bonding performance [166], and poor durability of the manufactured wood-based panels [167]. Therefore, improving the anti-mold properties of plant protein adhesives to extend the lifespan of wood-based panels is a significant research focus and challenge. While there is extensive mature research on antimicrobial and anti-mold mechanisms, the key research focus is on how to make these mechanisms compatible with the adhesive system, enhancing both the adhesive’s bonding performance and long-term anti-mold properties.
In the study of anti-mold properties of plant protein adhesives, researchers have found that introducing natural polyphenols, metal ions, quaternary ammonium salts, and disulfide bonds into the adhesive can enhance its anti-mold properties. Natural polyphenols can react with anions on the surface of bacterial and fungal cell membranes or with thiol groups, thereby disrupting the proteins of the microorganisms or affecting the synthesis of biofilms [168]. Tannins, naturally occurring polyphenolic compounds in plants, exhibit excellent antibacterial and antioxidant properties [169]. In the study by Liu et al. [170], hyperbranched polyamide (HP) was grafted onto 3-hydroxyphenylphosphinylpropionic acid (PPA) to form a PPA@HP complex, which was then combined with epoxidized tannin (ETA) to modify soy protein adhesives. The phenolic substances in ETA reduced the physiological functions of microorganisms, extending the anti-mold duration from 24 to 120 h. Chen et al. [169] used soybean meal as the main raw material and constructed a highly cross-linked waterproof structure with triglycidylamine and larch tannin to prepare soy protein adhesives. The phenolic hydroxyl groups in tannin extended the shelf life of the modified adhesive to 60 h.
Metal ions possess excellent antibacterial and bactericidal properties. They can interact with the negative charges on cell membranes, inhibit the action of cell synthases, and disrupt DNA replication, thereby providing antibacterial effects and significantly improving the anti-mold properties of plant protein adhesives [168]. Inspired by the cuticle of arthropods, Xu et al. [157] introduced dopamine-functionalized waste paper fibers (WPF-PDA) into the soy protein adhesive system to form a hydrogen bond network. Then, by chelating copper hydroxide with WPF-PDA, they constructed an ionic bond network, extending the anti-mold duration from 1 day to over 40 days. Xu et al. [156] constructed a triple network structure in soy protein adhesives using 1,2,3-propanetriol-diglycidyl ether, tannic acid, and Zn2+. The antibacterial properties of Zn2+ extended the anti-mold duration of the adhesive to over 15 days. Huang et al. [171] prepared chitosan grafted with 3,4-dihydroxybenzaldehyde and introduced it into soy protein isolate adhesives. Using this system, they in situ reduced silver ions to silver nanoparticles, providing the adhesive with anti-mold properties lasting up to 40 days.
Quaternary ammonium salts and disulfide bonds are also ideal methods for improving the anti-mold properties of plant protein adhesives. Quaternary ammonium salts can disrupt the integrity of bacterial and fungal cell membranes. Their long-chain structure can interact with soy protein, helping to form a stable antibacterial layer within the adhesive, effectively inhibiting mold growth and reproduction [168]. Inspired by the structure of oysters, Xu et al. [172] synthesized an antibacterial agent, quaternary ammonium salt-functionalized hyperbranched polyamide, which was modified with graphene nanosheets through cation-π interactions and combined with soy protein isolate and phytic acid. The resulting adhesive had a shelf life of up to 144 h. Disulfide bonds enhance the structural stability of proteins, helping to resist microbial erosion and destruction, thereby improving the anti-mold properties of the adhesive. Aladejana et al. [173] prepared an adhesive based on the recombination of thiol groups in chicken feather keratin and soy protein with boron-coordinated 3,3’-dithiodipropionic acid and 2,2’-dithiodibenzoic acid. This adhesive maintained its bonding ability even after 12 days of exposure to mold conditions.
6.7 Water Retention Modifications
In practical applications, during the production process of wood-based panels, there is a necessary transportation period between the coating, pre-pressing, and hot-pressing stages. Plant protein adhesives exhibit poor water retention when exposed to air [174]. During these transportation periods, the water content of plant protein-based adhesives significantly decreases, leading to a reduction in fluidity and a sharp decline in bonding strength, severely compromising product stability [175]. Unlike synthetic resins, which soften and then harden with increasing temperature due to their small molecular size, protein molecules have a high molecular weight. Upon losing water, their fluidity drastically decreases, making it difficult for them to penetrate the wood surface and form effective mechanical bonding, resulting in a sharp decline in adhesive performance. Traditional methods of adding humectants, such as glycerol, polyvinyl alcohol, and ethylene glycol, can increase the hydrophilicity of hydrogels [165]. However, adding large amounts of these substances significantly reduces the water resistance of plant protein-based adhesives. Therefore, constructing a water retention-enhancing structure within the adhesive is crucial.
Mimicking the skin structure of milk, constructing a moisture barrier film can effectively improve the water retention properties of adhesives. Inspired by lobster shells and milk skin, Liu and his team [175] used soybean flour, calcium phosphate (CaP) oligomers, and epoxy hyperbranched hydroxyethyl cellulose to simulate lobster shells, constructing an organic-inorganic hybrid composite adhesive based on a hyperbranched structure. Before the adhesive cured, the CaP oligomers mineralized to form an inorganic film similar to milk skin, effectively preventing water evaporation and increasing the adhesive’s water retention time to 100 min. In another study inspired by muscles, Liu et al. [176] synthesized a hyperbranched polyester using glycerol as a humectant. They grafted epoxy groups onto its structure to obtain an epoxy hyperbranched polyester, which was then combined with soy protein to prepare the adhesive. This modified soy protein-based adhesive exhibited superior initial viscosity and coating performance compared to traditional soy protein-based adhesives, significantly improving the water retention time from 0.5 to 2 h.
Enhancing the water molecule binding capacity within the adhesive system can effectively improve water retention properties, as well as coating and bonding performance. Huang et al. [165] applied this concept to the water retention of plant protein adhesives. Through a radical copolymerization reaction, they blended acrylic acid and methacryloylethyl sulfobetaine to form a polymer P(AA-SBMA), which was introduced into soy protein isolate to prepare the adhesive. Additionally, the adhesive could be combined with lithium chloride to enhance the system’s structure and water retention. This topological structure not only allowed the adhesive to retain over 75% of its mass after being stored in an open environment for 24 h but also enhanced its bonding and coating performance.
Developing and utilizing functional plant protein adhesives to produce functional wood composites as alternatives to metals, plastics, etc., offers advantages such as scalable production and environmental sustainability. These composites have potential applications in automotive manufacturing, aerospace, military, construction, and energy-saving sectors. However, traditional plant protein adhesives often require the addition of large amounts of functional fillers, which significantly reduce the adhesive bonding performance. Therefore, enhancing the adhesive bonding performance while incorporating high-functional fillers to achieve underwater adhesion, thermal conductivity, wave absorption, flame retardancy, and other functions is crucial for developing functional wood composites and expanding the applications of engineered wood products.
In the exploration of underwater bonding applications of plant protein adhesives, Li et al. [177] developed a strategy based on isochemical principles. They combined amino acid-like functional blocks with active AP-AH-AP group sequences and a hydrophilic epoxy backbone with instant water absorption properties capable of expelling interfacial water. The resulting adhesive exhibited effective instant water absorption, multiple molecular interactions enhancing interfacial adhesion, and rich covalent crosslinking increasing bulk cohesion, collectively improving underwater adhesion strength to 3.92 MPa. Li et al. [178] grafted allyl glycidyl ether onto soy protein and then grafted urushiol through radical polymerization to obtain soy protein molecules with catechol structures. They then mixed these with unsaturated calcium oxide to create an organic-inorganic hybrid adhesive. During curing, calcium oxide absorbed interfacial water, and the catechol structure generated strong interfacial adhesion, effectively improving underwater adhesion. The synergistic enhancement of the phenol-amine crosslinking structure and inorganic mineralization strategy increased the adhesive’s cohesion, enabling underwater curing performance.
In the exploration of enhancing the thermal conductivity of plant protein adhesives and their wood composites, Zhang et al. [179] used mechanical ball milling to exfoliate boron nitride (BN) and combined it with soy protein isolate (SPI) to improve the interfacial compatibility between BN and SPI. This significantly increased the adhesive’s thermal conductivity to 0.363 W/mK, a 171% improvement compared to the unmodified adhesive (0.134 W/mK). During hot pressing, BN oriented under pressure and fluid forces, constructing thermal conduction pathways in the particleboard system, resulting in a thermal conductivity of 1.308 W/mK for the prepared particleboard, ten times that of particleboard made with unmodified adhesive. Furthermore, inspired by the fiber pathways of the human nervous system, Zhang et al. [180] used natural wood fibers as a template and coated them with graphene nanosheets through a simple electrostatic self-assembly method. After hot pressing, this formed an efficient three-dimensional fiber “track” thermal conduction pathway, achieving metal-level thermal conductivity of 134 W/mK.
In the exploration of the electrical conductivity and electromagnetic shielding performance of plant protein adhesives, Zhang et al. [179,180] sequentially grafted pyrrole and dopamine hydrochloride onto protein molecules using methacrylic anhydride, forming polypyrrole and introducing silver nanoparticles to enhance the adhesive’s conductivity. The resulting adhesive’s conductivity increased by up to 260%, making it suitable for developing particleboard with electromagnetic shielding capabilities. Xu et al. [169], inspired by the organic-inorganic hybrid structure of oysters, synthesized a hyperbranched antibacterial agent (QHBPA) using quaternary ammonium salts and hyperbranched polyamide through an epoxy ring-opening reaction. Graphene nanosheets were introduced into QHBPA through cation-π interactions to prepare the G-co-Q hybrid. Combined with phytic acid and SPI, they formed a non-covalent crosslinked network through electrostatic interactions and strong hydrogen bonds, constructing a nano-phase reinforced organic-inorganic hybrid structure. Due to its unique multilayer isolation structure, the distance between conductive layers was closer to the wavelength of high-frequency waves, exhibiting stronger scattering and absorption of high-frequency waves. The addition of conductive G-co-Q endowed the adhesive with higher conductivity, which increased significantly with the amount of G-co-Q, reaching up to 275 times the conductivity of the previous adhesive and demonstrating an electromagnetic shielding effect of 43 dB.
In the exploration of flame retardant applications of plant protein adhesives, Zhang et al. [179] used mechanical ball milling to exfoliate boron nitride and combined it with SPI. Under the action of a crosslinking agent, they introduced terminal amino hyperbranched polyamide and phosphorus-nitrogen framework material hexachlorocyclotriphosphazene to synthesize a hyperbranched reactive substrate (CPA). The N and P atoms in CPA formed a uniform carbon layer during combustion, isolating oxygen. Phosphorus compounds formed a non-volatile liquid film on the polymer surface during combustion, protecting the carbon layer from oxidation. Meanwhile, boron nitride’s excellent thermal conductivity further enhanced the adhesive’s flame retardant performance, achieving a limiting oxygen index (LOI) of 36.5%. Liu et al. [160] used soy protein as the matrix, 4-carboxyphenylboronic acid as the crosslinking agent, self-synthesized hyperbranched catechol polymer as the catechol group donor, and silver nitrate as the Ag+ donor to develop a soy protein adhesive with excellent flame retardancy. The hyperbranched amine and polyphenol structures were rich in N and C, forming a C-N crosslinked structure after combustion, blocking heat and oxygen transfer and preventing combustion. Meanwhile, boron formed a glassy coating on the adhesive surface, promoting dehydration and forming a dense carbon layer. Compared to pure SM adhesive, the peak heat release rate of the resulting adhesive decreased by 52.1% to 79 kW/m2, and the LOI increased by 64.0% to 37.4%.
7 Other Bio-Adhesives for Wood Composites
There are other biomaterials that have been proposed for the preparation of bio-adhesives. None of the resins presented in this section has reached, at least up to now, industrial trial, but there are interesting and unusual concepts that are worthwhile to pursue and report.
First to be mentioned are urea and its derivate melamine both classifiable as biosourced and by elimination of the toxic formaldehyde usable as a bioadhesive in several combinations. For example, Oktay et al. [181] prepared a green urea-dialdehyde starch particleboard resin using copper sulfate-catalyzed, hydrogen peroxide-oxidized corn starch (OCS) and urea (U) (Figs. 6 and 34). The oxidized starch was laboratory prepared to obtain a kind of dialdehyde starch. The adhesive was strengthened by adding nanoparticles of titanium dioxide. The structure and characteristics of the adhesive synthesized were examined. A hybrid adhesive system was used to prepare laboratory particleboards, and the relative proportions of oxidized, dialdehyde starch to urea were determined. Testing of theses panels according to European norms EN 312 (2010) [76] resulted as satisfying the requirements for dry environment P2 type panels. The formaldehyde emission of these panels were also determined by perforator. These resins were shown to be used in an hybrid adhesive system coupled with commercial melamine-urea-formaldehyde resins (MUF) to obtain of much lower formaldehyde emission.
The approach was the same as the one shown in Fig. 6 proposed and prepared by Zhao et al. [29] but with the additional cross-linking obtained by mean of the nanoparticles of titanium oxide (Fig. 37).

Figure 37: Example of titanium dioxide nanoparticles cross-linking of urea-dialdehyde starch structures [181]
Zhang et al. have then extended the use of oxidized starch to melamine resins as a particleboard surface coating prepared by reaction of melamine with dialdehyde starch by Zhang et al. [182]. The existence of species as shown in Fig. 38 were determined confirming the cross-linking on curing of the resin system. The resins were prepared by two different procedures as dialdehyde starch is sensitive to higher temperatures when heated for relatively long times. Such resin coatings were then applied on particleboard panels surfaces either in liquid form or as a paper pre-impregnated by the resin. The best results of these surface coatings were determined for water repellence by sessile drop contact angle measurement, its progressive decrease as a function of time and by cross-cut test measured adhesion. The best results were achieved with 2% ammonium sulfate hardener by a resin-impregnated paper at 3 min press time at 200°C. Results at 180°C press temperature were also encouraging.

Figure 38: Example of a detected higher molecular weight melamine-dialdehyde starch tridimensional structure [182]
The approach to urea-oxidized carbohydrates adhesives has then been even further advanced by Zhang et al. [183] by reacting periodate specifically oxidized cellulose with a polyurea-hexanediamine dendrimer The high level of crystallinity of cellulose renders it a low accessibility reagent and especially because it is difficult to dissolve it thus being rather difficult to make it react. Zhang et al. then prepared a suitable wood adhesive by a first step of enzyme hydrolysis of cellulose hence increasing its solubility and rendering more available a larger number of its vicinal hydroxygroups, then oxidized by sodium periodate to yield a large proportion of bioaldehyde groups. The now soluble preoxidized cellulose is then reacted and cross-linked with a highly branched dendrimer obtained by prereacting a polyurea with hexanediamine by deamination. The oxidant type, its proportions, the reagents relative proportion and the reaction times and temperature were optimized, at 15% periodate on cellulose treated for 2 h at 90°C and at 1:1 mass ratio with the polyurea-hexanediamine dendrimer. Multiple instrumental analysis of the hardened adhesive detected C=N and –COH-NH- covalent linkages confirming cross-linking reactions between oxidized cellulose the polyurea-hexanediamine dendrimer. The plywood bonded with such an adhesive presented really good results with 1.71, 1.61 and 1.05 MPa dry, 24 h cold water soaking and 3 h hot water (63°C) soaking strengths.
Second approach to be mentioned for other bio-based adhesives is the use of cashew nut shell liquid, mainly composed of cardanol and cardol (Fig. 39) but containing also other compounds. Its dual nature, a phenol (cardanol) or resorcinol (cardol) aromatic ring with an unsaturated fatty acid chain, makes it a potential natural raw material for the synthesis of water resistant resins and polymers. The phenolic/resorcinol group and/or the double bonds in the chain can be directly used to form hardened networks, the former for reactions with aldehydes and the latter for radical cross-linking. Alternatively, more suitable functional groups such as aldehyde groups and others can be generated by ozonolysis from the alkenyl chain of cardanol itself. The first reaction step yields as major product a cardanol hydroperoxide; the following reduction by glucose or by zinc/acetic acid yields a high proportion of cardanol-derived aldehyde groups. These cross-link with the aromatic groups of cardanol itself, thus a self-condensation of the system yielding hardened networks occurs [184] (Fig. 40). The same approach was used by using unsaturated sunflower oil reacted with a reactive biopolyphenol, namely pine bark procyanidin tannin extract [185]. As regards wood adhesives all the cardanol approaches worked but they did not reach the strength requirements of relevant standards. A recent review on cardanol thermoset polymers other than for wood composites does exist [186].

Figure 39: Structural formulas of cardol and cardanol, main constituents of cashew nut shell liquid

Figure 40: Scheme of ozonolysis of cashew nut shell liquid (cardol and cardanol) generating aldehyde groups directly and by reduction to aldehydes of the hydroperoxide groups generated. The aldehydes then generated crosslinks then with the reactive phenolic nuclei [184]
Vegetable oils adhesive formulations have also been advanced based, equally on unsaturated oils and applicable toadhesives for wood fibers and wood itself [187]. Acrylics-epoxidized soy oil (AESO) addition to UF adhesives were used at 8% and 13% resin loads were used to bond particleboards of wheat straw. The panels yielded better internal bond strengths, cold water thickness swelling and other characteristics than the UF alone controls [188]. Soy bean oil as well as other vegetable oils also appear to have been used to prepare bioresins [189]. The resins were liquids and were derived by functionalizing animal and vegetable triglycerides to allow their polymerization. The functionalizing groups tried were amine, carboxyl, vinyl, hydroxyl and epoxy groups as well as others. The examples of natural fibers used to be bonded into composites were reported to be hemp, flax, straw, wood fibers, particles and flakes, however no claims of their effectiveness not results were reported. An epoxidized oil resin has been presented in an older research work, and tested as an adhesive for wood panels, resin preparation control being implemented by appropriately selecting triglycerides and polycarboxylic anhydrides [189]. The claims on this resin [189] were the capability of controlling and varying its level of cross-linking could be done by using some special catalysts. The article claims that panels were prepared in the 120°C–180°C range and that a number of laboratory samples had good tolerance to moisture and water at higher temperature too. Too long a hot press time appear to be their principal drawback, coupled with their high cost.
Other materials appear to have been used and suitable for bioadhesives, such as isorbides-derived polyacetals [190] (Fig. 41).

Figure 41: Structural formulas of isosorbide (right) and isosorbide polyacetals (left) [190]
The curing and adhesion of natural urushiol [191], a catechol derivative, under water were also studied (Fig. 42). The curing reaction of urushiol under water in the presence of Fe3+ (such as FeCl3) showed the possibility of utilizing this material as an underwater adhesive. Urushiol becomes a cross-linked network via the formation of chemical and Fe3+-coordination bonds. The adhesion strength of the cross-linked material formed by urushiol in a FeCl3 aqueous solution appears to depend on the pH and the film thickness. An adhesion strength of 0.14 MPa was found at a thickness of 100 μm under acidic conditions, showing some potential for the applicability of natural urushiol as an underwater adhesive.

Figure 42: Structure of urushiol [191]
It is now several decades that the research on wood adhesives has been focalized on bioadhesives, namely adhesives from renewable biosources. Starting from tannin and lignin based adhesives the focus of the research has been slowly enlarged to carbohydrates of all types, such as glucose, sucrose starch and cellulose and to vegetal proteins, mainly soy both in its form as soy flour and soy protein isolate. The oldest of these biosourced renewable material to be in industrial use are the tannin adhesives, commercialized since the early 1970’s. However, for many decades the hardener of choice was paraformaldehyde, while in much more recent times the problem linked to formaldehyde emission of the finished bonded products has shifted the focus of attention to developing adhesive formulations without any formaldehyde. A considerable variety of tannin adhesives formulations have thus been developed in this field. Similar trends have occurred in lignin for adhesives with the added focus to develop formulations overcoming the historical problem of low lignin reactivity. To overcome this last truly innovative approaches have been taken, relying on a number of different reactions, somewhat different from the usual ones in this field. Even more recently considerable interest in carbohydrate sources to prepare bioadhesives for wood have taken a considerable and rapid expansion, with fundamental developments never really sought before, with approaches extremely innovative.
As regards the use of proteins, the modification research of plant protein adhesives has become a research hotspot in the field of wood adhesives in recent years, owing to its significant potential in sustainable development and environmental protection. After more than a decade of scientific research, the water resistance, mold resistance, and processing performance of plant protein adhesives have been substantially improved, and costs have significantly decreased, enabling industrial applications in plywood and blockboard. However, issues such as poor water retention and production compatibility have emerged during industrial applications, leading to challenges such as stringent production process requirements, higher personnel skill requirements, and poor production stability. Additionally, the cost of plant protein adhesives remains higher than that of urea-formaldehyde resin, limiting their large-scale substitution for urea-formaldehyde resin.
Therefore, future research directions for plant protein adhesives should focus on improving water retention, low-cost modification, and low-temperature/ambient-temperature curing. By addressing these areas, the performance of adhesives can be practically enhanced, and costs can be reduced below those of urea-formaldehyde resin.
Furthermore, developing low-temperature curing technology can effectively promote the large-scale application of tannin, lignin, carbohydrates and plant protein adhesives, significantly contributing to the environmental upgrading, transformation, and high-quality development of the engineered wood industry.
Acknowledgement: The substantial help by discussion and corrections on the protein adhesives section by Prof. Jianzhang Li and Prof. Qiang Gao of Beijing Forestry University is gratefully acknowledged with thanks.
Funding Statement: The authors received no specific funding for assembling this review.
Author Contributions: The authors confirm contribution to the paper as follows: Consult materials and write papers: Hong Lei, Xiaojian Zhou, Antonio Pizzi, Guanben Du, Xuedong Xi; Coordinate papers: Antonio Pizzi; Organize papers: Antonio Pizzi, Guanben Du; Verifying papers: Xuedong Xi. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: In original articles in references.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present review.
References
1. Conner AH, River BH, Lorenz LF. Carbohydrates-modified PF resins for wood panels. J Wood Chem Technol. 1986;6(4):591–6. doi:10.1080/02773818608085246. [Google Scholar] [CrossRef]
2. Conner AH, Lorenz LF, River BH. Carbohydrate-modified PF resins formulated at neutral conditions. In: Adhesives from Renewable Resources, ACS Symposium Series. USA: American Chemical Society; 1989. Vol. 385, p. 355–69. [Google Scholar]
3. Moubarik A, Pizzi A, Charrier F, Allal A, Badia M, Mansouri HR, et al. Mechanical characterization of industrial particleboard panels glued with cornstarch-mimosa tannin-urea formaldehyde resins. J Adhesion Sci Technol. 2023;27(4):423–9. doi:10.1080/01694243.2012.711739. [Google Scholar] [CrossRef]
4. Moubarik A, Mansouri HR, Pizzi A, Charrier F, Allal A, Charrier B. Corn flour-mimosa tannin-based adhesives without formaldehyde for interior particleboard production. Wood Sci Technol. 2013;47(4):1–9. doi:10.1007/s00226-012-0525-4. [Google Scholar] [CrossRef]
5. Moubarik A, Allal A, Pizzi A, Charrier F, Charrier B. Characterization of a formaldehyde-free cornstarch-tannin wood adhesives for interior plywood. Eur J Wood Wood Prod. 2010;68(4):427–33. doi:10.1007/s00107-009-0379-0. [Google Scholar] [CrossRef]
6. Moubarik A, Charrier B, Allal A, Charrier F, Pizzi A. Development and optimisation of a new formaldehyde-free cornstarch and tannin adhesive. Eur J Wood Wood Prod. 2010;68(2):167–77. doi:10.1007/s00107-009-0357-6. [Google Scholar] [CrossRef]
7. Moubarik A, Pizzi A, Allal A, Charrier F, Charrier B. Cornstarch and tannin in phenol-formaldehyde resins for plywood production. Ind Crops Prod. 2009;30(2):188–93. doi:10.1016/j.indcrop.2009.03.005. [Google Scholar] [CrossRef]
8. Belgacem MN, Gandini A. Furan-based adhesives. In: Pizzi A, Mittal KL, editors. Handbook of adhesive technology. 2nd edition. Boca Raton, FL, USA: CRC Press; 2003. p. 615–34. [Google Scholar]
9. Xi X, Wu Z, Pizzi A, Gerardin C, Lei H, Du G. Furfuryl alcohol-aldehyde plywood adhesive resins. J Adhesion. 2020;96(9):814–38. doi:10.1080/00218464.2018.1519435. [Google Scholar] [CrossRef]
10. Wan H, He Z, Mao A, Liu X. Synthesis of polymers from liquefied biomass and their utilization in wood bonding. In: He Z, editors. Bio-based wood adhesives: preparation, characterization and testing. Boca Raton, FL, USA: CRC Press/Taylor & Francis; 2017. p. 239–59. [Google Scholar]
11. Jiang W, Kumar A, Adamopoulos S. Liquefaction of lignocellulosic materials and its applications in wood adhesives—a review. Ind Crops Prod. 2018;124:325–42. doi:10.1016/j.indcrop.2018.07.053. [Google Scholar] [CrossRef]
12. Alma MH, Yoshioka M, Yao Y, Shiraishi N. Preparation of sulfuric acid-catalyzed phenolated wood resin. Wood Sci Technol. 1998;32(4):297–308. doi:10.1007/BF00702897. [Google Scholar] [CrossRef]
13. Song J, Chen S, Zhang Q, Lei H, Xi X, Du G, et al. Developing on the well performance and eco-friendly sucrose-based wood adhesive. Ind Crops Prod. 2023;195:116298. doi:10.1016/j.indcrop.2023.116298. [Google Scholar] [CrossRef]
14. Bobbitt JM. Periodate oxidation of carbohydrates. Adanced Carbohyd Chem. 1956;11:1–41. doi:10.1016/S0096-5332(08)60115-0. [Google Scholar] [PubMed] [CrossRef]
15. Frihart CR, Pizzi A, Xi X, Lorenz L. Reactions of soy flour and soy protein by non-volatile aldehydes generation by specific oxidation. Polymers. 2019;11:1478. doi:10.3390/polym11091478. [Google Scholar] [PubMed] [CrossRef]
16. Frihart CR, Lorenz L. Specific oxidants improve the wood bonding strength of soy and other plant flours. J Polym Sci A: Polym Chemi. 2019;57:1017–23. doi:10.1002/pola.29357. [Google Scholar] [CrossRef]
17. Guigo N, Mazeau K, Putaux JL, Heux L. Surface modification of cellulose microfibrils by periodate oxidation and subsequent reductive amination with benzylamine: a topochemical study. Cellulose. 2014;21:4119–33. doi:10.1007/s10570-014-0459-0. [Google Scholar] [CrossRef]
18. Codou A, Guigo N, Heux L, Sbirrazzuoli N. Partial periodate oxidation and thermal cross-linking for the processing of thermoset all-cellulose composites. Compos Sci Technol. 2015;117:54–61. doi:10.1016/j.compscitech.2015.05.022. [Google Scholar] [CrossRef]
19. Xi X, Pizzi A, Frihart CR, Lorenz L, Gerardin C. Tannin plywood adhesives by non-volatile aldehydes generation from specific oxidation of mono- and disaccharides. Int J Adhes Adhes. 2020;98(1998):102499. doi:10.1016/j.ijadhadh.2019.102499. [Google Scholar] [CrossRef]
20. Chen X, Xi X, Pizzi A, Fredon E, Du G, Gerardin C. Oxidized demethylated lignin as a bio-based adhesive for wood bonding. J Adhesion. 2021;97(9):873–90. doi:10.1080/00218464.2019.1710830. [Google Scholar] [CrossRef]
21. Xi X, Pizzi A, Lei H, Chen X, Zhang B, Du G. Environmental friendly chitosan adhesives for plywood bonding. Int J Adhes Adhes. 2022;112(2–3):103027. doi:10.1016/j.ijadhadh.2021.103027. [Google Scholar] [CrossRef]
22. Yang H, Guanben Du G, Ni K, Yingchen Wu Y, Xin Ran X, Tan X, et al. Development of high-performance sucrose-based adhesives with high density cross-linking network inspired by Maillard reaction or Cuisine chemistry. Ind Crops Prod. 2023;195(1478):116416. doi:10.1016/j.indcrop.2023.116416. [Google Scholar] [CrossRef]
23. Zhi Li Z, Du G, Yang H, Liu T, Yuan J, Liu C, et al. Construction of a cellulose-based high-performance adhesive with a crosslinking structure bridged by Schiff base and ureido groups. Int J Biol Macromol. 2022;223:971–9. doi:10.1016/j.ijbiomac.2022.11.069. [Google Scholar] [PubMed] [CrossRef]
24. Liu S, Du G, Yang H, Su H, Ran X, Li J, et al. Developing high-performance cellulose-based wood adhesive with a cross-linked network. ACS Sustain Chem Eng. 2021;9(49):16849–61. doi:10.1021/acssuschemeng.1c07012. [Google Scholar] [CrossRef]
25. Zhang Q, Yan R, Xiong Y, Hong Lei H, Du G, Pizzi A, et al. Preparation and characterization of polymeric cellulose wood adhesive with excellent bonding properties and water resistance. Carbohydr Polym. 2025;347(6):122705. doi:10.1016/j.carbpol.2024.122705. [Google Scholar] [PubMed] [CrossRef]
26. Li Z, Du G, Yang H, Ni K, Liu S, Ran X, et al. Sugar-painting inspired branched ureido polymers as high-performance formaldehyde-free wood adhesive. Wood Sci Technol. 2023;57(9):483–505. doi:10.1007/s00226-023-01453-x. [Google Scholar] [CrossRef]
27. Xi X, Pizzi A, Delmotte L. Isocyanate-free polyurethane coatings and adhesives from mono- and di-saccharides. Polymers. 2018;10(4):402. doi:10.3390/polym10040402. [Google Scholar] [PubMed] [CrossRef]
28. Xi X, Wu Z, Pizzi A, Gerardin C, Lei H, Zhang B, et al. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci Technol. 2019;53(2):393–405. doi:10.1007/s00226-019-01083-2. [Google Scholar] [CrossRef]
29. Zhao X, Peng L, Wang H, Wang Y, Zhang H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohydr Polym. 2018;181(2):1112–8. doi:10.1016/j.carbpol.2017.11.035. [Google Scholar] [PubMed] [CrossRef]
30. Umemura K, Ueda T, Munawar S, Kawai S. Application of citric acid as natural adhesive for wood. J Appl Polym Sci. 2012;123(4):1991–6. doi:10.1002/app.34708. [Google Scholar] [CrossRef]
31. Umemura K, Kawai S. Development of wood-based materials bonded with citric acid. For Prod J. 2015;65(1-2):38–42. doi:10.13073/FPJ-D-14-00036. [Google Scholar] [CrossRef]
32. Amirou S, Pizzi A, Delmotte L. Citric acid as waterproofing additive in butt joints linear wood welding. Eur J wood Wood Prod. 2017;75(4):651–4. doi:10.1007/s00107-017-1167-x. [Google Scholar] [CrossRef]
33. Del Menezzi C, Amirou S, Pizzi A, Xi X, Delmotte L. Reactions with wood carbohydrates and lignin of citric acid as a bond promoter of wood veneer panels. Polymers. 2018;10(8):833. doi:10.3390/polym10080833. [Google Scholar] [PubMed] [CrossRef]
34. Umemura K, Sugihara O, Kawai S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J Wood Sci. 2013;59:203–8. doi:10.1007/s10086-013-1326-6. [Google Scholar] [CrossRef]
35. Widyorini R, Nugraha PA, Rahman MZA, Prayitno TA. Bonding ability of a new adhesive composed of citric acid-sucrose for particleboard. BioResources. 2016;11:4526–35. doi:10.15376/biores.11.2.4526-4535. [Google Scholar] [CrossRef]
36. Zhao Z, Sakai S, Wu D, Chen Z, Zhu N, Huang C, et al. Further exploration of sucrose-citric acid adhesive: investigation of optimal hot-pressing conditions for plywood and curing behavior. Polymers. 2019;11:1–14. doi:10.3390/polym11121996. [Google Scholar] [PubMed] [CrossRef]
37. Sun S, Zhao Z, Umemura K. Further exploration of sucrose-citric acid adhesive: synthesis and application on plywood. Polymers. 2019;11:1875. doi:10.3390/polym11111875. [Google Scholar] [PubMed] [CrossRef]
38. Ando D, Umemura K, Yamauchi H. New ester-type chemical bonding wood adhesion with a dicarboxylic acid compound. Wood Sci Technol. 2025;59:14. doi:10.1007/s00226-024-01621-7. [Google Scholar] [CrossRef]
39. Sakai S, S.Chen S, Matsuo-Ueda M, Umemura K. Curing behavior of sucrose with p-Toluenesulfonic acid. Polymers. 2023;15(23):4592. doi:10.3390/polym15234592. [Google Scholar] [PubMed] [CrossRef]
40. Xu D, Li C, Pizzi A, Xi X, Wang Z, Du G, et al. Self-neutralizing citric acid−corn starch wood adhesives. ACS Sustain Chem Eng. 2024;12(5):13382–91. doi:10.1021/acssuschemeng.4c05590. [Google Scholar] [CrossRef]
41. Pizzi A, Vosloo R, Cameron FA, Orovan E. Self-neutralizing acid-set PF wood adhesives. Holz als Roh- und Werkstoff. 1986;44(6):229–34. doi:10.1007/BF02612001. [Google Scholar] [CrossRef]
42. Xi X, Pizzi A, Lei H, Zhou X, Du G. Self-neutralizing melamine-urea-formaldehyde-citric acid resins for wood panels adhesives. Polymers. 2024;16(13):1819. doi:10.3390/polym16131819. [Google Scholar] [PubMed] [CrossRef]
43. Yang CQ. FT-IR spectroscopy study of the ester crosslinking mechanism of cotton cellulose. Text Res J. 1991;61(8):433–40. doi:10.1177/004051759106100801. [Google Scholar] [CrossRef]
44. Kurkowiak K, Hentges D, Dumarçay S, Géradin P, Militz H. Understanding the mode of action of sorbitol and citric acid. In: Proceedings of the Tenth European Conference on Wood Modification; 2022 Apr 25–26; Nancy, France. [Google Scholar]
45. Lee SH, Tahir PM, Lum WC, Tan LP, Bawon P, Park B-D, et al. A review on citric acid as green modifying agent and binder for wood. Polymers. 2020;12(8):1692. doi:10.3390/polym12081692. [Google Scholar] [PubMed] [CrossRef]
46. Xi X, Pizzi A. No-aldehydes glucose/sucrose-triacetin-diamine wood adhesives for particleboard. J Renew Mat. 2020;8(7):715–25. doi:10.32604/jrm.2020.010882. [Google Scholar] [CrossRef]
47. Zhang Q, Song J, Lei H, Pizzi A, Du G, Xi X. Preparation of the water-resistant eco-friendly carbohydrate wood adhesives by Schiff base reaction. Wood Mat Sci Eng. 2024;19(2):275–8. doi:10.1080/17480272.2023.2238261. [Google Scholar] [CrossRef]
48. Chen X, Pizzi A, Zhang B, Zhou X, Fredon E, Gerardin C, et al. Particleboard bioadhesive by glyoxalated lignin and oxidized dialdehyde starch cross-linked by urea. Wood Sci Technol. 2022;56(1):63–85. doi:10.1007/s00226-021-01344-z. [Google Scholar] [CrossRef]
49. Thébault M, Pizzi A, Dumarçay S, Gerardin P, Fredon E, Delmotte L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind Crops Prod. 2014;59(3):329–36. doi:10.1016/j.indcrop.2014.05.036. [Google Scholar] [CrossRef]
50. Tundo P, Selva M. The chemistry of dimethyl carbonate. Account Chem Res. 2002;35(9):706–16. doi:10.1021/ar010076f. [Google Scholar] [PubMed] [CrossRef]
51. Thébault M, Pizzi A, Essawy H, Baroum A, van Assche G. Isocyanate free condensed tannin-based polyurethanes. Eur Polym J. 2015;67(3):513–26. doi:10.1016/j.eurpolymj.2014.10.022. [Google Scholar] [CrossRef]
52. Thébault M, Pizzi A, Santiago-Medina FJ, Al-Marzouki FM, Abdalla S. Isocyanate-free polyurethanes by coreaction of condensed tannins with aminated tannins. J Renew Mater. 2017;5(1):21–9. doi:10.7569/JRM.2016.634116. [Google Scholar] [CrossRef]
53. Sarazin J, Pizzi A, Amirou S, Schmidt D, Sernek M. Organosolv lignin non-isocyanate based polyurethanes (NIPU) as Wood Adhesive. J Renew Mater. 2021;9(5):881–907. doi:10.32604/jrm.2021.015047. [Google Scholar] [CrossRef]
54. Braghiroli F, Fierro V, Pizzi A, Rode K, Radke W, Delmotte L, et al. Condensation reaction of flavonoid tannins with ammonia. Ind Crops Prod. 2013;44(4):330–5. doi:10.1016/j.indcrop.2012.11.024. [Google Scholar] [CrossRef]
55. Braghiroli FL, Fierro V, Izquierdo M-T, Parmentier J, Pizzi A, Delmotte L, et al. High surface-high N-content carbons prepared by hydrothermal treatment of aminated tannin. Ind Crops Prod. 2015;66:282–90. doi:10.1016/j.indcrop.2014.11.022. [Google Scholar] [CrossRef]
56. Santiago-Medina F, Pizzi A, Basso MC, Delmotte L, Celzard A. Polycondensation resins by flavonoid tannins reaction with amines. Polymers. 2017;9(2):37. doi:10.3390/polym9020037. [Google Scholar] [PubMed] [CrossRef]
57. Iranpoor N, Panahi F. Direct Nickel-catalyzed amination of phenols via C-O Bond activation using 2,4,6-Trichloro-1,3,5-triazine (TCT) as reagent. Adv Synth Catal. 2014;356(14-15):3067–73. doi:10.1002/adsc.201400460. [Google Scholar] [CrossRef]
58. Yu J, Wang Y, Zhang P, Wu J. Direct amination of phenols under metal-free conditions. Synlett. 2013;24:1448–54. doi:10.1055/s-00000083. [Google Scholar] [CrossRef]
59. Hashida K, Makino R, Ohara S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung. 2009;63(3):319–26. doi:10.1515/HF.2009.043. [Google Scholar] [CrossRef]
60. Santiago-Medina FJ, Pizzi A, Basso MC, Delmotte L, Abdalla S. Polycondensation resins by lignin reaction with (Poly) amines. J Renew Mater. 2017;5(5):399–9. doi:10.7569/JRM.2017.634142. [Google Scholar] [CrossRef]
61. Basso MC, Pizzi A, Delmotte L, S.Abdalla S. MALDI-TOF and 13C NMR analysis of the cross-linking reaction of lignin by triethyl phosphate. Polymers. 2017;9:206. doi:10.3390/polym9060206. [Google Scholar] [PubMed] [CrossRef]
62. Basso MC, Pizzi A, Polesel-Maris J, Delmotte L, Colin B, Rogaume Y. MALDI-TOF and 13C NMR analysis of the cross-linking reaction of condensed tannins by triethyl phosphate. Ind Crops Prod. 2017;95:621–31. doi:10.1016/j.indcrop.2016.11.031. [Google Scholar] [CrossRef]
63. Polesel-Maris J, Jutang I. Anti-adhesive coatings based on condensed tannins. PCT Patent WO 2017/037393 A1, assigned to SEB S.A; 2017 [cited 2024 Dec 10]. Available from: https://patents.google.com/patent/EP3344706A1/en. [Google Scholar]
64. Pizzi A. Recent developments in advanced lignin-based adhesives and binders. In: Pizzi A, editors. Advanced lignin technologies. London, UK: IntechOpen; 2024. p. 109–25. [Google Scholar]
65. Leban J-M, Pizzi A, Wieland S, Zanetti M, Properzi M, Pichelin F. X-ray microdensitometry analysis of vibration-welded wood. J Adhes Sci Technol. 2004;18(6):673–85. doi:10.1163/156856104839310. [Google Scholar] [CrossRef]
66. Zhang W, Liu C, Du Z, Wang H, Du G, Essawy H, et al. Soybean meal-oxidized lignin as bio-hybridized wood panel adhesives with increased water resistance. Polymers. 2024;15:1036. doi:10.3390/f15061036. [Google Scholar] [CrossRef]
67. Lin H, Chen X, Lei H, Zhou X, Du G, Essawy H, et al. Synthesis and characterization of a bio-aldehyde based lignin adhesive with desirable water resistance. Int J Biol Macromol. 2024;264:130020. doi:10.1016/j.ijbiomac.2024.130020. [Google Scholar] [PubMed] [CrossRef]
68. Ghahri S, Chen X, Pizzi A, Hajihassani R, Papadopoulos A. Tannins as new cross-linking materials for soy-based adhesives. Polymers. 2021;13(4):595. doi:10.3390/polym13040595. [Google Scholar] [PubMed] [CrossRef]
69. Ghahri S, Pizzi A, Mohebby B, Mirshoktaie A, Mansouri HR. Improving water resistance of soy-based adhesive by vegetable tannin. J Polym Environ. 2018;26(5):1881–90. doi:10.1007/s10924-017-1090-6. [Google Scholar] [CrossRef]
70. Ghahri S, Pizzi A. Improving soy-based adhesives for wood particleboard by tannins addition. Wood Sci Technol. 2018;52(1):261–79. doi:10.1007/s00226-017-0957-y. [Google Scholar] [CrossRef]
71. Ghahri S, Pizzi A, Mohebby B, Mirshokraie A, Mansouri HR. Soy-based, tannin-modified plywood adhesives. J Adhesion. 2018;94(3):218–37. doi:10.1080/00218464.2016.1258310. [Google Scholar] [CrossRef]
72. Pizzi A. Covalent and ionic bonding between tannin and collagen in leather making and shrinking: a MALDI-ToF study. J Renew Mater. 2021;9:1345–64. doi:10.32604/jrm.2021.015663. [Google Scholar] [CrossRef]
73. Ghahri S, Pizzi A, Hajihassani R. A study of concept to prepare totally biosourced wood adhesives from only soy protein and tannin. Polymers. 2022;14:1150. doi:10.3390/polym14061150. [Google Scholar] [PubMed] [CrossRef]
74. Chen X, Pizzi A, Fredon E, Gerardin C, Zhou X, Zhang B, et al. Low curing temperature tannin-based non-isocyanate polyurethane (NIPU) wood adhesives: preparation and properties evaluation. Int J Adhes Adhes. 2022;112:103001. doi:10.1016/j.ijadhadh.2021.103001. [Google Scholar] [CrossRef]
75. Chen X, Essawy H, Wu H, Pizzi A, Fredon E, Gerardin C, et al. Mimosa tannin based NIPU wood adhesive with significant substitution of hexamethylenediamine using polyethyleneimine. Int J Adhes Adhes. 2024;128:103549. doi:10.1016/j.ijadhadh.2023.103549. [Google Scholar] [CrossRef]
76. European norm EN 312. Particleboards, specifications. Bruxelles, Belgium: European Qtandardization Committee; 2010. [Google Scholar]
77. Ndiwe B, Pizzi A, Tibi B, Danwe R, Konai N, Amirou S. African tree bark exudate extracts as biohardeners of fully biosourced thermoset tannin adhesives for wood panels. Ind Crops Prod. 2019;132:253–68. doi:10.1016/j.indcrop.2019.02.023. [Google Scholar] [CrossRef]
78. Navarrete P, Mansouri HR, Pizzi A, Tapin-Lingua S, Benjelloun-Mlayah B, Rigolet S. Synthetic-resin-free wood panel adhesives from low molecular mass lignin and tannin. J Adhes Sci Technol. 2010;24(8-10):1597–1610. doi:10.1163/016942410X500972. [Google Scholar] [CrossRef]
79. Liu C, Zhang Y, Li X, Luo J, Gao J, Li Q, et al. Green bio-thermoset resins derived from soy protein isolate and condensed tannins. Ind Crops Prod. 2017;108:363–70. doi:10.1016/j.indcrop.2017.06.057. [Google Scholar] [CrossRef]
80. Xi X, Jian Y, Tian H, Liang J, Lei H, Du G, et al. Study on the preparation of tannin-hydroxymethylated furfuryl alcohol adhesives. Int J Adhes Adhes. 2024;129(8):103578. doi:10.1016/j.ijadhadh.2023.103578. [Google Scholar] [CrossRef]
81. Abdullah UHB, Pizzi A. Tannin-furfuryl alcohol wood panel adhesives without formaldehyde. Eur J Wood Wood Prod. 2013;71(1):131–2. doi:10.1007/s00107-012-0629-4. [Google Scholar] [CrossRef]
82. Ballerini A, Despres A, Pizzi A. Non-toxic, zero-emission tannin-glyoxal adhesives for wood panels. Holz als Roh- und Werkstoff. 2005;63(6):477–8. doi:10.1007/s00107-005-0048-x. [Google Scholar] [CrossRef]
83. Böhm R, Hauptmann M, Pizzi A, Friederich C, Laborie M-P. The chemical, kinetic and mechanical characterization of tannin-based adhesives with different crosslinking systems. Int J Adhes Adhes. 2016;68:1–8. doi:10.1016/j.ijadhadh.2016.01.006. [Google Scholar] [CrossRef]
84. Santiago-Medina FJ, Foyer G, Pizzi A, Calliol S, Delmotte L. Lignin-derived non-toxic aldehydes for ecofriendly tannin adhesives for wood panels. Int J Adhesion Adhesives. 2016;70:239–48. doi:10.1016/j.ijadhadh.2016.07.002. [Google Scholar] [CrossRef]
85. Trosa A, Pizzi A. A no-aldehyde emission hardener for tannin-based wood adhesives. Holz als Roh- und Werkstoff. 2001;59:266–71. doi:10.1007/s001070100200. [Google Scholar] [CrossRef]
86. Grigsby WJ, McIntosh CD, Warnes JM, Suckling ID, Anderson CR. Adhesives. U.S. Patent 7,319,115 B2. New Zealand Forestry Research Institute; 2008. [Google Scholar]
87. Trosa A, Pizzi A. Industrial hardboard and other panels binder from tannin/furfuryl alcohol in absence of formaldehyde. Holz als Roh- und Werkstoff. 1988;56:213–4. doi:10.1007/s001070050301. [Google Scholar] [CrossRef]
88. Kamoun C, Pizzi A. Mechanism of hexamine as a non-aldehyde polycondensation hardener, Part 1: hexamine decomposition and reactive intermediates. Holzforschung und Holzverwertung. 2000;52:16–9. [Google Scholar]
89. Kamoun C, Pizzi A. Mechanism of hexamine as a non-aldehyde polycondensation hardener, Part 2: recomposition of intermediate reactive compound. Holzforschung und Holzverwertung. 2000;52:66–7. [Google Scholar]
90. Kamoun C, Pizzi A, Zanetti M. Upgrading of MUF resins by buffering additives—part 1: hexamine sulphate effect and its limits. J Appl Polym Sci. 2003;90:203–14. [Google Scholar]
91. Pichelin F, Nakatani M, Pizzi A, Wieland S, Despres A, Rigolet S. Structural beams from thick wood panels bonded industrially with formaldehyde free tannin adhesives. For Prod J. 2006;56:31–6. [Google Scholar]
92. Pizzi A, Meikleham N, Dombo B, Roll W. Autocondensation-based, zero-emission, tannin adhesives for particleboard. Holz als Roh- und Werkstoff. 1995;53:201–4. doi:10.1007/BF02716424. [Google Scholar] [CrossRef]
93. Pizzi A, Meikleham N, Stephanou A. Induced accelerated autocondensation of polyflavonoid tannins for phenolic polycondensates—part II: cellulose effect and application. J Appl Polym Sci. 1995;55:929–33. doi:10.1002/app.1995.070550611. [Google Scholar] [CrossRef]
94. Garcia R, Pizzi A, Merlin A. Ionic polycondensation effects on the radical autocondensation of polyflavonoid tannins-An ESR study. J Appl Polym Sci. 1997;65:2623–32. doi:10.1002/(SICI)1097-4628(19970926)65:13<2623::AID-APP4>3.0.CO;2-D. [Google Scholar] [CrossRef]
95. Garcia R, Pizzi A. Polycondensation and autocondensation networks in polyflavonoid tannins, Part 1: final networks. J Appl Polym Sci. 1998;70:1083–92. doi:10.1002/(SICI)1097-4628(19981107)70:6<1083::AID-APP5>3.0.CO;2-K. [Google Scholar] [CrossRef]
96. Garcia R, Pizzi A. Polycondensation and autocondensation networks in polyflavonoid tannins, Part 2: polycondensation vs. autocondensation. J Appl Polym Sci. 1998;70:1093–1110. doi:10.1002/(SICI)1097-4628(19981107)70:6<1093::AID-APP6>3.0.CO;2-J. [Google Scholar] [CrossRef]
97. Garcia R, Pizzi A. Cross-linked and entanglement networks in thermomechanical analysis of polycondensation resins. J Appl Polym Sci. 1998;70:1111–6. doi:10.1002/(SICI)1097-4628(19981107)70:6<1111::AID-APP7>3.0.CO;2-R. [Google Scholar] [CrossRef]
98. Chen X, Pizzi A, Essawy H, Fredon E, Gerardin C, Guigo N, et al. Non-furanic humins-based non-isocyanate polyurethane (NIPU) thermoset wood adhesives. Polymers. 2021;13:372. doi:10.3390/polym13030372. [Google Scholar] [PubMed] [CrossRef]
99. Valenzuela J, von Leyser E, Pizzi A, Westermeyer C, Gorrini B. Industrial production of pine tannin-bonded particleboard and MDF. Eur J Wood Wood Prod. 2012;70(5):735–40. doi:10.1007/s00107-012-0610-2. [Google Scholar] [CrossRef]
100. Jiang S, Hui Niu H, Wang S, Qian Z, Du G, Zhou X, et al. Novel and high-performance tannin-polyamine adhesive: new insight into phenol-amine chemistry. Ind Crops Prod. 2023;192:116129. doi:10.1016/j.indcrop.2022.116129. [Google Scholar] [CrossRef]
101. Jiang S, Liu S, Du G, Wang S, Zhou X, Yang J, et al. Chitosan-tannin adhesive: fully biomass, synthesis-free and high performance for bamboo-based composite bonding. Int J Biol Macromol. 2023;230:123115. doi:10.1016/j.ijbiomac.2022.123115. [Google Scholar] [PubMed] [CrossRef]
102. Zhang B, Chen X, Pizzi A, Petrissans M, Dumarcay S, Petrissans A, et al. Highly branched Tannin-Tris(2-aminoethyl)amine-urea wood adhesives. Polymers. 2023;15(2):890. doi:10.3390/polym15040890. [Google Scholar] [PubMed] [CrossRef]
103. Tester RF, Karkalas J, Qi X. Starch—composition, fine structure and architecture. J Cereal Sci. 2004;39(2):151–65. doi:10.1016/j.jcs.2003.12.001. [Google Scholar] [CrossRef]
104. Ambigaipalan P, Hoover R, Donner E, Liu Q, Jaiswal S, Chibbar R, et al. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res Intern. 2011;44(9):2962–74. doi:10.1016/j.foodres.2011.07.006. [Google Scholar] [CrossRef]
105. Xie F, Pollet E, Halley PJ, Avérous L. Starch-based nano-biocomposites. Prog Polym Sci. 2013;38(10):1590–1628. doi:10.1016/j.progpolymsci.2013.05.002. [Google Scholar] [CrossRef]
106. Gérard C, Barron C, Colonna P, Planchot V. Amylose determination in genetically modified starches. Carbohydr Polym. 2001;44(1):19–27. doi:10.1016/S0144-8617(00)00194-6. [Google Scholar] [CrossRef]
107. Xie F, Liu H, Chen P, Xue T, Chen L, Yu L, et al. Starch Gelatinization under Shearless and Shear Conditions. Int J Food Eng. 2007;2(5):1–31. doi:10.2202/1556-3758.1162. [Google Scholar] [CrossRef]
108. Sun Y, Gu J, Tan H, Zhang Y, Huo P. Physicochemical properties of starch adhesives enhanced by esterification modification with dodecenyl succinic anhydride. Int J Biol Macromol. 2018;112:1257–63. doi:10.1016/j.ijbiomac.2018.01.222. [Google Scholar] [PubMed] [CrossRef]
109. Zhou J, Tong J, Su X, Ren L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int J Biol Macromol. 2016;91:1186–93. doi:10.1016/j.ijbiomac.2016.06.082. [Google Scholar] [PubMed] [CrossRef]
110. Adak S, Banerjee R. A green approach for starch modification: esterification by lipase and novel imidazolium surfactant. Carbohydr Polym. 2016;150:359–68. doi:10.1016/j.carbpol.2016.05.038. [Google Scholar] [PubMed] [CrossRef]
111. Tolvanen P, Sorokin A, Mäki-Arvela P, Murzin DY, Salmi T. Oxidation of starch by H2O2 in the presence of iron tetrasulfophthalocyanine catalyst: the effect of catalyst concentration, pH, solid-liquid ratio, and origin of starch. Indust Eng Chem Res. 2013;52(27):9351–8. doi:10.1021/ie4003969. [Google Scholar] [CrossRef]
112. Zuo Y, Liu W, Xiao J, Zhao X, Zhu Y, Wu Y. Preparation and characterization of dialdehyde starch by one-step acid hydrolysis and oxidation. Int J Biol Macromol. 2017;103:1257–64. doi:10.1016/j.ijbiomac.2017.05.188. [Google Scholar] [PubMed] [CrossRef]
113. Liu M, Yao W, Zheng H, Zhao H, Shao R, Tan H, et al. Preparation of a high-strength, hydrophobic performance starch-based adhesive with oxidative cross-linking via Fenton’s reagent. Int J Biol Macromol. 2023;253:126995. doi:10.1016/j.ijbiomac.2023.126995. [Google Scholar] [PubMed] [CrossRef]
114. Yang J, Wang M, Zhang Y, Jia X, Chen Y, Liu T, et al. Rapid preparation of oxidized starch with high carbonyl contents using NaBrO as oxidizer. Starch-Stärke. 2019;71(9–10):1900054. doi:10.1002/STAR.201900054. [Google Scholar] [CrossRef]
115. Hao J, Lu J, Xu N, Linhardt RJ, Zhang Z. Specific oxidation pattern of soluble starch with TEMPO-NaBr-NaClO system. Carbohydr Polym. 2016;146:238–44. doi:10.1016/j.carbpol.2016.03.040. [Google Scholar] [PubMed] [CrossRef]
116. Wang S, Wang Q, Fan X, Xu J, Zhang Y, Yuan J, et al. Synthesis and characterization of starch-poly(methyl acrylate) graft copolymers using horseradish peroxidase. Carbohydr Polym. 2016;136:1010–6. doi:10.1016/j.carbpol.2015.09.110. [Google Scholar] [PubMed] [CrossRef]
117. Zhang B, Wei B, Hu X, Jin Z, Xu X, Tian Y. Preparation and characterization of carboxymethyl starch microgel with different crosslinking densities. Carbohydr Polym. 2015;124:245–53. doi:10.1016/j.carbpol.2015.01.075. [Google Scholar] [PubMed] [CrossRef]
118. Yang M, Fu J, Alee M, Duan Q, Xie H, Ali A, et al. Improving water resistance property of starch straw by synergistic effects of chemical crosslinking, physical barrier, and surface modifications, Air oxidation and hyperbranched crosslinking approach for a boiling water-resistant sucrose-derived wood adhesive. J Appl Polym Sci. 2023;140(31):54210. doi:10.1002/app.54210. [Google Scholar] [CrossRef]
119. Song J, Chen S, Zhang Q, Xi X, Lei H, Du G, et al. Preparation and characterization of the bonding performance of a starch-based water resistance adhesive by Schiff base reaction. Int J Biol Macromol. 2023;251(4):126254. doi:10.1016/j.ijbiomac.2023.126254. [Google Scholar] [PubMed] [CrossRef]
120. Mei J-Q, Zhou D-N, Jin Z-Y, Xu X-M, Chen H-Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015;187(2):378–84. doi:10.1016/j.foodchem.2015.04.076. [Google Scholar] [PubMed] [CrossRef]
121. Hu A, Chen X, Wang J, Wang X, Zheng J, Wang L. Effects on the structure and properties of native corn starch modified by enzymatic debranching ED, microwave assisted esterification with citric acid (MCAE) and by the dual ED/MCAE treatment. Int J Biol Macromol. 2021;171:123–9. doi:10.1016/j.ijbiomac.2021.01.012. [Google Scholar] [PubMed] [CrossRef]
122. Reddy N, Yang Y. Citric acid cross-linking of starch films. Food Chem. 2010;118(3):702–11. doi:10.1016/j.foodchem.2009.05.050. [Google Scholar] [CrossRef]
123. Martins PC, Martins VG. Effect of rice starch hydrolysis and esterification processes on the physicochemical properties of biodegradable films. Starch-Stärke. 2021;73(9–10):2100022. doi:10.1002/star.202100022. [Google Scholar] [CrossRef]
124. Zhang K, Cheng F, Zhang K, Hu J, Xu C, Lin Y, et al. Synthesis of long-chain fatty acid starch esters in aqueous medium and its characterization. Eur Polym J. 2019;119:136–47. doi:10.1016/j.eurpolymj.2019.07.021. [Google Scholar] [CrossRef]
125. Zuo YF, Gu J, Qiao Z, Tan H, Cao J, Zhang Y. Effects of dry method esterification of starch on the degradation characteristics of starch/polylactic acid composites. Int J Biol Macromol. 2015;72:391–402. doi:10.1016/j.ijbiomac.2014.08.038. [Google Scholar] [PubMed] [CrossRef]
126. Li P, He X, Zuo Y, Li X, Wu Y. Synthesis and characterization of lactic acid esterified starch by an in-situ solid phase method. Int J Biol Macromol. 2020;156:1316–22. doi:10.1016/j.ijbiomac.2019.11.171. [Google Scholar] [PubMed] [CrossRef]
127. Jin J, Cheng L, Chen C, Li Z, Hong Y, Li C, et al. Synthesis, characterization, and application of starch-based adhesives modified with itaconic acid and N-hydroxyethyl acrylamide. Ind Crops Prod. 2023;196:116524. doi:10.1016/j.indcrop.2023.116524. [Google Scholar] [CrossRef]
128. Lu K, Zhu J, Bao X, Liu H, Yu L, Chen L. Effect of starch microstructure on microwave-assisted esterification. Int J Biol Macromol. 2020;164:2550–7. doi:10.1016/j.ijbiomac.2020.08.099. [Google Scholar] [PubMed] [CrossRef]
129. Lack S, Dulong V, Picton L, Cerf DL, Condamine E. High-resolution nuclear magnetic resonance spectroscopy studies of polysaccharides crosslinked by sodium trimetaphosphate: a proposal for the reaction mechanism. Carbohyd Res. 2007;342(7):943–53. doi:10.1016/j.carres.2007.01.011. [Google Scholar] [PubMed] [CrossRef]
130. Kim H-Y, Oh S-M, Bae J-E, Yeom J-H, Kim B-Y, Kim H-S, et al. Preparation and characterization of amorphous granular potato starches (AGPS) and cross-linked amorphous granular potato starches (CLAGPS). Carbohydr Polym. 2017;178(2):41–7. doi:10.1016/j.carbpol.2017.09.020. [Google Scholar] [PubMed] [CrossRef]
131. Haq F, Yu H, Wang L, Teng L, Haroon M, Khan RU, et al. Advances in chemical modifications of starches and their and their applications. Carbohyd Res. 2019;476:12–35. doi:10.1016/j.carres.2019.02.007. [Google Scholar] [PubMed] [CrossRef]
132. Zia ud D, Xiong H, Wang Z, Fei P, Ullah I, Javaid AB, et al. Effects of sucrose fatty acid esters on the stability and bonding performance of high amylose starch-based wood adhesive. Int J Biol Macromol. 2017;104(2006):846–53. doi:10.1016/j.ijbiomac.2017.06.090. [Google Scholar] [PubMed] [CrossRef]
133. Umemura K, Sugihara O, Kawai S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: effects of board density and pressing temperature. J Wood Sci. 2015;61(1):40–4. doi:10.1007/s10086-014-1437-8. [Google Scholar] [CrossRef]
134. Sucipto T, Widyorini R, Prayitno TA, Lukmandaru G. Properties of a new adhesive composed of gambir-sucrose. J Korean Wood Sci Technol. 2020;48(3):303–14. doi:10.5658/WOOD.2020.48.3.303. [Google Scholar] [CrossRef]
135. Wibowo ES, Kusumah S, Subyakto S, Umemura K. Modification of novel bio-based adhesive made from citric acid and sucrose by ZnCl2. Int J Adhes Adhes. 2021;108:102866. doi:10.1016/j.ijadhadh.2021.102866. [Google Scholar] [CrossRef]
136. Li C, Lei H, Wu Z, Xi X, Du G, Pizzi A. Fully biobased adhesive from glucose and citric acid for plywood with high performance. ACS Appl Mat Interf. 2022;14(20):23859–67. doi:10.1021/acsami.2c02859. [Google Scholar] [PubMed] [CrossRef]
137. Chinese standard GB/T 9846-2015. Plywood for general use. General Administration of Quality Supervision, Inspection of Quarantine of the People’s Republic of China. Standardization Administration of te People’s Republic of China. 2015 [cited 2024 Dec 10]. Available from: https://www.standardsofchina.com/standard/GBT9846-2015. [Google Scholar]
138. Li C, Hou D, Xi X, Lei H, Tondi G, Shi J, et al. Eco-friendly biobased adhesives prepared from different polyester-type glucose with high cross-linked structure. ACS Sustain Chem Eng. 2024;12(20):7831–43. doi:10.1021/acssuschemeng.4c00669. [Google Scholar] [CrossRef]
139. Widyorini R, Umemura K, Kusumaningtyas AR, Prayitno TA. Effect of starch addition on properties of citric acid-bonded particleboard made from bamboo. BioResources. 2017;12(4):8068–77. [Google Scholar]
140. Kusumah SS, Jayadi W, Pramasari D, Ayu Widyaningrum D, Darmawan B, Notowiharjo T, et al. Investigation of eco-friendly plywood bonded with citric acid-starch based adhesive. IOP Conf Ser: Earth Environ Sci. 2020;460(1):012009. doi:10.1088/1755-1315/460/1/012009. [Google Scholar] [CrossRef]
141. Amini M, Hashim R, Sulaiman NS, Mohamed M, Sulaiman O. Citric acid-modified starch as an environmentally friendly binder for wood composite making. BioResources. 2020;15(2):4234–8. doi:10.15376/biores.15.2.4234-4248. [Google Scholar] [CrossRef]
142. Li C, Hou D, Lei H, Xi X, Du G, Zhang H, et al. Effective and eco-friendly safe self-antimildew strategy to simultaneously improve the water resistance and bonding strength of starch-based adhesive. Int J Biol Macromol. 2023;248(13):125889. doi:10.1016/j.ijbiomac.2023.125889. [Google Scholar] [PubMed] [CrossRef]
143. Li X, Zhang F, Li J, Xia C, Li J. Evaluation performance of soybean meal and peanut meal blends-based wood adhesive. Polym Test. 2022;109:107543. doi:10.1016/j.polymertesting.2022.107543. [Google Scholar] [CrossRef]
144. Zhang J, Long C, Zhang X, Liu Z, Zhang X, Liu T, et al. An easy-coating, versatile, and strong soy flour adhesive via a biomineralized structure combined with a biomimetic brush-like polymer. Chem Eng J. 2022;450(6):138387. doi:10.1016/j.cej.2022.138387. [Google Scholar] [CrossRef]
145. Li Z, Niu W, Cai L, Li J, Chen H, Gao Q. Performance of eco-friendly soy protein adhesive reinforced by aldehyde sodium alginate. Int J Adhes Adhes. 2024;131(3):103649. doi:10.1016/j.ijadhadh.2024.103649. [Google Scholar] [CrossRef]
146. Xu Y, Han Y, Shi SQ, Gao Q, Li J. Preparation of a moderate viscosity, high performance and adequately-stabilized soy protein-based adhesive via recombination of protein molecules. J Clean Prod. 2020;255(6):120303. doi:10.1016/j.jclepro.2020.120303. [Google Scholar] [CrossRef]
147. Xu Y, Han Y, Li Y, Luo J, Li J, Li J, et al. Nacre-inspired construction of soft-hard double network structure to prepare strong, tough, and water-resistant soy protein adhesive. J Appl Polym Sci. 2022;139(21):52202. doi:10.1002/app.52202. [Google Scholar] [CrossRef]
148. Liu Z, Liu T, Jiang H, Zhang X, Li J, Shi QS, et al. Biomimetic lignin-protein adhesive with dynamic covalent/hydrogen hybrid networks enables high bonding performance and wood-based panel recycling. Int J Biol Macromol. 2022;214(5):230–40. doi:10.1016/j.ijbiomac.2022.06.042. [Google Scholar] [PubMed] [CrossRef]
149. Zeng G, Dong Y, Luo J, Zhou Y, Li C, Li Q, et al. Desirable strong and tough adhesive inspired by dragonfly wings and plant cell walls. ACS Nano. 2024;18(13):9451–69. doi:10.1021/acsnano.3c11160. [Google Scholar] [PubMed] [CrossRef]
150. Zhou Y, Luo J, Jing Q, Ge S, Chen S, Guo Z, et al. Tough, waterproofing, and sustainable bio-adhesive inspired by the dragonfly wing. Adv Funct Mater. 2024;34(42):2406557. doi:10.1002/adfm.202406557. [Google Scholar] [CrossRef]
151. Jiang S, Wei Y, Fu Z, Li J, Li X, Li J. Nacre-inspired high-performance multifunctional plant protein adhesive through vitrimer networks. Ind Crops Prod. 2023;192:116100. doi:10.1016/j.indcrop.2022.116100. [Google Scholar] [CrossRef]
152. Zhang J, Zhang M, Zhang Y, Shi SQ, Zhan X, Li J, et al. Improving bond performance and reducing cross-linker dosage for soy flour adhesives inspired by spider silk. ACS Sustain Chem Eng. 2021;9(1):168–79. doi:10.1021/acssuschemeng.0c06333. [Google Scholar] [CrossRef]
153. Zhang F, Li H, Wang T, Li X, Li K, Aladejana JT, et al. Advanced biomimetic soybean meal-based adhesive with high strength and toughness. ACS Sustain Chem Eng. 2022;10(51):17355–68. doi:10.1021/acssuschemeng.2c06464. [Google Scholar] [CrossRef]
154. Zhang Y, Liu Z, Xu Y, Li J, Shi SQ, Li J, et al. High performance and multifunctional protein-based adhesive produced via phenol-amine chemistry and mineral reinforcement strategy inspired by arthropod cuticles. Chem Eng J. 2021;426(7691):130852. doi:10.1016/j.cej.2021.130852. [Google Scholar] [CrossRef]
155. Cui Z, Xu Y, Sun G, Peng L, Li J, Luo J, et al. Improving bond performance and reducing cross-linker dosage of soy protein adhesive via hyper-branched and organic-inorganic hybrid structures. Nanomaterials. 2023;13(1):203. doi:10.3390/nano13010203. [Google Scholar] [PubMed] [CrossRef]
156. Xu Y, Han Y, Chen M, Peng L, Li J, Luo J, et al. Constructing a triple network structure to prepare strong, tough, and mildew resistant soy protein adhesive. Composit Part B: Eng. 2021;211:108677. doi:10.1016/j.compositesb.2021.108677. [Google Scholar] [CrossRef]
157. Xu Y, Han Y, Li Y, Li J, Li J, Gao Q. Preparation of a strong, mildew-resistant, and flame-retardant biomimetic multifunctional soy protein adhesive via the construction of an organic-inorganic hybrid multiple-bonding structure. Chem Eng J. 2022;437:135437. doi:10.1016/j.cej.2022.135437. [Google Scholar] [CrossRef]
158. Xu Y, Zhang X, Liu Z, Zhang X, Luo J, Li J, et al. Constructing SiO2 nanohybrid to develop a strong soy protein adhesive with excellent flame-retardant and coating ability. Chem Eng J. 2022;446:137065. doi:10.1016/j.cej.2022.137065. [Google Scholar] [CrossRef]
159. Liu Z, Liu T, Li Y, Zhang X, Xu Y, Li J, et al. Performance of soybean protein adhesive cross-linked by lignin and cuprum. J Clean Prod. 2022;366:132906. doi:10.1016/j.jclepro.2022.132906. [Google Scholar] [CrossRef]
160. Liu Z, Liu T, Gu W, Zhang X, Li J, Shi SQ, et al. Hyperbranched catechol biomineralization for preparing super antibacterial and fire-resistant soybean protein adhesives with long-term adhesion. Chem Eng J. 2022;449(35):137822. doi:10.1016/j.cej.2022.137822. [Google Scholar] [CrossRef]
161. Yang J, Bai R, Suo Z. Topological adhesion of wet materials. Adv Mater Deerfield. 2018;30(25):1800671. doi:10.1002/adma.201800671. [Google Scholar] [PubMed] [CrossRef]
162. Huang X, Chen Y, Li J, Li J, Gao Q, Zhan X. Improving the coating and prepressing properties of soybean meal adhesive by constructing a biomimetic topological structure. Mat Design. 2022;223:111163. doi:10.1016/j.matdes.2022.111163. [Google Scholar] [CrossRef]
163. Liu X, Bian Y, Zhang X, Liu Z, Weng T, Wang G, et al. Preparation of strong and mildew-resistant soybean meal adhesives with self-assembled core-shell structured nanospheres. Ind Crops Prod. 2023;205(5):117446. doi:10.1016/j.indcrop.2023.117446. [Google Scholar] [CrossRef]
164. Zeng G, Li K, Zhou Y, Wang T, Dong Y, Luo J, et al. Natural organic-inorganic hybrid structure enabled green biomass adhesive with desirable strength, toughness and mildew resistance. Int J Biol Macromol. 2023;236(98):123931. doi:10.1016/j.ijbiomac.2023.123931. [Google Scholar] [PubMed] [CrossRef]
165. Huang X, Chen Y, Lin X, Li J, Gao Q. A strong soy protein-based adhesive with excellent water retention. Chem Eng J. 2023;472(4):145037. doi:10.1016/j.cej.2023.145037. [Google Scholar] [CrossRef]
166. Aladejana JT, Zeng G, Zhang F, Li K, Li X, Dong Y, et al. Tough, waterproof, and mildew-resistant fully biobased soybean protein adhesives enhanced by furfuryl alcohol with dynamic covalent linkages. Ind Crops Prod. 2023;198:116759. doi:10.1016/j.indcrop.2023.116759. [Google Scholar] [CrossRef]
167. Zhang X, Liu Z, Cai L, Zhang X, Long C, Li J, et al. Development of a strong and conductive soy protein adhesive by building a hybrid structure based on multifunctional wood composite materials. J Clean Prod. 2023;412:137461. doi:10.1016/j.jclepro.2023.137461. [Google Scholar] [CrossRef]
168. Shao J, Li X, Aladejana JT, Chen S, Li J. Strengthening soybean protein adhesive anti-mildew properties: design strategies, enhancing mechanisms and application potential studies. Mater Today Commun. 2023;36:106426. doi:10.1016/j.mtcomm.2023.106426. [Google Scholar] [CrossRef]
169. Chen Y, Chen M, Luo J, Li J, Gao Q. Effect of tannin on the bonding performance and mildew resistance of soybean meal-based adhesive. Ind Crops Prod. 2022;189:115740. doi:10.1016/j.indcrop.2022.115740. [Google Scholar] [CrossRef]
170. Liu Z, Liu T, Zhang J, Li Y, Luo J, Li J, et al. Preparation of a strong, high prepressing intensity, and multifunction soybean protein adhesive by using hyperbranched functional polymer. Polym Test. 2023;119:107931. doi:10.1016/j.polymertesting.2023.107931. [Google Scholar] [CrossRef]
171. Huang X, Chen Y, Li J, Li J, Gao Q, Mao A. Development of a strong soy protein-based adhesive with excellent antibacterial and antimildew properties via biomineralized silver nanoparticles. Ind Crops Prod. 2022;188(49):115567. doi:10.1016/j.indcrop.2022.115567. [Google Scholar] [CrossRef]
172. Xu Y, Zhang X, Wang G, Zhang X, Luo J, Li J, et al. Preparation of a strong soy protein adhesive with mildew proof, flame-retardant, and electromagnetic shielding properties via constructing nanophase-reinforced organic-inorganic hybrid structure. Chem Eng J. 2022;447(1):137536. doi:10.1016/j.cej.2022.137536. [Google Scholar] [CrossRef]
173. Aladejana JT, Zhang F, Zeng G, Li K, Dong Y, Li X, et al. Dual crosslinked soybean protein adhesives with high strength, mold resistance, and extended shelf-life via dynamic covalent bonds. Eur Polym J. 2023;194(14):112150. doi:10.1016/j.eurpolymj.2023.112150. [Google Scholar] [CrossRef]
174. Usui K, Bonn M, Nagata Y. Molecular mechanism of water evaporation. Phys Rev Lett. 2015;115(23):236102. doi:10.1103/PhysRevLett.115.236102. [Google Scholar] [PubMed] [CrossRef]
175. Liu Z, Liu T, Zhang X, Long C, Li J, Gao Q. Preparation of strong, water retention, and multifunctional soybean flour-based adhesive inspired by lobster shells and milk skin. Composit Part B: Eng. 2023;266(7691):110989. doi:10.1016/j.compositesb.2023.110989. [Google Scholar] [CrossRef]
176. Liu Z, Zhang X, Zhou W, Wang G, Liu T, Luo J, et al. Bioinspired water-retaining and strong soybean flour-based adhesive for efficient preparation of plywood. J Clean Prod. 2023;414(1):137739. doi:10.1016/j.jclepro.2023.137739. [Google Scholar] [CrossRef]
177. Li F, Mo J, Zhang Z, Shi SQ, Li J, Cao J, et al. Achieving strong, stable, and durable underwater adhesives based on a simple and generic amino-acid-resembling design. Mater Horiz. 2023;10(8):2980–8. doi:10.1039/D3MH00301A. [Google Scholar] [PubMed] [CrossRef]
178. Li Y, Cai L, Zhang X, Li J, Li J, Gao Q. Development of a strong and multifunctional soy protein-based adhesive with excellent coating and prepressing in wet state by constructing a radical polymerization and organic-inorganic mineralization bionic structure. J Clean Prod. 2023;400(9):136730. doi:10.1016/j.jclepro.2023.136730. [Google Scholar] [CrossRef]
179. Zhang X, Long C, Zhu X, Chen Y, Cui Z, Luo J, et al. Preparation of strong and thermally conductive, spider silk-inspired, soybean protein-based adhesive for thermally conductive wood-based composites. ACS Nano. 2023;17(19):18850–63. doi:10.1021/acsnano.3c03782. [Google Scholar] [PubMed] [CrossRef]
180. Zhang X, Li J, Gao Q, Wang Z, Ye N, Li J, et al. Nerve-fiber-inspired construction of 3D graphene tracks supported by wood fibers for multifunctional biocomposite with metal-level thermal conductivity. Adv Funct Mater. 2023;33(18):2213274. doi:10.1002/adfm.202213274. [Google Scholar] [CrossRef]
181. Oktay S, Kızılcan N, Bengu B. Oxidized cornstarch—Urea wood adhesive for interior particleboard production. Int J Adhes Adhes. 2021;110:102947. doi:10.1016/j.ijadhadh.2021.102947. [Google Scholar] [CrossRef]
182. Zhang B, Pizzi A, Petrissans M, Petrissans A, Colin B. A melamine-dialdehyde starch wood surface finish without formaldehyde. J Renew Mater. 2023;11(11):3867–89. doi:10.32604/jrm.2023.028888. [Google Scholar] [CrossRef]
183. Zhang Q, Yan R, Lei H, Du G, Pizzi A, Puagsin B, et al. An easily-synthesized, environment friendly cellulose-based wood bioadhesive of excellent bonding properties: preparation and characterization. Carbohydrate Polymers. 2025;458(10):139688. [Google Scholar]
184. Tomkinson J. Adhesives based on natural resources. In: Dunky M, Pizzi A, Van Leemput M, editors. Wood adhesion and glued products: wood adhesives. Brussels: European Commission, Directorate General for Research; 2002. p. 46–65. [Google Scholar]
185. Thébault M, Pizzi A, Fredon E. Synthesis of resins with ozonized sunflower oil and Radiata Pine tannins. J Renew Mater. 2013;1(4):242–52. doi:10.7569/JRM.2013.634121. [Google Scholar] [CrossRef]
186. Jia P, Song F, Li Q, Xia H, Li M, Shu X, et al. Recent development of cardanol based polymer materials—a review. J Renew Mater. 2019;7(7):601–19. doi:10.32604/jrm.2019.07011. [Google Scholar] [CrossRef]
187. Tasooji M, Tabarsa T, Khazaeian A, Wool RP. Acrylated epoxidised soy oil as an alternative to urea-formaldehyde in making wheat straw particleboard. J Adhes Sci Technol. 2010;24(8–10):1717–27. doi:10.1163/016942410X507786. [Google Scholar] [CrossRef]
188. Wool RP, Kusefoglu SH, Khot SN, Zhao R, Palmese G, Boyd A, et al. Affordable bio-derived plastics and binders for the composite industry. In: Proceedings of the Second European Panel Products Symposium; 1998; Bangor, UK: Academic Press. p. 233–6. [Google Scholar]
189. Miller R, Shonfeld U. Company literature. Esbacher Weg 15, Feuchtwangen, Germany: Preform Raumgliederungssysteme GmBH; 2002. [Google Scholar]
190. Hammami N, Jarroux N, Robitzer M, Majdou M, Habas J-P. Optimized synthesis according to one-step process of a biobased thermoplastic polyacetal derived from isosorbide. Polymers. 2016;8(8):294. doi:10.3390/polym8080294. [Google Scholar] [PubMed] [CrossRef]
191. Je H, Won J. Natural urushiol as a novel under-water adhesive. Chem Eng J. 2021;404(1985):126424. doi:10.1016/j.cej.2020.126424. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools