 Open Access
Open Access
REVIEW
Performance Enhancement of Chitosan for Food Packaging: Impact of Additives and Nanotechnology
1 Magister of Nanotechnology, Graduate School, Institut Teknologi Bandung, Jalan Ganesha No. 10, Bandung, 40132, West Java, Indonesia
2 Nano and Quantum Technology Research Group, Faculty of Industrial Technology, Institut Teknologi Bandung, Jalan Ganesha No. 10, Bandung, 40132, West Java, Indonesia
3 Research Center for Nanoscience and Nanotechnology, Center for Advance Science, Institut Teknologi Bandung, Jalan Ganesha No. 10, Bandung, 40132, West Java, Indonesia
4 Materials Science and Engineering Research Group, Faculty of Mechanical and Aerospace Engineering, Institut Teknologi Bandung, Jalan Ganesha No. 10, Bandung, 40132, West Java, Indonesia
5 Physics of Instrumentation and Computation Research Group, Faculty of Mathematics and Natural Sciences, Institut Teknologi Bandung, Jalan Ganesha No. 10, Bandung, 40132, West Java, Indonesia
* Corresponding Author: Damar Rastri Adhika. Email:
(This article belongs to the Special Issue: Special issue from 1st International Conference of Natural Fiber and Biocomposite (1st ICONFIB) 2024 )
Journal of Renewable Materials 2025, 13(6), 1043-1070. https://doi.org/10.32604/jrm.2025.02024-0002
Received 30 September 2024; Accepted 09 January 2025; Issue published 23 June 2025
Abstract
The continuous increase in petroleum-based plastic food packaging has led to numerous environmental concerns. One effort to reduce the use of plastic packaging in food is through preservation using biopolymer-based packaging. Among the many types of biopolymers, chitosan is widely used and researched due to its non-toxic, antimicrobial, and antifungal properties. Chitosan is widely available since it is a compound extracted from seafood waste, especially shrimps and crabs. The biodegradability and biocompatibility of chitosan also showed good potential for various applications. These characteristics and properties make chitosan an attractive biopolymer to be implemented as food packaging in films and coatings. Chitosan has been tested in maintaining and increasing the shelf life of food, especially seafood such as fish and shrimp, and post-harvest products such as fruits and vegetables. In addition to its various advantages, the properties and characteristics of chitosan need to be improved to produce optimal preservation. The properties and characteristics of chitosan are improved by adding various types of additive materials such as biopolymers, plant extracts, essential oils, and metal nanoparticles. Research shows that material additives and nanotechnology can improve the quality of chitosan-based food packaging for various types of food by enhancing mechanical properties, thermal stability, antimicrobial activity, and antioxidant activity. This review provides a perspective on the recent development and properties enhancement of chitosan composite with additives and nanotechnology, as well as this material’s challenges and prospects as food packaging.Graphic Abstract
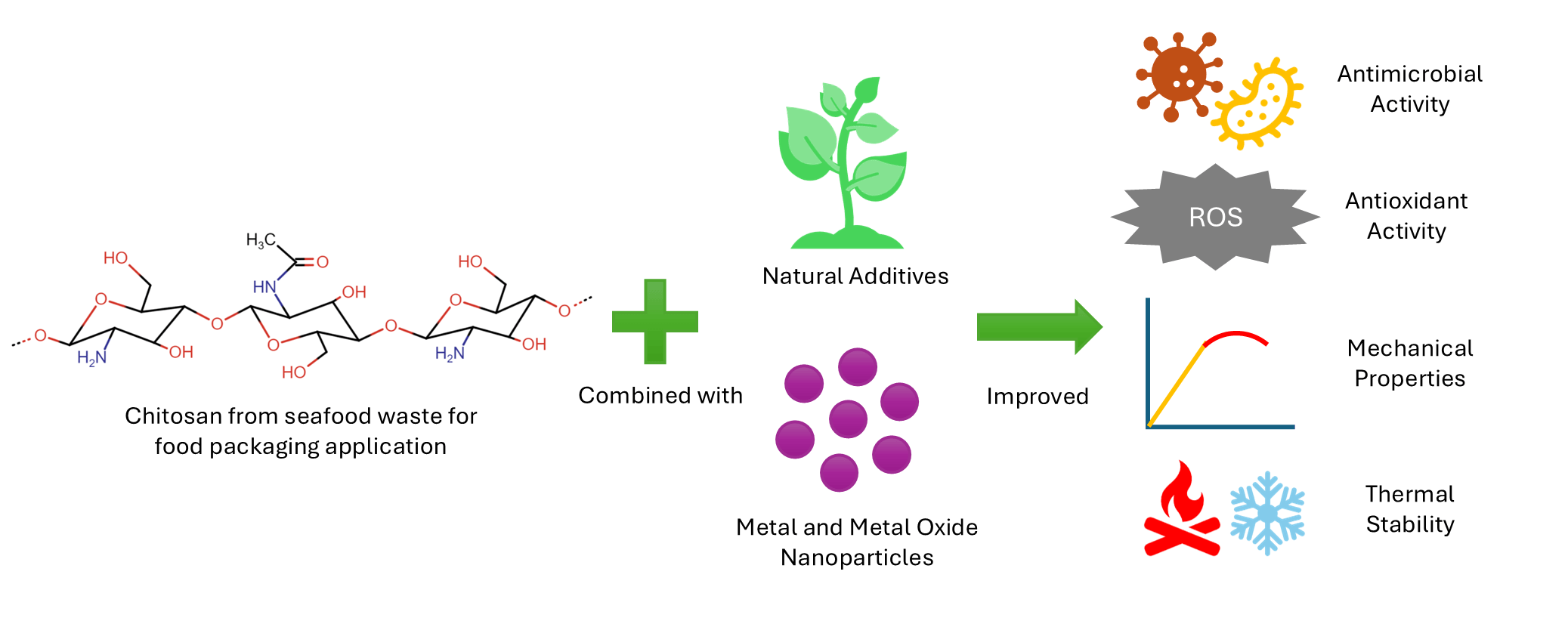
Keywords
Food products, especially plantation and marine products, are abundant and have a very good market potential, especially in tropical countries with fertile soil and large marine areas like Indonesia. However, food products such as fruits, vegetables, fish, and meat are highly susceptible to spoilage due to enzymatic activity, air circulation, and microbial growth. Microorganisms’ activity is one of the major contributors to the rapid spoilage of food products. They can produce various enzymes and biofilms that lead to undesirable changes in food, including pH, nutrients, physical properties, and moisture content [1]. These changes indicate that food spoilage is happening rapidly. Therefore, microbial control is essential. Refrigeration, freezing, dehydration, and smoking are common methods to preserve food quality [2]. Despite their widespread use, these techniques have limitations in long-term storage due to their incomplete inhibition of microbial growth. In order to protect and maintain the quality of food products, food packaging is widely used for preservation. Packaging is the most applied method for food processing and safety, as it effectively prevents oxygen-containing air in food products, which can lead to bacterial growth [3].
Glass, with its inherent properties, is well-suited for packaging. Nevertheless, the weight and cost of glass have led to a dominance of metal and plastic packaging in modern markets. Plastic has become the ubiquitous choice for food packaging due to its flexibility and affordability [4]. However, the environmental implications of plastic’s non-biodegradability have sparked a search for more sustainable alternatives. Biopolymers, derived from natural sources, represent a promising alternative to replace non-biodegradable plastic. Several methods of innovative food packaging that have recently been widely researched are edible film and coating. Biopolymers derived from polysaccharides, proteins, lipids, and their derivatives can be applied as coatings and films for various food products [5]. Generally, the synthesis of film and coating matrices can be carried out using a similar method. The difference lies in the packaging process. In film packaging, the thin film packaging is synthesized and dried until it forms a plastic-like solid packaging. The film is then applied to the food to be tested. In the coating technique, the matrix remains in a liquid form. The food can be dipped into the coating solution for a certain period, then removed and dried [4].
To reduce the environmental impact of conventional packaging materials, innovative biopolymer-based food packaging materials such as alginate, pectin, chitosan, and cellulose have gained significant attention. Chitosan is widely used due to its natural antimicrobial properties [6]. Biopolymer-based films and coatings, particularly those made from chitosan, have shown their effectiveness in extending the shelf life of a wide range of food products, including seafood like shrimp [7,8] and tuna fish [9,10], chicken meat [11,12], beef meat [13,14], and also post-harvest products such as cherries [15], apples [16,17], green chilli peppers [18], papayas [9,19], bananas [20–23] and strawberries [24,25].
However, the performance of chitosan-based food packaging needs to be further enhanced to more effectively inhibit bacterial growth. Besides that, chitosan also has limitations regarding its water retention and mechanical properties, resulting in brittle and rigid films forming [5]. Recently, a significant amount of research has focused on combining chitosan with other materials as additives to enhance its properties, particularly its antimicrobial activity [26]. Nanotechnology offers a promising approach to improving the antimicrobial efficacy of chitosan-based packaging [27]. This review explores various combinations of natural additives with chitosan and the role of nanotechnology on the performance of chitosan-based food packaging, as summarised in Fig. 1.
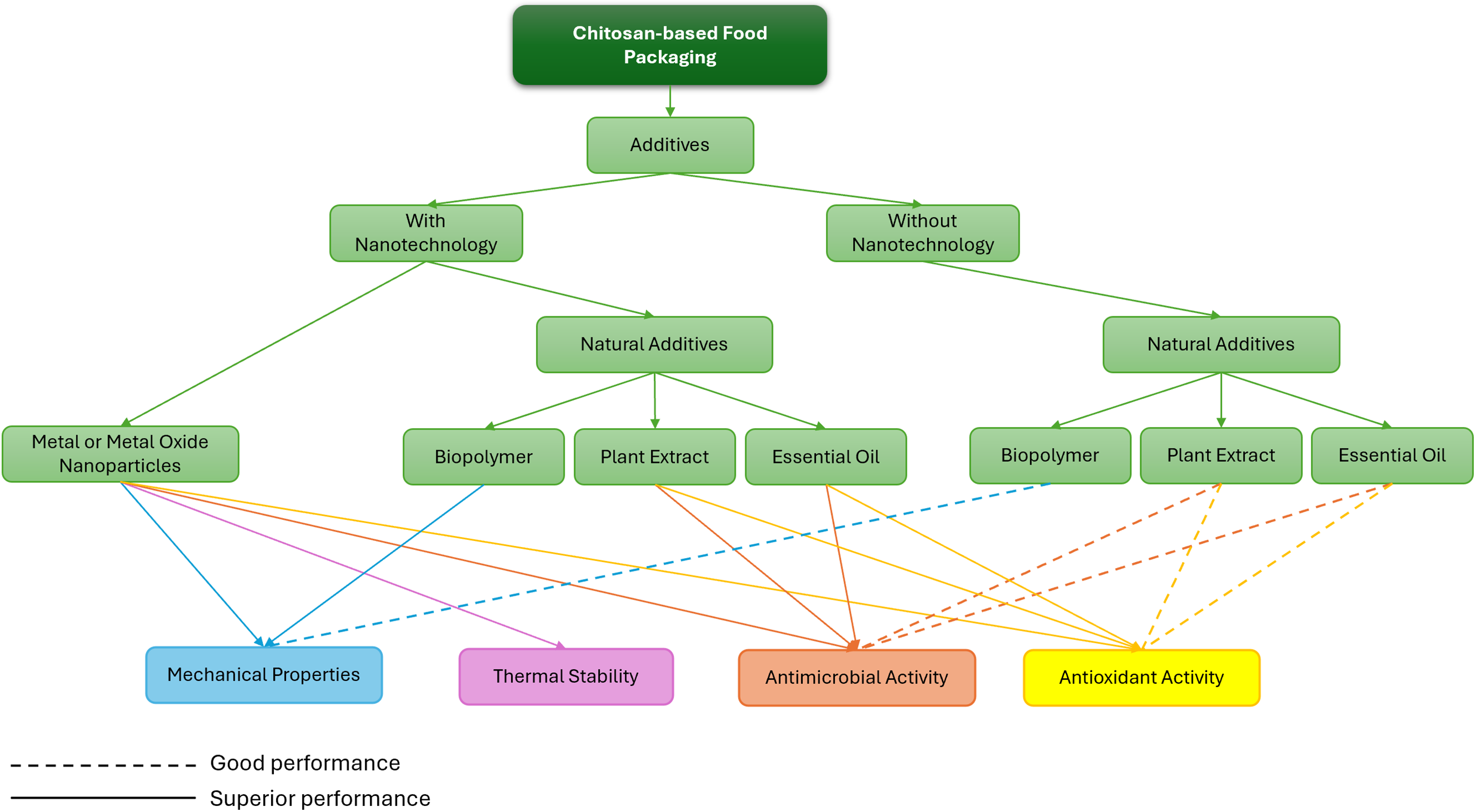
Figure 1: Effect of additives on the performance of chitosan-based food packaging
2 Chitosan Properties and Characteristics
Chitosan is a natural biopolymer synthesized through the deacetylation of chitin, which is extracted from natural sources using a two-step chemical extraction process involving deproteinization and demineralization [28]. Chitin can be obtained from crustaceans (crabs, shrimps, lobsters), arthropods (spiders, beetles, scorpions), mollusca (squids), and microorganisms (fungi and cell walls). Currently, the primary source of chitin production is seafood waste, especially the exoskeletons of crabs, lobsters, and shrimps [5]. Both chitin and chitosan are co-polymers of N-acetyl-D-glucosamine and D-glucosamine, linked together by β-(1-4)-glycosidic bonds [4,6]. The chemical structures of chitin and chitosan are illustrated in Fig. 2. Chitosan is a biopolymer that has been extensively studied compared to other polymers. Research on chitosan-based food packaging has steadily increased year after year [6]. Chitosan is currently applied in various industries, particularly in the food sector.
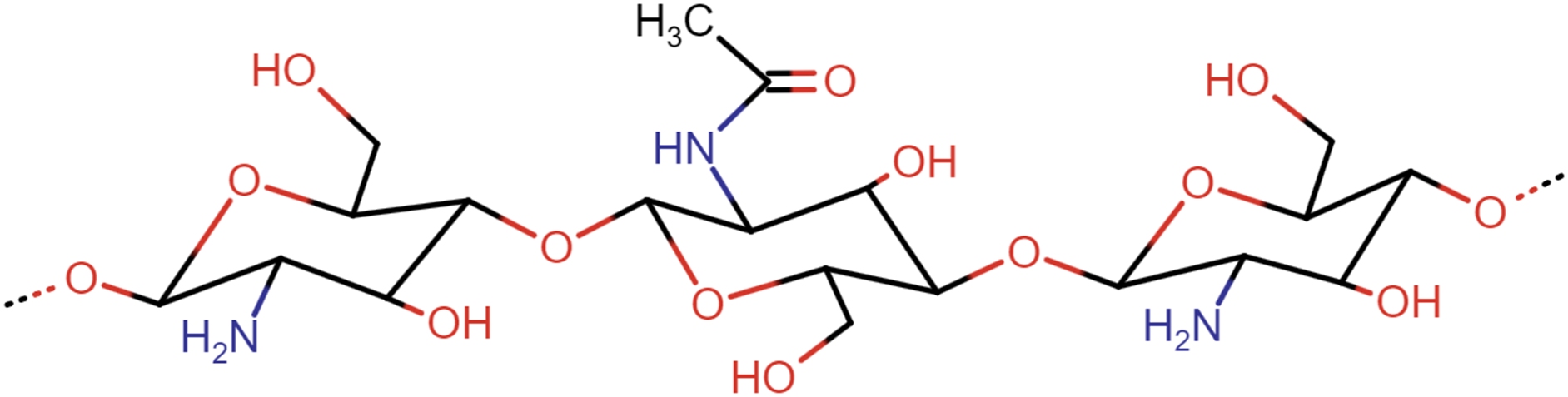
Figure 2: Chemical structures of chitosan (adapted from Kumar et al. [29])
2.1 Ionic Bonds and Solubility
Chitosan’s ability to form complex compounds with transition metals is an important discovery. Research has shown that it can ionically bind with Cu2+, Hg2+, Zn2+, Cd2+, Ni2+, and other metal ions [30]. This unique property is of utmost importance for active food packaging, as it can bind metal nanoparticles and regulate antimicrobial activity for extended periods, underlining the broad potential for its application in food safety. The ionic strength of the solvent, ion interactions in the solvent, and solution pH are important parameters that determine the solubility properties of chitosan in water [4]. Chitosan has a unique property of being soluble in acidic conditions but insoluble in basic conditions. However, chitosan with a medium molecular weight and a degree of acetylation (DA) of around 0.5 can be soluble at a pH of around 7. Differences in solubility values will significantly affect the biological properties of chitosan due to changes in enzyme interactions [31].
2.2 Antimicrobial and Antioxidant Activity
Chitosan is a natural polysaccharide with antimicrobial properties against bacteria, fungi, and yeast. This antimicrobial activity depends on its cationic nature, degree of acetylation, concentration, and the test organism [4]. The mechanism of chitosan’s antimicrobial activities is still being discussed. Chitosan can form a protective layer like cellophane on the food surface, which keeps microbes from physically attacking it [32]. The chitosan layer can limit gas exchange from food to the external environment, thus inhibiting oxygen transport for microbes to grow and develop [33]. According to its structure, the cationic groups of chitosan can easily interact with the anionic peptidoglycan in bacterial cell walls, causing the cell wall to disintegrate and damage to the cell wall results in the leakage of intracellular parts, leading to bacterial death [34]. Chitosan also forms a watertight layer around bacterial cells, preventing the exchange of solutes and thus disrupting bacterial metabolism [35]. Meanwhile, low molecular weight chitosan can go through the cell nucleus directly and inhibit the translation of DNA into RNA by binding to DNA molecules [36]. By incorporating various additives, including metal and metal oxide nanoparticles, essential oils, and plant extracts, the antibacterial properties of chitosan films can be significantly improved [37].
Besides that, one of the most essential properties that need more concern is antioxidant activity. Antioxidants are substances that prevent damage to biological processes caused by free radicals. They work by giving electrons to free radicals, rendering them harmless [38]. Free radicals are often associated with oxidative stress, which occurs when oxygen combines with certain chemicals. These harmful radicals can damage essential cell components like DNA, proteins, and membranes. Antioxidants neutralize free radicals before they can cause harm. Plants produce a variety of antioxidants as secondary metabolites. Chitosan has been shown to exhibit antioxidant activity [39], but further improvements are needed.
3 Natural Additives to Enhance Chitosan Properties
Although chitosan has good properties, the quality of chitosan-based food packaging needs further improvement for longer-term food preservation. With amine and hydroxyl groups, chitosan can facilitate its interaction with the other molecules to improve and modify its properties [4]. The combination between chitosan and the other molecules can also be classified as a composite. Incorporating nanomaterials into a composite blend produces a nanocomposite [40]. Additive materials include biopolymers, plant extracts, essential oils, and metal nanoparticles. These various types of materials can generally improve the performance of chitosan as food packaging.
Several studies have tested the performance of chitosan as food packaging combined with other biopolymers, showing an improvement in the properties and characteristics of chitosan-based composites [6]. Some biopolymers previously studied are starch, cellulose, alginate, gelatin, and pectin. Various types of biopolymers that can be combined with the chitosan matrix are shown in Table 1.
Starch-based biodegradable films share similar physical properties with synthetic polymers, such as being tasteless, transparent, odorless, and resistant to gases like oxygen and carbon dioxide [49]. However, starch’s strong intermolecular bonds make it difficult to melt, limiting its direct use in film production. Additionally, the high water content and low melting temperature of starch make film processing complex. Incorporating active agents into starch films can also be challenging, as these agents may degrade or lose efficacy during processing or storage [50]. Blending chitosan with starch produces food packaging films with enhanced antimicrobial and antioxidant properties due to improved water barrier properties. When added with thyme leaf extract, the chitosan-starch matrix also demonstrates improved mechanical properties [41].
Cellulose, another abundant natural biopolymer, is commonly derived from plant stems. Films formed from the chitosan-cellulose combination exhibit excellent mechanical properties due to electrostatic interactions between the two polymers [42]. The cationic nature of chitosan and the anionic nature of cellulose facilitate strong interactions, resulting in food packaging that has been shown to extend the shelf life of products like cheese [42]. Other forms of cellulose, including methylcellulose, hydroxypropyl methylcellulose, quaternized hemicellulose, carboxymethyl cellulose, and microfibrillated cellulose, can also be combined with chitosan to create biocomposite-based films. Research on cellulose derivatives, such as carboxymethyl cellulose, has demonstrated their potential as additives for chitosan to improve mechanical properties and enhance antibacterial activity. The chitosan-carboxymethyl cellulose matrix effectively inhibits the growth of Staphylococcus pneumonia, Bacillus subtilis, and Escherichia coli [43]. A study also revealed that avocados coated with moringa, chitosan, and carboxymethyl cellulose experienced slower respiration rates and maintained higher firmness than uncoated avocados throughout the storage period. These factors reduced moisture loss and improved overall fruit quality and shelf life [44]. Another form of cellulose, hydroxypropyl methylcellulose, exhibited excellent UV barrier properties when combined with chitosan [45].
Alginate, a biopolymer sourced from seaweed, is gaining significant attention as a sustainable alternative to traditional petroleum-based polymers. Seaweed, a plentiful marine resource, doesn’t require land for cultivation, making it an environmentally friendly choice [51]. Alginate can also interact electrostatically with cationic chitosan. This interaction significantly influences contact angle, microstructure, and thermal performance. Adding alginate to the chitosan matrix enhances water and gas barrier properties [46].
Pectin, another plant-based biopolymer, is rich in structural polysaccharides. Pectin is a complex carbohydrate extracted from plants, primarily composed of galacturonic acid units. These units can be chemically modified, with some carboxyl groups esterified with methyl groups or neutralized with bases [52]. Pectin possesses an anionic layer, facilitating stable intermolecular interactions with chitosan [53]. Chitosan-pectin composites exhibit improved properties and characteristics while preserving fruits’ physicochemical and sensory properties [47].
Carrageenan, a seaweed-based biopolymer, can work effectively with chitosan to improve food preservation. Carrageenan is a linear polysaccharide composed of galactose and ester sulfates, distinguished by the presence of sulfur groups. Lambda (λ), kappa (κ), and iota (ι) carrageenan are the three primary types, each characterized by a unique arrangement of sulfate groups in their repeating units [54]. According to Nguyen et al. (2021), with a specific combination of 1% chitosan and 0.2% κ-carrageenan could reduce weight loss in dragon fruit stored at 10°C and successfully delayed the yellowing of the fruit’s skin. A natural aging process like yellowing is a major visual sign that influences consumer choices [48].
Gelatin can also be added to chitosan matrices as a co-agent to enhance the quality of food packaging. Gelatin is a naturally occurring water-soluble protein obtained from the partial hydrolysis of collagen. A study applied a carboxymethyl chitosan matrix with added gelatin to cherries. The results indicated that this combination of biopolymers could improve food packaging quality. This study also demonstrated that the biopolymer blend supplemented with ascorbic acid could enhance the antioxidant properties of the packaging [55]. A specific study investigated food packaging from a chitosan matrix combined with gelatin from tuna fish skin as a preservative for papaya fruit. This matrix combination improved antioxidant properties and reduced mass loss, thereby successfully extending the shelf life of peeled papaya [19].
3.1.1 Nanotechnology Application on Biopolymer
Besides combining multiple types of biopolymers, improving the performance of food packaging can also be achieved by changing the size of the polymer to nanoscale. Biopolymer-based nanoparticles are a common type of biodegradable nanoparticles made from natural polymers in living organisms. Biopolymer-based nanoparticles are being developed to address toxicity, biocompatibility, and biodegradability issues. They can be applied in various industries. However, biopolymer-based nanoparticles are considered safer and more environmentally friendly than metal nanoparticles [56].
Using nanotechnology in food preservation offers numerous advantages. These include enhancing the properties of polymers in terms of mechanical characteristics and adding new functionalities, such as active packaging based on the characteristics of the nanomaterials [27]. Nanomaterials, which include nanoparticles, as defined by the European Commission, are substances composed of unbound or aggregated particles, where at least 50% of the particles in the size distribution have one or more external dimensions ranging from 1 to 100 nm. These materials can be composed of a single element or compound, or a combination thereof [57]. The other form, nanoemulsions, are tiny oil droplets in the water mixture or vice versa, with a size of around 50 to 1000 nm. Typically, they are between 100 and 500 nm in size and can be either oil-in-water or water-in-oil system [58].
Chitosan nanoparticles have been reported to be capable of increasing the mechanical, barrier, thermal, and antimicrobial properties of films. These results suggest that using chitosan nanoparticles can make an edible and biodegradable film better suited for food packaging [40]. Naskar et al. (2019) outlined various techniques for producing chitosan nanoparticles, including ionotropic gelation, emulsion droplet coalescence, emulsion solvent diffusion, and reverse micellization [59]. In addition, adding one of the nanocellulose types, cellulose nanofibers (CNF), to chitosan films enhanced the mechanical properties and prevented water vapor from passing through. The increased strength was likely due to the nanofibers evenly spreading throughout the chitosan and the good connection between the fibers and the polymers [60]. Nanocellulose, including cellulose nanofiber, cellulose nanocrystal, and bacterial nanocellulose possesses good oxygen, water vapor, and ultraviolet barrier properties [61].
Despite having antimicrobial and antioxidant properties, chitosan’s potential can be further maximized through improvements in these areas. One method to enhance the antioxidant and antimicrobial properties of chitosan-based food packaging is by adding extracts from plant parts such as leaves, fruit peels, stems, and seeds [6]. In addition to being non-toxic, plant extracts are widely available in nature. Adding extracts rich in polyphenols, like banana peel and mango peel extracts, can improve the antioxidant properties of chitosan-based films and coatings. Various plant extracts that have been studied as antioxidant agents are shown in Table 2.
Antioxidant properties are often tested using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent. The DPPH method for testing antioxidants is one of the most widely used methods for testing plant extracts. This method is based on measuring electron paramagnetic resonance spectroscopy of DPPH depletion during its reaction with antioxidants. Antioxidant activity is usually expressed as a percentage of free radical inhibition. Although widely used, the DPPH method needs to be standardized [68]. The kinetic properties of each sample greatly influence antioxidant activity. Plant extracts can react rapidly with DPPH, while essential oils undergo a slower reaction [71]. In addition to antioxidant properties, plant extracts also potentially have antimicrobial properties.
Banana peels, often considered waste, have shown potential as an additive in chitosan-based packaging. Research has indicated that banana peels can alter chitosan films’ physical and mechanical properties. Banana peels contain phenolic compounds such as carotenoids, ascorbic acid, and biogenic amines that function as antioxidants [62]. The addition of banana peel extracts significantly enhanced the antioxidant capacity of the films compared to pure chitosan films. As the banana peel extract concentration increased, so did the antioxidant activity. It shows a similar trend observed in the total phenolic content [62]. Meanwhile, this research also demonstrated that increasing the concentration of banana peel extract led to a decrease in mechanical properties, specifically tensile strength [62].
Green tea leaves (Camellia sinensis) are a rich source of phenolic compounds like catechin, theaflavin, and thearubigin [63]. These three compounds have been shown to inhibit microbial growth and possess good antioxidant properties. Studies have shown that adding green tea extract to chitosan films can enhance their antioxidant properties. Results indicate that the higher the concentration of the extract, the better the antioxidant properties [63]. The study also indicated that adding green tea extract to chitosan films improved their mechanical and barrier properties. The interactions between chitosan and the polyphenolic compounds present in green tea extracts likely contributed to these modifications in film properties [63].
Research on developing chitosan-based food packaging has also been conducted using extracts from red grape seeds combined with Zataria multiflora Boiss essential oil. Grape seeds have a high content of flavonoids. Research has proven that chitosan films with these natural compounds can improve their antioxidant and antimicrobial properties [64]. A comparative analysis revealed that chitosan films fortified with grape seed extract possessed a greater capacity to scavenge DPPH radicals than those fortified with Zataria multiflora Boiss essential oil [64].
Jujube leaves, also known as ziziphus leaves, have been shown to improve the antioxidant properties of chitosan films [65]. Jujube leaves exhibited the highest DPPH scavenging activity compared to pine nutshell and peanut, similarly, demonstrating the total phenolic content. The variation in antioxidant activity among the composite films could be attributed to the intricate interplay of the three plant extracts. The incorporation of the three plant extracts into chitosan films led to a significant decrease in the water swelling capacity and mechanical strength of the films. Conversely, it increased the permeability of the films to water vapor, oxygen, and carbon dioxide. These modified properties hold potential for the development of active modified atmosphere packaging systems for fruits and vegetables [65].
Propolis, a substance bees produce, can also be used as an antioxidant and antimicrobial agent. Propolis contains many beneficial compounds, such as flavonoids, phenolic acids, phenyl aldehydes, and other amino acids. The addition of propolis extract to chitosan films led to an increase in total phenolic content and DPPH scavenging activity. Furthermore, as the concentration of propolis extract increased, so did the antioxidant activity and total phenolic content [66]. Another study explored the use of propolis extract from three types of Malaysian stingless bees: T. apicalis, H. fimbriata, and T. binghami. These propolis samples were found to contain flavonoids and phenolic compounds, known for their potential in creating biologically active substances and exhibiting strong antioxidant properties. The results indicated that all three types of propolis demonstrated significant antioxidant activity, with H. fimbriata propolis exhibiting the highest performance [67]. Propolis has been studied as a compound that can inhibit bacterial growth such as Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus [66].
Mango leaf extract has been investigated as an additive in producing chitosan-based films implemented on cashew nuts. Research has shown an increase in antioxidant activity after adding mango leaf extract at a concentration of 5%, resulting in a maximum inhibition percentage in the DPPH test of 87.16% [68]. The increase in inhibition is due to the thickening of the chitosan composite film with the extract. The increase in antioxidant activity also suggests the involvement of other phenolic compounds present in mango leaf extract. In addition, the parameter of oxidation resistance was also shown to increase significantly after the film was supplemented with mango leaf extract at concentrations of 3% and 5%. The morphological results showed an interaction between the phenolic polymer of mango leaf extract and chitosan, resulting in a smooth film structure [68].
Purple sweet potato is a source of anthocyanins. Anthocyanins are phenolic compounds that play a role in antioxidant activity. Plants rich in anthocyanins exhibit various colours such as orange, red, purple, and blue, depending on the type of plant [72]. Research has reported that the addition of purple sweet potato extract can improve the antioxidant properties of chitosan. This antioxidant mechanism is related to the chelating efficiency that can inhibit lipid peroxidation. Due to these advantages, chitosan films supplemented with purple sweet potato extract can protect food from oxidative damage [69].
Olive leaves (Olea europaea L.) are recognized as a valuable source of various bioactive compounds, particularly a diverse range of phenolic compounds. Olive leaf extract exhibits potent antioxidant and antimicrobial properties, making it effective in combating diseases such as coronary heart disease, diabetes, and certain bacterial infections [73]. It was confirmed by a study demonstrating that a chitosan-olive leaf extract blend exhibited approximately 75% DPPH radical scavenging activity [70].
The addition of turmeric extract to chitosan films significantly enhanced their antimicrobial properties. Compared to pure chitosan films, turmeric extract-incorporated films demonstrated a significant reduction in Staphylococcus aureus and Salmonella counts [74]. This improved antimicrobial activity can be attributed to the presence of curcuminoids and terpenoids within the turmeric extract. The addition of turmeric extract to chitosan-based food packaging also increases the value of the tensile strength parameter and significantly improves the barrier properties against visible ultraviolet light [74]. Another study shows that the turmeric extract could inhibit fungal growth, especially for Botrytis cinerea [75].
A study has demonstrated synergistic performance between chitosan films and apple peel polyphenols. The high polyphenol content of apple peel extract enables it to inhibit the activity of Staphylococcus aureus, Salmonella typhimurium, Escherichia coli, and Bacillus cereus [76]. This was evidenced by the formation of inhibition zones on the agar medium. It was observed that as the concentration of apple extract increased, the size of the inhibition zone also increased. The addition of polyphenols led to a decrease in the mechanical properties of the chitosan film. This reduction in mechanical strength may be attributed to a disruption in the crystalline structure of the chitosan matrix. The incorporation of polyphenols could interfere with the formation of ordered crystalline structures, weakening the intermolecular hydrogen bonding within the matrix [76].
In a study, pork coated with a tomato extract and chitosan-based edible coating was able to reduce the population of aerobic mesophilic bacteria for up to 21 days [77]. In this study, bacterial analysis was calculated based on the growth of bacterial colonies, represented by log/CFU. The number of mesophilic and coliform bacteria analyzed over several days indicated that the combination of chitosan and tomato extract was able to inhibit bacterial growth [77].
Pomegranate peel can scavenge free radicals and has antimicrobial properties. Pomegranate peel extract has been tested to inhibit the exchange of oxygen and carbon dioxide and increase the antioxidant activity of chitosan-based food packaging. This extract can inhibit the growth of Staphylococcus aureus [78]. The addition of 10 g/L of pomegranate peel extract of film material resulted in a notable ability to kill Staphylococcus aureus bacteria. However, the same treatment did not affect Escherichia coli. This aligns with the general understanding that Gram-positive bacteria, are more susceptible to antimicrobial agents compared to Gram-negative bacteria [78].
Clove extract can also be used as an antibacterial agent, especially against Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli. This study showed that the addition of clove extract to the chitosan matrix can inhibit bacterial activity. This is indicated by the bacterial test results observed from the zone of inhibition formed. The largest zone of inhibition against bacteria was observed from the test using a chitosan matrix supplemented with clove extract [7].
Similar to clove extract, the combination between chitosan and peppermint (Mentha piperita L.) extract could be effective against bacteria (79). The chitosan demonstrated effectiveness against both gram-positive and gram-negative bacteria. It was particularly strong against Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli. When combined with peppermint extract, the mixture exhibited a synergistic effect, especially against Staphylococcus aureus, with a larger zone of bacterial growth inhibition compared to the other bacteria [79].
3.2.1 Nanotechnology Application on Plant Extract
As observed in biopolymers, reducing the size of plant extracts to the nanoscale can also enhance their performance, especially regarding antioxidant and antimicrobial activities. These nanoscale plant extracts, as antioxidant agents, can penetrate cells more easily than the original forms [80]. Plant extracts in the form of nanoemulsions offer numerous benefits. Additionally, the tiny droplets in nanoemulsions enhance the effectiveness of the extract against various pathogens [81], which also means that a smaller extract can be used efficiently. Different methods exist for creating nanoemulsions, such as high-pressure or ultrasound utilization. Ultrasonic homogenization has become a popular choice among food manufacturers because it is less expensive, more energy-efficient, and allows for better control of the process [82].
As an example, in 2024, Rahmani et al. revealed that the nanoemulsion form of essential oil could improve the inhibition process from biofilm and bacterial growth [83]. The antibacterial activities of the synthesized formulations were evaluated by measuring their minimum inhibitory concentration (MIC), zone of inhibition diameter, minimum bactericidal concentration (MBC), and their ability to inhibit biofilm formation by Salmonella typhimurium and Klebsiella pneumoniae. The highest inhibition of biofilm formation was observed with the combination of chitosan, nanoencapsulated nettle and wormwood leaves [83].
In addition to plant extracts, the properties of chitosan-based food packaging can be enhanced using essential oils. Numerous studies have investigated using essential oils as additives in chitosan, which can also influence its mechanical properties. The effect of essential oils depends on their composition. Generally, some research has shown an incompatibility between hydrophobic essential oils and chitosan, affecting the polymer’s microstructure. The interaction between essential oils and chitosan can lead to a more porous structure, reducing the tensile strength of the resulting film [6]. It could also happen due to the volatility of the essential oil [84]. Despite the limitations, chitosan and essential oil have a synergistic relationship. Chitosan can effectively slow down the evaporation rate of essential oils. Polymeric-based materials are effective in encapsulating and preserving essential oils. Various types of polymeric platforms, including hydrogels [85], nanoparticles, and nanoemulsions [86,87], have been studied to analyze the performance of essential oils.
Besides affecting the mechanical properties of chitosan, essential oils can improve their antimicrobial and antioxidant properties. Similar to plant extracts, the antioxidant properties of essential oils are often evaluated using the DPPH method. Compared to plant extracts, essential oils react more slowly with DPPH. The differences in reaction kinetics have been classified into three categories: rapid, intermediate, and slow [71]. Various types of essential oils that have been studied as antioxidants and antimicrobial agents are shown in Table 3, respectively.
Zataria multiflora Boiss, a fragrant medicinal plant, is part of the Lamiaceae. It grows in warm, mountainous regions of Iran, Pakistan, and Afghanistan [93]. The essential oil from this plant contains many oxygenated monoterpene phenols, enabling it to inhibit the activity of bacteria, fungi, and radicals [64]. Zataria multiflora Boiss essential oil-fortified chitosan exhibited improved antioxidant activity. The study revealed a synergistic effect when grape seed extract was combined with this formulation, leading to a higher overall antioxidant capacity [64]. Meanwhile, another study reveals that Zataria multiflora Boiss essential oil could more effectively inhibit Listeria monocytogenes growth than grape seed extracts [94].
Eucalyptus globulus essential oil has been shown to enhance the antioxidant properties of chitosan-based food packaging by 43% [88]. This study also demonstrated an increased inhibition rate against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Candida parapsilosis, and Candida albicans. As the concentration of Eucalyptus globulus essential oil increases, the zone of inhibition on the agar plate becomes larger. These findings demonstrate that Eucalyptus globulus essential oil can be effective against a broad range of microorganisms [88].
Chitosan-based food packaging has also been tested with apricot kernel essential oil as an additive. The results showed that this combination resulted in good antioxidant, antimicrobial, and water barrier properties [89]. The primary components of apricot kernel oil were oleic acid and linoleic acid. The antioxidant properties of chitosan films fortified with apricot kernel oil are likely attributed to the presence of N-methyl-2-pyrrolidone within the apricot kernel oil. Moreover, the incorporation of apricot kernel oil into chitosan films demonstrated inhibitory effects on bacterial growth, particularly against Bacillus subtilis and Escherichia coli [89].
Caraway can also be used as an antioxidant agent. A study showed that adding caraway to chitosan films increased the antioxidant properties of chitosan as measured by the DPPH method [90]. The addition of caraway essential oil to chitosan films significantly enhanced their antioxidant activity. The primary constituents of caraway essential oil, carvone and limonene, exhibit strong antioxidant properties [90].
Carum copticum essential oil has also been tested in combination with chitosan to assess its potential as a food packaging material. Carum copticum essential oil exhibits stronger antioxidant properties in its pure form compared to its encapsulated form within chitosan nanoparticles. This difference may be attributed to the limited release of Carum copticum essential oil of the nanoparticles in ethanol. The nanoparticles remain intact, preventing the full release of the encapsulated Carum copticum essential oil [91]. This essential oil encapsulated in chitosan nanoparticles demonstrated the strongest antibacterial activity against a range of bacteria, including Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus, Escherichia coli, Salmonella typhimurium, and Proteus vulgaris [91].
Another essential oil that can be used as an additive is Eryngium campestre. Eryngium campestre L., a perennial plant from the Umbrella family, is known for its medicinal properties. Its essential oil is valued for its potential to cure nerve pain, rheumatic conditions, inflammation, and heartburn [95]. Chitosan combined with Eryngium campestre essential oil can enhance antioxidant activity, particularly in neutralizing DPPH radicals. The antioxidant effect can be primarily attributed to the presence of phenolic compounds [92]. This study prepared chitosan as nano-chitosan and combined it with essential oil through encapsulation. Subsequently, this matrix was applied to cherries for 21 days of storage. The study demonstrated that Eryngium campestre essential oil could reduce aerobic bacterial counts, mass loss percentage, and titratable acidity [92].
Basil essential oil has also been investigated as an antimicrobial agent in chitosan films. Basil essential oil is often used as a food additive to prevent oxidation, as an antimicrobial agent, or as a flavoring agent in various products [96]. Testing chitosan films with basil oil has shown a good inhibitory effect on Staphylococcus saprophyticus and Escherichia coli [97]. Consequently, basil essential oil demonstrated potent antimicrobial activity against tested microorganisms, encompassing both gram-positive and gram-negative bacteria [97].
Tea tree oil (Melaleuca alternifolia) is well-known for its various benefits. Notably, it offers a promising alternative to conventional antifungal drugs [98]. Tea tree oil can also be used as an antimicrobial agent and is effective against Penicillium italicum. Tea tree oil composite films inhibited the growth of Penicillium italicum, with the rate of inhibition depending on the oil concentration. Lower tea tree oil concentrations had no observable antimicrobial effect. Research has shown that the addition of tea tree oil can also reduce the activity of Listeria monocytogenes even at a storage temperature of 10°C for 12 days [99].
Cinnamon is a natural material with excellent antioxidant properties. The primary active compound is cinnamaldehyde [100]. Incorporating cinnamon essential oil into chitosan films can enhance radical scavenging activity. Cinnamon essential oil combined with ginger essential oil is particularly suitable for chitosan films used to preserve meat. A common method for evaluating the antimicrobial efficacy of agents is to measure the total viable count of microorganisms on a sample, such as meat. In this case, a higher concentration of essential oils in chitosan-based films led to stronger antimicrobial activity against pork slices. This suggests that specific antimicrobial compounds present in ginger and cinnamon essential oil, including cinnamaldehyde, eugenol, limonene, and zingiberene, are responsible for this enhanced activity [100].
Extracted essential oil from lemongrass could also improve the antimicrobial activity of chitosan-based composite films and coatings. The essential oil contains various monoterpenoid groups of secondary metabolites such as geranial, neral, and citral, which essentially promote a good effect on bacteria growth inhibition [101,102]. Furthermore, a combination of chitosan and lemongrass essential oil can also improve the anticancer properties [103], antioxidant activity [104], antifungal properties [105], and mechanical properties [106]. Lemongrass essential oil combined with chitosan was reported to effectively inhibit the growth of Staphylococcus aureus, Staphylococcus epidermidis [107], Rhizopus stolonifera [105], Bacillus cereus, Escherichia coli, Listeria monocytogenes, and Salmonella typhi [101].
3.3.1 Nanotechnology Application on Essential Oils
The use of essential oils in their unaltered forms faces several significant challenges. Firstly, they exhibit low stability and are prone to degradation through volatilization and oxidation when exposed to external factors such as oxygen, light, and temperature. Secondly, their low solubility in water limits their application. The incorporation of essential oils directly into water-based matrices and environments is challenging because of their hydrophobic properties. Consequently, this limitation can reduce antimicrobial efficacy and restrict practical applications. Thirdly, there are potential interactions with components in food that could affect their efficacy. Notably, the presence of oxygen has been shown to cause substantial physicochemical and compositional changes in essential oils, thereby diminishing their stability, quality, and functional properties [108].
Therefore, it is necessary to incorporate other materials to mitigate evaporation. Nanoencapsulation or nanoemulsification using emulsifiers can be implemented to prolong the shelf life of essential oils. Nanoencapsulation appears to be a promising solution to these challenges. This method not only protects essential oils and their bioactive components from exposure and degradation by creating a physical barrier, but also allows for their controlled release, enhancing bioavailability and effectiveness. Various nanoencapsulation techniques have been developed, with nanoparticles and nanoemulsions being the most favorable and frequently investigated options. These encapsulation processes involve coating bioactive substances within a matrix, such as a synthetic or natural polymer, effectively isolating the active ingredients from external factors [109]. On the other hand, emulsifiers possessing both hydrophilic and hydrophobic regions, facilitate the formation of nanoemulsions and improve their stability [110]. Essential oil-based nanoemulsion could also be applied to a biopolymer matrix. Biopolymers maintain the efficacy of essential oils, and encapsulation using biopolymers can be adapted to gradually release their bioactive components [111]. Encapsulation also extends the duration of antimicrobial and antioxidant effects. Nanoemulsions possess unique properties and exhibit greater stability than traditional ones, making them highly promising for food, pharmaceutical, and cosmetic applications. Despite their potential, they are not entirely stable, necessitating further research to achieve stable nanoemulsions for production. Encapsulating essential oils in nanoemulsions offers numerous benefits, enhancing product performance as well as biological and physicochemical stability [112].
A study revealed that Alhagi maororum essential oil nanoemulsions exhibited superior antibacterial properties compared to its free forms. Additionally, this nanoemulsion also demonstrated antibiofilm activity [113]. Similar results were obtained from experiments using Syzygium aromaticum essential oil, where the percentage of biofilm inhibition in samples with nanoemulsions was significantly higher compared to those without nanoemulsions [114]. This enhanced performance is attributed to factors such as increased dissolution, wetting, and surface area, leading to improved cellular penetration and bioavailability. Nanoemulsions offer several advantages, including increased efficacy, reduced dosage, and sustained release of active ingredients. These benefits make nanoemulsions a promising approach for delivering essential oils and other bioactive compounds [113].
4 Metal or Metal Oxide Nanoparticles to Enhance Chitosan Properties
The utilization of nanotechnology to enhance food packaging performance, apart from being applied through nano-sized natural additives, can also be done by adding metal or metal oxide nanoparticles. The incorporation of metal or metal oxide nanoparticles into chitosan-based food packaging systems presents a novel approach for improving material performance. Metals and metal oxide nanoparticles can specifically target and disrupt bacterial metabolic processes. Like certain antibiotics, metal compounds can differentiate between bacterial and human cells, exploiting differences in how cells handle metal transport and proteins. This selectivity makes metals a promising long-term solution for both bacterial infections and biofilm control [115]. Metal nanoparticles possess intrinsic antimicrobial properties and can synergistically interact with chitosan, offering a promising strategy to improve the overall functionality of these materials. On the other hand, metallic ions can be introduced into a chitosan solution to increase the mechanical strength or thermal stability of food packaging materials. These ions are then transformed into nanoparticles and integrated into the polymer matrix [116]. Various types of metal nanoparticles studied as antimicrobial nanocomposites are presented in Table 4. Furthermore, a more detailed analysis of the antimicrobial and antioxidant activity, mechanical, and thermal properties enhancement of chitosan by metal nanoparticles will follow.
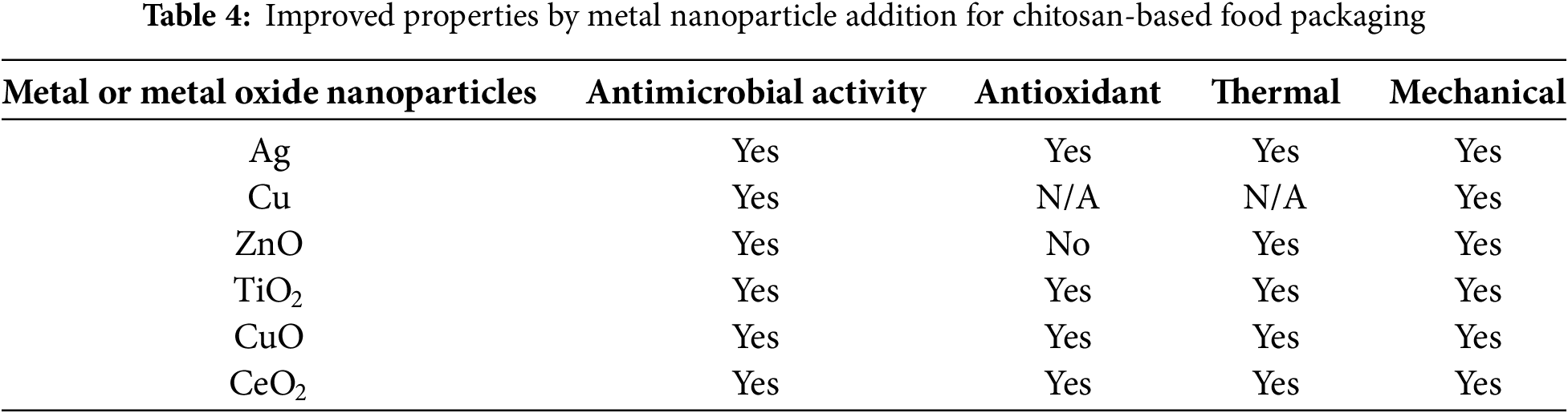
4.1 Antimicrobial and Antioxidant Activity
Metal nanoparticles such as silver and copper are effective antimicrobial nanomaterials against gram-positive and gram-negative bacteria, fungi, protozoa, and viruses. Antimicrobial agents in nanomaterials can electrostatically bind to the microbial cell wall, altering the membrane potential and leading to various metabolic disruptions, including impaired cellular respiration, cell membrane damage, and transport imbalances, ultimately causing cell death [6]. Another mechanism specific to metal nanoparticles involves the production of reactive oxygen species (ROS). Certain nanomaterials can stimulate inflammation, chemically converting toxic oxidants such as hydrogen peroxide into more reactive free radicals. Semiconductor nanomaterials can generate ROS through reactions between excited electrons and water molecules, producing substances toxic to microorganisms. ROS can cause damage to DNA and RNA, lipid peroxidation, and inhibit enzymes in microbes. The size and shape of nanoparticles can influence ROS production and antimicrobial activity. Small nanoparticles can penetrate cell membranes and other biological layers [6].
The green synthesis of metal nanoparticles has found widespread application in the food industry. Silver nanoparticles (AgNPs) are one example of metal nanoparticles used in chitosan-based food packaging. A study investigated the synthesis of chitosan-based films incorporated with AgNPs. The results indicated that adding AgNPs enhanced antimicrobial activity, particularly against Staphylococcus aureus, Escherichia coli, Salmonella, and Listeria monocytogenes [117]. Antimicrobial activity enhancement was demonstrated by the increased size of the inhibition zone observed during testing. The addition of AgNPs to the chitosan/polyvinyl alcohol film significantly improved its antioxidant properties, and this effect became more pronounced as the C-Ag concentration increased. The antioxidant mechanism of chitosan/polyvinyl alcohol films involves the pairing of oxygen atoms from C-Ag with nitrogen atoms in DPPH. This pairing leads to a decrease in absorbance at 517 nm and the disappearance of DPPH’s purple color [118].
Copper nanoparticles (CuNPs) combined with chitosan have also been studied and shown to exhibit excellent antifungal activity against tomato fruit [119]. CuNPs were effective in inhibiting the growth of Alternaria solani and Fusarium oxysporum. Cu ions are known to exhibit antifungal properties by generating highly reactive hydroxyl radicals that can damage biological molecules. In this study, the enhanced antifungal activity of Cu-chitosan nanoparticles arises from a synergistic effect between chitosan, which induces defense enzymes, and the toxic effects of released Cu ions [119].
Metal oxide nanoparticles, such as zinc oxide (ZnO), titanium dioxide (TiO2), and copper oxide (CuO), have also been investigated as additive materials. These metal oxide nanoparticles are commonly used as additives in biopolymer matrices to improve the quality of food packaging. The ZnO nanoparticles have been found to extend the shelf life of meat by up to 14 days [120]. This study tested ZnO nanoparticles as an inhibitor of bacterial activity, specifically Staphylococcus aureus and Escherichia coli. Additionally, ZnO has been tested in chitosan matrices for banana application. In this study, ZnO nanoparticles were proven to inhibit the activity of Bacillus subtilis also. The best results were shown in the inhibition rate of Staphylococcus aureus [121]. In a study reveals that adding ZnO nanoparticles to chitosan films did not significantly improve their antioxidant properties [122].
Other research has shown that using titanium dioxide nanoparticles can help chitosan inhibit microbes in food packaging. Antimicrobial testing was conducted on Staphylococcus aureus, Escherichia coli, Candida albicans, and Aspergillus niger [123]. The study proved that titanium dioxide nanoparticles could inhibit the activity of all these types of microbes. A study reveals that the antioxidant activity of the samples with added TiO2 NPs show a decrease with increasing TiO2 NPs concentration. Meanwhile, at certain concentrations, shows increased antioxidant activity. This is likely because the antioxidants were bound to the surface of TiO2, limiting their ability to interact with and neutralize free radicals [124].
Copper oxide nanoparticles could inhibit the microbes Escherichia coli, Pseudomonas aeruginosa, and Listeria monocytogenes [125]. A research study has demonstrated that copper oxide nanoparticles (CuONPs) exhibit enhanced antimicrobial activity against gram-positive bacteria compared to gram-negative bacteria. This increased susceptibility of gram-positive bacteria, particularly Staphylococcus aureus, to CuONPs is linked to the higher concentration of amine and carboxyl groups on their cell surfaces. These functional groups have a stronger affinity for copper ions, leading to increased toxicity and disruption of cellular processes in gram-positive bacteria [126]. The incorporation of CuO NPs into the chitosan/hydroxypropyl cellulose matrix resulted in a substantial increase in antioxidant activity as the nanoparticle concentration was raised. This improved antioxidant performance of the nanocomposite films is likely attributed to the increased electron density of the CuO NPs, which enhances their ability to scavenge free radicals [127].
In addition to the various metal and metal oxide nanoparticles mentioned previously, there is another type with high potential for improving the antimicrobial properties of chitosan. This type of nanoparticle is cerium oxide (CeO2) nanoparticles. Cerium oxide has a unique property derived from the cerium element, which can be reversibly converted from the Ce(III) to Ce(IV) form [128]. This property is particularly attractive because it plays an important role in inhibiting the activity of microorganisms. Meanwhile, cerium oxide nanoparticles could also inhibit ROS activity, which is good for improving the antioxidant activity of chitosan [129]. A study shown that the nanocomposite chitosan films containing 2% nanoceria effectively scavenged approximately 75% of ABTS and 50% of DPPH free radicals. This antioxidant activity is attributed to nanoceria’s unique ability to transition between different oxidation states (Ce3+ and Ce4+) due to oxygen vacancies within its crystal structure [130].
Mechanical properties are one of the criteria that need to be achieved for food packaging applications. The strength of the packaging can affect the durability and shelf life of food. Chitosan films offer a high degree of customization, allowing them to be tailored for specific applications, such as enhancing barrier properties or mechanical strength. A major focus of current research is developing chitosan-based edible films that can serve as primary food packaging, preventing moisture loss and oxygen access [131]. However, to increase its resistance to external disturbances, the mechanical properties of chitosan still need to be improved.
A study was shown that the addition of AgNPs enhances the hydrophobicity of the material, resulting in contact angle measurements for the chitosan/PVA layer that exceed 90 degrees [118]. Other studies have shown that the presence of AgNPs also enhances the mechanical properties of chitosan-PVA composites. For chitosan/PVA-Ag nanocomposite films, a significant increase in tensile strength, stress at break, and elongation at break was observed. This improvement can be attributed to the formation of an additional bonded network created by the incorporation of AgNPs within the polymer blend. This network is formed through hydrogen bonding and strong electrostatic interactions between the polymer blend and AgNPs [132].
The other study reveals that the tensile strength of chitosan and CuNPs films was higher than pure chitosan films. Both tensile strength and elongation at break showed an increasing trend with increasing CuNPs concentration up to a certain value. However, as the concentration of CuNPs increased, the tensile strength gradually decreased. The reduction in strength is likely caused by a weakening of the interaction between the nanoparticles and the chitosan matrix at higher concentration [133].
Typically, a higher elongation at break, coupled with a good tensile strength, indicates superior material quality. By incorporating ZnO nanoparticles into a blend of chitosan and xanthan gum, the mechanical properties of the resulting films were significantly enhanced. This improvement is attributed to the enhanced interaction between the biopolymers and the nanoparticles [134]. The ZnO nanoparticles could also significantly weaken the films’ mechanical properties. The nanoparticles interfere with the crystalline order of the chitosan, reducing the strength of the intermolecular bonds between the polymer chains [122].
The addition of TiO2 nanoparticles to chitosan significantly improved the tensile strength and elongation at break of the resulting nanocomposites. The tensile strength and deformation at break were both higher in samples containing TiO2 compared to those without [135]. As the concentration of TiO2 nanoparticles increased, so did the mechanical properties [136]. This enhancement is likely due to the electrostatic interaction between the positively charged TiO2 nanoparticles and the negatively charged carboxyl groups in the chitosan matrix. Additionally, the incorporation of nanoparticles into the chitosan chains may increase chain mobility, further contributing to the improved tensile strength and elongation. However, these molecular interactions can also strengthen the polymer network, potentially reducing its flexibility [136].
In the other study, the chitosan/hydroxypropyl cellulose film with CuO nanoparticles showed a significant increase in tensile strength and Young’s modulus compared to the control film without nanoparticles. This improvement is attributed to the nanoparticles reinforcing the polymer matrix, creating a denser network that restricts molecular movement [127]. Meanwhile, another study demonstrated that a combination of 1% CuO/NiO nanoparticles could enhance the tensile strength and elongation at break of polyvinyl alcohol/starch/chitosan films. However, further increasing the nanoparticles concentration to 2% led to a significant decrease in tensile strength and elongation at break. This decline suggests that the nanoparticles may have agglomerated, reducing the number of effective interactions with the polymer and potentially disrupting the hydrogen bonding network within the polymer matrix [137]. The optimal concentration of both nanoparticles and polymers must be carefully considered to ensure the quality of food packaging.
Chitosan-based nanocomposite with the gelatin and CeO4Zr nanoparticles showed the improvement of structural, morphological, mechanical, and thermal properties [138]. Although there is not much research on cerium oxide nanoparticles, the development of this metal oxide has a high potential for improving the properties and characteristics of chitosan as food packaging.
The addition of metal or metal oxide nanoparticles improves the thermal stability of chitosan. For example, adding silver nanoparticles to chitosan films enhanced their thermal resistance, especially at higher temperatures. This improvement is attributed to the interaction between the metal nanoparticles and the polymer chains. The specific interactions, such as van der Waals forces, hydrogen bonding, and other chemical bonds, influenced the thermal properties of the composite films. While the thermal profiles of the composite films were similar to pure chitosan at lower temperatures, a significant increase in thermal stability was observed at higher temperatures [139].
The thermal stability of the chitosan/polyvynil alcohol films was enhanced by increasing the AgNPs content. This improvement was due to the stable nature of the AgNPs. Polyvynil alcohol-Ag and chitosan-Ag nanocomposites exhibited higher thermal stability compared to pure polymers. This suggests that the incorporation of AgNPs into the chitosan/polyvynil alcohol mixture improved heat resistance and delayed thermal degradation [118].
On other hand, the thermal stability of chitosan, ZnO nanoparticles, and their composite was investigated under high temperatures by Aouadi et al. (2024). Chitosan was less thermally stable than both ZnO nanoparticles and the chitosan-ZnO nanoparticle composite. This is due to the heat sensitivity of chitosan’s polysaccharide structure, which is prone to degradation at elevated temperatures. In contrast, ZnO nanoparticles and the composite exhibited higher thermal stability, suggesting that the addition of chitosan did not significantly compromise their thermal properties. Interestingly, coating ZnO nanoparticles with chitosan enhanced their thermal stability, indicating a synergistic effect between the two materials [140]. Meanwhile, adding a small amount of ZnO nanoparticles to the chitosan films did not significantly affect their thermal stability. This may be because the limited number of nanoparticles hindered the interaction between the chitosan chains, leading to a slight decrease in thermal stability [141].
TiO2 nanoparticles to chitosan enhanced the thermal stability of the resulting composites. The addition of TiO2 nanoparticles to chitosan films led to the formation of TiO2 bonds, which strengthened the interactions between chitosan molecules. This enhanced interaction resulted in improved thermal properties of the composite film [142]. This improvement is attributed to the formation of hydrogen bonds between the functional groups of chitosan and the TiO2 crystallites. The presence of TiO2 nanoparticles altered the decomposition mechanism of the chitosan matrix. Interestingly, increasing the concentration of nanoparticles led to a further increase in thermal stability [136].
Another study reveals that incorporating NiO/CuONPs into the polyvinyl alcohol/starch/chitosan blend slightly enhanced the thermal stability of the resulting bio-nanocomposites. This improvement is attributed to the formation of stronger chemical bonds between the polymer chains, which slows down the degradation process. Given their enhanced thermal stability, these bio-nanocomposite films show potential for use in food packaging applications [137].
On the other hand, the incorporation of 1% nanoceria did not significantly affect the thermal stability of the chitosan film matrix. However, increasing the nanoceria concentration to 2% led to a notable decrease in thermal stability. This reduction is likely due to the lower concentration of nanoceria at this level, which may result in a more uniform distribution throughout the polymer matrix [130].
5 Properties Improvement by Natural Additives and Metal or Metal Oxide Nanoparticles
The various additive materials discussed earlier offer unique benefits for enhancing the properties of chitosan-based films and coatings. A comparative analysis of these additives, considering different evaluation parameters, is summarized in Table 5. Based on the analysis and comparison in Table 5, several aspects can be used for comparison, including the improved properties and characteristics of chitosan films and coatings, environmental and sustainability issues, economic aspects, availability and synthesis difficulty, and sensory properties. Compared to their influence on chitosan films and coatings, all additive materials have particular benefits depending on the type and concentration of additives and the specific application. Biopolymers are generally added to the chitosan matrix to create electrostatic interactions that can improve the mechanical strength of chitosan films and coatings, making them less susceptible to damage [42,46]. Plant extracts and essential oils, both plant-based additive materials, can improve the antioxidant and antimicrobial properties of chitosan films and coatings [143]. Metal nanoparticles are generally added to enhance antimicrobial properties because nanoparticles can inhibit microbial activity on chitosan films and coatings [144].
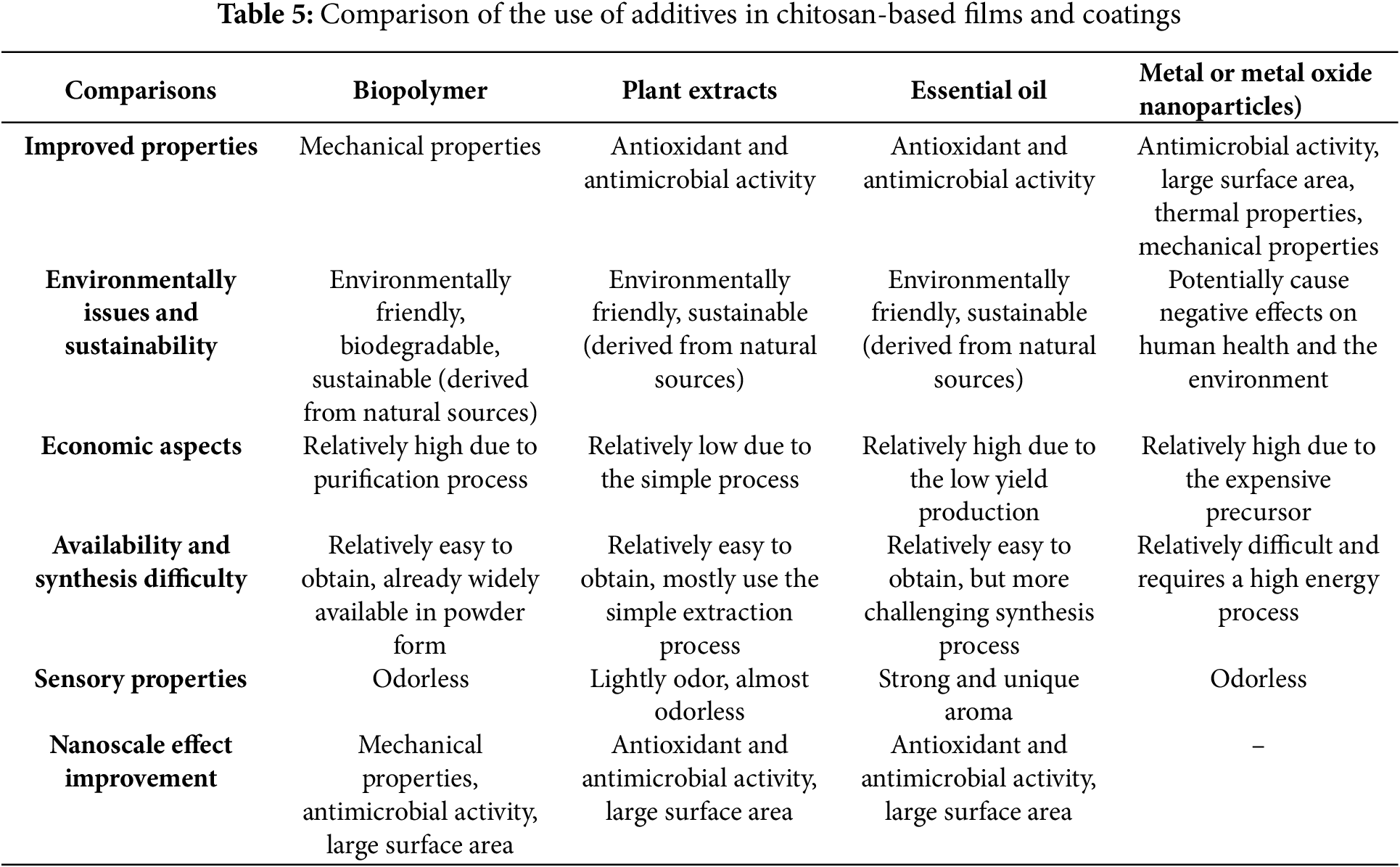
Another important aspect of comparison is environmental and sustainability issues. Environmental issues are currently a major focus in various sectors. Biopolymers are materials based on natural materials, so they are easily biodegradable [4]. Similarly to biopolymers, plant extracts and essential oils are derived from plants, which are easy to degrade due to the biodegradable natural material sources [143]. However, the production of metal nanoparticles is more complex and can produce chemicals that are toxic to the environment. Based on current technological developments, the term “green synthesis” is known, which is a more environmentally friendly method for producing metal nanoparticles using green materials such as microorganisms and extracts from flowers, fruits, peels, and seeds [145]. Furthermore, due to their potential toxicity, metal nanoparticles could negatively affect human health and the environment. However, there are still limited studies investigating the environmental impact and standardized exposure limit of nanoparticles of all types [146].
Economic aspects can also be used as a comparative parameter to select the most suitable additive material. The evaluation of economic aspects can be reviewed from capital costs, such as production costs, equipment, and materials, to operational costs, such as electricity costs. Most current biopolymer production is still quite costly compared to fossil-based plastics due to the need for various expensive raw materials, complex production processes, and small-scale production [147]. Plant extracts require relatively low costs because they generally come from natural materials that are easily obtained and have simple production processes. Unlike plant extracts, the production costs of essential oils are much higher due to complex production processes that require various special equipment and relatively low production yields [148]. The production costs of metal nanoparticles are also quite expensive because they require many chemicals and more complex production processes [145].
The next aspect to compare is the level of availability and the difficulty of the synthesis process. Biopolymers come from natural materials that are relatively easy to obtain [4], but the production process is still considered complex and requires many chemicals. However, biopolymers are now widely sold in powder form. Plant extracts can be obtained easily for certain types of plants. However, many types of plants may only be able to grow in certain places and at certain times of the year [149]. Even so, the plant extraction process can be done with simple methods. The synthesis process of essential oils is almost similar to that of plant extracts, but the production process is more difficult because it requires specialized equipment. In addition, the difficulty of producing metal nanoparticles is perhaps the most difficult compared to other types of additives because it requires many chemicals and various material characterizations [145].
Another aspect that can influence is the aroma. Films and coatings implemented on various types of food will need to be adjusted to determine whether the aroma of the additive material will affect the smell and properties of the food. Edible films and coatings are generally transparent and odorless so as not to interfere with the sensory properties of the coated food [6]. Mostly, biopolymers and metal nanoparticles are known odorless. Plant extracts produce a mild aroma and, even in some types of plants, are odorless. Unlike other additives, essential oils generally have a distinctive and intense aroma that has the potential to affect the taste of food [148]. From the comparison of various types of additives, each additive possesses its unique set of advantages and limitations. The compatibility between chitosan and these additives depends on the specific application. Therefore, the consideration of additives for chitosan-based food packaging should be more deeply learned.
While various additives are beneficial, proper mixing of materials is essential to produce biofilms or edible coatings with a uniform composition to ensure their enhancement effects are evenly distributed. Using bulk materials can lead to complete mixing if the mixing process is adequate. Therefore, one approach to address this issue is to convert the materials into a nanoscale form. Nanocomposites made by mixing nano-based materials into a polymer matrix offer superior properties to conventional composites. The key to unlocking their full potential is developing methods to distribute the nanoparticles evenly throughout the material. This even distribution improves the bond between the matrix components, improving overall performance [150]. Nanotechnology has offered new ways to food preservation techniques, improving its physical and chemical properties. Nanomaterials have unique properties like a large surface area, high reactivity, high strength, quantum effects, and increase flexibility when combined with matrices, making them valuable in various applications [27,151,152]. Nanoparticles, nanoemulsions, and other nanomaterials can enhance food packaging performance depending on their characteristics and interactions between each type of constituent composite material. These superior properties differ from bulk materials, which must be evaluated and researched more deeply. These superior properties could also advance the agricultural and food sectors, especially food packaging and preservation. Modifying materials at the nanoscale for food packaging applications can enlarge and increase surface area, improving their physical, antimicrobial, and antioxidant properties and extending shelf life for post-harvest products and meats [151]. While nanotechnology offers significant potential, there are safety concerns associated with nanomaterials, as their toxicological profiles may differ from conventional materials. Further research is necessary to address these concerns and promote the safe and widespread use of nanotechnology [153].
The exploration of chitosan, which has non-toxic, antimicrobial, and antifungal properties, as a sustainable alternative for food packaging has shown promising results. However, improving the limited properties and characteristics of chitosan as food packaging requires enhancement for optimal performance. Combining chitosan with various natural additives such as biopolymers, plant extracts, and essential oils is an effective way to improve the quality of chitosan as food packaging. Nanotechnology could further improve the properties and characteristics of chitosan food packaging. Comparative studies conducted on various essential oils and plant extracts show that by transforming natural additives into nanoscale, substantial improvements in antimicrobial and antioxidant properties could be achieved. The incorporation of metal and metal oxide nanoparticles into chitosan-based food packaging materials significantly improves antimicrobial, antioxidant, thermal stability, and mechanical properties. However, further studies investigating nanoparticles’ environmental impact and standardized exposure limit still must be performed. Meanwhile, further development on chitosan-based food packaging also needs to be performed to explore a lot of bio-based and other nanomaterials as additives suitable for a particular need of food products, and there are also challenges in achieving improved quality and performance of chitosan-based food packaging. The continued development of chitosan composites holds great potential for reducing the environmental impact of petroleum-based plastic food packaging and promoting sustainability in the food industry.
Acknowledgement: Not applicable.
Funding Statement: The grants are: Penelitian Tesis Magister (PTM) Research Grant from Indonesian Government Kemdikbudristek with contract number 036/E5/PG.02.00.PL/2024. PPMl 2024 Research Grant from Faculty of Industrial Technology, ITB.
Author Contributions: The authors confirm contribution to the paper as follows: manuscript structure and preparation: Panji Setya Utama Putra, Damar Rastri Adhika; study, analysis, and discussion: Panji Setya Utama Putra, Damar Rastri Adhika, Suprijadi Suprijadi, Lia Amelia Tresna Wulan Asri. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the articles.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Rebezov M, Chughtai MFJ, Mehmood T, Khaliq A, Tanweer S, Semenova A, et al. Novel techniques for microbiological safety in meat and fish industries. Appl Sci. 2022;12(1):319. doi:10.3390/app12010319. [Google Scholar] [CrossRef]
2. Hussain MA, Sumon TA, Mazumder SK, Ali MM, Jang WJ, Abualreesh MH, et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: a review. Food Control. 2021;129(3):108244. doi:10.1016/j.foodcont.2021.108244. [Google Scholar] [CrossRef]
3. Versino F, Ortega F, Monroy Y, Rivero S, López OV, García MA. Sustainable and bio-based food packaging: a review on past and current design innovations. Foods. 2023;12(5):1057. doi:10.3390/foods12051057. [Google Scholar] [PubMed] [CrossRef]
4. Priyadarshi R, Rhim JW. Chitosan-based biodegradable functional films for food packaging applications. Innovat Food Sci Emerg Technol. 2020;62(2):102346. doi:10.1016/j.ifset.2020.102346. [Google Scholar] [CrossRef]
5. Haghighi H, Licciardello F, Fava P, Siesler HW, Pulvirenti A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag Shelf Life. 2020;26(8):100551. doi:10.1016/j.fpsl.2020.100551. [Google Scholar] [CrossRef]
6. Flórez M, Guerra-Rodríguez E, Cazón P, Vázquez M. Chitosan for food packaging: recent advances in active and intelligent films. Food Hydrocoll. 2022;24(B):107328. doi:10.1016/j.foodhyd.2021.107328. [Google Scholar] [CrossRef]
7. Tayel AA, Elzahy AF, Moussa SH, Al-Saggaf MS, Diab AM. Biopreservation of shrimps using composed edible coatings from chitosan nanoparticles and cloves extract. J Food Qual. 2020;2020:8878452. doi:10.1155/2020/8878452. [Google Scholar] [CrossRef]
8. Wu G, Su W, Huo L, Guo Q, Wei J, Zhong H, et al. Sodium alginate/chitosan-based intelligent multifunctional bilayer film for shrimp freshness retention and monitoring. Int J Biol Macromol. 2024;277(2):133908. doi:10.1016/j.ijbiomac.2024.133908. [Google Scholar] [PubMed] [CrossRef]
9. Eranda DHU, Chaijan M, Panpipat W, Karnjanapratum S, Cerqueira MA, Castro-Muñoz R. Gelatin-chitosan interactions in edible films and coatings doped with plant extracts for biopreservation of fresh tuna fish products: a review. Int J Biol Macromol. 2024;280(2):135661. doi:10.1016/j.ijbiomac.2024.135661. [Google Scholar] [PubMed] [CrossRef]
10. Rasulu H, Praseptiangga D, Made Joni I, Handono Ramelan A. Introduction test edible coating fresh fish fillet of tuna and smoked fish using biopolymer nanoparticle chitosan coconut crab. Adv Eng Res. 2020;194:173–80. doi:10.2991/aer.k.200325.035. [Google Scholar] [CrossRef]
11. Musalem S, Hamdy MM, Mashaly MM, Kamoun EA, Malak NML. Extending the shelf-life of refrigerated chicken fillets by using polymeric coating layer composed of ginger Zingiber officinale essential oil loaded-chitosan nanoparticles. J Umm Al-Qura Univ Appll Sci. 2024;136(2):133. doi:10.1007/s43994-024-00172-8. [Google Scholar] [CrossRef]
12. Sheerzad S, Khorrami R, Khanjari A, Gandomi H, Basti AA, Khansavar F. Improving chicken meat shelf-life: coating with whey protein isolate, nanochitosan, bacterial nanocellulose, and cinnamon essential oil. LWT. 2024;197(7):115912. doi:10.1016/j.lwt.2024.115912. [Google Scholar] [CrossRef]
13. de Lima AF, de Leite RHL, Pereira MWF, Silva MRL, de Araújo TLAC, de Lima Júnior DM, et al. Chitosan coating with rosemary extract increases shelf life and reduces water losses from beef. Foods. 2024;13(9):1353. doi:10.3390/foods13091353. [Google Scholar] [PubMed] [CrossRef]
14. Isvand A, Karimaei S, Amini M. Assessment of chitosan coating enriched with Citrus limon essential oil on the quality characteristics and shelf life of beef meat during cold storage. Int J Food Microbiol. 2024;423(2):110825. doi:10.1016/j.ijfoodmicro.2024.110825. [Google Scholar] [PubMed] [CrossRef]
15. Zheng H, Deng W, Yu L, Shi Y, Deng Y, Wang D, et al. Chitosan coatings with different degrees of deacetylation regulate the postharvest quality of sweet cherry through internal metabolism. Int J Biol Macromol. 2024;254(1):127419. doi:10.1016/j.ijbiomac.2023.127419. [Google Scholar] [PubMed] [CrossRef]
16. Popescu PA, Palade LM, Nicolae IC, Popa EE, Miteluț AC, Drăghici MC, et al. Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods. 2022;11(21):3317. doi:10.3390/foods11213317. [Google Scholar] [PubMed] [CrossRef]
17. Sahraei Khosh Gardesh A, Badii F, Hashemi M, Ardakani AY, Maftoonazad N, Gorji AM. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz LWT. 2016;70(2):33–40. doi:10.1016/j.lwt.2016.02.002. [Google Scholar] [CrossRef]
18. Priyadarshi R, Sauraj, Kumar B, Negi YS. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr Polym. 2018;195(10):329–38. doi:10.1016/j.carbpol.2018.04.089. [Google Scholar] [PubMed] [CrossRef]
19. Sekarina AS, Supriyadi, Munawaroh HSH, Susanto E, Show PL, Ningrum A. Effects of edible coatings of chitosan - fish skin gelatine containing black tea extract on quality of minimally processed papaya during refrigerated storage. Carbohydr Polym Technol Appl. 2023;5(2):100287. doi:10.1016/j.carpta.2023.100287. [Google Scholar] [CrossRef]
20. Dwivany FM, Aprilyandi AN, Suendo V, Sukriandi N. Carrageenan edible coating application prolongs Cavendish banana shelf life. Int J Food Sci. 2020;2020:8861610. doi:10.1155/2020/8861610. [Google Scholar] [PubMed] [CrossRef]
21. Lustriane C, Dwivany FM, Suendo V, Reza M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J Plant Biotechnol. 2018;45(1):36–44. doi:10.5010/JPB.2018.45.1.036. [Google Scholar] [CrossRef]
22. Mwakalesi AJ, Umbayda TG. Chitosan enriched with ZnO-single bondnanoparticles fabricated using Synadenium glaucescens (Pax) aqueous leaf extract maintains postharvest quality of banana. Appl Food Res. 2024;4(2):100536. doi:10.1016/j.afres.2024.100536. [Google Scholar] [CrossRef]
23. Xiao F, Xiao Y, Ji W, Li L, Zhang Y, Chen M, et al. Photocatalytic chitosan-based bactericidal films incorporated with WO3/AgBr/Ag and activated carbon for ethylene removal and application to banana preservation. Carbohydr Polym. 2024;328(39):121681. doi:10.1016/j.carbpol.2023.121681. [Google Scholar] [PubMed] [CrossRef]
24. Phuong NTH, Koga A, Nkede FN, Tanaka F, Tanaka F. Application of edible coatings composed of chitosan and tea seed oil for quality improvement of strawberries and visualization of internal structure changes using X-ray computed tomography. Prog Org Coat. 2023;183:107730. doi:10.1016/j.porgcoat.2023.107730. [Google Scholar] [CrossRef]
25. Fu X, Chang X, Xu S, Xu H, Ge S, Xie Y, et al. Development of a chitosan/pectin-based multi-active food packaging with both UV and microbial defense functions for effectively preserving of strawberry. Int J Biol Macromol. 2024;254(2):127968. doi:10.1016/j.ijbiomac.2023.127968. [Google Scholar] [PubMed] [CrossRef]
26. Hameed AZ, Raj SA, Kandasamy J, Baghdadi MA, Shahzad MA. Chitosan: a sustainable material for multifarious applications. Polymers. 2022;14(12):2335. doi:10.3390/polym14122335. [Google Scholar] [PubMed] [CrossRef]
27. Biswas R, Alam M, Sarkar A, Haque MI, MdM Hasan, Hoque M. Application of nanotechnology in food: processing, preservation, packaging and safety assessment. Heliyon. 2022 Nov;8(11):e11795. doi:10.1016/j.heliyon.2022.e11795. [Google Scholar] [PubMed] [CrossRef]
28. Madhu S, Devarajan Y, Balasubramanian M, Prithivi Raj M. Synthesis and characterization of nano chitosan obtained using different seafood waste. Mater Lett. 2022;329(1):133195. doi:10.1016/j.matlet.2022.133195. [Google Scholar] [CrossRef]
29. Kumar S, Mukherjee A, Dutta J. Chitosan based nanocomposite films and coatings: emerging antimicrobial food packaging alternatives. Trends Food Sci Technol. 2020;97(4):196–209. doi:10.1016/j.tifs.2020.01.002. [Google Scholar] [CrossRef]
30. Varma AJ, Deshpande SV, Kennedy JF. Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym. 2004;55(1):77–93. doi:10.1016/j.carbpol.2003.08.005. [Google Scholar] [CrossRef]
31. Tømmeraas K, Vårum KM, Christensen BE, Smidsrød O. Preparation and characterisation of oligosaccharides produced by nitrous acid depolymerisation of chitosans [Internet]; 2001 [cited 2025 Jan 06]. Available from: www.elsevier.com/locate/carres. [Google Scholar]
32. Kumari P, Barman K, Patel VB, Siddiqui MW, Kole B. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Sci Hortic. 2015;197(5):555–63. doi:10.1016/j.scienta.2015.10.017. [Google Scholar] [CrossRef]
33. Dotto GL, Vieira MLG, Pinto LAA. Use of chitosan solutions for the microbiological shelf life extension of papaya fruits during storage at room temperature. LWT. 2015;64(1):126–30. doi:10.1016/j.lwt.2015.05.042. [Google Scholar] [CrossRef]
34. Latou E, Mexis SF, Badeka AV, Kontakos S, Kontominas MG. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT. 2014;55(1):263–8. doi:10.1016/j.lwt.2013.09.010. [Google Scholar] [CrossRef]
35. Díez-Pascual AM, Díez-Vicente AL. Antimicrobial and sustainable food packaging based on poly(butylene adipate-co-terephthalate) and electrospun chitosan nanofibers. RSC Adv. 2015;5(113):93095–107. doi:10.1039/C5RA14359D. [Google Scholar] [CrossRef]
36. Youssef AM, Abou-Yousef H, El-Sayed SM, Kamel S. Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int J Biol Macromol. 2015;76:25–32. doi:10.1016/j.ijbiomac.2015.02.016. [Google Scholar] [PubMed] [CrossRef]
37. Khubiev OM, Egorov AR, Kirichuk AA, Khrustalev VN, Tskhovrebov AG, Kritchenkov AS. Chitosan-based antibacterial films for biomedical and food applications. Int J Mol Sci. 2023;24(13):10738. doi:10.3390/ijms241310738. [Google Scholar] [PubMed] [CrossRef]
38. Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of ficus religiosa. Molecules. 2022;27(4):1326. doi:10.3390/molecules27041326. [Google Scholar] [PubMed] [CrossRef]
39. Ngo DH, Kim SK. Antioxidant effects of chitin, chitosan, and their derivatives. In: Advances in food and nutrition research. USA: Academic Press Inc.; 2014. p. 15–31. [Google Scholar]
40. Ferreira DCM, de Souza AL, da Silveira JVW, Pelissari FM, Marim BM, Giraldo GAG, et al. Chitosan nanocomposites for food packaging applications. In: Multifunctional hybrid nanomaterials for sustainable agri-food and ecosystems. Netherlands: Elsevier; 2020. p. 393–435. [Google Scholar]
41. Talón E, Trifkovic KT, Nedovic VA, Bugarski BM, Vargas M, Chiralt A, et al. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr Polym. 2017;157(5):1153–61. doi:10.1016/j.carbpol.2016.10.080. [Google Scholar] [PubMed] [CrossRef]
42. Xiao W, Xu J, Liu X, Hu Q, Huang J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J Mater Chem B. 2013;1(28):3477–85. doi:10.1039/c3tb20303d. [Google Scholar] [PubMed] [CrossRef]
43. Salama HE, Abdel Aziz MS, Sabaa MW. Development of antibacterial carboxymethyl cellulose/chitosan biguanidine hydrochloride edible films activated with frankincense essential oil. Int J Biol Macromol. 2019;139(1):1162–7. doi:10.1016/j.ijbiomac.2019.08.104. [Google Scholar] [PubMed] [CrossRef]
44. Tesfay SZ, Magwaza LS. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag Shelf Life. 2017;11(11):40–8. doi:10.1016/j.fpsl.2016.12.001. [Google Scholar] [CrossRef]
45. Bigi F, Haghighi H, Siesler HW, Licciardello F, Pulvirenti A. Characterization of chitosan-hydroxypropyl methylcellulose blend films enriched with nettle or sage leaf extract for active food packaging applications. Food Hydrocoll. 2021;120(4):106979. doi:10.1016/j.foodhyd.2021.106979. [Google Scholar] [CrossRef]
46. Maftoonazad N, Ramaswamy HS, Marcotte M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int J Food Sci Technol. 2008;43(6):951–7. doi:10.1111/j.1365-2621.2006.01444.x. [Google Scholar] [CrossRef]
47. Panahirad S, Dadpour M, Peighambardoust SH, Soltanzadeh M, Gullón B, Alirezalu K, et al. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: a review. Trends Food Sci Technol. 2021;110(4):663–73. doi:10.1016/j.tifs.2021.02.025. [Google Scholar] [CrossRef]
48. Nguyen HT, Boonyaritthongchai P, Buanong M, Supapvanich S, Wongs-Aree C. Chitosan- and κ-carrageenan-based composite coating on dragon fruit (Hylocereus undatus) pretreated with plant growth regulators maintains bract chlorophyll and fruit edibility. Sci Hortic. 2021;281(3):109916. doi:10.1016/j.scienta.2021.109916. [Google Scholar] [CrossRef]
49. Mary SK, Koshy RR, Arunima R, Thomas S, Pothen LA. A review of recent advances in starch-based materials: bionanocomposites, pH sensitive films, aerogels and carbon dots. Carbohydr Polym Technol Appl. 2022;3:100190. doi:10.1016/j.carpta.2022.100190. [Google Scholar] [CrossRef]
50. Dutta D, Sit N. Comprehensive review on developments in starch-based films along with active ingredients for sustainable food packaging. Sustain Chem Pharm. 2024;39(1):101534. doi:10.1016/j.scp.2024.101534. [Google Scholar] [CrossRef]
51. Xie F. Alginate-based nanocomposites for food preservation: recent progress showcasing heightened material properties and functionalities. Adv Nanocompos. 2024;1(1):248–74. doi:10.1016/j.adna.2024.07.002. [Google Scholar] [CrossRef]
52. Roy S, Priyadarshi R, Łopusiewicz L, Biswas D, Chandel V, Rhim JW. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: a review. Int J Biol Macromol. 2023;239(2):124248. doi:10.1016/j.ijbiomac.2023.124248. [Google Scholar] [PubMed] [CrossRef]
53. Maciel VBV, Yoshida CMP, Franco TT. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr Polym. 2015;132:537–45. doi:10.1016/j.carbpol.2015.06.047. [Google Scholar] [PubMed] [CrossRef]
54. Kokkuvayil Ramadas B, Rhim JW, Roy S. Recent progress of carrageenan-based composite films in active and intelligent food packaging applications. Polymers. 2024;16(7):1001. doi:10.3390/polym16071001. [Google Scholar] [PubMed] [CrossRef]
55. Zhang YL, Cui QL, Wang Y, Shi F, Liu YP, Liu JL, et al. Effect of carboxymethyl chitosan-gelatin-based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Sci Hortic. 2021;289(4):110462. doi:10.1016/j.scienta.2021.110462. [Google Scholar] [CrossRef]
56. Vodyashkin AA, Kezimana P, Vetcher AA, Stanishevskiy YM. Biopolymeric nanoparticles-multifunctional materials of the future. Polymers. 2022;14(11):2287. doi:10.3390/polym14112287. [Google Scholar] [PubMed] [CrossRef]
57. Rahman M, Islam KS, Dip TM, Chowdhury MFM, Debnath SR, Hasan SMM, et al. A review on nanomaterial-based additive manufacturing: dynamics in properties, prospects, and challenges. Prog Addit Manuf. 2024;9(4):1197–224. doi:10.1007/s40964-023-00514-8. [Google Scholar] [CrossRef]
58. Salcido A. Equilibrium properties of the cellular automata models for traffic flow in a single lane. In: Cellular automata-simplicity behind complexity. Austria: IntechOpen; 2011. [Google Scholar]
59. Naskar S, Sharma S, Kuotsu K. Chitosan-based nanoparticles: an overview of biomedical applications and its preparation. J Drug Delivery Sci Technol. 2019;49:66–81. doi:10.1016/j.jddst.2018.10.022. [Google Scholar] [CrossRef]
60. Azeredo HMC, Mattoso LHC, Avena-Bustillos RJ, Filho GC, Munford ML, Wood D, et al. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J Food Sci. 2010;75(1):N1–7. doi:10.1111/j.1750-3841.2009.01386.x. [Google Scholar] [PubMed] [CrossRef]
61. Xu Y, Wu Z, Li A, Chen N, Rao J, Zeng Q. Nanocellulose composite films in food packaging materials: a review. Polymers. 2024;16(3):423. doi:10.3390/polym16030423. [Google Scholar] [PubMed] [CrossRef]
62. Zhang W, Li X, Jiang W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int J Biol Macromol. 2020;154(2):1205–14. doi:10.1016/j.ijbiomac.2019.10.275. [Google Scholar] [PubMed] [CrossRef]
63. Siripatrawan U, Harte BR. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010;24(8):770–5. doi:10.1016/j.foodhyd.2010.04.003. [Google Scholar] [CrossRef]
64. Moradi M, Tajik H, Razavi Rohani SM, Oromiehie AR, Malekinejad H, Aliakbarlu J, et al. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT. 2012;46(2):477–84. doi:10.1016/j.lwt.2011.11.020. [Google Scholar] [CrossRef]
65. Zhang X, Lian H, Shi J, Meng W, Peng Y. Plant extracts such as pine nut shell, peanut shell and jujube leaf improved the antioxidant ability and gas permeability of chitosan films. Int J Biol Macromol. 2020 Apr 1;148(8):1242–50. doi:10.1016/j.ijbiomac.2019.11.108. [Google Scholar] [PubMed] [CrossRef]
66. Siripatrawan U, Vitchayakitti W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016;61(1):695–702. doi:10.1016/j.foodhyd.2016.06.001. [Google Scholar] [CrossRef]
67. Hanapiah NAM, Salleh SNAS, Johari WLW, Adzahan NM, Halimoon N, Osman NH. Biodegradable films incorporating Malaysian stingless bee propolis: development, characterization, and potential for food packaging. Appl Food Res. 2024;4(2):100594. doi:10.1016/j.afres.2024.100594. [Google Scholar] [CrossRef]
68. Rambabu K, Bharath G, Banat F, Show PL, Cocoletzi HH. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int J Biol Macromol. 2019;126:1234–43. doi:10.1016/j.ijbiomac.2018.12.196. [Google Scholar] [PubMed] [CrossRef]
69. Yong H, Wang X, Bai R, Miao Z, Zhang X, Liu J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019;90:216–24. doi:10.1016/j.foodhyd.2018.12.015. [Google Scholar] [CrossRef]
70. Zam W. Effect of alginate and chitosan edible coating enriched with olive leaves extract on the shelf life of sweet cherries (Prunus avium L.). J Food Qual. 2019;2019:8192964. doi:10.1155/2019/8192964. [Google Scholar] [CrossRef]
71. Fadda A, Serra M, Molinu MG, Azara E, Barberis A, Sanna D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J Food Compos Anal. 2014;35(2):112–9. doi:10.1016/j.jfca.2014.06.006. [Google Scholar] [CrossRef]
72. Yousuf B, Gul K, Wani AA, Singh P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: a review. Crit Rev Food Sci Nutr. 2016;56(13):2223–30. doi:10.1080/10408398.2013.805316. [Google Scholar] [PubMed] [CrossRef]
73. Sabry O, Sabry OMM. Review: beneficial health effects of olive leaves extracts [Internet]. J Nat Sci Res. 2014;4(19). [cited 2024 Jan 11]. Available from: https://www.researchgate.net/publication/305755353,www.iiste.org. [Google Scholar]
74. Kalaycıoğlu Z, Torlak E, Akın-Evingür G, Özen İ, Erim FB. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int J Biol Macromol. 2017;101:882–8. doi:10.1016/j.ijbiomac.2017.03.174. [Google Scholar] [PubMed] [CrossRef]
75. Yang C, Lu JH, Xu MT, Shi XC, Song ZW, Chen TM, et al. Evaluation of chitosan coatings enriched with turmeric and green tea extracts on postharvest preservation of strawberries. LWT. 2022;163(1):113551. doi:10.1016/j.lwt.2022.113551. [Google Scholar] [CrossRef]
76. Riaz A, Lei S, Akhtar HMS, Wan P, Chen D, Jabbar S, et al. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int J Biol Macromol. 2018;114:547–55. doi:10.1016/j.ijbiomac.2018.03.126. [Google Scholar] [PubMed] [CrossRef]
77. Chaparro-Hernández S, Ruiz-Cruz S, Márquez-Ríos E, de Jesús Ornelas-Paz J, Del-Toro-Sánchez CL, Gassos-Ortega LE, et al. Effect of chitosan-tomato plant extract edible coating on the quality, shelf life, and antioxidant capacity of pork during refrigerated storage. Coatings. 2019;9(12):827. doi:10.3390/coatings9120827. [Google Scholar] [CrossRef]
78. Yuan G, Lv H, Yang B, Chen X, Sun H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules. 2015;20(6):11034–45. doi:10.3390/molecules200611034. [Google Scholar] [PubMed] [CrossRef]
79. Morachis-Valdez AG, Santillán-Álvarez Á, Gómez-Oliván LM, García-Argueta I, Islas-Flores H, Dublán-García O. Effects of peppermint extract and chitosan-based edible coating on storage quality of common carp (Cyprinus carpio) fillets. Polymers. 2021;13(19):3243. doi:10.3390/polym13193243. [Google Scholar] [PubMed] [CrossRef]
80. Kashtiban AE, Okpala COR, Karimidastjerd A, Zahedinia S. Recent advances in nano-related natural antioxidants, their extraction methods and applications in the food industry. Explorat Foods Foodom. 2024;2(2):125–54. doi:10.37349/eff.2024.00030. [Google Scholar] [CrossRef]
81. Ghazy OA, Fouad MT, Morsy TA, Kholif AE. Nanoemulsion formulation of Lawsonia inermis extract and its potential antimicrobial and preservative efficacy against foodborne pathogens. Food Control. 2023;145(3):109458. doi:10.1016/j.foodcont.2022.109458. [Google Scholar] [CrossRef]
82. Ghazy OA, Fouad MT, Saleh HH, Kholif AE, Morsy TA. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021;341(2):128259. doi:10.1016/j.foodchem.2020.128259. [Google Scholar] [PubMed] [CrossRef]
83. Rahmani Z, Karimi M, Saffari I, Mirzaei H, Nejati M, Sharafati Chaleshtori R. Nanoemulsion and nanoencapsulation of a hydroethanolic extract of Nettle (Urtica dioica) and Wormwood (Artemisia absinthiumcomparison of antibacterial and anticancer activity. Front Chem. 2024;12:1266573. doi:10.3389/fchem.2024.1266573. [Google Scholar] [PubMed] [CrossRef]
84. Shetta A, Ali IH, Sharaf NS, Mamdouh W. Review of strategic methods for encapsulating essential oils into chitosan nanosystems and their applications. Int J Biol Macromol. 2024;259(2):129212. doi:10.1016/j.ijbiomac.2024.129212. [Google Scholar] [PubMed] [CrossRef]
85. Alven S, Peter S, Aderibigbe BA. Polymer-based hydrogels enriched with essential oils: a promising approach for the treatment of infected wounds. Polymers. 2022;14(18):3772. doi:10.3390/polym14183772. [Google Scholar] [PubMed] [CrossRef]
86. Marinković J, Nikolić B, Marković T, Radunović M, Ilić J, Bošković M, et al. Cymbopogon citratus essential oil: an active principle of nanoemulsion against Enterococcus faecalis root canal biofilm. Future Microbiol. 2021;16(12):907–18. doi:10.2217/fmb-2021-0081. [Google Scholar] [PubMed] [CrossRef]
87. Wang H, Ma Y, Liu L, Liu Y, Niu X. Incorporation of clove essential oil nanoemulsion in chitosan coating to control Burkholderia gladioli and improve postharvest quality of fresh Tremella fuciformis. LWT. 2022;170(1):114059. doi:10.1016/j.lwt.2022.114059. [Google Scholar] [CrossRef]
88. Hafsa J, Smach ali M, Ben Khedher MR, Charfeddine B, Limem K, Majdoub H, et al. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT. 2016;68:356–64. doi:10.1016/j.lwt.2015.12.050. [Google Scholar] [CrossRef]
89. Priyadarshi R, Sauraj, Kumar B, Deeba F, Kulshreshtha A, Negi YS. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018;85(10):158–66. doi:10.1016/j.foodhyd.2018.07.003. [Google Scholar] [CrossRef]
90. Hromiš NM, Lazić VL, Markov SL, Vaštag ŽG, Popović SZ, Šuput DZ, et al. Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. J Food Eng. 2015;158(3):86–93. doi:10.1016/j.jfoodeng.2015.01.001. [Google Scholar] [CrossRef]
91. Esmaeili A, Asgari A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int J Biol Macromol. 2015;81:283–90. doi:10.1016/j.ijbiomac.2015.08.010. [Google Scholar] [PubMed] [CrossRef]
92. Arabpoor B, Yousefi S, Weisany W, Ghasemlou M. Multifunctional coating composed of Eryngium campestre L. essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry fruits. Food Hydrocoll. 2021;111:106394. doi:10.1016/j.foodhyd.2020.106394. [Google Scholar] [CrossRef]
93. Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48(6):1562–7. doi:10.1016/j.fct.2010.03.025. [Google Scholar] [PubMed] [CrossRef]
94. Moradi M, Tajik H, Razavi Rohani SM, Oromiehie AR. Effectiveness of Zataria multiflora Boiss essential oil and grape seed extract impregnated chitosan film on ready-to-eat mortadella-type sausages during refrigerated storage. J Sci Food Agric. 2011;91(15):2850–7. doi:10.1002/jsfa.4531. [Google Scholar] [PubMed] [CrossRef]
95. Thiem B, Kikowska M, Krawczyk A, Wieckowska B, Sliwinska E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tissue Organ Cult. 2013;114(2):197–206. doi:10.1007/s11240-013-0315-1. [Google Scholar] [CrossRef]
96. Murbach Teles Andrade BF, Nunes Barbosa L, Da Silva Probst I, Fernandes Júnior A. Antimicrobial activity of essential oils. J Essent Oil Res. 2014;26(1):34–40. doi:10.1080/10412905.2013.860409. [Google Scholar] [CrossRef]
97. Amor G, Sabbah M, Caputo L, Idbella M, De Feo V, Porta R, et al. Basil essential oil: composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods. 2021;10(1):121. doi:10.3390/foods10010121. [Google Scholar] [PubMed] [CrossRef]
98. Hammer KA, Carson CF, Riley TV, Nielsen JB. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem Toxicol. 2006;44(5):616–25. doi:10.1016/j.fct.2005.09.001. [Google Scholar] [PubMed] [CrossRef]
99. Sánchez-González L, González-Martínez C, Chiralt A, Cháfer M. Physical and antimicrobial properties of chitosan-tea tree essential oil composite films. J Food Eng. 2010;98(4):443–52. doi:10.1016/j.jfoodeng.2010.01.026. [Google Scholar] [CrossRef]
100. Wang Y, Xia Y, Zhang P, Ye L, Wu L, He S. Physical characterization and pork packaging application of chitosan films incorporated with combined essential oils of cinnamon and ginger. Food Bioproc Tech. 2017;10(3):503–11. doi:10.1007/s11947-016-1833-8. [Google Scholar] [CrossRef]
101. Han Lyn F, Nur Hanani ZA. Effect of lemongrass (Cymbopogon citratus) essential oil on the properties of chitosan films for active packaging. J Packag Technol Res. 2020;4(1):33–44. doi:10.1007/s41783-019-00081-w. [Google Scholar] [CrossRef]
102. Oh YA, Oh YJ, Song AY, Won JS, Bin Song K, Min SC. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT. 2017;75(10):742–50. doi:10.1016/j.lwt.2016.10.033. [Google Scholar] [CrossRef]
103. Silva dos Se G, Marques Nátaly de JJ, Linhares EPM, Bonora CM, Costa Érico T, Saraiva MF. Review of anticancer activity of monoterpenoids: geraniol, nerol, geranial and neral. Chem-Biol Interact. 2022;362:109994. doi:10.1016/j.cbi.2022.109994. [Google Scholar] [PubMed] [CrossRef]
104. Faheem F, Liu ZW, Rabail R, Haq IU, Gul M, Bryła M, et al. Uncovering the industrial potentials of lemongrass essential oil as a food preservative: a review. Antioxidants. 2022;11(4):720. doi:10.3390/antiox11040720. [Google Scholar] [PubMed] [CrossRef]
105. Athayde AJAA, de Oliveira PDL, Guerra ICD, da Conceição ML, de Lima MAB, Arcanjo NMO, et al. A coating composed of chitosan and Cymbopogon citratus (Dc. Ex Nees) essential oil to control Rhizopus soft rot and quality in tomato fruit stored at room temperature. J Hortic Sci Biotechnol. 2016;91(6):582–91. doi:10.1080/14620316.2016.1193428. [Google Scholar] [CrossRef]
106. Imawan C, Yunilawati R, Fauzia V, Rahmi D, Umar A. Physical and mechanical properties of antimicrobial film form lemongrass oil incorporated with chitosan/ascorbic acid. Adv Eng Res. 2022;210. doi:10.2991/aer.k.220131.056. [Google Scholar] [CrossRef]
107. Istiqomah A, Prasetyo WE, Firdaus M, Kusumaningsih T. Valorisation of lemongrass essential oils onto chitosan-starch film for sustainable active packaging: greatly enhanced antibacterial and antioxidant activity. Int J Biol Macromol. 2022;210:669–81. doi:10.1016/j.ijbiomac.2022.04.223. [Google Scholar] [PubMed] [CrossRef]
108. Yammine J, Chihib NE, Gharsallaoui A, Ismail A, Karam L. Advances in essential oils encapsulation: development, characterization and release mechanisms. Polym Bull. 2024;81(5):3837–82. doi:10.1007/s00289-023-04916-0. [Google Scholar] [CrossRef]
109. Albuquerque Pícia M, Azevedo SG, de Andrade CP, D’Ambros Nália Cêa de S, Pérez MTM, Manzato L. Biotechnological applications of nanoencapsulated essential oils: a review. Polymers. 2022;14(24):5495. doi:10.3390/polym14245495. [Google Scholar] [PubMed] [CrossRef]
110. Chakraborty A, Dhar P. A review on potential of proteins as an excipient for developing a nano-carrier delivery system [Internet]. Criti Rev™ Therap Drug Carr Syst. 2017;34. [cited 2024 Jan 11]. Available from: www.begellhouse.com. [Google Scholar]
111. Raza ZA, Khalil S, Ayub A, Banat IM. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr Res. 2020;492:108004. doi:10.1016/j.carres.2020.108004. [Google Scholar] [PubMed] [CrossRef]
112. Sharma K, Babaei A, Oberoi K, Aayush K, Sharma R, Sharma S. Essential oil nanoemulsion edible coating in food industry: a review. Food Bioprocess Technol. Springer. 2022;15(11):2375–95. doi:10.1007/s11947-022-02811-6. [Google Scholar] [CrossRef]
113. Hassanshahian M, Saadatfar A, Masoumipour F. Formulation and characterization of nanoemulsion from Alhagi maurorum essential oil and study of its antimicrobial, antibiofilm, and plasmid curing activity against antibiotic-resistant pathogenic bacteria. J Environ Health Sci Engineer. 2020;18(2):1015–27. doi:10.1007/s40201-020-00523-7. [Google Scholar] [PubMed] [CrossRef]
114. Shehabeldine AM, Doghish AS, El-Dakroury WA, Hassanin MMH, Al-Askar AA, AbdElgawad H, et al. Antimicrobial, antibiofilm, and anticancer activities of syzygium aromaticum essential oil nanoemulsion. Molecules. 2023;28(15):5812. doi:10.3390/molecules28155812. [Google Scholar] [PubMed] [CrossRef]
115. Hancharova M, Halicka-Stępień K, Dupla A, Lesiak A, Sołoducho J, Cabaj J. Antimicrobial activity of metal-based nanoparticles: a mini-review. BioMetals. 2024;37(4):773–801. doi:10.1007/s10534-023-00573-y. [Google Scholar] [PubMed] [CrossRef]
116. Ben Amor I, Hemmami H, Grara N, Aidat O, Ben Amor A, Zeghoud S, et al. Chitosan: a green approach to metallic nanoparticle/nanocomposite synthesis and applications. Polymers. 2024;16(18):2662. doi:10.3390/polym16182662. [Google Scholar] [PubMed] [CrossRef]
117. Qin Y, Liu Y, Yuan L, Yong H, Liu J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019;96:102–11. doi:10.1016/j.foodhyd.2019.05.017. [Google Scholar] [CrossRef]
118. Feng Q, Fan B, He YC, Ma C. Antibacterial, antioxidant and fruit packaging ability of biochar-based silver nanoparticles-polyvinyl alcohol-chitosan composite film. Int J Biol Macromol. 2024;256(2):128297. doi:10.1016/j.ijbiomac.2023.128297. [Google Scholar] [PubMed] [CrossRef]
119. Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, et al. Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol. 2015;75(3):346–53. doi:10.1016/j.ijbiomac.2015.01.027. [Google Scholar] [PubMed] [CrossRef]
120. Suo B, Li H, Wang Y, Li Z, Pan Z, Ai Z. Effects of ZnO-nanoparticle-coated packaging film on pork meat quality during cold storage. J Sci Food Agric. 2017;97(7):2023–9. doi:10.1002/jsfa.8003. [Google Scholar] [PubMed] [CrossRef]
121. La DD, Nguyen-Tri P, Le KH, Nguyen PTM, Nguyen MDB, Vo ATK, et al. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum arabic edible coating for post-harvest banana preservation. Prog Org Coat. 2021;151:106057. doi:10.1016/j.porgcoat.2020.106057. [Google Scholar] [CrossRef]
122. Liu J, Huang J, Hu Z, Li G, Hu L, Chen X, et al. Chitosan-based films with antioxidant of bamboo leaves and ZnO nanoparticles for application in active food packaging. Int J Biol Macromol. 2021;189(4):363–9. doi:10.1016/j.ijbiomac.2021.08.136. [Google Scholar] [PubMed] [CrossRef]
123. Zhang X, Xiao G, Wang Y, Zhao Y, Su H, Tan T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr Polym. 2017;169(20):101–7. doi:10.1016/j.carbpol.2017.03.073. [Google Scholar] [PubMed] [CrossRef]
124. Dordevic S, Dordevic D, Tesikova K, Sedlacek P, Kalina M, Vapenka L, et al. Nanometals incorporation into active and biodegradable chitosan films. Heliyon. 2024;10(7):e28430. doi:10.1016/j.heliyon.2024.e28430. [Google Scholar] [PubMed] [CrossRef]
125. Almasi H, Jafarzadeh P, Mehryar L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: characterization, antimicrobial and release properties. Carbohydr Polym. 2018;186(2):273–81. doi:10.1016/j.carbpol.2018.01.067. [Google Scholar] [PubMed] [CrossRef]
126. Christopher D, Anbalagan A, Govindarajan VU, Muthuraman MS. Biofabrication of copper oxide nanoparticles incorporated chitosan/gelatin films for food packaging applications. Biomass Convers Biorefin. 2024;78(9):1–18. doi:10.1007/s13399-024-05442-3. [Google Scholar] [CrossRef]
127. Gunaki MN, Masti SP, D’souza OJ, Eelager MP, Kurabetta LK, Chougale RB, et al. Fabrication of CuO nanoparticles embedded novel chitosan/hydroxypropyl cellulose bio-nanocomposites for active packaging of jamun fruit. Food Hydrocoll. 2024;152(5):109937. doi:10.1016/j.foodhyd.2024.109937. [Google Scholar] [CrossRef]
128. Zhang M, Zhang C, Zhai X, Luo F, Du Y, Yan C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci China Mater. 2019;62(11):1727–39. doi:10.1007/s40843-019-9471-7. [Google Scholar] [CrossRef]
129. Sathiyaseelan A, Saravanakumar K, Wang MH. Cerium oxide decorated 5-fluorouracil loaded chitosan nanoparticles for treatment of hepatocellular carcinoma. Int J Biol Macromol. 2022;216(10):52–64. doi:10.1016/j.ijbiomac.2022.06.112. [Google Scholar] [PubMed] [CrossRef]
130. Purohit SD, Priyadarshi R, Bhaskar R, Han SS. Chitosan-based multifunctional films reinforced with cerium oxide nanoparticles for food packaging applications. Food Hydrocoll. 2023;143(4):108910. doi:10.1016/j.foodhyd.2023.108910. [Google Scholar] [CrossRef]
131. Barik M, BhagyaRaj GVS, Dash KK, Shams R. A thorough evaluation of chitosan-based packaging film and coating for food product shelf-life extension. J Agric Food Res. 2024;16:101164. doi:10.1016/j.jafr.2024.101164. [Google Scholar] [CrossRef]
132. Ragab HM, Diab NS, Obeidat ST, Alghamdi AM, Khaled AM, Farea MO, et al. Improving the optical, thermal, mechanical, electrical properties and antibacterial activity of PVA-chitosan by biosynthesized Ag nanoparticles: eco-friendly nanocomposites for food packaging applications. Int J Biol Macromol. 2024;264(2):130668. doi:10.1016/j.ijbiomac.2024.130668. [Google Scholar] [PubMed] [CrossRef]
133. Marin-Silva DA, Romano N, Damonte L, Giannuzzi L, Pinotti A. Hybrid materials based on chitosan functionalized with green synthesized copper nanoparticles: physico-chemical and antimicrobial analysis. Int J Biol Macromol. 2023;242(2):124898. doi:10.1016/j.ijbiomac.2023.124898. [Google Scholar] [PubMed] [CrossRef]
134. Tabassum Z, Girdhar M, Kumar A, Malik T, Mohan A. ZnO nanoparticles-reinforced chitosan-xanthan gum blend novel film with enhanced properties and degradability for application in food packaging. ACS Omega. 2023;8(34):31318–32. doi:10.1021/acsomega.3c03763. [Google Scholar] [PubMed] [CrossRef]
135. Sundareva Yu A, Dumina IS, Salomatina EV, Smirnova ON, Bulanov EN, Zarubin DM, et al. Properties of chitosan films modified with TiO2 nanoparticles promising as biodegradable food packaging. J Phys.: Conf Ser. 2024;2845(1):012035. doi:10.1088/1742-6596/2845/1/012035. [Google Scholar] [CrossRef]
136. Hussein EM, Desoky WM, Hanafy MF, Guirguis OW. Effect of TiO2 nanoparticles on the structural configurations and thermal, mechanical, and optical properties of chitosan/TiO2 nanoparticle composites. J Phys Chem Solid. 2021;152:109983. doi:10.1016/j.jpcs.2021.109983. [Google Scholar] [CrossRef]
137. Momtaz F, Momtaz E, Mehrgardi MA, Momtaz M, Narimani T, Poursina F. Enhanced antibacterial properties of polyvinyl alcohol/starch/chitosan films with NiO-CuO nanoparticles for food packaging. Sci Rep. 2024;14(1):7356. doi:10.1038/s41598-024-58210-8. [Google Scholar] [PubMed] [CrossRef]
138. M.Ahmed E, Saber D, Abd Elaziz K, Alghtani AH, Felemban BF, Ali HT, et al. Chitosan-based nanocomposites: preparation and characterization for food packing industry. Mater Res Express. 2021;8(2):025017. doi:10.1088/2053-1591/abe791. [Google Scholar] [CrossRef]
139. De Matteis V, Cascione M, Costa D, Martano S, Manno D, Cannavale A, et al. Aloe vera silver nanoparticles addition in chitosan films: Improvement of physicochemical properties for eco-friendly food packaging material. J Mater Res Technol. 2023;24(24):1015–33. doi:10.1016/j.jmrt.2023.03.025. [Google Scholar] [CrossRef]
140. Aouadi A, Hamada Saud D, Rebiai A, Achouri A, Benabdesselam S, Mohamed Abd El-Mordy F, et al. Introducing the antibacterial and photocatalytic degradation potentials of biosynthesized chitosan, chitosan-ZnO, and chitosan-ZnO/PVP nanoparticles. Sci Rep. 2024;14(1):14753. doi:10.1038/s41598-024-65579-z. [Google Scholar] [PubMed] [CrossRef]
141. Sun X, Wang H, Liang H, Meng N, Zhou N. Fabrication of antimicrobial chitosan/ZnO nanoparticles/lecithin-montmorillonite films for food packaging application. Food Hydrocoll. 2025;159(4):110686. doi:10.1016/j.foodhyd.2024.110686. [Google Scholar] [CrossRef]
142. Xing Y, Li X, Guo X, Li W, Chen J, Liu Q, et al. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials. 2020;10(7):1365. doi:10.3390/nano10071365. [Google Scholar] [PubMed] [CrossRef]
143. Mir SA, Dar BN, Wani AA, Shah MA. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci Technol. 2018;80(4):141–54. doi:10.1016/j.tifs.2018.08.004. [Google Scholar] [CrossRef]
144. Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, et al. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10(2):292. doi:10.3390/nano10020292. [Google Scholar] [PubMed] [CrossRef]
145. Ying S, Guan Z, Ofoegbu PC, Clubb P, Rico C, He F, et al. Green synthesis of nanoparticles: current developments and limitations. Environ Technol Innovation. 2022;26(1):102336. doi:10.1016/j.eti.2022.102336. [Google Scholar] [CrossRef]
146. Kumah EA, Fopa RD, Harati S, Boadu P, Zohoori FV, Pak T. Human and environmental impacts of nanoparticles: a scoping review of the current literature. BMC Pub Heal. 2023;23(1):1059. doi:10.1186/s12889-023-15958-4. [Google Scholar] [PubMed] [CrossRef]
147. Rosenboom JG, Langer R, Traverso G. Bioplastics for a circular economy. Nat Rev Mater. 2022;7(2):117–37. doi:10.1038/s41578-021-00407-8. [Google Scholar] [PubMed] [CrossRef]
148. Anuranj PR, Harisankaran PS, Adithya Krishna S, Parvathy S, Prakash G, Vishnu Savanth V, et al. Essential oils as valuable feed additive: a narrative review of the state of knowledge about their beneficial health applications and enhancement of production performances in poultry. J Exp Biol Agric Sci. 2022;10(6):1290–317. doi:10.18006/2022.10(6).1290.1317. [Google Scholar] [CrossRef]
149. Sultan R, Majid N, Nissar S, Rather AM. Seasonal variation of phytochemicals. Inter J Pharmac Biologic Sci (IJPBS) | [Internet]. 2018;8:2230–7605. [cited 2024 Jan 11]. Available from: www.ijpbs.comorwww.ijpbsonline.com. [Google Scholar]
150. Rashid Bin A, Haque M, Islam SMM, Uddin Labib KMR. Nanotechnology-enhanced fiber-reinforced polymer composites: recent advancements on processing techniques and applications. Heliyon. 2024;10(2):e24692. doi:10.1016/j.heliyon.2024.e24692. [Google Scholar] [PubMed] [CrossRef]
151. Omerović N, Djisalov M, Živojević K, Mladenović M, Vunduk J, Milenković I, et al. Antimicrobial nanoparticles and biodegradable polymer composites for active food packaging applications. Compr Rev Food Sci Food Saf. 2021;20(3):2428–54. doi:10.1111/1541-4337.12727. [Google Scholar] [PubMed] [CrossRef]
152. Demiroglu S, Singaravelu V, Seydibeyoğlu MÖ, Misra M, Mohanty AK. The use of nanotechnology for fibre-reinforced polymer composites. Fiber Technol Fiber-Reinf Compos. 2017;2017:277–97. doi:10.1016/B978-0-08-101871-2.00013-8. [Google Scholar] [CrossRef]
153. Barradas TN, de Holanda e Silva KG. Nanoemulsions of essential oils to improve solubility, stability and permeability: a review. Environ Chem Lett. 2021;19(2):1153–71. doi:10.1007/s10311-020-01142-2. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
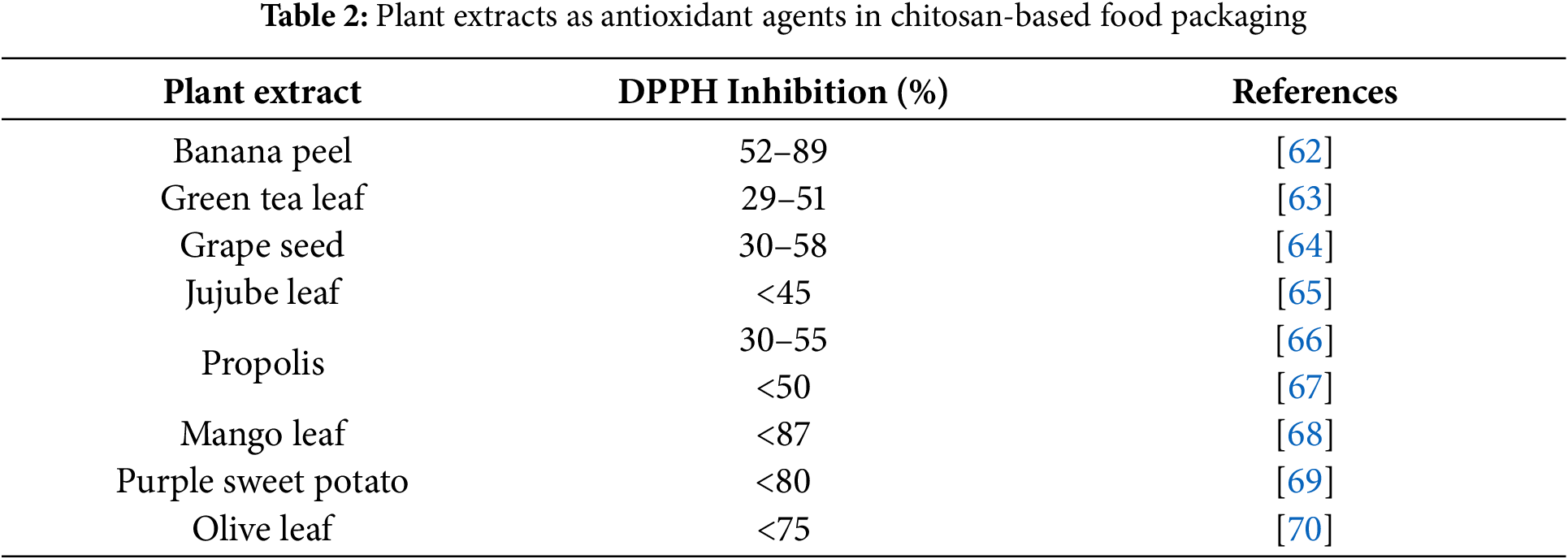
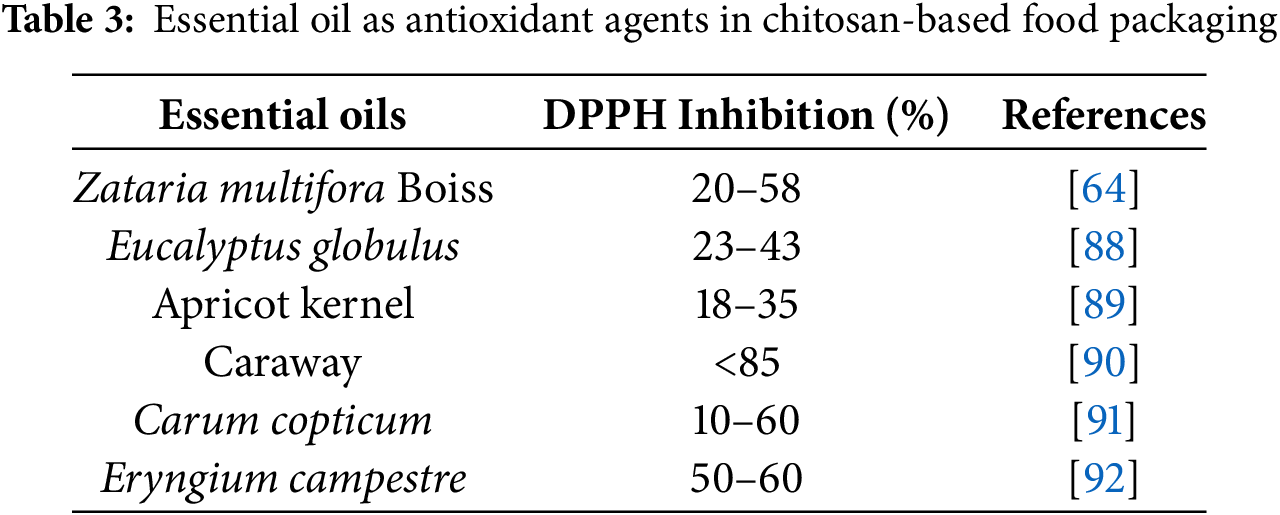
 Downloads
Downloads
 Citation Tools
Citation Tools