 Open Access
Open Access
ARTICLE
Saccharification of Paper Sludge and Fiber Dust Wastes from the Tissue Paper Industry by Maximyze® Enzymes
1 Cellulose and Paper Department, National Research Centre, Dokki, Giza, 12622, Egypt
2 Advanced Materials and Nanotechnology Group, Centre of Excellence for Advanced Sciences, National Research Centre, Dokki, Giza, 12622, Egypt
3 Chemical Engineering and Pilot Plant Department, National Research Centre, Dokki, Giza, 12622, Egypt
* Corresponding Author: Mohammad Hassan. Email:
Journal of Renewable Materials 2025, 13(6), 1169-1187. https://doi.org/10.32604/jrm.2025.02024-0030
Received 05 November 2024; Accepted 31 December 2024; Issue published 23 June 2025
Abstract
Saccharification of lignocellulosic wastes is the bottleneck of different bio-based chemical industries. Using enzymes for saccharification of lignocellulosic materials has several advantages over using chemicals. In the current work, the application of the Maximyze® enzyme system, which is industrially used in papermaking, was investigated in the saccharification of paper sludge and fiber dust wastes from the tissue paper industry. For optimizing the saccharification process, the effects of the consistency %, enzyme loading, and incubation time were studied and optimized using the Response Surface Methodology. The effect of these factors on the weight loss of paper sludge and total sugars in the hydrolyzate was studied. High-Performance Liquid Chromatography (HPLC) was used to measure the sugars composition of the hydrolyzate. Under the optimized conditions, ~90% and ~66% of the fiber dust and paper sludge could be hydrolyzed into sugars, respectively. The sugar composition was 80.23% glucose, 10.99% xylose, and 8.65% arabinose based on the total sugars in the case of fiber dust. In comparison, 80.63% glucose, 8.43% xylose, and 10.75% arabinose were detected in the case of paper sludge. The results showed the applicability of the Maximyze® commercial enzymes used in the paper industry for efficient saccharification of paper sludge and fiber dust. The presence of non-cellulosic materials in the paper sludge (residual ink, paper additives, and ash) didn’t affect the activity of the enzymes. The study also showed the potential use of fiber dust as a valuable and clean source of sugars that can be used to prepare different bio-based chemicals.Graphic Abstract
Keywords
Enzymes are widely used in many industries as an attractive green technology. They are non-toxic to living creatures and the environment, biodegradable, and derived from renewable resources. In the papermaking industry, different enzyme systems have been developed to modify the quality of pulp fibers and thus the properties of paper products [1–3]. Using enzymes also assists in reducing the cost of paper production. The development of enzymes used in papermaking has led to new generations of enzyme formulations with better effectiveness and lower costs. The main component of these formulations is the cellulase enzymes. These enzymes hydrolyze cellulose parts, leading to the cell walls’ delamination and facilitating their collapse [4]. The enzyme-treated fibers can then be refined more easily and the paper strength properties are improved without increasing the applied refining energy [5]. In addition, a reduction in the amount of pulp needed to produce the paper and an increase in production rate can be achieved using the aforementioned enzyme technology. Buckman’s second and third-generation enzyme technology, Maximyze®, is a family of products based on cellulase enzymes. These enzymes are effective for use with bleached hardwood and softwood pulps to improve the properties of tissue paper [6,7]. These new generation enzymes are a blend of single component enzymes combined with so-called potentiators, which increase the enzyme’s activity and facilitate their action and contact of the enzyme to the fibers.
Paper sludge is a common waste in papermaking. The characteristics of the paper sludge depend on the kind of paper and pulp that the paper mills produce. For example, paper sludge produced from kraft pulp and paper production differs in composition from that produced from recycled paper used for writing and printing paper or tissue paper. Therefore, the utilization and treatment of paper sludge differs from one paper mill to another.
Several approaches are followed to utilize the large amounts of paper sludge worldwide [8]. Among these approaches is to hydrolyze the carbohydrate part of the sludge, i.e., cellulose and hemicelluloses, into sugars to be utilized in the production of different chemicals such as ethanol, lactic acid, and polyhydroxy butyrate. The hydrolysis process, the so-called saccharification process, can be carried out using mineral acids or enzymes. The acid hydrolysis involves using mineral acids such as sulfuric, hydrochloric, or phosphoric acid [9,10]. Using enzymes for the saccharification of different kinds of paper sludge has been studied as summarized in Table 1 [11–41].
The tissue paper industry is one of the largest paper sectors. The tissue paper can be manufactured from virgin pulp or recycled pulp. The recycled pulp used in some grades of tissue paper comes from writing and printing waste paper, which contains high amounts of fillers and inks, in addition to the other commonly used paper additives. After several processes of repulping, de-inking, bleaching, and kneading of waste paper to get recycled fibers, the sludge generated contains high amounts of ash (~ up to 35%), residual inks and paper additives, in addition to the very short pulp fibers. Therefore, the saccharification of paper sludge generated from the recycling of writing and printing paper is a challenge due to the presence of the aforementioned non-cellulosic materials. As Table 1 shows, the saccharification of paper sludge from the tissue paper industry has been studied to a much lesser extent than the other kinds of paper sludge.
Another waste, but in much lower quantities than paper sludge, from the tissue paper industry is fiber dust. The fiber dust is generated from wrapping and trimming the tissue paper reels by the high-speed machines [42]. According to the estimation made by the tissue paper mills, the fiber dust is about 1%–2% of the produced tissue paper. The fiber dust is collected by filters installed above the tissue-making machines and dumped without use due to its very short length. The fiber dust is therefore a pure pulp, especially when the virgin pulp is used in tissue paper production. In this case, the fiber dust contains small amounts of additives, mainly the wet strength agent (from 0.5 to 2 wt.% of tissue paper). To the best of our knowledge, there are no previous studies in the literature regarding the utilization of fiber dust generated from the tissue paper industry.
Since hydrolysis of cellulosic fibers into sugars is the bottle-neck process for different bio-based chemicals, the current work aims to study the saccharification of paper sludge generated from the recycling of printed writing and printing paper using a second-generation Maximyze® enzyme system, which is used in papermaking to improve the properties of pulp fibers and decrease refining time/energy. As seen in Table 1, different commercial enzymes have been evaluated in the saccharification of different kinds of paper sludge. The use of the commercial Maximyze® enzyme system is evaluated for the first time for the saccharification of paper sludge. In addition, the saccharification of fiber dust waste from tissue paper making is investigated for the first time using the same enzyme system to investigate the utilization of this kind of waste for further utilization in bio-based chemicals such as ethanol, lactic acid, etc., and also as a comparison to the paper sludge to see how the other non-cellulosic materials in the paper sludge can affect the saccharification process using the Maximyze® enzyme system. For optimizing the saccharification conditions of paper sludge and fiber dust, the Response Surface Methodology was employed to create prediction models. The derived models were analyzed using ANOVA analysis to verify the models’ terms and the models’ accuracy.
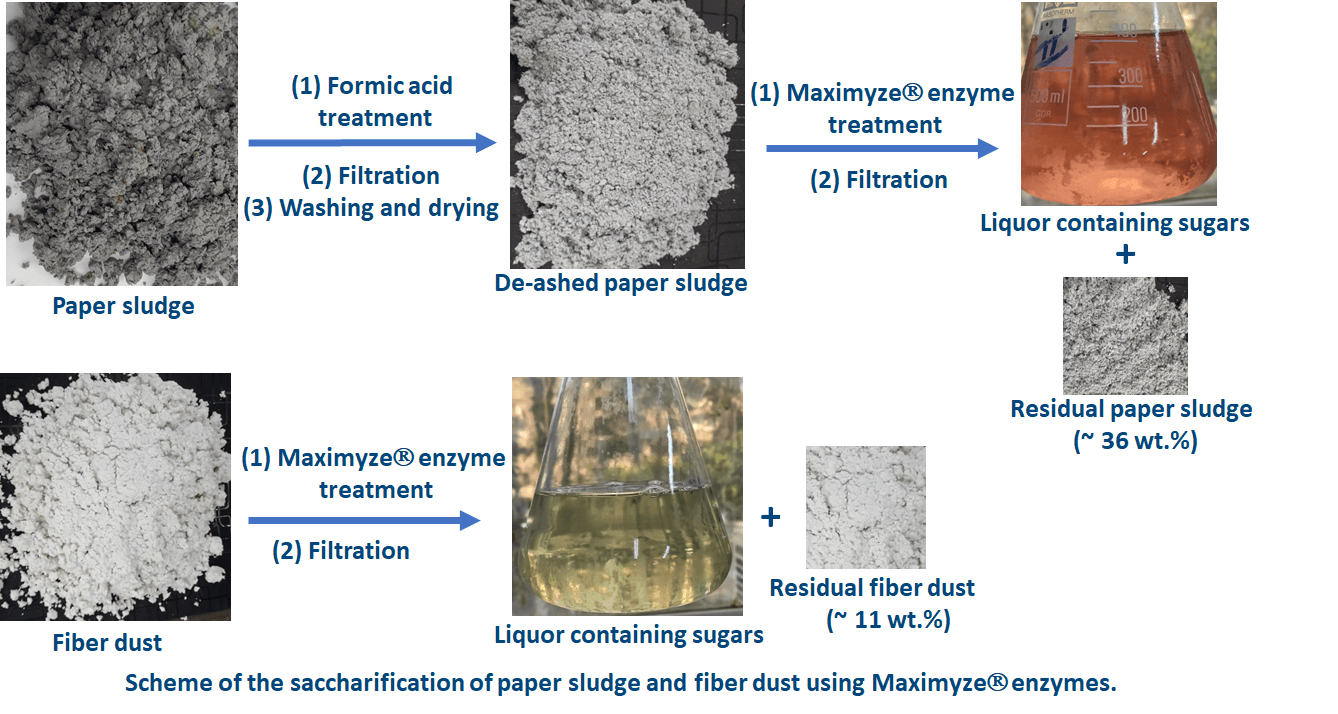
Interstate Paper Industries, Sadat City in Egypt kindly supplied the paper sludge and fiber dust used in this study. The paper sludge was generated from the recycling and de-inking processes of white writing and printing paper. The moisture content of the sludge was about 70%. Before use, the paper sludge was dried in an oven with air circulation at 40°C for 12 h; the moisture content after drying was 9.41%. The fiber dust was collected from the filters installed above the tissue-making machines and used as received; the moisture content of the fiber dust was 6.50%.
The enzymes used in the saccharification were a second-generation Maximyze® solution produced by Buckman (Buckman Laboratories, Inc., Memphis, MO, USA) and used as received. The enzymes exhibited an FPase activity of 14.8 (µmole/mL/min) as assayed using Whatman number 1 filter paper as a substrate, a xylanase activity of 478.03 (µmole/mL/min) was assayed using 1% (w/v) birch wood xylan (Sigma, St. Louis, USA) as a substrate, and carboxymethyl cellulase (CMC) activity of 60.35 (µmole/mg/min). The FPase activity was determined at 50°C and an incubation time of one hour in citrate buffer (pH 4.8) while the xylanase and CMC activities were determined at 50°C and an incubation time of 0.5 h in citrate buffer (pH 4.8).
Formic acid, sodium citrate, sulfuric acid (98%), phenol (crystals), absolute ethanol (95%), sodium hydroxide, sodium chlorite, glacial acetic acid, toluene, and 3,5 dinitro salicylic acid were purchased from Thermo Scientific Chemicals (Thermo Fisher Scientific Inc., Waltham, MA, USA) and used as received. Carboxymethyl cellulose was purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA).
2.2.1 Chemical Analyses of Paper Sludge and Fiber Dust
Total carbohydrates (as total sugars) were determined according to the standard phenol/sulfuric acid hydrolysis method [43] for both fiber dust and paper sludge after hydrolysis using 72% sulfuric acid (TAPPI standard T222). The concentration of the hydrolyzed sugars was determined using Jenway 7305 UV-visible spectrophotometer (Jenway, Staffordshire, England); the results were expressed as glucose. The ash content was determined using a muffle furnace at 900°C for 45 min according to the TAPPI T 413 standard method. The acid non-hydrolyzable material was determined by hydrolysis of the sample using 72% sulfuric acid according to the TAPPI T222 standard method. Hollocellulose was determined using ASTM-D1104 standard method, α-cellulose content was determined using the TAPPI standard method (TAPPI T 429), and hemicelluloses were estimated from the difference in weight due to the treatment of the paper sludge by 17.5% NaOH. Organo-soluble extractives were determined using the TAPPI T204 standard method using a 1:1 ethanol/toluene mixture. In the case of paper sludge, the results of holocellulose, α-cellulose, and hemicelluloses were corrected for the ash content that remained in the samples.
2.2.2 De-Ashing of Paper Sludge
Before being used in the saccharification experiments, the paper sludge was treated with dilute formic acid at a liquor ratio 1:10 for 1 h at 40°C with stirring to remove the acid-soluble ash, e.g., calcium carbonate [44].
2.2.3 Enzymatic Saccharification
Optimization of the enzymatic saccharification of paper sludge and fiber dust was carried out according to an experimental design to reach the optimum conditions that result in maximum hydrolysis (expressed as weight loss). Three main factors were selected to study their effect on the weight loss % (X) as a consequence of the enzymatic hydrolysis. The studied factors were the consistency (A), the enzyme load (B), and the duration of hydrolysis (C). The central composite design was used to design the experiments by Response Surface Methodology using Design Expert software version 6.8. Each factor was studied over five levels; two levels for the maximum and minimum of the factor range, two levels for the axial points, and a level for the center point. These five levels navigated 28 experiments in addition to 4 central experiments (the total number of experiments was 32 since they were carried out in duplicates). The factorial points and axial points were duplicated while the axial points were face-centered to expand the area of navigation. The selected ranges of the three parameters were 1% to 7.5% for factor A, 5 to 50 U/g for factor B, and 24 to 186 h for factor C. The design of experiments of the enzymatic hydrolysis of paper sludge and fiber dust is shown in Table 2.

All the saccharification experiments were conducted in 250-mL capped Erlenmeyer flasks. The substrates (paper sludge or fiber dust) were first sterilized by autoclaving at 121°C, 2 atm, for 15 min. After cooling to room temperature, different volumes of sterilized 0.05 M sodium citrate buffer pH 4.8 were added to the substrates to achieve different consistencies. These substrate suspensions were incubated with different loading of the enzyme solution at 50°C in a shaking incubator at 140 rpm for different incubation times according to the experimental design.
After the saccharification, the residual paper sludge or fiber dust was removed from the hydrolyzate by centrifugation at 7000 rpm for 20 min, washed with distilled water using vacuum filtration until neutral pH, and dried in an oven with circulating air at 45°C for 18 h. The residual weight of the paper sludge or fiber dust (Yield, Y) was determined gravimetrically from the weight of the paper sludge or fiber dust before and after saccharification as in Eq. (1).
where X1 and X2 are the weight of the paper sludge or fiber dust before and after saccharification, respectively.
The supernatant containing the sugars was collected, centrifuged at 7000 rpm, and further vacuum-filtered using a 45 μm cellulose nitrate membrane, and kept in the fridge until used.
2.2.4 Determination of the Total Sugars
The total sugars in the hydrolyzate were measured by the phenol/sulfuric acid standard method using glucose as a standard [43].
2.2.5 HPLC Analysis of the Hydrolyzate
High-performance liquid chromatography (HPLC) was used to determine the exact sugars obtained in the hydrolyzate. An Agilent Technologies 1100 series liquid chromatography instrument (Agilent Technologies, Santa clara, CA, USA) equipped with a refractive index detector was used. The analytical column was a Shim-pack SCR-101N. The mobile phase consisted of ultrapure water. The flow rate was 0.7 mL/min, the mobile phase was ultrapure water, and the column temperature was 25°C.
3.1 Chemical Composition of the De-Ashed Paper Sludge and Fibers Dust
The chemical composition of the de-ashed paper sludge was 11.87% ash, 9.80% acid non-hydrolyzable materials (lignin and other organic materials), 2.95% ethanol/toluene extractives, 75.65% holocellulose, 54.76% α-cellulose, 21.17% hemicelluloses, and sugar content of 0.58 g/g sample. The ash remained after the de-ashing using formic acid is mainly kaolin which couldn’t be hydrolyzed by formic acid [44] while the original ash content of the paper sludge was 33.5%. On the other hand, the fiber dust had the typical chemical composition of bleached paper pulp: 1.48% ash, 0.80% acid non-hydrolyzable materials (lignin), 0.46% ethanol/toluene extractives, 72.33% α-cellulose, 25.4% hemicelluloses, and a high sugars content (1.16 g glucose/g of sample) since it is mainly pulp fibers.
3.2 Enzymatic Saccharification of the Fiber Dust and De-Ashed Paper Sludge
Enzymatic saccharification of the fiber dust and de-ashed paper sludge was carried out according to the experimental design mentioned in the experimental part to reach the optimum conditions that result in the maximum hydrolysis (expressed as weight loss). Table 2 shows the results of the experimental design experiments regarding the effect on weight loss of paper sludge and fiber dust. The data was statistically analyzed to get the best conditions for hydrolysis and also to set a mathematical model for the prediction of the extent of saccharification by the enzymes and compare it with the experimental results. This is shown in Tables 3 and 4, and Figs. 1 to 6.



Figure 1: Normal plot of residuals for enzymatic hydrolysis of fiber dust (a) and paper sludge (b)

Figure 2: Predicted values vs. Actual values of weight loss % of the fibers dust (a) and paper sludge (b) enzymatic hydrolysis experiments

Figure 3: Simultaneous effect of enzyme loading and consistency on weight loss % for the model describing the enzymatic hydrolysis for fiber dust at the central point of time

Figure 4: Simultaneous effect of time of reaction and consistency on weight loss % for the model describing the enzyme hydrolysis for fiber dust at the central point of enzyme loading

Figure 5: Simultaneous effect of enzyme loading and consistency on weight loss % for enzyme hydrolysis for paper sludge at the central point of time

Figure 6: Simultaneous effect of time of reaction and consistency on weight loss % for enzyme hydrolysis for paper sludge at the central point of enzymes loading
The results in Table 2 show that increasing the incubation time and enzymes concentration generally increased the weight loss of both fiber dust and paper sludge, which is expected. Interestingly, at the highest enzyme loading used (50 U/g) and at 24 h incubation time and 1% consistency (Run # 3), the enzymes could hydrolyze 60% and 57% of the fiber dust and paper sludge, respectively. Increasing the incubation time to 186 h (the maximum incubation time studied) at the same enzyme loading and consistency (Run # 7) resulted in the hydrolysis of 89% and 64% of the fiber dust and paper sludge, respectively. This indicates the high activity of the Maximyze® enzymes to hydrolyze both samples, especially the paper sludge which contains 11.87% ash (mostly kaolin) and 9.80% acid nonhydrolyzable materials. The cellulases and hemicellulases enzymes in the Maximyze® formulation could efficiently hydrolyze cellulose and hemicelluloses in the fiber dust and paper sludge. The cellulase enzymes contain the endoglucanases which break the internal bonds of cellulose and disrupt the crystalline cellulose structure, the exocelluloses which attack the non-reducing end groups and separate oligomers of two to four sugar monomers from the exposed cellulose chains, and the cellobiases which hydrolyze these small fragments into glucose [45]. On the other hand, the hemicellulases can hydrolyze the hemicelluloses, which are amorphous and with much shorter chain lengths, into the constituting sugars of hemicelluloses [46].
In fact, it is difficult to compare the saccharification results of different kinds of sludges in other studies due to the various compositions of the sludges and the saccharification conditions used (enzyme dose, incubation time, consistency, and temperature). A tentative comparison of the current work’s results to some selected previously published results indicates the high activity of the Maximyze® enzymes in the hydrolysis of paper sludge. For example, a recent study on the saccharification of sludge from recycled paper by different commercial enzymes showed that the maximum hydrolysis achieved was 71% after 3 days of incubation [14]. The sludge contained lower ash content and acid-nonhydrolyzable materials (8% ash and 3% lignin) than that in the current work, and a mixture of commercial cellulase (~147 U/g of sludge) and hemicellulases (368 U/g of sludge) enzymes were used in that previous study. Another study on saccharification of a primary sludge using Cellic C2tec enzymes from Novozymes assisted by PEG-4000 surfactant showed optimum saccharification of about 57% using 2% enzyme loading (based on the weight of paper sludge) and 1% surfactant for 48 h [16]. The sludge had 62% cellulose and hemicelluloses, 13.9% ash, and 11% lignin. A study on saccharification of paper sludge with high fiber content produced from sulfite pulp (glucan content 89.7%, lignin 0.8%, and ash 1.7%) using a recombinant cellulase enzyme cocktail [(mono-components: S. cerevisiae-derived PcBGL1B (BGL), TeCel7A (CBHI), ClCel6A (CBHII) and TrCel5A (EGII) mono-component cellulase enzymes)] showed 80% yield of hydrolysis [24]. The high yield in this study could be attributed to the high cellulose content of the sludge used compared to the paper sludges used in the aforementioned ones. Another study on saccharification of a subcritical water-pretreated paper sludge using Acinetobacter cellulolyticus cellulase (Meiji Seika Co., Ltd., Tokyo, Japan) for 4 days gave 71 wt.% glucose yield (based on the cellulose content of the sludge) [11]. A study on saccharification of paper sludge from recycled paper containing 34% cellulose, 8% xylan, 29% ash, and 20% Klason lignin using Celluclasts 1.5 L and Novozyms 188 showed that 100% of cellulose in the sludge could be hydrolyzed after 72 h [25].
Analysis of the saccharification results
Analysis of the data of fiber dust and paper sludge saccharification shows that the models describe the relationship between the consistency % (A), the enzyme load (B), and the time spent for the process (C) and their effect on the weight loss % is a surface reduced quadratic model. The model terms A, B, C, A2, B2, AB, AC were significant. In the case of enzymatic hydrolysis of fiber dust, the model is represented by the following equation:
On the other hand, in the case of enzymatic hydrolysis of paper sludge, the model is represented by the following equation:
where X represents Weight loss; A represents Consistency; B represents Enzyme loading; and C represents Time.
The predicted R2 values (Pred R-Squared) from analysis of the model equations mentioned above in the case of the enzymatic hydrolysis of both fiber dust and paper sludge are in reasonable agreement with the adjusted R2 (Adj R-Squared) as illustrated in Tables 3 and 4. In addition, the adequate precision values (Adeq Precision) in the tables indicate an adequate signal; these results elucidated the ability of model navigation through the design space.
The model equations of the enzymatic hydrolysis of fiber dust and paper sludge were also verified by the normal plot of residuals (Fig. 1), and the actual values resulting from the experiment vs. the predicted values (Fig. 2). The figures show good fitting of the model equations for predicting the weight loss after enzymatic hydrolysis of fiber dust and paper sludge.
To estimate the range of different conditions to get the maximum weight loss by the enzymatic saccharification of fiber dust and paper sludge, the model graph of the simultaneous effect of the enzyme load and the consistency on weight loss, and the effect of consistency and time on weight loss are presented in Figs. 3 to 6. In the case of fiber dust (Figs. 3 and 4), the maximum weight loss could be achieved at low consistency in the range of 1%–2.6% conjugated with a high enzyme load in the range of 27.5–50 U/g (Fig. 3) while the simultaneous study of the consistency and time in Fig. 4 shows that the maximum value of weight loss could be achieved at a low consistency in the range of 1%–2.6% during a range of time 105–186 h.
On the other hand, in the case of paper sludge, the simultaneous study of the concistency and time (Fig. 5) showed that the maximum weight loss % could be achieved at low concistecy in the range of 1%–3% conjucated with high enzyme load in the range of 30–50 U/g while the simultaneous study of the consistency and time (Fig. 6) shows that the maximum value of weight loss % could be achieved at low consistency in the range of 1%–2.6% during the range of time 105–186 h; the enzyme load affects this relationship since the maximum weight loss is recorded at the maximum enzyme load 50 U/g as shown in Fig. 6a, while the weight loss decreases as the enzyme load decreased as illustrated in Fig. 6b.
The above results indicate that the conditions for obtaining the highest weight loss in the case of fiber dust and paper sludge are generally similar.
Applying the criteria of consistency, enzyme load, and time to obtain the maximum weight loss, the result revealed that the predicted maximum weight loss in the case of the fiber dust is 90.79% at consistency 1.27%, enzyme load 41.27 U/g, for time 183.88 h. On the other hand, in the case of the paper sludge, the maximum weight loss predicted is 65.27% at consistency 1%, enzyme load 50 U/g, for time 126.47 h. In both cases of fiber dust and paper sludge, the predicted value was evaluated with 95% confidence interval (CI) and 95% predicted interval (PI) as shown in Table 5.

3.3 Total Sugars Determination
Determination of the total sugars in the hydrolyzate produced from the enzymatic saccharification of fiber dust and paper sludge was also carried out for the samples used in the experimental design; the results are shown in Table 6. The results in the table are consistent with the weight loss results in Table 2. The maximum total sugars in the hydrolyzate (estimated as glucose) were about 1050 and 631 mg/g in the case of fiber dust and paper sludge, respectively. The maximum total sugars in both cases were obtained at the same enzymatic treatment conditions, e.g., 1% consistency, 50 U/g of the enzyme, and 186 h of the treatment. The higher total sugars obtained in the case of fiber dust than that of paper sludge is due to the chemical composition of both materials where the paper sludge contains 11.87% ash and 9.81% acid-nonhydrolyzable materials while the fiber dust contains 1.48% ash and 0.80% acid-non-hydrolyzable materials.
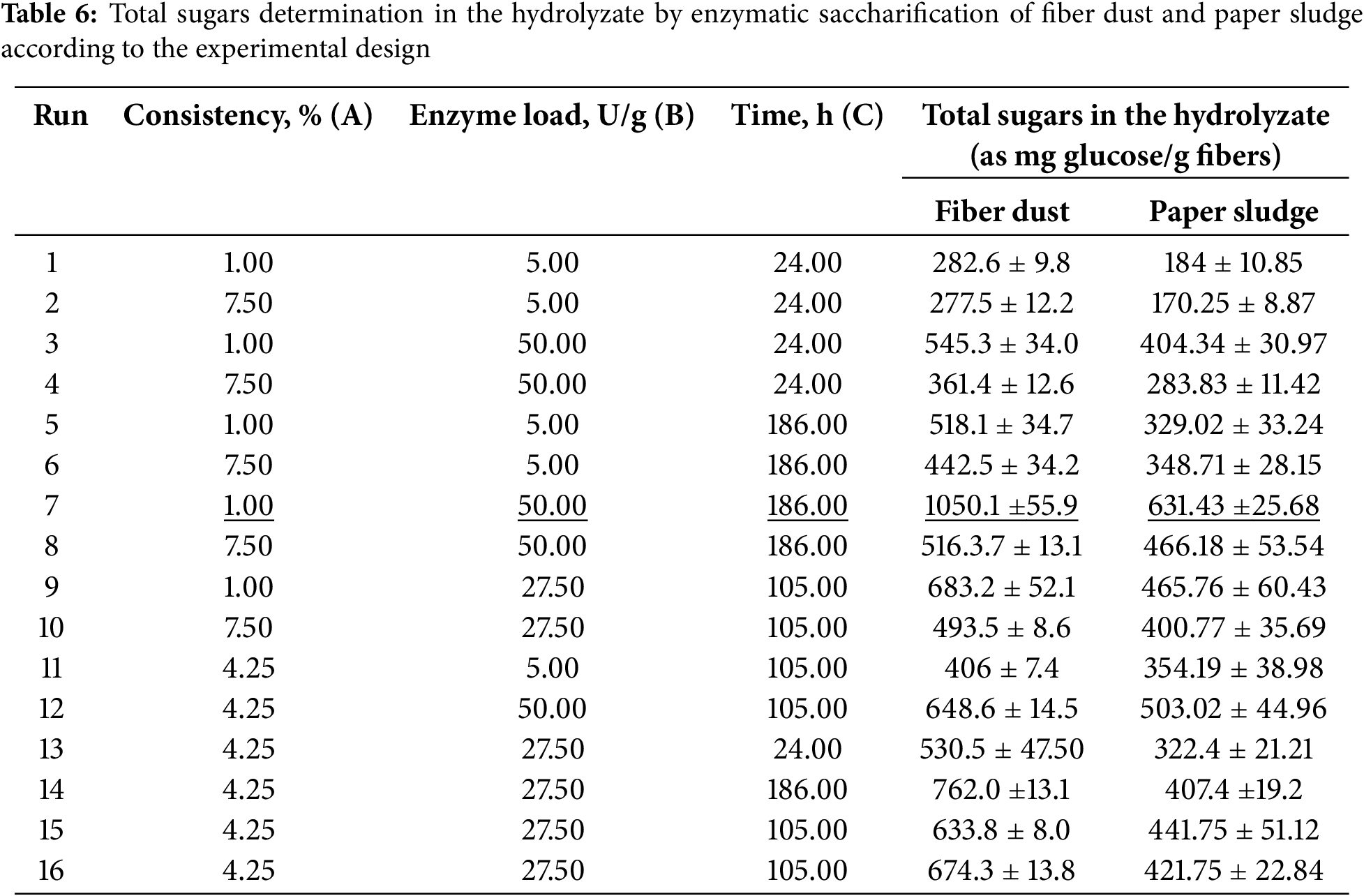
The crude hydrolyzates containing sugars obtained by the enzymatic saccharification of fiber dust and paper sludge samples with the highest total sugars (Run # 7: 1% consistency, enzymes loading 50 U/g, and time 186 h) were further analyzed by HPLC to determine the sugars exist in the hydrolyzate and their percentage. In the case of fiber dust, the major sugars detected in the hydrolyzate were glucose, xylose, and arabinose. The percentage of these sugars was 80.23% glucose, 10.99% xylose, and 8.65% arabinose based on the detected total sugars. On the other hand, in the case of paper sludge, the sugars detected were 80.63% glucose, 8.43% xylose, and 10.75% arabinose based on the total sugars.
The use of Maximyze® enzyme system for saccharification of paper sludge from recycled paper and fiber dust generated during tissue paper manufacturing was studied. To optimize the saccharification process by the enzymes, the factors affecting the saccharification (dose of enzymes, incubation time, and consistency) were studied using Response Surface Methodology and optimization models for the enzymatic hydrolysis of paper sludge and fiber dust could be reached. The models could predict the optimum conditions for getting the highest yield of saccharification. The enzymes showed high efficiency in hydrolyzing both the fiber dust and paper sludge (~ 90% and 66% saccharification, respectively). The presence of the non-cellulosic materials in the paper sludge didn’t affect the activity of the Maximyze® enzymes since the majority of the cellulosic materials could be hydrolyzed into sugars. Although the maximum saccharification according to the conditions used was recorded at the longest incubation time (186 h), the enzymes could hydrolyze 60% and 57% of the fiber dust and paper sludge, respectively, after 24 h which reveals the high activity of the Maximyze® enzymes as compared to some other studies which used other commercial enzymes.
Acknowledgement: The authors acknowledge the support of the National Research Centre (NRC) in Egypt by submitting the facilities required for completing the different experiments of the current work. The authors also acknowledge the support of Interstate Paper Industries, Sadat City, Egypt for kindly providing the paper sludge and dust fibers used in the study.
Funding Statement: The authors acknowledge funding of the current work by the Science, Technology, and Innovation Funding Authority (STDF), Egypt, project no. 46104: “Recycling of sludge wastes from paper industry via green technologies”.
Authors Contributions: The authors confirm their contribution to the paper as follows: Funding acquisition: Mohammad Hassan; Study conception and design: Mohammad Hassan, Samar El-Mekkawi, Enas Hassan, Wafaa Abou Elseoud; Methodology, analysis and interpretation of results: Mohammad Hassan, Samar El-Mekkawi, Enas Hassan, Wafaa Abou Elseoud; Project administration: Mohammad Hassan; Supervision: Mohammad Hassan; Writing original Draft: Mohammad Hassan, Enas Hassan, Samar El-Mekkawi; Writing, review and editing final manuscript: Mohammad Hassan, Samar El-Mekkawi, Enas Hassan, Wafaa Abou Elseoud. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Barrios N, Smith MM, Venditti RA, Pal L. Enzyme-assisted dewatering and strength enhancement of cellulosic fibers for sustainable papermaking: a bench and pilot study. J Clean Prod. 2024;434:140094. doi:10.1016/j.jclepro.2023.140094. [Google Scholar] [CrossRef]
2. Tanveer A, Gupta S, Dwivedi S, Yadav K, Yadav S, Yadav D. Innovations in papermaking using enzymatic intervention: an ecofriendly approach. Cellulose. 2023;30(12):7393–425. doi:10.1007/s10570-023-05333-2. [Google Scholar] [CrossRef]
3. Yakubu A, Vyas A. Industrial application of alkaline cellulase enzymes in pulp and paper recycling: a review. Cell Chem Technol. 2023;57(1–2):17–28. [Google Scholar]
4. Rahikainen J, Ceccherini S, Molinier M, Holopainen-Mantila U, Reza M, Väisänen S, et al. Effect of cellulase family and structure on modification of wood fibres at high consistency. Cellulose. 2019;26(8):5085–103. doi:10.1007/s10570-019-02424-x. [Google Scholar] [CrossRef]
5. Kumar A, Tazeb A, Ram C. Enzyme-assisted pulp refining: an energy saving approach. Phys Sci Rev. 2021;6(2):20190046. doi:10.1515/psr-2019-0046. [Google Scholar] [CrossRef]
6. Loosvelt I. Maximyze®-fiber modification enzyme for the paper industry. Przeglad Papierniczy. 2009;65(6):339–41. [Google Scholar]
7. Covarrubias RM. Buckman develops third generation Maximyze® for recycled packaging. IPPTA: Q J Indian Pulp Pap Tech Assoc. 2017;29(3):47–50. [Google Scholar]
8. De Azevedo ARG, Alexandre J, Pessanha LSP, Manhães RDST, de Brito J, Marvila MT. Characterizing the paper industry sludge for environmentally-safe disposal. Waste Manag. 2019;95:43–52. doi:10.1016/j.wasman.2019.06.001. [Google Scholar] [PubMed] [CrossRef]
9. Gnanasekaran L, Priya AK, Thanigaivel S, Hoang TK, Soto-Moscoso M. The conversion of biomass to fuels via cutting-edge technologies: explorations from natural utilization systems. Fuel. 2023;331:125668. doi:10.1016/j.fuel.2022.125668. [Google Scholar] [CrossRef]
10. El-Mekkawi S, Abou-Elseoud W, Fadel S, Hassan E, Hassan M. Phosphoric acid pretreatment and saccharification of paper sludge as a renewable material for cellulosic fibers. J Renew Mater. 2024;12(9):1573–91. doi:10.32604/jrm.2024.053589. [Google Scholar] [CrossRef]
11. Okajima I, Muto M, Morimoto S, Nauchi K, Kodama Y, Park EY, et al. Bioethanol production from paper sludge by subcritical water pretreatment and semi-simultaneous saccharifcation and fermentation. BioEnergy Res. 2024;17:1662–73. doi:10.1007/s12155-024-10755-2. [Google Scholar] [CrossRef]
12. Romaní A, Del-Río PG, Rubira A, Pérez MJ, Garrote G. Co-valorization of discarded wood pinchips and sludge from the pulp and paper industry for production of advanced biofuels. Ind Crops Prod. 2024;209:117992. doi:10.1016/j.indcrop.2023.117992. [Google Scholar] [CrossRef]
13. Dyk J, Gorgens JF, Rensburg E. Enhanced ethanol production from paper sludge waste under high-solids conditions with industrial and cellulase-producing strains of Saccharomyces cerevisiae. Bioresour Technol. 2024;394(5):130163. doi:10.1016/j.biortech.2023.130163. [Google Scholar] [PubMed] [CrossRef]
14. Rabemanolontsoa H, Phang ST, Kawamoto H. Evaluation of various commercial enzymes for paper sludge saccharification. IOP Conf Ser: Earth Environ Sci. 2024;1354(1):012020. doi:10.1088/1755-1315/1354/1/012020. [Google Scholar] [CrossRef]
15. Mendes CVT, Rocha JMS, Carvalho MGVS. Batch simultaneous saccharification and fermentation of primary sludge at very high solid concentrations for bioethanol production. Fermentation. 2023;9(10):888. doi:10.3390/fermentation9100888. [Google Scholar] [CrossRef]
16. Zambare V, Jaco S, Din MF, Ponraj M. Box-behnken design-based optimization of the saccharification of primary paper-mill sludge as a renewable raw material for bioethanol production. Sustainability. 2023;15(13):10740. doi:10.3390/su151310740. [Google Scholar] [CrossRef]
17. Rorke DCS, Lekha P, Kan GEB, Sithole BB. Effect of pharmaceutical wastewater as nitrogen source on the optimization of simultaneous saccharification and fermentation hydrogen production from paper mill sludge. Sustain Chem Pharm. 2022;25(15):100619. doi:10.1016/j.scp.2022.100619. [Google Scholar] [CrossRef]
18. Rabemanolontso H, Triwahyuni E, Takada M. Consolidated bioprocessing of paper sludge to acetic acid by clostridial co-culture. Bioresour Technol Rep. 2021;16(2):100842. doi:10.1016/j.biteb.2021.100842. [Google Scholar] [CrossRef]
19. Arthur W, Diedericks D, Coetzee G, Rensburg EV, Gorgens JF. Kinetic modelling of cellulase recycling in paper sludge to ethanol fermentation. J Environ Chem Eng. 2021;9(5):105981. doi:10.1016/j.jece.2021.105981. [Google Scholar] [CrossRef]
20. Li J, Suan S, Wang Y, Jiang Z. Integrated production of optically pure L-lactic acid from paper mill sludge by simultaneous saccharification and co-fermentation (SSCF). Waste Manag. 2021;129(465):35–46. doi:10.1016/j.wasman.2021.05.008. [Google Scholar] [PubMed] [CrossRef]
21. Rorke DCS, Lekha P, Kana GEB, Sithole BB. Surfactant-assisted green liquor dregs pretreatment to enhance the digestibility of paper mill sludge. Int J Hydrogen Energy. 2021;46(41):21359–71. doi:10.1016/j.ijhydene.2021.04.018. [Google Scholar] [CrossRef]
22. Dey P, Rangarajan V, Nayak J, Diganta Bhusan Das DB, Wood SB. An improved enzymatic pre-hydrolysis strategy for efficient bioconversion of industrial pulp and paper sludge waste to bioethanol using a semi-simultaneous saccharification and fermentation process. Fuel. 2021;294:120581. doi:10.1016/j.fuel.2021.120581. [Google Scholar] [CrossRef]
23. Alkasrawi M, Al-Othman A, Tawalbeh M, Doncan S, Gurram R, Singsaas E, et al. A novel technique of paper mill sludge conversion to bioethanol toward sustainable energy production: effect of fiber recovery on the saccharification hydrolysis and fermentation. Energy. 2021;223:120018. doi:10.1016/j.energy.2021.120018. [Google Scholar] [CrossRef]
24. Malgas S, Rose SH, van Zyl WH, Pletschke B. Enzymatic hydrolysis of softwood derived paper sludge by an in vitro recombinant cellulase cocktail for the production of fermentable sugars. Catalysts. 2020;10(7):775. doi:10.3390/catal10070775. [Google Scholar] [CrossRef]
25. Marques S, Gírio FM, Santos JAL, Roseiro JC. Pulsed fed-batch strategy towards intensified process for lactic acid production using recycled paper sludge. Biomass Convers Biorefin. 2017;7:127–37. doi:10.1007/s13399-016-0211-0. [Google Scholar] [CrossRef]
26. Schroeder BG, Zanoni PRS, Magalhaes WLE, Hansel FA, Tavares LBB. Evaluation of biotechnological processes to obtain ethanol from recycled paper sludge. J Mater Cycles Waste Manag. 2017;19:463–72. doi:10.1007/s10163-015-0445-0. [Google Scholar] [CrossRef]
27. Takano M, Hoshino K. Lactic acid production from paper sludge by SSF with thermotolerant Rhizopus sp. Bioresour Bioprocess. 2016;3:29. doi:10.1186/s40643-016-0106-8. [Google Scholar] [CrossRef]
28. Mendes CVT, Cruz CHG, Reis DFN, Carvalho MGVS, Rocha JMS. Integrated bioconversion of pulp and paper primary sludge to second generation bioethanol using Saccharomyces cerevisiae ATCC 26602. Bioresour Technol. 2016;220:161–7. doi:10.1016/j.biortech.2016.07.140. [Google Scholar] [PubMed] [CrossRef]
29. Robus CLL, Gottumukkala LD, van Rensburg E, Görgens JF. Feasible process development and techno-economic evaluation of paper sludge to bioethanol conversion: south African paper mills scenario. Renew Energy. 2016;92:333–45. doi:10.1016/j.renene.2016.02.017. [Google Scholar] [CrossRef]
30. Boshoff S, Gottumukkala LD, van Rensburg E, Görgens J. Paper sludge (PS) to bioethanol: evaluation of virgin and recycle mill sludge for low enzyme, high-solids fermentation. Bioresour Technol. 2016;203:103–11. doi:10.1016/j.biortech.2015.12.028. [Google Scholar] [PubMed] [CrossRef]
31. Alkasrawi M, Al-Hamamre Z, Al-Shannag M, Abedin MJ, Singsaas E. Conversion of paper mill residuals to fermentable sugars. BioResources. 2016;11(1):2287–96. [Google Scholar]
32. Guan W, Shi S, Tu M, Lee YY. Acetone-butanol–ethanol production from Kraft paper mill sludge by simultaneous saccharification and fermentation. Bioresour Technol. 2016;200:713–21. doi:10.1016/j.biortech.2015.10.102. [Google Scholar] [PubMed] [CrossRef]
33. Shi S, Kang L, Lee YY. Production of lactic acid from the mixture of softwood pre-hydrolysate and paper mill sludge by simultaneous saccharification and fermentation. Appl Biochem Biotechnol. 2015;175:2741–54. doi:10.1007/s12010-014-1451-8. [Google Scholar] [PubMed] [CrossRef]
34. Cavka A, Alriksson B, Rose SH, van Zyl WH, Jönsson LJ. Production of cellulosic ethanol and enzyme from waste fiber sludge using SSF, recycling of hydrolytic enzymes and yeast, and recombinant cellulase–producing Aspergillus niger. J Ind Microbiol Biotechnol. 2014;41:1191–200. doi:10.1007/s10295-014-1457-9. [Google Scholar] [PubMed] [CrossRef]
35. Zheng H, liu Y, Liu X, Han Y, Wang J, Lu F. Overexpression of a Paenibacillus campinasensis xylanase in Bacillus megaterium and its applications to biobleaching of cotton stalk pulp and saccharification of recycled paper sludge. Bioresour Technol. 2012;125:182–7. doi:10.1016/j.biortech.2012.08.101. [Google Scholar] [PubMed] [CrossRef]
36. Kemppainen K, Ranta L, Sipila E, Östman A, Vehmaanpera J, Puranen T, et al. Ethanol and biogas production from waste fibre and fibre sludge—the FibreEtOH concept. Biomass Bioenerg. 2012;46:60–9. doi:10.1016/j.biombioe.2012.03.027. [Google Scholar] [CrossRef]
37. Dwiarti L, Boonchird C, Harashima S, Park EY. Simultaneous saccharification and fermentation of paper sludge without pretreatment using cellulase from Acremonium cellulolyticus and thermotolerant Saccharomyces cerevisiae. Biomass Bioenerg. 2012;42:114–22. doi:10.1016/j.biombioe.2012.02.019. [Google Scholar] [CrossRef]
38. Lin Y, Wang D, Wang T. Ethanol production from pulp & paper sludge and monosodium glutamate waste liquor by simultaneous saccharification and fermentation in batch condition. Chem Eng J. 2012;191:31–7. doi:10.1016/j.cej.2011.09.040. [Google Scholar] [CrossRef]
39. Prasetyo J, Naruse K, Kato T, Boonchird C, Harashima S, Park EY. Bioconversion of paper sludge to biofuel by simultaneous saccharification and fermentation using a cellulase of paper sludge origin and thermotolerant Saccharomyces cerevisiae TJ14. Biotechnol Biofuels Bioprod. 2011;4(1):35. doi:10.1186/1754-6834-4-35. [Google Scholar] [PubMed] [CrossRef]
40. Peng L, Chen Y. Conversion of paper sludge to ethanol by separate hydrolysis and fermentation (SHF) using Saccharomyces cerevisiae. Biomass Bioenergy. 2011;35:1600–6. doi:10.1016/j.biombioe.2011.01.059. [Google Scholar] [CrossRef]
41. Prasetyo J, Kato T, Park EY. Efficient cellulase-catalyzed saccharification of untreated paper sludge targeting for biorefinery. Biomass Bioenergy. 2010;34:1906–13. doi:10.1016/j.biombioe.2010.07.021. [Google Scholar] [CrossRef]
42. Frazier R, Zambrano F, Pawlak JJ, Gonzalez R. Methods to assess and control dusting and linting in the paper industry: a review. Int J Adv Manuf Technol. 2022;119(9–10):5511–28. doi:10.1007/s00170-021-08482-5. [Google Scholar] [CrossRef]
43. Nielsen SS. Phenol-sulfuric acid method for total carbohydrates, food analysis laboratory manual. In: Nielsen SS, editor. Food analysis laboratory manual. Food science texts series. Boston: Springer; 2010. p. 47–52. [Google Scholar]
44. Hassan ML, Hassan EA, Elseoud WSA, Moustafa AM. Utilization of paper sludge in preparation of high-purity calcium formate. Biomass Convers Biorefin. 2023;8(7):2333. doi:10.1007/s13399-023-05216-3. [Google Scholar] [CrossRef]
45. Bajpai P. Roles of cellulases in cellulose hydrolysis. In: Bajpai P, editor. Cellulases in the biofuel industry. Amsterdam: Elsevier; 2023. p. 119–38. [Google Scholar]
46. Xiao M, Liu Y-J, Bayer EA, Kosugi A, Cui Q, Feng Y. Cellulosomal hemicellulases: indispensable players for ensuring effective lignocellulose bioconversion. Green Carbon. 2024;2:57–69. doi:10.1016/j.greenca.2024.01.003. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools