 Open Access
Open Access
REVIEW
Extraction, Utilization, Functional Modification, and Application of Cellulose and Its Derivatives
Department of Polymer Material and Engineering, Guangdong University of Technology (GDUT), Guangzhou, 510006, China
* Corresponding Author: Haoqun Hong. Email:
Journal of Renewable Materials 2025, 13(9), 1707-1763. https://doi.org/10.32604/jrm.2025.02025-0005
Received 04 January 2025; Accepted 18 March 2025; Issue published 22 September 2025
Abstract
Under the background of the current energy crisis and environmental pollution, the development of green and sustainable materials has become particularly urgent. As one of the most abundant natural polymers on earth, cellulose has attracted wide attention due to its green recycling, sustainable development, degradability, and low cost. Therefore, cellulose and its derivatives were used as the starting point for comprehensive analysis. First, the basic structural properties of cellulose were discussed, and then the extraction and utilization methods of cellulose were reviewed, including Sodium Hydroxide based solvent system, N, N-Dimethylacetamide/Lithium Chloride System, N-Methylmorpholine-N-Oxide (NMMO) system, ionic liquids (ILs) system, and deep eutectic solvent (DES) system. Then, the functional modification techniques of cellulose are introduced, including nano-modification, small molecule modification, and macromolecular modification. Finally, the potential applications of cellulose in the fields of reinforcement materials, self-healing materials, radioactive cooling, nanogenerators, and biomedicine were discussed. At the end of this paper, the challenges and future development direction of cellulose materials are prospectively analyzed, aiming at providing guidance and inspiration for the research and application in related fields.Graphic Abstract
Keywords
Nowadays, in this challenging era, environmental issues and energy crisis have increasingly become the focus of global attention, and it is urgent for us to find new solutions to deal with these challenges. Compared with traditional petroleum-based materials, bio-based materials have attracted much attention due to their degradability, renewability, and environmental friendliness. Among the many bio-based materials, cellulosic materials have attracted much attention due to their abundant sources, excellent biodegradability, contribution to sustainable development, excellent mechanical strength, excellent biocompatibility, and relatively low cost. These characteristics make cellulosic materials of great significance in resource recycling.
Among various biomass-derived materials, cellulose-based materials have garnered considerable attention in the scientific community. Primarily, this preference stems from two critical advantages: First, compared with protein-based materials, cellulose is a kind of green natural polymer material with the most abundant reserves on the earth, which makes its raw material supply sufficient. Secondly, cellulose and its derivatives exhibit superior physical and mechanical properties relative to starch-based materials, including exceptional tensile strength, enhanced toughness, and remarkable thermal stability [1]. In addition, cellulose material contains active hydroxyl groups on its surface, which enables us to endow cellulose with new functions through chemical modification (such as molecule modification and large molecule modification), and then develop diversified cellulose derivatives. Nanocellulose, as a refined cellulose material, has injected new impetus to the development of cellulose materials because of its high specific surface area, excellent heat resistance and excellent mechanical properties.
Currently, the mainstream products of cellulose materials include cellulose films, cellulose elastomers, cellulose hydrogels, and cellulose microspheres. Through functional modification or combination with other materials, these cellulose materials are widely used in several fields such as energy, biomedicine, and intelligent manufacturing, promoting green transformation in these fields. This review focuses on cellulose materials, discusses their structure and properties, summarizes the extraction and utilization methods of cellulose, combs the ways of cellulose functionalization and modification, and summarizes its application in different fields. Finally, the advantages and challenges of cellulosic materials are summarized, and their future development prospects are prospected.
2 Cellulose Structure and Properties
The chemical structural formula of cellulose is (C6H10O5)n, and its basic chemical structure is D-Galactose. The specific structure as shown in Fig. 1a. The bond between the structures is formed by the condensation reaction between the hydroxyl group on C-1 of the glucose unit and the hydroxyl group on C-4 of the adjacent glucose unit to form 1-4 glycosidic bond, that is, β-(1→4)-glycosidic bond to form a natural linear polymer [2]. In general, the repeating unit of cellulose polymers is cellobiose (a dimer of glucose), because in space two adjacent glucose units rotate 180 degrees with each other along the fiber axis in the polymer chain.
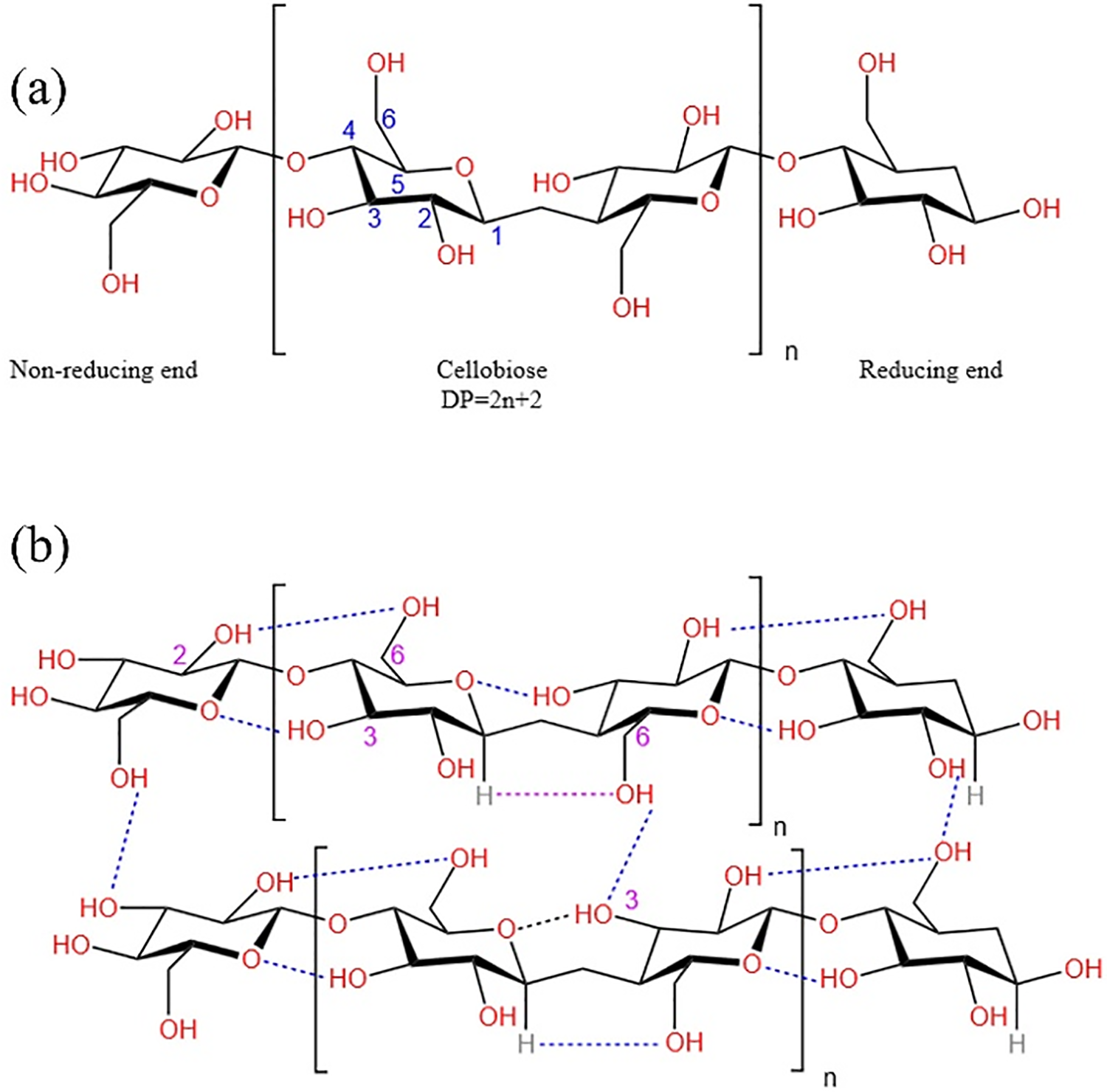
Figure 1: (a) Structure of cellulose. (b) Hydrogen bond network of cellulose
Cellulose has abundant hydroxyl groups, with each glucose unit containing three hydroxyls, which are located in carbon atoms C-2, C-3, and C-6, thus contributing to the establishment of an extensive hydrogen bond network. The specific hydrogen bond network is shown in Fig. 1b. In the molecular chain, because the H on O-3(oxygen at C-3) on the glucose unit forms a stable hydrogen bond (O(3)-H······O) with the epoxy on another glucose or the H on O-2(oxygen at C-2) with O-6(oxygen at C-6) (O (2)-H······O (6)), make the structure of cellulose molecular chain rigid. As for the molecular chains, the hydrogen bonds formed between the H on the O-3 of the glucose units and the O-6, as well as between the H on the unconventional C and the O (O(3)-H······O(6) and C-H······O), lead to close packing of the cellulose molecular chains, which contributes to the development of crystallization. The specific structure and hydrogen bond interaction of cellulose is shown in Fig. 1.
The different positions of the H atom and hydroxy -OH at the two ends of the C atom endow cellulose with different physicochemical properties. The hydroxyl group on the chain unit of dehydrated glucose was located at C-2, C-3, and C-6, respectively. The reaction activity was also different according to the different structural positions. Among them, the most representative reaction is the reaction of primary and secondary alcohols, and the adjacent primary hydroxyl group shows a typical diol structure [3]. In addition, the hydroxyl group at the end of the cellulose chain also has different behaviors: the hydroxyl group at the C-1 end is reducing, while the hydroxyl group at the C-4 end is oxidizing.
3 Utilization and Extraction of Cellulose
The primary source of cellulose is plants, with a smaller portion derived from microorganisms. Compared to plant cellulose, bacterial cellulose exhibits superior mechanical properties, polymerization rates, and crystallinity [4]. Natural plant fibers consist of multiple fiber bundles, which are adhered together by adhesives. The main chemical components of natural plant fibers are cellulose, hemicellulose, and lignin, along with trace amounts of waxes and lipids [5]. Cellulose, as the primary component, reinforces the material, while hemicellulose and lignin act as fillers and binders. Although these components protect the cell walls, they are not conducive to the utilization of cellulose materials. Additionally, within the cellulose microfibrils, there are variations in the degree of molecular chain packing, which leads to the classification of “crystalline regions” and “amorphous regions.” This alternating arrangement endows cellulose with unique properties: good flexibility, high thermal stability, and excellent mechanical strength [6,7]. However, it also makes it difficult for solvents to penetrate the crystalline regions, resulting in poor solubility of cellulose. The degree of reaction, reaction rate, and reaction uniformity are all adversely affected. Therefore, to utilize cellulose as a material, it must undergo treatment beforehand.
To effectively utilize cellulose, it typically needs to be dissolved. Therefore, breaking the tightly packed molecular structure is required to separate the cellulose chain. In general, it is necessary to destroy its hydrogen bond network, thus destroying its crystalline regions, and achieving the dissolution of cellulose. Generally, cellulose can be swollen by water, acid, alkali or salt solution can be used to enter the crystallization zone of fiber by osmosis, so that cellulose can be swelled infinitely and further dissolved. It is worth noting that cellulose exhibits different reactions under various conditions. When exposed to a high concentration of inorganic acid, cellulose undergoes hydrolysis to produce glucose. In contrast, when it encounters a high concentration of caustic soda solution, it forms alkali cellulose. Furthermore, in the presence of a strong oxidizing agent, cellulose is oxidized to produce oxidized cellulose.
Generally, the factors affecting cellulose dissolution are mainly divided into molecular weight, crystallinity, and hydrophobic interaction [2]:
(1) The effect of molecular weight:
Molecular weight is a key factor affecting the physical properties of polymers, and this value is inversely proportional to the entropy of dissolution [2]. In general, cellulose is insoluble in water, whereas its hydrolysate glucose, cellobiose (the basic unit of cellulose), and cellulose oligomers with a degree of polymerization (DP) less than 10 all appear water-soluble. Therefore, reducing the molecular weight of cellulose may be an effective strategy to achieve its enhanced dissolution [8–10]. The DP of cellulose depends on the extraction process and source of cellulose. Normally, the change of DP can be affected by adjusting reaction time, controlling temperature, changing pressure, and adding polar aprotic cosolvent [11]. In addition, in strong polar solvents (such as highly acidic or basic environments), the degradation of the cellulose chain due to environmental influences leads to a decrease in DP, which increases its solubility. If the product does not require high mechanical properties, reducing the molecular weight is an effective strategy to achieve the dissolution and utilization of cellulose [12–14].
(2) The effect of crystallinity:
Since cellulose is a highly crystalline polymer, its amorphous region is easily permeated by solvents, whereas parts of its crystalline region are difficult to dissolve, which makes it difficult for solvents to enter cellulose. Therefore, selecting an appropriate solvent is crucial. Usually, cellulose dissolution includes crystal removal and molecular chain unwinding [15]. If crystallization removal is the decisive step, reducing crystallinity facilitates the dissolution of cellulose fibers. On the other hand, if the unwinding of the molecular chain is the decisive step, then the diameter of the fiber is more important than the crystallinity [16]. Ghasemi et al. [15] showed that both crystallization removal and chain untangling play an important role in the dissolution process of cellulose. They pointed out that during the dissolution process of cellulose, only crystal detachment occurred, with no chain untangling taking place. Although it can reduce the crystallinity of cellulose, it does not dissolve well. In contrast, if there is only disentanglement and no crystal removal occurs, only the amorphous region of cellulose dissolves. Therefore, for cellulose to dissolve fully, the solvent system must be equipped with the ability to remove the crystallization of cellulose and untangle the molecular chains.
(3) The effects of hydrophobic effect:
Research has shown that the solubility of cellulose is related to the hydrophobic interaction [17]. It has been found that the accumulation of cellulose tends to form crystalline structures under hydrophobic action, and the contribution of hydrophobic interaction is about 8 times that of hydrogen bonding [18]. Medronho et al. [17,19,20] improved the solubility of cellulose in ILs and NaOH/ urea/water systems by reducing its hydrophobic interaction. This is because, in ILs, amphiphilic cations in the system will change the hydrophobic interaction between cellulose. In the NaOH/H2O solution system, the polarity of the solvent system can be reduced by adding urea, and the hydrophobic effect can be weakened, to improve the solubility of cellulose.
In addition, to make cellulose dissolve well, it is necessary to equip the corresponding solvent system. In general, cellulose solvents can be divided into two categories: cellulose-derived solvent system and non-derived solvent system [2]. The so-called derived solvent system refers to the chemical reaction between the solvent and the OH group on the cellulose chain to destroy the strong interaction between the molecular chains, especially the crystalline part of the cellulose [21]. However, the non-derived solvent system can dissolve cellulose by breaking the strong interaction force between the OH groups of cellulose and establishing a new interaction relationship [22]. In addition to the above two solvent systems, researchers have also found that some metal salt hydrates can affect the interaction between cellulose chains, establishing a new interaction between the OH group of cellulose and the cations and anions of metal salts [11], thereby replacing the original hydrogen bond network and realizing the dissolution of cellulose.
3.1 Extraction of Cellulose Based on NaOH Aqueous Solution by Alkali Method
The solvent system using NaOH to dissolve cellulose has developed rapidly due to its low cost and easy recovery. Early studies found that NaOH concentration between 7% and 10% was associated with lower temperature (<10°C), and cellulose can show good dissolution [23]. Since the solubility of cellulose is affected by molecular weight, crystallinity, and hydrophobic interaction, only the oligosaccharides with DP less than 250 can be dissolved, and the solubility is limited to 5% in the case of high crystallinity cellulose. Therefore, to improve the dissolution ability of cellulose in NaOH, people changed the composition of the original system to improve the dissolution ability. However, the aqueous solution of NaOH has a major disadvantage as a solvent. This system can only dissolve cellulose at low temperatures and within a limited concentration range of NaOH [24]. Moreover, the solubility of cellulose in this system and the stability of the solution obtained is low. With the development of time, people have a deeper understanding of the dissolution of cellulose by NaOH. He et al. [25] conducted a systematic study on the dissolution performance of cellulose based on the three parameters of NaOH concentration, temperature, and pretreatment. They found that the purity of cellulose was higher after drying and crushing pretreatment. In addition, they found that high temperature and high concentration of NaOH had more advantages in the dissolution and purification of cellulose than low temperature and low concentration of NaOH solvent. This is because the high temperature and high concentration conditions help to loosen the surface structure of cellulose, so that the solution can penetrate its interior, dissolve impurities and achieve the purpose of purification. Moreover, they also found that the yield of cellulose extracted at 30°C alkaline solution temperature was higher than that at 90°C alkaline solution temperature, indicating that high temperature had a slight degradation effect on cellulose.
As for the dissolution mechanism of NaOH on cellulose, it is generally believed that NaOH molecules in aqueous solution system form sodium hydroxide hydrate at low temperature. These hydrates can dissolve cellulose by forming a new interaction force with the hydroxyl group on the cellulose chain unit, thus replacing the hydrogen bond between the original molecular chains [19,26]. Xiong et al. [27] pointed out that OH−, as a strong hydrogen bond receptor, replaced the intramolecular and intermolecular hydrogen bonds of cellulose by forming a new hydrogen bond force with the hydroxyl group in cellulose. Meanwhile Na+ ions played a role in preventing cellulose chains from being close to each other to achieve stability in aqueous solution. The reason for the poor water solubility of cellulose at low concentration of NaOH can be explained as that the hydrodynamic radius of sodium hydroxide hydrate is too large in the environment of low concentration of NaOH so that the hydrate cannot penetrate the hydrogen bond network of cellulose, thus replacing the corresponding force. When urea is added to the system, the solubility of cellulose will be improved, due to the urea hydrate occurring in NaOH and cellulose of complex surface form clathrate self-assembly behavior. Urea, as a hydrogen bond donor, can prevent cellulose chains from trying to get close, thereby improving the stability of the aqueous solution [28]. Peng et al. [29] systematically summarized the mechanism and influence of an alkaline solution system to dissolve cellulose through designed experiments. Through polarizing microscopy, they found that as the concentration of NaOH increased, the spots gradually decreased, because the volume of hydrate molecules formed was smaller when the concentration of NaOH was higher. However, smaller molecules can better penetrate the internal structure of cellulose molecules and form new hydrogen bonds with cellulose molecular chains, thus destroying the original hydrogen bond interaction and affecting the optical properties. They then carried out a systematic analysis of the sediment and found that the sediment decreased as the concentration of NaOH increased, and the mass of the sediment decreased significantly at a NaOH concentration of 7%. They concluded that the hydrate network was the smallest and most likely to penetrate the cellulose. In addition, they also analyzed the samples by FTIR and found that the change in NaOH concentration did not affect the intrinsic structure of cellulose. However, they found that the peak intensity of 3350 cm−1(-OH) increased when the concentration of NaOH increased. Combined with the previous polarizing microscope pictures, it was proved that the size of the crystal structure of cellulose would gradually decrease during the dissolution process. This indicates that the cellulose structure is opened. The reduction of the size means that the increase of the specific surface area of cellulose easily exposes more hydroxyl groups, thus enhancing the strength of the tensile vibration absorption peak of -OH. In addition, they concluded by XRD tests that when NaOH concentration was low, the volume of the solvent hydration network was large, and difficult to penetrate the tight crystalline region. On the contrary, these solvents are more likely to enter the loose amorphous region and form hydrogen bonds with the cellulose molecular chains, resulting in the preferential dissolution of the amorphous region of cellulose and an increase in the proportion of crystalline regions, thereby improving the crystallinity of cellulose. However, as the concentration of NaOH increases, the crystalline region is dissolved, resulting in a decrease in the crystallinity of the cellulose.
To improve the solubility of cellulose in NaOH, new variant systems have been developed, such as adding different hydrogen bond donors and acceptors (such as urea, thiourea, zinc oxide (ZnO), and polyethylene glycol with different molecular weights) to the original system or pretreating the raw material before adding NaOH aqueous solution. These pretreatment techniques include mechanical pretreatment (e.g., steam blasting and ultrasonic treatment), chemical pretreatment (e.g., ethanol and hydrochloric acid treatment), and enzymatic pretreatment (e.g., cellulase) [30].
3.2 Extraction of Cellulose Based on N, N-Dimethylacetamide (DMAc)/Lithium Chloride (LiCl) System
Initially, Dawsey et al. [31] found that the mixed solvent system of DMAc/LiCl could dissolve cellulose. Although the solvent system has good solubility, DMAc is physiologically toxic and does not have the characteristics of environmental friendliness. People conducted a series of gradient experiments on this system and found that 8%(w:v) LiCl was the most suitable and commonly used in DMAc [32]. It was also found that the system not only has a good ability to dissolve cellulose but also can derivate cellulose. Due to the good solubility of this system, it is often used to measure the molecular weight of cellulose in GPC.
Although this system has good solubility, there are two points to pay attention to: one is high moisture absorption, because DMAc and LiCl components are easy to absorb moisture. Hence, the solvent solubility will be seriously reduced in the presence of water [33]; The other is the formation of keteniminium ion, which forms an active intermediate in a two-component solvent system under high-temperature conditions, resulting in the induced degradation of cellulose [34].
For cellulose to exist stably in DMAc, the following factors should be considered: the amount of LiCl, the content of cellulose, the storage time, and the presence of water [33], among which the presence of water and the content of LiCl are the key parameters affecting the dissolution stability of cellulose. From the mechanism, the solvation shell of Li+ absorbs the water in the system [34], which leads to the hydrolysis of DMAc, and the additional water also enters DMAc. This accumulation of water limits the complexation of the Li+ solvated shell with cellulose [34]. Therefore, it is necessary to dry the cellulose and solvent system before dissolving the cellulose. For example, flame drying can be used for LiCl, and distillation and water catcher can be used together for DMAc to keep the system dry [33].
As for the dissolution mechanism of cellulose by the DMAc/LiCl system, it is speculated that a new interaction relationship is established between Cl− and cellulose, thus replacing the original hydrogen bond network, as shown in Fig. 2. It has been reported that about 80% of the dipole-dipole interaction between DMAc and cellulose is due to the Cl-cellulose interaction. In addition, due to the solvation of Li+ by DMAc molecules, a large cationic complex ([Li−(DMAc) x]) is formed, which forms new interactions with cellulose, accounting for 10% of the total dipole interactions [35]. Sen et al. [36] added through the study that the strong hydrogen bond formed between the hydroxyl proton and the Cl− of the salt caused the original hydrogen bond to break, thus allowing cellulose to dissolve, and pointed out that the hydroxyl proton of cellulose did not form a new interaction relationship with the carbonyl group in DMAc molecule.
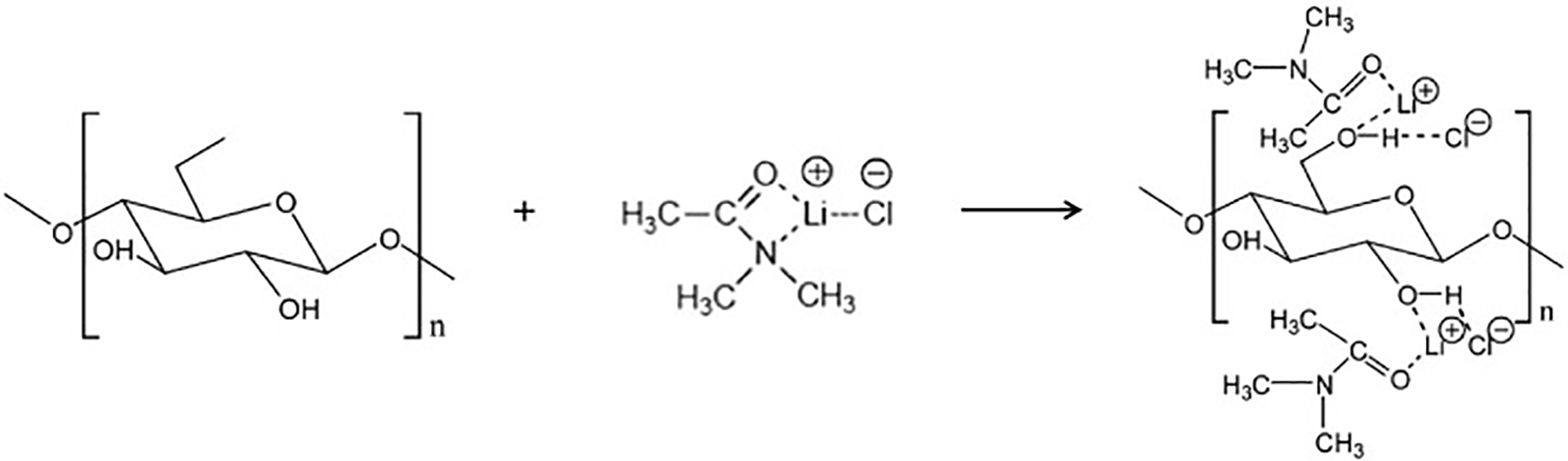
Figure 2: Diagram of hydrogen bond fracture mechanism of cellulose dissolved in LiCl/dimethylacetamide (LiCl/DMAc) solvent system. Adapted with permission from Reference [19], Copyright © 2015, Elsevier
Ma et al. [37] found that LiCl was the most important for cellulose dissolution in the LiCl/DMAc system through a series of characterization methods. Their analysis showed that the Li bond plays a major role in the formation of the Lix (DMAc)yClz complex. They found through FMO analysis that the splitting of Li+-Cl− ion pairs increases the HOMO energy level, which facilitates the insertion of the [Li (DMAc)4] Cl complex into the cellulose chain and facilitates the transfer of Cl− ion electrons to unoccupied orbitals of the cellulose molecule. In addition, by combining the DFT calculation results with the nuclear magnetic test, they found that when the [Li (DMAc)4] Cl complex is inserted into the cellulose dimer, it can effectively weaken the hydrogen bond interaction and promote the separation of the two cellulose dimers. Based on these results, they deduced that the two cellulose chains are separated at the non-reducing end by the combination of the Cl− ion and the steric hindrance of the [Li (DMAc)] + unit. The hydrogen proton is terminated by the Cl− ion and blocked by the steric hindrance of the tetrahedron-arranged [Li (DMAc)4] + unit, effectively preventing the hydrogen bond recombination of the two cellulose chains. Therefore, the Li bond plays an important role in DMAc solvent.
To enhance the dissolution of cellulose in the DMAc/LiCl system, the general treatment method is to pretreat the raw material to activate the cellulose. By pretreatment, the structure and properties of cellulose can be changed, so that it is more conducive to dissolution in solvents. One of the methods is the solvent exchange method, by adding a polar solvent (such as water) to make the cellulose swell, remove the original solvent, dry, and then adding to the DMAc/LiCl system, this method can improve the solubility of cellulose in the solvent system. Another method is to expand in water and then freeze dry. The result of this method is to increase the porosity, voidness, and surface area of cellulose [38]. These structures facilitate the rapid diffusion of solvent into cellulose to achieve dissolution. Another common method is the high temperature activated cellulose method. At high temperatures, the solvent diffuses into the fiber, and the solvent vapor causes the fiber to expand. Although this method can process a large amount of cellulose, high temperatures may lead to cellulose degradation [34].
Although the DMAc/LiCl system has good solubility, DMAc is physiologically toxic and not environmentally friendly. In addition, the combination of DMAc and LiCl is highly toxic, corrosive, and volatile, leading to a higher degree of health and safety-related issues. Therefore, the technology has yet to achieve successful commercialization [39].
3.3 Extraction of Cellulose Based on N-Methylmorpholine-N-Oxide (NMMO)
The NMMO solvent system belongs to the non-derived solvent system of cellulose solvent. Cellulose can be directly dissolved in 13% NMMO aqueous solution at 80°C~120°C, and the cellulose concentration in its aqueous solution can reach 23% [40]. The Lyocell process is an important process for cellulose production in the industry, which uses an NMMO solvent system to dissolve cellulose. It does not need to be pre-treated or derivatized in advance to dissolve well like the above solution system, so it can shorten the process route in industrial production and improve production efficiency thanks to its unique advantages. Compared with the viscose method for obtaining cellulose, the lyocell method is considered an environmentally friendly process in the fiber production route because it is environmentally friendly and does not release toxic gases [41]. It is worth noting that the maximum recovery rate of NMMO solvent in this process can reach 99% [42].
Generally, the cellulose needs to be swelled before dissolving, so the cellulose needs to be put into a dilute solution of NMMO (60% NMMO and 20%–30% water) [43]. However, when the water content is high (>15%), NMMO will not dissolve cellulose [41], so to achieve the dissolution of cellulose, the process needs to use the vacuum heating evaporation method to remove water, which leads to the shortcoming of high energy consumption of this process. In addition, the process also causes oxidation of solvents and polymers [39].
So why does NMMO dissolve cellulose? From the perspective of the dissolution mechanism, due to the strong dipolar nature of the active NO part in NMMO, its oxygen atoms form a new hydrogen bond force with cellulose, thus replacing the original hydrogen bond network. Finally, water-soluble cellulose-NMMO hydrogen bonding complexes are formed [44]. The reason why cellulose is difficult to dissolve when the water content exceeds 15% can be explained by the coordination between water and the O atom of NMMO, which hinders the formation of the interaction between cellulose and NMMO [45]. Fig. 3 shows the corresponding mechanism of cellulose dissolution in NMMO:
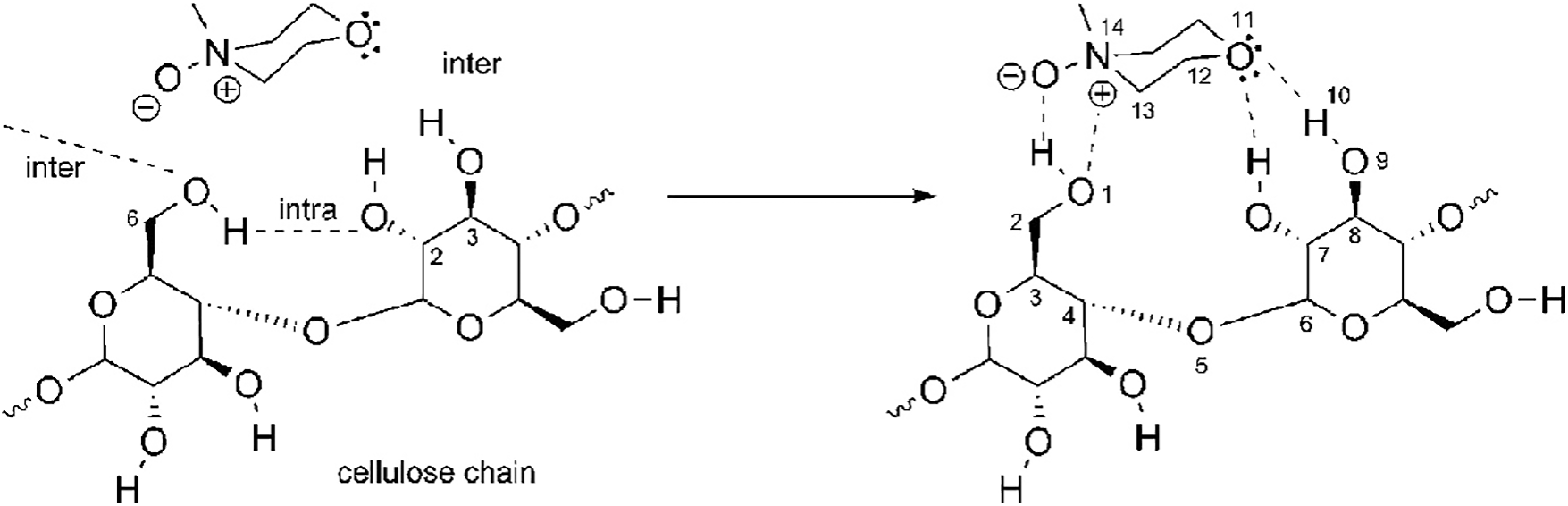
Figure 3: Possible hydrogen bond interaction between NMMO and cellulose. Adapted with permission from Reference [46], Copyright © 2010, American Chemistry Society
However, in actual production, NMMO is prone to degradation and oxidation side reactions due to its poor thermal stability and high N-O bond energy, which will lead to degradation and color change of the obtained cellulose products. Therefore, it is necessary to add stabilizer in the production to prevent the impact of side reactions. N-Methylmorpholine (NMM) and morpholine are common by-products of NMMO degradation. The main degradation mechanisms of NMMO are free radical reaction (main) and two heterodegradation processes (including Polonowski-type reaction and autocatalytic decomposition of NMMO catalyzed by Mannich intermediates). To prevent these side reactions, it is necessary to add the corresponding stabilizer. For the type of free radical reaction stabilizer, it is crucial to play the role of free radical scavenger and stabilize the subsequent products by forming stable free radicals, to achieve the blocking effect. For the heterodegradation reaction, the stabilizer needs to be captured after the formation of N-(methylene)morpholinium ions to remove the degradation catalyst, or by consuming formaldehyde so that the degradation catalyst cannot be formed [47]. Therefore, to eliminate the above three side reactions, the optimal stabilizer must be able to well capture the three reactive substances in the system: free radicals, formaldehyde, and N-(methylene)iminium ions.
3.4 Extraction of Cellulose Based on Ionic Liquid (ILs)
Ionic liquids are defined as molten salts that remain liquid at or below 100°C [48]. These ionic liquids have good thermal stability and do not release toxic gases. Therefore, ILs can be used not only for the design and synthesis of novel polymer ionic liquid-based biomaterials [49] but also for the extraction of cellulosic materials. However, ILs are highly absorbent when dissolving cellulose, so this solvent is difficult to recycle [50].
At present, ILs can be used to dissolve and deconstruct cellulose. The dissolution of cellulose is achieved through the interaction of anion and cation with cellulose. In terms of mechanism study, Swatloski et al. first proposed that the anions in ILs interact with the hydroxyl group of cellulose molecules, destroying the original hydrogen bond network [51], and the solubility of cellulose seems to follow its alkalinity [52]. Zhao et al. found that when the anions in ILs have the following characteristics: hydrogen bond acceptor with higher electron density (the order of interaction strength is Cl−> [CH3COO]− > [(CH3O)2PO2]− > [SCN]− > [PF6]−), shorter alkyl chain and no electron-withdrawing group, the dissolution capacity of ionic liquids will increase [53].
For cation, its role after anion. Zhang et al. [54] pointed out that cations can also form hydrogen bonds with cellulose, while Li et al. [55] believe that the van der Waals forces between cations and cellulose contribute to the formation of hydrogen bonds. In conclusion, the type of anion (such as imidazolyl or pyridyl) and the length of the alkyl chain have an important impact on the dissolution of cellulose by ILs [53]. Rahman et al. [56] used imidazolyl double salt ionic liquid (DSIL) to dissolve and extract cellulose. The relevant mechanism of dissolving cellulose is shown in Fig. 4 below.
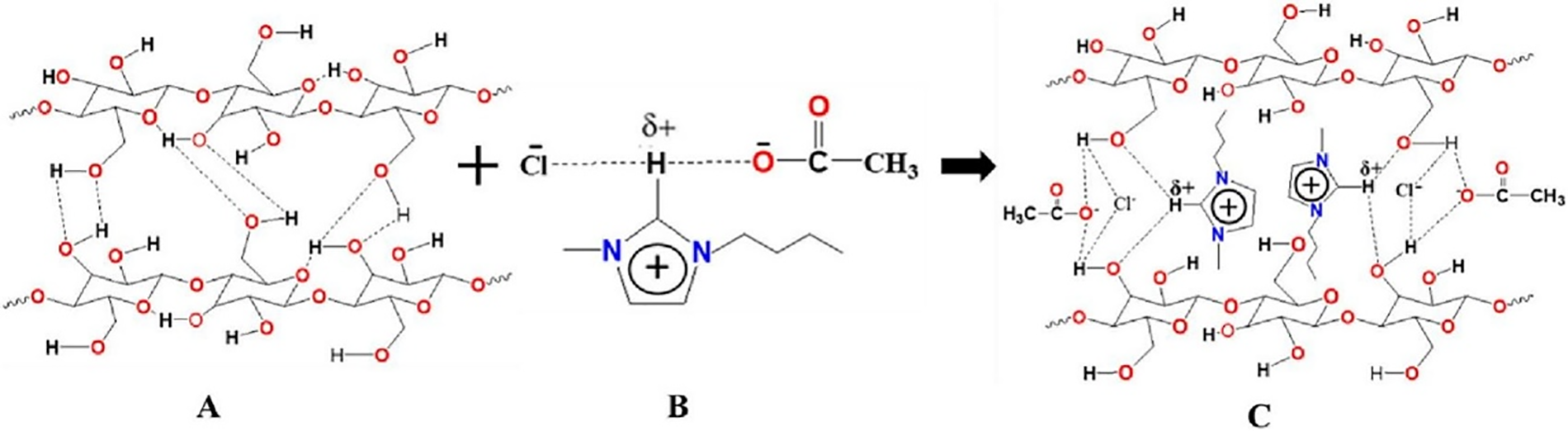
Figure 4: Mechanism diagram of cellulose dissolution by imidazole-based double salt ionic liquid (A–C). Adapted with permission from Reference [56], Copyright © 2023 Elsevier
Although ILs have good solubility, their high-water absorption has seriously affected its application in cellulose. This is because the presence of water competes with ILs and prevents cellulose from forming hydrogen bonds with ILs [51]. Therefore, dehydration is required when using the ILs system to dissolve cellulose. To make this technology widely used in the industrial production of cellulose, appropriate heating can be used to increase the dissolution rate [57]. However, when the method of heating is used to improve the dissolution rate, it is inevitable to consider the influence of excessive temperature on cellulose, and excessive heating will induce cellulose degradation [58]. Therefore, new methods are needed to improve the dissolution rate. It has been found that the addition of aprotic cosolvent to the system can help improve the dissolution ability of ILs. As for the dissolution mechanism, some researchers believe that the addition of cosolvent to the system can promote the solvation of cations in ILs, thereby releasing anions, to improve the interaction between anions and cellulose [57]. Other studies have shown that the main function of cosolvents is to promote the better dispersion and dissolution of cellulose in solvents by changing the mass transfer characteristics of the solution (such as ionic conductivity or viscosity) [59].
To reduce the cost of ionic liquids and minimize their impact on the environment, several technologies have been proposed to recover ionic liquids from different solutions. These methods include distillation, extraction, adsorption, membrane separation, aqueous two-phase extraction, crystallization, and external force field separation [50]. Although some single methods can achieve good recovery of ionic liquids, the efficiency of most methods is limited, which leads to low product purity and high cost and energy consumption. Therefore, in terms of recycling, how to optimize the recycling process is the focus of future research. The existing separation technology needs to be studied more deeply and comprehensively. It is also necessary to summarize and evaluate the selection and design of the recycling process.
3.5 Extraction of Cellulose by Deep Eutectic Solvent Method (DES)
Although ionic liquids have a stable chemical system, due to various factors such as high cost and negative environmental impacts, the new ionic liquid DES has a great advantage over ionic liquids in these aspects [60]. DES is mainly composed of hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA), which can be prepared by mixing by simple heating [61]. DES is utilized to extract cellulose mainly based on the DES system’s difficult solubility of cellulose [62] and the priority of lignin dissolved [63]. In this case, the dense hydrogen bond network of DES only led to the swelling between hydrogen bonds of cellulose, keeping the relatively complete structure of cellulose. At the same time, DES dissolves lignin, separating cellulose from lignin and degumming the original biomass [64]. In addition to being used to extract cellulose, DES can also be used to adjust the thermal behavior of heat-responsive polymer [65].
At present, there have been quite a lot of studies on the extraction of cellulose by DES, and most of the studies have focused on changing the composition of DES, adopting more efficient mechanical combined DES treatment to improve the treatment effect, and expanding the source of cellulose. In Table 1, the research achievements in this field in recent years are listed. Surprisingly, this carboxylic acid type of DES seems to have become the focus of current research.
This is because, in all kinds of DES, carboxylic acids DES promote further hydrolysis and carboxylation between cellulose due to the existence of active hydrogen ions [73]. Compared with other DES, carboxylate DES can pretreat cellulose more efficiently [74]. Liu et al. [75] applied different carboxylic acids DES to prepare CNF from pretreatment cellulose raw materials, combined with spiral extrusion and colloidal grinding and carried out esterification modification of cellulose nanofibers (CNF) at the same time. The results show that the combination of DES pretreatment with mechanical treatment can swell and esterify the cellulose material to produce CNF with a width of less than 100 nm.
In addition, DES pretreatment temperature plays an important role in the esterification modification and nano-fibrillation of cellulose. The esterification modification of cellulose prevented excessive hydrolysis and dissolution of cellulose during DES pretreatment, ensuring a high CNF yield of 72% to 88% and maintaining the cellulose I crystal structure. Ma et al. [76] explored a ternary DES system composed of chloroacetic acid, urea, and choline chloride to pretreat bamboo fiber and then grind it to prepare carboxymethylated modified cellulose nanofibers. This ternary DES pretreatment was modified while maintaining the crystal structure of cellulose. In addition, it also has a wetting and swelling effect on the fibers, resulting in the successful destruction of strong hydrogen bonds during mechanical treatment and the rapid production of cellulose nanofibril. Carboxymethylation enhanced the dispersion of CNF and increased the stability of the suspension. In addition, prolonging the duration of DES pretreatment can enhance the fibril fabrication process and reduce fibril polymerization. The produced CMCNF exhibits a high aspect ratio, with diameters ranging from 10 to 50 nm and lengths extending to several microns.
In addition, due to the stable physical and chemical characteristics of DES, DES has good recycling characteristics. By separating and purifying DES after pretreatment, it can be recycled and reused many times. Usually, the pretreated plant residue can be filtered to remove impurities such as lignin from the DES solvent using the antisolvent method or acid gradient method. Then the reaction system was purified by rotating evaporation [77], freeze-drying [78], electrodialysis [79], and membrane separation [80], and the obtained DES solvent could be recycled continuously. In a word, the main separation and purification ideas of DES can be divided into two categories, one is to remove impurities from the solvent, and the other is to transfer the solvent. At present, the evaluation of DES process performance is mainly based on recovery times and pretreatment efficiency. However, it is worth noting that the pretreatment efficiency of DES decreases with the increase in the number of recoveries because some plant fibers will be dissolved in DES during the reaction, occupying a part of the solvent space. In addition, these impurities also affect the hydrogen bond network of DES. As a result, its processing efficiency decreases [81].
3.6 Comparison of Solvent Systems
There are many methods to extract cellulose, and the yield of cellulose obtained by different extraction methods will also be affected. Therefore, to make a reasonable comparison, this paper tries to unify the raw materials and summarizes the advantages and disadvantages of five cellulose extraction methods by comparing them. As shown in Table 2.
The first four items are derived from wood pulp, and the last four are sourced from MCC. From example 1, it can be seen that different materials will lead to changes in cellulose yield. Compared with 1 and 5 in Table 2, the same NaOH system has a relatively large impact on cellulose yield with or without pretreatment. Based on the above examples, it can be roughly concluded that the ionic liquid system and DES system have more advantages in the extraction of cellulose. The traditional NaOH system requires material pretreatment to achieve good extraction efficiency. The dissolution capacity of the DMAc/LiCl system is limited, which is easily affected by the content of raw materials in the system. In addition, in terms of environmental problems, NaOH is easy to affect the environment due to the need for a large amount of alkali solution, while DMAc/LiCl system and NMMO system have certain impacts on human health and the environment due to toxic organic solvents. As for the ionic liquid and DES system, their solvents can be recycled and reused repeatedly, which has a good circular economic effect. It is worth noting that DES solvents are generally green and low toxic solvents, which are environmentally friendly and pollution-free.
In addition, by comparing the five systems, it can be found that there are some potential obstacles in the field of cellulose extraction, which will affect the promotion and application of cellulose materials. The main reason for these obstacles is that the sources of raw materials are different, and the content of cellulose contained in different raw materials will be different, which will more or less affect the extraction efficiency of cellulose. The different structures of raw materials (such as soft and hard plants) will lead to changes in process conditions, which will affect the cost. In addition, cellulose extracted from different plants using the same process will have certain differences in structure, size, surface topography, and other aspects, which will directly affect the performance of cellulose products. Therefore, it is critical to solve the batch stability problem of cellulose or bio-based materials.
4 Modification of Cellulose and the Derivatives
Due to the presence of a large number of free active hydroxyl groups at C-2, C-3, and C-6 on the surface cellulose structural units, strong hydrogen bonding exists between linear cellulose molecular chains. To make cellulose can be used efficiently, people have modified its structure to expand its application range. Since cellulose is composed of linear chains and has abundant reactive sites, it can be transformed into many cellulose derivatives, such as cellulose acetate, carboxymethyl cellulose, hydroxypropyl methylcellulose, etc., by surface modification with small or large molecular reagents. Cellulose nanofibers (CNF) and cellulose nanocrystals (CNC) with smaller structural scales can be obtained by mechanical treatment or chemical acid and enzyme treatment, which can meet the application of cellulose in various related fields. The cellulose-related modification process is shown in Fig. 5.
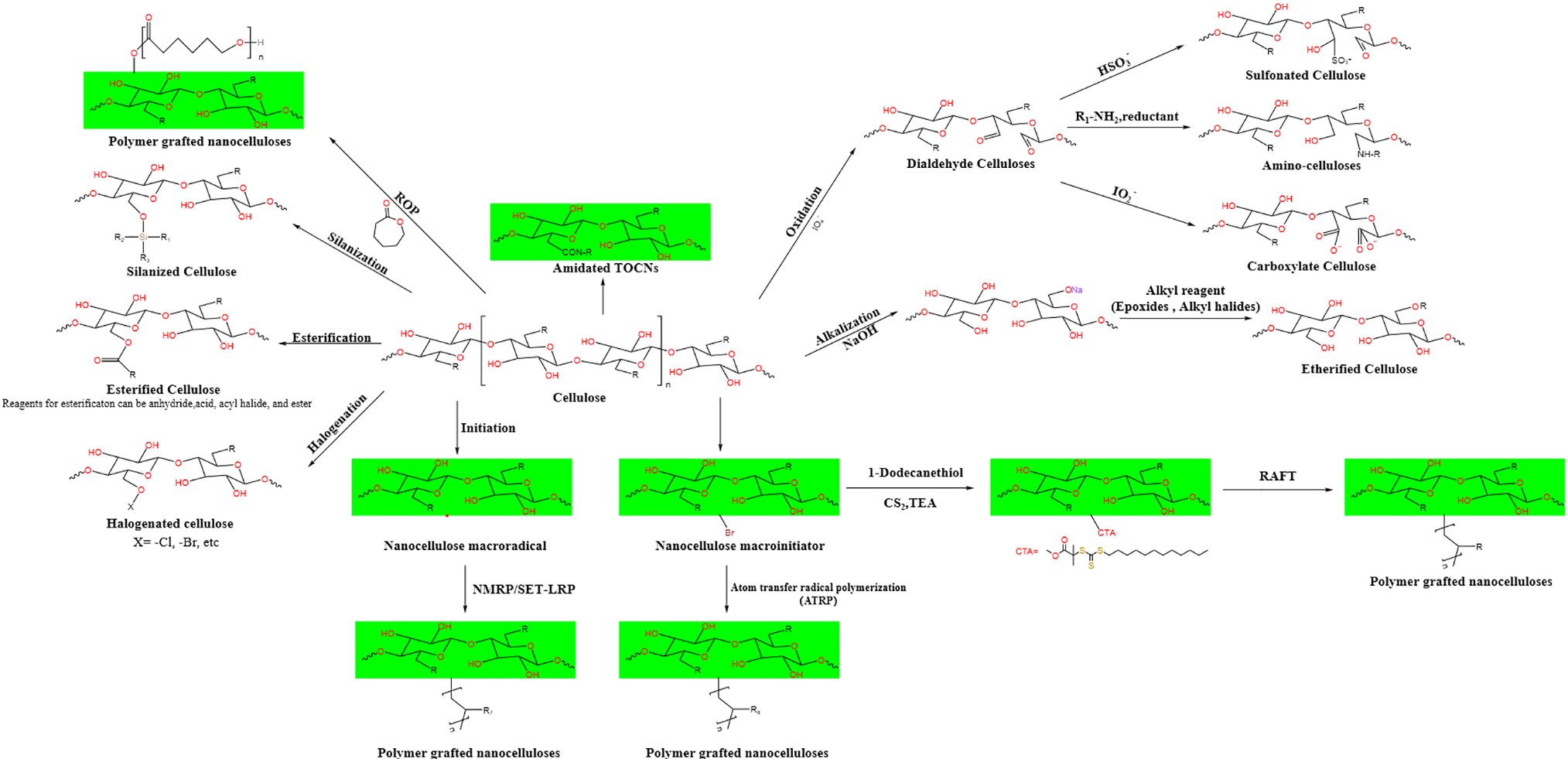
Figure 5: Modification process of cellulose (including molecule modification and macromolecule modification)
Regardless of the source of cellulose, only two forms of nanoscale cellulose exist after the cellulose is dissolved and extracted. The first is a semi-crystalline type of fiber with a high aspect ratio and flexibility, known as cellulose nanofibers or nanofibril (CNF). The other is the rigid fibril with high crystallization and low aspect ratio, which is obtained by destroying the amorphous region of cellulose, and is called cellulose nanocrystalline whiskers or nanorods or nanocrystals (CNC). In general, the composition of fibrils includes crystalline regions and amorphous regions. The fibrils and fibril bundles extracted by solvent are collectively called CNF. The crystalline part extracted from the fiber is called CNC [89].
4.1.1 Cellulose Nanofibers (CNF)
The so-called cellulose nanofibers (CNF) are materials with a semi-crystalline structure, usually with a diameter of 1 to 100 nm and a length of approximately 500 nm or more. Generally, CNF can be obtained in two approaches: the physical method and the chemical method.
Physical Method
The physical method generally allows CNF to be extracted longitudinally from the pretreated fibers through mechanical shear forces. The common physical methods for extracting CNF include steam blasting [90], high-pressure homogenization [91], microfluidization [92], ball milling [93], grinding [94], etc. These processes have in common the use of strong mechanical forces, such as shear forces, to break the hydrogen bond network and hydrolyze the glycosidic bonds in cellulose to produce CNF materials. To obtain CNF more efficiently, chemical or biological enzyme pretreatment can be carried out before mechanical treatment, which makes the structure of cellulose loose and reduces the hydrogen bond force, which is helpful for further treatment to obtain CNF. Liu et al. [95] combined a high-pressure homogenization method with ultrasonic assistance to obtain CNF from potato residue. According to the experiment, the yield of CNF is the highest (19.81%) when the ultrasonic power is 125 W for 15 min and the high-pressure homogenization treatment is 40 MPa for 4 times. However, the shortcoming of this method is that the crystallinity of CNF will be reduced.
Chemical Method
In the chemical treatment of cellulose to obtain CNF, the most common methods are TEMPO(2,2,6,6-tetramethylpethidine-1-oxyradical) reagent guided oxidation, and carboxymethyl method [96]. By modifying the surface of cellulose, this method brings charge, which helps the cellulose to undergo electrostatic repulsion and realize the expansion of the cell wall, thus promoting the generation of CNF. In general, CNF oxidized by TEMPO can be written as TOCNs for distinction. Huang et al. [97] used office waste paper as raw material and adopted the TEMPO oxidation method to prepare CNF materials. Before preparation, three different pretreatment methods (low acid treatment, alkali treatment, and bleaching treatment) were used to treat raw materials to explore the influence of different pretreatment methods on the preparation of CNF. The experimental results show that: (1) Using low acid treatment (1% sulfuric acid solution) to treat the office wastepaper can remove part of the hemicellulose and lignin, to expose more cellulose. This process will help to follow up the TEMPO oxidation process, making cellulose more effective in the preparation of nanofibers (CNF). In addition, the crystallinity of CNFs (WCNF1) treated with low acid was increased, which helped to improve the mechanical properties and tensile strength of CNFs. (2) The alkali treatment is by using a 12% sodium hydroxide solution to deal with office wastepaper, the purpose is to further remove impurities such as lignin and ink so that more pure cellulose. After alkali treatment, CNFs (WCNF2) showed a relatively concentrated size distribution in particle size distribution, which helped improve the uniformity and dispersion stability of CNF. (3) Bleaching was performed by using the D0(EP)D1 bleaching sequence (where D represents the chlorine dioxide stage and (EP) represents the hydrogen peroxide enhanced alkali extraction stage) to selectively remove lignin and improve the purity of cellulose. The bleaching treatment of CNF showed higher crystallinity, which in turn enhanced the optical properties of CNF and increased its transmission in the visible light range.
Besides the TEMPO oxidation process for CNF, a deep eutectic solvent system (DES) is also available on CNF. Due to the dense hydrogen bond network of DES, the long chain of cellulose will expand, and the hydrogen bond within the amorphous regions of cellulose will be destroyed to promote fiber liberation [98]. This results in DES still needing mechanical treatment, such as ultrasonic crushing, high-pressure homogenization, ball milling, extruder extrusion, etc., to further refine the cellulose after the treatment of cellulose. Yu et al. [99] prepared CNF by treating ramie fiber (RF) with a choline chlorine-urea (CU)DES system and ball milling technology. The CNF yield reached 94.06%, the crystallinity reached 66.51%, and the thermal stability was
4.1.2 Cellulose Nanocrystals (CNC)
Cellulose nanocrystalline (CNC), a rod-like nanomaterial, can be extracted from lignocellulose. By removing the amorphous areas in the CNC, the degree of crystallinity can be enhanced. In general, the diameter of CNC is 3 to 50 nm, and the length is 50 to 500 nm. Compared with CNF, CNC has a lower aspect ratio and a higher crystallinity. To be able to prepare CNC, it is generally necessary to remove lignin and hemicellulose in cellulose to obtain CNC with high cellulose content. The usual pretreatment methods mainly include physical, chemical, and biological pretreatment.
Physical Method
The first physical pretreatment method involves the use of mechanical shearing forces to disrupt the cellulose cell walls, thereby transforming cellulose from its fibrillar state to a nano-structured form. Wet disk milling (WDM) can refine biomass into bent microfibers through high-speed rotation, and then obtain CNC materials through hydrolysis [100]. Another common method is the ultrasonic method, which uses high-frequency oscillations to separate cellulose fibers. Generally, ultrasonic (>20 kHz) acting under the liquid, using the alternating change of low pressure and high-pressure waves to achieve the generation and rupture of small vacuum bubbles. With powerful mechanical oscillation power, high-intensity waves are generated, which contribute to the formation, expansion, and implosion of microscopic bubbles as molecules absorb ultrasonic energy. This treatment will produce a strong hydrodynamic shear force, which can destroy the hydrogen bond force between cellulose molecules, thus achieving the destruction of the fiber cell wall. Under the action of low-concentration acid, the destruction and hydrolysis of amorphous region can be realized, to prepare CNC. Gui et al. [101] successfully produced CNC with microcrystalline cellulose as the basic raw material under the combined action of ultrasonic and phosphotungstic acid (PTA). Compared with the traditional sulfuric acid method, the crystallinity of the CNC prepared by this method can reach 86.93% under the condition of 20°C, and the required acid concentration is lower (13.7%), which has little damage to the CNC. Zianor Azrina et al. [102] used empty fruit bunch pulp (EFBP) as raw material to obtain CNC through ultrasonic-assisted acid hydrolysis. It is worth noting that, compared with the raw empty fruit bunch fiber (REFB), the CNC prepared from EFBP as raw material has a spherical structure, and the spherical CNC has higher crystallinity and better thermal stability.
Chemical Method
Acid hydrolysis is generally used to prepare cellulose nanocrystals by chemical methods. In the process of acid hydrolysis, the surface properties of CNC are also different according to the types of inorganic acids. By hydrochloric acid hydrolysis method of CNC surface contains a small amount of negative charge, so prone to reunite phenomenon between CNC particles [103]. However, the surface of CNC prepared by hydrolysis with sulfuric acid will react with sulfuric acid to modify the sulfate group, thus providing a large amount of negative charge on the surface of CNC [104]. Due to the strong mutual repulsion between the charges, the CNC suspension prepared by the sulfuric acid method has strong colloidal stability.
Unfortunately, the use of large amounts of acid can lead to inevitable damage to the environment. In recent years, researchers have also tried to use DES to prepare nanocellulose. As with the preparation of CNF, this mild treatment method cannot directly prepare CNC but requires a combination of different mechanical or chemical treatments. Zhang et al. [88] proposed and evaluated a method for preparing CNC by combining hydrated DES with a mechanical high-shear force method. When the processing time was increased, the CNC output increased from 27.2% (0.5 h) to 65.2% (2.5 h). The average diameter of the CNC is 25.1–33.3 nm, and the length ranges from 281.3 to 404.2 nm. Wang et al. [105] reported the preparation of CNC from cotton and other biomass feedstocks using DES and high-pressure homogenization (HPH). The diameter range of the CNC is 50–100 nm and the length range is 500–800 nm. The obtained CNC remained stable even after one month of storage. It is a good choice to use carboxylic acid DES to prepare CNC, and the processed cellulose can directly obtain CNC without post-processing [106]. Zhu et al. [107] prepared CNC by using purple potato peel as raw material by ultrasound-assisted maleic acid hydrolysis of cellulose. After acid hydrolysis, the CNC retains the cellulose type I structure and carries out esterification modification on the CNC surface.
Biological Method
Biological methods generally involve the separation of cellulose structures using cellulase. This method mainly involves the conversion of the carbonyl group to the carboxyl group and the subsequent modification by the sulfate group in the hydrolysis process with the acid. This helps to increase the charge density of the CNC surface, thus achieving a stable dispersion of the CNC suspension. The combination of biological methods with chemical methods can reduce the acid concentration required for the hydrolysis of CNC in subsequent chemical methods. In addition, biological methods can be combined with physical methods and chemical methods to achieve efficient preparation of CNC materials. Cui et al. [108] used wheat microcrystalline cellulose as raw material to prepare CNC by combining ultrasonic-assisted and enzymatic hydrolysis. Under the condition of 120 h of enzymatic hydrolysis and 10 times of ultrasonic treatment for 1 h, the maximum yield of CNC can reach 22.57%. In addition, without ultrasonic treatment, the yield of the CNC was reduced by 6.81%. Ren et al. [109] used MCC as raw material and carried out enzymatic hydrolysis in the way of complex enzymes to prepare spherical CNC. Spherical CNC with a particle size of 24~76 nm can be extracted under the condition of lye (pH = 9) and centrifugation speed of 3000 rpm, and then washed and purified three times under the condition of pH = 4 acid, pure spherical CNC materials can be obtained. The crystal structure of the spherical CNC material is cellulose Iβ type, but the degree of crystallization is low.
Hydrophilic modification:
2,2,6,6-tetramethylpethidine-1-oxyradical (TEMPO) is a good agent for promoting the separation of nanocelluloses, which selectively introduces carboxyl groups at the glucose unit C-6. In this process, an additional catalyst (NaBr), and an initial oxidizer (NaClO) are generally added at a PH of 9 to 11. In addition to the carboxylation modification of cellulose by the TEMPO oxidation method, the carboxylation treatment of cellulose can also be realized by using a periodate-sodium chlorite system to oxidize cellulose, and the conversion of cellulose secondary alcohol to carboxyl group can be realized [110]. Liimatainen et al. [110] used a periodate-sodium chlorite system to oxidize cellulose. In this process, secondary alcohol was first oxidized to the aldehyde group by periodate and then oxidized to the carboxyl group by sodium chlorite, and the oxidation process is shown in Fig. 6. The degree of nano fibrillation of hardwood cellulosic pulp was enhanced by applying a periodate-chlorite system for region-selective oxidation, coupled with mechanical homogenization treatment.
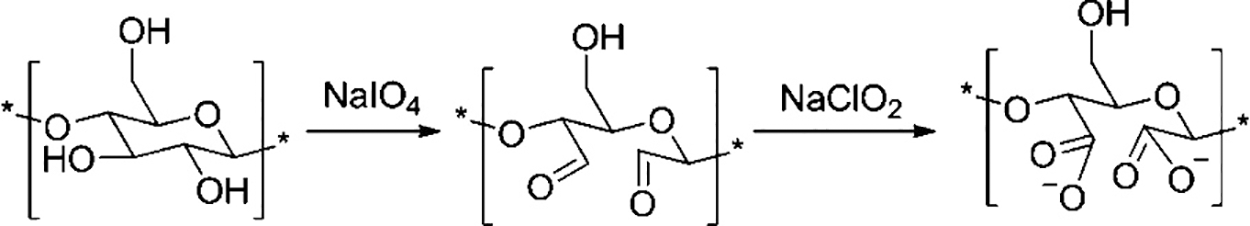
Figure 6: Oxidation of cellulose with periodate and chlorite. Adapted with permission from References [110], Copyright © 2012 American Chemistry Society
Zhou et al. [111] prepared carboxylate cellulose nanocrystals (CNCs-COOH) using potassium permanganate and oxalic acid as oxidant and reducing agent, respectively, with the reaction temperature of 50°C and the concentration of sulfuric acid of 1wt% from paper pulp. Compared with Tempo oxidation, the amount of oxidant is greatly reduced, and the yield can reach 68.0%. Ammonium persulfate (APS) can also carboxylate cellulose materials, but the yield of this method is low in industry. Therefore, Liu et al. [112] improved the APS method. In this paper, a method was proposed to optimize the preparation process of cellulose carboxylate nanocrystals by APS using the activation of N, N, N’, N’-tetramethylethylenediamine (TMEDA) and ultrasonic assistance. The yield of carboxylate cellulose nanocrystals prepared by this method can reach 62.5%, and the crystallinity of carboxylate cellulose nanocrystals prepared by this method is high (93%), and the content of carboxylic acid groups is also high (1.45 mmol/g).
The sulfonation reaction is one of the methods to attach negative groups to the surface of cellulose. The sulfonation reagent is combined with the hydroxyl group on the surface of cellulose to achieve surface sulfonation modification. Thiangtham et al. [113] first oxidized MCC with sodium periodate to obtain dialdehyde cellulose, and then sulfonated dialdehyde cellulose with K2S2O5 to prepare sulfonated cellulose (SC). Through the experimental characterization test, it was found that when the content of the sulfonic acid group was 528~689 μmol/g, the water solubility of SC was improved, and the transmittance of SC could reach 80% in the wavelength range of 400~800 nm. Mayer et al. [114] to create sodium 4-((4,6-dichloro-1,3,5-triazin-2-yl)amino)benzenesulfonate (SDTAB). The reagent was mixed with sodium sulfate and the target cellulose for 30 min, and then sodium carbonate was added for one hour. Finally, the cellulose with the sulfonic acid group was prepared by rotating under the shaker for 30 min at a speed of 80 revolutions per minute, which established the basis for subsequent conductive modification through interface interaction between ionic bonds and conductive polymers. After extracting MCC from bagasse, Kanbua et al. [115] carried out sulfonation modification of cellulose by gamma-ray and potassium sulfite successively. The increase of γ radiation will increase the oxidation degree of cellulose. The prepared sulfonated cellulose (SC) can be used as filler to fill the polyether block amide/polyethylene glycol diacrylate (PEBAX/PEGDA) polymer matrix, thereby improving the electrolyte affinity and thermal stability of the composite film.
Etherified cellulose is prepared by homogeneous reaction with alkyl reagents such as epoxides and alkyl halides under alkaline conditions [116]. At present, ether cellulose such as methylcellulose (MC), carboxymethyl cellulose (CMC), hydroxyethyl cellulose (HEC), hydroxypropyl methylcellulose (HPC), and carboxyethyl cellulose (CEC) have been developed.
Etherified cellulose can be classified as single ether and mixed ether according to the substitution situation. The single ether can be subdivided into alkyl ether (such as ethyl cellulose, propyl cellulose, etc.), hydroxyalkyl ether (such as hydroxymethyl cellulose, hydroxyethyl cellulose, etc.), and carboxyalkyl ether (such as carboxymethyl cellulose, carboxyethyl cellulose, etc.) three large pieces.
In addition, it can be divided into four categories according to the types of substituted ionic groups: non-ionic cellulose ethers, anionic cellulose ethers, cationic cellulose ethers, and amphoteric cellulose ethers (that is, both cationic and anionic groups). Among them, cellulose ether with quaternary ammonium cationic group has good flocculation and decolorization efficiency and has been widely used. Aguado et al. [117] prepared water-soluble cationic cellulose derivatives (WSCC) by cationizing cellulose with (3-chloro-2-hydroxypropyl) trimethyl ammonium chloride. This material is usually used as a dynamic coating material for capillary, which can effectively inhibit the adsorption of basic protein on the tube wall and improve the separation efficiency of basic protein. Matsumoto et al. [118] introduced amyl ether on the side chain of ethyl cellulose (EC). The degree of substitution of amyl ether is affected by the concentration of solvent and base in the process. The EC modified by side chain etherification can be dissolved in methacrylic acid (MAA), and liquid crystal with cholesteric photonic crystal structure (CLC) can be prepared.
In addition, under alkaline conditions, due to the different positions of the three hydroxyl groups of cellulose in the glucose group, they are affected by the neighboring substituents and the spatial obstruction. The dissociation degree of the three hydroxyl groups is C-2 > C-3 > C-6. Therefore, when etherification is carried out under alkaline conditions, the reaction order is that the hydroxyl group on C-2 is the first reaction, followed by C-3, and finally C-6, while esterification under acidic conditions is the opposite.
Hydrophobic modification:
Esterification of hydroxyl groups on cellulose chains with acids, acyl halides, and anhydrides is used to make esters, including aliphatic and aromatic [119]. Generally, this type of cellulose can be divided into inorganic and organic acid esters. The so-called inorganic acid ester refers to the product obtained by esterification of the hydroxyl group in the cellulose molecular chain with the inorganic acid. Among them, cellulose nitrate and cellulose sulfate are widely used. By adjusting the proportion of the mixture of sulfuric acid and nitric acid cellulose nitrate processing.
Among the inorganic ester types, cellulose nitrate esters can be divided into fire cotton and gum cotton based on the amount of nitrogen content. Because of its high nitrogen content, fire cotton is usually used to make smokeless gunpowder, while rubber cotton is low in nitrogen content and is usually used in artificial leather, etc. As for sulfate, because of its low price and degradable, it is widely used as a drilling fluid treatment agent in the petrochemical field. In the field of industrial coatings, it can be used as a thickening agent in the preparation process.
In organic acid esters, the preparation idea is to generate the hydroxyl group in the cellulose molecular chain by reacting with organic acids, acyl halides, or acid anhydrides, mainly including cellulose formats, acetic esters, and so on. For example, the traditional transesterification reaction occurs under heterogeneous conditions, when the catalyst is an inorganic base, it needs to react at high temperatures for a long time, and the reaction is insufficient. Therefore, preprocessing is required to solve the problem. Chen et al. [120] pretreated cellulose with CO2/DBU/DMSO to induce transesterification between cellulose and vinyl ester, increasing the degree of substitution of cellulose to 0.58~3.0. The transesterification reaction conditions are relatively mild, without adding a catalyst, the reaction degree is relatively sufficient, and the steps are simple, clean, and pollution-free. It is worth noting that the physical and chemical properties of organic acid esters will be changed by the degree of substitution. Physical properties such as strength, melting point, density, and hygroscopic properties decrease as the molecular weight of the substituent increases. In addition, organic acid groups also have great effects on its performance characteristics. The esterified cellulose derivatives produced by esterification reactions can be used in the chemical industry and other fields.
Using different types of reagents (such as acetyl chloride, acetic anhydride, and vinyl acetate) can realize the acetylation of cellulose. These reagents have solubilization effects on cellulose and can even be used as organic catalysts for the reaction. Gao et al. [121] used
Besides cellulose can react with isocyanate and can achieve ammonia formylation, preparation of ammonia by formylation cellulose can be expected to replace traditional esterification agents. Siqueira et al. [122] used octadecyl isocyanate to modify the surface of CNC and CNF and then compared the effects of the two modified cellulose on the thermal and mechanical properties of polycaprolactone composites. The results showed that the substitution degree of carbamylated CNC and CNF reached 0.07 and 0.09, respectively. Since the incorporation of isocyanate improves the dispersion of CNC and CNF in organic solvents, the final properties of the composites are enhanced. The study by Beaumont [123] studied the solid-phase reaction between cellulose and N-acetylimidazole under different moisture conditions (anhydrous, 7%, 20%, and 30%). When the water content of cellulose is 7%, the acetylation reaction is the most active. They believed that the presence of water promoted the diffusion of reactants through the hydration layer of cellulose, and the imidazole generated during the reaction played the role of an alkaline catalyst, realizing the autocatalytic effect. By using nuclear magnetic resonance technology, they explored the effect of confined water on the chemical activity of hydroxyl groups on cellulose surface, revealing that changes in water content can regulate the reactivity of different hydroxyl groups, and then control the regional selectivity of acetylation on cellulose surface. The team hypothesized that the localized water facilitates the cellulose surface acetylation because it facilitates the proton transfer process from the cellulose acyl imidazolium intermediate to the imidazole base catalyst. In addition, the surface wettability of cellulose after acetylation changed significantly, and the contact Angle of water increased from 12° to 134°, indicating that its hydrophobicity was significantly enhanced.
The so-called amino cellulose (AC) is a chemical reaction that converts the hydroxyl group on cellulose into a derivative of the amino group. The replacement of the hydroxyl group on the molecular chain with amino groups results in the destruction of the inherent crystalline structure of cellulose. Consequently, amino cellulose exhibits enhanced water solubility, biocompatibility, and degradability. In the actual production situation, the hydroxyl group on cellulose is difficult to directly ammoniate, so the common way to produce amino cellulose is to synthesize active intermediates to prepare amino cellulose. There are four methods to synthesize amino cellulose by active intermediate: p-toluene sulfonic acid method, halogenated cellulose method, cyanoethyl cellulose method, and oxidized cellulose method.
(1) P-toluene sulfonic acid method:
The active intermediate was obtained by the reaction of p-toluenesulfonyl chloride with cellulose. Then, with the p-toluenesulfonic acid group as the leaving group, a suitable nucleophilic reagent was selected to carry out the substitution reaction, and the amino group was attached to the cellulose to obtain 6-deoxyaminocellulose. Heinze et al. [124] synthesized cellulose p-toluene sulfonyl chloride by esterifying cellulose with p-toluenesulfonyl chloride in the DMAc/LiCl system. The resulting intermediates are dispersed in ethylenediamine and heated to dissolve gradually, resulting in 6-deoxy-6 -(2-amino-alkyl) amino cellulose derivatives. The method has good solvent recovery ability and is environmentally friendly and harmless. This method involves the direct reaction of amino compounds with p-toluenesulfonate, which is simple and convenient. However, it is challenging to achieve complete replacement of the p-toluenesulfonate group. Zarth et al. [125] prepared amino cellulose hydrochloride in the presence of human p-toluenesulfonic acid chloride, using lactam as a nucleophile. By changing the reaction solvent conditions, the degree of substitution of amino cellulose can be controlled (between 0.24 and 1.17).
The preparation of amino cellulose by using p-toluenesulfonate as an intermediate is the most common method at present, which has the advantage of a quick and convenient reaction. However, when the intermediate is replaced, many p-toluenesulfonate ions are produced, which will be combined with the amino group, so the resulting product is generally amino-cellulose p-toluenesulfonate.
(2) Halogenated cellulose method:
The halogenated cellulose method is to synthesize halogenated cellulose intermediates and then convert them into amino cellulose. Deng et al. [126] prepared chlorinated cellulose intermediates by reacting cellulose with sulfoxide chloride. Amino-cellulose (ADC) was prepared by dissolving the intermediate in DMSO and adding ethylenediamine for an amination reaction. Gao et al. [127] prepared 6-amino-6-deoxy cellulose by using cellulose bromide as the base material through an aside reduction reaction. The results showed that the azide group was almost completely reduced and the degree of amino substitution reached 0.97 when DMSO was used as the solvent and reacted with 20 times excessive NaBH4 at 115°C for 7 h.
The halogenated cellulose method uses sulfoxide chloride to activate the C-6 position on cellulose, which makes the reaction selective. At the same time, the chlorine atom has a good departure, so that the intermediate can be replaced by an amino group so that the amino cellulose derivatives with relatively high purity can be obtained. However, sulfoxide chloride is toxic and easily decomposed, so the development of the chlorinated cellulose method is limited.
(3) Cyanoethyl cellulose method:
In the early stage, it was proposed that cyanoethyl cellulose could be prepared by reacting cellulose with acrylonitrile under alkaline conditions. Hassan et al. [128] pointed out that cyanoethyl cellulose was reduced by BH3 to the amino group and p-aminopropyl cellulose was prepared. It was found that the nitrogen content of aminopropyl cellulose was 12.43%, indicating that the hydroxyl group on cellulose was almost completely replaced. However, this method can only synthesize aminopropyl cellulose, resulting in a relatively simple type of amino cellulose synthesized.
(4) Oxidized cellulose method:
The main idea of this method is that aldehyde cellulose is used as an intermediate, and ethylene diamine is added to react with the intermediate to obtain the Schiff base. On this basis, it is further reduced with sodium borohydride to obtain amino ethylamine deoxy cellulose. The synthesis of aldehyde cellulose intermediates is based on the ring-opening reaction of periodate salts (such as sodium periodate NaIO4) on cellulose, which oxidizes C-2 and C-3 on glucose units to aldehyde groups, thus obtaining dialdehyde cellulose intermediates [129]. Dash et al. [130] used sodium periodate to oxidize cellulose to dialdehyde cellulose, and then grafted methylamine and butylamine on dialdehyde cellulose by Schiff base reaction to prepare amino cellulose derivatives. Su et al. [131] firstly oxidized cellulose with sodium periodate to obtain dialdehyde cellulose by using the above ideas. Subsequently, the DAC undergoes dynamic crosslinking with long-chain vegetable oil diamine (Schiff base reaction) at ambient temperature. This process entails partial replacement of the hydrogen bond network within the cellulose with a dynamic imine crosslinking network. This method significantly improves the stress relaxation ability and free movement ability of cellulose molecular chains and produces cellulose-based plastics with excellent thermoplastic processing. Its mechanism is shown in Fig. 7.
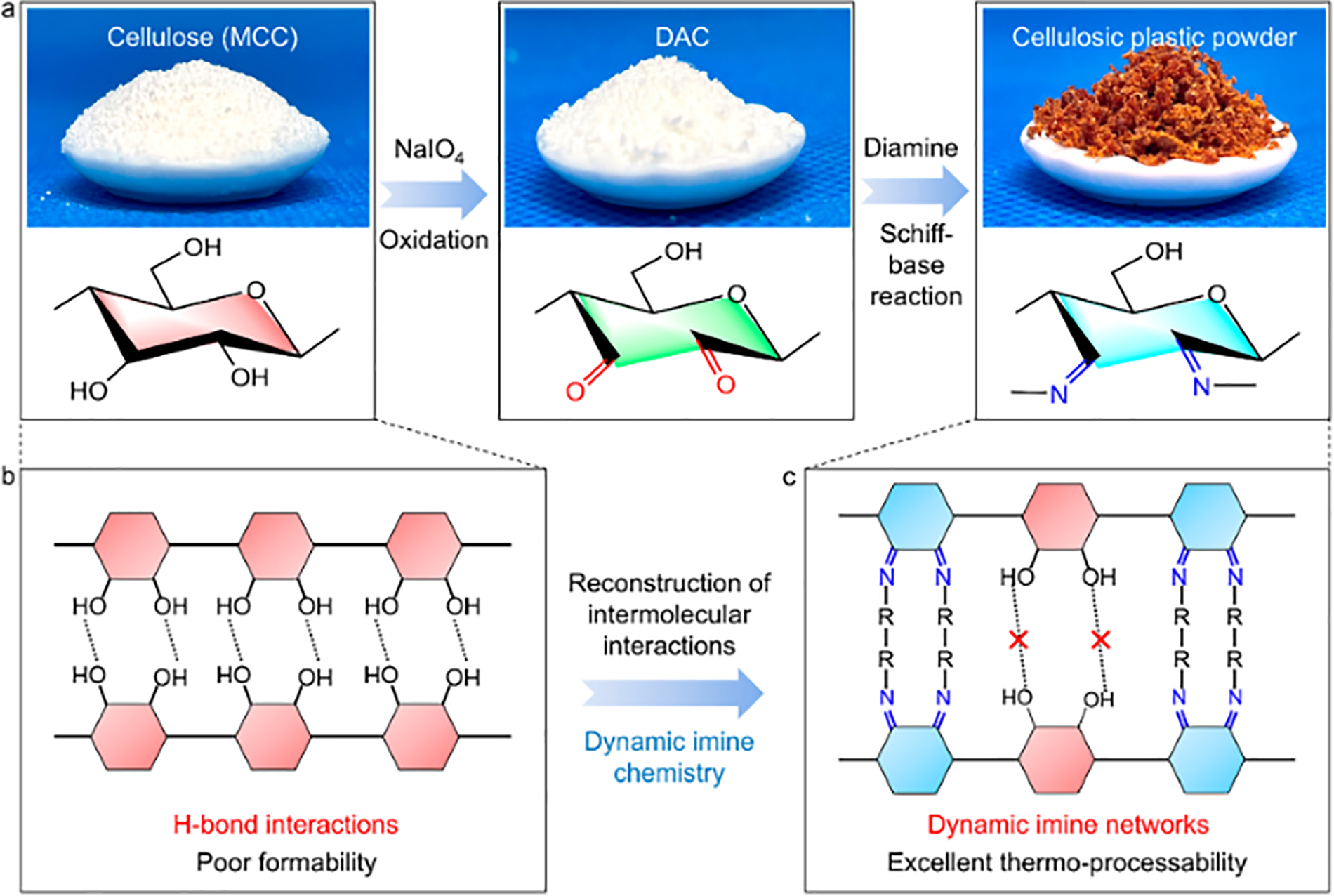
Figure 7: (a) Flow chart of cellulose plastic powder preparation; (b) and (c) Diagrams of reconstructing cellulose hydrogen bond networks using dynamic imine covalent bonds. Adapted with permission from Reference [131], Copyright © 2023 American Chemistry Society
Lasseuguette [132] used Tempo, sodium hypochlorite, and sodium bromide to selectively oxidize primary alcohols on cellulose into carboxyl groups under the condition of water as a solvent. Amino cellulose derivatives were then prepared by coupling the amine derivatives with oxidized cellulose by adding carbodiimide (catalyst) and hydroxysuccimide (amidation agent).
Silanization modification of cellulose is a method of surface hydrophobic modification. Generally, Si-OR does not react with the cellulose hydroxyl group but can react with the lignin hydroxyl group at high temperatures without water. Under high temperatures, when water is introduced into the system, it can trigger a reaction between the silanol and hydroxyl groups that promote cellulose. By adding a silane coupling agent to cellulose suspension for mixing reaction, silyl groups can be introduced on cellulose surfaces.
The commonly used silane coupling agents are alkyl alkyloxysilane, vinyltriethoxysilane, etc. The reason for choosing this type of coupling agent is that the presence of hydrocarbon chains in silane enhances the wettability of the fiber, which can pave the way for subsequent chemical reactions. The reason for choosing this type of coupling agent is that the presence of hydrocarbon chains in the silane enhances the wettability of the fibers, paving the way for subsequent chemical reactions. Wang et al. [133] used two different organ silane coupling agents to functionalize and modify the CNF suspension before spray drying and applied 1 wt.%, 3 wt.%, and 5 wt.% organ silane solutions according to the gradient design and the solid content of CNF, respectively. After modification, Wang et al. determined the surface morphology and properties of the modified CNF. The experimental results show that there is agglomeration behavior between large particles and small rectangular particles in the system, and there is a local difference in CNF before and after treatment. By connecting different functional groups on the surface of CNF, not only can the acid-base properties be changed, but also the surface energy of CNF can be reduced. Pacaphol et al. [134] studied the effects of different silyl groups (amino group, epoxy group, and methacryloxy group) on the adhesion properties of nanocellulose films on glass and aluminum substrates. The experimental results show that amino silanized nanocellulose is better in bonding properties. In terms of elasticity, the performance of epoxy silanized nanocellulose is more significant. Amino silanized cellulose showed the worst performance in elongation and crack resistance. In the transmission of light, all below the glass, the aspect of hydrophobicity is slightly higher than that of pure aluminum.
Halogenated cellulose refers to the halogenated modification of cellulose by reacting with hydroxyl groups on the surface of cellulose. Chlorine stands as the most prevalent halogen in polymers, with the option to introduce halogen atoms through the selection of distinct chemical feedstocks as precursors. Due to the reactivity between hydroxyl groups and the diversity of commonly used chlorinating agents, the chlorination selectivity of cellulose regions is affected. Moreover, the halogenation of cellulose provides a precursor for subsequent nucleophilic substitution reactions, so that cellulose can be incorporated into new functional groups. Gao et al. [135] prepared 6-chloro-6-deoxycellulose ester and its derivatives by halogenation reaction. The primary alcohol group of cellulose acetate with a substitution degree of 1.75 was selected to react with methylsulfonyl chloride for quantitative chlorination to prepare 6-chloro-6 deoxy cellulose acetate. The experimental results show that chemical and regionally selective chlorination occurs at C-6. In addition, the study also showed that cellulose chloride acetate is a useful intermediate for the preparation of functional cellulose ester derivatives by substitution reaction with nucleophiles such as sodium azide, amine, and mercaptan. Du et al. [136] developed a new type of cellulose paper by impregnating nanocellulose paper (CNP) in CS and then impregnating CNP/CS in 0.6 wt% NaClO aqueous solution to achieve simple acyl chlorination modification to obtain CNP/CS-Cl. The CNP/CS-Cl had a good bactericidal effect on Staphylococcus aureus and Escherichia coli (bactericidal rate reached 100% within 10 min). Therefore, the new material has potential application value in food packaging.
In addition to chlorine, the modification of cellulose by fluorine has also attracted attention. Khanjani et al. [137] used 2H,2H,3H,3H-perfluorononanoyl chloride and 2H,2H,3H,3H-perfluorodecanoyl chloride to perform regional fluorine-containing modification of CNC. Then nano spherical cellulose fluoride ester dispersion was prepared by nanoprecipitation method. Finally, the superhydrophobic paper is prepared by coating the dispersion in the paper by the spinning coating method. The superhydrophobic paper has good hydrophobic properties, which the contact angles are all greater than 150°. Therefore, it has potential application value in the field of packaging.
4.3 Macromolecular Modification
Although the surface structure and properties of cellulose can be well changed by molecule modification, the molecular weight and structure on the main chain do not change much. Therefore, molecule modification has little effect on other properties of cellulose, such as mechanical properties. Macromolecular modification is a new method to change the physical and chemical properties of cellulose. Under the premise of maintaining the original properties of cellulose, the introduction of macromolecules can give cellulose new and unique properties. In the modification of macromolecules, grafting copolymerization is the most used method. By grafting other designed polymers on cellulose macromolecules, unique properties can be conferred to form functionalized cellulose materials.
Three methods are commonly used to synthesize grafted cellulose, namely “grafting from”, “grafting onto” and “grafting through” [138]. “Grafting from” refers to the polymerization and growth of other monomers on the active site of the polymer backbone by the initiator according to its own active reaction point, to form a new graft polymer. “Grafting onto” means that the active groups are present on the two different polymers. When the third substance is added, a new covalent bond can be formed with the active groups on the other two polymers to form a new grafting polymer. “Grafting through” means because the owner chain polymerization is available for reaction activity of double bond. Consequently, it can act as a polymerization monomer, facilitating copolymerization.
In the “grafting from” method, the reaction site on the backbone can be directly initiated by chemical treatment or irradiation, and then the monomer is added to generate the graft copolymer. Although the grafting density of this method is high, there are also many shortcomings, such as the molecular weight of the graft polymer cannot be determined, the molecular weight of the graft polymer is low, and the homopolymer exists in the polymerization system. Therefore, the above shortcomings can be overcome by the mechanism of reversible chain termination and reversible chain transfer through active polymerization.
(1) ATRP
The initiation system of ATRP is mainly composed of halides, metal catalysts, and ligands. The polymerization mechanism is the formation of the complex by free radicals through a reversible REDOX reaction. This complex first undergoes oxidation to lose electrons while capturing halogen atoms from halides of dormant species [139]. The reaction mechanism is shown in Fig. 8.
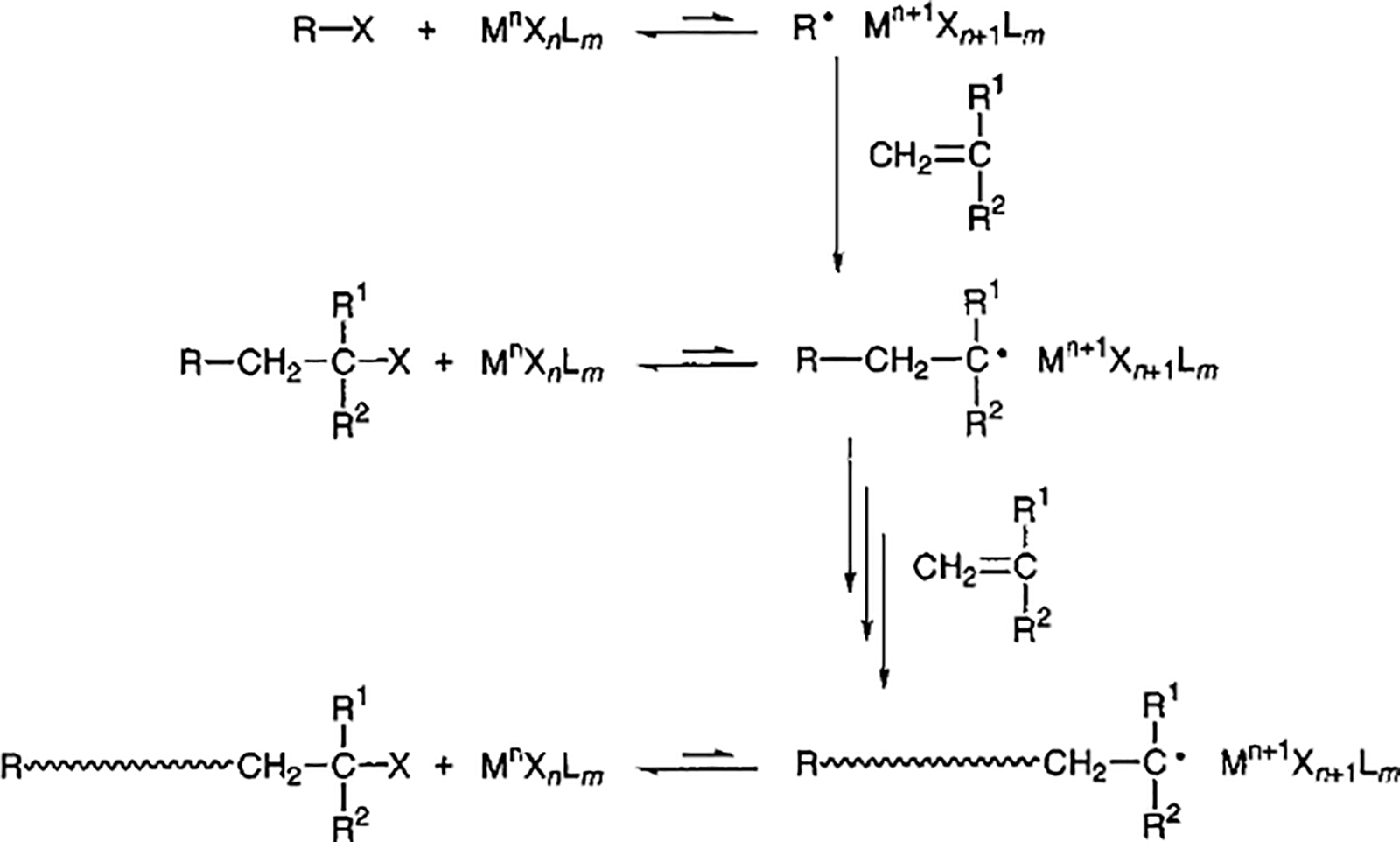
Figure 8: Mechanism of metal-catalyzed active radical polymerization. Adapted with permission from Reference [139], Copyright © 2001 American Chemistry Society
Usually, the initiator of ATRP is a compound containing halogen atoms, and halogen atomic dissociation can be activated due to A-carbonyl, phenyl, vinyl, or cyanide groups on the initiator. Therefore, the bond dissociation energy of alkyl halides has an important effect on the reactivity of the initiator [140]. According to current studies, the initiatory activity of ATRP initiator is affected by three structural factors [141]: (1) the degree of initiator substitution (primary < secondary < tertiary), (2) the departing atom/group, and (3) the radical stabilization group (-Ph~-C(O)OR <<-CN).
When ATRP is used to synthesize graft copolymers, it is necessary to perform homogeneous solution reactions or heterogeneous surface modification on cellulosic materials to prepare cellulose macromolecular initiators with ATRP initiators. Zhang et al. [142] used surface-initiated atomic transfer radical polymerization (SI-ATRP) and Surface-Initiated Activator ReGenerated by Electron Transfer ATRP (SI-ARGET-ATRP) to attach poly4-vinylpyridine and polystyrene to the surface of CNC. The results show that the electron transfer activator is more beneficial to the grafting modification of long-chain and low graft-density polymer than the conventional atomic transfer radical polymerization. In addition, the surface initiation efficiency of brominated cellulose nanocrystals in the electron transfer activator system is much lower than that in the atom transfer radical polymerization system, which is mainly caused by the lower content of catalyst and the faster propagation speed in the electron transfer activator system.
However, for active polymerization, the presence of water will undoubtedly affect the progress of the reaction. In this regard, Glaied et al. [143] developed a method to prepare cellulose-cationic polymer using ATRP in aqueous solution. They first grafted the surface of cellulose with a brominated initiator, and then initiated polymerization on the surface of the cellulose, thereby densely grafting the cationic polymer poly [2-(methacryloyloxy)ethyl]-trimethylammoniumchloride (PMeDMA) onto the surface of the cellulose fiber. The paper prepared by the synthesized polymer is water-soluble and has important potential application value in the field of paper making.
Traditional ATRP can produce cellulose copolymers with residual metal catalysts, and these residues limit the applications of the synthesized cellulose copolymers in microelectronics and biology. In recent years, with the continuous advancement of technology, metal-free ATRP has also emerged. Lu et al. [144] prepared macromolecular initiators by modifying ethyl cellulose with 2-bromo-2-phenylacetyl ester, and then prepared cellulose grafted polymers with block side chain structure by photoinduction of metal-free ATRP. The key to controlling the activity is that the activation and deactivation of the polymerization of dodecyl methacrylate on the surface of cellulose can be controlled by ultraviolet irradiation in the presence of an organic photo-redox catalyst.
(2) RAFT
In the free radical system, disulfide is usually selected as the transfer agent to achieve RAFT. RAFT reagent is a kind of organic compound containing disulfide carbonyl groups. The mechanism of RAFT active polymerization is that the growing free radical (active species) is inserted into the C=S part of the RAFT reagent to form an intermediate free radical, which can form both the original active species and a new active radical that can continue to be polymerized by splitting the intermediate [145]. Its structure and reaction mechanism are shown in Fig. 9. Chain transfer reagents can effectively regulate molecular weight by equilibrium transfer between dormant species and active species to avoid the occurrence of termination reactions.
Figure 9: (a) RAFT chain transfer agent; (b) The general structure of the MADIX chain transfer agent (“RAFT agent”); (c) General RAFT polymerization mechanism. Adapted with permission from Reference [145], Copyright © 2009 American Chemistry Society
Jiang et al. [146] designed a new cellulose-based macromolecular chain transfer agent (Cell-CTA) and synthesized Cell-g-P(BA-co-AM) copolymer thermoplastic elastomer by RAFT polymerization. Because the cellulose chain of the relatively rigid skeleton is an elastomer, and the side chain of thermoplastic elastomer is relatively soft can be used as a rubber matrix. The experimental results show that the polymer has strong tensile strength and high toughness. At the same time, it is worth noting that the amide groups randomly distributed in the acrylamide unit can be used as supramolecular crosslinking points in the graft copolymer to form strong self-complementary hydrogen bonds. Gong et al. [147] first prepared an ethyl cellulose-based large initiator through an esterification reaction, and then polymerized vanillin methacrylate and lauryl methacrylate on a cellulose surface to form cellulose graft copolymer via RAFT. The prepared copolymer formed a good self-healing adhesive network by virtue of its bottle brush structure and Schiff base dynamic bond. Through testing, it was found that the self-healing efficiency could reach 98.7%, which had good and efficient self-healing ability.
However, the traditional RAFT polymerization based on disulfide ester requires high environmental requirements and requires anhydrous and oxygen-free conditions. Due to the hydroxyl group of cellulose itself, it is easy to absorb water in the air and hydrolyze the disulfide ester, so the application of this technology in cellulose is limited. In recent years, due to the evolution of technology, we have developed a new active RAFT polymerization based on trithioester, which can overcome the effect of water on the reaction system. Stenzel et al. [148] developed a trithiocarbonate chain transfer agent based on cellulose derivatives (a-D-glucose, b-cyclodextrin, and modified cellulose), and a polystyrene polymer with comb structure was synthesized based on the chain transfer agent. The CTA reagent based on trithiocarbonate is different from traditional CTA reagent in that the Z group is replaced by the S group. By changing the groups, the stability of the CTA reagent in the water environment can be improved, which can continue to maintain the RAFT polymerization process. Zhou et al. [149] successfully synthesized cellulose nanocrystals/fluorinated polyacrylate soap-free emulsion by RAFT-assisted Pickering emulsion method based on trithioester. They adopt reactive first three glucosinolates carbonate based linear block copolymer poly(2-(dimethylamino) ethyl methacrylate)-b-poly (glycidyl methacrylate)-b-poly (2,2,3,4,4,4-Hexafluorobutyl acrylate) (PDMAEMA-b-PGMA-b-PHFBA) was grafted to CNC to prepare modified CNC stabilizer. Then, cellulose nanocrystalline/fluorinated polyacrylate soap-free emulsion was prepared by Pickering emulsion polymerization in the presence of a stabilizer.
(3) NMRP
The NMRP method mainly uses nitrogen oxygen free radicals to form a dormant active species inverse system, such as 2,2,6,6-tetramethylpiperidine oxide (TEMPO), which is the most used free radical trapping agent. In this method, the chain-growing active species (
Figure 10: Diagram of the dynamic equilibrium mechanism between dormant and active species of NMRP. Adapted with permission from Reference [150], Copyright © 2007 Elsevier
The active polymerization of free radicals can be achieved by introducing an alkoxyamine system. In general, the polymerization reaction is usually carried out at high temperatures. Daly et al. [151] prepared a cellulose-based comb copolymer by grafting nitrogen oxides under homogeneous conditions. N-hydroxypyridine-2-thione ester or carbonate (Barton ester) is added to carboxypropyl cellulose, and the Barton ester is anchored to the cellulose by the reaction. Then, in the environment of tetrahydrofuran as a solvent, TEMPO and styrene were irradiated to initiate grafting on hydroxypropyl cellulose to form the graft copolymer of polystyrene grafted cellulose. Experiments show that the length of the grafted polymer chain segment increases with the prolongation of polymerization time. The polydispersity of polystyrene grafts ranged from 1.3 to 1.5.
(4) SET-LRP
The SET-LRP method activates the initiator or dormant species through the transfer of outer electrons. The initiator system mainly consists of a halide initiator, copper (fine powder or filament) with zero valence state, and appropriate ligands need to be added. Different from ATRP, copper can undergo rapid disproportionation reaction with nitrogen-containing ligands in polar solvents due to the low activation energy in the outer electron transfer mechanism [152], and its reaction mechanism is shown in Fig. 11. Jiang et al. [153] proposed a strategy for surface modification of CNF by combining SET-LRP and CuI-catalyzed azidine-alkyne click chemistry (CuAAC). This strategy can be used to graft di (ethylene glycol) ethyl ether acrylate (DEGEEA) and acrylic acid 3-trimethylsilyl-prop-2-ynyl ester (TMSPgA) on CNF to form cellulosic graft copolymers.
Figure 11: The mechanism of SET-LRP. Adapted with permission from Reference [154], Copyright © 2009 John Wiley & Sons
Jiang et al. [155] grafted stearyl acrylate polymer (PSA) and upconversion nanoparticles (UCNPs) onto CNF using the SET-LRP method to form a highly crosslinking CNF system structure. Before grafting, the original hydrophobic ligands of UCNPs need to be replaced by hydrophilic ligands through a two-step method. This procedure enables UCNPs can adapt to the SET-LRP process. It is worth noting that the crosslinking UCNPs changed the structure of the modified CNF from the original oblong and round structure to a spherical structure, reducing the agglomeration of grafted CNF. By adding UCNPs, CNF has potential application value in the fields of safety ink and biomedicine.
(5) Ring-opening polymerization (ROP)
The so-called ring-opening polymerization is to form a linear polymer with a certain chain length by ring-opening polymerization of cyclic monomer through a catalyst or initiator. The monomers of choice are usually cyclic lactone or lactide. Due to the reaction initiator often choosing alcohol or hydroxyl class, cellulose or its derivatives on the macromolecular and just have many hydroxyl groups. Therefore, when cellulose and its derivatives are modified as a polymer matrix, they can also act as initiators to initiate cyclic monomer grafting polymerization on cellulose. Hafrén et al. [156] initiated the ring-opening polymerization of ε-caprolactone in bulk using organic acid catalysis and solid cotton and paper cellulose as initiators, as depicted in the ring-opening mechanism shown in Fig. 12. The ring-opening polymerization was carried out in a solvent-free/inert atmosphere, in which the non-grafted polycaprolactone was recovered. Due to the advantages of simple operation, low cost, and environmental protection, the ring-opening polymerization process provides a new method for preparing good biocompatible nanomaterials.
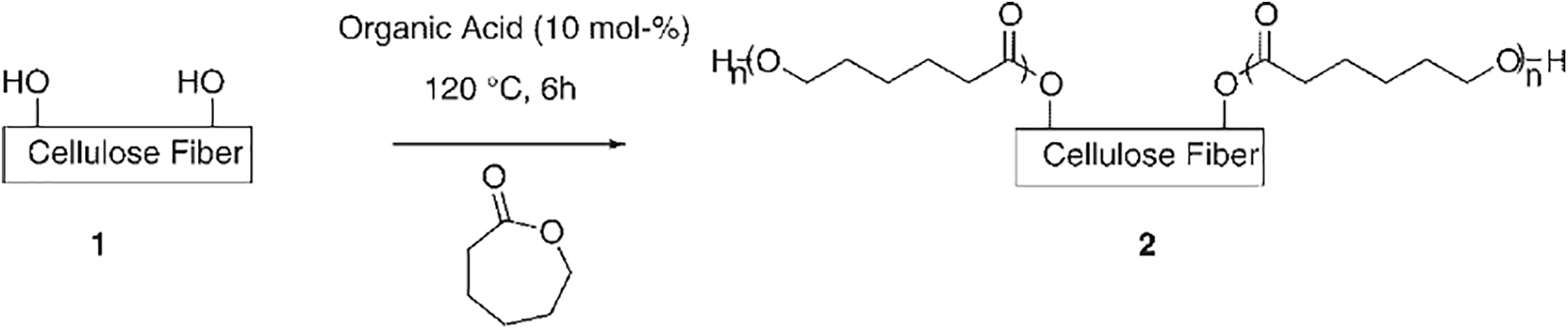
Figure 12: Ring-opening polymerization of cellulose and polycaprolactone catalyzed by organic acids. Adapted with permission from Reference [156], Copyright 2004 © John Wiley & Sons
Lee et al. [157] prepared a series of alkyl branched-chain cellulose copolymers with different lengths by grafting the cyclic ester monomers on the cellulose backbone by direct ring-opening polymerization. The results show that the influence of the branched copolymer on the crystal structure is greater than that of the linear copolymer. Furthermore, as the number of branches increases, the Tg temperature of the copolymer exhibits a continuous decline. This phenomenon can be attributed to the elevated concentration of branch chain end groups, which results in an increased free volume and consequently leads to a reduction in Tg. This work provides a good design idea for the future design of thermoplastic cellulose copolymers. Zhu et al. [158] prepared a cellulose paper for oil-water separation by ROP. Cellulose-g-polysulfonamides were prepared by ring-opening polymerization of N-sulfonyl aziridines on cellulose surface with 7-methyl-1,5,7-triazabicyclo [4.4.0] dec-5-ene (MTBD) biocatalyst.
The “grafting onto” method is to connect two macromolecular chains through covalent bonds, but the “grafting onto” method usually has low grafting efficiency, due to the activity of macromolecular reaction is low, and the steric hindrance is large.
Mano et al. [159] successfully prepared modified CNC(P(Cl-B-LLA)-g-CNC) by grafting the block copolymer (P(Cl-B-LLA)) of ε-caprolactone (CL) and L-lactic acid (LA) onto the surface of cellulose nanocrystals (CNC) through covalent bonding. This modification significantly improves the dispersion of CNC in polar substrates, making it an ideal filler and compatibilizer in the field of bio-nano composites. Sperandeo et al. [160] grafted antimicrobial peptides onto microcrystalline cellulose through the C-terminus or N-terminus by covalent bonding. To graft the peptide onto the cellulose, both need to be chemically modified, so they fixed the cysteine on the surface of the cellulose and modified the peptide with thioester. The prepared cellulose-peptide complex has a good antibacterial effect and has potential application value in the field of biomedicine.
4.3.3 Modification by Crosslinking
The so-called crosslinking modification is to add a multi-functional (usually functional degree >2) crosslinking agent in a cellulose system to achieve covalent binding between molecules, or with other polymers (such as hyaluronic acid, polyvinyl alcohol, chitosan, etc.) crosslinking reaction, which is a commonly used method of cellulose modification. After crosslinking, the mechanical properties and hydrophilicity of the materials can be significantly improved. Munster et al. [161] successfully synthesized dialdehyde cellulose (DAC) through periodate oxidation by using α-cellulose as the starting material. Subsequently, DAC was introduced into the preparation of polyvinyl alcohol (PVA) hydrogel as an efficient crosslinking agent. Under the action of acid catalyst, the active aldehyde group in DAC and the hydroxyl group in the PVA molecular chain crosslinking on the anhydrous glucose unit at positions C-2 and C-3. The experimental results show that compared with the traditional glutaraldehyde crosslinking agent, the hydrogel after DAC crosslinking reaction shows a unique network topology.
5 Regeneration and Application of Cellulose
Whether it is by pretreatment methods and different solvent systems to dissolve the cellulose, or the use of physical or chemical methods to modify the cellulose chemical, the purpose is to be able to make the cellulose can be recycled, to be used in the actual production and life. Modified cellulose solution can be used to prepare a variety of products, such as plastics, elastomers, hydrogels, coatings, and so on. At present, the two most widely used directions of cellulose materials are thermoplastics and thermoplastic elastomers. Bio-based plastics and elastomers, represented by cellulose, contribute to the solution of petroleum-based nondegradable and nonrenewable materials. Therefore, this section mainly summarizes and introduces the relevant application fields of cellulose-based elastomers and plastics.
5.1 Application as Reinforcement Material
In the early stage when cellulose material extraction technology and modification technology are not mature, people usually use wood flour, rice bran [162], and other biomass as reinforcing agents, to prepare wood-plastic composite materials and achieve good results, which is a green and environmentally sustainable development material. This kind of wood-plastic composite material is mainly polyethylene-based wood-plastic composite material and polypropylene-based wood-plastic composite material. However, the polarity of the matrix and reinforcement of the two kinds of wood-plastic composites is too different, and the interface compatibility is not good. It is necessary to improve the interface compatibility, especially the graft copolymer of maleic anhydride and polyolefin.
Hong et al. [163] adopted the grafted copolymer of maleic anhydride, methyl methacrylate, butyl acrylate, and polymer as the interface compatibilizer of WPC and achieved good results. The trimonomer graft copolymer has a high grafting rate, excellent physicochemical properties, and excellent capacity enhancement effect on polyolefin or polylactic acid-based wood plastic composites. This graft copolymer also has a good effect on cellulose-reinforced composites. In addition, a silane coupling agent can be used to enhance the capacity. In terms of the compatibilization of bagasse fiber (BF) and polylactic acid (PLA), Hong et al. [164] compared the effect of alkali treatment, silane coupling agent treatment, and combined treatment on the compatibilization of BF in PLA. It has been shown that alkali treatment will destroy the microstructure of BF but will remove small molecules to increase the crystallinity of cellulose and will make BF fibrosis provide more active sites on the BF surface for the good binding of silane coupling agents. The addition of a silane coupling agent has better BF compatible with the PLA’s interface.
With the development of cellulose utilization and modification technology, people gradually shift their attention from the original cellulose raw materials to modified cellulose and nanocellulose. Cellulose and its derivatives can be used as reinforcing agents to enhance the strength or other mechanical properties of resin matrix materials in some special applications. Especially for rubber tires and other fields, in the context of energy saving, environmental protection, and sustainable development, replacing the original carbon black and silicon dioxide such as high pollution, high energy consumption, high density, and non-degradable fillers have great application potential. Cellulose is the most abundant natural polymer in the world and has the characteristics of green and sustainable regeneration, so it has a unique advantage in the replacement of reinforcing fillers.
Common cellulose-based reinforcing materials include cellulose nanocrystals (CNC), cellulose nanofibers (CNF), and cellulose derivatives (such as EC, AC, etc.). CNC is a good reinforcing material, but because of its high specific surface area, it is easy to aggregate and difficult to disperse well in the rubber matrix. CNF is a hydrophilic material, and there is an interface barrier between it and hydrophobic rubber, which is not conducive to the combination of CNF and hydrophobic rubber.
The common reinforcement method is to compound with the matrix resin. There are many kinds of composite methods, which are generally divided into direct blending methods and in-situ polymerization methods. In-situ polymerization can be divided into nanoparticle in situ polymerization and polymer matrix in situ polymerization.
If direct blending is used, it is necessary to solve the problem of interface compatibility. Generally, CNC or CNF surface modification is required to ensure good compatibility. According to compatibility problems, different scholars have adopted different methods to solve them. Magana et al. [165] solved the composite problem of polyphenylene and cellulose nanocrystals (CNC) employing plasma surface treatment. Through the rheological study, it is found that the storage modulus of the composite is improved by adding the surface-modified CNC, which has better performance than the unmodified CNC composite. In terms of compatibility, Zhu et al. [166] introduced active sulfhydryl groups on the surface of CNF while retaining surface hydroxyl groups, thus forming a permeation network in the composite material. They blended dispersed modified CNF and NR in the form of latex and then evaporated by casting to obtain nanocomposites. Sun et al. [167] proposed a nonsolvent-induced phase separation method to coat cellulose nanocrystals (GLCNCs) with 3-Glycidyloxypropyltrimethoxysilane to solve the interface compatibility problem between fillers and thermoplastic polyurethane (TPU). The tensile strain and toughness of the composite films are enhanced due to the enhanced interfacial interaction between the matrix resin and the filler. Among them, the photochemically induced mercaptan reaction between the double bond of NR and the sulfur group forms a covalent crosslinking, which helps to improve the modulus and toughness of the material. Pajares et al. [168] combined cellulose derivatives (such as CA, EC, MCC, etc.) as reinforcing agents with high-performance styrene block copolymers to improve structure and surface properties. The effects of different blend compounds and blend proportions on mechanical properties, thermal properties, and biological toxicity of the materials were investigated.
5.1.2 In-Situ Polymerization of Nanoparticles
The so-called in-situ polymerization of nanoparticles means that the nano-filler is dissolved first, and then the imbalance of dissolution balance is used to precipitate the nanoparticles in the polymer matrix as growth to achieve composite. Yuan et al. [169] proposed a bottom-up self-assembly method, which broke the dissolution equilibrium by mixing cellulose solution with natural rubber (NR) latex so that cellulose crystals could be regenerated locally in the NR matrix. By regenerating cellulose in the NR matrix, the rubber can form a rigid RRC-RRC network, which significantly enhances the tensile modulus of rubber. Li et al. [170] chose ionic liquid (IL) to dissolve cellulose and then mixed it with other components such as dry rubber and silica. Based on the interaction force between IL and silica, the dissolution balance of cellulose /IL was disturbed, to realize the regeneration of cellulose crystals in the rubber matrix. The structure of the regenerated cellulose is rod-like, which has a strengthening effect on rubber. This process is green and environmentally friendly, and it is a new process design idea for the rubber strengthening field.
5.1.3 In-Situ Polymerization of Polymer Matrix
In-situ polymerization of polymer matrix refers to the polymerization and growth of monomer molecules on the surface of nano-filler to achieve composite. Mahadi [171] prepared the polytrimethylene ether glycol-based thermoplastic polyurethane (TPU)/cellulose nanocrystal (CNC) composite by in-situ polymerization. The composite has good self-healing performance, and the addition of CNC greatly improves the physical and mechanical properties of TPU/CNC composite. In the presence of CNC, Miao [172] used ring-opening in-situ polymerization to open-ring L-lactide grafted on CNC to form CNC-PLLA nanomaterials, and PLLA homopolymer was also obtained. According to the study, the presence of PLLA homopolymer can not only prevent the aggregation of CNC-PLLA but also help CNC-PLLA to be compatible with other polymers as a compatibilizer. This nanomaterial can be used in medical or other engineering applications. Rosa [173] used CNC as an initiator and grafted ε-caprolactone (CL) onto the surface of CNC through in-situ ring-opening polymerization, thereby preparing polycaprolactone grafted cellulose nanocrystalline copolymer (PCL-g-CNC). Through mathematical modeling and calculation, they concluded that part of the hydroxyl group on the CNC surface served as an initiator to promote the in-situ polymerization and growth of PCL on the CNC surface.
In a word, by adding cellulose-based reinforcement agents to the matrix resin, it can not only realize reinforcement, but also help to promote energy conservation and environmental protection and develop sustainable regeneration economy.
However, in the field of reinforcement, compared with other materials, cellulose materials also have certain limitations. Gitari et al. [174] compared the study of nanocellulose (CNF) and bacterial nanocellulose (BNC) in enhancing the mechanical properties of polylactic acid (PLA) films. Both CNF/PLA and BNC/PLA nanocomposite films exhibited higher tensile strength and Young’s modulus than pure PLA without sacrificing their toughness. However, it was found that the tensile strength of CNF/PLA (36.7 MPa (CNF = 0.5 wt.%) and 44.2 MPa (CNF = 1.0 wt.%)) was lower than that of BNC/PLA (49.9 MPa (BNC = 0.5 wt.%) and 48.7 MPa (BNC = 1.0 wt.%)). Therefore, the mechanical properties of BNC/PLA are superior to those of CNF/PLA nanocomposites. Similarly, Delgado et al. [175] studied the effects of rice husk cellulose nanofibers (RHCNF) and bacterial nanocellulose (BNC) on the mechanical properties of yeast biomass membranes at pH 6 and 11. Through experimental tests, they found that the addition of nanofibers to the yeast matrix can improve the tensile strength, but BNC can improve the mechanical properties of the yeast membrane more than RHCNF. However, only BNC (5 wt.%) simultaneously increased the Young’s modulus (36 MPa), tensile strength (3.1 MPa), elongation at break (44 MPa), and tensile toughness (1 MPa) of the yeast matrix. But in terms of packaging, the cost-benefit relationship should be considered, because the production cost is higher than RHNCF BNC.
In addition, Kim et al. [176] compared cellulose materials (cellulose based flax) with animal protein materials (protein-based wool). They added both to polypropylene for reinforcement modification and found that flax had superior mechanical properties (the tensile modulus of flax fiber (46.9 GPa) was higher than that of wool (4.8 GPa)), making flax composite have better tensile and bending properties than wool composite.
In summary, although cellulose materials can strengthen polymers, cellulose materials may not be the best reinforcer. Therefore, to obtain higher strength cellulose based materials, it is necessary to functionally modify cellulose materials to achieve the desired purpose.
Self-healing materials are a class of polymer material that contain dynamic covalent bonds, including linear and crosslinking dynamic covalent bond polymers. The characteristics of these materials are that under the action of external stimuli (such as heat, light, force, pH, etc.), the dynamic covalent bonds inside the materials can be reversible broken and formed, thus inducing the dynamic adjustment of the polymer network structure. When the external stimulation is eliminated, the dynamic covalent bond can be restored to the high stability of the general covalent polymer. Based on the dynamic crosslinking network, it can realize multiple repairs of itself.
In the field, cellulose-based material has an important role. Since cellulose itself has many hydroxyl groups, hydrogen bond networks can be formed. The hydrogen bond network itself is a reversible dynamic physical crosslinking network, and the fracture and regeneration of hydrogen bonds can be realized by controlling the temperature, which can make the material have good thermoplastic processing performance. Therefore, based on the above principle, Utrera-Barrios et al. [177] prepared bio-based self-healing elastomers by blending cellulose propionate (CP) with epoxidized natural rubber (ENR) and adding CNF as a reinforcing agent. Due to the synergistic interaction between the mobility of the molecular chains and hydrogen bond formation, the elastic body has a good self-healing ability (healing efficiency is 75%). Shen et al. [178] used amino cellulose (AC), pimelic acid-modified epoxy soybean oil (ESOPA), natural rubber, and liquid metal (LM) as raw materials to prepare a crosslinking metal-polymer composite elastomer with multiple hydrogen bonds. With the active groups of AC and ESOPA, the elastomer can not only improve its self-healing ability (97.28%) by constructing a poly hydrogen bond network but also enhance its mechanical strength (1.51 MPa) and tensile properties (515.3%). In addition, the composite elastomer also has stable photothermal conversion performance, which has potential application value in the field of energy.
Although cellulose has a strong hydrogen bond network, strong hydrogen bond interaction will affect the temperature of thermoplastic processing, resulting in material processing difficulties. Therefore, it is necessary to adjust the original hydrogen bond network to improve the machining performance of composite materials. Fan [179] added new dynamic covalent bonds to the original hydrogen bond network to adjust the processing and self-healing properties of materials. They developed a self-healing supramolecular composite based on polyurethane elastomer and cellulose nanocrystals, and the synthesis process is shown in Fig. 13. Due to the rich hydroxyl group on the surface of CNC, the material will form a hydrogen bond with PU to form a dynamic physical crosslinking network, coupled with the oxime-carbamate bond, which gives the composite material a good self-healing ability. The experimental results show that the material has good tensile strength and toughness, and its mechanical properties can remain unchanged even after three repeated machining.
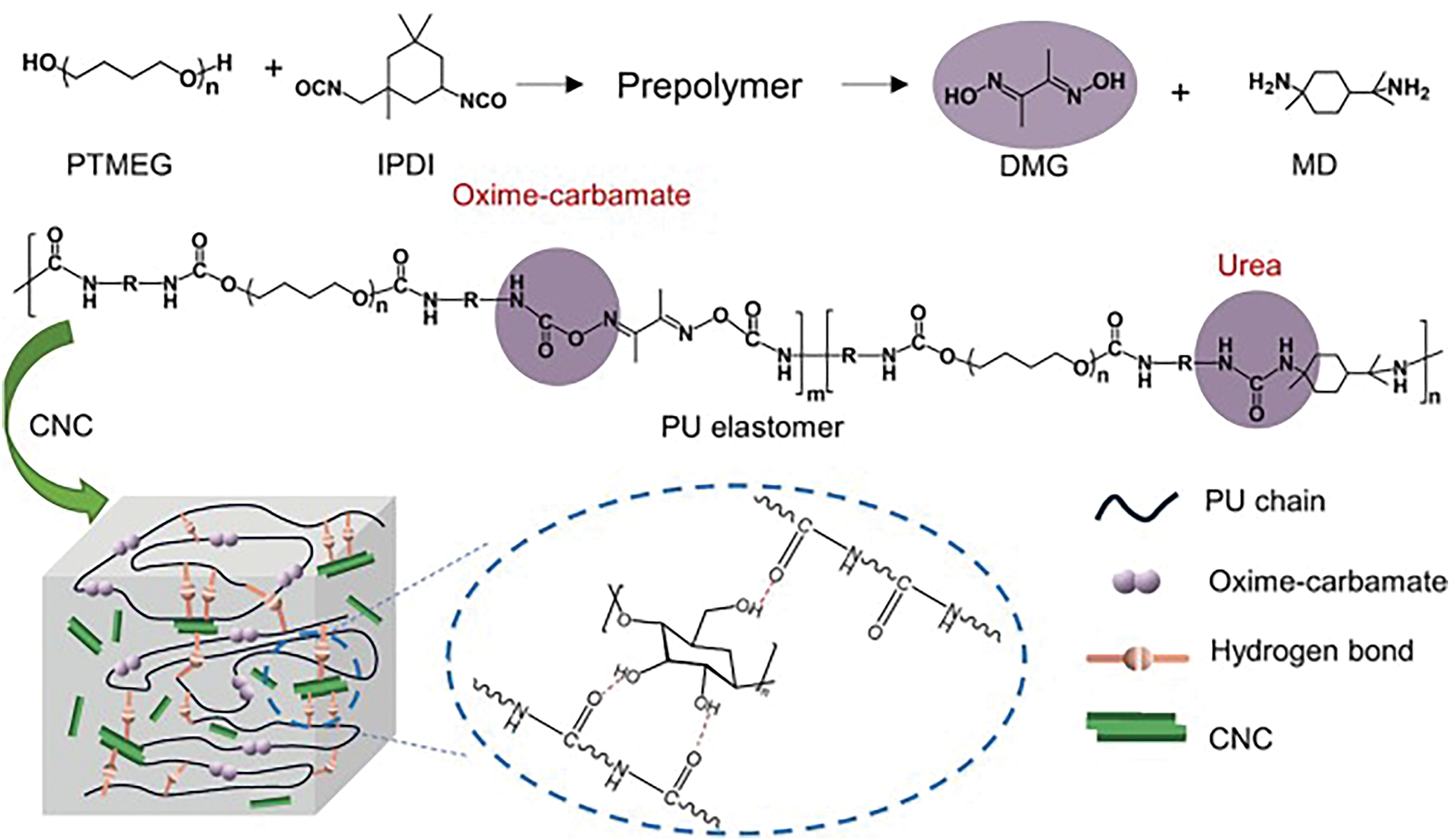
Figure 13: Synthesis route of DOU PU and DOU PU CNC. Adapted with permission from Reference [179], Copyright © 2023 Elsevier
Su et al. [131] first oxidized cellulose into dialdehyde cellulose (AC) and then carried out a Schiff base reaction to establish a dynamic covalent bond network, thus replacing the original hydrogen bond network of cellulose. By changing the interaction between the molecules of the cellulose chain, the Tg temperature of the original cellulose plastic was reduced, and the cellulosic plastic was endowed with good recyclable and processable properties. Moreover, it has been found that the plastic modified by Schiff base also exhibits good water resistance, chemical resistance, and high-temperature resistance. These properties offer a promising and effective approach for the development of high-performance cellulose plastics.
Nowadays, the excessive consumption of fossil energy such as oil and gas has led to environmental pollution, global warming, and other crises. To cope with the energy crisis and global environmental challenges, radiative cooling, with its zero-energy consumption and green environmental protection characteristics, helps to alleviate the greenhouse effect and solve energy problems. This passive radiative cooling (PRC) reduces the temperature of the object below the ambient temperature by its highly transparent characteristics in the atmospheric window band of 8–13 μm, which effectively suppresses the solar absorption and realizes radiative heat exchange in the atmospheric window band of high emission (that is, strong infrared absorption). Due to the large number of C-C and C-O structures, the strong molecular vibration of cellulose-based radiative cooling materials endows cellulose with high infrared emissivity in the atmospheric window. At the same time, cellulose has a multi-level macro and microstructure, which can carry out multiple scattering of sunlight [180], so it has potential application in the field of radiative cooling. Moreover, as research on cellulose becomes more and more mature, it has been increasingly recognized that nanocellulose materials play an important role in radiative cooling. CNC and CNF provide new design ideas for the preparation of sustainable radiative cooling materials by their unique structure and surface modification characteristics.
In general, cellulose-based radiative cooling materials can be divided into two categories, namely, multi-pore radiative cooling materials with white pores, and the other is a new type of radiative cooling materials with reflection ability or color.
5.3.1 Porous Radiative Cooling Material
For porous radiative cooling materials, the design of microscopic holes (micro and nano size) can scatter sunlight, to improve the performance of radiative cooling. In addition, the radiative cooling performance of cellulose-based composites can be improved by adding optical nanoparticles, such as TiO2 [181], SiO2 [182], and other nanoparticles. In addition, the addition of nanoparticles not only enhances the optical properties of the cellulose film but also endows the film with high mechanical strength. Yang et al. [183] used wood powder as raw material to obtain acetylated lignocellulose through lignin removal, bleaching, and acetylation modification. Then the obtained material was mixed with silica microspheres and a double-layer composite structure of radiative cooling film with silica as the bottom layer was obtained by evaporative deposition technology. Through the experimental characterization, it is found that the composite film not only has a high infrared emissivity in the range of 8–13 um (98.7%) but also shows a high reflectivity in the visible light band (98.44%). When the content of silica microspheres is 30%, the cooling performance of the composite film is the best, and the cooling coefficient can reach 9. At the same time, the cooling effect of 6 degrees can be achieved in the outdoor radiative cooling test.
In general, the preparation methods of porous radiative cooling materials are usually divided into two categories: one is to directly modify the cellulose paper by physical or chemical methods, to obtain films with high scattering ability. Tian et al. [184] developed a new cellulose-based radiative cooling material, which sprayed ethanol polytetrafluoroethylene (PTFE) particulate suspension on cellulose paper by air spraying method. Some of the PTFE particles are embedded in the micropores of the cellulose fibers, thus forming a double-layer structure. The addition of PTFE makes the cellulose paper have good hydrophobicity and self-cleaning and can also enhance its solar reflectivity through sunlight backscattering. Through the radiative cooling performance test, it is found that the cooling performance of the composite material can reach 5°C when the solar irradiance is 834 W/m2. In the case of solar intensity of 671 W/m2, its radiative cooling power can reach 104 W/m2.
The other is to use the phase transition method to prepare the structure of ordered, porous white cellulose-based radiative cooling materials. Cai et al. [185] prepared cellulose composite film (CCF) with a well-defined, hierarchical micro/nanostructure by rational solvent-induced phase separation technology. On this basis, TiO2@PT dual-function modifier was prepared by ball milling technology. When combined with CCF, the optical, mechanical, and UV resistance properties of the film can be improved and enhanced. In addition, due to the micro/nanostructure of the film itself, the CCF not only has an ultra-high solar reflectance (97.6%) but also increases Young’s modulus (13 times) and tensile strength (4.4 times). And the most important thing is that the reflectivity of CCF remains unchanged under continuous 720 h UV irradiation.
5.3.2 Color/Transreflective Radiative Cooling Materials
In traditional PRC devices, to improve the reflectance of sunlight as much as possible, people will prepare the device into a white or porous structure, and the heat exchange window is mostly concentrated in the far-infrared window (8–13 um). Therefore, the color adjustment of most devices has great limitations, and the direct addition of traditional dyes will produce necessary visible light absorption and near-infrared absorption [186]. So, people began to attention to the color radiative cooling (ColPRC) field and strive to resolve how to implement color at the same time as effective radiative cooling. People found ColPRC systems with structural color wavelength selective reflection/transmission to avoid this problem while maintaining low solar absorption. Shanker et al. [187] mixed CNC and glucose (GLU) into a suspension in proportion and built a photonic crystal composite film with a spiral periodic structure through evaporation-induced self-assembly. The composite film can not only realize the selective structural color reflection of the photonic crystal structure but also transmit other parts of the solar spectrum. By combining the composite film with a silicon substrate, the temperature can be reduced to 9°C under solar irradiation. In contrast, Zhu et al. [186] applied CNC suspension onto porous ethyl cellulose (EC) with high scattering. The composite structure can not only scatter all sunlight passing through the CNC layer through the underlying porous EC layer but also realize broadband solar reflection and structural color display with the help of the structural reflection of the CNC layer. Its structure design is shown in Fig. 14. Moreover, Zhu et al. also achieved mass production of the color radiative cooling film through a roll-to-roll process, making an important contribution to promoting the application of color radiative cooling. He et al. [188] used carbon dioxide-based waterborne polyurethane (WPU) and cellulose nanocrystals (CNC) as raw materials and obtained photonic crystal composite films by self-assembly induced by evaporation after mixing. Based on the original, the multistage photonic crystal structure film is constructed by pouring different proportions of mixed solution for evaporation self-assembly. The experimental results show that, compared with the single photonic crystal structure, the reflection ability of the multistage photonic crystal structure is improved in the visible light range, and its reflection curve can cover the whole visible light band. The composite film can achieve a maximum cooling temperature difference of 6°C in the outdoor radiative cooling test.
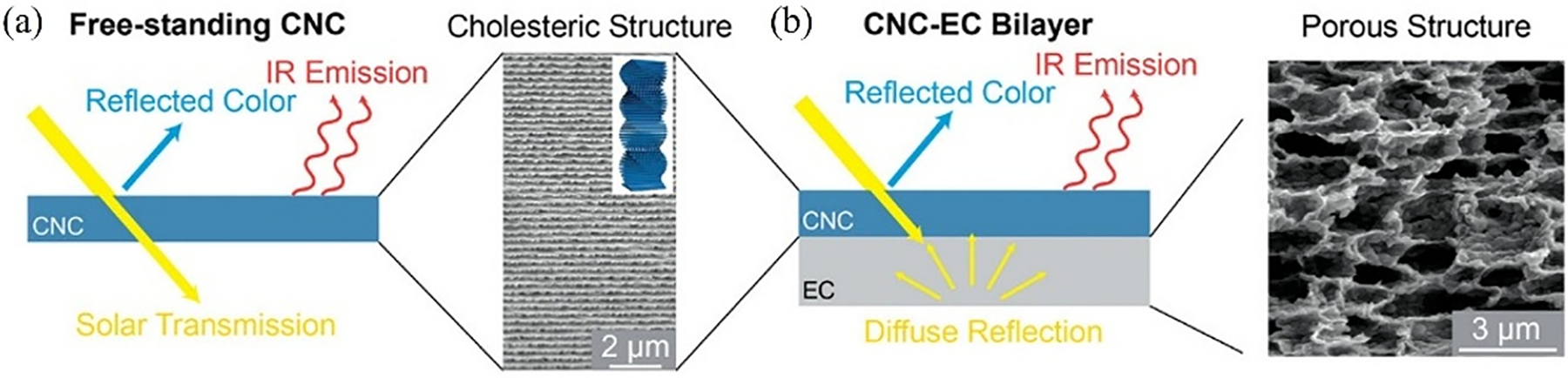
Figure 14: (a) Schematic diagram of radiative energy transport in cholesteric CNC films and scanning electron microscope (SEM) images of the corresponding sections. (b) Schematic diagram of the radiative energy transport of the CNC-EC bilayer composite membrane, and SEM cross section of the porous EC substrate. Adapted with permission from Reference [186], Copyright © 2022 John Wiley & Sons
To better distinguish and highlight the characteristics of reflective and transmission radiative cooling devices, Gamage et al. [189] prepared a reflective radiative cooler and a transparent radiative cooler by electrospinning and casting manufacturing technology. They control the microstructure to achieve high reflectance (>90%, porous structure) structure transition to a highly transparent structure (≈90%, uniform structure). Through experimental testing it was found that both cellulosic materials showed high thermal emissivity and minimal solar energy absorption. Reflective and transparent cellulosic coolers could passively reduce sample temperatures by up to 15°C and 5°C, respectively. They suggest that transparent cellulosic devices can be used as coating materials for photovoltaic and solar harvesting devices to reduce heat-induced performance degradation, while reflective cellulosic devices are expected to be used as coatings for cooling other potential objects, such as cooling water harvesting systems in building spaces.
In summary, cellulose-based radiative cooling materials have great application potential in the field of radiative cooling due to their own infrared vibration groups (C-C and C-O) and multi-level micro/nanostructures.
Nanogenerators are devices capable of collecting and converting tiny amounts of mechanical energy into electrical energy. Such devices are usually implemented based on two effects, namely piezoelectric and triboelectric effects. Therefore, according to these two benefits, the corresponding nanogenerators can be prepared, which are piezoelectric nanogenerators (PENG) and triboelectric nanogenerators (TENG), respectively.
5.4.1 Piezoelectric Nanogenerator (PENG)
The piezoelectric effect refers to the phenomenon that the material will produce charge separation under the action of mechanical stress, and this charge separation will cause the generation of voltage and current. Generally, PENG is mainly composed of materials with piezoelectric properties. The process works as follows: when these materials are subjected to mechanical deformation, such as squeezing, stretching, or bending, the dipole moments inside them change, causing the charge density to be redistributed. Electrons are transferred through the external circuit to re-balance the charge, generate current output, and realize the conversion of external force into electric energy for harvesting [190]. Therefore, it is commonly used to collect small mechanical energy in the environment such as footsteps, vibration, or sound, and convert it into electrical energy.
In general, since regenerated cellulose belongs to type II cellulose, its monoclinic and triclinic eutectic structures have no symmetry center. Moreover, due to the high polarity of the hydroxyl group, all kinds of cellulose crystals have asymmetric dipoles, which leads to the necessary structural conditions of cellulose crystals as piezoelectric materials [191]. In the
In addition to generating voltage by changing the polarization of cellulose materials under the action of mechanical forces, it is also possible to generate charge through the internal spontaneous polarization change of materials under the change of time and temperature through the pyroelectric effect [194,195]. Li et al. [196] first used concentrated NaOH to extract CNF and then used Tempo for oxidation to enhance the negative charge density of their CNF, to enhance the thermoelectric conversion ability of their materials. Then, a NaOH-based polymer electrolyte was used to penetrate the cellulose membrane to obtain the ionic conductor material. Thanks to the effective insertion of sodium ions into the charged molecular chains of the Type II cellulose film, the material exhibits excellent thermoelectric properties (24 mV k−1 thermal gradient ratio). Maity et al. [197] prepared a pyro-piezoelectric nanogenerator (Py-PNG) based on CNC material for mixed energy (mechanical energy and thermal energy) recovery by 3D printing. The nanogenerator has excellent mechanical energy and thermal energy harvesting ability, and it can be prepared as a sensor and applied to the medical field, such as skin sensing and cardiopulmonary detection, which can show high sensitivity. It is worth noting that the sensor assembled by the generator can be equipped without any battery or external power supply. Liao et al. [198] innovatively combined radiative cooling with pyroelectric materials. First, they design two kinds of materials based on cellulose. (1) Heater: a heater material used for solar heating (ITO is deposited on black cellulose film by magnetron sputtering (ITO/BC)). (2) Cooler: a cooler material for radiative cooling (a porous cellulose acetate film prepared by electrospinning (IME Technologies EC-CLI)). These two materials have opposite optical properties in the solar and mid-infrared (MIR) thermal spectrum and will produce a huge temperature difference under the irradiation of sunlight, providing a temperature difference environment for pyroelectric materials. The Heater and Cooler are used as two poles (the two materials are connected to the gold electrode), and an ionic thermoelectric gel-like IL-HEC electrolyte is added between the two poles to form a closed pyroelectric device, as shown in Fig. 15.
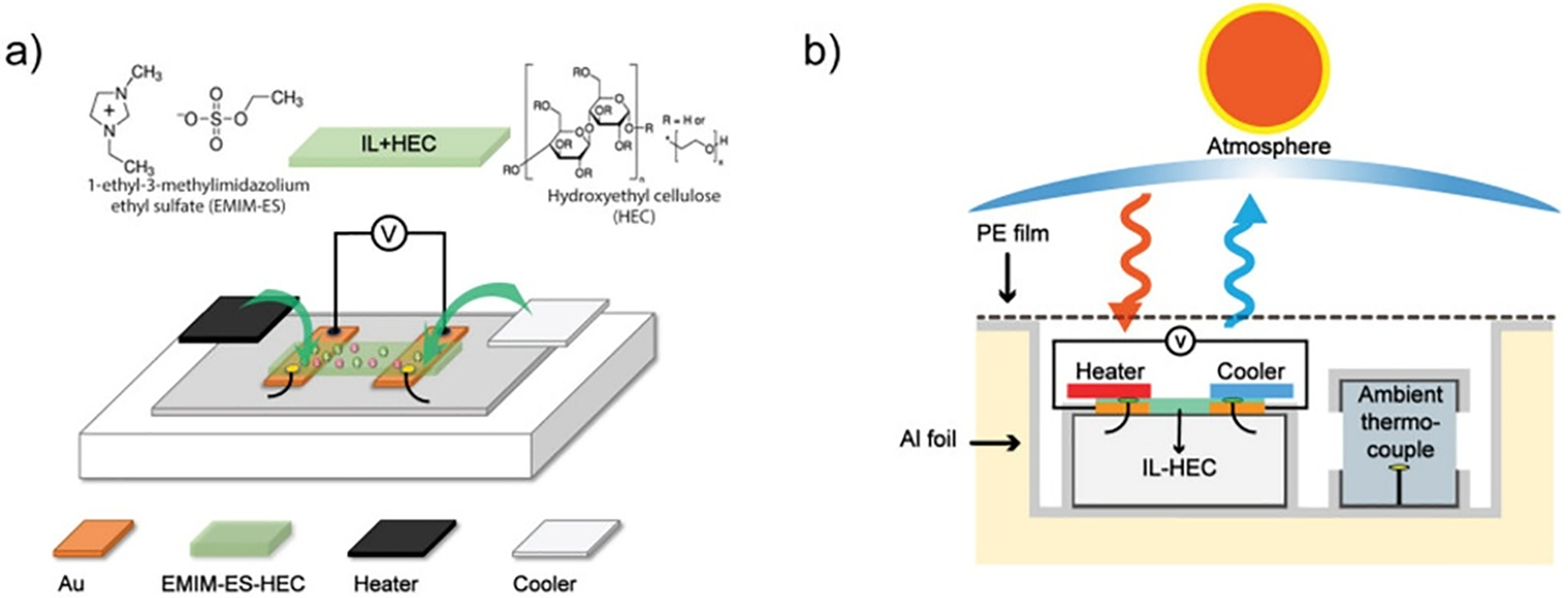
Figure 15: (a) Schematic diagram of the lateral thermoelectric device. (b) Schematic diagram of outdoor device. Adapted with permission from Reference [198], Copyright © 2023 John Wiley & Sons
5.4.2 Triboelectric Nanogenerator (TENG)
The mechanism of TENG is implemented based on the coupling of triboelectrification and electrostatic coupling effects. TENG is mainly composed of two materials with different dielectric constants. When these two materials come into contact under mechanical force, electrons are transferred from one layer to the other through the contact charge effect. One material will lose electrons and become positively charged, and the other material will gain electrons and become negatively charged, resulting in positively and negatively charged surfaces with equivalent heterogeneous charges. When the two materials are separated, a strong electrostatic field is generated between the two materials with dissimilar charges, which drives the flow of electrons to regain potential balance, thus generating tribological energy [199]. This type of generator can collect mechanical energy from the environment, such as wind, water flow, and human motion, and convert it into electrical energy.
Since cellulose contains three hydroxyl groups per repeat unit, these hydroxyl groups can cause the formation of hydrogen bond networks inside the cellulose molecules, leading to the formation of a highly ordered crystalline structure of cellulose. At the same time, the lone electron group of the oxygen atom on the hydroxyl group has the electron-giving effect and the tendency to lose electrons, which is the prerequisite for the frictional electrogeneration of cellulose materials [191]. In the case of cellulose-based TENG, the positive electrode material generally contains oxygen-rich or amino-rich groups. Therefore, cellulosic materials have potential application value in the positive electrode materials of TENG.
Generally, cellulose-based TENG materials can be divided into four categories, namely “A-B” type, rough machining enhanced type, filler enhanced type, and chemical modification type.
The so-called A-B type requires only cellulose and another material, one serving as A positive electrode and the other as a negative electrode. Parandeh [200] used polycaprolactone (PCL)/graphene oxide (GO) as the negative electrode material and cellulose paper as the positive electrode material. They found that thanks to the graphene oxide fiber layer structure and oxygen-containing functional groups forming nanopores on the PCL and promoting the accumulation of negative charges, the triboelectric nanogenerator has good power generation performance (open circuit voltage of 120 V at 4%GO content, current density of 2.5 mA/m2, maximum power density of 72.5 mW/m2). To solve the problem of non-degradability and environmental pollution of traditional TENG, Park [201] designed A new type of degradable A-B bilayer TENG based on hydroxyethyl cellulose (HEC) and gelatin. Due to the large difference in relative permittivity between HEC and gelatin, this difference induces interfacial polarization, effectively inhibiting charge recombination and thus enhancing the triboelectric properties. The open circuit voltage of the optimized TENG can reach 93 V and the maximum power density can reach 57.8 µW/cm2.
“Rough Processing Enhancement” Type
Since the cellulose film is relatively smooth and not very friendly to friction generation, it is necessary to roughen its surface to improve its friction properties. The general methods to improve the roughness include etching, plasma treatment, template back printing, 3D printing, electrospinning, freeze drying, etc., to achieve the performance of TENG by improving its contact area. Mule et al. [202] designed a flexible wearable TENG. They used microfiber mesh cotton fabric as the support skeleton by in-situ polymerization method and then deposited it to form a polypyrrole-coated cotton fabric (PPy@CT). Through the adoption of PPy @ CT as an electrode to build the flexible PENG. In addition, they further performed soft print lithography on PPy@CT, adding a layer of polydimethylsiloxane with microstructure (like abrasive with auxiliary friction) on the top of PPy@CT to build a high-performance single-electrode TENG(PPy-WSEM-TENG). Human skin as a positive friction layer, while PPy-WSEM-TENG as a negative friction layer, through repeated contact separation, can effectively convert mechanical energy into electrical energy. Seol [203] designed a fully printed triboelectric nanogenerator (AP-TENG) by combining 3D printing and 2D printing technology. The shell structure is formed by 3D printing on the structural frame so that the contact layer can effectively contact friction in motion. In the aspect of the contact layer, the 2D inkjet technology is used to form a nano-scale printed contact layer on the nano-cellulose paper, to ensure a high output power.
By adding functional fillers to the cellulose film, the output performance of cellulose-based TENG is thus improved. For example, adding electron transfer filler to improve the electron transfer and electron supply capacity of the film; Alternatively, dielectric, or ferroelectric materials can be added to improve the output efficiency of TENG by improving the electron trapping ability of the film. He et al. [204] took cellulose microfiber (CMF) as the skeleton and constructed an ideal nanopore-micropore two-dimensional structure through CNF on both sides of the skeleton to form CMF/CNF paper. CMF/CNF was used as a template and silver was deposited on both sides to obtain a typical nanostructure composed of Ag nanofibers, and a self-powered cellulose fiber triboelectric nanogenerator (cf-TENG) was constructed. Due to the ideal porous nanostructure and unique power generation characteristics of the cf-TENG, the cf-TENG can remove PM2.5 and monitor respiratory status with 98.83% efficiency without a power supply. Shi et al. [205] chose porous cellulose and polydimethylsiloxane to assemble TENG. To improve its output power, Shi et al. added nanoparticles (such as BaTiO3, Ag) to perform dielectric regulation on the material, thus improving the surface charge density and electron trapping ability of the material and realizing the improvement of the dielectric constant. As a result, the output power of the TENG is enhanced (output voltage up to 88 V, current up to 8.3 μA, output power of 141 μW). The TENG can act as a sensor to achieve remote mechanical monitoring and transmit relevant signals. In addition, their research also found that the material has great potential in the synthesis of supercapacitors. TENG can act as an efficient power source for the electro-polymerization of polyaniline on CNT electrodes.
Although the output capacity of cellulose films can be improved by physically mixing fillers, the inevitable problem in blending is the compatibility of fillers and substrates. Therefore, based on the hydroxyl group on the surface of cellulose material or the carboxyl group on the surface of TOCN, the group that promotes electron transfer is connected to the surface of cellulose material through chemical modification, which can improve the surface polarity, electron giving ability and surface roughness of the material. It is also possible to improve the performance of TENG by changing the electronic orientation of cellulose to improve the triboelectric properties. Zhu et al. [206] adopt dopamine (PDA) to modify cellulose. Polydopamine nanoparticles were prepared by oxidative self-polymerization and then attached to CNF. The introduction of amino and membrane surface microstructure of synergy can effectively improve the output performance of TENG. Through experimental testing, the optimized TENG can achieve the best output performance under the condition of 205 V voltage, 20 μA current, and 48.75 μW·cm−2 power density. The treatment method of Yao et al. [207] was cationic modified the cellulose on the surface by impregnating the wood in 3-Chloro-2-hydroxypropyltrimethyl ammonium chloride (CHPTAC) solution, to achieve twice the increase of surface potential. The experimental results show that the modified TENG can produce a peak current of 9.74 μA, a voltage of 335 V, and a transfer charge density of 71.45 μC/m2.
Cellulose-based materials can also play an important role in biomedical applications. At present, natural polymer materials such as cellulose are made into biomedical polymer materials because their characteristics meet the specific needs of the organism and are similar to the extracellular matrix of the human body [208]. In addition, cellulose has attracted much attention in the field of biomedicine due to its hydrophilic and structurally stable properties. Cellulose derivatives, due to the good biocompatibility of fiber materials, better solubility, and functional abilities, widen the cellulose materials in the field of biomedical applications.
Wound dressings are materials that are coated on the surface of wounds, which protect the wound, block bacterial infection, and promote wound healing. General wound dressings are required to have good air permeability, moisture absorption, antibacterial and biocompatibility, and low adhesion to the wound to avoid secondary trauma [209]. Therefore, cellulosic materials need to be processed and compounded to achieve the functional requirements.
The most common method is blending, where cellulose is mixed with other functional materials. Mayer [210] prepared bio-based films by blending dialdehyde cellulose with corn protein and gelatin. It was proved that the composite film has good bacteriostatic ability (against Gram-positive and Gram-negative bacteria) and no cytotoxicity and can be used in wound dressing. Pitpisutkul [211] added carboxymethyl starch (CMS) and zinc oxide nanoparticles (ZnO-NPs) to hydroxypropyl methylcellulose (HPMC) films to prepare nanocomposite films. The composite film has good resistance to Gram-positive and Gram-negative bacteria and has great potential in wound dressing. Dang [212] first grafted 1H-Indole-3-acetic acid (IAA) into MCC through chemical modification to prepare a soluble cellulose-based nonionic biopolymers (CIs) and then blended it with polycaprolactone (PCL) to obtain PCL-CI membrane. The composite film has good antibacterial and bactericidal effects against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) and has a promising application in wound dressing.
In addition to the blending method, multi-layer assembly is also an effective means of realization. Guo [213] first prepared Bai@TA nanoparticles by solvent exchange method. Then the microgel was prepared by blending sodium carboxymethyl cellulose (CMC) with hydroxypropyl trimethyl ammonium chloride chitosan (HACC). Through layered assembly technology, the gel and nanoparticles are alternately deposited into a film in a medical bandage to prepare an antibacterial dressing. The dressing can have a good antibacterial effect against Staphylococcus aureus, Escherichia coli, and Methicillin-resistant Staphylococcus aureus (MRSA), helping to reduce inflammation and accelerate wound healing.
In addition to traditional wound dressings, intelligent wound dressings have also emerged to meet the needs of people for different occasions. By controlling the conditions of the external environment, the timed and quantitative release of the drug can be achieved. Baptista [214] first functionalized fiber cloth and electro-spun cellulose acetate fiber membrane by polypyrrole (Ppy), then impregnated fiber cloth in ibuprofen and added IBU solution to CA fiber membrane to obtain Ppy/ ibuprofen /Ppy membrane. Electronically controlled drug release films can be prepared by combining the composite films with Ag electrodes. The film can be used as a wound dressing to maintain ibuprofen non-release at a voltage of 1.5 V, while rapidly releasing the drug at −0.5 V. Xu et al. [215] prepared a smart dressing to solve the problem of diabetic wound healing. The dressing is composed of a cellulose covering layer and an antibacterial hydrophobic polycaprolactone layer, which can unidirectionally and irreversibly drain the exudate from the wound. Moreover, the dressing can detect the degree of wound healing through the color response to PH, which has important application value.
So, what are the unique advantages of cellulose-based wound dressings over non-cellulose-based ones? As cellulose nanocrystals (CNC) serve as a specialized functional filler, they demonstrate distinctive hemostatic and reparative capabilities in wound management. This study conducts a comparative analysis between CNC-based dressings and other dressing types to highlight the exceptional merits of cellulose-based systems. Poonguzhali et al. [216] incorporated CNC into a chitosan/polyvinylpyrrolidone (CS/PVP) matrix. Experimental results revealed that the CS/PVP biocomposite film containing 3% CNC achieved superior wound closure efficiency compared to the control group (povidone-iodine ointment). Notably, the CNC-modified dressing achieved near-complete wound healing within 21 days, whereas the control group and pure CS/PVP membranes displayed inferior healing performance.
Regarding biological functionality, Cheng et al. [217] conducted in vivo hemostatic evaluations comparing commercial gauze, pure alginate dressing, and TOCNC-containing composite dressings. Compared with gauze and alginate dressings, the amount of bleeding and hemostasis time of the composite for liver and ear injuries were lower. In addition, when the content of TOCNC reaches 30%, its composite gauze has the best effect. Furthermore, they found that the composite containing TOCNC was fully absorbed after 21 days and stimulated the growth of new tissue. These findings collectively demonstrate that CNC-based wound dressings possess distinctive therapeutic advantages over pure polymeric systems and commercial dressings, particularly in accelerating wound healing through enhanced hemostasis, biodegradability, and tissue regeneration capabilities.
Although CNC materials have a good application in wound dressing, it has been found that when CNC is applied in vivo and in vitro models, it may also cause inflammatory reactions. This may be due to the high aspect ratio, insolubility, and biological persistence of CNC [218]. Therefore, the safety of this nanomaterial must be examined and evaluated before clinical studies are conducted.
5.5.2 Drug Delivery and Release
The so-called drug delivery refers to the safe and accurate delivery of drugs to the target area while achieving the purpose of treatment and reducing the impact of side effects and biological toxicity. Through the modification and compounding of cellulose materials, it can not only improve the drug loading capacity of cellulose materials but also control the time of drug release, to accurately deliver the drug to the disease.
Shaikh et al. [219] added CNC to the PVA/guar-gum-based phase separation composite film, and the addition of CNC improved the mechanical properties of the composite film. The composite film can carry moxifloxacin, and the drug can be released by changing the content of CNC in the phase separation film. To solve the problem of oral mucosal drug delivery, Site [220] designed a multilayer nanofiber-foam-to-film (NFF) drug delivery system. The fiber layer is composed of bonded chitosan-based nanofibers, the foam layer is a peptide-loaded hydroxypropyl methylcellulose foam, and the film layer is composed of ethyl cellulose. Experimental results show that the NFF system has good adhesion properties, can improve depressing release, and has important application potential in drug delivery. Narh et al. [221] synthesized cellulose bags from inulin and fructose, which can be used for drug delivery and storage. It also has good resistance to Gram-positive and Gram-negative bacteria. Carmona [222] prepared porous phase separation ethyl cellulose/hydroxypropyl cellulose (EC/HPC) film by continuous spin coating method. They also analyzed the relationship between different mixing behaviors and the structural evolution of the multilayer through Cahn-Hilliard simulations. The porous multilayer composite film can be used in the field of drug delivery.
A human health detector is a wearable, implantable, and flexible electronic device that can be used to detect human physio-physical and physio-chemical information. Cellulose base material due to its good biocompatibility, low cost, portability, and other characteristics, is a functional carrier, and tests are widely applied to human body health.
In terms of human motion, sensors made of cellulose composite materials can monitor human motion conditions in real-time, such as heartbeat and pulse, and collect human activity information like electronic skin to monitor human health in real time. The most common approach is to combine cellulosic materials with carbon nanotubes or conductive silver materials, which can make the material conductive and thus produce a sensor. Xu et al. [223] designed a 3D printable flexible composite based on modified cellulose nanofibers/carbon nanotubes/silicone rubber (MCNF/CNT/SR). The surface of CNF was modified by silane to improve its interface compatibility and mechanical properties of the composite film. Because CNT has good thixotropic properties, it can be used to detect human motion. Lu et al. [224] prepared a series of thermoplastic cellulose grafted copolymers with furfural groups by selecting cellulose, fatty acids, and furfural as raw materials and using a metal-free ATRP system. Then, epoxidized soybean oil bearing 6-maleimidohexanoic group (ESOM) was used as a crosslinking agent to react with the furfural group in cellulose graft copolymer, and the elastomer with dynamic crosslinking was prepared. The elastomer could be used as a wearable sensor after being combined with carbon nanotubes (CNT). Fan [179] chose AgNW conductors as filler to be combined with the prepared CNC/PU composite material to prepare a healing conductive composite material with an electrical conductivity of 0.79 ± 0.02 S/m, which can be used as a sensor to detect human movement.
In addition to the addition of common conductive fillers, the cellulose material can also be endowed with conductive ability by functional modification to prepare a human body sensor. Han et al. [225] prepared polypyrrole (Ppy) to cover CNC and CNF materials through the oxidation of ferric chloride (FeCl3) and used it as a conductive filler for the enhancement of polyvinyl alcohol. In addition, by virtue of the weak hydrogen bond and coordination bond of the fillers, the PVA composite film can also self-heal. The film has good mechanical recovery and conductive recovery properties, and the material is very sensitive to small strain (<48.5%). Therefore, this film can be used to detect human motion and has great potential for applications in skin sensing and body detection. Lu [226] used a polymerizable deep eutectic solvent for photopolymerization to prepare cellulose derived ionic conductive elastomers (ICEs) with high electrical conductivity and environmental stability. The carboxylate cellulose nanocrystals (C-CNC) can be used as a template for in situ polymerization of aniline. Through hydrogen bonds and strong coordination bonds, ICEs have high mechanical strength, toughness, and elasticity, which can be used to prepare multifunctional strain, humidity, and temperature sensors to detect human physiological conditions.
In addition to human activity detection, cellulose-based sensors can also detect the physiological chemical situation inside the human body. By detecting and collecting the concentration of specific biomarkers, a relevant assessment of physical health can be made. Abdel Rahman et al. [227] prepared nano-functional films by mixing cellulose acetate (CA) with ionic liquid glycerol (IL), polypyrrole, and tungsten oxide nanoparticles. The functional film can sensitively detect H2S gas (minimum working temperature 20°C, minimum gas concentration 1 ppm), and has a high response speed (31.7 s), which can be used to detect H2S gas exhaled from the human mouth to understand human health. Jia et al. [228] used the hydroxyl group on cellulose acetate (CA) to react with the indicator fluorescein isothiocyanate (FITC) and the reference protoporphyrin IX (PpIX) to bind to CA, respectively, to obtain CA-FITC emitting green light and CA-PpIX emitting red light. The double-emission solid fluorescent material can be obtained by mixing them in different proportions. This material responses quickly in the ammonia concentration range of 5.0 ppm to 2.5 × 104 ppm and can be used for human health detection.
5.5.6 Human Body Surface Environment Detection
Cellulose-based bio detectors can also detect changes in the body’s surface environment, such as body temperature, PH, and moisture. By understanding the real-time situation of the body’s surface, it can determine whether the body is healthy or not. Kim et al. [229] prepared a PVA/CNC composite film, which has a certain self-healing ability and is affected by CNC content, temperature, and PH value. By combining the composite film with gold nanosheet (AuNS) and polyaniline/multiwalled nanotube (PANI/MWCNT) electrodes, supercapacitors, and temperature sensors can be made, which play an important role in human body temperature health detection. The device is shown in Fig. 16.
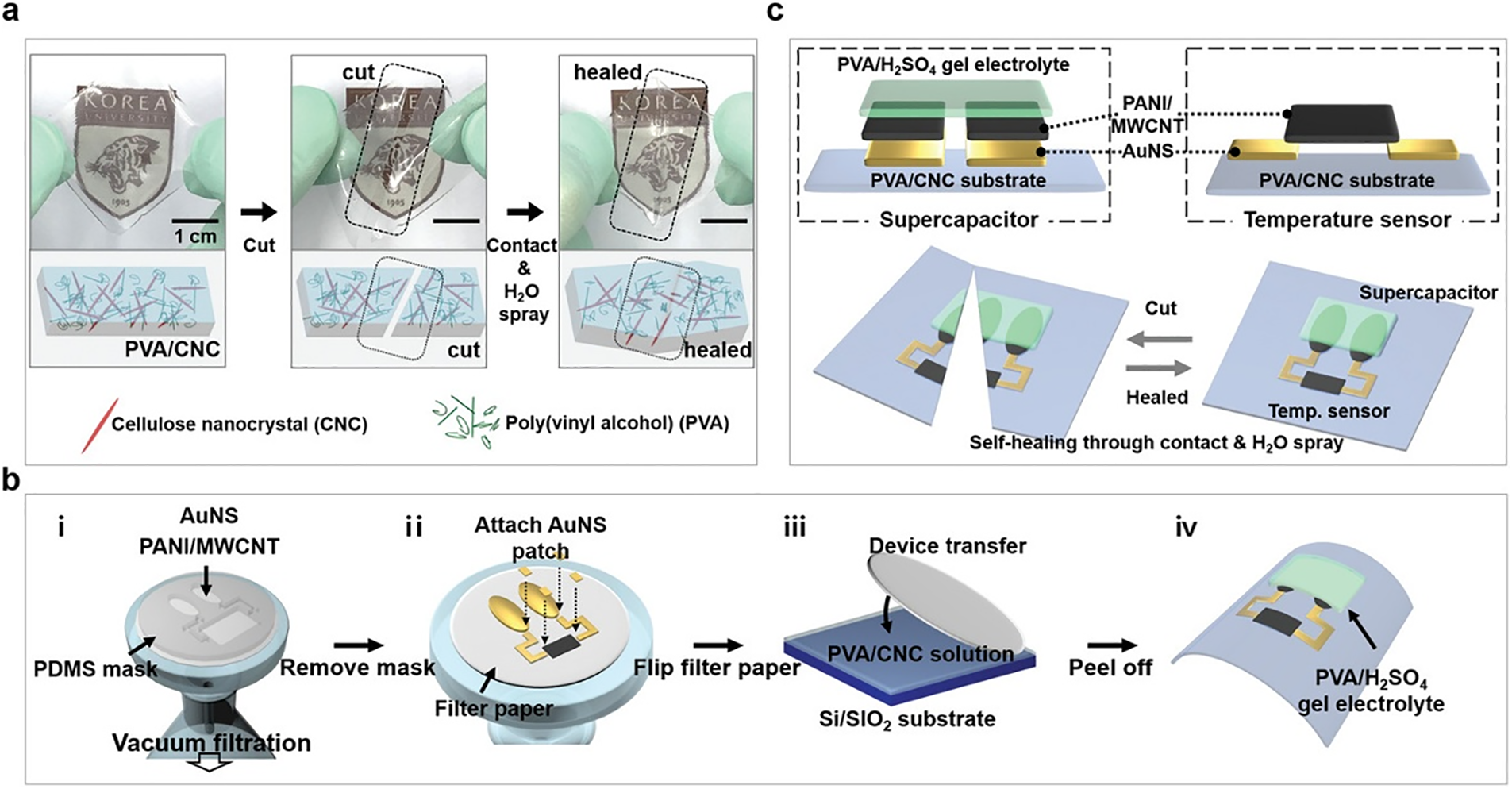
Figure 16: (a) Photographs of the original PVA/CNC composite film with the self-healing process. (b) Supercapacitors and temperature sensors are prepared by vacuum filtration transfer method. (c) Detailed structure of the self-healing mechanism. Adapted with permission from Reference [229], Copyright © 2019 John Wiley & Sons
Wang et al. [230] prepared a transparent and flexible cellulose/KOH composite ionic film (CKF), which has the characteristics of humidity sensing. They assembled the CKF into a skin moisture detector, which can be used to detect the real-time moisture of human skin. Xu et al. [231] designed a pencil-paper-based skin electronic device that can detect biophysical and chemical information about the human body. They can detect human health information in real time by using a pencil to draw graphite patterns on cellulose paper as conductive traces and electrodes. In addition, they have developed a self-powered skin transdermal drug delivery system on a pencil-to-paper basis.
5.5.7 Biological Separation Membrane Field
Cellulose-based materials have important applications in the pharmaceutical field and human organ replacement, such as kidney metabolism due to their green, natural, non-cytotoxic, and biocompatible properties.
In the pharmaceutical field, due to the chiral characteristics of the synthesized drugs, the purity of the target drugs is not high, which limits the application of drugs in medicine. In response to this situation, Ke [232] first synthesized diethylamino-beta-cyclodextrin (EDA-β-CD) chiral monomer, and then polymerized the monomer and trimesoyl chloride (TMC) on the surface of cellulose acetate membrane by in situ interfacial polymerization to obtain CTFC membrane. Through experiments, they showed that the compound can separate some chiral drugs (such as ibuprofen, ketoprofen, nefopam, etc.), so it has important application value in the field of pharmaceutical separation. Luo [233] carried out physicochemical modification of cellulose acetate by using β-cyclodextrin covalent organic framework (β-CD COF) as a chiral selector. Thus, two types of β-CD COF films (β-CD COF mixed matrix membrane (β-CD COF MMM) and β-CD COF thin film nanocomposite membrane (β-CD COF TFN)) were prepared. Moreover, β-CD COF TFN showed better enantioselectivity than β-CD COF MMM, and it had better enantiomeric separation effect on D, L-tryptophan (e.e % = 100%), D, L-phenylalanine (e.e % = 36.3%), and (RS) -propranolol (e.e % = 18.0%).
In terms of human metabolism, for some patients with uremia or kidney damage, it is necessary to eliminate the toxins in the body through hemodialysis. The qualified dialysis membrane needs to have good biocompatibility and selective permeability. Faria et al. [234] proposed a new method for the synthesis of separation membranes. They combined phase transformation and sol-gel technology to prepare cellulose acetate/silica (CASiO2) membranes. The film has good blood compatibility and permeability characteristics, can be used in human kidney metabolism, can ensure urea preferential penetration and protein retention, and has potential application value in organ replacement. Azhar et al. [235] modified the CA hemodialysis membrane by adding polyvinyl alcohol (PVA) and polyethylene glycol (PEG) and prepared the CA-PVA membrane by the phase transition method. Through experimental tests, it was found that the film could reject the passage of 95% bovine serum albumin (BSA), separate 93% urea and 89% creatinine, and the blood compatibility was better than the traditional CA membrane. Therefore, the film has better biocompatibility and permeability than the traditional CA film.
The extracellular matrix of cell culture is essential for cell attachment, differentiation, and growth. Therefore, the carrier of cell culture needs to have good physical and chemical stability, good biocompatibility (such as cells, tissues, or microorganisms), and not cause toxic reactions or immune reactions.
Cellulosic composites have been widely used in the field of cell culture because of their natural and non-toxic characteristics and good biocompatibility. Pazzi et al. [236] proposed a technique using cellulose paper (composed of nanocellulose) to facilitate the simplified and efficient assembly of giant unilamellar vesicles (GUVs). The technology addresses barriers that hinder the delivery of GUVs drugs, synthetic cells, and artificial tissue assembly vectors. Hua et al. [237] studied the behavior of monocyte/macrophage (MM) by preparing nano-fibrotic fibers with different surface modifications (anionic (a-NFC), cationic (c-NFC) and unmodified (u-NFC)). It was demonstrated that MM cells cultured on A-NFC (carboxymethylated) were activated with a proinflammatory phenotype, whereas cells cultured on u-NFC showed mild activation. As for c-NFC (hydroxypropyl trimethylammonium ylation), the material did not promote MM cell activation. Therefore, NFC materials have great potential for application in the culture and control of MM cells.
In this review, the structural properties and chemical properties of cellulosic materials are explored in depth. Cellulose materials, due to their abundance of hydroxyl groups, with three unbound hydroxyl groups on each glucose unit, contribute to an extensive network of hydrogen bonds. This hydrogen bond network endows cellulose materials with superior structural rigidity and thermal stability. At the same time, the hydroxyl group on the surface of cellulose has high reactivity, which lays a solid foundation for the functionalization of cellulose.
Although the hydrogen bonding network of cellulose materials gives them unique physical and chemical properties, this network also increases the complexity of cellulosic processing and application. Therefore, this review summarizes the various solution systems developed by predecessors for the efficient utilization of cellulose, including the alkali system of NaOH aqueous solution, DMAc/LiCl, NMMO, ionic liquid (ILs), and DES system. Despite their use in industry, the alkaline and DMAc/LiCl systems have raised concerns about their impact on the environment due to their toxicity and corrosiveness, which limits their application prospects in sustainability. In contrast, the NMMO, ionic liquid, and DES system show good future development potential in cellulose extraction and utilization due to their green and low toxicity characteristics. Finding a suitable solvent system with high efficiency of cellulose extraction, low cost and energy consumption, and green and recyclable cellulose industrialization is the direction that still needs to continue to strive in the future. Moreover, the stability of the obtained cellulose products needs to be solved continuously.
In order to broaden the application scope of cellulose materials, based on the activity of hydroxyl groups on their surface, researchers have carried out a variety of modification treatments on cellulose materials to obtain corresponding derivatives. This review summarizes the main directions of cellulose material modification technology, including nanocellulose processing, molecule modification, and large molecule modification. These functional modification technologies enable cellulose materials to be customized for different application scenarios. Although these modification technologies have their advantages and disadvantages, in the context of green chemistry in the future, it is still necessary to further explore and solve the problems such as the cost of modification technology, the simplicity of the preparation method, and the degradability of materials. In the future, the green functional modification and application of cellulose will become the focus of research, and improving the green conversion efficiency of cellulose and its derivatives will be the key to research. Through functional modification, the functionalization of cellulosic materials (such as CNC, CNF, etc.) to meet the needs of a variety of different application fields is the focus of future research.
Cellulose material, with its renewable, biodegradable, environmentally friendly, and cost effective has become an important research object in the field of green chemistry. They show unique advantages in several application fields. This review lists a variety of application scenarios for cellulosic materials. In addition to the traditional applications in the field of reinforcement materials, the application of cellulosic materials in self-healing technology is also presented. Besides, we summarize and analyze the application cases of cellulosic materials in emerging technologies such as radiative cooling, nanogenerators, and biomedicine. The utilization of cellulose materials for high-value-added applications is a current and prospective research hotspot.
With the technological development of cellulosic materials, it has promoted the development of various fields in the direction of sustainable green chemistry. The versatility and customizability of cellulosic materials, combined with their contribution to the bioeconomy, offer new possibilities for sustainable development. Therefore, the research on the extraction and utilization, functional modification, and application of cellulose materials is of strategic significance in achieving global sustainable development goals and providing new possibilities for alleviating energy and environmental pressure.
Acknowledgement: Not applicable.
Funding Statement: This work is financially supported by Research Fund for the Doctoral Program of Higher Education of China (20134420120009), Science and Technology Planning Project of Guangdong (2014A010105047) and Science and Technology Planning Project of Guangzhou City (201707010367).
Author Contributions: Wohua He and Haoqun Hong designed the structural framework of the paper. Wohua He and Fangji Wu collected the literature, summarized, and wrote the content. Haoqun Hong acquired funding for this work. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data is available upon request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Li X, Wan C, Tao T, Chai H, Huang Q, Chai Y, et al. An overview of the development status and applications of cellulose-based functional materials. Cellulose. 2024;31:61–99. doi:10.1007/s10570-023-05616-8. [Google Scholar] [CrossRef]
2. Acharya S, Liyanage S, Parajuli P, Rumi SS, Shamshina JL, Abidi N. Utilization of cellulose to its full potential: a review on cellulose dissolution, regeneration, and applications.Polymers.2021;13(24):4344. doi:10.3390/polym13244344. [Google Scholar] [CrossRef]
3. Varaprasad K, Raghavendra GM, Jayaramudu T, Seo J. Nano zinc oxide-sodium alginate antibacterial cellulose fibres. Carbohydr Polym. 2016;135:349–55. doi:10.1016/j.carbpol.2015.08.078. [Google Scholar] [PubMed] [CrossRef]
4. Zhao D, Zhu Y, Cheng W, Chen W, Wu Y, Yu H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv Mater Deerfield. 2021;33:2000619. doi:10.1002/adma.202000619. [Google Scholar] [PubMed] [CrossRef]
5. Sharma A, Thakur M, Bhattacharya M, Mandal T, Goswami S. Commercial application of cellulose nano-composites—a review. Biotechnol Rep. 2019;21:e00316. doi:10.1016/j.btre.2019.e00316. [Google Scholar] [PubMed] [CrossRef]
6. Ling S. Biopolymer nanofibrils: structure, modeling, preparation, and applications. Prog PolymSci. 2018;85:1–56. [Google Scholar] [PubMed]
7. Joseph B, K. SV, Sabu C, Kalarikkal N, Thomas S. Cellulose nanocomposites: fabrication and biomedical applications. J Bioresources Bioproducts. 2020;5:223–37. doi:10.1016/j.jobab.2020.10.001. [Google Scholar] [CrossRef]
8. Shi Z, Yang Q, Kuga S, Matsumoto Y. Dissolution of wood pulp in aqueous NaOH/Urea solution via dilute acid pretreatment. J Agric Food Chem. 2015;63:6113–9. doi:10.1021/acs.jafc.5b01714. [Google Scholar] [PubMed] [CrossRef]
9. Trygg J, Fardim P. Enhancement of cellulose dissolution in water-based solvent via ethanol-hydrochloric acid pretreatment. Cellulose. 2011;18:987–94. doi:10.1007/s10570-011-9550-y. [Google Scholar] [CrossRef]
10. Gong X, Wang Y, Tian Z, Zheng X, Chen L. Controlled production of spruce cellulose gels using an environmentally “green” system. Cellulose. 2014;21:1667–78. doi:10.1007/s10570-014-0200-z. [Google Scholar] [CrossRef]
11. Nawaz H, He A, Wu Z, Wang X, Jiang Y, Ullah A, et al. Revisiting various mechanistic approaches for cellulose dissolution in different solvent systems: a comprehensive review. Int J Biol Macromol. 2024;273:133012. doi:10.1016/j.ijbiomac.2024.133012. [Google Scholar] [PubMed] [CrossRef]
12. Satari B, Karimi K, Kumar R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: a review. Sustainable Energy Fuels. 2019;3:11–62. doi:10.1039/C8SE00287H. [Google Scholar] [CrossRef]
13. Acharya S, Hu Y, Abidi N. Cellulose dissolution in ionic liquid under mild conditions: effect of hydrolysis and temperature. Fibers. 2021;9:5. doi:10.3390/fib9010005. [Google Scholar] [CrossRef]
14. Kuo C-H, Lee C-K. Enhancement of enzymatic saccharification of cellulose by cellulose dissolution pretreatments. Carbohydr Polym. 2009;77:41–6. doi:10.1016/j.carbpol.2008.12.003. [Google Scholar] [CrossRef]
15. Ghasemi M, Tsianou M, Alexandridis P. Assessment of solvents for cellulose dissolution. Bioresour Technol. 2017;228:330–8. doi:10.1016/j.biortech.2016.12.049. [Google Scholar] [PubMed] [CrossRef]
16. Ghasemi M, Alexandridis P, Tsianou M. Dissolution of cellulosic fibers: impact of crystallinity and fiber diameter. Biomacromolecules. 2018;19:640–51. doi:10.1021/acs.biomac.7b01745. [Google Scholar] [PubMed] [CrossRef]
17. Medronho B, Lindman B. Competing forces during cellulose dissolution: from solvents to mechanisms. Curr Opinion Coll Interface Sci. 2014;19:32–40. doi:10.1016/j.cocis.2013.12.001. [Google Scholar] [CrossRef]
18. Bergenstråhle M, Wohlert J, Himmel ME, Brady JW. Simulation studies of the insolubility of cellulose. Carbohydr Res. 2010;345:2060–6. doi:10.1016/j.carres.2010.06.017. [Google Scholar] [PubMed] [CrossRef]
19. Medronho B, Lindman B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv Colloid Interface Sci. 2015;222:502–8. doi:10.1016/j.cis.2014.05.004. [Google Scholar] [PubMed] [CrossRef]
20. Medronho B, Romano A, Miguel MG, Stigsson L, Lindman B. Rationalizing cellulose (in)solubility: reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose. 2012;19:581–7. doi:10.1007/s10570-011-9644-6. [Google Scholar] [CrossRef]
21. Lindman B, Karlström G, Stigsson L. On the mechanism of dissolution of cellulose. J Mol Liq. 2010;156:76–81. doi:10.1016/j.molliq.2010.04.016. [Google Scholar] [CrossRef]
22. Alves L, Medronho B, Antunes FE, Topgaard D, Lindman B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose. 2016;23:247–58. doi:10.1007/s10570-015-0809-6. [Google Scholar] [CrossRef]
23. Kamide K, Okajima K, Kowsaka K. Dissolution of natural cellulose into aqueous alkali solution: role of super-molecular structure of cellulose. Polym J. 1992;24:71–86. doi:10.1295/polymj.24.71. [Google Scholar] [CrossRef]
24. Davidson GF. 12—The dissolution of chemically modified cotton cellulose in alkaline solutions. Part I—In solutions of sodium hydroxide, particularly at temperatures below the normal. J Text Inst Trans. 1934;25:T174–96. doi:10.1080/19447023408661621. [Google Scholar] [CrossRef]
25. He H, An F, Wang Y, Wu W, Huang Z, Song H. Effects of pretreatment, NaOH concentration, and extraction temperature on the cellulose from Lophatherum gracile Brongn. Int J Biol Macromol. 2021;190:810–8. doi:10.1016/j.ijbiomac.2021.09.041. [Google Scholar] [PubMed] [CrossRef]
26. Cai J, Zhang L, Liu S, Liu Y, Xu X, Chen X, et al. Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules. 2008;41:9345–51. doi:10.1021/ma801110g. [Google Scholar] [CrossRef]
27. Xiong B, Zhao P, Cai P, Zhang L, Hu K, Cheng G. NMR spectroscopic studies on the mechanism of cellulose dissolution in alkali solutions. Cellulose. 2013;20:613–21. doi:10.1007/s10570-013-9869-7. [Google Scholar] [CrossRef]
28. Lu A, Liu Y, Zhang L, Potthast A. Investigation on metastable solution of cellulose dissolved in NaOH/Urea aqueous system at low temperature. J Phys Chem B. 2011;115:12801–8. doi:10.1021/jp206643f. [Google Scholar] [PubMed] [CrossRef]
29. Peng J, Fu R, Huang Y, Lu J, Xie X, Xue Z, et al. Influence and mechanism of NaOH concentration on the dissolution of cellulose and extraction of CNF in alkaline solvents at 15°C. Carbohydr Polym. 2025;353:123265. doi:10.1016/j.carbpol.2025.123265. [Google Scholar] [PubMed] [CrossRef]
30. Wang Y, Zhao Y, Deng Y. Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr Polym. 2008;72:178–84. doi:10.1016/j.carbpol.2007.08.003. [Google Scholar] [CrossRef]
31. Dawsey TR, McCormick CL. The lithium chloride/dimethylacetamide solvent for cellulose: a literature review. J Macromol Sci, Part C. 1990;30(3–4):405–40. doi:10.1080/07366579008050914. [Google Scholar] [CrossRef]
32. Rumi SS, Liyanage S, Abidi N. Conversion of low-quality cotton to bioplastics. Cellulose. 2021;28:2021–38. doi:10.1007/s10570-020-03661-1. [Google Scholar] [CrossRef]
33. Röder T, Potthast A, Rosenau T, Kosmsa P, Baldinger T, Morgenstern B, et al. The effect of water on cellulose solutions in DMAc/LiCl. Macromol Symp. 2002;190:151–60. doi:10.1002/masy.200290011. [Google Scholar] [CrossRef]
34. Potthast A, Rosenau T, Sixta H, Kosma P. Degradation of cellulosic materials by heating in DMAc/LiCl. Tetrahedron Lett. 2002;43:7757–9. doi:10.1016/S0040-4039(02)01767-7. [Google Scholar] [CrossRef]
35. Shi Z, Liu Y, Xu H, Yang Q, Xiong C, Kuga S, et al. Facile dissolution of wood pulp in aqueous NaOH/urea solution by ball milling pretreatment. Ind Crops Prod. 2018;118:48–52. doi:10.1016/j.indcrop.2018.03.035. [Google Scholar] [CrossRef]
36. Sen S, Martin JD, Argyropoulos DS. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustainable Chem Eng. 2013;1:858–70. [Google Scholar]
37. Ma Y-Y, Lu Z-L, Xing Y-Z, Zheng W-S, Liu C-G. A fresh perspective on dissociation mechanism of cellulose in DMAc/LiCl system based on Li bond theory. Int J Biol Macromol. 2024;268:131729. [Google Scholar] [PubMed]
38. Acharya S, Hu Y, Moussa H, Abidi N. Preparation and characterization of transparent cellulose films using an improved cellulose dissolution process. J Appl Polym Sci. 2017;134:44871. [Google Scholar]
39. Sayyed AJ, Deshmukh NA, Pinjari DV. A critical review of manufacturing processes used in regenerated cellulosic fibres: viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose. 2019;26:2913–40. [Google Scholar]
40. Gao Q, Shen X, Lu X. Regenerated bacterial cellulose fibers prepared by the NMMO·H2O process. Carbohydr Polym. 2011;83:1253–6. [Google Scholar]
41. Zhang S, Chen C, Duan C, Hu H, Li H, Li J, et al. Regenerated cellulose by the Lyocell process, a brief review of the process and properties. BioResources. 2018;13:4577–92. doi:10.15376/biores.13.2.Zhang. [Google Scholar] [CrossRef]
42. Ruan D, Zhang L, Zhou J, Jin H, Chen H. Structure and properties of novel fibers spun from cellulose in NaOH/Thiourea aqueous solution. Macromol Biosci. 2004;4:1105–12. doi:10.1002/mabi.200400120. [Google Scholar] [PubMed] [CrossRef]
43. Rosenau T, Potthast A, Sixta H, Kosma P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog Polym Sci. 2001;26:1763–837. doi:10.1016/S0079-6700(01)00023-5. [Google Scholar] [CrossRef]
44. Hudson SM, Cuculo JA. The solubility of unmodified cellulose: a critique of the literature. J Macromol Sci, Part C. 1980;18:1–82. doi:10.1080/00222358008080915. [Google Scholar] [CrossRef]
45. Chanzy H, Peguy A, Chaunis S, Monzie P. Oriented cellulose films and fibers from a mesophase system. J Polym Sci Polym Phys Ed. 1980;18:1137–44. doi:10.1002/pol.1980.180180517. [Google Scholar] [CrossRef]
46. Pinkert A, Marsh KN, Pang S. Reflections on the solubility of cellulose. Ind Eng Chem Res. 2010;49:11121–30. doi:10.1021/ie1006596. [Google Scholar] [CrossRef]
47. Rosenau T, Potthast A, Adorjan I, Hofinger A, Firgo H, Kosma P. Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)—degradation processes and stabilizers. Cellulose. 2002;9:283–91. doi:10.1023/A:1021127423041. [Google Scholar] [CrossRef]
48. Rogers RD, Seddon KR. Ionic liquids–solvents of the future? Science. 2003;302:792–3. doi:10.1126/science.1090313. [Google Scholar] [PubMed] [CrossRef]
49. Umapathi R, Kumar K, Ghoreishian SM, Rani GM, Huh YS, Venkatesu P. Interactions between a biomedical thermoresponsive polymer and imidazolium-based ionic liquids: a comprehensive biophysical investigation. Coll Surfaces A: Physicochem Eng Aspects. 2022;641:128619. doi:10.1016/j.colsurfa.2022.128619. [Google Scholar] [CrossRef]
50. Zhou J, Sui H, Jia Z, Yang Z, He L, Li X. Recovery and purification of ionic liquids from solutions: a review. RSC Adv. 2018;8:32832–64. doi:10.1039/C8RA06384B. [Google Scholar] [PubMed] [CrossRef]
51. Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Dissolution of cellose with ionic liquids. J Am Chem Soc. 2002;124:4974–5. doi:10.1021/ja025790m. [Google Scholar] [PubMed] [CrossRef]
52. Wang H, Gurau G, Rogers RD. Ionic liquid processing of cellulose. Chem Soc Rev. 2012;41:1519. doi:10.1039/c2cs15311d. [Google Scholar] [PubMed] [CrossRef]
53. Zhao Y, Liu X, Wang J, Zhang S. Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation. Carbohydr Polym. 2013;94:723–30. doi:10.1016/j.carbpol.2013.02.011. [Google Scholar] [PubMed] [CrossRef]
54. Zhang J, Zhang H, Wu J, Zhang J, He J, Xiang J. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys Chem Chem Phys. 2010;12:1941. doi:10.1039/b920446f. [Google Scholar] [PubMed] [CrossRef]
55. Li Y, Liu X, Zhang Y, Jiang K, Wang J, Zhang S. Why only ionic liquids with unsaturated heterocyclic cations can dissolve cellulose: a simulation study. ACS Sustainable Chem Eng. 2017;5:3417–28. doi:10.1021/acssuschemeng.7b00073. [Google Scholar] [CrossRef]
56. Rahman MM, Jahan MS, Islam MDM, Susan MDABH. Dissolution of cellulose in imidazolium-based double salt ionic liquids. Int J Biol Macromol. 2024;267:131331. doi:10.1016/j.ijbiomac.2024.131331. [Google Scholar] [PubMed] [CrossRef]
57. Xu A, Zhang Y, Zhao Y, Wang J. Cellulose dissolution at ambient temperature: role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr Polym. 2013;92:540–4. doi:10.1016/j.carbpol.2012.09.028. [Google Scholar] [PubMed] [CrossRef]
58. Turner MB, Spear SK, Holbrey JD, Rogers RD. Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules. 2004;5:1379–84. doi:10.1021/bm049748q. [Google Scholar] [PubMed] [CrossRef]
59. Dissanayake N. Dissolution of cotton cellulose in 1: 1 mixtures of 1-butyl-3-methylimidazolium methylphosphonate and 1-alkylimidazole co-solvents. Carbohydrate Polymers. 2019;221:63–72. doi:10.1016/j.carbpol.2019.05.071. [Google Scholar] [PubMed] [CrossRef]
60. Lin Z, Liu X, Jiao B. Deep eutectic solvents-modified advanced functional materials for pollutant detection in food and the environment. Trends Analyt Chem. 2023;159:116923. doi:10.1016/j.trac.2023.116923. [Google Scholar] [CrossRef]
61. Lai P, Zhou H, Niu Z, Li L, Zhu W, Dai L. Deep eutectic solvent-mediated preparation of solvothermal carbon with rich carboxyl and phenol groups from crop straw for high-efficient uranium adsorption. Chem Eng J. 2023;457:141255. doi:10.1016/j.cej.2022.141255. [Google Scholar] [CrossRef]
62. Chen Y-L, Zhang X, You T-T, Xu F. Deep eutectic solvents (DESs) for cellulose dissolution: a mini-review. Cellulose. 2019;26:205–13. doi:10.1007/s10570-018-2130-7. [Google Scholar] [CrossRef]
63. Zhang L, Yu H, Liu S, Wang Y, Mu T, Xue Z. Kamlet—Taft parameters of deep eutectic solvents and their relationship with dissolution of main lignocellulosic components. Ind Eng Chem Res. 2023;62:11723–34. doi:10.1021/acs.iecr.3c01309. [Google Scholar] [CrossRef]
64. Lynam JG, Kumar N, Wong MJ. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour Technol. 2017;238:684–9. doi:10.1016/j.biortech.2017.04.079. [Google Scholar] [PubMed] [CrossRef]
65. Kumar K, Bisht M, Arora H, Ghoreishian SM, Umapathi R, Huh YS, et al. Temperature-switchable polymer: uniting deep eutectic solvents with Poly(N-isopropylacrylamide) and Poly(N-vinyl caprolactam). ACS Sustainable Chem Eng. 2022;10:9991–10002. doi:10.1021/acssuschemeng.2c02613. [Google Scholar] [CrossRef]
66. Ma H, Fu P, Zhao J, Lin X, Wu W, Yu Z, et al. Pretreatment of wheat straw lignocelluloses by deep eutectic solvent for lignin extraction. Molecules. 2022;27:7955. doi:10.3390/molecules27227955. [Google Scholar] [PubMed] [CrossRef]
67. Xiong Y, Li W, Qin Z, Su T, Xie X, Ji H. A green extraction technology of lignocellulose from cassava residue by mechanical activation-assisted ternary deep eutectic solvent. Int J Biol Macromol. 2024;281:136339. doi:10.1016/j.ijbiomac.2024.136339. [Google Scholar] [PubMed] [CrossRef]
68. Al Ragib A, Alanazi YM, El-Harbawi M, Yin C-Y, Khiari R. Sustainable reuse of date palm biomass via extraction of cellulose using natural deep eutectic solvent (NaDES) and microwave-assisted process. Int J Biol Macromol. 2024;279:135558. doi:10.1016/j.ijbiomac.2024.135558. [Google Scholar] [PubMed] [CrossRef]
69. Huang H, Tang Q, Lin G, Yu C, Wang H, Li Z. High-efficiency and recyclable ramie cellulose fiber degumming enabled by deep eutectic solvent. Ind Crops Prod. 2021;171:113879. doi:10.1016/j.indcrop.2021.113879. [Google Scholar] [CrossRef]
70. Xie J, Chen J, Cheng Z, Zhu S, Xu J. Pretreatment of pine lignocelluloses by recyclable deep eutectic solvent for elevated enzymatic saccharification and lignin nanoparticles extraction. Carbohydr Polym. 2021;269:118321. doi:10.1016/j.carbpol.2021.118321. [Google Scholar] [PubMed] [CrossRef]
71. Li P, Dong C, Pang Z, Chen X. Utilization of benzoic acid-based green deep eutectic solvents for the fractionation of lignocellulosic biomass. Int J Biol Macromol. 2024;282:137062. doi:10.1016/j.ijbiomac.2024.137062. [Google Scholar] [PubMed] [CrossRef]
72. Xu Y, Zhu B, Ge H, Wang S, Li B, Xu H. Microwave-assisted extraction of cellulose and aromatic compounds from rose petals based on deep eutectic solvent. Int J Biol Macromol. 2024;258:129058. doi:10.1016/j.ijbiomac.2023.129058. [Google Scholar] [PubMed] [CrossRef]
73. Wu F, He W, Hong H, Wei T. Sustainable method to prepare nanocellulose with enhanced stability and structural uniformity by using deep eutectic solvents. Cellulose Chem Technol. 2024;58:959–71. doi:10.35812/CelluloseChemTechnol.2024.58.83. [Google Scholar] [CrossRef]
74. Wang Y. Production of nanocellulose using acidic deep eutectic solvents based on choline chloride and carboxylic acids: a review. Int J Biol Macromol. 2023;245:125227. doi:10.1016/j.ijbiomac.2023.125227. [Google Scholar] [PubMed] [CrossRef]
75. Liu S, Zhang Q, Gou S, Zhang L, Wang Z. Esterification of cellulose using carboxylic acid-based deep eutectic solvents to produce high-yield cellulose nanofibers. Carbohydr Polym. 2021;251:117018. doi:10.1016/j.carbpol.2020.117018. [Google Scholar] [PubMed] [CrossRef]
76. Ma G, Yin W, Shi Z, Xu Y, Yang G. Direct carboxymethylation of cellulose fibers by ternary reactive deep eutectic solvents for the preparation of high-yield cellulose nanofibrils. ACS Sustainable Chem Eng. 2024;12:5871–83. [Google Scholar]
77. Yan G, Zhou Y, Zhao L, Wang W, Yang Y, Zhao X, et al. Recycling of deep eutectic solvent for sustainable and efficient pretreatment of corncob. Ind Crops Prod. 2022;183:115005. [Google Scholar]
78. Wang J, Zhang S, Ma Z, Yan L. Deep eutectic solvents eutectogels: progress and challenges. Green Chem Eng. 2021;2:359–67. [Google Scholar]
79. Liang X, Fu Y, Chang J. Effective separation, recovery and recycling of deep eutectic solvent after biomass fractionation with membrane-based methodology. Sep Purif Technol. 2019;210:409–16. [Google Scholar]
80. Yang X, Xie H, Du H, Zhang X, Zou Z, Zou Y, et al. Facile extraction of thermally stable and dispersible cellulose nanocrystals with high yield via a green and recyclable FeCl3-catalyzed deep eutectic solvent system. ACS Sustainable Chem Eng. 2019;7:7200–8. [Google Scholar]
81. Xiao T, Hou M, Guo X, Cao X, Li C, Zhang Q, et al. Recent progress in deep eutectic solvent (DES) fractionation of lignocellulosic components: a review. Renew Sustain Energ Rev. 2024;192:114243. doi:10.1016/j.rser.2023.114243. [Google Scholar] [CrossRef]
82. Yamashiki T, Matsui T, Saitoh M, Okajima K, Kamide K, Sawada T. Characterisation of cellulose treated by the steam explosion method. Part 1: influence of cellulose resources on changes in morphology, degree of polymerisation, solubility and solid structure. Brit Poly J. 1990;22:73–83. doi:10.1002/pi.4980220111. [Google Scholar] [CrossRef]
83. Jerosch H, Lavédrine B, Cherton J-C. Study of the stability of cellulose-holocellulose solutions in N,N-dimethylacetamide-lithium chloride by size exclusion chromatography. J Chromatogr A. 2001;927:31–8. doi:10.1016/S0021-9673(01)01094-9. [Google Scholar] [PubMed] [CrossRef]
84. Zhang H, Wang ZG, Zhang ZN, Wu J, Zhang J, He JS. Carbon-Nanotube composite fibers with enhanced mechanical properties prepared with the Ionic Liquid 1-Allyl-3-methylimidazolium Chloride. Adv Mater. 2007;19:698–704. doi:10.1002/adma.200600442. [Google Scholar] [CrossRef]
85. Sayyed AJ, Mohite LV, Deshmukh NA, Pinjari DV. Structural characterization of cellulose pulp in aqueous NMMO solution under the process conditions of lyocell slurry. Carbohydr Polym. 2019;206:220–8. doi:10.1016/j.carbpol.2018.11.004. [Google Scholar] [PubMed] [CrossRef]
86. Egal M, Budtova T, Navard P. Structure of aqueous solutions of microcrystalline cellulose/sodium hydroxide below 0°C and the limit of cellulose dissolution. Biomacromolecules. 2007;8:2282–7. doi:10.1021/bm0702399. [Google Scholar] [PubMed] [CrossRef]
87. Kadokawa J, Murakami M, Kaneko Y. A facile preparation of gel materials from a solution of cellulose in ionic liquid. Carbohydr Res. 2008;343:769–72. doi:10.1016/j.carres.2008.01.017. [Google Scholar] [PubMed] [CrossRef]
88. Zhang H, Wu Y, Zhang J, Wu Z, Zhan X. Separation cellulose nanocrystals from microcrystalline cellulose using hydrated deep eutectic solvent and high shear force. Ind Crops Prod. 2022;189:115781. doi:10.1016/j.indcrop.2022.115781. [Google Scholar] [CrossRef]
89. Yang X, Biswas SK, Han J, Tanpichai S, Li M, Chen C, et al. Surface and interface engineering for nanocellulosic advanced materials. Adv Mater Deerfield. 2021;33:2002264. doi:10.1002/adma.202002264. [Google Scholar] [PubMed] [CrossRef]
90. Damay J, Duret X, Ghislain T, Lalonde O, Lavoie J-M. Steam explosion of sweet sorghum stems: optimisation of the production of sugars by response surface methodology combined with the severity factor. Ind Crops Prod. 2018;111:482–93. doi:10.1016/j.indcrop.2017.11.006. [Google Scholar] [CrossRef]
91. Nechyporchuk O, Belgacem MN, Bras J. Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod. 2016;93:2–25. doi:10.1016/j.indcrop.2016.02.016. [Google Scholar] [CrossRef]
92. Li J, Wang Y, Wei X, Wang F, Han D, Wang Q, et al. Homogeneous isolation of nanocelluloses by controlling the shearing force and pressure in microenvironment. Carbohydr Polym. 2014;113:388–93. doi:10.1016/j.carbpol.2014.06.085. [Google Scholar] [PubMed] [CrossRef]
93. Nagarajan KJ, Balaji AN, Ramanujam NR. Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment. Carbohydr Polym. 2019;212:312–22. doi:10.1016/j.carbpol.2019.02.063. [Google Scholar] [PubMed] [CrossRef]
94. Chen W, Abe K, Uetani K, Yu H, Liu Y, Yano H. Individual cotton cellulose nanofibers: pretreatment and fibrillation technique. Cellulose. 2014;21:1517–28. doi:10.1007/s10570-014-0172-z. [Google Scholar] [CrossRef]
95. Liu X, Sun H, Mu T, Fauconnier ML, Li M. Preparation of cellulose nanofibers from potato residues by ultrasonication combined with high-pressure homogenization. Food Chem. 2023;413:135675. doi:10.1016/j.foodchem.2023.135675. [Google Scholar] [PubMed] [CrossRef]
96. Osong SH, Norgren S, Engstrand P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose. 2016;23:93–123. doi:10.1007/s10570-015-0798-5. [Google Scholar] [CrossRef]
97. Huang D, Hong H, Huang W, Zhang H, Hong X. Scalable preparation of cellulose nanofibers from office waste paper by an environment-friendly method. Polymers. 2021;13:3119. doi:10.3390/polym13183119. [Google Scholar] [PubMed] [CrossRef]
98. Liu S, Tian Z, Ji X-X, Ma M-G. Preparation and modification of nanocellulose using deep eutectic solvents and their applications. Cellulose. 2024;31:2175–205. doi:10.1007/s10570-024-05738-7. [Google Scholar] [CrossRef]
99. Yu W, Wang C, Yi Y, Zhou W, Wang H, Yang Y, et al. Choline chloride-based deep eutectic solvent systems as a pretreatment for nanofibrillation of ramie fibers. Cellulose. 2019;26:3069–82. doi:10.1007/s10570-019-02290-7. [Google Scholar] [CrossRef]
100. Teixeira RSS, Silva ASD, Jang J-H, Kim H-W, Ishikawa K, Endo T, et al. Combining biomass wet disk milling and endoglucanase/β-glucosidase hydrolysis for the production of cellulose nanocrystals. Carbohydr Polym. 2015;128:75–81. doi:10.1016/j.carbpol.2015.03.087. [Google Scholar] [PubMed] [CrossRef]
101. Gui X, Wan Z, Zhang H, Niu M, Guo Y, Li H. Preparation of cellulose nanocrystals by ultrasonication-assisted phosphotungstic acid method: an effective method of high yield and friendly environment. Ind Crops Prod. 2024;222:119780. doi:10.1016/j.indcrop.2024.119780. [Google Scholar] [CrossRef]
102. Zianor Azrina ZA, Beg MDH, Rosli MY, Ramli R, Junadi N, Alam AKMM. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr Polym. 2017;162:115–20. doi:10.1016/j.carbpol.2017.01.035. [Google Scholar] [PubMed] [CrossRef]
103. Rosa MF, Medeiros ES, Malmonge JA, Gregorski KS, Wood DF, Mattoso LHC, et al. Cellulose nanowhiskers from coconut husk fibers: effect of preparation conditions on their thermal and morphological behavior. Carbohydr Polym. 2010;81:83–92. doi:10.1016/j.carbpol.2010.01.059. [Google Scholar] [CrossRef]
104. Edgar CD, Gray DG. Smooth model cellulose I surfaces from nanocrystal suspensions. Cellulose. 2003;10:299–306. doi:10.1023/A:1027333928715. [Google Scholar] [CrossRef]
105. Wang H, Li J, Zeng X, Tang X, Sun Y, Lei T, et al. Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent. Cellulose. 2020;27:1301–14. doi:10.1007/s10570-019-02867-2. [Google Scholar] [CrossRef]
106. Sirviö JA, Visanko M, Liimatainen H. Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules. 2016;17:3025–32. doi:10.1021/acs.biomac.6b00910. [Google Scholar] [PubMed] [CrossRef]
107. Zhu S, Sun H, Mu T, Li Q, Richel A. Preparation of cellulose nanocrystals from purple sweet potato peels by ultrasound-assisted maleic acid hydrolysis. Food Chem. 2023;403:134496. doi:10.1016/j.foodchem.2022.134496. [Google Scholar] [PubMed] [CrossRef]
108. Cui S, Zhang S, Ge S, Xiong L, Sun Q. Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind Crops Prod. 2016;83:346–52. doi:10.1016/j.indcrop.2016.01.019. [Google Scholar] [CrossRef]
109. Ren R-W, Chen X-Q, Shen W-H. Preparation and separation of pure spherical cellulose nanocrystals from microcrystalline cellulose by complex enzymatic hydrolysis. Int J Biol Macromol. 2022;202:1–10. doi:10.1016/j.ijbiomac.2022.01.009. [Google Scholar] [PubMed] [CrossRef]
110. Liimatainen H, Visanko M, Sirviö JA, Hormi OEO, Niinimaki J. Enhancement of the nanofibrillation of wood cellulose through sequential periodate-chlorite oxidation. Biomacromolecules. 2012;13:1592–7. doi:10.1021/bm300319m. [Google Scholar] [PubMed] [CrossRef]
111. Zhou L, Li N, Shu J, Liu Y, Wang K, Cui X, et al. One-pot preparation of carboxylated cellulose nanocrystals and their liquid crystalline behaviors. ACS Sustainable Chem Eng. 2018;6:12403–10. doi:10.1021/acssuschemeng.8b02926. [Google Scholar] [CrossRef]
112. Liu Y, Liu L, Wang K, Zhang H, Yuan Y, Wei H, et al. Modified ammonium persulfate oxidations for efficient preparation of carboxylated cellulose nanocrystals. Carbohydr Polym. 2020;229:115572. doi:10.1016/j.carbpol.2019.115572. [Google Scholar] [PubMed] [CrossRef]
113. Thiangtham S, Runt J, Manuspiya H. Sulfonation of dialdehyde cellulose extracted from sugarcane bagasse for synergistically enhanced water solubility. Carbohydr Polym. 2019;208:314–22. doi:10.1016/j.carbpol.2018.12.080. [Google Scholar] [PubMed] [CrossRef]
114. Mayer P, Netzer F, Bechtold T, Pham T. Enhancing the attraction of positively charged precursors of conductive polymer poly(3,4-ethylenedioxythiophene) by anchoring sulfonate groups in cellulose fibres. Polymer. 2024;303:127122. doi:10.1016/j.polymer.2024.127122. [Google Scholar] [CrossRef]
115. Kanbua C, Rattanawongwiboon T, Khamlue R, Ummartyotin S. Green synthesis of sulfonated cellulose/polyether block amide/polyethylene glycol diacrylate (SC/PEBAX/PEGDA) composite membrane by gamma radiation and sulfonation techniques for battery application. Int J Biol Macromol. 2023;248:125844. doi:10.1016/j.ijbiomac.2023.125844. [Google Scholar] [PubMed] [CrossRef]
116. Nguyen DT, Pham QT. A theoretical and experimental study on etherification of primary alcohols with the hydroxyl groups of cellulose chain (n = 1–3) in acidic condition. J Mol Struct. 2021;1236:130314. [Google Scholar]
117. Aguado R, Lourenço AF, Ferreira PJ, Moral A, Tijero A. Cationic cellulosic derivatives as flocculants in papermaking. Cellulose. 2017;24:3015–27. [Google Scholar]
118. Matsumoto K, Iwata N, Furumi S. Cholesteric liquid crystals with thermally stable reflection color from mixtures of completely etherified ethyl cellulose derivative and methacrylic acid. Polymers. 2024;16:401. [Google Scholar] [PubMed]
119. Barthel S, Heinze T. Acylation and carbanilation of cellulose in ionic liquids. Green Chem. 2006;8:301–6. [Google Scholar]
120. Chen H, Yang F, Du J, Xie H, Zhang L, Guo Y, et al. Efficient transesterification reaction of cellulose with vinyl esters in DBU/DMSO/CO2 solvent system at low temperature. Cellulose. 2018;25:6935–45. [Google Scholar]
121. Gao E, Li Q, Zhao X, Zhang C, Hua Z, Wu Z, et al. Reaction behavior of solid acid catalytic cellulose acetylation. Cellulose. 2024;31:10285–302. doi:10.1007/s10570-024-06213-z. [Google Scholar] [CrossRef]
122. Siqueira G, Bras J, Dufresne A. Cellulose whiskers versus microfibrils: influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules. 2009;10:425–32. doi:10.1021/bm801193d. [Google Scholar] [PubMed] [CrossRef]
123. Beaumont M. Unique reactivity of nanoporous cellulosic materials mediated by surface-confined water. Nat Commun. 2021;12:2513. doi:10.1038/s41467-021-22682-3. [Google Scholar] [CrossRef]
124. Heinze T, Pfeifer A, Koschella A, Schaller J, Meister F. Solvent-free synthesis of 6-deoxy-6-(ω-aminoalkyl)amino cellulose. J Appl Polym Sci. 2016;133:43987. doi:10.1002/app.43987. [Google Scholar] [CrossRef]
125. Zarth CSP, Koschella A, Pfeifer A, Dorn S, Heinze T. Synthesis and characterization of novel amino cellulose esters. Cellulose. 2011;18:1315–25. doi:10.1007/s10570-011-9557-4. [Google Scholar] [CrossRef]
126. Deng X, Zhi Y, Shan S, Miao Y, Jia Q, Ni Y. Effective and reusable microcrystalline cellulosic Salen complexes for epoxidation of alpha-pinene. Cellulose. 2018;25:1281–9. doi:10.1007/s10570-018-1650-5. [Google Scholar] [CrossRef]
127. Gao H, Fu L-L, Cai M-L, Chen W, Bai Z-W. Preparation of 6-amino-6-deoxy cellulose and its derivatives used as chiral separation materials. Carbohydr Polym. 2021;259:117756. doi:10.1016/j.carbpol.2021.117756. [Google Scholar] [PubMed] [CrossRef]
128. Hassan ML. Preparation and thermal stability of new cellulose-based poly(propylene imine) and poly(amido amine) hyperbranched derivatives. J Appl Polym Sci. 2006;101:2079–87. doi:10.1002/app.23828. [Google Scholar] [CrossRef]
129. Jin L, Li W, Xu Q, Sun Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose. 2015;22:2443–56. doi:10.1007/s10570-015-0649-4. [Google Scholar] [CrossRef]
130. Dash R, Elder T, Ragauskas AJ. Grafting of model primary amine compounds to cellulose nanowhiskers through periodate oxidation. 2012. doi:10.1007/s10570-012-9769-2. [Google Scholar] [CrossRef]
131. Su Z, Yu L, Cui L, Zhou G, Zhang X, Qiu X, et al. Reconstruction of cellulose intermolecular interactions from hydrogen bonds to dynamic covalent networks enables a thermo-processable cellulosic plastic with tunable strength and toughness. ACS Nano. 2023;17:21420–31. doi:10.1021/acsnano.3c06175. [Google Scholar] [PubMed] [CrossRef]
132. Lasseuguette E. Grafting onto microfibrils of native cellulose. Cellulose. 2008;15:571–80. doi:10.1007/s10570-008-9200-1. [Google Scholar] [CrossRef]
133. Wang L, Sanders JE, Gardner DG, Han Y. In-situ modification of cellulose nanofibrils by organosilanes during spray drying. Ind Crops Prod. 2016;93:129–35. [Google Scholar]
134. Pacaphol K, Aht-Ong D. The influences of silanes on interfacial adhesion and surface properties of nanocellulose film coating on glass and aluminum substrates. Surf Coat Technol. 2017;320:70–81. [Google Scholar]
135. Gao C, Liu S, Edgar KJ. Regioselective chlorination of cellulose esters by methanesulfonyl chloride. Carbohydr Polym. 2018;193:108–18. [Google Scholar] [PubMed]
136. Du H, Parit M, Liu K, Zhang M, Jiang Z, Huang T-S, et al. Engineering cellulose nanopaper with water resistant, antibacterial, and improved barrier properties by impregnation of chitosan and the followed halogenation. Carbohydr Polym. 2021;270:118372. [Google Scholar] [PubMed]
137. Khanjani P, King AWT, Partl GJ, Johansson L-S, Kostiainen MA, Ras RHA. Superhydrophobic paper from nanostructured fluorinated cellulose esters. ACS Appl Mater Interfaces. 2018;10:11280–8. [Google Scholar] [PubMed]
138. Kang H, Liu R, Huang Y. Graft modification of cellulose: methods, properties and applications. Polymer. 2015;70:A1–16. doi:10.1016/j.polymer.2015.05.041. [Google Scholar] [CrossRef]
139. Kamigaito M, Ando T, Sawamoto M. Metal-catalyzed living radical polymerization. Chem Rev. 2001;101:3689–746. doi:10.1021/cr9901182. [Google Scholar] [PubMed] [CrossRef]
140. Gillies MB, Matyjaszewski K, Norrby P-O, Pintauer T, Poli R, Richard P. A DFT study of R−X bond dissociation enthalpies of relevance to the initiation process of atom transfer radical polymerization. Macromolecules. 2003;36:8551–9. doi:10.1021/ma0351672. [Google Scholar] [CrossRef]
141. Tang W, Tsarevsky NV, Matyjaszewski K. Determination of equilibrium constants for atom transfer radical polymerization. J Am Chem Soc. 2006;128:1598–604. doi:10.1021/ja0558591. [Google Scholar] [PubMed] [CrossRef]
142. Zhang Z, Wang X, Tam KC, Sèbe G. A comparative study on grafting polymers from cellulose nanocrystals via surface-initiated atom transfer radical polymerization (ATRP) and activator re-generated by electron transfer ATRP. Carbohydr Polym. 2019;205:322–9. doi:10.1016/j.carbpol.2018.10.050. [Google Scholar] [PubMed] [CrossRef]
143. Glaied O, Dubé M, Chabot B, Daneault C. Synthesis of cationic polymer-grafted cellulose by aqueous ATRP. J Coll Interface Sci. 2009;333:145–51. doi:10.1016/j.jcis.2009.01.050. [Google Scholar] [PubMed] [CrossRef]
144. Lu C, Wang C, Yu J, Wang J, Chu F. Metal-free ATRP “grafting from” technique for renewable cellulose graft copolymers. Green Chem. 2019. [Google Scholar]
145. Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, Perrier S. Bioapplications of RAFT polymerization. Chem Rev. 2009;109:5402–36. doi:10.1021/cr9001403. [Google Scholar] [PubMed] [CrossRef]
146. Jiang F, Pan C, Zhang Y, Fang Y. Cellulose graft copolymers toward strong thermoplastic elastomers via RAFT polymerization. Appl Surface Sci. 2019;480:162–71. doi:10.1016/j.apsusc.2019.02.210. [Google Scholar] [CrossRef]
147. Gong X, Cheng Z, Gao S, Zhang D, Ma Y, Wang J, et al. Ethyl cellulose based self-healing adhesives synthesized via RAFT and aromatic schiff-base chemistry. Carbohydr Polym. 2020;250:116846. doi:10.1016/j.carbpol.2020.116846. [Google Scholar] [PubMed] [CrossRef]
148. Stenzel MH, Davis TP, Fane AG. Honeycomb structured porous films prepared from carbohydrate based polymers synthesized via the RAFT process. J Mater Chem. 2003;13:2090. doi:10.1039/b304204a. [Google Scholar] [CrossRef]
149. Zhou J, Li Y, Li H, Yao H. Cellulose nanocrystals/fluorinated polyacrylate soap-free emulsion prepared via RAFT-assisted Pickering emulsion polymerization. Coll Surfaces B: Biointerfaces. 2019;177:321–8. [Google Scholar]
150. Braunecker WA, Matyjaszewski K. Controlled/living radical polymerization: features, developments, and perspectives. Prog Polym Sci. 2007;32:93–146. [Google Scholar]
151. Daly WH, Evenson TS, Iacono ST, Jones RW. Recent developments in cellulose grafting chemistry utilizing Barton ester intermediates and nitroxide mediation. Macromol Symp. 2001;174:155–64. [Google Scholar]
152. Raus V, Štěpánek M, Uchman M, Šlouf M, Látalová P, Čadová E, et al. Cellulose-based graft copolymers with controlled architecture prepared in a homogeneous phase. J Polym Sci A Polym Chem. 2011;49:4353–67. [Google Scholar]
153. Jiang X, Mietner JB, Navarro JRG. A combination of surface-initiated controlled radical polymerization (SET-LRP) and click-chemistry for the chemical modification and fluorescent labeling of cellulose nanofibrils: STED super-resolution imaging of a single fibril and a single fibril embedded in a composite. Cellulose. 2023;30:2929–50. [Google Scholar]
154. Rosen BM, Jiang X, Wilson CJ, Nguyen NH, Monteiro MJ, Percec V. The disproportionation of Cu(I)X mediated by ligand and solvent into Cu(0) and Cu(II)X2 and its implications for SET-LRP. J Polym Sci A Polym Chem. 2009;47:5606–28. [Google Scholar]
155. Jiang X, Mietner JB, Harder C, Komban R, Chen S, Strelow C, et al. 3D printable hybrid gel made of polymer surface-modified cellulose nanofibrils prepared by surface-initiated controlled radical polymerization (SI-SET-LRP) and upconversion luminescent nanoparticles. ACS Appl Mater Interfaces. 2023;15(4):5687–700. [Google Scholar] [PubMed]
156. Hafrén J, Córdova A. Direct organocatalytic polymerization from cellulose fibers. Macromol Rapid Commun. 2005;26:82–6. [Google Scholar]
157. Lee W, Ahn Y, Chung JW, Kwak S-Y. Remarkable thermoplasticity of branched cellulose copolymers: graft-chain-dependent structural transition and thermoplasticity. Carbohydr Polym. 2021;261:117862. [Google Scholar] [PubMed]
158. Zhu L, Chen Q, Wang Y, Huang H, Luo W, Li Z, et al. Grafting polysulfonamide from cellulose paper through organocatalytic ring-opening polymerization of N-sulfonyl aziridines. Carbohydr Polym. 2021;261:117903. [Google Scholar] [PubMed]
159. Mano V, Chimenti S, Ruggeri G, Pereira FV, De Paula EL. P(CL-b-LLA) diblock copolymers grafting onto cellulosic nanocrystals. Polym Bull. 2017;74:3673–88. doi:10.1007/s00289-017-1919-0. [Google Scholar] [CrossRef]
160. Sperandeo P, Bosco F, Clerici F, Polissi A, Gelmi ML, Romanelli A. Covalent grafting of antimicrobial peptides onto microcrystalline cellulose. ACS Appl Bio Mater. 2020;3(8):4895–901. doi:10.1021/acsabm.0c00412. [Google Scholar] [CrossRef]
161. Münster L, Vícha J, Klofáč J, Masař M, Hurajová A, Kuřitka I. Dialdehyde cellulose crosslinked poly(vinyl alcohol) hydrogels: influence of catalyst and crosslinker shelf life. Carbohydr Polym. 2018;198:181–90. doi:10.1016/j.carbpol.2018.06.035. [Google Scholar] [PubMed] [CrossRef]
162. Hong H, Li X, Liu H, Zhang H, He H, Xu H, et al. Transform rice husk and recycled polyethylene into high performance composites: using a novel compatibilizer to infiltratively enhance the interfacial interactions. Progress Rubber, Plastics Recycling Technol. 2016;32:253–68. doi:10.1177/147776061603200405. [Google Scholar] [CrossRef]
163. Hong H, Guo Q, Zhang H, He H. Effect of interfacial modifiers and wood flour treatment on the rheological properties of recycled polyethylene/wood flour composites. 2019. doi:10.1177/1477760619895014. [Google Scholar] [CrossRef]
164. Hong H, Xiao R, Guo Q, Liu H, Zhang H. Quantitively characterizing the chemical composition of tailored bagasse fiber and its effect on the thermal and mechanical properties of polylactic acid-based composites. 2019. doi:10.3390/polym11101567. [Google Scholar] [CrossRef]
165. Magaña I, Georgouvelas D, Handa R, Neira Velázquez MG, López González HR, Enríquez Medrano FJ, et al. Fully bio-based elastomer nanocomposites comprising polyfarnesene reinforced with plasma-modified cellulose nanocrystals. Polymers. 2021;13:2810. doi:10.3390/polym13162810. [Google Scholar] [PubMed] [CrossRef]
166. Zhu G, Dufresne A. Synergistic reinforcing and cross-linking effect of thiol-ene-modified cellulose nanofibrils on natural rubber. Carbohydr Polym. 2022;278:118954. doi:10.1016/j.carbpol.2021.118954. [Google Scholar] [PubMed] [CrossRef]
167. Sun X, Yang X, Zhang J, Shang B, Lyu P, Zhang C, et al. Fabrication of silane-grafted cellulose nanocrystals and their effects on the structural, thermal, mechanical, and hysteretic behavior of thermoplastic polyurethane. Int J Mol Sci. 2023;24:5036–50. [Google Scholar] [PubMed]
168. Pajares E, Maestu JF, Fernandez-de-Mendiola I, Silvan U, Costa P, Agirrezabal-Telleria I, et al. Strategies for improving sustainability in the development of high-performance styrenic block copolymers by developing blends with cellulose derivatives. Polymers. 2024;16:856. [Google Scholar] [PubMed]
169. Yuan H, Li P, Wang X, Zhao H, Sun J. Rod-like cellulose regenerated by bottom-up assembly in natural rubber latex and its reinforcement. Int J Mol Sci. 2023;24:6457–67. [Google Scholar] [PubMed]
170. Li P, Yuan H, Wang X, Yu C, Wang X, Sun J. Green and efficient utilization of cellulose by in situ regenerating rod-like cellulose crystals in dry blending rubber compounds. Polym Compos. 2024;45:4839–49. [Google Scholar]
171. Mahadi N. Synergistic effects of cellulose nanocrystal on the mechanical and shape memory properties of TPU composites. Int J Biol Macromol. 2024;278:134842–56. [Google Scholar] [PubMed]
172. Miao C. In-situ polymerized cellulose nanocrystals (CNC)—poly(l-lactide) (PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr Polym. 2016;153:549–58. [Google Scholar] [PubMed]
173. Rosa RP. Cellulose nanocrystals as initiator of ring-opening polymerization of ε-caprolactone: mathematical modeling and experimental verification. Eur Polym J. 2022;170:111171–7. [Google Scholar]
174. Gitari B, Chang BP, Misra M, Navabi A, Mohanty AK. A comparative study on the mechanical, thermal, and water barrier properties of PLA nanocomposite films prepared with bacterial nanocellulose and cellulose nanofibrils. BioRes. 2019;14:1867–89. [Google Scholar]
175. Delgado JF, De La Osa O, Salvay AG, Cavallo E, Cerrutti P, Foresti ML, et al. Reinforcement of yeast biomass films with bacterial cellulose and rice husk cellulose nanofibres. J Polym Environ. 2021;29:3242–51. [Google Scholar]
176. Kim NK, Lin RJT, Bhattacharyya D. Flammability and mechanical behaviour of polypropylene composites filled with cellulose and protein based fibres: a comparative study. Compos Part A: Appl Sci Manuf. 2017;100:215–26. [Google Scholar]
177. Utrera-Barrios S, Pinho Lopes O, Mas-Giner I, Verdejo R, López-Manchado MA, Hernández Santana M. Sustainable composites with self-healing capability: epoxidized natural rubber and cellulose propionate reinforced with cellulose fibers. Polym Compos. 2024;45:7918–31. [Google Scholar]
178. Shen Y, Wang X, Xu S, Huang C, Yu J, Yong Q, et al. Multiple hydrogen bonds network enabled sustainable and reproducible cellulose/liquid metal composite elastomer for clean energy collection and conversion. Compos Sci Technol. 2023;242:110201. [Google Scholar]
179. Fan X. Room-temperature self-healing polyurethane-cellulose nanocrystal composites with strong strength and toughness based on dynamic bonds. Carbohydr Polym. 2023;308:120654. [Google Scholar] [PubMed]
180. Geng A. Strong double networked hybrid cellulosic foam for passive cooling. Int J Biol Macromol. 2024;264:130676. [Google Scholar] [PubMed]
181. Chen X. Cellulose-based porous polymer film with auto-deposited TiO2 as spectrally selective materials for passive daytime radiative cooling. Opt Mater. 2021;120:111431. [Google Scholar]
182. Xiang B, Zhang R, Luo Y, Zhang S, Xu L, Min H, et al. 3D porous polymer film with designed pore architecture and auto-deposited SiO2 for highly efficient passive radiative cooling. Nano Energy. 2021;81:105600. [Google Scholar]
183. Yang X, Hong H, Zhang H, Hong X. Highly spectrally selective dual-layer cellulose-based composite material for daytime radiative cooling. Express Polym Lett. 2022;16:665–76. [Google Scholar]
184. Tian Y, Shao H, Liu X, Chen F, Li Y, Tang C, et al. Superhydrophobic and recyclable cellulose-fiber-based composites for high-efficiency passive radiative cooling. ACS Appl Mater Interfaces. 2021;13:22521–30. [Google Scholar] [PubMed]
185. Cai C, Chen F, Wei Z, Ding C, Chen Y, Wang Y, et al. Large scalable, anti-ultraviolet, strong cellulose film with well-defined dual-pores for longtime daytime radiative cooling. Chem Eng J. 2023;476:146668. [Google Scholar]
186. Zhu W, Droguet B, Shen Q, Zhang Y, Parton TG, Shan X, et al. Structurally colored radiative cooling cellulosic films. Adv Sci. 2022;9:2202061. [Google Scholar]
187. Shanker R, Ravi Anusuyadevi P, Gamage S, Hallberg T, Kariis H, Banerjee D, et al. Structurally colored cellulose nanocrystal films as transreflective radiative coolers. ACS Nano. 2022;16:10156–62. doi:10.1021/acsnano.1c10959. [Google Scholar] [CrossRef]
188. He W, Huang X, Wu F, Hong H. Preparation of CO2-based waterborne polyurethane/cellulose nanocrystal composite films and research of their radiative cooling properties. 2024. doi:10.1002/app.55755. [Google Scholar] [CrossRef]
189. Gamage S, Banerjee D, Alam MM, Hallberg T, Åkerlind C, Sultana A, et al. Reflective and transparent cellulose-based passive radiative coolers. Cellulose. 2021;28:9383–93. doi:10.1007/s10570-021-04112-1. [Google Scholar] [CrossRef]
190. Wan C, Bowen CR. Multiscale-structuring of polyvinylidene fluoride for energy harvesting: the impact of molecular-, micro- and macro-structure. J Mater Chem A. 2017;5:3091–128. doi:10.1039/C6TA09590A. [Google Scholar] [CrossRef]
191. Song Y. Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: a review. J Mater Chem A. 2021;9:1910–37. doi:10.1039/D0TA08642H. [Google Scholar] [CrossRef]
192. Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941. doi:10.1039/c0cs00108b. [Google Scholar] [PubMed] [CrossRef]
193. Choi HY, Jeong YG. Microstructures and piezoelectric performance of eco-friendly composite films based on nanocellulose and barium titanate nanoparticle. Compos Part B: Eng. 2019;168:58–65. doi:10.1016/j.compositesb.2018.12.072. [Google Scholar] [CrossRef]
194. Bowen CR. Pyroelectric materials and devices for energy harvesting applications. Environ Sci. 2014;7:3817–4148. [Google Scholar]
195. Bowen CR, Kim HA, Weaver PM, Dunn S. Piezoelectric and ferroelectric materials and structures for energy harvesting applications. Environ Sci. 2014;7:25–44. doi:10.1039/C3EE42454E. [Google Scholar] [CrossRef]
196. Li T, Zhang X, Lacey SD, Mi R, Zhao X, Jiang F, et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat Mater. 2019;18:608–13. doi:10.1038/s41563-019-0315-6. [Google Scholar] [PubMed] [CrossRef]
197. Maity K, Mondal A, Saha MC. Cellulose nanocrystal-based All-3D-printed pyro-piezoelectric nanogenerator for hybrid energy harvesting and self-powered cardiorespiratory monitoring toward the human-machine interface. ACS Appl Mater Interfaces. 2023;15:acsami.2c21680. [Google Scholar]
198. Liao M, Banerjee D, Hallberg T, Åkerlind C, Alam MM, Zhang Q, et al. Cellulose-based radiative cooling and solar heating powers ionic thermoelectrics. Adv Sci. 2023;10:2206510. doi:10.1002/advs.202206510. [Google Scholar] [PubMed] [CrossRef]
199. Wang ZL. On Maxwell’s displacement current for energy and sensors: the origin of nanogenerators. Mater Today. 2017;20(2):74–82. doi:10.1016/j.mattod.2016.12.001. [Google Scholar] [CrossRef]
200. Parandeh S. An eco-friendly triboelectric hybrid nanogenerators based on graphene oxide incorporated polycaprolactone fibers and cellulose paper. Nano Energy. 2019;59:412–21. doi:10.1016/j.nanoen.2019.02.058. [Google Scholar] [CrossRef]
201. Park Y-J. Biodegradable, stretchable, and high-performance triboelectric nanogenerators through interfacial polarization in bilayer structure. Nano Energy. 2024;132:110411. doi:10.1016/j.nanoen.2024.110411. [Google Scholar] [CrossRef]
202. Mule AR, Dudem B, Patnam H, Graham SA, Yu JS. Wearable single-electrode-mode triboelectric nanogenerator via conductive polymer-coated textiles for self-power electronics. ACS Sustainable Chem Eng. 2019;7:16450–8. doi:10.1021/acssuschemeng.9b03629. [Google Scholar] [CrossRef]
203. Seol M-L. All-printed triboelectric nanogenerator. Nano Energy. 2018;44:82–8. doi:10.1016/j.nanoen.2017.11.067. [Google Scholar] [CrossRef]
204. He X, Zou H, Geng Z, Wang X, Ding W, Hu F, et al. A hierarchically nanostructured cellulose fiber-based triboelectric nanogenerator for self-powered healthcare products. Adv Funct Materials. 2018;28:1805540. doi:10.1002/adfm.201805540. [Google Scholar] [CrossRef]
205. Shi K, Zou H, Sun B, Jiang P, He J, Huang X. Dielectric modulated cellulose paper/PDMS-based triboelectric nanogenerators for wireless transmission and electropolymerization applications. Adv Funct Mater. 2020;30:1904536. doi:10.1002/adfm.201904536. [Google Scholar] [CrossRef]
206. Zhu Q, Wang T, Wei Y, Sun X, Zhang S, Wang X, et al. Low-cost, environmentally friendly and high-performance cellulose-based triboelectric nanogenerator for self-powered human motion monitoring. Cellulose. 2022;29:8733–47. doi:10.1007/s10570-022-04800-6. [Google Scholar] [CrossRef]
207. Yao L, Zhou Z, Zhang Z, Du X, Zhang Q-L, Yang H. Dyeing-inspired sustainable and low-cost modified cellulose-based TENG for energy harvesting and sensing. ACS Sustain Chem Eng. 2022;10(12):3909–19. [Google Scholar]
208. Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL. The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers. 2010;2:522–53. doi:10.3390/polym2040522. [Google Scholar] [CrossRef]
209. Dong R, Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41:101290. doi:10.1016/j.nantod.2021.101290. [Google Scholar] [CrossRef]
210. Mayer S. Antimicrobial and physicochemical characterization of 2,3-dialdehyde cellulose-based wound dressings systems. Carbohydr Polym. 2021;272:118506. doi:10.1016/j.carbpol.2021.118506. [Google Scholar] [PubMed] [CrossRef]
211. Pitpisutkul V. Hydroxypropyl methylcellulose/carboxymethyl starch/zinc oxide porous nanocomposite films for wound dressing application. Carbohydr Polym. 2022;298:120082. doi:10.1016/j.carbpol.2022.120082. [Google Scholar] [PubMed] [CrossRef]
212. Dang X. Sustainable one-pot synthesis of novel soluble cellulose-based nonionic biopolymers for natural antimicrobial materials. Chem Eng J. 2023;468:143810. doi:10.1016/j.cej.2023.143810. [Google Scholar] [CrossRef]
213. Guo J. Bandage modified with antibacterial films of quaternized chitosan & sodium carboxymethyl cellulose microgels/baicalein nanoparticles for accelerating infected wound healing. Int J Biol Macromol. 2023;250:126274. doi:10.1016/j.ijbiomac.2023.126274. [Google Scholar] [PubMed] [CrossRef]
214. Baptista AC. Electronic control of drug release from gauze or cellulose acetate fibres for dermal applications. J Mater Chem B. 2021;9:3515–22. [Google Scholar] [PubMed]
215. Xu Z, Fan J, Tian W, Ji X, Cui Y, Nan Q, et al. Cellulose-based pH-responsive janus dressing with unidirectional moisture drainage for exudate management and diabetic wounds healing. Adv Funct Mater. 2024;34:2307449. [Google Scholar]
216. Poonguzhali R, Khaleel Basha S, Sugantha Kumari V. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. Int J Biol Macromol. 2018;112:1300–9. [Google Scholar] [PubMed]
217. Cheng F, Liu C, Wei X, Yan T, Li H, He J, et al. Preparation and characterization of 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-oxidized cellulose nanocrystal/alginate biodegradable composite dressing for hemostasis applications. ACS Sustainable Chem Eng. 2017;5:3819–28. [Google Scholar]
218. Ventura C, Pinto F, Lourenço AF, Ferreira PJT, Louro H, Silva MJ. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose. 2020;27:5509–44. [Google Scholar]
219. Shaikh HM, Anis A, Poulose AM, Madhar NA, Al-Zahrani SM. Date-palm-derived cellulose nanocrystals as reinforcing agents for Poly(vinyl alcohol)/Guar-gum-based phase-separated composite films. Nanomater. 2022;12:1104. doi:10.3390/nano12071104. [Google Scholar] [PubMed] [CrossRef]
220. Stie MB. Mucoadhesive chitosan- and cellulose derivative-based nanofiber-on-foam-on-film system for non-invasive peptide delivery. Carbohydr Polym. 2023;303:120429. doi:10.1016/j.carbpol.2022.120429. [Google Scholar] [PubMed] [CrossRef]
221. Narh C, Badoe W, Howard EK, Lin NX, Mensah A, Wang T, et al. Synthesized OH-radical rich bacteria cellulosic pockets with photodynamic bacteria inactivation properties against S. ureus and E. coli. Mater Sci Eng: C. 2020;116:111230. doi:10.1016/j.msec.2020.111230. [Google Scholar] [PubMed] [CrossRef]
222. Carmona P. Controlling the structure of spin-coated multilayer ethylcellulose/hydroxypropylcellulose films for drug release. Int J Pharm. 2023;644:123350. doi:10.1016/j.ijpharm.2023.123350. [Google Scholar] [PubMed] [CrossRef]
223. Xu M, Yue Y, Lu Y, Xiang K, Wang J, Lu W, et al. Three-dimensional printed cellulose nanofibers/carbon nanotubes/silicone rubber flexible strain sensor for wearable body monitoring. J Mater Chem C. 2024;12:5972–84. doi:10.1039/D3TC04595A. [Google Scholar] [CrossRef]
224. Lu C, Guo X, Wang C, Wang J, Chu F. Integration of metal-free ATRP and diels-alder reaction toward sustainable and recyclable cellulose-based thermoset elastomers. Carbohydr Polym. 2020;242:116404. doi:10.1016/j.carbpol.2020.116404. [Google Scholar] [PubMed] [CrossRef]
225. Han L, Cui S, Yu H-Y, Song M, Zhang H, Grishkewich N, et al. Self-healable conductive nanocellulose nanocomposites for biocompatible electronic skin sensor systems. ACS Appl Mater Interfaces. 2019;11:44642–51. doi:10.1021/acsami.9b17030. [Google Scholar] [PubMed] [CrossRef]
226. Lu C. 3D printed mechanical robust cellulose derived liquid-free ionic conductive elastomer for multifunctional electronic devices. Carbohydr Polym. 2024;324:121496. doi:10.1016/j.carbpol.2023.121496. [Google Scholar] [PubMed] [CrossRef]
227. Abdel Rahman NS, Greish YE, Mahmoud ST, Qamhieh NN, El-Maghraby HF, Zeze D. Fabrication and characterization of cellulose acetate-based nanofibers and nanofilms for H2S gas sensing application. Carbohydr Polym. 2021;258:117643. doi:10.1016/j.carbpol.2021.117643. [Google Scholar] [PubMed] [CrossRef]
228. Jia R, Tian W, Bai H, Zhang J, Wang S, Zhang J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat Commun. 2019;10:795. doi:10.1038/s41467-019-08675-3. [Google Scholar] [PubMed] [CrossRef]
229. Kim JW, Park H, Lee G, Jeong YR, Hong SY, Keum K, et al. Paper-like, thin, foldable, and self-healable electronics based on PVA/CNC nanocomposite film. Adv Funct Mater. 2019;29:1905968. doi:10.1002/adfm.201905968. [Google Scholar] [CrossRef]
230. Wang Y, Zhang L, Zhou J, Lu A. Flexible and transparent cellulose-based ionic film as a humidity sensor. ACS Appl Mater Interfaces. 2020;12(6):7631–8. doi:10.1021/acsami.9b22754. [Google Scholar] [PubMed] [CrossRef]
231. Xu Y, Zhao G, Zhu L, Fei Q, Zhang Z, Chen Z, et al. Pencil-paper on-skin electronics. Proc Natl Acad Sci U S A. 2020;117:18292–301. [Google Scholar]
232. Ke J. Novel chiral composite membrane prepared via the interfacial polymerization of diethylamino-beta-cyclodextrin for the enantioseparation of chiral drugs. J Membr Sci. 2020;597:117635. doi:10.1016/j.memsci.2019.117635. [Google Scholar] [CrossRef]
233. Luo H. β-Cyclodextrin covalent organic framework modified-cellulose acetate membranes for enantioseparation of chiral drugs. Sep Purif Technol. 2022;285:120336. doi:10.1016/j.seppur.2021.120336. [Google Scholar] [CrossRef]
234. Faria M, Moreira C, Eusébio T, Brogueira P, De Pinho MN. Hybrid flat sheet cellulose acetate/silicon dioxide ultrafiltration membranes for uremic blood purification. Cellulose. 2020;27:3847–69. doi:10.1007/s10570-020-02985-2. [Google Scholar] [CrossRef]
235. Azhar O, Jahan Z, Sher F, Niazi MBK, Kakar SJ, Shahid M. Cellulose acetate-polyvinyl alcohol blend hemodialysis membranes integrated with dialysis performance and high biocompatibility. Mater Sci Eng: C. 2021;126:112127. doi:10.1016/j.msec.2021.112127. [Google Scholar] [PubMed] [CrossRef]
236. Pazzi J, Subramaniam AB. Nanoscale curvature promotes high yield spontaneous formation of cell-mimetic giant vesicles on nanocellulose paper. ACS Appl Mater Interfaces. 2020;12:56549–61. doi:10.1021/acsami.0c14485. [Google Scholar] [PubMed] [CrossRef]
237. Hua K, Ålander E, Lindström T, Mihranyan A, Strømme M, Ferraz N. Surface chemistry of nanocellulose fibers directs monocyte/macrophage response. Biomacromolecules. 2015;16:2787–95. doi:10.1021/acs.biomac.5b00727. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF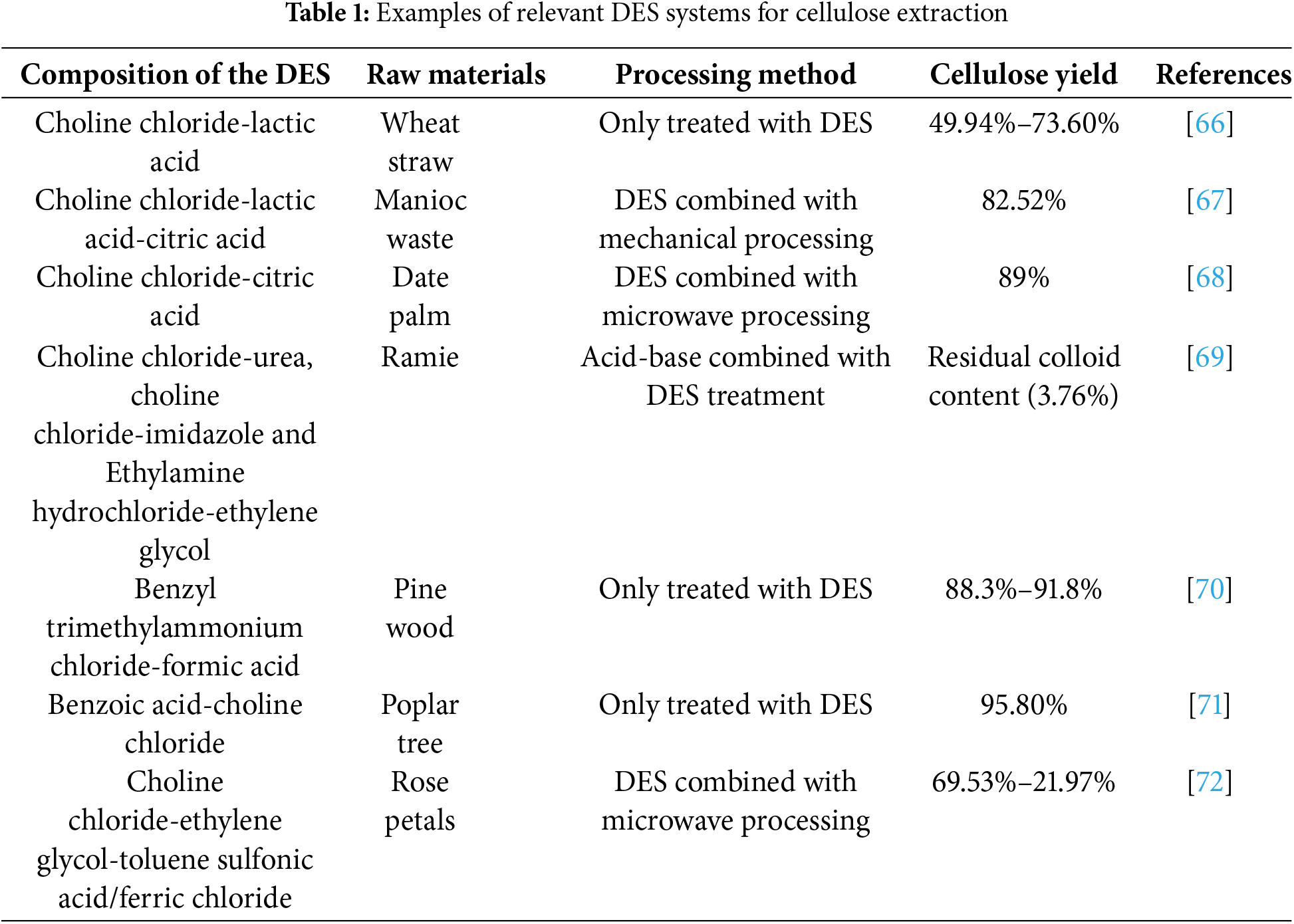
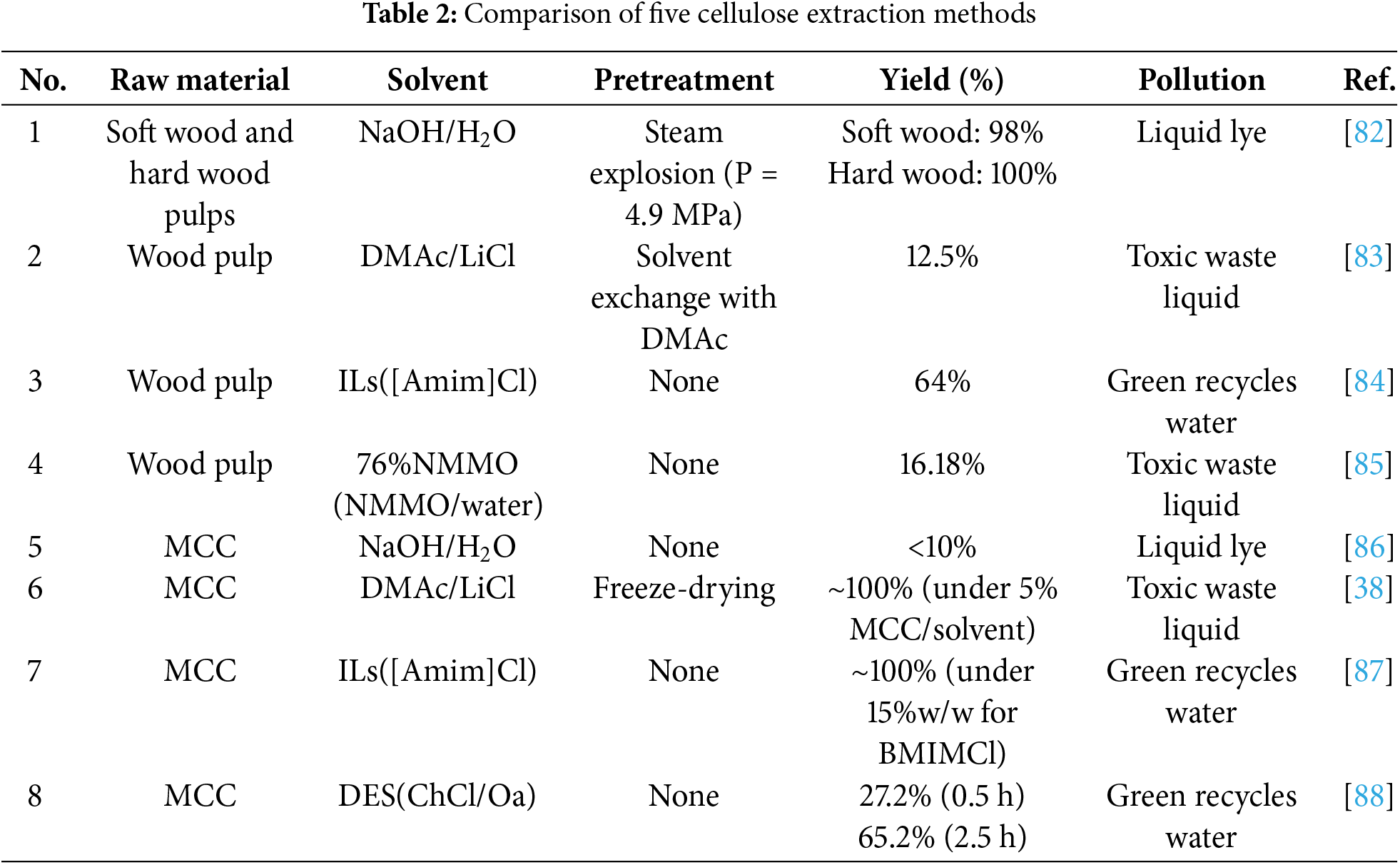
 Downloads
Downloads
 Citation Tools
Citation Tools