 Open Access
Open Access
REVIEW
Unveiling the Anticancer Potential of Urolithin A in Colorectal Cancer: A Systematic Review
1 Polytechnic University of Coimbra, ESTESC, UCPCBL, Rua 5 de Outubro, SM Bispo, Apartado, Coimbra, 3046-854, Portugal
2 H&TRC—Health & Technology Research Center, Coimbra Health School, Polytechnic University of Coimbra, Coimbra, 3045-043, Portugal
3 Coimbra Institute for Clinical and Biomedical Research (iCBR) Area of Environment Genetics and Oncobiology (CIMAGO), Biophysics Institute of Faculty of Medicine, University of Coimbra, Coimbra, 3045-043, Portugal
4 Center for Innovative Biomedicine and Biotechnology (CIBB), University of Coimbra, Coimbra, 3045-043, Portugal
5 European Association of Biomedical Scientists, Brussels, 1000, Belgium
* Corresponding Author: Fernando Mendes. Email:
(This article belongs to the Special Issue: Advances and Innovations in Colorectal Cancer Research and Treatment)
Oncology Research 2026, 34(2), 3 https://doi.org/10.32604/or.2025.070276
Received 11 July 2025; Accepted 24 November 2025; Issue published 19 January 2026
Abstract
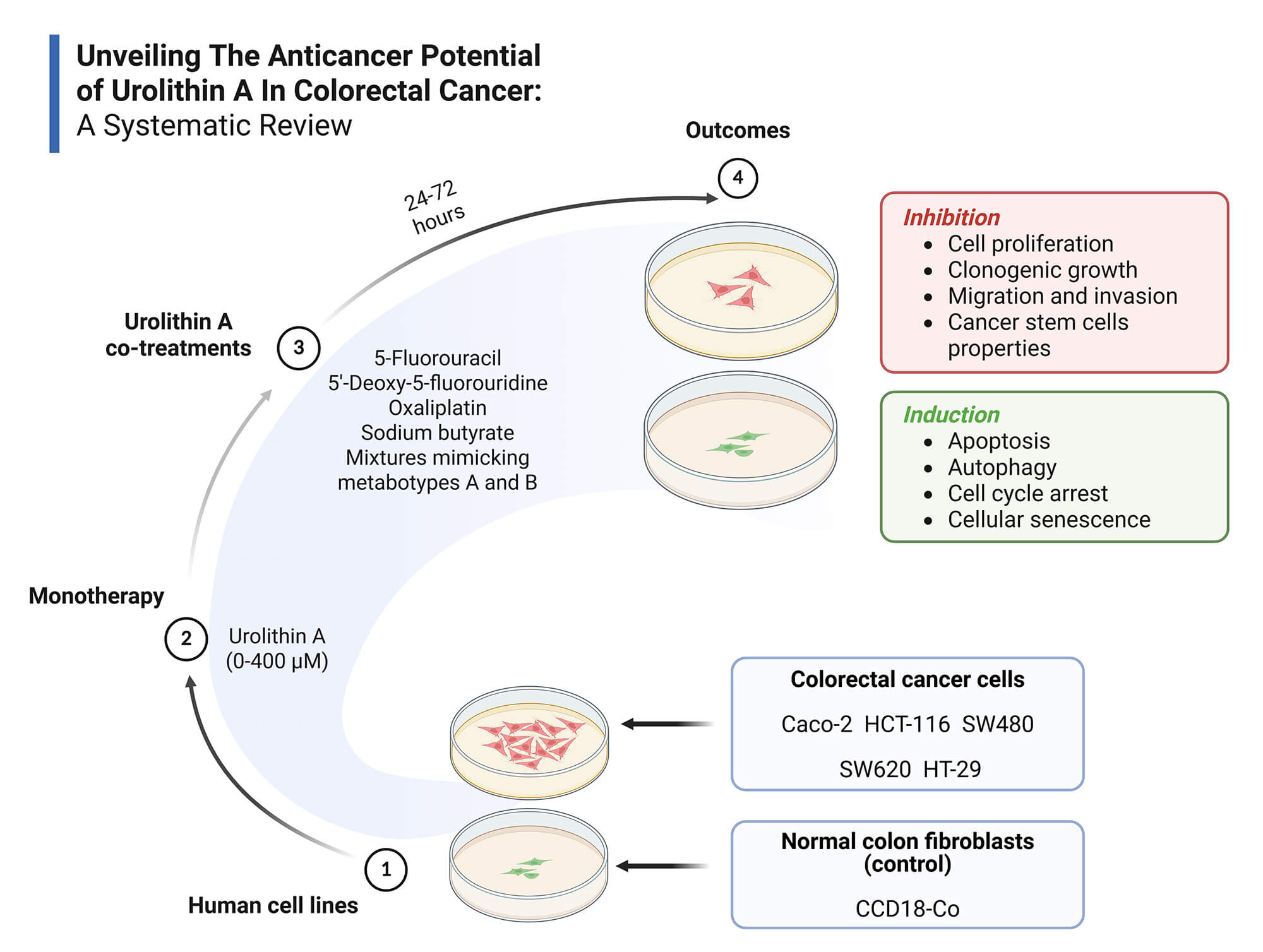
Objectives: Colorectal cancer (CRC) is a major global health burden, and Urolithin A (Uro-A) has emerged as a promising anticancer agent. This systematic review aims to synthesize current in vitro evidence on the anticancer effects of Uro-A in CRC, highlighting effective concentration ranges, exposure times, relevant outcomes, and underlying molecular mechanisms. Methods: Following PRISMA 2020 guidelines, a systematic search was conducted in PubMed, Scopus, and Web of Science using the following strategy: (colorectal cancer) AND (urolithin a) OR (3,8-dihydroxy-6H-dibenzo(b,d)pyran-6-one). Eligibility criteria were defined by the PICO framework: (P) in vitro CRC cell models; (I) Uro-A alone or combined treatments; (C) No intervention, vehicle or other treatments; (O) Relevant anticancer outcomes of Uro-A in CRC. Only original, full-text, in vitro studies in English were included. Risk of bias was assessed using ToxRTool. A qualitative synthesis was performed due to the heterogeneity of the included studies. Results: Fifteen studies met inclusion criteria, involving CRC cell lines (Caco-2, HCT-116, HT-29, SW480, SW620) and normal colon fibroblasts (CCD18-Co). Uro-A inhibited CRC cell proliferation, clonogenic growth, cancer stem cells properties, migration, and invasion, and induced cell cycle arrest, apoptosis, autophagy, and senescence, through modulation of key signaling pathways and proteins. Co-treatments with conventional chemotherapeutics and microbiota-derived metabolites showed additive or synergistic effects. Discussion: The findings support Uro-A’s potential as a preventive or adjuvant agent in CRC treatment. However, preclinical nature of the evidence and methodological heterogeneity hinder clinical extrapolation to in vivo contexts. Human clinical trials are necessary to overcome these limitations. Other: This review was registered in PROSPERO (CRD420251070874) and supported by FCT/MCTES UIDP/05608/2020 and UIDB/05608/2020. Institutional.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools