 Open Access
Open Access
REVIEW
Nanotechnology-Driven Treatment Strategies for Breast Cancer: Recent Advances and Innovations
1 Centre for Global Health Research, Saveetha Medical College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, 602105, India
2 Department of Pharmaceutics, School of Pharmaceutical Sciences, Delhi Pharmaceutical Sciences and Research University (DPSRU), New Delhi, 110017, India
3 Department of Pharmaceutics, Nitte College of Pharmaceutical Sciences, Bangalore, 560064, India
4 Centre for Inflammation, Faculty of Science, School of Life Sciences, Centenary Institute and University of Technology Sydney, Sydney, NSW 2050, Australia
5 Department of Pharmaceutics, Guru Ramdas Khalsa Institute of Science and Technology, Jabalpur, 483001, India
6 Centre of Excellence in Computational Research and Drug Discovery, Department of Bio-Sciences and Technology, Maharishi Markandeshwar Engineering College, Maharishi Markandeshwar (Deemed to Be University), Mullana, Ambala, India
7 Bio-nanotechnology and Nanomedicine Lab., Dept. of Applied Nutrition and Food Technology, Islamic University, Kushtia, 7003, Bangladesh
8 Division of Pharmacology, Faculty of Medical and Life Sciences, Sunway University, Bandar Sunway, 47500, Malaysia
* Corresponding Authors: Keshav Raj Paudel. Email: ; Madhu Gupta. Email:
(This article belongs to the Special Issue: Breast Cancer Biomarkers and Drug Targets Discoveries Towards a More Personalized Treatment Setting)
Oncology Research 2025, 33(10), 2787-2831. https://doi.org/10.32604/or.2025.066624
Received 13 April 2025; Accepted 07 August 2025; Issue published 26 September 2025
Abstract
Breast cancer is among the most prevalent cancers in females globally and has the highest mortality rate. The emergence of pharmacologic resistance in breast cancer is a significant challenge for researchers in the pursuit of effective treatment. Investigations in cancer nanotechnology have been transformed by the advancement of smart polymers, lipids, and inorganic materials. Research is now being conducted in the field of innovative nano-pharmaceutical formulations aimed at enhancing the efficacy and durability of chemotherapy. Nanotechnology-based delivery systems are beneficial for combating breast cancer due to theranostic applications, augmented drug encapsulation, decreased degradation, and minimal adverse effects. This review discusses breast cancer and its stages, various risk factors, and pathogenesis, in addition to diagnosis and treatment. Novel nanocarriers are included with the most recent findings in this area and the potential use of these nanocarriers in cancer therapy that centers on their clinical usage for improved treatment. Patents and promising clinical trial results are also explained in detail, with nanotoxicity, ethical concerns, and regulations. This study underscores the importance of treatment strategies using nanotechnology, highlighting the advancing paradigm of breast cancer care. This article examines the prospects, obstacles, and future trajectories of nanomedicines.Keywords
Cancer represents a proliferative disorder with a multifaceted character and is considered the leading cause of significant demises worldwide [1]. Now, it is regarded as the most common barrier to life, shortening the life span worldwide [2]. Mutational changes in the genetic material will result in uncontrolled growth of cells and tissue due to malfunction and metastasis to different body parts [3]. Among all, Breast cancer (BC) is responsible for the maximum number of fatalities in females [4]. The increasing rate of death of females due to BC makes it the second-highest form of cancer, giving more importance to its cure [5]. Among women, annually, BC is the leading cause of mortality [6], around 2.5%, corresponding to a 1 in 39 chance of death [7,8]. Furthermore, according to the reports of 2020 Globocan data, BC recorded 10.6% of cancer-related fatalities, covering almost 13.5% of all cancer diagnoses in India, with a cumulative risk of 2.81 [9]. The prevalence of BC is higher in developed nations with high Human Development Index (HDI) and transitioning republics.
It is categorized into four stages (from stage 0 to stage 4) depending upon the size and type of the tumor (Fig. 1), as well as the penetrability of tumor cells within breast tissues [10–12]. BC is primarily categorized into two main types: invasive or non-invasive breast cancer. By the histopathological or biochemical assessment of BC cases, invasive BC (IBC) may be categorized into three primary subtypes, namely Luminal, Non-Luminal (human epidermal growth factor receptor 2 (HER2)+), and Triple-negative BC (TNBC). Luminal BC refers to a subtype of IBC that exhibits hormone receptor expression. This kind of IBC sometimes presents in histological forms, including mucinous, invasive lobular, tubular, invasive cribriform, etc. [13–15]. Furthermore, based on the proliferation pathway and luminal regulation, luminal BC is further classified into subtypes A and B [16,17]. TNBC is a highly aggressive form of breast cancer, and its prevalence continuously increases. It is associated with the worst prognosis and significantly decreased patient life span compared to other types of breast cancer [11,12]. Population- and epidemiology-based studies prove that TNBC mainly occurs in women under the age of 40 and accounts for approximately 25% of all breast cancer diagnoses [16]. The mortality rate among individuals with TNBC approaches 40% during the first five years following diagnosis. The mortality rate during the third month following TNBC recurrence may reach up to 75%, reflecting the disease’s aggressive and refractory nature [17]. TNBC is characterized by a lack of useful therapy options. This is largely due to the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, making endocrine and targeted therapy useless. Therefore, chemotherapy remains the main treatment method for dealing with TNBC [18,19]. Over the past few years, nanotechnology has offered several advantages in the treatment of breast cancer or TNBC, addressing key challenges such as drug resistance, metastasis, and toxicity. One of its primary benefits is enhanced targeted drug delivery, where nanocarriers enable precise accumulation of therapeutic agents in TNBC cells while minimizing side effects on healthy tissues [20]. Nanotechnology also plays a crucial role in overcoming drug resistance by bypassing efflux pumps, ensuring better intracellular drug retention, and improving treatment efficacy. These advancements demonstrate how nanotechnology is revolutionizing TNBC treatment by offering more precise, effective, and less toxic therapeutic options [21,22].
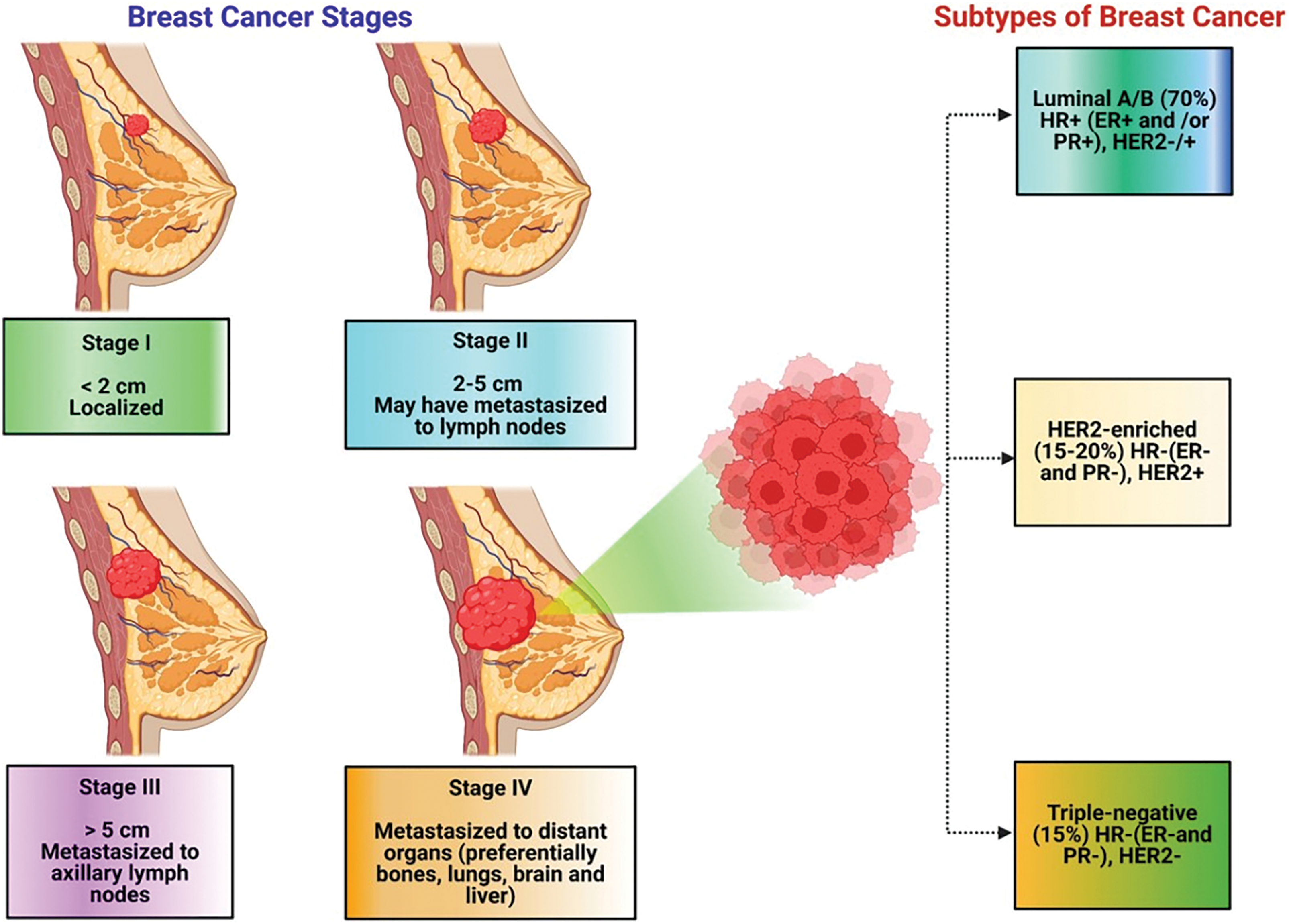
Figure 1: Clinical classification of breast cancer
This paper critically reviews the recent developments in nanocarriers for breast cancer. This detailed literature review discusses the stages of breast cancer, pathogenesis, various risk factors with diagnosis, nanocarrier-based treatment approaches, and the most recent findings in this area, with their limitations, nanotoxicity, and ethics are highlighted in the manuscript. Patents and promising clinical trials and regulatory aspects are stated in this review paper, along with prospects. This study underscores the importance of treatment strategies using nanotechnology, highlighting the advancing paradigm of breast cancer care.
2 Breast Cancer: Pathogenesis, Diagnosis and Risk Factors
The essential clinical factors promoting the progression of BC comprise high hormonal status (ER, PR & HER2), cellular factors, microRNAs (miRNAs), and specific gene mutations [23]. BC Stem Cells (BCSCs) are considered central cancer-driving cells. BCSCs are responsible for tumor initiation and facilitate therapeutic resistance and tumor malignancy. Highly active cytokines like Transforming growth factor beta receptor (TGFBR), Chemokines, and interleukins contribute to their role in the onset of BC malignancy. Approximately fifty distinct chemokines mainly induce chemotaxis of immune cells via twenty-five distinct seven-transmembrane G-protein coupled receptors. Plasma levels of IL8 and IL6 (also called CXCL8) correlate with the stage and mortality of BC and the resistance to chemotherapeutics [24]. Xenobiotic chemicals target breast tissue and generate reactive free radicals, causing breast cancer. These carcinogenic chemicals are metabolism-resistant, fat-soluble, and stored in breast adipose tissue [24]. CG protein-coupled receptor, e.g., Ca2+-sensing receptor (CaSR), gained attention as an oncogene and acts as an oncoprotein in the case of BC following skeletal metastases pathogenic pathway [25].
Cancer development is facilitated by oncogenic miRNAs, which impede tumor suppressor gene expression. Conversely, some miRNAs can downregulate oncogenic genes, demonstrating tumor suppressor characteristics [26]. Biomarkers are significant in managing BC therapy as they predict treatment outcomes and also have great importance in the early diagnosis of breast carcinoma [27]. Different types of biomarkers used for the prognosis of BC comprise BRCA1/2 gene mutations, serum biomarkers like carcinoembryonic antigen (CEA) or cancer antigen 15-3 (CA 15-3), circulating tumor-derived DNA, circulating tumor cells, and a multi-analyte profile of Oncotype DX or uPA/PAI-1, etc. [28]. Exosomes with various tumor antigens have become a great hotspot in diagnosis because of the elevated secretion level on the cancer cell’s surface. Furthermore, Aptamer probe hybridization (APH) and fluorescence in situ hybridization (FISH) are the two methods in nucleic acid hybridization that can identify unique tumor biomarker fragments and look for novel tumor biomarkers. CA125, HER-2, CA153, and CEA are partial oncogene proteins related to BC, which are used primarily as predictive markers of BC detection [29,30].
Mucinous carcinoma represents a distinct and infrequent variant of breast cancer, accounting for approximately 2% of the overall incidence of this disease [31]. The differential expression of mucins in BC has been investigated to explore its potential diagnostic and prognostic significance. The expression of MUC1 is notably increased in around 59% of healthy breast tissues, and this elevated level of expression is likewise observed in the malignant ducts near the normal tissue. As compared to normal breast epithelium, BC tissues are diagnosed with upregulated secretion and membrane-bound mucins in ductal adenocarcinoma of the breast [32,33]. Combining the currently employed blood tests and scanning procedures with novel nanoparticle-based techniques has been anticipated to improve the diagnosis [34].
Diagnostic imaging and image-guided needle biopsies are integral components in diagnosing, planning treatment, and determining the stage of BC in patients. The mammograms are conducted to detect a mass and those with a documented history of BC within the last five years [35]. BC incidences become more common even after the research is carried out on a laboratory and commercial scale and remain an economic burden for women. The risk factors responsible for BC among women can be genetic or non-genetic [36]. The genetic background of a woman plays a significant role in determining her susceptibility to developing BC. Birth weight is a risk factor for BC [37]. Pathogenetic alterations in genes like “SNPs, CHEK2, BRCA1, and BRCA2” are at a high risk of inducing tumors and fall under the category of genetic factors [38]. Inherited alterations in the genes (BRCA1 & BRCA2) involved in the DNA strand repair caused approximately 2.5% of all BC incidences [39]. Other genes that rarely carry the mutations from parents to offspring are PTEN, TP53, STK11 or LKB1, CDH1, ataxia telangiectasia mutated, NBN, CHEK2, and BRCA2 [40].
Fibroadenoma (FA) is a prevalent lesion in the breast, accounting for 25% of women who do not exhibit any symptoms. FA is a collective term used to describe a cluster of hyperplastic breast lobules that are regarded as deviations from typical patterns of development and involution. Multiple studies have provided evidence suggesting that familial aggregation constitutes a genetic predisposition for developing breast cancer. FA exhibiting significant alterations is recognized as a precancerous lesion, with a relatively low incidence of malignant transformation ranging from 0.12% to 0.3%. The most common form of malignancy that arises from such lesions is lobular carcinoma in situ [41]. Non-genetic risk factors of BC are age, high mammary gland density of the breast, a higher index of body mass (BMI), personal pathological history of breast, exposure of chest to radiation like X-ray, exogenous intake of female hormones, reproductive factors, alcohol and insufficient physical activity [36]. The primary origin of estrogen in women after menopause arises from converting androstenedione to estrone by adipose tissue. Consequently, obesity is linked to a sustained elevation in estrogen exposure over an extended period [41]. BC is additionally linked to lifestyle factors. Based on existing evidence, there is a positive link between daily alcohol intake and the risk of developing BC. According to research, silicone implants may have a reduced likelihood of developing BC [42].
As per WHO, the breastfeeding rate is much lower than the recommended rate, which becomes the relative risk factor of BC incidents, especially in high-income countries. Breastfeeding for 12 months can effectively reduce the risk of BC incidences by up to 4% [43]. However, the mechanism of BC protection due to breastfeeding is not completely clear; still, it is believed that increased concentration of an oncogene named pregnancy-associated plasma protein A (PAPP-A) during the pregnancy period is inhibited due to increased stanniocalcin glycoproteins (STC1 and STC2) secretion in the lactation process. This PAPP oncogene, in case of low breast-feeding rate, accelerates the process of tumor formation [43]. Like age and BMI of women, mammographic density is taken into consideration as a non-genetic cancer-causing risk factor. Women with high mammographic density are more prone to BC risk as compared to women with low mammographic density. Men, in comparison to women, have a low mammographic density, so they are less prone to BC [44].
3 Treatment Strategies for Breast Cancer
BC treatment includes immunotherapy, hormonal therapy, chemotherapy, radiation therapy, and surgery [45], where grade, molecular subtype, and stage of BC are the basis of BC management selection [46]. Currently, targeted treatment of BC aiming at HER2+ is the most commonly used therapy. Personalized treatment is used for treating metastatic breast cancer; however, it has no benefit in triple-negative BC and is fraught with problems due to drug resistance [47]. With time, the use of chemotherapy causes resistance and serious side effects like alopecia and a negative impact on healthy cells. So, to overcome this, radiation therapy, hormonal therapy, immunotherapy, and nanotechnology are highly emerging treatment methods for BC, either along with chemotherapy or alternative cytotoxic drugs. BC treatment strategies include endocrine therapy, chemotherapy, radiation, immunotherapy, and surgery (Fig. 2). Surgery is done when the tumor is in its initial stage of non-invasiveness and surgically removes the mass of tumor cells located in the breast and lymph nodes. Drugs used in conventional chemotherapy cause cell death in cancerous cells as well as normal and healthy cells. They also require high doses for their efficient therapeutic actions. Therefore, all conventionally used BC therapies involve several limitations [48].
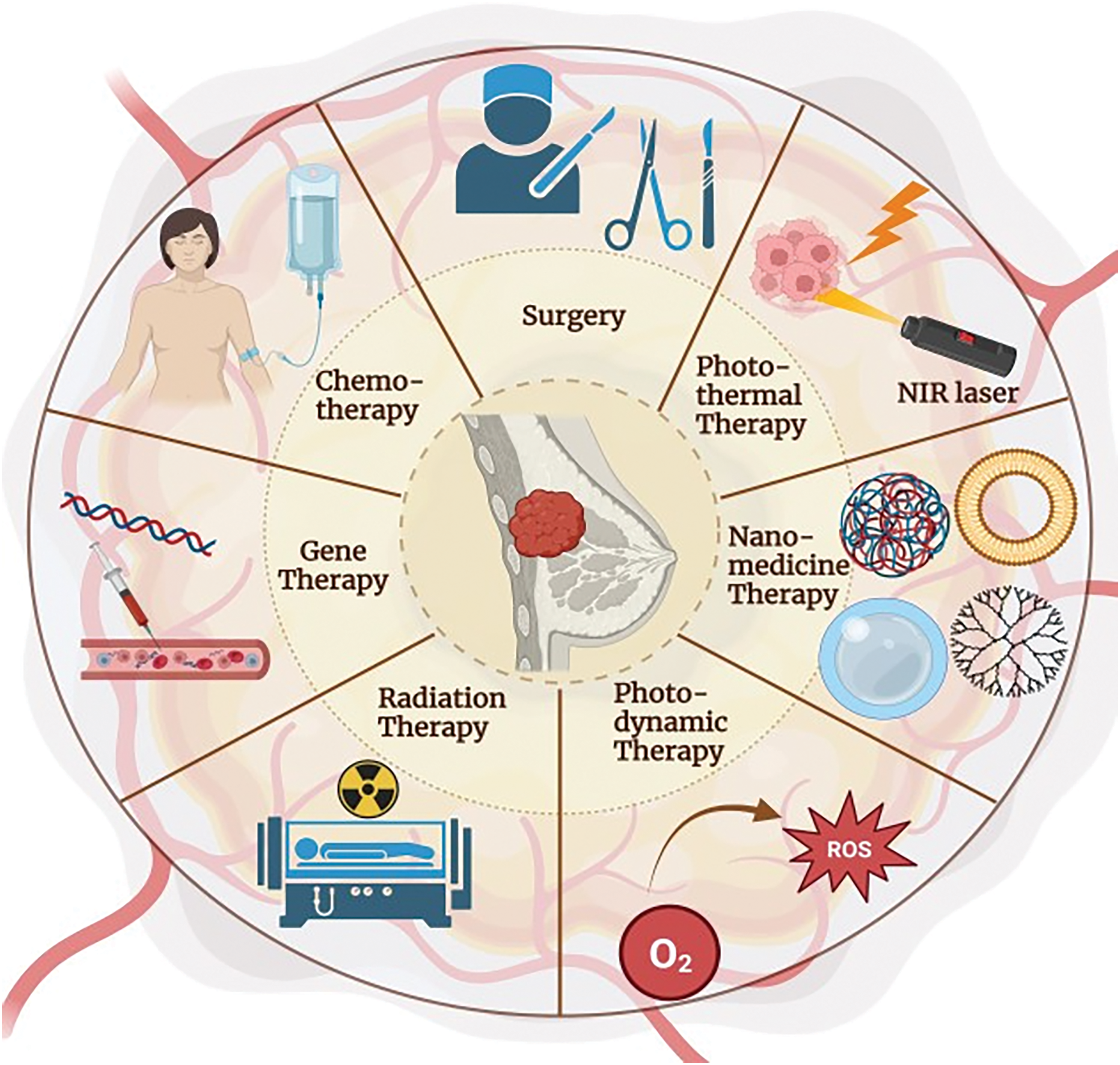
Figure 2: Treatment strategy for breast cancer
BC involves a different pathogenesis type that requires selective treatment to avoid serious side effects. Newer therapies have evolved as a combined therapy of traditional chemotherapeutic drugs with monoclonal antibodies for treating BC, which provide satisfactory clinical outcomes by immune cell death (Table 1). The dose used is sufficient to trigger immune responses against the tumor and prevent it from destroying the immune response. Due to the dose limitation of traditional chemotherapy, new targeted therapies that target the signaling mechanism responsible for BC occurrence are discussed in this section. These signaling mechanisms include checkpoint inhibitors, microRNA, apoptotic cell death pathway, Poly (ADP-ribose) polymerase (PARP) inhibitors, mitogen activated protein kinase (MAPK) inhibitors, notch signaling pathway, chimeric antigen receptor T-cell therapy (CART) therapy, vascular endothelial growth factor (VEGF), tumor protein p53 (P53 proteins), C-X-C chemokine receptor 4 (CXCR4), and G protein coupled receptor (GPER) receptors targeting [49]. Programmed cell death receptors (PD-1) and check proteins (CTLA-4) prevent the regular mechanism of the body’s defense system from responding to any antigen in case of breast cancer. Targeted inhibition of both PD-1 receptors and checkpoint proteins is employed as an advanced therapy for treating metastatic and advanced BC types [50,51]. Inhibition of miRNA-21 resulted in the anti-proliferation and non-invasiveness of BC cells and the downregulation of oncogenes involved in breast cancer. Therefore, this miRNA-targeted treatment is a suitable choice for treating BC [52].
In the case of people in a state of good health, the process of homeostasis is regulated by the controlled mechanisms of apoptosis and proliferation of cells. This balance is achieved through the presence of certain genes, namely BCLX, BCL-W, and BCL-2, which are located on the mitochondria’s outer membrane and contribute to the cellular count regulation inside the body. However, in the case of breast cancer, anti-apoptotic genes get overexpressed and cause cancer by inducing miRNA degradation and suppressing miRNA transcription [53,54]. Targeted suppression of the BRCA-1 gene signaling pathway and poly (ADP) ribose polymerase (PARP) prevents mutations and non-repairing of breakdown DNA, thereby reducing the chances of cancer progression [55,56]. Tumor proliferation and tumor metabolism are mediated by P13K, an important protein in the phosphoinositide 3-kinase pathway (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway. Therefore, inhibition of the PI3K/AKT/mTOR signaling pathways plays an essential step in a good therapy strategy for breast cancer [57]. Epigenetic modifications cause various cancers; therefore, Histone deacetylase (HDAC) inhibitor, as well as demethylation inhibitors, provides promising and beneficial strategies to overcome TNBC [57]. Inhibition of PARP proteins is an important treatment strategy for BC as it prevents the repair of injured cancerous cells [58]. The inhibition and mutation of MAPK dysregulate the proliferation of residual metastatic cancer cells, specifically BC cells [59]. Furthermore, they are accountable for cell proliferation and growth rate [60]. Notch signaling pathway inhibition prevents mutations in the notch receptors and breaks the association between angiogenesis and the renewal of cancer cells. Hence, it provides anticancer activity and relief from exaggerated BC tumor conditions [61]. Inhibition of Hedgehog (Hh) protein downregulated the embryonic and homeostasis developmental processes in the BC cells [62]. Chimeric antigen receptor (CAR) T-cell (CART) therapy is also known as genetically engineered T-cell therapy. The process in which inactivated T-cells are taken out from the BC patient by apheresis. After that, a chimeric antigen receptor DNA is introduced into the T-cells to activate them, which is then reinserted into the patient’s body. These genetically modified T-cells consider the cancer cells as foreign particles and actively inhibit cancerous cell proliferation [63]. Vascular endothelial growth factors (VEGF) play a crucial role in the multiplication of cellular growth in individuals’ bodies. Moreover, an overly expressed VEGF pathway is critically responsible for the multiplication of cancerous cells. Hence, inhibiting VEG factors like VEGFD, VEGFC, VEGFB, and VEGFA provides effective therapy for BC [61].
4 Nano-Size Carrier Systems for Breast Cancer
Recently from the last decades, nanotechnology has been broadly employed in the area of cancer treatment because of several advantages of nanotechnology, for instance, minuscule size, high capacity of drug loading, sustained drug release, improved stability, efficacy, reduced toxicity due to site-specific drug delivery and increased tolerability of nanocarriers encapsulated with drug particles [78]. Nanocarriers play a crucial role in targeting the tumor microenvironment (TME), which is a complex system influencing cancer progression, immune evasion, and drug resistance (Table 2). TME is a very complicated and heterogeneous environment that consists of cancer cells, stromal cells (e.g., fibroblasts, immune cells, endothelial cells), extracellular matrix (ECM), aberrant vasculature, hypoxia, acidic pH, and high interstitial fluid pressure (IFP) [79]. Thick ECM and abnormal vasculature restrict deep tumor tissue penetration of nanocarriers [80]. Hypoxia and acidic pH may decrease drug efficacy and modify release profiles [81] tumor cells tend to utilize anti-immune mechanisms, which lower the efficiency of immune-based targeting approaches [82]. Smart nanocarriers are designed with surface modification to avoid immune recognition. Camouflaging with cell membranes (e.g., red blood cells, platelets, or tumor cells) offers a natural disguise, evading immune detection [83]. Active targeting ligands help in the functionalization of nanocarriers using antibodies, peptides, aptamers, or small molecules to enhance the selective binding to tumor-associated receptors (e.g., folate receptor, HER2, transferrin receptor) (Fig. 3). pH- or hypoxia-sensitive systems are structured to take advantage of the TME’s specific acidic or hypoxic microenvironment; these systems change their structure or function to facilitate increased cellular uptake [84]. In ECM-cleaving nanocarriers, like Co-delivering hyaluronidase or collagenase assist in breaking down ECM components, allowing greater penetration [85]. In stimulus-responsive release systems, TME-specific triggers (pH, enzymes, reactive oxygen species) trigger smart carriers to deliver the drug exactly at the tumor site, minimizing off-target toxicity [86]. Oxygen-generating nanocarriers help in treating oxygen in hypoxic areas and can improve treatments such as photodynamic therapy (PDT) [87]. Novel drug delivery systems (NDDSs) are inorganic, organic, and hybrid (prepared with two nanomaterials). The design Strategies for integrating nanomaterials with other substances encompass hybrid nanoparticles, the amalgamation of organic and inorganic nanoparticles improves therapeutic effectiveness and stability. Secondly, surface functionalization that modifies nanoparticle surfaces with ligands or antibodies enhances targeted specificity (Fig. 4). Utilizing biodegradable polymers guarantees safe elimination from the body following therapy. Lastly, it includes the biologically controlled release of active materials and should be colloidally stable in physiologically stable [88]. Nanocarriers allow therapeutic drugs to be directly delivered to the cancer cells, which improves treatment outcomes, reduces damage to healthy cells, and combats drug resistance. Nanomaterials surmounted drug resistance by circumventing multidrug resistance mechanisms and enhancing intracellular drug accumulation. Nanoparticles show increased permeability in addition to the retention effect (EPR). The nanocarrier selection for a particulate nano-based system for BC is the most important aspect of the drug delivery systems (Fig. 5), which includes particle size ranging from 10–200 nm, encapsulation of active molecules, and reasonable circulation time. Nanocarriers facilitate the continuous and regulated release of chemotherapeutic drugs, therefore reducing adverse effects [88] (Table 3).
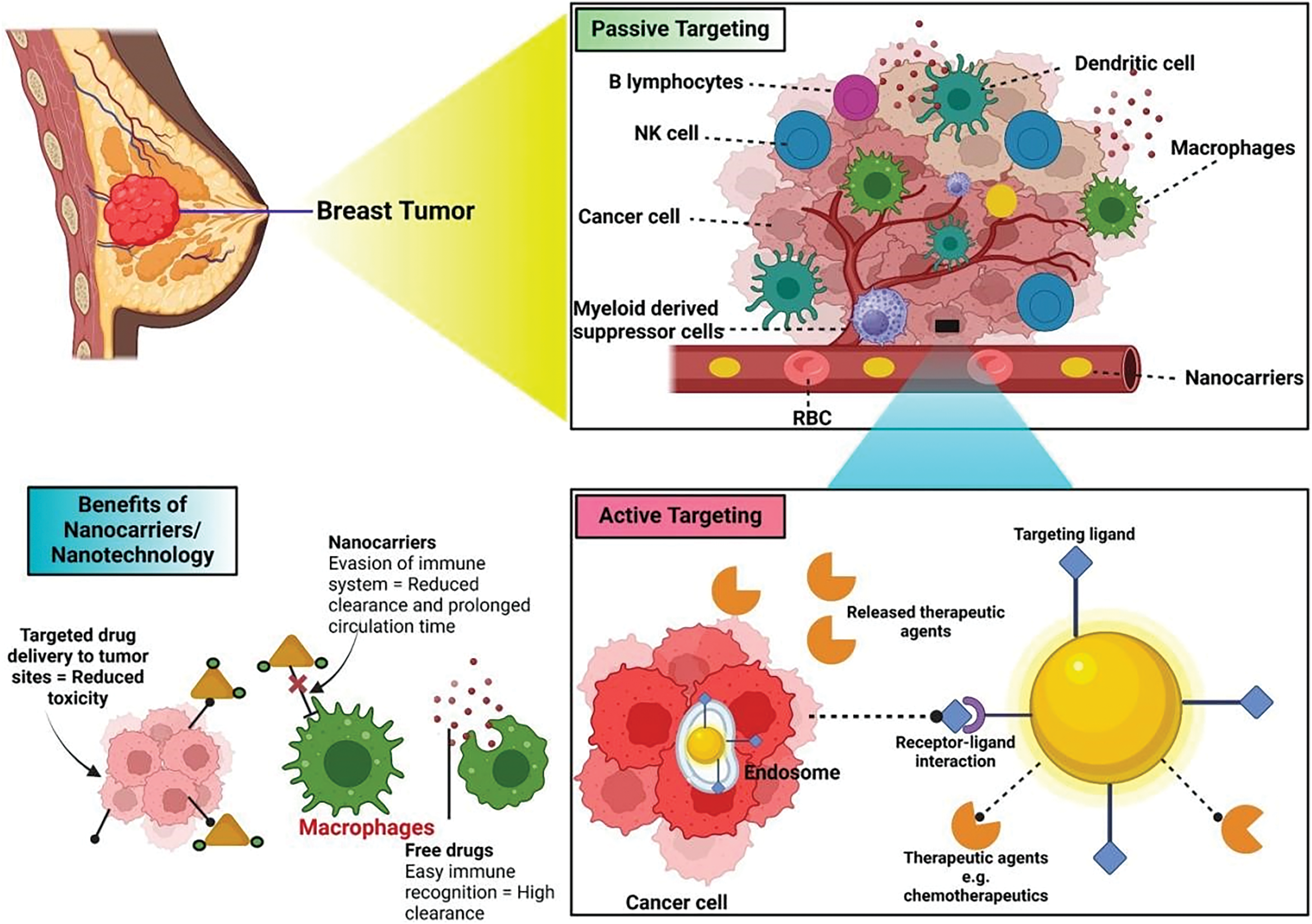
Figure 3: Tumor removal by active and passive targeting in breast cancer
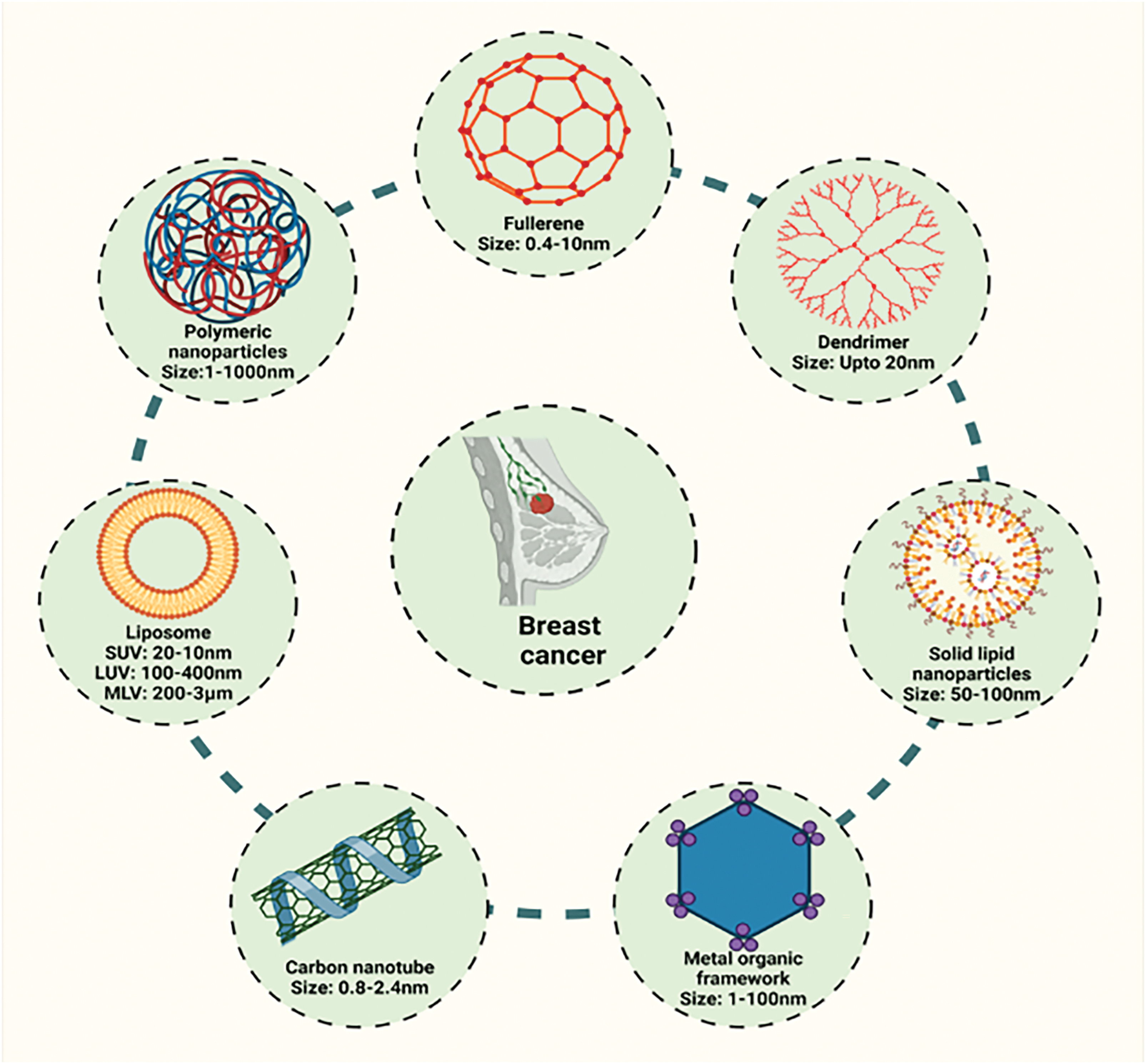
Figure 4: Different nano-based systems for the management of breast cancer
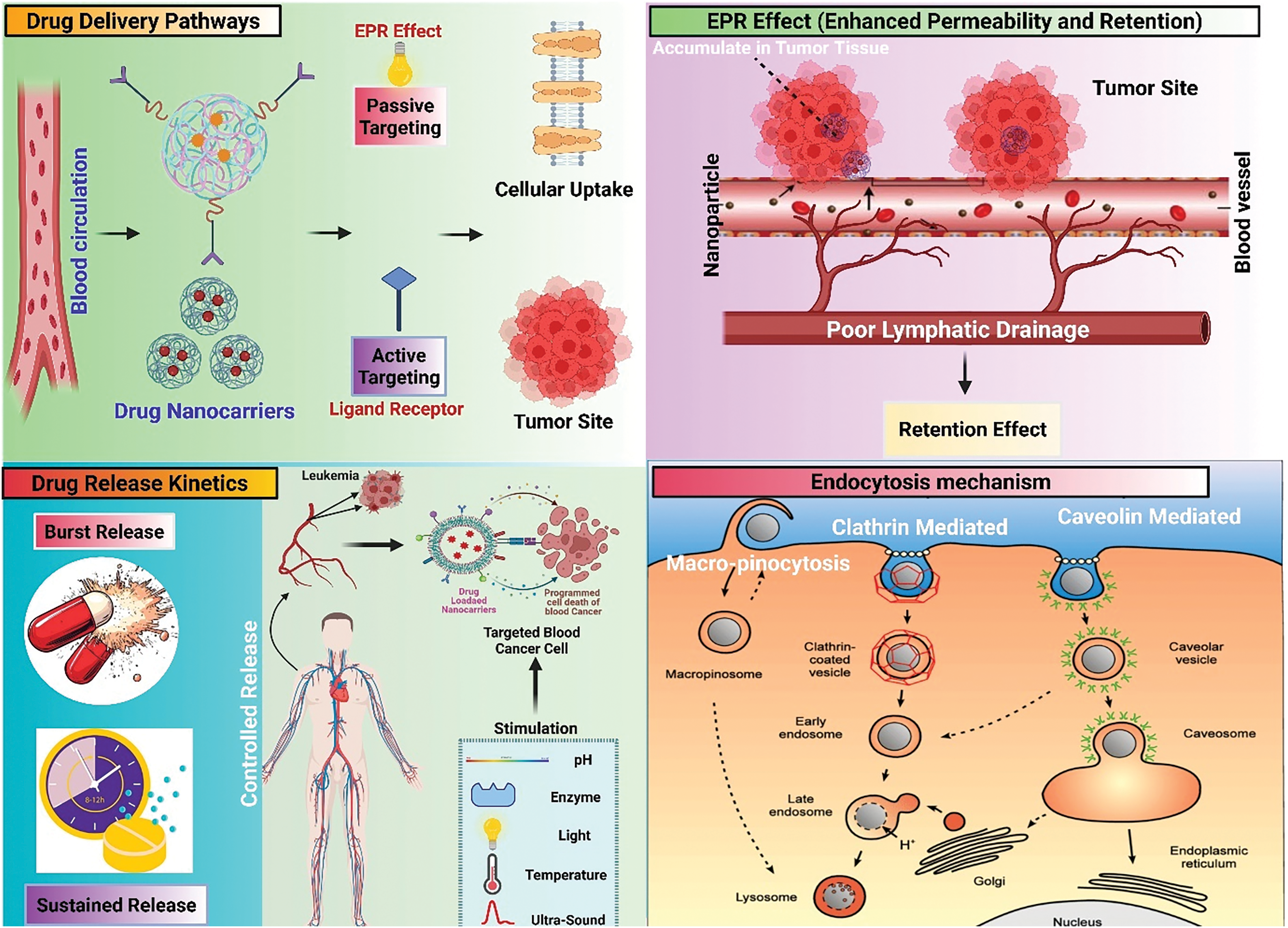
Figure 5: Schematic diagram showing EPR effect, endocytosis mechanisms, and drug release kinetics
Dendrimers have very efficient branching arrangements and are distinguished as three-layered polymeric macromolecules. An asymmetric core nucleus, internal shell, and exterior surface comprise a complicated dendrimer. Dendrimers offer biological and therapeutic applications because of their exact atomic weight, bio-similarity, monodispersed, high solubility in aqueous medium, and high natural border susceptibility (e.g., gene therapy, imaging, and drug delivery) [107]. Dendrimers are considered drug carriers with multifunctional properties since they can change specific drug delivery and regulate release of drugs while improving a dissolution profile, effectiveness, stability, bioavailability, adsorption, and solubility of the drug [45,108]. Medical researchers are researching a variety of dendrimer types as drug carriers, including dendrimers supported by two 2-bis(hydroxymethyl) propanoic acids, and dendrimers based on melamine poly (amidoamine) (PAMAM) dendrimers. According to some reports, dendrimers’ surfaces can be modified to hinder charges and reduce their toxicity. One such modification is PEGylation [109–111]. The findings of the Phase I study indicate that the utilization of a PEGylated PLL dendrimer as a nanoformulation for docetaxel demonstrated enhanced efficacy, safety, and pharmacokinetic properties compared to conventional docetaxel. Similarly, a nanoformulation of SN-38 based on PEGylated PLL dendrimers has progressed to Phase II clinical trials after Phase I trials involving breast, colorectal, and pancreatic cancer patients. These trials demonstrated enhanced anticancer efficacy and improved safety profile in comparison to the conventional irinotecan treatment [112–114]. Zhou et al. developed a polyester dendrimer nanoparticle loaded with hypericin and manganese (MHD) to improve magnetic resonance imaging (MRI) and photodynamic therapy (PDT) using hypericin. They found that the utilization of MHD resulted in a substantial improvement in MRI contrast, owing to the presence of an Mn-based paramagnetic dendrimer carrier. The author also suggested that the suppression of breast tumors through MRI-guided PDT can be accomplished using MHD-carrying hypericin, and the effectiveness of this approach can be further enhanced using Mn2+ to increase cellular ROS and alleviate the hypoxic microenvironment [115]. Researchers introduced a new type of dendrimer, called tumor microenvironment (TME)-responsive core-shell tecto dendrimer (CSTD), which demonstrated the effective binding capabilities of CSTD towards Cu2+ ions using the anticancer agent disulfiram (DSF) and the further processed complex named CSTD-Cu (II)
Chuang et al. created highly adaptable polyamidoamine (PAMAM) dendrimer-based gel nanoparticles that can target both urokinase-type plasminogen activator receptor (uPAR) and ribonucleotide reductase R2 (R2). These nanoparticles were made to attack both TNBC cells and cancer-associated stromal cells by using uPA-uPAR connections and sending the antisense oligonucleotide GTI-2040 (GTI) against R2. The resultant dual-functional dendrimer gel nanoparticles were exceedingly biocompatible. They were around 16.45 nm in size and delivered GTI 3.4 times better in TNBC cells (MDA-MB-231) and 4.8 times better in stromal cells (HCC2218) than GTI alone. It lowered R2 expression by 83.1% and caused about 30% of TNBC cells to die. In a TNBC xenograft model, GDP-uPA/GTI stopped tumor development by 50.5%. These results show that dendrimer gel nanoparticles as promising treatment option for TNBC [118]. Gholamrezazadeh et al. developed and characterized two new nitrophenol-substituted phosphazene dendrimers: [N3P3(OC6H5NO2)6] (III) exhibited reduced fluorescence due to its six 2-nitrophenol substitutions and [N3P3(OC6H3(NO2)2)5O]−. C6H16N+ · H2O (IV) showed elevated fluorescence intensity and toxicity. These findings suggest potential biomedical applications for these cyclophosphamide derivatives, warranting further research into their therapeutic potential [119]. Huang et al. developed a modified generation-5 PAMAM dendrimer to incorporate copper peroxide that which inhibits tumor metastasis, lowers intracellular pH, increases ROS generation, and depletes glutathione (GSH), leading to effective therapeutic outcomes. In vivo experiments showed that CuO2@G5-BS/TF nano complexes inhibit 4T1 breast tumor growth and metastasis without significant toxicity, offering a novel approach for treating triple-negative BC and enhancing targeted MR imaging [120]. Therefore, rigorous research on rationally crafted dendrimers for breast cancer can open doors to the creation of multiphase dendrimers with outstanding characteristics for combating TNBC in breast cancer therapy.
Nanocarriers based on lipids such as solid lipid nanparticles (SLN), niosomes, and liposomes) have gained considerable attention in drug administration due to simplicity in development, high stability, large-scale manufacture at a cheap cost, biocompatibility, and targetability. Additionally, by providing regulated drug release and increasing drug half-life, they can prolong the effects of drugs. Lipid-based nanocarriers have revolutionized cancer therapy because they have been reported to increase anticancer drug efficacies while lowering toxicities, therapeutic doses, and drug resistance [121,122]. Liposomes are the first developed nanocarriers that are lipid-based for drug delivery. These are sphere-shaped lipid vesicles with at least one phospholipid bilayer surrounding them and an aqueous center. Because phospholipids are amphipathic, they can transport hydrophobic and hydrophilic medications into the phospholipid bilayer and the inner aqueous compartments. PEGylated liposomal doxorubicin was developed in 1995 for treating AIDS-related Kaposi’s sarcoma and was the first FDA-approved nanomedicine. It is now licensed to treat recurrent ovarian cancer, metastatic BC, and multiple myeloma [123–126]. Researchers have designed to deliver site-specific anticancer drug trastuzumab-conjugated Paclitaxel (PTX) and Elacridar (ELA)-loaded PEGylated pH-sensitive liposomes (TPPLs), which resulted in a more significant reduction in tumor burden in the in vivo tumor regression investigation with minimal toxicity and hemolysis, along with more drug release percent at an acidic pH = 5 [127]. Ahamad and coworkers designed nanostilbenes that can deliver loaded bioactives to targeted sites and greatly enhance the therapeutic efficiency of encapsulated bioactives, resulting in improved water solubility, decreased breakdown, and enhanced cellular uptake at the site of breast tumors [128]. Lu and his colleague developed a polydopamine (PDA)-coated liposome-based nanoplatform, utilizing both chemotherapy and laser-induced photodynamic and photothermal therapies. This liposomal system consists of Indocyanine green (ICG) and doxorubicin (DOX), resulting in the construction of PDA-liposome nanoparticles, which suggests that it is a versatile nanosystem that utilizes PDA-coated liposomes to facilitate precise and effective combined cancer treatment [129]. This study looks at a new neoadjuvant therapy that uses folate surface-modified liposomes to make an oncolytic Ad with human telomerase reverse transcriptase (Ad-hTERT) work better in CAR-low TNBC tumors. This medication helps lower the level of treatment by lowering or getting rid of the requirement for hazardous chemotherapy combinations or checkpoint inhibitors. In vitro investigations using CAR-low TNBC murine 4T1-eGFP cells, CAR-high TNBC human MDA-MB-231-GFP cells, and numerous additional TNBC human cancer cell lines with different levels of CAR expression showed that encapsulated Ad-hTERT was far more hazardous than Ad-hTERT. The same findings were seen in primary TNBC cells taken from patients. In vivo studies in immunocompetent mice with CAR-low 4T1-eGFP tumors showed that encapsulated Ad-hTERT, given as neoadjuvant therapy, led to stable or smaller tumors, higher survival rates, more cancer cell death, less cancer cell growth, and more T-cell infiltration in tumors that had been removed. So, liposomal encapsulation of Ad might be a good way to treat TNBC [130]. Maghsoudi et al. synthesized a novel nanocarrier, DOX@m-Lip/PEG, by encapsulating doxorubicin in liposomes containing PEG and Fe3O4. This nanocarrier was investigated for its potential in treating BC, and it was found that the system is more effective and less harmful to the heart. Moreover, the magnetic characteristic of the m-Lip@PEG nanocarrier renders it a promising material for hyperthermia and MRI investigations [131]. George and his colleague introduce a novel formulation of a newly developed anthraquinone derivative, LipoRV, modified with polyethylene glycol at the nanoscale level. The formulation has demonstrated significant efficacy against the TNBC cell line. The administration of LipoRV effectively impeded the development of the cell cycle, triggered cellular apoptosis, and diminished the extended proliferative capacity of TNBC Cells. In a xenograft animal model, LipoRV successfully cleared tumors and demonstrated a good safety profile, without detrimental effects on biochemical markers. Finally, RNA sequencing of LipoRV-treated TNBC cells was carried out, indicating that LipoRV may have immunomodulatory properties [132]. Li et al. designed a co-loaded crizotinib (Cri) and F7 into a thermosensitive liposome (TSL) to create F7-Cri-TSL. The F7-Cri-TSL was found with high entrapment efficiency (>95%), significant thermosensitive properties, and good stability. On the MCF-7 xenograft mice model, also a therapeutic synergism of Crizotinib, F7, and hyperthermia. Meanwhile, it was shown that the TSL reduced the systemic toxicity of the chemotherapy drug and can function as a viable mechanism for BC therapy that is activated by changes in temperature [133]. Shemesh et al. evaluated a liposomal nano-delivery system in TNBC that used indocyanine green (ICG) as a photosensitizer, which showed that photo-activating liposomal ICG with NIR radiation significantly reduced cell viability by 96% and improved tumor accumulation because of its luminous characteristics. The nano-based liposomal ICG could deliver pharmacological effects and real-time formulation monitoring [134]. Metformin-loaded liposomes, with the help of the Bangham method, were developed. Met-liposomes’ diameter and negative surface charge were 170 and −20 mV, demonstrating reduced TNBC cell proliferation because of the encapsulation of the drug [135]. Li et al. and their colleagues constructed a thermo-sensitive folate receptor-targeted liposome (BI-FA-LP) consisting of Berberine and the photothermal agent Indocyanine green for BC therapy to attenuate the PTT-induced inflammation and inhibit tumor metastasis. BI-FA-LP utilized enhanced permeability and retention (EPR) effect and FA receptor-mediated endocytosis to selectively accumulate at the tumor, reducing off-target toxicity during the treatment. Moreover, BBR could suppress the PTT-induced inflammation, thus inhibiting tumor metastasis and ameliorating tissue injury [136]. This study explores effects of all-trans-retinoic acid (ATRA) and cinnamaldehyde (CA) work together with cisplatin (CPT) in MDA-MB-231 breast cancer cells. Compared to controls, the liposomal formulation CPT_ATRA_CA cut down on cell growth by a lot to 25.9 ± 2.8%, and it also stopped angiogenesis. Also, it caused apoptosis, as shown by flow cytometry, DAPI staining, and a higher Bax/Bcl-2 gene expression ratio. The experimental results, which were backed up by computer data, show that this pharmacological trio has strong anti-tumor effects. These results show that liposomal administration of ATRA, CA, and CPT might improve breast cancer treatment by targeting many pathways at the same time [137]. Various present discoveries have highlighted the role of liposomes as a critical player in the effective treatment of breast cancer. Therefore, a major research effort should go into creating future liposomes that could potentially decrease tumors successfully in breast cancer.
Spherical, closed-bilayer structures called niosomes form when non-ionic surfactants, including cholesterol, self-organize into aggregates in aqueous solution [121,138,139]. In contrast, niosomes exhibit enhanced stability compared to liposomes, necessitate less complex manufacturing procedures, and offer a more cost-effective production approach. Niosomes are thus considered a viable alternative for liposomal delivery of anticancer drugs [121]. Sabale et al. developed Capecitabine-loaded niosomes to enhance promising treatment approaches in breast cancer. The optimized batch (F8) exhibited a particle size of 118 nm, a zeta potential of 24.1 mV, an entrapment efficiency of 93%, and a polydispersity index (PDI) of 0.25. The cumulative drug release at a pH of 6.8 indicated that 86.46 ± 0.45% of the drug was released in 24 h. Cytotoxicity testing using MTT assay on MCF-7 breast cancer cell lines showed that the capecitabine niosomes were 2.6 times more cytotoxic than the pure drug. The study demonstrates that capecitabine-niosomes significantly enhanced the anticancer activity of capecitabine, suggesting a promising approach for breast cancer treatment [140].
In this study, a targeted and pH-sensitive niosome (pH-SN) formulation, incorporating quantum dot (QD)-labeled Trastuzumab (Trz) molecules for the specific delivery of Palbociclib (Pal) to cells overexpressing human epidermal growth factor receptor 2 (HER2). Pal encapsulation reached ∼86%, and the release pattern followed a two-phase pH-dependent mechanism. MTT assessments demonstrated enhanced apoptosis induction, particularly in HER2-positive cells, by Trz-Pal-pHSNs. Fluorescence imaging further validated the internalization of particles into cells. In conclusion, Trz-Pal-pHSNs emerge as a promising platform for personalized medicine in the treatment of HER2-positive breast cancer [141]. In the recent investigation by Sharafshadeh et al. (2023), developed niosomes coated with alginate for delivery of cisplatin (Cis) and doxorubicin (Dox), called alginate-coated niosomes (Nio-Cis-Dox-AL), in an attempt to decrease drug doses and overcome multidrug resistance for the effective management of breast cancer. MTT assay showed the IC50 of Nio-Cis-Dox-AL was much lower than the Nio-Cis-Dox formulations and free drugs. Cellular and molecular assays demonstrated that Nio-Cis-Dox-AL caused a significant increase in apoptosis induction rate and cell cycle arrest in MCF-7 and A2780 cancer cells and suggested that Nio-Cis-Dox-AL was effectively co-delivered Cis and Dox for the treatment of ovarian and BC [142]. The recent development of niosome nanoparticles as a delivery system for Cyclophosphamide (CYC) and Sodium Oxamate enhances cytotoxicity, induces apoptosis, elevates NRF2 protein levels, and increases expression of caspase-3 and Bax, confirming the activation of apoptotic pathways, leading to effective strategies for managing breast cancer [143]. Jawale et al. developed Plumbagin-encapsulated niosomes to improve the solubility and bioavailability of plumbagin, revealing an extended-release profile. In contrast, niosomes, such as encapsulated plumbagin, are highly effective nanocarriers for enhancing cancer therapy and are promising in drug delivery applications [144]. Moammeri et al. designed niosomal nanocarriers loaded with cisplatin (CIS) and epirubicin (EPI), also consisting of folic acid and polyethylene glycol, to improve endocytosis in the BALB/c mice model. They revealed that the co-administration of CIS and EPI via FA-PEGylated niosomes enhanced the apoptosis rate and cell migration, exhibiting promising prospects for the management of BC [145]. Recent investigational studies reported in the literature have suggested that niosomes can be a potential carrier in the coming years based on their intrinsic characteristics for breast cancer therapy.
A novel class of nanocarriers with enormous therapeutic potential is solid lipid nanoparticles (SLNs). Numerous studies have shown minimal toxicity, good encapsulation, stability, and efficiency. SLNs are submicron-sized colloidal particles with a diameter ranging from 50 to 1000 nm. They comprise a matrix of solid crystalline lipids at room temperature. Antineoplastic drug encapsulation in SLNs has allowed a targeted, regulated, and prolonged release, improving therapeutic efficacy and lowering side effects. Additionally, SLNs’ surfaces can be altered to boost effectiveness [146]. Curcumin Co-Loaded Lawsone SLN with enhanced anticancer efficacy against MCF-7 BC Cell Lines. This SLN was prepared by hot emulsification and probe sonification, whereas TEM studies revealed spherical particles. MTT assay of MCF-7 cells demonstrated increased CUR-LS-SLN cell inhibition and effectiveness, and when combining both drugs with nanocarriers, it produced better inhibition. Moreover, it showed drug synergistic action and sustained drug release for the formulation, and the optimized SLN remained stable for six months as per the stability studies [147]. Chin et al. and their coworkers designed abemaciclib solid lipid nanoparticles to target BC cell lines. The formulation was prepared by Melt emulsification and ultrasonication, followed by Quality-by-Design (QbD) optimization. The study showed enhanced anticancer activity in the MDA-MB-231 and T47D cell lines. In addition, the improved cellular uptake and cytotoxicity, as well as high entrapment efficiency and prolonged release in ABE-SLNs, the application of QbD is a revolutionary strategy that may establish a new benchmark for drug delivery systems based on nanoparticles [148]. Researchers designed atorvastatin and quercetin-loaded solid lipid nanoparticles to combat breast cancer. The studies showed a sustained release profile that performed better than drugs derived from the SLN matrix. The combined action of atorvastatin and quercetin SLNs against MDA-MB-231 was demonstrated in the in vitro cytotoxicity studies to be more effective than the two drugs alone. SLNs are a promising drug delivery technology against BC that employs an innovative approach to repurposing well-known drugs [149]. The advent of new SLN technologies for treating breast cancer will provide reliable and selective therapies to patients with BC.
4.5 Nanostructured-Lipid Carriers (NLCs)
Nanostructured lipid carriers (NLCs) are an advanced type of lipid-based drug delivery system designed to improve drug stability, bioavailability, and controlled release. They are an evolution of solid lipid nanoparticles (SLNs), incorporating both solid and liquid lipids to enhance drug loading capacity and reduce limitations like drug expulsion during storage.
Passos et al. designed a NLCs for in situ thermosensitive drug formulations to prolong drug retention and facilitate ductal administration for BC treatment. Tibutyrin-containing NLCs significantly reduced IC50 values for paclitaxel and 5-fluorouracil in BC cells compared to tricaprylin NLCs. This combination increased the retention of hydrophilic and lipophilic markers in mammary tissue and enhanced cytotoxicity in monolayer and spheroid models, highlighting their potential for improved local BC treatment [150].
Researchers developed a Capecitabine-loaded NLCs to reduce toxicity while treating BC. Results revealed that the niosomes had dramatically increased cytotoxic efficacy, around ten times greater than free capecitabine for a much longer time, as it showed sustained and slow release of capecitabine, effective treatment targeting to tumor site, reducing toxicity, and enhanced therapeutic effects at lower doses, which is therefore considered the preferred cancer treatment [151]. Rodero et al. designed a NLCs loaded with rapamycin with folic acid to overcome drug resistance and the side effects observed also promoted targeting to BC cells, which displayed a particle size of 100 nm, indicating reduced tumor cell viability. In vitro studies suggested that FA-NLC-RAP exhibited a higher degree of internalization in cancer cells (MCF-7) than in normal cells (MCF-10A), demonstrating the potential of folic acid as a ligand for promoting active targeting of RAP for breast cancer cells through folate receptors overexpressed in tumor cells FA-NLC-RAP significantly reduced tumor cell viability, similarly to that observed with the RAP solution. The release profile of the formulation was prolonged. Finally, studies in Caenorhabditis elegans evidenced the safety of FA-NLC-RAP, characterized by a complete absence of toxicity in this animal model. Therefore, the findings imply that FA-NLC-RAP holds considerable promise for the treatment of breast cancer [152]. Nehal et al. developed a Palbociclib NLCs for BC treatment with reduced adverse effects related to the drug. Intestinal permeation studies demonstrated a 3.76-fold increase in gut permeation with PB-NLC compared to that with PB-Sus. The lipolysis study indicated an enhanced drug availability at the site of absorption. Confocal studies revealed improved drug penetration depth in the intestine with PB-NLC compared to that with PB-Sus. In vivo pharmacokinetic studies demonstrated that incorporating PB into a lipidic nanocarrier (PB-NLC) significantly enhanced its bioavailability by approximately 5.9-fold (p < 0.05) compared to PB suspension. Additionally, acute toxicity studies in Wistar rats confirmed the safety of the developed NLCs for oral administration in managing breast cancer. Therefore, the PB-loaded NLCs shows significant promise for breast cancer treatment, providing improved drug delivery and minimized side effects [153].
Elkholy et al. repurposed niclosamide for BC treatment but faced issues with its hydrophobicity and low oral bioavailability. They developed NLCs and chitosan-coated NLCs to enhance oral delivery. Furthermore, in vivo studies on Ehrlich Carcinoma-bearing mice showed that both NLCs formulations, especially the chitosan-coated NLCs, significantly enhanced niclosamide’s antiproliferative activity compared to the drug suspension [154]. The excellent results on NLCs in the above section demonstrate that NLCs can be satisfactorily manufactured for TNBC as a therapeutic approach.
4.6 Polymeric Drug Delivery Systems
Polymer-based nanocarriers provide targeted drug administration and sustained drug release while protecting pharmaceuticals from RES, liver, and renal metabolism and clearance that occurs quickly [155]. They can be made either from natural and/or synthetic polymers. Synthetic polymers are more prevalent than natural ones, have higher mechanical and thermal stability, and may be treated more efficiently to get the necessary scaffold shape and pore size. Natural polymers typically offer higher biocompatibility and biodegradability than synthetic polymers, which can contain impurities that impact their properties [156,157]. He et al. and colleagues utilized a PLGA nanoparticulate platform to encapsulate saturated FA palmitic acid (PA), with DOX or alone, to investigate the potential of PA as a monotherapy or combination therapy for BC and suggest that the PLGA-PA-NPs were as effective in reducing primary tumor growth and metastasis reduced the expression of genes associated with multi-drug resistance and inhibition of apoptosis, and induced apoptosis via a caspase-3-independent pathway in breast cancer cells. In addition, immunohistochemical analysis of residual tumors showed a reduction in M2 macrophage content and infiltration of leukocytes after treatment of PLGA-PA NPs and PLGA-PA-DOX NPs, suggesting immunomodulatory properties of PA in the tumor microenvironment. In conclusion, the use of PA alone or in combination with DOX may represent a promising novel strategy for the treatment of breast cancer [158].
Wang et al. designed a nano-fluorescent polymer drug delivery for BC treatment, where heterometallic coordination polymer (CPI) was synthesized by solvothermal reaction. This novel nanocarrier significantly increased bioavailability and reduced cytotoxicity by exhibiting outstanding fluorescence responsiveness and enabling prolonged drug release in aqueous conditions. The study findings demonstrated that Dimethylamine cations stabilize the compound’s three-dimensional anionic structure, which keeps it thermally stable up to 480°C. Colorless polyimide (CP1) is an excellent option for blue luminescent materials because of its broad blue emission band at 452 nm and exceptional heat stability [159]. Polymeric nanoparticles loaded with Brazilian red propolis were developed by Justino et al. and characterized using Transmission Electron Microscopy (TEM) and Fourier Transform Infrared Spectroscopy (FTIR). While FTIR showed that the extract had been successfully encapsulated, TEM verified the spherical shape and anticipated size of the nanoparticles. Cytotoxicity tests revealed that nanoparticle-encapsulated BRPE (NCBRPE) exhibited reduced toxicity to normal breast cells (MCF-10) but increased toxicity to breast cancer cells (MCF-7) compared to free BRPE, suggesting targeted therapeutic potential. Notably, NCBRPE demonstrated enhanced efficacy in acidic tumor microenvironments, further supporting its targeted therapeutic potential for breast cancer therapy [160]. Chaudhari et al. developed poly (lactic-co-glycolic acid) (PLGA) loaded with paclitaxel (PTX) and conjugated with adenosine (ADN) to target triple-negative BC (TNBC), in which the ADN component of the nanoparticles serves as a substrate for adenosine receptors (AR), which are known to lie on the surface of TNBC. The study’s findings indicate that the optimized particles exhibited biocompatibility and enhanced anti-TNBC activity [161]. PLGA nanoparticles co-loaded with the anticancer agents, namely curcumin and paclitaxel, and the system referred to as PLGA-CUR-PTX nanoparticles. The result of this investigation suggested that the optimized nanoparticles exhibited a superior cytotoxic effect, as evidenced by reduced IC50 values, against the MCF-7 and 4T1 BC cell lines compared to the unbound drugs [162]. Sarpoli et al. synthesized a novel nanostructure PLGA-polyethyleneimine (PEI) nanosystem with the purpose of co-delivering curcumin and siRNA to BC cells and suggested that the nanosystem exhibited notable cytotoxic effects on the T47D cell line by delivering an anticancer drug for the effective management of BC cells [163]. Hu et al. and their team synthesized hyaluronic acid-coated Olaparib-loaded PEI-PLGA nanoparticles. They evaluated their efficiency as a site-effective anticancer agent, and sustained drug release behavior exhibited efficient in vitro and in vivo antitumor activities. HA-Ola-PPNPs induced cell apoptosis by upregulating Bax, Cytochrome C, and Caspase 3, downregulating Bcl-2 in breast cancer-bearing mice against tumors and suggested that nanoparticles loaded with Olaparib potently target triple-negative BC cells [164].
Malarz et al. developed pH-sensitive phthalocyanine-loaded polymeric nanoparticles as a novel treatment strategy for combating BC. However, Polymeric nanoparticles coated with pH-sensitive phthalocyanines selectively inhibit the growth of BC cells while leaving healthy cells unaltered. Photocytotoxicity experiments demonstrated their excellent efficacy and selectivity for the SK-BR-3 cell line, confirming their high antiproliferative activity [165].
Behl and his research team created a novel nanoformulation, called DM-PEG-PCL NPs, comprising a polyethylene glycol-polycaprolactone (PEG-PCL) polymer that is packed with a MUC1 inhibitor and a potent anticancer medication, doxorubicin (DOX), for the treatment of TNBC. The delivery technology under control exhibits biodegradability, non-toxicity, and anti-multidrug-resistant properties. Furthermore, the customized smart nanoformulation has demonstrated notable efficacy in treating TNBC [166].
Liaqat et al. synthesized zinc selenide quantum dots (ZnSe QDs) and combined them with a doxorubicin sitagliptin-lignin biopolymer (SL) for a drug delivery system. Optimal drug release occurred at pH 6.5 and 45°C, achieving 81.75% drug encapsulation. SLQD-Doxo demonstrated 24.4% anti-inflammatory activity and 71.45% lipoxygenase inhibition. Tested on MCF-7 BC cells, it resulted in 74.39% cell viability and 24.48% cell death, significant therapeutic, anti-inflammatory, antioxidant, cytotoxic, and increased antioxidant activity. The lignin’s polyphenolic nature contributed to vigorous antioxidant activity, making SLQD-Doxo effective for tumor cells’ high temperatures and acidic pH [167].
Odeniyi et al. designed thiolated and carboxymethylated Jackfruit seed starch nanocrystals as polymeric drug carriers for the delivery of curcumin to cancer Cells. This study assessed modified jackfruit starch nanocrystals as potential delivery systems for curcumin to MCF-7 BC cell lines. The cytotoxicity and in vitro drug release of curcumin-loaded starches on MCF-7 cancer cells were assessed. The produced nanocrystals showed a modified morphology and an average particle size of 6.15 ± 0.80 nm. Carboxymethylated nanocrystals had the highest drug loading (70.23% ± 0.23%), followed by thiolated nanocrystals (63.37% ± 0.40%), while native starch had the lowest (49.31% ± 0.13%). Therefore, modified jackfruit starch nanocrystals are acceptable alternatives for the efficient delivery of curcumin to cancer cells because they have desirable qualities [168].
Nasr et al. developed folic acid (FA) grafted polymeric micelles loaded with tamoxifen citrate (TMXC) to enhance antitumor activity in breast tissues. In vitro evaluation showed that the FA-conjugated micelles (FA-P123/P84) had an encapsulation efficiency of 87.83%, a size of 35.01 nm, and a surface charge of −20.50 mV. These micelles released 67.58% of TMXC after 36 h and demonstrated 2.48 times better cytotoxicity and higher cellular uptake in MCF-7 cells. In vivo studies showed significant tumor volume reduction in tumor-bearing mice treated with TMXC-loaded FA-P123/P84, suggesting these micelles are a promising carrier for TMXC in BC treatment [169].
Sah and Kumar designed PLGA-SPC3 functionalized gefitinib mesoporous silica nano-scaffolds for BC by using a heat-assisted method. In optimized MSN, albino Wistar rats were used for the in vivo biodistribution investigations, cytotoxicity measurement, and in vitro drug release kinetics. The outcome indicates that MSN’s surface area, pore volume, % encapsulation efficiency (EE), and drug loading increased. Moreover, in vitro cytotoxicity showed more anticancer activity than free drug (43.84% ± 0.63%, p < 0.05. However, its effectiveness in treating BC was demonstrated by the rise in cell viability observed in the MCF-7 cell line during in vitro tests; therefore, a novel approach for combating BC [170].
Lv et al. designed folate-functionalized carboxymethyl chitosan (CMCS)/calcium phosphate hybrid nanoparticles (CF/CaP) with Ca2+ production to treat BC by combining them with encapsulated curcumin (Cur). The spherical C@CF/CaP nanoparticles, about 179 nm, showed strong stability, acid-responsive drug release, and good biocompatibility. At pH 5.0, over 70% of Cur was released after 36 h. These nanoparticles targeted tumor tissues via folate receptor-mediated endocytosis, causing mitochondrial Ca2+ overload, initiating the mitochondrial apoptotic pathway, and damaging the mitochondrial structure. The study demonstrated that CMCS-based Ca2+ nano-modulators could be a viable organelle-targeting approach for cancer treatment [171]. Rakhshani et al. designed doxorubicin PLGA nanoparticles and simvastatin loaded in silk films (SV/DOX PLGA/SF films) for local treatment of BC. The study revealed uniform size and spherical morphology. In female Balb/c mice, SV/DOX PLGA/SF films showed the most significant tumor suppression effect and the lowest tumor recurrence, with only around 52% ± 10.3% of the original tumor remaining. Furthermore, SF-based localized therapies dramatically decreased the mitotic index, according to histological investigation using H&E staining. Therefore, it is biocompatible, biodegradable, and used for post-surgical cancer combination treatment [172].
4.7 Protein-Based Drug Delivery Systems
Multiple protein subunits make protein-based nanocarriers, which can self-assemble precisely and spontaneously to create hollow cavities with internal nanocarriers [173]. Due to the unique characteristics of protein-based nanocarriers, their practical applications (such as biocatalysis, diagnostic imaging, drug administration, and vaccine development) have rapidly increased during the past few years [174]. In addition to their biocompatibility and biodegradability, protein-based nanocarriers are also easy to synthesize, control size, are inexpensive, have strong stability, can be modified on the surface for targeted drug delivery, and can be used to control drug release. Nevertheless, the utilization of nanocarriers derived from diverse proteins is not without its limitations. These drawbacks encompass the considerable expense associated with specific proteins like albumin and ferritin, the possible risk of prion transmission from animal sources, like collagen and gelatin, the poor mechanical integrity of gelatin, and the slow or fast degradation of silk protein fibroin [175,176]. The field of oncology has demonstrated the most significant utilization of protein-based nanocarriers.
Wang et al. developed cinchonine and immunoglobulin G nanoparticles in order to improve anti-BC activity. This study revealed that the produced Cin-IgG NPs exhibit a pH-sensitive drug release behavior, a hydrodynamic size of 190 nm, and an EE% of 72.38%. Furthermore, Cin-IgG NPs have a more substantial effect on the downregulation of PI3K/p-AKT and the overexpression of the Bax/Bcl-2 ratio compared to the free drug. This study concludes that Cin can bind IgG as a human plasma protein and that complexing with IgG to generate an NP form can enhance its anti-BC properties [177]. Deng et al. developed silk protein-based nanoporous microspheres using an Ionic Liquids (ILs)-induced self-assembly process blended with poly (d,l-lactic acid). These microspheres exhibited effective drug loading (up to 88.7%) and sustained release kinetics, with over 53.5% drug release in the first four hours. The ILs facilitated hydrophobic and electrostatic interactions, enabling drug entry into the nanoporous matrix and inducing reversible changes in protein structure during delivery. The system shows promise as an efficient drug delivery vehicle for various biomaterial applications [178].
Are and her coworkers designed nanoparticles using human serum albumin (HSA), which was coupled with vitamin E and loaded with a tyrosine kinase inhibitor (TKI) or an aromatase inhibitor (AI) with the help of the desolvation method. Some physicochemical tests, such as Infrared spectroscopy (IR), gel permeation chromatography (GPC), ultraviolet, infrared, and CD spectroscopy, were used to study HSA (VE) binding and drug incorporation into nanoparticles, assessing their drug entrapment and release efficiency. Eventually, cell viability tests and in vitro studies on resistant and sensitive cell lines. The study showed efficient drug encapsulation and absorption. Studies conducted in vitro and in vivo showed that a 75:2 lapatinib-loaded nib-loaded HAS-VE NPs to Letrozole loaded HAS-VE NPs reduced tumor growth and increased apoptosis compared to individual NP therapy and free drug. In addition, when VE and HSA were conjugated, the outcome was self-assembly into nanoaggregates with enhanced cellular absorption, decreased IC50 values, and a high drug loading capacity [179].
Safwat et al. developed bioinspired caffeic acid-laden milk protein-based nanoparticles targeting folate receptors for BC treatment prepared using a simple coacervation method and lyophilization. This study demonstrated that conjugated NPs achieved IC50 = 40 ± 2.9 μg/mL, and PS = 157.23 ± 2.64 nm. In the tumor-induced animals treated with conjugated NPs, there was a significant decrease in the biochemical marker levels of malondialdehyde, carbohydrate antigen 15−3, and carcinoembryonic antigen, and an increase in superoxide dismutase. Histopathological analyses revealed significant improvement in the necrotic and mammary areas. Moreover, these findings highlight the most promising platform for showcasing advanced therapeutic applications for combating various diseases, including breast cancer [180]. As research continues to develop, these novel approaches, like protein-drug delivery, show a promising arena in the creation of customized, next-generation cancer treatment.
Carbon-based nanocarriers have also been made because of their features, notably high chemical stability, large surface area, preferential tumor accumulation, and high cellular penetration. They are now potentially promising medication carriers for cancer treatment [181,182]. Bovine serum albumin (BSA) and folic acid-coated platinum-functionalized oxygenated single-walled carbon nanotubes (O-SWCNTs-Pt to create O-SWCNTs-Pt-BSA-FA. The resulting nanotubes were then utilized as radiosensitizers to enhance the therapeutic effectiveness of X-rays in an in vitro mouse model of BC (4T1). The novel nano-sensitizer can be utilized with radiotherapy technology for cancer treatment, as it showed very strong cell-killing activity in the 4T1 cell line, controlled release, and easy penetration into biological membranes [183]. Badea et al. investigated the fabrication of a nanoconjugate utilizing single-walled carbon nanotubes with single-walled that were functionalized with cisplatin (CDDP) and carboxyl groups (SWCNT-COOH-CDDP) were compared them with conventional 2D- and 3D-spheroid cultures of human breast cancer cells. The SWCNT–COOH–CDDP complex proved to have high anti-cancer efficiency on 2D and 3D cultures by inhibiting cell proliferation and activating cell death. A dose of 0.632 μg/mL complex triggered different pathways of apoptosis in 2D and 3D cultures, by intrinsic, extrinsic, and endoplasmic reticulum pathways. Overall, the 2D cultures showed higher susceptibility to the action of complex compared to 3D cultures, and SWCNT–COOH–CDDP proved enhanced anti-tumoral activity compared to free CDDP [184].
Xie et al. developed a carbon nanoparticle with Fe (II) complex (CNSI-Fe) combined with sorafenib for ferroptosis-induced (SRF) antitumor effects in triple-negative breast cancer. In this study, CNSI-Fe, in conjunction with sorafenib (SRF), a ferroptosis inducer, can provide adequate treatment for TNBC. With a maximal adsorption capacity of 31 mg/g, CNSI-Fe could adsorb SRF through hydrophobic contact and π-π stacking. Compared to CNSI-Fe or SRF alone, the CNSI-Fe+SRF combination significantly reduced the cell viability of 4T1 cells during the in vitro tests. The oxidative damage, hydroxyl radical production, and elevated Fe absorption confirmed that 4T1 cells underwent ferroptosis following CNSI-Fe+SRF therapy. SRF improved the therapeutic impact of CNSI-Fe during the in vivo tests, as evidenced by the greater survival rate and tumor growth suppression rate of 67.8%. Therefore, it is a promising and efficient treatment for TNBC through ferroptosis [185].
Graphene oxide nanoparticles (HA-GO-Met) loaded with metformin grafted with hyaluronic acid (HA) showed antitumor effectiveness at far lower doses than metformin alone, according to a 2021 study. Cell migration was inhibited by the reduction of pFAK/integrinβ1 expression. Treatment inhibited epithelial-mesenchymal transition (EMT) and reduced stemness as evidenced by the increase in E-cadherin expression, inhibition of mammosphere formation, and low expression levels of stemness markers, including nanog, oct4, and sox2, as compared to control. Moreover, tumor regression was studied in chick embryo xenograft and BALB/c mice models. HA-GO-Met nanoparticle treatment reduced tumor load and nullified toxicity in peripheral organs imparted by the tumor [186]. Carbon dots doped with gadolinium, Fe3+, and Mn2+ have been demonstrated to have been developed from N-hydroxyphthalimide. Cell viability was assessed after doped carbon dots were applied to normal and cancerous cell lines. It was found that Mn2+ doped Carbon Dots (Mn-CDs-NHF) had antitumor properties without compromising the viability of normal cells and decreased the size of primary mammary tumors while permitting magnetic resonance imaging, implying that they can be employed in preclinical models as theranostic agents [187]. Radzi et al. prepared an oxidized multi-walled carbon nanotube (ox-MWCNTs) developed by acid washing and hypothermic breast cancer treatment. The prepared formulation was found to eliminate the tumor when tested in vivo in mice. Histopathology analysis revealed that tumors treated with the intended treatment underwent necrosis. Also, there was a significant increase in natural killer cells, macrophages, and CD8+ and CD84+ T cells in the tumor cells. The study’s overall findings point to the potential of ox-MWCNTs-mediated HT as an anticancer therapeutic agent, which may prove advantageous for the treatment of BC in the future [188].
Tiwari et al. developed a method to reduce chemotherapy toxicity by encapsulating dacarbazine (DC) in fucose-based carbon quantum dots (CQDs) and then coating them with exosomes from BC cells (Ex-DC@CQDs). Western blotting and nanoparticle tracking analysis confirmed that Ex-DC@CQDs retained exosome properties. Exosomes enhanced delivery to cancer cells via HSPG receptors, increasing mitochondrial membrane potential depolarization, ROS production, and apoptosis. In vivo, Ex-DC@CQDs showed more significant tumor accumulation due to sustained release and exosome-mediated uptake. This approach suggested biologically sourced nanocarriers as a promising future for BC treatment [189]. Varvarà et al. designed targeted NIR-triggered doxorubicin release using carbon dots–poly(ethylene glycol)–folate conjugates for effective treatment. Doxorubicin-loaded CDs (CDs-bAPAE-PEG-FA/Dox) exhibited on-demand NIR-boosted drug release, which is increased by 50% after localized NIR exposure, whereas in vitro studies on BC cells MCF-7 and MDA-MB-231 exhibited NIR-enhanced antitumor efficacy, which reveals selective and remote-controlled synergistic therapy. According to data, CDs-bAPAE-PEG-FA/Dox could carry out effective image-guided and selective BC therapy, improving the therapeutic results [190].
Carbon nanocarriers, including graphene, carbon nanotubes, and fullerenes, hold promise for drug delivery but also present challenges in terms of off-target toxicity, biodistribution, and immune response. Carbon nanocarriers may accumulate in non-target organs, resulting in unwanted cytotoxicity. Certain carbon-based materials cause oxidative stress, which hurts normal cells. Nonhomogeneous distribution of nanoparticles may lead to suboptimal delivery of drugs, decreasing therapeutic efficacy. Some nanocarriers show extended retention in the spleen and liver, impacting clearance. Carbon-based nanocarriers can interact with immune cells, and this may result in immunosuppression or overstimulation. Surface modifications are under investigation to prevent immune system activation and enhance biocompatibility.
Nanocomposites are a subset of deliberately engineered materials known as nanomaterials, which integrate nanoscale particles inside a matrix of conventional materials. Nanosized particles may possess one, two, or three dimensions measuring less than 100 nm. The incorporation of nanoscale components confers distinctive properties to the composite material, rendering nanocomposites advantageous for many applications, including healthcare.
Silica-based nanocomposites are used for biosensing and targeted therapy due to their porous structure. Nanomaterials interact with cell membranes, facilitating targeted drug delivery [191].
This investigation used green synthesis of a bi-metallic nanocomposite of nickel and zinc using an aqueous extract of Citrus sinensis in the presence of chitosan (Ni/Zn@orange/chitosan). The NPs were spherical in shape and size of 17.34–90.51 nm., The composite nanoparticles were studied by the MTT method for the anti-breast adenocarcinoma potential in malignant cell lines. The results revealed that the newly synthesized nanocomposite is a potent photocatalyst and an acceptable new drug to treat breast cancer [192].
Hybrid nanomedicine means combination therapies that include chemotherapeutics, and multiple chemotherapeutics, along with biologicals, polydynamic, and chemotherapeutics, radiation, and chemotherapeutics. It contains both inorganic and organic components. The potential to combine with mass organic and inorganic components to achieve the desired hybrid nano-formulation that allows the systemic alteration to achieve the desired results. Hybrid nanomaterials and gold-coated iron oxide nanoparticles are used for imaging and drug delivery. Hybrid nanogels integrate organic and inorganic components for controlled drug release [193].
ISL-loaded zein phosphatidylcholine hybrid nanoparticles (ISL@ZLH NPs) were constructed by a one-step solvent evaporation method. Optimization and characterization of ISL@ZLH NPs were performed. Cellular uptake in vitro and biodistribution of ZLH NPs in vivo were investigated. The pharmacokinetics of ISL@ZLH NPs in terms of ISL content in the plasma, organs, and tumor tissues were validated, and the anti-TNBC efficacy was evaluated. Encapsulation efficiency (96.75 ± 1.41%) and drug loading efficiency (6.56 ± 0.83%) were found in ISL@ZLH NPs. ISL@ZLH NPs were able to enhance the absorption of ISL in the tumor sites. Moreover, the upregulation of p27 and downregulation of EGFR and CDK4 were observed in ISL@ZLH NPs-treated tumors. Collectively, oral intake of ISL@ZLH NPs would be translated into a potential clinical therapy strategy against TNBC [194].
Designing multiphase nanomaterials can be done by doping with metals like silver or gold to enhance antibacterial and photocatalytic properties by doping with nitrogen or boron to improve conductivity and biocompatibility. Combining polymers with metal oxides for improved drug delivery and stability.
4.11 Nanotoxicity and Associated Risks
Numerous nanocarriers encounter difficulties in accurately targeting breast cancer cells, resulting in off-target consequences and diminished therapeutic efficiency. Certain nanoparticles are unable to surmount multidrug resistance processes, hence constraining their efficacy in prolonged therapy. The synthesis and scalability of nanocarriers present significant challenges, obstructing their extensive use. Some nanomaterials demonstrate cytotoxicity, prompting questions over their safety for therapeutic use. Many inorganic NDDSs exhibit cytotoxicity, limiting their clinical applications. Organic NDDSs often suffer from rapid degradation, reducing drug efficacy. Hybrid NDDSs sometimes fail to achieve optimal targeting due to inconsistent surface modifications. The synthesis of NDDSs requires precise control over size, shape, and composition, making large-scale production challenging. The long-term effects of NDDSs on human health and the environment remain inadequately studied. The interaction between NDDSs and biological systems is not fully understood, affecting their predictability in drug delivery. There is a lack of standardized protocols for evaluating NDDS efficacy and safety. Despite promising preclinical results, few NDDSs have successfully transitioned to clinical applications.
Nanotoxicology, an essential component of nanoscience, examines the possible detrimental consequences of manufactured nanoparticles with diverse mechanisms, particularly in biological applications. Nanoparticles (NPs) are extensively utilized in nanomedicine; yet, their nanosize and unique properties raise concerns over their safety, along with some other factors like size, shape, surface functional groups, and dose-dependent characteristics that also influence toxicity. Chemically synthesized nanoparticles may have elevated toxicity due to synthetic compounds, whereas biosynthesized nanoparticles with biocompatible surface groups indicate reduced toxicity [195]. About human health, dose-dependent nanoparticle toxicity refers to the escalation of a particle’s harmful effects with higher concentrations or extended exposure. At lower quantities, they may be benign or even beneficial; nevertheless, at elevated concentrations, they can induce oxidative stress, inflammation, and DNA damage, and even result in cellular death. The toxicity of a nanoparticle is contingent upon its method of exposure (ingestion, inhalation, etc.) and the degradation of nanoparticles into simpler forms or their accumulation within cells. Prolonged or elevated exposure heightens the risk of cancer and adversely affects the respiratory, cardiovascular, and neurological systems [196,197]. Nanotoxicology aims to detect possible risks and evaluate the safety of nanomedicines. The subsequent section reviewed contemporary research that offered recent insights into the toxicological consequences linked to nanoparticles and the principal processes driving nanoparticle toxicity. Specifically, concentrating on the potential of nanoparticles for direct contact with the cell membrane, their capacity to dissolve and release harmful ions, and their propensity to trigger oxidative stress. Research indicates that nanoparticles (NPs) can infiltrate the human body by inhalation, ingestion, and topical application, potentially causing harm to adjacent cells, tissues, and organs. Recent studies have emphasized the exploration of the processes behind the toxicity of nanomaterials, particularly their adhesion to the cell membrane. The interaction of nanoparticles with cells or cellular components, including the cell membrane, metabolic processes, ribosomes, mitochondrial activity, and the electron transport chain, influences their toxicity [198].
The morphology of nanoparticles influences their interaction with cellular membranes, behavior in the circulatory system, and their dispersion throughout various tissues. For example, rod-shaped or needle-like nanoparticles may infiltrate cell membranes more readily than spherical nanoparticles, potentially resulting in more damage and heightened toxicity. Various geometries can affect the mechanism and efficacy of cellular absorption. Shapes that are more easily absorbed may result in elevated intracellular concentrations of nanoparticles, hence increasing toxicity [199]. Non-spherical nanoparticles may exhibit modified clearance rates and tissue penetration, possibly resulting in a buildup in certain organs and heightened local toxicity [200]. Various nanoparticle morphologies may obstruct their elimination from the organism, causing extended circulation durations, resulting in bioaccumulation and possible chronic health repercussions [201]. Shape management is essential, as various shapes interact with cell membranes and tissues distinctly, influencing cellular uptake, membrane integrity, and biodistribution, all of which contribute to nanotoxicity.
To ensure the safe use of nanoparticles, researchers are developing strategies such as coating nanoparticles with biocompatible materials, controlling nanoparticle size to improve biodistribution and minimize unintended effects, and establishing safety protocols for nanoparticle exposure in medical and industrial applications. Nanotechnology offers immense potential, but understanding its risks is essential for responsible innovation.
5 Clinical Trials, Ethical and Regulatory Considerations in Nanomedicine for Breast Cancer
Clinical trials are human research studies carried out by different commercial organizations throughout the globe to develop new treatments with more potential and less toxicity. Various clinical trials of drug moieties, biological compounds, and their combinations are running for the BC treatment. For BC therapy, clinical trials of different stages and FDA-approved drugs are listed below (Tables 4 and 5). The patented technology is also discussed in tabular form (Table 6). Nanomedicine is transforming breast cancer therapy by providing precision-targeted medicines and improved drug delivery systems. Before these improvements can be used in clinical practice, they must traverse a multifaceted terrain of ethical concerns, clinical trial mandates, and regulatory approvals. Researchers and physicians must confront the following ethical issues: patient safety and risk mitigation, due to the potential accumulation of nanoparticles in unexpected organs, and their long-term consequences are indeterminate. Ethical principles emphasize thorough preclinical safety evaluations to reduce health hazards [202].
Nanotherapeutics sometimes entail substantial research and production expenses, prompting apprehensions over affordability and accessibility for disadvantaged patients. The equitable allocation of sophisticated cancer therapies is an increasingly significant ethical concern. Nonetheless, its clinical application needs rigorous regulatory supervision to guarantee safety, effectiveness, and ethical adherence [203]. The Food and Drug Administration (FDA), European Medicines Agency (EMA), and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are pivotal in formulating criteria for the approval of nanomedicine. The FDA offers explicit recommendations for medicinal products utilizing nanotechnology, emphasizing manufacturing processes, pharmacokinetics, and toxicity evaluations. Guidelines for assessing nanoparticle interactions with biological systems to mitigate toxicity concerns. Focus on extensive biodistribution studies and assessments of immune responses for nanomedicine-based therapies. The EMA has created horizon-scanning reports to evaluate developing nanomedicines, focusing on categorization, regulatory readiness, and ethical issues. Essential factors encompass the continuous assessment of nanoparticle stability, toxicity, and therapeutic effectiveness. Assessing the effects of nanomedicines on ecosystems and human health. Protocols for connecting preclinical discoveries with human trials to improve regulatory approval procedures [204]. The ICH formulates worldwide harmonization guidelines to guarantee uniform safety, effectiveness, and quality criteria for nanomedicines across various regulatory jurisdictions. Essential elements comprise standardized methodologies for the assessment of nanoparticle toxicity. Guidelines for ensuring uniformity and repeatability in nanomedicine formulations. Enhancing international collaboration in nanomedicine authorization to expedite clinical translation [205]. Effective communication of the dangers and advantages of nanomedicine is crucial for regulatory approval and public trust. As nanomedicine progresses in breast cancer therapy, ethical integrity, rigorous clinical studies, and regulatory uniformity will be essential for guaranteeing patient safety and broad accessibility. By tackling these obstacles, researchers and governments may promote responsible innovation and worldwide implementation of nanomedicine-based medicines.
6 Conclusion and Future Prospects
The intricate clinical manifestations and multifaceted origins of breast cancer continue to establish it as a predominant worldwide health concern. Although tumor treatment is becoming increasingly challenging, BC chemotherapy, as advanced nanotechnology, will remain helpful. This review seeks to elucidate the significance of pathophysiology and molecular subtypes of breast cancer, various risk factors with diagnosis, nanocarrier-based treatment approaches and the most recent findings in this area, with their limitations, nanotoxicity and ethical considerations are highlighted in the manuscript. Patents and promising clinical trials and regulatory aspects are also covered. It is now well acknowledged that only targeting traditional oncogenes in cancer cells is insufficient for treating all cancer patients. The majority of cancer patients benefit from medicines that target various components inside the tumor microenvironment, particularly immune cells, which are crucial for augmenting the body’s inherent antitumor immune response. Immunotherapeutics, similar to other pharmaceuticals, utilize nanotechnology to prolong their circulation half-lives, protect their integrity from circulating enzymes, and enhance their targeted delivery to tumors or tissues in patients. The successful clinical translation of nano-immune-therapeutics depends on several essential factors, including feasibility, toxicity, manufacturability, and cost-effectiveness. Despite extensive efforts to passively target different nanocarriers for breast cancer treatment, their anticancer effectiveness and accumulation in the targeted tissue remain constrained. Significantly, there have been considerable breakthroughs in molecular analysis methods in both preclinical and clinical environments, shown by the use of the serial measurements of molecular and architectural responses to treatment (SMMART) platform. The nanoparticles encapsulate genetic tools to safeguard them from degradation and augment their concentration in tumor cells, hence enhancing the efficiency of gene expression control and inhibiting breast cancer growth. Consequently, the simultaneous delivery of genes and pharmaceuticals can enhance breast cancer treatment. Despite the advancements in breast cancer treatment using gene therapy, its efficacy in vivo and in clinical trials is constrained by inadequate accumulation at the tumor site and the potential for enzymatic destruction in the bloodstream. Nanomaterials can be investigated in the delivery of genes for breast cancer suppression in the future, overcoming the associated limitations. To enhance the prevalence of nanoparticle-mediated drug delivery systems in clinical settings, a greater emphasis must be placed on therapeutic development and preclinical testing within the drug development process. Beyond lipid and basic polymeric nanoparticles, few additional nanoparticle-based therapeutics have successfully progressed to clinical assessment, despite encouraging preclinical results. This constraint mostly arises from the substantial development expenses and regulatory hurdles linked to intricate nanoparticle systems. Formulations with several components have challenges in elucidating processes, bioactivities, and toxicological characteristics. Moreover, they have difficulties pertaining to manufacturability (such as scalability and batch-to-batch repeatability) and stability, requiring adherence to stringent FDA regulatory standards. Moreover, to foster innovation and advancement in nanotechnology for breast cancer therapy, it is essential to establish interdisciplinary cooperation platforms that facilitate the seamless integration of scientific findings with clinical trials.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Conceptualization, Neha Raina, Keshav Raj Paudel and Madhu Gupta; validation, Neha Raina, Keshav Raj Paudel, Madhu Gupta, Radha Rani, Mahika Kanojia and Avril Mathias; investigation, Neha Raina and Hardeep Singh Tuli; resources, Madhu Gupta, Neha Raina and Keshav Raj Paudel; data curation, Neha Raina and Keshav Raj Paudel; writing—original draft preparation, Neha Raina, Radha Rani, Ashish Garg, Vetriselvan Subramaniyan, Madhu Gupta and A. T. M. Mijanur Rahman; writing—review and editing, Madhu Gupta, Neha Raina, Hardeep Singh Tuli, Mahika Kanojia, Vetriselvan Subramaniyan, Radha Rani, A. T. M. Mijanur Rahman, Avril Mathias and Keshav Raj Paudel; visualization, Neha Raina, Radha Rani, Mahika Kanojia, Keshav Raj Paudel, Ashish Garg and Madhu Gupta; supervision, Madhu Gupta, Vetriselvan Subramaniyan, Hardeep Singh Tuli, A. T. M. Mijanur Rahman and Keshav Raj Paudel; project administration, Neha Raina, Keshav Raj Paudel and Madhu Gupta; funding acquisition, Keshav Raj Paudel. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, et al. Breast cancer: pathogenesis and treatments. Signal Transduct Target Ther. 2025;10(1):49 doi:10.1038/s41392-024-02108-4. [Google Scholar] [PubMed] [CrossRef]
2. Bai J, Gao Y, Zhang G. The treatment of breast cancer in the era of precision medicine. Cancer Biol Med. 2025;22(4):1–26. [Google Scholar]
3. Qiao R, Liu C, Liu M, Hu H, Liu C, Hou Y, et al. Ultrasensitive in vivo detection of primary gastric tumor and lymphatic metastasis using upconversion nanoparticles. ACS Nano. 2015;9(2):2120–9. doi:10.1021/nn507433p. [Google Scholar] [PubMed] [CrossRef]
4. Mehrotra R, Yadav K. Breast cancer in India: present scenario and the challenges ahead. World J Clin Oncol. 2022;13(3):209–18 doi:10.5306/wjco.v13.i3.209. [Google Scholar] [PubMed] [CrossRef]
5. Wynder EL, Bross IJ, Hirayama T. A study of the epidemiology of cancer of the breast. Cancer. 1960;13(3):559–601. [Google Scholar] [PubMed]
6. Kanoujia J, Das A, Raina N, Kaur G, Singh SK, Tuli HS, et al. Recent advances in BCRP-induced breast cancer resistance treatment with marine-based natural products. IUBMB Life. 2023;75(11):896–910. doi:10.1002/iub.2764. [Google Scholar] [PubMed] [CrossRef]
7. Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. doi:10.1093/jnci/dju055. [Google Scholar] [PubMed] [CrossRef]
8. Breast Cancer Statistics. How common is breast cancer? American Cancer Society [Internet]. [cited 2025 May 23]. Available from: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html. [Google Scholar]
9. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2024;74(3):229–63. doi:10.3322/caac.21834. [Google Scholar] [PubMed] [CrossRef]
10. Huang J, Chan PS, Lok V, Chen X, Ding H, Jin Y, et al. Global incidence and mortality of breast cancer: a trend analysis. Aging. 2021;13(4):5748–803. doi:10.18632/aging.202502. [Google Scholar] [PubMed] [CrossRef]
11. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers. 2021;13(17):4287. doi:10.3390/cancers13174287. [Google Scholar] [PubMed] [CrossRef]
12. Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):33. doi:10.1186/s40659-017-0140-9. [Google Scholar] [PubMed] [CrossRef]
13. Leyrer CM, Berriochoa CA, Agrawal S, Donaldson A, Calhoun BC, Shah C, et al. Predictive factors on outcomes in metaplastic breast cancer. Breast Cancer Res Treat. 2017;165(3):499–504. doi:10.1007/s10549-017-4367-5. [Google Scholar] [PubMed] [CrossRef]
14. Sandhu PS, Kumar R, Beg S, Jain S, Kushwah V, Katare OP, et al. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: systematic approach for improved breast cancer therapeutics. Nanomed. 2017;13(5):1703–13. doi:10.1016/j.nano.2017.03.003. [Google Scholar] [PubMed] [CrossRef]
15. Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84(1):106535. doi:10.1016/j.intimp.2020.106535. [Google Scholar] [PubMed] [CrossRef]
16. Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4(3):192–208. doi:10.1016/j.molonc.2010.04.004. [Google Scholar] [PubMed] [CrossRef]
17. Makki J. Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol. 2015;8(1):23–31. doi:10.4137/cpath.s31563. [Google Scholar] [PubMed] [CrossRef]
18. Eroles P, Bosch A, Alejandro Pérez-Fidalgo J, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38(6):698–707. doi:10.1016/j.ctrv.2011.11.005. [Google Scholar] [PubMed] [CrossRef]
19. Prat A, Cruz C, Hoadley KA, Díez O, Perou CM, Balmaña J, et al. Molecular features of the basal-like breast cancer subtype based on BRCA1 mutation status. Breast Cancer Res Treat. 2014;147(1):185–91. doi:10.1007/s10549-014-3056-x. [Google Scholar] [PubMed] [CrossRef]
20. Phi XA, Houssami N, Obdeijn IM, Warner E, Sardanelli F, Leach MO, et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥50 years: evidence from an individual patient data meta-analysis. J Clin Oncol. 2015;33(4):349–56. doi:10.1200/jco.2014.56.6232. [Google Scholar] [PubMed] [CrossRef]
21. Torres Quintas S, Canha-Borges A, Oliveira MJ, Sarmento B, Castro F. Special issue: nanotherapeutics in women’s health emerging nanotechnologies for triple-negative breast cancer treatment. Small. 2024;20(41):e2300666. doi:10.1002/smll.202300666. [Google Scholar] [PubMed] [CrossRef]
22. Dubey SK, Kali M, Hejmady S, Saha RN, Alexander A, Kesharwani P, et al. Recent advances of dendrimers as multifunctional nano-carriers to combat breast cancer. Eur J Pharm Sci. 2021;164(3):105890. doi:10.1016/j.ejps.2021.105890. [Google Scholar] [PubMed] [CrossRef]
23. Resendiz-Hernández M, García-Hernández AP, Silva-Cázares MB, Coronado-Uribe R, Hernández-de la Cruz ON, Arriaga-Pizano LA, et al. MicroRNA-204 regulates angiogenesis and vasculogenic mimicry in CD44+/CD24− breast cancer stem-like cells. Non-coding RNA. 2024;10(1):14. doi:10.3390/ncrna10010014. [Google Scholar] [PubMed] [CrossRef]
24. Saad Abed A, Saad Abed Karkosh I, Saad Abed Karkosh Z. Molecular and physiological facts affected breast cancer development [Internet]. [cited 2025 Jun 1]. Available from: https://wahj-dna.com.iq/en/molecular-and-physiological-facts-affected-breast-cancer-development/. [Google Scholar]
25. Das S, Clézardin P, Kamel S, Brazier M, Mentaverri R. The CaSR in pathogenesis of breast cancer: a new target for early stage bone metastases. Front Oncol. 2020;10:69. doi:10.3389/fonc.2020.00069. [Google Scholar] [PubMed] [CrossRef]
26. Corsini LR, Bronte G, Terrasi M, Amodeo V, Fanale D, Fiorentino E, et al. The role of microRNAs in cancer: diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012;16(Suppl 2):S103–9. doi:10.1517/14728222.2011.650632. [Google Scholar] [PubMed] [CrossRef]
27. Shah R, Rosso K, David Nathanson S. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol. 2014;5(3):283–98. [Google Scholar] [PubMed]
28. Duffy MJ, Walsh S, McDermott EW, Crown J. Biomarkers in breast cancer: where are we and where are we going? Adv Clin Chem. 2015;71:1–23. [Google Scholar] [PubMed]
29. He Z, Chen Z, Tan M, Elingarami S, Liu Y, Li T, et al. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020;53(7):e12822. [Google Scholar] [PubMed]
30. Burrai GP, Tanca A, De Miglio MR, Abbondio M, Pisanu S, Polinas M, et al. Investigation of HER2 expression in canine mammary tumors by antibody-based, transcriptomic and mass spectrometry analysis: is the dog a suitable animal model for human breast cancer? Tumor Biol. 2015;36(11):9083–91. doi:10.1007/s13277-015-3661-2. [Google Scholar] [PubMed] [CrossRef]
31. Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK, et al. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta Rev Cancer. 2011;1815(2):224–40. doi:10.1016/j.bbcan.2011.01.001. [Google Scholar] [PubMed] [CrossRef]
32. Limaiem F, Ahmad F. Mucinous breast carcinoma. StatPearls [Internet]. [cited 2025 Jun 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538334/. [Google Scholar]
33. Ahmad A, Das S, Kharade V, Gupta M, Pandey VP, Anju KV, et al. Dosimetric study comparing 3D conformal radiotherapy (3D-CRTintensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) in hypofractionated one-week radiotherapy regimen in breast cancer. Cureus. 2022;14(11):e31860. doi:10.7759/cureus.31860. [Google Scholar] [PubMed] [CrossRef]
34. Oehler JB, Rajapaksha W, Albrecht H. Emerging applications of nanoparticles in the diagnosis and treatment of breast cancer. J Pers Med. 2024;14(7):723. doi:10.3390/jpm14070723. [Google Scholar] [PubMed] [CrossRef]
35. Shah PD, Patil S, Dickler MN, Offit K, Hudis CA, Robson ME, et al. Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: differences based on germline mutation status. Cancer. 2016;122(8):1178–84. doi:10.1002/cncr.29903. [Google Scholar] [PubMed] [CrossRef]
36. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20(8):417–36. doi:10.1038/s41568-020-0266-x. [Google Scholar] [PubMed] [CrossRef]
37. Michels KB, Willett WC, Rosner BA, Manson JE, Hunter DJ, Colditz GA, et al. Prospective assessment of breast-feeding and breast cancer incidence among 89 887 women. Lancet. 1996;347(8999):431–6. doi:10.1016/s0140-6736(96)90010-0. [Google Scholar] [PubMed] [CrossRef]
38. Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. [Google Scholar] [PubMed]
39. Ligtenberg MJL, Hogervorst FBL, Willems HW, Arts PJW, Brink G, Hageman S, et al. Characteristics of small breast and/or ovarian cancer families with germline mutations in BRCA1 and BRCA2. Br J Cancer. 1999;79(9–10):1475–8. [Google Scholar] [PubMed]
40. Easton DF, Pharoah PDP, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequenc-ing and the prediction of breast-cancer risk. New Engl J Med. 2015;372(23):2243–57. doi:10.1056/nejmsr1501341. [Google Scholar] [PubMed] [CrossRef]
41. Martin AM, Weber BL. Genetic and hormonal risk factors in breast cancer. J Natl Cancer Inst. 2000;92(14):1126–35. [Google Scholar] [PubMed]
42. Berkel H, Birdsell DC, Jenkins H. Breast augmentation: a risk factor for breast cancer? New Engl J Med. 1992;326(25):1649–53. doi:10.1056/nejm199206183262501. [Google Scholar] [PubMed] [CrossRef]
43. Terefe B, Belachew TB, Asmamaw DB, Wassie GT, Azene AG, Eshetu HB, et al. Determinants of early initiation of breastfeeding following birth in West Africa: a multilevel analysis using data from multicountry national health surveys. PLoS One. 2024;19(5):e0302143. doi:10.1371/journal.pone.0302143. [Google Scholar] [PubMed] [CrossRef]
44. McCormack VA, Dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159–69. doi:10.1158/1055-9965.epi-06-0034. [Google Scholar] [PubMed] [CrossRef]
45. Abbas Z, Rehman S. An overview of cancer treatment modalities. In: Neoplasm. London, UK: IntechOpen; 2018. p. 139–57 doi:10.5772/intechopen.76558. [Google Scholar] [CrossRef]
46. Elston CW, Ellis IO, Parham DM. Method for grading breast cancer. J Clin Pathol. 1993;46(2):189–90. [Google Scholar] [PubMed]
47. Burguin A, Diorio C, Durocher F. Breast cancer treatments: updates and new challenges. J Pers Med. 2021;11(8):808. doi:10.3390/jpm11080808. [Google Scholar] [PubMed] [CrossRef]
48. Jain V, Kumar H, Anod HV, Chand P, Gupta NV, Dey S, et al. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Control Release. 2020;326(2007):628–47. doi:10.1016/j.jconrel.2020.07.003. [Google Scholar] [PubMed] [CrossRef]
49. Singh B, Sarli VN, Lucci A. Inhibition of resistant triple-negative breast cancer cells with low-dose 6-mercaptopurine and 5-azacitidine. Oncotarget. 2021;12(7):626–37. doi:10.18632/oncotarget.27922. [Google Scholar] [PubMed] [CrossRef]
50. Athreya K, Ali S. Advances on immunotherapy in breast cancer. Transl Cancer Res. 2017;6(1):30–7. doi:10.21037/tcr.2017.01.09. [Google Scholar] [CrossRef]
51. Dias K, Dvorkin-Gheva A, Hallett RM, Wu Y, Hassell J, Pond GR, et al. Claudin-low breast cancer; clinical & pathological characteristics. PLoS One. 2017;12(1):e0168669. [Google Scholar] [PubMed]
52. Wang H, Li Y, Khan SA, Luo Y. Prediction of breast cancer distant recurrence using natural language processing and knowledge-guided convolutional neural network. Artif Intell Med. 2020;110:101977. doi:10.1016/j.artmed.2020.101977. [Google Scholar] [PubMed] [CrossRef]
53. Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18(4):157–64. doi:10.1016/j.tcb.2008.01.007. [Google Scholar] [PubMed] [CrossRef]
54. Teo WW, Merino VF, Cho S, Korangath P, Liang X, Wu RC, et al. HOXA5 determines cell fate transition and impedes tumor initiation and progression in breast cancer through regulation of E-cadherin and CD24. Oncogene. 2016;35(42):5539–51. doi:10.1038/onc.2016.95. [Google Scholar] [PubMed] [CrossRef]
55. Jie H, Ma W, Huang C. Diagnosis, prognosis, and treatment of triple-negative breast cancer: a review. Breast Cancer (Dove Med Press). 2025;17:265–74. doi:10.2147/BCTT.S516542. [Google Scholar] [PubMed] [CrossRef]
56. Livraghi L, Garber JE. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC Med. 2015;13(1):188. doi:10.1186/s12916-015-0425-1. [Google Scholar] [PubMed] [CrossRef]
57. Zhu S, Wu Y, Song B, Yi M, Yan Y, Mei Q, et al. Recent advances in targeted strategies for triple-negative breast cancer. J Hematol Oncol. 2023;16(1):100. doi:10.1186/s13045-023-01497-3. [Google Scholar] [PubMed] [CrossRef]
58. Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: american society of clinical oncology guideline. J Clin Oncol. 2016;34(25):3069–103. doi:10.1200/jco.2016.67.1487. [Google Scholar] [PubMed] [CrossRef]
59. Wei Z, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. doi:10.1038/sj.cr.7290105. [Google Scholar] [PubMed] [CrossRef]
60. Alboabdullah AKA, Goodarzi MT, Homayouni Tabrizi M. The Lawson-loaded β-cyclodextrin nanocarriers (LB-NCs) a novel targeted cancer cell in stomach and breast cancer as a drug delivery system. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(9):6623–31. doi:10.1007/s00210-024-03042-6. [Google Scholar] [PubMed] [CrossRef]
61. Venkatesh V, Nataraj R, Thangaraj GS, Karthikeyan M, Gnanasekaran A, Kaginelli SB, et al. Targeting notch signaling pathway of cancer stem cells. Stem Cell Investig. 2018;5(2):5. doi:10.21037/sci.2018.02.02. [Google Scholar] [PubMed] [CrossRef]
62. Gonnissen A, Isebaert S, Haustermans K. Targeting the hedgehog signaling pathway in cancer: beyond smoothened. Oncotarget. 2015;6(16):13899–913. doi:10.18632/oncotarget.4224. [Google Scholar] [PubMed] [CrossRef]
63. Wang M, Hou L, Chen M, Zhou Y, Liang Y, Wang S, et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Sci Rep. 2017;7(1):44673. doi:10.1038/srep44673. [Google Scholar] [PubMed] [CrossRef]
64. Akbarzadeh I, Farid M, Javidfar M, Zabet N, Shokoohian B, Arki MK, et al. The optimized formulation of tamoxifen-loaded niosomes efficiently induced apoptosis and cell cycle arrest in breast cancer cells. AAPS PharmSciTech. 2022;23(1):57. doi:10.1208/s12249-022-02212-0. [Google Scholar] [PubMed] [CrossRef]
65. Zhu J, Li Q, Wu Z, Xu Y, Jiang R. Curcumin for treating breast cancer: a review of molecular mechanisms, combinations with anticancer drugs, and nanosystems. Pharmaceutics. 2024;16(1):79. doi:10.3390/pharmaceutics16010079. [Google Scholar] [PubMed] [CrossRef]
66. Zhang J, Wu Y, Li Y, Li S, Liu J, Yang X, et al. Natural products and derivatives for breast cancer treatment: from drug discovery to molecular mechanism. Phytomedicine. 2024;129:155600. doi:10.1016/j.phymed.2024.155600. [Google Scholar] [PubMed] [CrossRef]
67. Kommineni N, Saka R, Bulbake U, Khan W. Cabazitaxel and thymoquinone co-loaded lipospheres as a synergistic combination for breast cancer. Chem Phys Lipids. 2019;224(7):104707. doi:10.1016/j.chemphyslip.2018.11.009. [Google Scholar] [PubMed] [CrossRef]
68. Sharma L, Nambiar SS, Mohanty A, Patra A, Kumari S, Saini GK. Elucidating the anticancer properties of posaconazole in triple-negative breast cancer through in-silico and in-vitro analysis. Biochem Biophys Res Commun. 2025;771(4):152001. doi:10.1016/j.bbrc.2025.152001. [Google Scholar] [PubMed] [CrossRef]
69. Hsieh CJ, Kuo PL, Hou MF, Hung JY, Chang FR, Hsu YC, et al. Wedelolactone inhibits breast cancer-induced os-teoclastogenesis by decreasing Akt/mTOR signaling. Int J Oncol. 2015;46(2):555–62. doi:10.3892/ijo.2014.2769. [Google Scholar] [PubMed] [CrossRef]
70. Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X, Jiang H, et al. Systemic delivery of tumor-targeting siRNA Nanoparticles against an oncogenic LncRNA facilitates effective triple-negative breast cancer therapy. Bioconjug Chem. 2019;30(3):907–19. doi:10.1021/acs.bioconjchem.9b00028. [Google Scholar] [PubMed] [CrossRef]
71. Liu CG, Han YH, Kankala RK, Wang S, Bin , Chen AZ. Subcellular performance of nanoparticles in cancer therapy. Int J Nanomed. 2020;15:675–704. [Google Scholar]
72. Sinha D, Sarkar N, Biswas J, Bishayee A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin Cancer Biol. 2016;40–41:209–32. [Google Scholar] [PubMed]
73. Indini A, Rijavec E, Grossi F. Trastuzumab deruxtecan: changing the destiny of HER2 expressing solid tumors. Int J Mol Sci. 2021;22(9):4774. doi:10.3390/ijms22094774. [Google Scholar] [PubMed] [CrossRef]
74. Cataldo ML, De Placido P, Esposito D, Formisano L, Arpino G, Giuliano M, et al. The effect of the alpha-specific PI3K inhibitor alpelisib combined with anti-HER2 therapy in HER2+/PIK3CA mutant breast cancer. Front Oncol. 2023;13:1108242. doi:10.3389/fonc.2023.1108242. [Google Scholar] [PubMed] [CrossRef]
75. Zhang Y, Wei S, Zhang Q, Zhang Y, Sun C. Paris saponin VII inhibits triple-negative breast cancer by targeting the MEK/ERK/STMN1 signaling axis. Phytomedicine. 2024;130:155746. doi:10.1016/j.phymed.2024.155746. [Google Scholar] [PubMed] [CrossRef]
76. Arun B, Akar U, Gutierrez-Barrera AM, Hortobagyi GN, Ozpolat B. The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. Int J Oncol. 2015;47(1):262–8. doi:10.3892/ijo.2015.3003. [Google Scholar] [PubMed] [CrossRef]
77. Shi M, Ho K, Keating A, Shoichet MS. Doxorubicin-conjugated immuno-nanoparticles for intracellular anti-cancer drug delivery. Adv Funct Mater. 2009;19(11):1689–96. [Google Scholar]
78. Tran P, Lee SE, Kim DH, Pyo YC, Park JS. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J Pharm Investig. 2020;50(3):261–70. doi:10.1007/s40005-019-00459-7. [Google Scholar] [CrossRef]
79. Nikitin MP, Zelepukin IV, Shipunova VO, Sokolov IL, Deyev SM, Nikitin PI. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat Biomed Eng. 2020;4(7):717–31. doi:10.1038/s41551-020-0581-2. [Google Scholar] [PubMed] [CrossRef]
80. Ding J, Chen J, Gao L, Jiang Z, Zhang Y, Li M, et al. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29:100800. [Google Scholar]
81. Soltani M, Souri M, Moradi Kashkooli F. Effects of hypoxia and nanocarrier size on pH-responsive nano-delivery system to solid tumors. Sci Rep. 2021;11(1):1–12. doi:10.1038/s41598-021-98638-w. [Google Scholar] [PubMed] [CrossRef]
82. Roerden M, Spranger S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat Rev Immunol. 2025;25(5):353–69. doi:10.1038/s41577-024-01111-8. [Google Scholar] [PubMed] [CrossRef]
83. Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ, et al. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18. doi:10.1016/j.jare.2018.06.005. [Google Scholar] [PubMed] [CrossRef]
84. Morales-Cruz M, Delgado Y, Castillo B, Figueroa CM, Molina AM, Torres A, et al. Smart targeting to improve cancer therapeutics. Drug Des Devel Ther. 2019;13:3753–72. [Google Scholar] [PubMed]
85. Zhang Y, Dong X, Zhang Y, Chen Z, Zhou G, Chen N, et al. Biomaterials to regulate tumor extracellular matrix in immunotherapy. J Control Release. 2024;376:149–66. doi:10.1016/j.jconrel.2024.10.010. [Google Scholar] [PubMed] [CrossRef]
86. Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules. 2021;26(19):5905. doi:10.3390/molecules26195905. [Google Scholar] [PubMed] [CrossRef]
87. Shang T, Jia Z, Li J, Cao H, Xu H, Cong L, et al. Unraveling the triad of hypoxia, cancer cell stemness, and drug resistance. J Hematol Oncol. 2025;18(1):32. doi:10.1186/s13045-025-01684-4. [Google Scholar] [PubMed] [CrossRef]
88. Tang X, Loc WS, Dong C, Matters GL, Butler PJ, Kester M, et al. The use of nanoparticulates to treat breast cancer. Nanomed. 2017;12(19):2367–88. doi:10.2217/nnm-2017-0202. [Google Scholar] [PubMed] [CrossRef]
89. Ghafari M, Haghiralsadat F, Khanamani Falahati-Pour S, Zavar Reza J. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J Cell Biochem. 2020;121(7):3584–92. doi:10.1002/jcb.29651. [Google Scholar] [PubMed] [CrossRef]
90. Gilani SJ, Bin-Jumah MN, Fatima F. Development of statistically optimized piperine-loaded polymeric nanoparticles for breast cancer: in vitro evaluation and cell culture studies. ACS Omega. 2023;8(46):44183–94. doi:10.1021/acsomega.3c06605. [Google Scholar] [PubMed] [CrossRef]
91. Sarkar T, Sarkar T, Banerjee S, Goswami M, Das P, Bhakat A, et al. Formulation of curcumin loaded solid lipid nano particles following response surface methodology to improve the anti-cancer activity in triple-negative breast cancer cells. Mater Today Commun. 2025;45(2):112220. doi:10.1016/j.mtcomm.2025.112220. [Google Scholar] [CrossRef]
92. Peleje IR, di Filippo LD, Luiz MT, Fortunato GC, Portuondo DL, et al. Design and in vitro cytotoxicity of docetaxel-loaded hyaluronic acid-coated nanostructured lipid carriers in breast cancer cells. J Drug Deliv Sci Technol. 2025;110:107077. doi:10.1016/j.jddst.2025.107077. [Google Scholar] [CrossRef]
93. Gaikwad DS, Chougale RD, Patil KS, Disouza JI, Hajare AA. Design, development, and evaluation of docetaxeloaded niosomes for the treatment of breast cancer. Future J Pharm Sci. 2023;9(1):43. doi:10.1186/s43094-023-00494-0. [Google Scholar] [CrossRef]
94. Saleh T, Soudi T, Shojaosadati SA. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nano-particles for targeted delivery to HER-2 positive breast cancer cells. Int J Biol Macromol. 2019;130(5):109–16. doi:10.1016/j.ijbiomac.2019.02.129. [Google Scholar] [PubMed] [CrossRef]
95. Honarvari B, Karimifard S, Akhtari N, Mehrarya M, Moghaddam ZS, Ansari MJ, et al. Folate-targeted curcumin-loaded niosomes for site-specific delivery in breast cancer treatment in: silico and in vitro study. Molecules. 2022;27(14):4634. [Google Scholar] [PubMed]
96. Sakhi M, Khan A, Iqbal Z, Khan I, Raza A, Ullah A, et al. Design and characterization of paclitaxel-loaded polymeric nanoparticles decorated with trastuzumab for the effective treatment of breast cancer. Front Pharmacol. 2022;13:855294. doi:10.3389/fphar.2022.855294. [Google Scholar] [PubMed] [CrossRef]
97. Cabeza L, El-Hammadi MM, Ortiz R, Cayero-Otero MD, Jiménez-López J, Perazzoli G, et al. Evaluation of poly (lactic-co-glycolic acid) nanoparticles to improve the therapeutic efficacy of paclitaxel in breast cancer. Bioimpacts. 2022;12(6):515. doi:10.34172/bi.2022.23433. [Google Scholar] [PubMed] [CrossRef]
98. Khazaei MR, Bozorgi M, Khazaei M, Aftabi M, Bozorgi A. Resveratrol nanoformulation inhibits invasive breast cancer cell growth through autophagy induction: an in vitro study. Cell Journal. 2024;26(2):112. [Google Scholar] [PubMed]
99. Cé R, Couto GK, Pacheco BZ, Dallemole DR, Paschoal JD, Pacheco BS, et al. Folic acid-doxorubicin polymeric nanocapsules: a promising formulation for the treatment of triple-negative breast cancer. Eur J Pharm Sci. 2021;165:105943. doi:10.1016/j.ejps.2021.105943. [Google Scholar] [PubMed] [CrossRef]
100. Ma W, Zhao Q, Zhu S, Wang X, Zhang C, Ma D, et al. Construction of glutathione-responsive paclitaxel prodrug nanoparticles for image-guided targeted delivery and breast cancer therapy. RSC Adv. 2024;14(18):12796–806. doi:10.1039/d4ra00610k. [Google Scholar] [PubMed] [CrossRef]
101. Xu Z, Zhou Z, Yang X, Thakur A, Han N, Li HT, et al. Determining M2 macrophages content for the anti-tumor effects of metal-organic framework-encapsulated pazopanib nanoparticles in breast cancer. J Nanobiotechnol. 2024;22(1):429. doi:10.1186/s12951-024-02694-z. [Google Scholar] [PubMed] [CrossRef]
102. Gu Y, Fei Z. Mesoporous silica nanoparticles loaded with resveratrol are used for targeted breast cancer therapy. J Oncol. 2022;2022:8471331. doi:10.1155/2022/8471331. [Google Scholar] [PubMed] [CrossRef]
103. Ghanbari N, Fam AM, Salehi Z, Khodadadi AA, Safarian S. Synergistic effects of graphene quantum dots nanocarriers and folic acid targeting agent on enhanced killing of breast cancer cells by tamoxifen. Canadian J Chem Eng. 2024;102(9):3029–38. doi:10.1002/cjce.25267. [Google Scholar] [CrossRef]
104. Rahdari T, Mahdavimehr M, Ghafouri H, Ramezanpour S, Ehtesham S, Asghari SM. Advancing triple-negative breast cancer treatment through peptide decorated solid lipid nanoparticles for paclitaxel delivery. Sci Rep. 2025;15(1):6043. doi:10.1038/s41598-025-90107-y. [Google Scholar] [PubMed] [CrossRef]
105. Gómez-Pastor S, Maugard A, Walker HR, Elies J, Børsum KE, Grimaldi G, et al. CD-44 targeted nanoparticles for combination therapy in an in vitro model of triple-negative breast cancer: targeting the tumour inside out. Col-loids Surf B Biointerfaces. 2025;249:114504. doi:10.1016/j.colsurfb.2025.114504. [Google Scholar] [PubMed] [CrossRef]
106. Marwah H, Pant J, Shah K, Alam P, Dewangan HK. Anticancer efficacy of dual-loaded SLNs with doxorubicin (DOX) and pterostilbene (PTSformulation, characterization, and evaluation for breast cancer. Nanomedicine. 2025;20(11):1249–65. doi:10.1080/17435889.2025.2501526. [Google Scholar] [PubMed] [CrossRef]
107. Sharma AK, Gothwal A, Kesharwani P, Alsaab H, Iyer AK, Gupta U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov Today. 2017;22(2):314–26. doi:10.1016/j.drudis.2016.09.013. [Google Scholar] [PubMed] [CrossRef]
108. Madan J, Gundala SR, Kasetti Y, Bharatam PV, Aneja R, Katyal A, et al. Enhanced noscapine delivery using estrogen-receptor-targeted nanoparticles for breast cancer therapy. Anti-cancer Drugs. 2014;25(6):704–16. doi:10.1097/cad.0000000000000098. [Google Scholar] [PubMed] [CrossRef]
109. Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev. 2008;60(9):1037–55. doi:10.1016/j.addr.2008.02.012. [Google Scholar] [PubMed] [CrossRef]
110. Chauhan AS. Dendrimers for drug delivery. Molecules. 2018;23(4):938. [Google Scholar] [PubMed]
111. Aurelia Chis A, Dobrea C, Morgovan C, Arseniu AM, Rus LL, Butuca A, et al. Applications and limitations of dendrimers in biomedicine. Molecules. 2020;25(17):3982. doi:10.3390/molecules25173982. [Google Scholar] [PubMed] [CrossRef]
112. Mendes LP, Pan J, Torchilin VP. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):1401. doi:10.3390/molecules22091401. [Google Scholar] [PubMed] [CrossRef]
113. Janaszewska A, Lazniewska J, Trzepiński P, Marcinkowska M, Klajnert-Maculewicz B. Cytotoxicity of dendrimers. Biomolecules. 2019;9(8):330. doi:10.3390/biom9080330. [Google Scholar] [PubMed] [CrossRef]
114. Yap KM, Sekar M, Fuloria S, Wu YS, Gan SH, Rani NNIM, et al. Drug delivery of natural products through nanocarriers for effective breast cancer therapy: a comprehensive review of literature. Int J Nanomed. 2021;16:7891–941. doi:10.2147/ijn.s328135. [Google Scholar] [PubMed] [CrossRef]
115. Zhou X, Xu X, Hu Q, Wu Y, Yu F, He C, et al. Novel manganese and polyester dendrimer-based theranostic na-noparticles for MRI and breast cancer therapy. J Mater Chem B. 2022;11(3):648–56. doi:10.1039/d2tb01855a. [Google Scholar] [PubMed] [CrossRef]
116. Ni C, Ouyang Z, Li G, Liu J, Cao X, Zheng L, et al. A tumor microenvironment-responsive core-shell tecto den-drimer nanoplatform for magnetic resonance imaging-guided and cuproptosis-promoted chemo-chemodynamic therapy. Acta Biomater. 2023;164(44):474–86. doi:10.1016/j.actbio.2023.04.003. [Google Scholar] [PubMed] [CrossRef]
117. Lewińska A, Wróbel K, Błoniarz D, Adamczyk-Grochala J, Wołowiec S, Wnuk M, et al. Lapatinib- and fulves-trant-PAMAM dendrimer conjugates promote apoptosis in chemotherapy-induced senescent breast cancer cells with different receptor status. Biomater Adv. 2022;140:213047. doi:10.1016/j.bioadv.2022.213047. [Google Scholar] [PubMed] [CrossRef]
118. Chuang HY, Huang D, Qi L, Singh V, Chernatynskaya A, Huang YW, et al. Highly adaptable dendrimer gel nanoparticles with dual targeting of uPAR and ribonucleotide reductase R2 for better retention and improved therapeutic outcomes in triple-negative breast cancer. ACS Appl Mater Interfaces. 2025;17(23):33439–50. doi:10.1021/acsami.5c03560. [Google Scholar] [PubMed] [CrossRef]
119. Gholamrezazadeh C, Hakimi M, Dadmehr M. Synthesis, characterization and biological activities of nitrophe-nol substituted phosphazene dendrimers. J Mol Struct. 2024;1312:138526. doi:10.1016/j.molstruc.2024.138526. [Google Scholar] [CrossRef]
120. Huang H, Guo H, Liu J, Ni C, Xia L, Cao X, et al. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO2 for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumor microenvironment. Acta Biomater. 2024;183(6):252–63. doi:10.1016/j.actbio.2024.05.035. [Google Scholar] [PubMed] [CrossRef]
121. Arias JL, Clares B, Morales ME, Gallardo V, Ruiz MA. Lipid-based drug delivery systems for cancer treat-ment. Curr Drug Targets. 2011;12(8):1151–65. doi:10.2174/138945011795906570. [Google Scholar] [PubMed] [CrossRef]
122. García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, Sarabia F, Prados J, Melguizo C, et al. Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomater. 2019;9(4):638. doi:10.3390/nano9040638. [Google Scholar] [PubMed] [CrossRef]
123. Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. doi:10.1016/j.jconrel.2012.03.020. [Google Scholar] [PubMed] [CrossRef]
124. Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68(3):701–87. doi:10.1124/pr.115.012070. [Google Scholar] [PubMed] [CrossRef]
125. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. P T. 2017;42(8):514–21. [Google Scholar] [PubMed]
126. Singh M, Habib S, Daniels A, Ariatti M. Anti-c-myc cholesterol based lipoplexes as onco-nanotherapeutic agents in vitro. F1000Res. 2021;9:770. doi:10.12688/f1000research.25142.2. [Google Scholar] [PubMed] [CrossRef]
127. Raikwar S, Yadav V, Jain S, Jain SK. Antibody-conjugated pH-sensitive liposomes for HER-2 positive breast cancer: development, characterization, in vitro and in vivo assessment. J Liposome Res. 2024;34(2):239–63. doi:10.1080/08982104.2023.2248505. [Google Scholar] [PubMed] [CrossRef]
128. Ahamad J, Ismail SA, Jalal AZ, Akhtar MS, Ahmad J. Stilbenes in breast cancer treatment: nanostilbenes an ap-proach to improve therapeutic efficacy. Pharmacol Res-Nat Prod. 2025;6(8):100173. doi:10.1016/j.prenap.2025.100173. [Google Scholar] [CrossRef]
129. Lu W, Liu W, Hu A, Shen J, Yi H, Cheng Z, et al. Combinatorial polydopamine-liposome nanoformulation as an effective anti-breast cancer therapy. Int J Nanomed. 2023;18:861–79. doi:10.2147/ijn.s382109. [Google Scholar] [PubMed] [CrossRef]
130. Shah JR, Dong T, Phung AT, Khan S, Aisagbonhi O, Blair SL, et al. Liposomal oncolytic adenovirus as a neoadjuvant therapy for triple-negative breast cancer. Sci Rep. 2025;15(1):16737. doi:10.1038/s41598-025-00211-2. [Google Scholar] [PubMed] [CrossRef]
131. Maghsoudi S, Hosseini SA, Soraya H, Roosta Y, Mohammadzadeh A. Development of doxorubicin-encapsulated magnetic liposome@PEG for treatment of breast cancer in BALB/c mice. Drug Deliv Transl Res. 2023;13(10):2589–603. doi:10.1007/s13346-023-01339-2. [Google Scholar] [PubMed] [CrossRef]
132. George TA, Chen MM, Czosseck A, Chen H-P, Huang H-S, Lundy DJ. Liposome-encapsulated anthraquinone improves efficacy and safety in triple negative breast cancer. J Control Release. 2022;342(2018):31–43. doi:10.1016/j.jconrel.2021.12.001. [Google Scholar] [PubMed] [CrossRef]
133. Li M, Li Y, Li S, Jia L, Du C, Li M, et al. Co-delivery of F7 and crizotinib by thermosensitive liposome for breast cancer treatment. J Liposome Res. 2022;32(3):265–75. doi:10.1080/08982104.2021.2001499. [Google Scholar] [PubMed] [CrossRef]
134. Shemesh CS, Moshkelani D, Zhang H. Thermosensitive liposome formulated indocyanine green for near-infrared triggered photodynamic therapy: in vivo evaluation for triple-negative breast cancer. Pharm Res. 2015;32(5):1604–14. doi:10.1007/s11095-014-1560-7. [Google Scholar] [PubMed] [CrossRef]
135. Vozgirdaite D, Hervé-Aubert K, Uzbekov R, Chourpa I, Allard-Vannier E. Design, optimization, characterization, and in vitro evaluation of metformin-loaded liposomes for triple negative breast cancer treatment. J Liposome Res. 2024;34(4):547–61. doi:10.1080/08982104.2024.2321528. [Google Scholar] [PubMed] [CrossRef]
136. Li Z, Lan J, Liu L, Wang Y, Chen L, Zeng R, et al. Versatile thermo-sensitive liposomes with HSP inhibition and anti-inflammation for synergistic chemo-photothermal to inhibit breast cancer metastasis. Int J Pharm. 2024;664(9):124583. doi:10.1016/j.ijpharm.2024.124583. [Google Scholar] [PubMed] [CrossRef]
137. Valinezhadi N, Dehghan G, Yaghoubzad-Maleki M, Mohammadi M, Alizadeh AA, Hamishehkar H. Liposome-assisted combination chemotherapy improves the anti-proliferation and anti-angiogenesis response of cisplatin in breast cancer; experimental and computational study. J Chemother. 2025;9(1):1–18. doi:10.1080/1120009X.2025.2484078. [Google Scholar] [PubMed] [CrossRef]
138. Bartelds R, Nematollahi MH, Pols T, Stuart MCA, Pardakhty A, Asadikaram G, et al. Niosomes, an alternative for liposomal delivery. PLoS One. 2018;13(4):e0194179. doi:10.1371/journal.pone.0194179. [Google Scholar] [PubMed] [CrossRef]
139. Chen S, Hanning S, Falconer J, Locke M, Wen J. Recent advances in non-ionic surfactant vesicles (niosomesfab-rication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm. 2019;144(117):18–39. doi:10.1016/j.ejpb.2019.08.015. [Google Scholar] [PubMed] [CrossRef]
140. Sabale V, Ingole A, Pathak R, Sabale P. Optimization of capecitabine-loaded niosomes using factorial design: an approach for enhanced drug release and cytotoxicity in breast cancer. AAPS PharmSciTech. 2025;26(2):62. doi:10.1208/s12249-025-03037-3. [Google Scholar] [PubMed] [CrossRef]
141. Saharkhiz S, Nasri N, Naderi N, Dini G, Ghalehshahi SS, Firoozbakht F. Evaluating a targeted Palbo-ciclib-Trastuzumab loaded smart niosome platform for treating HER2 positive breast cancer cells. Int J Pharm X. 2024;7(1):100237. doi:10.1016/j.ijpx.2024.100237. [Google Scholar] [PubMed] [CrossRef]
142. Safari Sharafshadeh M, Tafvizi F, Khodarahmi P, Ehtesham S. Preparation and physicochemical properties of cisplatin and doxorubicin encapsulated by niosome alginate nanocarrier for cancer therapy. Int J Biol Macromol. 2023;235(6):123686. doi:10.1016/j.ijbiomac.2023.123686. [Google Scholar] [PubMed] [CrossRef]
143. Fakeri M, Haghi M, Jahanban Esfahlan R, Fathi M, Hosseinpour Feizi MA. Targeted apoptosis in breast cancer cells via niosome-mediated delivery of cyclophosphamide and sodium oxamate. Mol Biol Rep. 2025;52(1):139. doi:10.1007/s11033-025-10241-8. [Google Scholar] [PubMed] [CrossRef]
144. Jawale JK, Kashikar VS. Niosomes: a promising nonionic surfactant vesicular system for enhancement of anticancer effect of phytochemicals. Ijhs. 2022;6(S5):8809–33. doi:10.53730/ijhs.v6ns5.11794. [Google Scholar] [CrossRef]
145. Moammeri A, Chegeni MM, Sahrayi H, Ghafelehbashi R, Memarzadeh F, Mansouri A, et al. Current advances in niosomes applications for drug delivery and cancer treatment. Mater Today Bio. 2023;23(12):100837. doi:10.1016/j.mtbio.2023.100837. [Google Scholar] [PubMed] [CrossRef]
146. Llaguno-Munive M, Vazquez-Lopez MI, Garcia-Lopez P. Solid lipid nanoparticles, an alternative for the treatment of triple-negative breast cancer. Int J Mol Sci. 2024;25(19):10712. doi:10.3390/ijms251910712. [Google Scholar] [PubMed] [CrossRef]
147. Penugonda S, Beesappagari P, Repollu M, Badiginchala P, Qudsiya S, Mala CUS, et al. Enhanced anticancer efficiency of curcumin co-loaded lawsone solid lipid nanoparticles against MCF-7 breast cancer cell lines: optimization by statistical JMP software-based experimental approach. Assay Drug Dev Technol. 2025;23(5):269–79. doi:10.1089/adt.2024.125. [Google Scholar] [PubMed] [CrossRef]
148. Chin B, Meng Lim W, Almurisi SH, Madheswaran T. A quality-by-design approach to develop abemaciclib solid lipid nanoparticles for targeting breast cancer cell lines. Ther Deliv. 2025;16(2):123–37. doi:10.1080/20415990.2025.2457314. [Google Scholar] [PubMed] [CrossRef]
149. Lalchandani DS, Chenkual L, Sonpasare K, Rajdev B, Naidu VGM, Chella N, et al. Optimization of atorvastatin and quercetin-loaded solid lipid nanoparticles using Box-Behnken design. Nanomedicine. 2024;19(17):1541–55. doi:10.1080/17435889.2024.2364585. [Google Scholar] [PubMed] [CrossRef]
150. Passos JS, Apolinario AC, Ishida K, Martins TS, Lopes LB. Nanostructured lipid carriers loaded into in situ gels for breast cancer local treatment. Eur J Pharm Sci. 2024;192(12):106638. doi:10.1016/j.ejps.2023.106638. [Google Scholar] [PubMed] [CrossRef]
151. Hadi Sultan M, Almoshari Y, Mohan S, Ahmed Al-Kasim M, Alyami HS, Azam Ansari M, et al. Capecita-bine-loaded NLC for breast cancer treatment: preparation, characterization, and in vitro evaluation. Curr Drug Deliv. 2024;22(7):968–82. doi:10.2174/0115672018309370240708113038. [Google Scholar] [PubMed] [CrossRef]
152. Rodero CF, Luiz MT, Sato MR, Boni F, Fernandes GFS, dos Santos JL, et al. Rapamycin-loaded nanostructured lipid carrier modified with folic acid intended for breast cancer therapy. Int J Pharm. 2025;668:124954. doi:10.1016/j.ijpharm.2024.124954. [Google Scholar] [PubMed] [CrossRef]
153. Nehal N, Unnithan D, Emad NA, Aashish N, Sartaj A, Baboota S, et al. Development and preclinical assessment of a palbociclib nanostructured lipid carrier for potential breast cancer management. Mol Pharm. 2025;22(3):1419–36. doi:10.1021/acs.molpharmaceut.4c01122. [Google Scholar] [PubMed] [CrossRef]
154. Elkholy NE, Sultan AA, Abu-Risha SE, El Maghraby GM. Chitosan coated lipid carriers as nanoplatform for repurposed anti-breast cancer activity of niclosamide. J Drug Deliv Sci Technol. 2024;93:105414. doi:10.1016/j.jddst.2024.105414. [Google Scholar] [CrossRef]
155. Pandey P, Satija S, Wadhwa R, Mehta M, Purohit D, Gupta G, et al. Emerging trends in nanomedicine for topical delivery in skin disorders: current and translational approaches. Dermatol Ther. 2020;33(3):e13292. doi:10.1111/dth.13292. [Google Scholar] [PubMed] [CrossRef]
156. Sithole MN, Choonara YE, du Toit LC, Kumar P, Pillay V. A review of semi-synthetic biopolymer complexes: modified polysaccharide nano-carriers for enhancement of oral drug bioavailability. Pharm Dev Technol. 2017;22(2):283–95. doi:10.1080/10837450.2016.1212882. [Google Scholar] [PubMed] [CrossRef]
157. Dadwal A, Baldi A, Kumar Narang R. Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol. 2018;46(sup2):295–305. doi:10.1080/21691401.2018.1457039. [Google Scholar] [PubMed] [CrossRef]
158. He Y, de Araújo Júnior RF, Cavalcante RS, Yu Z, Schomann T, Gu Z, et al. Effective breast cancer therapy based on palmitic acid-loaded PLGA nanoparticles. Biomater Adv. 2023;145(1024–1036):213270. doi:10.1016/j.bioadv.2022.213270. [Google Scholar] [PubMed] [CrossRef]
159. Wang T, Cao Y, Xiong J, Li L. Construction of a nano-fluorescent polymer drug delivery system for breast cancer treatment. J Mol Struct. 2025;1321:139820. doi:10.1016/j.molstruc.2024.139820. [Google Scholar] [CrossRef]
160. Justino IA, Ferreira IRS, Botteon CA, Tucci LFF, Marincek A, Amaral R, et al. Cytotoxic potential of polymeric nanoparticles loaded with Brazilian red propolis in breast cancer. J Drug Deliv Sci Technol. 2025;105:106663. doi:10.1016/j.jddst.2025.106663. [Google Scholar] [CrossRef]
161. Chaudhari D, Kuche K, Yadav V, Ghadi R, Date T, Bhargavi N, et al. Exploring paclitaxel-loaded adenosine-conjugated PEGylated PLGA nanoparticles for targeting triple-negative breast cancer. Drug Deliv Transl Res. 2023;13(4):1074–87. doi:10.1007/s13346-022-01273-9. [Google Scholar] [PubMed] [CrossRef]
162. Hu H, Liao Z, Xu M, Wan S, Wu Y, Zou W, et al. Fabrication, optimization, and evaluation of paclitaxel and curcumin coloaded PLGA nanoparticles for improved antitumor activity. ACS Omega. 2023;8(1):976–86. doi:10.1021/acsomega.2c06359. [Google Scholar] [PubMed] [CrossRef]
163. Mohammad Gholinia Sarpoli L, Zare-Karizi S, Heidari E, Hasanzadeh A, Bayandori M, Azedi F, et al. Co-delivery of curcumin and Bcl-2 siRNA to enhance therapeutic effect against breast cancer cells using PEI-functionalized PLGA nanoparticles. Pharm Dev Technol. 2022;27(7):785–93. doi:10.1080/10837450.2022.2120003. [Google Scholar] [PubMed] [CrossRef]
164. Hu H, Zhang Y, Ji W, Mei H, Wu T, He Z, et al. Hyaluronic acid-coated and Olaparib-loaded PEI–PLGA nano-particles for the targeted therapy of triple negative breast cancer. J Microencapsul. 2022;39(1):25–36. doi:10.1080/02652048.2021.2014586. [Google Scholar] [PubMed] [CrossRef]
165. Malarz K, Borzęcka W, Ziola P, Domiński A, Rawicka P, Bialik-Wąs K, et al. pH-sensitive phthalocyanine-loaded polymeric nanoparticles as a novel treatment strategy for breast cancer. Bioorg Chem. 2025;155(35):108127. doi:10.1016/j.bioorg.2025.108127. [Google Scholar] [PubMed] [CrossRef]
166. Behl A, Solanki S, Paswan SK, Datta TK, Saini AK, Saini RV, et al. Biodegradable PEG-PCL nanoparticles for codelivery of MUC1 inhibitor and doxorubicin for the confinement of triple-negative breast cancer. J Polym Environ. 2023;31(3):999–1018. doi:10.1007/s10924-022-02654-4. [Google Scholar] [PubMed] [CrossRef]
167. Liaqat S, Fatima B, Hussain D, Imran M, Zahra Jawad SE, Saeed A, et al. Doxorubicin encapsulated blend of sitagliptin-lignin polymeric drug delivery system for effective combination therapy against cancer. Int J Biol Macromol. 2024;269(Pt 2):132146. doi:10.1016/j.ijbiomac.2024.132146. [Google Scholar] [PubMed] [CrossRef]
168. Balogun-Agbaje OA, Odeniyi MA. Thiolated and carboxymethylated jackfruit seed starch nanocrystals as polymeric drug carriers for curcumin delivery to cancer cells. Starch-Stärke. 2025;7(5):e202400207. doi:10.1002/star.202400207. [Google Scholar] [CrossRef]
169. Nasr M, Hashem F, Teiama M, Tantawy N, Abdelmoniem R. Folic acid grafted mixed polymeric micelles as a tar-geted delivery strategy for tamoxifen citrate in treatment of breast cancer. Drug Deliv Transl Res. 2024;14(4):945–58. doi:10.1007/s13346-023-01443-3. [Google Scholar] [PubMed] [CrossRef]
170. Sah RK, Kumar BS. Development of PLGA-SPC3 functionalized gefitinib mesoporous silica nano-scaffolds for breast cancer targeting: biodistribution and cytotoxicity analysis. Pharm Dev Technol. 2025;30(2):160–76. doi:10.1080/10837450.2025.2460732. [Google Scholar] [PubMed] [CrossRef]
171. Lv Y, Chen X, Shen Y. Folate-modified carboxymethyl chitosan-based drug delivery system for breast cancer specific combination therapy via regulating mitochondrial calcium concentration. Carbohydr Polym. 2024;323(9):121434. doi:10.1016/j.carbpol.2023.121434. [Google Scholar] [PubMed] [CrossRef]
172. Rakhshani A, Maghsoudian S, Motasadizadeh H, Fatahi Y, Malek-Khatabi A, Ghahremani MH, et al. Synergistic effects of doxorubicin PLGA nanoparticles and simvastatin loaded in silk films for local treatment of breast cancer. J Drug Deliv Sci Technol. 2025;104:106431. doi:10.1016/j.jddst.2024.106431. [Google Scholar] [CrossRef]
173. Sandra F, Khaliq NU, Sunna A, Care A. Developing protein-based nanoparticles as versatile delivery systems for cancer therapy and imaging. Nanomater. 2019;9(9):1329. doi:10.3390/nano9091329. [Google Scholar] [PubMed] [CrossRef]
174. Díaz C, González-Olmedo C, Díaz-Beltrán L, Camacho J, Mena García P, Martín-Blázquez A, et al. Predicting dynamic response to neoadjuvant chemotherapy in breast cancer: a novel metabolomics approach. Mol Oncol. 2022;16(14):2658–71. doi:10.1002/1878-0261.13216. [Google Scholar] [PubMed] [CrossRef]
175. Jain S, Narang V, Jain K, Paul D, Singh J, Sohi AS, et al. Prevalence of molecular subtypes in operated cases of breast cancer and its clinicopathological correlation: a single institute study from a tertiary cancer centre in North India. Indian J Surg Oncol. 2021;12(3):538–44. doi:10.1007/s13193-021-01374-w. [Google Scholar] [PubMed] [CrossRef]
176. Hong R, Xu B. Breast cancer: an up-to-date review and future perspectives. Cancer Commun. 2022;42(10):913–36. doi:10.1002/cac2.12358. [Google Scholar] [PubMed] [CrossRef]
177. Wang C, Li Q, Hou Y, Sun M, Sun J, Lou Z, et al. The interaction of cinchonine and immunoglobulin G and the development of a nanocomplex with improved anti-breast cancer activity. Int J Biol Macromol. 2025;287(6):138152. doi:10.1016/j.ijbiomac.2024.138152. [Google Scholar] [PubMed] [CrossRef]
178. Deng Q, Lin P, Gu H, Zhuang X, Wang F. Silk protein-based nanoporous microsphere for controllable drug delivery through self-assembly in ionic liquid system. Biomacromolecules. 2024;25(3):1527–40. doi:10.1021/acs.biomac.3c01104. [Google Scholar] [PubMed] [CrossRef]
179. Are V, Das S, P SS, Biswas S. Combination therapy of Lapatinib/Letrozole-based protein-vitamin nanoparticles to enhance the therapeutic effectiveness in drug-resistant breast cancer. Colloids Surf B Biointerfaces. 2025;247:138152. doi:10.1016/j.colsurfb.2024.114399. [Google Scholar] [PubMed] [CrossRef]
180. Safwat S, Ishak RAH, Hathout RM, Mortada ND. Bioinspired caffeic acid-laden milk protein-based nanoparticles targeting folate receptors for breast cancer treatment. Ther Deliv. 2024;16(1):43–61. doi:10.1080/20415990.2024.2433938. [Google Scholar] [PubMed] [CrossRef]
181. Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A. Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano. 2013;7(4):2891–7. doi:10.1021/nn401196a. [Google Scholar] [PubMed] [CrossRef]
182. Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9:9. doi:10.3389/fphar.2018.01401. [Google Scholar] [PubMed] [CrossRef]
183. Aghaei A, Shaterian M, Danafar H, Likozar B, Šuligoj A, Gyergyek S. Synthesis of single-walled carbon nanotubes functionalized with platinum nanoparticles to sense breast cancer cells in 4T1 model to X-ray radiation. Mikrochim Acta. 2023;190(5):184. doi:10.1007/s00604-023-05761-8. [Google Scholar] [PubMed] [CrossRef]
184. Badea MA, Balas M, Ionita D, Dinischiotu A. Carbon nanotubes conjugated with cisplatin activate different apoptosis signaling pathways in 2D and 3D-spheroid triple-negative breast cancer cell cultures: a comparative study. Arch Toxicol. 2024;98(9):2843–66. doi:10.1007/s00204-024-03779-2. [Google Scholar] [PubMed] [CrossRef]
185. Xie P, Qu T, Tang K, Huang Y, Zeng G, Yuan H, et al. Carbon nanoparticles-Fe(II) complex combined with soraf-enib for ferroptosis-induced antitumor effects in triple-negative breast cancer. Colloids Surf B Biointerfaces. 2025;250(8):114562. doi:10.1016/j.colsurfb.2025.114562. [Google Scholar] [PubMed] [CrossRef]
186. Basu A, Upadhyay P, Ghosh A, Bose A, Gupta P, Chattopadhyay S, et al. Hyaluronic acid engrafted metformin loaded graphene oxide nanoparticle as CD44 targeted anti-cancer therapy for triple negative breast cancer. Biochim Et Biophys Acta BBA-Gen Subj. 2021;1865(3):129841. doi:10.1016/j.bbagen.2020.129841. [Google Scholar] [PubMed] [CrossRef]
187. Tiron A, Stan CS, Luta G, Uritu CM, Vacarean-Trandafir IC, Stanciu GD, et al. Manganese-doped n-hydroxyphthalimide-derived carbon dots—theranostics applications in experimental breast cancer models. Pharmaceutics. 2021;13(11):13–1. doi:10.3390/pharmaceutics13111982 1982. [Google Scholar] [PubMed] [CrossRef]
188. Radzi MRM, Johari NA, Zawawi WFAWM, Zawawi NA, Latiff NA, Malek NANN, et al. In vivo evaluation of oxi-dized multiwalled-carbon nanotubes-mediated hyperthermia treatment for breast cancer. Biomat Adv. 2022;134(6):112586. doi:10.1016/j.msec.2021.112586. [Google Scholar] [PubMed] [CrossRef]
189. Tiwari P, Shukla RP, Yadav K, Singh N, Marwaha D, Gautam S, et al. Dacarbazine-primed carbon quantum dots coated with breast cancer cell-derived exosomes for improved breast cancer therapy. J Controll Release. 2024;365:43–59. doi:10.1016/j.jconrel.2023.11.005. [Google Scholar] [PubMed] [CrossRef]
190. Varvarà P, Mauro N, Cavallaro G. Targeted NIR-triggered doxorubicin release using carbon dots-poly(ethylene glycol)–folate conjugates for breast cancer treatment. Nanoscale Adv. 2025;7(3):862–75. doi:10.1039/d4na00834k. [Google Scholar] [PubMed] [CrossRef]
191. Andoh V, Ocansey DKW, Naveed H, Wang N, Chen L, Chen K, et al. The advancing role of nanocomposites in cancer diagnosis and treatment. Int J Nanomed. 2024;19:6099–126. doi:10.2147/IJN.S471360. [Google Scholar] [PubMed] [CrossRef]
192. Li J, Mahdavi B, Baghayeri M, Rivandi B, Lotfi M, Mahdi Zangeneh M, et al. A new formulation of Ni/Zn bimetallic nanocomposite and evaluation of its applications for pollution removal, photocatalytic, electrochemical sensing, and anti-breast cancer. Environ Res. 2023;233:116462. doi:10.1016/j.envres.2023.116462. [Google Scholar] [PubMed] [CrossRef]
193. Afzal M, Ameeduzzafar, Alharbi KS, Alruwaili NK, Al-Abassi FA, Al Labeed Al-Malki A, et al. Nanomedicine in treatment of breast cancer-A challenge to conventional therapy. Semin Cancer Biol. 2021;69(10):279–92. doi:10.1016/j.semcancer.2019.12.016. [Google Scholar] [PubMed] [CrossRef]
194. Wang Y, Zhang C, Xiao M, Ganesan K, Gao F, Liu Q, et al. A tumor-targeted delivery of oral isoliquiritigenin through encapsulated zein phosphatidylcholine hybrid nanoparticles prevents triple-negative breast cancer. J Drug Deliv Sci Technol. 2023;79:103922. doi:10.1016/j.jddst.2022.103922. [Google Scholar] [CrossRef]
195. Waris A, Sharif S, Naz S, Manzoor F, Rashid F, Tabassum S, et al. Review on metallic nanoparticles induced toxicity on renal function and overall health of kidneys. Environ Eng Res. 2024;29(4):230549. doi:10.4491/eer.2023.549. [Google Scholar] [CrossRef]
196. Graham UM, Jacobs G, Yokel RA, Davis BH, Dozier AK, Birch ME, et al. From dose to response: in vivo nano-particle processing and potential toxicity. Adv Exp Med Biol. 2017;947(5):71–100. doi:10.1007/978-3-319-47754-1_4. [Google Scholar] [PubMed] [CrossRef]
197. Abonyi HN, Peter IE, Onwuka AM, Achile PA, Obi CB, Akunne MO, et al. Nanotoxicology: developments and new insights. Nanomed. 2025;20(2):225–41. doi:10.1080/17435889.2024.2443385. [Google Scholar] [PubMed] [CrossRef]
198. Ali M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv Colloid Interface Sci. 2023;314(10):102881. doi:10.1016/j.cis.2023.102881. [Google Scholar] [PubMed] [CrossRef]
199. Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomed. 2011;6(4):715–28. doi:10.2217/nnm.11.19. [Google Scholar] [PubMed] [CrossRef]
200. Foroozandeh P, Aziz AA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018;13(1):339. doi:10.1186/s11671-018-2728-6. [Google Scholar] [PubMed] [CrossRef]
201. John AT, Wadhwa S, Mathur A. Nanotoxicology: exposure, mechanism, and effects on human health. In: New frontiers in environmental toxicology. Cham, Switzerland: Springer; 2022. p. 35–77. doi:10.1007/978-3-030-72173-2_5. [Google Scholar] [CrossRef]
202. Sarwal A, Singh G, Singh K, Garg S. Recent interventions for nanotechnology based drug products: insights into the regulatory aspects. Curr Pharm Des. 2019;24(43):5219–28. doi:10.2174/1381612825666190117094250. [Google Scholar] [PubMed] [CrossRef]
203. Rahman Z, Charoo NA, Akhter S, Beg S, Reddy IK, Khan MA, et al. Nanotechnology-based drug products: Science and regulatory considerations. In: Nanoscale fabrication, optimization, scale-up and biological aspects of pharmaceutical nanotechnology. Amsterdam, The Netherlands: Elsevier; 2018. p. 619–55. doi:10.1016/b978-0-12-813629-4.00016-4. [Google Scholar] [CrossRef]
204. Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360. doi:10.3389/fchem.2018.00360. [Google Scholar] [PubMed] [CrossRef]
205. Satalkar P, Elger BS, Hunziker P, Shaw D. Challenges of clinical translation in nanomedicine: a qualitative study. Nanomed. 2016;12(4):893–900. doi:10.1016/j.nano.2015.12.376. [Google Scholar] [PubMed] [CrossRef]
206. NIH. ClinicalTrials.gov [Internet]. [cited 2025 Mar 12]. Available from: https://clinicaltrials.gov/. [Google Scholar]
207. Telli ML. A phase 1/2 Trial of ARV-471 alone and in combination with Palbociclib (IBRANCE®) in patients with ER+/HER2- locally advanced or metastatic breast cancer [Internet]. Trial ID: NCT04072952. [cited 2025 Mar 12]. Available from: https://stanfordhealthcare.org/trials/a/NCT04072952.html. [Google Scholar]
208. Riaz F. A Phase-3, open-label, randomized study of Dato-DXd versus investigator’s choice of chemotherapy (ICC) in participants with inoperable or metastatic HR-Positive, HER2-negative breast cancer who have been treated with one or two prior lines of systemic chemotherapy (TROPION-Breast01) [Internet]. Trial ID: NCT05104866. [cited 2025 Mar 13]. Available from: https://clinicaltrials.stanford.edu/trials/a/NCT05104866.html. [Google Scholar]
209. NIH. A safety study of SGN-LIV1A in breast cancer patients [Internet]. Trial ID: NCT01969643. [cited 2025 Mar 13]. Available from: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2013-02245. [Google Scholar]
210. Klimberg VS. Axillary reverse mapping in preventing lymphedema in patients with breast cancer undergoing axillary lymph node dissection. division of cancer prevention [Internet]. NCT ID: NCT03927027. [cited 2025 Mar 13]. Available from: https://prevention.cancer.gov/clinical-trials/clinical-trials-search/nct03927027. [Google Scholar]
211. Georgia’s Online Cancer Information Center. I-SPY TRIAL: neoadjuvant and personalized adaptive novel agents to treat breast cancer. NCT ID: NCT01042379. [cited 2025 Mar 13]. Available from: https://www.georgiacancerinfo.org/clinical-trials/detail/2422. [Google Scholar]
212. A study of tucatinib plus trastuzumab deruxtecan in HER2+ Breast Cancer (HER2CLIMB-04) [Internet]. NCT ID: NCT04539938. [cited 2025 Mar 13]. Available from: https://ctv.veeva.com/study/a-study-of-tucatinib-plus-trastuzumab-deruxtecan-in-her2-breast-cancer. [Google Scholar]
213. Atezolizumab + Sacituzumab govitecan to prevent recurrence in TNBC (ASPRIA). [Internet]. NCT ID: NCT04434040. [cited 2025 Mar 13]. Available from: https://www.smartpatients.com/trials/NCT04434040. [Google Scholar]
214. Avelumab with Binimetinib, Sacituzumab Govitecan, or Liposomal Doxorubicin in Treating Patients with Stage IV or Unresectable, Recurrent Triple Negative Breast Cancer - NCI [Internet]. NCT ID: NCT03971409. [cited 2025 Mar 13]. Available from: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2019-01531. [Google Scholar]
215. Fujiwara Y, Mukai H, Saeki T, Ro J, Lin YC, Nagai SE, et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer. 2019;120(5):475–80. doi:10.1038/s41416-019-0391-z. [Google Scholar] [PubMed] [CrossRef]
216. Subramanian S, Prasanna R, Biswas G, Das Majumdar SK, Joshi N, Bunger D, et al. Nanosomal docetaxel lipid suspension-based chemotherapy in breast cancer: results from a multicenter retrospective study. Breast Cancer. 2020;12:77–85. doi:10.2147/BCTT.S236108. [Google Scholar] [PubMed] [CrossRef]
217. Treatment of Triple-negative Breast Cancer With Albumin-bound Paclitaxel as Neoadjuvant Therapy. Treatment of triple-negative breast cancer with albumin-bound paclitaxel as neoadjuvant therapy: a prospective RCT [Internet]. NCT ID: NCT04137653. [cited 2025 Mar 13]. Available from: https://clinicaltrials.gov/study/NCT04137653. [Google Scholar]
218. Single-arm, multi-center clinical study of pyrotinib maleate tablets combined with albu-min-bound paclitaxel and trastuzumab in neoadjuvant treatment of Her2-positive early or locally advanced breast cancer [Internet]. NCT ID: NCT04917900. [cited 2025 Mar 13]. Available from: https://clinicaltrials.gov/study/NCT04917900. [Google Scholar]
219. Efficacy and safety of paclitaxel polymeric micelles for injection in the treatment of metastatic breast cancer [Internet]. NCT ID: NCT06143553. [cited 2025 Mar 13]. Available from: https://clinicaltrials.gov/study/NCT06143553. [Google Scholar]
220. Clinical efficacy and safety of paclitaxel polymeric micelles for injection in the treatment of pa-tients with taxans-resistant pancreatic adenocarcinoma, cholangiocarcinoma, lung cancer, gastric cancer, esophageal carcinoma, or breast cancer [Internet]. NCT ID: NCT06199895. [cited 2025 Mar 13]. Available from: https://clinicaltrials.gov/study/NCT06199895. [Google Scholar]
221. OCTANE: Adjuvant Liposomal Doxorubicin and Carboplatin for Early-stage Triple-negative Breast Cancer [Internet]. NCT ID: NCT05949021. [cited 2025 Mar 13]. Available from: https://clinicaltrials.gov/study/NCT05949021. [Google Scholar]
222. The efficacy and safety of eutideron, etoposide, and bevacizumab in patients with brain metas-tases from breast cancer. [Internet]. NCT ID: NCT05781633. [cited 2025 Mar 13]. Available from: https://clinicaltrials.gov/study/NCT05781633?cond=Breast%20Cancer&rank=5. [Google Scholar]
223. UCSF BRCA 1 Gene Mutation Trial→Denosumab on preventing breast cancer in women with a brca1 germline mutation [Internet]. NCT ID: NCT04711109. [cited 2025 Mar 13]. Available from: https://clinicaltrials.ucsf.edu/trial/NCT04711109. [Google Scholar]
224. FDA. Drugs trials snapshot: iTOVEBI [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/drugs-trials-snapshot-itovebi. [Google Scholar]
225. FDA. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutatFDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer. [Google Scholar]
226. FDA. FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HR-positive, HER2-low or HER2-ultralow breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-hr-positive-her2-low-or-her2. [Google Scholar]
227. FDA. FDA approves datopotamab deruxtecan-dlnk for unresectable or metastatic, HR-positive, HER2-negative breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-datopotamab-deruxtecan-dlnk-unresectable-or-metastatic-hr-positive-her2-negative-breast. [Google Scholar]
228. FDA. FDA approves capivasertib with fulvestrant for breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capivasertib-fulvestrant-breast-cancer. [Google Scholar]
229. Endocrine therapy with or without abemaciclib (LY2835219) following surgery in participants with breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://clinicaltrials.gov/study/NCT03155997. [Google Scholar]
230. Study assessing the efficacy and safety of alpelisib plus fulvestrant in men and postmenopausal women with advanced breast cancer wich progressed on or after aromatase inhibitor treatment [Internet]. [cited 2025 Jun 1]. Available from: https://clinicaltrials.gov/study/NCT02437318#study-overview. [Google Scholar]
231. FDA. FDA approves sacituzumab govitecan-hziy for HR-positive breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sacituzumab-govitecan-hziy-hr-positive-breast-cancer. [Google Scholar]
232. FDA. FDA approves Kisqali with an aromatase inhibitor and Kisqali Femara Co-Pack for early high-risk breast cancer [Internet]. [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-kisqali-aromatase-inhibitor-and-kisqali-femara-co-pack-early-high-risk-breast-cancer. [Google Scholar]
233. Johnson F, Golub L, inventors; Chem-Master International Inc., Research foundation of the State University of New York, assignee. Curcumin analogues as zinc chelators and their uses. United States patent US20240360068A1. 2024. [Google Scholar]
234. Blair W, Bulik-Sullivan B, Busby J, Derti A, Gitlin L, Grotenbreg G, et al., inventors; Seattle Project Corp., assignee. Viral delivery of neoantigens. Australia patent AU2024259755A1. 2024. [Google Scholar]
235. Abudayyeh O, Turitz Cox DB, Gootenberg J, Kannan S, Zhang F, inventors; Massachusetts Institute of Technology Broad Institute Inc., Harvard University, assignee. Systems, methods, and compositions for targeted nucleic acid editing. Australia patent AU2024259800A1. 2024. [Google Scholar]
236. Choi J, Juneja V, inventors; Biontech US Inc., assignee. T cell receptor constructs and uses thereof. Australia patent AU2024227645A1. 2024. [Google Scholar]
237. Jessen K, Lannutti B, Vo T, Watkins JD, inventors; Velosbio Inc., assignee. ROR1 antibody immunoconjugates. Australia patent AU2024227446A1. 2024. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF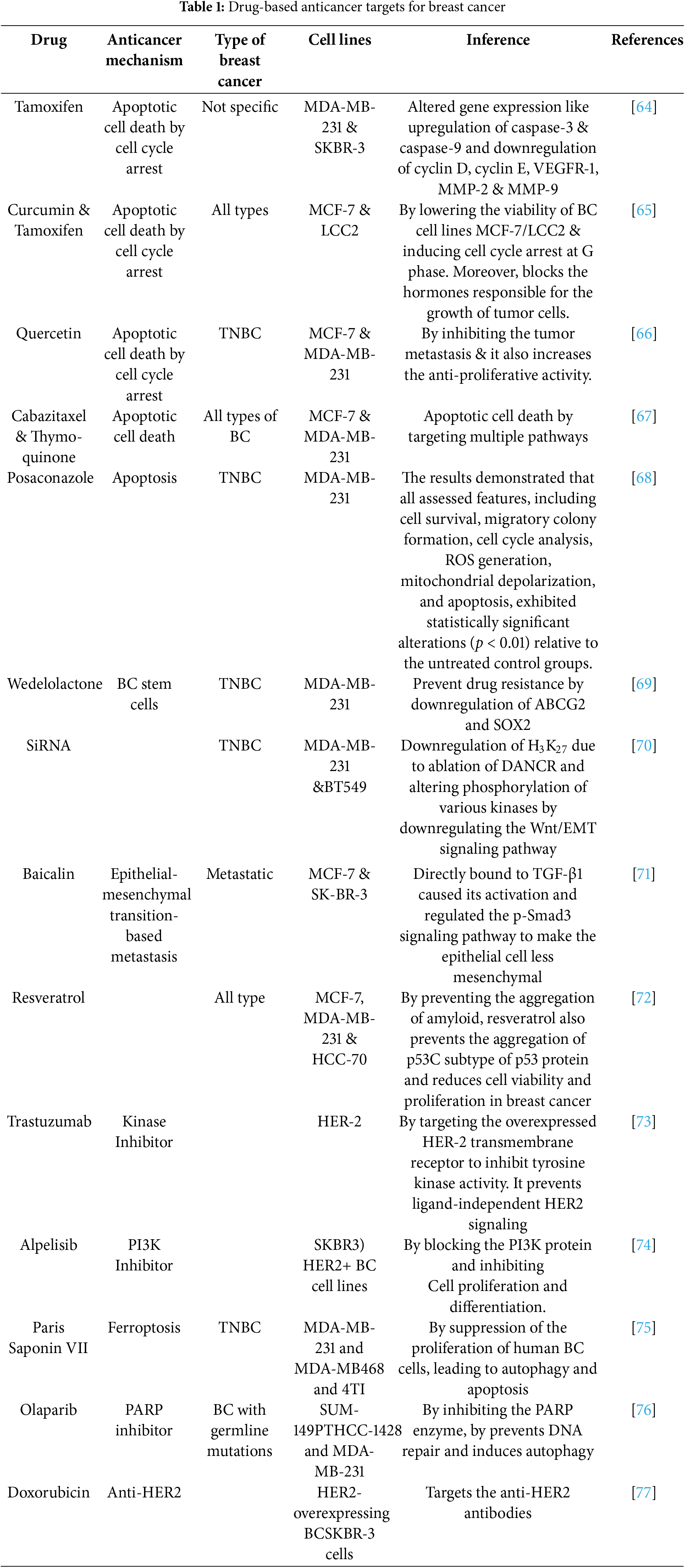
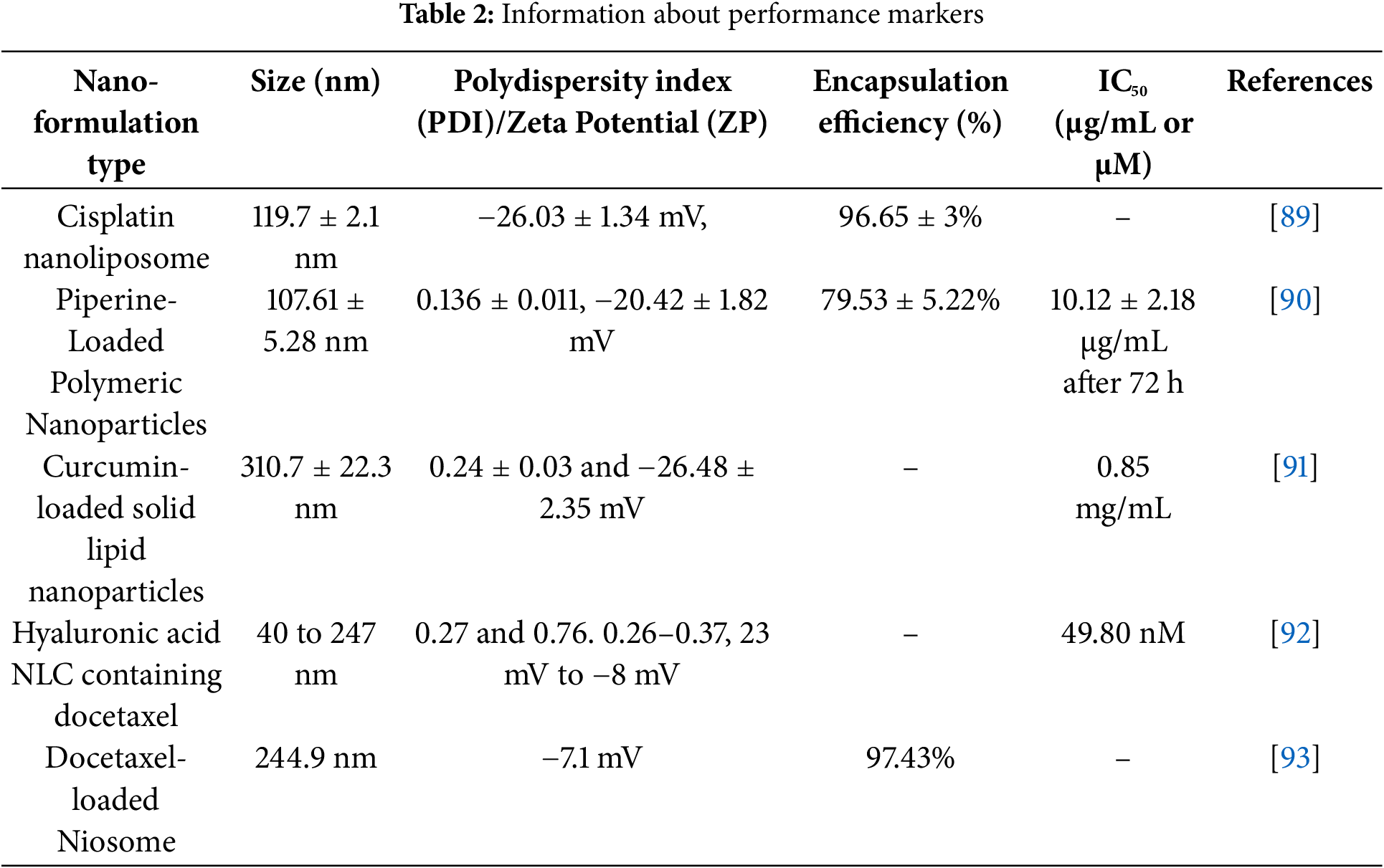
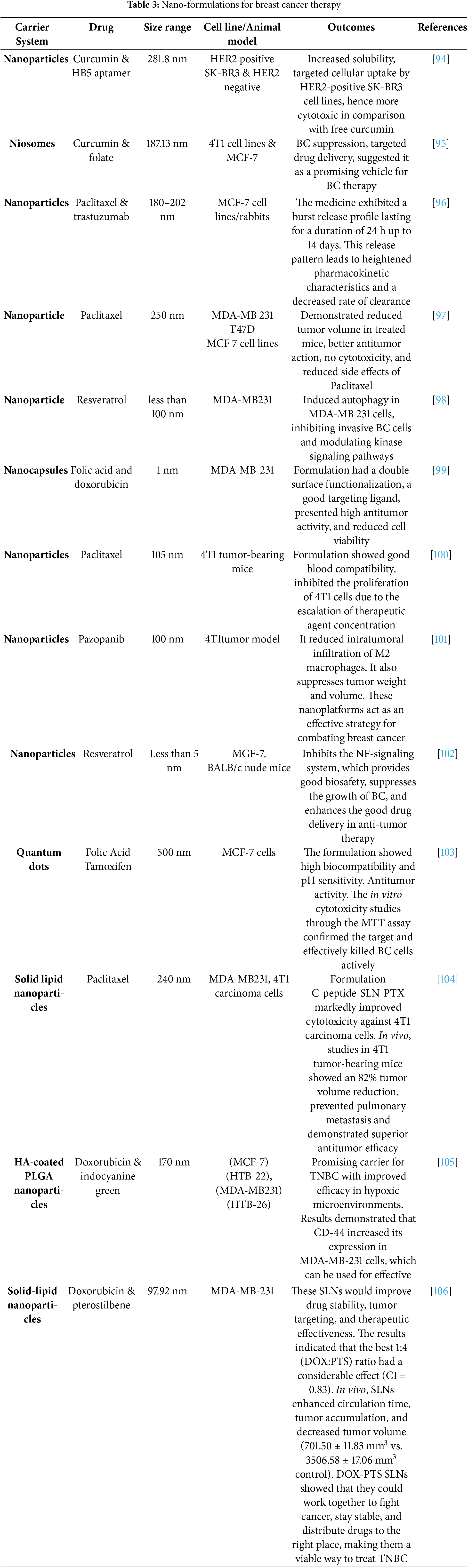
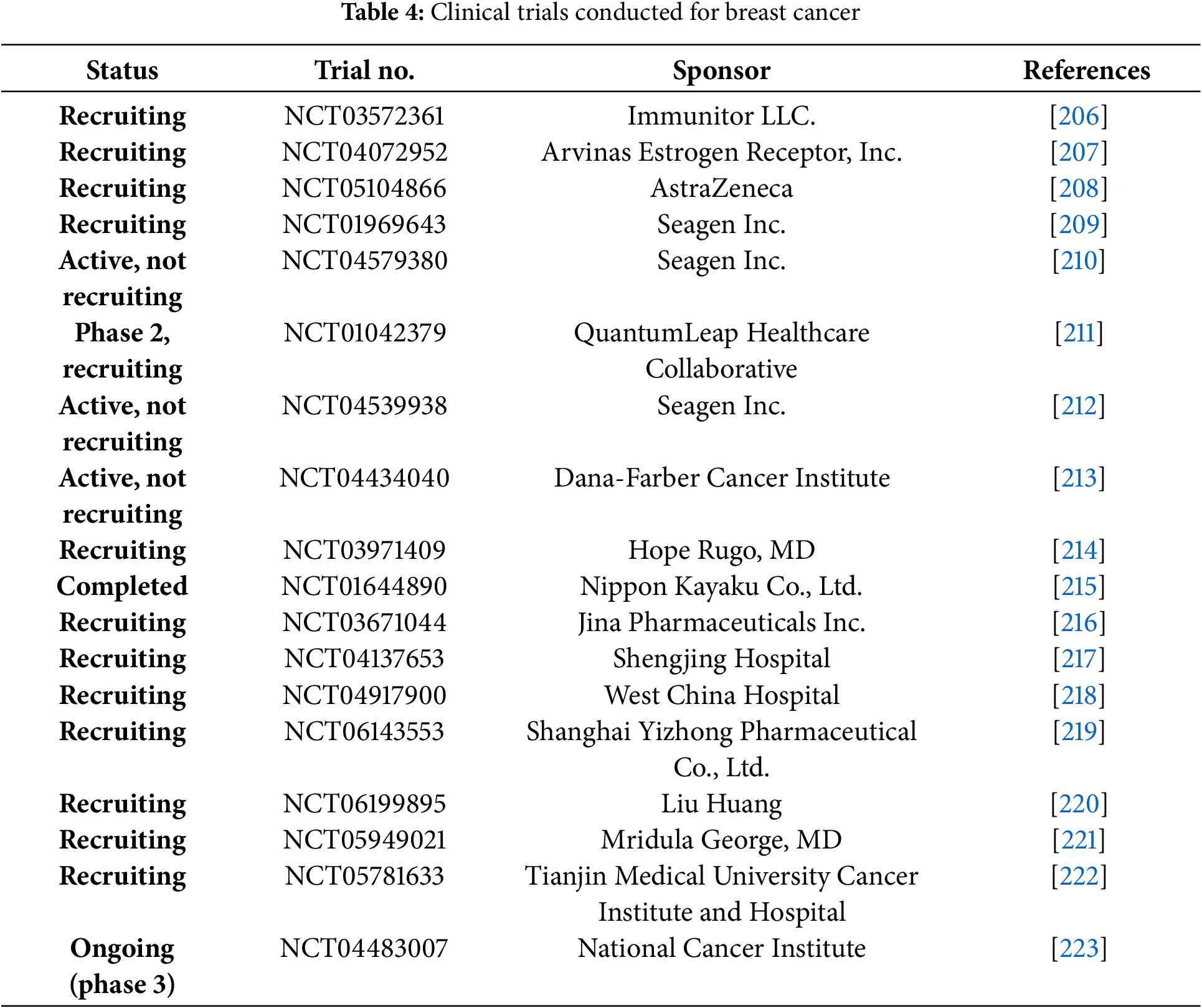
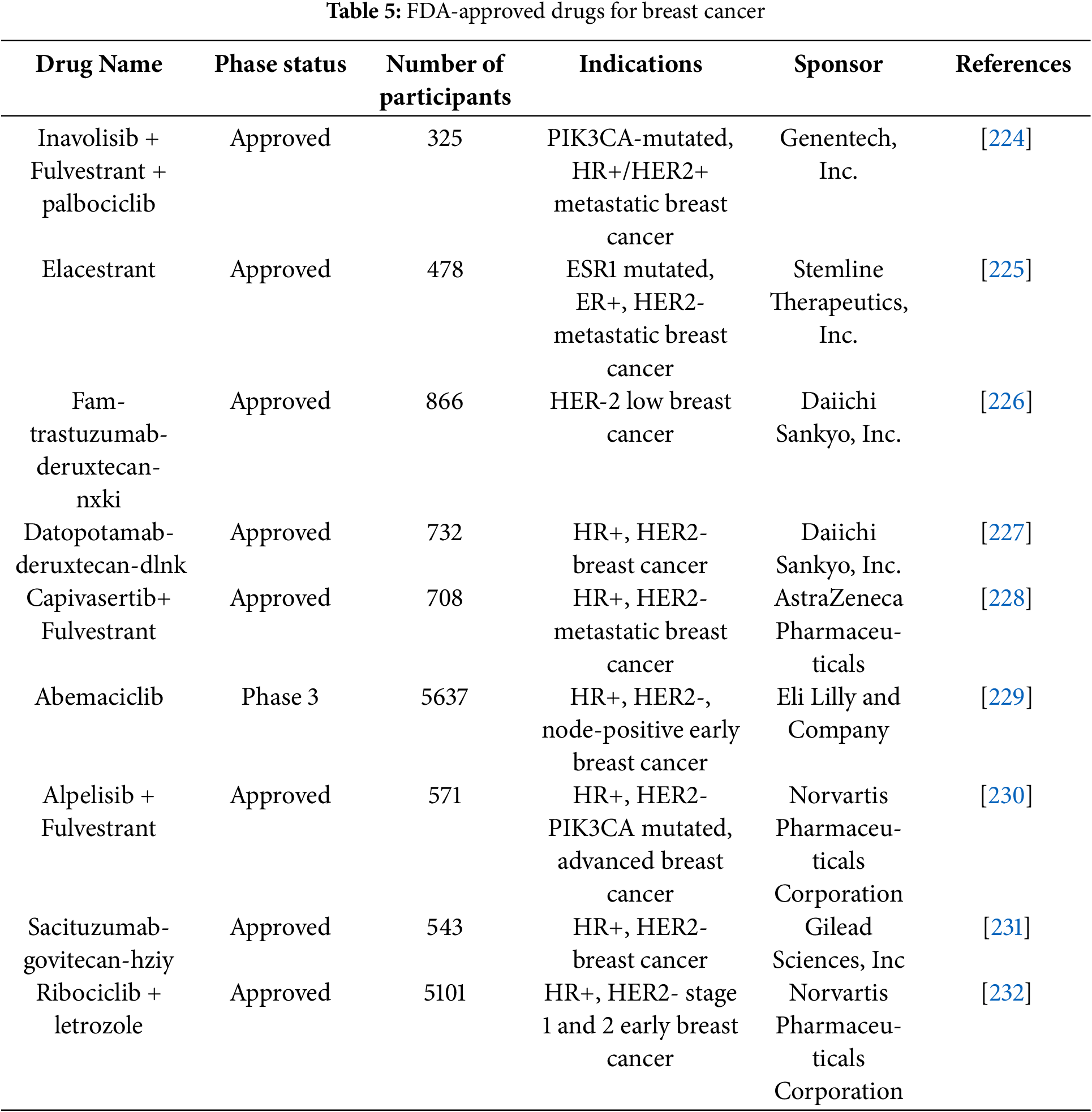
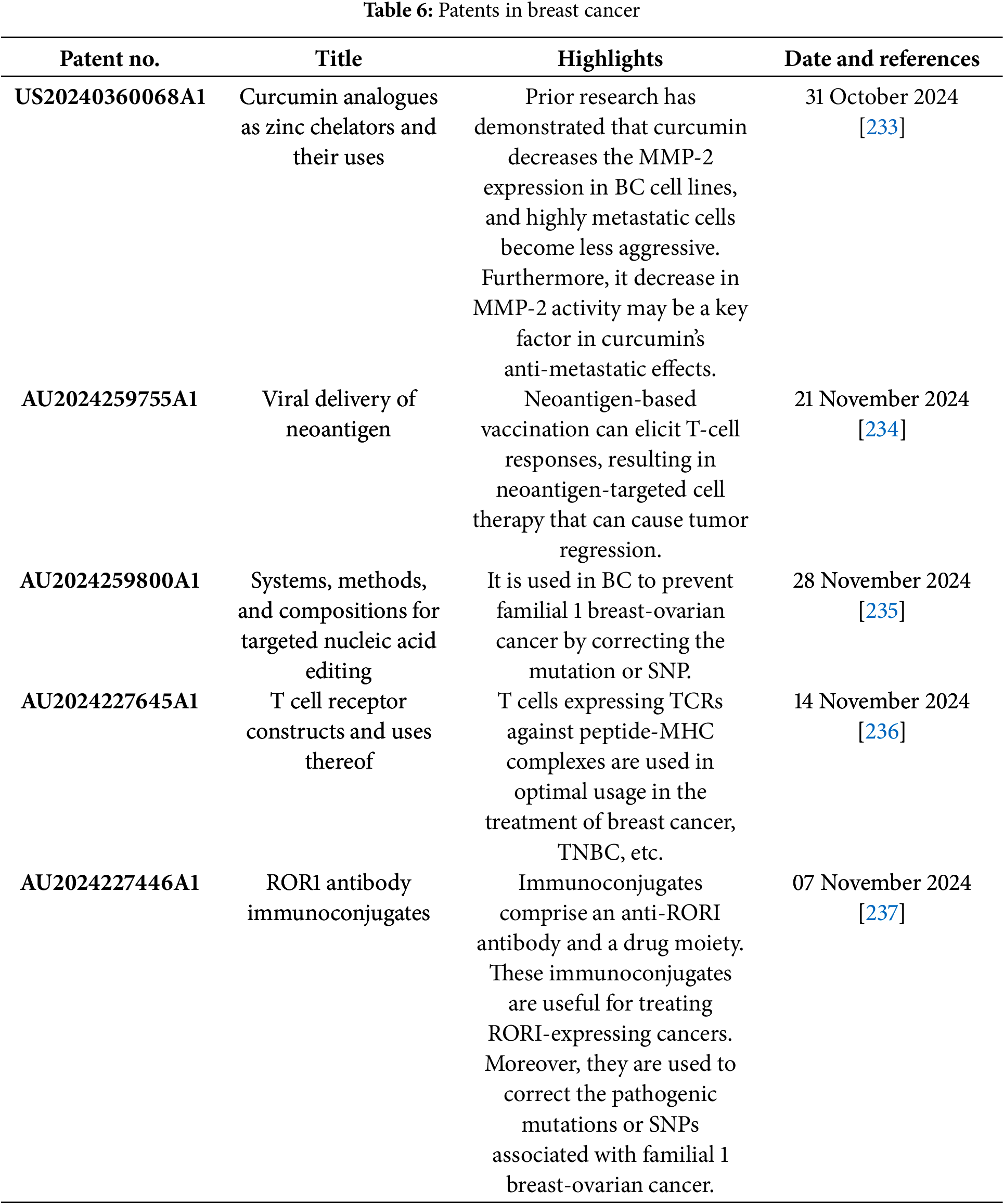
 Downloads
Downloads
 Citation Tools
Citation Tools