 Open Access
Open Access
REVIEW
Universal CAR-T Cell Therapy for Cancer Treatment: Advances and Challenges
School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, 310053, China
* Corresponding Author: Jianan Lei. Email:
(This article belongs to the Special Issue: Advances in Cancer Immunotherapy)
Oncology Research 2025, 33(11), 3347-3373. https://doi.org/10.32604/or.2025.067445
Received 04 May 2025; Accepted 20 June 2025; Issue published 22 October 2025
Abstract
This review aims to explore the development, challenges, and future directions of UCAR cell therapy as a scalable alternative to autologous CAR-T for cancer treatment. Consequently, limitations of autologous CAR-T, including long production, variable quality, and cost, drive off-the-shelf UCAR development to standardize manufacturing and improve access. Current UCAR-T cell strategies focus on mitigating the risks of graft-vs.-host disease and host-vs.-graft rejection through advanced gene editing technologies, including clustered regularly interspaced short palindromic repeat-associated system Cas9-mediated knockout of the T cell receptor, human leukocyte antigen, and cluster of differentiation 52 (CD52). Beyond conventional T cells, cell types such as double-negative T cells, γδT cells, and virus-specific T cells are being engineered with CARs to improve tumor targeting and minimize off-tumor toxicity. UCAR-T therapy is frequently used for hematologic malignancies, including acute lymphoblastic leukemia, non-Hodgkin lymphoma, and multiple myeloma, with efficacy and safety supported by numerous clinical studies. Although trials for solid tumors (e.g., CYAD-101, CTX130) show modest responses, challenges such as tumor heterogeneity and T cell exhaustion remain. Future research should focus on optimizing gene editing precision, integrating combination therapies, and advancing scalable manufacturing platforms. With expanded targets and cell types, UCAR therapies show promise for both hematologic and solid tumors, reshaping cancer treatment and patient outcomes.Keywords
Chimeric antigen receptor (CAR)-T cell therapy was first proposed in 1989. This chimeric receptor provides T cells with antibody-like specificity and can effectively transmit signals that activate T cells to perform their effector functions, representing a revolutionary advance in the field of cancer immunotherapy [1,2]. This innovative approach involves the genetic engineering of a patient’s T cells to express CARs on their surface. CARs are designed to recognize and bind to specific antigens present on the surface of cancer cells, thereby enabling T cells to identify and eliminate them with high precision [3]. CAR-T cell therapy typically begins with the collection of a patient’s T cells, usually from peripheral blood [4]. These cells are then genetically modified in the laboratory to express CARs. CARs are constructed by combining a portion of the T cell receptor (TCR) that recognizes the tumor antigen with an immunoglobulin (Ig) domain, allowing T cells to bind to the antigen [5]. T cell redirection involves genetic modification with the CAR or the use of recombinant proteins designated as bispecific T cell engagers (BiTEs) [5]. The modified T cells are then expanded in culture to increase their number before being infused back into the patient [6]. CAR-T cells are designed to activate and proliferate upon encountering target antigens, leading to the destruction of cancer cells. This process is highly specific because CARs are tailored to recognize particular antigens expressed by tumor cells. This specificity minimizes the risk of off-target effects, which can cause damage to healthy cells [7,8]. The development of CAR-T cell therapy has been particularly successful in treating hematological malignancies, such as acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma (NHL), and multiple myeloma, with overall response rates (ORRs) of 44%–91% and complete response (CR) rates of 28%–68% [9]. However, the application of CAR-T cell therapy to solid tumors remains a significant challenge because these tumors often have complex antigen expression patterns and an immunosuppressive tumor microenvironment, which can hinder the efficacy of CAR-T cells [10,11]. Despite these challenges, ongoing research and development efforts have focused on improving the efficacy, safety, and accessibility of CAR-T cell therapy. These include the development of novel CAR designs, optimization of manufacturing processes, and exploration of combination therapies with other immunotherapies and targeted therapies. The goal is to make CAR-T cell therapy a viable treatment option for a wider range of patients with cancer, including those with solid tumors [12]. Mesothelin (MSLN) has become a new immune target, employing regional routes of delivery, introducing novel modifications leading to enhanced tumor infiltration and persistence, and improved safety profiles and combining anti-MSLN CAR-T cells with standard therapies, which could render them more efficacious in the treatment of solid malignancies [13,14].
Traditional autologous CAR-T cell therapy has complex production and cost limitations that are not conducive to its widespread popularization. In this context, the systematic advantage of replacing autologous CAR-T cells with universal chimeric antigen receptor-T (UCAR-T) cells is obvious [15]. UCAR-T cell therapy can be produced in batches at lower costs and without the need to wait for individual patients. This review explores its concept, development, and application in cancer treatment, with particular attention to its underlying mechanisms, therapeutic advantages, and existing challenges. We compare UCAR-T with conventional CAR-T therapies in terms of biological mechanisms, manufacturing processes, and clinical applications. Additionally, we examine its current research progress and clinical status across multiple cancer types, analyze key technical, safety, and ethical concerns, and discuss potential strategies to overcome these limitations. Ultimately, this study aims to support the advancement of UCAR-T cell therapy, stimulate further research, and improve clinical outcomes for cancer patients.
2 Comparison of CAR-T and UCAR-T Cell Therapies
2.1 Mechanism of CAR-T Cell Therapy
2.1.1 CAR Structure and Function
The CAR cell surface receptor is a synthetic protein that interacts with traditional T cell activation. CAR activation of T cells usually does not require major histocompatibility complex (MHC)-peptide binding to T cell surface antigens via antigen-presenting cells, but activates T cells directly. The structure of CARs plays a crucial role in the efficacy and specificity of CAR-T cell therapy [16]. This therapy involves genetic modification of T cells to express tumor-specific receptors, enabling T cells to produce a tumor-specific immune response when returned to the patient [17]. CAR usually consists of four domains: the extracellular antigen-binding domain, spacer or hinge region, transmembrane domain, and intracellular signaling domain [18].
The extracellular domain is responsible for recognizing and binding tumor-associated antigens (TAAs) expressed on the surface of cancer cells [19]. This domain is typically derived from an antibody that provides high affinity and specificity for a target antigen. The intracellular signaling domain transmits signals from the antigen-binding domain to T cells, leading to T cell activation and subsequent tumor cell death [11]. This domain is often derived from TCR or cluster of differentiation 28 (CD28)/4-1BB co-stimulatory molecules, which activate T cells and enhance their effector functions. The antigen-binding domain of CARs can be engineered to target a wide range of TAAs, allowing for the development of CAR-T cell therapies for various types of cancer. For example, CARs targeting cluster of differentiation 19 (CD19), a protein expressed on the surface of B-cell malignancies, have been successfully used to treat patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) and NHL [20]. Additionally, CARs targeting B cell maturation antigen (BCMA), a protein expressed on multiple myeloma cells, have demonstrated encouraging outcomes in clinical studies for the treatment of this hematologic malignancy. The intracellular signaling domain of CARs is also a critical factor in determining the antitumor activity of CAR-T cell therapy [21]. Early generations of CARs contained only the TCR signaling domain, which is associated with limited efficacy and increased toxicity. To address this issue, subsequent generations of CARs have incorporated costimulatory domains, such as CD28 or 4-1BB, to enhance T cell activation and improve the therapeutic response [22]. This has resulted in improved efficacy and reduced toxicity in clinical trials. The hinge region of the CARs serves as a flexible linker between the extracellular and intracellular domains. This region allows for conformational changes in the CAR, which are important for antigen binding and T cell activation [23]. The hinge region can also be engineered to improve CAR stability and reduce the risk of CAR internalization and shedding, leading to antigen loss and reduced efficacy [24]. Ongoing research is focused on optimizing the design of CARs to enhance their specificity, affinity, and stability as well as to reduce toxicity and improve the therapeutic response in patients with various types of cancer [25].
2.1.2 Food and Drug Administration (FDA) Approval and Commercialization of Traditional CAR-T
The FDA approval of CAR-T cell therapies has marked significant milestones in the development and commercialization of this innovative cancer treatment. Since the first approval of Kymriah (Tisagenlecleucel) for the treatment of B-ALL in 2017 [26], the FDA has approved several CAR-T cell therapies targeting various hematologic malignancies. These approvals include Yescarta (Axicabtagene Ciloleucel), which is used for the treatment of relapsed/refractory large B cell lymphoma (r/r LBCL) and relapsed/refractory follicular lymphoma [27]; Tecartus (Brexucabtagene Autoleucel), which is used to treat relapsed/refractory mantle cell lymphoma and relapsed/refractory precursor B cell acute lymphocyte leukemia [28]; Breyanzi (Lisocabtagene Maraleucel), which is used to treat r/r LBCL [29]; ABECMA (Idecabtagene vicleucel) for r/r multiple myeloma who have had at least four prior therapies [30]; Carvykti (Ciltacabtagene autoleucel) for r/r multiple myeloma who have had at least four prior therapies [31]; and Aucatzyl (Obecabtagene Autoleucel), which is used to treat adult patients with relapsed/refractory precursor B cell acute lymphocyte leukemia [32]. These approvals have not only validated the efficacy of CAR-T cell therapy but have also paved the way for its commercialization. The commercialization of CAR-T cell therapy is a complex process that involves various challenges [33]. One primary challenge is the high cost of manufacturing CAR-T cells [34]. The process of isolating, engineering, and expanding T cells ex vivo is time-consuming and requires specialized facilities and skilled personnel. This has led to the use of high-priced tags for CAR-T cell therapies, making them accessible to only a limited number of patients. However, efforts are being made to optimize manufacturing processes and reduce costs, thereby making CAR-T cell therapies more affordable [35]. Another challenge in the commercialization of CAR-T cell therapies is the complexity of regulatory approval [36]. The FDA approval process for CAR-T cell therapies is rigorous and requires extensive preclinical and clinical data to demonstrate safety and efficacy [37]. This process can be time-consuming and expensive, further delaying the availability of these therapies to patients. Despite these challenges, the commercialization of CAR-T cell therapies has led to significant advancements in the treatment of hematologic malignancies [38]. The success of CAR-T cell therapies has sparked considerable interest in the development of UCAR-T cell therapies, which aim to overcome the challenges associated with current CAR-T cell therapies, such as finite production capacity and the need for personalized treatment. The development of UCAR-T cell therapies could potentially make this innovative cancer treatment more accessible and affordable for a broader patient population.
2.2 Why Develop UCAR-T Cell Therapy?
2.2.1 Mechanism of UCAR-T Cell Therapy
The mechanism of UCAR-T cell therapy is fundamentally similar to that of conventional CAR-T cell therapy (Fig. 1), involving the genetic engineering of T cells to express a CAR that specifically recognizes TAAs [39–41]. However, the key distinction lies in the origin and availability of the T cells. In UCAR-T cell therapy, the T cells are derived from healthy donors and are genetically modified to prevent graft-vs.-host disease (GvHD) and host-vs.-graft (HvG) reactions, enabling their use in multiple patients without the need for individualized production. To generate UCAR-T cells, several gene-editing strategies, such as the disruption of the T-cell receptor (TCR) and human leukocyte antigen (HLA) class I molecules, are employed. This minimizes immune rejection and allows these allogeneic T cells to be administered as “off-the-shelf” products. One of the major advantages of UCAR-T therapy is the potential to streamline manufacturing, reduce treatment delays, and enhance accessibility, particularly for patients with rapidly progressing cancers or those lacking sufficient autologous T cells [42]. Additionally, it provides a scalable and economical approach to expanding global access to CAR-T cell therapy. In essence, UCAR-T therapy utilizes gene-edited allogeneic T cells engineered to express a single tumor-specific CAR, offering a universal and readily accessible immunotherapy alternative that circumvents the complexities associated with autologous cell preparation.
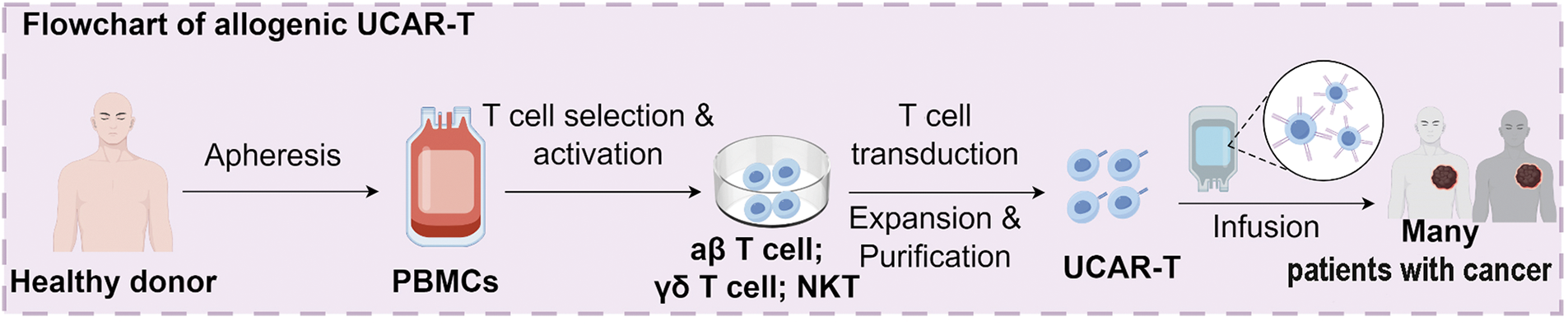
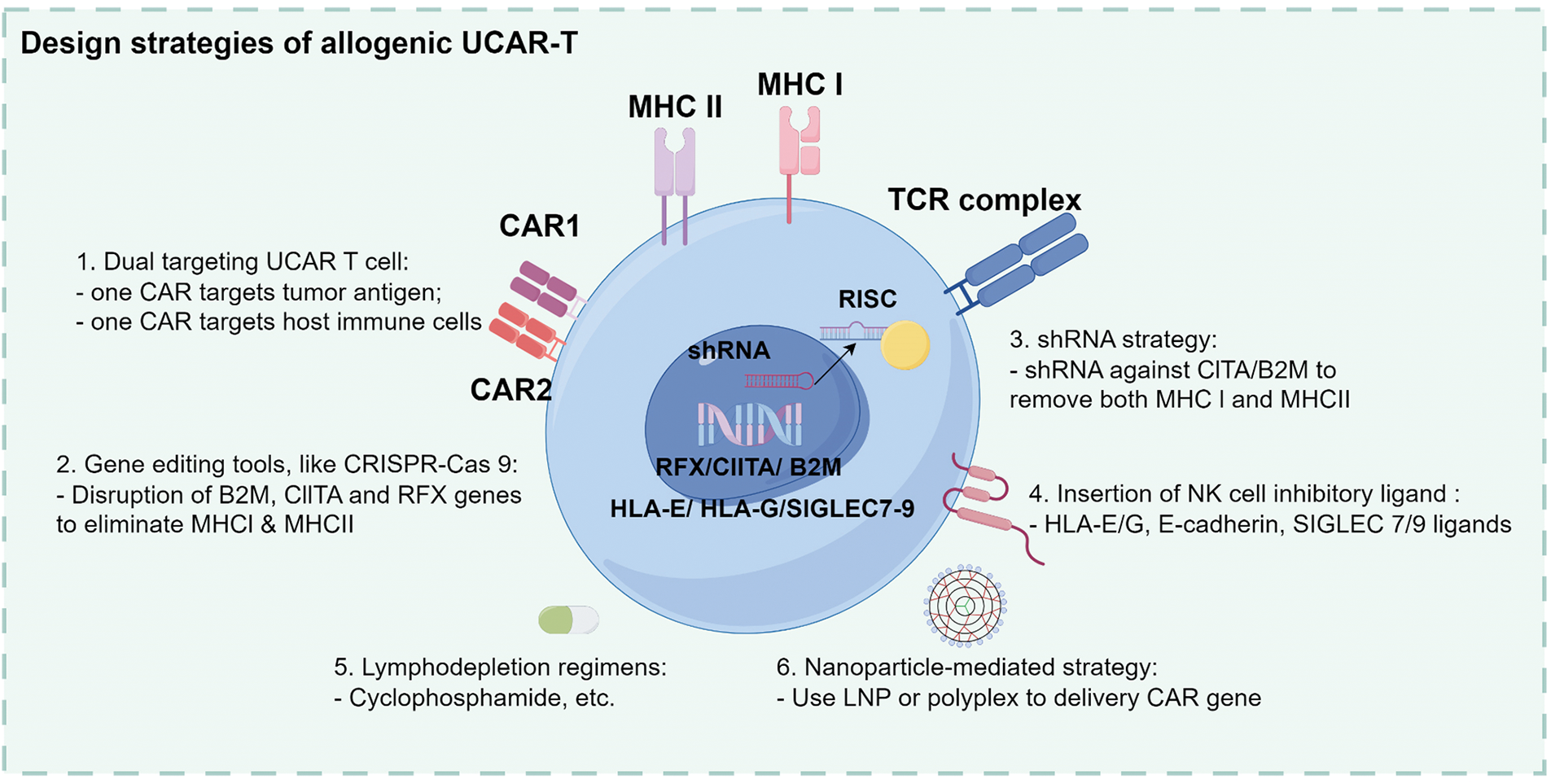
Figure 1: Overview of the manufacturing and design strategies for allogeneic UCAR-T cells. UCAR-T cells are derived from healthy donor peripheral blood mononuclear cells (PBMCs) through T cell selection, activation, CAR transduction, and in vitro expansion. Design strategies include dual-targeting CARs (tumor and host immune cells), gene editing using CRISPR-Cas9 (targeting B2M, CIITA, and RFX), shRNA-mediated silencing, insertion of NK cell inhibitory ligands (e.g., HLA-E/G, SIGLEC 7/9), lymphodepletion regimens, and nanoparticle-based CAR gene delivery. UCAR-T, universal chimeric antigen receptor T cells; PBMC, peripheral blood mononuclear cells; CAR, chimeric antigen receptor; CRISPR-Cas, clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9; B2M, Beta-2 microglobulin; CIITA, class II major histocompatibility complex transactivator; RFX, regulatory factor X; shRNA, short hairpin RNA; NK, natural killer
2.2.2 Comparison of CAR-T and UCAR-T Cell Therapies
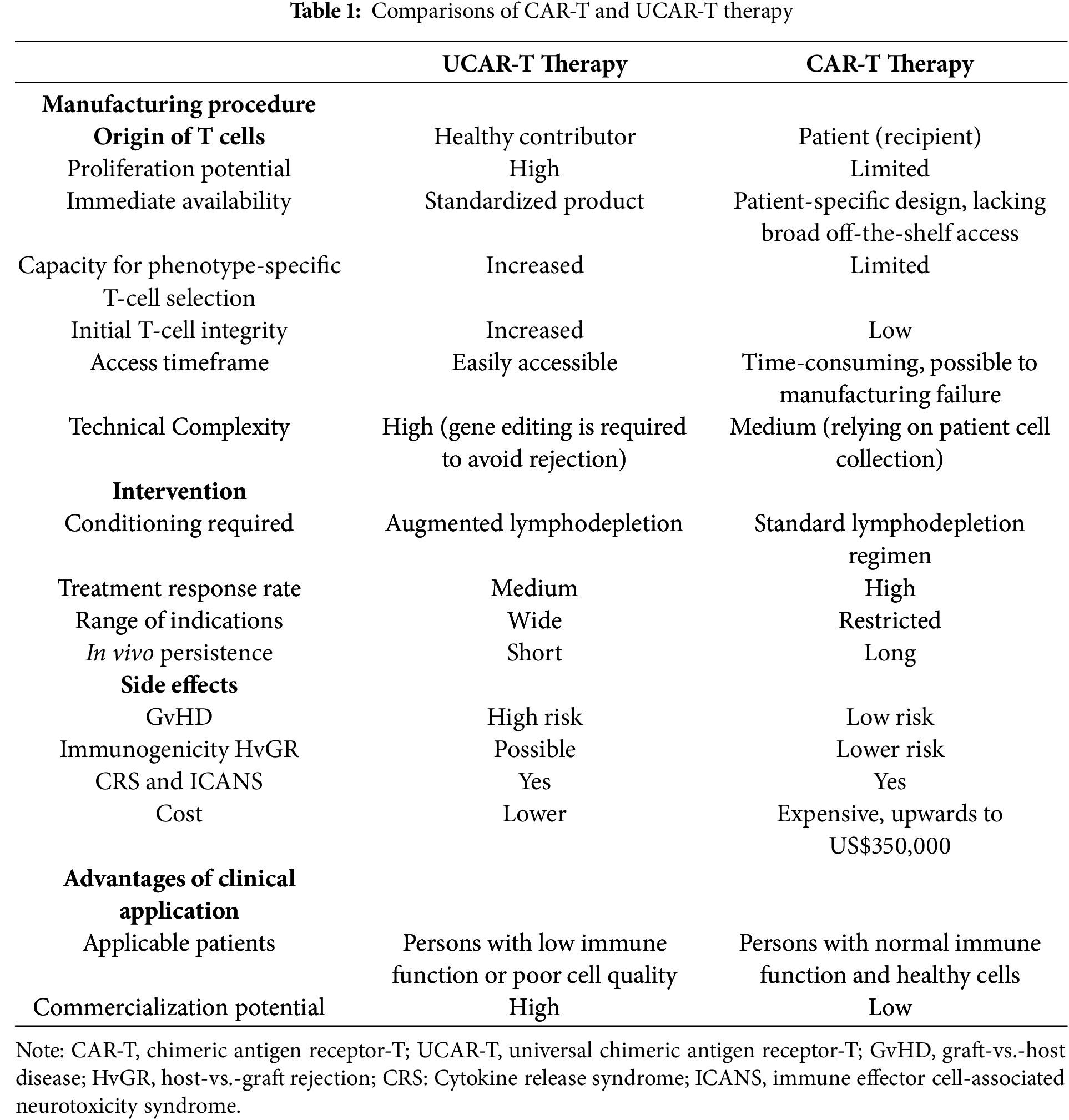
CAR-T cell therapy has demonstrated remarkable efficacy in treating hematologic malignancies such as B-ALL, diffuse large B-cell lymphoma (DLBCL), and multiple myeloma [10,43–45]. This approach involves collecting a patient’s own T cells, modifying them to express CARs that recognize specific tumor antigens, growing the cells in vitro and then administering them back to the patient. Despite its success, autologous CAR-T therapy has notable limitations, including high production costs, manufacturing delays, and reduced feasibility in heavily pretreated or immunocompromised patients. In contrast, UCAR-T cell therapy uses T cells from healthy donors that are genetically engineered and manufactured in advance (Table 1). These cells can be stored and administered to multiple patients as an off-the-shelf product, significantly reducing the time and resources required for individualized preparation. Moreover, gene-editing techniques help minimize immunologic complications by removing endogenous TCRs and HLA molecules. While current CAR-T therapies are mostly limited to hematologic cancers, UCAR-T therapies are being explored for both hematologic and solid tumors. However, further clinical validation is needed to address challenges such as persistence, efficacy in the tumor microenvironment, and long-term safety. In summary, although autologous CAR-T therapy has transformed the treatment landscape for certain blood cancers, UCAR-T therapy offers a promising universal alternative with improved scalability, faster availability, and the potential to expand access to CAR-T therapy for a wider patient population [46,47].
3 Advances in UCAR-T Cell Therapy for Cancer Treatment
3.1.1 Gene Editing Technologies
Gene editing technologies have revolutionized the field of CAR-T cell therapy, offering unprecedented opportunities to enhance the efficacy, safety, and accessibility of this innovative treatment. Among the various gene editing tools available, clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 has emerged as a game changer because of its high efficiency, specificity, and ease of use [48–50]. This technology enables precise modification of the T cell genome, allowing for the introduction of the desired genetic changes without the need for viral vectors, thereby reducing the risk of insertional mutagenesis and off-target effects [51]. CRISPR/Cas9 gene editing can be used to address several critical challenges in CAR-T cell therapy. For instance, it can be used to knock out genes encoding inhibitory receptors such as programmed death receptor 1 (PD-1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) [52,53], thereby enhancing the antitumor activity of CAR-T cells and mitigating the risk of immune exhaustion [54,55]. Using CRISPR/Cas9 to knockdown CD38, TCR, human leukocyte antigen (HLA)-I, and HLA-II genes, followed by lentiviral transduction, can enable lectin-like transcript 1 (LLT1) overexpression, enhance UCAR-T cell activity, and prevent allogeneic rejection, providing the necessary insights for the development of UCAR-T cell therapy [56]. In addition to CRISPR/Cas9, other gene editing tools such as transcription activator-like effector (TALE) nucleases (TALENs) and zinc-finger nucleases (ZFNs) have also been employed in CAR-T cell therapy [57,58]. Although less efficient and more complex than CRISPR/Cas9, these technologies remain valuable for enhancing the functionality of CAR-T cells. Gene editing as a whole has propelled significant progress in CAR-T cell therapy, offering innovative solutions to some of its most critical challenges. As these tools continue to evolve, further advances in the efficacy, safety, and accessibility of CAR-T treatments are anticipated, ultimately improving outcomes for cancer patients worldwide.
3.1.2 Overcoming Alloreactivity
Alloreactivity, an immune response against foreign cells, is a significant hurdle in the development of UCAR-T cell therapy [59]. Unlike autologous CAR-T cells derived from a patient’s T cells, UCAR-T cells are designed as prebuilt products for use in multiple patients. This approach requires the use of allogeneic T cells, which can trigger an immune reaction against donor cells, leading to reduced therapeutic efficacy. Researchers have explored several strategies for addressing alloreactivity. One involves engineering UCAR-T cells to express costimulatory molecules, such as CD80 or CD40 ligands, enhancing the interactions between antigen-presenting cells (APCs) and T cells [60]. Additionally, targeting non-self-antigens with CARs minimizes alloreactivity risks because these antigens are less likely to be present in recipient cells [61]. Another approach modifies T cells to express inhibitory receptors such as PD-1 or CTLA-4, thereby dampening immune responses [62]. Preclinical research has demonstrated that engineered T cells display diminished alloreactivity alongside enhanced antitumor efficacy. Gene editing technologies, particularly CRISPR/Cas9, have been instrumental in achieving these modifications by precisely altering T cells to minimize immune rejection. For instance, CRISPR/Cas9 can knockout genes encoding alloreactive TCR or introduce genes encoding inhibitory receptors [63]. This innovation holds promise for the development of more robust UCAR-T cell therapies with fewer side effects. In summary, overcoming alloreactivity remains critical for advancing UCAR-T cell therapy. By leveraging strategies involving co-stimulatory molecules, inhibitory receptors, and gene editing technologies, researchers aim to enhance safety and efficacy in patients with cancer. These advancements underscore the transformative potential of combining CRISPR/Cas9 with CAR-T cell therapy in modern oncology [63].
3.2 Preclinical and Clinical Successes
Preclinical studies have played a pivotal role in the development of UCAR-T cell therapies. These studies provide valuable insights into the efficacy, safety, and feasibility of this innovative approach. One of the key preclinical studies involved the use of CRISPR/Cas9 technology to engineer UCAR-T cells. This technology has allowed researchers to precisely edit the genome of T cells by introducing desired genetic modifications without the need for viral vectors [64]. UCAR-T cell therapy faces the challenge of quickly clearing allogeneic cells from the host immune system. Another significant preclinical study focused on enhancing the antitumor activity of UCAR-T cells. For example, a study screened a safe and effective anti-CD70scFv for constructing anti-CD70CAR-T cells and then produced anti-CD70UCAR-T cells by knocking out the T cell receptor alpha constant (TRAC), beta 2-microglobulin (B2M) and human leukocyte antigen-DR alpha (HLA-DRA) [65]. To overcome the limitations of UCAR-T cell therapy, there is an “integrated” self-activation and protection module was developed for integration into CAR scaffolds. The SAP module comprises the CD47 extracellular domain, a mutated interleukin 7 receptor α (IL7Rα) transmembrane region, and its intracellular domain, designed to shield UCAR-T cells from host immune responses and improve their survival [65]. Furthermore, preclinical studies have investigated the application of UCAR-T cells in various cancer types. For instance, one study demonstrated the efficacy of UCAR-T cells in treating glioblastoma, a challenging solid tumor. By engineering CAR-T cells resistant to PD-1 inhibition, prolonged survival has been achieved in mice with intracranial tumors [66]. An experimental study developed a clinically feasible culture method to generate IL-15-expressing allogeneic CAR natural killer T (NKT) cells from human hematopoietic stem/progenitor cells (HSPCs), targeting seven types of cancer; a clinical trial was conducted to evaluate the efficacy of this method. These cells showed antitumor activity, expansion capacity, and persistence in multiple myeloma models [67]. These preclinical findings have laid the groundwork for the clinical translation of UCAR-T cell therapy, offering hope to patients with a wide range of malignancies. However, although preclinical studies have shown promising results, further research is needed to address the challenges associated with translating these findings into clinical practice.
3.2.2 Clinical Trials of UCAR-T in Various Cancer Types
In recent years, advancements in gene editing technologies and fundamental cell biology have led to the exploration of various new approaches addressing the challenges mentioned above. Some of these novel discoveries have already progressed to the clinical stage, as outlined in the list of clinical trials of UCAR-T therapy for hematologic (Table 2) and solid (Table 3) tumors. Clinical trials are key contributors in advancing the development and validation of UCAR-T cell therapies. These trials were designed to assess the safety, efficacy, and optimal dose of the therapy in diverse patient populations. To date, several clinical trials have been conducted to evaluate the potential of UCAR-T cells for the treatment of various types of cancer. These trials have provided valuable insights into the safety, efficacy, and optimal dosing of the therapy, laying the groundwork for its further development (Table 2) and potential approval for clinical use. As research continues to advance, we expect to see more clinical trials exploring the potential of UCAR-T cell therapy for treating a wider range of cancer types.
Hematological malignancies, including ALL, acute myeloid leukemia (AML), and lymphoma, have been the primary focus of CAR-T cell therapy because of their unmet medical needs and the significant advancements made in this field [64,101,102]. The advancement of UCAR-T cell therapy has further expanded the potential of this treatment modality for hematological malignancies. In these malignancies, the genetic and molecular heterogeneity of tumors poses a significant challenge for targeted therapies. CAR-T cell therapy, with its ability to target tumor-specific antigens, has shown promising results in the treatment of hematological malignancies [103,104].
Numerous therapies targeting CD19 or BCMA are being investigated for R/r B cell malignancies, including B cell lymphoma, B-ALL, and NHL. ALLO-501 [68], a CD19-targeted therapy utilizing TALEN-mediated disruption of TRAC and CD52 genes, demonstrated an ORR of 75% and a CR rate of 50% in 32 efficacy-assessed patients, with safety evaluated in 46 participants. Similarly, CTX110 [71], which employs CRISPR/Cas9 to disrupt the TRAC gene, reported a 56.3% ORR and 34.4% CR in 32 patients. UCART22 [77], targeting CD22 via TALEN-edited TRAC and CD52 inactivation, achieved a 66.7% ORR and 33.3% CR in a small cohort of three patients. Notably, TruUCARTM GC502 [76], developed in China to disrupt both TRAC and CD7 loci for dual targeting of CD19 and CD7, showed a 100% ORR and 75% CR in four patients. Additional China-developed therapies, such as CTA101 CRISPR/Cas9-mediated TRAC and CD52 knockout, and GC007g with CD19-targeted [81,83], achieved 100% ORR in six and nine patients, respectively, although CR rates varied from 33.3% to 77.8%. Other therapies, including FT819 and PBCAR0191, have shown preliminary safety but lack efficacy data [72,80]. Nathali-01, targeting CD20 and CD22 via TRAC/CD52 inactivation, reported a 100% ORR and 66.7% CR in three patients [74]. A phase I dose-escalation trial (NCT03774654) was conducted to evaluate the safety and preliminary efficacy of allogeneic CD19-specific CAR-NKT cells in patients with relapsed or refractory B-cell malignancies. This study used NKT cells from healthy donors, engineered to express CD19-specific CAR, IL-15, and downregulated β2-microglobulin and CD74 by shRNA to reduce the expression of HLA class I and II molecules. The effects of CD19-specific CAR, IL-15, and CD74 on the expression of HLA class I and II molecules, thereby reducing immunogenicity, were further investigated. Although no formal clinical studies on allogeneic CAR-NKT cells have been published, the ongoing studies described above demonstrate the potential of this therapy for the treatment of B-cell malignancies. More data on its safety and efficacy are expected in the future.
BCMA remains the central focus of r/r multiple myeloma therapy. ALLO-715 [69], combining lymphodepletion with the anti-CD52 antibody ALLO-647 achieved a 75% ORR and 50% CR in 36 efficacy-evaluated patients, with safety assessed in 52 participants. In contrast, CYAD-211 [70], which uses shRNA to silence CD3ζ mRNA in TCRs, reported a limited efficacy of 12.6% ORR and no CR in nine patients. P-BCMA-ALLO1 [73], leveraging the Cas-CLOVER™ system to disrupt T-cell receptor constant β chain (TRBC) and B2M genes, demonstrated safety in 24 patients but lacked efficacy outcomes. ADI-001 [93], a γδT cell therapy targeting CD20 in B cell lymphoma, achieved a 78% ORR and 78% CR in nine patients, suggesting potential cross-indication utility. Additionally, CAR Epstein-Barr virus (EBV)-specific T cells (EBV-VSTs) and CAR EBV-specific cytotoxic T lymphocytes (EBV-CTLs) [91,94], which utilize Epstein-Barr virus-specific T cells modified with CD19-targeted CARs, were safe in 16 patients but showed pending efficacy results. These findings underscore the need to optimize gene editing platforms, such as TALENs, CRISPR, and combination strategies, to enhance BCMA-directed responses.
For T cell malignancies, CTX130 [75], a CD70-targeted therapy using CRISPR/Cas9 to disrupt TRAC, reported a 46.2% ORR and 19.4% CR in 39 patients across the US, Australia, and Canada. WU-CART-007 [81], targeting CD7 and TRAC via CRISPR achieved a 58.3% CR in 12 patients with r/r T-ALL or lymphoblastic lymphoma. Base-edited CAR7 T cells [89], developed in the UK, inactivate CD52, CD7, and TCR genes via CRISPR/nCas9 base editing, although efficacy data remain unreported. For AML, CD33CART [88], a CD33-targeted therapy, demonstrated an 11% complete response rate in 19 patients.
CAR-T, targeting CD4 and CD8, achieved a 100% objective response rate in 12 patients, but lacked complete response data. AVC-101 [87], an adapter CAR-T system combining UCAR-T cells with a CD123-targeting module, exhibited a 53% objective response rate in 15 patients. Emerging strategies, such as CD30 CAR EBV-specific T cells achieving a 69.2% objective response rate and 38.5% CR rate in CD30-positive lymphoma, and CD19CAR/virus-specific T cells, demonstrating a 33.3% objective and CR rate in CD19-positive malignancies, highlight innovative approaches to enhance precision and safety [95]. Additionally, RD13-01 [84], a CRISPR-edited therapy disrupting CD7 and TCR/CD3 in T cell ALL or lymphoblastic lymphoma, reported an 88.8% objective response rate and 63.6% complete response rate in 11 patients, further underscoring the transformative potential of gene editing technologies in addressing T cell malignancies.
Solid tumors present a significant challenge for CAR T-cell therapy because of their complex and heterogeneous nature, including dense stroma, hypoxia, and multiple immune checkpoints. However, because of the intricate location of solid tumors, CAR-T cell therapy for solid tumors encounters numerous challenges such as a hostile tumor microenvironment, supra/exotumor toxicity, and unwanted antigen specificity [105]. Many strategies and methods have been attempted to overcome these obstacles, including knocking out PD-1 expression or secreting cytokines/chemokines to arm CAR-T cells, and using CAR-T cells in combination with other therapies [106–108]. Despite these efforts, CAR-T cells have not been approved for the treatment of solid tumors. Encouraging and optimistic, more than 40 clinical trials on CAR-T cell treatment of solid tumors have been registered in China alone [109]. To combat T cell dysfunction in the tumor microenvironment, CAR-T therapy has made progress in solid tumor treatment, in which CAR-T cells are metabolized by modifying them to secrete interleukin 10 (IL-10) to eradicate solid tumors and maintain immune protection. IL-10 secretion promotes the proliferation and effector function of CAR-T cells, resulting in the complete regression of solid tumors and metastatic cancers in various cancer types in homogeneous and xenograft mouse models (including colon, breast, melanoma, and pancreatic cancer). IL-10CAR-T cells also induce stem cell-like memory responses in lymphoid organs, providing lasting protection against tumor reattack [110].
Three UCAR-based investigational therapies have demonstrated different results in clinical trials targeting solid tumors. CYAD-101 [98], which targets NK cells by inhibiting the T-cell receptor signaling pathway in donor T cells, was evaluated in a Phase 1 trial involving 15 patients with unresectable metastatic colorectal cancer. The therapy demonstrated a 13.33% objective response rate, although no complete responses were reported. ALLO_GD2-CART01 [99] designed to target GD2 using CD4 or CD8 central memory CAR-positive T cells, achieved a 60% objective response rate and 40% complete response rate in a small cohort of five patients with relapsed/refractory high-risk neuroblastoma, highlighting its potential in pediatric solid tumors. CTX130 [100], which employs CRISPR/Cas9-mediated disruption of the TRAC gene to target CD70 in clear cell renal cell carcinoma, reported a 6.3% complete response rate among 16 patients in a Phase 1 trial, although objective response data were not disclosed. These results underscore the challenges and early promise of UCAR therapies for solid tumors, particularly for optimizing targeting strategies, enhancing response durability, and addressing the heterogeneity of solid tumor microenvironments. Further refinement of gene editing technologies and combination regimens may improve clinical outcomes in difficult-to-treat malignancies.
4 Primary Barriers in UCAR-T Cell Therapy
4.1 Challenge of Immune Rejection in Transplantation
4.1.1 Graft-Versus-Host Disease (GvHD)
UCAR-T, as a “ready-to-use” cell therapy approach, is designed to address the prolonged manufacturing timelines and high costs associated with autologous CAR-T therapy. However, its allogeneic origin introduces GvHD as a significant safety concern. GvHD occurs when donor T cells mount an immune response against recipient tissues—a phenomenon commonly observed following allogeneic stem cell transplantation. In the context of UCAR-T therapy, donor T cells may recognize the recipient’s major histocompatibility complex (MHC) molecules through their TCRs, thereby initiating the GvHD response. Alloreactivity of αβ-TCRS is the main cause of GvHD development, and therefore, knockout of TCR genes in donor T cells is considered an effective strategy to reduce GvHD risk [111]. A retrospective study analyzed 25 patients undergoing hematopoietic stem cell allograft with CD19-directed CAR-T cell therapy, 11 of whom developed symptoms of suspected GvHD, and three patients (12%) were diagnosed with GvHD induced by CAR-T cell therapy [112]. This study underscores the risk of GvHD following CAR-T cell therapy, especially concerning donor compatibility and the design of the CAR construct. In a mouse model, allogeneic CD19 CAR-T cells initiate fatal GvHD in the presence of CD19-positive leukemia, whereas GvHD was not observed in the absence of leukemia [113]. This indicates that the presence of tumor antigens enhances CAR-T cell activity, thereby increasing the likelihood of GvHD.
HvG is a key barrier to the efficacy and persistence of UCAR-T cell therapy. This mechanism involves the recognition and clearance of foreign CAR-T cells by the host immune system, leading to transient survival and reduced efficacy in vivo [114]. Allogeneic CAR-T cells expressing donor MHC molecules, particularly HLA-I and HLA-II, may be recognized as “non-self” by the host’s CD8+ and CD4+ T cells, triggering an immune response that results in the clearance of CAR-T cells. Even in partial HLA matching, minor antigenic differences can trigger immune rejection [114]. To reduce T cell-mediated rejection, researchers tried to reduce CAR-T cell surface HLA expression by knocking out genes such as β2-microglobulin. However, deletion of HLA-I may activate the host’s NK cells, which recognize the “missing self” signal and attack CAR-T cells [23]. The host may produce antibodies against donor HLA molecules, forming anti-donor-specific antibodies (DSA), which may lead to the clearance of CAR-T cells and limit subsequent cell therapy [115].
4.2.1 Cytokine Release Syndrome
Cytokine release syndrome (CRS) remains a major safety challenge in UCAR-T therapy, particularly in hematological malignancies [116,117]. CRS occurs when CAR-T cells recognize and attack healthy cells, leading to the release of large amounts of cytokines, such as interleukin-2 (IL-2) and interferon-gamma (IFN-γ), into the bloodstream. CRS is the predominant adverse event associated with CAR T-cell immunotherapy and exhibits variable incidence rates across clinical studies. As evidenced by comprehensive trial data, this systemic inflammatory condition manifests in 42%–100% of treated patients, with severe presentations (grade ≥3) occurring in 0%–46% of cases. A meta-analysis of 2592 patients from 84 clinical investigations revealed an aggregate CRS-associated mortality rate of 1% [118] underscoring the generally manageable nature of this complication when employing appropriate interventions. Molecular pathogenesis involves a three-phase cascade: initial CAR T-cell activation induces bystander immune cell stimulation, particularly in the monocyte/macrophage lineage, followed by cytokine-driven endothelial activation [119]. Endothelial dysregulation ultimately mediates the characteristic hemodynamic instability and capillary leak syndrome observed in patients with severe CRS. Due to their allogeneic origin, UCAR-T cells may elicit a stronger immune response in the host, thereby increasing the risk of CRS. In addition, to reduce the risk of GvHD, UCAR-T cells often knock out TCR and HLA molecules through gene editing, which may affect their interaction with the host immune system and reduce the risk of GvHD, indirectly affecting the occurrence of CRS [120].
Off-target effects represent a significant challenge in UCAR-T cell therapy, particularly when considering the potential of these therapies for a broader range of cancer types [121]. The potential for off-target effects is heightened by the fact that many cancer antigens are also expressed on normal cells such as hematopoietic stem cells, endothelial cells, and epithelial cells. For example, CD19, a common target in B cell malignancies, is also expressed in some normal B cells, which can lead to the destruction of these cells, resulting in severe autoimmune reactions. Similarly, the CD133 antigen, which is often targeted in solid tumors, is also expressed on normal stem cells, raising concerns about the potential for off-target effects. Various strategies have been employed to mitigate the risk of off-target effects. CAR-T cells targeting CLL-1 or CD123 escape the off-target tumor effect and immunosuppressive tumor microenvironment and have good efficacy in AML [122]. Despite these efforts, off-target effects remain a significant concern in CAR-T cell therapy. Clinical trials have reported severe adverse events, such as encephalitis and myocarditis, which are believed to be related to off-target effects [123]. To address this challenge, ongoing research is focused on developing more sophisticated CAR constructs and manufacturing processes that minimize the risk of off-target effects while maintaining the efficacy of CAR-T cell therapy.
4.3 Regulatory and Ethical Issues
The path to developing and approving UCAR-T cell therapy has significant regulatory challenges that have hindered its widespread adoption. One of the primary obstacles is the lengthy and intricate approval process, which requires extensive preclinical and clinical data to establish safety and efficacy. This process becomes even more complex because of the autologous nature of UCAR-T cells, which are derived from the patient’s cells, and introduce additional variables and uncertainties into clinical trials. Regulatory bodies, such as the U.S. FDA and the European Medicines Agency (EMA), set rigorous criteria for the approval of cellular therapies, such as CAR-T cells [124,125]. These requirements include demonstrating the consistency and reproducibility of the manufacturing process, ensuring the quality and purity of the final product, and providing evidence of its long-term safety and efficacy. The unique characteristics of CAR-T cell therapy, particularly the use of gene editing technologies such as CRISPR/Cas9, further complicate the regulatory landscape. The potential for off-target effects and unintended genetic modifications necessitates a rigorous evaluation of the safety profile of the therapy, considering not only the risks associated with the gene editing process itself but also the possibility of modified cells causing harm to the patient. Collaboration between academic institutions, biotechnology companies, and collaboration with regulatory agencies is crucial for the effective development and approval of UCAR-T cell therapy. However, such collaboration introduces additional complexities and challenges. Coordinated approaches to data sharing, intellectual property rights, and clinical trial design are crucial but can slow down the approval process and increase costs. Addressing these challenges will be pivotal for the successful development and broad adoption of UCAR-T cell therapy as a cancer treatment. Despite these hurdles, ongoing advancements in gene editing technologies and innovative strategies to enhance the safety and efficacy of CAR-T cells offer hope for overcoming these barriers. By refining the manufacturing processes, improving target specificity, and reducing adverse effects, researchers aim to make UCAR-T cell therapy a more accessible and effective option for patients with cancer.
The ethical dimensions of UCAR-T cell therapy are complex and multifaceted. One primary concern is the potential for unintended consequences and off-target effects, particularly given the intricate nature of the human immune system and the implications of genetic modifications. There is a risk that these therapies could inadvertently trigger immune responses against healthy cells or tissues, leading to unforeseen complications. Ensuring the safety and efficacy of UCAR-T cells requires rigorous preclinical and clinical testing to address these risks. Another critical consideration pertains to equity and accessibility. The high cost of CAR-T cell therapy raises concerns that UCAR-T cells might become a luxury treatment accessible only to those with financial resources, potentially exacerbating existing health disparities. Efforts must be made to ensure affordability and accessibility for all patients in need, in line with the broader ethical principles of fairness and justice. Additionally, the use of gene editing technologies in developing UCAR-T cells introduces ethical questions regarding unintended genetic modifications and their long-term implications. The potential for heritable changes in the genome and unintended genetic drift necessitate careful oversight to ensure the responsible and ethical application of these technologies. This underscores the importance of robust regulatory frameworks and the ongoing evaluation of the safety and efficacy of gene editing tools. Finally, patient autonomy and informed consent are fundamental ethical considerations. Patients must be fully informed of the potential risks and benefits of UCAR-T cell therapy to make autonomous decisions regarding their treatment. Upholding these principles ensures that the development and implementation of UCAR-T cell therapy align with the highest ethical standards [126].
5 Future Directions and Perspectives
Improving the therapeutic effectiveness and safety profile of UCAR-T cell therapy remains a critical focus for advancing this innovative cancer treatment. Researchers are exploring multiple strategies to achieve these goals, starting with the optimization of the CAR structure. Fine-tuning of the antigen recognition domain can enhance specificity while minimizing off-target effects, which is crucial for improving therapeutic outcomes. Additionally, incorporating costimulatory domains boosts T cell activation and proliferation, leading to a more robust antitumor response. The development of bispecific CARs that simultaneously target multiple antigens represents another promising approach to increase the likelihood of successful tumor eradication. Addressing T cell exhaustion is vital for improving the persistence and overall effectiveness of CAR-T cells. This challenge can be addressed using various methods, such as engineering CAR-T cells to express inhibitory receptors capable of neutralizing signals from the immunosuppressive tumor microenvironment. Modulating the tumor microenvironment itself by reducing factors that suppress immunity further supports T cell activation. Improving the manufacturing process of UCAR-T cells is essential to reduce costs and ensure scalability. Innovations in production methods are also important. For example, constitutive expression of mutant B2M-HLA-E (MBE) and B2M-HLA-G (MBG) fusion proteins in anti-CD19 UCART (UCART-19) cells was carried out to prevent allogeneic NK cell-mediated lysis, and UCART-19 cells constitutively expressing MBE and MBG fusion proteins were created, showing effective and specific antitumor activity. Constitutive expression of MBE and MBG fusion proteins in UCART-19 cells prevents allogeneic NK cell-mediated lysis [127]. By focusing on these multifaceted strategies, researchers and clinicians aim to enhance both the efficacy and safety of UCAR-T cell therapy, making it a more accessible and effective treatment option for patients with cancer globally.
Expanding the application of UCAR-T cell therapy is essential for advancing cancer treatment. Although this therapy has primarily been applied to hematological malignancies such as ALL, NHL, and CLL, its potential extends far beyond these diseases [64,101,128]. Researchers are actively exploring the use of UCAR-T cells to treat solid tumors, which account for a significant proportion of cancer-related deaths. The challenges of applying CAR-T cells to solid tumors are complex. Solid tumors often exhibit heterogeneous microenvironments that hinder CAR T-cell infiltration and effectiveness. Moreover, immunosuppressive factors within the tumor microenvironment further compromise the efficacy of therapy. In recent years, UCAR-T therapy has made breakthroughs in the field of solid tumor treatment; however, it still faces multiple biological challenges. Unlike hematological tumors, factors such as the heterogeneity of solid tumors, immunosuppressive microenvironments (TMEs), and lack of ideal target antigens significantly limit the infiltration, activity, and durability of CAR-T cells. In response to these bottlenecks, researchers optimized UCAR-T design through multi-dimensional strategies: 1) target selection optimization: develop CAR structures for solid tumor-specific antigens or tumor microenvironment-related targets [99], and explore dual-target/logical gating CAR design to reduce off-target toxicity [129,130]; 2) microenvironment regulation: gene editing technology confers UCAR-T cells the ability to secrete immunomodulatory factors such as IL-12, PD-1 inhibitors [66], or knock out TGF-β receptors to resist TME inhibition; and 3) allogeneic rejection control: using gene editing to eliminate TCR and HLA molecule expression, and in combination with an immunosuppressive regimen to reduce the risk of GvHD [131]. In terms of clinical translation, early trials showed some responses. For example, UCAR-T cells targeting GD2 achieved objective remission in neuroblastoma (NCT04539366), whereas allogeneic CAR-T cells combined with an RNA vaccine against claudin-6 (CLDN6) showed initial antitumor activity in testicular cancer (NCT04503278). However, their efficacy is limited by insufficient CAR-T cell expansion and TME-mediated depletion. To improve its efficacy, researchers are exploring combined treatment strategies such as the use of checkpoint inhibitors [132], oncolytic viruses, or local radiotherapy to enhance tumor immunogenicity.
UCAR-T cell therapy was originally used to treat malignant tumors of the blood and has recently been used to treat autoimmune diseases. The mechanism mainly involves resetting the patient’s immune system by targeting and removing abnormally activated B cells. CRISPR-Cas9 gene editing technology was used to genetically engineer healthy donor-derived CD19-targeting CAR-T cells to address immune rejection, which is a promising strategy for the treatment of CD19-related diseases, leading to the development of a new generation of UCAR-T therapy and the successful treatment of one patient with refractory immune-mediated necrotizing myopathy and two patients with diffuse cutaneous systemic sclerosis [133]. During the 6-month follow-up after treatment, all three patients experienced deep remission of symptoms, significant improvement in the disease clinical response index score, and reversal of inflammation and organ fibrosis; there were no CRS or other serious adverse events.
As an important breakthrough in tumor immunotherapy, CAR-T cell therapy faces multidimensional equity challenges in its global promotion, forming a new pattern of health inequality. Current barriers to implementation are primarily economic, with personalized preparation processes resulting in single-treatment costs of up to US$400,000–500,000, resulting and less than 5% patient coverage in low- and middle-income countries (LMICs). Most (75%) of the world’s CAR-T production centers are in North and Western Europe, which highlights a serious imbalance in the regional distribution of resources. This dilemma is exacerbated by a lack of technological infrastructure, and developing countries generally lack standardized cold-chain logistics, quality control systems, and specialist medical teams; no indigenous CAR-T production facilities have been established in Africa. Fragmentation of the regulatory system is also a key constraint, with an average delay of 3–5 years for FDA-/EMA-approved products in LMICs. Approximately 80% of LMICs do not yet have a dedicated regulatory framework, leading to a lengthy and uncertain approval process. To overcome these bottlenecks, the international community is exploring solutions through technological innovation and collaborative models. In terms of technological cost reduction, UCAR-T eliminates the TCR/HLA-I gene through CRISPR editing technology, which reduces the production cost by 60%–70%. Automated closed manufacturing systems, such as CliniMACS Prodigy, reduce the lead time from 14 days to 7 days [134], significantly improving accessibility. Regional coordinated innovation is beginning to bear fruit, with companies such as India’s Bharat Biotech pushing for localized production through technology transfer through their Asian CAR-T hub programmer [135], The multi-country Procurement Consortium (MPPA) uses the model of the Global Alliance for Vaccine Immunization (Gavi) for central bargaining to reduce prices. At the policy level, the pay-for-effects model implemented by the UK NHS and the WHO-led stepped approval path provide operational access schemes for resource-limited areas.
The development of UCAR-T cell therapy represents a significant advancement in cancer immunotherapy. Although CAR-T cell therapy has revolutionized the treatment of hematological malignancies, its application to solid tumors remains challenging. The development of UCAR-T cells with the potential to target a wide range of cancer antigens offers a promising solution to this problem. Advancements in gene editing technologies such as CRISPR/Cas9 have facilitated the engineering of UCAR-T cells, overcoming the limitations of traditional CAR-T cell therapy. However, the development of UCAR-T cell therapies is challenging. Technical challenges such as manufacturing complexity and ensuring persistence and efficacy need to be addressed. Safety concerns, including CRS and off-target effects, should be carefully managed. Additionally, regulatory and ethical issues pose significant hurdles in the development and implementation of UCAR-T cell therapy. Despite these challenges, the potential of UCAR-T cell therapy for the treatment of various types of cancers cannot be overlooked. Future research should focus on enhancing the efficacy and safety of UCAR-T cells, expanding their application to a broader range of cancer types and ensuring equitable global access to this innovative therapy. By addressing these obstacles and progressing UCAR-T cell therapy, we can open the door to a new chapter in cancer treatment, bringing hope and enhancing patient outcomes globally.
In conclusion, while UCAR-T cell therapy offers a promising “off-the-shelf” alternative to autologous CAR-T cell approaches, its interactions within the complex immunological landscape of individual patients remain incompletely understood. Continued research is essential to elucidate the immunobiological dynamics between UCAR-T cells and the host immune system, and to refine gene-editing techniques and manufacturing protocols that ensure consistent safety and efficacy across diverse patient populations.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Conceptualization, Jianan Lei; original draft preparation and editing, Jianan Lei, Zhuona Ni, Ruidi Zhang; supervision, Jianan Lei; each author (Jianan Lei, Zhuona Ni, Ruidi Zhang) participated sufficiently in drafting the article and revising the article for important rational content. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Glossary
| CAR | Chimeric antigen receptor |
| UCAR | Universal CAR |
| CD52 | Cluster of differentiation 52 |
| Ig | Immunoglobulin |
| TCR | T cell receptors |
| HLA | Human leukocyte antigen |
| CRISPR/Cas | Clustered regularly interspaced short palindromic repeat/Associated system |
| MSLN | Mesothelin |
| MHC | Major histocompatibility complex |
| DNT | Double-negative T |
| NK | Natural killer |
| ALL | Acute lymphoblastic leukemia |
| NHL | Non-Hodgkin lymphoma |
| ORR | Overall response rate |
| CR | Complete response |
| TAA | Tumor-associated antigens |
| B-ALL | B-cell acute lymphoblastic leukemia |
| BCMA | B-cell maturation antigen |
| LBCL | Large B cell lymphoma |
| DLBCL | Diffuse large B-cell lymphoma |
| TALEN | Transcription activator-like effector nucleases |
| ZFNs | Zinc-finger nucleases |
| PD-1 | Programmed death receptor 1 |
| LLT1 | Lectin-like transcript 1 |
| CTLA-4 | Cytotoxic T lymphocyte-associated antigen-4 |
| TRAC | T cell receptor alpha constant |
| B2M | Beta 2-microglobulin |
| HLA-DRA | Human leukocyte antigen-DR alpha |
| IL7Rα | Interleukin 7 receptor α |
| R/r | Relapsed/refractory |
| mCRC | Metastatic colorectal cancer |
| HR-NB | High-risk neuroblastoma |
| T-ALL | T-cell acute lymphoblastic leukemia |
| LBL | Lymphoblastic lymphoma |
| CLL | Chronic lymphocytic leukemia |
| CIK | Cytokine-induced killer |
| AML | Acute myeloid leukemia |
| EBV | Epstein-Barr virus |
| EBV-VSTs | Epstein-Barr virus-specific T cells |
| EBV-CTLs | EBV-specific cytotoxic T lymphocytes |
| IL-10 | Interleukin 10 |
| IL-7 | Interleukin 7 |
| IL-15 | Interleukin 15 |
| NKT | Natural killer T |
| CRS | Cytokine release syndrome |
| MBE | B2M-HLA-E |
| MBG | B2M-HLA-G |
| CLDN6 | Claudin 6 |
| TME | Immunosuppressive microenvironment |
| B2M | Beta-2 microglobulin |
| HSPCs | Hematopoietic stem/progenitor cells |
| CIITA | Class II major histocompatibility complex transactivator; |
| RFX | Regulatory factor X |
| shRNA | Short hairpin RNA |
| NK | Natural killer |
| ccRCC | Clear cell renal cell carcinoma |
| r/r LBCL | Relapsed/refractory large B cell lymphoma |
References
1. Dabas P, Danda A. Revolutionizing cancer treatment: a comprehensive review of CAR-T cell therapy. Med Oncol. 2023;40(9):275. doi:10.1007/s12032-023-02146-y. [Google Scholar] [PubMed] [CrossRef]
2. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86(24):10024–8. doi:10.1073/pnas.86.24.10024. [Google Scholar] [PubMed] [CrossRef]
3. Kiesgen S, Messinger JC, Chintala NK, Tano Z, Adusumilli PS. Comparative analysis of assays to measure CAR T-cell-mediated cytotoxicity. Nat Protoc. 2021;16(3):1331–42. doi:10.1038/s41596-020-00467-0. [Google Scholar] [PubMed] [CrossRef]
4. Qin YT, Li YP, He XW, Wang X, Li WY, Zhang YK. Biomaterials promote in vivo generation and immunotherapy of CAR-T cells. Front Immunol. 2023;14:1165576. doi:10.3389/fimmu.2023.1165576. [Google Scholar] [PubMed] [CrossRef]
5. Slaney CY, Wang P, Darcy PK, Kershaw MH. CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov. 2018;8(8):924–34. doi:10.1158/2159-8290.CD-18-0297. [Google Scholar] [PubMed] [CrossRef]
6. Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2(6):377–91. doi:10.1038/s41551-018-0235-9. [Google Scholar] [PubMed] [CrossRef]
7. Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–503. doi:10.1038/s41591-018-0201-9. [Google Scholar] [PubMed] [CrossRef]
8. Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99. doi:10.1038/s41573-019-0051-2. [Google Scholar] [PubMed] [CrossRef]
9. Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359–71. doi:10.1038/s41571-023-00754-1. [Google Scholar] [PubMed] [CrossRef]
10. Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15(12):2548–60. doi:10.7150/ijbs.34213. [Google Scholar] [PubMed] [CrossRef]
11. Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68:139–52. doi:10.1146/annurev-med-062315-120245. [Google Scholar] [PubMed] [CrossRef]
12. Chen K, Wang S, Qi D, Ma P, Fang Y, Jiang N, et al. Clinical investigations of CAR-T cell therapy for solid tumors. Front Immunol. 2022;13:896685. doi:10.3389/fimmu.2022.896685. [Google Scholar] [PubMed] [CrossRef]
13. Klampatsa A, Dimou V, Albelda SM. Mesothelin-targeted CAR-T cell therapy for solid tumors. Expert Opin Biol Ther. 2021;21(4):473–86. doi:10.1080/14712598.2021.1843628. [Google Scholar] [PubMed] [CrossRef]
14. Chen J, Hu J, Gu L, Ji F, Zhang F, Zhang M, et al. Anti-mesothelin CAR-T immunotherapy in patients with ovarian cancer. Cancer Immunol Immunother. 2023;72(2):409–25. doi:10.1007/s00262-022-03238-w. [Google Scholar] [PubMed] [CrossRef]
15. Wang Y, Zhao G, Wang S, Li N. Deleting SUV39H1 in CAR-T cells epigenetically enhances the antitumor function. MedComm. 2024;5(5):e552. doi:10.1002/mco2.552. [Google Scholar] [PubMed] [CrossRef]
16. Peng L, Sferruzza G, Yang L, Zhou L, Chen S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol Immunol. 2024;21(10):1089–108. doi:10.1038/s41423-024-01207-0. [Google Scholar] [PubMed] [CrossRef]
17. Uscanga-Palomeque AC, Chávez-Escamilla AK, Alvizo-Báez CA, Saavedra-Alonso S, Terrazas-Armendáriz LD, Tamez-Guerra RS, et al. CAR-T cell therapy: from the shop to cancer therapy. Int J Mol Sci. 2023;24(21):15688. doi:10.3390/ijms242115688. [Google Scholar] [PubMed] [CrossRef]
18. Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021;20(7):531–50. doi:10.1038/s41573-021-00189-2. [Google Scholar] [PubMed] [CrossRef]
19. Sadelain M. CD19 CAR T cells. Cell. 2017;171(7):1471. doi:10.1016/j.cell.2017.12.002. [Google Scholar] [PubMed] [CrossRef]
20. Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94(S1):S3–9. doi:10.1002/ajh.25418. [Google Scholar] [PubMed] [CrossRef]
21. Haslauer T, Greil R, Zaborsky N, Geisberger R. CAR T-cell therapy in hematological malignancies. Int J Mol Sci. 2021;22(16):8996. doi:10.3390/ijms22168996. [Google Scholar] [PubMed] [CrossRef]
22. Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20(1):49–62. doi:10.1038/s41571-022-00704-3. [Google Scholar] [PubMed] [CrossRef]
23. Wagner DL, Fritsche E, Pulsipher MA, Ahmed N, Hamieh M, Hegde M, et al. Immunogenicity of CAR T cells in cancer therapy. Nat Rev Clin Oncol. 2021;18(6):379–93. doi:10.1038/s41571-021-00476-2. [Google Scholar] [PubMed] [CrossRef]
24. Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–83. doi:10.1038/nrclinonc.2016.36. [Google Scholar] [PubMed] [CrossRef]
25. Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–88. doi:10.1016/j.ccell.2020.07.005. [Google Scholar] [PubMed] [CrossRef]
26. Fournier C, Martin F, Zitvogel L, Kroemer G, Galluzzi L, Apetoh L. Trial watch: adoptively transferred cells for anticancer immunotherapy. Oncoimmunology. 2017;6(11):e1363139. doi:10.1080/2162402X.2017.1363139. [Google Scholar] [PubMed] [CrossRef]
27. Bouchkouj N, Zimmerman M, Kasamon YL, Wang C, Dai T, Xu Z, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory follicular lymphoma. Oncologist. 2022;27(7):587–94. doi:10.1093/oncolo/oyac054. [Google Scholar] [PubMed] [CrossRef]
28. Administration USFaD. FDA approves brexucabtagene autoleucel for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. [Internet] [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-b-cell-precursor-acute-lymphoblastic. [Google Scholar]
29. Administration USFaD. FDA approves lisocabtagene maraleucel for relapsed or refractory mantle cell lymphoma. [Internet] [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-mantle-cell-lymphoma. [Google Scholar]
30. Sharma P, Kanapuru B, George B, Lin X, Xu Z, Bryan WW, et al. FDA approval summary: idecabtagene vicleucel for relapsed or refractory multiple myeloma. Clin Cancer Res. 2022;28(9):1759–64. doi:10.1158/1078-0432.CCR-21-3803. [Google Scholar] [PubMed] [CrossRef]
31. Natrajan K, Kaushal M, George B, Kanapuru B, Theoret MR. FDA approval summary: ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. Clin Cancer Res. 2024;30(14):2865–71. doi:10.1158/1078-0432.CCR-24-0378. [Google Scholar] [PubMed] [CrossRef]
32. Administration USFaD. FDA approves obecabtagene autoleucel for adults with relapsed or re-fractory B-cell precursor acute lymphoblastic leukemia. [Internet] [cited 2025 Jun 1]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-obecabtagene-autoleucel-adults-relapsed-or-refractory-b-cell-precursor-acute. [Google Scholar]
33. Watanabe N, Mo F, McKenna MK. Impact of manufacturing procedures on CAR T cell functionality. Front Immunol. 2022;13:876339. doi:10.3389/fimmu.2022.876339. [Google Scholar] [PubMed] [CrossRef]
34. Zhang X, Zhang H, Lan H, Wu J, Xiao Y. CAR-T cell therapy in multiple myeloma: current limitations and potential strategies. Front Immunol. 2023;14:1101495. doi:10.3389/fimmu.2023.1101495. [Google Scholar] [PubMed] [CrossRef]
35. Liu Y, An L, Huang R, Xiong J, Yang H, Wang X, et al. Strategies to enhance CAR-T persistence. Biomark Res. 2022;10(1):86. doi:10.1186/s40364-022-00434-9. [Google Scholar] [PubMed] [CrossRef]
36. Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8(4):539–51. doi:10.1016/j.apsb.2018.03.001. [Google Scholar] [PubMed] [CrossRef]
37. Mitra A, Barua A, Huang L, Ganguly S, Feng Q, He B. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. 2023;14:1188049. doi:10.3389/fimmu.2023.1188049. [Google Scholar] [PubMed] [CrossRef]
38. Tomasik J, Jasiński M, Basak GW. Next generations of CAR-T cells—new therapeutic opportunities in hematology? Front Immunol. 2022;13:1034707. doi:10.3389/fimmu.2022.1034707. [Google Scholar] [PubMed] [CrossRef]
39. Brudno JN, Maus MV, Hinrichs CS. CAR T cells and T-cell therapies for cancer. JAMA. 2024;332(22):1924. doi:10.1001/jama.2024.19462. [Google Scholar] [PubMed] [CrossRef]
40. Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. 2023;22(1):20. doi:10.1186/s12943-023-01723-z. [Google Scholar] [PubMed] [CrossRef]
41. Marei HE, Althani A, Afifi N, Hasan A, Caceci T, Pozzoli G, et al. Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme. Cancer Med. 2021;10(15):5019–30. doi:10.1002/cam4.4064. [Google Scholar] [PubMed] [CrossRef]
42. Monje M, Mahdi J, Majzner R, Yeom KW, Schultz LM, Richards RM, et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature. 2025;637(8046):708–15. doi:10.1038/s41586-024-08171-9. [Google Scholar] [PubMed] [CrossRef]
43. Zhang X, Zhu L, Zhang H, Chen S, Xiao Y. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front Immunol. 2022;13:927153. doi:10.3389/fimmu.2022.927153. [Google Scholar] [PubMed] [CrossRef]
44. Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for B-cell lymphoma. Curr Probl Cancer. 2022;46(1):100826. doi:10.1016/j.currproblcancer.2021.100826. [Google Scholar] [PubMed] [CrossRef]
45. Chen R, Chen L, Wang C, Zhu H, Gu L, Li Y, et al. CAR-T treatment for cancer: prospects and challenges. Front Oncol. 2023;13:1288383. doi:10.3389/fonc.2023.1288383. [Google Scholar] [PubMed] [CrossRef]
46. Wei W, Chen ZN, Wang K. CRISPR/Cas9: a powerful strategy to improve CAR-T cell persistence. Int J Mol Sci. 2023;24(15):12317. doi:10.3390/ijms241512317. [Google Scholar] [PubMed] [CrossRef]
47. Lin H, Cheng J, Mu W, Zhou J, Zhu L. Advances in universal CAR-T cell therapy. Front Immunol. 2021;12:744823. doi:10.3389/fimmu.2021.744823. [Google Scholar] [PubMed] [CrossRef]
48. Ma Y, Zhang L, Huang X. Genome modification by CRISPR/Cas9. FEBS J. 2014;281(23):5186–93. doi:10.1111/febs.13110. [Google Scholar] [PubMed] [CrossRef]
49. Wang SW, Gao C, Zheng YM, Yi L, Lu JC, Huang XY, et al. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol Cancer. 2022;21(1):57. doi:10.1186/s12943-022-01518-8. [Google Scholar] [PubMed] [CrossRef]
50. Hryhorowicz M, Lipiński D, Zeyland J, Słomski R. CRISPR/Cas9 immune system as a tool for genome engineering. Arch Immunol Ther Exp. 2017;65(3):233–40. doi:10.1007/s00005-016-0427-5. [Google Scholar] [PubMed] [CrossRef]
51. Guo C, Ma X, Gao F, Guo Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol. 2023;11:1143157. doi:10.3389/fbioe.2023.1143157. [Google Scholar] [PubMed] [CrossRef]
52. Shi L, Meng T, Zhao Z, Han J, Zhang W, Gao F, et al. CRISPR knock out CTLA-4 enhances the anti-tumor activity of cytotoxic T lymphocytes. Gene. 2017;636:36–41. doi:10.1016/j.gene.2017.09.010. [Google Scholar] [PubMed] [CrossRef]
53. Vackova J, Polakova I, Johari SD, Smahel M. CD80 expression on tumor cells alters tumor microenvironment and efficacy of cancer immunotherapy by CTLA-4 blockade. Cancers. 2021;13(8):1935. doi:10.3390/cancers13081935. [Google Scholar] [PubMed] [CrossRef]
54. Xu Y, Chen C, Guo Y, Hu S, Sun Z. Effect of CRISPR/Cas9-edited PD-1/PD-L1 on tumor immunity and immunotherapy. Front Immunol. 2022;13:848327. doi:10.3389/fimmu.2022.848327. [Google Scholar] [PubMed] [CrossRef]
55. Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. doi:10.1126/science.aba7365. [Google Scholar] [PubMed] [CrossRef]
56. Zhu S, Zuo S, Li C, You X, Jiang E, Feng X, et al. LLT1 overexpression renders allogeneic-NK resistance and facilitates the generation of enhanced universal CAR-T cells. J Exp Clin Cancer Res. 2025;44(1):25. doi:10.1186/s13046-025-03273-2. [Google Scholar] [PubMed] [CrossRef]
57. Dharani S, Cho H, Fernandez JP, Juillerat A, Valton J, Duchateau P, et al. TALEN-edited allogeneic inducible dual CAR T cells enable effective targeting of solid tumors while mitigating off-tumor toxicity. Mol Ther. 2024;32(11):3915–31. doi:10.1016/j.ymthe.2024.08.018. [Google Scholar] [PubMed] [CrossRef]
58. Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188(4):773–82. doi:10.1534/genetics.111.131433. [Google Scholar] [PubMed] [CrossRef]
59. Nagy ZA. Alloreactivity: an old puzzle revisited. Scand J Immunol. 2012;75(5):463–70. doi:10.1111/j.1365-3083.2012.02680.x. [Google Scholar] [PubMed] [CrossRef]
60. Polito VA, Cristantielli R, Weber G, Del Bufalo F, Belardinilli T, Arnone CM, et al. Universal ready-to-use immunotherapeutic approach for the treatment of cancer: expanded and activated polyclonal γδ memory T cells. Front Immunol. 2019;10:2717. doi:10.3389/fimmu.2019.02717. [Google Scholar] [PubMed] [CrossRef]
61. Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13(1):168. doi:10.1186/s13045-020-00998-9. [Google Scholar] [PubMed] [CrossRef]
62. Cassady K, Martin PJ, Zeng D. Regulation of GVHD and GVL activity via PD-L1 interaction with PD-1 and CD80. Front Immunol. 2018;9:3061. doi:10.3389/fimmu.2018.03061. [Google Scholar] [PubMed] [CrossRef]
63. Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer. 2022;21(1):78. doi:10.1186/s12943-022-01559-z. [Google Scholar] [PubMed] [CrossRef]
64. Chen X, Tan B, Xing H, Zhao X, Ping Y, Zhang Z, et al. Allogeneic CAR-T cells with of HLA-A/B and TRAC disruption exhibit promising antitumor capacity against B cell malignancies. Cancer Immunol Immunother. 2024;73(1):13. doi:10.1007/s00262-023-03586-1. [Google Scholar] [PubMed] [CrossRef]
65. Zhang Z, Zhao L, Huang T, Chen Z, Zhao Y, Liang J, et al. A self-activated and protective module enhances the preclinical performance of allogeneic anti-CD70 CAR-T cells. Front Immunol. 2025;15:1531294. doi:10.3389/fimmu.2024.1531294. [Google Scholar] [PubMed] [CrossRef]
66. Choi BD, Yu X, Castano AP, Darr H, Henderson DB, Bouffard AA, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7(1):304. doi:10.1186/s40425-019-0806-7. [Google Scholar] [PubMed] [CrossRef]
67. Li YR, Zhou Y, Yu J, Kim YJ, Li M, Lee D, et al. Generation of allogeneic CAR-NKT cells from hematopoietic stem and progenitor cells using a clinically guided culture method. Nat Biotechnol. 2025;43(3):329–44. doi:10.1038/s41587-024-02226-y. [Google Scholar] [PubMed] [CrossRef]
68. Anonymous. First allogeneic CAR T-Cell therapy impresses in relapsed/refractory lymphoma. Oncologist. 2020;25(Suppl 1):S4–5. doi:10.1634/theoncologist.2020-0570. [Google Scholar] [PubMed] [CrossRef]
69. Mailankody S, Matous JV, Liedtke M, Sidana S, Oluwole OO, Mohan M, et al. Universal updated phase 1 data highlights role of allogeneic anti-BCMA ALLO-715 therapy for relapsed/refractory multiple myeloma. Blood. 2022;140:4620–2. doi:10.1182/blood-2022-158231. [Google Scholar] [CrossRef]
70. Al-Homsi AS, Anguille S, Deeren D, Nishihori T, Meuleman N, Abdul-Hay M, et al. Immunicy-1: targeting BCMA with cyad-211 to establish proof of concept of an shRNA-based allogeneic CAR T cell therapy platform. Blood. 2021;138:2817. doi:10.1182/blood-2021-147738. [Google Scholar] [CrossRef]
71. McGuirk JP, Tam CS, Kröger N, Riedell PA, Murthy HS, Ho PJ, et al. CTX110 allogeneic CRISPR-Cas9-engineered CAR T cells in patients (pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCLresults from the phase 1 dose escalation carbon study. Blood. 2022;140(Suppl 1):10303–6. doi:10.1182/blood-2022-166432. [Google Scholar] [CrossRef]
72. Mehta A, Farooq U, Chen A, McGuirk JP, Ly T, Wong L, et al. Interim phase I clinical data of FT819-101, a study of the first-ever, off-the-shelf, iPSC-derived TCR-less CD19 CAR T-cell therapy for patients with relapsed/refractory B-cell malignancies. Blood. 2022;140(Suppl 1):4577–8. doi:10.1182/blood-2022-167194. [Google Scholar] [CrossRef]
73. Dholaria B, Kocoglu MH, Kin A, Asch AS, Ramakrishnan A, Bachier C, et al. Early safety results of P-BCMA-ALLO1, a fully allogeneic chimeric antigen receptor T-cell (CAR-Tin patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2023;142(Suppl 1):3479. doi:10.1182/blood-2023-182430. [Google Scholar] [CrossRef]
74. Abramson JS, Ramakrishnan A, Pierola AA, Braunschweig I, Cartron G, Thieblemont C, et al. Preliminary results of nathali-01: a first-in-human phase I/IIa study of UCART20 × 22, a dual allogeneic CAR-T cell product targeting CD20 and CD22, in relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL). Blood. 2023;142(Suppl 1):2110. doi:10.1182/blood-2023-186570. [Google Scholar] [CrossRef]
75. Iyer SP, Sica RA, Ho PJ, Prica A, Zain J, Foss FM, et al. Safety and activity of CTX130, a CD70-targeted allogeneic CRISPR-Cas9-engineered CAR T-cell therapy, in patients with relapsed or refractory T-cell malignancies (COBALT-LYMa single-arm, open-label, phase 1, dose-escalation study. Lancet Oncol. 2025;26(1):110–22. doi:10.1016/S1470-2045(24)00508-4. [Google Scholar] [PubMed] [CrossRef]
76. Li S, Wang X, Yuan Z, Liu L, Li Y, Liu J, et al. Abstract CT196: early results of a safety and efficacy study of allogeneic TruUCAR™ GC502 in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL). Cancer Res. 2022;82(12 Suppl):CT196. doi:10.1158/1538-7445.am2022-ct196. [Google Scholar] [CrossRef]
77. Jain N, Chevallier P, Liu H, Schiller GJ, Méar JB, DeAngelo DJ, et al. Updated results of the phase I BALLI-01 trial of UCART22 process 2 (P2an anti-CD22 allogeneic CAR-T cell product manufactured by cellectis biologics, in patients with relapsed or refractory (R/R) CD22+ B-cell acute lymphoblastic leukemia (B-ALL). Blood. 2023;142(Suppl 1):4847. doi:10.1182/blood-2023-187252. [Google Scholar] [CrossRef]
78. Hu Y, Wei G, Fu S, Xiao P, Feng J, Zhang M, et al. Intracellular retention of tcrαβ/CD3 to generate novel allogeneic CAR-T cells (ThisCART19A) with enhanced antitumor potency for treating B-ALL. Blood. 2023;142(Suppl 1):2111. doi:10.1182/blood-2023-189052. [Google Scholar] [CrossRef]
79. Benjamin R, Graham C, Yallop D, Jozwik A, Ciocarlie O, Jain N, et al. Preliminary data on safety, cellular kinetics and anti-leukemic activity of UCART19, an allogeneic anti-CD19 CAR T-cell product, in a pool of adult and pediatric patients with high-risk CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia. Blood. 2018;132(Suppl 1):896. doi:10.1182/blood-2018-99-111356. [Google Scholar] [CrossRef]
80. Jain N, Kantarjian H, Solomon SR, He F, Sauter CS, Heery CR, et al. Preliminary safety and efficacy of PBCAR0191, an allogeneic ‘off-the-shelf’ CD19-directed CAR-T for patients with relapsed/refractory (R/R) CD19+ B-ALL. Blood. 2021;138(Suppl 1):650. doi:10.1182/blood-2021-153166. [Google Scholar] [CrossRef]
81. Ghobadi A, Aldoss I, Maude SL, Bhojwani D, Wayne AS, Bajel A, et al. Phase 1/2 dose-escalation/dose-expansion study of anti-CD7 allogeneic CAR-T cells (WU-CART-007) in relapsed or refractory (R/R) T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/LBL). Blood. 2023;142(Suppl 1):770. doi:10.1182/blood-2023-178723. [Google Scholar] [CrossRef]
82. Ottaviano G, Georgiadis C, Gkazi SA, Syed F, Zhan H, Etuk A, et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med. 2022;14(668):eabq3010. doi:10.1126/scitranslmed.abq3010. [Google Scholar] [PubMed] [CrossRef]
83. Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, et al. The safety and efficacy of a CRISPR/Cas9-engineered universal CAR-T cell product (CTA101) in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Blood. 2020;136(Suppl 1):52. doi:10.1182/blood-2020-142262. [Google Scholar] [CrossRef]
84. Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32(11):995–1007. doi:10.1038/s41422-022-00721-y. [Google Scholar] [PubMed] [CrossRef]
85. Magnani CF, Gaipa G, Lussana F, Belotti D, Gritti G, Napolitano S, et al. Sleeping beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest. 2020;130(11):6021–33. doi:10.1172/jci138473. [Google Scholar] [PubMed] [CrossRef]
86. Neelapu SS, Budde LE, McGuirk JP, Dahiya S, Deol A, Thompson PA, et al. Phase 1 study of SC291, a hypoimmune, allogeneic CD19-directed CAR T cell therapy for relapsed/refractory B-cell malignancies (ARDENT)—initial clinical data. Blood. 2023;142(Suppl 1):6852. doi:10.1182/blood-2023-179441. [Google Scholar] [CrossRef]
87. Wermke M, Metzelder S, Kraus S, Sala E, Vucinic V, Fiedler W, et al. Updated results from a phase I dose escalation study of the rapidly-switchable universal CAR-T therapy UniCAR-T-CD123 in relapsed/refractory AML. Blood. 2023;142(Suppl 1):3465. doi:10.1182/blood-2023-177867. [Google Scholar] [CrossRef]
88. Shah NN, Tasian SK, Kohler ME, Hsieh EM, Baumeister SHC, Summers C, et al. CD33 CAR T-cells (CD33CART) for children and young adults with relapsed/refractory AML: dose-escalation results from a phase I/II multicenter trial. Blood. 2023;142(Suppl 1):771. doi:10.1182/blood-2023-179667. [Google Scholar] [CrossRef]
89. Chiesa R, Georgiadis C, Syed F, Zhan H, Etuk A, Gkazi SA, et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N Engl J Med. 2023;389(10):899–910. doi:10.1056/NEJMoa2300709. [Google Scholar] [PubMed] [CrossRef]
90. Zhang M, Zhang X, Wang J, Lu W, Xiao X, Lyu H, et al. Utilization of donor CLL-1 CAR-T cells for the treatment of relapsed of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation. Front Immunol. 2025;15:1491341. doi:10.3389/fimmu.2024.1491341. [Google Scholar] [PubMed] [CrossRef]
91. Shahid S, Prockop SE, Flynn GC, Mauguen A, White CO, Bieler J, et al. Allogeneic off-the-shelf CAR T-cell therapy for relapsed or refractory B-cell malignancies. Blood Adv. 2025;9(7):1644–57. doi:10.1182/bloodadvances.2024015157. [Google Scholar] [PubMed] [CrossRef]
92. Luo Y, Gao L, Liu J, Yang L, Wang L, Lai X, et al. Donor-derived Anti-CD19 CAR T cells GC007g for relapsed or refractory B-cell acute lymphoblastic leukemia after allogeneic HSCT: a phase 1 trial. eClinicalMedicine. 2023;67:102377. doi:10.1016/j.eclinm.2023.102377. [Google Scholar] [PubMed] [CrossRef]
93. Neelapu SS, Stevens DA, Hamadani M, Frank MJ, Holmes H, Jacobovits A, et al. A phase 1 study of ADI-001: anti-CD20 CAR-engineered allogeneic gamma Delta1 (γδ) T cells in adults with B-cell malignancies. Blood. 2022;140:4617–9. doi:10.1182/blood-2022-157400. [Google Scholar] [CrossRef]
94. Shahid S, Flynn GC, Mauguen A, Bieler J, Prockop SE, Scaradavou A, et al. Long term follow-up after treatment with allogeneic off-the-shelf CAR T cell therapy for relapsed or refractory B-cell malignancies. Blood. 2023;142:3476. doi:10.1182/blood-2023-180753. [Google Scholar] [CrossRef]
95. Quach DH, Ramos CA, Lulla PD, Sharma S, Ganesh HR, Nouraee N, et al. CD30.CAR-modified epstein-barr virus-specific T cells (CD30.CAR EBVSTs) provide a safe and effective off-the-shelf therapy for patients with CD30-positive lymphoma. Blood. 2022;140:412–4. doi:10.1182/blood-2022-160244. [Google Scholar] [CrossRef]
96. Cruz CRY, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–73. doi:10.1182/blood-2013-06-506741. [Google Scholar] [PubMed] [CrossRef]
97. Xiao X, Liu H, Qiu X, Chen P, Li X, Wang D, et al. CD19-CAR-DNT cells (RJMty19) in patients with relapsed or refractory large B-cell lymphoma: a phase 1, first-in-human study. eClinicalMedicine. 2024;70:102516. doi:10.1016/j.eclinm.2024.102516. [Google Scholar] [PubMed] [CrossRef]
98. Prenen H, Dekervel J, Hendlisz A, Anguille S, Awada A, Cerf E, et al. Updated data from alloSHRINK phase I first-in-human study evaluating CYAD-101, an innovative non-gene edited allogeneic CAR-T in mCRC. J Clin Oncol. 2021;39(3 suppl):74. doi:10.1200/jco.2021.39.3_suppl.74. [Google Scholar] [CrossRef]
99. Quintarelli C, Del Bufalo F, De Ioris MA, Guercio M, Algeri M, Pagliara D, et al. Donor-derived GD2-specific CAR T cells in relapsed or refractory neuroblastoma. Nat Med. 2025;31(3):849–60. doi:10.1038/s41591-024-03449-x. [Google Scholar] [PubMed] [CrossRef]
100. Pal SK, Tran B, Haanen JBAG, Hurwitz ME, Sacher A, Tannir NM, et al. CD70-targeted allogeneic CAR T-cell therapy for advanced clear cell renal cell carcinoma. Cancer Discov. 2024;14(7):1176–89. doi:10.1158/2159-8290.CD-24-0102. [Google Scholar] [PubMed] [CrossRef]
101. Grauwet K, Berger T, Kann MC, Silva H, Larson R, Leick MB, et al. Stealth transgenes enable CAR-T cells to evade host immune responses. J Immunother Cancer. 2024;12(5):e008417. doi:10.1136/jitc-2023-008417. [Google Scholar] [PubMed] [CrossRef]
102. Xie L, Gu R, Yang X, Qiu S, Xu Y, Mou J, et al. Universal anti-CD7 CAR-T cells targeting T-ALL and functional analysis of CD7 antigen on T/CAR-T cells. Hum Gene Ther. 2023;34(23–24):1257–72. doi:10.1089/hum.2023.029. [Google Scholar] [PubMed] [CrossRef]
103. Maldonado-Pérez N, Tristán-Manzano M, Justicia-Lirio P, Martínez-Planes E, Muñoz P, Pavlovic K, et al. Efficacy and safety of universal (TCRKO) ARI-0001 CAR-T cells for the treatment of B-cell lymphoma. Front Immunol. 2022;13:1011858. doi:10.3389/fimmu.2022.1011858. [Google Scholar] [PubMed] [CrossRef]
104. Mitwasi N, Arndt C, Loureiro LR, Kegler A, Fasslrinner F, Berndt N, et al. Targeting CD10 on B-cell leukemia using the universal CAR T-cell platform (UniCAR). Int J Mol Sci. 2022;23(9):4920. doi:10.3390/ijms23094920. [Google Scholar] [PubMed] [CrossRef]
105. Zhang H, Ye ZL, Yuan ZG, Luo ZQ, Jin HJ, Qian QJ. New strategies for the treatment of solid tumors with CAR-T cells. Int J Biol Sci. 2016;12(6):718–29. doi:10.7150/ijbs.14405. [Google Scholar] [PubMed] [CrossRef]
106. Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. 2017;25(9):2214–24. doi:10.1016/j.ymthe.2017.05.012. [Google Scholar] [PubMed] [CrossRef]
107. Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi:10.1038/s41598-017-00462-8. [Google Scholar] [PubMed] [CrossRef]
108. Hegde UP, Mukherji B. Current status of chimeric antigen receptor engineered T cell-based and immune checkpoint blockade-based cancer immunotherapies. Cancer Immunol Immunother. 2017;66(9):1113–21. doi:10.1007/s00262-017-2007-x. [Google Scholar] [PubMed] [CrossRef]
109. Luo C, Wei J, Han W. Spotlight on chimeric antigen receptor engineered T cell research and clinical trials in China. Sci China Life Sci. 2016;59(4):349–59. doi:10.1007/s11427-016-5034-5. [Google Scholar] [PubMed] [CrossRef]
110. Zhao Y, Chen J, Andreatta M, Feng B, Xie YQ, Wenes M, et al. IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases. Nat Biotechnol. 2024;42(11):1693–704. doi:10.1038/s41587-023-02060-8. [Google Scholar] [PubMed] [CrossRef]
111. Tang C, Zhang Y. Potential alternatives to αβ-T cells to prevent graft-versus-host disease (GvHD) in allogeneic chimeric antigen receptor (CAR)-based cancer immunotherapy: a comprehensive review. Pathol Res Pract. 2024;262:155518. doi:10.1016/j.prp.2024.155518. [Google Scholar] [PubMed] [CrossRef]
112. Farid KMN, Bug G, Schmitt A, Lang F, Schubert ML, Haberkorn U, et al. GVHD after CAR T-cell therapy post allogeneic hematopoietic cell transplantation-successfully treated by extracorporeal photopheresis. Front Immunol. 2024;15:1500177. doi:10.3389/fimmu.2024.1500177. [Google Scholar] [PubMed] [CrossRef]
113. Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood. 2016;127(10):1361–70. doi:10.1182/blood-2015-08-664250. [Google Scholar] [PubMed] [CrossRef]
114. An Y, Jin X, Zhang H, Zhang M, Mahara S, Lu W, et al. Off-the-shelf allogeneic CAR cell therapy-neglected HvG effect. Curr Treat Options Oncol. 2023;24(5):409–41. doi:10.1007/s11864-023-01061-8. [Google Scholar] [PubMed] [CrossRef]
115. Chen S, van den Brink MRM. Allogeneic “off-the-shelf” CAR T cells: challenges and advances. Best Pract Res Clin Haematol. 2024;37(3):101566. doi:10.1016/j.beha.2024.101566. [Google Scholar] [PubMed] [CrossRef]
116. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. doi:10.1016/j.bbmt.2018.12.758. [Google Scholar] [PubMed] [CrossRef]
117. Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146(5):940–8. doi:10.1016/j.jaci.2020.07.025. [Google Scholar] [PubMed] [CrossRef]
118. Lei W, Xie M, Jiang Q, Xu N, Li P, Liang A, et al. Treatment-related adverse events of chimeric antigen receptor T-cell (CAR T) in clinical trials: a systematic review and meta-analysis. Cancers. 2021;13(15):3912. doi:10.3390/cancers13153912. [Google Scholar] [PubMed] [CrossRef]
119. Wu Z, Wang Y, Jin X, Wang L. Universal CAR cell therapy: challenges and expanding applications. Transl Oncol. 2025;51:102147. doi:10.1016/j.tranon.2024.102147. [Google Scholar] [PubMed] [CrossRef]
120. Li W, Zhu X, Xu Y, Chen J, Zhang H, Yang Z, et al. Simultaneous editing of TCR, HLA-I/II and HLA-E resulted in enhanced universal CAR-T resistance to allo-rejection. Front Immunol. 2022;13:1052717. doi:10.3389/fimmu.2022.1052717. [Google Scholar] [PubMed] [CrossRef]
121. Miao L, Zhang Z, Ren Z, Li Y. Reactions related to CAR-T cell therapy. Front Immunol. 2021;12:663201. doi:10.3389/fimmu.2021.663201. [Google Scholar] [PubMed] [CrossRef]
122. Saito S, Nakazawa Y. CAR-T cell therapy in AML: recent progress and future perspectives. Int J Hematol. 2024;120(4):455–66. doi:10.1007/s12185-024-03809-w. [Google Scholar] [PubMed] [CrossRef]
123. Afzal A, Farooque U, Gillies E, Hassell L. T-cell therapy-mediated myocarditis secondary to cytokine release syndrome. Cureus. 2020;12(8):e10022. doi:10.7759/cureus.10022. [Google Scholar] [PubMed] [CrossRef]
124. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi:10.1016/j.blre.2018.11.002. [Google Scholar] [PubMed] [CrossRef]
125. Wat J, Barmettler S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions. J Allergy Clin Immunol Pract. 2022;10(2):460–6. doi:10.1016/j.jaip.2021.10.037. [Google Scholar] [PubMed] [CrossRef]
126. Kron F, Franz J, Kron A, Hallek M. Economics and management of CAR T-cell therapy: status quo and future perspectives. Internist. 2021;62(6):620–6. doi:10.1007/s00108-021-01042-9. [Google Scholar] [PubMed] [CrossRef]
127. Guo Y, Xu B, Wu Z, Bo J, Tong C, Chen D, et al. Mutant B2M-HLA-E and B2M-HLA-G fusion proteins protects universal chimeric antigen receptor-modified T cells from allogeneic NK cell-mediated lysis. Eur J Immunol. 2021;51(10):2513–21. doi:10.1002/eji.202049107. [Google Scholar] [PubMed] [CrossRef]
128. Gauthier J, Maloney DG. Allogeneic transplantation and chimeric antigen receptor-engineered T-cell therapy for relapsed or refractory mantle cell lymphoma. Hematol Oncol Clin North Am. 2020;34(5):957–70. doi:10.1016/j.hoc.2020.06.010. [Google Scholar] [PubMed] [CrossRef]
129. Nagy L, Mezősi-Csaplár M, Rebenku I, Vereb G, Szöőr Á. Universal CAR T cells targeted to HER2 with a biotin-trastuzumab soluble linker penetrate spheroids and large tumor xenografts that are inherently resistant to trastuzumab mediated ADCC. Front Immunol. 2024;15:1365172. doi:10.3389/fimmu.2024.1365172. [Google Scholar] [PubMed] [CrossRef]
130. Zhao Y, Li Y, Wang S, Han J, Lu M, Xu Y, et al. CAR-γδ T cells targeting Claudin18.2 show superior cytotoxicity against solid tumor compared to traditional CAR-αβ T cells. Cancers. 2025;17(6):998. doi:10.3390/cancers17060998. [Google Scholar] [PubMed] [CrossRef]
131. Naeem M, Hazafa A, Bano N, Ali R, Farooq M, Razak SIA, et al. Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in tumor immunotherapy. Life Sci. 2023;316:121409. doi:10.1016/j.lfs.2023.121409. [Google Scholar] [PubMed] [CrossRef]
132. Das S, Valton J, Duchateau P, Poirot L. Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy. Front Immunol. 2023;14:1172681. doi:10.3389/fimmu.2023.1172681. [Google Scholar] [PubMed] [CrossRef]
133. Wang X, Wu X, Tan B, Zhu L, Zhang Y, Lin L, et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell. 2024;187(18):4890–904.e9. doi:10.1016/j.cell.2024.06.027. [Google Scholar] [PubMed] [CrossRef]
134. Palani HK, Arunachalam AK, Yasar M, Venkatraman A, Kulkarni U, Lionel SA, et al. Decentralized manufacturing of anti CD19 CAR-T cells using CliniMACS Prodigy®: real-world experience and cost analysis in India. Bone Marrow Transplant. 2023;58(2):160–7. doi:10.1038/s41409-022-01866-5. [Google Scholar] [PubMed] [CrossRef]
135. Tommasi A, Cappabianca D, Bugel M, Gimse K, Lund-Peterson K, Shrestha H, et al. Efficient nonviral integration of large transgenes into human T cells using Cas9-CLIPT. Mol Ther Methods Clin Dev. 2025;33(1):101437. doi:10.1016/j.omtm.2025.101437. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF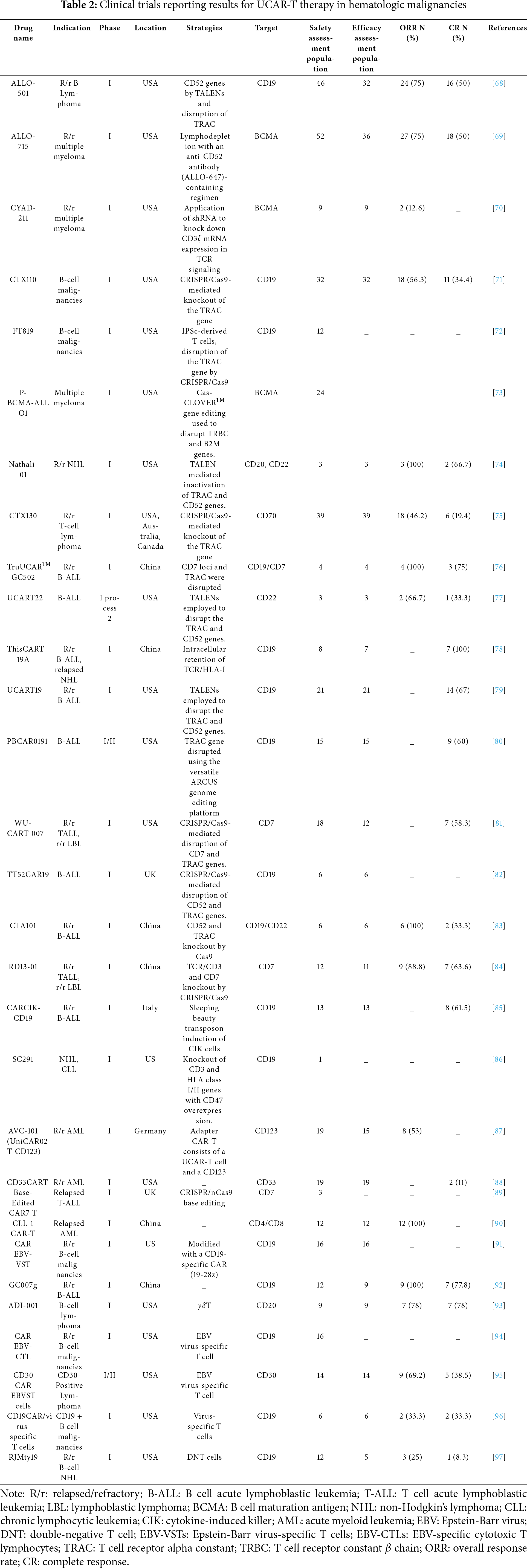
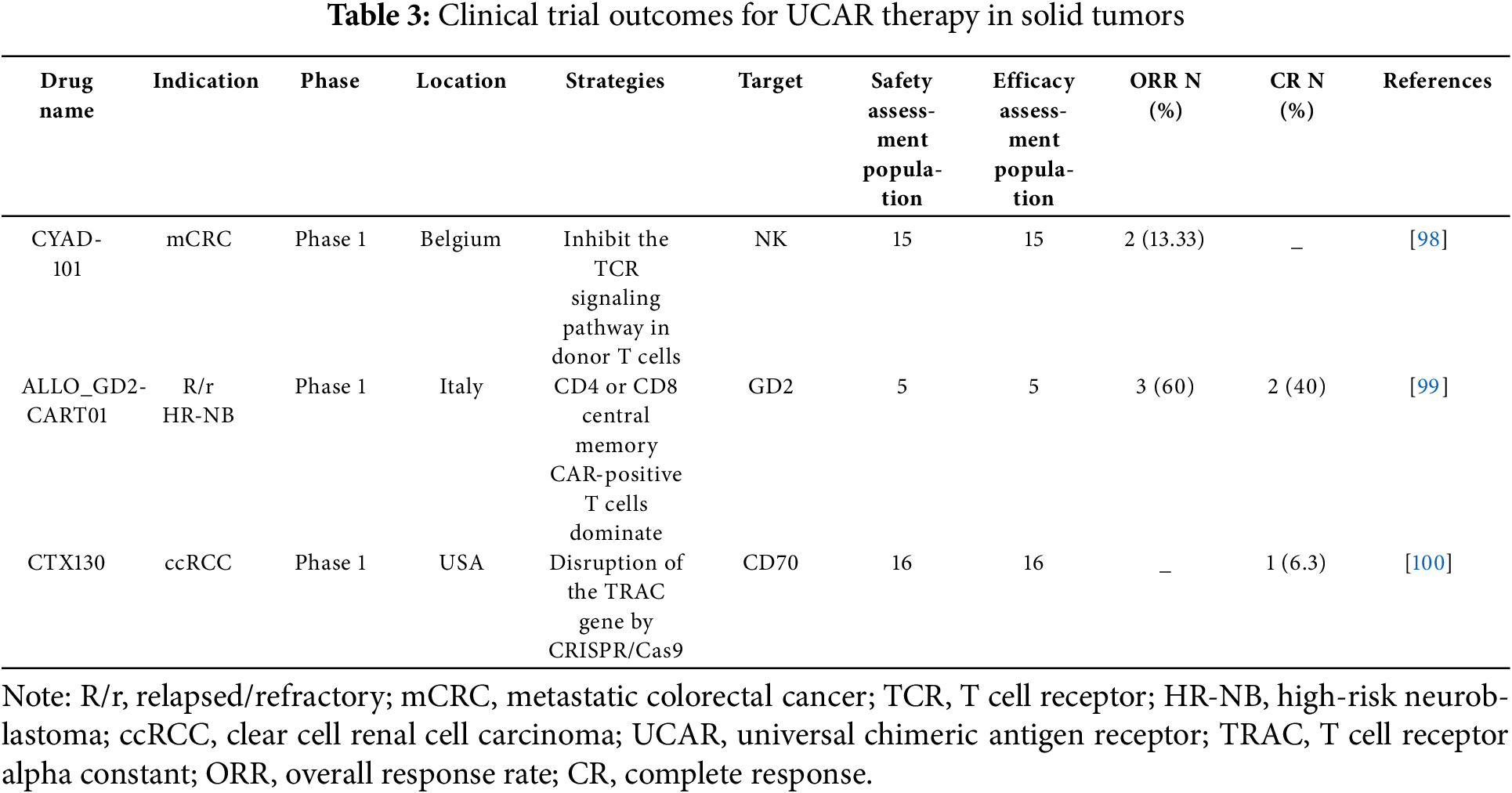
 Downloads
Downloads
 Citation Tools
Citation Tools