 Open Access
Open Access
ARTICLE
Preparation of Tartary Buckwheat Seed Coating Agent and Its Effect on Germination
1 Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, College of Food and Biological Engineering, Chengdu University, Chengdu, 610106, China
2 Agricultural Service Center, People’s Government of Yanglu Town, Yunyang County, Chongqing, 404512, China
* Corresponding Author: Dabing Xiang. Email:
Phyton-International Journal of Experimental Botany 2024, 93(4), 699-712. https://doi.org/10.32604/phyton.2024.048469
Received 08 December 2023; Accepted 12 March 2024; Issue published 29 April 2024
Abstract
To mitigate the wastage of seed resources and reduce the usage of pesticides and fertilizers, seed coating agents have gained popularity. This study employs single-factor and multi-index orthogonal experimental design methods to investigate the seed coating formula and physical properties of Tartary buckwheat. The specific effects of each component on Tartary buckwheat seed germination are analyzed. The findings reveal that the seed coating agent formulated with 1.5% polyvinyl alcohol, 0.15% sodium alginate, 0.2% op-10, 0.1% polyacrylamide, 8% colorant, 3% ammonium sulfate, 1% potassium dihydrogen phosphate, and 0.15% carbendazim exhibits the most effective coating. It demonstrates optimal physical properties and promotes seed germination efficiently. The suspension rate of this seed coating agent reaches 91.12%, with a mere 2.13% coating shedding rate and 2.5% coating seed rot rate. Furthermore, it achieves a germination percentage of 99.17%, which is 20.84% higher than the lowest group. The germination potential and index are also significantly higher than the lowest group, with an increase of 20.84% and 26.56%, respectively. Additionally, the vitality index is 553.08, a 15.75% increase compared to the lowest group. The application of seed coating agents helps reduce seed resource loss, increase plant numbers, and ultimately enhance agricultural yields. This finding holds practical significance in agricultural production.Keywords
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) is an annual herbaceous plant [1]. It is rich in lysine [2], phenols [3], flavonoids [4], and D-Chiral inositol [5]. These components can improve the body’s antioxidant system function, potentially lower blood sugar and lipids, and exhibit anti-cancer properties [6]. Due to these reasons, Tartary buckwheat holds significant development value and prospects. The cultivation technology to enhance its yield and quality has been gaining increasing attention. Tartary buckwheat has a short growth period and wide adaptability, making it a valuable crop with superior planting advantages. It has been extensively studied and shows potential for producing and commercializing related products. However, buckwheat is sensitive to abiotic stress, particularly during seedling [7]. Through years of research, we have identified the urgent issue of low seed emergence rate in Tartary buckwheat. Our germination experiments revealed a high mold rate, which directly affects the seedlings’ resistance, seedling rate, and yield.
Seed coating agent is a chemical pesticide preparation that can be used for coating crop seeds or plant seeds and has film-forming characteristics. It can directly deliver essential compounds (symbiotic microorganisms, micronutrients, growth regulators, etc.) and is filled with protective chemicals (pesticides, herbicides, fungicides, etc.), which is more conducive to seed germination and survival under unfavorable environmental conditions [8,9]. At present, the application of seed coating agents has been more common in the international market, and most of the agricultural and horticultural seeds sold in the market have been coated with seeds [10], especially the film seed coating agent, which was widely popularized and applied in the 1980s [11]. European and American countries have developed patented seed coating agent products for agricultural production. The research focus on coated crops in developed countries is different, but most focus on wheat, corn, cotton, flowers, seedlings, rice, vegetables, and other crops [12]. Coated seeds can also be stored for a long time without losing their characteristics [13], which has outstanding advantages and application value.
In order to apply the seed coating agent to the field of Tartary buckwheat more effectively, it is necessary to study the basic formula of conventional seed coating agents in detail. In this study, Xiqiao No. 1 was selected as the research material, and the germination of seeds was studied using different coating treatments to develop a conventional seed coating agent formula suitable for Tartary buckwheat.
This experiment was conducted from November 2021 to February 2022, and the Tartary buckwheat “Xiqiao No.1 (XQ1)” was provided by the Key Laboratory of Coarse Grains Processing of the Ministry of Agriculture and Rural Affairs of Chengdu University.
According to the preliminary experiment, the drug-seed ratio was set to 1:40, polyvinyl alcohol and sodium alginate were used as film-forming agents, op-10, polyacrylamide, and colorant were used as auxiliaries, and ammonium sulfate, potassium dihydrogen phosphate, and carbendazim were used as active ingredients.
The film-forming agent was screened by single-factor experimental design. The concentration of the film-forming agent (all concentrations in this paper refer to mass concentration) was set as follows: polyvinyl alcohol: 0.5%, 1%, 1.5%, 2%, and 2.5%, respectively; Sodium alginate: 0.05%, 0.1%, 0.15%, 0.2%, and 0.25%.
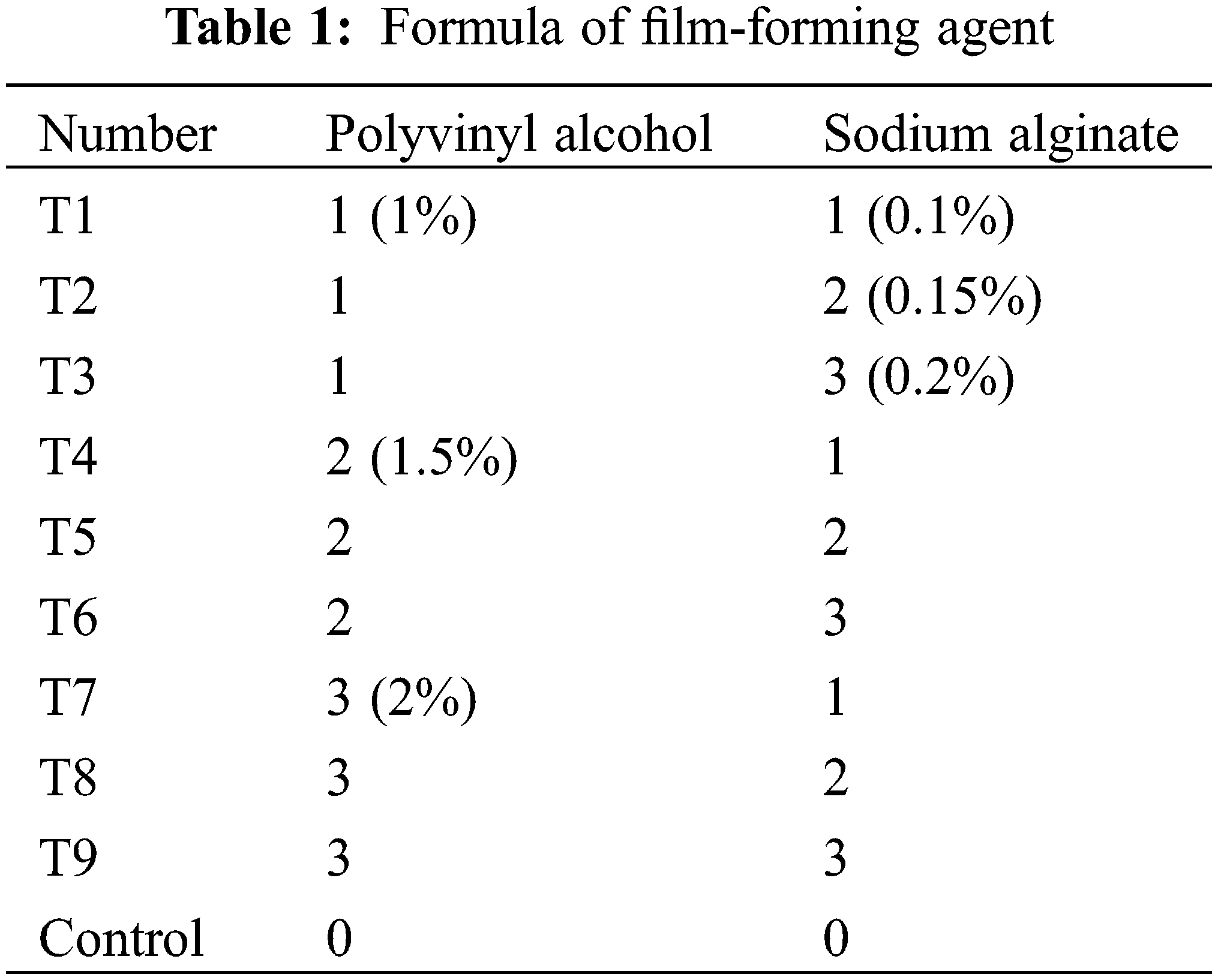
Polyvinyl alcohol (PVA) is an excellent film-forming agent. It was found that sodium alginate not only has the film-forming ability but also has the function of increasing the viscosity of solution so that the seed coating agent can better adhere to the surface of seeds. The single factor pre-experiment in the early stage showed that the film-forming ability of polyvinyl alcohol solution with 1.5%, 2%, and 2.5% concentration was qualified; 0.15%, 0.2%, and 0.25% sodium alginate solutions are qualified for film formation. Considering that if they are compounded, the concentration of the film-forming agent cannot form a film after the two film-forming agents are compounded, so the concentration range of polyvinyl alcohol is set to 1%, 1.5%, and 2%. The concentration range of sodium alginate is set to 0.1%, 0.15%, and 0.2%, and further experiments are made, as shown in Table 1.
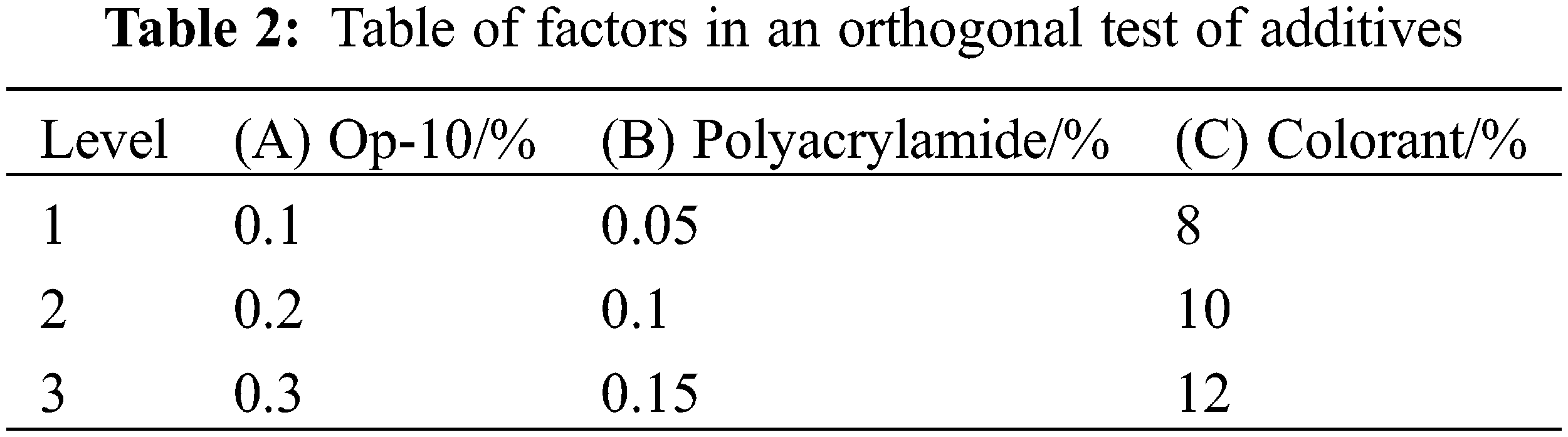
The selection of additives adopts a factor experimental design and multi-index orthogonal experimental design, and the factors and concentrations are set as OP-10: 0.1%, 0.2%, 0.3%, 0.4%, and 0.5%, respectively. Polyacrylamide: 0.05%, 0.1%, 0.15%, 0.2%, and 0.25%; Colorant: 6%, 8%, 10%, 12%, and 14%. Op-10, polyacrylamide, and colorant are set at three levels, as shown in Table 2.
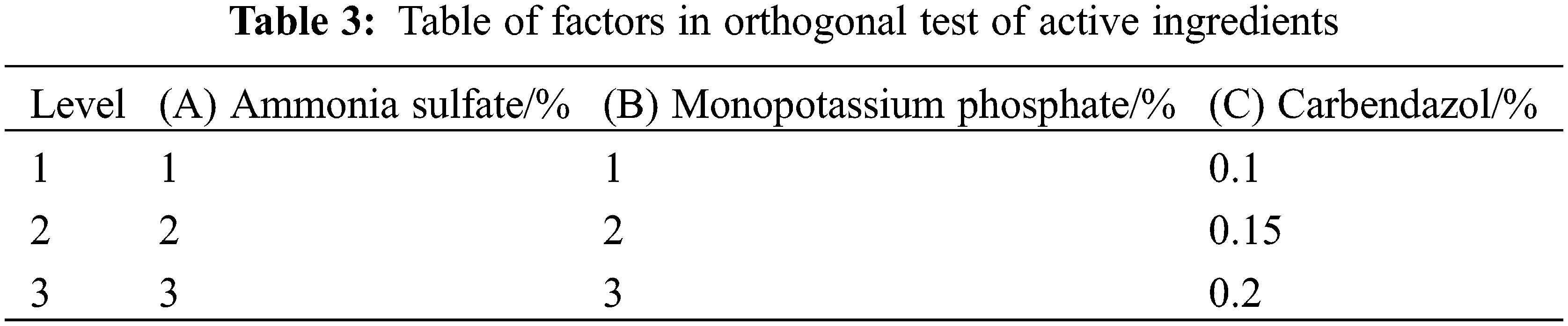
The active ingredients were screened by single-factor experimental design and multi-index orthogonal experimental design, and the factors and concentrations were set as follows: ammonium sulfate: 1%, 2%, 3%, 4% and 5%, respectively; Potassium dihydrogen phosphate: 1%, 2%, 3%, 4%, and 5%; Carbendazim: 0.05%, 0.1%, 0.15%, 0.2%, and 0.25%. There are three levels for ammonium sulfate, potassium dihydrogen phosphate, and carbendazim, as shown in Table 3.
2.3 Plant Growth and Sample Preparation
The seed germination test adopts the filter paper germination method, and two layers of filter paper are placed in a Petri dish with a diameter of 11 cm as a germination bed to ensure that the filter paper is saturated with water and the Petri dish is free of water at any time. Forty seeds were placed in each dish, and three replicates were set. The Petri dish was placed in a light incubator, and the culture conditions were set at 25°C and light for 12 h/day. Observe the seed germination at 6 pm daily and replenish the lost water.
Taking the seed implantation day as the first day, taking the hypocotyl length as the germination standard, measuring the germination potential on the fourth day, and ending the germination on the seventh day, randomly selecting three seedlings with similar growth and measuring the primary root length (cm) for each repetition, and calculating the germination percentage, germination potential, germination index, vigor index, and seed mildew rate.
According to the quality inspection method of suspension seed coating agent in GB/T 17768-1999 [14], the following physical performance indices of seed coating agent were determined: Film-forming property: after the coated seeds were laid flat for 5 min, the surface of the seeds was stirred and observed. If all seed coating agents on the surface of the seeds had solidified into films, and the seeds did not stick or agglomerate, the film-forming agent was qualified.
ma: the mass (g) of 25 mL suspension left at the bottom of measuring cylinder A evaporated to constant weight, mb: the mass (g) of 25 mL suspension left at the bottom of measuring cylinder B evaporated to constant weight.
m0: the mass of coated seeds in preparation solution A, m1: the mass of coated seeds in preparation solution B, A0: the absorbance of solution A, and A1: the absorbance of solution B.
n: number of coated seeds with absorbance a ranging from 0.7 to 1.3Aa, 20: number of coated seeds tested.
n: number of seeds germinated on the 7th day, N: total number of seeds [15].
n: the number of seeds germinated on the fourth day, N: the total number of seeds [16].
Germination index (GI) was estimated by using the formula described in the Association of Official Seed Analysts.
Gt: number of seeds germinated on day t, Dt: number of days in calculation [17].
GI: germination index, h: taproot length (cm).
n: number of seeds mildew on the 7th day, N: total number of seeds [17].
All the data were statistically sorted by Excel 2016 software and statistically analyzed by SPSS Statistics 26.0 with ANOVA analysis of variance. The data were expressed by the mean standard deviation (SD), significantly analyzed by the least significant difference method (LSD).
3.1 Screening of Film Forming Agents
As shown in Table 4, the film-forming ability of the solution is qualified for different combinations, and the compound film-forming agent has little effect on the germination percentage of Tartary buckwheat seeds. By observing the data, compared with other treatments, the germination percentage, germination potential, germination index, and vigor index of buckwheat seeds in T5 are relatively higher, which are 97.5%, 97.5%, 70.01%, and 475.36%, respectively. Considering comprehensively, the compound concentration of T5 film-forming agent was selected, that is, 1.5% polyvinyl alcohol and 0.15% sodium alginate.
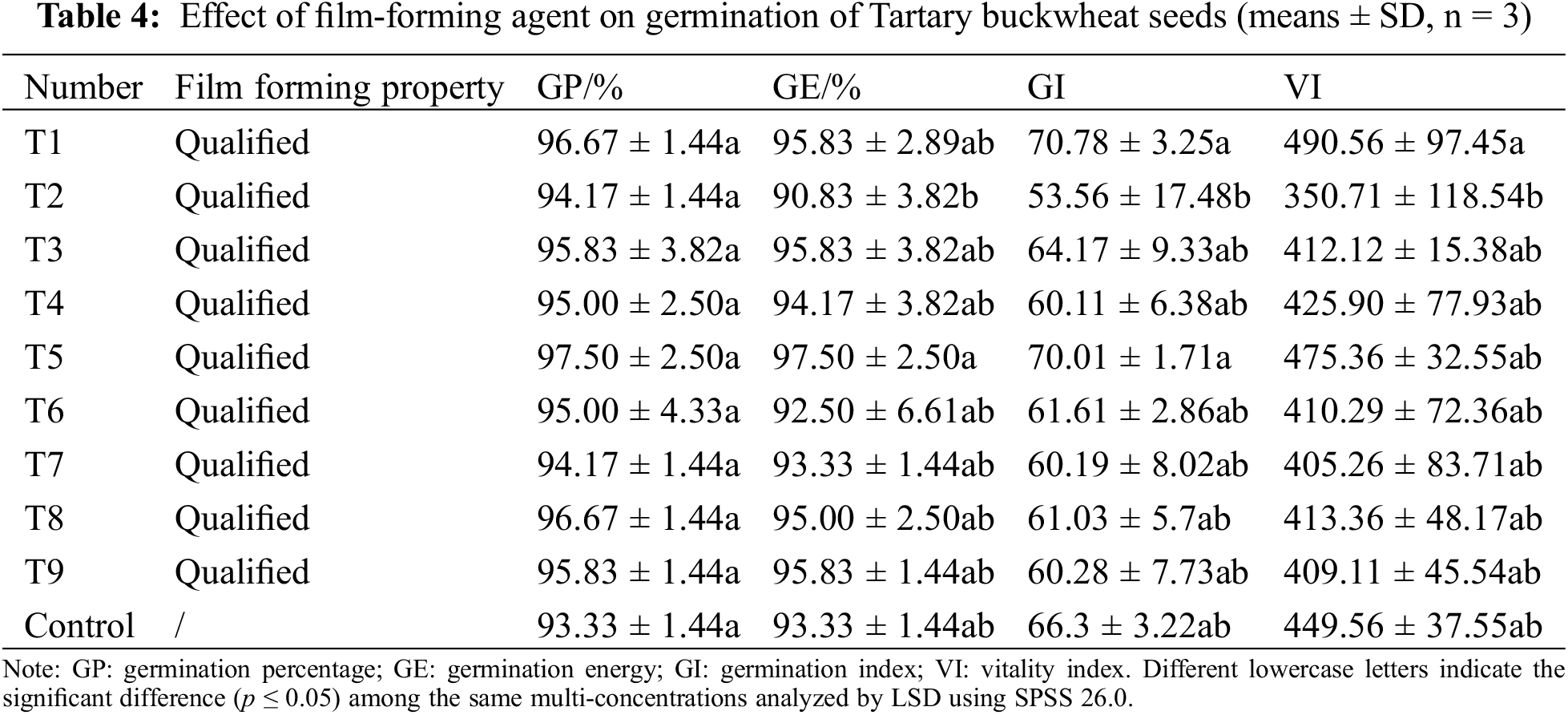
Considering the stable physical properties, environmental protection, low cost, and other aspects, the selected auxiliaries are 0p-10, polyacrylamide, and colorant, which are mixed with 1.5% polyvinyl alcohol and 0.15% sodium alginate, respectively, and the single factor test of auxiliaries is carried out.
As shown in Table 5, compared with control, 0.5% op-10 significantly reduced the germination percentage by 13.33%, and there was no significant difference under other concentrations. The appearance of the seed coating was uniform, without obvious impurities and bumps. Therefore, according to the comprehensive consideration of data and cost, 0.1%, 0.2%, and 0.3% are selected as the concentration range of op-10 in the orthogonal test. Compared with control, 0.25% polyacrylamide significantly reduced the germination percentage by 5.83%, and polyacrylamide had a uniform coating effect. According to the comprehensive consideration of data and cost, 0.05%, 0.1%, and 0.15% were selected as the concentration range of polyacrylamide in the orthogonal test. Compared with the control, different concentrations of colorant had no significant effect on the germination percentage. The coverage of 6% colorant on the seed surface was weak, and the coating was uneven. After coating it with 14% colorant, the seed surface was rough, and the coloring thickness was uneven. Therefore, the concentration range of colorant is 8%, 10%, and 12%.
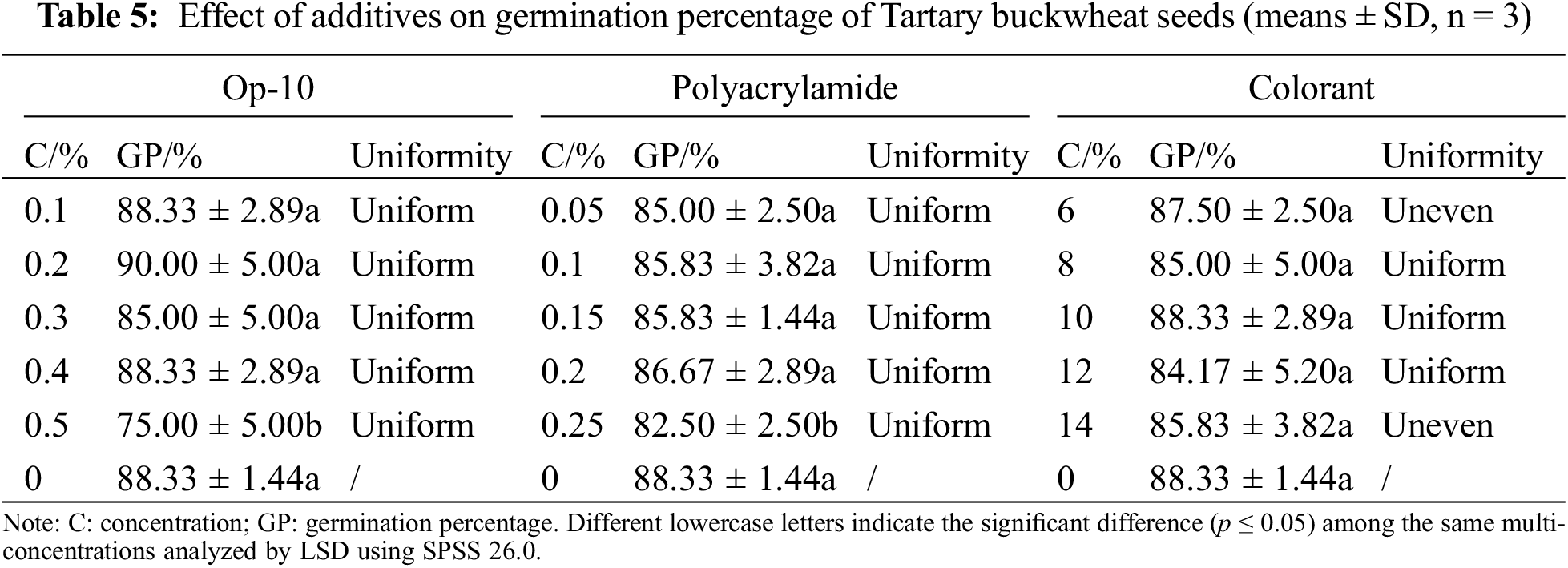
Regardless of the interaction between factors, the optimal formula of the seed coating agent was obtained by the comprehensive balance method. Orthogonal table L9(34) is selected to arrange the experiment. Table 6 shows the experimental scheme and results. It can be seen from Table 7 that the influence degree of different factors is different for different indicators, and the optimal scheme corresponding to different indicators is also different. The comprehensive balance is as follows:
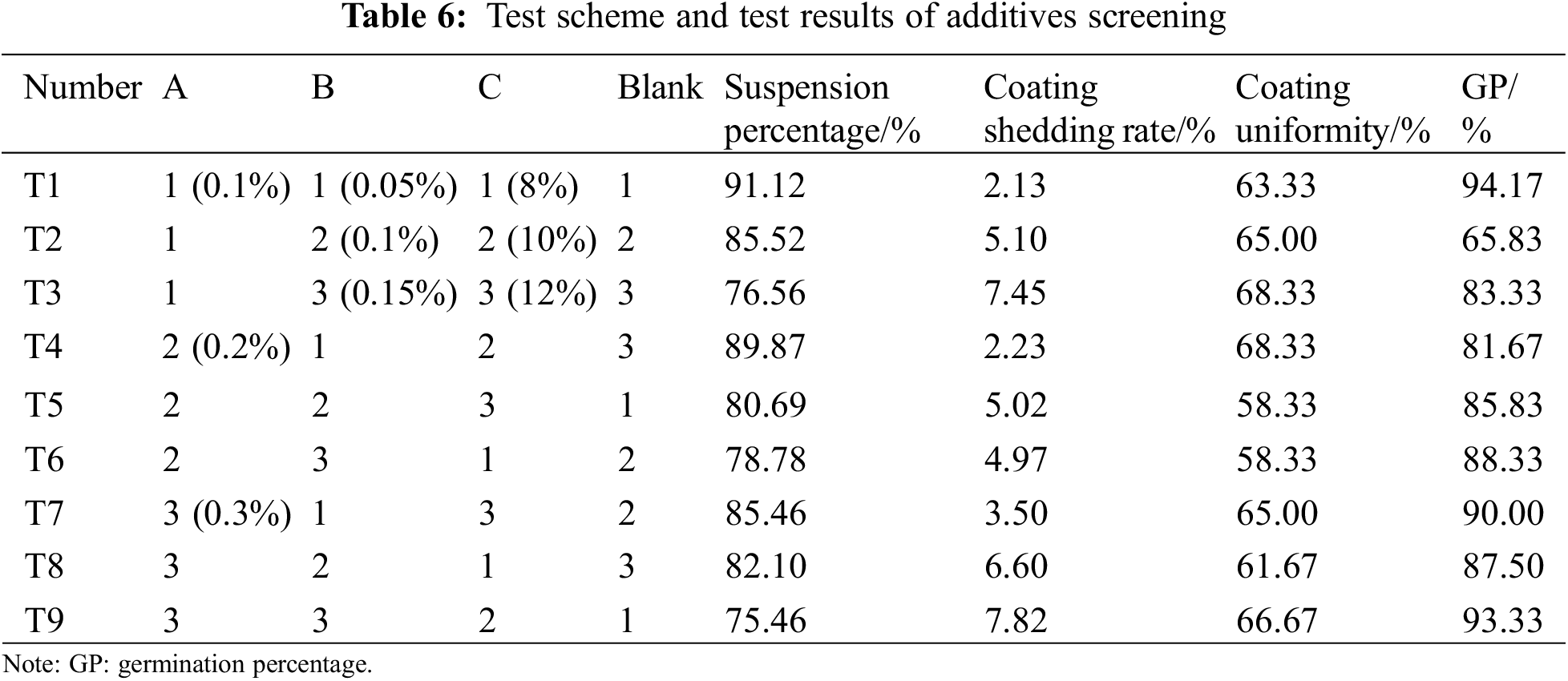
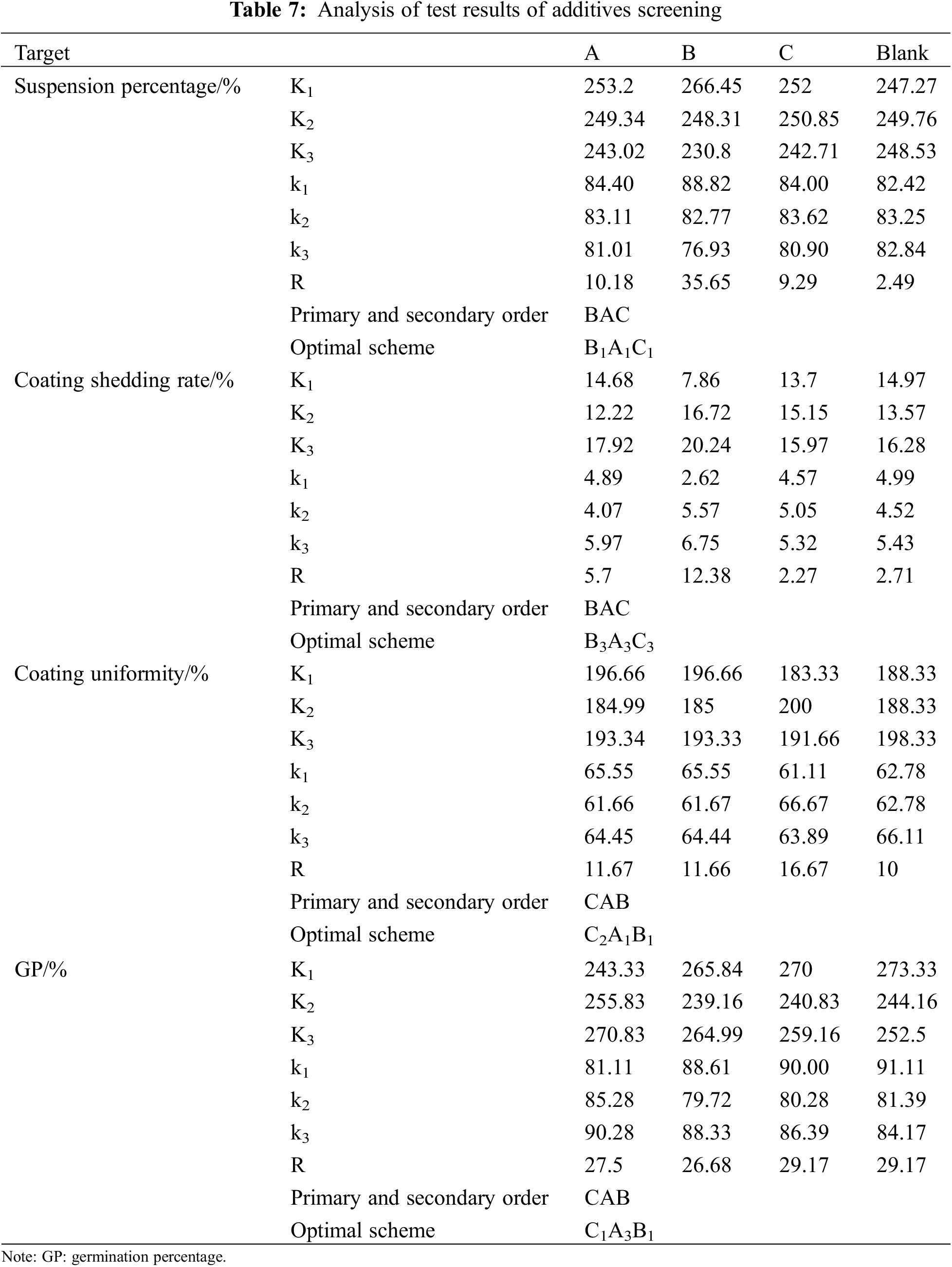
Factor A: In terms of suspension rate and coating uniformity, A1 is the best choice. In terms of coating shedding rate and germination percentage, A3 is the best choice. In addition, factor A is the second factor for these four indicators, so the influence of which level is selected on the four indicators is relatively tiny. A1 is selected to reduce solvent consumption based on the principle of reducing consumption.
Factor B: In terms of suspension rate, coating uniformity, and germination percentage, B1 is the best choice. From the suspension rate, B1 is the most crucial factor, and from Ki(ki), we know that the suspension rates of B1 and B3 are pretty different. B3 is the best choice for the coating shedding rate, and B3 is the most crucial factor. From Ki(ki), we know little difference between the coating shedding rates when B1 and B3 are selected. So choose B1.
Factor C: From the perspective of suspension rate, C1 is the best choice. From the perspective of germination percentage, C1 is also the best choice, and C1 is the most crucial factor. It is essential to consider when determining the superior level. From Ki(ki), it can be seen that the germination percentage is much higher when C1 is selected than C2. From the perspective of coating shedding rate, C3 is the best choice, the secondary factor at the bottom. From the perspective of coating uniformity, C2 is the best choice, and C2 is the most crucial factor. From Ki(ki), it can be seen that there is little difference in coating uniformity when C1, C2, and C3 are selected. So choose C1.
Based on the above analysis, the optimal scheme is A1B1C1, 0.2% op-10, 0.1% polyacrylamide and 8% colorant.
3.3 Screening of Active Ingredients
The optimal concentrations of polyvinyl alcohol, sodium alginate, op-10, polyacrylamide, and colorant were mixed with different concentrations of active ingredients, and the single-factor tests of active ingredients were carried out after coating. As shown in Table 8, compared with control, the germination percentage was significantly increased by 7.67% when the concentration of ammonium sulfate was 2%, so 1%, 2%, and 3% ammonium sulfate were selected as the concentration range of the orthogonal test. Compared with control, when the concentration of potassium dihydrogen phosphate is 2%, the germination percentage is most significantly increased by 9.17%, so 1%, 2%, and 3% are selected as the concentration range of potassium dihydrogen phosphate in the orthogonal experiment. Compared with the control, the germination percentage of Tartary buckwheat seeds was significantly improved when the concentration of carbendazim was 0.15%, 0.2%, and 0.25%, especially when it was 0.15%. The mildew rate of seeds treated with carbendazim was significantly reduced (the seeds were not disinfected in advance), and the mildew rate of seeds reached the lowest when the concentration of carbendazim was 0.2% or 0.25%. According to the germination and seed mildew rates, 0.1%, 0.15%, and 0.2% were selected as the concentration range of potassium dihydrogen phosphate in the orthogonal test.
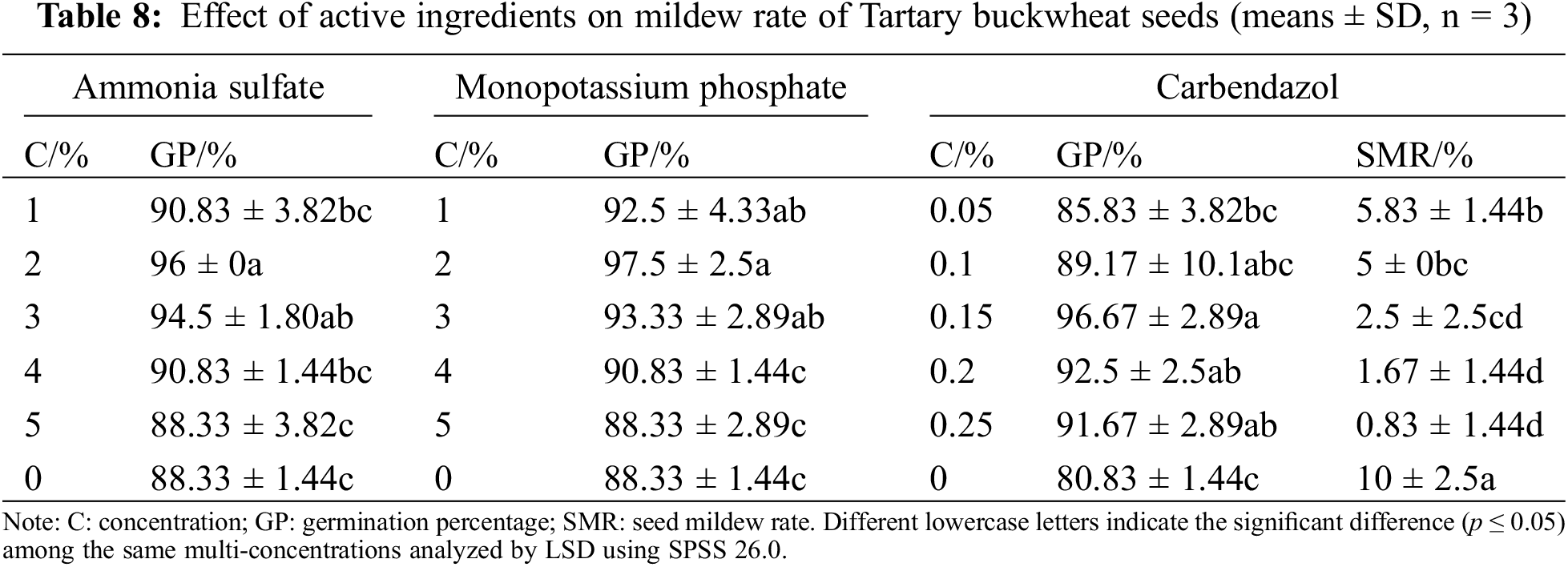
Regardless of the interaction between factors, the optimal formula of the seed coating agent was obtained by the comprehensive balance method. Orthogonal table L9(34) is selected to arrange the experiment. Table 9 shows the experimental scheme and results. As can be seen from Table 10, the influence degree of different factors is different for different indicators, and the optimal scheme corresponding to different indicators is also different. After the comprehensive balance, the specific balance is as follows:
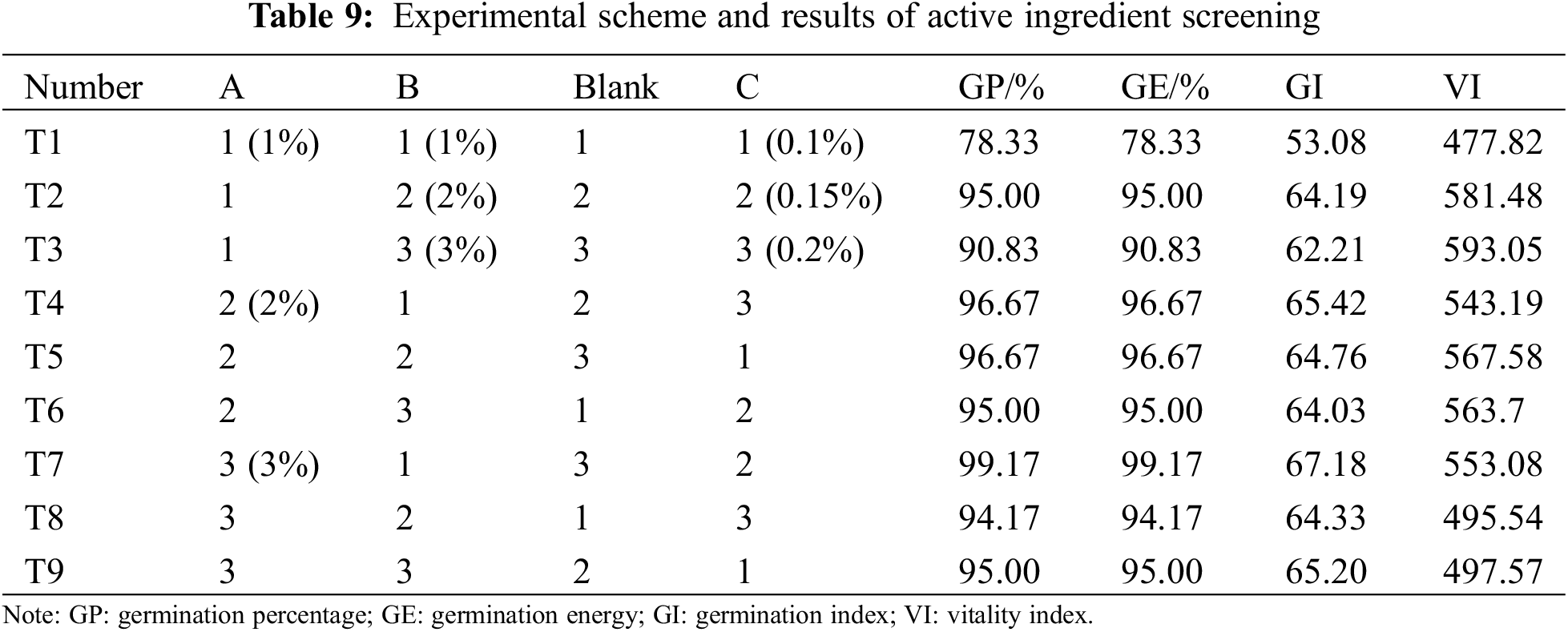
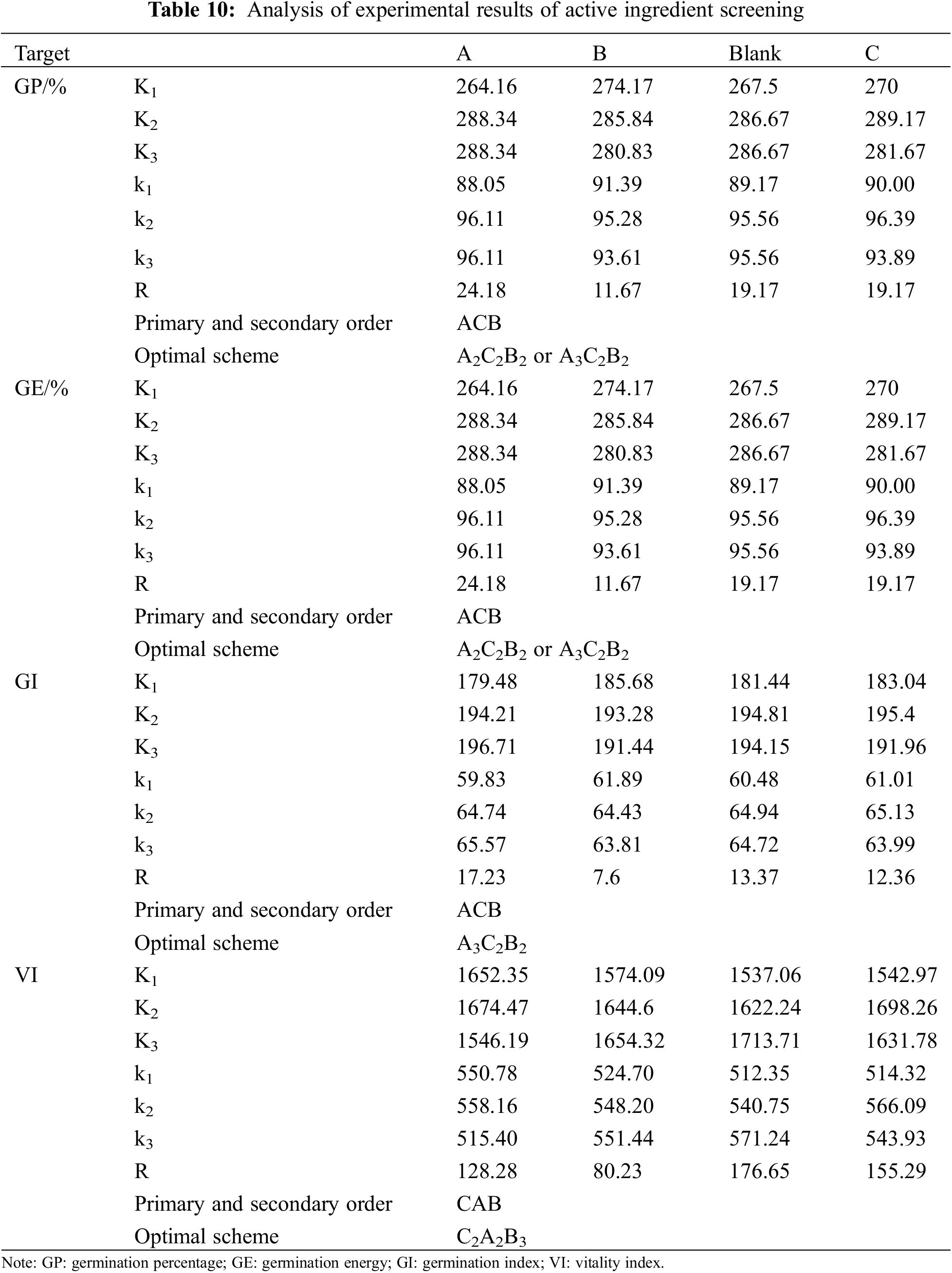
Factor A: Regarding germination percentage and potential, A2 and A3 are the best choices, and their factor A is the main factor. From the germination index, A3 is the best choice, and factor A is the main factor. Judging from the vitality index, A2 is the best choice. From Ki(ki), it can be seen that there is little difference between A2 and A3, and from the extreme range, A is the less crucial factor. Therefore, according to the majority tendency and the importance of factor A to different indicators, A3 is selected.
Factor B: In terms of germination percentage, germination potential, and germination index, B2 is the best choice, and from Ki(ki), it can be seen that there is little difference in germination percentage, germination potential, and germination index when B1, B2, and B3 are selected. Judging from the vitality index, B3 is the best choice. In addition, factor B is the last factor for these four indicators, so the influence of which level is selected on the four indicators is relatively tiny. B1 is selected to reduce solvent consumption based on the principle of reducing consumption.
Factor C: For the four indicators, the best level is C2, so C2 is selected.
Based on the above analysis, the optimal scheme is A3B1C2, namely 3% ammonium sulfate, 1% potassium dihydrogen phosphate, and 0.15% carbendazim.
To sum up, we suggest that the best formula for seed coating agent is 1.5% polyvinyl alcohol, 0.15% sodium alginate, 0.2% op-10, 0.1% polyacrylamide, 8% colorant, 3% ammonium sulfate, 1% potassium dihydrogen phosphate and 0.15% carbendazim. At this time, the germination percentage is 99.17%.
Polyvinyl alcohol (PVA) is a biodegradable synthetic polymer [18] with hydrophilic characteristics approved by the FDA, which has good film-forming, adhesion, and excellent thermal stability [19]. It is also called “green polymer” because of its solubility and biodegradability [20]. Sodium alginate is a natural polysaccharide, which can also be obtained by biosynthesis [21] and has the advantages of non-toxicity, biodegradability, and easy preparation [22]. In agricultural applications, sodium alginate is mainly used as a carrier of nutrients [23] or as a component of slow-release coating [24]. Because of their excellent stability and biodegradability, polyvinyl alcohol and sodium alginate were chosen as the preferred materials for the film-forming seed-coating agent. Finally, the optimal mass concentration of their combination was 1.5% polyvinyl alcohol and 0.15% sodium alginate. Op-10 is a stable emulsifier, which avoids flocculation of all components. This experiment found that ammonium sulfate and polyacrylamide will partially flocculate into coated spheres, so adding op-10 and designing a preparation process can make them blend well. Polymer is one of the promising materials recently applied to soil stabilization. Polyacrylamide (PAM) is one of the most commonly used superabsorbent polymers (SAP), which can stabilize the soil surface by aggregating fine soil particles, thus reducing soil erosion caused by irrigation [25]. Because of its long-term water retention capacity, PAM is usually used as an agricultural water-retaining agent [26]. Other studies have reported that PAM can strengthen nutrient retention and inhibit weed growth [27].
Seeds with low germination potential lead to low competition potential of seedlings in the field [28]. Germination percentage represents seed vigor and is an important index to evaluate seed quality [29]. Studies have pointed out that nitrogen, phosphorus, and potassium, especially nitrogen, play a vital role in plants’ physiological and biochemical metabolism [30]. In the single-factor experiment of this study, 2% ammonium sulfate has the best effect, and the seed germination percentage is 7.67% higher than that of the blank control; 2% potassium dihydrogen phosphate has the best effect, and the seed germination percentage is 9.17% higher than that of the blank control. According to reports, compared with applying fertilizer to soil or foliage, using limited nutrient elements can more effectively overcome the problem of crop nutrient deficiency and improve plant growth in poor soil [31]. Some studies show that applying potassium dihydrogen phosphate can reduce malondialdehyde’s content and reduce plants’ oxidative damage [32]. Carbendazim is a broad-spectrum fungicide used to control fungal pathogens in cereals and fruit crops [33], reducing the probability of fungal invasion of seeds during storage and germination. In the single-factor experiment of this study, carbendazim significantly improved the germination percentage of seeds, which was 15.84% higher than that of the blank control. It also significantly reduced the number of moldy seeds, and the moldy rate of seeds was at most 9.17% lower than that of the blank control, consistent with the research results obtained by the above scholars. Germination is the main characteristic that determines the maximum potential of seed quality, and it evaluates the percentage of normal seedlings under the best development conditions [34].
In good cultivation practice, seed quality is one of the main factors to be considered to establish good field seedlings and achieve good grain yield. The research results provide a technical reference for applying Tartary buckwheat in the seed coating agent industry. On this basis, some targeted functional components, such as pesticides, hormones, and beneficial fungi, are added to achieve the expected results, which expands the potential application of seed coating agents in miscellaneous grains and other fields. At the same time, further research on the subsequent growth of Tartary buckwheat-coated seeds is necessary to prove this coating agent’s practicability in agricultural production.
The optimal formulation of the Tartary buckwheat seed coating agent is 1.5% polyvinyl alcohol, 0.15% sodium alginate, 0.2% op-10, 0.1% polyacrylamide, 8% colorant, 3% ammonium sulfate, 1% potassium dihydrogen phosphate and 0.15% carbendazim. The seed coating agent has the advantages of stable performance, good film-forming performance, short film-forming time, low shedding rate, high coating uniformity, suspension rate of 91.12%, convenient use, and good promotion effect on seed germination, with the germination percentage as high as 99.17%. The combination of various components can reduce the mildew rate of seeds, thus effectively reducing the loss of resources, and has no adverse impact on the safety of Tartary buckwheat seeds, which has a good promotion prospect.
Acknowledgement: Not applicable.
Funding Statement: This work was funded by the Sichuan Science and Technology Program (2023NSFSC0214), China Agriculture Research System (CARS-07-B-1), and National Natural Sciences Foundation of China (Nos. 32301850; 31771716).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: D.B. Xiang, X. Zou; data collection: J.Y. Zhang; analysis and interpretation of results: X. Zou, T. Cheng, Y.Y. Guo, X. Han; draft manuscript preparation: H. Liu, Y.X. Qin, J. Li. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Fan Y, Jin Y, Ding M, Tang Y, Cheng J, Zhang K et al. The complete chloroplast genome sequences of eight fagopyrum species: insights into genome evolution and phylogenetic relationships. Front Plant Sci. 2021;12:3389. doi:10.3389/fpls.2021.799904. [Google Scholar] [PubMed] [CrossRef]
2. Huda MN, Lu S, Jahan T, Ding M, Jha R, Zhang K, et al. Treasure from garden: bioactive compounds of buckwheat. Food Chem. 2021;335:127653. doi:10.1016/j.foodchem.2020.127653. [Google Scholar] [PubMed] [CrossRef]
3. Peng LX, Wei LJ, Yi Q, Chen GH, Yao ZD, Yan ZY, et al. In vitro potential of flavonoids from Tartary buckwheat on antioxidants activity and starch digestibility. Int J Food Sci Tech. 2019;54:2209–18. doi:10.1111/ijfs.14131. [Google Scholar] [CrossRef]
4. Saeed F, Afzaal M, Ikram A, Imran A, Hussain S, Mohamed AA, et al. Exploring the amino acid composition and vitamin-B profile of buckwheat varieties. J Food Process Pres. 2021;45:1–8. doi:10.1111/jfpp.15743. [Google Scholar] [CrossRef]
5. Cheng FE, Ge XH, Gao CF, Li YL, Wang M. The distribution of D-chiro-inositol in buckwheat and its antioxidative effect in HepG2. J Cereal Sci. 2019;89:1–29. doi:10.1016/j.jcs.2019.102808. [Google Scholar] [CrossRef]
6. Huda MN, Lu S, Jahan T, Ding M, Jha R, Zhang K et al. Treasure from garden: bioactive compounds of buckwheat. Food Chem. 2020;335:127653. doi:10.1016/j.foodchem.2020.127653. [Google Scholar] [PubMed] [CrossRef]
7. Yao X, Zhou M, Ruan J, Peng Y, Yang H, Tang Y, et al. Pretreatment with H2O2 alleviates the negative impacts of NaCl stress on seed germination of tartary buckwheat (Fagopyrum tataricum). Plants. 2021;10(9):1784. [Google Scholar] [PubMed]
8. Pedrini S, Merritt DJ, Stevens JC, Dixon KW. Seed coating: science or marketing spin? Trends Plant Sci. 2017;22(2):106–16. doi:10.1016/j.tplants.2016.11.002. [Google Scholar] [PubMed] [CrossRef]
9. Zeng D, Fan Z, Tian X, Wang W, Zhou M, Li H. Preparation and mechanism analysis of an environment-friendly maize seed coating agent. J Sci Food Agr. 2017;98(8):2889–97. doi:10.1002/jsfa.8783. [Google Scholar] [PubMed] [CrossRef]
10. Pedrini S, Bhalsing K, Cross AT, Dixon KW. Protocol development tool (PDT) for seed encrusting and pelleting. Seed Sci Technol. 2018;46(2):393–405. doi:10.15258/sst.2018.46.2.21. [Google Scholar] [CrossRef]
11. Rocha I, Ma Y, Souza-Alonso P, Vosátka M, Freitas H, Oliveira RS. Seed coating: a tool for delivering beneficial microbes to agricultural crops. Front Plant Sci. 2019;10:1357. doi:10.3389/fpls.2019.01357. [Google Scholar] [PubMed] [CrossRef]
12. Ren XX, Chen C, Ye ZH, Su XY, Xiao JJ, Liao M, et al. Development and application of seed coating agent for the control of major soil-borne diseases infecting wheat. Agronomy. 2019;9(8):413. doi:10.3390/AGRONOMY9080413. [Google Scholar] [CrossRef]
13. de la Torre-Roche R, Cantu J, Tamez C, Zuverza-Mena N, Hamdi H, Adisa IO, et al. Seed biofortification by engineered nanomaterials: a pathway to alleviate malnutrition? J Agric Food Chem. 2020;68(44):12189–202. doi:10.1021/acs.jafc.0c04881. [Google Scholar] [PubMed] [CrossRef]
14. GB/T 17768-1999. Guidelines on drafting standards of suspension concentrates for seed dressing. Beijing: Standards Press of China; 1999 (In Chinese). [Google Scholar]
15. Tan M, Liao F, Hou L, Wang J, Wei L, Jian H, et al. Genome-wide association analysis of seed germination percentage and germination index in Brassica napus L. under salt and drought stresses. Euphytica. 2017;213:40. [Google Scholar]
16. Benedetti S, González M, Garcia EC, Quiroz IA. An analysis of the physical and germination parameters of the sweet Chestnut (Castanea sativa). Cienc Investig Agrar. 2012;39:185–92. doi:10.4067/S0718-16202012000100015. [Google Scholar] [CrossRef]
17. Wang X, Zheng H, Tang Q, Mo W, Ma J. Effects of gibberellic acid application after anthesis on seed vigor of indica hybrid rice (Oryza sativa L.). Agronomy. 2019;9:861. doi:10.3390/agronomy9120861. [Google Scholar] [CrossRef]
18. Abedi-Firoozjah R, Chabook N, Rostami O, Heydari M, Kolahdouz-Nasiri A, Javanmardi F et al. PVA/starch films: an updated review of their preparation, characterization, and diverse applications in the food industry. Polym Test. 2022;118:107903. doi:10.1016/j.polymertesting.2022.107903. [Google Scholar] [CrossRef]
19. Azahari N, Othman N, Ismail H. Biodegradation studies of polyvinyl alcohol/corn starch blend films in solid and solution media. J Phys Sci. 2011;22(2):15–31. [Google Scholar]
20. Barui A. Synthetic polymeric gel. In: Polymeric gels. The Netherlands, Amsterdam: Elsevier; 2018. [Google Scholar]
21. Szabó L, Gerber-Lemaire S, Wandrey C. Strategies to functionalize the anionic biopolymer Na-alginate without restricting its polyelectrolyte properties. Polymers. 2020;12(4):919. doi:10.3390/polym12040919. [Google Scholar] [PubMed] [CrossRef]
22. Hernández-González AC, Téllez-Jurado L, Rodríguez-Lorenzo LM. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: a review. Carbohydr Polym. 2020;229:115514. doi:10.1016/j.carbpol.2019.115514. [Google Scholar] [PubMed] [CrossRef]
23. Huang Y, Liu M, Jasmi II, Chen S, Tang Y, Lin S. Enhanced adsorption and slow release of phosphate by dolomite-alginate composite beads as potential fertilizer. Water Environ. Res. 2019;91(8):797–804. doi:10.1002/wer.1122. [Google Scholar] [PubMed] [CrossRef]
24. Peretiatko CD, Hupalo EA, Campos JR, Parabocz CR. Efficiency of zinc and calcium ion crosslinking in alginate-coated nitrogen fertilizer. Orbital Electron J Chem. 2018;10:218–25. doi:10.17807/orbital.v10i3.1103. [Google Scholar] [CrossRef]
25. Lu S, Wang Z, Hu Y, Liu B, Liu J. Effectiveness and durability of polyacrylamide (PAM) and polysaccharide (Jag C 162) in reducing soil erosion under simulated rainfalls. Water. 2018;10:257. doi:10.3390/w10030257. [Google Scholar] [CrossRef]
26. Yang K, Tang Z, Feng J. Coal fly ash and polyacrylamide influence transport and redistribution of soil nitrogen in a sandy sloping land. Agriculture. 2021;11(1):47. doi:10.3390/agriculture11010047. [Google Scholar] [CrossRef]
27. Ao C, Yang P, Zeng W, Chen W, Xu Y, Xu H, et al. Impact of raindrop diameter and polyacrylamide application on runoff, soil and nitrogen loss via raindrop splashing. Geoderma. 2019;353:372–81. doi:10.1016/j.geoderma.2019.07.026. [Google Scholar] [CrossRef]
28. França-neto JB, Krzyzanowski FC, Henning AA. Importance of using high quality soybean seeds. Available from: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/661047/a-importancia-do-uso-de-semente-de-soja-de-alta-qualidade. [Accessed 2023]. [Google Scholar]
29. Pan L, Wei H, Zhang H, Wang Y. Effects of chitosan on seed germination and seedling growth of trifolium repens under salt stress. Mol Plant Breed. 2018;16:3740–4. [Google Scholar]
30. Shilpa, Sharma AK, Chauhan M, Bijalwan P. Plant growth promoting rhizobacteria, organic manures, and chemical fertilizers: impact on crop productivity and soil health of capsicum (Capsicum annuum L.) in North Western Himalayan region. J Plant Nutr. 2023;47(3):448–67. doi:10.1080/01904167.2023.2280120. [Google Scholar] [CrossRef]
31. Meng Y, Wu K, Gong P, Zhang Z, Han M, Wei Z et al. Effects of corn stalks returning on soil microbial carbon use efficiency and corn yield in semi-arid cropland. BioResources. 2023;19(1):103–15. doi:10.15376/biores.19.1.103-115. [Google Scholar] [CrossRef]
32. M’Sehli W, Kallala N, Jaleli K, Bouallegue A, Mhadhbi H. Monopotassium phosphate (KH2PO4) and salicylic acid (SA) as seed priming in Vicia faba L. and Vicia sativa L. Biosci J. 2020;36(6):2078–91. doi:10.14393/BJ-v36n6a2020-51267. [Google Scholar] [CrossRef]
33. Zhang S, Luo T, Weng Y, Wang D, Sun L, Yu Z et al. Toxicologic effect and transcriptome analysis for sub-chronic exposure to carbendazim, prochloraz, and their combination on the liver of mice. Environ Sci Pollut R. 2023;31:5500–12. doi:10.1007/s11356-023-31412-9. [Google Scholar] [PubMed] [CrossRef]
34. Wang G, Kang Y, Li X, Zhang L, Xu G, Zheng Y. Effects of seed coating and priming with exogenous brassinosteroid on tobacco seed germination. J Plant Interact. 2024;19(1):353. doi:10.1080/17429145.2023.2299546. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools