 Open Access
Open Access
ARTICLE
Exogenous Alpha-Ketoglutarate (AKG) Modulate Physiological Characteristics, Photosynthesis, Secondary Metabolism and Antioxidant Defense System in Peganum Harmala L. under Nickel Stress
1 Department of Biology, University of Tunis El Manar, Laboratory of Vegetable Productivity and Environmental Constraints, Faculty of Sciences of Tunis (LR18ES04), University of Tunis El Manar (UTM), Tunis, 1060, Tunisia
2 State Key Laboratory of Desert and Oasis Ecology, Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, 830011, China
3 University of Chinese Academy of Sciences, Beijing, 100049, China
4 Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, 11451, Saudi Arabia
* Corresponding Author: Marwa Rezgui. Email:
# These authors contributed equally
(This article belongs to the Special Issue: Integrative Approaches to Plant Stress Responses under Changing Climate Conditions)
Phyton-International Journal of Experimental Botany 2025, 94(1), 137-155. https://doi.org/10.32604/phyton.2025.058851
Received 23 September 2024; Accepted 04 December 2024; Issue published 24 January 2025
Abstract
Nickel (Ni) toxicity significantly impairs plant growth, photosynthesis, and metabolism by inducing oxidative stress. This study evaluates the potential of exogenous Alpha-Ketoglutarate (AKG) in mitigating Ni-induced stress in Peganum harmala L. Seedlings were exposed to 0, 200, 500, and 750 μM NiCl2, with or without AKG supplementation. Under 750 μM Ni stress, dry weight (DW) decreased by 33.7%, tissue water content (TWC) by 39.9%, and chlorophyll a and total chlorophyll levels were reduced by 17% and 15%, respectively. Ni exposure also significantly increased secondary metabolite production, with leaf anthocyanin content rising by 131%, and superoxide dismutase (SOD) and catalase (CAT) activities increasing by 228% and 53%, respectively, in roots at 500 μM Ni. AKG treatment alleviated Ni toxicity by enhancing TWC by 39% and promoting root and shoot growth. Additionally, AKG treatment boosted the synthesis of phenolic compounds and flavonoids, contributing to improved tolerance against Ni stress. These findings demonstrate the potential of AKG in enhancing Ni tolerance in P. harmala, suggesting its promising role in bioremediation of metal-contaminated soils. This is the first study to report the beneficial effects of exogenous AKG in alleviating nickel toxicity in P. harmala L., offering a new approach for improving plant resilience to heavy metal stress.Keywords
Heavy metals pose a significant challenge to the health of crops, animals, and humans. However, trace amounts of these metals can be beneficial, enhancing plant growth and productivity [1]. For instance, nickel (Ni) plays a crucial role in plant metabolism, but it becomes harmful when present in excessive amounts [2]. High concentrations of nickel can disrupt various enzymatic activities vital for plant metabolism, and a deficiency of this metal can impede urease activity, leading to symptoms like leaf tip necrosis and accelerated plant aging [3,4]. Previous studies have shown that elevated levels of nickel can cause phytotoxicity, evident through a reduction in relative water content and impairment of photosynthetic pigments, enzymatic functions, and osmolyte balance [5–7]. Additionally, excessive nickel can stifle plant growth, leading to shorter shoots and roots [8], and can adversely affect seed germination and seedling development [9], particularly in crops like rice [10].
Alpha-ketoglutarate (AKG), also known as 2-ketoglutarate, 2-oxoglutarate, or oxoglutarate, is a critical intermediate in the tricarboxylic acid (TCA) cycle and plays an essential role in amino acid and protein metabolism [11,12]. Emerging evidence suggests that the external application of AKG can enhance ammonia utilization, boost carbon and nitrogen metabolism, and increase crop yields [13,14]. Furthermore, AKG has been shown to bolster plant resistance to stress by reducing oxidative stress markers and enhancing antioxidant defenses, thereby promoting improved growth and adaptability under various environmental stresses [15,16]. Various studies have indicated that abiotic stresses, such as extreme temperatures, heavy metal exposure, salinity, can affect AKG levels in plants [17–19]. For instance, Zhu et al. [20] demonstrated that potassium deficiency under drought stress alters metabolic pathways in rapeseed, including significant changes in alpha-ketoglutarate (AKG) levels. These findings emphasize the role of AKG as a key metabolic intermediate involved in plant stress responses, aligning with its broader role in mitigating the adverse effects of abiotic stresses. Additionally, providing organic carbon sources like AKG can improve plant growth, functioning as a crucial growth regulator and a potential foliar fertilizer [13]. For example, research by Liu et al. [12] has shown that AKG, in conjunction with zinc (Zn), regulates plant growth, gas exchange properties, and chlorophyll fluorescence in rice. Other studies, such as that by Du et al. [21] have reported that exogenous AKG application enhances the 2-acetyl-1-pyrroline content in grains, thereby improving the quality of fragrant rice. Similarly, AKG has been shown to alleviate the negative effects of drought and low nitrogen stress in wheat [22].
Peganum harmala L., commonly referred to as “Harmel” in North Africa, is a glabrous plant belonging to the Zygophyllaceae family. Native to Central Asia and the Mediterranean region, this plant is notable for its ethnobotanical and medicinal significance. Rashid et al. [23] describe its traditional use and therapeutic potential, highlighting its alkaloid composition and bioactive properties. Their work also confirms the plant’s distribution across these regions, aligning with its classification as a native species of the Mediterranean basin and Central Asia., Peganum harmala L., widely used in traditional medicine, has been employed to treat various ailments, including hemorrhoids, eczema, malaria fever, respiratory and nervous system disorders, and infected skin areas. Jaradat et al. [24] comprehensively reviewed the pharmacological activities of Peganum harmala extracts, emphasizing its bioactive potential and ethnopharmacological applications. Their study highlights the plant’s historical and current significance in addressing a broad spectrum of medical conditions, aligning with its prominent role in traditional medicine across various cultures.
P. harmala is rich in bioactive compounds such as flavonoids, phenolics, alkaloids, fatty acids, triterpenoids, anthraquinones, and essential oils. However, the content of these antioxidant products can vary based on genetic and environmental factors. Although P. harmala is found in both contaminated and non-contaminated soils, studies on its response to abiotic stresses, particularly heavy metal stress, are limited. Previous research has largely focused on the chemistry, biological, and pharmacological activities of this species, with little attention given to its antioxidative response under heavy metal stress or its potential for bioremediation. Recently, El Hasnaoui et al. [25] investigated the effects of lead (Pb) and zinc (Zn) stress on P. harmala, concluding that the species shows promise for phytostabilization strategies. To date, no study has examined the response of P. harmala L. to nickel stress. Therefore, the current study aims to explore, for the first time, the effects of nickel stress on the growth, secondary metabolite synthesis, and antioxidative system response of P. harmala. This investigation is crucial for better utilizing this underexplored species and for rehabilitating polluted areas. Given AKG’s widespread use in industry and its under-researched effects on plant biochemistry and physiology, particularly in mitigating heavy metal toxicity, this study hypothesizes that exogenous AKG can enhance the nickel tolerance of P. harmala L. and modulate its antioxidant activity.
2.1 Plant Material and Growth Conditions
The seeds of Peganum harmala L. were collected from the locality of Kasserine in in the west-central Tunisia. (latitude 35°10′00″ (N), longitude 8°50′00″ (E), altitude 656 m a.s.l.). The climate of the sampling site is mild and continental with hot and dry summers, while it is cold and humid in winter. It is also characterized by an average annual precipitation of 239 mm and a mean temperature of 18.1°C. The plant material was authenticated by Professor Chiraz Chaffei Haouari (Director of Laboratory Plant Productivity & Environmental Constraints, Faculty of Sciences of Tunisia). The seeds were first sterilized using a 10% (v/v) hydrogen peroxide solution for 20 min, followed by thorough rinsing with double distilled water (DDW). The sterilized seeds were then wrapped in muslin cloth, placed in Petri dishes, and incubated in darkness at 25°C to promote sprouting. After 7 days, the sprouted seedlings were transferred to sterilized sand for further growth, and subsequently moved to a hydroponic system. The seedlings were provided with half-strength Hoagland’s nutrient solution [26] either daily or as needed. The growth chamber was maintained at 26°C with 70% relative humidity during the day and 20°C with 90% relative humidity at night. A 16-h photoperiod was implemented with a light intensity of 150 μmol m−2·s−1 at the canopy level.
2.2 Nickel (Ni) and Alpha-Ketoglutarate (AKG) Treatments
After 30 days of growth, the seedlings were subjected to three different concentrations of NiCl2·6H2O (200, 500, and 750 μM) alongside an untreated control group that received only the half-strength Hoagland’s nutrient solution. The range of nickel concentrations (200, 500 and 750 μM) was chosen based on existingstudies on the tolerance of heavy metals in plants. Moderate to high levels of nickel, such as those chosen here, are known to induce measurable physiological and biochemical responses to stress, allowing us to observe the potential attenuating effects of AKG under realistic stress conditions. Similar studies have shown that concentrations above 200 μM effectively induce oxidative stress and growth inhibition in plants such as Brassica napus without causing acute toxicity that can lead to rapid death of the plants [27]; Thus, the chosen concentration range allows a thorough analysis of the response mechanisms of Peganum harmala to nickel stress and the possible modulatory effect of AKG. Each NiCl2 concentration was tested both with and without the addition of 1 mM Alpha-Ketoglutarate (AKG). The experimental setup included combinations where plants were divided into leaves and roots for further analysis. The samples were either snap-frozen in liquid nitrogen for later examination or dried at 70°C for a minimum of three days to prepare for additional analysis.
2.3 Plant Growth, Biomass, and Tissue Water Content (TWC)
Harvested seedlings were carefully washed with deionized water, and any residual surface water was removed using absorbent paper. The seedlings were then separated into leaves and roots, after which the fresh weight (FW) was immediately measured. The samples were then oven-dried at 65°C for seven days to obtain a constant mass and determine the dry weight (DW). Tissue water content (TWC), expressed as ml H2O per gram of DW, was calculated using the following equation:
2.4 Chlorophyll Content Measurement
Fresh leaves were weighed and ground in 80% acetone. The resulting mixture was centrifuged for 5 min at 3000 rpm. The chlorophyll content in the supernatant was then estimated following the method described by Arnon [28].
2.5 Determination of Anthocyanin Content
Anthocyanin content was measured based on the spectrophotometric method developed by Gould et al. [29], which quantifies anthocyanins. This method is well-suited for plant tissue analysis and provides reliable measurements of anthocyanin concentrations, a critical indicator of stress response in plants. Its application in our study allowed us to assess the role of anthocyanins in mitigating nickel stress effects and enhancing antioxidant defense. A sample of 200 mg of fresh tissue was macerated in a 2 mL solvent mixture (HCl/H2O/MeOH in a 1/3/16 ratio). The extraction was conducted in darkness at 4°C for 72 h. The absorbance of the supernatant was recorded at 530 and 653 nm to determine the anthocyanin concentration.
2.6 Extraction and Quantification of Total Phenolic Content (TPC)
The extraction process involved mixing 1 g of each sample with 10 mL of 100% methanol and allowing it to stand at room temperature for 24 h. The dry material was extracted three times, with the extracts being combined and filtered using Whatman filter paper No. 4. The solvent was then removed through vacuum distillation. The resulting extract was stirred in darkness for 24 h using a shaker, filtered with ashless filter paper, and stored at 4°C for subsequent analysis. The TPC was quantified colorimetrically as outlined by Dewanto et al. [30] with absorbance measured at 760 nm.
2.7 Estimation of Total Flavonoid Content (TFC)
TFC was assessed using a colorimetric assay, also described by Dewanto et al. [30]. An aliquot of the diluted sample or a standard solution of (+)-catechin was mixed with 75 μL of sodium nitrite solution (NaNO2) and allowed to react for 6 min before adding 0.15 mL of aluminum chloride hexahydrate solution (AlCl3·6H2O). After 5 min, 0.5 mL of sodium hydroxide (NaOH, 1 M) was added, and the final volume was adjusted to 2.5 mL with distilled water. Absorbance was recorded at 510 nm against a blank.
2.8 Antioxidative Enzyme Assay
Both leaves and roots of P. harmala L. (from control and treated plants) were homogenized in liquid nitrogen using a mortar. The resulting powder was mixed with 50 mM potassium phosphate buffer (pH 7), 1 mM EDTA, and 5% (v/v) polyvinylpolypyrrolidone (PVP) to prepare extracts for superoxide dismutase (SOD) [31] and catalase (CAT) assays. For ascorbate peroxidase (APX), 5 mM ascorbate was included in the extraction buffer. Crude extracts were centrifuged at 14,000 g for 30 min at 4°C, and the supernatants were used for enzyme assays. SOD activity was determined by measuring absorbance at 340 nm [31], CAT activity by tracking the decomposition of H2O2 at 240 nm [32], APX activity by following the oxidation of ascorbate at 290 nm [33], and guaiacol peroxidase (GPX) activity was measured using the spectrophotometric method described by Urbanek et al. [34]. This method monitors the oxidation of guaiacol to tetraguaiacol, resulting in a colored product with an absorbance peak at 470 nm. This well-established technique was chosen for its reliability in quantifying peroxidase activity, particularly in studies of plant responses to biotic and abiotic stresses, aligning with the objectives of our investigation into the effects of nickel stress on Peganum harmala L.
Results are presented as the mean ± standard error (S.E.) from at least three independent replicates. Results were examined statistically using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. The differences between individual means were considered to be significant at p < 0.05.
3.1 AKG Significantly Alleviates Nickel Toxicity in P. harmala Growth
This study investigated the effects of Alpha-Ketoglutarate (AKG) on the growth characteristics of P. harmala under nickel (Ni) stress, and the results are depicted in Fig. 1. A dose-dependent reduction in fresh weight (FW) and dry weight (DW) was observed in Ni-treated plants, indicating a significant negative impact on growth. Nickel stress resulted in a marked reduction in growth across all examined parameters, affecting both leaves and roots. At increasing concentrations of NiCl2 (200, 500, and 750 µM), a delay in the development of the fourth foliar stage was noted, along with symptoms of chlorosis and wilting. Specifically, exposure to 750 µM NiCl2 led to a significant reduction in DW by 26.88% in leaves and 33.70% in roots compared to control plants, as shown in Fig. 2. The inhibitory effects of nickel on plant growth and biomass are consistent with previous reports [35], where high metal concentrations were found to suppress cell division, prolong the cell cycle, slow down growth, reduce metabolism, and inhibit the absorption of essential nutrients [36].

Figure 1: Effect of AKG application on the growth of P. harmala subjected to different concentrations of NiCl2 (0, 200, 500, 100, and 750 mM)
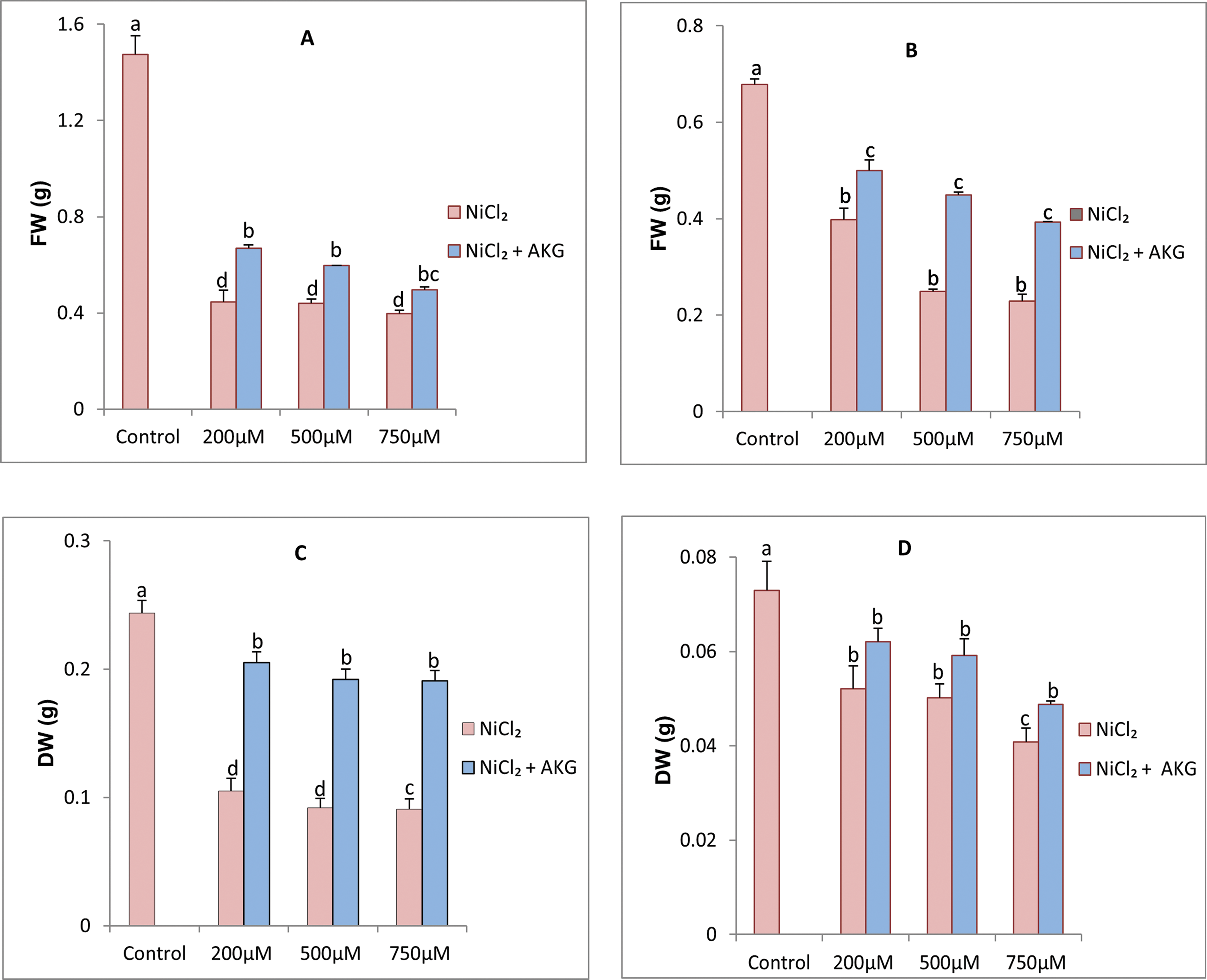
Figure 2: Effect of Nickel stress at different concentrations (0, 200, 500, 750 µM NiCl2) and AKG on (A) leaves fresh weight, (B) Roots fresh weight, (C) Roots dry weight, (D) Leaves fresh weight of P.harmala L. Values are the means ± SD (n = 3). Letters indicate that values are significantly different at p < 0.05
Conversely, the application of exogenous AKG significantly mitigated the negative effects of Ni-induced stress, leading to substantial improvements in all growth parameters of P. harmala seedlings. As presented in Fig. 2, FW in plants treated with 1 mM AKG combined with 500 µM NiCl2 was nearly double that of plants exposed to 500 µM NiCl2 without AKG.
The beneficial effects of AKG have been demonstrated in various crops. For instance, Zhang et al. [37] showed that foliar application of AKG improved maize growth under short-term drought stress, and a similar increase in soybean FW under cold stress following AKG application has been reported [38]. Additionally, AKG priming in tomato seedlings has been shown to alleviate arsenic toxicity [39]. Notably, the roots of P. harmala exhibited a concentration-dependent response under nickel stress combined with AKG treatment, as shown in Fig. 3.
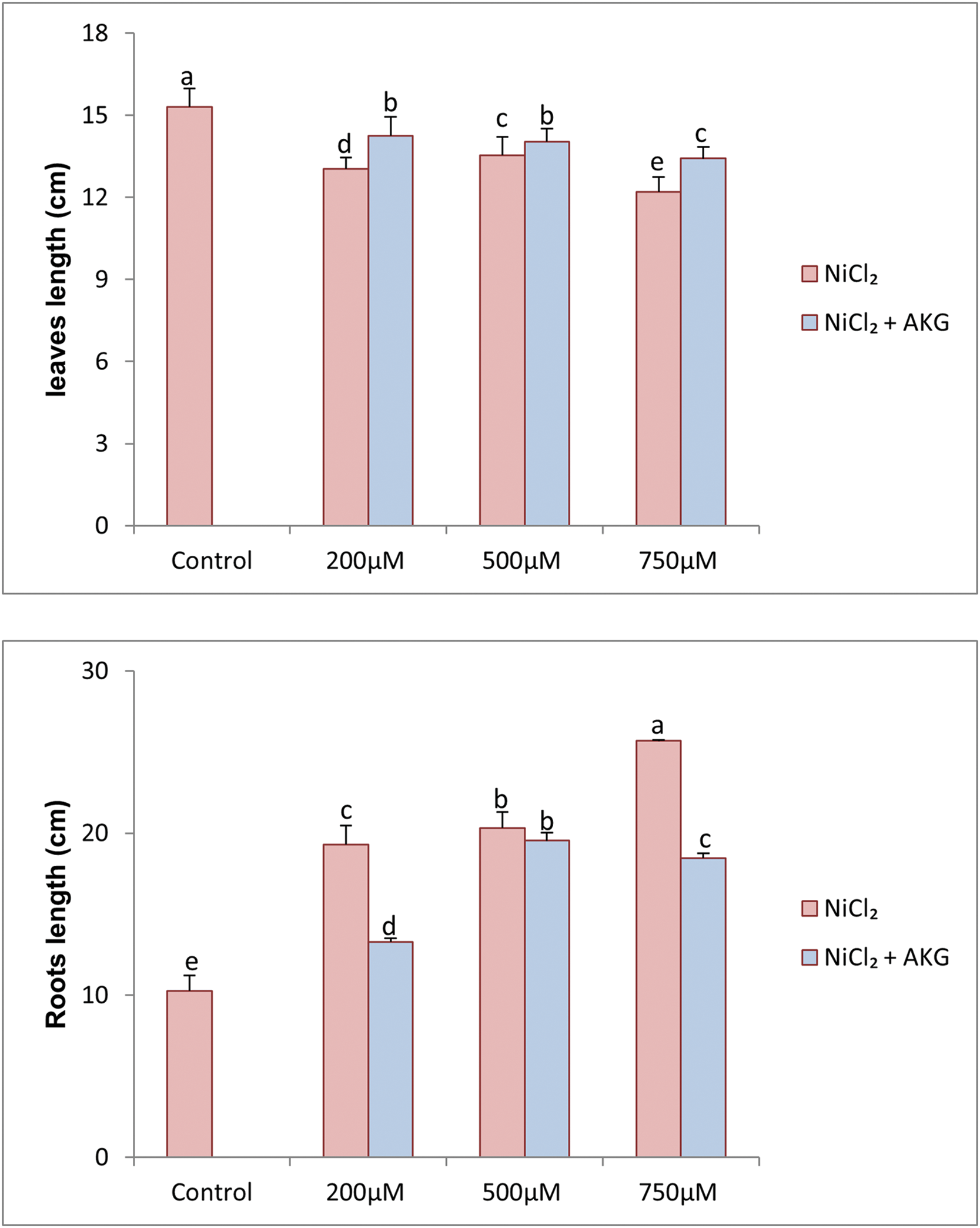
Figure 3: Effect of Nickel stress at different concentrations (0, 200, 500, 750 µM NiCl2) and AKG on on leaves and roots length of P. harmala L. Values are the means ± SD (n = 3). Letters indicate that values are significantly different at p < 0.05
Nickel exposure significantly enhanced root length, with increases of approximately 1.88 to 2.5-fold in plants subjected to 200, 500, and 750 µM NiCl2, respectively. AKG treatment further reduced the detrimental effects of nickel, resulting in increased root length by 1.2 to 1.22 cm compared to plants treated with NiCl2 alone. These morphometric and morphological changes in roots are consistent with indicators used for evaluating Ni toxicity.
In the case of leaves, Ni stress led to a reduction in leaf length compared to control plants. However, AKG supplementation effectively promoted growth, with untreated plants showing the highest leaf length (15.3 cm), followed by those treated with AKG + 200, AKG + 500, and AKG + 750 µM NiCl2, with values ranging from 14.25 to 13.42 cm. These findings align with previous studies demonstrating the growth-promoting effects of exogenous AKG in various crops [15,16,40].
3.2 AKG Mitigates the Negative Impact of Nickel on Tissue Water Content (TWC) in P. harmala
Tissue water content (TWC) is a reliable measure of plant water status, and the results in Fig. 4 indicate that exposure to Ni significantly reduced the relative water content in P. harmala roots, particularly at 500 and 750 µM NiCl2, where TWC was reduced by approximately 2.09 and 1.8-fold, respectively, compared to the control. In leaves, TWC decreased by up to 39.93% at 750 µM Ni. Nickel toxicity is known to alter water status in plant tissues by decreasing their water content, a phenomenon attributed to changes in plasma membrane permeability, including water uptake. However, the application of exogenous AKG notably improved TWC in roots by 5.77%, 39.84%, and 34.67% at 200, 500, and 750 µM NiCl2, respectively (Fig. 4). In a similar context, Hadi et al. [41] concluded that plant growth regulators enhance root strength, leading to increased nutrient and water uptake.
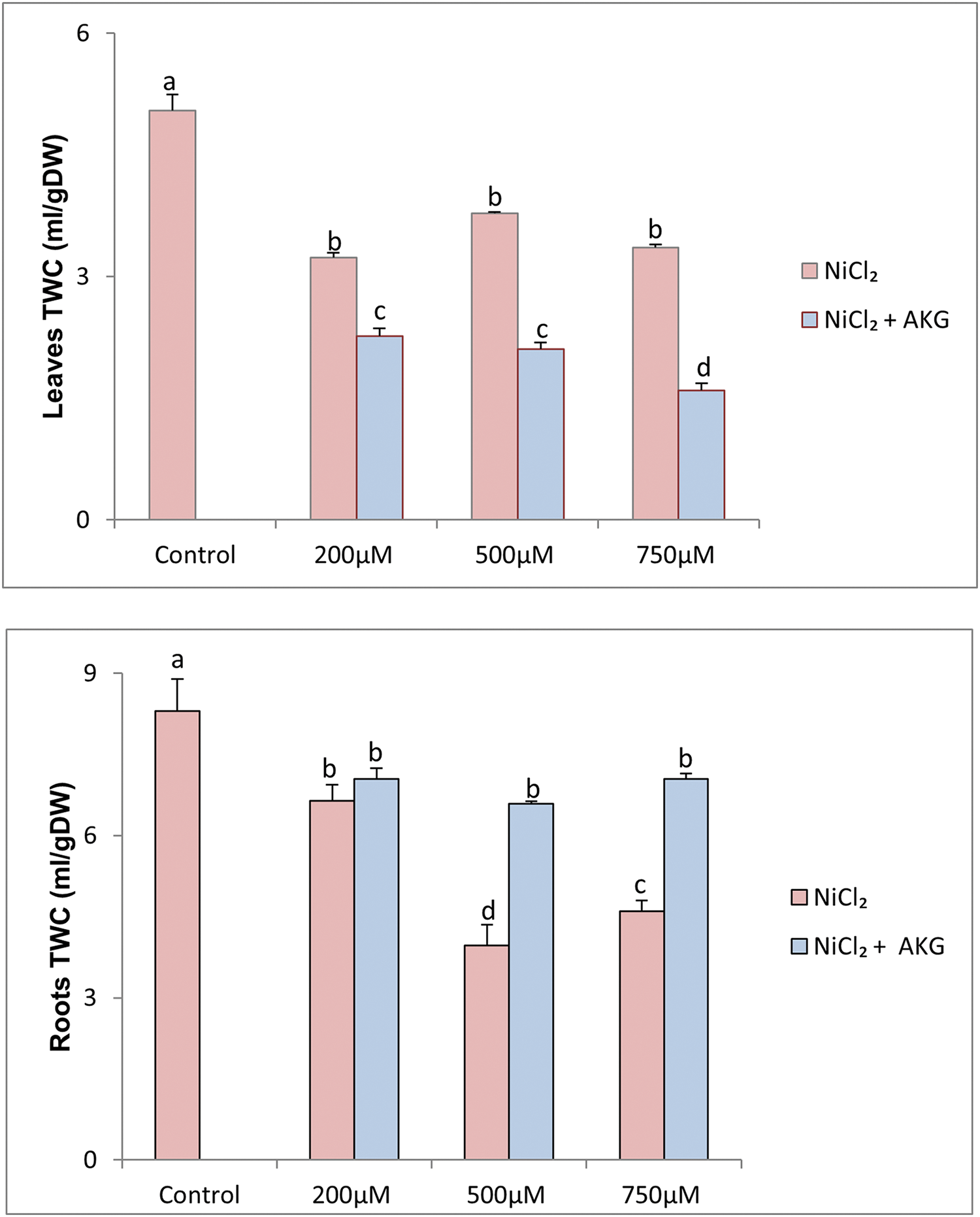
Figure 4: Effect of Nickel stress at different concentrations (0, 200, 500, 750 µM NiCl2) and AKG on leaves and roots TWC of P. harmala L. Values are the means ± SD (n = 3). Letters indicate that values are significantly different at p < 0.05
3.3 AKG Reduces Chlorophyll Content in P. harmala under Ni Stress
Photosynthesis is closely linked to plant growth, and the study investigated photosynthesis-related parameters such as chlorophyll a, chlorophyll b, and total chlorophyll content, as shown in Table 1.
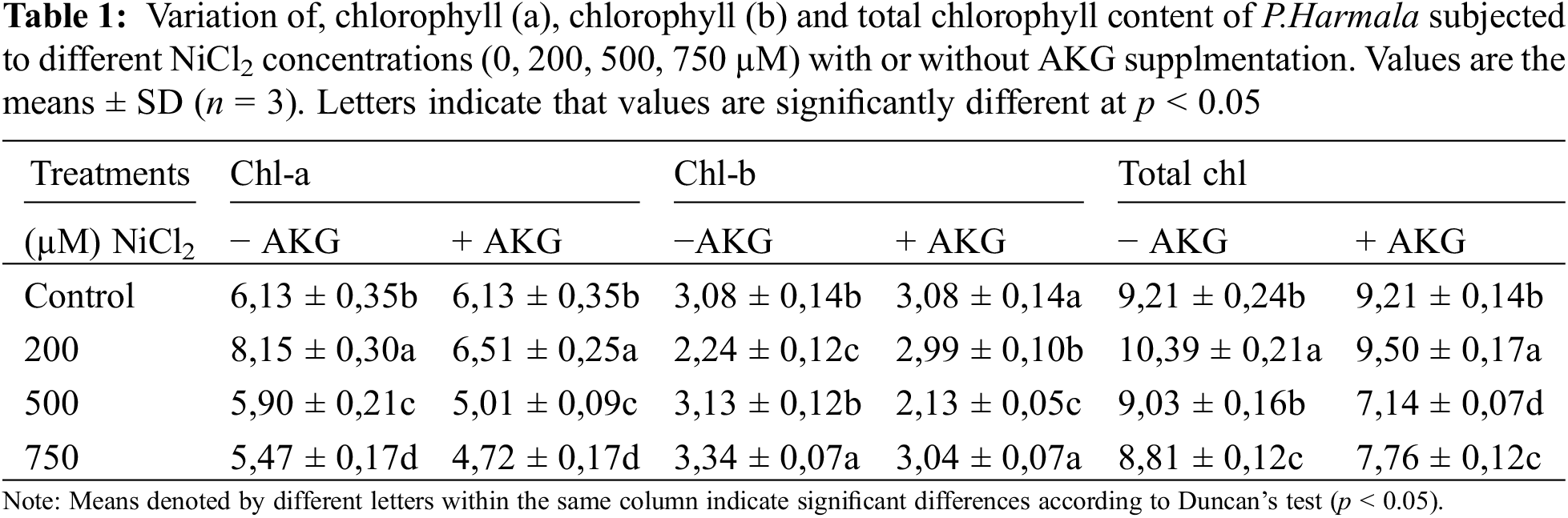
The results demonstrated that chlorophyll content is significantly affected by environmental conditions. Nickel stress significantly enhanced chlorophyll a content at 200 µM NiCl2, but a reduction was observed in plants treated with 500 and 750 µM NiCl2, with levels dropping to 5.9 and 507 mg/L, respectively. Similarly, chlorophyll b content increased markedly in plants exposed to 750 µM NiCl2, with a 108.41% increase compared to control plants. The highest total chlorophyll content was found in plants treated with 200 µM NiCl2 (10.39 mg/mL), followed by control plants treated with 500 (9.03 mg/mL) and 750 µM NiCl2 (8.81 mg/mL). The findings suggest that P. harmala’s scavenging system functions effectively, but total chlorophyll content declines with increasing Ni concentration. Low-dose stimulation of chlorophyll production may represent an adaptive response that allows plants to cope with more challenging environmental conditions [42–45]. This behavior has been observed in other studies, such as those on Urginea maritima, where heavy metal exposure adversely affected photosynthesis. The authors hypothesized that one of the most significant consequences of heavy metal excess is damage to the photosynthetic apparatus, which is countered by increased pheophytin levels and the activation of adaptive mechanisms, as evidenced by increased chlorophyll content [46].
However, the presence of AKG led to a decrease in chlorophyll levels throughout the soaking period [47]. Similar results have been reported in other studies, where engineered nanomaterials such as CuO, Cu, MoO3, and CeO2 nanoparticles negatively impacted photosynthesis by reducing chlorophyll and carotenoid contents in rice [48], soybean [49], corn [50], and tomato [51], or by interfering with photosynthesis-related genes [52]. This trend contrasts with findings in soybean seedlings [38], winter wheat under drought stress, and Gossypium hirsutum under salt stress [53], where AKG application enhanced chlorophyll content [54].
3.4 AKG Modulates Antioxidant Production in P. harmala under Ni Toxicity
Anthocyanins, which are secondary metabolites produced in response to oxidative stress, play a vital role in stress protection [55]. The study found that anthocyanin levels in P. harmala increased significantly after exposure to Ni stress, with concentrations ranging from 2.25 to 11.18 mg/L in leaves and 1.2 to 7.57 mg/L in roots (Table 2). The ability to adapt to nickel stress is linked to higher anthocyanin concentrations. However, the addition of exogenous AKG resulted in a notable decrease in anthocyanin levels. Anthocyanins protect plants from abiotic stress, and their synthesis is induced by different types of stress, with varying physiological roles. For example, higher anthocyanin accumulation has been suggested as a protective trait under salt stress in black glutinous rice [56] and has been observed in ripening grapevine fruits under water deficit conditions [57]. Polyphenols, another class of secondary metabolites with diverse biological activities, are synthesized by plants under harsh environmental conditions [58]. As shown in Table 2, TPC accumulation was more pronounced in leaves than roots, consistent with previous findings in tomato, where TPC levels were higher in leaves than in roots under cadmium stress [59]. The response pattern varied depending on the treatment concentration and the organ exposed to Ni stress. In leaves, an increase in TPC was observed, with the highest levels recorded at 750 µM NiCl2 (246 mg GAE/g DW). However, a decrease in TPC was observed in roots, with the lowest levels recorded at 750 µM NiCl2 treatment. P. harmala reacted differently to AKG treatment, with a clear increase in TPC in leaves treated with Ni and AKG, reaching a peak of 191.2 mg GAE/g DW at 750 µM. Conversely, a significant decrease in TPC was recorded in roots as NiCl2 concentration increased, with the lowest value observed at 750 µM NiCl2 + AKG. The plant’s response to stress is closely linked to the species and the level of heavy metals in the environment. Literature indicates that heavy metal stress can induce secondary metabolism, promoting the synthesis of antioxidants like polyphenols and flavonoids, which accumulate primarily in leaves rather than roots [60]. Nickel stress has been shown to increase TPC in sweet potato [61], which can be attributed to the accumulation of phenolic compounds such as gallic acid, rutin, salicylic acid, and quercetin [62,63]. This induced accumulation of TPC in P. harmala could enhance tolerance to nickel toxicity. Other studies suggest that TPC stimulation results from increased enzyme activity in the phenylpropanoid pathway [64,65]. However, some studies have demonstrated that the application of heavy metals like Cu, Cd, and Pb can significantly reduce TPC in tomato leaves. Similar to TPC, the study found that flavonoid accumulation was higher in leaves than in roots under Ni stress, with TFC levels gradually decreasing in roots as Ni concentration increased. These results align with previous findings that show a positive correlation between TPC and TFC in leaves and roots [66]. The decrease in TPC and TFC in roots under Ni stress may be due to reduced activity of phenylalanine ammonia-lyase (PAL), the first enzyme in the phenylpropanoid pathway, potentially resulting from toxic Ni accumulation. Supporting evidence comes from studies where salt stress caused a significant reduction in TPC in Marrubium vulgare [67], and decreased phenol accumulation in wheat was associated with lower PAL activity [68]. In this case, the decline in TPC and TFC in roots could be due to an imbalance between reactive oxygen species (ROS) and antioxidant formation, leading to oxidative stress [69].
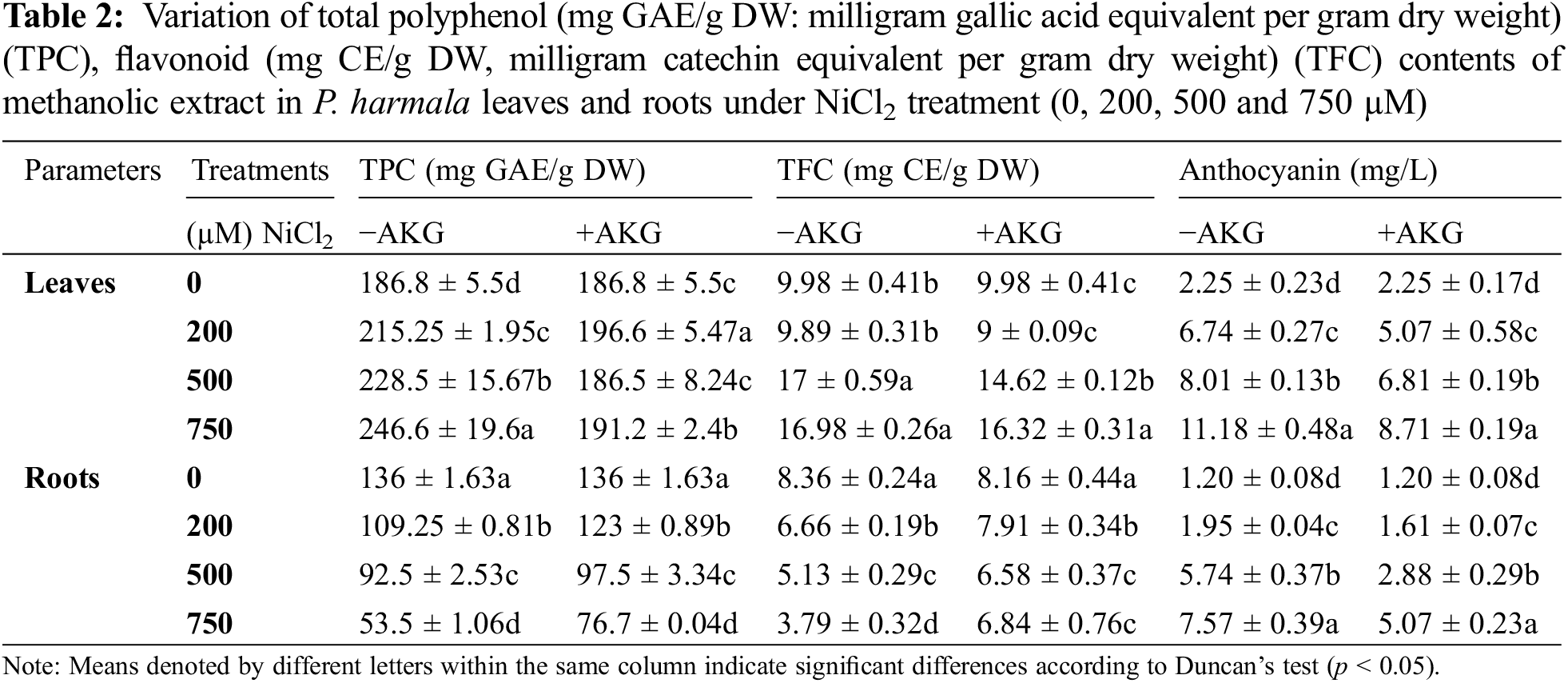
However, AKG supplementation stabilized TFC levels in P. harmala leaves, while AKG pre-treatment with Ni stress resulted in a significant increase in root TFC. Similar findings have been reported where heavy metal stress led to a decrease in TPC due to reduced enzyme activity involved in phenolic compound biosynthesis [70]. Various studies have examined the impact of exogenous applications on plant tolerance to heavy metals, concluding that such treatments can improve the production of antioxidant metabolites like proline, which regulates flavonoid biosynthesis and accumulation under nickel stress in wheat seedlings [71]. The effects of phytohormones like jasmonic acid on TPC and TFC in Solanum lycopersicum under sodium chloride stress [72], as well as the effects of nitric oxide [73] or exopolysaccharide [74] supplementation on tomato under cadmium stress, have also been observed.
3.5 AKG Differentially Regulates Antioxidant Enzyme Activity in P. harmala Leaves and Roots under Ni Stress
Fig. 5 shows the activities of antioxidative enzymes (SOD, CAT, APX, and GPX) in P. harmala leaves and roots under nickel stress, with or without AKG supplementation. An increase in antioxidant enzyme activity was observed in response to Ni treatments, with significant enhancements (p < 0.05). However, NiCl2 treatment reduced APX activity in leaves, particularly at the highest concentration, where a decrease of 80.20% was noted. In roots, APX activity increased with higher NiCl2 concentrations, with a 5.27-fold increase at 750 µM NiCl2. AKG supplementation caused a slight improvement in leaf APX activity, particularly at 750 µM NiCl2, but resulted in a notable decrease in root APX activity at 200 and 500 µM NiCl2, with a subsequent increase at 750 µM NiCl2.
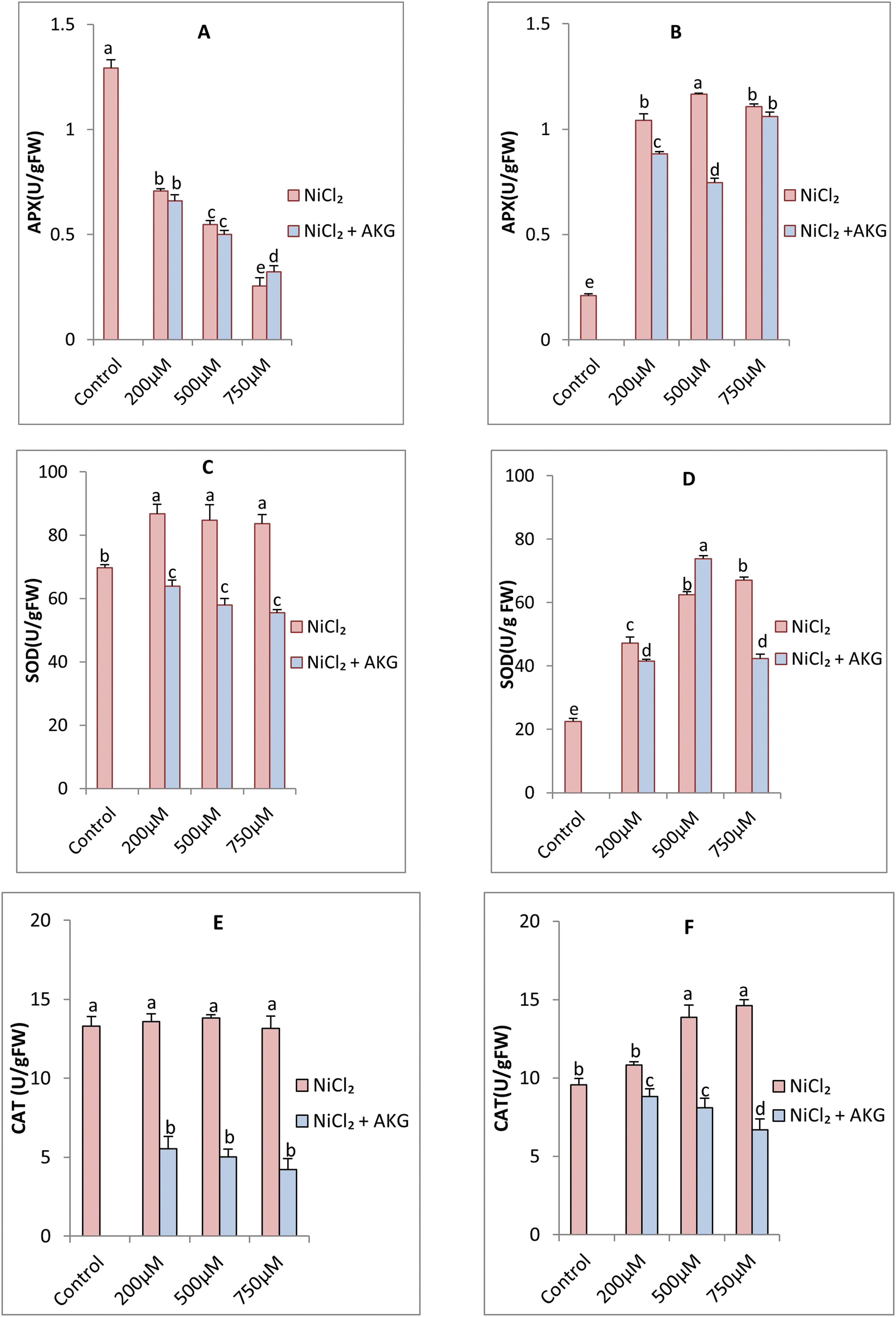
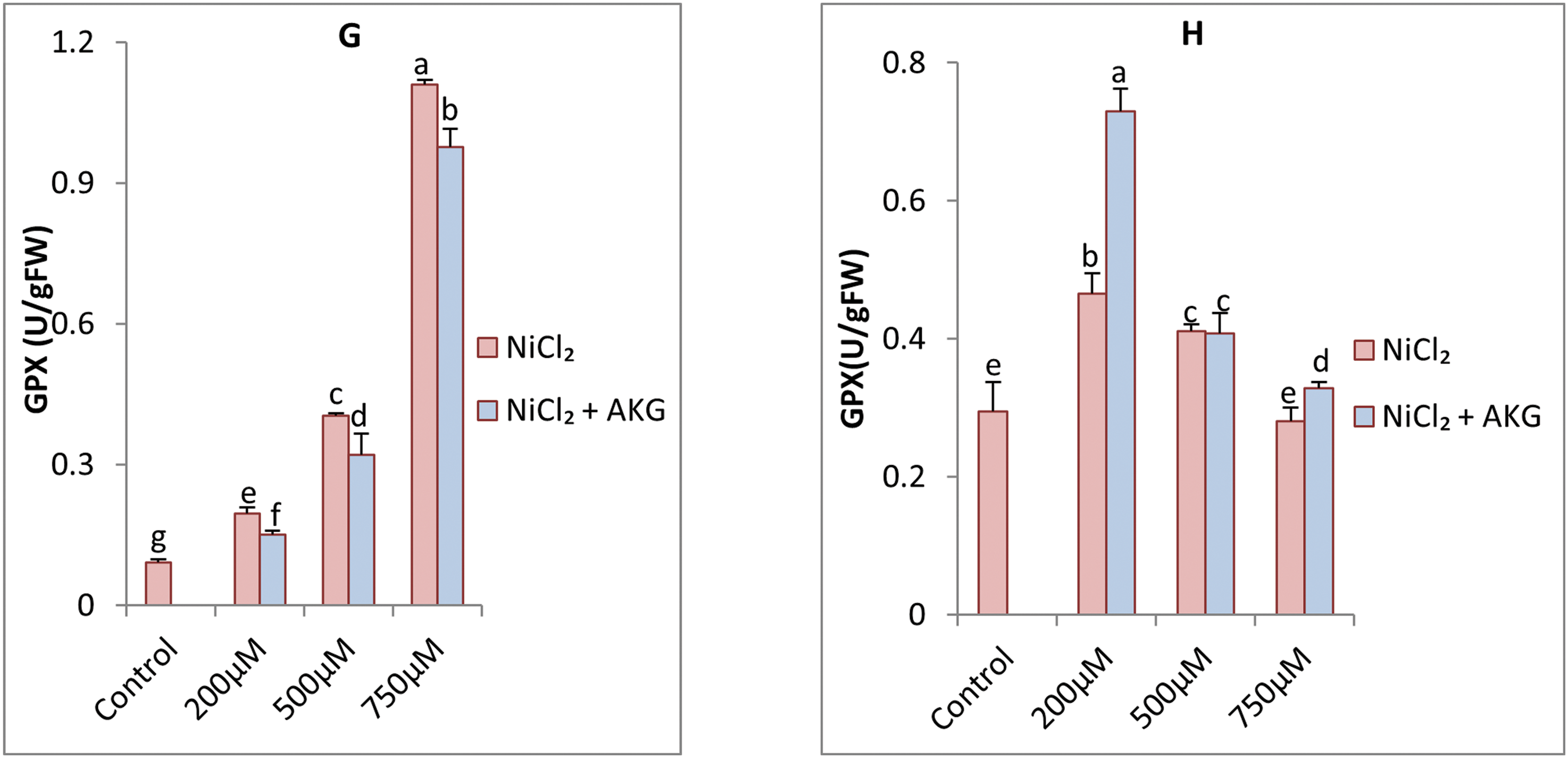
Figure 5: Effect of Nickel stress at different concentrations (0, 200, 500, 750 μM NiCl2) and AKG on the activities of ascorbate peroxidase (APX) on leaves (A), roots (B), superoxide dismutase (SOD) on leaves (C), roots (D), catalase (CAT) on leaves (E) and roots (F), guaiacol peroxidase (GPX) on leaves (G), roots (H) of P.harmala L. Values are the means ± SD (n = 3). Letters indicate that values are significantly different at p < 0.05
SOD activity was about three times higher in control plant leaves than in roots. After Ni application, both organs showed increased SOD activity, more pronounced in leaves. However, AKG supplementation in Ni-treated plants decreased SOD activity in leaves by 26.43%, 31.53%, and 33.54% at 200, 500, and 750 µM NiCl2, respectively. SOD plays a crucial role in the plant’s defense mechanism, transforming superoxide radicals into hydrogen peroxide, which is then broken down by CAT. This coordinated activity suggests that P. harmala uses a powerful ROS scavenging system in response to high nickel levels, thereby preventing oxidative damage to cellular components.
Studies on other Ni-tolerant plants, such as soybean and pepper (Capsicum annuum) [75,76], reveal similar trends where SOD and CAT activities increase in response to Ni exposure, highlighting the role of these enzymes in improving stress tolerance. This response not only helps to eliminate ROS, but also helps to maintain cellular integrity and stabilize metabolic functions under Ni stress. However, enzyme activity may vary depending on Ni concentration and duration of exposure. For example, while Low to moderate levels of Ni generally increase the activity of SOD and CAT, excess Ni can overwhelm the antioxidant system, potentially reducing the effectiveness of these enzymes over time. The variability in SOD and CAT responses among different plant species highlights how genetic factors, developmental stages and environmental conditions affect Ni tolerance.
In roots, SOD activity slightly decreased with AKG treatment at 200 and 750 µM NiCl2, but a significant increase of 228.17% was observed at 500 µM NiCl2 compared to the control. Nickel stress did not significantly affect CAT activity in leaves, but in roots, CAT activity increased progressively with Ni stress, reaching a 52.88% stimulation at 750 µM NiCl2 compared to the control. However, AKG supplementation reduced CAT activity in both leaves and roots, with the most significant reduction observed at the highest Ni concentration (750 µM NiCl2), by 7% and 70%, respectively, compared to the controls.
GPX activity was notably higher in roots than in leaves, with a 3-fold higher constitutive level in roots. Ni application increased GPX activity in both organs, with a more pronounced effect in roots, particularly at higher concentrations, resulting in a 12.06-fold increase. Interestingly, the activities of APX and GPX were more pronounced in leaves subjected to high concentrations of nickel, which potentially indicates the specific regulation of the tissue of antioxidant defenses. APX uses ascorbate as a substrate, which can explain the observed increase of nonenzymatic antioxidants (for example, phenols and flavonoids) in response to nickel This suggests a dual strategy that P. harmala not only regulates enzymatic antioxidants, but also strengthens non-enzymatic defenses effectively detoxifies ROS. However, AKG supplementation reduced GPX activity in leaves treated with 750 µM NiCl2, with a decrease of 12.4%. Despite these variations, all enzymes exhibited higher constitutive activities in roots than in leaves. Differential enzyme activity between roots and leaves implies a spatially regulated response that minimizes energy expenditure while maximizing defense. This adaptive mechanism may confer resistance by maintaining cellular redox homeostasis in various tissue types. Further studies may elucidate how these enzymatic and non-enzymatic pathways interact to facilitate long-term tolerance to heavy metal stress.
When plants are exposed to biotic or abiotic stress, including environmental factors, an increase in ROS levels is a common response [77], leading to the oxidation of biomolecules such as lipids, proteins, and DNA, and potentially causing cell death [78]. To counteract this, plants activate their defense mechanisms, including antioxidative enzymes like APX, SOD, CAT, and GPX, which are crucial for mitigating oxidative stress [67]. However, high Ni concentrations can disrupt the balance between ROS generation and detoxification [79], leading to oxidative damage [80]. The current study demonstrated that different Ni concentrations, along with AKG supplementation, influenced antioxidative enzyme activities in P. harmala. The increased enzyme activities indicate that they function together to provide better protection against nickel-induced oxidative stress, strengthen the plant cell wall, and improve P. harmala’s overall tolerance to Ni toxicity. Our results showed agreement with those concluded in tomato [81]. However, the inhibition of CAT activity in leaves at high Ni concentrations may lead to an accumulation of O2•− and H2O2, contributing to oxidative stress [82–84].
The exogenous application of AKG in P. harmala under Ni stress generally led to decreased activities of antioxidative enzymes (APX, SOD, CAT, GPX) in both leaves and roots, with the exception of GPX in roots. These findings differ from earlier research that demonstrated increased enzyme activities following AKG treatment in tomato under arsenic stress and soybean under drought stress. Specifically, Alamri et al. [39] observed that priming tomato seedlings with 2-oxoglutarate enhanced arsenic toxicity alleviation by increasing enzyme activities linked to nitric oxide signaling. Similarly, Gai et al. [16] showed that 2-oxoglutarate contributed to foliar nitrogen’s role in boosting drought tolerance during soybean flowering, which was associated with increased enzymatic activity and improved grain yield. This divergence underscores the variability in AKG’s effects and suggest that AKG may regulate antioxidant enzyme activities and cellular redox through different mechanisms. Discrepancies between studies may be attributed to differences in plant species, their tolerance levels to metal stress, the intensity and duration of stress, and varying experimental conditions. In our study, the results suggest that AKG might exert its protective effects through other redox regulatory mechanisms rather than directly enhancing antioxidant activities. The modulation of these enzyme activities under AKG treatment also suggests that AKG may act as a signaling molecule, optimizing antioxidative responses. These findings open up potential applications for using AKG as a protective treatment in crops grown in heavy metal-contaminated soils. Liu et al. [85] proposed that the balance between oxidants and antioxidants is crucial for physiological functions in cells, and AKG may act as a source of energy and an antioxidant, scavenging ROS and combating oxidative stress through nonenzymatic oxidative decarboxylation in H2O2 decomposition.
A limitation of the current study is the absence of a control group treated only with AKG (without nickel). The inclusion of such a group would allow a clearer interpretation of the specific effects of AKG on physiological responses of P. harmala. Due to resource limitations, we could not perform these additional experiments; however, we consider this a priority for future research to validate and extend our results.
The findings from this study suggest that nickel-induced oxidative stress in Peganum harmala (both leaves and roots) is complex and involves a sophisticated antioxidant defense network that relies on both enzymatic and non-enzymatic mechanisms, as well as phytochemicals like polyphenols, flavonoids, and anthocyanins. The results indicate that P. harmala is a tolerant species, as evidenced by a significant increase in the majority of its antioxidant enzymes when exposed to nickel stress. Alpha-ketoglutarate (AKG) plays a crucial role in cellular energy metabolism and antioxidative stress responses. This research provides valuable insights into AKG’s potential to modulate biochemical pathways, helping P. harmala to withstand heavy metal toxicity. However, it’s important to note that AKG supplementation led to a reduction in the activity of these antioxidant enzymes. Despite these findings, the full range of AKG’s actions and mechanisms remains not fully understood, and further research is necessary to unravel the metabolic and molecular basis of AKG-induced modulations in P. harmala.
Nickel poses significant environmental threats, particularly to soil and water quality. Therefore, appropriate regulation and legislation are required to utilize nickel-tolerant species as effective bioremediation tools. Phytoremediation and phytoextraction offer sustainable approaches to safeguarding our environment and human health, especially by preventing contamination of the food chain with heavy metals. One promising strategy for managing nickel pollution involves screening potential plant species, such as P. harmala, which has shown the ability to tolerate nickel stress. The use of AKG could further enhance the effectiveness of phytoextraction by promoting P. harmala growth under nickel exposure. These research avenues warrant further exploration and investigation.
Acknowledgement: We are grateful for the support provided by the Tunisian Ministry of Higher Education, Scientific Research, and the University of Tunis El Manar.
Funding Statement: This research was funded by the Researchers Supporting Project No. (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: Conceptualization, Chiraz Chaffei Haouari; methodology, Marwa Rezgui and Chiraz Chaffei Haouari; software, Wided Ben Ammar; validation, Muhammad Nazim; formal analysis, Marwa Rezgui; investigation, Marwa Rezgui; resources data curation, Wided Ben Ammar; writing—original draft preparation, Marwa Rezgui; writing—review and editing, Marwa Rezgui and Chiraz Chaffei Haouari; visualization, Muhammad Nazim and Marwa Rezgui; supervision, Chiraz Chaffei Haouari; project administration, Chiraz Chaffei Haouari, Muhammad Nazim and Walid Soufan; funding acquisition, Walid Soufan and Chiraz Chaffei Haouari. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Amjad M, Raza H, Murtaza B, Abbas G, Imran M, Shahid M, et al. Nickel toxicity induced changes in nutrient dynamics and antioxidant profiling in two maize (Zea mays L.) hybrids. Plants. 2019;9(1):5. doi:10.3390/plants9010005. [Google Scholar] [PubMed] [CrossRef]
2. Ahmad K, Rani S, Khan ZI, Akhtar S, Ashfaq A. Effects of fertilizers on copper and nickel accumulation and human health risk assessment of vegetables and food crops. J Biores Manag. 2023;(1):84–96. [Google Scholar]
3. Alomran MM, Noman A, Khalid N, Naila H, Fatmah MA, Naila H, et al. Harnessing melatonin protective efficacy in capsicum plants against nickel-contaminated soil. J Soil Sci Plant Nutr. 2024;24(1):408–20. doi:10.1007/s42729-023-01551-6. [Google Scholar] [CrossRef]
4. Fabiano CC, Tezotto T, Favarin JL, Polacco JC, Mazzafera P. Essentiality of nickel in plants: a role in plant stresses. Front Plant Sci. 2015;6:754. [Google Scholar] [PubMed]
5. Rizwan M, Usman K, Alsafran M. Ecological impacts and potential hazards of nickel on soil microbes, plants, and human health. Chemosphere. 2024;357:142028. [Google Scholar] [PubMed]
6. Naeem M, Aftab T, Khan MMA. Intimidating effects of heavy metals on mentha species and their mitigation using scientific approaches. In: Contaminants in agriculture: sources, impacts and management. Cham, Switzerland: Springer; 2020. p. 305–25. doi:10.1007/978-3-030-41552-5_15. [Google Scholar] [CrossRef]
7. Nabi A, Naeem M, Aftab T, Khan MMA. Alterations in photosynthetic pigments, antioxidant machinery, essential oil constituents and growth of menthol mint (Mentha arvensis L.) upon nickel exposure. Rev Bras Bot. 2020;43(4):721–31. doi:10.1007/s40415-020-00649-w. [Google Scholar] [CrossRef]
8. Rahi AA, Younis U, Ahmed N, Ali MA, Fahad S, Sultan H, et al. Toxicity of cadmium and nickel in the context of applied activated carbon biochar. Sci Total Environ. 2022;29(2):743–50. [Google Scholar]
9. Prajapati K, Shah B, Parmar A, Patel R. Effect of nickel toxicity on the seedling growth of guar (Cyamopsis tetragonoloba L.). Int Res J Plant Sci. 2022;13:1–5. [Google Scholar]
10. Maheshwari R, Dubey R. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Reg. 2009;59:37–49. [Google Scholar]
11. Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Sig. 2013;18(5):2145–65. doi:10.1089/ars.2012.5118. [Google Scholar] [CrossRef]
12. Liu XW, Huang ZL, Fan PS, Zhang L, Yan YF, Yang YH, et al. Zinc and α-ketoglutaric acid modulates plant growth, gas exchange attributes, chlorophyll fuorescence and Zn content in rice. Int J Agric Biol. 2020b;23:155–63. [Google Scholar]
13. Gui P, Chen X, Liao Z, Wang L. Effect of organic carbon on carbon and nitrogen metabolism and the growth of water spanich as affected by nitrogen levels. Acta Pedol Sin. 2016;53:746–56. [Google Scholar]
14. Legendre F, MacLean A, Appanna VP, Appanna VD. Biochemical pathways to α-Ketoglutarate, a multi-faceted metabolite. World J Microbiol Biotechnol. 2020;36:1–11. [Google Scholar]
15. Singh M, Singh P, Prasad SM. α-Ketoglutarate enhanced Solanum melongena L. growth: acceleration of nitrogen assimilating enzymes and antioxidant system under arsenate toxicity. J Plant Growth Regul. 2022;41:1699–713. [Google Scholar]
16. Gai Z, Zhang M, Zhang P, Zhang J, Liu J, Cai L, et al. 2-oxoglutarate contributes to the effect of foliar nitrogen on enhancing drought tolerance during flowering and grain yield of soybean. Sci Rep. 2023;13:7274. [Google Scholar] [PubMed]
17. Lei S, Huang B. Metabolic regulation of α-Ketoglutarate associated with heat tolerance in perennial ryegrass. Plant Physiol Biochem J. 2022;190:164–73. [Google Scholar] [PubMed]
18. Liu J, Fu P, Wang L, Lin X, Enayatizamir N. A fungus (Trametes pubescens) resists cadmium toxicity by rewiring nitrogen metabolism and enhancing energy metabolism. Front Microbiol. 2022;13:1040579. [Google Scholar] [PubMed]
19. Wu YL, Hu JX, Chen Y, Zheng BS, Yan DL. Effects of external application of α-Ketoglutarate on growth, carbon, nitrogen and phosphorus accumulation and their stoichiometric relationships in Kosteletzkya virginica under salt stress. J Agric Sci Technol. 2023;25(7):170–7. [Google Scholar]
20. Zhu B, Xu Q, Zou Y, Ma S, Zhang X, Xie X, et al. Effect of potassium deficiency on growth, antioxidants, ionome and metabolism in rapeseed under drought stress. Plant Growth Regul. 2020;90:455–66. [Google Scholar]
21. Du B, Wu Q, Jiang S, Zhang D, Qiao Y, Xie Y. Effects of exogenous α-ketoglutaric acid on 2-acetyl-1-pyrroline, yield formation and grain quality characters of aromatic rice. Phyton-Int J Exp Bot. 2021;90(2):437–47. doi:10.32604/phyton.2021.012903. [Google Scholar] [CrossRef]
22. Sun Q, Liang W, Jia L, Wang ZQ, Lin T. Effects of exogenous α-oxoglutarate on yield traits of wheat under low water potential and low nitrogen stress. J Anhui Agric Sci. 2014;42(3):671–74, 676 (In Chinese). [Google Scholar]
23. Rashid S, Sameti M, Alqarni MH, Bar FMA. In vivo investigation of the inhibitory effect of Peganum harmala L. and its major alkaloids on ethylene glycol-induced urolithiasis in rats. J Ethnopharmacol. 2023;300:115752. [Google Scholar] [PubMed]
24. Jaradat N, Hawash M, Sharifi-Rad M, Shakhshir A, Sobuh S, Hussein F, et al. Insights into free radicals scavenging, α-amylase inhibition, cytotoxic and antifibrotic activities unveiled by Peganum harmala extracts. BMC Complement Med Ther. 2024;24(1):299. [Google Scholar] [PubMed]
25. El Hasnaoui S, Diallo A, Wandan EN, Colin F, Smouni A, Fahr M. Lead and zinc tolerance and accumulation in metallicolous and non-metallicolous populations of Peganum harmala L.: potential use in phytostabilization. Bioremediat J. 2024;28(3):302–24. [Google Scholar]
26. Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. 2nd edDavis, CA, USA: Agricultural Experiment Station; 1950. vol. 347, p. 1–32. [Google Scholar]
27. Dhaliwal SS, Singh J, Taneja PK, Mandal A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ Sci Pollut Res. 2020;27(2):1319–33. [Google Scholar]
28. Arnon DL. A copper enzyme is isolated chloroplast polyphenol oxidase in Beta vulgaries. Plant Physiol. 1949;24:1–15. [Google Scholar] [PubMed]
29. Gould WD, King M, Mohapatra BR, Cameron R, Kapoor A, Koren DW. A critical review on destruction of thiocyanate in mining effluents. Miner Eng. 2012;34:38–47. [Google Scholar]
30. Dewanto V, Wu X, Adom KK, Liu RH. Termal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–4. [Google Scholar] [PubMed]
31. Beyer WF, Fridovich Y. Assaying for superoxide dismutase activity some large consequence of minor changes in conditions. Anal Biochem. 1987;161:559–66. [Google Scholar] [PubMed]
32. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamidegels. Anal Biochem. 1971;44:276–87. [Google Scholar] [PubMed]
33. Chen GX, Asada K. Ascorbate peroxidase in pea diferences in theirenzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–98. [Google Scholar]
34. Urbanek H, Kuzniak-Gebarowska E, Herka K. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol Plant. 1991;13:43–50. [Google Scholar]
35. Naqqash T, Aziz A, Baber M, Shahid M, Sajid M, Emanuele R, et al. Metal-tolerant morganella morganii isolates can potentially mediate nickel stress tolerance in Arabidopsis by upregulating antioxidative enzyme activities. Plant Sig Behav. 2024;19(1):2318513. [Google Scholar]
36. Samantary S. Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere. 2002;47:1065–72. [Google Scholar] [PubMed]
37. Zhang LX, Li SX, Liang ZS, Li SQ. Effect of foliar nitrogen application on nitrogen metabolism, water status, and plant growth in two maize cultivars under short-term moderate stress. J Plant Nutr. 2009;32(11):1861–81. [Google Scholar]
38. Gai ZJ, Liu L, Zhang JT, Liu JQ, Cai LJ. Effects of exogenous α-oxoglutarate on proline accumulation, ammonium assimilation and photosynthesis of soybean seedling (Glycine max L.) exposed to cold stress. Sci Rep. 2022a;10:17017. [Google Scholar]
39. Alamri S, Alsubaie QD, Al-Amri AA, Al-Munqedi B, Ali HM. Priming of tomato seedlings with 2-oxoglutarate induces arsenic toxicity alleviatory responses by involving endogenous nitric oxide. Physiol Plant. 2021;173:45–57. [Google Scholar] [PubMed]
40. Fu X, Gui R, Li W, Gao Z, Ashraf U, Tan J, et al. Nitrogen and α-ketoglutaric acid application modulate grain yield, aroma, nutrient uptake and physiological attributes in fragrant rice. J Plant Growth Regul. 2021;40:1613–28. [Google Scholar]
41. Hadi F, Bano A, Fuller MP. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere. 2010;80:457–62. [Google Scholar] [PubMed]
42. Slimani N, Arraouadi S, Hajlaoui H, Borgi MA, Boughattas NEH, De Feo V, et al. The impact of greenhouse and field growth conditions on Chenopodium quinoa willd accessions’ response to salt stress: a comparative approach. Agronomy. 2023;13(9):2303. [Google Scholar]
43. Haghpanah M, Hashemipetroudi S, Arzani A, Araniti F. Drought tolerance in plants: physiological and molecular responses. Plants. 2024;13(21):2962. [Google Scholar] [PubMed]
44. Elbasan F, Arikan-Abdulveli B, Ozfidan-Konakci C, Yildiztugay E, Tarhan İ, Çelik B. Exploring the defense strategies of benzalkonium chloride exposures on the antioxidant system, photosynthesis and ROS accumulation in lemna minor. Chemosphere. 2024;363:142924. [Google Scholar] [PubMed]
45. Moustakas M, Dobrikova A, Sperdouli I, Hanć A, Adamakis IDS, Moustaka J, et al. A hormetic spatiotemporal photosystem II response mechanism of salvia to excess zinc exposure. Int J Mol Sci. 2022;23(19):11232. [Google Scholar] [PubMed]
46. Houri T, Khairallah Y, Zahab AA, Osta B, Romanos D, Haddad G. Heavy metals accumulation effects on the photosynthetic performance of geophytes in mediterranean reserve. J King Saud Univ Sci. 2020;32(1):874–80. [Google Scholar]
47. Inanç AL. Chlorophyll: structural properties, health benefits and its occurrence in virgin olive oils. Acad Food J/Akad GIDA. 2011;9:90–344. [Google Scholar]
48. Du WC, Gardea-Torresdey JL, Ji R, Yin Y, Zhu JG, Peralta-Videa JR, et al. Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: a life cycle field study. Environ Sci Technol. 2015;49(19):11884–93. doi:10.1021/acs.est.5b03055. [Google Scholar] [PubMed] [CrossRef]
49. Yang Z, Xiao Y, Jiao T, Zhang Y, Chen J, Gao Y. Effects of copper oxide nanoparticles on the growth of rice (Oryza sativa L.) seedlings and the relevant physiological responses. Int J Env Res Pub He. 2020b;17(4):1260. [Google Scholar]
50. Rai P, Singh VP, Peralta-Videa J, Tripathi DK, Sharma S, Corpas FJ. Hydrogen sulfide (H2S) underpins the beneficial silicon effects against the copper oxide nanoparticles (CuO NPs) phytotoxicity in Oryza sativa seedlings. J Hazard Mater. 2021;415:124907. doi:10.1016/j.jhazmat.2020.124907. [Google Scholar] [PubMed] [CrossRef]
51. Sutulienė R, Brazaitytė A, Małek S, Jasik M, Samuoliene G. Biochemical responses of pea plants to drought stress and in the presence of molybdenum trioxide nanoparticles. Plant Soil. 2023;492:381–97. [Google Scholar]
52. Wang X, Yang X, Chen S, Li Q, Wei W, Hou C, et al. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front Plant Sci. 2016;6:1243. doi:10.3389/fpls.2015.01243. [Google Scholar] [PubMed] [CrossRef]
53. Luo Z, Kong XQ, Dai JL, Dong HZ. Soil plus foliar nitrogen application increases cotton growth and salinity tolerance. J Plant Nutr. 2015;38(3):443–55. [Google Scholar]
54. Jabeen R, Ahmad R. Alleviation of the adverse efects of salt stress by foliar application of sodium antagonistic essential minerals on cotton (Gossypium hirsutum L.). Pak J Bot. 2009;41:2199–08.55. [Google Scholar]
55. Juszczuk IM, Wiktorowska A, Malusá E, Rychter AM. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.). Plant Soil. 2004;267:41–9. [Google Scholar]
56. Chunthaburee S, Dongsansuk A, Sanitchon J, Pattanagul W, Theerakulpisut P. Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J Biol Sci. 2016;23(4):467–77. [Google Scholar] [PubMed]
57. Castellarin SD, Pfeiffer A, Silviotti P, Degan M, Peterlunger E, Di Gaspero G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007;30:1381–99. [Google Scholar] [PubMed]
58. Abbas M, Saeed F, Anjum FM, Afzaal M, Tufail T, Bashir MS, et al. Natural polyphenols: an overview. Int J Food Prop. 2017;20(8):1689–99. [Google Scholar]
59. Natarajan A, Vijayarengan P, Vijayaragavan M. Influence of cadmium on growth and biochemical contents of tomato plants. J Plant Stress Physiol. 2018;4:4–6. [Google Scholar]
60. Badiaa O, Yssaad HAR, Topcuoglu B. Effect of heavy metals (copper and zinc) on proline, polyphenols and flavonoids content of tomato (Lycopersicon esculentum Mill.). Plant Arch. 2020;20(2):2125–37. [Google Scholar]
61. Kumar S, Wang M, Liu Y, Fahad S, Qayyum A, Jadoon SA, et al. Nickel toxicity alters growth patterns and induces oxidative stress response in sweetpotato. Front Plant Sci. 2022;13:1054924. [Google Scholar] [PubMed]
62. Sarker U, Oba S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected amaranthus tricolor under salinity stress. Sci Rep. 2018;8:12349. [Google Scholar] [PubMed]
63. El-Beltagi HS, El-Sayed SM, Abdelhamid AN, Hassan KM, Elshalakany WA, Nossier MI, et al. Potentiating biosynthesis of alkaloids and polyphenolic substances in Catharanthus roseus plant using ĸ-carrageenan. Molecules. 2023;28(8):3642. doi:10.3390/molecules28083642. [Google Scholar] [PubMed] [CrossRef]
64. André CM, Schafleitner R, Legay S, Lefèvre I, Aliaga CAA, Nomberto G, et al. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry. 2009;70:1107– 16. [Google Scholar]
65. Kisa D, Kayir Ö, Saglam N, Şahin S, Öztürk L, Elmastaş M. Changes of phenolic compounds in tomato associated with the heavy metal stress. Int J Appl Nat Sci. 2019;2(1):35–43. [Google Scholar]
66. Singh OS, Pant NC, Laishram ML, Tewari RD, Joshi K, Pandey C. Effect of CuO nanoparticles on polyphenols content and antioxidant activity in Ashwagandha (Withania somnifera L. Dunal). J Pharmacogn Phytochem. 2018;7(2):3433–9. [Google Scholar]
67. Rezgui M, Hosni K, Majdoub N, Ben Kaâb S, Marzouk B, Gouia H, et al. Effect of long-term salinity on compatible solutes and antioxidant status of horehound (Marrubium vulgare L.). Agrochimica. 2016;60(3):162–72. [Google Scholar]
68. Saleh AM, Madany MMY. Coumarin pretreatment alleviates salinity stress in wheat seedlings. Plant Physiol Biochem. 2015;88:27. [Google Scholar] [PubMed]
69. Taârit MB, Msaada K, Hosni K, Marzouk B. Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. J Sci Food Agric. 2012;92(8):1614–9. [Google Scholar]
70. Kebert M, Kostić S, Vuksanović V, Gavranović Markić A, Kiprovski B, Zorić M, et al. Metal-and organ-specific response to heavy metal-induced stress mediated by antioxidant enzymes’ activities, polyamines, and plant hormones levels in Populus deltoides. Plants. 2022;11(23):3246. doi:10.3390/plants11233246. [Google Scholar] [PubMed] [CrossRef]
71. Atta N, Shahbaz M, Farhat F, Maqsood MF, Zulfqar U, Naz N, et al. Proline-mediated redox regulation in wheat for mitigating nickel-induced stress and soil decontamination. Sci Rep. 2024;14:456. [Google Scholar] [PubMed]
72. Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P, Ashraf M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact. 2018a;13(1):64–72. [Google Scholar]
73. Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma. 2018b;255(1):79–93. [Google Scholar] [PubMed]
74. Arroussi HEL, Benhima R, Elbaouchi A, Sijilmassi B, Mernissi NEL, Aafsar A, et al. Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum L.). J Appl Phycol. 2018;30:2929–41. doi:10.1007/s10811-017-1382-1. [Google Scholar] [CrossRef]
75. Einhardt AM, Oliveira LM, Ferreira S, Araújo WL, Medeiros DB, Fernie AR, et al. Defense responses and oxidative metabolism of glyphosateresistant soybean plants infected by Phakopsora pachyrhizi modulated by glyphosate and nickel. Physiol Mol Plant Pathol. 2020;118:101817. doi:10.1016/j.pmpp.2022.101817. [Google Scholar] [CrossRef]
76. Altaf MA, Hao Y, He C, Mumtaz MA, Shu H, Fu H, et al. Physiological and biochemical responses of pepper (Capsicum annuum L.) seedlings to nickel toxicity. Front Plant Sci. 2022;13:950392. [Google Scholar] [PubMed]
77. Kumar S, Li G, Yang J, Huang X, Ji Q, Zhou K, et al. Investigation of an antioxidative system for salinity tolerance in oenanthe javanica. Antioxidants. 2020;9:940. [Google Scholar] [PubMed]
78. Suman S, Bagal D, Jain D, Singh R, Singh IK, Singh A. Biotic stresses on plants: reactive oxygen species generation and antioxidant mechanism. In: Frontiers in plant-soil interaction. Cambridge, MA, USA: Academic Press; 2021. p. 381–411. [Google Scholar]
79. Kumar S, Li G, Yang J, Huang X, Ji Q, Liu Z, et al. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front Plant Sci. 2021;12:660409. [Google Scholar] [PubMed]
80. Barcelos JPQ, Reis HPG, Godoy CV, Gratão PL, Furlani Junior E, Putti F, et al. Impact of foliar nickel application on urease activity, antioxidant metabolism and control of powdery mildew (Microsphaera diffusa) in soybean plants. Plant Pathol. 2018;67:1502–13. [Google Scholar]
81. Amjad M, Ameen N, Murtaza B, Imran M, Shahid M, Abbas G, et al. Comparative physiological and biochemical evaluation of salt and nickel tolerance mechanisms in two contrasting tomato genotypes. Physiol Plant. 2020;168:27–37. [Google Scholar] [PubMed]
82. Zhou X, An Y, Qu T, Jin T, Zhao L, Guo H, et al. Effects of Ni and Cu stresses on morphological and physiological characteristics of Euphorbia marginata Pursh seedlings. Agronomy. 2024;14(6):1223. doi:10.3390/agronomy14061223. [Google Scholar] [CrossRef]
83. Rajput VD, Harish S, Verma RK, Sharma KK, Quiroz-Figueroa L, Mandzhieva FR, et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology. 2021;10(4):267. [Google Scholar] [PubMed]
84. Yusuf M, Fariduddin Q, Varshney P, Ahmad A. Salicylic acid minimizes nickel and/or salinity-induced toxicity in Indian mustard (Brassica juncea) through an improved antioxidant system. Environ Sci Pollut Res Int. 2012;19:8–18. [Google Scholar] [PubMed]
85. Liu S, He L, Yao K. The antioxidative function of alpha-ketoglutarate and its applications. BioMed Res Int. 2018;2018(1):3408467. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools