 Open Access
Open Access
ARTICLE
Optimizing Silver Nanoparticle Concentrations to Improve the In Vitro Regeneration and Growth of Phalaenopsis Orchids
1 Department of Horticulture and Life Science, Yeungnam University, Gyeongsan, 38541, Republic of Korea
2 Department of Horticultural Science, Kyungpook National University, Daegu, 41566, Republic of Korea
* Corresponding Authors: Aung Htay Naing. Email: ; Kyeung II Park. Email:
(This article belongs to the Special Issue: Advances in Ornamental Plants: Micropropagation, Plant Biotechnology, Chromosome Doubling, Mutagenesis, Plant Breeding, Environmental Stress Tolerance, and Postharvest Physiology)
Phyton-International Journal of Experimental Botany 2025, 94(9), 2719-2727. https://doi.org/10.32604/phyton.2025.068713
Received 04 June 2025; Accepted 08 August 2025; Issue published 30 September 2025
Abstract
Phalaenopsis orchids are economically important ornamental crops; however, their commercial micropropagation is often limited by poor rooting efficiency and inconsistent growth. In this study, we investigated the effects of silver nanoparticles (Ag-NPs) on the in vitro regeneration and growth of Phalaenopsis cultivar 611B to determine the optimal concentration of Ag-NPs for improved micropropagation outcomes. Shoot tip explants (2–3 mm)—derived from protocorm-like bodies were cultured on a regeneration medium containing Hyponex (20:20:20 and 6.5:6.5:19), 18 g/L sugar, 2 g/L peptone, 0.8 g/L activated charcoal, 12.5 g/L potato extract, 50 mL/L apple juice, and 10 mg/L 6-benzylaminopurine (6-BA), with varying concentrations of Ag-NPs (0, 0.5, 1.0, 2.0, and 2.5 mg/L). After 10–12 weeks, shoot and root formation, plant height, fresh weight, leaf number, and chlorophyll contents were evaluated. At 1.0 mg/L Ag-NPs, shoot regeneration (5.4 vs. 2.9 shoots per explant), root induction (2.1 vs. 1.4 roots per explant), and shoot formation frequency (100% vs. 55%) were significantly higher than the control (0 mg/L). Fresh weight (592.4 mg) and leaf number (9.7) also showed notable increases at this concentration. Although chlorophyll a and b levels peaked at 2.0 mg/L, the difference from 1.0 mg/L was not statistically significant. These results suggest that 1.0 mg/L Ag-NPs is the optimal concentration for enhancing shoot and root development and improving overall plantlet quality in Phalaenopsis. The findings highlight the potential of nanomaterials to improve the efficiency of orchid tissue culture systems.Keywords
Phalaenopsis orchids are among the most extensively cultivated species in the global orchid market, valued for their ornamental appeal and economic significance as both cut flowers and potted plants. Despite this, their vegetative propagation remains challenging due to genetic variability in seedlings and the extended time required to produce market-ready plants. Consequently, plant tissue culture has become a vital alternative for propagating Phalaenopsis [1]. Several studies have reported the successful in vitro regeneration of Phalaenopsis orchids using tissue culture techniques [2–6]. However, the efficiency of these methods depends on numerous factors, including the genotype, physiological condition of the donor plant, explant type, surface sterilization procedures, culture medium composition, and use of plant growth regulators. These factors highlight the importance of developing cultivar-specific protocols to improve regeneration outcomes.
Among available cultivars, Phalaenopsis 611B has shown promise in preliminary evaluations due to its vigorous growth, morphological stability, and potential for desirable floral traits-qualities that make it a strong candidate for future commercial introduction. However, like many elite genotypes, cultivar 611B suffers from low multiplication rates and poor root development when propagated conventionally. To support large-scale production, an efficient in vitro regeneration protocol tailored to this cultivar is required. Although we initially attempted regeneration of Phalaenopsis 611B using published protocols [2–6] shoot regeneration and overall in vitro efficiency remained suboptimal. In our preliminary trials, a medium containing 1 g/L Hyponex (20:20:20), 2 g/L Hyponex (6.5:6.5:19), 18 g/L sucrose, 2 g/L peptone, 0.8 g/L activated charcoal, 12.5 g/L potato extract, 7 g/L agar, 50 mL/L apple juice, and 1.0 mg/L 6-benzylaminopurine (6-BA) produced an average of only 2.9 shoots and 1.0 root per explant. Moreover, the frequencies of shoot and root formation remained low. Therefore, in vitro shoot regeneration and rooting remain critical bottlenecks in the micropropagation of Phalaenopsis cultivar 611B.
Recent advances in nanotechnology have demonstrated considerable potential to enhance in vitro plant regeneration across various species, including orchids [7–10]. In particular, silver nanoparticles (Ag-NPs) have been reported to enhance shoot proliferation, rooting, and acclimatization during the in vitro regeneration of Phalaenopsis orchids [8,9]. However, the specific effects of Ag-NPs on shoot and root development in Phalaenopsis cultivar 611B remain unexplored.
Therefore, we evaluated the impact of various Ag-NP concentrations on shoot and root formation frequency, the number of shoots and roots per explant, and overall plant growth in Phalaenopsis cultivar 611B to develop an optimized micropropagation protocol for this elite cultivar.
2.1 Plant Materials and Culture Conditions
In this study, in vitro-grown shoots of Phalaenopsis cultivar 611B (purchased from Orchitech Co., Taipei City, Taiwan), derived from protocorm-like bodies, served as the experimental material. These shoots were sub-cultured bi-monthly and maintained on hormone-free Orchimax medium (Duchefa Biochemie, Haarlem, The Netherlands) supplemented with 7 g/L agar. The medium was adjusted to pH 5.7 before sterilization by autoclaving at 121°C for 15 min. Cultures were incubated in a controlled growth room at 24 ± 2°C under a 16-h photoperiod and a light intensity of 2000 lux (27 μmol·m−2·s−1).
2.2 Effects of Silver Nanoparticles on In Vitro Shoot and Root Regeneration
To investigate the effects of Ag-NPs on in vitro shoot and root formation, shoots approximately 1 cm in length were selected. Shoot tips (2–3 mm) were excised and cultured on a regeneration medium containing 1 g/L Hyponex 2 (20:20:20), 2 g/L Hyponex 1 (6.5:6.5:19), 18 g/L sugar, 2 g/L peptone, 0.8 g/L activated charcoal, 12.5 g/L potato extract, 50 mL/L apple juice, 10 mg/L 6-benzylaminopurine (6-BA), 7 g/L agar, and varying concentrations of Ag-NPs (0, 0.5, 1, 2, and 2.5 mg/L). The Ag-NPs used had an average diameter of 10 nm. The medium was adjusted to pH 5.7 and sterilized by autoclaving at 121°C for 15 min. The excised shoot tips were cultured in test tubes containing the treatment media and maintained under the same environmental conditions described above. Each treatment contained 20 explants and was replicated three times.
After a 10-week culture period, data were collected on shoot and root formation frequency, fresh weight (FW), the average number of shoots and leaves per explant, and chlorophyll a and b contents. The FW was recorded after removing residual medium. The mean number of roots per explant was determined after 12 weeks of culture.
2.3 Chlorophyll Content Analysis
Chlorophyll content was determined following the method described in a previous study [9]. Chlorophyll a and b were extracted from 0.2 g of fresh leaf tissue and measured spectrophotometrically at 663 nm and 645 nm, respectively. Chlorophyll a content was calculated as [12.7 × A663 − 2.69 × A645] × V/1000 × W and chlorophyll b content as [22.9 × A645 − 4.86 × A663] × V/1000 × W. Here, V is the volume of the extract in milliliters, and W is the fresh leaf weight in grams. Chlorophyll measurements were performed on three biological replicates for each treatment.
Data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) and presented as means of three replicates. Mean comparisons were conducted using the least significant difference test (LSDT) at a significance level of p < 0.05.
3.1 Influence of Silver Nanoparticles on Shoot Regeneration and Root Induction
The application of Ag-NPs at varying concentrations significantly influenced shoot and root formation percentages, the number of shoots and roots per explant, and overall in vitro growth of Phalaenopsis orchid 611B. Shoot formation increased markedly with Ag-NP treatment, rising from approximately 55% in the control to 90% at 0.5 mg/L, and reaching 100% at 1.0 mg/L. Although the rate remained high at 2.0 mg/L (100%), it declined to below 80% at 2.5 mg/L (Fig. 1A). Root formation showed a similar pattern (Fig. 1B), starting at 10% in the control, increasing to 30% at 0.5 mg/L, peaking at 70% at 1.0 mg/L, then decreasing to 50% at 2.0 mg/L and returning to 10% at 2.5 mg/L.
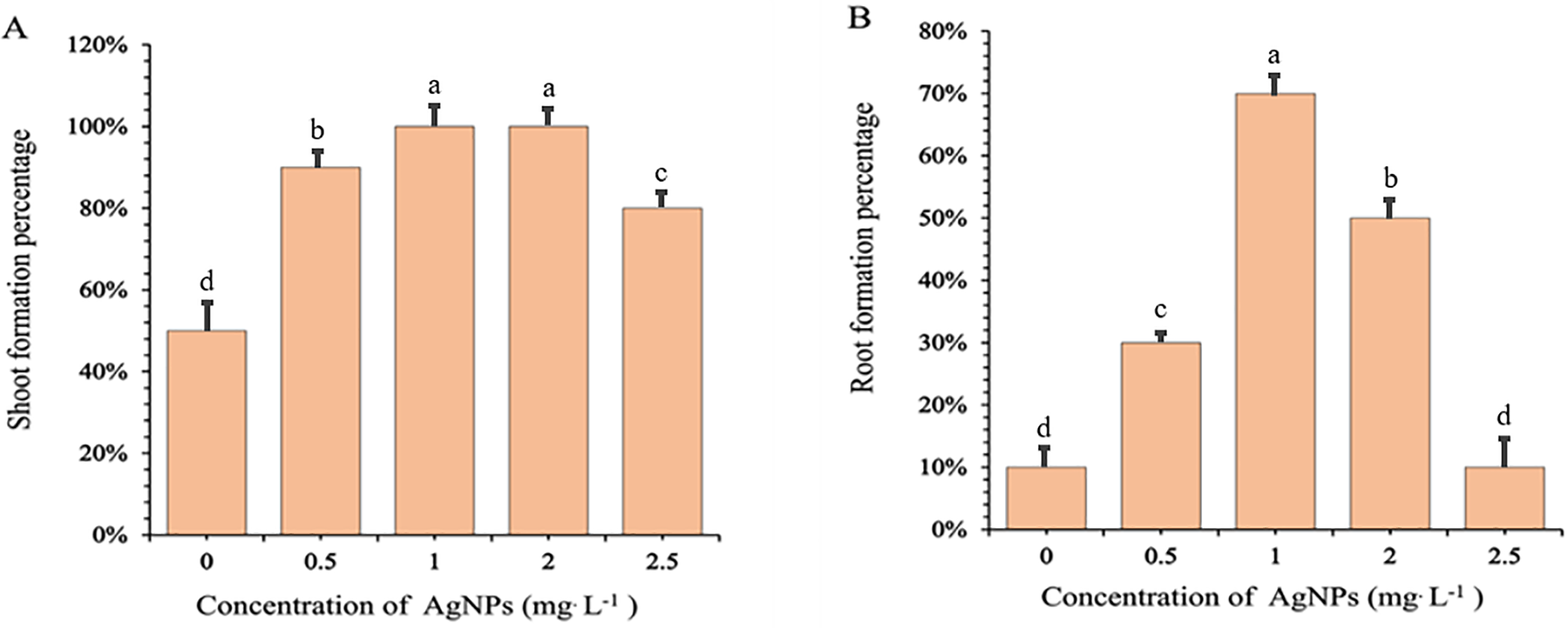
Figure 1: Effects of different concentrations of silver nanoparticles (Ag-NPs) on the percentages of shoots (A) and roots (B) formation in in vitro-regenerated Phalaenopsis orchids (cultivar 611B). Shoot formation percentages were recorded after 10 weeks of culture, while root formation percentages were recorded after 12 weeks. Means followed by the same letters are not significantly different according to the LSDT (p < 0.05). Error bars represent standard errors
The number of shoots per explant also increased significantly with Ag-NP concentrations up to 1.0 mg/L. Explants in the control group (0 mg/L) produced an average of 2.9 shoots, compared to 4.9 at 0.5 mg/L and a peak of 5.4 at 1.0 mg/L. However, shoot numbers declined at higher concentrations, dropping to 4.1 and 3.0 at 2.0 and 2.5 mg/L, respectively (Fig. 2A). Statistical analysis revealed that shoot regeneration was significantly enhanced at 0.5 and 1.0 mg/L, moderately effective at 2.0 mg/L, and not significantly different from the control at 2.5 mg/L. These trends were visually confirmed (Fig. 3A), with explants cultured at 0.5 and 1.0 mg/L displaying vigorous and compact shoot clusters. In contrast, plantlets exposed to 2.5 mg/L closely resembled the control in both shoot size and number.
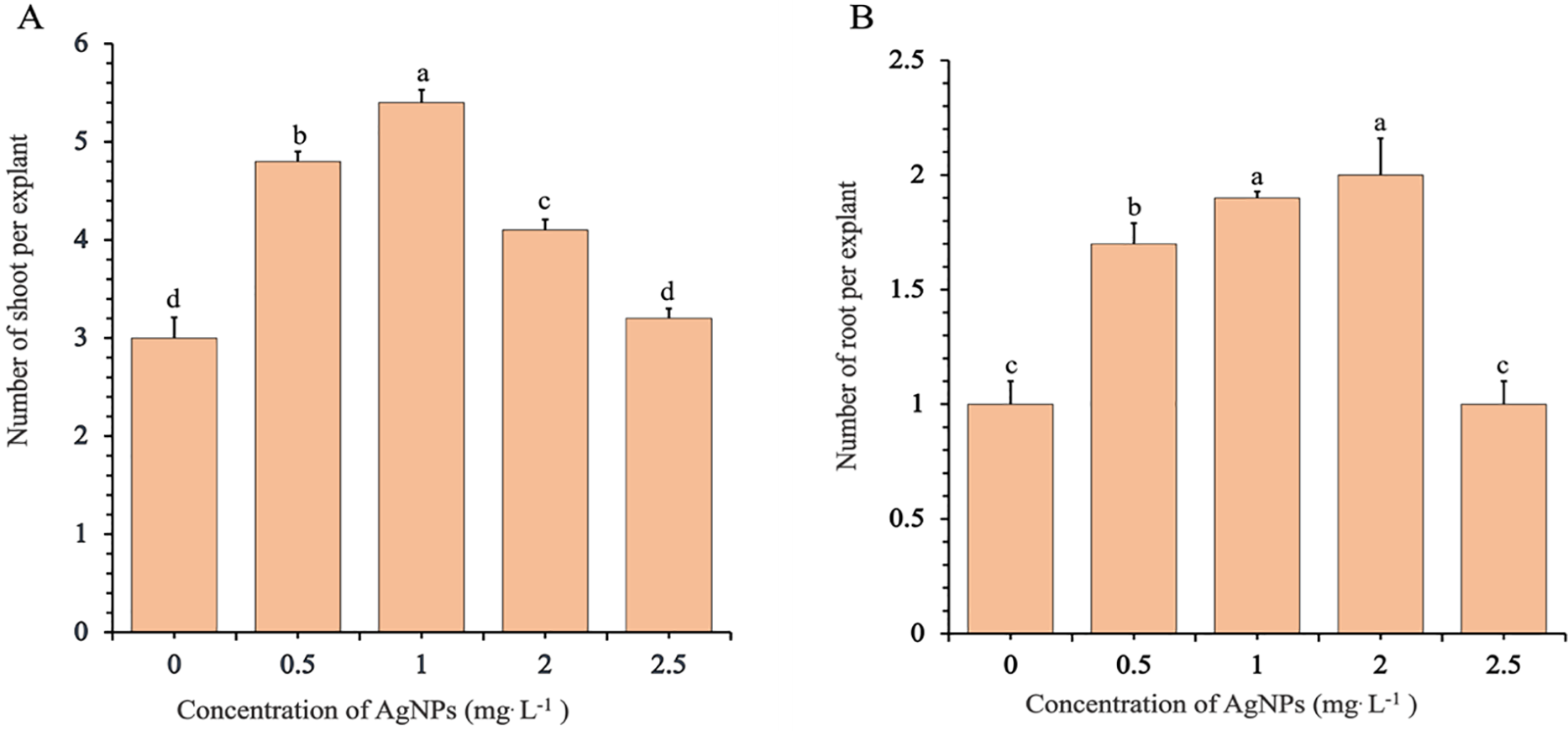
Figure 2: Effects of different concentrations of silver nanoparticles (Ag-NPs) on the mean number of shoots (A) and roots (B) in in vitro-regenerated Phalaenopsis orchids (cultivar 611B). The mean number of shoots was recorded after 10 weeks of culture, and the mean number of roots after 12 weeks. Means sharing according to the LSDT (p < 0.05). Error bars represent standard errors

Figure 3: Illustration of shoot formation (A) and root formation (B) in in vitro-regenerated Phalaenopsis orchids (cultivar 611B). Images of shoot formation were captured after 10 weeks of culture, and images of root formation after 12 weeks
Root induction followed a similar concentration-dependent pattern (Fig. 2B). The control group produced an average of 1.4 roots per explant, which increased to 1.9 and 2.1 roots at 0.5 and 1.0 mg/L Ag-NPs, respectively. A comparable root count (2.0) was observed at 2.0 mg/L, while a sharp decline occurred at 2.5 mg/L, with root numbers dropping to 1.4—statistically indistinguishable from the control. These results were consistent with visual observations of root development shown in Fig. 3B, where well-developed root systems were evident at 1.0 and 2.0 mg/L, while weaker root systems were observed at 2.5 mg/L and in the control.
Collectively, these results demonstrate that Ag-NP concentrations between 0.5 and 1.0 mg/L significantly enhanced shoot and root formation in Phalaenopsis orchids, with 1.0 mg/L consistently yielding the highest frequency and number of shoots and roots. In contrast, the highest concentration tested (2.5 mg/L) resulted in substantial reductions in both parameters, indicating potential phytotoxic effects at excessive Ag-NP levels. These findings highlight the importance of precise concentration control when applying Ag-NPs to optimize in vitro regeneration efficiency.
3.2 Influence of Silver Nanoparticleson In Vitro Plant Growth
Ag-NPs significantly affected several vegetative growth parameters in in vitro-regenerated Phalaenopsis plants, including leaf number, plant height, FW, and chlorophyll content. The number of leaves per explant was markedly affected by Ag-NP concentration (Fig. 4A). The control group produced an average of 5.5 leaves per explant, which increased significantly to 8.5 at 0.5 mg/L and peaked at 9.7 at 1.0 mg/L. However, higher concentrations resulted in a decline, with average leaf numbers dropping to 7.2 and 7.0 at 2.0 and 2.5 mg/L, respectively. These results indicate that elevated Ag-NP levels inhibit leaf development. Plant height followed a similar trend (Fig. 4B). The tallest plants were observed at 0.5 mg/L (1.66 cm), followed closely by 1.0 mg/L (1.64 cm), both of which were significantly taller than the control (1.58 cm). A notable reduction in height occurred at 2.0 mg/L (1.49 cm), with a slight recovery at 2.5 mg/L (1.58 cm), although this value was still lower than that at optimal concentrations. These findings suggest that low to moderate Ag-NP concentrations promote elongation, while higher concentrations suppress it.
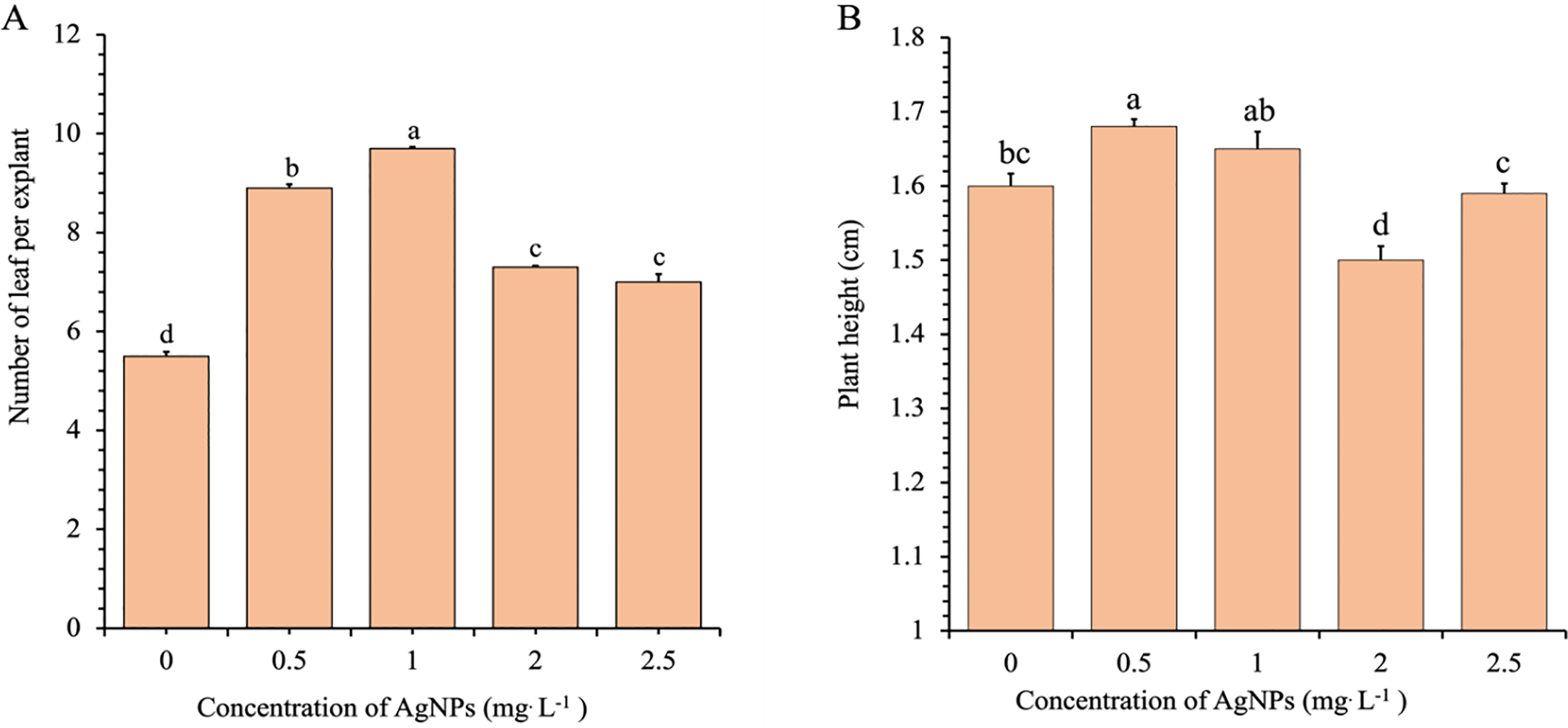
Figure 4: Effects of different concentrations of Ag-NPs on the number of leaves per explant (A) and plant height (B) of in vitro-regenerated Phalaenopsis orchids (cultivar 611B). Means sharing the same letters are not significantly different according to the LSDT (p < 0.05). Error bars represent standard errors
Fresh biomass accumulation also responded positively to moderate Ag-NP treatments (Fig. 5). The control group had a mean FW of 340.2 mg, which increased significantly to 573.8 mg and 592.4 mg at 0.5 and 1.0 mg/L, respectively. However, biomass declined substantially at 2.0 (390.5 mg) and 2.5 mg/L (370.6 mg), indicating that elevated Ag-NP concentrations may hinder biomass development.
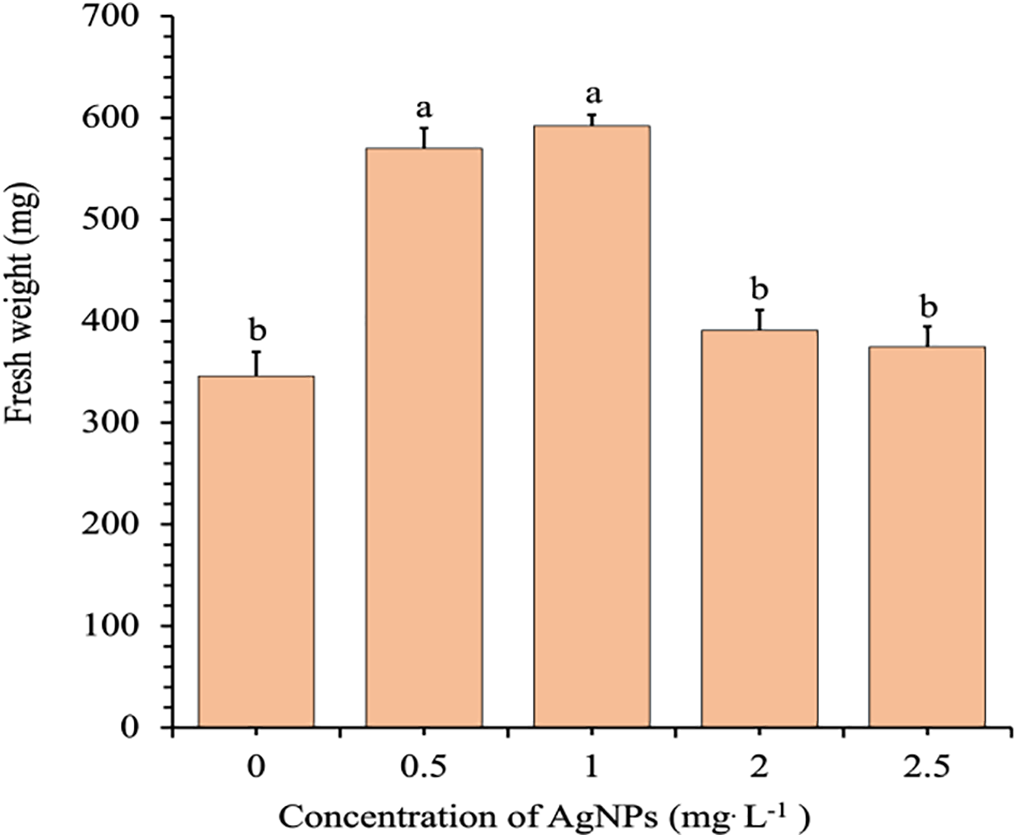
Figure 5: Effects of different concentrations of Ag-NPs on the FW of in vitro-regenerated Phalaenopsis orchid (cultivar 611B). Means sharing the same letters are not significantly different according to the LSDT (p < 0.05). Error bars represent standard errors
3.3 Chlorophyll Content Analysis
Chlorophyll content analysis revealed that Ag-NPs significantly influenced photosynthetic pigment accumulation (Fig. 6A,B). The highest chlorophyll a level (91.46 μg/L) was observed at 2.0 mg/L, which was significantly greater than that of the control (73.18 μg/L) and the 2.5 mg/L treatment (75.23 μg/L). Chlorophyll b content followed a similar pattern, peaking at 46.58 μg/L at 2.0 mg/L compared to 37.51 μg/L in the control. Although the 0.5 and 1.0 mg/L treatments also resulted in increased chlorophyll content relative to the control, these increases were lower than those observed at 2.0 mg/L. Notably, both chlorophyll a and b levels declined at 2.5 mg/L.
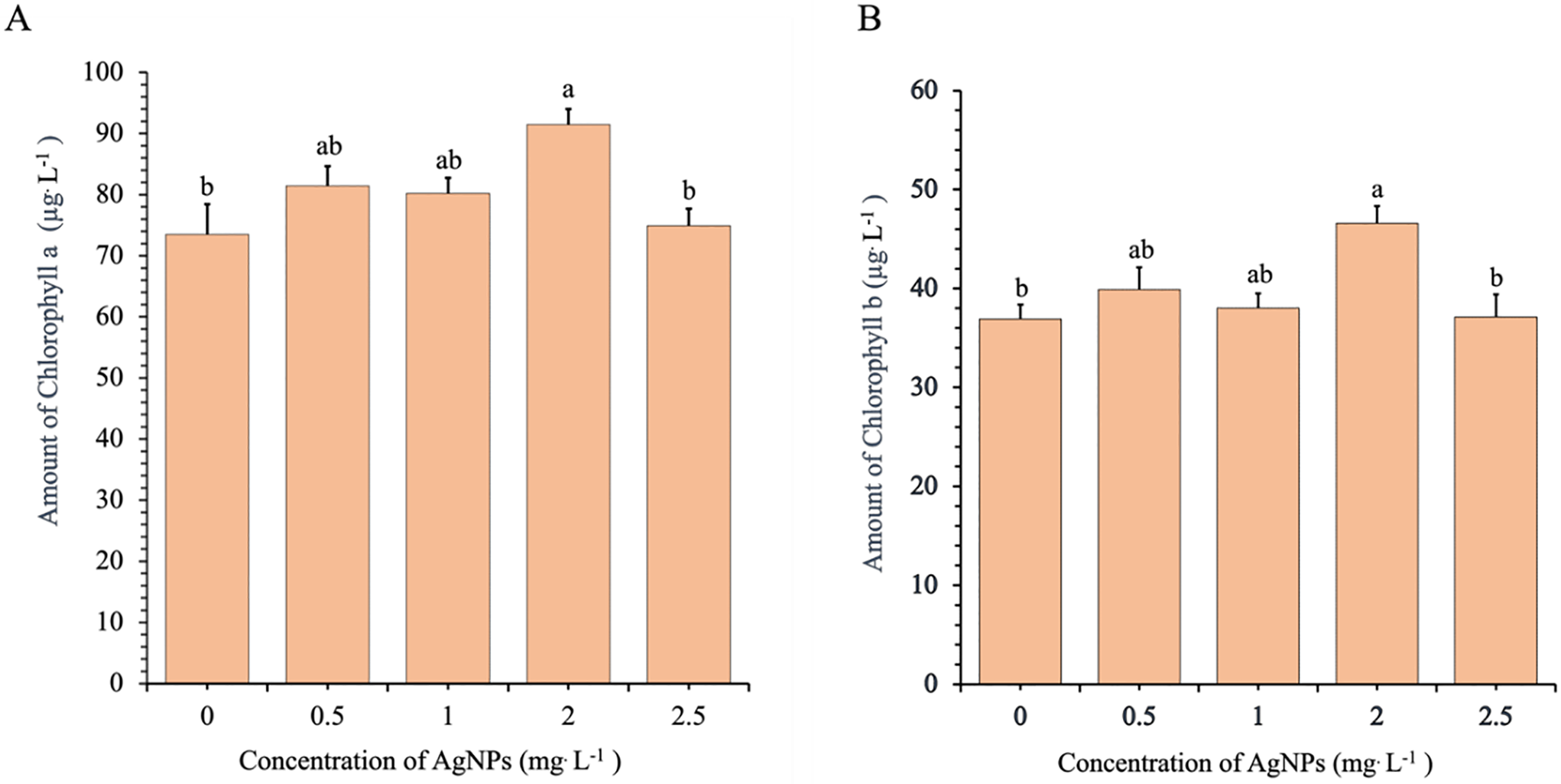
Figure 6: Effects of different concentrations of Ag-NPs on chlorophyll content: chlorophyll a (A) and chlorophyll b (B) in in vitro-regenerated Phalaenopsis orchid (cultivar 611B). Means sharing the same letters are not significantly different according to the LSDT (p < 0.05). Error bars represent standard errors
Overall, the data indicate that Ag-NP concentrations between 0.5 and 1.0 mg/L effectively promote vegetative growth, as indicated by increased leaf number, plant height, and biomass. In contrast, the highest chlorophyll accumulation was recorded at 2.0 mg/L, although the difference was not statistically significant compared to the lower concentrations. The consistent decline in all measured parameters at 2.5 mg/L indicates potential phytotoxic effects at elevated Ag-NP levels.
Ag-NPs are increasingly investigated for their potential to enhance in vitro plant regeneration. In this study, we demonstrated that Ag-NPs, when applied at specific concentrations, significantly improved shoot and root formation, plant growth, and physiological responses in Phalaenopsis cultivar 611B. However, these effects were clearly concentration-dependent: the most favorable outcomes were observed at 1.0 mg/L, while a notable decline in regeneration and growth occurred at 2.5 mg/L. This biphasic response aligns with previous findings in Phalaenopsis amabilis, where 0.5–1.0 mg/L Ag-NPs enhanced regeneration, but higher concentrations were either ineffective or inhibitory [9]. These inhibitory effects at elevated concentrations have been linked to oxidative stress, as higher Ag-NP levels can increase the accumulation of Reactive Oxygen Species (ROS), such as H2O2, and lipid peroxidation products, such as malondialdehyde. Similarly, Ref. [11] reported that excessive silver ions disrupted cellular redox homeostasis, triggering stress responses in plant tissues. Although ROS indicators were not measured in our study, the reduced growth performance at 2.5 mg/L suggests a comparable phytotoxic effect likely associated with oxidative stress. Notably, the optimal Ag-NP concentration varies considerably across species. For example, Vanilla planifolia responded positively to much higher Ag-NP concentrations (25–50 mg/L), with growth suppression only occurring at 100–200 mg/L [12]. Such interspecific variation may be attributed to differences in nanoparticle uptake efficiency, tissue permeability, antioxidant capacity, and metabolic activity. Orchids like Phalaenopsis may exhibit greater sensitivity to silver ions, necessitating lower concentrations to prevent toxicity. These findings underscore the importance of identifying genotype-specific concentration windows to maximize the beneficial effects of Ag-NPs while minimizing potential phytotoxicity.
Ag-NPs also markedly enhanced rooting efficiency, with 1.0 mg/L resulting in a sevenfold increase in root formation compared with the control. Similar root-promotion effects have been reported in other species [10,13–16]. One proposed mechanism involves the inhibition of ethylene signaling, as silver ions are known antagonists of ethylene receptors. Ref. [17] suggested that Ag-NPs promote rooting in Crocus sativus by inhibiting ethylene perception. Based on this, it is plausible that a similar hormonal modulation occurred in Phalaenopsis. However, this hypothesis remains speculative, as our study did not include hormone assays or gene expression analyses. Future studies using molecular or biochemical approaches are needed to validate this hypothesis. Another important consideration is the uptake and localization of Ag-NPs. In vitro, nanoparticles may enter plant tissues through passive diffusion, endocytosis, or wounds and cut surfaces [18,19]. Although the observed phenotypic improvements in our study suggest successful nanoparticle uptake, internalization was not directly confirmed. Techniques such as inductively coupled plasma mass spectrometry or transmission electron microscopy could be used in future research to verify Ag-NP distribution and assess potential accumulation or toxicity at the cellular level [20,21].
Although numerous studies have evaluated the effects of Ag-NPs in plant tissue culture, most have focused on generalized protocols or non-orchid species. To our knowledge, this is the first study to systematically optimize Ag-NP concentrations for in vitro regeneration in Phalaenopsis cultivar 611B—a genotype known for its low multiplication and rooting performance under conventional conditions. Our results provide a clear, cultivar-specific concentration–response profile, demonstrating that 1.0 mg/L Ag-NPs significantly enhance shoot regeneration, root development, vegetative growth, and overall plantlet quality. These findings provide a practical advancement in the micropropagation of this elite cultivar and contribute to the broader understanding of nanomaterial applications in orchid tissue culture.
This study demonstrates that Ag-NPs can effectively enhance the in vitro regeneration and growth of Phalaenopsis orchid (cultivar 611B), a genotype known for its low propagation efficiency. Ag-NPs significantly improved shoot and root formation, leaf number, fresh biomass, and chlorophyll content in a concentration-dependent manner. Among the concentrations tested, 1.0 mg/L consistently yielded the highest regeneration rates and plantlet vigor, identifying it as the optimal concentration for the micropropagation of this cultivar. In contrast, the highest concentration (2.5 mg/L) failed to improve growth or regeneration, likely due to phytotoxic effects associated with oxidative stress. This study is the first to establish a systematic, concentration-specific Ag-NP protocol for Phalaenopsis cultivar 611B. The findings highlight the importance of species- and genotype-specific nanoparticle application strategies and support the broader potential of nanotechnology to enhance the efficiency and scalability of orchid micropropagation.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Aung Htay Naing conceptualized and designed the study. Hay Mon Aung conducted the experiments. Chang Kil Kim assisted with the experimental procedures. Hay Mon Aung wrote the original draft. Aung Htay Naing and Kyeung II Park reviewed and edited the manuscript. Aung Htay Naing and Kyeung II Park also supervised the project. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Park SY, Murthy HN, Paek KY. Rapid propagation of Phalaenopsis from floral stalk-derived leaves. In Vitro Cell Dev Biol-Plant. 2002;38:168–72. doi:10.1079/IVP.2001274. [Google Scholar] [CrossRef]
2. Niknejad A, Kadir MA, Kadzimin SB. In vitro plant regeneration from protocorms-like bodies (PLBs) and callus of Phalaenopsis gigantea (Epidendroideae: orchidaceae). Afr J Biotechnol. 2011;10:11808–16. doi:10.5897/AJB10.2597. [Google Scholar] [CrossRef]
3. Balilashaki K, Vahedi M, Karimi R. In vitro direct regeneration from node and leaf explants of Phalaenopsis cv. Surabaya. Plant Tissue Cult Biotechnol. 2015;25(2):193–205. doi:10.3329/ptcb.v25i2.26254. [Google Scholar] [CrossRef]
4. Li YY, Hao ZG, Miao S, Zhang X, Li JQ, Guo SX, et al. Profiles of cytokinins metabolic genes and endogenous cytokinins dynamics during shoot multiplication in vitro of Phalaenopsis. Int J Mol Sci. 2022;23(7):3755. doi:10.3390/ijms23073755. [Google Scholar] [CrossRef]
5. Cruz KZ, da Silva Alencar AA, Santana JG, Alves LE. The auxin and cytokinin promotes the regeneration of shoots in Phalaenopsis Golden Peoker ‘BL’HCC/AOS (Orchidaceae). Braz J Dev. 2024;10(4):1–15. doi:10.34117/bjdv10n4-033. [Google Scholar] [CrossRef]
6. Sarmah D, Mohapatra PP, Seleiman MF, Mandal T, Mandal N, Pramanik K, et al. Efficient regeneration of in vitro derived plants and genetic fidelity assessment of Phalaenopsis orchid. Front Sustain Food Syst. 2024;8:1359486. doi:10.3389/fsufs.2024.1359486. [Google Scholar] [CrossRef]
7. Thangavelu RM, Gunasekaran D, Jesse MI, Mohammed Riyaz SU, Sundarajan D, Krishnan K. Nanobiotechnology approach using plant rooting hormone synthesized silver nanoparticle as nanobullets for the dynamic applications in horticulture—an in vitro and ex vitro study. Arab J Chem. 2016;11(1):48–61. doi:10.1016/j.arabjc.2016.09.022. [Google Scholar] [CrossRef]
8. Seliem MK, Abdalla N, El-Ramady HR. Response of Phalaenopsis orchid to selenium and bio-nano-selenium: in vitro rooting and acclimatization. Environ Biodivers Soil Secur. 2020;4(0):277–90. doi:10.21608/jenvbs.2020.42806.1107. [Google Scholar] [CrossRef]
9. Farrokhzad Y, Babaei A, Yadollahi A, Kashkooli AB, Mokhtassi-Bidgoli A, Hessami S. Informative title: development of lighting intensity approach for shoot proliferation in Phalaenopsis amabilis through combination with silver nanoparticles. Sci Hortic. 2021;292(2):110582. doi:10.1016/j.scienta.2021.110582. [Google Scholar] [CrossRef]
10. Tymoszuk A, Miler N. Silver and gold nanoparticles impact on in vitro adventitious organogenesis in chrysanthemum, gerbera and Cape Primrose. Sci Hortic. 2019;257(1):108766. doi:10.1016/j.scienta.2019.108766. [Google Scholar] [CrossRef]
11. Zareei A, Abbaspour H, Peyvandi M, Majd A. Physiological and molecular responses of basil (Ocimum basilicum) to silver stress: a comparison between silver nanoparticles and silver nitrate treatments. Biologia. 2024;79(6):1611–21. doi:10.1007/s11756-024-01627-3. [Google Scholar] [CrossRef]
12. Spinoso-Castillo JL, Chavez-Santoscoy RA, Bogdanchikova N, Pérez-Sato JA, Morales-Ramos V, Bello-Bello JJ. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. 2017;129(2):195–207. doi:10.1007/s11240-017-1169-8. [Google Scholar] [CrossRef]
13. Jadczak P, Kulpa D, Bihun M, Przewodowski W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia mill. Plant Cell Tissue Organ Cult. 2019;139(1):191–7. doi:10.1007/s11240-019-01656-w. [Google Scholar] [CrossRef]
14. Zia M, Yaqoob K, Mannan A, Nisa S, Raza G, Rehman RU. Regeneration response of carnation cultivars in response of silver nanoparticles under in vitro conditions. Vegetos. 2020;33(1):11–20. doi:10.1007/s42535-019-00074-9. [Google Scholar] [CrossRef]
15. Tung HT, Thuong TT, Cuong DM, Luan VQ, Hien VT, Hieu T, et al. Silver nanoparticles improved explant disinfection, in vitro growth, runner formation and limited ethylene accumulation during micropropagation of strawberry (Fragaria × ananassa). Plant Cell Tissue Organ Cult. 2021;145(2):393–403. doi:10.1007/s11240-021-02015-4. [Google Scholar] [CrossRef]
16. Elsayh SA, Arafa RN, Ali GA, Abdelaal WB, Sidky RA, Ragab TI. Impact of silver nanoparticles on multiplication, rooting of shoots and biochemical analyses of date palm Hayani cv. by in vitro. Biocatal Agric Biotechnol. 2022;43(26):102400. doi:10.1016/j.bcab.2022.102400. [Google Scholar] [CrossRef]
17. Rezvani N, Sorooshzadeh A, Farhadi N. Effect of nano-silver on growth of saffron in flooding stress. World Acad Sci Eng Technol. 2012;1:517–22. [Google Scholar]
18. Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408(16):3053–61. doi:10.1016/j.scitotenv.2010.03.031. [Google Scholar] [PubMed] [CrossRef]
19. Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, Wiesner MR. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—critical review. Nanotoxicology. 2016;10(3):257–78. doi:10.3109/17435390.2015.1048326. [Google Scholar] [PubMed] [CrossRef]
20. Bao D, Oh ZG, Chen Z. Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis. Front Plant Sci. 2016;7(37):32. doi:10.3389/fpls.2016.00032. [Google Scholar] [PubMed] [CrossRef]
21. Wang J, Yue L, Zhao J, Cao X, Wang C, Chen F, et al. Uptake and bioaccumulation of nanoparticles by five higher plants using single-particle-inductively coupled plasma-mass spectrometry. Environ Sci Nano. 2022;9(8):3066–80. doi:10.1039/D1EN01195B. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools