 Open Access
Open Access
REVIEW
A Review: Sources, Preparation and Application of Nanocellulose
1 College of Mechanical Engineering, Jiamusi University, Jiamusi, 154007, China
2 College of Materials Science and Engineering, Jiamusi University, Jiamusi, 154007, China
* Corresponding Author: Qiang He. Email:
(This article belongs to the Special Issue: Sustainable Development and Multifunctional Application of Cellulose Composites)
Journal of Polymer Materials 2025, 42(2), 379-409. https://doi.org/10.32604/jpm.2025.066695
Received 15 April 2025; Accepted 06 June 2025; Issue published 14 July 2025
Abstract
Nanocellulose, derived from abundant lignocellulosic biomass, has emerged as a transformative material with unparalleled versatility across industries. This review systematically analyzes its sources, extraction methods, and multidimensional applications. Key findings include: (1) Plant fiber hierarchy dictates nanocellulose properties, with wood-derived cellulose offering high crystallinity and agricultural waste enabling cost-effective production. (2) Acid hydrolysis remains dominant for cellulose nanocrystals (CNCs), while mechanical methods yield high-aspect-ratio cellulose nanofibrils (CNFs). (3) Nanocellulose’s mechanical strength, biocompatibility, and tunable surface chemistry drive innovations in energy storage (e.g., supercapacitors), biosensors (e.g., glucose monitoring), and biomedical engineering (e.g., 3D-printed scaffolds). Challenges in scalability and sustainability persist, necessitating green synthesis and standardized protocols. This work underscores nanocellulose’s potential as a cornerstone of the circular bioeconomy.Keywords
Contemporary technological progress and rapid economic expansion have yielded substantial benefits for human society. However, these developments have concurrently accelerated environmental degradation through direct and indirect pathways. Consequently, sustainable resource management and environmental preservation have garnered significant global attention. Transitioning to clean, renewable energy sources as alternatives to fossil fuels has emerged as a critical imperative for achieving a sustainable development paradigm [1]. Cellulose, a polysaccharide composed of D-glucopyranose units linked by β-1,4-glycosidic bonds, constitutes the primary structural component of plant cell walls in biomass such as wood, bamboo, and cotton [2]. As an industrially relevant biopolymer, cellulose possesses unique attributes including biodegradability and renewability, distinguishing it from synthetic polymers [3]. Despite these advantages, cellulose exhibits inherent limitations in mechanical strength, thermal stability, and chemical resistance, constraining its broader industrial utilization [4]. Nanoscale processing of cellulose materials presents a viable strategy for enhancing their functional characteristics and broadening application potential. The extraction of nanocellulose from agricultural and forestry byproducts offers dual ecological and economic advantages, enabling high-value product development while mitigating pollution from biomass incineration.
Nanocellulose, the fundamental structural constituent of cellulose, exhibits nanoscale cross-sectional dimensions and tunable aspect ratios. This material comprises three primary variants: (1) Cellulose nanocrystals (CNCs), typically rod-like nanoparticles with diameters of 5–70 nm and lengths of 100–250 nm, characterized by high crystallinity (70%–85%) due to acid hydrolysis-induced removal of amorphous regions; (2) Cellulose nanofibers (CNFs), elongated fibrils with diameters of 2–20 nm and lengths exceeding 1 μm, retaining both crystalline and amorphous domains, resulting in high aspect ratios (L/D > 100) and mechanical flexibility; and (3) Bacterial nanocellulose (BNC), synthesized by microbial fermentation, with diameters <100 nm and ultra-high purity [5,6]. Nanocellulose exhibits distinctive physicochemical properties such as exceptional mechanical strength, high crystallinity, extensive surface area, and robust adsorption capabilities. Its abundant surface hydroxyl moieties facilitate chemical functionalization, thereby enabling the design of advanced nanocomposites with tailored properties [7]. Current research highlights its applicability in biomedical engineering and environmental remediation. For instance, nanocellulose derived from lignocellulosic biomass is utilized in fabricating reinforced composites, selective filtration membranes, oil-absorbent substrates, and flexible electronics [8].
Despite these advances, challenges persist in reconciling scalability with sustainability. Conventional acid hydrolysis generates acidic waste, while mechanical fibrillation consumes substantial energy (20–50 kWh/kg) [9]. Recent trends emphasize green synthesis methods, such as enzymatic hydrolysis and ionic liquid-assisted extraction, which minimize environmental footprints [10]. This review focuses on the fabrication and functionalization of nanocellulose, highlighting their transformative potential in sustainable technology. We systematically analyze: (1) the structural hierarchy of plant fibers and their implications for nanocellulose isolation; (2) state-of-the-art extraction techniques (chemical, mechanical, and biological), and (3) emerging applications across zero-dimensional to three-dimensional (0D to 3D materials) [11–13]. By synthesizing these insights, this review aims to guide researchers and industry stakeholders in optimizing nanocellulose production and unlocking its full potential in the circular bioeconomy.
2 Hierarchical Structure of Plant Fibres
Trees, as higher plants, possess a distinct cellular hierarchical structure. As depicted in Fig. 1, the walls of wood’s tubular cells are composed of the primary wall, secondary wall, middle lamella, and lumen, with the lumen ranging from 10 to 80 μm [14,15]. The middle lamella, typically 0.5–2 μm thick, connects neighboring cells during cell expansion and differentiation. Initially composed of pectin, this layer becomes highly lignified (up to 70%) as the plant matures. Lignin in the middle lamella retains water in the fibers and stabilizes the cell wall, enabling plant stems to withstand gravitational and environmental forces. In contrast, pectin acts as an adhesive between cell walls, facilitating their interaction [16]. The lumen, which forms as organelles degrade and fibers collapse, functions as the water-transporting tissue. The primary wall, formed during cell division and growth, is a thin, loose network of fibers, approximately 0.03–0.1 μm thick. Due to the difficulty in distinguishing the middle lamella from the primary wall, they are collectively referred to as the compound middle lamella (CML).
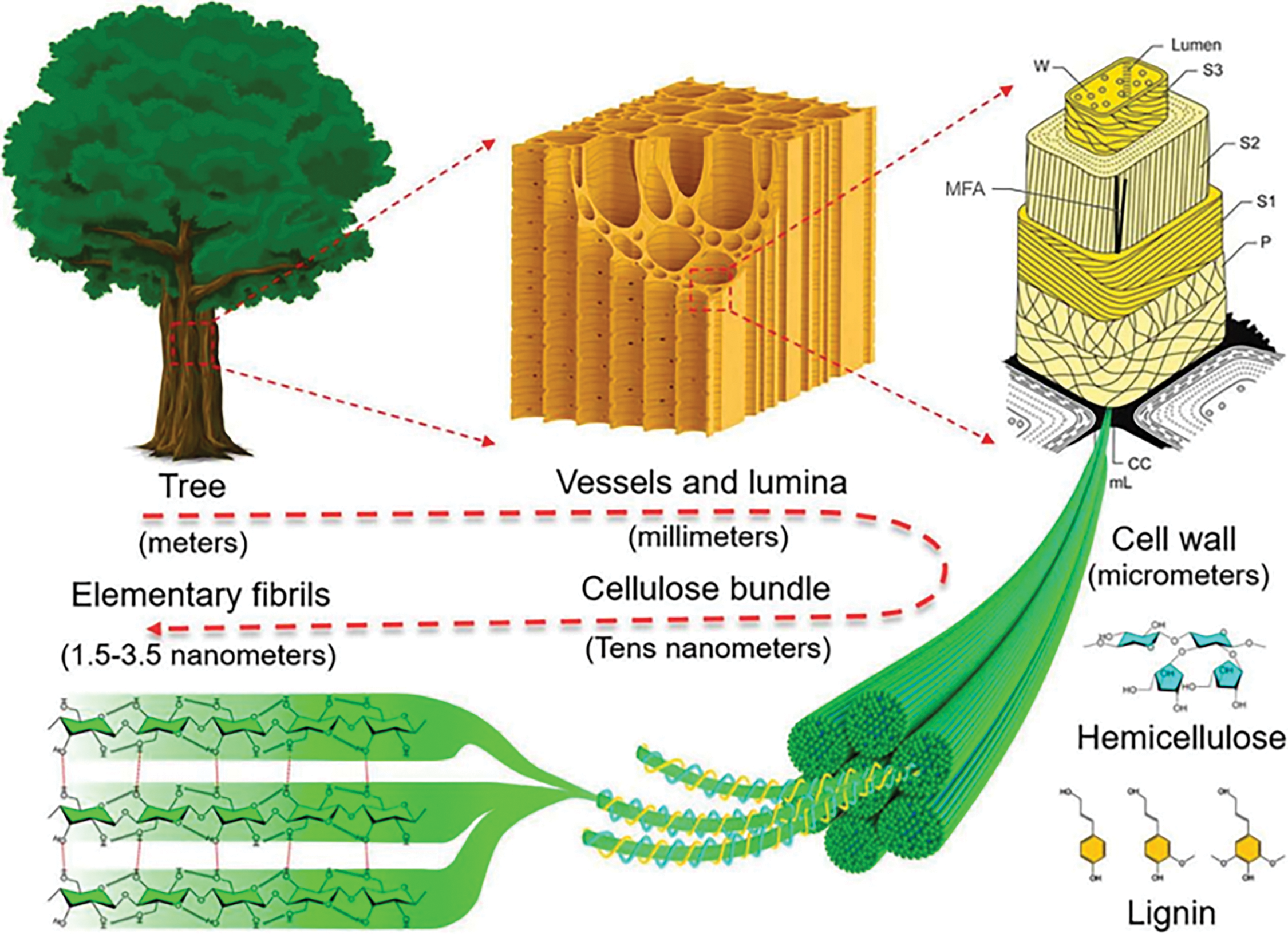
Figure 1: Schematic illustration of hierarchical structure of wood, from macroscopic to molecular scale
The secondary wall (S-layer), which forms after the primary wall, is the primary structural component of plant fibers and is composed of three layers with varying cellulose content. The outermost layer (S1) has a thickness of 0.1–0.3 μm, while the middle layer (S2), containing the highest cellulose content, measures 1–5 μm. The innermost layer (S3), adjacent to the lumen, has a thickness of 0.04–0.1 μm. In each of these layers, the microfibrils are arranged in a characteristic helical pattern relative to the fiber axis. The angle between the fiber axis and the microfibrils, known as the Microfibril Angle (MFA), varies depending on the specific cell wall layer and the plant species [17,18].
The wood cell wall consists primarily of three components: 40–50 wt% cellulose, 20–35 wt% hemicellulose, and approximately 20–30 wt% lignin. Fibers derived from the wood cell wall, known as cellulose bundles, consist of microfibrillated cellulose with diameters ranging from 20 to 50 nm. Through further refinement, nanofibrillated cellulose with diameters between 5 and 20 nm can be isolated, while smaller fibers, ranging from 1.5 to 3.5 nm in diameter, are also present in nanofibrillated cellulose. On the molecular level, cellulose is a linear, rigid macromolecule composed of repeating β-1,4-pyranose units that interact through covalent, intramolecular, and intermolecular hydrogen bonds [19].
Cellulose (C6H10O5)n is a thermoplastic natural polymer characterized by crystalline and amorphous regions [20]. It is a homopolymer composed of linear chains of β-glucan units linked by (1 → 4)-glycosidic bonds (Fig. 2). These (1 → 4) bonds contribute significantly to the strength and rigidity of cellulose. The average degree of polymerization ranges from 8000 to 10,000, with the molecular chains typically extending to an average length of approximately 5000 nm, depending on the plant species. The cellulose fiber structure comprises two primary regions: crystalline cellulose, which is highly ordered, and amorphous cellulose, which exhibits lower order [21–23]. The degree of crystallinity can be quantified using techniques such as X-ray diffraction (XRD) and solid-state 13C nuclear magnetic resonance (NMR) [24,25]. Notably, cellulose remains structurally stable in most solvents, except under acidic, alkaline, or ionic liquid-mediated conditions. This stability is primarily attributed to its fibrous architecture and extensive hydrogen bonding, which confer exceptional tensile strength [26].
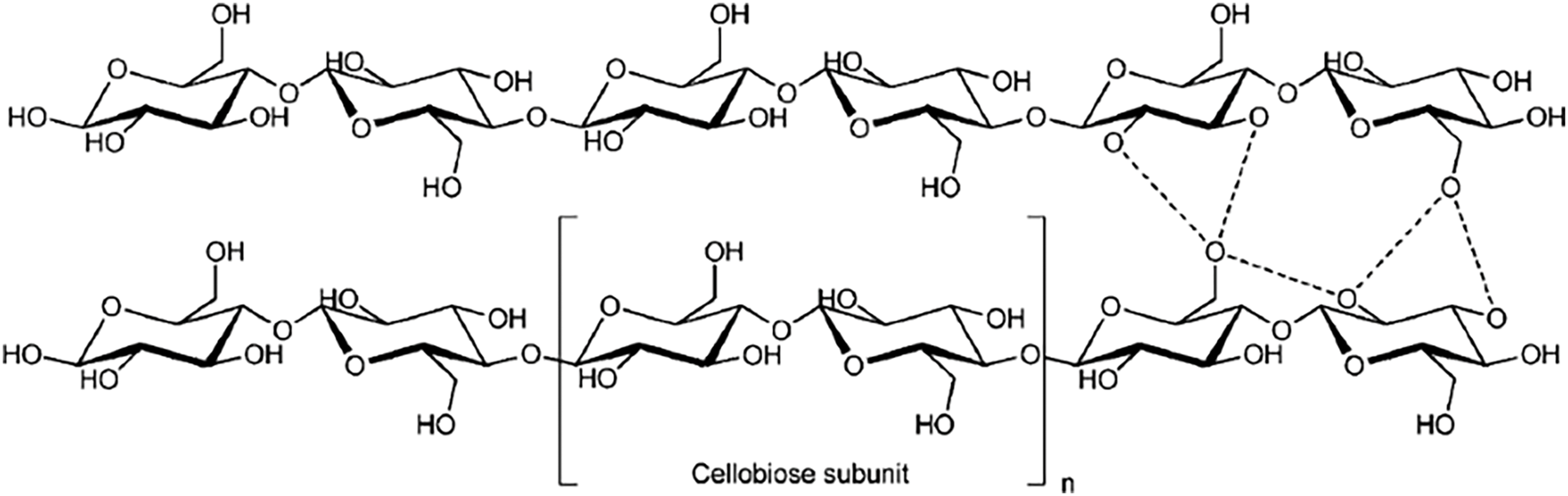
Figure 2: Structure of cellulose
Hemicellulose is a heterogeneous polysaccharide synthesized through a biosynthetic pathway distinct from that of cellulose. This biopolymer can exhibit both linear and branched structures. Functioning primarily as a supporting material, hemicellulose is closely associated with lignin in the plant cell wall. Fig. 3 illustrates the primary monomeric components of hemicellulose. It comprises sugar units containing either five-carbon (pentose) or six-carbon (hexose) structures, along with minor quantities of sugar acids such as rhamnose, glucuronide, methylglucuronide, and galacturonide [27]. Six-carbon sugars, including mannose, glucose, and galactose, are the most prevalent. Due to its heterogeneous and amorphous nature, hemicellulose is highly susceptible to decomposition under heat and acidic conditions. The most common pentose sugars in hemicellulose are xylose and arabinose, with approximately 70% of hemicellulose structures (xylose residues) acetylated at the 2 or 3 positions of their sugar rings [28]. Hemicellulose composition varies between hardwoods and softwoods. In hardwoods, it primarily consists of xylans with a degree of polymerization ranging from 150 to 200. Conversely, in softwoods, glucans dominate, with a degree of polymerization between 70 and 130.
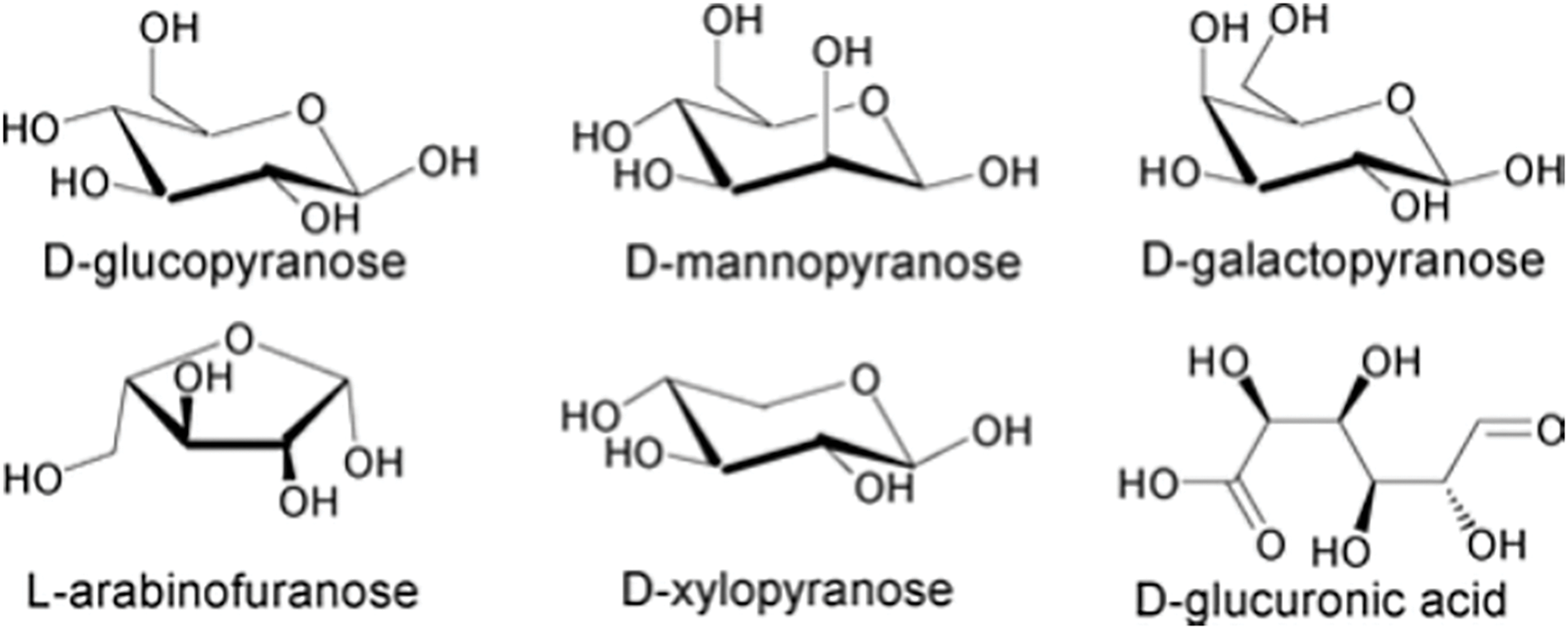
Figure 3: Major monomers in hemicellulose
Lignin is the predominant polymer in the secondary cell walls of woody biomass and possesses a chemical structure more complex than that of cellulose and hemicellulose. It is a three-dimensional, high-molecular-weight amorphous polymer composed of methoxyphenylpropane units, constituting approximately 17%–30% of wood by weight [29]. Lignin is typically isolated as an insoluble residue through hydrolysis, a process that removes polysaccharides from non-extracted wood. Functionally, lignin acts as a structural component within the cell wall, coexisting with cellulose and forming a physical barrier that protects plant cells. One of lignin’s primary roles is to shield cellulose and other carbohydrates from enzymatic degradation [30]. The polymer is derived from three main types of hydroxycinnamic alcohol monomers, each with varying degrees of methoxylation: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (commonly referred to as monolignols) (Fig. 4) [31].
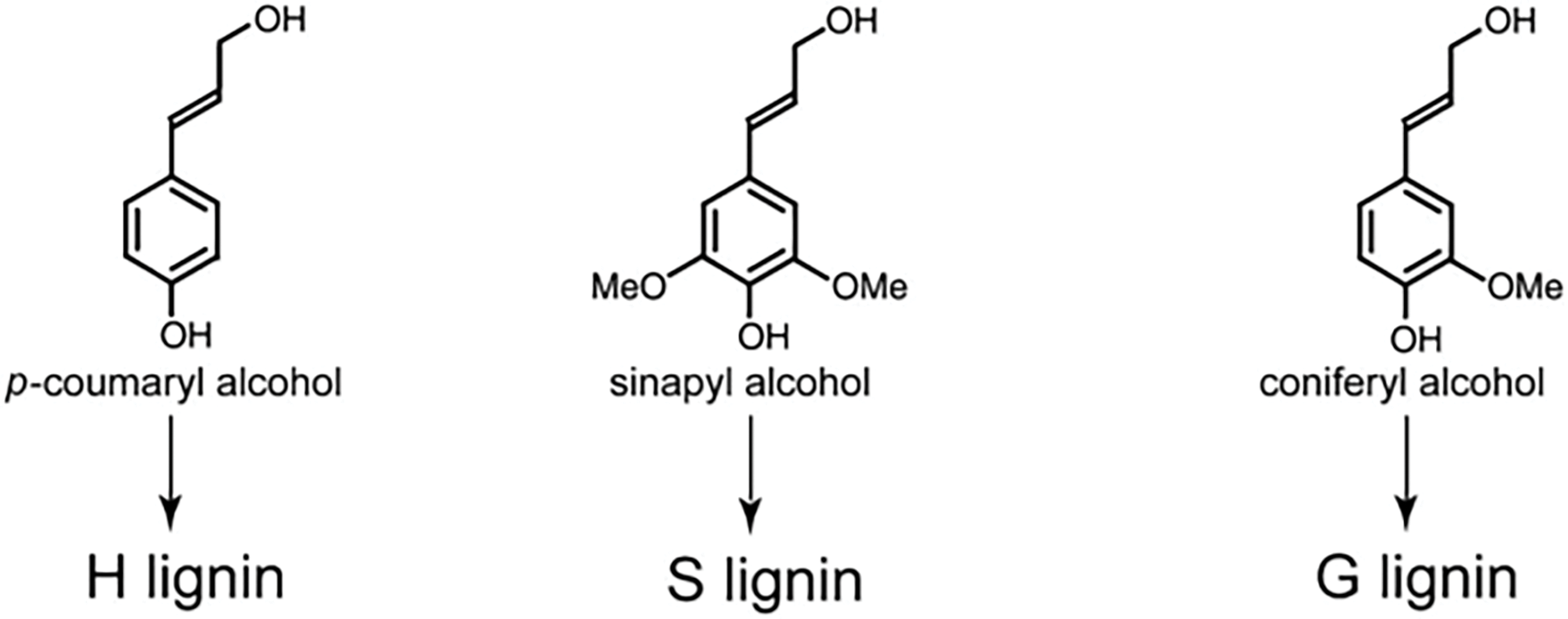
Figure 4: Major alcohol monomer-type structures present in the lignin structure
The composition of lignin varies across plant species, with softwoods, hardwoods, and grasses containing different proportions of these monolignols [32].
3.1 Sources and Classification of Cellulose
Cellulose can be isolated from various biomass sources, including wood, agricultural wastes, marine animals (e.g., periphyton), and algae. The choice of biomass significantly impacts nanocellulose properties: wood-derived cellulose exhibits higher crystallinity (60%–80%) and microfibril alignment due to its dense hierarchical structure, whereas agricultural residues like banana peels or pistachio shells require extensive purification to remove lignin and hemicellulose, often resulting in lower crystallinity (40%–60%) but higher surface charge post-acid hydrolysis. These variations influence mechanical strength, thermal stability, and compatibility with polymer matrices [33–36]. There are approximately 370,000 plant species worldwide, and differentiating, studying, and utilizing these plants are crucial for maximizing resource efficiency. In China’s pulp and paper industry, plant raw materials are broadly classified into two categories: wood and non-wood fibres [37,38]. Cellulose is classified based on its origin into plant-based, bacterial, and novel sources (e.g., algae, fungi). This classification reflects structural and compositional variations that critically influence nanocellulose properties. Fig. 5 illustrates the hierarchical categorization of natural cellulose sources, emphasizing their distinct extraction protocols and application potentials.
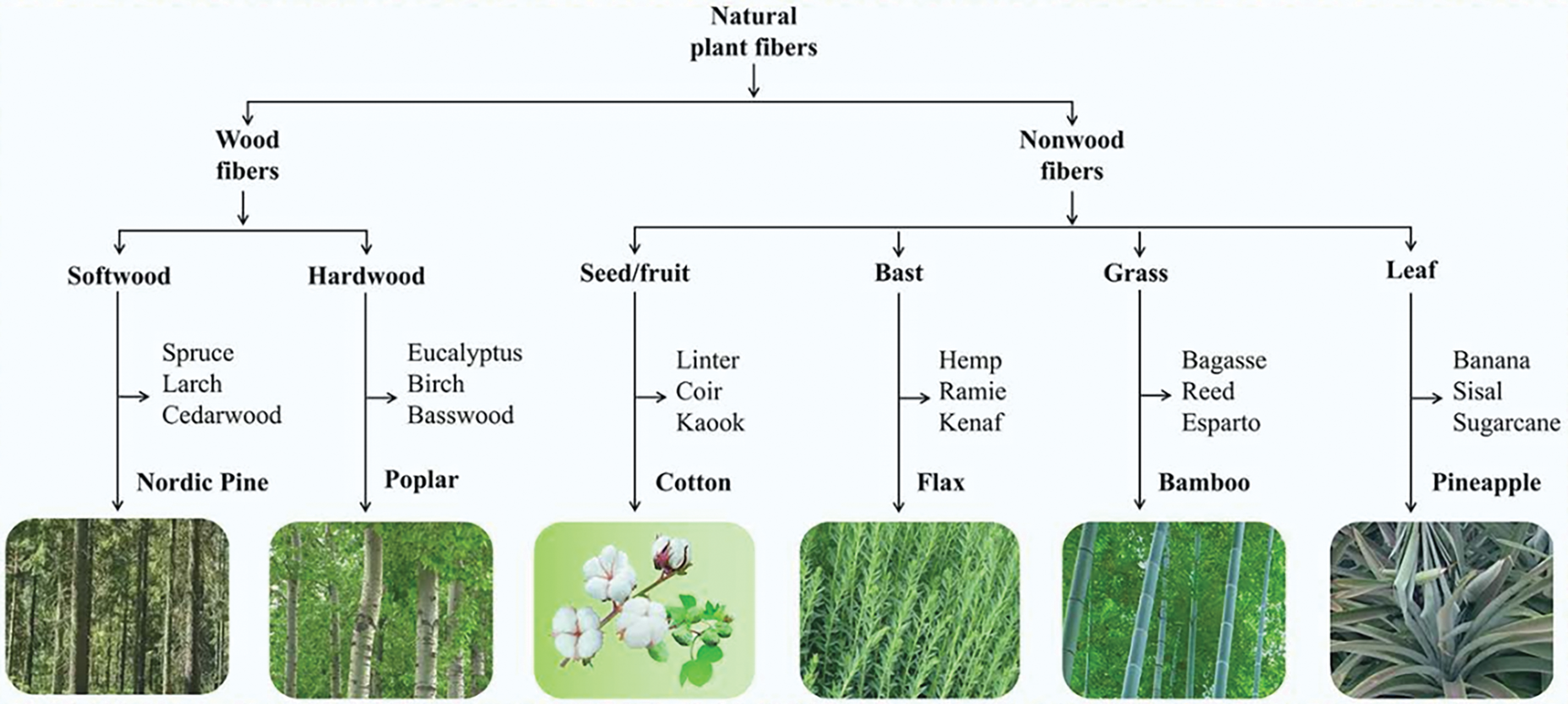
Figure 5: Hierarchy graph for natural plant fiber classification
Plant-derived cellulose accounts for >90% of global nanocellulose production. It is further subclassified based on feedstock type: Wood Pulp: Hardwoods (e.g., poplar, eucalyptus): Shorter fibers (1–2 mm), high crystallinity (70%–85%), and low lignin content, yielding thermally stable nanocellulose for optical films [33,38]. Softwoods (e.g., pine, spruce): Longer fibers (3–5 mm), higher microfibril angles (MFAs), and mechanical flexibility, ideal for composite reinforcement [37]. Agricultural Residues (e.g., sugarcane bagasse, rice straw, corn husks): Require intensive delignification but offer cost-effective nanocellulose with high aspect ratios (L/D > 100) for emulsion stabilization [34,39]. Non-Wood Fibers: Bast fibers (e.g., hemp, flax): High cellulose purity (80%–90%), long fibers (5–55 mm), and superior mechanical strength for textiles [40]. Leaf fibers (e.g., sisal): Short fibers (1–8 mm) with high lignin content, suited for rigid composites [41].
3.1.2 Bacterial Cellulose (BC)
Synthesized by bacteria such as Komagataeibacter hansenii, BC lacks lignin/hemicellulose, enabling high purity (99%) and crystallinity (84%–89%). Its 3D nanofibrillar network (5–20 nm diameter) is tailored for biomedical scaffolds and flexible electronicsv [42,43].
Algae-Derived Cellulose: Extracted from Cladophora spp., featuring high crystallinity (70%–80%) and colloidal stability for drug delivery [44]. Fungal Cellulose: Produced by Aspergillus niger via fermentation, offering rapid synthesis and moderate crystallinity (50%–60%) for sustainable packaging [45].
Cellulose nanocrystals (CNCs), rod-like nanoparticles derived from acid hydrolysis, typically exhibit with a smaller aspect ratio than cellulose nanofibrils (CNFs), produced via mechanical fibrillation, retain both crystalline and amorphous regions. During CNC preparation, the amorphous regions of natural cellulose are significantly disrupted, while the crystalline regions remain intact, resulting in high crystallinity. CNCs were first synthesized in 1949 through sulfuric acid hydrolysis of wood pulp cellulose, a method still widely used today. Currently, CNCs are primarily produced via acid hydrolysis using either inorganic or organic acids [6]. Compared to CNFs, CNC production through acid hydrolysis yields lower quantities (20%–35%) due to the degradation of the amorphous cellulose zones [40]. CNC suspensions are considered “all-nano,” meaning most plant-based CNCs have diameters of 5–10 nm and lengths of 100–300 nm, leading to a narrower and more uniform size distribution compared to other nanocellulose types [46,47].
Nanocellulose preparation methods are generally classified into “top-down” and “bottom-up” approaches. The “top-down” approach involves processing cellulose-containing raw materials, primarily plant fibers, through biological, physical, and chemical treatments to achieve nanoscale dimensions, as illustrated in Fig. 6. In contrast, the “bottom-up” approach synthesizes nanocellulose from low-molecular-weight saccharides via bacterial synthesis or from dissolved cellulose (or cellulose derivatives) through electrostatic spinning. While bacterial cellulose (BC) is synthesized via a “bottom-up” approach, its unique biosynthesis process and niche applications (e.g., biomedical scaffolds) are beyond the scope of this review’s focus on plant-derived nanocellulose. Thus, BC is discussed only briefly in Section 3.3 for comparative context. The hierarchical classification of natural plant fibers (Fig. 5) directly correlates with the structural and functional properties of derived nanocellulose. For instance, softwoods (e.g., pine, spruce) yield cellulose with high microfibril angles (MFAs) and longer fibers, contributing to nanocellulose with enhanced mechanical strength and flexibility, ideal for composite reinforcement [33,37]. In contrast, hardwoods (e.g., eucalyptus, poplar) possess lower MFAs and higher cellulose crystallinity, resulting in nanocellulose with superior thermal stability and uniform morphology, suitable for optical and barrier applications [6,38]. Non-wood fibers, such as bast fibers (flax, ramie) and seed hairs (cotton), are rich in cellulose content (80%–90%) and exhibit low lignin residues post-purification, enabling high-purity nanocellulose with exceptional hydrophilicity and biocompatibility for biomedical uses [40,46]. Agricultural residues (e.g., banana peel, sugarcane bagasse) often contain amorphous regions that facilitate efficient fibrillation, producing nanocellulose with high aspect ratios (L/D > 100) for Pickering emulsion stabilization [48,49]. Bacterial cellulose, though not plant-derived, offers unique advantages such as ultra-high crystallinity (84%–89%) and tunable nanostructures, making it valuable for advanced electronics and tissue engineering [50]. This diversity in fiber sources underscores the importance of selecting appropriate raw materials to tailor nanocellulose properties for targeted applications. Following the classification of nanocellulose variants, the next section systematically examines the sources of nanocellulose, emphasizing how structural differences in raw materials influence extraction efficiency and final properties.
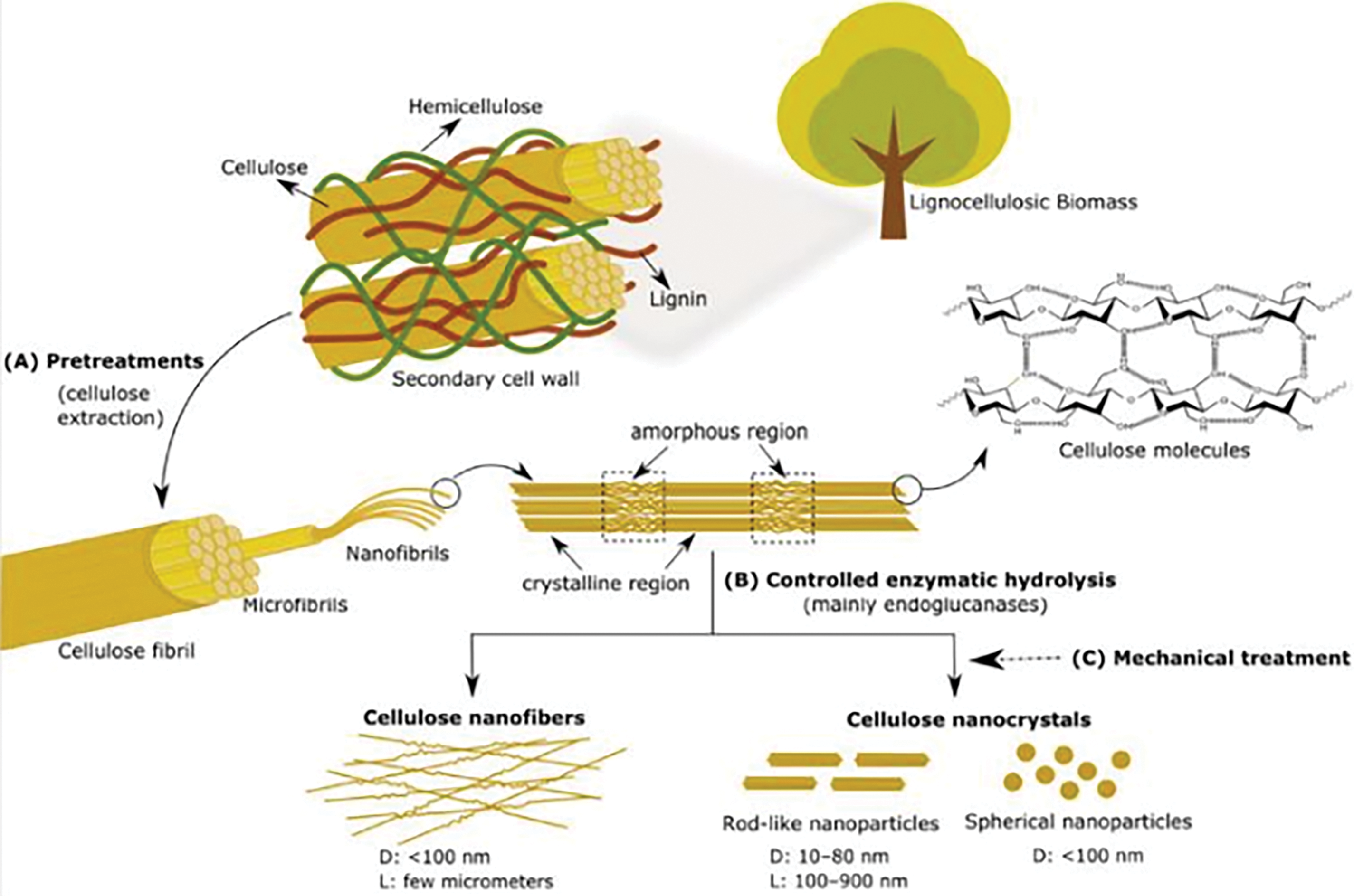
Figure 6: “Top-bottom” preparation of cellulose nanofibers and cellulose nanocrystal [48]
Nanocellulose can theoretically be derived from any cellulosic material. Wood fibres (softwoods: 3–5 mm; hardwoods: 1–2 mm) are longer than agricultural waste fibres (e.g., banana peel: 335–455 μm) but require similar mechanical/chemical processing. Unique sources like hemp (bast fiber, 5–55 mm) and sisal (leaf fiber, 1–8 mm) offer distinct advantages in nanocellulose properties due to their high crystallinity and mechanical strength [41]. Additionally, cellulose is also sourced from bacteria (e.g., Acetobacter xylinum) and certain marine organisms [51]. Agricultural and forestry waste also serve as significant sources of cellulose. With rapid population growth and urbanization, the global demand for food has surged, resulting in substantial agricultural processing waste. Traditionally, such waste is managed by using it as animal feed, organic fertilizer, or by incineration, dumping, and landfilling [41]. However, methods like incineration and landfilling are resource-intensive and environmentally damaging. In contrast, extracting cellulose from agricultural waste is more efficient and less energy-intensive compared to natural cellulosic materials, making it a cost-effective alternative. The choice of biomass source critically influences nanocellulose properties. Wood-derived cellulose, owing to its higher cellulose content (40–50 wt%) and structural homogeneity, typically yields nanocellulose with higher crystallinity (70%–85%) and mechanical strength compared to agricultural waste sources [33,49]. Agricultural residues, such as banana peel or pistachio shells, often contain higher lignin, hemicellulose, and inorganic impurities (e.g., silica), necessitating harsher pretreatment steps (e.g., alkali/bleaching) that may degrade cellulose chains, reduce crystallinity, and broaden particle size distributions (Table 1) [34,39]. For example, nanocellulose from wood pulp exhibits narrower diameter ranges (5–20 nm) and higher thermal stability (>300°C), whereas agricultural waste-derived counterparts (e.g., garlic straw, walnut shells) show wider variations (6–50 nm) and lower degradation temperatures due to residual lignin and ash [52,53]. These differences underscore the importance of source selection and customized processing for target applications. Research has demonstrated successful extraction of nanocellulose from agricultural waste, as detailed in Table 1. This process is crucial for both environmental protection and effective waste utilization.
Key takeaways from Table 1 reveal that sulfuric acid hydrolysis (50%–64% concentration) combined with alkali pretreatment (e.g., NaOH) is the most widely adopted method for agricultural waste-derived nanocellulose, yielding rod-like cellulose nanocrystals (CNCs) with diameters of 5–50 nm [34–36]. However, this method generates acidic waste and requires intensive post-treatment (e.g., dialysis), raising environmental concerns. In contrast, mechanical methods such as high-pressure homogenization (HPH, a mechanical method involving high-energy shear forces) and ultrasonication produce cellulose nanofibrils (CNFs) with higher aspect ratios (e.g., 3000–3500 nm length for banana peel CNFs [39]), albeit with higher energy consumption (20–50 kWh/kg). Enzymatic hydrolysis, though less common, offers a greener alternative by selectively degrading lignin/hemicellulose with minimal chemical waste, as seen in poplar pulp-derived CNFs [58]. For instance, walnut shell nanocellulose prepared via phosphoric acid hydrolysis achieved a crystallinity of 72% with lower toxicity compared to sulfuric acid [53]. Overall, sulfuric acid hydrolysis remains the most efficient for high-crystallinity CNCs (yield: 20%–35%), while HPH and enzymatic methods are preferable for CNFs with lower environmental impact, despite trade-offs in yield (15%–25%). In summary, wood-derived nanocellulose remains the gold standard for high crystallinity and mechanical robustness, while agricultural waste offers cost-effective alternatives despite requiring intensive purification. Source selection must align with application-specific requirements, balancing purity, energy input, and environmental impact.
3.4 Direct and Indirect Products Obtained from Agrowastes
Agro-processing waste is predominantly composed of lignocellulosic material, consisting of cellulose, lignin, hemicellulose, and other components. Among these, non-cellulosic components decrease the crystallinity of nanocellulose and negatively affect its thermal and mechanical properties. Therefore, raw materials must be cleaned and purified before nanocellulose preparation [49]. The purification process typically involves the following steps: (1) cleaning and crushing the raw materials to facilitate subsequent reactions; (2) using alkali to remove hemicellulose and lignin; and (3) delignification or bleaching under acidic conditions to enhance cellulose purity and whiteness [49,60]. Alkali treatment is crucial for exposing the internal structure of fibers and purifying cellulose, with NaOH and KOH being the most commonly used alkali solutions. Bleaching agents such as sodium chlorite (NaClO2) and hydrogen peroxide (H2O2) are frequently employed in the bleaching process. The success of pretreatment is typically confirmed through structural and compositional analyses. For instance, Fourier-transform infrared spectroscopy (FTIR) is employed to monitor the removal of lignin and hemicellulose by detecting the disappearance of characteristic peaks (e.g., lignin’s aromatic C=C stretching at 1505–1600 cm−1) [61,62]. X-ray diffraction (XRD) quantifies crystallinity indices, where higher values (e.g., 75%–85% for wood pulp) indicate effective removal of amorphous components [34,62]. Scanning electron microscopy (SEM) reveals morphological changes, such as the transition from compact fiber bundles to individualized fibrils, as demonstrated in alkali-treated banana peel fibers [39]. Ni et al. (2020) utilized SEM and XRD to validate the elimination of non-cellulosic layers in ginkgo seed shells, achieving a crystallinity increase from 48% to 72% after pretreatment [61]. These techniques collectively ensure that purified cellulose meets the quality standards required for subsequent nanocellulose production.
3.5 Nanocellulose Preparation Methods
3.5.1 Chemical Preparation Method
Acid hydrolysis is one of the commonly employed chemical methods for isolating nanocellulose. The purified cellulose typically consists of elongated protofibrils, as depicted in Fig. 7, which contain crystalline regions that remain after the non-crystalline regions are degraded by acid hydrolysis [63]. Under the action of acid, the non-crystalline regions are easily degraded, leaving behind crystalline regions, which results in the formation of short, rod-like (or needle-like) cellulose nanocrystals (CNCs) with a more homogeneous structure [54,62,64]. Common acids used for hydrolysis include sulfuric acid [34], hydrochloric acid [65], phosphoric acid [66], and hydrobromic acid [67], with sulfuric acid being the most commonly applied. The properties of CNCs, such as crystallinity, morphology, surface charge, and mechanical characteristics, are influenced by factors such as acid type and concentration, hydrolysis time and temperature, and the cellulose-to-acid ratio. Mahmud et al. successfully prepared CNCs from medical-grade cotton using sulfuric (50%, 50°C, 1 h), hydrochloric (37%, 50°C, 1 h), and phosphoric acids (85%, 50°C, 12 h) [68]. They found that sulfuric and hydrochloric acids were the most effective for CNC preparation, while phosphoric acid hydrolysis yielded spherical CNCs. Hydrolysis with hydrochloric acid revealed cellulose type I, while phosphoric acid resulted in cellulose type II, and sulfuric acid hydrolysis produced both cellulose crystal types.
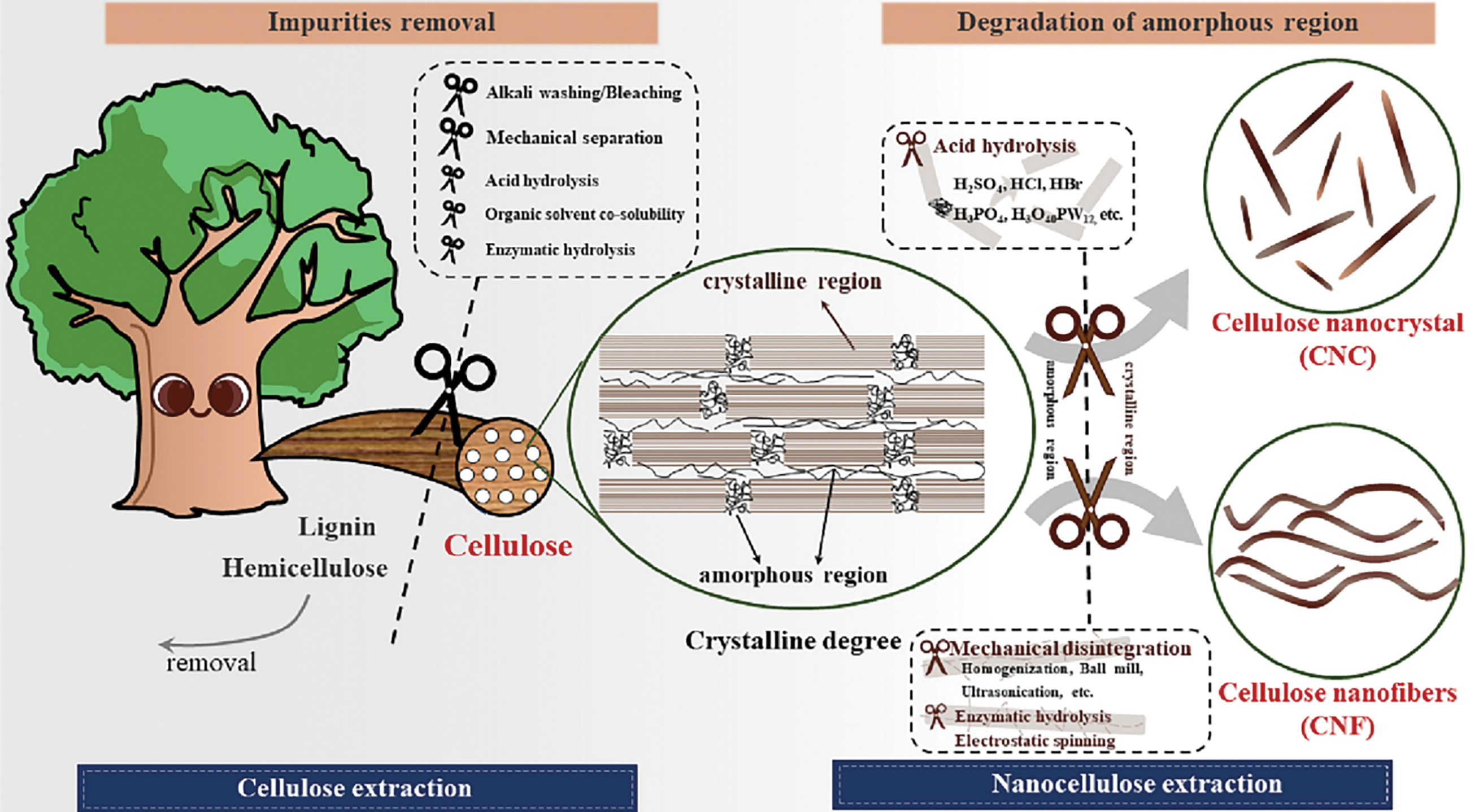
Figure 7: Schematic of isolation CNCs and CNFs from cellulose sources [69]. Reprinted with permission from Reference [69]. Copyright 2025, Elsevier and Copyright clearance, License number (6038621094941)
The surface functional groups of CNCs are influenced by the type of acid used in hydrolysis. Typically, CNCs prepared through sulfuric acid hydrolysis exhibit superior suspension stability due to the electrostatic repulsion provided by negatively charged sulfonyl groups [63]. CNCs hydrolyzed using phosphoric acid, which introduces phosphate groups on their surface, also demonstrate good colloidal stability and greater thermal stability compared to those prepared using sulfuric acid [70]. In contrast, CNCs obtained via hydrochloric acid hydrolysis are generally uncharged, resulting in particle agglomeration and poor suspension dispersion [71]. The duration of acid hydrolysis and the concentration of acid significantly impact the characteristics of CNCs, with longer hydrolysis times or higher acid concentrations producing shorter CNCs with lower crystallinity and higher surface charge density [72]. Consequently, the properties of CNCs can be tailored by adjusting the hydrolysis conditions. Beyond traditional inorganic acids, researchers have explored the use of organic acids [73], ionic liquids [74], and solid acids [75] as alternatives to mitigate issues such as equipment corrosion and environmental pollution associated with conventional inorganic acids.
More recently, green pretreatment routes have emerged to further reduce energy consumption and chemical waste. Deep eutectic solvents (DESs), such as choline chloride-oxalic acid and choline chloride-urea mixtures, selectively remove lignin and hemicellulose under mild conditions, achieving high cellulose yields while minimizing effluent toxicity [76–78]. Organic acid pretreatments (e.g., phytic, malic, and lactic acids) likewise facilitate effective delignification and swelling at 80–150°C without harsh alkali steps. Microwave-assisted ionic-liquid pretreatments accelerate cellulose dissolution-completing in under 10 min at 130°C-enabling high-crystallinity nanofibril recovery with minimal processing time [79–81]. Mechanochemical methods, including ball milling combined with ultrafine grinding or electrospray dispersions, produce nanofibrils with tailored aspect ratios directly in the solid state, often in tandem with polymerizable ionic liquids to form functional composite precursors in a single step [82–84]. Having discussed chemical methods for nanocellulose isolation, we now turn to physical preparation techniques, which prioritize mechanical fibrillation over chemical modification, albeit with trade-offs in energy consumption.
3.5.2 Physical Preparation Method
Physical preparation methods for nanocellulose primarily involve mechanical treatments that utilize high-intensity energy to break the strong hydrogen bonding interactions between cellulose chains, resulting in fibre fracture and fine fibrillation, thereby producing nanoscale cellulose [85]. This mechanical approach is the predominant method for producing cellulose nanofibrils (CNFs). Unlike cellulose nanocrystals (CNCs), which are typically prepared via acid hydrolysis, CNFs produced through mechanical treatments retain both crystalline and amorphous regions, exhibit longer lengths, and possess a more flexible, curved morphology, as illustrated in Fig. 7. Additionally, CNFs experience minimal alterations in their crystal structure and chemical properties during processing. Common physical treatment techniques include high-pressure homogenization (HPH), microjet processing, grinding, and ultrasonic methods [86].
High-pressure homogenization is one of the most straightforward and commonly used techniques for nanocellulose production. Major companies such as Rettenmaier (Germany) and Daicel (Japan) employ HPH as a key step in cellulose deconstruction. In this process, cellulose suspensions are subjected to high-pressure conditions in narrow chambers, where various forces—such as crushing, shear, turbulence, and cavitation—are generated due to the high-pressure gradients. This combination of forces effectively reduces cellulose protofibrils to the nanoscale [87]. For example, Winuprasith and Suphantharika produced elongated CNFs from mangosteen shells after 20 cycles of homogenization at 50 MPa [56], while Pelissari et al. extracted CNFs with a diameter of 22 nm from banana peels using a combination of chemical purification, homogenization, and a two-stage high-pressure process [39]. Despite its effectiveness, HPH has some limitations, including high energy consumption (20–50 kWh/kg) and equipment instability [86]. Additionally, cellulose suspensions are prone to clogging in the narrow orifices of homogenizers, complicating maintenance and impeding the preparation process. These issues must be addressed for the successful industrial-scale application of HPH in cellulose production [88].
Microjet technology is similar to high-pressure homogenization (HPH) in that it subjects suspensions to high pressure, forcing them through Z- or Y-shaped channels at speeds reaching several Mach. During this process, the material undergoes high-speed shearing and impaction, resulting in nanoscale cellulose particles [86]. Due to differences in flow channel structure and higher pressure, microjet technology offers higher single-pass processing efficiency compared to HPH. As a result, microjet technology is a key method for the industrial production of cellulose nanofibrils (CNFs) [89]. Ferrer et al. successfully produced CNFs from empty palm fruit bunch cellulose at a constant flow rate under microjet conditions, maintaining a pressure of 55 MPa [90]. However, studies have indicated that the crystallinity of CNFs decreases under ultra-high pressure (UHP) and that the creep flexibility and fibre network structure of cellulose relax with repeated processing [91,92]. Like HPH, microjet technology is also characterized by high energy consumption and the potential for clogging.
The grinding method involves the mechanical shearing and friction of cellulose fibres using equipment such as grinding discs, ball mills, and fixed-slot discs. The size of the resulting nanocellulose particles depends on the distance between the grinding discs [60]. As the gap between the grinding discs narrows, the cellulose fibres are increasingly squeezed and sheared, reducing their size. For instance, Lu et al. successfully reduced cellulose particles from sizes ranging between 10 to 90 µm down to nanocellulose particles measuring between 38 and 671 nm using media milling [93]. Compared to high-pressure techniques, milling is less productive and requires frequent adjustments to the disc clearance. Once the cellulose particles are significantly reduced in size, further reduction becomes challenging, even with additional adjustments to the disc gap. If the spacing between the discs is not properly managed, the high-speed rotating discs can collide, introducing metal or quartz debris into the nanocellulose and reducing its purity [94]. Furthermore, grooves on the grinding discs’ surface can trap cellulose during the process, leading to inconsistent particle sizes. Disc mills also generate substantial heat, limiting their continuous operation. Despite these drawbacks, grinding is advantageous for pre-processing large amounts of cellulose prior to enzymatic digestion or homogenization, as it reduces particle size in advance and avoids frequent clogging [95,96].
The preparation of cellulose nanofibers (CNFs) through ultrasonication primarily depends on the acoustic cavitation effect induced by high-frequency (20 kHz) sound waves in aqueous suspensions [97]. This effect is characterized by the formation and subsequent violent collapse of small bubbles created by the transmission of sound waves through the medium [98]. The violent collapse of these bubbles generates micro-jets and shock waves on the natural fiber surfaces, causing surface erosion and axial splitting. The size and yield of nanocellulose produced by ultrasonication are closely related to both ultrasonic power and duration. Ni et al. investigated the effects of varying ultrasonic power (150–600 W) and durations (10–60 min) on the physical properties of nanocellulose [99]. They found that ultrasonic duration had a more significant impact on nanocellulose properties than ultrasonic power. Additionally, elevated temperatures further enhance the sonication process, and combining ultrasonication with homogenization or chemical treatments can result in more homogeneous CNF production [100].
Mechanical treatment can effectively extract flexible and elongated nanocellulose from cellulose protofibers, though its high energy consumption and associated equipment wear limit its broader application. To mitigate energy consumption and equipment wear, chemical pretreatment can be applied to the fibrous raw material prior to mechanical treatment. TEMPO (2,2,6,6-tetramethylpiperidine-1-yloxy radical) oxidation is one of the most commonly used chemical pretreatment methods. The chemically treated cellulose can then be further mechanically processed to produce well-dispersed CNFs. Additionally, alternative methods such as low-temperature pressing, steam blasting, and electrostatic spinning have been explored for the production of cellulose nanoparticles [86].
3.5.3 Biological Preparation Method
Biological preparation methods utilize microorganisms (e.g., Acetobacter xylinus) or enzymes to synthesize nanocellulose through a “bottom-up” approach. Bacterial cellulose (BC) is produced via aerobic fermentation, where glucose is metabolized into extracellular cellulose fibrils (5–20 nm diameter) with high crystallinity (80–90%) and purity (no lignin/hemicellulose) [59,101]. For instance, Chen et al. (2018) achieved BC yields of 6.8 g/L using optimized Komagataeibacter hansenii strains in a bioreactor, demonstrating scalability for biomedical applications like wound dressings [102]. Enzymatic hydrolysis, employing cellulases (e.g., endoglucanases), selectively degrades amorphous cellulose regions, producing CNFs with low energy input (5–10 kWh/kg) and minimal chemical waste [48]. However, biological methods face challenges such as long fermentation cycles (5–7 days for BC) and high substrate costs, limiting industrial adoption compared to chemical/mechanical approaches.
The choice of preparation method hinges on target application requirements. Chemical methods (e.g., acid hydrolysis) are preferred for rigid, crystalline CNCs in reinforced composites, while physical methods (e.g., HPH) suit flexible CNFs for films or textiles. Biological methods, though niche, offer unparalleled biocompatibility for medical devices but require cost reductions to compete industrially (Table 2). Collectively, the choice of preparation method hinges on targeted functionalities: chemical routes for rigid composites, mechanical methods for flexible films, and biological synthesis for niche biomedical uses. Hybrid approaches may bridge scalability and sustainability gaps in future work.

3.6 Post-Treatment of Nanocellulose Preparation
In most instances of acid hydrolysis for preparing cellulose nanocrystals (CNCs), the target nanocellulose is not immediately obtained upon completion of the hydrolysis process, necessitating post-treatment. As shown in Table 1, researchers often employ high-energy mechanical devices, such as ultrasound or high-pressure homogenization, to further process the acid hydrolysis products and achieve the final nanocellulose form. It is well established that purified cellulose microfibers contain both crystalline and amorphous regions. Cellulose chains in the amorphous regions are randomly oriented, forming less dense, non-crystalline structures. During acid hydrolysis, hydrated hydrogen ions penetrate cellulose chains in these amorphous domains, facilitating glycosidic bond cleavage and releasing cellulose crystals [103,104]. However, after hydrolysis, the released microcrystals tend to aggregate into particles larger than the original cellulose microfibers. Mechanical post-treatment reduces the size of microcrystalline particles and facilitates the production of nanocellulose suspensions with uniform particle size [105].
Despite this, many researchers often overlook the changes induced by post-treatment on the target nanocellulose properties. Post-treatment success is rigorously validated through multi-scale characterization. Dynamic light scattering (DLS) quantifies particle size distribution, where narrower profiles (e.g., 10–50 nm for ultrasonicated CNCs [61]) indicate effective deagglomeration. X-ray diffraction (XRD) monitors crystallinity retention; for instance, sulfuric acid-hydrolyzed CNCs maintain crystallinity indices of 70%–85% after homogenization [34]. Scanning electron microscopy (SEM) or transmission electron microscopy (TEM) visualizes morphological uniformity, as demonstrated by Winuprasith and Suphantharika (2013), who observed individualized CNFs (13 nm width) from mangosteen husks after high-pressure homogenization [56]. Surface chemistry alterations, such as sulfonation from acid hydrolysis, are confirmed via Fourier-transform infrared spectroscopy (FTIR) through the appearance of S=O stretching bands (~1210 cm−1) [68]. Additionally, thermogravimetric analysis (TGA) assesses thermal stability, with post-treated nanocellulose typically showing degradation temperatures above 250°C, suitable for high-temperature composites [53]. He et al. developed a strategy for preparing nano-powders that can be efficiently redispersed by co-freeze-drying cellulose nanocrystals with gelatin (Fig. 8). They confirmed that gelatin can inhibit dehydration agglomeration and improve redispersion performance [106]. These analyses collectively ensure that post-treatment optimizes nanocellulose for target applications, such as uniform Pickering emulsions (DLS/TEM) or thermally stable aerogels (TGA/XRD).
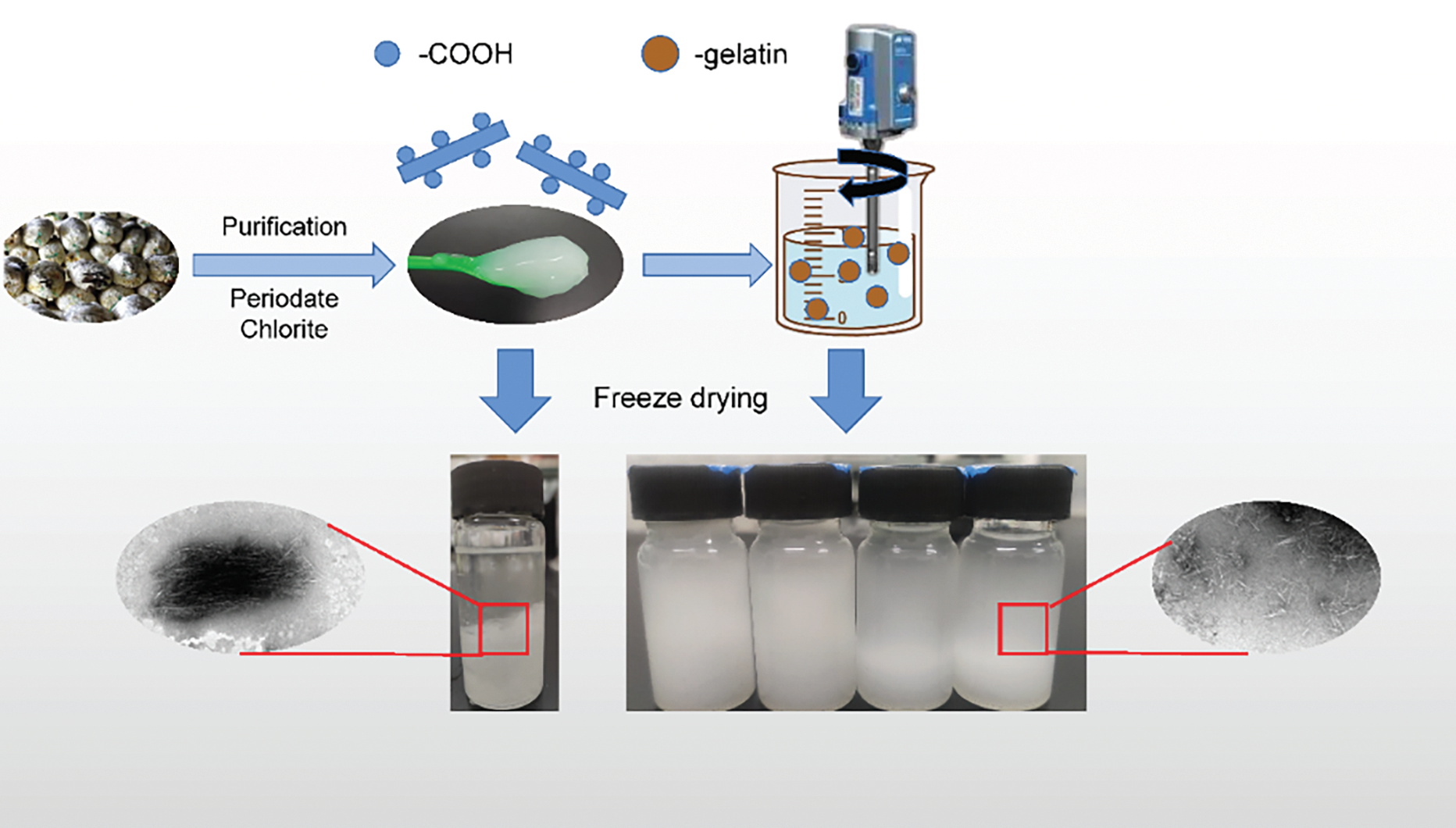
Figure 8: Post-treatment of redispersion of nanocellulose with gelatin [106]. Reprinted with permission from Reference [106]. Copyright 2025, Elsevier and Copyright clearance, License number (6038621355446)
4 Properties of Nanocellulose and Its Applications
Nanocellulose possesses several unique properties, including low density, high crystallinity, high modulus, significant strength, and strong hydrophilicity due to its abundant surface hydroxyl groups (-OH), which form extensive hydrogen bonds with water molecules [107]. This hydrophilicity enables nanocellulose to disperse readily in aqueous media and interact with polar matrices, making it ideal for biomedical and environmental applications such as hydrogels or water purification membranes [108]. The property is further enhanced by its large specific surface area (discussed below), which amplifies interfacial interactions. Nanocellulose possesses several unique properties, including low density, high crystallinity, high modulus, significant strength, and strong hydrophilicity due to its abundant surface hydroxyl groups (-OH), which form extensive hydrogen bonds with water molecules [13]. Furthermore, its exceptionally large specific surface area (ranging from 100 to 500 m2/g depending on the source and extraction method) amplifies interfacial interactions, enabling high adsorption capacities, efficient reinforcement in composites, and enhanced catalytic activity in functionalized forms [109]. These combined properties drive innovations in energy storage, biosensors, and environmental remediation. In contrast to ordinary cellulose, nanocellulose fibers exhibit near-perfect molecular chain alignment at the nanoscale, with abundant hydroxyl groups forming extensive hydrogen bonds [110,111]. This tighter bonding enables nanocellulose to maintain low density while exhibiting superior mechanical properties. Studies report that the Young’s modulus of nanocellulose can reach up to 220 GPa, significantly exceeding that of most engineering materials currently in use [112]. Nanocellulose is widely employed as a reinforcement material for polymers due to its large specific surface area and high aspect ratio [113]. The length-to-diameter (L/D) ratio of CNCs obtained via acid hydrolysis typically ranges from several dozen to a few hundred, while that of CNFs produced by mechanical treatment or bacterial synthesis can exceed tens of thousands. These high aspect ratios facilitate the efficient transfer of internal stresses between nanocellulose and the polymer matrix, thereby significantly enhancing the mechanical strength of composites. Nanocellulose also exhibits remarkable optical, rheological, electrical, and magnetic properties [114,115].
Due to its unique properties, nanocellulose has found applications in both traditional and emerging fields, including packaging, water treatment, and medical materials, as well as flexible electronic displays, smart sensing devices, and energy storage and conversion systems, as illustrated in Fig. 9 [116]. To better understand the application potential of nanocellulose, a review of the literature reveals that its applications can be classified into four categories based on dimensionality: zero-dimensional, one-dimensional, two-dimensional, and three-dimensional nanofibrillar cellulose-based materials.
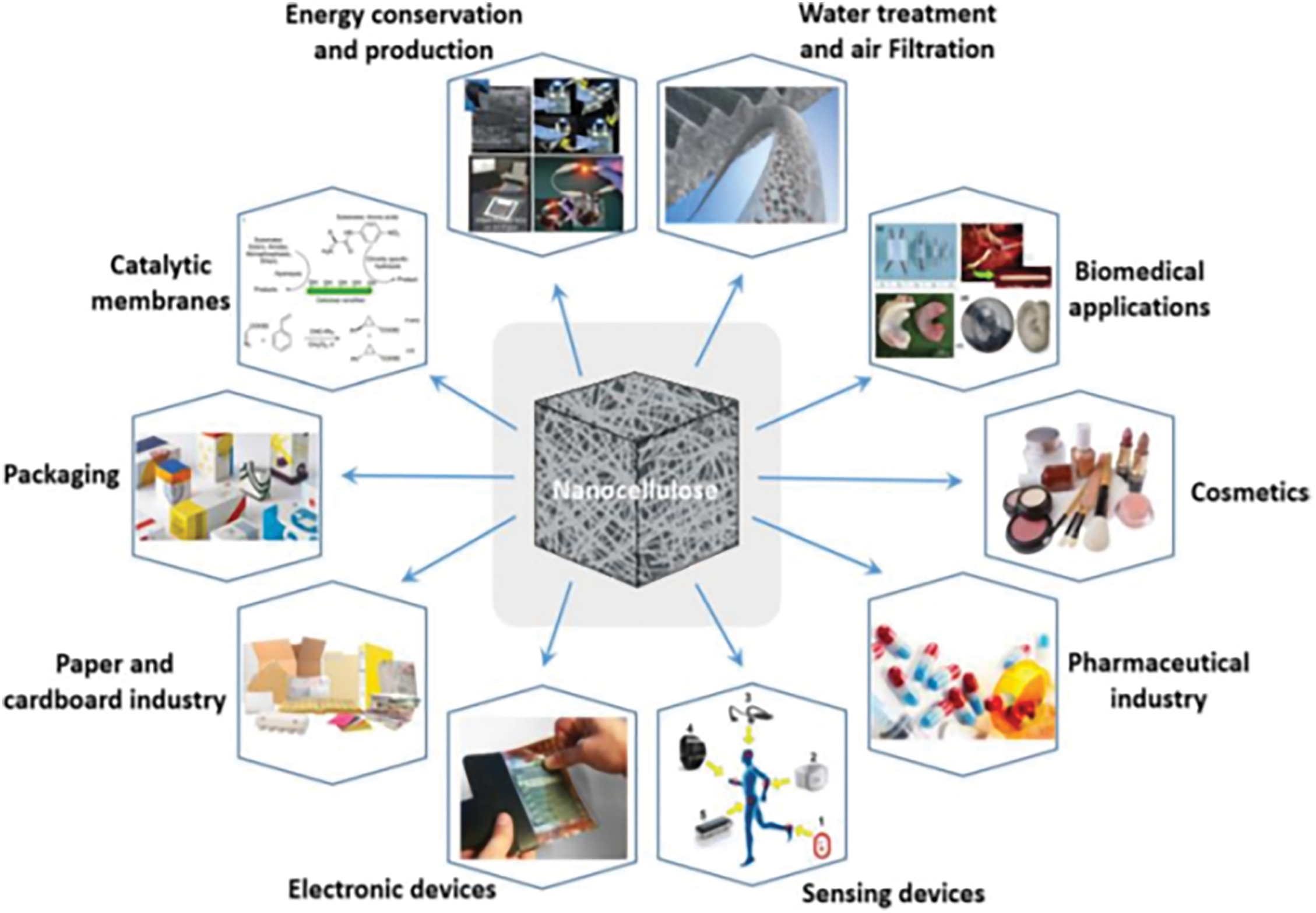
Figure 9: State of nanocellulose based materials for applications [117]. Copyright © 2020, Springer Nature B.V
4.1 Zero-Dimensional Nanocellulose-Based Materials and Their Applications
Zero-dimensional (0D) nanomaterials refer to structures with all dimensions at the nanoscale (1–100 nm), exhibiting particle-like morphology without significant elongation or planar extension. These materials are typically isolated as discrete nanoparticles, such as cellulose nanocrystals (CNCs) or spherical nanocellulose aggregates. Their high surface-to-volume ratio and tunable surface chemistry enable unique functionalities in colloidal systems. The most prominent 0D application is nanocellulose-stabilized Pickering emulsions, where CNCs or CNFs act as solid stabilizers at oil-water interfaces due to their hydrophilic nature and ability to adsorb at the interface [118]. Historically, most particles used in Pickering emulsions were inorganic, such as silica particles, due to silica’s controllable particle shape, size, and narrow size distribution. However, biocompatibility and biodegradability are often required for Pickering emulsions, limiting the use of inorganic particles. As a result, recent research has shifted toward using biologically sourced particles to stabilize Pickering emulsions. Nanocellulose, with its non-toxicity, sustainability, and superior biocompatibility, addresses the limitations of inorganic particles, enabling widespread applications in industries such as food, pharmaceuticals, and cosmetics [119,120].
Guo et al. conducted a systematic investigation into how nanocellulose length-to-diameter (L/D) ratios affect its performance as a stabilizer in Pickering emulsions. Their findings revealed that at the same concentration, CNCs with lower aspect ratios exhibited superior emulsification properties compared to CNFs with higher aspect ratios. However, emulsions stabilized by CNFs demonstrated superior storage stability compared to those stabilized by CNCs, as indicated by minimal changes in particle size over time. Building on this theory, researchers investigated the properties of Pickering emulsions when nanocellulose was used to stabilize the paper-sizing agent alkenyl succinic anhydride (ASA). The study found that increasing the nanocellulose concentration resulted in higher Zeta potentials, smaller particle sizes, and an increased emulsion phase volume. Rheological tests revealed that CNF-stabilized emulsions exhibited higher viscosities, and at a concentration of 2%, they showed significant gelation, which contributed to the formation of stable ASA emulsions [121].
Fujisawa et al. described a straightforward method for synthesizing polystyrene (PS)/cellulose nanofiber (CNF) composites with nanostructures using CNF-stabilized Pickering emulsions under aqueous conditions, as illustrated in Fig. 10. Polystyrene (PS) nanoparticles with a narrow size distribution were synthesized in water via emulsion radical polymerization, with CNFs serving as the stabilizer. The nanoparticles were readily collected by filtration, forming a composite in which PS nanoparticles were embedded within a CNF matrix. Following hot pressing, the PS/CNF nanocomposites demonstrated high optical transparency, mechanical strength, and dimensional thermal stability. This technique offers a simple and environmentally sustainable method for producing novel CNF/polymer nanocomposites. Similarly, this process can be extended to other types of nanocellulose, such as cellulose nanocrystals (CNCs) and lignocellulose nanofibers (LCNFs), to improve the mechanical properties of composites [122]. Li et al. demonstrated a novel method for preparing heat storage materials based on CNF-stabilized Pickering emulsions. The strong emulsification properties of CNFs allowed for the encapsulation of over 72 wt.% paraffin, a typical thermal phase change material. The encapsulated paraffin melts upon heating, enabling the material to absorb heat without leakage during heating/cooling cycles. This property makes the material highly effective for thermal regulation, with potential applications as a smart building insulation material [123]. Furthermore, Huan et al. investigated the application of CNF-stabilized Pickering emulsions in 3D printing. Their findings showed that incorporating polylactic acid (PLA) into the dispersed phase of the emulsions allowed for precise control over the properties of 3D-printed materials [124]. The interaction between the hydrophilic groups of CNFs and PLA enhanced the ability to control the shape of the printed materials. This integration of CNF-stabilized Pickering emulsions with 3D printing technology enables the customized fabrication of specific biomedical scaffolds [125]. While zero-dimensional nanocellulose excels in emulsion stabilization, one-dimensional fibrillar structures unlock advanced applications in flexible electronics and wearable devices, as detailed below.
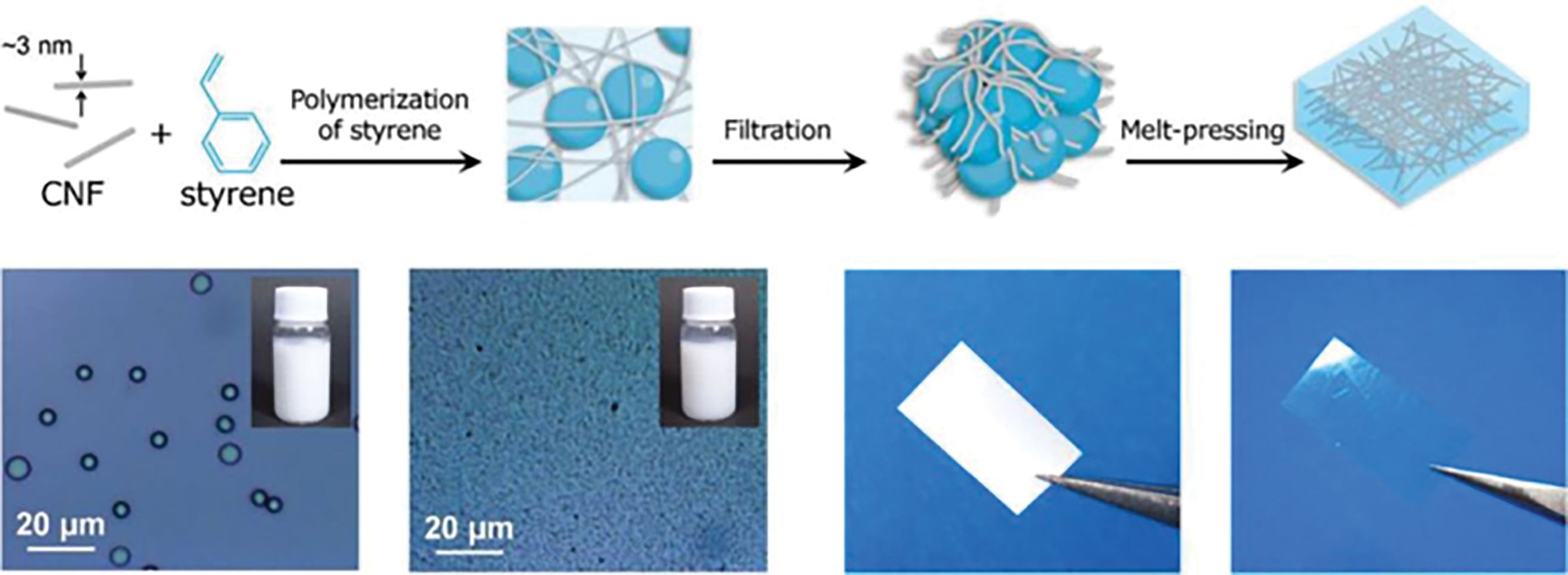
Figure 10: Facile route to CNFs/PS composite material from an aqueous Pickering emulsion [122]. Copyright © 2020 American Chemical Society
4.2 One-Dimensional Nanocellulose-Based Materials and Their Applications
One-dimensional (1D) nanomaterials are characterized by a high aspect ratio (length > diameter), including nanofibers, nanowires, or nanotubes. Nanocellulose fibrils (CNFs) and aligned CNC assemblies exemplify 1D structures. Their anisotropic properties, such as directional mechanical strength and electron transport, make them ideal for load-bearing or conductive applications. The theoretical Young’s modulus of individual nanocellulose fibers has been reported to be as high as 220 GPa. However, conventional cellulose fiber products have not reached such high mechanical properties, with even highly oriented natural plant fibers failing to achieve a modulus of 160 GPa. To fabricate high-performance cellulose fibers, researchers have employed wet spinning, dry spinning, and wet-dry spinning techniques to transform nanocellulose into macroscopic fibers [126]. Nitesh et al. demonstrated a method using microfluidics to spin cellulose nanofibers (CNFs) into macroscopic fibers with nearly perfect unidirectional alignment. The crosslinked and highly ordered structure enabled efficient stress transfer from the macroscopic scale to the individual CNFs, resulting in nanocellulose fibers with a Young’s modulus of 86 GPa and a tensile strength of 1.57 GPa, surpassing the mechanical properties of all known natural and synthetic biopolymer fibers [127].
Other research groups have also reported the preparation of nanocellulose fibers with enhanced mechanical properties through techniques such as ionic cross-linking, UV-cured cross-linking, and pre-stretching orientation [128–130]. For instance, Niu et al. utilized a nanohybridization strategy by adding unmodified single-walled carbon nanotubes (SWCNTs) to a CNF suspension to prepare a hybrid dispersion. This hybrid was then extruded into a coagulation bath (ethanol) using a syringe-based spinning technique, resulting in nanocellulose/SWCNT hybrid fibers with an average diameter of approximately 50 μm after air drying [131]. These fibers were woven into a mat via a braiding process to create a novel wearable supercapacitor with excellent electrochemical performance, stability, and damage resistance. Similarly, such conductive hybrid fibers have shown potential for use as sensors in detecting stress-strain changes, muscle movements, and other dynamic systems [132].
4.3 Two-Dimensional Nanocellulose-Based Materials and Their Applications
Two-dimensional (2D) materials possess nanoscale thickness (1–100 nm) and macroscopic lateral dimensions, forming films, membranes, or sheets. Nanocellulose-based 2D materials leverage hydrogen bonding and fibril entanglement to achieve dense, planar architectures. Their high transparency, barrier properties, and surface smoothness drive innovations. These 2D materials typically exhibit densities between 0.8–1.5 g cm−3, with notable properties including excellent flexibility, nanoscale surface roughness, high thermal stability, adjustable transparency, high tensile strength, and effective barrier properties [133–135]. Due to the unique characteristics of nanocellulose, traditional fabrication methods for 2D cellulose-based materials (such as paper) are not suitable for producing nanocellulose-based equivalents. Instead, techniques like vacuum filtration, press filtration, cast coating, and spray deposition are employed [136]. Thanks to these properties, 2D nanocellulose materials have demonstrated unique applications across a range of fields, often outperforming conventional materials [137,138]. For instance, Xu et al. developed a series of cellulose nanocrystal (CNC) membranes that emit circularly polarized light and fluorescence via evaporation-induced self-assembly. These membranes exhibited the ability to emit left circularly polarized light and right circularly polarized fluorescence due to their left-handed chiral nematic structures [139]. Furthermore, the researchers demonstrated that left- and right-rotating circularly polarized patterns, as well as chiral inversion of polarized light, could be achieved through the combination of reaction dynamics and kinetic trapping. Leveraging these circularly polarized optical properties, the team highlighted the potential of these nanofibrous films for advanced optical anti-counterfeiting applications, as shown in Fig. 11 [140].
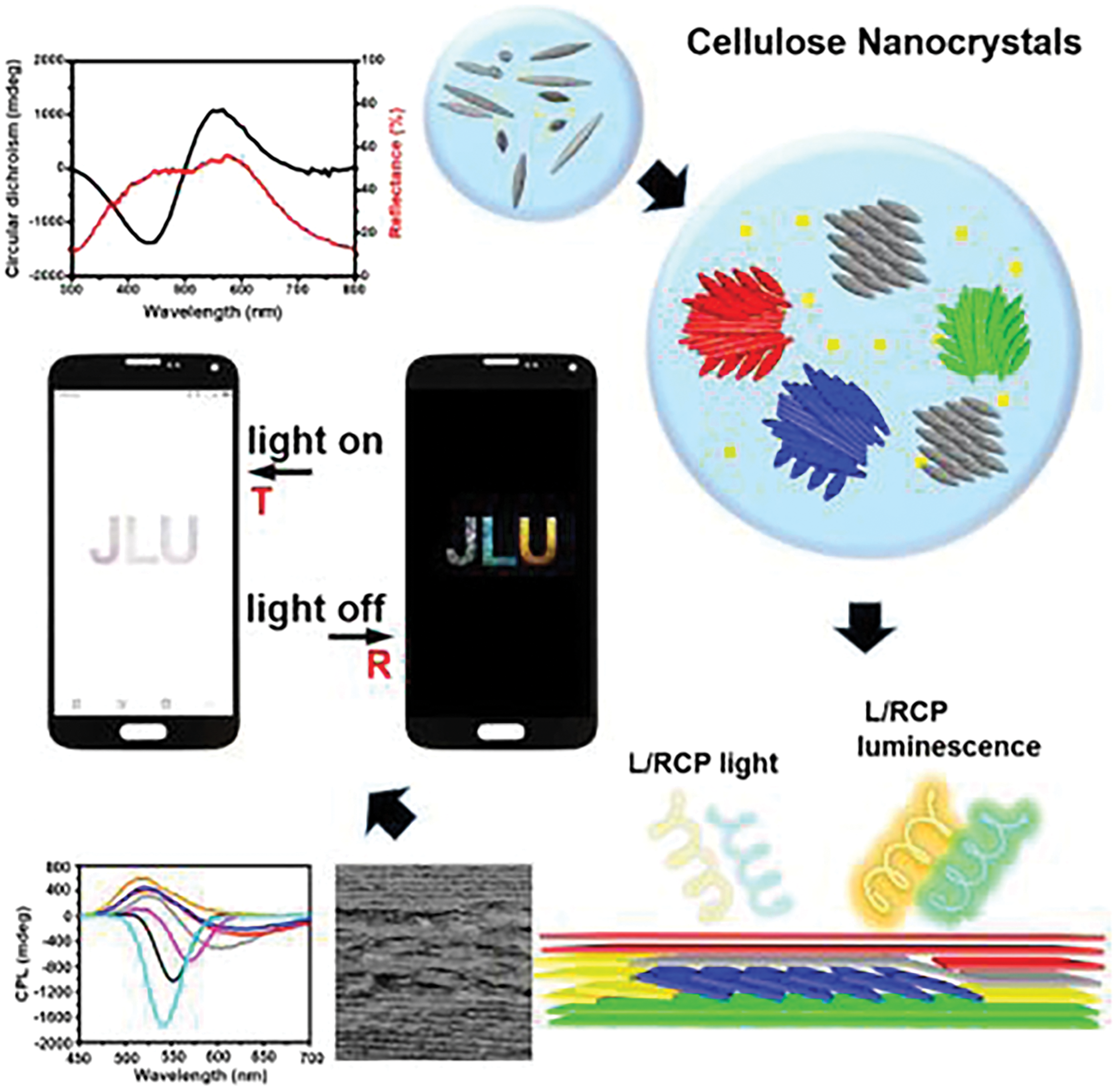
Figure 11: Nanocellulose-based optical anti-counterfeiting material [140]
Additionally, Zhu et al. created chiral plasmonic functional composite membranes by co-assembling surface plasmon-active gold nanorods with CNCs. By embedding gold nanorods of different charges and concentrations into the chiral nematic CNC matrix, they produced composite films with enhanced surface fluorescence and plasmon resonance-modified chiral optical properties [141]. This co-assembly technique has also been successfully used to fabricate other cellulose-based nanocomposite optical films [142,143].
Additionally, nanocellulose has emerged as a critical component in energy storage systems, particularly in supercapacitors (SCs). Its high surface area, chemical stability, and tunable surface chemistry enable its use in electrodes, electrolytes, and separators [144]. For instance, Ding et al. (2023) developed a cellulose nanofibril (CNF) membrane separator with 59% porosity and ionic conductivity of 0.265 S cm−1, achieving an energy density of 3.85 mWh cm−3 in SCs. Chen et al. (2019) demonstrated a self-healing polyvinyl alcohol/nanocellulose hydrogel electrolyte with strong ionic conductivity, enhancing SC performance. Nanocellulose’s role in miniaturized and flexible SCs further supports applications in microelectronics and wearable devices [15,145–147].
The utilization of nanocellulose as a food packaging material is costeffective and non-toxic, as it is derived from lignocellulosic waste Adding nano-clay and polylactic acid (PLA) matrix to the nano-bio composite film can enhance its water and oxygen barrier properties [148]. Silver nanoparticles were added to PLA films through melt extrusion using CNCs [17,149]. The inhibition of the development of gram-positive and gram-negative bacteria by silver nanoparticles acting as an antibacterial agent [150,151]. TEMPO oxidation was applied to CNFs by Dong et al. in order to impart carboxylate groups that were easily bindable with silver nanoparticles [152]. Since the carboxylate-Ag binding reduces the water solvation between the CNFs, the nanoparticles can be exploited for food packaging purposes to compact the CNF coalescence in the films [153]. Nanocellulose has been functionalized in many studies, resulting in the addition of additional properties. Food safety is receiving increasing attention as people become more aware of how to determine the freshness of food and how to extend the shelf life of food. The freshness of food is likely to be affected by many factors during transportation, including temperature, humidity, and external influences. Research into smart packaging materials has become an important area in the packaging field and holds great promise for improving food safety. The detection of product freshness in cold chain packaging has been successfully achieved through the use of time-temperature indicator (TTI) labels. In the future, the use of active labels and smart labels, which are both cost effective and highly effective, will be essential in the further development of product freshness testing [154].
In addition to these fundamental uses, recent studies have demonstrated the versatility of nanocellulose in advanced treatment technologies. For micro and nano-scale contaminant removal, engineered nanocellulose membranes and nanopapers with precisely tuned pore structures achieve removal efficiencies exceeding 99% via size-exclusion mechanisms, effectively intercepting plastic particles and metal nanoparticles [155]. Functionalized nanocellulose aerogels and hydrogels, when combined with photocatalysts or redox-active groups, enable rapid adsorption and catalytic degradation of dyes and organic pollutants, attaining over 95% removal of Rhodamine B and bisphenol A under visible light irradiation. Selective heavy-metal ion uptake is realized through thiol- and amine-modified CNCs and CNF composites, with capacities surpassing 2000 mg g−1 for Pb2+ and Cu2+, while maintaining fast adsorption kinetics [156,157]. Sun et al. regioselectively modified CNF aerogel using sodium periodate oxidation and continuous sodium sulfite sulfonation. The sulfonated aerogel exhibited underwater superoleophobicity and had high oil-water separation efficiency by completely repelling nonpolar oil phase and making water easy to penetrate with the oil content in filtrated water lower than 0.03% [158]. Zhang et al. produced a recyclable anisotropic CNF/chitosan (CS) aerogel by directed freeze-dying and cross-linked processes. The oil absorption of aerogel steadily increased as the CS concentration increased, when the CS content reached 0.8 wt%, the oil absorption of aerogel reached 253 g/g [159].
4.4 Three-Dimensional Nanocellulose-Based Materials and Their Applications
Three-dimensional (3D) materials exhibit nanoscale features within a macroscopic porous network, such as aerogels, hydrogels, or scaffolds. These structures combine high porosity (>90%) with mechanical resilience through cross-linking or freeze-drying, which represent the most typical examples of three-dimensional nanocellulose materials. Nanocellulose hydrogels are stable, three-dimensional network structures formed through the physical entanglement of nanocellulose, intermolecular hydrogen bonding, or other cross-linking mechanisms. The hydrophilic nature and large surface area of nanocellulose enable these structures to retain significant amounts of water, resulting in hydrogel formation. When water is removed from nanocellulose hydrogels using techniques such as freeze-drying or supercritical drying, the result is a highly porous three-dimensional scaffold known as nanocellulose aerogel [160].
Nanocellulose hydrogels and aerogels exhibit significant potential for a range of applications, including biomedical, energy storage, construction, separation processes, cosmetics, and food industries [161]. Recently, Zeng et al. combined silver nanowires (AgNWs) with cellulose nanofibrils (CNFs) of high aspect ratios to create ultralight, flexible CNF/AgNW composite aerogels via unidirectional freeze-drying. The composite aerogels displayed excellent electromagnetic shielding (EMI) properties (>70 dB), which were attributed to the effective conductive network provided by the AgNWs, the porous structure formed by freeze-drying, and the strong hydrogen bonding between the CNFs and polyvinylpyrrolidone adsorbed onto the AgNW surfaces (Fig. 12) [162].
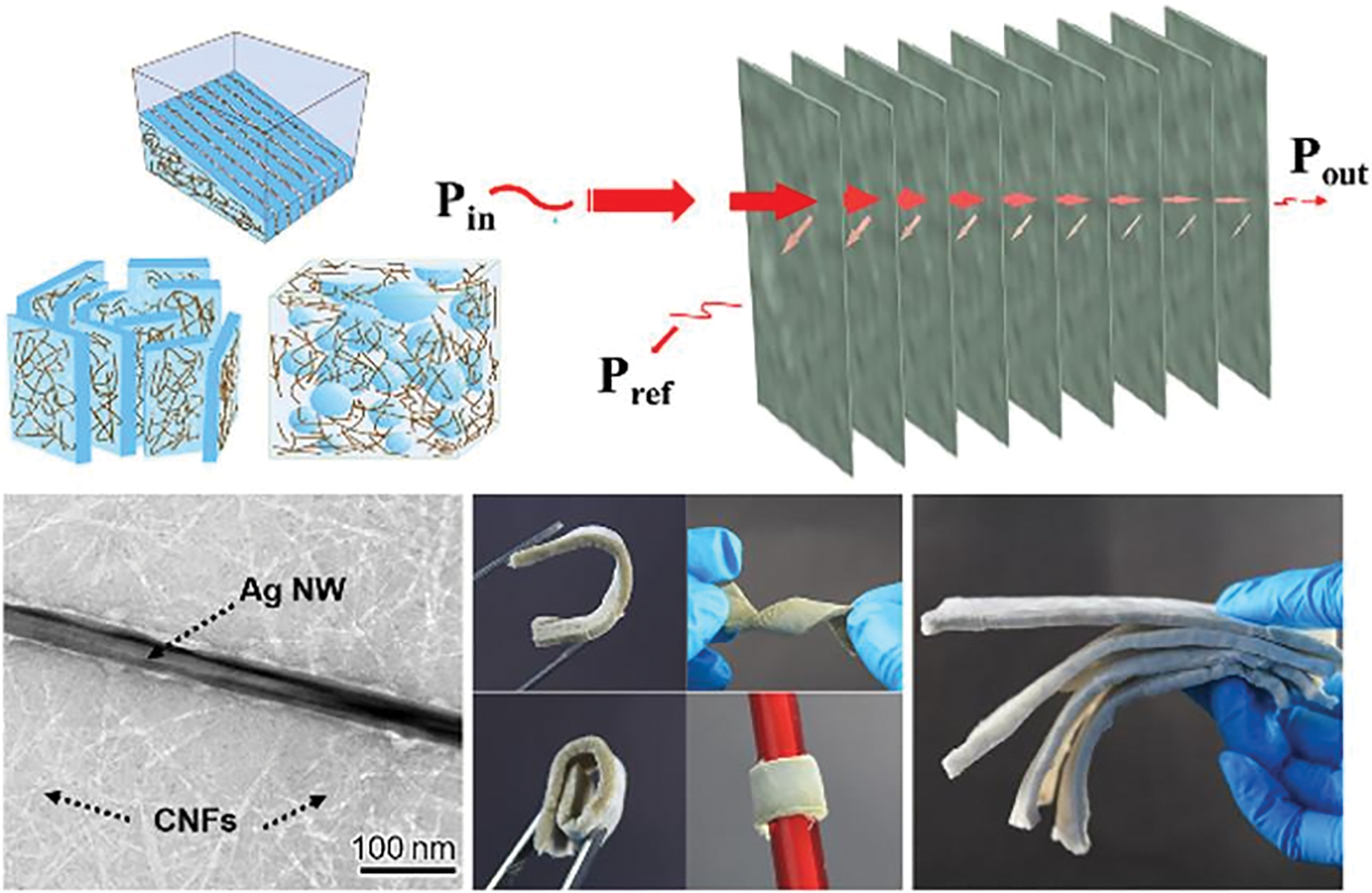
Figure 12: Lamellar and flexible unidirectional freeze-dried CNFs/AgNWs composite Aerogel [162]. Copyright © 2020 American Chemical Society
Xu et al. proposed a novel approach for preparing nanocellulose hydrogel scaffolds by employing a double cross-linking strategy combined with 3D printing technology. First, cellulose hydrogels were formed through in situ cross-linking with Ca2+, followed by chemical cross-linking with 1,4-butanediol diglycidyl ether (BDDE) at a 1% concentration. These double-cross-linked hydrogels were then used to 3D print scaffolds, which exhibited adjustable mechanical strength, ranging from 3 to 8 kPa [163]. Cellular testing demonstrated that these scaffolds not only supported fibroblast proliferation but also improved cell proliferation with increasing scaffold stiffness. These 3D-printed scaffolds represent a significant advance in nanofibrillar cellulose-based bio-tissue engineering, offering new possibilities for cell regeneration, wound healing, and tissue repair.
In biomedical applications, nanocellulose hydrogels and aerogels are extensively explored for drug delivery, tissue engineering, and wound healing. Xu et al. (2018) developed 3D-printed nanocellulose scaffolds with adjustable mechanical strength (3–8 kPa) that promote fibroblast proliferation, highlighting their potential in tissue regeneration. Nanocellulose’s biocompatibility and high surface area also enable its use in biosensors. Functionalized with enzymes or antibodies, nanocellulose-based biosensors detect glucose, pathogens, and environmental pollutants with high sensitivity. For bioimaging, nanocellulose conjugated with superparamagnetic iron oxide nanoparticles (SPIONs) serves as an MRI contrast agent, enhancing diagnostic accuracy [45,164–166].
5 Challenges and Future Perspectives
Despite the exceptional properties of nanocellulose and its wide range of potential applications, the nanocellulose market has yet to realize its full potential. One significant challenge is the lack of process-adapted measurement equipment capable of accurately characterizing nanocellulose at the speed necessary for industrial-scale production. Additionally, the high energy consumption associated with nanocellulose production presents a barrier to its commercialization. Nevertheless, the future of nanocellulose is promising, driven by its versatility in advanced applications. Emerging trends include its integration into flexible electronics (e.g., wearable sensors, foldable displays) due to its optical transparency and mechanical robustness, as well as its use in 3D-printed biomedical scaffolds for tissue engineering, leveraging its biocompatibility and tunable porosity. Green synthesis methods, such as enzymatic hydrolysis and bacterial fermentation, are expected to reduce energy demands and environmental footprints Furthermore, hybrid nanocomposites combining nanocellulose with conductive polymers or inorganic nanoparticles (e.g., graphene, AgNWs) could revolutionize energy storage systems (e.g., supercapacitors, batteries) and smart packaging. Standardization of production protocols and lifecycle assessments will be critical to ensure scalability and sustainability. Collaborative efforts between academia and industry must address these challenges to unlock nanocellulose’s full potential in the circular bioeconomy.
Nanocellulose, derived from abundant lignocellulosic biomass, has emerged as a transformative material with unparalleled versatility across industries. This review systematically analyzed its sources, extraction methods, and multidimensional applications. Key findings include: (1) Plant fiber hierarchy dictates nanocellulose properties, with wood-derived cellulose offering high crystallinity and agricultural waste enabling cost-effective production. (2) Acid hydrolysis remains dominant for cellulose nanocrystals (CNCs), while mechanical methods yield high-aspect-ratio cellulose nanofibrils (CNFs). (3) Nanocellulose’s mechanical strength, biocompatibility, and tunable surface chemistry drive innovations in energy storage (e.g., supercapacitors), biosensors (e.g., glucose monitoring), and biomedical engineering (e.g., 3D-printed scaffolds). In conclusion, this review underscores nanocellulose’s transformative potential across industries, driven by its tunable properties and renewable sourcing. Key advancements include optimized extraction protocols, multidimensional applications (0D–3D), and emerging hybrid composites. However, challenges in scalability, energy efficiency, and standardization persist. Future research must prioritize green synthesis, lifecycle assessments, and industry-academia collaboration to fully integrate nanocellulose into the circular bioeconomy, ensuring its role as a cornerstone of sustainable material innovation.
Acknowledgement: The authors would like to thank the College of Mechanical Engineering, Jiamusi University.
Funding Statement: This work was supported by the Basic Scientific Research Funds Project of Heilongjiang Universities [grant number 2024-KYYWF-0554] and the Heilongjiang Province 2024 Innovation Training Programme for University Students [grant number S202410222123].
Author Contributions: Haoquan Xue, Yujie Zhang, Haoran Gao and Zhuang Zhao: Investigation, Data Curation, Writing—Original Draft, Visualization. Wanlin Bao, Jiaxuan Li, Zhiheng Zhang and Qi Wang: Writing—Original Draft, Writing—Review & Editing, Visualization. Qiang He: Writing—Review & Editing, Supervision, Funding. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Ji Q, Zhou C, Li Z, Boateng ID, Liu X. Is nanocellulose a good substitute for non-renewable raw materials? A comprehensive review of the state of the art, preparations, and industrial applications. Ind Crops Prod. 2023;202(8):117093. doi:10.1016/j.indcrop.2023.117093. [Google Scholar] [CrossRef]
2. Chen W, Yu H, Lee SY, Wei T, Li J, Fan Z. Nanocellulose: a promising nanomaterial for advanced electrochemical energy storage. Chem Soc Rev. 2018;47(8):2837–72. doi:10.1039/C7CS00790F. [Google Scholar] [PubMed] [CrossRef]
3. Ji Q, Yu X, Chen L, Mustapha AT, Okonkwo CE, Zhou C, et al. Comprehensive depolymerization of lignin from lignocellulosic biomass: a review. Crit Rev Environ Sci Technol. 2023;53(21):1866–87. doi:10.1080/10643389.2023.2190314. [Google Scholar] [CrossRef]
4. Iskandar MA, Yahya EB, Abdul Khalil HS, Rahman AA, Ismail MA. Recent progress in modification strategies of nanocellulose-based aerogels for oil absorption application. Polymers. 2022;14(5):849. doi:10.3390/polym14050849. [Google Scholar] [PubMed] [CrossRef]
5. Wan C, Jiao Y, Wei S, Zhang L, Wu Y, Li J. Functional nanocomposites from sustainable regenerated cellulose aerogels: a review. Chem Eng J. 2019;359:459–75. doi:10.1016/j.cej.2018.11.115. [Google Scholar] [CrossRef]
6. Vanderfleet OM, Cranston ED. Production routes to tailor the performance of cellulose nanocrystals. Nat Rev Mater. 2021;6(2):124–44. doi:10.1038/s41578-020-00239-y. [Google Scholar] [CrossRef]
7. Ding Q, Xu X, Yue Y, Mei C, Huang C, Jiang S, et al. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl Mater Interfaces. 2018;10(33):27987–8002. doi:10.1021/acsami.8b09656. [Google Scholar] [PubMed] [CrossRef]
8. Xiong R, Hu K, Grant AM, Ma R, Xu W, Lu C, et al. Ultrarobust transparent cellulose nanocrystal-graphene membranes with high electrical conductivity. Adv Mater. 2016;28(7):1501–9. doi:10.1002/adma.201504438. [Google Scholar] [PubMed] [CrossRef]
9. Wang Y, Wei X, Li J, Wang F, Wang Q, Zhang Y, et al. Homogeneous isolation of nanocellulose from Eucalyptus pulp by high pressure homogenization. Ind Crops Prod. 2017;104:237–41. doi:10.1016/j.indcrop.2017.04.032. [Google Scholar] [CrossRef]
10. Mbisana M, Keroletswe N, Nareetsile F, Mogopodi D, Chibua I. Nanocellulose composites: synthesis, properties, and applications to wastewater treatment. Cellulose. 2024;31(18):10651–78. doi:10.1007/s10570-024-06268-y. [Google Scholar] [CrossRef]
11. Cho Y, Ninh PTT, Hwang S, Choe S, Myung J. Sustainability meets functionality: green design approaches to cellulose-based materials. ACS Mater Lett. 2025;7(4):1563–92. doi:10.1021/acsmaterialslett.4c02591. [Google Scholar] [CrossRef]
12. El-Sakhawy M, Kamel S, Tohamy HS. A greener future: carbon nanomaterials from lignocellulose. J Renew Mater. 2025;13(1):21–47. doi:10.32604/jrm.2024.058603. [Google Scholar] [CrossRef]
13. Ma H, Cheng Z, Li X, Li B, Fu Y, Jiang J. Advances and challenges of cellulose functional materials in sensors. J Bioresour Bioprod. 2023;8(1):15–32. doi:10.1016/j.jobab.2022.11.001. [Google Scholar] [CrossRef]
14. Kumar A, Jyske T, Petrič M. Delignified wood from understanding the hierarchically aligned cellulosic structures to creating novel functional materials: a review. Adv Sustain Syst. 2021;5(5):2000251. doi:10.1002/adsu.202000251. [Google Scholar] [CrossRef]
15. Chen C, Hu L. Nanocellulose toward advanced energy storage devices: structure and electrochemistry. Acc Chem Res. 2018;51(12):3154–65. doi:10.1021/acs.accounts.8b00391. [Google Scholar] [PubMed] [CrossRef]
16. Hazarika D, Gogoi N, Jose S, Das R, Basu G. Exploration of future prospects of Indian pineapple leaf, an agro waste for textile application. J Clean Prod. 2017;141:580–6. doi:10.1016/j.jclepro.2016.09.092. [Google Scholar] [CrossRef]
17. Xue Y, Mou Z, Xiao H. Nanocellulose as a sustainable biomass material: structure, properties, present status and future prospects in biomedical applications. Nanoscale. 2017;9(39):14758–81. doi:10.1039/c7nr04994c. [Google Scholar] [PubMed] [CrossRef]
18. Kalia S, Dufresne A, Cherian BM, Kaith BS, Avérous L, Njuguna J, et al. Cellulose-based bio- and nanocomposites: a review. Int J Polym Sci. 2011;2011(8):837875. doi:10.1155/2011/837875. [Google Scholar] [CrossRef]
19. Lavoine N, Desloges I, Dufresne A, Bras J. Microfibrillated cellulose—its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym. 2012;90(2):735–64. doi:10.1016/j.carbpol.2012.05.026. [Google Scholar] [PubMed] [CrossRef]
20. Saikia P. Chapter 1—cellulose and its derivatives: fundamental chemistry, structure, properties, and applications. In: Deshmukh K, Barua S, Baruah S, Mustansar Hussain C, editors. Cellulose-based hydrogells. Amsterdam, The Netherlands: Elsevier; 2025. p. 1–21. doi:10.1016/b978-0-443-22049-4.00020-5. [Google Scholar] [CrossRef]
21. Watanabe A, Morita S, Ozaki Y. Temperature-dependent changes in hydrogen bonds in cellulose Iα studied by infrared spectroscopy in combination with perturbation-correlation moving-window two-dimensional correlation spectroscopy: comparison with cellulose Iβ. Biomacromolecules. 2007;8(9):2969–75. doi:10.1021/bm700678u. [Google Scholar] [PubMed] [CrossRef]
22. Cruz H, Fanselow M, Holbrey JD, Seddon KR. Determining relative rates of cellulose dissolution in ionic liquids through in situ viscosity measurement. Chem Commun. 2012;48(45):5620–2. doi:10.1039/c2cc31487h. [Google Scholar] [PubMed] [CrossRef]
23. Pu Y, Hallac B, Ragauskas AJ. Plant biomass characterization: application of solution- and solid-state NMR spectroscopy. In: Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2013. p. 369–90. doi:10.1002/9780470975831.ch18. [Google Scholar] [CrossRef]
24. Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels. 2010;3(1):10. doi:10.1186/1754-6834-3-10. [Google Scholar] [PubMed] [CrossRef]
25. Hansen NML, Plackett D. Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules. 2008;9(6):1493–505. doi:10.1021/bm800053z. [Google Scholar] [PubMed] [CrossRef]
26. Huber GW, Corma A. Synergies between bio- and oil refineries for the production of fuels from biomass. Angew Chem Int Ed. 2007;46(38):7184–201. doi:10.1002/anie.200604504. [Google Scholar] [PubMed] [CrossRef]
27. Converse AO, Kwarteng IK, Grethlein HE, Ooshima H. Kinetics of thermochemical pretreatment of lignocellulosic materials. Appl Biochem Biotechnol. 1989;20(1):63–78. doi:10.1007/BF02936473. [Google Scholar] [CrossRef]
28. Lee HV, Abu Yazid N, Bin Johan MR. Chapter two—cellulose and hemicellulose: types, cleavage, and depolymerization. In: Rahimpour MR, Bakhtyari A, Makarem MA, editors. Advances in hydrotreating for integrated biofuel production. Amsterdam, The Netherlands: Elsevier; 2024. p. 51–75. doi:10.1016/b978-0-443-19076-6.00003-0. [Google Scholar] [CrossRef]
29. Bocchini P, Galletti GC, Camarero S, Martinez AT. Absolute quantitation of lignin pyrolysis products using an internal standard. J Chromatogr A. 1997;773(1–2):227–32. doi:10.1016/S0021-9673(97)00114-3. [Google Scholar] [CrossRef]
30. Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. Lignin biosynthesis and structure. Plant Physiol. 2010;153(3):895–905. doi:10.1104/pp.110.155119. [Google Scholar] [PubMed] [CrossRef]
31. Heitner C, Dimmel DR, Schmidt JA. Lignin and lignans: advances in chemistry. Boca Raton, FL, USA: CRC Press; 2010. [Google Scholar]
32. Feofilova EP, Mysyakina IS. Lignin: chemical structure, biodegradation, and practical application (a review). Appl Biochem Microbiol. 2016;52(6):573–81. doi:10.1134/s0003683816060053. [Google Scholar] [CrossRef]
33. Rajinipriya M, Nagalakshmaiah M, Robert M, Elkoun S. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustainable Chem Eng. 2018;6(3):2807–28. doi:10.1021/acssuschemeng.7b03437. [Google Scholar] [CrossRef]
34. Kasiri N, Fathi M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int J Biol Macromol. 2018;106(12–13):1023–31. doi:10.1016/j.ijbiomac.2017.08.112. [Google Scholar] [PubMed] [CrossRef]
35. El Achaby M, El Miri N, Hannache H, Gmouh S, Ben Youcef H, Aboulkas A. Production of cellulose nanocrystals from vine shoots and their use for the development of nanocomposite materials. Int J Biol Macromol. 2018;117:592–600. doi:10.1016/j.ijbiomac.2018.05.201. [Google Scholar] [PubMed] [CrossRef]
36. Naduparambath S, Jinitha TV, Shaniba V, Sreejith MP, Balan AK, Purushothaman E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr Polym. 2018;180(4):13–20. doi:10.1016/j.carbpol.2017.09.088. [Google Scholar] [PubMed] [CrossRef]
37. Mwaikambo L. Review of the history, properties and application of plant fibres. Afr J Sci Technol. 2006;7:120–33. [Google Scholar]
38. Fan M, Fu F. Advanced high strength natural fibre composites in construction. Sawston, UK: Woodhead Publishing; 2016. [Google Scholar]
39. Pelissari FM, do Amaral Sobral PJ, Menegalli FC. Isolation and characterization of cellulose nanofibers from banana peels. Cellulose. 2014;21(1):417–32. doi:10.1007/s10570-013-0138-6. [Google Scholar] [CrossRef]
40. Tingaut P, Zimmermann T, Sèbe G. Cellulose nanocrystals and microfibrillated cellulose as building blocks for the design of hierarchical functional materials. J Mater Chem. 2012;22(38):20105–11. doi:10.1039/c2jm32956e. [Google Scholar] [CrossRef]
41. Malucelli LC, Lacerda LG, Dziedzic M, da Silva Carvalho Filho MA. Preparation, properties and futureperspectives of nanocrystals from agro-industrial residues: a review of recentresearch. Rev Environ Sci Bio/Technol. 2017;16(1):131–45. doi:10.1007/s11157-017-9423-4. [Google Scholar] [CrossRef]
42. Li Q, Wang Y, Wu Y, He K, Li Y, Luo X, et al. Flexible cellulose nanofibrils as novel Pickering stabilizers: the emulsifying property and packing behavior. Food Hydrocoll. 2019;88(5):180–9. doi:10.1016/j.foodhyd.2018.09.039. [Google Scholar] [CrossRef]
43. Utoiu E, Manoiu VS, Oprita EI, Craciunescu O. Bacterial cellulose: a sustainable source for hydrogels and 3D-printed scaffolds for tissue engineering. Gels. 2024;10(6):387. doi:10.3390/gels10060387. [Google Scholar] [PubMed] [CrossRef]
44. Jonoobi M, Oladi R, Davoudpour Y, Oksman K, Dufresne A, Hamzeh Y, et al. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose. 2015;22(2):935–69. doi:10.1007/s10570-015-0551-0. [Google Scholar] [CrossRef]
45. Kamel S, Khattab TA. Recent advances in cellulose-based biosensors for medical diagnosis. Biosensors. 2020;10(6):67. doi:10.3390/bios10060067. [Google Scholar] [PubMed] [CrossRef]
46. Grishkewich N, Mohammed N, Tang J, Tam KC. Recent advances in the application of cellulose nanocrystals. Curr Opin Colloid Interface Sci. 2017;29:32–45. doi:10.1016/j.cocis.2017.01.005. [Google Scholar] [CrossRef]
47. Dhall S, Acharya L. A systematic review of basic strategies for enhancing food storage life with nanotechnology products. ACS Food Sci Technol. 2025;5(4):1239–54. doi:10.1021/acsfoodscitech.4c01016. [Google Scholar] [CrossRef]
48. Michelin M, Gomes DG, Romaní A, Polizeli MD, Teixeira JA. Nanocellulose production: exploring the enzymatic route and residues of pulp and paper industry. Molecules. 2020;25(15):3411. doi:10.3390/molecules25153411. [Google Scholar] [PubMed] [CrossRef]
49. García A, Gandini A, Labidi J, Belgacem N, Bras J. Industrial and crop wastes: a new source for nanocellulose biorefinery. Ind Crops Prod. 2016;93(2):26–38. doi:10.1016/j.indcrop.2016.06.004. [Google Scholar] [CrossRef]
50. Wahid F, Zhong C. Chapter 13—production and applications of bacterial cellulose. In: Binod P, Raveendran S, Pandey A, editors. Biomass, biofuels, biochemicals. Amsterdam, The Netherlands: Elsevier; 2021. p. 359–90. doi: 10.1016/B978-0-12-821888-4.00010-1. [Google Scholar] [CrossRef]
51. Gallegos AMA, Herrera Carrera S, Parra R, Keshavarz T, Iqbal HMN. Bacterial cellulose: a sustainable source to develop value-added products—a review. BioResources. 2016;11(2):5641–55. doi:10.15376/biores.11.2.gallegos. [Google Scholar] [CrossRef]
52. Frone AN, Chiulan I, Panaitescu DM, Nicolae CA, Ghiurea M, Galan AM. Isolation of cellulose nanocrystals from plum seed shells, structural and morphological characterization. Mater Lett. 2017;194:160–3. doi:10.1016/j.matlet.2017.02.051. [Google Scholar] [CrossRef]
53. Hemmati F, Jafari SM, Kashaninejad M, Barani Motlagh M. Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int J Biol Macromol. 2018;120(Pt A):1216–24. doi:10.1016/j.ijbiomac.2018.09.012. [Google Scholar] [PubMed] [CrossRef]
54. Wang Z, Yao Z, Zhou J, He M, Jiang Q, Li S, et al. Isolation and characterization of cellulose nanocrystals from pueraria root residue. Int J Biol Macromol. 2019;129:1081–9. doi:10.1016/j.ijbiomac.2018.07.055. [Google Scholar] [PubMed] [CrossRef]
55. Vallejos ME, Felissia FE, Area MC, Ehman NV, Tarrés Q, Mutjé P. Nanofibrillated cellulose (CNF) from Eucalyptus sawdust as a dry strength agent of unrefined Eucalyptus handsheets. Carbohydr Polym. 2016;139(2):99–105. doi:10.1016/j.carbpol.2015.12.004. [Google Scholar] [PubMed] [CrossRef]
56. Winuprasith T, Suphantharika M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013;32(2):383–94. doi:10.1016/j.foodhyd.2013.01.023. [Google Scholar] [CrossRef]
57. Costa ALR, Gomes A, Tibolla H, Menegalli FC, Cunha RL. Cellulose nanofibers from banana peels as a Pickering emulsifier: high-energy emulsification processes. Carbohydr Polym. 2018;194(1–2):122–31. doi:10.1016/j.carbpol.2018.04.001. [Google Scholar] [PubMed] [CrossRef]
58. Li W, Zhao X, Liu S. Preparation of entangled nanocellulose fibers from APMP and its magnetic functional property as matrix. Carbohydr Polym. 2013;94(1):278–85. doi:10.1016/j.carbpol.2013.01.052. [Google Scholar] [PubMed] [CrossRef]
59. Chen W, Yu H, Liu Y, Chen P, Zhang M, Hai Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr Polym. 2011;83(4):1804–11. doi:10.1016/j.carbpol.2010.10.040. [Google Scholar] [CrossRef]
60. Phanthong P, Reubroycharoen P, Hao X, Xu G, Abudula A, Guan G. Nanocellulose: extraction and application. Carbon Resour Convers. 2018;1(1):32–43. doi:10.1016/j.crcon.2018.05.004. [Google Scholar] [CrossRef]
61. Ni Y, Li J, Fan L. Production of nanocellulose with different length from Ginkgo seed shells and applications for oil in water Pickering emulsions. Int J Biol Macromol. 2020;149(3):617–26. doi:10.1016/j.ijbiomac.2020.01.263. [Google Scholar] [PubMed] [CrossRef]
62. dos Santos RM, Flauzino Neto WP, Silvério HA, Martins DF, Dantas NO, Pasquini D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind Crops Prod. 2013;50(4):707–14. doi:10.1016/j.indcrop.2013.08.049. [Google Scholar] [CrossRef]
63. Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev. 2010;110(6):3479–500. doi:10.1021/cr900339w. [Google Scholar] [PubMed] [CrossRef]
64. Silvério HA, Flauzino Neto WP, Dantas NO, Pasquini D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind Crops Prod. 2013;44(12):427–36. doi:10.1016/j.indcrop.2012.10.014. [Google Scholar] [CrossRef]
65. Shang Z, An X, Seta FT, Ma M, Shen M, Dai L, et al. Improving dispersion stability of hydrochloric acid hydrolyzed cellulose nano-crystals. Carbohydr Polym. 2019;222(2):115037. doi:10.1016/j.carbpol.2019.115037. [Google Scholar] [PubMed] [CrossRef]
66. Vanderfleet OM, Osorio DA, Cranston ED. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Philos Trans A Math Phys Eng Sci. 2018;376(2112):20170041. doi:10.1098/rsta.2017.0041. [Google Scholar] [PubMed] [CrossRef]
67. Du H, Liu C, Mu X, Gong W, Lv D, Hong Y, et al. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose. 2016;23(4):2389–407. doi:10.1007/s10570-016-0963-5. [Google Scholar] [CrossRef]
68. Mahmud MM, Perveen A, Jahan RA, Matin MA, Wong SY, Li X, et al. Preparation of different polymorphs of cellulose from different acid hydrolysis medium. Int J Biol Macromol. 2019;130:969–76. doi:10.1016/j.ijbiomac.2019.03.027. [Google Scholar] [PubMed] [CrossRef]
69. Jiang H, Wu S, Zhou J. Preparation and modification of nanocellulose and its application to heavy metal adsorption: a review. Int J Biol Macromol. 2023;236(100557):123916. doi:10.1016/j.ijbiomac.2023.123916. [Google Scholar] [PubMed] [CrossRef]
70. Camarero Espinosa S, Kuhnt T, Foster EJ, Weder C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules. 2013;14(4):1223–30. doi:10.1021/bm400219u. [Google Scholar] [PubMed] [CrossRef]
71. Dong XM, Revol J-F, Gray DG. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose. 1998;5(1):19–32. doi:10.1023/A:1009260511939. [Google Scholar] [CrossRef]
72. Kalashnikova I, Bizot H, Cathala B, Capron I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules. 2012;13(1):267–75. doi:10.1021/bm201599j. [Google Scholar] [PubMed] [CrossRef]
73. Bian H, Chen L, Dai H, Zhu JY. Effect of fiber drying on properties of lignin containing cellulose nanocrystals and nanofibrils produced through maleic acid hydrolysis. Cellulose. 2017;24(10):4205–16. doi:10.1007/s10570-017-1430-7. [Google Scholar] [CrossRef]
74. Miao J, Yu Y, Jiang Z, Zhang L. One-pot preparation of hydrophobic cellulose nanocrystals in an ionic liquid. Cellulose. 2016;23(2):1209–19. doi:10.1007/s10570-016-0864-7. [Google Scholar] [CrossRef]
75. Liu Y, Wang H, Yu G, Yu Q, Li B, Mu X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr Polym. 2014;110(1):415–22. doi:10.1016/j.carbpol.2014.04.040. [Google Scholar] [PubMed] [CrossRef]
76. Panyamao P, Charumanee S, Ruangsuriya J, Saenjum C. Efficient isolation of cellulosic fibers from coffee parchment via natural acidic deep eutectic solvent pretreatment for nanocellulose production. ACS Sustainable Chem Eng. 2023;11(38):13962–73. doi:10.1021/acssuschemeng.3c02679. [Google Scholar] [CrossRef]
77. Wang L, Zhu X, Chen X, Zhang Y, Yang H, Li Q, et al. Isolation and characteristics of nanocellulose from hardwood pulp via phytic acid pretreatment. Ind Crops Prod. 2022;182(14):114921. doi:10.1016/j.indcrop.2022.114921. [Google Scholar] [CrossRef]
78. Wang Y, Liu H, Ji X, Wang Q, Tian Z, Fatehi P. Production of nanocellulose using acidic deep eutectic solvents based on choline chloride and carboxylic acids: a review. Int J Biol Macromol. 2023;245(11):125227. doi:10.1016/j.ijbiomac.2023.125227. [Google Scholar] [PubMed] [CrossRef]
79. Naseem S, Durrani AI, Rizwan M, Yasmeen F, Siddiqui S, Habib F. Sono-microwave assisted Chlorine free and Ionic liquid (SMACIL) extraction of cellulose from Urtica dioica: a benign to green approach. Int J Biol Macromol. 2024;259(Pt 1):129059. doi:10.1016/j.ijbiomac.2023.129059. [Google Scholar] [PubMed] [CrossRef]
80. Al Ragib A, Alanazi YM, El-Harbawi M, Yin CY, Khiari R. Sustainable reuse of date palm biomass via extraction of cellulose using natural deep eutectic solvent (NaDES) and microwave-assisted process. Int J Biol Macromol. 2024;279(Pt 2):135558. doi:10.1016/j.ijbiomac.2024.135558. [Google Scholar] [PubMed] [CrossRef]
81. Bahndral A, Shams R, Dash KK, Ali NA, Shaikh AM, Kovács B. Microwave assisted extraction of cellulose from lemon grass: effect on techno-functional and microstructural properties. J Agric Food Res. 2024;16:101170. doi:10.1016/j.jafr.2024.101170. [Google Scholar] [CrossRef]
82. Sui X, Yuan J, Yuan W, Zhou M. Preparation of cellulose nanofibers/nanoparticles via electrospray. Chem Lett. 2008;37(1):114–5. doi:10.1246/cl.2008.114. [Google Scholar] [PubMed] [CrossRef]
83. Wang J, Wang Z, Yun J, Chen S, Liu S, Li C, et al. Ball milling-induced disassembly of cellulose in coconut endosperm pomace: structural mechanism for enhanced Pickering emulsification. Int J Biol Macromol. 2025;310(Pt 1):143238. doi:10.1016/j.ijbiomac.2025.143238. [Google Scholar] [PubMed] [CrossRef]
84. Gao Y, Zhang W, Ali Asadollahi M, Liu C, Yu G, Li H, et al. The effect of combining mineral salt with ball milling for promoting enzymatic saccharification of cellulose. Biomass Bioenergy. 2025;193:107577. doi:10.1016/j.biombioe.2024.107577. [Google Scholar] [CrossRef]
85. Yan Z, Jiang S, Xi J, Ye W, Meng L, Xiao H, et al. Frost-resistant nanocellulose-based organohydrogel with high mechanical strength and transparency. J Colloid Interface Sci. 2024;661(39):879–87. doi:10.1016/j.jcis.2024.02.002. [Google Scholar] [PubMed] [CrossRef]
86. Yi T, Zhao H, Mo Q, Pan D, Liu Y, Huang L, et al. From cellulose to cellulose nanofibrils—a comprehensive review of the preparation and modification of cellulose nanofibrils. Materials. 2020;13(22):5062. doi:10.3390/ma13225062. [Google Scholar] [PubMed] [CrossRef]
87. Verma YK, Singh AK, Paswan MK, Gurmaita PK. Preparation and characterization of bamboo based nanocellulose by ball milling and used as a filler for preparation of nanocomposite. Polymer. 2024;308(3):127396. doi:10.1016/j.polymer.2024.127396. [Google Scholar] [CrossRef]
88. Boufi S, Gandini A. Triticale crop residue: a cheap material for high performance nanofibrillated cellulose. RSC Adv. 2015;5(5):3141–51. doi:10.1039/c4ra12918k. [Google Scholar] [CrossRef]
89. Yokota S, Kamada K, Sugiyama A, Kondo T. Pickering emulsion stabilization by using amphiphilic cellulose nanofibrils prepared by aqueous counter collision. Carbohydr Polym. 2019;226(10):115293. doi:10.1016/j.carbpol.2019.115293. [Google Scholar] [PubMed] [CrossRef]
90. Ferrer A, Filpponen I, Rodríguez A, Laine J, Rojas OJ. Valorization of residual empty palm fruit bunch fibers (EPFBF) by microfluidization: production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour Technol. 2012;125(1):249–55. doi:10.1016/j.biortech.2012.08.108. [Google Scholar] [PubMed] [CrossRef]
91. Taipale T, Österberg M, Nykänen A, Ruokolainen J, Laine J. Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose. 2010;17(5):1005–20. doi:10.1007/s10570-010-9431-9. [Google Scholar] [CrossRef]
92. Ling S, Chen W, Fan Y, Zheng K, Jin K, Yu H, et al. Biopolymer nanofibrils: structure, modeling, preparation, and applications. Prog Polym Sci. 2018;85:1–56. doi:10.1016/j.progpolymsci.2018.06.004. [Google Scholar] [PubMed] [CrossRef]
93. Lu X, Zhang H, Li Y, Huang Q. Fabrication of milled cellulose particles-stabilized Pickering emulsions. Food Hydrocoll. 2018;77(6):427–35. doi:10.1016/j.foodhyd.2017.10.019. [Google Scholar] [CrossRef]
94. Barakat A, Mayer-Laigle C, Solhy A, Arancon RAD, de Vries H, Luque R. Mechanical pretreatments of lignocellulosic biomass: towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014;4(89):48109–27. doi:10.1039/c4ra07568d. [Google Scholar] [CrossRef]
95. Jonoobi M, Mathew AP, Oksman K. Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind Crops Prod. 2012;40:232–8. doi:10.1016/j.indcrop.2012.03.018. [Google Scholar] [CrossRef]
96. Spence KL, Venditti RA, Rojas OJ, Habibi Y, Pawlak JJ. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose. 2011;18(4):1097–111. doi:10.1007/s10570-011-9533-z. [Google Scholar] [CrossRef]
97. Abral H, Lawrensius V, Handayani D, Sugiarti E. Preparation of nano-sized particles from bacterial cellulose using ultrasonication and their characterization. Carbohydr Polym. 2018;191(2):161–7. doi:10.1016/j.carbpol.2018.03.026. [Google Scholar] [PubMed] [CrossRef]
98. Paximada P, Dimitrakopoulou EA, Tsouko E, Koutinas AA, Fasseas C, Mandala IG. Structural modification of bacterial cellulose fibrils under ultrasonic irradiation. Carbohydr Polym. 2016;150(3):5–12. doi:10.1016/j.carbpol.2016.04.125. [Google Scholar] [PubMed] [CrossRef]
99. Ni Y, Li J, Fan L. Effects of ultrasonic conditions on the interfacial property and emulsifying property of cellulose nanoparticles from Ginkgo seed shells. Ultrason Sonochem. 2021;70(3):105335. doi:10.1016/j.ultsonch.2020.105335. [Google Scholar] [PubMed] [CrossRef]
100. Wang S, Cheng Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, part 1: process optimization. J Appl Polym Sci. 2009;113(2):1270–5. doi:10.1002/app.30072. [Google Scholar] [CrossRef]
101. Iguchi M, Yamanaka S, Budhiono A. Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci. 2000;35(2):261–70. doi:10.1023/A:1004775229149. [Google Scholar] [CrossRef]
102. Chen SQ, Lopez-Sanchez P, Wang D, Mikkelsen D, Gidley MJ. Mechanical properties of bacterial cellulose synthesised by diverse strains of the genus Komagataeibacter. Food Hydrocoll. 2018;81:87–95. doi:10.1016/j.foodhyd.2018.02.031. [Google Scholar] [CrossRef]
103. de Souza Lima MM, Borsali R. Rodlike cellulose microcrystals: structure, properties, and applications. Macromol Rapid Commun. 2004;25(7):771–87. doi:10.1002/marc.200300268. [Google Scholar] [CrossRef]
104. BeMiller JN. Carbohydrate chemistry for food scientists. Amsterdam, The Netherlands: Elsevier; 2018. [Google Scholar]
105. Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40(7):3941–94. doi:10.1039/c0cs00108b. [Google Scholar] [PubMed] [CrossRef]
106. He Q, Sun X, Bai Y, Meng X, Li C. Isolation of dicarboxy cellulose nanocrystal from spent fungi substrate and redispersion with gelatin. J Mol Liq. 2022;367(8):120397. doi:10.1016/j.molliq.2022.120397. [Google Scholar] [CrossRef]
107. Kupnik K, Primožič M, Kokol V, Leitgeb M. Nanocellulose in drug delivery and antimicrobially active materials. Polymers. 2020;12(12):2825. doi:10.3390/polym12122825. [Google Scholar] [PubMed] [CrossRef]
108. Seidi F, Jiang W, Yu Z, Deng C. Cellulose-MXene composites: new platforms with outstanding multifunctional characteristics. J Bioresour Bioprod. 2024;9(3):243–5. doi:10.1016/j.jobab.2024.05.002. [Google Scholar] [CrossRef]
109. Zhang M, Wang Y, Liu K, Liu Y, Xu T, Du H, et al. Strong, conductive, and freezing-tolerant polyacrylamide/PEDOT:pss/cellulose nanofibrils hydrogels for wearable strain sensors. Carbohydr Polym. 2023;305(11):120567. doi:10.1016/j.carbpol.2023.120567. [Google Scholar] [PubMed] [CrossRef]
110. Yu K, Yang L, Zhang S, Zhang N. Nanocellulose-based aerogels for the adsorption and removal of heavy-metal ions from wastewater: a review. Mater Today Commun. 2025;43:111744. doi:10.1016/j.mtcomm.2025.111744. [Google Scholar] [CrossRef]
111. Wei T, Zhu Y, Duan G, Han J, Han X, Zhang C, et al. Recent advances in nanocellulose-derived materials for dyes adsorption: a review. Int J Biol Macromol. 2025;306(Pt 4):141770. doi:10.1016/j.ijbiomac.2025.141770. [Google Scholar] [PubMed] [CrossRef]
112. Diddens I, Murphy B, Krisch M, Müller M. Anisotropic elastic properties of cellulose measured using inelastic X-ray scattering. Macromolecules. 2008;41(24):9755–9. doi:10.1021/ma801796u. [Google Scholar] [CrossRef]
113. Kargarzadeh H, Mariano M, Huang J, Lin N, Ahmad I, Dufresne A, et al. Recent developments on nanocellulose reinforced polymer nanocomposites: a review. Polymer. 2017;132(2016):368–93. doi:10.1016/j.polymer.2017.09.043. [Google Scholar] [CrossRef]
114. Zhu H, Parvinian S, Preston C, Vaaland O, Ruan Z, Hu L. Transparent nanopaper with tailored optical properties. Nanoscale. 2013;5(9):3787–92. doi:10.1039/c3nr00520h. [Google Scholar] [PubMed] [CrossRef]
115. Yadav C, Saini A, Zhang W, You X, Chauhan I, Mohanty P, et al. Plant-based nanocellulose: a review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int J Biol Macromol. 2021;166:1586–616. doi:10.1016/j.ijbiomac.2020.11.038. [Google Scholar] [PubMed] [CrossRef]
116. Thomas B, Raj MC, Athira KB, Rubiyah MH, Joy J, Moores A, et al. Nanocellulose, a versatile green platform: from biosources to materials and their applications. Chem Rev. 2018;118(24):11575–625. doi:10.1021/acs.chemrev.7b00627. [Google Scholar] [PubMed] [CrossRef]
117. Ventura C, Pinto F, Lourenço AF, Ferreira PJT, Louro H, Silva MJ. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose. 2020;27(10):5509–44. doi:10.1007/s10570-020-03176-9. [Google Scholar] [CrossRef]
118. Low LE, Siva SP, Ho YK, Chan ES, Tey BT. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv Colloid Interface Sci. 2020;277:102117. doi:10.1016/j.cis.2020.102117. [Google Scholar] [PubMed] [CrossRef]
119. Fujisawa S, Togawa E, Kuroda K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci Technol Adv Mater. 2017;18(1):959–71. doi:10.1080/14686996.2017.1401423. [Google Scholar] [PubMed] [CrossRef]
120. Li G, Bao L, Hu G, Chen L, Zhou X, Hong FF. Development and performance evaluation of a novel elastic bacterial nanocellulose/polyurethane small caliber artificial blood vessels. Int J Biol Macromol. 2024;268(Pt 2):131685. doi:10.1016/j.ijbiomac.2024.131685. [Google Scholar] [PubMed] [CrossRef]
121. Guo S, Li X, Kuang Y, Liao J, Liu K, Li J, et al. Residual lignin in cellulose nanofibrils enhances the interfacial stabilization of Pickering emulsions. Carbohydr Polym. 2021;253(6):117223. doi:10.1016/j.carbpol.2020.117223. [Google Scholar] [PubMed] [CrossRef]
122. Fujisawa S, Togawa E, Kuroda K. Facile route to transparent, strong, and thermally stable nanocellulose/polymer nanocomposites from an aqueous Pickering emulsion. Biomacromolecules. 2017;18(1):266–71. doi:10.1021/acs.biomac.6b01615. [Google Scholar] [PubMed] [CrossRef]
123. Li Y, Yu S, Chen P, Rojas R, Hajian A, Berglund L. Cellulose nanofibers enable paraffin encapsulation and the formation of stable thermal regulation nanocomposites. Nano Energy. 2017;34:541–8. doi:10.1016/j.nanoen.2017.03.010. [Google Scholar] [CrossRef]
124. Huan S, Ajdary R, Bai L, Klar V, Rojas OJ. Low solids emulsion gels based on nanocellulose for 3D-printing. Biomacromolecules. 2019;20(2):635–44. doi:10.1021/acs.biomac.8b01224. [Google Scholar] [PubMed] [CrossRef]
125. Liu K, Du H, Zheng T, Liu W, Zhang M, Liu H, et al. Lignin-containing cellulose nanomaterials: preparation and applications. Green Chem. 2021;23(24):9723–46. doi:10.1039/d1gc02841c. [Google Scholar] [CrossRef]
126. Wang Q, Yao Q, Liu J, Sun J, Zhu Q, Chen H. Processing nanocellulose to bulk materials: a review. Cellulose. 2019;26(13):7585–617. doi:10.1007/s10570-019-02642-3. [Google Scholar] [CrossRef]
127. Mittal N, Ansari F, Gowda VK, Brouzet C, Chen P, Larsson PT, et al. Multiscale control of nanocellulose assembly: transferring remarkable nanoscale fibril mechanics to macroscale fibers. ACS Nano. 2018;12(7):6378–88. doi:10.1021/acsnano.8b01084. [Google Scholar] [PubMed] [CrossRef]
128. Wang J, Gao Q, Wang Y, Liu X, Nie S. Strong fibrous filaments nanocellulose crystals prepared by self-twisting microfluidic spinning. Ind Crops Prod. 2022;178:114599. doi:10.1016/j.indcrop.2022.114599. [Google Scholar] [CrossRef]
129. Kim HC, Kim D, Lee JY, Zhai L, Kim J. Effect of wet spinning and stretching to enhance mechanical properties of cellulose nanofiber filament. Int J Precis Eng Manuf Green Technol. 2019;6(3):567–75. doi:10.1007/s40684-019-00070-z. [Google Scholar] [CrossRef]
130. Rosén T, Hsiao BS, Söderberg LD. Elucidating the opportunities and challenges for nanocellulose spinning. Adv Mater. 2021;33(28):e2001238. doi:10.1002/adma.202001238. [Google Scholar] [PubMed] [CrossRef]
131. Niu Q, Gao K, Shao Z. Cellulose nanofiber/single-walled carbon nanotube hybrid non-woven macrofiber mats as novel wearable supercapacitors with excellent stability, tailorability and reliability. Nanoscale. 2014;6(8):4083–8. doi:10.1039/c3nr05929d. [Google Scholar] [PubMed] [CrossRef]
132. Wan Z, Chen C, Meng T, Mojtaba M, Teng Y, Feng Q, et al. Multifunctional wet-spun filaments through robust nanocellulose networks wrapping to single-walled carbon nanotubes. ACS Appl Mater Interfaces. 2019;11(45):42808–17. doi:10.1021/acsami.9b15153. [Google Scholar] [PubMed] [CrossRef]
133. Sehaqui H, Zhou Q, Ikkala O, Berglund LA. Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules. 2011;12(10):3638–44. doi:10.1021/bm2008907. [Google Scholar] [PubMed] [CrossRef]
134. González I, Alcalà M, Chinga-Carrasco G, Vilaseca F, Boufi S, Mutjé P. From paper to nanopaper: evolution of mechanical and physical properties. Cellulose. 2014;21(4):2599–609. doi:10.1007/s10570-014-0341-0. [Google Scholar] [CrossRef]
135. Huang J, Zhu H, Chen Y, Preston C, Rohrbach K, Cumings J, et al. Highly transparent and flexible nanopaper transistors. ACS Nano. 2013;7(3):2106–13. doi:10.1021/nn304407r. [Google Scholar] [PubMed] [CrossRef]
136. Barhoum A, Samyn P, Öhlund T, Dufresne A. Review of recent research on flexible multifunctional nanopapers. Nanoscale. 2017;9(40):15181–205. doi:10.1039/c7nr04656a. [Google Scholar] [PubMed] [CrossRef]
137. Wang Q, Feng S, Liu J, Liu H, Tu W, Zhu Q. Engineering nanocellulose percolation network for flexible strain sensor. Cellulose. 2024;31(9):5381–417. doi:10.1007/s10570-024-05955-0. [Google Scholar] [CrossRef]
138. Kang X, Jiang K, Ge S, Wei K, Zhou Y, Xu BB, et al. Frontier in advanced luminescent biomass nanocomposites for surface anticounterfeiting. ACS Nano. 2025;19(12):11547–75. doi:10.1021/acsnano.4c17883. [Google Scholar] [PubMed] [CrossRef]
139. Zheng H, Li W, Li W, Wang X, Tang Z, Zhang SX, et al. Uncovering the circular polarization potential of chiral photonic cellulose films for photonic applications. Adv Mater. 2018;30(13):e1705948. doi:10.1002/adma.201705948. [Google Scholar] [PubMed] [CrossRef]
140. Tao J, Zou C, Jiang H, Li M, Lu D, Mann S, et al. Optically ambidextrous reflection and luminescence in self-organized left-handed chiral nematic cellulose nanocrystal films. CCS Chem. 2021;3(3):932–45. doi:10.31635/ccschem.020.202000248. [Google Scholar] [CrossRef]
141. Cheng Z, Ma Y, Yang L, Cheng F, Huang Z, Natan A, et al. Plasmonic-enhanced cholesteric films: coassembling anisotropic gold nanorods with cellulose nanocrystals. Adv Opt Mater. 2019;7(9):1801816. doi:10.1002/adom.201801816. [Google Scholar] [CrossRef]
142. Chen T, Zhao Q, Meng X, Li Y, Peng H, Whittaker AK, et al. Ultrasensitive magnetic tuning of optical properties of films of cholesteric cellulose nanocrystals. ACS Nano. 2020;14(8):9440–8. doi:10.1021/acsnano.0c00506. [Google Scholar] [PubMed] [CrossRef]
143. Parker RM, Guidetti G, Williams CA, Zhao T, Narkevicius A, Vignolini S, et al. The self-assembly of cellulose nanocrystals: hierarchical design of visual appearance. Adv Mater. 2018;30(19):e1704477. doi:10.1002/adma.201704477. [Google Scholar] [PubMed] [CrossRef]
144. Zhao X, Zhang Y, Lv Z, Ding Z, Wang Y, Yang H, et al. Nanocellulose and its composites toward flexible supercapacitor. Adv Mater Technol. 2025;10(10):2401709. doi:10.1002/admt.202401709. [Google Scholar] [CrossRef]
145. Shrestha M, Amatya I, Wang K, Zheng B, Gu Z, Fan QH. Electrophoretic deposition of activated carbon YP-50 with ethyl cellulose binders for supercapacitor electrodes. J Energy Storage. 2017;13(2):206–10. doi:10.1016/j.est.2017.07.015. [Google Scholar] [CrossRef]
146. Chen M, Chen J, Zhou W, Xu J, Wong CP. High-performance flexible and self-healable quasi-solid-state zinc-ion hybrid supercapacitor based on borax-crosslinked polyvinyl alcohol/nanocellulose hydrogel electrolyte. J Mater Chem A. 2019;7(46):26524–32. doi:10.1039/c9ta10944g. [Google Scholar] [CrossRef]
147. Ding Z, Yang X, Tang Y. Nanocellulose-based electrodes and separator toward sustainable and flexible all-solid-state supercapacitor. Int J Biol Macromol. 2023;228(22):467–77. doi:10.1016/j.ijbiomac.2022.12.224. [Google Scholar] [PubMed] [CrossRef]
148. Gao W, Tu Q, Wang P, Zeng J, Li J, Wang B, et al. Conductive polymer/nanocellulose composites as a functional platform for electronic devices: a mini-review. Polym Rev. 2024;64(1):162–91. doi:10.1080/15583724.2023.2220018. [Google Scholar] [CrossRef]
149. Sacui IA, Nieuwendaal RC, Burnett DJ, Stranick SJ, Jorfi M, Weder C, et al. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl Mater Interfaces. 2014;6(9):6127–38. doi:10.1021/am500359f. [Google Scholar] [PubMed] [CrossRef]
150. Wise HG, Takana H, Dichiara AB. Dynamic assembly of strong and conductive carbon nanotube/nanocellulose composite filaments and their application in resistive liquid sensing. ACS Appl Mater Interfaces. 2023;15(30):36647–56. doi:10.1021/acsami.3c03906. [Google Scholar] [PubMed] [CrossRef]
151. Ghasemlou M, Daver F, Ivanova EP, Murdoch BJ, Adhikari B. Use of synergistic interactions to fabricate transparent and mechanically robust nanohybrids based on starch, non-isocyanate polyurethanes, and cellulose nanocrystals. ACS Appl Mater Interfaces. 2020;12(42):47865–78. doi:10.1021/acsami.0c14525. [Google Scholar] [PubMed] [CrossRef]
152. Dong C, Zhang H, Pang Z, Liu Y, Zhang F. Sulfonated modification of cotton Linter and its application as adsorbent for high-efficiency removal of lead(II) in effluent. Bioresour Technol. 2013;146:512–8. doi:10.1016/j.biortech.2013.07.108. [Google Scholar] [PubMed] [CrossRef]
153. Capadona JR, Van Den Berg O, Capadona LA, Schroeter M, Rowan SJ, Tyler DJ, et al. A versatile approach for the processing of polymer nanocomposites with self-assembled nanofibre templates. Nat Nanotechnol. 2007;2(12):765–9. doi:10.1038/nnano.2007.379. [Google Scholar] [PubMed] [CrossRef]
154. Arivendan A, Chen X, Zhang YF, Gao W. Recent advances in nanocellulose pretreatment routes, developments, applications and future prospects: a state-of-the-art review. Int J Biol Macromol. 2024;281(Pt 2):135925. doi:10.1016/j.ijbiomac.2024.135925. [Google Scholar] [PubMed] [CrossRef]
155. Mishra K, Siwal SS, Sithole T, Singh N, Hart P, Thakur VK. Biorenewable materials for water remediation: the central role of cellulose in achieving sustainability. J Bioresour Bioprod. 2024;9(3):253–82. doi:10.1016/j.jobab.2023.12.002. [Google Scholar] [CrossRef]
156. Das R, Lindström T, Sharma PR, Chi K, Hsiao BS. Nanocellulose for sustainable water purification. Chem Rev. 2022;122(9):8936–9031. doi:10.1021/acs.chemrev.1c00683. [Google Scholar] [PubMed] [CrossRef]
157. Aoudi B, Boluk Y, Gamal El-Din M. Recent advances and future perspective on nanocellulose-based materials in diverse water treatment applications. Sci Total Environ. 2022;843(6):156903. doi:10.1016/j.scitotenv.2022.156903. [Google Scholar] [PubMed] [CrossRef]
158. Sun F, Liu W, Dong Z, Deng Y. Underwater superoleophobicity cellulose nanofibril aerogel through regioselective sulfonation for oil/water separation. Chem Eng J. 2017;330(2):774–82. doi:10.1016/j.cej.2017.07.142. [Google Scholar] [CrossRef]
159. Zhang M, Jiang S, Han F, Li M, Wang N, Liu L. Anisotropic cellulose nanofiber/chitosan aerogel with thermal management and oil absorption properties. Carbohydr Polym. 2021;264(2):118033. doi:10.1016/j.carbpol.2021.118033. [Google Scholar] [PubMed] [CrossRef]
160. De France KJ, Hoare T, Cranston ED. Review of hydrogels and aerogels containing nanocellulose. Chem Mater. 2017;29(11):4609–31. doi:10.1021/acs.chemmater.7b00531. [Google Scholar] [CrossRef]
161. Chen Y, Zhang L, Yang Y, Pang B, Xu W, Duan G, et al. Recent progress on nanocellulose aerogels: preparation, modification, composite fabrication, applications. Adv Mater. 2021;33(11):e2005569. doi:10.1002/adma.202005569. [Google Scholar] [PubMed] [CrossRef]
162. Zeng Z, Wu T, Han D, Ren Q, Siqueira G, Nyström G. Ultralight, flexible, and biomimetic nanocellulose/silver nanowire aerogels for electromagnetic interference shielding. ACS Nano. 2020;14(3):2927–38. doi:10.1021/acsnano.9b07452. [Google Scholar] [PubMed] [CrossRef]
163. Xu C, Molino BZ, Wang X, Cheng F, Xu W, Molino P, et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J Mater Chem B. 2018;6(43):7066–75. doi:10.1039/c8tb01757c. [Google Scholar] [PubMed] [CrossRef]
164. Zieliński PS, Gudeti PKR, Rikmanspoel T, Włodarczyk-Biegun MK. 3D printing of bio-instructive materials: toward directing the cell. Bioact Mater. 2022;19:292–327. doi:10.1016/j.bioactmat.2022.04.008. [Google Scholar] [PubMed] [CrossRef]
165. Petersen N, Gatenholm P. Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol. 2011;91(5):1277–86. doi:10.1007/s00253-011-3432-y. [Google Scholar] [PubMed] [CrossRef]
166. Ferreira FV, Souza AG, Ajdary R, de Souza LP, Lopes JH, Correa DS, et al. Nanocellulose-based porous materials: regulation and pathway to commercialization in regenerative medicine. Bioact Mater. 2023;29:151–76. doi:10.1016/j.bioactmat.2023.06.020. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools