 Open Access
Open Access
ARTICLE
In Vitro Anti-Bacterial and Anti-Fungal Activities of Extracts from Different Parts of 7 Zingiberaceae Plants
1
College of Horticulture and Landscape Architecture, Zhongkai University of Agriculture and Engineering, Guangzhou, 510225,
China
2
College of Chemistry and Chemical Engineering, Zhongkai University of Agriculture and Engineering, Guangzhou, 510225, China
* Corresponding Authors: Hui Zhang. Email: ; Xiu Hu. Email:
# Lixian Wu and Yongquan Li contributed equally to this work
Journal of Renewable Materials 2023, 11(2), 975-989. https://doi.org/10.32604/jrm.2022.023547
Received 01 May 2022; Accepted 12 July 2022; Issue published 22 September 2022
Abstract
This study aimed to explore the anti-bacterial and anti-fungal activities of extracts from different parts of plants in the Zingiberaceae family. The inhibitory rate, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) of leaf and stem, and root and rhizome extracts from Alpinia katsumadai Hayata, Alpinia oxyphylla Miq × Alpinia henryi K. Schumann, Alpinia oblongifolia Hayata, Alpinia nigra (Gaertn.) Burtt, Amomum villosum Lour, Alpinia zerumbet (Pers.) Burtt. et Smith and Alpinia oxyphylla Miq were determined using the fungus cake method and double dilution method. The seven Zingiberaceae plants exhibited characteristic antibacterial activities against pathogenic bacteria and fungi. At a 1.5 mg mL−1, A. zerumbet root and rhizome extracts exhibited strong inhibitory activity against S. aureus and E. coli, with 83.23% and 79.62%, respectively. In addition, A. zerumbet leaf and stem extracts had an inhibitory rate of 90.85% against P. aeruginosa. At the same concentration, the leaf and stem, root and rhizome extracts of A. katsumadai had the best antibacterial effect against F. oxysporum, with inhibition rates of 84.46% and 84.73%, respectively. Moreover, A. katsumadai and A. zerumbet leaf and stem extracts had the most significant antibacterial effect against S. aureus, with a MIC of 0.063 mg mL−1 . Thus, both A. katsumadai and A. zerumbet extracts had significant antibacterial activity. In addition, by comparing the inhibitory effect of extracts from different parts, it was found that the inhibitory rate and average inhibitory rate of extracts from leaf and stem were higher than those from root and rhizome. The chemical constituents of A. katsumadai and A. zerumbet, determined by the high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), revealed that citric acid (CA), alpinetin, and pinocembrin (PNCB) were the functional constituents yielding the antibacterial activity. Overall, A. katsumadai and A. zerumbet have the potential to be developed as new plant fungicides and bactericides.Keywords
Nomenclature
| CA | citric acid |
| PNCB | pinocembrin |
| UPLC-MS/MS | high-performance liquid chromatography-tandem mass spectrometry |
Zingiberaceae consists of perennial herbaceous plants with a characteristic fragrance, and some plants are used as raw materials for a few Chinese herbal medicines [1,2]. But mostly, it has prostrate and horizontal rhizomes, massively expanding at the root ends. Zingiberaceae is mainly distributed in the tropical and subtropical regions and mostly in Asia, Africa, and tropical America, with about 57 genera and 1600 species identified worldwide [3,4]. The Zingiberaceae family is commonly used as medicinal materials, food, spices, cosmetics, or ornamental plants [5,6]. Specifically, it is rich in pharmacological activities, including antibacterial [7], anti-tumor [8], anti-oxidation [9], anti-allergy [10] and anti-inflammatory [11]. At present, many studies in China, Brazil and Japan have focused on the bacteriostasis of Zingiberaceae with respect to food hygiene [12], human health [13], animal and plant disease prevention [14,15]. Through research report shows that many Zingiberaceae plants on Gram-positive bacteria and Gram-negative bacteria have obvious growth inhibition. Among them, Alpinia galanga [16] and Kaempferia galanga [17] ethanol extracts, Hedychium spicatum [18] and Amomum subulatum [19] methanol extracts on Staphylococcus aureus have the obvious bacteriostatic action. At the same time, Hedychium spicatum [18] and Amomum subulatum [19] methanol extracts, Zingiber officinale [20] ethanol extracts also had a good growth inhibition effect on Gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa and Salmonella. In addition, the methanol extracts, ar-turmerone, and curcumin isolated from the Curcuma longa roots have antibacterial activities against Phytophthora infestans, Fusarium solani, and Alternaria alternata [21]. At present, it is known that Zingiberaceae plants contain chemical components with antibacterial effect such as linalool [22], beta pinene [22], kaempferol, gingerol [23], alpinetin [24], etc. These components may make Zingiberaceae plant extracts have an antibacterial effect, which is of development value as antibacterial agents.
Since Zingiberaceae plants contain flavonoids, alkaloids, phenolic acids, diarylheptanoids and other complex bioactive ingredients [9], many researchers have carried out various chemical and biological studies on Zingiberaceae. The roots, leaves, leaf stems, fruits, and flowers of plants in the Zingiberaceae family have different chemical components and pharmacological activities [11]. However, there are no reports that evaluated the antibacterial activity among Zingiberaceae plants’ different parts. In the present study, the antibacterial activities of Alpinia katsumadai Hayata, Alpinia oxyphylla Miq × Alpinia henryi K. Schumann, Alpinia oblongifolia Hayata, Alpinia nigra (Gaertn.) Burtt, Amomum villosum Lour, Alpinia zerumbet (Pers.) Burtt. et Smith, Alpinia oxyphylla Miq were screened against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Fusarium oxysporum, and Pseudomonas solanacearum in vitro. In addition, chemical constituents of Zingiberaceae were determined by UPLC-MS/MS. The findings in this study will provide data to support and a theoretical basis for developing natural plant bacteriostatic agents in the food industry to enhance their application value.
2.1 Test Plant Materials and Pathogen
The whole plant with rhizome of A. katsumadai, A. oxyphylla × A. henryi, A. oblongifolia, A. nigra, A. villosum, A. zerumbet, A. oxyphylla were obtained from the plant Germplasm Resources Base of Zhongkai University of Agriculture and Engineering. The samples were identified by Associate Professor Hu Xiu of Zhongkai University of Agriculture and Engineering. Then, the voucher specimens were deposited in the ZKYDL herbarium with the following herbarium numbers; ZKYDL 041401 for A. katsumadai, ZKYDL 041402 for A. oxyphylla × A. henryi, ZKYDL 041403 for A. oblongifolia, ZKYDL 041404 for A. nigra, ZKYDL 041405 for A. villosum, ZKYDL 041406 for A. zerumbet, ZKYDL 041407 for A. oxyphylla for future references; We procured stocks of S. aureus, E. coli, P. aeruginosa, F. oxysporum, and P. solanacearum were purchased from Microbial Species Preservation Center.
The whole plants with rhizome were washed and dried at room temperature (24°C) for five days, and further oven-dried at 40°C. The dried plants were divided into two parts. One part was leaves and stems, and the other part was roots and rhizomes. Materials were independently crushed, and passed through a 30-mesh sieve (Lvruo screen mesh). Next, refer to the Yakubu et al. [25] extraction method slightly modified, 0.05 kg of crushed leaves and stems, and roots and rhizomes were weighed and soaked in three changes of 70% ethanol in the ratio of 1:10 (m:V) at room temperature for 1 h each time. During the first extraction, we used ultrasonic-assisted extraction for 5 min. After extraction, we used rotate evaporation to paste the shape, and store in refrigerator at 4°C for future studies as a reserve.
2.3 Determination of Antibacterial and Antifungal Activity
According to the method of El-Tarabily [26], the growth inhibition activity of the plant extracts against the five pathogens was tested using the fungus cake method. Specifically, 50, 100, and 150 mg of extracts were dissolved in 1 mL dimethyl sulfoxide (DMSO), filtered using a 0.22 μm microporous membrane, then mixed in 99 mL medium without extracts to prepare medium containing extracts concentration of 0.5, 1.0, 1.5 mg mL−1, poured into a petri dish with a diameter of 8.5 cm and waited for it to cool and solidify.
After 5 days incubation, AGAR plugs with a diameter of 5 mm were cut from the edge of the colony and inoculated in the medium containing extracts for 48 h (bacteria cultured at 37°C and fungi cultured at 28°C). We used medium without extracts as control. Then measure the diameter of the pathogen on the culture medium. Each treatment was replicated thrice. The inhibition rate was calculated using the formula below.
Colony diameter (cm) = measurement diameter – fungus/bacteria cake diameter (0.5 cm)
Inhibition rate = [(control colony diameter – treatment colony diameter)/control colony diameter] × 100%.
2.4 Preparation of Pathogens Suspension
A total of 3 mL of sterile water was added to the solid cultured media inoculated with the bacterial and fungal pathogens, and the colonies were scraped off using an inoculation ring. The scraped bacterial and fungal colonies were transferred into test tubes. An appropriate amount of sterile water was added to each test tube. The concentrations were adjusted to 1 × 108 CFU/mL using a McFarland turbidimetric tube after uniformly shaking the test tubes [27].
2.5 Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC and MBC were determined using the double dilution method [28]. Precisely, 100 μL medium and 100 μL extracts (1.0 mg mL−1) were added to 96 well plates. The concentration gradient was adjusted to 1.0, 0.5, 0.25, 0.125, 0.063, 0.031, 0.016, 0.008, 0.004, 0.002 and 0.001 mg mL−1 per well plate. Next, 20 μL bacterial/fungal solution was added to each well. In addition, 100 μL bacterial solution was added to the 12th well plate as the control. Finally, 15 μL of 0.5% triphenyl tetrazole chloride (TTC) was added to each well and incubated at a constant temperature for 24 h. The MIC for each extract was determined at the point when no red color was displayed in the well with the minimum concentration. From each well where no bacterial/fungal growth was observed, 100 μL of the well content was inoculated into an AGAR medium and incubated 24 h (bacteria cultured at 37°C and fungi cultured at 28°C). The MBC for each extract was defined as the colony growth of less than 5.
2.6 Phytochemical Analysis of Extract by UPLC-MS/MS
Extraction of plant components: Extracts from leaves, stems, roots and rhizomes were freeze-dried in vacuum (ScientZ-100F) and ground (30 Hz, 1.5 min) with a grinder (MM 400, Retsch) to powder form. Powder (100 mg) was put in 1.2 ml 70% methanol to extract with 30 s vortex every 30 min for 6 times. The samples were put in the refrigerator at 4°C overnight. The supernatant was obtained after centrifuge (12000 RPM, 10 min), then filtered with microporous membrane for UPLC-MS/MS analysis. The effluent after UPLC was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.
UPLC Conditions: The sample extracts were analyzed using a UPLC-ESI-MS/MS system (UPLC, SHIMADZU Nexera X2, MS, Applied Biosystems 6500 Q TRAP). The analytical conditions were used chromatographic column (Agilent SB-C18 1.8 µm, 2.1 mm * 100 mm) with mobile phase (A phase was ultrapure water (0.1% formic acid added), B phase was acetonitrile (0.1% formic acid added)). Elution gradient: the proportion of B phase was 5% (0.00 min), the proportion of B phase increased linearly to 95% (9.00 min), and remained at 95% (1 min), the proportion of B phase decreased to 5% (10.00–11.10 min), and balanced at 5% (14 min). The flow rate was 0.35 mL/min at 40°C of the column temperature with 2 μL of the injection volume.
The mass spectrum conditions: LIT and triple quadrupole (QQQ) scans were used on a triple quadrupole linear ion trap mass spectrometer (Q TRAP). AB6500 Q TRAP UPLC/MS/MS system was performed with ESI Turbo Ion Spray interface. Both positive and negative ion modes can be executed by Analyst 1.6.3 software (AB Sciex). Operating parameters of ESI source: ion source, turbine spray; source temperature 550°C; ion spray voltage (IS) 5500 V (positive ion mode)/−4500 V (negative ion mode); the ion source gas I (GSI), gas II (GSII) and curtain gas (CUR) (50 psi, 60 psi and 25.0 psi, respectively), and the collision-induced ionization parameter (high). Instrument tuning and quality calibration were executed with 10 and 100 μmol/L polypropylene glycol solution in QQQ and LIT mode, respectively. QQQ scan employs MRM mode and uses collision gas (nitrogen) as medium. DP and CE of each MRM ion pair were achieved by further DP and CE optimization. A specific set of MRM ion pairs was monitored at each period based on the eluted metabolites in each period.
All statistical analyses were performed using the Statistical Package for the Social Science (SPSS) software. Data were statistically described as frequencies and percentages. Origin 2022 was used for data graphing and mapping.
3.1 Inhibition Effects of Zingiberaceae Extracts to Pathogens
As can be seen from Figs. 1 and 2, the tested plant extracts had different antibacterial activities against the tested pathogens. On the one hand, the plant extracts had a higher inhibitory ability against food-borne pathogens than against plant-derived pathogens. The plant extracts had better antibacterial activities against P. solanacearum than the other four pathogens. Through data analysis, it also showed that plant extracts had certain inhibitory ability on S. aureus, E. coli and P. aeruginosa, indicating that the tested plant extracts had certain inhibitory effect on Gram-positive bacteria and Gram-negative bacteria. Moreover, the inhibitory ability of extracts on bacteria was stronger than that of fungi. Among them, plant extracts had the best inhibitory effect on P. aeruginosa, with an average inhibitory rate of 63.47% (1.0 mg mL−1), and the inhibitory rate of leaf and stem extracts of A. zerumbet on P. aeruginosa was as high as 90.5%. In addition, the growth inhibition effect of A. zerumbet root and rhizome extracts on S. aureus and E. coli was also significant, 83.23% and 79.62%, respectively. However, most of the extracts showed poor inhibitory effect on P. solanacearum, and some even showed no inhibitory activity. The inhibitory degrees of plant extracts on the tested pathogens were P. aeruginosa > S. aureus > E. coli > F. oxysporum > P. solanacearum.
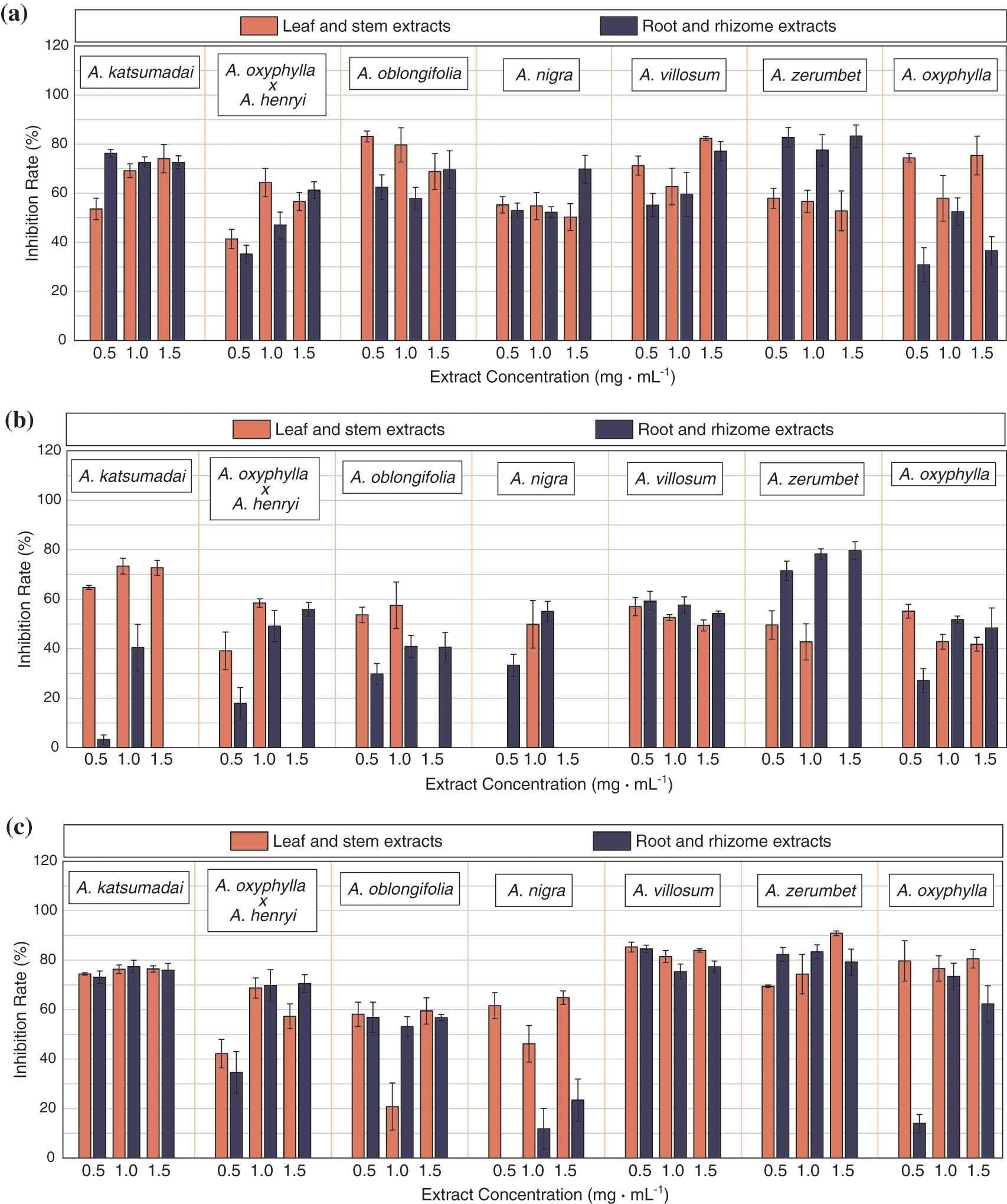

Figure 1: (a): Inhibitory effect of Zingiberaceae extracts against S. aureus, (b): Inhibition effect of Zingiberaceae extracts against E. coli, (c): Inhibition effect of Zingiberaceae extracts against P. aeruginosa, (d): Inhibition effect of Zingiberaceae extracts against F. oxysporum, (e): Inhibition effect of Zingiberaceae extracts against P. solanacearum

Figure 2: Effect on antimicrobial activity of A. katsumadai leaf and stem extracts. (A,a): F. oxysporum, (B,b): S. aureus, (C,c): E. coli, (D,d): P. aeruginosa, (E,e): P. solanacearum, (A~E): Culture medium without added extracts, (a~e): Culture medium supplemented with 1.5 mg mL−1 A. katsumadai leaf and stem extracts
On the other hand, the same extract also had different antibacterial activities against different pathogens, and there was no similarity in antibacterial spectrum and antibacterial activities of the same genus of plants. According to the comparison, the antibacterial effects of A. katsumadai, A. villosum and A. zerumbet were more outstanding, and their inhibition rate on S. aureus and P. aeruginosa can reach more than 80%, and the overall inhibitory effect was relatively average. Under the three concentration gradients, only a few extracts showed gradient changes in inhibition rate. For example, the inhibitory effect of extracts from leaf and stem, root and rhizome of A. katsumadai reached the best when the concentration was 1.5 mg mL−1, and the inhibitory rate was 84.46% and 84.73%, respectively. The inhibitory rates of the extracts from leaf and stem, root and rhizome of A. villosum on E. coli and F. oxysporum also varied with the concentration. The inhibitory rates of E. coli were 57.01% and 59.27%, respectively, when the concentration was 0.5 mg mL−1. But the inhibitory rates on F. oxysporum were the most significant when the concentration was 1.5 mg mL−1. The inhibition rates were 75.79% and 77.35%, respectively. This indicated that it was not the higher the concentration of the extracts, the better the inhibitory effect of the pathogen, which may be related to the gradient span was not obvious. Furthermore, there were some differences in antibacterial effect between extracts from different parts of the plant (Fig. 3). The inhibitory rates of leaf and stem were higher than those of root and rhizome, and the inhibitory rates of leaf and stem extracts on S. aureus and P. aeruginosa were relatively concentrated, while the inhibitory rates of root and rhizome extracts were relatively dispersed.

Figure 3: Comparison of antibacterial effects between leaf and stem, and root and rhizome extracts
3.2 The MIC and MBC of Zingiberaceae Extracts
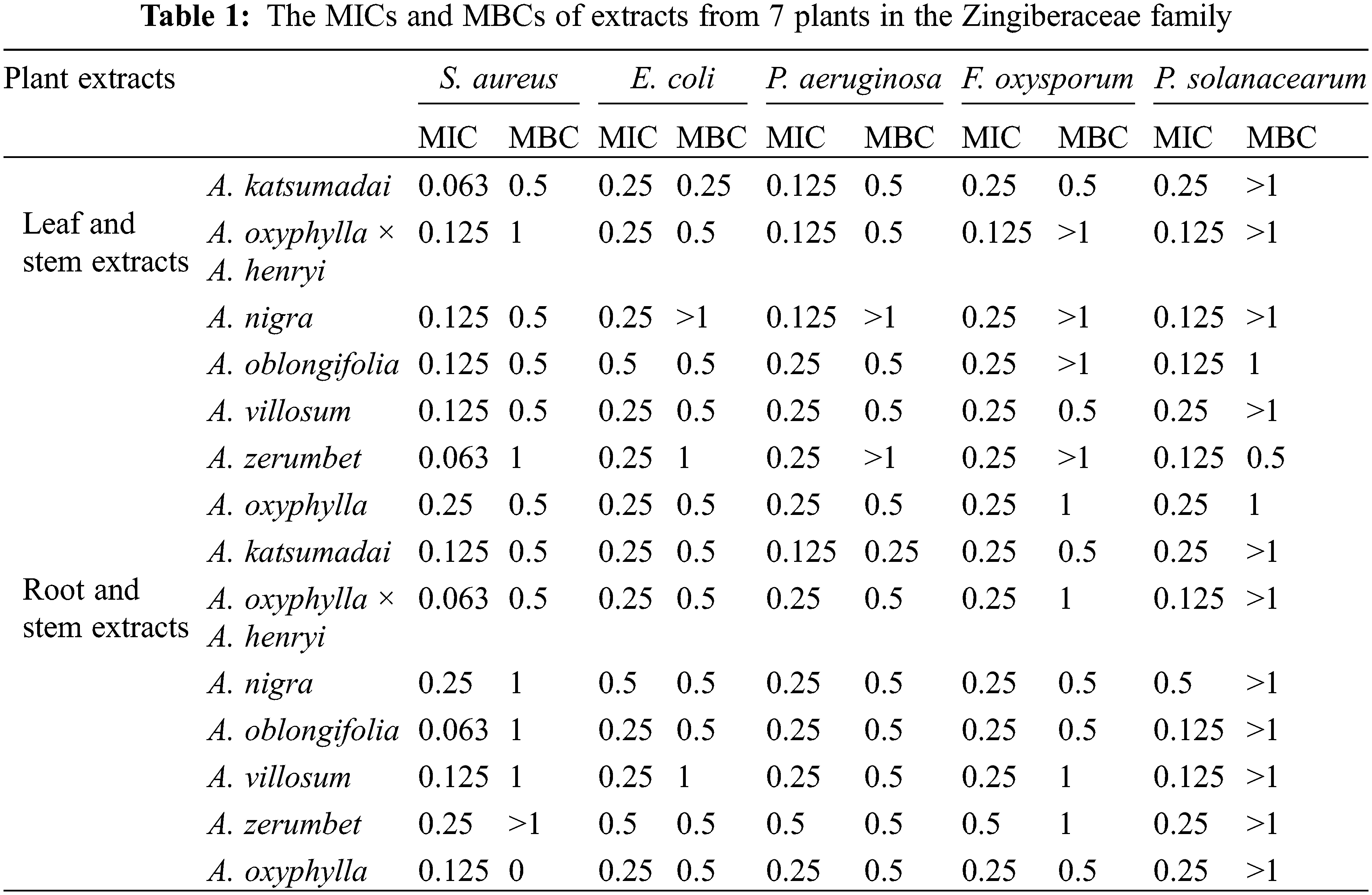
The MIC of the 14 Zingiberaceae plant extracts differed across the different extracts (Table 1). The A. katsumadai leaf and stem extracts and A. oxyphylla × A. henryi root and rhizome extracts had the best antibacterial effect on S. aureus, the MIC and MBC of the two extracts were 0.063 and 0.5 mg mL−1, respectively. Not only that, E. coli was sensitive to the leaf and stem extracts of A. katsumadai, with MIC of 0.25 mg mL−1 and MBC of 0.25 mg mL−1. The root and rhizome extracts of A. katsumadai had the best antibacterial effect against P. aeruginosa, with MIC of 0.125 mg mL−1 and MBC of 0.25 mg mL−1. However, the extracts from the leaf and stem of A. oxyphylla × A. henryi had a better antibacterial effect on F. oxysporum, with MIC and MBC of 0.125 and 0.5 mg mL−1, respectively. The best antibacterial effect on P. solanacearum was expressed by the leaf and stem extracts of A. zerumbet, with MIC of 0.125 mg mL−1 and MBC of 0.5 mg mL−1. Overall, a comprehensive analysis of MIC and MBC revealed that A. katsumadai, A. oxyphylla × A. henryi, and A. zerumbet leaf and stem extracts had a good antibacterial effect in vitro.
3.3 Phytochemical Composition of A. katsumadai and A. zerumbet Extracts by UPLC-MS/MS
The results of 3.1 and 3.2 showed that A. katsumadai and A. zerumbet had significant antibacterial effect, analysis of their chemical components using UPLC-MS/MS technology were presented in Table 2. Their total ions current were shown in Figs. 4 and 5.
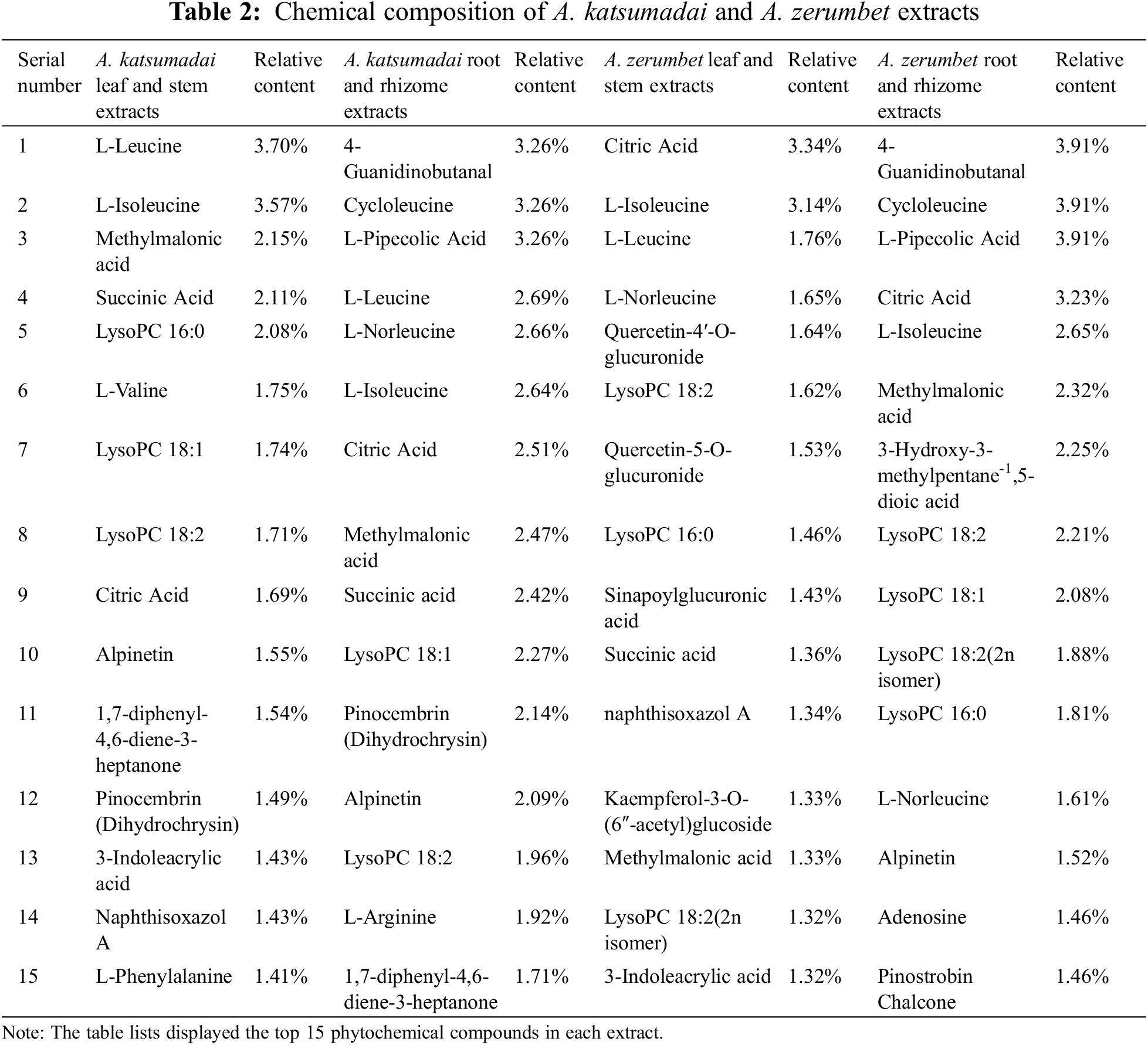

Figure 4: Total ions current (Anion mode)
Figure 5: Total ions current (Positive ion mode)
The results showed that flavonoids, organic acids, lipids, amino acids and derivatives were the main components of the plant extracts (Fig. 6). Among them, flavonoids and organic acids have been proved to have significant antibacterial effects by most reports [29,30]. They were present in all kinds of plants, with high concentrations in vegetables and fruits, where they existed as secondary metabolites. In addition, they were highly valuable bioactive compounds with antioxidant, anti-inflammatory, neuroprotective, anti-cancer, and anti-diabetes effects [31,32].
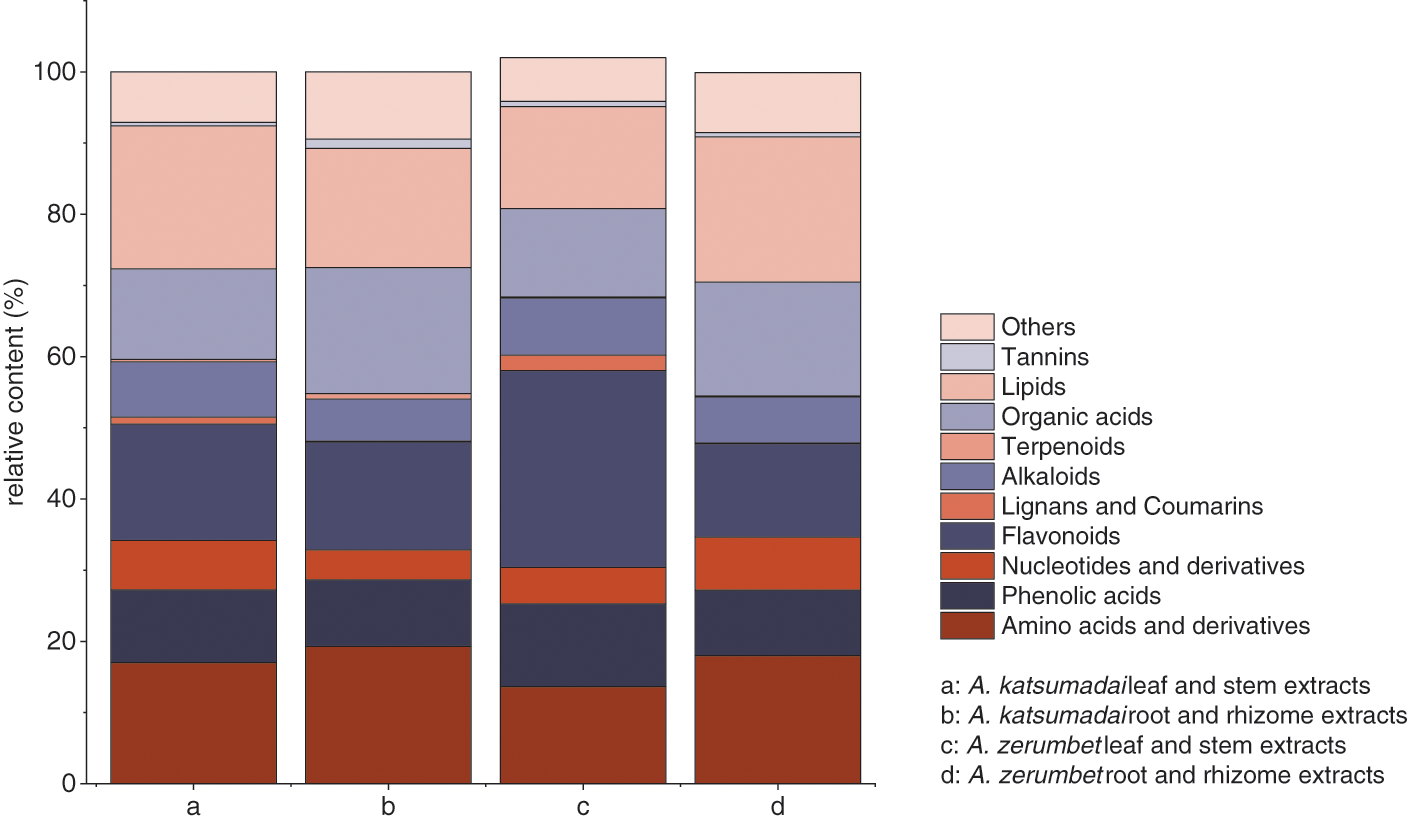
Figure 6: The proportion of each component in the extracts
Among the chemicals tested, CA, Alpinetin (Fig. 7) and PNCB (Fig. 8) were the ones with antibacterial activity. CA is an important organic acid, mostly existing in citrus plants, and has an antibacterial effect against E. coli, Salmonella enterica, Shigella flexneri, and Enterococcus faecalis [33–35]. Zhao et al. [36] observations under a scanning electron microscopy (SEM) revealed that S. aureus cells treated with CA lost their original shape, resulting in distorted and irregular shapes with holes on the surface, accounting for 29.2% of the damage to the cells. In addition, the microbial membrane structure was damaged by the natural CA compounds; a typical weak acid used as a food preservative by lowering the pH and killing or inhibiting the microbial growth. In addition, CA is used as a bivalent ions isolation agent for calcium and magnesium ions. However, the outer membrane of Gram-negative bacteria has an interference effect. PNCB has also been reported to have anti-Staphylococcus aureus effects in vitro and in vivo [37]. It inhibits cell damage mediated by S. aureus α-toxin, a porogen toxin expressed by most Staphylococci [38]. Similarly, alpinetin has broad-spectrum antibacterial activity, exhibiting inhibitory effects against most Gram-negative bacteria such as E. coli [24]. In addition, Chen et al. [39] found in vitro that alpinetin had good antibacterial activity against drug-resistant Aeromonas Hydrophila. Alpinetin could cause Aeromonas Hydrophila to shrink, damage cell wall and cell membrane, and cause loss of cytoplasm and internal cavitation. So it can be speculated that the antibacterial effect of alpinetin may be achieved by damaging bacterial cell walls and promoting cell membrane permeability.
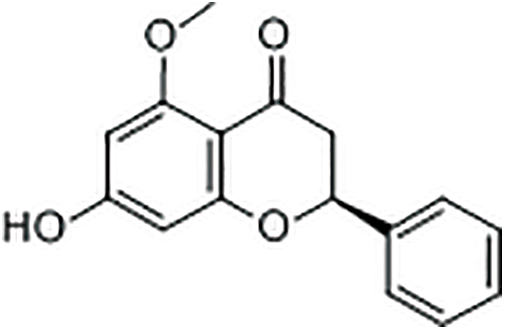
Figure 7: Chemical formula for alpinetin

Figure 8: Chemical formula for PNCB
In recent advances, studies on the antibacterial activity of plants in the Zingiberaceae family have mostly focused on essential oils and single plant parts. However, in the present study, several Zingiberaceae plants’ parts were studied for the first time, and their antibacterial activities were compared. In addition, the plant extracts with better antibacterial activity were screened to determine their phytochemical composition. Seven Zingiberaceae plants exhibited different degrees of antibacterial activity against five pathogens. In the case of pathogens, the inhibitory effect of all the extracts against bacterial pathogens was higher than that of the fungal pathogen. Among the tested plants, A. zerumbet extracts had the best inhibitory effect on bacteria(P. aeruginosa), with an inhibition rate of 90.85%. The A. katsumadai extracts had the best inhibitory effect on fungi (F. oxysporum), with an inhibition rate of 84.73%. Moreover, The MIC of bacteria (0.063 mg mL−1) was lower than that of fungi (0.25 mg mL−1).
There was no obvious gradient deviation in the inhibition rate of plant extracts on the pathogens at the three concentration gradients, which might be due to the insufficient gradient span. Further study could be conducted by increasing the concentration gradient. Antibacterial activity of extracts from leaf and stem was somewhat higher than that of extracts from root and rhizome in studies on different portions of Zingiberaceae plants, which should be paid more attention to in the future. At the same time, the pathogen inhibition rate and the matching MIC value are not always negatively correlated due to the varied activities of the components in the extracts.
According to the experimental results of inhibition rate and MIC, A. katsumadai and A. zerumbet were screened out to have prominent antibacterial activities. Specifically, the inhibitory effect of A. katsumadai and A. zerumbet extracts against E. coli was stronger than that of Alpinia purpurta extracts (MIC 21.12 mg mL−1) [40]. In addition to Zingiberaceae plants, compared with plant extracts with a strong inhibitory effect on pathogens such as cinnamon (MIC 55 mg mL−1) [41] and garlic (MIC 65 mg mL−1) [41], the MIC of E. coli is also higher than that of A. katsumadai and A. zerumbet leaf and stem extracts. It can be seen that the antibacterial effect of A. katsumadai and A. zerumbet extracts is more significant. Through the determination of chemical composition, the extract can be identified as rich in flavonoids and organic acids. Among them, CA, Alpinetin and PNCB may be functional components with antibacterial effects. Hence, it is important to further investigate how the mixture of these chemicals is special for awarding antibacterial activities in these plants. However, the effects of different extraction conditions, including different solvents, solvent ratio, and extraction temperature, on compound yield necessitate further investigations [42]. In addition, the thermal stability and antibacterial activity of CA, alpinetin, and PNCB against pathogens should be explored to understand their antibacterial mechanism for the exploitation of plant resources [43].
Overall, more attention should be paid to the utilization of leaves and stems of Zingiaceae in the future, which can not only prevent the waste of resources on leaves and stems, but also promote the research and development of a new, safer and more natural antibacterial agent [44,45]. They have the potential to replace chemical synthetic antibacterial agents and have high application value.
Funding Statement: This work was funded by the Forestry Science and Technology Innovation Project of Guangdong Province, China (2020KJCX010).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Appalasamy, S., Arumugam, N. (2020). Four new records of Zingiberaceae in gunung telapak burok, berembun forest reserve (frnegeri sembilan. IOP Conference Series: Earth and Environmental Science, 596(1), 012059. DOI 10.1088/1755-1315/596/1/012059. [Google Scholar] [CrossRef]
2. Elim, H. I., Mapanawang, A. L. (2018). The attractive differences of two types of herbal medicine from Zingiberaceae fruit (Golobe Halmahera). International Journal of Health Medicine and Current Research, 3(1), 826–834. DOI 10.22301/IJHMCR.2528-3189.826. [Google Scholar] [CrossRef]
3. Christenhusz, M. J., Byng, J. W. (2016). The number of known plants species in the world and its annual increase. Phytotaxa, 261(3), 201–217. DOI 10.11646/phytotaxa.261.3.1. [Google Scholar] [CrossRef]
4. Ragsasilp, A., Saensouk, P., Saensouk, S. (2022). Ginger family from Bueng Kan Province, Thailand: Diversity, conservation status, and traditional uses. Biodiversitas Journal of Biological Diversity, 23(5), 2739–2752. DOI 10.13057/biodiv/d230556. [Google Scholar] [CrossRef]
5. Saensouk, S., Saensouk, P., Pasorn, P., Chantaranothai, P. (2016). Diversity and uses of Zingiberaceae in Nam Nao National Park, Chaiyaphum and Phetchabun Provinces, Thailand, with a new record for Thailand. Agriculture and Natural Resources, 50(6), 445–453. DOI 10.1016/j.anres.2016.08.002. [Google Scholar] [CrossRef]
6. Zhang, M., Zhao, R., Wang, D., Wang, L., Zhang, Q. et al. (2021). Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytotherapy Research, 35(2), 711–742. DOI 10.1002/ptr.6858. [Google Scholar] [CrossRef]
7. Irayanti, A., Putra, A. G. R. Y. (2020). A narrative review of Zingiberaceae family as antibacterial agent for traditional medication based on balinese local wisdom. Journal of Pharmaceutical Science and Application, 2(2), 66–76. DOI 10.24843/JPSA.2020.v02.i02.p04. [Google Scholar] [CrossRef]
8. Gharge, S., Hiremath, S. I., Kagawad, P., Jivaje, K., Palled, M. S. et al. (2021). Curcuma zedoaria Rosc (ZingiberaceaeA review on its chemical, pharmacological and biological activities. Future Journal of Pharmaceutical Sciences, 7(1), 1–9. DOI 10.1186/s43094-021-00316-1. [Google Scholar] [CrossRef]
9. Ivanović, M., Makoter, K., Islamčević Razboršek, M. (2021). Comparative study of chemical composition and antioxidant activity of essential oils and crude extracts of four characteristic Zingiberaceae herbs. Plants, 10(3), 501. DOI 10.3390/plants10030501. [Google Scholar] [CrossRef]
10. Pham, N. K., Nguyen, H. T., Nguyen, Q. B. (2021). A review on the ethnomedicinal uses, phytochemistry and pharmacology of plant species belonging to Kaempferia L. genus (Zingiberaceae). Pharmaceutical Sciences Asia, 48(1), 1–24. DOI 10.29090/psa.2021.01.19.070. [Google Scholar] [CrossRef]
11. Ji, Y. P., Shi, T. Y., Zhang, Y. Y., Lin, D., Linghu, K. G. et al. (2019). Essential oil from Fructus Alpinia zerumbet (fruit of Alpinia zerumbet (Pers.) Burttet Smith) protected against aortic endothelial cell injury and inflammation in vitro and in vivo. Journal of Ethnopharmacology, 237, 149–158. DOI 10.1016/j.jep.2019.03.011. [Google Scholar] [CrossRef]
12. Huang, X., Lao, Y., Pan, Y., Chen, Y., Zhao, H. et al. (2021). Synergistic antimicrobial effectiveness of plant essential oil and its application in seafood preservation: A review. Molecules, 26(2), 307. DOI 10.3390/molecules26020307. [Google Scholar] [CrossRef]
13. Kodjio, N., Atsafack, S. S., Fodouop, S. P., Kuiate, J. R., Gatsing, D. (2016). In vitro antisalmonellal and antioxidant activities of extracts and fractions of Curcuma longa L. Rhizomes (Zingiberaceae). International Journal of Biochemistry Research & Review, 11(3), 1–14. DOI 10.9734/IJBCRR/2016/25106. [Google Scholar] [CrossRef]
14. Yin, Y. J., Chen, C. J., Guo, S. W., Li, K. M., Ma, Y. N. et al. (2018). The fight against panax notoginseng root-rot disease using Zingiberaceae essential oils as potential weapons. Frontiers in Plant Science, 9, 1346. DOI 10.3389/fpls.2018.01346. [Google Scholar] [CrossRef]
15. Narusaka, M., Hatanaka, T., Narusaka, Y. (2021). Inactivation of plant and animal viruses by proanthocyanidins from Alpinia zerumbet extract. Plant Biotechnology 25, 38(4), 453–455. DOI 10.5511/plantbiotechnology.21.0925a. [Google Scholar] [CrossRef]
16. Imchen, P., Ziekhrü, M., Zhimomi, B. K., Phucho, T. (2022). Biosynthesis of silver nanoparticles using the extract of Alpinia galanga rhizome and Rhus semialata fruit and their antibacterial activity. Inorganic Chemistry Communications, 142(1), 109599. DOI 10.1016/j.inoche.2022.109599. [Google Scholar] [CrossRef]
17. Fikriah, I., Yuniati, Y., Ismail, S., Kosala, K. (2018). Evaluation of synergistic effect of Kaempferia galanga L. Rhizome extracts on the antibiotic activity against bacterial pathogens. Journal of Tropical Pharmacy and Chemistry, 4(3), 108–113. DOI 10.25026/jtpc.v4i3.148. [Google Scholar] [CrossRef]
18. Arora, R., Mazumder, A. (2017). Phytochemical screening and antimicrobial activity of rhizomes of hedychium spicatum. Pharmacognosy Journal, 9(6s), 64–68. DOI 10.5530/pj.2017.6s.159. [Google Scholar] [CrossRef]
19. Subba, B., Seling, T. R., Kandel, R. C., Phuyal, G. P. (2017). Assessment of antimicrobial and antioxidant activities of Amomum subulatum Roxb. of Nepal. Assessment, 10(4), 95–97. DOI 10.22159/ajpcr.2017.v10i4.16018. [Google Scholar] [CrossRef]
20. Hai, P. V., van, H. T. H., van Chao, N., Khuong, N. D. T., Le, T. T. T. et al. (2019). Antimicrobial activity of chives and ginger extract on Escherichia coli and Salmonella spp. isolated from broiler chickens. Hue University Journal of Science: Agriculture and Rural Development, 128(3B), 105–111. DOI 10.26459/hueuni-jard.v128i3B.5310. [Google Scholar] [CrossRef]
21. Abdelgaleil, S. A. M., El-Bakry, A., Zoghroban, A. A. M., Kassem, S. M. I. (2019). Insecticidal and antifungal activities of crude extracts and pure compounds from rhizomes of Curcuma longa L.(Zingiberaceae). Journal of Agricultural Science and Technology, 21(4), 1049–1061. DOI 20.1001.1.16807073.2019.21.4.6.1. [Google Scholar]
22. Peng, W., Li, P., Ling, R., Wang, Z., Feng, X. et al. (2022). Diversity of volatile compounds in ten varieties of zingiberaceae. Molecules, 27(2), 565. DOI 10.3390/molecules27020565. [Google Scholar] [CrossRef]
23. Deng, M., Yun, X., Ren, S., Qing, Z., Luo, F. (2022). Plants of the genus zingiber: A review of their ethnomedicine, phytochemistry and pharmacology. Molecules, 27(9), 2826. DOI 10.3390/molecules27092826. [Google Scholar] [CrossRef]
24. Zhao, G., Tong, Y., Luan, F., Zhu, W., Zhan, C. et al. (2022). Alpinetin: A review of its pharmacology and pharmacokinetics. Frontiers in Pharmacology, 13, 814370. DOI 10.3389/fphar.2022.814370. [Google Scholar] [CrossRef]
25. Yakubu, M. I., Enoch, T., Abbas, M. Y., Abah, J. O., Jimoh, A. A. et al. (2022). Comparative anticonvulsant studies on ethanol and ethyl acetate extracts of Zingiber Officinale Roscoe rhizome in mice and chicks. Journal of Current Biomedical Research, 2(2), 122–132. DOI 10.54117/jcbr.v2i2.8. [Google Scholar] [CrossRef]
26. El-Tarabily, K. A., Nassar, A. H., Hardy, G. S. J., Sivasithamparam, K. (2009). Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. Journal of Applied Microbiology, 106(1), 13–26. DOI 10.1111/j.1365-2672.2008.03926.x. [Google Scholar] [CrossRef]
27. Nwinee, Z., Bako, G. D., Aliyu, S., Yusuf, J. S., Korfii, U. (2022). Antibacterial activity of herbal spices (Garlic and Turmeric) on Staphylococcus aureus and Escherichia coli. IOSR Journal of Pharmacy and Biological Sciences, 17(2), 39–43. DOI 10.9790/3008-1702013943. [Google Scholar] [CrossRef]
28. Yu, Z., Wu, X., He, J. (2022). Study on the antifungal activity and mechanism of tea saponin from Camellia oleifera cake. European Food Research and Technology, 248(3), 783–795. DOI 10.1007/s00217-021-03929-1. [Google Scholar] [CrossRef]
29. Kahraman, H. A., Tutun, H., Keyvan, E., Balkan, B. M. (2022). Investigation of chemical, antibacterial and antiradical properties of home-made apple and grape vinegars. Ankara Üniversitesi Veteriner Fakültesi Dergisi, 69, 139–148. DOI 10.33988/auvfd.865309. [Google Scholar] [CrossRef]
30. Shahrajabian, M. H., Sun, W., Cheng, Q. (2022). The importance of flavonoids and phytochemicals of medicinal plants with antiviral activities. Mini-Reviews in Organic Chemistry, 19(3), 293–318. DOI 10.2174/1570178618666210707161025. [Google Scholar] [CrossRef]
31. Mileo, A. M., Nisticò, P., Miccadei, S. (2019). Polyphenols: Immunomodulatory and therapeutic implication in colorectal cancer. Frontiers in Immunology, 10, 729. DOI 10.3389/fimmu.2019.00729. [Google Scholar] [CrossRef]
32. Kumar Singh, A., Cabral, C., Kumar, R., Ganguly, R., Kumar Rana, H. et al. (2019). Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients, 11(9), 2216. DOI 10.3390/nu11092216. [Google Scholar] [CrossRef]
33. Kim, S. A., Rhee, M. S. (2015). Synergistic antimicrobial activity of caprylic acid in combination with citric acid against both Escherichia coli O157:H7 and indigenous microflora in carrot juice. Food Microbiology, 49, 166–172. DOI 10.1016/j.fm.2015.02.009. [Google Scholar] [CrossRef]
34. Scott, B. R., Yang, X., Geornaras, I., Delmore, R. J., Woerner, D. R. et al. (2015). Antimicrobial efficacy of a lactic acid and citric acid blend against shiga toxin—producing Escherichia coli, Salmonella, and nonpathogenic Escherichia coli biotype I on inoculated prerigor beef carcass surface tissue. Journal of Food Protection, 78(12), 2136–2142. DOI 10.4315/0362-028X.JFP-15-194. [Google Scholar] [CrossRef]
35. Adamczak, A., Ożarowski, M., Karpiński, T. M. (2019). Antibacterial activity of some flavonoids and organic acids widely distributed in plants. Journal of Clinical Medicine, 9(1), 109. DOI 10.3390/jcm9010109. [Google Scholar] [CrossRef]
36. Zhao, X., Zhen, Z., Wang, X., Guo, N. (2017). Synergy of a combination of nisin and citric acid against Staphylococcus aureus and Listeria monocytogenes. Food Additives & Contaminants: Part A, 34(12), 2058–2068. DOI 10.1080/19440049.2017.1366076. [Google Scholar] [CrossRef]
37. Tundis, R., Frattaruolo, L., Carullo, G., Armentano, B., Badolato, M. et al. (2019). An ancient remedial repurposing: Synthesis of new pinocembrin fatty acid acyl derivatives as potential antimicrobial/anti-inflammatory agents. Natural Product Research, 33(2), 162–168. DOI 10.1080/14786419.2018.1440224. [Google Scholar] [CrossRef]
38. Soromou, L. W., Zhang, Y., Cui, Y., Wei, M., Chen, N. et al. (2013). Subinhibitory concentrations of pinocembrin exert anti-Staphylococcus aureus activity by reducing α-toxin expression. Journal of Applied Microbiology, 115(1), 41–49. DOI 10.1111/jam.12221. [Google Scholar] [CrossRef]
39. Chen, J. H., Miao, Y. J., Liang, C., Tao, Y., Ouyang, P. et al. (2021). Study on the antibacterial mechanism of alpinetin against fish-derived drug-resistant aeromonas hydrophila in vitro. Biotechnology Bulletin, 37(2), 103. DOI 10.13560/j.cnki.biotech.bull.1985.2020-0743. [Google Scholar] [CrossRef]
40. de Cuffa Matusaiki, C., Ferreira, R. G., Otutumi, L. K., dos Santos, I. C., Ramos, F. A. P. et al. (2021). Antibiotic resistance profile of gram-negative bacteria isolated from dog nasal swab samples, and antibacterial and antioxidant activities of aqueous extracts of Alpinia purpurta (Vieill.) K. Schum (Zingiberaceae). Semina: Ciências Agrárias, 42(1), 179–192. DOI 10.5433/1679-0359.2021v42n1p179. [Google Scholar] [CrossRef]
41. Habib, M. A., Abozid, M. M., El Sofany, S. A., Faragalla, S. F. (2021). Antimicrobial potentials of cinnamon and garlic extracts against some foodborne pathogenes. Zagazig Journal of Agricultural Research, 48(3), 805–815. DOI 10.21608/zjar.2021.191318. [Google Scholar] [CrossRef]
42. Li, Q., Gong, S., Yan, J., Hu, H., Shu, X. et al. (2020). Synthesis and kinetics of hydrogenated rosin dodecyl ester as an environmentally friendly plasticizer. Journal of Renewable Materials, 8(3), 289–300. DOI 10.32604/jrm.2020.08897. [Google Scholar] [CrossRef]
43. Li, Q., Yan, X., Chen, J., Shu, X., Jia, P. et al. (2021). Preparation and characterization of potassium monopersulfate/ethyl cellulose microcapsules and their sustained release performance. Journal of Renewable Materials, 9(10), 1673–1684. DOI 10.32604/jrm.2021.014695. [Google Scholar] [CrossRef]
44. Yang, X., Wang, S., Liu, X., Huang, Z., Huang, X. et al. (2021). Preparation of non-isocyanate polyurethanes from epoxy soybean oil: Dual dynamic networks to realize self-healing and reprocessing under mild conditions. Green Chemistry, 23(17), 6349–6355. DOI 10.1039/D1GC01936H. [Google Scholar] [CrossRef]
45. Dong, F., Yang, X., Guo, L., Wang, Y., Shaghaleh, H. et al. (2022). Self-healing polyurethane with high strength and toughness based on a dynamic chemical strategy. Journal of Materials Chemistry A, 10(18), 10139–10149. DOI 10.1039/D2TA00802E. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools