 Open Access
Open Access
ARTICLE
Characterization of Physico-Chemical Changes of Lignin Obtained under Different Conditions of Enzymatic Hydrolysis on an Industrial Scale
1 Latvian State Institute of Wood Chemistry, Riga, LV-1006, Latvia
2 Fibenol OÜ, Tallinn, 13522, Estonia
* Corresponding Author: Maris Lauberts. Email:
(This article belongs to the Special Issue: Advances in Biorefinery Technologies and Products – 2024)
Journal of Renewable Materials 2025, 13(1), 115-126. https://doi.org/10.32604/jrm.2024.056815
Received 31 July 2024; Accepted 01 November 2024; Issue published 20 January 2025
Abstract
Research-based on lignin as a bioproduct has grown due to its high availability, reactivity, physicochemical stability, and abundance of different aromatic units. Lignin consists of various functional groups, which can react in various chemical reactions and serve as a raw material in various processes to obtain multiple products. These characteristics make lignin suitable for synthesizing products from natural raw materials, replacing fossil ones. Due to a high aromatic variety and complex structural arrangement, lignin isolation and fractionation are still challenging. The aim and novelty of this work was the modification of severity and enzymatic hydrolysis procedure on an industrial pre-treatment to improve by-products of birch processing as a raw material for the potential production of different products. Lignin from birch wood enzymatic hydrolysis was obtained and marked accordingly: HS (high severity), MS (medium severity), and LS (low severity) lignin. Samples were characterized by ash content, analytical pyrolysis, solubility, and viscosity. HS lignin was characterized by a relatively high carbohydrate content (16%) and lower lignin content (77%). Meanwhile, LS lignin showed increased lignin content (83%) and reduced carbohydrate content (9%). It can be concluded that the delignification process greatly influences the properties of the obtained lignin. HS lignin resulted in a lower polydispersity index (PDI) and more condensed structure, while LS lignin showed a higher PDI but a lower content of carbohydrates. Therefore, looking for a golden middle way is necessary while finding the conditions according to the use field.Keywords
Nomenclature
| PF | Phenol-formaldehyde resin |
| LPF | Lignin-based phenol formaldehyde resins |
| Py/GC/MS | Analytical pyrolysis |
| SEC-UV/RID | Size-exclusion chromatography with UV and refractive index detector |
| FTIR | Fourier transform infrared spectroscopy |
| GC-FID | Gas chromatography with flame ionization detection |
| KL | Klason lignin |
| ASL | Acid-soluble lignin |
| Ara | Arabinose |
| Xyl | Xylose |
| Man | Mannose |
| Gal | Galactose |
| Glc | Glucose |
| Mw | Mass average molar mass |
| Mn | Number average molar mass |
| PDI | Polydispersity index |
| LOD | Limit of detection |
| LSIWC | Latvian State Institute of Wood Chemistry |
With the beginning of the industrial revolution in the 18th century the continued development of modern chemical processing processes, and the intensive use of fossil sources as raw materials, the demand for this has grown beyond comprehension. For example, the beginning of the production of phenol-formaldehyde resin is the beginning of the 20th century, when the population of the world had not even reached 2 billion, but in a little more than 100 years, it reached 8 billion in 2022, and it is predicted that by 2040 it will reach already 9 billion [1]. Consequently, the need for various raw materials has grown and will continue to grow, but considering the world’s attitude towards fossil raw materials and the transition to environmentally friendly raw materials and care for the environment, the 21st century is challenging in this transitional period.
Currently, petroleum is the main raw material used in the production of various consumer products, as most chemicals and various products obtained from them, various polymers—plastic synthetic materials, etc., and, of course, a very large segment is also occupied by energy extraction from fossil raw materials [2]. However, growing concerns about environmental pollution and the depletion of fossil fuel reserves have driven industries to seek sustainable alternatives to petroleum-based resources [3]. Phenol is a commonly used chemical from petroleum in various areas, including chemistry, biology, medicine, etc. It is highly toxic to living cells and can easily be absorbed through intact skin. Therefore, it is crucial for users to be aware of phenol’s properties and adhere to proper handling procedures. Adequate training and the use of personal protective equipment, such as aprons and eye protection, are recommended when working with phenol. Additionally, phenols are prevalent in household products. It is used as a raw material to make phenolic resins and bisphenol A, which in turn is a raw material for epoxy resins. It is also used as a raw material for a variety of dyes, surfactants, disinfectants, agricultural chemicals, pharmaceuticals, and intermediate chemicals [4]. The substitution of phenol with renewable and cost-effective bio-phenols offers significant advantages in terms of cost reduction. The search for alternative sources of phenol from renewable resources plays a crucial role in promoting sustainable development. Over the past few decades, numerous studies have explored the utilization of renewable phenol alternatives obtained from lignocellulosic biomass materials such as bark, wood, and lignin in the production of phenol-formaldehyde (PF) resin [5]. Lignin is a natural polyaromatic, amorphous, three-dimensional, formed through the polymerization of three phenylpropane monomers: sinapyl (S), coniferyl (G), and coumaryl (H) alcohols highly branched, and second most abundant macromolecule after cellulose (see Fig. 1). Lignin is found in almost all plants cell walls and it makes up approximately 15%–35% of plant cell walls by weight [6]. Lignin is of natural origin; therefore, lignin is a viable alternative to fossil nonrenewable resources [7], has very high physical-chemical stability, and is available in large quantities, as it is obtained as a by-product of cellulose or bioethanol production. As the world increasingly abandons the use of oil products to produce various products and seeks green alternatives, lignin will definitely play an important role in the future as a raw material for the production of various products and chemicals in various sectors of the economy. Research based on lignin as a bio-based feedstock has grown due to its high availability, reactivity, and abundance of different aromatic units. Lignin is composed of 3 building blocks: guaiacyl (G), syringyl (S), and p-hydroxyphenol propane (H) units. The relative proportions of these units in the lignin structure are influenced by the original source of lignin and the delignification process [8–11]. Consequently, the G and H-type units possess reactive sites (ortho to the phenolic hydroxyl group) that readily interact with other reactive groups. Conversely, the S-type unit has both C3 and C5 positions occupied by a methoxy group, resulting in lower reactivity compared to the G-type and H-type units.
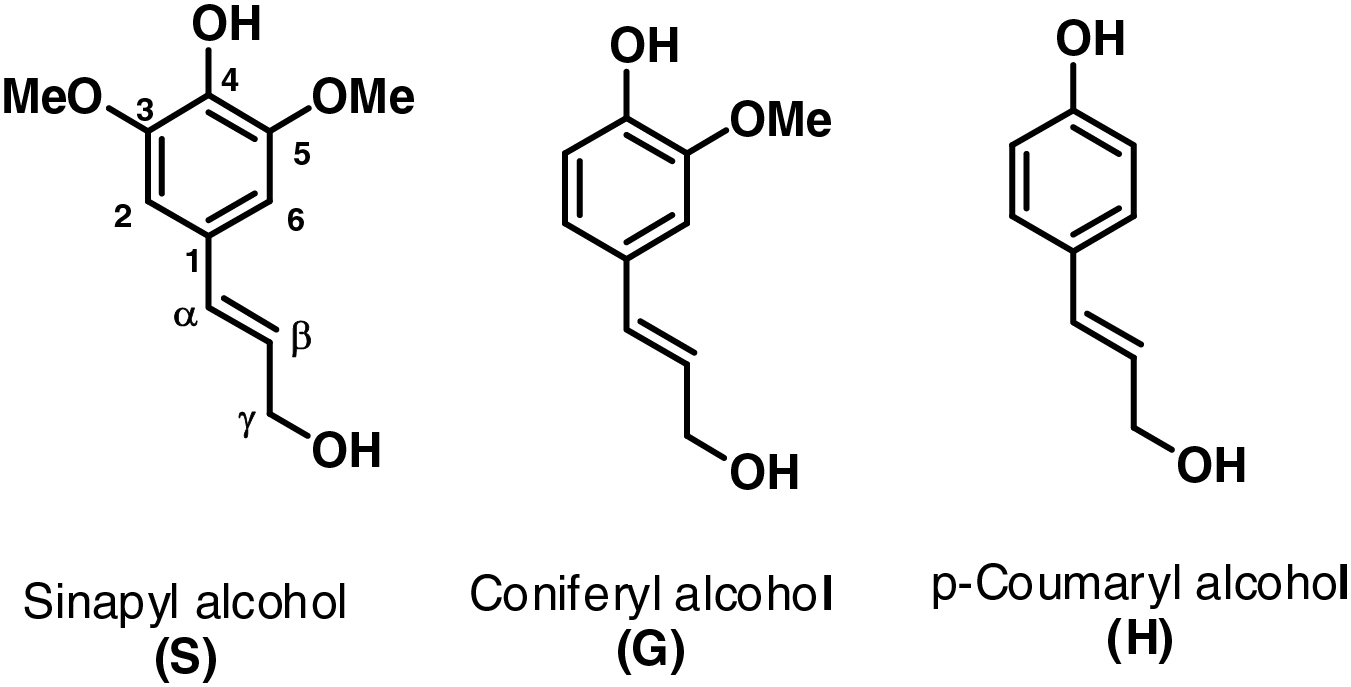
Figure 1: Lignin phenolic structure. Adapted with permission from Ref. [12]. Copyright ©2023, ETA-florence renewable energies publishing
The composition of lignin units varies among plant species due to the positioning of methoxy groups on the phenolic moieties of phenylpropanoid units, which are formed through photosynthesis in plants. These phenolic moieties are biosynthesized and polymerized with different C-C and C-O bonds, particularly β-O-4, β-O-5, α-O-4, 5-5, β-5, β-1, 4-O-5, and β-β linkages. Among these, β-O-4 linkages are relatively weak and are primary targets for cleavage, while the other linkages are more complex and harder to degrade [13–15]. For example, these characteristics potentially make lignin suitable for the synthesis/production of lignin-based phenol formaldehyde (LPF) resins as an alternative to phenol-formaldehyde resins—the most widely used binder for glued wood composite materials. The substitution of lignin for phenol can be achieved through the following process (see Fig. 2): lignin and phenol are subjected to hydroxymethylation, which involves their reaction with formaldehyde in the presence of NaOH as a catalyst. Subsequently, the hydroxymethylated lignin and phenol derivatives undergo copolymerization, resulting in the formation of a LPF copolymer [16].
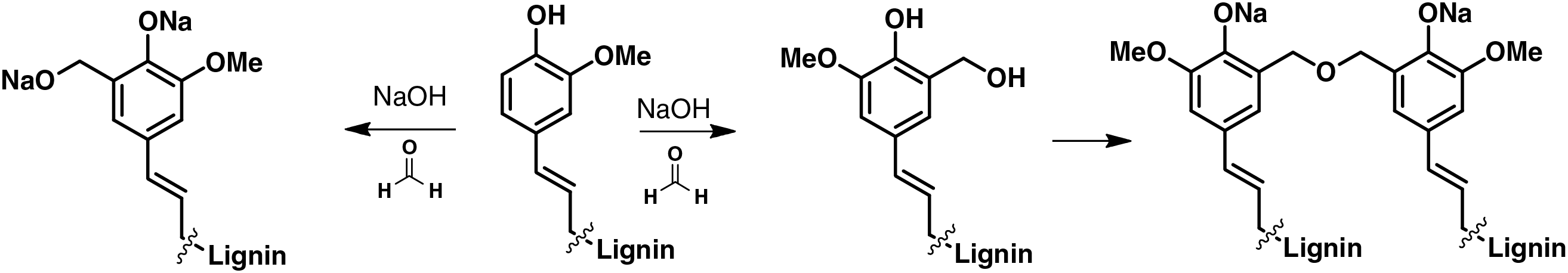
Figure 2: The possible reaction mechanism of lignin-based formaldehyde formation
Due to a high aromatic variety and complex structural arrangement, lignin isolation and fractionation is still a great challenge. Different sources and pulping processes can produce various types of lignin, each containing varying amounts of monomers and bonds. The lignin in hardwood consists mainly of G and S units, while softwood consists of G and H units [11]. These types include milled wood lignin, alkali lignin, Kraft lignin, and enzymatic lignin [15]. Despite this, lignin’s structure is difficult and complex to characterize. The delignification process significantly impacts lignin’s chemical structure, molecular weight, thermal properties, functional groups, and color [17,18]. In the pulp and paper industries, lignin is entirely removed through the sulfite process and is considered an unwanted by-product. Lignin is known to be a rich source of phenol and its derivatives. However, it is challenging to extract lignin from its source and break it down into phenol and its derivatives. Common methods for lignin removal and decomposition include enzymatic processes, the organosolv process, selective pretreatment with ionic liquids, etc. [19].
In both traditional and modern biomass pretreatment and fractionation processes, the breaking of chemical bonds between lignin-lignin and lignin-polysaccharides allows the production of lignin (delignification). The nature and degree of delignification affect the size of the resulting lignin macromolecules, the modifications they undergo during extraction, and the resulting chemical composition. In addition, the chemical structure of lignin varies with biomass source, species, and geographic location. This results in samples with varying degrees of heterogeneity, which directly affect the quality of lignin for further conversion and commercialization [20]. Indeed, the complexity and variability of extracted lignin present a common challenge across the spectrum of lignin valorization, from extraction to conversion and final processing. Thus, the development of new strategies to selectively process lignin into more homogeneous fractions is likely to be a significant challenge in successfully converting lignin into desired aromatic bioproducts.
The aim and novelty of this work were to modify the enzymatic hydrolysis procedure on an industrial scale to improve the raw material that could potentially be used for the production of LPF resin. The main criteria for successful LPF resin production were the solubility of lignin in NaOH solution, admixture content (organic/inorganic matter), functionality, and viscosity. Enzymatic hydrolysis lignin samples obtained with different severity were characterized and compared.
Birch wood lignin (provided by Fibenol OÜ, Estonia) was obtained after a modification of severity and an enzymatic hydrolysis procedure during an industrial pre-treatment. Severity is a combination of temperature, pH, and mechanical grinding in the extrusion-based Sunburst pretreatment. It is patented technology, which provides rapid pretreatment of woody biomass by heating the wood for a short time in a dilute acid solution. Because the pretreatment is quick, minimal inhibitory chemicals are produced, resulting in materials with distinct properties [21]. Due to patented technology and confidential changes in the industrial parameters, it was not allowed to inform about the precise conditions. Therefore, the lignin from hardwood enzymatic hydrolysis was obtained and marked accordingly: HS lignin—(high severity, standard hydrolysis); MS lignin—(medium severity, long hydrolysis followed by a standard washing step) and LS lignin—(low severity, long hydrolysis followed by a specific washing procedure).
2.2 Ash, Klason Lignin (KL), and Acid Soluble Lignin (ASL) Content
The inorganic (ash) content of the lignin samples was determined through dry mineralization following the Tappi standard 211om-22, conducted at a temperature of 525°C [20]. Approximately 1.5 g of lignin was weighed into a quartz crucible and heated on an electric stove for 3 h for initial mineralization. The partially mineralized sample was then transferred to a muffle furnace and heated at 550°C for 4 h until white ash was formed. The quantities of acid-insoluble lignin (KL) and acid-soluble lignin (ASL) were measured using the TAPPI T-222 standard method. A 0.5 g. lignin sample (free of extracts) was treated with 7.5 mL of 72% H2SO4 and maintained at 25°C for two hours [21]. The sample was then diluted with 260 mL of deionized water, refluxed for 4 h, and filtered through a P100 glass filter. The resulting precipitate was washed with hot deionized water until a neutral reaction pH = 7, then dried at 105°C until a constant mass was obtained.
2.3 Analytical Pyrolysis–Gas Chromatography–Mass Spectrometry (Py-GC/MS)
The gas chromatography–mass spectrometry analysis was performed using a Frontier Lab (Japan) Micro Double-shot Pyrolyser Py-2020iD (pyrolysis temperature 500°C, heating rate 600°C s−1) directly coupled with the Shimadzu GC/MS-QP 2010 apparatus (Japan) with capillary column RTX-1701 (Restec, USA), as described in [22] Identification of individual compounds was performed using a GC/MS chromatogram using library MS NIST 147.LI13. The relative peak areas of the compounds were calculated using Shimadzu software based on the GC/FID data. The molar areas of the respective peaks were summed and normalized to 100%, and the results were averaged from five pyrolysis experiments.
Total monomeric carbohydrates were determined by gas chromatography with flame ionization detection (GC-FID) using the alditol acetate derivatization method [23,24].
2.5 Determination of Total Hydroxyl Groups
The total quantity of hydroxyl groups was determined by acetylation according to the methodology developed by LSIWC [25]. The method is based on an aliphatic and phenolic hydroxyl group’s reaction with acetic anhydride in the presence of a pyridine catalyst, resulting in a free acetic ion.
2.6 Fourier Transform Infrared Spectroscopy (FTIR) Analysis
Fourier transform infrared (FTIR) spectra of the samples were recorded in KBr pellets by a Spectrum One (PerkinElmer, UK) FTIR spectrometer in the range of 4000–450 cm−1 (resolution, 4 cm−1; number of scans—64). The resulting spectra were normalized to the maximum intensity of 1510 cm−1, which was assigned to the aromatic skeletal vibrations in lignin samples.
2.7 Size-Exclusion Chromatography with UV and Refractive Index Detector (SEC-UV/RID) Analysis
Weight average molecular weight and number average molecular weight of lignin samples were determined by size-exclusion chromatography (SEC) using a Waters Instrument System. 5 mg of dried lignin sample was dissolved in HPLC-grade DMF, and the solution was filtered through a 45 μm syringe filter. HPLC-grade DMF, filtered through a 45 μm polytetrafluoroethylene membrane filter and degassed, was used as the mobile phase. The flow rate of the mobile phase was set to 0.3 mL/min. Separation was achieved on Waters APC XT 450 2.5 µm, APC XT 200 2.5 µm, and APC XT 45 1.7 µm columns. A sample volume of 20 μL was injected into the SEC instrument, and UV signal was recorded at different wavelengths 254 and 280 nm and RI detector at the same time. Calibration was performed using polystyrene standards with nominal MW ranging from 480 to 130,000 Da.
Lignin (2 wt%) was mixed with a freshly prepared 1.5% NaOH solution and left at room temperature for 24 h under vigorous stirring. The mixture was then filtered through a glass filter with a pore size of P100, and the resulting precipitate on the filter was washed to get rid of NaOH residues. The washed precipitates were dried to constant weight in a vacuum oven under phosphorus pentoxide, and a sample was obtained, which is labeled as the NaOH insoluble part.
When analyzing the ash content of all industrially obtained lignin samples, it did not show any significant changes between themselves. An average value of 0.44% ± 0.03% was for HS and MS lignin samples and 0.42% ± 0.03% for LS lignin (Table 1). Such values were within the limits of most data found in the scientific literature on the ash content of biorefinery lignin samples (Kraft, enzymatic hydrolysis, and organosolv) [26]. The industrial lignins analyzed were relatively pure in terms of inorganic impurities, with an ash content not exceeding 0.45%. This means that pure birch wood was used as a raw material for lignin production.

Results of Py/GC/MS showed that severity was directly proportional to the relative content of the lignin derivatives (see Fig. 3) and inversely proportional to the relative content of carbohydrates.
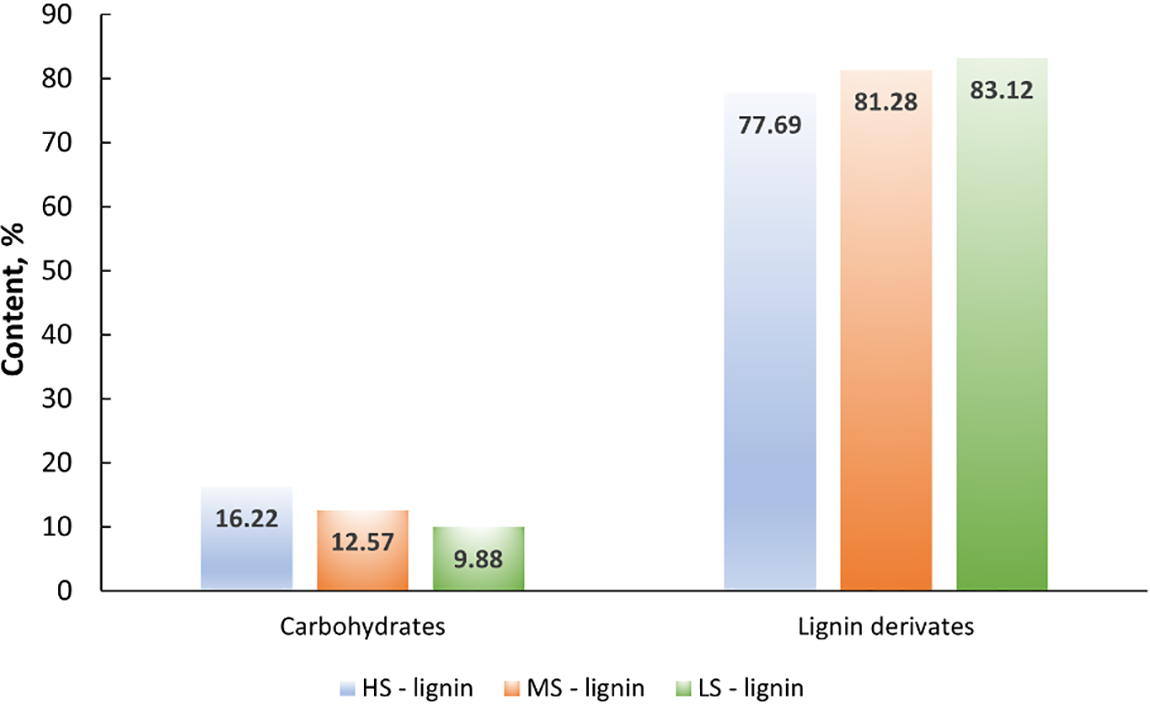
Figure 3: Lignin and carbohydrate derivatives in obtained lignin samples
The more severe the pre-treatment, the more carbohydrates were formed. LS lignin was obtained under low severity, but long hydrolysis followed by a specific washing procedure and there we can see that the carbohydrate relative content was only 9%, but lignin content was the highest (83%) in comparison to HS lignin where the standard pretreatment conditions were applied (carbohydrate content was 16% and lignin content was 77%). In addition, using Py/GC/MS, it was possible to obtain additional information about the sample characteristics in a faster period than KL + ASL, which are time-consuming analytical methods. KL + ASL analysis needs to be performed to determine the distribution of KL and ASL in lignins according to Tappi standard 222 [27]. Such distribution cannot be determined by Py/GC/MS. Classical lignin analyses are more appropriate for the samples delignified in mild conditions. The potential for pseudolignin formation from various impurities (such as proteins, carbohydrates, cellulose, and others) increases at pretreatment temperatures above 180°C, which can lead to an overestimation of the actual lignin content and misinterpret the results [28]. Pseudolignin is formed from a melted lignin-protein-carbohydrates complex. Therefore, KL and ASL will always be higher for technical lignins and should be evaluated in context with other impurities. Since these lignins were obtained by treating the samples with enzymes and the samples also contain nitrogen, this clearly indicates that the KL content will be increased. Therefore, Py/GC/MS is a more informative method than the classical KL method (Table 1).
This phenomenon was more extensive for the biorefinery lignins. The content of basic lignin monomer units in the samples was determined from the Py/GC/MS data (Table 2). By modifying the process conditions, the chemical composition of the obtained lignin samples was different (Fig. 4). It can also be observed that the basic units forming lignin, such as phenyl, guaiacyl, and syringyl, do not differ drastically between the samples (see Table 2), meaning that the same raw material was used for the process.

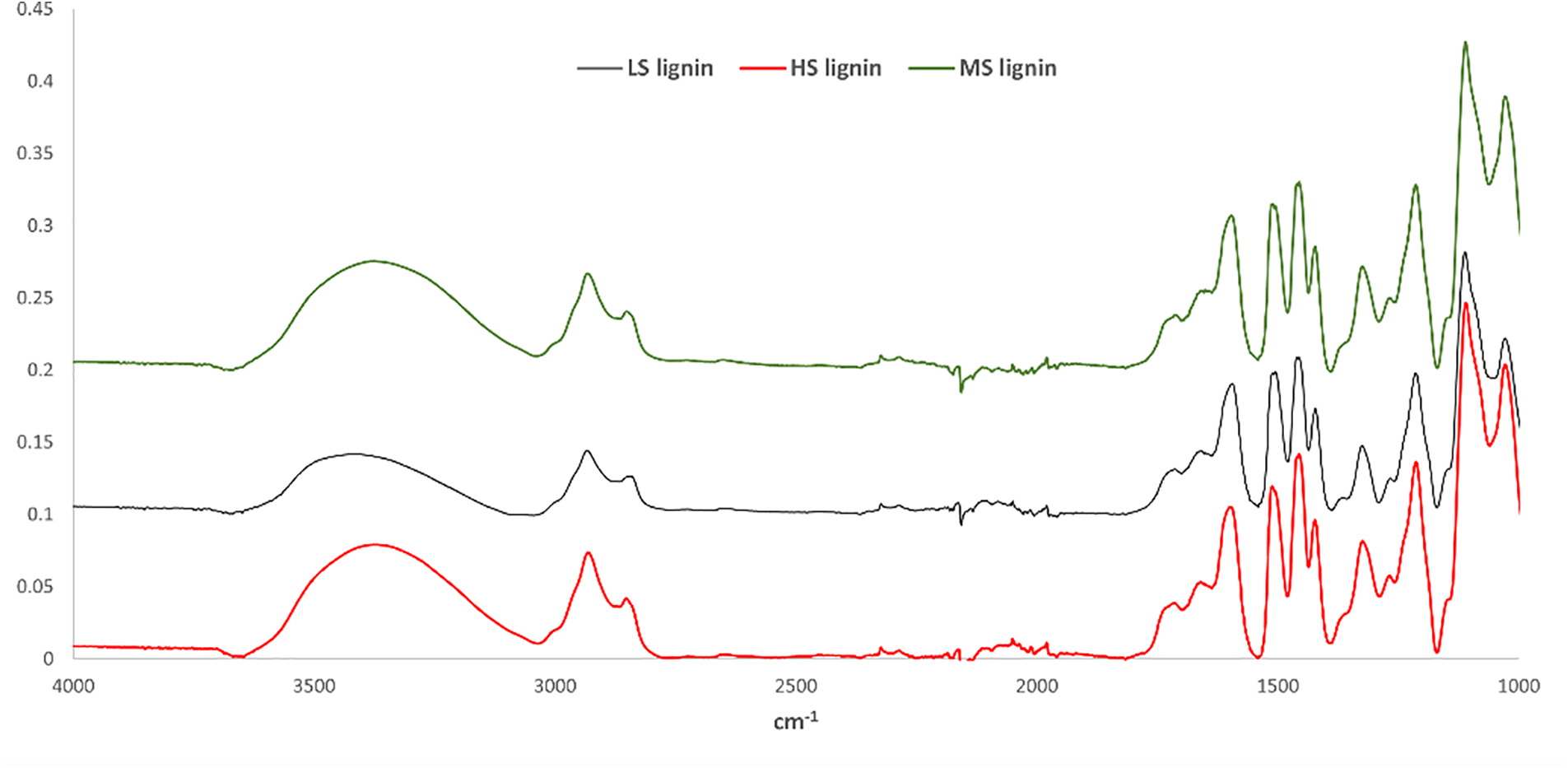
Figure 4: FTIR spectra of birch wood lignins obtained after modification of severity and enzymatic hydrolysis procedure on an industrial pre-treatment
To reveal details of lignin chemical structure, peak areas of lignin pyrolysis products were normalized to 100%, and peak areas of p-hydroxyphenyl- (H), guaicyl- (G), and syringyl- (S) derivatives were calculated as relative percentages. Natural lignin is mainly built from these three monomers. The ratio between these monomers differs among lignins of different plant species and in different parts of the plants. As such, the lignin in hardwood contains a higher quantity of S-lignin units, while softwood lignin contains more G-lignin units. H-lignin units are less abundant in wood; they can mainly be found in other plants. The most common bond connecting the monomers in all-natural lignins is the β-O-4 ether bond. About 45%–50% of the bonds in softwoods and 60%–69% in hardwoods are β-O-4 ether bonds [10].
The FTIR spectra for all three lignin samples examined in the fingerprint region exhibited significant variations in the OH, C-H, C-O, C=O groups, and aromatic rings, characteristic of the carbohydrate and lignin components found in plant cell walls [29]. The FTIR analysis results (Fig. 5) indicated a decrease in absorbance intensity at 1735 and 1242 cm−1, corresponding to carboxylic groups of acetylated xylans, a finding that was further supported by the yields of individual carbohydrates shown in Table 3. Notably, the HS lignin sample had the highest yield of xylose, which was also corroborated by the FTIR spectra.
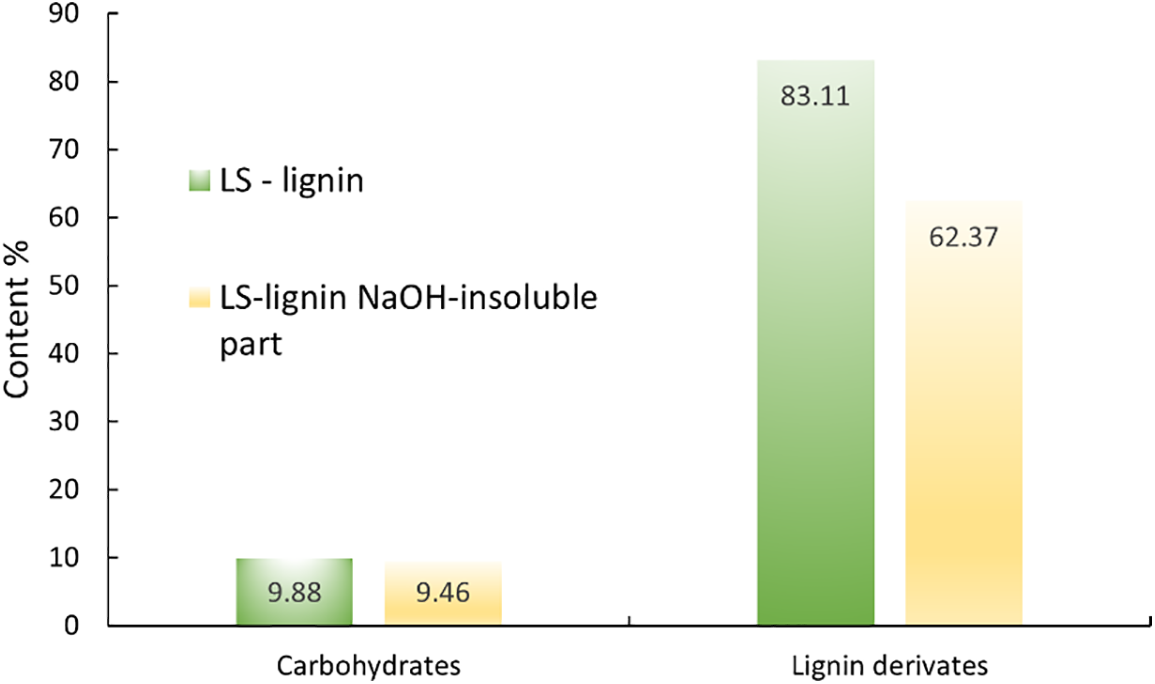
Figure 5: Lignin and carbohydrate derivatives in LS lignin and LS lignin NaOH insoluble part

At the same time, the proportion of carbohydrate components in the lignin samples decreased, leading to a reduction in absorbance intensity in the 1200–1000 cm−1 range, consistent with the component analysis data (Fig. 3). Following pretreatment under varying severity conditions, higher severity resulted in increased absorbance intensity at 1650 cm−1 (associated with conjugated C=O groups in lignin [19]), while lower severity showed a decrease in absorbance. Additionally, the increased absorbance at 1705 cm−1 (related to non-conjugated carbonyl stretching) and 1605 cm−1 (due to C=C bond vibrations) suggests that condensation processes were occurring within the lignin macromolecule. These FTIR spectroscopy results were corroborated by the changes in pentosanes and hexosanes content in the lignin samples obtained under different conditions, as discussed in Table 3. The solubility of lignin samples in an alkaline solution is important because LPF resins are produced in alkaline conditions. Despite the modification, it was not possible to completely dissolve lignin samples in 1.5% alkaline solution (Table 4). After testing the alkali-insoluble part using Py/GC/MS, it was found that the insoluble part of lignin is more condensed. This explains its poor solubility in alkali solution (see Fig. 5).

Likewise, the solubility experiments showed that the carbohydrates present in the LS—lignin did not dissolve in the alkali solution and remained in the insoluble part.
LS lignin was dissolved in NaOH, and its insoluble fraction was compared with the original LS lignin, and it contained much less C-O bonds in the side chains of the propane unit of the lignin molecule (3.37 and 11.37, respectively), which showed that the insoluble part has a condensed system, and it can also be observed by the lignin content from the Py/GC/MS data. This can explain why LS lignin has a higher lignin yield than HS lignin. In the pre-treatment process, lignin condenses due to different hydrolysis conditions. In addition, the insoluble part contains many lipophilic compounds, which also are insoluble in alkaline solutions. Aliphatic, aromatic, and cyclic monomers indicated the content of degradation products of both polar and lipophilic extracts, from which the heterogeneity of the sample from the point of view of the chemical composition can be characterized (see Table 4). For example, it may show higher levels of carbohydrate compounds in Py/GC/MS, as aldehydes and ketones are among the products of carbohydrate degradation and may also be formed from aliphatic, aromatic, and cyclic monomers in pyrolysis.
Also, a significant difference in the structure of lignin can be observed after dissolution in an alkaline solution (see Table 4). It can be seen that all lignin monomer units changed. Phenyl and guaiacyl derivatives are insoluble in alkaline solution because their content increases in the insoluble part. Meanwhile, syringyl derivatives are soluble, which can be seen by their decrease. Such monomer unit-forming derivatives like syringaldehyde contribute to the differences in condensed systems, which dissolve in the alkali solution and do not remain in the insoluble part.
As can be seen in Table 3, the content of arabinose (Ara), mannose (Man), and galactose (Gal) in the lignin samples were negligible and did not show any notable deviations. The most significant changes in the reduction of xylose (Xyl) and glucose (Glc) were observed for the LS lignin when a longer hydrolysis time in the pilot plant was used, followed by a specific washing procedure. While performing the molecular mass distribution Mw and Mn and calculating the polydispersity index (PDI), it can be seen that this particular sample was characterized by the highest PDI compared to HS-lignin (see Table 5).

Higher pre-treatment severity reduced PDI while having the highest content of the total carbohydrates in the sample. Low/medium severity produced similarly high PDI (low and medium severity were quite similar). Still, to be sure that lignin has the highest potential for LPF production, it must be tested in resin production, which is the main aim of future research on the project.
In conclusion, it can be stated that alterations in the process conditions of enzymatic hydrolysis, as well as the severity and washing procedures, significantly impacted the structure of lignin. The purity of lignin, particularly its separation from carbohydrates like cellulose, was influenced by both the duration of enzymatic hydrolysis and the washing technique used on the filter cake. The effectiveness of enzymatic hydrolysis was evidenced by the reduction of monosaccharide xylose in the carbohydrate results. Despite varying modes in industrial-scale enzymatic hydrolysis processes, the primary monolignols of lignin—specifically, the amounts and ratios of sinapyl (S), coniferyl (G), and coumaryl (H) alcohols—remained consistent. Notably, specific washing procedures had the most significant impact on reducing carbohydrate content in the lignin samples. The degree of delignification was altered by the disruption of lignocellulosic bonds, which affected lignin’s structure and, thus, potential reactivity as well.
It can be concluded that the delignification process plays a crucial role in determining the properties of the resulting lignin. HS lignin was found to have a lower PDI and a more condensed structure, whereas LS lignin exhibited a higher PDI but a lower carbohydrate content. Therefore, it is essential to strike a balance and optimize the conditions according to the intended application. Achieving effective delignification does not necessarily imply that the resulting lignin is suitable as a raw material for obtaining other products. Moreover, additional changes in lignin during processing can adversely affect the production processes of various products by increasing viscosity and creating heterogeneity, which must be taken into account choosing lignin as raw material for further processes.
Acknowledgement: We express our gratitude to Fibenol OÜ for the delivery of the raw material from several industrial processes for evaluation, as well as to the European Union’s Horizon 2020 Research and Innovation Programme and the Bio-Based Industries Consortium for supporting the project VIOBOND (https://viobond.eu/) (accessed on 31 October 2024).
Funding Statement: This research was funded by the VIOBOND project “Upscaling New Lignin-Phenol-Formaldehyde Resin Production with Biorefinery Lignin. VIOBOND—Sustainable Binder”, which received funding from the Bio-Based Industries Joint Undertaking (JU) under the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 101022987. The JU receives support from the European Union’s Horizon 2020 Research and Innovation Programme and the Bio-Based Industries Consortium.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Maris Lauberts, Janis Rizikovs, Karl Pebo; data collection: Maris Lauberts, Matiss Pals, Karl Pebo; analysis and interpretation of results: Maris Lauberts, Matiss Pals, Karl Pebo; draft manuscript preparation: Maris Lauberts, Janis Rizikovs. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Population Connection. World population milestones through history. 2024. Available from: https://populationconnection.org/learn/population-milestones/. [Accessed 2024]. [Google Scholar]
2. Council NR. Biobased industrial products: priorities for research and commercialization. Washington, DC, USA: The National Academies Press. 2000. Available from: https://nap.nationalacademies.org/catalog/5295/biobased-industrial-products-priorities-for-research-and-commercialization. [Accessed 2024]. [Google Scholar]
3. Sarika PR, Nancarrow P, Khansaheb A, Ibrahim T. Bio-based alternatives to phenol and formaldehyde for the production of resins. Polymers. 2020;12(10):2237. doi:10.3390/polym12102237. [Google Scholar] [PubMed] [CrossRef]
4. Mumtaz F, Li B, Al Shehhi MR, Feng X, Wang K. Treatment of phenolic-wastewater by hybrid technologies: a review. J Water Process Eng. 2024;57:104695. doi:10.1016/j.jwpe.2023.104695. [Google Scholar] [CrossRef]
5. Danielson B, Simonson R. Kraft lignin in phenol formaldehyde resin. Part 1. Partial replacement of phenol by kraft lignin in phenol formaldehyde adhesives for plywood. J Adhes Sci Technol. 1998;12(9):923–39. doi:10.1163/156856198X00542. [Google Scholar] [CrossRef]
6. Gan L, Pan X. Phenol-enhanced depolymerization and activation of kraft lignin in alkaline medium. Ind Eng Chem Res. 2019;58(19):7794–800. doi:10.1021/acs.iecr.9b01147. [Google Scholar] [CrossRef]
7. Vasile C, Baican M. Lignins as promising renewable biopolymers and bioactive compounds for high-performance materials. Polymers. 2023;15(15):3177. doi:10.3390/polym15153177. [Google Scholar] [PubMed] [CrossRef]
8. Melro E, Antunes FE, Valente AJM, Duarte H, Romano A, Medronho B. On the development of phenol-formaldehyde resins using a new type of lignin extracted from pine wood with a levulinic-acid based solvent. Molecules. 2022;27(9):2825. doi:10.3390/molecules27092825. [Google Scholar] [PubMed] [CrossRef]
9. Pals M, Lauberte L, Arshanitsa A, Vevere L, Jurkjane V, Telysheva G. Organosolv delignification of residual plantation willow bark after extractive removal. Res Rural Dev. 2020;71–6. doi:10.22616/RRD. [Google Scholar] [CrossRef]
10. Pals M, Lauberts M, Zijlstra DS, Ponomarenko J, Arshanitsa A, Deuss PJ. Mild organosolv delignification of residual aspen bark after extractives isolation as a step in biorefinery processing schemes. Molecules. 2022;27(10):3185. doi:10.3390/molecules27103185. [Google Scholar] [PubMed] [CrossRef]
11. Dizhbite T, Ponomarenko J, Andersone A, Dobele G, Lauberts M, Krasilnikova J, et al. Role of paramagnetic polyconjugated clusters in lignin antioxidant activity (in vitro). IOP Conf Ser Mater Sci Eng. 2012;38:012033. doi:10.1088/1757-899X/38/1/012033. [Google Scholar] [CrossRef]
12. Lauberts M, Rizikovs J, Pals M. Chemical characterization and reactivity studies of various industrially obtained lignins for their potential use in lignin-phenol-formaldehyde resin production. In: European Biomass Conference and Exhibition 2023, Bologna, Italy, 2023; p. 1089–93. [Google Scholar]
13. De Santi A, Monti S, Barcaro G, Zhang Z, Barta K, Deuss PJ. New mechanistic insights into the lignin β-O-4 linkage acidolysis with ethylene glycol stabilization aided by multilevel computational chemistry. ACS Sustain Chem Eng. 2021;9(5):2388–99. doi:10.1021/acssuschemeng.0c08901. [Google Scholar] [PubMed] [CrossRef]
14. Shen X, Xin Y, Liu H, Han B. Product-oriented direct cleavage of chemical linkages in lignin. ChemSusChem. 2020;13(17):4367–81. doi:10.1002/cssc.v13.17. [Google Scholar] [CrossRef]
15. Zhang X, Abushammala H, Puglia D, Lu B, Xu P, Yang W, et al. One-step to prepare lignin based fluorescent nanoparticles with excellent radical scavenging activity. J Renew Mater. 2024;12(5):895–908. doi:10.32604/jrm.2024.049810. [Google Scholar] [CrossRef]
16. Yang S, Zhang Y, Yuan T, Sun R. Lignin-phenol–formaldehyde resin adhesives prepared with biorefinery technical lignins. J Appl Polym Sci. 2015;132(36):1–8. [Google Scholar]
17. Gao B, Sun C, Yang T, Wen Q, You S, Yang Q, et al. Biphasic solvent systems enabled lignocellulosic biomass fractionation: a pathway towards comprehensive biomass utilization. Ind Crops Prod. 2023;202:117036. doi:10.1016/j.indcrop.2023.117036. [Google Scholar] [CrossRef]
18. Chang C, Gupta P. Exploring the oxidative effects of the microbial electro-fenton process on the depolymerization of lignin extracted from rice straw in a bio-electrochemical system coupled with wastewater treatment. Biomacromolecules. 2023;24(3):1220–32. doi:10.1021/acs.biomac.2c01281. [Google Scholar] [PubMed] [CrossRef]
19. Marques FP, Colares AS, Cavalcante MN, Almeida JS, Lomonaco D, Silva LMA, et al. Optimization by response surface methodology of ethanosolv lignin recovery from coconut fiber, oil palm mesocarp fiber, and sugarcane bagasse. Ind Eng Chem Res. 2022;61(11):4058–67. doi:10.1021/acs.iecr.1c04362. [Google Scholar] [CrossRef]
20. Amit TA, Roy R, Raynie DE. Thermal and structural characterization of two commercially available technical lignins for potential depolymerization via hydrothermal liquefaction. Curr Res Green Sustain Chem. 2021;4:100106. doi:10.1016/j.crgsc.2021.100106. [Google Scholar] [CrossRef]
21. Sweetwater Energy I. Rapid pretreatment AU 2015360513 B2. 2015. Available from: https://patents.google.com/patent/AU2015360513B2/en. [Accessed 2024]. [Google Scholar]
22. Lauberts M, Sevastyanova O, Ponomarenko J, Dizhbite T, Dobele G, Volperts A, et al. Fractionation of technical lignin with ionic liquids as a method for improving purity and antioxidant activity. Ind Crops Prod. 2017;95:512–20. doi:10.1016/j.indcrop.2016.11.004. [Google Scholar] [CrossRef]
23. Pals M, Lauberte L, Ponomarenko J, Lauberts M, Arshanitsa A. Microwave-assisted water extraction of aspen (Populus tremula) and pine (Pinus sylvestris L.) barks as a tool for their valorization. Plants. 2022;11(12):1544. doi:10.3390/plants11121544. [Google Scholar] [PubMed] [CrossRef]
24. Blakeney AB, Harris PJ, Henry RJ, Stone BA. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr Res. 1983 Mar;113(2):291–9. doi:10.1016/0008-6215(83)88244-5. [Google Scholar] [CrossRef]
25. Zakis GF. Functional analysis of lignins and their derivatives. Atlanta, USA: TAPPI Press; 1994. Available from: http://lib.ugent.be/catalog/rug01:001647975. [Accessed 2024]. [Google Scholar]
26. Ház A, Jablonský M, Šurina I, Kačík F, Bubeníková T, Ďurkovič J. Chemical composition and thermal behavior of kraft lignins. Forests. 2019;10(6):483. doi:10.3390/f10060483. [Google Scholar] [CrossRef]
27. Technical Association of Pulp and Paper Industry. T222 Om-02 lignin in wood and pulp. In: TAPPI test methods; 2011. p. 1–7. [Google Scholar]
28. Kim H, Padmakshan D, Li Y, Rencoret J, Hatfield RD, Ralph J. Characterization and elimination of undesirable protein residues in plant cell wall materials for enhancing lignin analysis by solution-state nuclear magnetic resonance spectroscopy. Biomacromolecules. 2017;18(12):4184–95. doi:10.1021/acs.biomac.7b01223. [Google Scholar] [PubMed] [CrossRef]
29. Pandey KK, Pitman AJ. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int Biodeterior Biodegradation. 2003;52(3):151–60. doi:10.1016/S0964-8305(03)00052-0. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools