 Open Access
Open Access
REVIEW
Essential Oils Usage on Vitis vinifera L., from the Vineyard to Post-Harvest: Advantages, Limitations, and Future Perspectives
1 Department of Agriculture, Food, Environment and Forestry (DAGRI), University of Florence, Sesto Fiorentino, 50019, Italy
2 Mendeleum-Institute of Genetics, Mendel University in Brno, Valtická 334, Lednice, 691 44, Czech Republic
3 Viticulture and Enology Research Center (CREA-Ve), Arezzo, 52100, Italy
* Corresponding Author: Eleonora Cataldo. Email:
(This article belongs to the Special Issue: Adaptation Mechanisms of Grapevines to Growing Environments and Agricultural Strategies)
Phyton-International Journal of Experimental Botany 2025, 94(4), 1047-1072. https://doi.org/10.32604/phyton.2025.064272
Received 10 February 2025; Accepted 14 April 2025; Issue published 30 April 2025
Abstract
The search for environmentally friendly approaches in viticulture is increasing, driven by the need to minimize the ecological footprint of conventional methods while ensuring high grape quality and stable yields. Among the various alternatives explored, essential oils (EOs) have drawn attention due to their natural origin and bioactive properties, including antimicrobial, antifungal, and insect-repellent effects. They are characterized by numerous utilisations, from managing diseases and pests in vineyards to post-harvest applications to preserve and prolong storage duration. This innovative review examines, for the first time, the topic of EOs on viticulture, embracing their multiple uses and considering their potential influence on key quality indicators such as fruit firmness, total soluble solids, and phenolic composition. Research findings indicate that EOs can contribute to suppressing fungal development and pest invasions, thereby reducing post-harvest deterioration. However, their effectiveness is influenced by factors such as chemical composition, mode of application, and environmental conditions. Although EOs align well with the principles and broader sustainability goals of integrated pest management (IPM), several obstacles remain, including issues related to their stability, degradation rate, potential phytotoxic effects, and regulatory constraints. In addition to the undoubtedly advantageous aspect for the vineyard, the final chapter of this review focuses right on these obstacles, emphasizing the need to have long-term post-application scientific data on wine organoleptic quality and thus their presence or absence in the must.Keywords
Glossary
| EOs | Essential oils |
| IPM | Integrated Pest Management |
| Cv | Cultivar |
| EU | European Union |
| AUDPC | Area Under the Disease Progress Curve |
| LC50 | Lethal Concentration 50 |
| LC90 | Lethal Concentration 90 |
| TSS | Total Soluble Solids |
| TA | Titratable Acidity |
| ROS | Reactive oxygen species |
| RS | Relative selectivity |
| RH | Relative humidity |
| CMC | Carboxymethyl cellulose |
| VOCs | Volatile Organic Compounds |
Essential oils (EOs) are complex mixtures of volatile compounds obtained from various parts of aromatic plants through steam distillation or cold pressing. Due to their broad spectrum of biological activities, they have been widely used in industries such as cosmetics, pharmaceuticals, food preservation, and agriculture [1]. Their antimicrobial, antifungal, insecticidal, and antioxidant properties have made them particularly attractive as natural alternatives to synthetic chemicals in different sectors, including sustainable agriculture [2–4]. Several studies have demonstrated their potential in plant protection, highlighting their effectiveness as biopesticides, herbicides, and antimicrobial agents in post-harvest management [1,5].
In viticulture, the increasing demand for sustainable and eco-friendly solutions has driven research toward the use of natural compounds to reduce the environmental impact of conventional practices [6]. Grapevine growing involves numerous contingencies, including fungal diseases, insect infestations, and post-harvest spoilage, which would traditionally require preventative or curative chemical treatments at scheduled intervals [7,8].
For the first time, this review aims to provide a comprehensive overview of the use of essential oils in viticulture. It explores their bioactive properties, advantages over conventional chemical products, and their efficacy in controlling key fungal diseases and arthropod pests affecting grapevines. Furthermore, the review aims to fully embrace the viticultural supply chain, providing an innovative overview of the impact of EOs both in the vineyard (i.e., grape quality and yield) and post-harvest (i.e., grape conservation). Comprehensively, it also addresses the limitations associated with the use of these natural products, offering ideas on where to direct future research. Moreover, this review attempts to highlight existing gaps in the literature and critically analyze conflicting findings, providing a balanced perspective on the current state of research on essential oils in viticulture.
2 Essential Oils: Definition and Properties
Essential oils are volatile compounds manufactured by many plant species and involved in their defense mechanisms against pathogens [9]. The main components of EOs are low molecular weight compounds: terpenoids, constituted by isoprene units: monoterpenes (two isoprene units), sesquiterpenes (three isoprene units), and diterpene (four isoprene units), although phenylpropanoids can also be present. However, both categories included phenolic compounds [10,11]. Additionally, a non-volatile residue, accounting for around 5%–10% of the composition, includes hydrocarbons, fatty acids, waxes, flavonoids, steroids, carotenoids, sterols, coumarins, and psoralens [12]. Typically, EOs contain over 300 dissimilar compounds [11] but are mainly constituted by two-three main components, accounting for high concentrations (between 20%–85%) and other components in traces [13]. For example, rosemary EO’s main components are 1,8-cineole (43.6%), camphor (12.3%) and α-pinene (7.4%); ginger EO’s main components are β-bisabolene (22.11%), AR-curcumin (14.5%) and camphene (14.1%); and orange EO’s main component is limonene (91.5%) (Fig. 1). Terpenes, terpenoids, and phenylpropanoids account for a wide variety of chemical compounds, resulting in a large variety of EOs properties, depending on the specific composition and the plant from which is extracted [11].
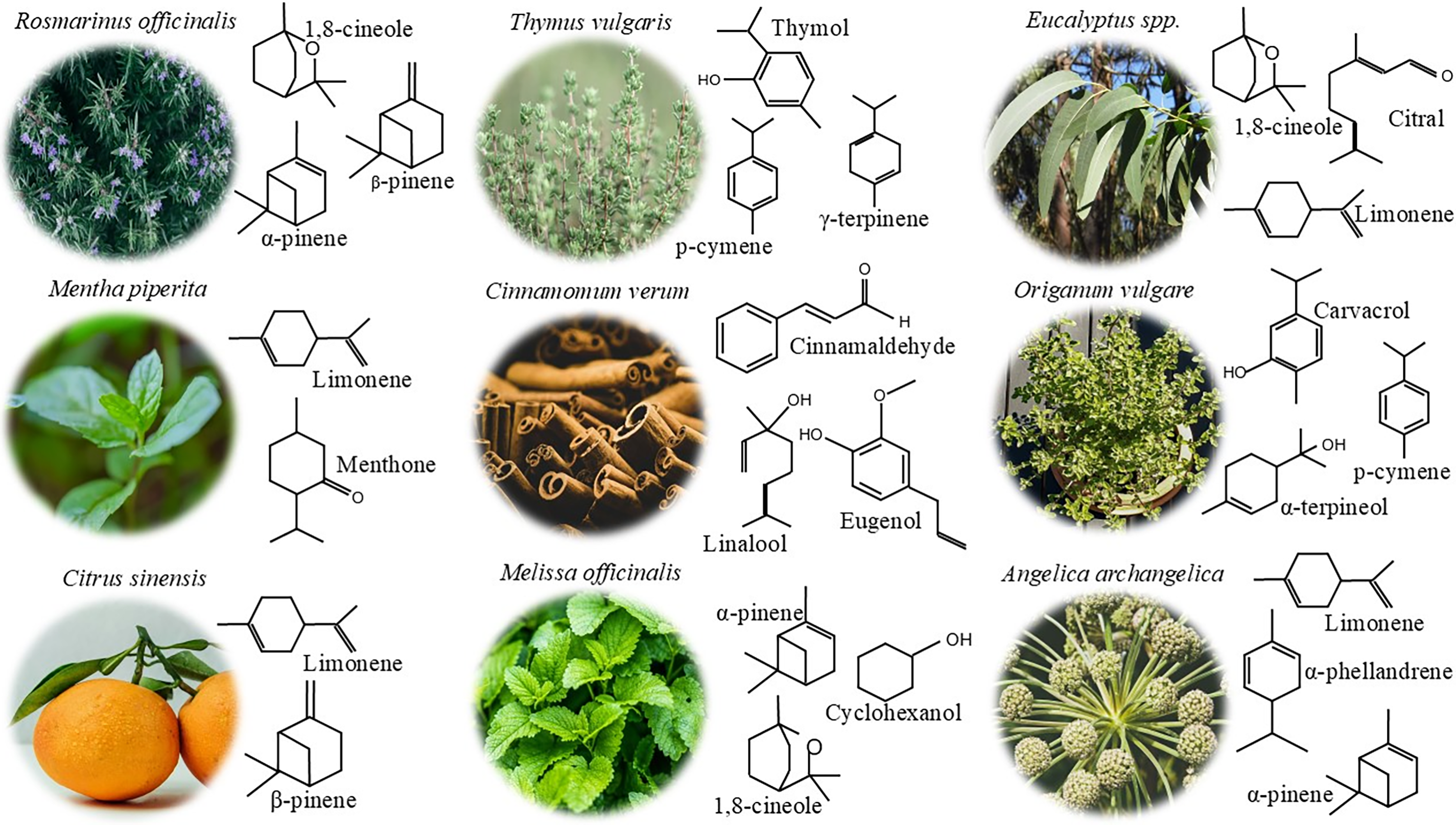
Figure 1: A selection of plants used for essential oil extraction, along with their corresponding key bioactive compounds
EOs are typically extracted from different plants through steam distillation or volatile organic solvents [14]. Steam distillation involves mixing plant material with water and heating the system until it boils; the vapour is then collected and condensed to separate the water from the oil [15]. In contrast, solvent extraction uses compounds such as ethanol, acetone, or hexane. However, the resulting oil cannot be truly classified as “essential” due to solvent residues that may compromise its purity [16]. The term “essential oils” derives from Paracelsus’ concept of “quintessence”, which laid the foundation for future research on EOs; indeed, the term “essential”, reflects the concept of the most potent part of a substance [17].
The use of EOs dates back to ancient Eastern civilizations, particularly in Egypt, Persia, and India. However, their true development occurred during the Middle Ages in Europe, thanks to the physician Arnald de Villanova, who introduced distillation into European medical practices [18]. During the 13th century, European pharmacies started to produce EOs for medicinal purposes, but a significant development took place in the 19th century when the production process was industrialized to meet the high demand for perfumes. During this period, the use of EOs for medical purposes became secondary, as they were increasingly used as fragrances [19]. During the last 50 years, the interest in EOs has grown, driven by the need for environmentally friendly agricultural techniques. Monoterpenes, the primary constituents of EOs, have been explored as control agents against plant phytophagous pests and pathogens [20,21].
2.1 Bioactive Properties of EOs
EOs are considered secondary metabolites in plants. The production of these compounds provides an evolutionary advantage, allowing plants to defend against pathogens, parasites, and weeds, protect stored foods, attract animals for pollination and seed dispersal, communicate with other plants, act as antioxidants, and stimulate soil microorganisms [1,22–25]. The bioactive properties of EOs are largely attributed to their complex composition and the presence of bioactive volatile compounds [26]. In summary, their properties can be categorized as antifungal, antibacterial, insecticidal, antioxidant, and plant-strengthening [23,27,28] (Table 1).
Regarding antimicrobial properties, the exact mechanisms of action are not fully understood. This is because EOs are composed of numerous components that may act synergistically, making it difficult to attribute their antimicrobial effects to a single compound [56]. However, studies suggest that EOs can penetrate bacterial membranes causing cellular dysfunction, while phenolic components alter cell permeability and damage internal structures, ultimately leading to bacterial cell death [57]. Similar mechanisms are thought to underline their antifungal effects. Overall, the interaction between EOs and microbial cells, particularly their membranes and mitochondria, seems to be key to their antimicrobial activity [27]. Plant disease causes around 10%–13% of losses in food production and bacterial infections cause significant losses in agricultural production [58]. For this reason, this important EOs property should be emphasized in plant defense strategies.
Besides the antimicrobial properties, EOs exhibit biostimulant activity for soil bacteria and, consequently, enhance soil metabolism [23]. Indeed, some aromatic plants can be used as soil amendments, releasing EOs that stimulate microbial communities in the soil [59].
The insecticidal properties of EOs can be attributed to several factors: firstly, some EOs compounds, such as monoterpenes, are neurotoxic for insects; moreover, some EOs can inhibit cytochrome P450, a key enzyme in pesticide metabolism, making them effective synergistic agents when used alongside other insecticides [21]. Additionally, some monoterpenes commonly found in EOs, such as α-pinene, citronellal, or thymol, also possess repellent properties [60]. With increasing concerns about the health and environmental risks of chemical insecticides and repellents [61], EOs represent a promising alternative for greener and more sustainable agriculture.
EOs also demonstrate potential in weed management due to their phytotoxic effects which cause chlorosis in leaves by reducing chlorophyll, removing the waxy cuticle, depolarizing membranes, and inhibiting mitosis and respiration [33]. This powerful effect is due to the extraction of terpenoids in their pure form during the EOs production process, compared to aqueous extract. Terpenoids and phenols, acting as allelochemicals, are especially effective during sensitive growth stages, such as pre- and post-emergence [62] (Fig. 2).
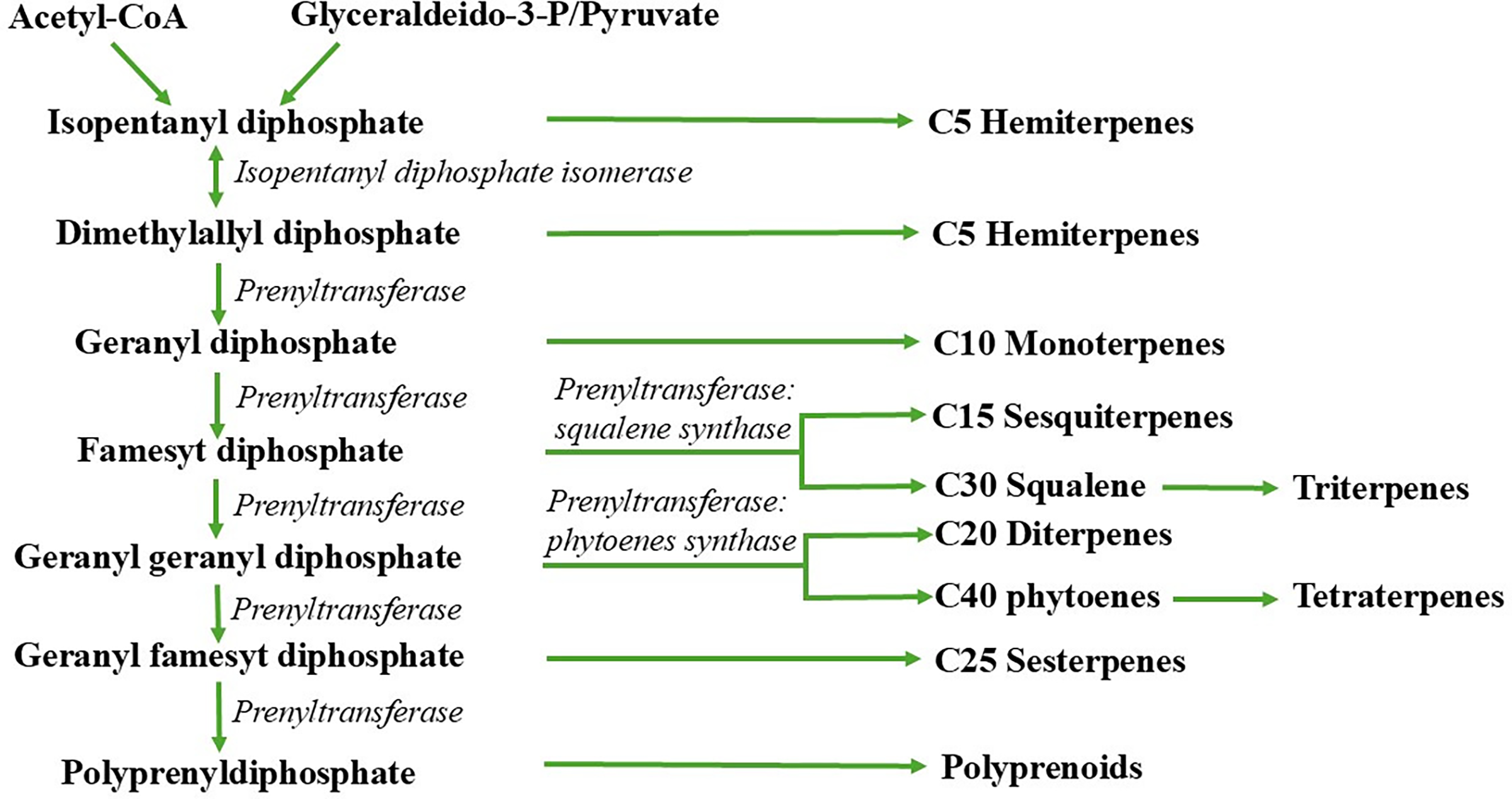
Figure 2: Terpenoid biosynthesis pathway (adapted from Ootani et al. [5])
Finally, some EOs show antioxidant activities due to the presence of phenolic compounds, which are highly reactive with peroxyl radicals [63]. This property is valuable for postharvest preservation, enhancing oxidative stability [64]. For instance, crops like grapes often face postharvest issues caused by diseases such as Botrytis cinerea. The use of EOs, both preventively and curatively, offers an alternative strategy to polish up the quality of the final product [41].
2.2 Regulatory Framework for EOs in Some Important Viticulture Regions
The regulatory status of EOs in agriculture varies across different regions, reflecting diverse approaches to their classification, approval, and use.
In the European Union (EU), the regulatory framework for the use of EOs in agriculture is primarily defined by Regulation (EC) No 1107/2009. According to this regulation, EOs can be exploited as active ingredients in plant protection products, provided they are approved by the European Food Safety Authority (EFSA), which evaluates their safety and efficacy [65]. Furthermore, the use of EOs in organic farming is governed by Regulation (EU) 2018/848 [66].
In the United States (USA), the Environmental Protection Agency (EPA) regulates pesticide products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). However, some natural substances, including certain EOs, are classified as “minimum risk pesticides” and listed in 40 CFR 152.25(f). These products are considered low risk to human and environmental health and are therefore exempt from the expensive and time-consuming toxicological and environmental tests required for standard pesticide registration. The list includes several plant-derived oils, such as castor oil, cedar oil, cinnamon oil, citronella oil, clove oil, eugenol, corn oil, cottonseed oil, garlic oil, geranium oil, geraniol, lemongrass oil, linseed oil, mint oil, peppermint oil, rosemary oil, soybean oil, and thyme oil [2,67,68].
In Australia, the Australian Pesticides and Veterinary Medicines Authority (APVMA) is responsible for registering agricultural and veterinary chemical products. Despite their natural origin, EOs are classified as chemical products and must meet the safety criteria required for agricultural chemical registration. However, many EOs have a long history of use in Australian agriculture, and in some cases, well-documented evidence of their safe application can support their approval as agricultural chemical products [69,70].
Overall, while EOs offer promising alternatives to conventional pesticides, their regulatory acceptance remains a complex process influenced by region-specific legislation. Due to these regulatory barriers, few EO-based biopesticides are available on the market, except in the USA, where exemptions from registration have contributed to the development of EO-based products [71]. Continued research and standardized evaluation criteria will be essential to facilitate their broader adoption in sustainable agriculture.
2.3 Advantages of EOs over Conventional Products
The use of EOs in agriculture offers several benefits when compared to the use of chemical agents. Indeed, EOs are natural products that generally pose a minimum risk to the environment, due to their biodegradability [21,72] which reduces the risk of soil and water contamination [73]. In addition, synthetic chemicals typically rely on a single active component that generates resistance in microorganisms and pests [74], EOs conversely contain multiple compounds that work synergistically giving a structural complexity to achieve antimicrobial effects. Against insects, in particular, EOs appear to target multiple physiological systems, thus making it more difficult for insects to develop multiple resistances [21].
The widespread use of chemicals poses an additional risk to the safety of agricultural operators and consumers. These substances can be inhaled, ingested, or absorbed through the skin, leading to health issues ranging from dermatological problems to carcinogenic and neurological conditions [75]. In contrast, EOs offer a safer alternative for reducing health risks. However, despite being natural substances, some EO components—such as terpenes and phenols—can exhibit toxic, allergenic, or mutagenic properties. Nevertheless, it was demonstrated that the LD50 (Lethal Dose 50, an indicator of acute toxicity) of EOs are always higher than the ones of chemical herbicides [76]. To make a comparison, the LD50 of EOs typically ranges between 1–20 g/kg of body weight, while commonly used copper-based fungicides in vineyards have LD50 values of 450–878 mg/kg of body weight [25,77]. Additionally, many EOs employed in pest control are selectively toxic to arthropods, without posing risks to mammals or fish [25]. Given their numerous health and environmental benefits, EOs are a valuable tool for IPM programs, warranting further research and application.
3 Application of EOs in Viticulture
3.1 Control of Fungal Diseases
Diseases such as downy mildew (Plasmopara viticola (Berk. & M.A. Curtis) Berl. & De Toni, 1888) [78] and gray mold (Botrytis cinerea (Pers., 1794)) [29] are prevalent in vineyards worldwide leading to substantial economic losses [79]. To combat these diseases, viticulturists often rely heavily on fungicides. However, this intensive use of fungicides raises environmental concerns and contributes to the developing fungicide-resistant strains [80].
EOs are effective against fungal pathogens through multiple mechanisms. They can stimulate plant defense systems, such as the production of secondary metabolites (e.g., resveratrol and/or flavonoids), which reduce the severity of infections by reinforcing leaf cell walls [37]. For instance, a recent work by Fandino et al. [81] demonstrated that EOs, such as oregano EO, can modulate key metabolic pathways in plants, including the phenylpropanoid pathway, and induce oxidative stress responses. This is evidenced by the upregulation of ROS-related genes, depletion of L-glutathione, and accumulation of organic metabolites like terpenes and phytoalexins, which may strengthen the plant’s innate immune response to pathogens such as Plasmopara viticola. Additionally, EOs are lipophilic substances, a property that allows them to interact with and permeate fungal cell walls and membranes. This interaction disrupts the protective layer composed of phospholipids, fatty acids, and polysaccharides, increasing cell permeability. The resulting loss of selective membrane activity, coupled with the inhibition of ATP-mediated H+ efflux, leads to osmotic imbalance and ultimately causes fungal cell death [82].
After the 19th century, the introduction of pathogens from America, such as downy mildew, nicknamed “the most critical vineyard disease”, posed a serious threat to European viticulture [83]. In conventional and organic viticulture, copper-based fungicides are widely used to control fungal diseases. However, repeated application leads to copper accumulation in vineyard soils damaging soil health and biodiversity. Average concentrations can reach 49.26 mg/kg, significantly higher than the European soil average of 16.85 mg/kg. To mitigate these effects, the European Union enforces limits on copper use, allowing a maximum of 28 kg/ha over seven years (4 kg/ha annually) under Regulation (EU) 2018/1981, prompting the need for sustainable alternatives [84]. EOs have demonstrated promising antifungal activity against P. viticola, particularly during early infection stages. Although the precise mechanisms remain unclear, they may involve the inhibition of haustoria formation, disruption of zoospore or mycelium viability, or stimulation of the plant’s defence system [78].
Rienth et al. [78] continuously vapourised oregano and thyme EOs on grapevine cuttings of Chasselas, artificially infected with P. viticola in a controlled growth chamber for ten days. At vapour concentrations of 0.015%–0.023% (oregano) and 0.06%–0.1% (thyme), both EOs reduced disease severity by approximately 98%. However, prolonged exposure (longer than 2 days at such vapour concentration) caused phytotoxic effects, prompting the authors to test shorter exposure times. When plants were exposed to EO vapours for only 24 h post-infection, disease severity was reduced by 95%, indicating the potential utility of early-stage treatments. However, the mode of action remains unclear. Similarly, in a two-year vineyard experiment in Brazil, Maia et al. [37] tested lemongrass EO on cv. Isabel, applying it weekly to the canopy at concentrations of 0.5, 1, and 2 mL/L from the beginning of sprouting. In the first year, the three doses reduced the AUDPC (area under the disease progress curve) by 45%, 48% and 46%, while in the second year, the two higher doses achieved reductions of 43% and 48%. These outcomes were comparable to standard chemical treatments (e.g., Bordeaux mixture), highlighting the efficacy of lemongrass EO as a potential alternative. However, Pedrotti et al. [85] conducted an on-field experiment in Brazil using Eucalyptus staigeriana EO at 0.5 mL/L dose applied every seven days, a consortium treatment, obtained by combining the same dose of EO with a synthetical fungicide (Mancozeb) with a 50% substitution, and a conventional treatment (only Mancozeb). While EO alone initially reduced downy mildew incidence, it was ineffective under fungi-favorable temperature and humidity conditions. Conversely, the consortium treatment significantly reduced downy mildew incidence by approximately 50% and severity by over 90% compared to the control and EO-only treatments. These results indicate that while this EO alone is insufficient, it can be effectively used in combination with synthetic fungicides, reducing their application by 50%.
From another Italian field trial on Vitis vinifera (cv. Malvasia di Candia) was found the ability of formulated products based on EOs (BM-608, 25% tea tree-0.75% applied concentration and Sporatec, 15% clove EO at a 1% rate) to reduce the incidence of the pathogen compared to conventional copper-based treatment [49]. By September, disease incidence on leaves was approximately 36% in control, 25% in the EO treatments, and 15% in the copper treatment. Similarly, disease severity was around 9% in the control, 5% with EO treatments, and 2.5% with conventional treatment. The authors suggest that, despite their lower efficacy compared to copper products, these reductions could be considered acceptable, particularly for organic farming systems where strict regulations limit the use of conventional chemical products.
These findings collectively indicate that while EOs alone may have limited efficacy under unfavorable conditions, their integration into combined strategies with synthetic fungicides offers a promising avenue for reducing fungicide use and mitigating environmental impacts without compromising disease control. Further research into the optimization of EO application methods and their modes of action could unlock their full potential in sustainable viticulture.
Among the various fungal pathogens, B. cinerea, the causative agent of gray mold, is particularly notorious for its ability to severely compromise directly grape quality and indirectly wine aroma [86].
Machado et al. [29] conducted an experiment on post-harvest grapes of the Isabel cv. in Brazil. Berries were artificially wounded, incubated under favorable temperature and humidity conditions, and inoculated with B. cinerea. Four hours later, rosemary EO was applied at concentrations of 5 and 10 mL/L. Both preventive and curative treatments were tested. The first was performed by treating the berries with EO 24 h before the inoculation; the second was performed by treating the berries 4 h after the inoculation. Preventive treatments reduced the incidence from 75.7% ± 6.7% to 26.7% ± 2.8% and 15.3% ± 2.7% for the two doses, respectively, and the severity from 13.8% ± 3.5% to 3.6% ± 1.5% and 1.0% ± 0.6%. Curative treatments achieved even more impressive results, reducing the incidence to 13.0% ± 2.6% and 0.0% ± 0.0%, and severity to 3.9% ± 1.8% and 0.0% ± 0.0%, for the two doses, respectively. El-Abbasy et al. [34] explored the effectiveness of oregano and thyme EOs in controlling B. cinerea during grape storage spraying them 48 h before harvest (2 and 4 mL/L) to replace the environmentally unsustainable sulphur dioxide (SO2) conventional treatment. After 45 days of storage, both EOs showed high efficacy in reducing disease incidence (3%–4% oregano and 2%–3.5% thyme) demonstrating superior performance to SO2 (5%–6%).
The findings were remarkable for eucalyptus essential oil too (weekly applications; 1 and 5 mL/L) on cv. Tannat in a Brazilian vineyard [87] comparing the results with conventional treatments (thiophanate-methyl, Mancozeb, Captan, Tebuconazole, copper sulphate, and sulphur). The incidence of disease was similar between the control (13.33% ± 1.07%) and the conventional treatment (14.42% ± 2.04%), whereas it was significantly lower with the EO treatments, at 2.92% ± 0.73% and 2.08% ± 0.52% for the lower and higher doses, respectively. A comparable trend was observed for disease severity, which was 20.83% ± 1.78% and 15.62% ± 1.63% in the control and conventional treatments, respectively, but reduced to 1.79% ± 0.98% and 0.92% ± 0.83% for the EO treatments. Importantly, no significant differences were found in the chemical parameters of must or wine, indicating that the use of EOs does not negatively affect the quality of the final product. Finally, Das et al. [55] investigated the effects of Angelica EO, an environmentally friendly alternative, applied either alone (1–5.5 g/L) or encapsulated in a chitosan nanoemulsion, across various concentrations (0.5–2.5 g/L). Their study revealed that both treatments effectively inhibited fungal growth, with reductions ranging from 15% at the lowest EO dose to nearly complete inhibition (100%) at the highest dose. The nanoemulsion, disrupting ergosterol biosynthesis in fungal membranes, demonstrated greater efficiency in reducing fungal growth (around 100%) compared to the EO alone (35% to 55%).
Research efforts have demonstrated the efficacy of EOs like rosemary, oregano, and thyme in managing B. cinerea both post-harvest and during storage. Field experiments further corroborate their potential as environmentally friendly replacements for conventional fungicides. Despite promising outcomes, scaling these findings for field use requires additional exploration into application methods, dosage optimisation, and cost-effectiveness to ensure their feasibility as part of integrated disease management strategies in viticulture. Additionally, it is crucial to investigate their impact on grape quality, including potential alterations in the chemical composition of the berries, as well as any effects on the sensory characteristics of the final wine. Such analyses would ensure that the use of EOs does not compromise the quality and marketability of the wine, maintaining consumer acceptance and compliance with industry standards.
Among arthropod pests, due to their widespread distribution in Europe and damage caused, particular attention is given to pests belonging to the classes Insecta and Arachnida, including the vine mealybug (Planococcus ficus) [88] and the European grapevine moth (Lobesia botrana) [89] (both Insecta), as well as the two-spotted spider mite (Tetranychus urticae) [90] (Arachnida).
Cultural practices and the judicious use of chemical treatments are essential to mitigate their and to preserve biodiversity in the vineyard [91]. EOs demonstrate neurotoxic effects against insects and mites [47]. Key components such as monoterpenoids target the octopaminergic receptors in insects, which regulate essential functions as neurotransmitters, neurohormones, and neuromodulators. Disruption of octopamine activity leads to nervous system failure, making the octopaminergic system a prime target for pest control (high-level selectivity). Since vertebrates, including mammals, do not have octopamine receptors, these compounds do not interfere with their physiological processes, reducing potential adverse effects on humans and animals [92]. Additionally, EOs exhibit strong penetrative abilities, enhancing their bioavailability as well as that of co-applied substances. This effect is linked to their ability to disrupt the lipid bilayers of cellular membranes [21]. Furthermore, some EOs act as synergists by inhibiting insect P450 cytochrome, which are enzymes critical for phase I xenobiotic metabolism. The P450 system enables insects to detoxify harmful substances, including insecticides, by chemically modifying them to facilitate their elimination. By disrupting this detoxification pathway, EOs can increase the efficacy of insecticides, making them valuable tools in pest control strategies [21,44]. Moreover, EOs can influence arthropod behavior and exert anti-nutritional effects when ingested, further contributing to their effectiveness in pest management [50].
The vine mealybug, P. ficus, is an insect belonging to the order Rhyncota and family Pseudococcidae. Briefly, the damage caused by P. ficus arises from its sap-sucking activity, causing the perfect photosynthetic machine to collapse; in fact, this phytophagous damages the leaf causing it to fall and leading to a general weakening of the plant [88]. Certain components of EOs demonstrate toxic effects against this pest. Interestingly, the efficacy of EOs is higher when used as a whole rather than as isolated components. This phenomenon is attributed to minor terpenes in EOs, which appear to inhibit the cytochrome P450 enzyme involved in detoxification systems [44].
Karamaouna et al. [93] tested various EOs against P. ficus in a laboratory trial on mealybugs grown on Soultanina cultivar. They recorded insect mortality using Lethal Concentration 50 (LC50) against 3rd instar nymphs and adults and compared these results with the reference product, paraffin oil. The study found that lemon EO (LC50 3.6–2.7 mg/mL), orange EO (LC50 5.4 mg/mL), thyme-leaved savoury EO (LC50 2.7–6.3 mg/mL), and peppermint EO (LC50 5.4–8.1 mg/mL) exhibited lower LC50 values than paraffin oil (LC50 9.1–10.9 mg/mL). Due to the absence of phytotoxic effects, the authors recommended Lemon and Orange EOs as the most promising candidates for controlling vine mealybugs. However, the potential side effects of these “friendly products” on beneficial coccinellid beetles should be considered.
Orange oil reconfirmed its efficacy against P. ficus in migration phase on cv Merlot [45] in New Zealand using a double dose of 5 mL/L on cv. Merlot. Specifically, adult counts per leaf decreased from 16.98 ± 39.10 (control) to 5.49 ± 8.04 (treatment) after the first application and from 15.73 ± 52.33 (control) to 3.09 ± 6.50 (treatment) after the second. This approach maximised the efficacy of the EO as a contact product with low persistence. However, its possible toxicity to Phytoseiidae mites should be noted.
Laboratory and field studies highlight the potential of citrus-based EOs for controlling mealybug populations. Despite their efficacy, concerns about their impact on beneficial organisms, such as predatory mites, necessitate further research to refine application strategies and minimise ecological risks. Incorporating EOs into IPM systems could strike a balance between effective pest control and environmental sustainability.
The European grapevine moth, L. botrana, is a critical pest that directly damages grapevines by feeding on blossoms or berries (larval stage) [94] and indirectly promotes fungal infections [95].
Although there is limited research on the effects of EOs on L. botrana, promising results have emerged from laboratory studies. Avgın et al. [96] tested peppermint, rosemary, thyme, fennel, and caraway EOs at 6.5, 12.5, 25, or 50 µL doses indicating an increase in insecticidal properties as the dose increases. Carum carvi EO performed best, achieving mortality rates of 68%–83% at 6.5 µL, 58%–72% at 12.5 µL, and 88%–100% at the two higher doses. Similarly, Benelli et al. [13] evaluated the effect of carline thistle (Carlina acaulis) EO on L. botrana larvae by incorporating various doses (1, 2.5, 6, 7.5, 8, 10, 30 µL/mL) directly into a semi-synthetic diet, recording mortality after three days. Significant larvicidal activity was observed from doses as low as 2.5 µL/mL, with an LC50 of 7.29 ± 0.25 µL/mL.
These findings underscore the potential of Asteraceae EOs as a likelihood alternative larvicidal products. However, scaling these results to field conditions is essential to validate their practical application. Investigations into their stability, cost, and impact on non-target organisms will determine their feasibility as sustainable alternatives within integrated pest management strategies.
The two-spotted spider mite (T. urticae) is a dangerous phytophagous mite that causes significant damage to many crops, including grapevines. It is commonly controlled using commercial products, such as Azamax, which contains 1.2% azadirachtin as its active ingredient, a natural compound extracted from Azadirachta indica (neem tree). Despite its natural origin, Azamax may still pose concerns regarding toxic residues in the environment, particularly when misused or over-applied [90].
Fidelis et al. [97] evaluated the effectiveness of different EOs in a laboratory setting at concentrations ranging from 0.009 to 5.40 µL/mL against T. urticae collected from various vineyards and compared them to Azamax solutions from 0.009 to 10 µL/mL. Fortunately, this study also took into consideration EOs toxicity on Neoseiulus californicus, a predatory mite important for pest control. Among the tested EOs, lemon EO, orange EO, Croton grewioides EO, and Lippia sidoides EO exhibited notable results. Lemon EO had an LC50 of 0.13 µL/mL and 0.19 µL/mL for adults and eggs, respectively, while showing a relatively high LC50 of 2.26 µL/mL for N. californicus. Orange EO exhibited an LC50 of 0.28 µL/mL and 0.15 µL/mL for T. urticae adults and eggs, respectively, with 3.80 µL/mL for the predator.
Doğu and Zobar [47] further tested different EOs on grapevine leaf disks sprayed at 1%, 3%, 6%, and 12% concentrations. Thyme, peppermint, and rosemary EOs yielded the most encouraging results. At 1% concentration, the mortality rates were approximately 33%, 27%, and 25%, respectively. At 3%, mortality increased to around 48%, 42%, and 33%, respectively. At 6%, mortality rates were approximately 70%, 67%, and 57%, respectively, while at 12%, the rates reached 85%, 71%, and 61%, respectively. Thyme EO consistently demonstrated the highest efficacy among the tested oils.
While these laboratory results highlight the potential of EOs, particularly thyme and L. sidoides EO, in controlling T. urticae and minimizing the impact on beneficial predatory mites, field studies are urgently needed to validate these findings under real agricultural conditions. Such studies should also evaluate application methods, persistence, and potential side effects on non-target organisms, ensuring the feasibility and safety of EO-based treatments in IPM strategies. In fact, it is essential to identify alternative products that are selective for phytoseiid mites, which are key predators of T. urticae [98].
3.3 Effects on Grape Quality and Yield in the Vineyard
The potential effects of EOs on grape yield and quality represent an area of growing interest in viticulture. While several studies have investigated their pest control properties, few have explored the impact on these agronomic and oenological parameters. Existing research provides mixed results, highlighting the need for further investigation to fully understand the implications of EO use in vineyards.
Maia et al. [37], in a previously analyzed study conducted in a Brazilian vineyard, evaluated the effects of lemongrass EO at 0.5, 1, 2, and 4 mL/L doses on grape yield and quality. They observed no significant effects on bunch weight but noted an increase in overall productivity during the second year (2 mL/L EO = 4.3 t/ha, Bordeaux mixture = 3.4, Acibenzolar-S-methyl = 3.17 t/ha, and Mancozeb treatment = 2.4 t/ha). Regarding qualitative parameters, they reported no significant effects on berry pH in the first year. However, differences were noted in total soluble solids (TSS), with the control registering 15.03 °Brix and the treatments registering 16, 16.55, 13.63, and 15.63 °Brix for the 0.5, 1, 2, and 4 mL/L doses, respectively. A reduction in titratable acidity (TA) was also observed, which influenced the maturity index (TSS/TA). However, these qualitative effects were not replicated in the second year, where no statistical differences were noted. Similarly, Machado et al. [29] conducted an on-field experiment in a Brazilian vineyard using rosemary EO at 0.5 and 1 mL/L doses. They noted no remarkable effects on either grape yield or quality, suggesting no interference on grape characteristics. Pedrotti et al. [87], in a previously presented study, also observed minimal differences in must pH between control, Eucalyptus EO treatment, and conventional treatment, with values consistently ranging between 2.9 and 3. They reported slight variations in TSS, with the conventional treatment achieving 19.74 ± 0.36 °Brix, while the control and EO treatments (1 and 5 mL/L) reached 17.18 ± 0.27, 17.50 ± 0.65, and 16.61 ± 0.54 °Brix, respectively. No differences were observed in wine total or volatile acidity.
While these studies suggest that EOs can be integrated into pest management strategies with minimal negative impacts on grape yield and quality, the variability in results, particularly concerning yield improvements, requires further exploration. The effectiveness of EOs may depend on factors such as concentration, EO origin, grapevine cultivar, and environmental conditions. Long-term field experiments are necessary to validate these findings and assess potential interactions with other vineyard practices. The scarcity and heterogeneity of results found in the bibliography on the technological and phenolic parameters of grapes lead to think of an intrinsic variability of the studies analysed (cultivars, agronomic techniques, and soil types).
3.4 Effects on Grape Quality in Post-Harvest Conservation
Although limited research exists on the on-field application of essential oils, numerous studies highlight their potential in the postharvest conservation of table grapes. As a non-climacteric fruit, table grapes are particularly vulnerable to postharvest handling practices, which significantly influence their quality during storage [99]. Extending the conservation period of table grapes holds economic value, as offering these fruits beyond their typical season can command higher market prices [100]. Synthetic fungicides are commonly used to combat fungal pathogens that reduce postharvest longevity; however, eco-friendly alternatives that ensure consumer health are increasingly necessary [99].
Dehestani-Ardakani and Mostofi [101] investigated the effects of thyme EO on cv. Shahroudi grapes in Iran by dipping clusters in EO solutions at 0.15 and 0.3 mL/L (stored in darkness at 0°C and 90% RH for 90 days). The decay index (fruit loss due to fungal diseases) was significantly reduced from 46.53% in the control group to 17.4% and 11.3% in the lower and higher doses, respectively. TSS increased from 14.22 °Brix in the control to 14.76 and 15.25 °Brix in the treated samples. Berry appearance was improved with EO application. Cracking and cracked berries n° per kg, decreased from 1.3 berries/kg in the control to approximately 0.9 berries/kg for both EO doses. Similarly, Guerra et al. [100] evaluated the post-harvest effects of a treatment combining 4 mg/mL chitosan with peppermint EO at concentrations of 2.5 or 5 mL/L on cv. Isabella table grapes. Their findings demonstrated that grapes stored at 25°C exhibited reduced weight loss (i.e., 6.5% in the control and 5% for both EO doses after 12 days) and a firmness increase (i.e., 4.7 N/mm in the control to 5.4 and 5.5 N/mm for the lower and higher EO doses, respectively, after 12 days). A similar trend was observed for grapes stored at 12°C, where weight loss was reduced over 24 days and firmness increase (i.e., 5.4 N/mm in the control to 6.6 and 6.4 N/mm for the lower and higher doses after 24 days). Similar results were obtained using Mentha villosa EO, indicating the potential of peppermint EO and related essential oils to enhance post-harvest conservation in grapes.
Mei et al. [102] investigated the efficacy of carboxymethyl cellulose (CMC) film combined with 1%, 2%, and 3% doses of Chinese fir EO as well as the EO alone in enhancing the post-harvest conservation (25°C and 40%–60% RH) of Shine Muscat grapes. After 15 and 18 days, the combined CMC and EO treatments significantly reduced weight loss (0.56%) and delayed decay (10%) compared to the control. The authors concluded that the most effective treatment was the combination of CMC and 1% Chinese fir EO, as the EO alone lacked sufficient coating properties to protect the grape surface.
After 45 days of storage (1° C and 90%–95% RH), Flame Seedless grapes treated with oregano EO showed significantly reduced weight loss, at approximately 3.5% for 2 mL/L dose and 5% for 4 mL/L dose compared with sulphur dioxide SO2 treatment (6%–7%). Thyme EO resulted in weight loss of around 4% and 4.5% for the two doses, respectively [34]. The two oils also proved to be effective decay reduction (2.5% and 6% oregano; 4% and 4.5% thyme) respect to SO2 (14.5%). These treatments generated a buildup in the percentage of marketable fruit and firmness with respect to ctrl. Contrary to other studies analysed, EOs reduced TSS content: the control and SO2 treatments exhibited TSS levels of around 22 °Brix, whereas all EO treatments showed reduced levels of approximately 20–20.5 °Brix. For TA, the control and SO2 treatments showed lower values (approximately 0.5% and 0.55%, respectively) compared to the higher doses of oregano and thyme EOs (around 0.6% and 0.65%). The lower EO doses recorded even higher TA values (approximately 0.85% and 0.75%). Regarding anthocyanin content, all EO treatments significantly increased levels to approximately 39–42 mg/100 g fresh weight (fw), compared to 27 mg/100 g fw in the control and SO2 treatments. The authors concluded that EO applications reduced grape respiration rates, slowing down the metabolic conversion of acids into sugars, which positively impacted the extension of conservation periods.
In another interesting Iranian study conducted by Kavoosi et al. [103] concerning Askari table grapes, the fruit firmness, highlighted by reduced berry shattering (+4%), was increased after cumin EO at 0.8 and 1 mL/L and lemongrass EO at 0.02 and 0.04 mL/L applications. In addition, rachis browning was decreased in EO treatments. However, TSS, TA, and juice pH were unaffected by the EO treatments. Elsayed et al. [52] studied Taify table grapes (Saudi Arabia) by spray applications of thyme, cinnamon, and oregano EOs (0.5% and 1% concentrations). After four weeks of storage (2°C with 90%–95% RH), the average weight loss in the control was 4.05%, whereas the EO treatments showed significant differences: 1.30% and 1.27% for thyme EO, 1.20% and 0.61% for cinnamon EO, and 0.61% and 0.28% for oregano EO. As observed in previous studies, EO treatments improved grape firmness, with increases of up to 1.6 N, compared to the control, especially for oregano and cinnamon EOs at higher dose, similar to the findings of Guerra et al. [100] and Kavoosi et al. [103]. In line with Kavoosi, no significant differences were found for TSS and TA, though a slight increasing trend in TSS was noted. Regarding phenolic compounds, total phenols remained unchanged except for the 1% oregano EO treatment, which exhibited a notable increase. Flavonoid content improved in all EO treatments, with the highest levels recorded for cinnamon and oregano EO (1%). Notably, vapourisation of EOs was less effective than direct application.
Liu et al. [104] developed an edible coating using a mixture of sodium alginate, magnolia EO, and β-cyclodextrin at concentrations of 0.5% and 1% to extend the shelf life of Kyoho grapes in China stored at room temperature (20°C) for 10 days. Their findings further confirmed the beneficial effects of EO-based coatings, demonstrating a significant reduction in decay rates and weight loss compared to the control. The firmness decline observed in untreated grapes was mitigated by magnolia EO coatings, particularly at the 1% concentration, which provided the most effective preservation. Unlike Elsayed et al. [52], who found no significant changes in TSS, Liu et al. [104] reported that EO-based coatings helped maintain higher sugar content throughout storage.
The studies collectively highlight the effectiveness of EOs and edible coatings in improving the post-harvest quality and shelf life of table grapes. These treatments significantly reduce weight loss, decay rates, and rachis browning, while enhancing firmness and, in some cases, preserving bioactive compounds. Notably, the combination of EOs with edible films or carriers, such as CMC, sodium alginate, or chitosan, proved more effective than EOs applied alone, likely due to improved coating adherence and a more uniform protective barrier. However, the impact on other quality parameters, such as TSS and TA, varied across studies. In some cases, EOs slowed metabolic changes, maintaining higher acidity and slightly reducing sugar content, while others reported no significant differences or increase in these attributes. The influence of EOs on phenolic and flavonoid content was generally positive. Overall, the use of EOs appears promising for extending the marketability of grapes, offering an alternative to conventional treatments like sulphur dioxide, with additional benefits for consumer health and reduced environmental impact.
The use of EOs in viticulture raises questions about their potential impact on wine quality. However, research suggests that, when properly dosed, they may not significantly alter wine composition while offering antimicrobial benefits. Pedrotti et al. [41] found that applying E. stigeriana EO (1–5 mL/L) in vineyards did not affect key parameters such as pH, acidity, alcohol content, and sugar levels, with fermentation proceeding normally. The only noticeable difference was a higher concentration of 1,8-cineole, a natural EO compound, which could subtly influence wine aroma without necessarily compromising its quality.
EOs have also been studied as natural preservatives, that can be used as an alternative to traditionally used preservative such as sulphur dioxide (E220) and potassium metabisulfite (E224) which are toxic chemicals [105,106]. Mitropoulou et al. [106] tested citrus and cinnamon EOs (0.010% v/v) in low-alcohol wines prone to microbial spoilage and found that they improved wine stability, with cinnamon EO proving the most effective. However, sensory analysis showed that while citrus EO enhanced fruity notes, higher doses became pungent, and cinnamon EO introduced a sweet, warming sensation. These results suggest that EOs can be effective preservatives if carefully dosed to avoid unwanted sensory changes. In terms of spoilage prevention, Chavan and Tupe [107] demonstrated that thymol and carvacrol (from oregano EO) were more effective than sulphur dioxide against most spoilage yeasts, except for Saccharomyces cerevisiae, which is crucial for fermentation. While their impact on wine aroma was not analysed, the authors suggest that, given the low concentrations required, they are unlikely to cause significant sensory alterations. To improve EO stability and reduce volatility, García-Ríos et al. [108] explored immobilised EO formulations, where EO components were bound to silica or cellulose supports. This method enhanced their antimicrobial efficiency while minimising their effect on aroma.
Overall, these studies highlight EOs as a promising alternative for vineyard treatments and wine preservation. However, further research is needed to refine optimal dosages and application methods to maximise their benefits without affecting wine quality.
The use of EOs in agriculture, particularly in viticulture, has attracted significant interest due to its natural origin and bioactivity. However, despite their promising potential, several limitations hinder their widespread adoption. These limitations encompass issues related to their physicochemical properties, potential phytotoxic effects, impact on non-target organisms, and economic viability.
1. Issues noted include their low persistence, low solubility, volatility, and susceptibility to oxidation due to environmental factors such as sunlight, humidity, and air. These factors make their use in field conditions difficult. To avoid this problem, microencapsulation has emerged as a promising solution for improving the chemical stability and controlled release of EOs [13,109]. Microencapsulation involves enclosing the active ingredient within a biodegradable polymer capsule. Techniques such as emulsion, coacervation, interfacial polymerisation, or extrusion are used to protect the EO from external environmental influences, ensuring a gradual release of the active compound and thereby extending its protective effect on crops [92,110].The delivery method plays a crucial role in determining EOs efficacy. Direct spray application on plant leaves is the most common approach; however, it is highly influenced by temperature, sunlight, humidity, evaporation rates, and wind, which can cause uneven deposition and rapid dissipation of the solution. Additionally, during field applications, factors such as water temperature, pH, impurities, and emulsion stability in the tank can further impact EO effectiveness [111].Several studies have explored alternative delivery strategies to enhance EO performance. Ponce et al. [112] tested three EOs (clove, tea tree, and rosemary) at different concentrations on stored lettuce leaves using three delivery methods: spray, immersion, and encapsulation in lactose capsules. Their results demonstrated that only the encapsulated EOs were effective in reducing microbial populations, while the other two methods showed limited efficacy. An alternative approach suggested by Rienth et al. [78] is the vapour-phase application of EOs, which has been shown to be more effective against fungal pathogens. Continuous fumigation using EO vapour emitters could provide a sustained protective effect, addressing issues related to low stability and rain wash-off. Additionally, the reduced contact between plant tissue and EOs can reduce the phytotoxic effects of direct application [113]. However, while this method may be feasible in controlled environments, its practical implementation in open fields remains challenging due to potentially high costs and logistical difficulties. To further improve the efficacy of vapour-phase EOs, Janatova et al. [114] investigated the encapsulation of volatile EOs components in mesoporous silica supports. Their results showed that encapsulated EOs had a prolonged antifungal effect against Aspergillus niger, with higher efficacy even after 14 days compared to pure EOs. This was attributed to the slow and continuous release of active vapours, reinforcing the idea that microencapsulation improves the controlled release over time for a better efficacy.These findings highlight that microencapsulation and vapour-phase release are among the most promising strategies to enhance EO stability and efficacy in agriculture. However, further field trials on grapevine are required to confirm their practical applicability and optimize delivery methods for viticulture.
2. Another issue is their potential to cause phytotoxic effects on both target and non-target plants [54]. For example, phytotoxic effects were observed in V. vinifera plants inoculated with Plasmopara viticola when exposed to the continuous vapour of thyme and oregano EOs in a growth chamber by showing acute symptoms of phytotoxicity, such as browning of young leaves, reduced growth, and decreased photosynthesis (from 7 µmol/m2s to 1.5 µmol/m2s) and nitrogen content in the leaves [78]. However, Ghuffar et al. [115] applied thyme EO on brunches in post-harvest storage, without any phytotoxic effect reported on the fruit skin, indicating that this EO may be suitable for post-harvest application. Further, Furet-Gavallet et al. [116] noted slight phytotoxic effects after 2 days, even without fungal inoculation. Karamaouna et al. [93] evaluated the potential phytotoxicity of various EOs against V. vinifera also by testing six different EOs (lemon, orange, thyme-leaved savoury, peppermint, and lavender) at varying concentrations (0.9, 4.5, 9, 13.5, 18, 27, 36, 45, and 63 mg/mL). Their results indicated that only citrus EOs did not cause phytotoxicity, while thyme-leaved savoury EO caused slight phytotoxicity (less than 25% of the leaf surface showing symptoms) at concentrations starting from 13.5 mg/mL, peppermint EO at 9 mg/mL, and lavender EO at 27 mg/mL. Pedrotti et al. reported no phytotoxic effects of eucalyptus EO on V. vinifera, assessing that this can be suitable as alternative control for grapevine phytopathogens [41,85,87]; in contrast, Ferreira et al. [117] found that Eucalyptus globulus EO caused phytotoxicity in Vitis labrusca plants, however, they replaced it with Eucalyptus citriodora EO to avoid the problem.Phytotoxicity resulting from EO application on plants occurs through several mechanisms [54]. The monoterpenes of EOs can disrupt stomatal guard cells, leading to desiccation [118]. Additionally, the solubilisation of the plasma membrane by EOs can cause membrane depolarisation and subsequent disruption [54]. The overproduction of reactive oxygen species (ROS) can also lead to oxidative stress in plants. Indeed, some EOs rich in phenolic compounds, such as thymol, can induce oxidative stress in plants, especially during germination [119]. Bioassays, such as those performed by Karamaouna et al. [93], are crucial for determining the appropriate concentration of EOs that will achieve the desired effect without causing phytotoxic damage.
3. Despite the large number of studies evaluating the toxic properties of EOs against pests, few have focused on their effects on non-target species. Predators or parasitic organisms play an important role in pest control and are valuable allies in biological control, which makes it essential to protect them [120].From the few findings on relative selectivity (RS), it is suggested that EOs could be commercially viable, as their selectivity might allow for combining the toxicity of EOs against harmful phytophagous mites by simultaneously protecting N. californicus (i.e., 3.61 RS Citrus aurantifolia, 9.5 RS Croton rhamnifolioides, 13.57 C. sinensis, 15.6 RS Lippia sidoides, 16.27 RS Piper divaricatum, 17.38 RS C. limon, and 23.28 RS C. grewioides compared to 4.22 RS common Azamax).In another study, Kader et al. [121] tested Melissa officinalis EO on the eggs and females of two phytoseiid mites (Typhlodromips swirskii and Neoseiulus barkeri) at doses corresponding to the respective LC50 and LC90 for T. urticae: 0.38% and 1.28% for eggs, and 0.36% and 1.3% for females. They observed that the eggs were not affected by these doses, but the females showed high mortality. The LC50 dose caused a mortality rate of 62% in T. swirskii and 46% in N. barkeri, while the LC90 dose resulted in 98.5% and 64% mortality, respectively. Furthermore, at a sublethal dose (LC25) for T. urticae, the two phytoseiid mites exhibited changes in behavior, with a 100% and 30.13% reduction in food consumption for T. swirskii and N. barkeri, respectively, and a 100% and 54% reduction in egg deposition for T. swirskii and N. barkeri, respectively. These results highlight the importance of carefully selecting EOs for use in IPM programs, particularly when the distribution of EOs is associated with natural enemies. However, Momen et al. [50] tested the same doses of M. officinalis EO on the N. californicus phytoseiid mite and found low mortality (8.5% and 12.5% at LC50 and LC90 for T. urticae, respectively). In this case, M. officinalis EO was selective for the predator mite, suggesting that it could be used in combination with EO treatments without adverse effects.Moreover, the use of EOs in agriculture can significantly impact soil microbial communities, although this aspect remains underexplored and requires further investigation. Research findings on this topic are sometimes contradictory. For instance, Vokou and Liotiri [122] observed that, despite their well-known antimicrobial properties, certain ubiquitous soil microorganisms, particularly those found in Mediterranean soils. can metabolize EOs as a source of energy. In their study, they applied oregano, rosemary, mint, lavender, and Coridothymus capitatus EOs to soil samples and measured soil respiration. The increase in respiration rates suggested that these compounds were actively degraded by the microbial community, supporting the idea that EOs do not accumulate in the environment but are instead integrated into soil microbial metabolism. However, other studies raise concerns about potential adverse effects. Thiele-Bruhn et al. [123] investigated the impact of thymol and carvacrol (two common EO components) on soil microbial activity and found that these monoterpenes can persist in the soil for up to one year, remaining bioavailable to soil microorganisms. Their toxic effects were shown to be dose- and time-dependent, with carvacrol exerting a stronger inhibitory effect on soil enzymatic activity than thymol. Specifically, enzyme inhibition levels of 10%–25% can be achieved with less than 1 µg to several grams per grams of soil. Interestingly, at lower concentrations, some stimulatory effects on enzyme activity were also noted. These findings suggest that while some soil microbial communities may adapt to and even benefit from EOs applications, others may experience functional disruptions, particularly at high doses or with prolonged exposure. Given these contrasting effects, further research is necessary to fully assess the risks and benefits of large-scale EO applications in agriculture. Long-term field studies are needed to determine whether EO treatments can be sustainably integrated into agroecosystems without compromising soil health and functionality.
4. The use of EOs in viticulture and, more broadly, in agriculture is attracting increasing interest as a sustainable alternative to conventional pesticides. However, optimal dosages and specific formulations remain far from standardised, as they can vary significantly based on multiple factors. These include the chemical composition of the EO itself, since different plant sources yield distinct compositions [11], the target species, and the application method. For instance, vapourisation techniques have been found to be more effective against fungal pathogens [78], and citrus, basil, thyme, and oregano EOs seems to be more effective on fungi [31].One of the key challenges in agricultural EO application, as noted by Dunan et al. [124], is the lack of optimised formulations that can adequately protect the volatile organic compounds (VOCs) present in EOs. To address this issue, they tested various formulations of artemisia and rosemary EOs. Their findings revealed that nanoemulsions exhibited the highest toxicity against Bemisia tabaci, followed by formulations prepared using natural deep eutectic solvents and pure EOs, while atomized powder formulations proved ineffective against the insect. Another major limitation is the significant discrepancy between laboratory and field conditions. While many laboratory experiments demonstrate promising results, the required doses in open-field applications often differ considerably from those tested in controlled environments [125]. Indeed, no universally accepted dosage has been established, as different studies employ widely varying concentrations for similar purposes. For example, El-Abbasy et al. [34] applied 2 and 4 mL/L of Thyme EO to combat Botrytis cinerea in post-harvest conservation, while Gebel and Magurno [126] used significantly higher doses, ranging from 200 to 500 mL/L for similar fungal control applications. This inconsistency highlights the urgent need for further research to determine effective, standardised dosages tailored to real-world agricultural settings.These findings emphasize the necessity of refining EO formulation strategies to improve their stability, bioavailability, and efficacy in agricultural applications. Establishing standardized doses and application methods is essential to fully harness the potential of EOs as viable, environmentally friendly alternatives to synthetic pesticides.
5. Due to their chemical structure, EO components are subject to cyclisation, oxidation, isomerisation, or dehydrogenation, triggered by enzymatic and/or chemical reaction. The volatility and thermolability of terpenoids leads them to be casually oxidised or hydrolysed (i.e., autoxidation deterioration) [24]. Light and temperature play an important role in their stability. Possible alterations could lead to the presence of unpleasant odours (such as the aromatic monoterpene p-cymene) which would affect the grapes during storage.
6. EOs can have cytotoxic effects on eukaryotic cells leading to ion homeostasis disfunction. This cytotoxic property is employed in chemotherapeutic usages against viruses or bacteria. Toxic consequences of hazard-like irritation, percutaneous absorption, and toxicity to organ system restrict their medicinal uses [127]. It was found that Mentha pulegium EO can provoke interior bleeding or injury of the lungs in canids [128]. In light of these considerations, it would be important to undertake studies on their implications for sedentary fauna.
7. The last concern regards economic viability. Currently, biological compounds are generally less cost-effective than chemical ones, due to the expensive extraction methods required and the need for large areas of land with specific climatic conditions to cultivate the plant sources. Additionally, harvesting must occur at the right time to ensure the quality of the product [82].The pesticide market is highly competitive, and for a product to succeed, it must be cost-competitive with other available pesticides, even if it is environmentally friendly and sustainable. Currently, the EO market is dominated by the flavouring and fragrance industries, while their application as pesticides remains a niche sector [68,129]. According to International Trade Centre statistics on yearly imported unit values in the USA (expressed in USD/kg), the 2024 prices for key EOs are as follows: orange EO ($13/kg), lemon EO ($27/kg), mint EO ($28/kg), peppermint EO ($28/kg), eucalyptus EO ($11/kg), rosemary EO ($33/kg), citronella EO ($11/kg), lemongrass EO ($16/kg), and lime EO ($79/kg). In contrast, some widely used synthetic agricultural products have significantly lower prices, such as copper sulphate ($3.06/kg), sulphur ($1.62/kg), and copper hydroxide ($11/kg) [130]. Despite their potential benefits, the relatively high cost of EOs compared to conventional pesticides remains a major limitation to their large-scale adoption in agriculture. To enhance their competitiveness, further research is needed to optimise extraction methods, improve formulation techniques, and develop cost-effective production strategies. Moreover, through well-designed informational programs aimed at educating farmers on the benefits of environmentally friendly products, such as promoting biodiversity, conservation, and the avoidance of resistance development, coupled with financial support from institutions, farmers may be more encouraged to adopt these alternatives to conventional products.
While EOs present significant advantages for sustainable pest and disease management in viticulture, their limitations must be addressed to maximise their utility. Future research should focus on developing more stable formulations, refining application techniques, and conducting comprehensive studies on their interactions with plants, pests, and non-target organisms. Moreover, detailed assessments of their impact on grape quality and potential modifications in the final product are essential to ensure their integration into modern viticulture. A holistic approach, combining scientific innovation, farmer education, and institutional support, can pave the way for the successful implementation of EOs as key components of environmentally friendly IPM programs.
Like other agricultural sectors, viticulture faces significant challenges related to climate change, pest resistance, and sustainability. The need to reduce reliance on synthetic products while preserving grape quality and yield has led to the exploration of alternative solutions.
Essential oils have shown promising potential in managing key fungal diseases and pests that affect grapevines, both in the vineyard and during post-harvest storage. Additionally, their ability to minimise decay and weight loss during storage enhances their value in viticulture. However, several obstacles remain, including inconsistencies in efficacy due to variations in EO composition, application techniques, and environmental influences. The rapid breakdown of these natural compounds, their possible phytotoxic effects, and regulatory constraints must also be addressed before they can be widely adopted.
Despite these limitations, existing research indicates that EOs could contribute significantly to sustainable vineyard management. Further investigations are necessary to refine their application methods, evaluate long-term impacts on grape production and wine quality, and develop standardized formulations that ensure reliable performance. Additionally, future studies should explore complementary strategies, such as combining EOs with other natural substances or advanced delivery systems to enhance their stability and effectiveness. By closing these knowledge gaps, EOs have the potential to become a practical, environmentally friendly solution for vineyard protection, supporting both ecological sustainability and the economic interests of wine producers.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Eleonora Cataldo conceived and planned the review structure. Pamela Lippi and Eleonora Cataldo wrote the original draft manuscript. Aleš Eichmeier, Giovan Battista Mattii, and Sergio Puccioni contributed to the bibliographic search, discussion, and revision. Eleonora Cataldo supervised the work. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analysed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Blázquez M. Role of natural essential oils in sustainable agriculture and food preservation. J Sci Res Rep. 2014;3(14):1843–60. doi:10.9734/JSRR/2014/11376. [Google Scholar] [CrossRef]
2. Isman MB, Miresmailli S, Machial C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev. 2011;10(2):197–204. doi:10.1007/s11101-010-9170-4. [Google Scholar] [CrossRef]
3. Zanellato M, Masciarelli E, Casorri L, Boccia P, Sturchio E, Pezzella M, et al. The essential oils in agriculture as an alternative strategy to herbicides: a case study. Int J Environ Health. 2009;3(2):198. doi:10.1504/IJENVH.2009.024878. [Google Scholar] [CrossRef]
4. Ammad F, Bentoumi Y, Gharnaout ML, Zebib B, Merah O. Inhibitory effect of eucalyptus globulus essential oil against Botryosphaeria dothidea and Fomitiporia mediterranea fungi causing wood diseases in viticulture. Vegetos. 2024;12(3):71. doi:10.1007/s42535-024-01081-1. [Google Scholar] [CrossRef]
5. Ootani MA, Aguiar RWDS, Ramos ACC, Brito DR, Silva JBD, Cajazeira JP. Utilização de Óleos Essenciais na Agricultura. J Biotechnol Biodivers. 2013;4(2):162–74. doi:10.20873/jbb.uft.cemaf.v4n2.ootani. [Google Scholar] [CrossRef]
6. Tucker S, Dumitriu (Gabur) GD, Teodosiu C. Pesticides identification and sustainable viticulture practices to reduce their use: an overview. Molecules. 2022;27(23):8205. doi:10.3390/molecules27238205. [Google Scholar] [PubMed] [CrossRef]
7. La Guerche S, Dauphin B, Pons M, Blancard D, Darriet P. Characterization of some mushroom and earthy off-odors microbially induced by the development of rot on grapes. J Agric Food Chem. 2006;54(24):9193–200. doi:10.1021/jf0615294. [Google Scholar] [PubMed] [CrossRef]
8. Pedrotti C, Ribeiro RTS, Schwambach J. Control of postharvest fungal rots in grapes through the use of Baccharis trimera and Baccharis dracunculifolia essential oils. Crop Prot. 2019;125(10):104912. doi:10.1016/j.cropro.2019.104912. [Google Scholar] [CrossRef]
9. Liu WT, Chu CL, Zhou T. Thymol and acetic acid vapors reduce postharvest brown rot of apricots and plums. Hort Sci. 2002;37(1):151–6. doi:10.21273/HORTSCI.37.1.151. [Google Scholar] [CrossRef]
10. Sharifi-Rad J, Sureda A, Tenore GC, Daglia M, Sharifi-Rad M, Valussi M, et al. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules. 2017;22(1):70. doi:10.3390/molecules22010070. [Google Scholar] [PubMed] [CrossRef]
11. Jugreet BS, Suroowan S, Rengasamy RRK, Mahomoodally MF. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci Technol. 2020;101:89–105. doi:10.1016/j.tifs.2020.04.025. [Google Scholar] [CrossRef]
12. Luque CMD, Jiménez-Carmona MM, Fernández-Pérez V. Towards more rational techniques for the isolation of valuable essential oils from plants. TrAC Trends Anal Chem. 1999;18(11):708–16. doi:10.1016/S0165-9936(99)00177-6. [Google Scholar] [CrossRef]
13. Benelli G, Pavoni L, Zeni V, Ricciardi R, Cosci F, Cacopardo G, et al. Developing a highly stable carlina acaulis essential oil nanoemulsion for managing Lobesia botrana. Nanomater. 2020;10(9):1867. doi:10.3390/nano10091867. [Google Scholar] [PubMed] [CrossRef]
14. Elgayyar M, Draughon FA, Golden DA, Mount JR. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot. 2001;64(7):1019–24. doi:10.4315/0362-028X-64.7.1019. [Google Scholar] [PubMed] [CrossRef]
15. Masango P. Cleaner production of essential oils by steam distillation. J Clean Prod. 2005;13(8):833–9. doi:10.1016/j.jclepro.2004.02.039. [Google Scholar] [CrossRef]
16. Yap PSX, Yiap BC, Ping HC, Lim SHE. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J. 2014;8(1):6–14. doi:10.2174/1874285801408010006. [Google Scholar] [PubMed] [CrossRef]
17. Carson CF, Hammer KA. Chemistry and bioactivity of essential oils. In: Thormar H, editor. Lipids and essential oils as antimicrobial agents. 1st ed. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2011. p. 203–38. doi:10.1002/9780470976623.ch9. [Google Scholar] [CrossRef]
18. Guenther E. The essential oils. In: History-origin in plants-production-analysis. Vol. 1. Atlantic Beach, FL, USA: Read Books Ltd.; 1948. [Google Scholar]
19. Bauer K, Garbe D, Surburg H. Common fragrance and flavor materials: preparation, properties and uses. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2008. [Google Scholar]
20. Ibrahim MA, Kainulainen P, Aflatuni A. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: with special reference to limonene and its suitability for control of insect pests. Agric Food Sci. 2001;10(3):243–59. doi:10.23986/afsci.5697. [Google Scholar] [CrossRef]
21. Regnault-Roger C, Vincent C, Arnason JT. Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol. 2012;57(1):405–24. doi:10.1146/annurev-ento-120710-100554. [Google Scholar] [PubMed] [CrossRef]
22. Sadgrove N, Jones G. A contemporary introduction to essential oils: chemistry, bioactivity and prospects for australian agriculture. Agriculture. 2015;5(1):48–102. doi:10.3390/agriculture5010048. [Google Scholar] [CrossRef]
23. Karamanoli K, Ainalidou A, Bouzoukla F, Vokou D. Decomposition profiles of leaf essential oils in the soil environment. Ind Crops Prod. 2018;124:397–401. doi:10.1016/j.indcrop.2018.07.082. [Google Scholar] [CrossRef]
24. Turek C, Stintzing FC. Stability of essential oils: a review. Compr Rev Food Sci Food Saf. 2013;12(1):40–53. doi:10.1111/1541-4337.12006. [Google Scholar] [CrossRef]
25. Kuttan R, Liju VB. Safety evaluation of essential oils. In: Hashemi SMB, Khaneghah AM, Sant AS, editors. Essential oils in food processing: chemistry, safety and applications. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2017. p. 339–58. doi:10.1002/9781119149392.ch12. [Google Scholar] [CrossRef]
26. Mahmoud SS, Croteau RB. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002;7(8):366–73. doi:10.1016/S1360-1385(02)02303-8. [Google Scholar] [PubMed] [CrossRef]
27. Mutlu-Ingok A, Devecioglu D, Dikmetas DN, Karbancioglu-Guler F, Capanoglu E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules. 2020;25(20):4711. doi:10.3390/molecules25204711. [Google Scholar] [PubMed] [CrossRef]
28. Digilio MC, Mancini E, Voto E, De Feo V. Insecticide activity of mediterranean essential oils. J Plant Interact. 2008;3(1):17–23. doi:10.1080/17429140701843741. [Google Scholar] [CrossRef]
29. Machado CC, Silvestre WP, Touguinha LBA, Pauletti GF, Schwambach J. Use of Rosmarinus officinalis essential oil and its fractions in the alternative control of grapevine phytopathogens. Braz Arch Biol Technol. 2024;67(4):e24240022. doi:10.1590/1678-4324-2024240022. [Google Scholar] [CrossRef]
30. Eslahi H, Fahimi N, Sardarian A. Chemical composition of essential oils. In: Hashemi SMB, Khaneghah AM, Sant AS, editors. Essential oils in food processing: chemistry, safety and applications. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2017. p. 119–71. doi:10.1002/9781119149392.ch4. [Google Scholar] [CrossRef]
31. Catani L, Grassi E, Cocozza MA, Guidi L, Sandulli R, Manachini B, et al. Essential oils and their applications in agriculture and agricultural products: a literature analysis through VOSviewer. Biocatal Agric Biotechnol. 2022;45(1):102502. doi:10.1016/j.bcab.2022.102502. [Google Scholar] [CrossRef]
32. Miresmailli S, Isman MB. Efficacy and persistence of rosemary oil as an acaricide against twospotted spider mite (Acari: Tetranychidae) on greenhouse tomato. J Econ Entomol. 2006;99(6):2015–23. [Google Scholar] [PubMed]
33. Raveau R, Fontaine J, Lounès-Hadj SA. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: a review. Foods. 2020;9(3):365. doi:10.3390/foods9030365. [Google Scholar] [PubMed] [CrossRef]
34. El-Abbasy UK, Abdel-Hameed MA, Hatterman-Valenti HM, El-Shereif AR, Abd El-Khalek AF. Effectiveness of oregano and thyme essential oils as alternatives for sulfur dioxide in controlling decay and gray mold and maintaining quality of ‘Flame Seedless’ table grape (Vitis vinifera L.) during cold storage. Agronomy. 2023;13(12):3075. doi:10.3390/agronomy13123075. [Google Scholar] [CrossRef]
35. Oliva MM, Carezzano ME, Giuliano M, Daghero J, Zygadlo J, Bogino P, et al. Antimicrobial activity of essential oils of thymus vulgaris and origanum vulgare on phytopathogenic strains isolated from soybean. Plant Biol. 2015;17(3):758–65. doi:10.1111/plb.12282. [Google Scholar] [PubMed] [CrossRef]
36. Vitoratos A, Bilalis D, Karkanis A, Efthimiadou A. Antifungal activity of plant essential oils against Botrytis cinerea, penicillium italicum and penicillium digitatum. Not Bot Horti Agrobot Cluj-Napoca. 2013;41(1):86–92. [Google Scholar]
37. Maia AJ, Oliveira JSB, Schwan-Estrada KRF, Faria CMR, Batista AF, Costa WF, et al. The control of isariopsis leaf spot and downy mildew in grapevine cv. Isabel with the essential oil of lemon grass and the activity of defensive enzymes in response to the essential oil. Crop Prot. 2014;63:57–67. doi:10.1016/j.cropro.2014.05.005. [Google Scholar] [CrossRef]
38. Lee LT, Martinazzo AP, Teodoro CEDS, Berbert PA. Potential use of lemongrass essential oil as fungicide against Aspergillus brasiliensis and as post-harvest protectant of wheat. Acta Sci Biol Sci. 2021;43:e56763. doi:10.4025/actascibiolsci.v43i1.56763. [Google Scholar] [CrossRef]
39. Eke P, Adamou S, Fokom R, Dinango NV, Tsouh FPV, Nana WL, et al. Arbuscular mycorrhizal fungi alter antifungal potential of lemongrass essential oil against Fusarium solani, causing root rot in common bean (Phaseolus vulgaris L.). Heliyon. 2020;6(12):e05737. doi:10.1016/j.heliyon.2020.e05737. [Google Scholar] [PubMed] [CrossRef]
40. El-Mohamedy RSR, El-Gamal NG, Bakeer ART. Application of chitosan and essential oils as alternatives fungicides to control green and blue moulds of citrus fruits. Int J Curr Microbiol Appl Sci. 2015;4:629–43. [Google Scholar]
41. Pedrotti C, Marcon ÂR, Sérgio, Echeverrigaray L, Ribeiro RTS, Schwambach J. Essential oil as sustainable alternative for diseases management of grapes in postharvest and in vineyard and its influence on wine. J Environ Sci Health Part B. 2020;56(1):73–81. doi:10.1080/03601234.2020.1838827. [Google Scholar] [PubMed] [CrossRef]
42. Dhakad AK, Pandey VV, Beg S, Rawat JM, Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J Sci Food Agric. 2018;98(3):833–48. doi:10.1002/jsfa.8600. [Google Scholar] [PubMed] [CrossRef]
43. Tomazoni EZ, Pauletti GF, da Silva RRT, Moura S, Schwambach J. In vitro and in vivo activity of essential oils extracted from Eucalyptus staigeriana, Eucalyptus globulus and Cinnamomum camphora against Alternaria solani Sorauer causing early blight in tomato. Sci Hortic. 2017;223(2):72–7. doi:10.1016/j.scienta.2017.04.033. [Google Scholar] [CrossRef]
44. Peschiutta ML, Pizzolitto RP, Ordano MA, Zaio YP, Zygadlo JA. Laboratory evaluation of insecticidal activity of plant essential oils against the vine mealybug, Planococcus ficus. VITIS—J Grapevine Res. 2017;56(2):79–83. doi:10.5073/VITIS.2017.56.79-83. [Google Scholar] [CrossRef]
45. Tacoli F, Bell VA, Cargnus E, Pavan F. Insecticidal activity of natural products against vineyard mealybugs (Hemiptera: Pseudococcidae). Crop Prot. 2018;111:50–7. doi:10.1016/j.cropro.2018.04.020. [Google Scholar] [CrossRef]
46. El Khetabi A, Ezrari S, El Ghadraoui L, Tahiri A, Ait Haddou L, Belabess Z, et al. In Vitro and in vivo antifungal activities of nine commercial essential oils against brown rot in apples. Horticulturae. 2021;7(12):545. doi:10.3390/horticulturae7120545. [Google Scholar] [CrossRef]
47. Doğu DM, Zobar D. Effects of some plant essential oils against Botrytis cinerea and Tetranychus urticae on Grapevine. Türk Tarım Ve Doğa Bilim Derg. 2014;1:1268–73. [Google Scholar]
48. Hsouna AB, Touj N, Hammami I, Dridi K, Al-Ayed AS, Hamdi N. Chemical composition and in vivo efficacy of the essential oil of Mentha piperita L. in the suppression of crown gall disease on tomato plants. J Oleo Sci. 2019;68(5):419–26. doi:10.5650/jos.ess18261. [Google Scholar] [PubMed] [CrossRef]
49. La Torre A, Mandalà C, Pezza L, Caradonia F, Battaglia V. Evaluation of essential plant oils for the control of Plasmopara viticola. J Essent Oil Res. 2014;26(4):282–91. doi:10.1080/10412905.2014.889049. [Google Scholar] [CrossRef]
50. Momen F, Rahman HA, Samour E, Aly S, Fahim S. Acaricidal activity of melissa officinalis oil and its formulation on Tetranychus urticae and the predatory mite Neoseiulus californicus (Acari: Tetranychidae and Phytoseiidae). Acta Phytopathol Et Entomol Hung. 2014;49(1):95–115. doi:10.1556/APhyt.49.2014.1.10. [Google Scholar] [CrossRef]
51. Bastas KK. Management of Erwinia amylovora by potential bio-pesticides in vitro and in vivo conditions. Turk J Agric Food Sci Technol. 2020;8:38–45. doi:10.24925/turjaf.v8isp1.38-45.3933. [Google Scholar] [CrossRef]
52. Elsayed MI, Al-Qurashi AD, Almasaudi NM, Abo-Elyousr KAM. Efficacy of essential oils against gray mold and effect on fruit quality during cold storage in table grapes. S Afr J Bot. 2022;146(4):481–90. doi:10.1016/j.sajb.2021.11.046. [Google Scholar] [CrossRef]
53. Kowalska J, Tyburski J, Krzymińska J, Jakubowska M. Cinnamon powder: an in vitro and in vivo evaluation of antifungal and plant growth promoting activity. Eur J Plant Pathol. 2020;156(1):237–43. doi:10.1007/s10658-019-01882-0. [Google Scholar] [CrossRef]
54. Werrie PY, Durenne B, Delaplace P, Fauconnier ML. Phytotoxicity of essential oils: opportunities and constraints for the development of biopesticides. A Rev Foods. 2020;9(9):1291. doi:10.3390/foods9091291. [Google Scholar] [PubMed] [CrossRef]
55. Das S, Chaudhari AK, Singh VK, Dwivedy AK, Dubey NK. Angelica archangelica essential oil loaded chitosan nanoemulsion as edible coating for preservation of table grape fruit against Botrytis cinerea contamination and storage quality deterioration. Postharvest Biol Technol. 2023;205(4):112482. doi:10.1016/j.postharvbio.2023.112482. [Google Scholar] [CrossRef]
56. Bajpai VK, Baek KH, Kang SC. Control of Salmonella in foods by using essential oils: a review. Food Res Int. 2012;45(2):722–34. doi:10.1016/j.foodres.2011.04.052. [Google Scholar] [CrossRef]
57. Calo JR, Crandall PG, O.’Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems—a review. Food Control. 2015;54(4):111–9. doi:10.1016/j.foodcont.2014.12.040. [Google Scholar] [CrossRef]
58. Sharma A, Abrahamian P, Carvalho R, Choudhary M, Paret ML, Vallad GE, et al. Future of bacterial disease management in crop production. Annu Rev Phytopathol. 2022;60(1):259–82. doi:10.1146/annurev-phyto-021621-121806. [Google Scholar] [PubMed] [CrossRef]
59. Chalkos D, Kadoglidou K, Karamanoli K, Fotiou C, Pavlatou-Ve AS, Eleftherohorinos IG, et al. Mentha spicata and Salvia fruticosa composts as soil amendments in tomato cultivation. Plant Soil. 2010;332(1–2):495–509. doi:10.1007/s11104-010-0315-4. [Google Scholar] [CrossRef]
60. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101(1):372–8. doi:10.1016/j.biortech.2009.07.048. [Google Scholar] [PubMed] [CrossRef]
61. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018(1):6860271. doi:10.1155/2018/6860271. [Google Scholar] [PubMed] [CrossRef]
62. De Mastro G, El Mahdi J, Ruta C. Bioherbicidal potential of the essential oils from mediterranean Lamiaceae for weed control in organic farming. Plants. 2021;10(4):818. doi:10.3390/plants10040818. [Google Scholar] [PubMed] [CrossRef]
63. Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem. 2013;61(46):10835–47. doi:10.1021/jf403496k. [Google Scholar] [PubMed] [CrossRef]
64. Yasin M, Younis A, Javed T, Akram A, Ahsan M, Shabbir R, et al. River tea tree oil: composition, antimicrobial and antioxidant activities, and potential applications in agriculture. Plants. 2021;10(10):2105. doi:10.3390/plants10102105. [Google Scholar] [PubMed] [CrossRef]
65. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC 2009. [cited 2025 Mar 19]. Available from: https://www.legislation.gov.uk/eur/2009/1107. [Google Scholar]
66. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007 2018. [cited 2025 Mar 19]. Available from: https://www.legislation.gov.uk/eur/2007/834/introduction. [Google Scholar]
67. Code of Federal Regulations. Section 152.25—exemptions for pesticides of a character not requiring FIFRA regulation; 2013. [cited 2025 Mar 19]. Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-152/subpart-B/section-152.25. [Google Scholar]
68. Isman MB. Pesticides based on plant essential oils: phytochemical and practical considerations. In: Jeliazkov VD, Cantrell CL, editors. Medicinal and aromatic crops: production, phytochemistry, and utilization. Vol. 1218. Washington, DC, USA: American Chemical Society; 2016. p. 13–26. doi:10.1021/bk-2016-1218.ch002. [Google Scholar] [CrossRef]
69. APVMA. Guideline for the regulation of biological agricultural products | Australian pesticides and veterinary medicines authority; 2022. [cited 2025 Mar 19]. Available from: https://www.apvma.gov.au/registrations-and-permits/data-requirements/agricultural-data-guidelines/biological. [Google Scholar]
70. APVMA. Chemistry and manufacture (Part 2) | Australian pesticides and veterinary medicines authority; 2014. [cited 2025 Mar 19]. Available from: https://www.apvma.gov.au/registrations-and-permits/data-requirements/agricultural-data-guidelines/chemistry-manufacture-part-2. [Google Scholar]
71. Sharma M, Haider SZ, Andola H, Purohit V. Essential oils as green pesticides: for sustainable agriculture. Res J Pharm Bio Chem Sci. 2011;2:100–6. [Google Scholar]
72. Can-Başer KH. Some thoughts on the future of essential oils. J Essent Oil Res. 2024;36(6):525–31. doi:10.1080/10412905.2024.2432743. [Google Scholar] [CrossRef]
73. Kookana RS, Baskaran S, Naidu R. Pesticide fate and behaviour in Australian soils in relation to contamination and management of soil and water: a review. Soil Res. 1998;36(5):715. doi:10.1071/S97109. [Google Scholar] [CrossRef]
74. Georghiou GP. The evolution of resistance to pesticides. Annu Rev Ecol Syst. 1972;3(1):133–68. doi:10.1146/annurev.es.03.110172.001025. [Google Scholar] [CrossRef]
75. Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4(3):148. doi:10.3389/fpubh.2016.00148. [Google Scholar] [PubMed] [CrossRef]
76. Maes C, Meersmans J, Lins L, Bouquillon S, Fauconnier ML. Essential oil-based bioherbicides: human health risks analysis. Int J Mol Sci. 2021;22(17):9396. doi:10.3390/ijms22179396. [Google Scholar] [PubMed] [CrossRef]
77. Burandt QC, Deising HB, von Tiedemann A. Further limitations of synthetic fungicide use and expansion of organic agriculture in europe will increase the environmental and health risks of chemical crop protection caused by copper-containing fungicides. Environ Toxicol Chem. 2024;43(1):19–30. doi:10.1002/etc.5766. [Google Scholar] [PubMed] [CrossRef]
78. Rienth M, Crovadore J, Ghaffari S, Lefort F. Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis Vinifera) and primes plant immunity mechanisms. PLoS One. 2019;14(9):e0222854. doi:10.1371/journal.pone.0222854. [Google Scholar] [PubMed] [CrossRef]
79. Sambucci OS, Alston JM, Fuller KB. The costs of powdery mildew management in grapes and the value of resistant varieties: evidence from California. Robert Mondavi Inst Cent Wine Econ. 2014;1402. Available from: https://vinecon.ucdavis.edu/sites/g/files/dgvnsk15031/files/inline-files/CWE1402.pdf. [Google Scholar]
80. Kölbl C, Diedrich M, Ellingen E, Duschek F, Selim M, Berkelmann-Löhnertz B. Remote detection of fungal pathogens in viticulture using laser-induced fluorescence: an experimental study on infected potted vines. Front Hortic. 2023;2:1185468. doi:10.3389/fhort.2023.1185468. [Google Scholar] [CrossRef]
81. Fandino ACA, Vigneron N, Alfonso E, Burdet JP, Remolif E, Cattani AM, et al. Priming grapevines with oregano essential oil vapour results in a metabolomic shift eliciting resistance against downy mildew. BMC Plant Biol. 2024;24(1):1180. doi:10.1186/s12870-024-05875-y. [Google Scholar] [PubMed] [CrossRef]
82. Gupta I, Singh R, Muthusamy S, Sharma M, Grewal K, Singh HP, et al. Plant essential oils as biopesticides: applications, mechanisms, innovations, and constraints. Plants. 2023;12(16):2916. doi:10.3390/plants12162916. [Google Scholar] [PubMed] [CrossRef]
83. Boso S, Gago P, Santiago JL, de la Fuente M, Martínez MC. Factors affecting the vineyard populational diversity of plasmopara viticola. Plant Pathol J. 2019;35(2):125–36. doi:10.5423/PPJ.OA.09.2018.0194. [Google Scholar] [PubMed] [CrossRef]
84. European Commission. Copper in topsoils; 2018. [cited 2025 Mar 19]. Available from: https://esdac.jrc.ec.europa.eu/themes/copper-topsoils#:~:text=The%20European%20Commission%20has%20adopted,copper%20use%20in%20agricultural%20soils. [Google Scholar]
85. Pedrotti C, Franzoi C, Sandi R, Grohs D, Schwambach J. Eucalyptus staigeriana essential oil can control downy mildew in grapevine. Pesqui Agropecuária Gaúcha. 2021;28(1):1–14. doi:10.36812/pag.20222811-14. [Google Scholar] [CrossRef]
86. Steel CC, Blackman JW, Schmidtke LM. Grapevine bunch rots: impacts on wine composition, quality, and potential procedures for the removal of wine faults. J Agric Food Chem. 2013;61(22):5189–206. doi:10.1021/jf400641r. [Google Scholar] [PubMed] [CrossRef]
87. Pedrotti C, Marcon Â.R, Delamare APL, Echeverrigaray S, da Silva RRT, Schwambach J. Alternative control of grape rots by essential oils of two eucalyptus species. J Sci Food Agric. 2019;99(14):6552–61. doi:10.1002/jsfa.9936. [Google Scholar] [PubMed] [CrossRef]
88. Cocco A, Pacheco SVC, Benelli G, Botton M, Lucchi A, Lentini A. Sustainable management of the vine mealybug in organic vineyards. J Pest Sci. 2021;94(2):153–85. doi:10.1007/s10340-020-01305-8. [Google Scholar] [CrossRef]
89. Di Giovanni F, Ricciardi R, Loni A, Scaramozzino PL, Benelli G, Lucchi A. Back to the wild: the parasitoid community of Lobesia botrana (Lepidoptera: Tortricidae) in a grapevine-free natural environment. Insects. 2022;13(7):627. doi:10.3390/insects13070627. [Google Scholar] [PubMed] [CrossRef]
90. Born FS, da Camara CAG, de Melo JPR, de Moraes MM. Acaricidal property of the essential oil from Lippia gracilis against Tetranychus urticae and a natural enemy, Neoseiulus californicus, under greenhouse conditions. Exp Appl Acarol. 2018;75(4):491–502. doi:10.1007/s10493-018-0286-3. [Google Scholar] [PubMed] [CrossRef]
91. Pertot I, Caffi T, Rossi V, Mugnai L, Hoffmann C, Grando MS, et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017;97(3):70–84. doi:10.1016/j.cropro.2016.11.025. [Google Scholar] [CrossRef]
92. Malešević VK, Vaštag Ž, Radulović-Popović L, Senka MP, Peričin-Starčević I. Chapter 12—microencapsulation technology and essential oil pesticides for food plant production. In: Preedy VR, editor. Essential oils in food preservation, flavor and safety. Cambridge, MA, USA: Academic Press; 2016. p. 123–9. doi:10.1016/B978-0-12-416641-7.00012-2. [Google Scholar] [CrossRef]
93. Karamaouna F, Kimbaris A, Michaelakis A, Papachristos D, Polissiou M, Papatsakona P, et al. Insecticidal activity of plant essential oils against the vine mealybug, Planococcus ficus. J Insect Sci. 2013;13(142):142. doi:10.1673/031.013.14201. [Google Scholar] [CrossRef]
94. Delbac L, Thiéry D. Damage to grape flowers and berries by Lobesia botrana larvae (Denis & Schiffernüller) (Lepidoptera: Tortricidaeand relation to larval age. Aust J Grape Wine Res. 2016;22(2):256–61. doi:10.1111/ajgw.12204. [Google Scholar] [CrossRef]
95. Cozzi G, Pascale M, Perrone G, Visconti A, Logrieco A. Effect of Lobesia botrana damages on black aspergilli rot and ochratoxin a content in grapes. Int J Food Microbiol. 2006;111:S88–92. doi:10.1016/j.ijfoodmicro.2006.03.012. [Google Scholar] [PubMed] [CrossRef]
96. Avgın SS, Karaman Ş, Bahadıroğlu C. Laboratory assays of plant extracts against Lobesia botrana (Denis & Schiffermüller) (Lepidoptera: Tortricidae) larvae. J Entomol Sci. 2008;43(4):423–5. doi:10.18474/0749-8004-43.4.423. [Google Scholar] [CrossRef]
97. Fidelis SM, Câmara CG, Barbosa MV, Ramos MJP, Martins MM. Bioactivity of essential oils for the management of Tetranychus urticae Koch and selectivity on its natural enemy Neoseiulus californicus (McGregora promising combination for agroecological systems. Acarologia. 2021;61(3):564–76. doi:10.24349/acarologia/20214451. [Google Scholar] [CrossRef]
98. Badii MH, Hernández-Ortiz E, Flores AE, Landeros J. Prey stage preference and functional response of Euseius hibisci to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp Appl Acarol. 2004;34(3):263–73. doi:10.1023/B:APPA.0000049222.65883.77. [Google Scholar] [CrossRef]
99. Kaya O, Yilmaz T, Ates F, Kustutan F, Hatterman-Valenti H, Hajizadeh HS, et al. Improving organic grape production: the effects of soil management and organic fertilizers on biogenic amine levels in Vitis vinifera cv., royal grapes. Chem Biol Technol Agric. 2024;11(1):38. doi:10.1186/s40538-024-00564-2. [Google Scholar] [CrossRef]
100. Guerra ICD, de Oliveira PDL, Santos MMF, Lúcio ASSC, Tavares JF, Barbosa-Filho JM, et al. The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov Food Sci Emerg Technol. 2016;34:112–21. doi:10.1016/j.ifset.2016.01.008. [Google Scholar] [CrossRef]
101. Dehestani-Ardakani M, Mostofi Y. Postharvest application of chitosan and Thymus essential oil increase quality of the table grape cv. ‘Shahroudi’. J Hortic Postharvest Res. 2019;2:1015. doi:10.22077/jhpr.2018.1444.1015. [Google Scholar] [CrossRef]
102. Mei L, Shi L, Song X, Liu S, Cheng Q, Zhu K, et al. Characterization of carboxymethyl cellulose films incorporated with chinese fir essential oil and their application to quality improvement of shine muscat grape. Coatings. 2021;11(1):97. doi:10.3390/coatings11010097. [Google Scholar] [CrossRef]
103. Kavoosi B, Dastyaran M, Zade BM, Saeidi K. Effect of cumin and lemon grass essential oils on physicochemical traits of Askari table grape. Agric Commun. 2014;2(1):X-X. [Google Scholar]
104. Liu Y, Weng L, Dai S, Liu M, Guo X, Meng F. Alginate-based edible coating with magnoliae essential oil/β-cyclodextrin inclusion complex for Kyoho grape preservation. LWT. 2024;210(18):116874. doi:10.1016/j.lwt.2024.116874. [Google Scholar] [CrossRef]
105. Ferrer-Gallego R, Puxeu M, Martín L, Nart E, Hidalgo C, Andorrà I, et al. Microbiological, physical, and chemical procedures to elaborate high-quality SO2-free wines. In: Jordão AM, Cosme F, editors. Grapes and wines-advances in production, processing, analysis and valorization. London, UK: IntechOpen; 2017. doi:10.5772/intechopen.71627. [Google Scholar] [CrossRef]
106. Mitropoulou G, Nikolaou A, Santarmaki V, Sgouros G, Kourkoutas Y. Citrus medica and cinnamomum zeylanicum essential oils as potential biopreservatives against spoilage in low alcohol wine products. Foods. 2020;9(5):577. doi:10.3390/foods9050577. [Google Scholar] [PubMed] [CrossRef]
107. Chavan PS, Tupe SG. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control. 2014;46(1):115–20. doi:10.1016/j.foodcont.2014.05.007. [Google Scholar] [CrossRef]
108. García-Ríos E, Ruiz-Rico M, Guillamón JM, Pérez-Esteve É, Barat JM. Improved antimicrobial activity of immobilised essential oil components against representative spoilage wine microorganisms. Food Control. 2018;94(3):177–86. doi:10.1016/j.foodcont.2018.07.005. [Google Scholar] [CrossRef]
109. Veiga RDSD, Aparecida SBR, Corso MP, Canan C. Essential oils microencapsulated obtained by spray drying: a review. J Essent Oil Res. 2019;31(6):457–73. doi:10.1080/10412905.2019.1612788. [Google Scholar] [CrossRef]
110. Perlatti B, Bergo PLS, Silva MF, Fernandes GF, Forim JB, Perlatti MR, et al. Polymeric nanoparticle-based insecticides: a controlled release purpose for agrochemicals. In: Trdan S, editor. Insecticides-development of safer and more effective technologies. London, UK: IntechOpen; 2013. doi:10.5772/53355. [Google Scholar] [CrossRef]
111. Rasheed E, Libs S, Salim R. Formulation of essential oil pesticides technology and their application. Agricultural Res Technol Open Access J. 2017;9(2):555759. doi:10.19080/ARTOAJ.2017.09.555759. [Google Scholar] [CrossRef]
112. Ponce A, Roura SI, Moreira MR. Essential oils as biopreservatives: different methods for the technological application in lettuce leaves. J Food Sci. 2011;76(1):M34–40. doi:10.1111/j.1750-3841.2010.01880.x. [Google Scholar] [PubMed] [CrossRef]
113. Burggraf A, Rienth M. Origanum vulgare essential oil vapour impedes Botrytis cinerea development on grapevine (Vitis vinifera) fruit. Phytopathol Mediterr. 2020;59:331–44. doi:10.14601/Phyto-11605. [Google Scholar] [CrossRef]
114. Janatova A, Bernardos A, Smid J, Frankova A, Lhotka M, Kourimská L, et al. Long-term antifungal activity of volatile essential oil components released from mesoporous silica materials. Ind Crops Prod. 2015;67(12):216–20. doi:10.1016/j.indcrop.2015.01.019. [Google Scholar] [CrossRef]
115. Ghuffar S, Irshad G, Naz F, Khan MA, Ahmed MZ, Gleason ML. Efficacy of plant essential oils against Alternaria alternata causing bunch rot of grapes in Pakistan. J Anim Plant Sci. 2022;32(1):150–62. doi:10.36899/JAPS.2022.1.0411. [Google Scholar] [CrossRef]
116. Furet-Gavallet C, Burdet JP, Remolif E, Cléroux M, Pernet A, Rienth M. Impact of the vaporization of essential oils on vine physiology. In: International Vintage Symposium: New Tools for Management of Vineyards and Improvementof Quality Wines; 2018 Jun 15; Valencia, Espagne. [Google Scholar]
117. Ferreira GM, Moreira RR, Jarek TM, Nesi CN, Biasi LA, May MLL. Alternative control of downy mildew and grapevine leaf spot on Vitis labrusca. Australas Plant Pathol. 2022;51(2):193–201. doi:10.1007/s13313-021-00836-7. [Google Scholar] [CrossRef]
118. Kriegs B, Jansen M, Hahn K, Peisker H, Samajová O, Beck M, et al. Cyclic monoterpene mediated modulations of Arabidopsis thaliana phenotype: effects on the cytoskeleton and on the expression of selected genes. Plant Signal Behav. 2010;5(7):832–8. doi:10.4161/psb.5.7.12032. [Google Scholar] [PubMed] [CrossRef]
119. Ricci D, Epifano F, Fraternale D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic activity in vitro against various weed seed. Molecules. 2017;22(2):222. doi:10.3390/molecules22020222. [Google Scholar] [PubMed] [CrossRef]
120. Giunti G, Benelli G, Palmeri V, Laudani F, Ricupero M, Ricciardi R, et al. Non-target effects of essential oil-based biopesticides for crop protection: impact on natural enemies, pollinators, and soil invertebrates. Biol Control. 2022;176(8):105071. doi:10.1016/j.biocontrol.2022.105071. [Google Scholar] [CrossRef]
121. Kader MMA, Momen FM, Sammour EA, Aly SM, Fahim SF. Influence of Melissa officinalis essential oil and its formulation on Typhlodromips swirskii and Neoseiulus barkeri (Acari: Phytoseiidae). Acta Phytopathologica et Entomologica Hungarica. 2015;50(1):139–48. doi:10.1556/038.50.2015.1.13. [Google Scholar] [CrossRef]
122. Vokou D, Liotiri S. Stimulation of soil microbial activity by essential oils. Chemoecology. 1999;9(1):41–5. doi:10.1007/s000490050032. [Google Scholar] [CrossRef]
123. Thiele-Bruhn S, Shikuku V, Dittrich F, Torjir DN, Saini M, Getenga Z. Soil sorption and effects on soil microorganisms of thymol and carvacrol monoterpenes from essential oils of aromatic plants. Front Environ Sci. 2024;12:1379018. doi:10.3389/fenvs.2024.1379018. [Google Scholar] [CrossRef]
124. Dunan L, Malanga T, Benhamou S, Papaiconomou N, Desneux N, Lavoir AV, et al. Effects of essential oil-based formulation on biopesticide activity. Ind Crops Prod. 2023;202(2004):117006. doi:10.1016/j.indcrop.2023.117006. [Google Scholar] [CrossRef]
125. Proto M, Biondi E, Di Vito M, Modesto M, Baldo D, Filippini G, et al. Oli essenziali in patologia vegetale: potenziale mezzo per la difesa dai batteri fitopatogeni. NATURAL 1. 2019;186:83–4. [Google Scholar]
126. Gebel MP, Magurno F. Assessment of the antifungal potential of the essential oil from Thymus vulgaris against Botrytis cinerea causative agent of postharvest grey mould on strawberry fruits. Columella—J Agric Environ Sci. 2014;1(2):17–23. doi:10.18380/SZIE.COLUM.2014.1.2.17. [Google Scholar] [CrossRef]
127. Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crops Prod. 2014;62:250–64. doi:10.1016/j.indcrop.2014.05.055. [Google Scholar] [CrossRef]
128. Sudekum M, Poppenga RH, Raju N, Braselton WE. Pennyroyal oil toxicosis in a dog. J Am Vet Med Assoc. 1992;200(6):817–8. doi:10.2460/javma.1992.200.06.817. [Google Scholar] [CrossRef]
129. Schmidt B. Regulatory and quality issues with the essential oils supply chain. In: Jeliazkov VD, Cantrell CL, editors. Medicinal and aromatic crops: production, phytochemistry, and utilization. Washington, DC, USA: American Chemical Society; 2016. p. 27–48. doi:10.1021/bk-2016-1218.ch003. [Google Scholar] [CrossRef]
130. International Trade Centre. Trade map—trade statistics for international business development. [cited 2025 Mar 20]. Available from: https://www.trademap.org/Index.aspx?nvpm=1%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c%7c. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF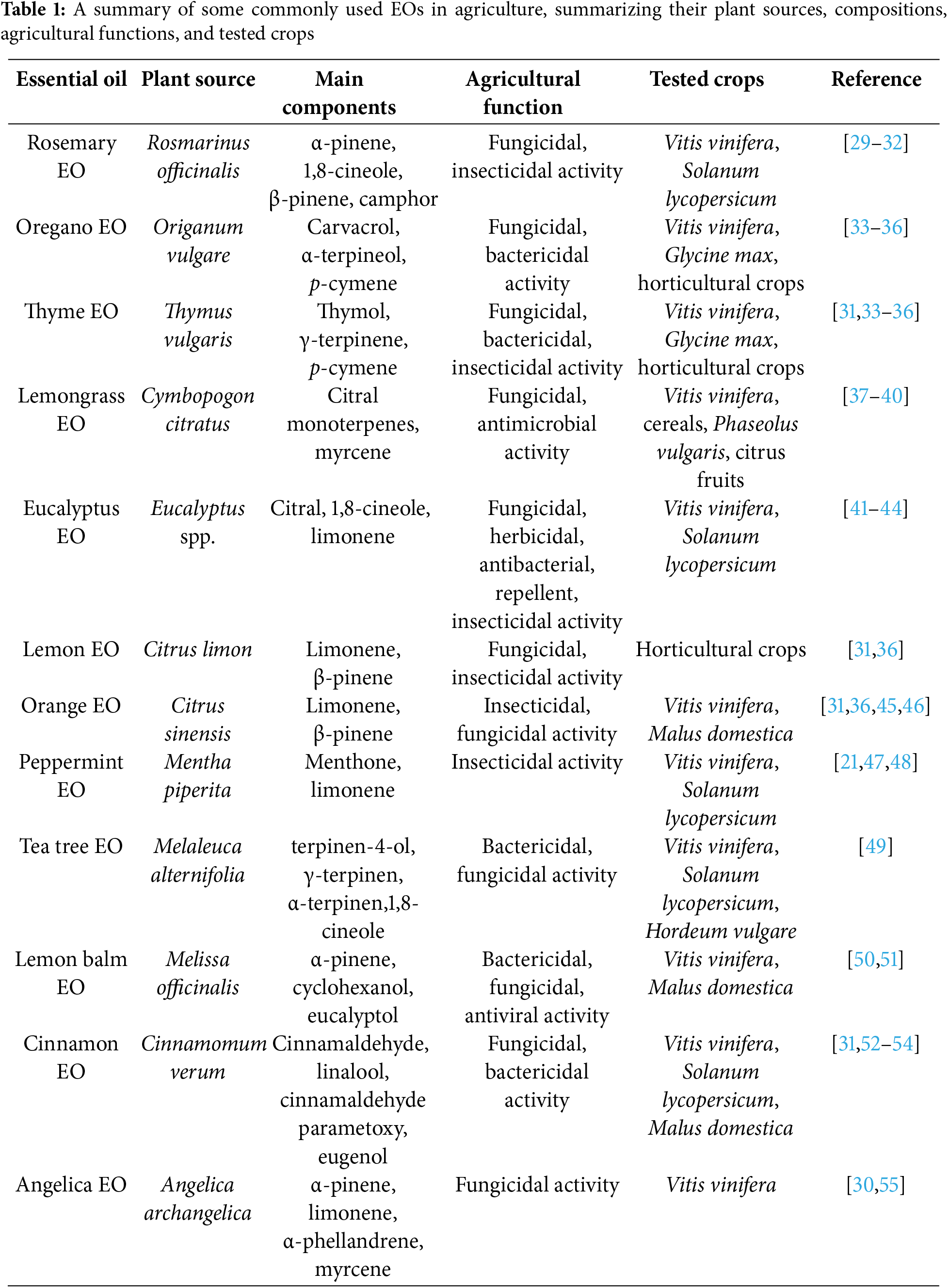
 Downloads
Downloads
 Citation Tools
Citation Tools