 Open Access
Open Access
REVIEW
Exosomes in viral infection: Effects for pathogenesis and treatment strategies
1 Anatomy Department, Faculty of Medicine, Qom University of Medical Sciences, Qom, Iran
2 Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran
3 Department of Molecular Genetics, College of Arts and Sciences, The Ohio State University, Columbus, OH, 43210, USA
4 Tissue Engineering Department, Faculty of Medicine, Qom University of Medical Sciences, Qom, Iran
5 Department of Mesenchymal Stem Cells, Academic Center for Education, Culture, and Research (ACECR), Qom Branch, Qom, Iran
6 The Persian Gulf Marine Biotechnology Research Center, The Persian Gulf Biomedical Sciences Research Institute, Bushehr University of Medical Sciences, Bushehr, Iran
7 Department of Molecular Medicine and Genetics, Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
8 Department of Medical Laboratory Sciences, Khomein University of Medical Sciences, Khomein, Iran
9 Molecular and Medicine Research Center, Khomein University of Medical Sciences, Khomein, Iran
* Corresponding Authors: MOHSEN SHEYKHHASAN. Email: ; MARYAM AZIMZADEH. Email:
(This article belongs to the Special Issue: Perspectives on Stem Cells and Regenerative Medicine)
BIOCELL 2023, 47(12), 2597-2608. https://doi.org/10.32604/biocell.2023.043351
Received 29 June 2023; Accepted 11 October 2023; Issue published 27 December 2023
Abstract
Exosomes are small vesicles that carry molecules from one cell to another. They have many features that make them interesting for research, such as their stability, low immunogenicity, size of the nanoscale, toxicity, and selective delivery. Exosomes can also interact with viruses in diverse ways. Emerging research highlights the significant role of exosomes in viral infections, particularly in the context of diseases like COVID-19, HIV, HBV and HCV. Understanding the intricate interplay between exosomes and the human immune system holds great promise for the development of effective antiviral therapies. An important aspect is gaining clarity on how exosomes influence the immune system and enhance viral infectivity through their inherent characteristics. By leveraging the innate properties of exosomes, viruses exploit the machinery involved in exosome biogenesis to set replication, facilitate the spread of infection, and eliminate immune responses. They can either help or hinder viral infection by modulating the immune system. This review summarizes the recent findings on how exosomes mediate viral infection and how they can be used for diagnosis or therapy. This could lead to new clinical applications of exosomes in disease management.Keywords
Exosomes are tiny sacs with two layers of membrane that measure 100 nm across, but can range from 30 to 150 nm (Sheykhhasan et al., 2022; Sheykhhasan et al., 2021b; Khoei et al., 2020). They can be found in many kinds of body fluids, such as blood, saliva, urine, bile, cerebrospinal fluid, semen, breast milk, pleural effusion, and amniotic fluid (Zhou et al., 2021; Yu et al., 2022). Exosomes carry over 1600 different proteins that have various functions in living cells, such as regulating metabolism and maintaining structure (Kalluri and LeBleu, 2020). Exosomes are involved in intercellular communication via transferring molecules between cells (Fayazi et al., 2021). They also have the great potential to serve as drug delivery vehicles, due to their high stability in circulation, low immunogenicity/toxicity, high penetration ability to blood brain barrier and selective targeting of cell types (Fig. 1) (Murphy et al., 2019; Sil et al., 2020).
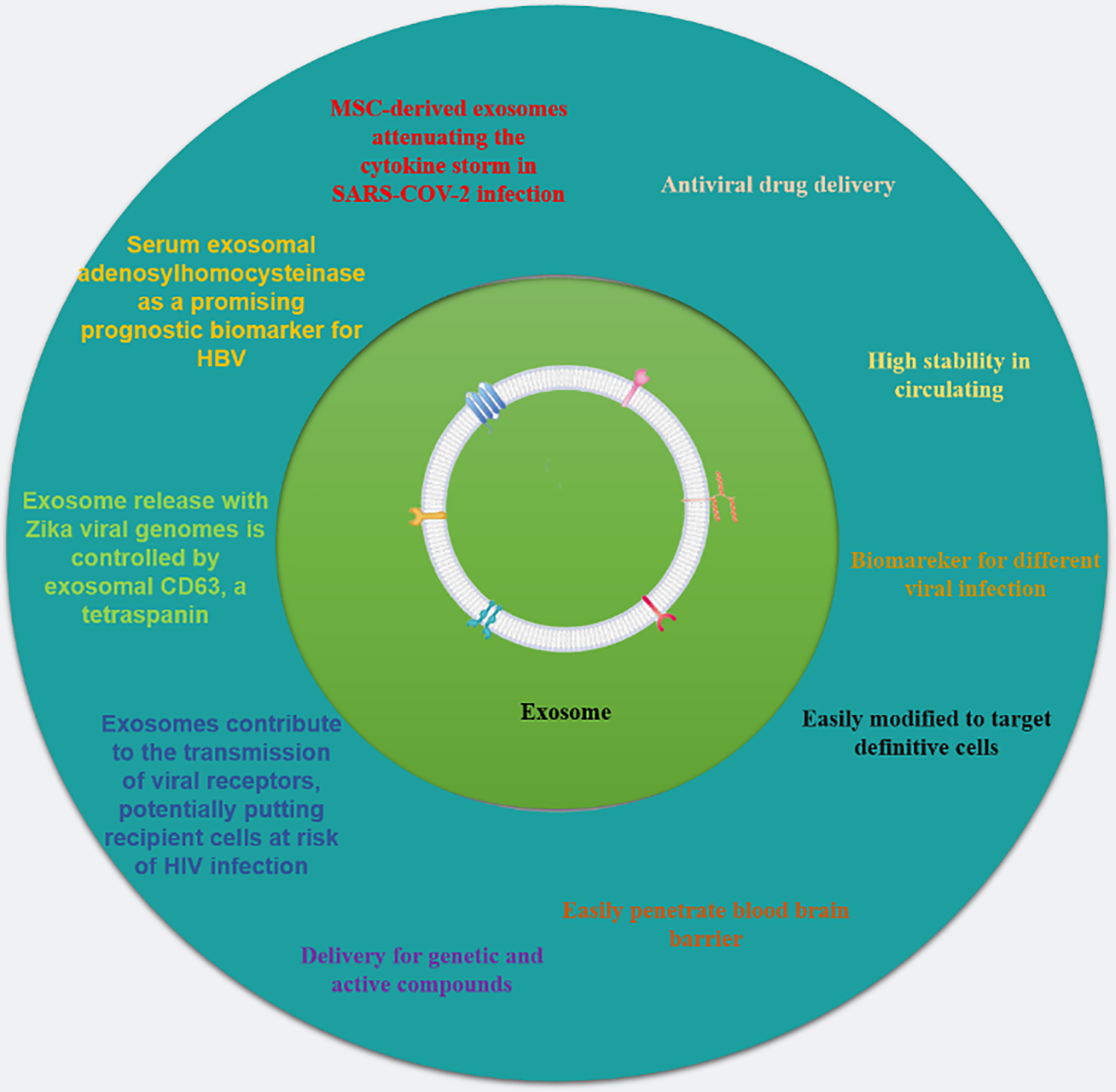
Figure 1: The potential exosomal properties and their use in various viral treatments. The advantageous features of exosomes, including their ability to efficiently transport genetic material, proteins, and medications are sumarized. Exosomes also possess the ability to target specific cells, exhibit biocompatibility and non-immunogenic properties, and can be readily absorbed by cells. The application of exosomes in treating various viral infections, such as HBV, HIV, and SARS-CoV-2 are showcased. The diagram was adapted from Saad et al. (2021) with some modifications and updates.
Exosomes have different functions depending on where they come from. For example, exosomes from tumor cells help them to spread and invade other tissues (Deb et al., 2021). Some cells, such as those that are infected by viruses, can produce exosomes that have different proteins on their surface or inside them. These proteins are derived from the virus that infects the cell. The exosomes can then carry these viral proteins to other cells and infect them with the same virus. This is one way that exosomes can mediate viral infection (Peng et al., 2023). Exosomes and viruses have many things in common, such as how they are made and what they carry. Exosomes that are linked to viruses can also affect the immune system in different ways. They can either help the virus to escape the immune system or activate the immune system to fight the virus. This means that exosomes can regulate the immune response in both directions (Peng et al., 2023) (Fig. 2). The aim of this review is to summarize the multifunctional role of exosomes in the infection of SARS-CoV-2, hepatitis, and nervous system viruses.
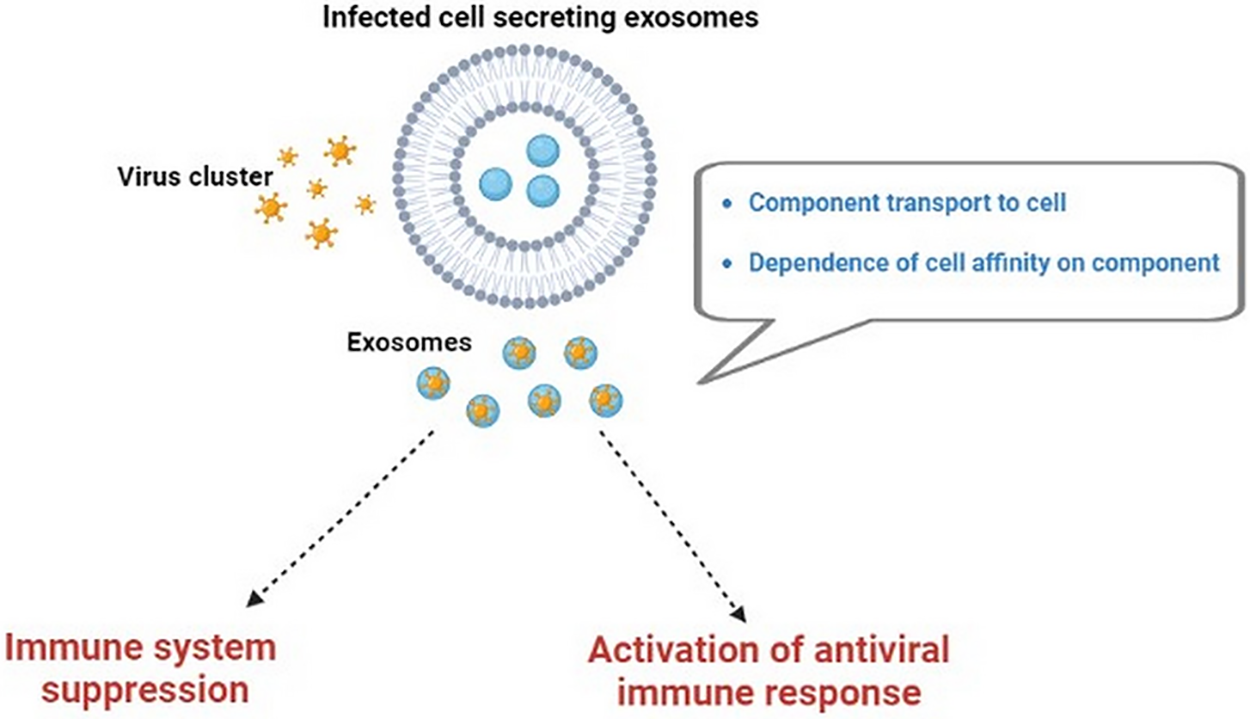
Figure 2: The dual function of exosomes in viral infection. Viruses employ the exosome biogenesis machinery to start replication, propagate infection, and circumvent the immune system. However, exosomes also take part in defense measures by activating the innate immune system.
The PubMed and Medline databases were systematically searched. The inclusion criteria were studies that were published up to April 2023. The Medical Subject Headings (MeSH) terms “exosome” and “viral infection” were utilized. The phrase “exosome” or “COVID-19” or “Hepatitis virus” or “Zika” or “HIV” was also used.
Similarities between viral infection and exosomes
Viruses can use exosomes to make themselves more infectious. For example, exosomes can carry transcription factors, which are proteins that help viruses make more copies of themselves (Subra et al., 2007).
Exosomes and viral infection can deliver their molecules, such as proteins, RNA, or DNA, to other cells and alter their gene expression and function. Their molecules have a key role for attaching to the cell membrane (for example, tetraspanins are a family of proteins that are involved in cell adhesion and signaling) (Andreu and Yáñez-Mó, 2014). In addition, the cell affinity to bind viral protein are mostly determined by its secreted exosome. Furthermore, the affinity of the cell to bind viral protein is largely influenced by the type and amount of exosome that it secretes. However, this phenomenon has only been investigated in vitro studies experimentally (Urbanelli et al., 2019) and it could be an interesting topic for future research. Therefore, there is a bidirectional interaction between viruses and exosomes in the human body that could affect the occurrence or development of various virus-related diseases (Fig. 2).
The COVID-19 definition, diagnosis, and immunopathology
The initial symptoms of SARS-CoV-2 infection are often mild and resemble those of other respiratory virus diseases, such as dry cough, tiredness, sore throat, anorexia, high fever, myalgia, and nasal congestion (Ashour et al., 2020; Krajewska et al., 2020; Mohammadi et al., 2022; Sheykhhasan et al., 2020). However, these symptoms may worsen over time and cause respiratory distress that requires hospitalization (Al Balushi et al., 2020). It has been estimated that about 80% of symptomatic patients do not need medical assistance or hospitalization, but the mortality rate among patients admitted to the ICU varies from 39% to 72% (Verity et al., 2020). Furthermore, the COVID-19 virus particles can impair the function of sensory receptor cells (blocking olfaction) by binding to the angiotensin-converting enzyme 2 or ACE2 receptors, which are highly expressed in the olfactory epithelial cells and oral mucosa (Fotuhi et al., 2020).
As expected, old adults with COVID-19 infection tend to have higher levels of production of cytokines, including IL-2, IL-6, IL-7, IL-10, IP-10, and TNFα, than young or healthy adults (Palanques-Pastor et al., 2020; Zhong et al., 2020). Furthermore, excessive levels of pro-inflammatory cytokines can cause higher blood viscosity and fatal thromboembolism in some elderly adults with COVID-19 infection (Soy et al., 2020). In these regards, multiple organ dysfunction may result from hyper-activation of pro-inflammatory markers and vascular damage in the heart, brain, kidney, and liver. Besides, the COVID-19-infected patients experience neurological symptoms such as anosmia or stroke (Ahmad and Rathore, 2020).
Several molecular tests have been approved for in vitro diagnostics of COVID-19, such as RT-PCR (Allan et al., 2020; Wang et al., 2020), Next-Generation Sequencing (NGS) (Chen et al., 2020), and loop-mediated isothermal amplification (LAMP) (Jiang et al., 2020). RT-PCR can detect high levels of SARS-CoV-2 viral RNA in patients with asymptomatic and pre-symptomatic SARS-CoV-2 infections (Nile et al., 2020).
SARS-CoV-2 antibodies that recognize prior infection and immunity are useful for ongoing surveillance, epidemiologic/vaccine studies, and estimating the risk of health care workers (Cheng et al., 2020; Huang et al., 2020; Krammer and Simon, 2020). Specifically, SARS-CoV-2 IgG appears ~10 days post-infection and peaks at day 49, while IgM antibodies appear ~7 days after symptom onset and peak at day 28 (Huang et al., 2020).
The mechanisms and functional roles of exosomes in the COVID-19 pandemic
It is known that the immune system is a natural defense mechanism that fights against invasive microbial infections. Exosomes mediate various types of biological processes during micro-organism infections and stimulate immune responses (Chitra and Harikumar, 2018). Briefly, immuno-exosomes are formed by the budding of sub-cellular endosomal membranes inward from immune effector cells (Wu et al., 2019) and delivered extracellularly to various cell types, such as T and B cells, dendritic cells (DCs), platelets, NK cells, mast cells, and epithelial cells (Admyre et al., 2007). Recent studies suggested that exosomes can be a promising specimen for the detection of COVID-19. SARS-CoV-2-infected cells can produce exosomes with more virus particles that these exosomes that transport viral and self-antigens, triggering the host immune response (Wan et al., 2020). It was proved that exosomes are important not only for sorting ACE2, but also for sorting proteins, miRNAs, and other cargo that can be transmitted to healthy cells like other viruses (Gunasekaran et al., 2017). Exosomes also alter the cargo of proteins involved in the pro-inflammatory, coagulopathy, and endothelial damage. Furthermore, it was reported that some exosomes can transfer ACE2 to receipt cells and facilitate the SARS-CoV2 internalization and infection (Wang et al., 2020), suggesting a link between exosomes and COVID-19 diagnosis (Hassanpour et al., 2020). The treatment of COVID-19 infection with antibody-mediated exosomes involves targeting the spike protein with monoclonal antibodies and internalizing them into the endosomes, then fusing the membranes to transfer genetic materials and prevent viral replication (Salvatori et al., 2020; Lee et al., 2021). It has been shown that exosomes and other extracellular vesicles (EVs) are released by infected cells and can adjust the immune response (Gurunathan et al., 2021). Barberis et al., 2021 found that SARS-CoV-2 infection changes exosome content and affects their role in disease progression and their potential application as biomarkers of disease severity (Barberis et al., 2021). For example, Fujita et al. (2021) found three potential early COVID-19 biomarkers and found that COPB2 extracellular vesicle had the optimal predictive value for the severe deterioration of COVID-19 patients (Fujita et al., 2021). It has been demonstrated that exosomes produced by SARS-CoV-2-infected cells contain specific proteins, such as fibrinogen, complement C1r subcomponent, and serum amyloid P-component, that act as important disease biomarkers (Barberis et al., 2021). Moreover, exosomes from virus-infected cells can release cytokines and chemokines from human monocytes and/or airway epithelial cells, which then trigger an innate immune response (Chahar et al., 2018).
Exosomes can also enhance antiviral responses in infected cells by various mechanisms (Rezabakhsh et al., 2022). For example, the secretion and synthesis of interferons can be increased by the transfer of interferon-stimulated genes (ISGs) through exosomes (Yao et al., 2018). Studies indicate that MSC-Exo contains high amounts of anti-inflammatory cytokines such as IL-10 and TGF-β (Rezabakhsh et al., 2022). MSC-Exo can produce specific growth factors, including vascular endothelial growth factor and basic fibroblast growth factor, that can restore the function of the alveolar-blood barrier and limit the infiltration of immune cells into the infected areas (Rezabakhsh et al., 2022). Exosome has cytoprotective properties against viral infection and high levels of antibacterial components, according to various studies. Exosome may have antibacterial effects by inhibiting bacterial growth and promoting phagocytosis in local immune cells (Russell et al., 2020). It appears that intranasal administration of exosome at the early stages of COVID-19 may reduce the replication and dissemination of certain SARS-CoV-2 strains into lower regions (Barberis et al., 2021). In fact, intranasal administration of exosomes is a more effective route for treating pulmonary disease and neurodegenerative disorders, as this way delivers more exosomes to the target site, while intravenous injection reduces the number of exosomes significantly within a few hours (Moss et al., 2021). However, intravenous administration of purified exosomes may reduce the need for invasive mechanical ventilation for respiratory failure in those patients with severe COVID-19 (Tsuchiya et al., 2020).
In an earlier study, exosomes derived from BM-MSCs were safe in improving pneumonia parameters, attenuating the cytokine storm, and restoring COVID-19 patients’ immunity (Sengupta et al., 2020). However, recent reports suggested further clinical research on exosomes derived from MSCs and ADSCs for the treatment of COVID-19 patients (Rogers et al., 2020; Mahida et al., 2020; Worthington and Hagood, 2020).
Hepatitis is a type of viral infection that induces inflammation of the liver and can cause serious health complications and death. The infection caused by the hepatitis virus continues to be major public health challenge globally, resulting in a high number of deaths worldwide (approximately 2 billion peoples) (Matičič et al., 2020; Razavi-Shearer et al., 2018). According to the World Health Organization, hepatitis B and C are the most common types of viral hepatitis. It is estimated that over 350 million people worldwide have contracted the hepatitis B virus (HBV) annually (Pungpapong et al., 2007). Chronic hepatitis B (CHB) is a lifelong infection caused by the HBV, which can damage the liver and increase the risk of liver cancer. CHB can be transmitted through even unaware contact with blood, semen, and/or other body fluids from an infected person. The prevalence of hepatitis B infection varies widely across different regions of the world, depending on the modes of transmission. The World Health Organization classifies regions as high, intermediate, or low prevalence based on the percentage of people with chronic hepatitis B infection. Those high prevalence regions (>8%) include most of sub-Saharan Africa and some parts of Asia. Intermediate prevalence regions (2%–7%) include North Africa, Middle East, Europe, Latin America, and South Asia. Low prevalence regions (<2%) include North America, Australia, New Zealand, and Japan (https://www.who.int/westernpacific/health-topics/hepatitis/regional-hepatitis-data) (Razavi-Shearer et al., 2018).
A comprehension of the existing diagnostic tests for HBV is essential to effectively screen, diagnose, and provide treatment to patients with HBV. The condition of viral pathogenesis is mainly caused by alterations in the growth factors and cytokines that regulate the extracellular matrix (ECM). These changes enhance the survival of the ECM and prevent its degradation, leading to liver damage over time (Devhare et al., 2017). This work underlines the role of the miR-222/transferrin receptor axis in liver fibrosis and offers fresh perspectives on therapeutic treatment choices. Hepatitis C virus (HCV) is a member of hepatitis virus family that affects around 130–170 million people worldwide. HCV can lead to serious health problems such as cirrhosis and hepatocellular carcinoma (HCC) (Kim et al., 2016). Recent studies have shown that HCV can spread by mechanisms that bypass the receptors. Therefore, the receptor-mediated therapeutic strategies (antibodies) have become less effective (Yin et al., 2022).
Exosome as carrier for hepatitis virus
Exosomes are small vesicles that can carry substances from infected cells to uninfected cells and facilitate the transmission of HBV. Most liver cells, including hepatocytes and Kupffer cells, have the ability to release or be targeted by exosomes (Jiao et al., 2021).
Ramakrishnaiah et al. (2013) were the first to demonstrate in 2013 that exosomes from HCV-infected hepatoma cells could contain viral particles (Ramakrishnaiah et al., 2013). On this subject, exosome markers (such as the tetraspanin CD63) have a crucial role in the formation and distribution of HBV particles (Ninomiya et al., 2021). In fact, exosomes act as a protective shield for the viral components to evade the immune system (Aydin et al., 2021). In this respect, exosomes can shield HBV particles from being destroyed. This, in turn, facilitates the infection/dysfunction of hepatocytes and immune cells such as NK cells (Yang et al., 2017). One of the mechanisms for weakening the body’s ability to fight viruses is that non-coding RNA (e.g., miR-155, miR-221, and miR-192) present in HBV-infected exosomes can directly target the human IL-21 gene and subsequently impair the function of T cells, which are essential for antiviral immunity (Enomoto et al., 2017). Wu et al. (2023) demonstrated the presence of a significant mechanism involving the hepatitis B virus (surface antigen) and Na+-taurocholate (co-transporting polypeptide) on the surface of exosomes derived from HBV-expressing cells (Wu et al., 2023). Exosome-encapsulated HBV was able to initiate infection in susceptible cells, demonstrating its capability for transmission. However, non-permissive cells exhibited a significantly lower efficiency in absorbing exosomal HBV (Wu et al., 2023). In a study conducted by Zhang et al. (2023), it was discovered that liver fibrosis is exacerbated by exosomal miR-222 derived from HBV-infected hepatocytes, which occurs through the blockade of the transferrin receptor and the induction of ferroptosis (Zhang et al., 2023). Recent findings reveal that exosomes derived from serum infected with HBV have been found to diminish NK cell function. These exosomes adversely affect various aspects of NK cell activity, such as interferon (IFN) production, lytic function, proliferation, and viability. Moreover, they also impede cellular responses to poly (I:C) stimulation (Yang et al., 2017). In HBV transgenic mice, exosomes produced by HBV hindered the immune response and expedited the progression of chronic hepatitis B (CHB). The presence of HBV-derived exosomes resulted in the inhibition of HBV-replicating cell elimination in HBV-infected mice, thereby exacerbating the course of the disease. These findings indicate that HBV infection leads to alterations in exosomal contents, which have a significant impact on disease progression (Liu et al., 2023). In recent years, there have been significant advancements in the discovery of diagnostic and prognostic biomarkers for various types of viral infection (Bodaghi et al., 2023; Tribolet et al., 2020; Hasham et al., 2020; Kramvis et al., 2022). Notably, microRNAs, lnc RNAs, and cRNAs in exosomes have emerged as promising biomarkers. A recent study explored the possibility of serum exosomal adenosylhomocysteinase as a novel predictive biomarker for (HBV-induced) liver cirrhosis (Tong et al., 2021a), sheding light on the importance of identifying and utilizing new biomarkers in the diagnosis and prognosis of diseases. In individuals with chronic hepatitis B who have not undergone prior treatment, there exists a favorable correlation between serum exosome HBV-miR-3 and markers such as HBV DNA, pgRNA, and notably, hepatitis B e antigen (Gan et al., 2022). Moreover, the utilization of exosomal HBV-DNA has been identified as beneficial for individuals exhibiting a high level of suspicion for HBV infection, despite yielding negative results in serum HBV-DNA tests (Xu et al., 2023). The serum exosomal AHCY level has been identified as a potential unique predictive biomarker in patients with HBV-LC. Its presence holds significant implications for the prognosis of HBV-LC patients (Tong et al., 2021a). Furthermore, research findings have indicated the potential of exosomes in treating HBV infection by inhibiting HBV transcription and replication (Liu et al., 2023).
HCV infection stimulates increased production of miR-155 in liver tissues, leading to abnormal growth of liver cells and the formation of tumors (Zhang et al., 2012).
According to recent findings, it has been observed that RNA present in exosomes infected with HCV has the ability to stimulate immune cells, particularly monocytes, to increase the production of Galactin-9 (gal-9). Gal-9 is a molecule that binds to T cells and facilitates the persistence of HCV infection within the body (Mengshol et al., 2010). The above discovery holds great potential in the early diagnosis and treatment of HCV infection. Adenosylhomocysteinase, a crucial enzyme in transmethylation reactions and a component of serum exosomal, emerges as a promising prognostic biomarker for liver cirrhosis induced by HBV (Tong et al., 2021b). In addition, it has been observed that individuals with chronic HCV exhibit elevated levels of CD81 protein in their serum exosomes. This finding suggests a potential link between CD81 protein, inflammation, and the severity of fibrosis (Welker et al., 2012).
Interferon-α (IFN-α) holds significant importance in HBV treatment strategies as it possesses inhibitory potential against the hepatitis B surface antigen. As a result, it is widely considered a valuable candidate for HBV therapy. Furthermore, the utilization of exosomes loaded with IFN-α has emerged as a promising approach to sustain the drug's efficacy. These exosomes facilitate the delivery of IFN-α from healthy liver cells to the infected ones, aiding in the continuity of the treatment process (Wu et al., 2021). exosomes have demonstrated effective capabilities in antigen presentation for recombinant vaccines. A notable example is the utilization of exosomes derived from CD4+ T cells, which have shown the ability to enhance B cell activity and improve the effectiveness of the HBsAg vaccine (Lu et al., 2019; Jesus et al., 2018). Pradhan et al. (2022) discovered a significant increase in miR-375 levels within exosomes isolated from individuals with cirrhosis and hepatocellular carcinoma (HCC). Further evidence supporting the involvement of miR-375 in HCV pathogenesis comes from observations of inhibited cell migration and proliferation in HCV-infected cells when miR-375 is depleted. Additionally, exosomes derived from HCV-infected cells have the ability to release miR-375, which can then be transferred to uninfected Huh7.5 cells, resulting in enhanced proliferation and migration capabilities (Pradhan et al., 2022).
Viral infection of nerveus system and exosomes involvement
Moreover, the weakened immune system in immunosuppressed people may make it easier for viruses to infect different parts of the body, such as the nervous system (Kennedy, 2021). Of this, Zika virus (ZIKV) was first identified as a yellow fever virus in the Zika Forest of Uganda. In 2015, ZIKV reemerged as a potential cause of severe developmental problems in the central nervous system (CNS) (Dick et al., 1952). ZIKV can impair the formation of new neurons in the CNS. For example, in an experimental model, Zika virus caused neuronal death and small brain size by activating a stress-induced response to misfolded proteins (Gladwyn-Ng et al., 2018). Especially, it is essential to monitor pregnant women who were exposed to ZIKV to detect any possible neurological problems early (Marbán-Castro et al., 2021). On the other hand, ZIKV infection was found to cause autonomic neuropathy and meningoencephalitis complications in the adult nervous system (Acosta-Ampudia et al., 2018).
Exosomes can pass through the BBB, which means they can carry viral signals to the brain. This is important for viral infection of the nervous system. By studying exosomes from infected cells, researchers found that exosomes are associated with non-structural protein 1 (NS1), which is a viral toxin (El Safadi et al., 2022). Moreover, ZIKV infections can affect the miRNAs in exosomes from host neural cells (such as miR-4792), which are involved in biological processes like oxidative stress (Tabari et al., 2020). Neutral Sphingomyelinase SMPD3is a crucial molecule for exosome production or release in neural cells. Zhou et al. (2019) reported that ZIKV can change the activity of this molecule, which may help it to spread through exosomes and cause severe neuronal damage (Zhou et al., 2019). Zika virus-infected cells secrete exosomes with specific viral protein profiles, and more exosomes are released during ZIKV infection than in noninfected cells. Also, the exosome release with Zika viral genomes is controlled by CD63, a tetraspanin that is abundant in exosomes (York et al., 2021).
Among viruses, the most widely recognized retrovirus is HIV-1 (Lu et al., 2011; Poondla et al., 2023; Sheykhhasan et al., 2021a). Exosomes play a role in facilitating the transmission of HIV-related proteins to target cells, thereby accelerating the spread of infection by rendering recipient cells more susceptible to HIV (Arenaccio et al., 2015). Specifically, the protein Nef is transmitted via exosomes, which subsequently activate cells and heighten their vulnerability to HIV infection upon reaching latent cells (Arenaccio et al., 2015). Furthermore, Nef possesses the capacity to inhibit the production of CD4+ exosomes by T cells, thereby diminishing the immune cells’ capability to identify viruses (Lu et al., 2019). Previous studies have demonstrated that the presence of Nef in exosomes leads to the apoptosis or senescence of CD4+ T cells (Lenassi et al., 2010; Arenaccio et al., 2014). Based on Schaefer et al. (2008), it has been observed that macrophages release exosomes containing Nef, which hinder T cell function by breaking down MHC-I and CD4+ molecules through a beta-COP-dependent mechanism (Schaefer et al., 2008). Furthermore, it has been observed that Nef+ exosomes possess the capability to hinder the adaptive immune response by decreasing the production of IgA and IgG in B cells. This evasion mechanism employed by the virus allows it to evade the humoral immune response, as documented (Xu et al., 2009). In addition, Dubrovsky et al. (2022) conducted research revealing that when human monocyte-derived macrophages, generated from monocytes pre-treated with extracellular vesicles containing the HIV-1 protein Nef (exNef), were stimulated by lipopolysaccharide, they exhibited an increased release of pro-inflammatory cytokines (Dubrovsky et al., 2022; Rezaie et al., 2021). Also, it was observed that the quantity of plasma extracellular vesicles significantly increased during the onset of an acute infection, correlating with the viral load. Following the simian immunodeficiency virus (SIV) or macaque model of HIV infection, specific microRNAs such as miR-181a, miR-342-3p, and miR-29a, which are present in extracellular vesicles, exhibited a decrease and remained downregulated during the latency phase (Huang et al., 2023).
Exosomes released from HIV-1-infected cells carry viral receptors, potentially rendering target cells more vulnerable to infection. Notably, the transmission of CCR5 to CCR5 null cells through exosomes derived from CCR5+ ovary cells and peripheral blood mononuclear cells (PBMNCs) has been observed, resulting in an increased susceptibility to HIV-1 infection (Mack et al., 2000). According to research conducted by Rozmyslowicz et al. (2003), exosomes produced by megakaryocytes and platelets contain HIV co-receptors CXCR4. This presence of CXCR4 makes CXCR4-null cells more susceptible to X4-HIV infection (Rozmyslowicz et al., 2003). These findings indicate that exosomes contribute to the transmission of viral receptors, potentially putting recipient cells at risk of HIV infection. However, the impact of these findings on animal models remains uncertain (Rezaie et al., 2021).
Furthermore, it has been observed that viral nucleic acids can be packaged into exosomes, leading to the transfer of the viral genome to healthy cells and consequently enhancing the rate of infection. For example, exosomes derived from HIV-infected cells have been found to transmit the specific transactivation response element (TAR) RNA (Zhou et al., 2019). TAR RNA, located at the 5' end of HIV transcript copies, interacts with the Tat protein to generate viral RNAs. The presence of TAR-RNA in exosomes can suppress a Bcl-2 interacting protein, finally promoting viral development and resistance to apoptosis (Zhou et al., 2019; Marbán-Castro et al., 2021). In fact, TAR plays a crucial role in preventing apoptosis, thereby facilitating the production of the virus within infected cells (Tabari et al., 2020).
Exosomes derived from infected cells can potentially exert an antiviral influence. Specifically, exosomes from infected cells contain APOBEC3G molecules, which serve as antiviral proteins that impede virus formation. These proteins effectively convert cytosine residues to uracil in the negative strand of viral DNA during reverse transcription. Notably, the exclusion of Vif, an opposing viral protein, from exosomes enhances this advantageous trait (Khatua et al., 2009). Additionally, the exosomes released by infected cells contain cGAMP, a molecule capable of triggering the production of interferon and activating innate immune responses. This, in turn, leads to the initiation of an antiviral defense mechanism (Bridgeman et al., 2015; Gentili et al., 2015). Based on the research conducted by Bernard et al., it has been found that certain HIV-related miRNAs, such as miRNA-88 and miRNA-99, play a role in activating the endosomal NFkB and TLR8 signaling pathways. These signaling pathways, in turn, stimulate the immune system’s response to combat HIV by inducing the production of TNF-α in macrophages (Bernard et al., 2014). Based on research findings, it has been observed that when NLRP3, a specific protein, is silenced in microglia, it leads to the production of Tat-microglia-derived-EVs. These EVs have shown to possess protective effects on various aspects of neuronal function, including miniature excitatory postsynaptic currents, spine density, and synaptic proteins. While the role of NLRP3 in inflammation is widely acknowledged, an intriguing discovery has suggested that targeting NLRP3 could be beneficial in treating HIV-associated neurological disorders, as it plays a role in EV-mediated neuronal injury (Muthukumar et al., 2022).
The potential of exosome-derived miRNA-21 as a predictive biomarker was proposed in studying HIV-1-infected elite controllers’ patients. This proposition arose from the observation that exosomes obtained from a specific subgroup of these patients demonstrated a decrease in miRNA-21 levels. This decrease correlated with the progression of HIV infection, as reflected by a decline in T CD4 lymphocyte count (Ruiz-de-León et al., 2019).
Kodidela et al. (2018) conducted a proteome analysis on exosomes derived from the plasma of HIV-positive individuals who were frequent smokers, drinkers, or both. Their findings revealed different concentrations of hemopexin, alpha-2-macroglobulin, properdin, IL-8, and IL-10 in the exosomes of the HIV-positive groups who smoked and drank. These altered exosomal proteins and chemokines hold potential as biomarkers for identifying HIV-positive individuals with substance dependence (Kodidela et al., 2018; Kodidela et al., 2020). A research study conducted by Poveda et al. (2023) demonstrated the significant effectiveness of IL-18 levels in distinguishing individuals with HIV from uninfected controls. It also proved to be a reliable differentiator between elite controllers and those receiving antiretroviral therapy (ART), as well as between individuals with durable control and those with transitory control (Poveda et al., 2023). Exosomes have been discovered to carry immunologically activating proteins and contribute to oxidative stress. They also exhibit immunomodulatory effects on myeloid cells, and in HIV patients receiving antiretroviral therapy (ART), they may potentially have pro-inflammatory and redox effects during pathogenesis (Chettimada et al., 2018). Marques de Menezes’ study revealed a correlation between EVs exhibiting activation of monocyte and neural markers and HIV-related cognitive impairment. This discovery suggests that specific subsets of EVs could serve as novel biomarkers for assessing brain damage in HIV infection. Furthermore, the study highlighted a potential interaction between circulating platelet EV levels and monocyte activation, indicating a previously unrecognized aspect in the pathophysiology of HIV-induced cognitive impairment (de Menezes et al., 2022). Based on research findings, it has been observed that in individuals with HIV who are using cART (combination antiretroviral therapy), there is a connection between extracellular vesicles expressing monocyte markers and carotid intima-media thickness (cIMT). Moreover, EV fractions derived from HIV-positive individuals have been found to induce endothelial cell death through necrotic pathways. These discoveries suggest that EVs could potentially serve as both indicators and therapeutic targets in understanding the development of cardiovascular disease (CVD) within the context of treated HIV infection (Menezes et al., 2022).
Exosomes come from inside the body, so they can be used for therapeutic purposes such as delivering proteins, peptides, RNA, genes, vaccines, and immunotherapy. The main challenge for using exosomes is to find ways to isolate and purify them. Different uses need different levels of recovery and specificity. Standard methods are needed for separating and purifying exosomes. The methods should be fast, efficient, specific, viable, and reproducible. Exosomes from inside the body are stable in the blood and can avoid being eaten by phagocytes and macrophages. Exosomes only last in the body for 2 to 20 min, though. The existing content inside exosomes limits how much external drug can be loaded, making it less effective (Chaudhari et al., 2022).
Various techniques have been employed to load drugs into exosomes, such as preloading and post-loading; however, these techniques require further optimization to achieve optimal outcomes. Large-scale production of exosomes entails serious concerns regarding aspects such as purity, potency, scalability, variability, and repeatability. It will entail considerable expenses to produce on a massive scale and introduce it to clinical settings. To elucidate how to utilize exosomes in biomedical applications, the function of exosomes must be investigated thoroughly (Chaudhari et al., 2022).
Exosomes that are loaded with drugs or not may have different effects and behaviors in living organisms. The pharmacokinetics and pharmacodynamics of therapeutic exosomes, which are the processes of how the drug moves and acts in the body, may differ from those of endogenous exosomes, which are the ones that are naturally present in the body (Chaudhari et al., 2022). The influence of exosomes on the exposure of drugs in vivo, which is the amount and duration of drug presence in the body, has to be extensively evaluated through long-term safety and toxicity studies. The impact of drug-loaded exosomes on the recipient cells, which are the cells that take up the exosomes, the absorption process, which is how the exosomes enter the cells, the intracellular and extracellular behavior, which is how the exosomes act inside and outside the cells, and the drug unloading mechanisms, which are how the exosomes release the drug, can all be studied using in vitro models, which are experiments done outside living organisms. The PK of therapeutic exosomes can be further investigated using additional in vivo methods, which are experiments done inside living organisms, and physiologically based pharmacokinetic models, which are mathematical models that simulate how the body works (Chaudhari et al., 2022). The creation of artificial exosomes is a promising solution for translational medicine, which is the process of applying research findings to clinical practice, because it can address the critical issues listed above and result in homogeneous exosomes, which are exosomes that have consistent properties and quality. Exosome-based biological applications have a bright future as synthetic exosomes are currently being developed (Chaudhari et al., 2022).
The present study offers a detailed analysis of the role of exosomes, a subtype of extracellular vesicles, in the pathogenesis of viral infections. Exosomes are nanoscale, bilayered vesicles that originate from the endosomal compartment of most eukaryotic cells. They serve as important regulators and mediators of intercellular communication, as they can deliver their cargo of proteins, lipids, and nucleic acids to recipient cells. The structure and composition of exosomes depend on the tissue and cell type of origin, and they can also vary in response to different clinical conditions. Several viruses have the ability to exploit exosomes and manipulate their functions for various objectives, such as immune evasion, viral dissemination, and host cell reprogramming (Saad et al., 2021).
Viruses exploit the biogenesis mechanisms of the exosomes they hijack to facilitate their capsid assembly, virion production, and viral particle secretion. They also employ exosomes as nano-carriers to transport viral proteins and/or viral miRNAs to uninfected cells, thereby modulating their cellular functions. Exosomes have multiple roles in biological processes, such as mediating intercellular communication, regulating immune responses, and transferring various materials from one cell to another. SARS-CoV-2, similar to other viruses, utilizes exosomes as a viral vehicle for intra-host transmission and viral amplification. Exosomes can be a potential option for designing various viral vaccines for the treatment and prevention of pandemic diseases, including COVID-19 (Saad et al., 2021).
The various biological functions of exosomes are largely influenced by their cellular origin and cargo composition. Exosomes can serve as important carriers of viruses, enhancing viral infection and antiviral immunity in different host cells. They are expected to offer a novel research platform for the diagnosis and treatment of viral disease.
Acknowledgement: We are grateful to all those who have contributed to this paper.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contributions to the paper as follows: study conception and design: MS, MA; data collection: RS; analysis and interpretation of results: PY, ME, SA; draft manuscript preparation: NK, HM. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Acosta-Ampudia Y, Monsalve DM, Castillo-Medina LF, Rodríguez Y, Pacheco Y, Halstead S, Willison HJ, Anaya JM, Ramírez-Santana C (2018). Autoimmune neurological conditions associated with Zika virus infection. Frontiers in Molecular Neuroscience 11: 116. [Google Scholar] [PubMed]
Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S (2007). B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. Journal of Allergy and Clinical Immunology 120: 1418–1424. [Google Scholar] [PubMed]
Ahmad I, Rathore FA (2020). Neurological manifestations and complications of COVID-19: A literature review. Journal of Clinical Neuroscience 77: 8–12. [Google Scholar] [PubMed]
Al Balushi L, Al Fahdi F, Al Ghafri T, Amin M, Singh J, Al Siyabi B, Al Kalbani M, Al Kindi M, Al Balushi F, Al Ghazaili H (2020). Epidemiological characteristics of COVID-19 confirmed cases in muscat governorate, sultanate of oman. Open Journal of Epidemiology 11: 56–69. [Google Scholar]
Allan D, Tieu A, Lalu M, Burger D (2020). Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: Progress and challenges toward clinical application. Stem Cells Translational Medicine 9: 39–46. [Google Scholar] [PubMed]
Andreu Z, Yáñez-Mó M (2014). Tetraspanins in extracellular vesicle formation and function. Frontiers in Immunology 5: 442. [Google Scholar] [PubMed]
Arenaccio C, Anticoli S, Manfredi F, Chiozzini C, Olivetta E, Federico M (2015). Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology 12: 87. https://doi.org/10.1186/s12977-015-0216-y [Google Scholar] [PubMed] [CrossRef]
Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Affabris E, Baur A, Federico M (2014). Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. Journal of Virology 88: 11529–11539. https://doi.org/10.1128/jvi.01712-14 [Google Scholar] [PubMed] [CrossRef]
Ashour HM, Elkhatib WF, Rahman M, Elshabrawy HA (2020). Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens 9: 186. [Google Scholar] [PubMed]
Aydin Y, Koksal AR, Reddy V, Lin D, Osman H, Heidari Z, Rhadhi SM, Wimley WC, Parsi MA, Dash S (2021). Extracellular vesicle release promotes viral replication during persistent HCV infection. Cells 10: 984. [Google Scholar] [PubMed]
Barberis E, Vanella VV, Falasca M, Caneapero V, Cappellano G et al. (2021). Circulating exosomes are strongly involved in SARS-CoV-2 infection. Frontiers in Molecular Biosciences 8: 632290. [Google Scholar] [PubMed]
Bernard MA, Zhao H, Yue SC, Anandaiah A, Koziel H, Tachado SD (2014). Novel HIV-1 miRNAs stimulate TNFα release in human macrophages via TLR8 signaling pathway. PLoS One 9: e106006. [Google Scholar] [PubMed]
Bodaghi A, Fattahi N, Ramazani A (2023). Biomarkers: Promising and valuable tools towards diagnosis, prognosis and treatment of COVID-19 and other diseases. Heliyon 9: e13323. https://doi.org/10.1016/j.heliyon.2023.e13323 [Google Scholar] [PubMed] [CrossRef]
Bridgeman A, Maelfait J, Davenne T, Partridge T, Peng Y, Mayer A, Dong T, Kaever V, Borrow P, Rehwinkel J (2015). Viruses transfer the antiviral second messenger cGAMP between cells. Science 349: 1228–1232. https://doi.org/10.1126/science.aab3632 [Google Scholar] [PubMed] [CrossRef]
Chahar HS, Corsello T, Kudlicki AS, Komaravelli N, Casola A (2018). Respiratory syncytial virus infection changes cargo composition of exosome released from airway epithelial cells. Scientific Reports 8: 387. https://doi.org/10.1038/s41598-017-18672-5 [Google Scholar] [PubMed] [CrossRef]
Chaudhari P, Ghate V, Nampoothiri M, Lewis S (2022). Multifunctional role of exosomes in viral diseases: From transmission to diagnosis and therapy. Cellular Signalling 94: 110325. https://doi.org/10.1016/j.cellsig.2022.110325 [Google Scholar] [PubMed] [CrossRef]
Chen L, Liu W, Zhang Q, Xu K, Ye G, Wu W, Sun Z, Liu F, Wu K, Zhong B (2020). RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerging Microbes & Infections 9: 313–319. [Google Scholar]
Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, Dittrich S, Yansouni CP (2020). Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: A narrative review. Annals of Internal Medicine 172: 726–734. [Google Scholar] [PubMed]
Chettimada S, Lorenz DR, Misra V, Dillon ST, Reeves RK, Manickam C, Morgello S, Kirk GD, Mehta SH, Gabuzda D (2018). Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Scientific Reports 8: 7227. https://doi.org/10.1038/s41598-018-25515-4 [Google Scholar] [PubMed] [CrossRef]
Chitra R, Harikumar KB (2018). The origin and functions of exosomes in cancer. Frontiers in Oncology 8: 66. [Google Scholar]
de Menezes EGM, Liu JS, Bowler SA, Giron LB, D’Antoni ML, Shikuma CM, Abdel-Mohsen M, Ndhlovu LC, Norris PJ (2022). Circulating brain-derived extracellular vesicles expressing neuroinflammatory markers are associated with HIV-related neurocognitive impairment. Frontiers in Immunology 13: 1033712. https://doi.org/10.3389/fimmu.2022.1033712 [Google Scholar] [PubMed] [CrossRef]
Deb A, Gupta S, Mazumder P (2021). Exosomes: A new horizon in modern medicine. Life Sciences 264: 118623. [Google Scholar] [PubMed]
Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB (2017). Exosome-mediated intercellular communication between hepatitis C virus-infected hepatocytes and hepatic stellate cells. Journal of Virology 91: e02225–02216. [Google Scholar]
Dick GW, Kitchen SF, Haddow AJ (1952). Zika virus (I). Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene 46: 509–520. [Google Scholar] [PubMed]
Dubrovsky L, Brichacek B, Prashant NM, Pushkarsky T, Mukhamedova N et al. (2022). Extracellular vesicles carrying HIV-1 Nef induce long-term hyperreactivity of myeloid cells. Cell Reports 41: 111674. https://doi.org/10.1016/j.celrep.2022.111674 [Google Scholar] [PubMed] [CrossRef]
El Safadi D, Lebeau G, Lagrave A, Mélade J, Grondin L, Rosanaly S, Begue F, Hoareau M, Veeren B, Roche M (2022). Extracellular vesicles are conveyors of the NS1 toxin during flavivirus infection. Viruses 15: 364. [Google Scholar]
Enomoto Y, Takagi R, Naito Y, Kiniwa T, Tanaka Y, Hamada-Tsutsumi S, Kawano M, Matsushita S, Ochiya T, Miyajima A (2017). Identification of the novel 3′ UTR sequences of human IL-21 mRNA as potential targets of miRNAs. Scientific Reports 7: 7780. [Google Scholar] [PubMed]
Fayazi N, Sheykhhasan M, Soleimani Asl S, Najafi R (2021). Stem cell-derived exosomes: A new strategy of neurodegenerative disease treatment. Molecular Neurobiology 58: 3494–3514. [Google Scholar] [PubMed]
Fotuhi M, Mian A, Meysami S, Raji CA (2020). Neurobiology of COVID-19. Journal of Alzheimer’s Disease 76: 3–19. [Google Scholar] [PubMed]
Fujita Y, Hoshina T, Matsuzaki J, Yoshioka Y, Kadota T et al. (2021). Early prediction of COVID-19 severity using extracellular vesicle COPB2. Journal of Extracellular Vesicles 10: e12092. https://doi.org/10.1002/jev2.12092 [Google Scholar] [PubMed] [CrossRef]
Gan W, Chen X, Wu Z, Zhu X, Liu J, Wang T, Gao Z (2022). The relationship between serum exosome HBV-miR-3 and current virological markers and its dynamics in chronic hepatitis B patients on antiviral treatment. Annals of Translational Medicine 10: 536. https://doi.org/10.21037/atm-22-2119 [Google Scholar] [PubMed] [CrossRef]
Gentili M, Kowal J, Tkach M, Satoh T, Lahaye X et al. (2015). Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science 349: 1232–1236. https://doi.org/10.1126/science.aab3628 [Google Scholar] [PubMed] [CrossRef]
Gladwyn-Ng I, Cordón-Barris L, Alfano C, Creppe C, Couderc T, Morelli G, Thelen N, America M, Bessières B, Encha-Razavi F (2018). Stress-induced unfolded protein response contributes to Zika virus-associated microcephaly. Nature Neuroscience 21: 63–71. [Google Scholar] [PubMed]
Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T (2017). Donor-derived exosomes with lung self-antigens in human lung allograft rejection. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons 17: 474–484. https://doi.org/10.1111/ajt.13915 [Google Scholar] [PubMed] [CrossRef]
Gurunathan S, Kang MH, Kim JH (2021). Diverse effects of exosomes on COVID-19: A perspective of progress from transmission to therapeutic developments. Frontiers Immunology 12: 716407. https://doi.org/10.3389/fimmu.2021.716407 [Google Scholar] [PubMed] [CrossRef]
Hasham K, Ahmed N, Zeshan B (2020). Circulating microRNAs in oncogenic viral infections: Potential diagnostic biomarkers. SN Applied Sciences 2: 442. https://doi.org/10.1007/s42452-020-2251-0 [Google Scholar] [CrossRef]
Hassanpour M, Rezaie J, Nouri M, Panahi Y (2020). The role of extracellular vesicles in COVID-19 virus infection. Infection, Genetics and Evolution 85: 104422. [Google Scholar] [PubMed]
Huang AT, Garcia-Carreras B, Hitchings MD, Yang B, Katzelnick LC, Rattigan SM, Borgert BA, Moreno CA, Solomon BD, Trimmer-Smith L (2020). A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nature Communications 11: 1–16. [Google Scholar]
Huang Y, Liao Z, Dang P, Queen S, Abreu CM, Gololobova O, Zheng L, Witwer KW (2023). Longitudinal characterization of circulating extracellular vesicles and small RNA during simian immunodeficiency virus infection and antiretroviral therapy. AIDS 37: 733–744. https://doi.org/10.1097/QAD.0000000000003487 [Google Scholar] [PubMed] [CrossRef]
Jesus S, Soares E, Cruz MT, Borges O (2018). Exosomes as adjuvants for the recombinant hepatitis B antigen: First report. European Journal of Pharmaceutics and Biopharmaceutics 133: 1–11. [Google Scholar] [PubMed]
Jiang M, Pan W, Arasthfer A, Fang W, Ling L, Fang H, Daneshnia F, Yu J, Liao W, Pei H (2020). Development and validation of a rapid, single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. Frontiers in Cellular and Infection Microbiology 10: 331. [Google Scholar] [PubMed]
Jiao Y, Xu P, Shi H, Chen D, Shi H (2021). Advances on liver cell-derived exosomes in liver diseases. Journal of Cellular and Molecular Medicine 25: 15–26. [Google Scholar] [PubMed]
Kalluri R, LeBleu VS (2020). The biology, function, and biomedical applications of exosomes. Science 367: eaau6977. [Google Scholar] [PubMed]
Kennedy PG (2021). An overview of viral infections of the nervous system in the immunosuppressed. Journal of Neurology 268: 3026–3030. [Google Scholar] [PubMed]
Khatua AK, Taylor HE, Hildreth JE, Popik W (2009). Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. Journal of Virology 83: 512–521. https://doi.org/10.1128/jvi.01658-08 [Google Scholar] [PubMed] [CrossRef]
Khoei SG, Dermani FK, Malih S, Fayazi N, Sheykhhasan M (2020). The use of mesenchymal stem cells and their derived extracellular vesicles in cardiovascular disease treatment. Current Stem Cell Research and Therapy 15: 623–638. https://doi.org/10.2174/1574888x15666200501235201 [Google Scholar] [PubMed] [CrossRef]
Kim S, Han KH, Ahn SH (2016). Hepatitis C virus and antiviral drug resistance. Gut and Liver 10: 890–895. [Google Scholar] [PubMed]
Kodidela S, Ranjit S, Sinha N, McArthur C, Kumar A, Kumar S (2018). Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers. PLoS One 13: e0201144. https://doi.org/10.1371/journal.pone.0201144 [Google Scholar] [PubMed] [CrossRef]
Kodidela S, Wang Y, Patters BJ, Gong Y, Sinha N et al. (2020). Proteomic profiling of exosomes derived from plasma of HIV-infected alcohol drinkers and cigarette smokers. Journal of Neuroimmune Pharmacology 15: 501–519. https://doi.org/10.1007/s11481-019-09853-2 [Google Scholar] [PubMed] [CrossRef]
Krajewska J, Krajewski W, Zub K, Zatoński T (2020). COVID-19 in otolaryngologist practice: A review of current knowledge. European Archives of Oto-Rhino-Laryngology 277: 1885–1897. [Google Scholar] [PubMed]
Krammer F, Simon V (2020). Serology assays to manage COVID-19. Science 368: 1060–1061. [Google Scholar] [PubMed]
Kramvis A, Chang KM, Dandri M, Farci P, Glebe D et al. (2022). A roadmap for serum biomarkers for hepatitis B virus: Current status and future outlook. Nature Reviews Gastroenterology & Hepatology 19: 727–745. https://doi.org/10.1038/s41575-022-00649-z [Google Scholar] [PubMed] [CrossRef]
Lee E, Sandgren K, Duette G, Stylianou V V, Khanna R et al. (2021). Identification of SARS-CoV-2 nucleocapsid and spike T-cell epitopes for assessing T-cell immunity. Journal of Virology 95: e02002–02020. [Google Scholar]
Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM (2010). HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11: 110–122. https://doi.org/10.1111/j.1600-0854.2009.01006.x [Google Scholar] [PubMed] [CrossRef]
Liu Z, Li Y, Wang Y, Bai X, Zhang Y (2023). Exosomes in HBV infection. Clinica Chimica Acta 538: 65–69. https://doi.org/10.1016/j.cca.2022.11.012 [Google Scholar] [PubMed] [CrossRef]
Lu K, Heng X, Summers MF (2011). Structural determinants and mechanism of HIV-1 genome packaging. Journal of Molecular Biology 410: 609–633. https://doi.org/10.1016/j.jmb.2011.04.029 [Google Scholar] [PubMed] [CrossRef]
Lu J, Wu J, Xie F, Tian J, Tang X, Guo H, Ma J, Xu P, Mao L, Xu H (2019). CD4+ T cell-released extracellular vesicles potentiate the efficacy of the HBsAg vaccine by enhancing B cell responses. Advanced Science 6: 1802219. [Google Scholar] [PubMed]
Mack M, Kleinschmidt A, Brühl H, Klier C, Nelson PJ, Cihak J, Plachý J, Stangassinger M, Erfle V, Schlöndorff D (2000). Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nature Medicine 6: 769–775. https://doi.org/10.1038/77498 [Google Scholar] [PubMed] [CrossRef]
Mahida RY, Matsumoto S, Matthay MA (2020). Extracellular vesicles: A new frontier for research in acute respiratory distress syndrome. American Journal of Respiratory Cell and Molecular Biology 63: 15–24. [Google Scholar] [PubMed]
Marbán-Castro E, Goncé A, Fumadó V, Romero-Acevedo L, Bardají A (2021). Zika virus infection in pregnant women and their children: A review. European Journal of Obstetrics & Gynecology and Reproductive Biology 265: 162–168. [Google Scholar]
Matičič M, Lombardi A, Mondelli MU, Colombo M (2020). Elimination of hepatitis C in Europe: Can WHO targets be achieved? Clinical Microbiology and Infection 26: 818–823. [Google Scholar] [PubMed]
Menezes EGMd, Deng X, Liu J, Bowler SA, Shikuma CM, Stone M, Hunt PW, Ndhlovu LC, Norris PJ (2022). Plasma CD16+ extracellular vesicles associate with carotid artery intima-media thickness in HIV+ adults on combination antiretroviral therapy. mBio 13: e03005-03021. https://doi.org/10.1128/mbio.03005-21 [Google Scholar] [PubMed] [CrossRef]
Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M (2010). A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One 5: e9504. [Google Scholar] [PubMed]
Mohammadi M, Akhoundi M, Malih S, Mohammadi A, Sheykhhasan M (2022). Therapeutic roles of CAR T cells in infectious diseases: Clinical lessons learnt from cancer. Review in Medical Virology 32: e2325. https://doi.org/10.1002/rmv.2325 [Google Scholar] [PubMed] [CrossRef]
Moss LD, Sode D, Patel R, Lui A, Hudson C, Patel NA, Bickford PC (2021). Intranasal delivery of exosomes from human adipose derived stem cells at forty-eight hours post injury reduces motor and cognitive impairments following traumatic brain injury. Neurochemistry International 150: 105173. [Google Scholar] [PubMed]
Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P (2019). Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Experimental & Molecular Medicine 51: 1–12. [Google Scholar]
Muthukumar K, Seema S, Divya TC, Abiola AO, Dinesh YG, Shashank MD, Shilpa B, Susmita S (2022). HIV-1 Tat induced microglial EVs leads to neuronal synaptodendritic injury: Microglia-neuron cross-talk in NeuroHIV. HIV-1 Tat induced microglial EVs leads to neuronal synaptodendritic injury: Microglia-neuron cross-talk in NeuroHIV 3. Extracellular Vesicles and Circulating Nucleic Acids 3: 133–149. https://doi.org/10.20517/evcna.2022.14 [Google Scholar] [PubMed] [CrossRef]
Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G (2020). COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine & Growth Factor Reviews 53: 66–70. [Google Scholar]
Ninomiya M, Inoue J, Krueger EW, Chen J, Cao H, Masamune A, McNiven MA (2021). The exosome-associated tetraspanin CD63 contributes to the efficient assembly and infectivity of the hepatitis B virus. Hepatology Communications 5: 1238–1251. [Google Scholar] [PubMed]
Palanques-Pastor T, López-Briz E, Andrés JLP (2020). Involvement of interleukin 6 in SARS-CoV-2 infection: Siltuximab as a therapeutic option against COVID-19. European Journal of Hospital Pharmacy 27: 297–298. [Google Scholar] [PubMed]
Peng Y, Yang Y, Li Y, Shi T, Luan Y, Yin C (2023). Exosome and virus infection. Frontiers in Immunology 14: 1154217. [Google Scholar] [PubMed]
Poondla N, Sheykhhasan M, Ahmadyousefi Y, Akbari M, Seyedebrahimi R, Farsani ME, Kalhor N (2023). Dendritic cells—winning the fight against HIV. Current Stem Cell Research and Therapy 18: 174–185. https://doi.org/10.2174/1574888X17666220401102718 [Google Scholar] [PubMed] [CrossRef]
Poveda E, Fitzgerald W, Reglero C, Pérez-González A, Mariño A et al. (2023). Interleukin 18 (IL-18) and IL-3 in extracellular vesicles: Biomarkers for durable elite control of HIV-1. The Journal of Infectious Diseases 227: 1381–1385. https://doi.org/10.1093/infdis/jiad042 [Google Scholar] [PubMed] [CrossRef]
Pradhan A, Shivaprasad S, Dey S, Goel A, Aggarwal R, Das S (2022). Exosome-associated microRNA-375 induces cell proliferation by regulating IGFBP4 upon hepatitis C virus infection. Molecular Microbiology 118: 570–587. https://doi.org/10.1111/mmi.14986 [Google Scholar] [PubMed] [CrossRef]
Pungpapong S, Kim WR, Poterucha JJ (2007). Natural history of hepatitis B virus infection: An update for clinicians. In: Mayo Clinic Proceedings, pp. 967–975. Amsterdam: Elsevier. [Google Scholar]
Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G (2013). Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proceedings of the National Academy of Sciences of the United States of America 110: 13109–13113. [Google Scholar] [PubMed]
Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, Abdulla M, Abou Rached A, Adda D, Aho I (2018). Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. The Lancet Gastroenterology & Hepatology 3: 383–403. [Google Scholar]
Rezabakhsh A, Mahdipour M, Nourazarian A, Habibollahi P, Sokullu E, Avci ÇB, Rahbarghazi R (2022). Application of exosomes for the alleviation of COVID-19-related pathologies. Cell Biochemistry and Function 40: 430–438. https://doi.org/10.1002/cbf.3720 [Google Scholar] [PubMed] [CrossRef]
Rezaie J, Aslan C, Ahmadi M, Zolbanin NM, Kashanchi F, Jafari R (2021). The versatile role of exosomes in human retroviral infections: From immunopathogenesis to clinical application. Cell & Bioscience 11: 19. https://doi.org/10.1186/s13578-021-00537-0 [Google Scholar] [PubMed] [CrossRef]
Rogers CJ, Harman RJ, Bunnell BA, Schreiber MA, Xiang C, Wang FS, Santidrian AF, Minev BR (2020). Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. Journal of Translational Medicine 18: 1–19. https://doi.org/10.1186/s12967-020-02380-2 [Google Scholar] [PubMed] [CrossRef]
Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ (2003). Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 17: 33–42. https://doi.org/10.1097/00002030-200301030-00006 [Google Scholar] [PubMed] [CrossRef]
Ruiz-de-León MJ, Jiménez-Sousa MA, Moreno S, García M, Gutiérrez-Rivas M et al. (2019). Lower expression of plasma-derived exosome miR-21 levels in HIV-1 elite controllers with decreasing CD4 T cell count. Journal of Microbiology, Immunology and Infection 52: 667–671. https://doi.org/10.1016/j.jmii.2018.07.007 [Google Scholar] [PubMed] [CrossRef]
Russell KA, Garbin LC, Wong JM, Koch TG (2020). Mesenchymal stromal cells as potential antimicrobial for veterinary use—A comprehensive review. Frontiers in Microbiology 11: 606404. [Google Scholar] [PubMed]
Saad MH, Badierah R, Redwan EM, El-Fakharany EM (2021). A Comprehensive insight into the role of exosomes in viral infection: Dual faces bearing different functions. Pharmaceutics 13: 1405. https://doi.org/10.3390/pharmaceutics13091405 [Google Scholar] [PubMed] [CrossRef]
Salvatori G, Luberto L, Maffei M, Aurisicchio L, Roscilli G, Palombo F, Marra E (2020). SARS-CoV-2 SPIKE PROTEIN: An optimal immunological target for vaccines. Journal of Translational Medicine 18: 1–3. [Google Scholar]
Schaefer MR, Wonderlich ER, Roeth JF, Leonard JA, Collins KL (2008). HIV-1 Nef targets MHC-I and CD4 for degradation via a final common β-COP-dependent pathway in T cells. PLoS Pathogens 4: e1000131. https://doi.org/10.1371/journal.ppat.1000131 [Google Scholar] [PubMed] [CrossRef]
Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells and Development 29: 747–754. [Google Scholar] [PubMed]
Sheykhhasan M, Amini R, Soleimani Asl S, Saidijam M, Hashemi SM, Najafi R (2022). Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomedicine & Pharmacotherapy 152: 113224. https://doi.org/10.1016/j.biopha.2022.113224 [Google Scholar] [PubMed] [CrossRef]
Sheykhhasan M, Foroutan A, Manoochehri H, Khoei SG, Poondla N, Saidijam M (2021a). Could gene therapy cure HIV? Life Sciences 277: 119451. https://doi.org/10.1016/j.lfs.2021.119451 [Google Scholar] [PubMed] [CrossRef]
Sheykhhasan M, Kalhor N, Sheikholeslami A, Dolati M, Amini E, Fazaeli H (2021b). Exosomes of mesenchymal stem cells as a proper vehicle for transfecting miR-145 into the breast cancer cell line and its effect on metastasis. BioMed Research International 2021: 5516078. https://doi.org/10.1155/2021/5516078 [Google Scholar] [PubMed] [CrossRef]
Sheykhhasan M, Manoochehri H, Saidijam M (2020). New therapeutic strategies in treating the new Coronavirus 2019. Tehran University Medical Journal 78: 261–273. [Google Scholar]
Sil S, Dagur RS, Liao K, Peeples ES, Hu G, Periyasamy P, Buch S (2020). Strategies for the use of extracellular vesicles for the delivery of therapeutics. Journal of Neuroimmune Pharmacology 15: 422–442. [Google Scholar] [PubMed]
Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S (2020). Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clinical Rheumatology 39: 2085–2094. [Google Scholar] [PubMed]
Subra C, Laulagnier K, Perret B, Record M (2007). Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 89: 205–212. [Google Scholar] [PubMed]
Tabari D, Scholl C, Steffens M, Weickhardt S, Elgner F, Bender D, Herrlein ML, Sabino C, Semkova V, Peitz M (2020). Impact of Zika virus infection on human neural stem cell microRNA signatures. Viruses 12: 1219. [Google Scholar] [PubMed]
Tong L, Yan C, Wang M, Yang J, Wang H, Wang Y (2021a). Prognostic value of serum exosomal AHCY expression in hepatitis B-induced liver cirrhosis. Frontiers in Medicine 8: 777452. https://doi.org/10.3389/fmed.2021.777452 [Google Scholar] [PubMed] [CrossRef]
Tong L, Yan C, Wang M, Yang J, Wang H, Wang Y (2021b). Prognostic value of serum exosomal AHCY expression in hepatitis B-induced liver cirrhosis. Frontiers in Medicine 8: 2080. [Google Scholar]
Tribolet L, Kerr E, Cowled C, Bean AGD, Stewart CR, Dearnley M, Farr RJ (2020). MicroRNA biomarkers for infectious diseases: From basic research to biosensing. Frontiers in Microbiology 11: 1197. https://doi.org/10.3389/fmicb.2020.01197 [Google Scholar] [PubMed] [CrossRef]
Tsuchiya A, Takeuchi S, Iwasawa T, Kumagai M, Sato T, Motegi S, Ishii Y, Koseki Y, Tomiyoshi K, Natsui K (2020). Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflammation and Regeneration 40: 1–6. [Google Scholar]
Urbanelli L, Buratta S, Tancini B, Sagini K, Delo F, Porcellati S, Emiliani C (2019). The role of extracellular vesicles in viral infection and transmission. Vaccines 7: 102. [Google Scholar] [PubMed]
Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PG, Fu H (2020). Estimates of the severity of coronavirus disease 2019: A model-based analysis. The Lancet Infectious Diseases 20: 669–677. [Google Scholar] [PubMed]
Wan Y, Shang J, Graham R, Baric RS, Li F (2020). Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS Coronavirus. Journal of Virology 94: e00127–00120. https://doi.org/10.1128/JVI.00127-20 [Google Scholar] [PubMed] [CrossRef]
Wang Y, Kang H, Liu X, Tong Z (2020). Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. Journal of Medical Virology 92: 538–539. [Google Scholar] [PubMed]
Welker MW, Reichert D, Susser S, Sarrazin C, Martinez Y, Herrmann E, Zeuzem S, Piiper A, Kronenberger B (2012). Soluble serum CD81 is elevated in patients with chronic hepatitis C and correlates with alanine aminotransferase serum activity. PLoS One 7: e30796. [Google Scholar] [PubMed]
Worthington EN, Hagood JS (2020). Therapeutic use of extracellular vesicles for acute and chronic lung disease. International Journal of Molecular Sciences 21: 2318. [Google Scholar] [PubMed]
Wu R, Gao W, Yao K, Ge J (2019). Roles of exosomes derived from immune cells in cardiovascular diseases. Frontiers in Immunology 10: 648. [Google Scholar] [PubMed]
Wu Q, Glitscher M, Tonnemacher S, Schollmeier A, Raupach J, Zahn T, Eberle R, Krijnse-Locker J, Basic M, Hildt E (2023). Presence of intact hepatitis B virions in exosomes. Cellular and Molecular Gastroenterology and Hepatology 15: 237–259. https://doi.org/10.1016/j.jcmgh.2022.09.012 [Google Scholar] [PubMed] [CrossRef]
Wu W, Wu D, Yan W, Wang Y, You J, Wan X, Xi D, Luo X, Han M, Ning Q (2021). Interferon-induced macrophage-derived exosomes mediate antiviral activity against hepatitis B virus through miR-574-5p. The Journal of Infectious Diseases 223: 686–698. [Google Scholar] [PubMed]
Xu W, Santini PA, Sullivan JS, He B, Shan M et al. (2009). HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nature Immunology 10: 1008–1017. https://doi.org/10.1038/ni.1753 [Google Scholar] [PubMed] [CrossRef]
Xu X, Zhang L, Liu J, Kong X, Yin Y, Jia Z, Zhang X, Peng B, Ji M, Pan W (2023). Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B. Open Life Sciences 18: 20220585. https://doi.org/10.1515/biol-2022-0585 [Google Scholar] [PubMed] [CrossRef]
Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J (2017). Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cellular & Molecular Immunology 14: 465–475. https://doi.org/10.1038/cmi.2016.24 [Google Scholar] [PubMed] [CrossRef]
Yao Z, Jia X, Megger DA, Chen J, Liu Y, Li J, Sitek B, Yuan Z (2018). Label-free proteomic analysis of exosomes secreted from THP-1-derived macrophages treated with IFN-α identifies antiviral proteins enriched in exosomes. Journal of Proteome Research 18: 855–864. [Google Scholar]
Yin Y, Zhao Y, Chen Q, Chen Y, Mao L (2022). Dual roles and potential applications of exosomes in HCV infections. Frontiers in Microbiology 13: 1044832. [Google Scholar] [PubMed]
York SB, Sun L, Cone AS, Duke LC, Cheerathodi MR, MeckesJr, DG (2021). Zika virus hijacks extracellular vesicle tetraspanin pathways for cell-to-cell transmission. mSphere 6: e00192–00121. [Google Scholar]
Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, Fang X, Zhang X (2022). Exosomes as a new frontier of cancer liquid biopsy. Molecular Cancer 21: 56. [Google Scholar] [PubMed]
Zhang Q, Qu Y, Zhang Q, Li F, Li B, Li Z, Dong Y, Lu L, Cai X (2023). Exosomes derived from hepatitis B virus-infected hepatocytes promote liver fibrosis via miR-222/TFRC axis. Cell Biology and Toxicology 39: 467–481. https://doi.org/10.1007/s10565-021-09684-z [Google Scholar] [PubMed] [CrossRef]
Zhang Y, Wei W, Cheng N, Wang K, Li B, Jiang X, Sun S (2012). Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 56: 1631–1640. [Google Scholar] [PubMed]
Zhong J, Tang J, Ye C, Dong L (2020). The immunology of COVID-19: Is immune modulation an option for treatment? The Lancet Rheumatology 2: e428–e436. [Google Scholar] [PubMed]
Zhou L, Wang W, Wang F, Yang S, Hu J, Lu B, Pan Z, Ma Y, Zheng M, Zhou L (2021). Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Molecular Cancer 20: 1–6. [Google Scholar]
Zhou W, Woodson M, Sherman MB, Neelakanta G, Sultana H (2019). Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerging Microbes & Infections 8: 307–326. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools