 Open Access
Open Access
VIEWPOINT
Analysis of tumor-draining vein secretome: A direct access to tumor-derived extracellular vesicles in surgical lung cancer patients
1 Molecular Oncology and Embryology Laboratory, Department of Surgery and Surgical Specializations, Human Anatomy Unit, Faculty of Medicine and Health Sciences, Universitat de Barcelona (UB), Barcelona, 08036, Spain
2 School of Basic Medical Sciences, Chengdu University, Chengdu, 610106, China
3 Department of Thoracic Surgery, Hospital Clínic de Barcelona, Universitat de Barcelona (UB), Barcelona, 08036, Spain
4 Thoracic Oncology Unit, Hospital Clinic, Barcelona, 08036, Spain
5 Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, 08036, Spain
6 Department of Pneumology, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Universitat de Barcelona (UB), Barcelona, 08036, Spain
7 Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, 28029, Spain
* Corresponding Author: ALFONS NAVARRO. Email:
BIOCELL 2023, 47(5), 951-957. https://doi.org/10.32604/biocell.2023.027718
Received 11 November 2022; Accepted 31 January 2023; Issue published 10 April 2023
Abstract
Tumor-secreted extracellular vesicles (EVs) participate in the metastasis process through different mechanisms, including the preparation of the pre-metastatic niche to grant circulating tumor cells (CTCs) implantation and growth. The study of the metastasis process through the analysis of CTCs and tumor-derived EVs is difficult because of the dilution grade of these elements in peripheral blood. In early-stage lung cancer patients, the tumor-secreted products are even more diluted. An attractive strategy in surgical lung cancer patients is to purify them from a pulmonary tumor-draining vein where they are enriched. The information obtained from the analysis of EVs and CTCs purified from this source could give more accurate information about tumor biology and could be an important source of biomarkers to identify patients at high risk of relapse after curative surgery.Keywords
Lung cancer remains one of the top leading causes of cancer-related deaths in 2022. According to GLOBOCAN statistics, lung cancer caused 18% of cancer-related deaths worldwide in 2020 (Sung et al., 2021). Most of the patients are diagnosed with non-small cell lung cancer (NSCLC), the most common type of lung cancer (Jemal et al., 2011). The 5-year survival of patients diagnosed with NSCLC cancer is still poor with only a median of 26% of survival (Allemani et al., 2018). Treatment strategy depends mainly on the disease stage and in localized tumors, which can be treated with surgery, and the 5-year survival can increase up to 64%. Surgical resection is possibly the most curative therapeutic option for early and even locally-advanced stages (Vansteenkiste et al., 2013). Nevertheless, around 35% of resected NSCLC patients develop recurrence and die of their disease (Hofman et al., 2011; Vokes, 2000). So surgical treatment is less than perfect, even if a complete macroscopic resection can be performed (Hashimoto et al., 2014). Numerous studies have tried to determine the elements involved in post-surgical relapse and recently the focus is on circulating tumor cells (CTCs) and tumor-secreted small extracellular vesicles (EVs). Small EVs term is used to define a group of vesicles ranging from 30 to 150 nm purified only according to physical characteristics like size (f.i. by ultracentrifugation) and includes two different populations: exosomes and microvesicles (Théry et al., 2018). Small EVs are a heterogeneous group of cell-derived membranous structures, which originate from the endosomal system in the case of exosomes or are shed from the plasma membrane in the case of microvesicles (Théry et al., 2009; van Niel et al., 2018). Now, it has been proved that EVs, especially exosomes, play a crucial role in both local and distant intercellular communication.
Circulating tumor cells, extracellular vesicles, and metastasis
CTCs and tumor-derived EVs are the main actors in the metastasis process (Jerabkova-Roda et al., 2022). During cancer progression, some tumoral cells detach from the main tumor and can attach to endothelial cells to enter the circulation. Likewise, the primary tumor can communicate with CTCs and “control” them by releasing EVs to the circulation that can target the CTCs (Fu et al., 2018; Ghoroghi et al., 2021a). Moreover, EVs themselves participate in the development of the premetastatic niche (Ghoroghi et al., 2021a; Peinado et al., 2017). In fact, it is widely accepted that priming with EVs precedes secondary tumor growth and is an important step in the cancer metastasis process (Peinado et al., 2017).
Recently, it has been shown that tumors exploit transmembrane cell adhesion molecules (CAMs), which participate in cell-to-cell interactions (Berrier and Yamada, 2007; Geiger et al., 2009), to direct CTCs and EVs migration toward a specific organ (organotropism). EVs and CTCs share similar CAMs that allow their own interaction and at the same time direct both to the same metastatic site. Moreover, these CAMs participate in the adhesion to the endothelium during the extravasation process (Ghoroghi et al., 2021b; Osmani et al., 2019).
Taking into account all this information, it is clear that characterizing the CTCs and tumor-secreted EVs can allow a better comprehension of the metastasis process and they can act as a source of relapse biomarkers to identify patients at high risk of relapse, and even identify the potential metastatic site before it is clinically detectable (Gold et al., 2015). But the question is, can this be properly performed in peripheral blood?
Tumor-draining vein as an enriched source of circulating tumor cells and extracellular vesicles
Despite great efforts in the development of more sensitive and efficient CTC isolation platforms, due to their ambiguity, rarity, and heterogeneity, isolation of CTCs remains a challenge due to their low abundance in peripheral blood (Bu et al., 2016). Despite great efforts in the development of more sensitive and efficient CTC isolation platforms, due to their ambiguity, rarity, and heterogeneity, isolation of CTCs remains a challenge due to their low abundance in peripheral blood (Bu et al., 2016). This is even more problematic in early-stage patients with no disseminated disease (Murlidhar et al., 2017). Similarly, EVs, are released by virtually all cell types and not only by tumor cells (Ludwig and Giebel, 2012) producing an important dilution effect on peripheral blood (Sharma et al., 2018) and requiring the identification of appropriate surface markers to allow the correct purification of the tumoral ones (Beltraminelli et al., 2021; Hoshino et al., 2020). So far, between the pool of identified surface exosomal proteins, the integrin family has emerged as one of the important elements involved in direct organ-specific colonization and pre-metastatic niche development (Hoshino et al., 2015). However, their role in the specific purification of tumor-derived EVs has not been properly explored. Several authors, including our group, have explored the effectiveness of using tumor-draining vein (TDV) as an enriched source of tumor-secreted products for refining the detection of CTCs and tumor-derived EVs. To understand the importance of TDV, we need to review how pulmonary circulation works. Anatomically, the lung can be divided into lobules and subdivided into segments. The venous blood from each segment is collected by a segmental vein. Segmental veins converge towards pulmonary veins. There are four pulmonary veins: right superior pulmonary vein (collects blood from the upper and middle lobes of the right lung), right inferior pulmonary vein (collects blood from the lower lobe of the right lung), left superior pulmonary vein (collects blood from the upper lobe and lingula of left lung), and the left inferior pulmonary vein (collects blood from the lower lobe of the left lung). Finally, pulmonary veins drain blood to the left atrium of the heart from where it is widely distributed peripherally (Porres et al., 2013). When a tumor is found in a specific lung lobule/segment, most cancer cells will disseminate through its specific pulmonary vein (Dudek and Louis, 2013). Therefore, the collection of blood from the pulmonary vein draining from the lobe where the tumor is located would allow the collection of tumor-secreted products (Buscail et al., 2019) (Fig. 1). Confirming this hypothesis, numerous studies have shown that blood obtained from TVD is enriched in tumor-secreted products, including proteins (Geary et al., 2019), CTCs (Crosbie et al., 2016; Hashimoto et al., 2014; Hattori et al., 2019; Murlidhar et al., 2017; Reddy et al., 2016) and even small EVs (Castellano et al., 2020; Han et al., 2022; Navarro et al., 2019) (Fig. 2). Geary et al. (2019) used blood from pulmonary TDV to profile the tumor secreted proteins and observed that the identified proteins correlated with features of the natural history of the tumor in situ. Many proteins were observed enriched in the TVD in comparison to a pulmonary vein from a non-cancerous lobe. They used the identified proteins to generate a panel biomarker of utility in the early diagnosis of lung cancer (Geary et al., 2019). Several groups have quantified CTCs in TDV to evaluate their potential as a prognostic biomarker, especially to identify patients at high risk of relapse after a curative surgery. Sienel et al. (2003) analyzed CTCs in TDV in a cohort of 62 patients; Funaki et al. (2011) studied 92 patients; Franco et al. (2012) analyzed 45 patients; and Crosbie et al. (2016) 30 patients. All these studies validate the superiority of the TDV in front of the peripheral vein at both the detection of CTCs and the capacity of relapse prediction.
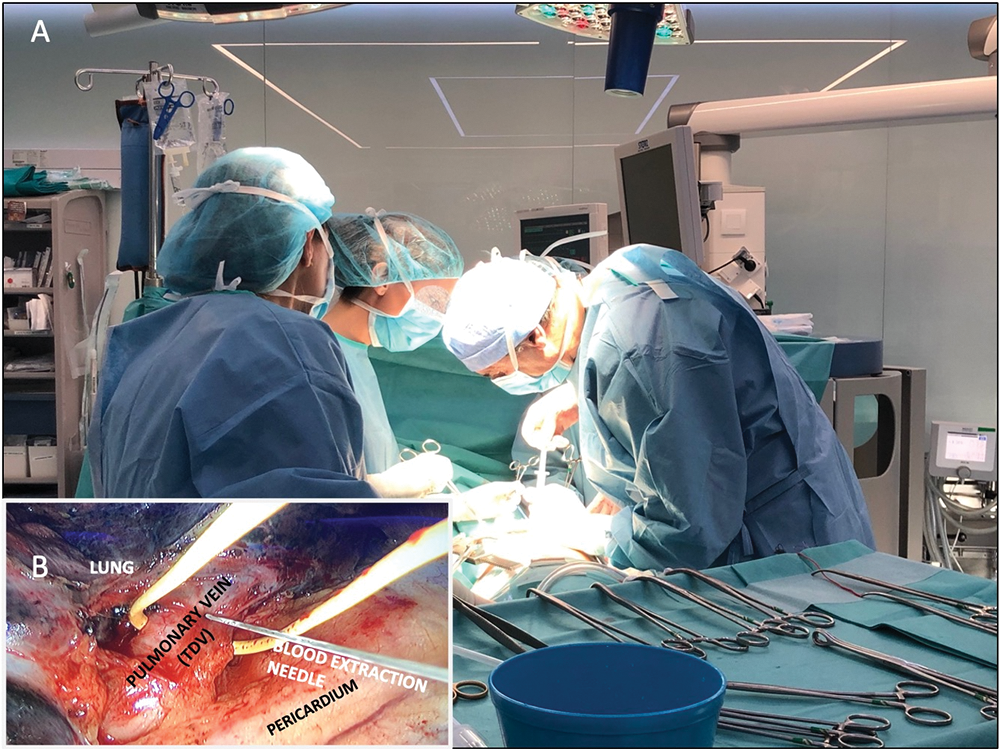
Figure 1: Intraoperative picture of the surgical procedure. (A) The image shows the moment when the surgeon is performing the extraction of blood from the pulmonary tumor-draining vein at the time of operation on the lung cancer to perform tumor resection. (B) Detail of the surgical field showing the pulmonary vein, the extraction needle, and the lung lobule where the tumor to be resected is located.
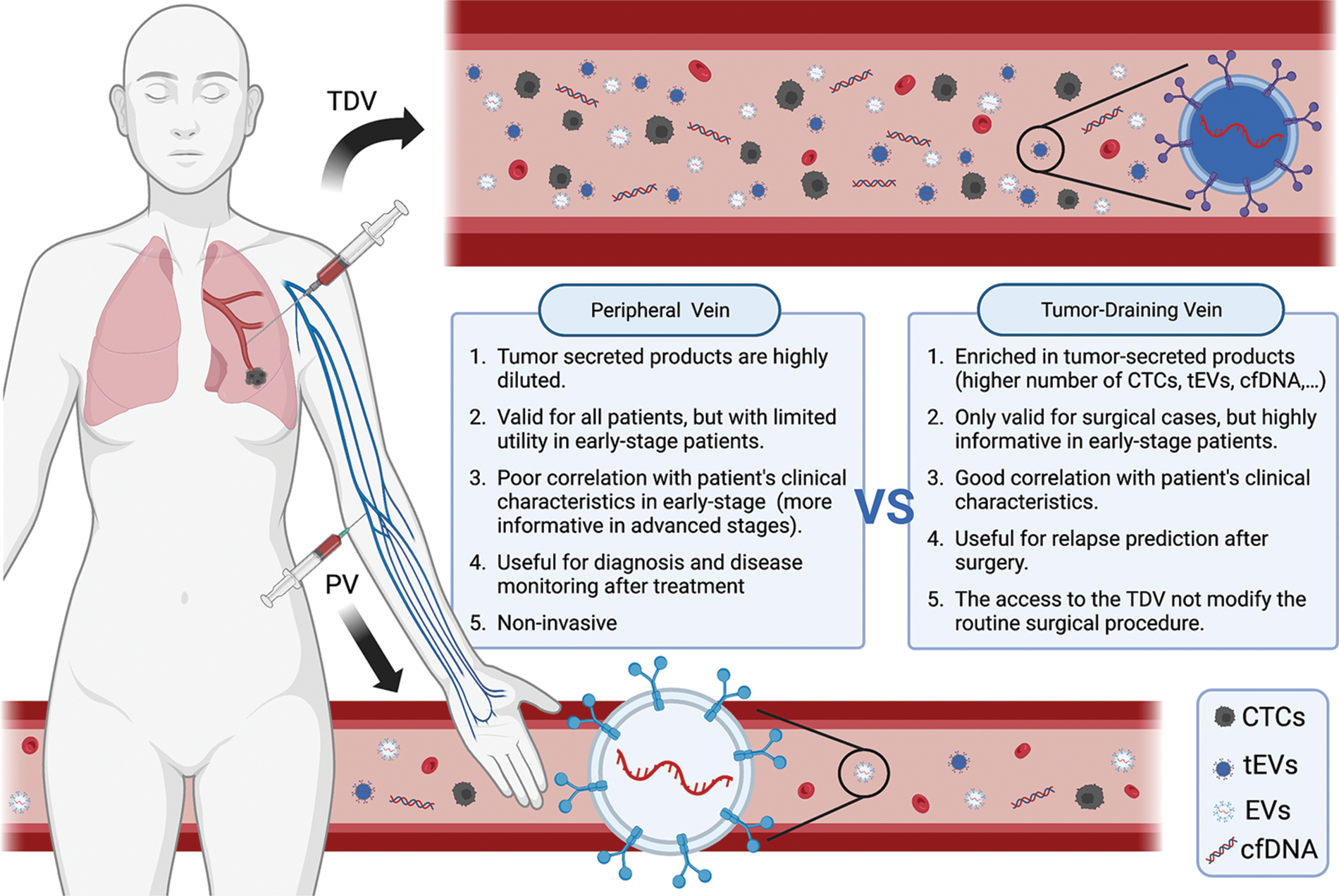
Figure 2: Scheme representing the main differences between peripheral and tumor-draining vein blood, especially in relation to circulating tumor cells (CTCs) and tumor-derived extracellular vesicles (EVs) content. Created with BioRender.com.
Analysis of extracellular vesicles in the tumor-draining vein
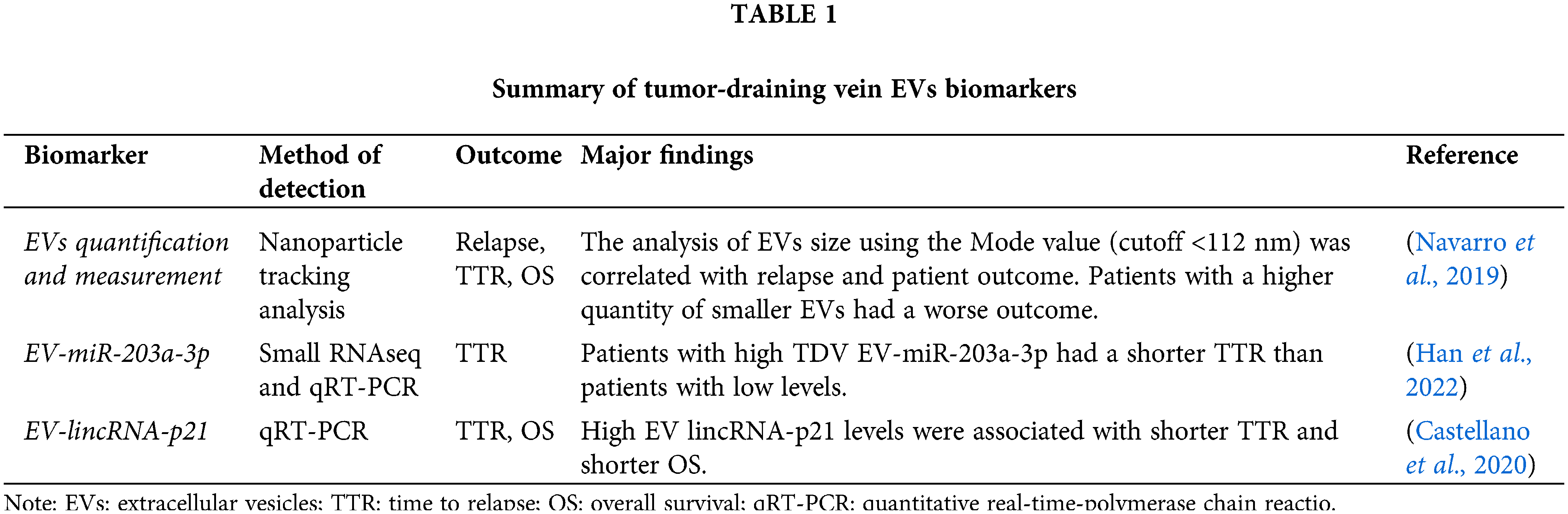
Although the analysis of CTCs in TDV has been extensively analyzed, few groups have studied this source EVs. Our group (Navarro et al., 2019), first quantified and analyzed the EVs size distribution in TDV in comparison with the paired peripheral vein. While we expected to find higher levels of EVs in TDV than in PV, we did not observe significant differences in quantity, but otherwise, differences in the size distribution of EVs were observed. A higher proportion of small-size EVs (30–50 nm) was found in TDV. Assessment of the relationship of the specific levels in each patient and the main clinical characteristics requires to be performed in the operating room and it is shown that the level of EVs in TDV were highly correlated with the disease stage, while no correlation was observed in PV. Additionally, the size of EVs from the pulmonary vein was significantly smaller in patients with relapsed compared to patients with non-relapsed lung cancer, while those differences were not detected in the peripheral vein (Navarro et al., 2019). Choi et al. (2020) confirmed that quantification of EVs in pulmonary TDV showed a higher correlation with the disease stage compared to those in the peripheral vein. In their study, Choi et al. (2020) performed both in vivo (rabbits with lung cancer) and ex-vivo studies to validate the properties of TDV in relation to EVs quantification. Their study showed that EVs levels from TDV in both animals and patients having lung cancer were significantly higher than those in preoperative peripheral blood (Caivano et al., 2015; Choi et al., 2020). In both studies, Navarro and Choi showed that EV levels in the pulmonary TDV blood reflect more strictly the tumor stage and could be used as a superior prognostic tool for lung cancer patients who had undergone surgery (Choi et al., 2020; Navarro et al., 2019).
The study of the pulmonary TDV-derived EVs cargo is of great interest. EVs cargo contains assorted molecules like DNA, mRNAs, microRNA (miRNA), circRNAs, proteins, enzymes, and many more molecules, that can potentially cause epigenetic manipulations in the target cells (Kanada et al., 2016; Vahabi et al., 2022). One of the most enriched products in EVs are miRNAs (representing around 40% of RNA content) (Yuan et al., 2016), which have recently gained significant attention as they were found to cause epigenetic regulation through translational silencing and mRNA instability (Ekstrom et al., 2012; Qing et al., 2018). Our group recently analyzed in a study by Han et al. the miRNA cargo of EVs purified from pulmonary TDV by small RNAseq and identified an miRNA signature composed of 17 EV-miRNAs able to predict relapse in NSCLC patients who had undergone surgery. The study of the miRNA with the highest levels, EV-miR-203a-3p, allowed its validation in an independent cohort of 70 patients the prognostic role of this EV-miRNA in TDV. When this EV miRNA was quantified in the paired peripheral blood, we observed that in almost one-third of the patients, EV-miR-203a-3p was even not detected in peripheral blood and when detected, the levels in TDV were significantly higher (Han et al., 2022). Before this screening analysis, our group in Castellano et al. (2020) analyzed the presence of a long non-coding RNA (lincRNA-p21) in TDV and also demonstrated its utility as a post-surgical relapse biomarker. We examined the role of lincRNA-p21 since previously we observed its utility as a biomarker when quantified in tumor tissues by regulating microvessel formation in the context of hypoxia (Castellano et al., 2016). Unlike miRNAs, lncRNAs are RNA molecules longer than 200 nt with little or no protein-coding capacity (Wilusz et al., 2009). Usually, lncRNA genes comprise fewer exons than mRNAs, since they are less constrained by selection during evolution; their expression is highly cell type/tissue-dependent showing a high tissue specificity (Cabili et al., 2011) and a high cancer type specificity (Yan et al., 2015). On analyzing lincRNA-p21 in EVs from lung cancer cell lines, we observed an overexpression under hypoxic conditions (Castellano et al., 2016; Huarte and Rinn, 2010). Analysis of EVs purified from pulmonary TDV showed that patients with high EV-lincRNA-p21 had a worse outcome after surgery. Our in vitro studies demonstrated that EV-lincRNA-p21 promoted angiogenesis and modulated the EVs cargo, enriching EVs with pro-angiogenin miRNAs that might be transferred from tumor to endothelial cells through EVs (Castellano et al., 2020; Castellano et al., 2016). The main biomarkers described in tumor-draining vein EVs are summarized in Table 1.
The tumor-derived EVs cargo is enhanced in TDV because it is enriched in tumor-secreted products. However, much remains to be done in this promising area of research (Castellano et al., 2020; Han et al., 2022; Navarro et al., 2019), which could be of special interest in early-stage tumors with low tumor dissemination grade. Nevertheless, some limitations need to be considered, especially when comparing its use with peripheral vein blood (Fig. 2). Compared to peripheral vein analysis, obtaining blood from the TDV can be considered invasive because requires to be performed in the operating room and it is limited to patients treated with surgery as a first treatment option. Yet, it is important to clarify that obtaining the TDV blood sample does not modify the routine surgical procedure. To obtain the blood, a gauge needle is used to puncture the visible pulmonary vein draining from the lobule/segment where the tumor is located. The surgeons need to locate and ligate the pulmonary vein prior to the process of the tumor resection anyway, irrespective of whether is going to extract pulmonary blood or not. Additionally, we must consider that this analysis is limited to NSCLC patients who undergo surgery and cannot be easily extended to advanced and even less to non-surgical NSCLC patients. However, as we have commented previously, TDV adds important information, especially in early-stage tumors, most of which are surgically treated, while in advanced stages the peripheral blood is already a valuable tool because of the higher dissemination grade of the disease in advanced stages.
Focusing on EVs, another limitation that is not exclusively associated with TDV, is that we need to consider the lack of a standard and reliable method for purifying EVs (Caivano et al., 2015; Gardiner et al., 2016). The EVs purification methodology needs to be standardized to improve its clinical application. The stability of EVs under different conditions in terms of maintaining viability and sample processing, allows them to be used in various fields without limitations (Kang et al., 2017) being a good source for biomarker identification, although clinical standardization is required. Additionally, the increasing interest in the study of EVs isolated directly from tumors (Beltraminelli et al., 2021; Gardiner et al., 2016; Shi et al., 2020) makes their study in TDV an excellent source, at least until the identification of appropriate surface markers that will allow specific purification of tumor-derived EVs. The clinical utility of quantification and characterization of EVs in TDV need further validation since most of the studies performed using TDV are retrospective studies that need to be confirmed in a prospective study, such as the ongoing clinical trial (NCT04939324) by the French group from the University Hospital of Limoges, that are recruiting early-stage NSCLC patients—who have undergone surgery—with the primary objective of evaluating size distribution, concentration and the molecular profiling of pulmonary vein exosomes and correlating them with patient characteristics and outcome. We will need to wait for the results of this and other clinical trials to establish the clinical role of EVs on NSCLC patients having undergone surgery.
Funding Statement: Ministry of Economy and Competition (MINECO) Co-Financed with the European Union FEDER Funds (SAF2017-88606-P, 2017); SEPAR-AstraZeneca Ayudas Investigación PII Oncología 2021; Becas SEPAR 2022 (Proyecto 1326).
Author Contributions: YH contributed to the conceptualization and prepared the manuscript draft. DSL, MAP, MB, AG, RMM, and LM contributed to the critical evaluation of the manuscript. AN contributed to conceptualization, and critical evaluation, and coordinated throughout the manuscript writing. All authors approved the final version of the manuscript.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Allemani C, Matsuda T, di Carlo V, Harewood R, Matz M et al. (2018). Global surveillance of trends in cancer survival 2000-14 (CONCORD-3Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet 391: 1023–1075. https://doi.org/10.1016/S0140-6736(17)33326-3 [Google Scholar] [PubMed] [CrossRef]
Beltraminelli T, Perez CR, de Palma M (2021). Disentangling the complexity of tumor-derived extracellular vesicles. Cell Reports 35: 108960. https://doi.org/10.1016/j.celrep.2021.108960 [Google Scholar] [PubMed] [CrossRef]
Berrier AL, Yamada KM (2007). Cell-matrix adhesion. Journal of Cellular Physiology 213: 565–573. https://doi.org/10.1002/(ISSN)1097-4652 [Google Scholar] [CrossRef]
Bu J, Kang YT, Kim YJ, Cho YH, Chang HJ, Kim H, Moon BI, Kim HG (2016). Dual-patterned immunofiltration (DIF) device for the rapid efficient negative selection of heterogeneous circulating tumor cells. Lab on a Chip 16: 4759–4769. https://doi.org/10.1039/C6LC01179A [Google Scholar] [PubMed] [CrossRef]
Buscail E, Chiche L, Laurent C, Vendrely V, Denost Q et al. (2019). Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Molecular Oncology 13: 1811–1826. https://doi.org/10.1002/1878-0261.12534 [Google Scholar] [PubMed] [CrossRef]
Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL (2011). Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes & Development 25: 1915–1927. https://doi.org/10.1101/gad.17446611 [Google Scholar] [PubMed] [CrossRef]
Caivano A, Laurenzana I, de Luca L, La Rocca F, Simeon V et al. (2015). High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumor Biology 36: 9739–9752. https://doi.org/10.1007/s13277-015-3741-3 [Google Scholar] [PubMed] [CrossRef]
Castellano JJ, Marrades RM, Molins L, Viñolas N, Moises J et al. (2020). Extracellular vesicle lincRNA-p21 expression in tumor-draining pulmonary vein defines prognosis in NSCLC and modulates endothelial cell behavior. Cancers 12: 734. https://doi.org/10.3390/cancers12030734 [Google Scholar] [PubMed] [CrossRef]
Castellano JJ, Navarro A, Viñolas N, Marrades RM, Moises J et al. (2016). LincRNA-p21 impacts prognosis in resected non-small cell lung cancer patients through angiogenesis regulation. Journal of Thoracic Oncology 11: 2173–2182. https://doi.org/10.1016/j.jtho.2016.07.015 [Google Scholar] [PubMed] [CrossRef]
Choi BH, Quan YH, Rho J, Hong S, Park Y et al. (2020). Levels of extracellular vesicles in pulmonary and peripheral blood correlate with stages of lung cancer patients. World Journal of Surgery 44: 3522–3529. https://doi.org/10.1007/s00268-020-05630-y [Google Scholar] [PubMed] [CrossRef]
Crosbie PA, Shah R, Krysiak P, Zhou C, Morris K, Tugwood J, Booton R, Blackhall F, Dive C (2016). Circulating tumor cells detected in the tumor-draining pulmonary vein are associated with disease recurrence after surgical resection of NSCLC. Journal of Thoracic Oncology 11: 1793–1797. https://doi.org/10.1016/j.jtho.2016.06.017 [Google Scholar] [PubMed] [CrossRef]
Dudek RW, Louis TM (2013). High-yieldTM gross anatomy. USA: Lippincott Williams & Wilkins. [Google Scholar]
Ekstrom K, Valadi H, Sjostrand M, Malmhall C, Bossios A, Eldh M, Lotvall J (2012). Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. Journal of Extracellular Vesicles 1: 18389. https://doi.org/10.3402/jev.v1i0.18389 [Google Scholar] [PubMed] [CrossRef]
Franco R, Pirozzi G, Scala S, Cantile M, Scognamiglio G, Camerlingo R, Botti G, Rocco G (2012). CXCL12-binding receptors expression in non-small cell lung cancer relates to tumoral microvascular density and CXCR4 positive circulating tumoral cells in lung draining venous blood. European Journal of Cardio-Thoracic Surgery 41: 368–375. https://doi.org/10.1016/j.ejcts.2011.05.009 [Google Scholar] [PubMed] [CrossRef]
Fu Q, Zhang Q, Lou Y, Yang J, Nie G et al. (2018). Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene 37: 6105–6118. https://doi.org/10.1038/s41388-018-0391-0 [Google Scholar] [PubMed] [CrossRef]
Funaki S, Sawabata N, Nakagiri T, Shintani Y, Inoue M, Kadota Y, Minami M, Okumura M (2011). Novel approach for detection of isolated tumor cells in pulmonary vein using negative selection method: Morphological classification and clinical implications. European Journal of Cardio-Thoracic Surgery 40: 322–327. https://doi.org/10.1016/j.ejcts.2010.11.029 [Google Scholar] [PubMed] [CrossRef]
Gardiner C, Vizio DD, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF (2016). Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. Journal of Extracellular Vesicles 5: 32945. https://doi.org/10.3402/jev.v5.32945 [Google Scholar] [PubMed] [CrossRef]
Geary B, Walker MJ, Snow JT, Lee DCH, Pernemalm M et al. (2019). Identification of a biomarker panel for early detection of lung cancer patients. Journal of Proteome Research 18: 3369–3382. https://doi.org/10.1021/acs.jproteome.9b00287 [Google Scholar] [PubMed] [CrossRef]
Geiger B, Spatz JP, Bershadsky AD (2009). Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology 10: 21–33. https://doi.org/10.1038/nrm2593 [Google Scholar] [PubMed] [CrossRef]
Ghoroghi S, Mary B, Asokan N, Goetz JG, Hyenne V (2021a). Tumor extracellular vesicles drive metastasis (it’s a long way from home). FASEB BioAdvances 3: 930–943. https://doi.org/10.1096/fba.2021-00079 [Google Scholar] [PubMed] [CrossRef]
Ghoroghi S, Mary B, Larnicol A, Asokan N, Klein A et al. (2021b). Ral GTPases promote breast cancer metastasis by controlling biogenesis and organ targeting of exosomes. eLife 10: e61539. https://doi.org/10.7554/eLife.61539 [Google Scholar] [PubMed] [CrossRef]
Gold B, Cankovic M, Furtado LV, Meier F, Gocke CD (2015). Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. The Journal of Molecular Diagnostics 17: 209–224. https://doi.org/10.1016/j.jmoldx.2015.02.001 [Google Scholar] [PubMed] [CrossRef]
Han B, Molins L, He Y, Viñolas N, Sánchez-Lorente D et al. (2022). Characterization of the MicroRNA cargo of extracellular vesicles isolated from a pulmonary tumor-draining vein identifies miR-203a-3p as a relapse biomarker for resected non-small cell lung cancer. International Journal of Molecular Sciences 23: 7138. https://doi.org/10.3390/ijms23137138 [Google Scholar] [PubMed] [CrossRef]
Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S et al. (2014). Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interactive CardioVascular and Thoracic Surgery 18: 775–783. https://doi.org/10.1093/icvts/ivu048 [Google Scholar] [PubMed] [CrossRef]
Hattori M, Nakanishi H, Yoshimura M, Iwase M, Yoshimura A et al. (2019). Circulating tumor cells detection in tumor draining vein of breast cancer patients. Scientific Reports 9: 18195. https://doi.org/10.1038/s41598-019-54839-y [Google Scholar] [PubMed] [CrossRef]
Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM et al. (2011). Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clinical Cancer Research 17: 827–835. https://doi.org/10.1158/1078-0432.CCR-10-0445 [Google Scholar] [PubMed] [CrossRef]
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335. https://doi.org/10.1038/nature15756 [Google Scholar] [PubMed] [CrossRef]
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M et al. (2020). Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 182: 1044–1061.e1018. https://doi.org/10.1016/j.cell.2020.07.009 [Google Scholar] [PubMed] [CrossRef]
Huarte M, Rinn JL (2010). Large non-coding RNAs: Missing links in cancer? Human Molecular Genetics 19: R152–R161. https://doi.org/10.1093/hmg/ddq353 [Google Scholar] [PubMed] [CrossRef]
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians 61: 69–90. https://doi.org/10.3322/caac.20107 [Google Scholar] [PubMed] [CrossRef]
Jerabkova-Roda K, Dupas A, Osmani N, Hyenne V, Goetz JG (2022). Circulating extracellular vesicles and tumor cells: Sticky partners in metastasis. Trends in Cancer 8: 799–805. https://doi.org/10.1016/j.trecan.2022.05.002 [Google Scholar] [PubMed] [CrossRef]
Kanada M, Bachmann MH, Contag CH (2016). Signaling by extracellular vesicles advances cancer hallmarks. Trends in Cancer 2: 84–94. https://doi.org/10.1016/j.trecan.2015.12.005 [Google Scholar] [PubMed] [CrossRef]
Kang YT, Kim YJ, Bu J, Cho YH, Han SW, Moon BI (2017). High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale 9: 13495–13505. https://doi.org/10.1039/C7NR04557C [Google Scholar] [PubMed] [CrossRef]
Ludwig AK, Giebel B (2012). Exosomes: Small vesicles participating in intercellular communication. The International Journal of Biochemistry & Cell Biology 44: 11–15. https://doi.org/10.1016/j.biocel.2011.10.005 [Google Scholar] [PubMed] [CrossRef]
Murlidhar V, Reddy RM, Fouladdel S, Zhao L, Ishikawa MK et al. (2017). Poor prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancerscirculating tumor cell clusters in early lung cancer. Cancer Research 77: 5194–5206. https://doi.org/10.1158/0008-5472.CAN-16-2072 [Google Scholar] [PubMed] [CrossRef]
Navarro A, Molins L, Marrades RM, Moises J, Viñolas N, Morales S, Canals J, Castellano JJ, Ramírez J, Monzo M (2019). Exosome analysis in tumor-draining pulmonary vein identifies NSCLC patients with higher risk of relapse after curative surgery. Cancers 11: 249. https://doi.org/10.3390/cancers11020249 [Google Scholar] [PubMed] [CrossRef]
Osmani N, Follain G, León MJG, Lefebvre O, Busnelli I, Larnicol A, Harlepp S, Goetz JG (2019). Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell Reports 28: 2491–2500.e5. https://doi.org/10.1016/j.celrep.2019.07.102 [Google Scholar] [PubMed] [CrossRef]
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A et al. (2017). Pre-metastatic niches: Organ-specific homes for metastases. Nature Reviews Cancer 17: 302–317. https://doi.org/10.1038/nrc.2017.6 [Google Scholar] [PubMed] [CrossRef]
Porres DV, Morenza ÓP, Pallisa E, Roque A, Andreu J, Martínez M (2013). Learning from the pulmonary veins. Radiographics 33: 999–1022. https://doi.org/10.1148/rg.334125043 [Google Scholar] [PubMed] [CrossRef]
Qing L, Chen H, Tang J, Jia X (2018). Exosomes and their MicroRNA cargo: New players in peripheral nerve regeneration. Neurorehabil Neural Repair 32: 765–776. https://doi.org/10.1177/1545968318798955 [Google Scholar] [PubMed] [CrossRef]
Reddy RM, Murlidhar V, Zhao L, Grabauskiene S, Zhang Z et al. (2016). Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. The Journal of Thoracic and Cardiovascular Surgery 151: 852–858. https://doi.org/10.1016/j.jtcvs.2015.09.126 [Google Scholar] [PubMed] [CrossRef]
Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, Whiteside TL (2018). Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. Journal of Extracellular Vesicles 7: 1435138. https://doi.org/10.1080/20013078.2018.1435138 [Google Scholar] [PubMed] [CrossRef]
Shi A, Kasumova GG, Michaud WA, Cintolo-Gonzalez J, Díaz-Martínez M et al. (2020). Plasma-derived extracellular vesicle analysis and deconvolution enable prediction and tracking of melanoma checkpoint blockade outcome. Science Advances 6: eabb3461. https://doi.org/10.1126/sciadv.abb3461 [Google Scholar] [PubMed] [CrossRef]
Sienel W, Seen-Hibler R, Mutschler W, Pantel K, Passlick B (2003). Tumour cells in the tumour draining vein of patients with non-small cell lung cancer: Detection rate and clinical significance. European Journal of Cardio-Thoracic Surgery 23: 451–456. https://doi.org/10.1016/S1010-7940(02)00865-5 [Google Scholar] [PubMed] [CrossRef]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 71: 209–249. https://doi.org/10.3322/caac.21660 [Google Scholar] [PubMed] [CrossRef]
Théry C, Ostrowski M, Segura E (2009). Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology 9: 581–593. https://doi.org/10.1038/nri2567 [Google Scholar] [PubMed] [CrossRef]
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 7: 1535750. https://doi.org/10.1080/20013078.2018.1535750 [Google Scholar] [PubMed] [CrossRef]
Vahabi A, Rezaie J, Hassanpour M, Panahi Y, Nemati M, Rasmi Y, Nemati M (2022). Tumor cells-derived exosomal CircRNAs: Novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochemical Pharmacology 200: 115038. https://doi.org/10.1016/j.bcp.2022.115038 [Google Scholar] [PubMed] [CrossRef]
van Niel G, D’Angelo G, Raposo G (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology 19: 213–228. https://doi.org/10.1038/nrm.2017.125 [Google Scholar] [PubMed] [CrossRef]
Vansteenkiste J, de Ruysscher D, Eberhardt W, Lim E, Senan S, Felip E, Peters S (2013). Early and locally advanced non-small-cell lung cancer (NSCLCESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 24: vi89–vi98. https://doi.org/10.1093/annonc/mdt241 [Google Scholar] [PubMed] [CrossRef]
Vokes E (2000). Lung cancer. The Lancet 355: 479–485. https://doi.org/10.1016/S0140-6736(00)82038-3 [Google Scholar] [PubMed] [CrossRef]
Wilusz JE, Sunwoo H, Spector DL (2009). Long noncoding RNAs: Functional surprises from the RNA world. Genes & Development 23: 1494–1504. https://doi.org/10.1101/gad.1800909 [Google Scholar] [PubMed] [CrossRef]
Yan X, Hu Z, Feng Y, Hu X, Yuan J et al. (2015). Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell 28: 529–540. https://doi.org/10.1016/j.ccell.2015.09.006 [Google Scholar] [PubMed] [CrossRef]
Yuan T, Huang X, Woodcock M, Du M, Dittmar R et al. (2016). Plasma extracellular RNA profiles in healthy and cancer patients. Scientific Reports 6: 1–11. https://doi.org/10.1038/srep19413 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools