 Open Access
Open Access
ARTICLE
Revealing the Roles of the SH3GLB1-Hydrogen Peroxide Axis in Glioblastoma Multiforme Cells
1 Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, 70101, Taiwan
2 National Institute of Cancer Research, National Health Research Institutes, Tainan, 70456, Taiwan
3 Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, 70101, Taiwan
4 Department of Chinese Medicine, E-Da Dachang Hospital, Kaohsiung, 80706, Taiwan
5 The School of Chinese Medicine for Post-Baccalaureate, I-Shou University, Kaohsiung, 82445, Taiwan
6 Department of Chinese Medicine, E-Da Hospital, Kaohsiung, 82445, Taiwan
7 Department of Surgery, E-Da Cancer Hospital, Kaohsiung, 82445, Taiwan
8 School of Medicine, I-Shou University, Kaohsiung, 82445, Taiwan
* Corresponding Author: Chia-Hung Chien. Email:
# These authors contributed equally to this work
Oncology Research 2026, 34(2), 15 https://doi.org/10.32604/or.2025.071258
Received 03 August 2025; Accepted 12 November 2025; Issue published 19 January 2026
Abstract
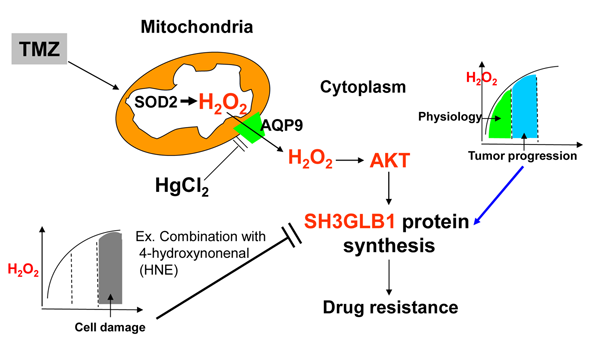
Objectives: Glioblastoma (GBM) is a prevalent malignant brain tumor prone to drug resistance. We previously found a strong correlation between SH3 domain GRB2-like endophilin B1 (SH3GLB1) and superoxide dismutase 2 (SOD2), which converts O2 to hydrogen peroxide (H2O2). Prior studies show that H2O2 redox signaling is vital for physiological processes and can drive tumor progression. Therefore, we aim to define how H2O2 signaling regulates SH3GLB1 and AKT (protein kinase B) pathways in GBM and to assess whether modulating H2O2 reverses temozolomide (TMZ) resistance. Methods: We used cultured cells and pharmacological inhibitors and activators to confirm the significance of H2O2 signaling. GBM cells were used to verify the role of H2O2 signaling in cell state transitions and animal experiments identified optimal treatment strategies. Results: We found that SOD2 acts as an upstream regulator of SH3GLB1. When SOD inhibitors and TMZ were combined, cells showed reduced SH3GLB1 and autophagy levels. SH3GLB1 was found to be regulated by H2O2 via AKT signaling using redox homeostasis-regulating experiments. Although treatment-induced changes in mitochondrial H2O2 levels mirrored those in the cytosol, parental and resistant cells exhibited divergent fates, highlighting cell-fate plasticity. TMZ combined with a redox modulator reduced resistant tumor cell growth (about 2/3 reduction of tumor size; p < 0.05) and suppressed SH3GLB1 and autophagy levels in animal models. The TMZ-induced increase in SH3GLB1 expression was reversed by HgCl2, which inhibited the aquaporin-9/AKT signaling. Conclusion: Overall, these findings underscore the importance of H2O2-SH3GLB1 signaling in GBM and may inform future therapeutic strategies for overcoming TMZ resistance.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools