 Open Access
Open Access
ARTICLE
The Physiological and Molecular Responses of Exogenous Selenium to Selenium Content and Fruit Quality in Walnut
1
Xinyang Agriculture and Forestry University, Xinyang, 464000, China
2
Tibet Plateau Walnut Industry Research Institute, Yangtze University, College of Horticulture and Gardening, Jingzhou, 434025,
China
3
Jingzhou Institute of Technology, Jingzhou, 434025, China
* Corresponding Author: Dejian Zhang. Email:
(This article belongs to the Special Issue: Integrating Agronomy and Plant Physiology for Improving Crop Production)
Phyton-International Journal of Experimental Botany 2023, 92(3), 851-860. https://doi.org/10.32604/phyton.2022.025147
Received 24 June 2022; Accepted 01 September 2022; Issue published 29 November 2022
Abstract
To study the effect of exogenous selenium on fruit quality in walnut (Juglans regia L.), 8-year-old walnut (Qingxiang) was taken as the research object. In the fruit expansion stage, 300 mg/L of sodium selenate, yeast selenium and sodium selenite solutions were applied on the leaf of walnut, and the selenium levels in leaves, pericarp and kernel were determined at the ripening stage. The fruit quality, mineral nutrient content, antioxidant enzyme activity, and related genes’ expression were analyzed. The results showed that the three exogenous selenium increased the selenium levels in leaves, pericarp and kernel of walnut. They also significantly increased fruit and kernel weights, and kernel linoleic acid, but markedly decreased kernel crude fat and saturated fatty acid. Selenium spraying promoted the absorption of mineral nutrients such as potassium and zinc, but inhibited the absorption of calcium, and had no significant effect on iron and magnesium in the kernel. Three exogenous selenium increased the activities of superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT) significantly in the kernel. Except for sodium selenate treatment significantly reduced malondialdehyde (MDA) content in the kernel, the other two selenium sources treatments had no significant effect on MDA and hydrogen peroxide (H2O2) levels. They also increased the expression of JrCu/Zn-SOD, Jr2-Cys POD, JrCyt-APX and JrCAT in kernel, to different extents. These implies that, in the walnut fruit enlargement period, the foliar spraying selenium could increase the selenium content of walnut, affect the mineral nutrient absorption, improve the antioxidant capacity and related genes’ expression, and reduce the degree of peroxide, and then improve the quality of fruit. Furthermore, yeast selenium showed the comprehensive effect of the best.Keywords
As a trace element in the human diet, Selenium affects all stages of human growth and development [1]. Selenium intake could enhance the ability of oxidation resistance in the humans and prevent some diseases, including hepatopathy, tumor, cardiovascular and Kaschin-Beck disease [2,3]. Selenium as an antidote to heavy metals, it detoxifies arsenic, lead, cadmium and mercury in the human body, and also can combine with these heavy metals to form compounds that be excreted from the body [4]. In addition, selenium can prevent and treat diseases, regulate human immunity, and inhibit the occurrence of tumors and cardiovascular diseases [2]. It has various biological effects on the human body, such as delaying aging [3]. As a protective agent of life, selenium cannot be made by people themselves and must be obtained from food [5]. Therefore, how to supplement selenium safely and effectively becomes an urgent problem to be solved. There are mainly 2 forms of inorganic selenium and organic selenium in nature. Inorganic selenium is more toxic, and organic selenium can be safely absorbed by the human body [6]. In consequence, the conversion of inorganic selenium into organic selenium for human absorption and utilization has become an essential way of human safety selenium supplement. In the aspect of selenium transformation, plants have significant advantages because of their high selenium conversion rate and rich organic selenium content. Especially as human food sources, crops can be used as a simple and effective carrier of human safe selenium supplementation [7].
In recent years, with the improvement of national economic strength and people’s living standard, the selenium-rich industry has received extensive attention from all walks of life. Australia, Denmark, the United States, Sweden, Germany and other countries have developed a variety of selenium-containing health products, selenium-rich agricultural products [8]. In China, Selenium-rich oil-tea, selenium-rich rice, selenium-rich potato, selenium-rich wheat, selenium-rich tea, selenium-rich edible fungus and other products have also been marketed and enjoyed by a vast number of consumers [9]. However, selenium-rich products and related research in the fruit field are still lacking. Therefore, it is particularly urgent to study the cultivation technology and regulation of selenium-rich fruit, explain the related mechanism, process selenium-rich fruit and research and develop high-value selenium-rich functional products.
The walnut, an important dried and oil fruit tree, is the most economically important member of the Juglandaceae family [10–13]. With the improvement of people’s consumption level, healthy walnut fruits are paid more and more attention by the market, such as selenium-rich pecan kernel. Selenium-enriched walnut cultivation can not only improve the selenium level in fruits, but also improve the fruit quality [14]. Therefore, this study took 8-year-old walnut (Qingxiang) as the research object, and studied the effects of three different selenium sources on the selenium content in leaves, pericarp and kernel by spraying during the fruit expansion period. The results of three selenium sources on fruit quality, mineral nutrient content and antioxidant enzyme activity were analyzed. Hence, this study aimed to investigate the physiological responses of exogenous selenium to selenium content and fruit quality in walnut. Thus, it may set a new perspective and provide a firm theoretical basis for future about selenium-fertilizer application in walnut production.
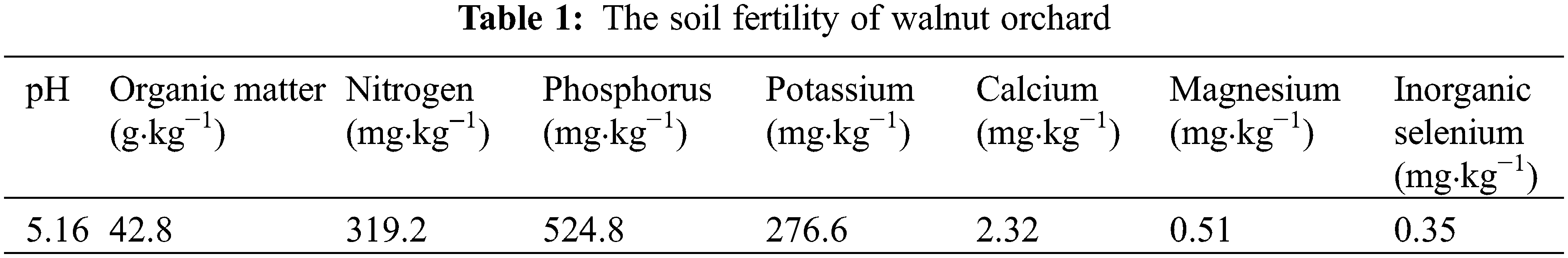
The 8-year-old walnut (Qingxiang) were provided by walnut demonstration orchard of Hubei Academy of Forestry, China. The soil fertility data were in Table 1.
Purified water, sodium selenate (Na2SeO4, 300 mg/L), yeast selenium (300 mg/L) and sodium Selenite (Na2SeO3, 300 mg/L) were sprayed on the leaf surface of walnut at its fruit enlargement period (May 20th, 2021). Na2SeO4, yeast selenium and Na2SeO3 were purchased from Sigma Co., Ltd., Vienna, Austria. Each treatment was arranged in a complete randomized block design and replicated 8 times, produced a total of 32 walnut trees. Spray once a week with 4 times from 05/20/2021, each tree spray 3 L solution.
Samples (leaves and fruits) were collected on September 20, 2021 (fruit ripening stage), while fruits were divided into pericarp and kernel. The leaf samples were selected from the top to bottom fourth leaf of the fruiting branch in the middle of peripheral branches of the tree crown, and the fruit samples were selected from the fruits in the middle of the tree crown with normal development and no diseases and insect pests. The collected samples were immediately placed in dry ice when washed with deionized water, brought back to the laboratory within 6 h, washed with deionized water, and stored in ultra cold storage freezer (−80°C) for later use.
Selenium contents in leaves, pericarp and kernel were determined by hydride-generation atomic fluorescence spectrometry (HG-AFS) (AFS8510; Beijing Haiguang Instrument Co., Beijing, China) according to Gui et al. [1]. Fruit and kernel weights were weighed by electronic balance. The rate of crude, linoleic, unsaturated fatty acid and saturated fatty acid in the kernel were determined as described by Velazquez et al. [15]. The concentrations of zinc (Zn), iron (Zn), potassium (K), calcium (Ca) and magnesium (Mg) in the kernel were measured by the inductively complied plasma-atomic emission spectrometry based on the protocol of Sun et al. [16].
Kernel samples (0.2 g) from 4 treatments were homogenized in 5 mL of 0.1 M phosphate buffer (pH 7.8) and centrifuged at 4,000 × g for 10 min at 4°C. And the supernatant was used to assay the concentrations of MDA and H2O2, and the activities of SOD, POD, APX and CAT, according to Liu et al. [17].
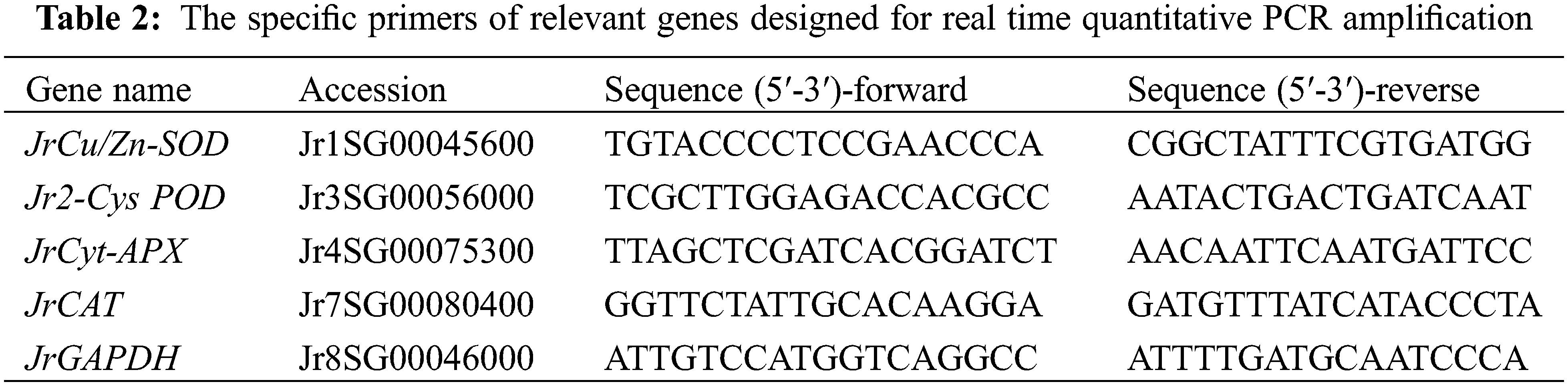
The kernel RNA was extracted in 0.1 g fresh sample using the RNA prep pure plant kit (DP441, TIANGEN Biotech Co., Ltd., China), and reverse transcription was done with the PrimescriptTM RT reagent kit with the gDNA Eraser (RR047A, TaKaRa Bio Inc., Japan). All the steps followed the manufacturer’s instructions. The specific primers (Table 2) of relevant genes for qRT-PCR analysis were designed using Primer Premier 5.0 software (Palo Alto, USA), according to the Walnut genome database (http://aegilops.wheat.ucdavis.edu/Walnut/data.php). A housekeeping gene, JrGAPDH, was magnifified as an endogenous control along with the target genes. The relative expression of genes was calculated by the 2−ΔΔCt method.
SAS software (9.1.3) was used to analyze the difference significance of data by Duncan’s new complex range method (P ≤ 0.05). The data were analyzed using one-way Anova. When F tests were significant, means were separated by Duncan’s new complex range method (P ≤ 0.05).
3.1 The Selenium Content in Walnut Leaves, Pericarp and Kernel
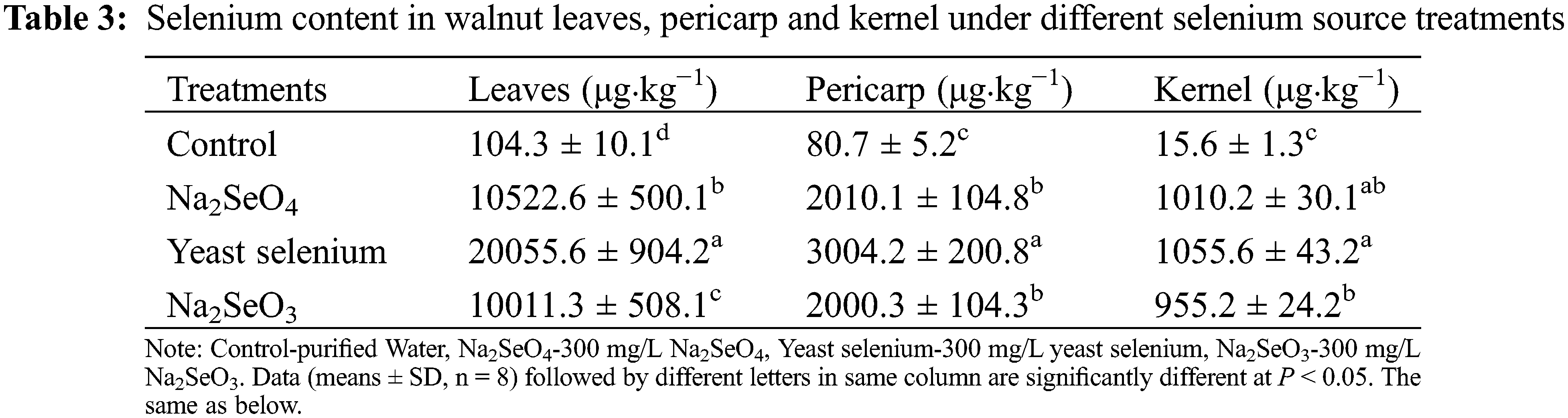
As shown in Table 3, three selenium sources improved the selenium content in varying degrees in leaves, pericarp and kernel of walnut. Compared with the water control group, yeast selenium treatment significantly increased selenium content by 191, 37 and 67 times in leaves, pericarp and kernel (Table 3). Na2SeO4 and Na2SeO3 treatments also dramatically increased its levels by 100 and 95 times in leaves, 24 and 23 times in pericarp, 64 and 60 times in kernel, respectively (Table 3).
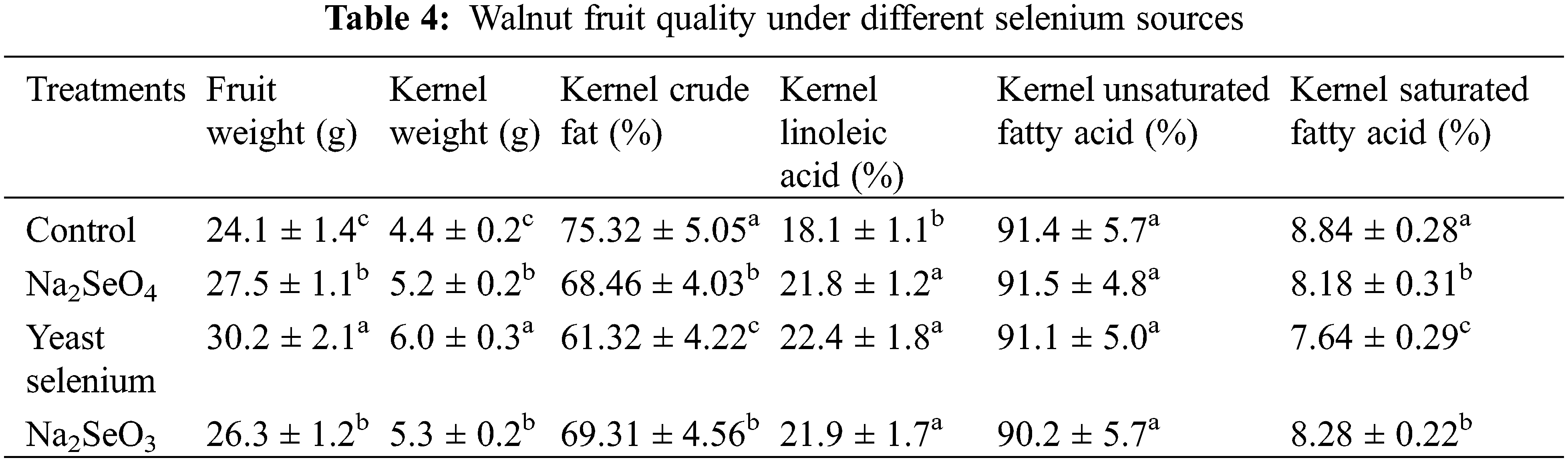
Fruit and kernel weights, the rate of crude, linoleic, unsaturated fatty acid and saturated fatty acid in the kernel were shown in Table 4. Na2SeO4, yeast selenium and Na2SeO3 notably increased the weight of fruit and kernel by 14.1%, 25.3%, 9.1%, and 18.2%, 36.4%, 20.5%, respectively, compared to the water control (Table 4). With regards to the quality of kernel, Na2SeO4, yeast selenium and Na2SeO3 dramatically decreased the kernel crude fat and kernel saturated fatty acid by 9.1%, 18.6%, 8.0%, and 7.5%, 13.6%, 6.3%, respectively, compared to the water control (Table 4). However, three exogenous seleniums markedly increased the rate of kernel linoleic acid at the same level. It is worth noting that there were no significant differences in the rate of kernel unsaturated fatty acid among all treatments.
3.3 The Mineral Nutrient Content in the Kernel
Table 5 shows the results of mineral nutrient contents in the kernel treated by three selenium sources. As can be seen from Table 3, the three selenium source treatments increased the contents of Zn and K in the kernel to varying degrees, but significantly reduced the level of Ca, while having no significant effect on Fe and Mg. Compared with water control, Na2SeO4, yeast selenium and Na2SeO3 treatments increased Zn and K content by 13.7%, 22.4% and 12.5%, 30.5%, 50.2% and 30.5%, respectively (Table 5). In the case of Zn, the yeast selenium was the only treatment significantly higher than the control. However, the Ca content in the kernel was reduced by 17.2%, 17.6% and 17.6%, respectively (Table 5).
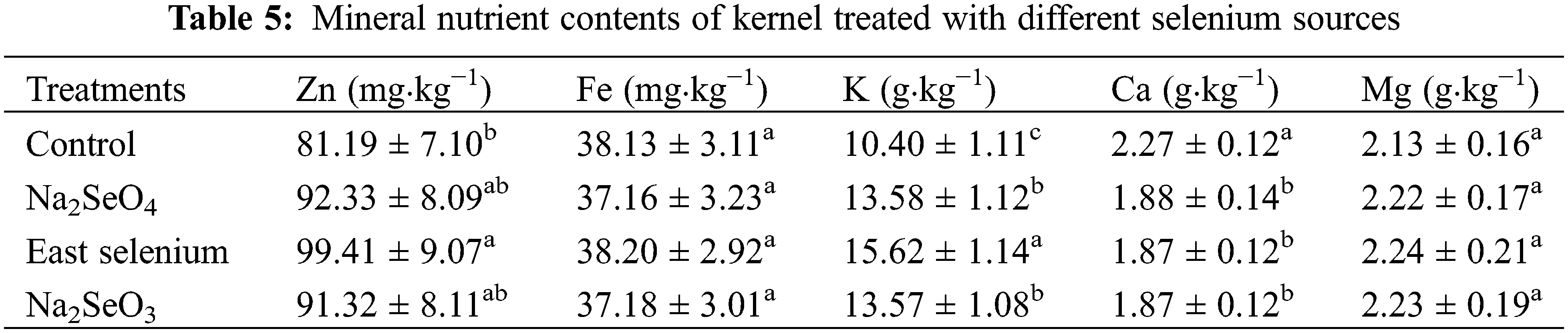
3.4 The Concatenation of MDA and the Activities of SOD, POD and APX in the Kernel
Table 6 shows the results of the activities of POD, SOD APX and CAT, and the contents of MDA and H2O2 in the kernel of walnut treated with three selenium sources. Compared to the appropriate control, Na2SeO4, yeast selenium and Na2SeO3 notably increased the activity of SOD, POD, APX and CAT by 28.9%, 76.3%, 28.1%, and 60.8%, 129.4%, 86.3%, and 9.7%, 24.5%, 17.8%, and 14.3%, 24.5%, and 12.9%, respectively (Table 6). However, yeast selenium and Na2SeO3 had no significant effects on MDA and H2O2 contents, while Na2SeO4 decreased the level of MDA by 26.9%, compared to the water control (Table 6). It is worth noting that there were no significant differences among the three treatments and the control for H2O2 (Table 6).
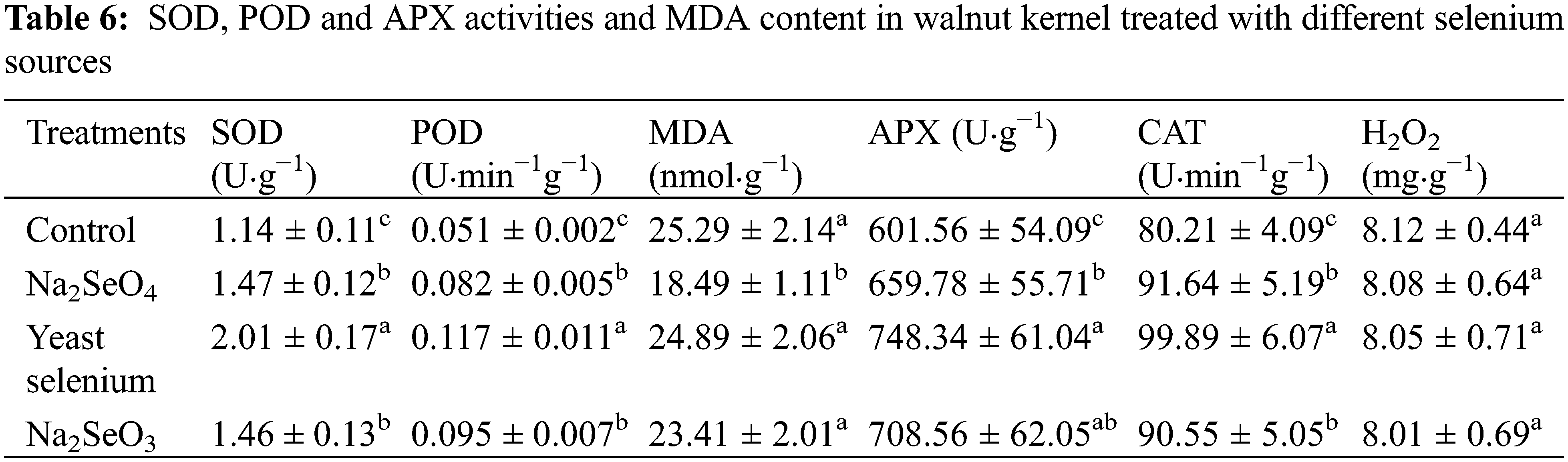
3.5 Relative Expression of Antioxidant System Responsive Genes
Fig. 1 shows the relative expression of antioxidant system responsive genes. Compared to the control, Na2SeO4, yeast selenium and Na2SeO3 notably increased the expression of JrCu/Zn-SOD, Jr2-Cys POD, JrCyt-APX and JrCAT by 50.2%, 156.3% and 55.8%, 80.2%, 159.3% and 62.7%, 54.5%, 127.8% and 61.3%, 114.5%, 168.2%, and 68.9% respectively. Furthermore, there has no significant change of the expression of JrCu/Zn-SOD, Jr2-Cys POD, JrCyt-APX and JrCAT in between Na2SeO4 and Na2SeO3 treatments (Fig. 1). Interestingly, yeast selenium showed the positive effect of the best of this four genes’ expression in this study (Fig. 1).
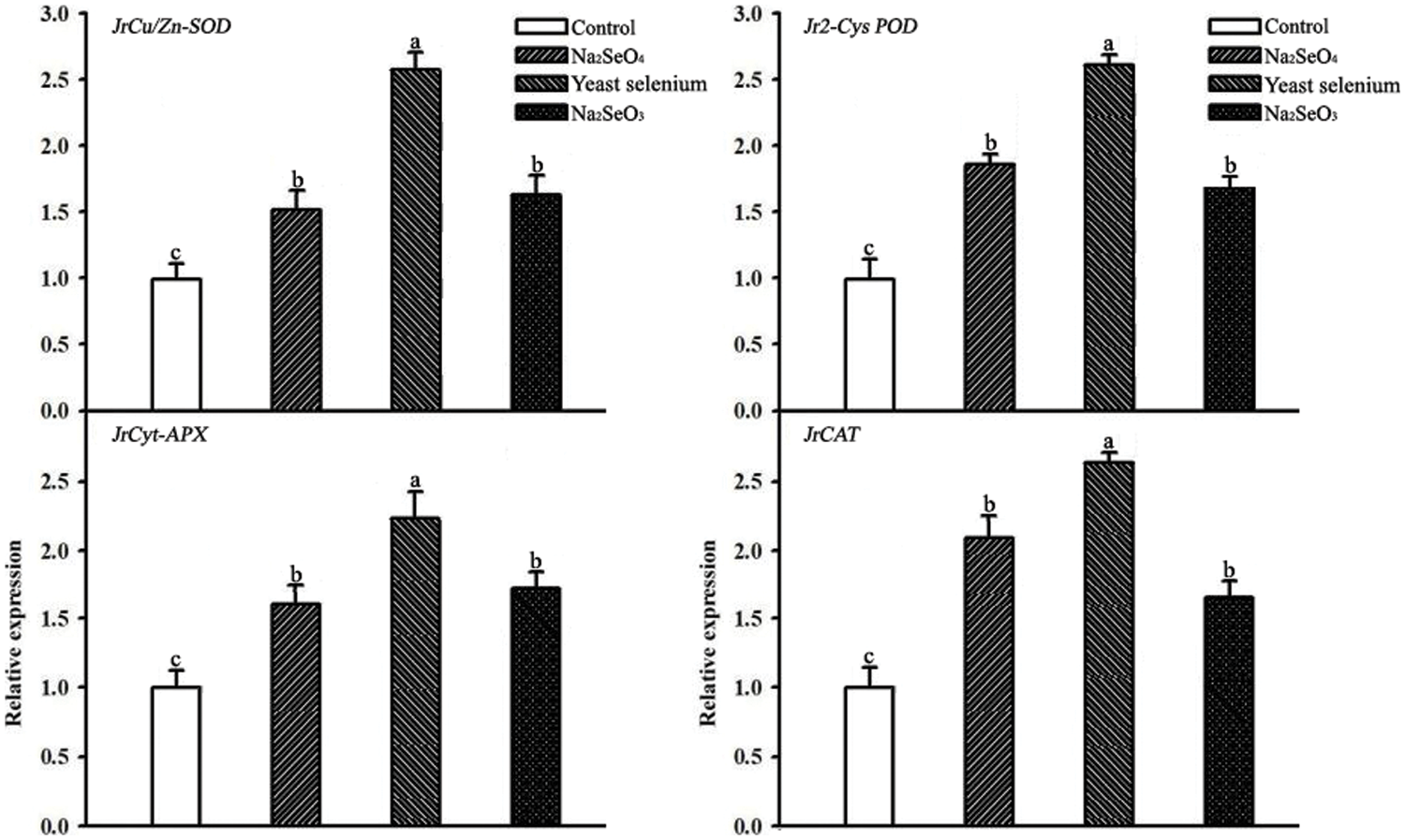
Figure 1: Effects of three selenium sources on relative expression of JrCu/Zn-SOD, Jr2-Cys POD, JrCyt-APX and JrCAT in walnut kernel
Compared with soil application of selenium fertilizer, foliar spraying of selenium can reduce selenium loss and effectively increase organic selenium content in plants, which is also a common method to increase organic selenium content in fruits or leaves in horticultural crop cultivation [18]. The suitable period and species of selenium spraying on leaves of different horticultural crops are different. For example, it is the best time to apply selenium fertilizer to grape and strawberry at the beginning of fruit expansion [19,20]. Previous study showed that spraying selenate or selenite during the blueberry fruit expansion could effectively increase the selenium content and the anthocyanin concentration in fruit [21]. Therefore, in this study, three selenium sources were sprayed on the leaves during the walnut fruit expansion stage to study the effects of different selenium sources on walnut selenium content and fruit quality.
Spraying sodium selenite solution not only increased the selenium content of lettuce leaves, but also increased the transpiration rate, the net photosynthetic rate, and the stomatal conductance of leaves, improved photosynthesis and antioxidant capacity, and promoted its growth [22]. Similar study [23] on passion fruit has demonstrated that selenium can improve the photosynthesis, thus promoting the accumulation of sugar, and has a significant effect on the increase of soluble solid content, which can further improve the fruit weight, which is consistent with this study. The study of selenium application on cotton showed that selenium mineral fertilizer could advance cotton boll opening period, improve the cotton nutrient level, and promote boll growth and development [24]. The application of bio-selenium in the tomato cultivation process can obtain higher organic selenium content in the fruit, and improve its fruit quality significantly [25]. Similar results were observed in Broccoli Florets [26,27], where there was induced selenium level upon yeast selenium addition. This study showed that spraying sodium selenate, yeast selenium and sodium selenite at the expansion stage of walnut fruit could increase the selenium content in its leaves, pericarp and kernel, and yeast selenium had the best effect, which could be widely used in the cultivation and production practice of selenium-rich walnut.
Exogenous selenium fertilizer not only increased the selenium content in plants, but also affected the development and growth in plants, and then affected the quality of fruit. Studies showed that spraying sodium selenite on leaves could significantly increase grape fruit quality (the single grain weight, grain length, Vc content, selenium content, etc.) and delay fruit and leaf senescence [28]. Selenium rich liquid fertilizer significantly increased the content of soluble solids, fruit weight, less skin lesions, better luster, and 7 days earlier the ripening stage of peach [29]. Zahedi et al. [30] demonstrated that foliar spraying of bio-nano selenium on a pomegranate could increase the soluble solid content of fruits, and its freshness and shelf life could be extended for 15 days. The results of this study were consistent with previous studies. three selenium sources all improved walnut fruit quality to varying degrees. In this study, fruit and kernel weights and the rate of kernel linoleic acid were increased, while the rates of kernel crude fat and kernel saturated fatty acid were decreased. Linoleic acid could reduce the risk of cardiovascular disease, as reflected by current dietary recommendations, while saturated fatty acid affects cholesterol metabolism [31,32]. So, exogenous selenium could improve walnut fruit’s health value. However, the kernel crude fat was decreased by three selenium treated, which is necessary to maintain normal metabolism. Relevant mechanisms of it need to be further explored.
With regards to the effects of selenium on the uptake of mineral nutrients in plants, the results of this research showed that the foliar spraying of selenium promoted the uptake of Zn and K in kernel fruits, inhibited the uptake of Ca, but had no significant effect on the content of Fe and Mg. The results of study on selenium application in watermelon showed that spraying selenium at a lower concentration could promote the absorption of Ca and Mg, while spraying selenium at a higher concentration could inhibit the absorption of Ca and Mg [33]. Related mechanisms and the relationship between selenium and mineral nutrient absorption remain to be further studied and discussed.
SOD, POD and APX are scavengers of reactive oxygen species (ROS) in plants, which can remove excessive ROS in plant cells in the process of stress or aging process [34]. H2O2 is one of the main components of ROS [34]. They can maintain the balance of cell metabolism, and improve the stress resistance of plants, which is related to the antioxidant capacity of plants [34,35]. MDA is one of the important products of membrane lipid peroxidation, and the higher content along with the stronger peroxidation degree [36]. In this study, the activities of SOD, POD and APX in walnut kernel treated with selenium spraying were significantly higher than those treated with clean water. At the same time, the contents of MDA and H2O2 were lower than that treated with clean water to varying degrees. JrCu/Zn-SOD, Jr2-Cys POD, JrCyt-APX and JrCAT are regulate the plant antioxidant system [34]. At present study, exogenous selenium induced these genes’ expression resulting in strengthen the activities of SOD, POD, APX and CAT in walnut kernel. It indicates that exogenous selenium can improve the antioxidant capacity of plants and protect the integrity of cell membrane by removing MDA and H2O2. This is consistent with the study in strawberry that selenium can not only protect the integrity of cell membrane, but also reduce the content of heavy metal ions, and effectively inhibit the absorption of heavy metal cadmium and lead in leaves and fruits [37]. In addition, among the three selenium source treatments in this study, yeast selenium had the strongest effect on improving the activities of SOD, POD, APX and CAT. So, yeast selenium can be applied to improve the antioxidant capacity of walnut.
In a word, in the walnut fruit enlargement period, spraying three kinds of selenium fertilizer (sodium selenate, yeast selenium and sodium selenite) on the leaves can increase the selenium content of leaf and fruit, thus affecting the absorption of mineral nutrient, improve the antioxidant capacity and related genes’ expression of the fruit, reduce the degree of peroxide, then improve the quality of walnut fruit. Among them, foliar spraying with yeast selenium had the best comprehensive effect. So it could be widely used in the cultivation of selenium-rich walnut. Future studies should pay more attention to the effect and mechanism of selenium on walnut quality.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: Yuan L. Y., Zhang D. J.; data collection: Sun M. F., Hui X. R.; analysis and interpretation of results: Tong C. L.; draft manuscript preparation: Sun M. F. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the Science and Technology Planning Project of Shannan Science and Technology Bureau of Tibet (2021Z21008) and the Hubei Province ‘14th Five-Year’ Major Science and Technology Aid Tibet Project (SCXX-XZCG-22016).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Gui, J. Y., Rao, S., Gou, Y. Y., Xu, F., Cheng, S. Y. (2022). Comparative study of the effects of selenium yeast and sodium selenite on selenium content and nutrient quality in broccoli florets (Brassica oleracea L. var. italica). Journal of the Science of Food and Agriculture, 102(4), 1707–1718. DOI 10.1002/jsfa.11511. [Google Scholar] [CrossRef]
2. Vinceti, M., Filippini, T., Wise, L. A. (2018). Environmental selenium and human health: An update. Current Environmental Health Reports, 5(4), 464–485. DOI 10.1007/s40572-018-0213-0. [Google Scholar] [CrossRef]
3. Wang, N., Tan, H. Y., Li, S., Xu, Y., Guo, W. et al. (2017). Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxidative Medicine and Cellular Longevity, 2017, 7478523. DOI 10.1155/2017/7478523. [Google Scholar] [CrossRef]
4. Farooq, M. U., Tang, Z. C., Zeng, R., Liang, Y. K., Zhang, Y. J. et al. (2019). Accumulation mobilization and transformation of selenium in rice grain provided with foliar sodium selenite. Journal of the Science of Food and Agriculture, 99(6), 2892–2900. DOI 10.1002/jsfa.9502. [Google Scholar] [CrossRef]
5. Emma, B., John, H., Bruce, S., John, K., Nicole, R. (2014). Selenium-enriched foods are more effective at increasing glutathione peroxidase activity compared with selenomethionine: A meta-analysis. Nutrients, 6(10), 4002–4031. DOI 10.3390/nu6104002. [Google Scholar] [CrossRef]
6. Zhou, X., Yang, J., Kronzucker, H. J., Shi, W. (2020). Selenium biofortification and interaction with other elements in plants: A review. Frontiers in Plant Science, 11, 586421. DOI 10.3389/fpls.2020.586421. [Google Scholar] [CrossRef]
7. Winkel, L. H., Vriens, B., Jones, G. D., Schneider, L. S., Pilon-Smits, E. et al. (2015). Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients, 7(6), 4199–4239. DOI 10.3390/nu7064199. [Google Scholar] [CrossRef]
8. Judit, E. S., Regina, M. K., Evana, A., Samar, K. H., Albert, V. et al. (2019). Food as medicine: Selenium enriched lentils offer relief against chronic arsenic poisoning in Bangladesh. Environmental Research, 122(7), 176–182. DOI 10.1016/j.envres.2019.108561. [Google Scholar] [CrossRef]
9. Zhang, L., Guo, Y., Liang, K., Hu, Z., Lu, B. (2020). Determination of selenium in common and selenium-rich rice from different areas in China and assessment of their dietary intake. International Journal of Environmental Research and Public Health, 17(12), 4596. DOI 10.3390/ijerph17124596. [Google Scholar] [CrossRef]
10. Milatovi, D., Nikoli, D., Jankovi, S., Jankovi, D., Stankovi, J. (2020). Morphological characteristics of male reproductive organs in some walnut (Juglans regia L.) genotypes. Scientia Horticulturae, 272(11), 109587. DOI 10.1016/j.scienta.2020.109587. [Google Scholar] [CrossRef]
11. Keshavarz, K., Vahdati, K., Samar, M., Azadegan, B., Brown, P. (2011). Foliar application of zinc and boron improves walnut vegetative and reproductive growth. HortTechnology, 21(2), 181–186. DOI 10.21273/HORTTECH.21.2.181. [Google Scholar] [CrossRef]
12. Sarikhani, S., Vahdati, K., Ligterink, W. (2021). Biochemical properties of superior Persian walnut genotypes originated from southwest of Iran. International Journal of Horticultural Science and Technology, 8(1), 13–24. DOI 10.22059/ijhst.2020.309363.392. [Google Scholar] [CrossRef]
13. Habibi, A., Yazdani, N., Chatrabnous, N., Saba, M. K., Vahdati, K. (2021). Inhibition of browning via aqueous gel solution of Aloe vera: A new method for preserving fresh fruits as a case study on fresh kernels of Persian walnut. Journal of Food Science and Technology, 59(7), 2784–2793. DOI 10.1007/s13197-021-05301-3. [Google Scholar] [CrossRef]
14. Ozrenk, K., Javidipour, I., Yarilgac, T., Balta, F., Gundogdu, M. (2012). Fatty acids, tocopherols, selenium and total carotene of pistachios (P. vera L.) from Diyarbakir (Southeastern Turkey) and walnuts (J. regia L.) from Erzincan (Eastern Turkey). Food Science and Technology International, 18(1), 55–62. DOI 10.1177/1082013211414174. [Google Scholar] [CrossRef]
15. Velazquez, J., Esparza-Rivera, J. R., Minjares-Fuentes, J. R., Luna, E., Sierra-Campos, E. (2021). Nutritional quality, fatty acids content and antioxidant capacity of nut fruits from criollo and improved walnut varieties. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 49(2), 12021. DOI 10.15835/nbha49212021. [Google Scholar] [CrossRef]
16. Sun, M. F., Yuan, D., Hu, X. C., Zhang, D. J., Li, Y. Y. (2020). Effects of mycorrhizal fungi on plant growth, nutrient absorption and phytohormones levels in tea under shading condition. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 48(4), 2006–2020. DOI 10.15835/nbha48412082. [Google Scholar] [CrossRef]
17. Liu, C. Y., Wang, Y. J., Wu, Q. S., Yang, T. Y., Kuča, K. (2020). Arbuscular mycorrhizal fungi improve the antioxidant capacity of tea (Camellia sinensis) seedlings under drought stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 48(4), 1993–2005. DOI 10.15835/nbha48412066. [Google Scholar] [CrossRef]
18. Azizi, I., Esmaielpour, B., Fatemi, H. (2020). Effect of foliar application of selenium on morphological and physiological indices of savory (Satureja hortensis) under cadmium stress. Food Science and Nutrition, 8(12), 6539–6549. DOI 10.1002/fsn3.1943. [Google Scholar] [CrossRef]
19. Gaglio, R., Pescuma, M., Albarrán, Y. M., Franciosi, E., Settanni, L. (2021). Selenium bio-enrichment of mediterranean fruit juices through lactic acid fermentation. International Journal of Food Microbiology, 354, 109248. DOI 10.1016/j.ijfoodmicro.2021.109248. [Google Scholar] [CrossRef]
20. Antoniou, O., Chrysargyris, A., Xylia, P., Tzortzakis, N. (2021). Effects of selenium and/or arbuscular mycorrhizal fungal inoculation on strawberry grown in hydroponic trial. Agronomy, 11(4), 721. DOI 10.3390/agronomy11040721. [Google Scholar] [CrossRef]
21. Li, M., Zhao, Z., Zhou, J., Zhou, D., Chen, B. et al. (2018). Effects of foliar spray of selenite or selenate at different growth stages on selenium distribution and quality of blueberries. Journal of the Science of Food and Agriculture, 98(12), 4700–4706. DOI 10.1002/jsfa.9004. [Google Scholar] [CrossRef]
22. Brazaitytė, A., Miliauskienė, J., Vaštakaitė-Kairienė, V., Sutulienė, R., Laužikė, K. (2021). The response of baby leaf lettuce to selenium biofortification under different lighting conditions. Biology and Life Sciences Forum, 3, 10. DOI 10.3390/IECAG2021-10010. [Google Scholar] [CrossRef]
23. Huang, W., Shen, M., Liu, Z., Zhang, Y., Wang, D. (2020). Effects of different application methods and application rates of biological nano-selenium on the quality of passion fruit. Medicinal Plant, 11(3), 72–73. [Google Scholar]
24. Saleem, M. F., Kamal, M. A., Shahid, M., Saleem, A., Shakeel, A. et al. (2020). Exogenous selenium-instigated physiochemical transformations impart terminal heat tolerance in Bt cotton. Journal of Soil Science and Plant Nutrition, 20(1), 274–283. DOI 10.1007/s42729-019-00139-3. [Google Scholar] [CrossRef]
25. Alves, L. R., Prado, E. R., Oliveira, R. D., Santos, E. F., Grato, P. L. et al. (2020). Mechanisms of cadmium-stress avoidance by selenium in tomato plants. Ecotoxicology, 29(2), 594–606. DOI 10.1007/s10646-020-02208-1. [Google Scholar] [CrossRef]
26. Rachmawati, Y. S., Hidayat, C., Supriadin, A., Imbarwati, S. (2021). Utilization of selenium in baglog waste to increase antioxidant and storability of Broccoli. IOP Conference Series: Materials Science and Engineering, 1098(5), 052079. DOI 10.1088/1757-899X/1098/5/052079. [Google Scholar] [CrossRef]
27. Rao, S., Gou, Y., Yu, T., Cong, X., Xu, F. (2021). Effects of selenate on Se, flavonoid, and glucosinolate in Broccoli Florets by combined transcriptome and metabolome analyses. Food Research International, 146, 110463. DOI 10.1016/j.foodres.2021.110463. [Google Scholar] [CrossRef]
28. Chen, H., Liu, L., Lin, L., Liao, M., Lv, X. (2019). Effects of different selenium concentrations on photosynthetic physiology of grape seedlings. IOP Conference Series: Earth and Environmental Science, 310(4), 042062. DOI 10.1088/1755-1315/310/4/042062. [Google Scholar] [CrossRef]
29. Zhang, H. Y., Han, T., Tian, L., Wang, Y. N., Jia, H. J. (2010). Accumulation of Se in peach, jujube and strawberry after spraying Sefertilizer on leaves. Journal of Fruit Science, 62(5), 547–566. DOI 10.13925/j.cnki.gsxb. [Google Scholar] [CrossRef]
30. Zahedi, S. M., Hosseini, M. S., Meybodi, N., Silva, J. (2019). Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. South African Journal of Botany, 124, 350–358. DOI 10.1016/j.sajb.2019.05.019. [Google Scholar] [CrossRef]
31. Harris, W. S. (2008). Linoleic acid and coronary heart disease. Prostaglandins, Leukotrienes and Essential Fatty Acids, 79(3–5), 169–171. DOI 10.1016/j.plefa.2008.09.005. [Google Scholar] [CrossRef]
32. Mensink, R. P. (2013). Fatty acids: Health effects of saturated fatty acid. In: Caballero, B. (Ed.Encyclopedia of human nutrition (Third Editionpp. 215–219. USA: Academic Press. DOI 10.1016/B978-0-12-375083-9.00101-X. [Google Scholar]
33. Du, S. P., Ma, Z. M., Xue, L. (2020). Effect of spraying with selenium fertilizer on yield, quality and nutrient absorption of watermelon in gravel-mulched field. Journal of Fruit Science, 37(5), 705–713. DOI 10.13925/j.cnki.gsxb.20190439. [Google Scholar] [CrossRef]
34. Swanson, S., Gilroy, S. (2010). ROS in plant development. Physiologia Plantarum, 138(4), 384–392. DOI 10.1111/j.1399-3054.2009.01313.x. [Google Scholar] [CrossRef]
35. Lanza, M., Reis, A. (2021). Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiology and Biochemistry, 164(6), 27–43. DOI 10.1016/j.plaphy.2021.04.026. [Google Scholar] [CrossRef]
36. Xu, P. L., Guo, Y. K., Bai, J. G., Shang, L., Wang, X. J. (2008). Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiologia Plantarum, 132(4), 467–478. DOI 10.1111/j.1399-3054.2007.01036.x. [Google Scholar] [CrossRef]
37. Huang, C., Qin, N., Sun, L., Yu, M., Hu, W. et al. (2018). Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. International Journal of Molecular Sciences, 19(7), 1913. DOI 10.3390/ijms19071913. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools