Sunitinib and Fenofibrate as Combination Therapy for MDR Glioblastoma: Insights from In Vitro and In Silico Studies
Saad Alobid1,#, Hussam Albassam1,#, Tebyan O. Mirgany2, Faris Almutairi1, Mohammed Mufadhe Alanazi1, Ahmed H. Bakheit2, Hanadi H. Asiri2, Eram Eltahir3, Gamaleldin I. Harisa3,*
1 Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
2 Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
3 Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia
* Corresponding Author: Gamaleldin I. Harisa. Email: 
# These authors contributed equally to this work
(This article belongs to the Special Issue: Pharmacological Bases of Anticancer Drug Therapies in Precision Oncology)
Oncology Research https://doi.org/10.32604/or.2025.073371
Received 17 September 2025; Accepted 19 November 2025; Published online 29 December 2025
Abstract
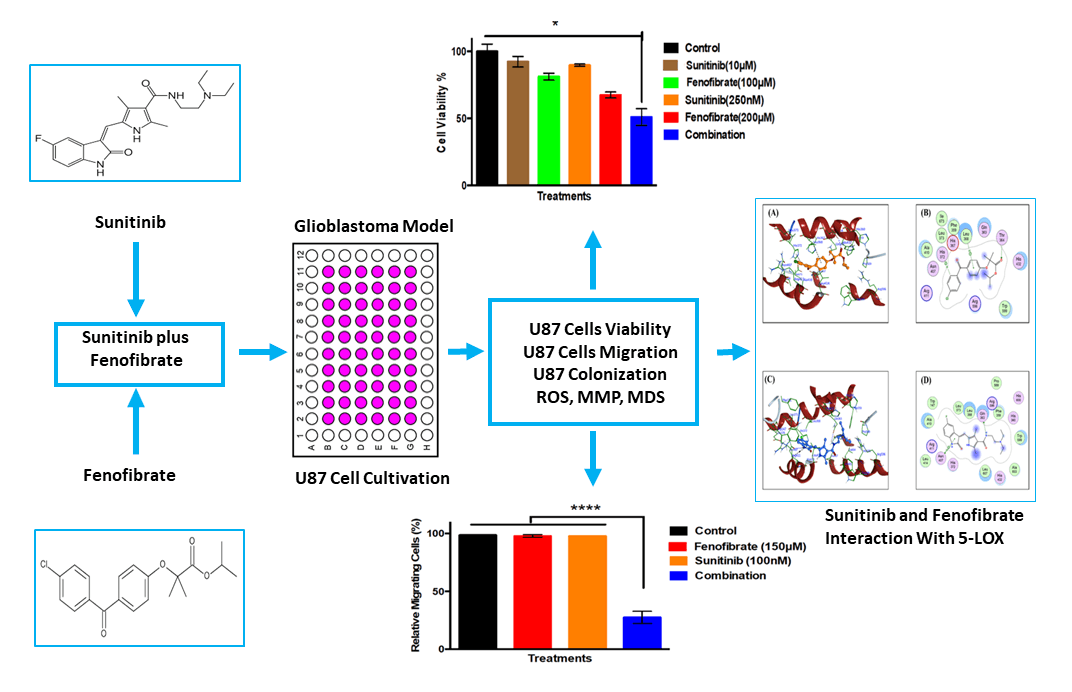
Objective: Glioblastoma (GB) therapy is challenged by tumor heterogeneity and multidrug resistance (MDR), highlighting the need for effective therapies. This study aimed to explore the combined anticancer effects of Sunitinib (SNB) and Fenofibrate (FEN) on U87 cells.
Methods: U87 cells were exposed to SNB, FEN, or their combination for 24 h, followed by evaluations of cell viability, migration, and clonogenic survival using MTT, scratch, and colony formation assays. Intracellular reactive oxygen species (ROS) were quantified via the 2
′, 7
′-dichlorofluorescein assay, while mitochondrial membrane potential (MMP) was assessed using JC-1 red/green fluorescence. Molecular docking was performed to investigate SNB and FEN interactions with multiple molecular targets, including topoisomerase II (TOP-II), c-Jun N-terminal kinase (JNK), histone deacetylase 2 (HDAC2), cyclooxygenase-2 (COX-2), matrix metalloproteinase-9 (MMP-9), cytochrome P450 3A4 (CYP3A4), glutathione peroxidase 4 (GPX4), glutathione S-transferase (GST), heme oxygenase-1 (HO-1), and 5-lipoxygenase (5-LOX).
Results: The results demonstrated that both SNB and FEN significantly reduced U87 cell viability, migration, and clonogenic potential, with the combination treatment exhibiting synergistic cytotoxicity. SNB alone markedly increased ROS levels, while FEN, individually or in combination, reduced oxidative stress. Although SNB diminished mitochondrial membrane potential, co-treatment with FEN restored MMP values close to control levels. Docking analyses revealed that SNB displayed strong affinities for TOP-II, JNK, and HDAC2, whereas FEN preferentially interacted with MMP-9, COX-2, CYP3A4, and GPX4, suggesting complementary mechanisms targeting oxidative stress, inflammation, and programmed cell death regulation.
Conclusion: The combination of SNB and FEN represents a promising multi-targeted therapeutic approach against GB. SNB and FEN combination capable of modulating and reprogramming key molecular pathways involved in GB progression and MDR.
Graphical Abstract
Keywords
Glioblastoma; drug repurposing; mitochondrial membrane potential; reactive oxygen species (ROS); topoisomerase II; matrix metalloproteinase-9; glutathione peroxidase 4
 Open Access
Open Access